Abstract
The contribution of immune cells in soft tissue sarcomas (STS) is not completely known and understanding their role is very essential for employing immunotherapy strategies. Here, we show that murine fibrosarcoma-conditioned medium promoted total spleen cell proliferation but inhibited T cell responses to mitogenic and allo-antigen-mediated stimulation. This increased proliferation was found to be in B cells resulting in generation of Breg further leading to Treg population. This was found to be the same in vitro and in vivo. The phenotype of these B cells was CD19+CD81+CD27+CD25+PD-L1hi and they secreted both IL-10 and TGF-β. These tumor evoked Bregs (tBreg), when co-cultured with B depleted T cells, suppressed their proliferation in response to anti-CD3/CD28 stimulation. tBreg-induced suppression of T cell responses was not abrogated by the inhibition or neutralization of IL-10 but by the small molecule inhibitor of TGFβ Receptor type I, SB431542. While SB531542 per se was not cytotoxic to tumor cells, administration of SB431542 in tumor-bearing mice (TBM) significantly reduced the tumor burden. In addition, the treatment significantly reduced Treg cells and rescued proliferation of T cells in response to mitogen and allo-antigen. Collectively, our results identify that tumor evoked Breg cells mediate T cell immune suppression through TGFβ-mediated pathway and that targeting the Breg–Treg axis can be potentially used as an immunotherapy agent.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02666-w) contains supplementary material, which is available to authorized users.
Keywords: Regulatory B cell, Breg–Treg axis, Immunosuppression, TGF-β signalling, SB431542, Fibrosarcoma
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of malignancies, comprising 1% of all adult cancers and arise from mesenchymal origin, mostly in connective and skeletal tissues with over 100 distinct histological subtypes [1] and [2]. STS can occur in any part of the body [2] mostly due to unknown aetiology and although metastasis is rarely seen in the initial stages, lung metastasis is often associated with the later stages of cancer [3]. Depending on the stage of cancer and the histological subtype, about 50% of the patients develop recurrence or metastasis [4]. The mainstay of treatment for sarcoma is surgery followed by radio-therapy. Chemotherapy has not been very successful in treating patients with metastatic STS [5].
The interaction between cancer cells and immune system has been well described in many tumour types. The role of tumour-associated T cells [6, 7], NK cells [8], dendritic cells and macrophages [9] in anti-tumor immunity has also been extensively studied. Though the evidence that B cells can facilitate the growth of experimental tumors in mice dates back to 1970s [10] and the presence of B cell, abundance, is often associated with poor prognosis [11], the biological significance of B cells in anti-tumor immunity is not very well understood.
In this study, we have investigated the mechanism by which suppression of T cell responses was induced by tumour-evoked B and T regulatory cells in a WEHI-164 fibrosarcoma model in BALB/c mice. Tumor-evoked Bregs skewed the TH cell differentiation in vitro towards Treg phenotype, produced IL-10 and TGF-β1 and suppressed T cell responses. However, treatment with AS-101, inhibitor of IL-10 production as well as antibody-mediated neutralisation of IL-10 did not restore regulatory B–T cell-induced suppression. TGF-β1 seems to be the major mediator since TGF-βR inhibitor SB431542 completely restored the immunosuppression resulting in immuno-competent T cells along with a statistically significant decrease in tumor burden.
Materials and methods
Reagents and chemicals
Fluorochrome-conjugated antibodies and ELISpot kits were purchased from BD Biosciences (San Diego, CA, USA). Anti-mouse IL-10 antibody, Concanvalin A (con A), SB431542 and carboxy fluorescein succinamidyl ester (CFSE) were from Sigma-Aldrich (St Louis, USA). AS-101 was from Cayman Chemicals (Ann Arbor, MI, USA). Anti-mouse CD4, IgG and CD19 beads were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). TGF-β1 ELISA kit and Dynabeads™ Mouse T-Activator CD3/CD28 were from Thermofisher Scientific (Waltham, MA USA). TGF-β2 quantikine ELISA set was from R&D Systems (Minneapolis,Minnesota, USA). One-step cDNA synthesis kit and Light Cycler 480 SYBR Green Master mix were from Roche Applied Science (Upper Bavaria, Penzberg, Germany). RPMI-1640, Fetal Bovine serum (FBS), antibiotic–antimycotic solution and RNA isolation kit were purchased from Himedia (Mumbai, Maharashtra, India). All other chemicals were from Sigma-Aldrich (St Louis, USA).
Animals and cell lines
Six- to eight-week-old BALB/c mice of either sex were bred and maintained in the animal house facility of Bhabha Atomic Research Centre (BARC), and given ad libitum access of water and food.
WEHI-164 cell line and maintained in RPMI-1640 medium supplemented with 10% FBS and 1% streptomycin–penicillin solution and grown at 37 °C in a humidified 5% CO2 incubator.
Generation of conditioned medium
The supernatants from WEHI-164 cells (1 × 106 cells/mL) cultured in complete medium for 72 h at 37 °C and 95% air was filtered using 0.22 µm membrane filter and used as the tumor-conditioned medium (TCM) for all the in vitro studies. TCM was aliquoted and stored at − 30 °C and used within 2 months. For all assays, 20% TCM was used (Fig. S1a).
Preparation of spleen cells
Spleens were removed aseptically and squeezed through a sterile nylon mesh (40 µm) into RPMI 1640 medium. Red blood cells were lysed using 0.83% ammonium chloride solution. The resulting cell suspension was thoroughly washed with medium and suspended in RPMI-1640 medium containing 10% FBS and 1% antibiotic–antimycotic solution (complete medium).
Antibody staining and flow cytometry
For surface marker labeling, cells (1 × 106 cells/mL) were incubated with the respective primary antibodies for 30 min at room temperature (RT) in dark. For intracellular staining, cells were fixed using 2% formaldehyde solution for 15–20 min followed by permeabilization (0.3% Triton X-100, 0.5% FBS in PBS) for 15 min. Cells were incubated with primary antibody for 45 min at 4 °C followed by secondary antibody staining. Appropriate isotype controls were used. Samples were acquired in Cyflowspace™ flow cytometer (Sysmex, Germany) using Flowmax software and data were analyzed using FCS express software.
Assay for proliferation
Proliferation was assessed by flow cytometric analysis of CFSE dye dilution. CFSE labeled cells (2 × 105) were seeded in 96-well plates and incubated for 72 h at 37 °C in a 5% CO2 incubator. Cells were stimulated with (a) mitogen con A (1 µg/mL) or (b) allo antigen (stimulator spleen cells) from C57BL/6 mice (MHC H-2b) exposed to 30 Gy of γ-radiation from a Co60 source blood irradiator (Board of Radiation and Isotope Technology, Mumbai, India). Cells were harvested and labeled with anti-CD4 and CD8 antibodies on days 3–5, and 20,000 cells were acquired in Cyflow space™ using Flowmax software and analyzed by FCS express software.
Purification of cells using magnetic assisted cell separation (MACS)
All cell purifications were done using MACS in VarioMACS™ (Miltenyi Biotec, Gladbach, Germany). CD4+ cells and CD19+ B cells were purified by positive selection method. Briefly, spleen cells from BALB/c mice were labeled with anti-mouse CD4 or anti-mouse CD19+ micro-beads as per manufacturer’s protocol and purified through MS or LS columns, respectively, in VarioMACS™ (Miltenyi Biotec, Gladbach, Germany). The cells retained in the column were eluted and purity was assessed by flow cytometry. The flow through of cells obtained during B cell purification was used as IgG depleted cells. The purity of the populations was always found to be > 95% (Fig. S1b, c and d).
T cell suppression assay
Purified B cells (1 × 106 cells/mL) were cultured for 72 h with (a) complete medium: (B-UT), (b) 10 μg/mL LPS: (B-LPS), (c) 20% TCM: (B-TCM), (d) 20% TCM and AS-101: (B AS101–TCM), (e) 20% TCM and SB431542: (B SB431542–TCM). After 72 h, these treated B cells were washed and co-cultured with CFSE labeled, B depleted responder T cells (1:1) along with anti-CD3/anti-CD28 beads (3:1). After co-culture for 72 h, the cells were washed and labeled with PE conjugated anti-CD4 antibody. The frequency of proliferating CD4+ cells was determined by flow cytometry.
Real-time PCR
Total RNA was isolated from 1 × 106 cells using RNA isolation kit (Himedia). RNA (20 ng) was transcribed to cDNA using random hexamer primers, dNTPs and reverse transcriptase using one-step cDNA synthesis kit. cDNA (5 ng) was used for PCR amplification using gene-specific primers for TH subtype specific genes (Table S1). Real-time PCR was carried out in Light Cycler® 480 (Roche Applied Science, Penzberg, Upper Bavaria, Germany). All reactions were performed with SYBR green in PCR mix and in triplicates. Results are represented as relative expression with reference to actin expression.
ELISpot assay
ELISpot assays were performed as per manufacturer’s protocol. Briefly, sterile poly vinylidene difluoride (PVDF) bottomed 96-well plates were coated overnight at 4 °C with capture antibodies. The plates were blocked for 2 h at 37 °C with complete medium. Spleen cells (1 × 105 per well) were added with or without 20% TCM and the plates were incubated overnight at 37 °C in a 5% CO2 incubator. The cells were then washed off and the plates incubated for 2 h at RT with the corresponding biotin conjugated detection antibodies followed by avidin-horse radish peroxidase conjugate for 1 h at RT. The spots were developed with substrate addition. The reaction was stopped by rinsing the plates with distilled water and the spots were counted using ELISpot reader (Immunospot, Cellular Technology Ltd, Cleveland, OH, USA). Results are reported as the number of spot-forming cells per 105 spleen cells.
Establishment of WEHI-164 fibrosarcoma tumor model and in vivo studies
A tumor model was established as follows (Fig. S1e); WEHI-164 cells (2 × 106) were injected i.m into right flank of BALB/c mice. Palpable tumors were observed on day 8. Tumors were measured on alternate days from day 10 till the end of experiments using vernier calipers. Tumor volume calculated as length × width2 × π/6, where length represents the largest diameter and width represents the perpendicular diameter of the tumor [12]. All Tumor-bearing mice (TBM) were sacrificed on day 20 and used for experiments. Non-tumor (NT) mice served as control.
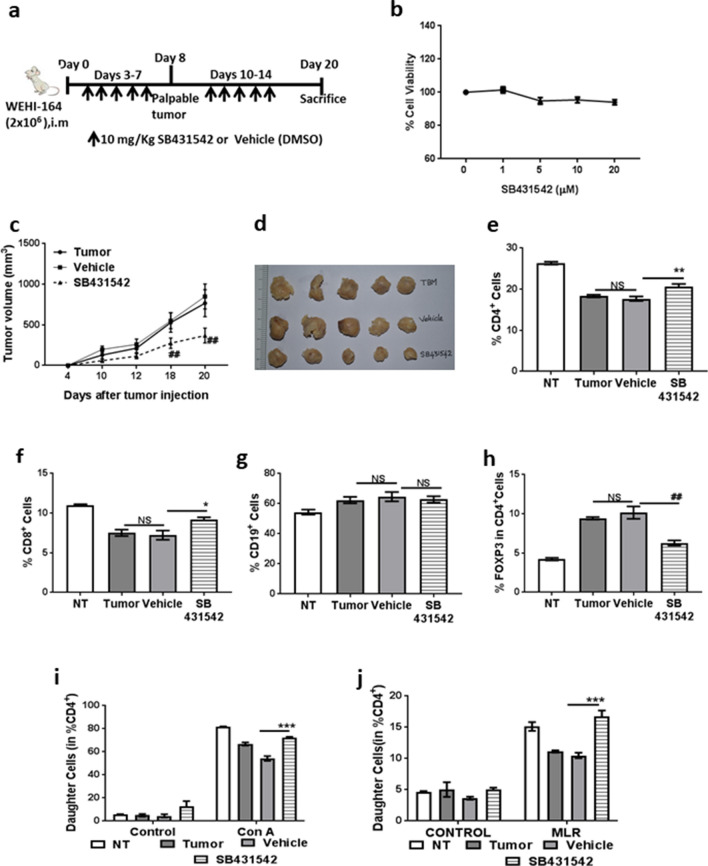
For SB431542 studies, mice were randomized into three groups (n = 5 per group) on day 2 after tumor inoculation and treated i.p with either 50 μL dimethyl sulfoxide (DMSO) or SB431542 (10 mg/kg body weight) (Fig. 6a). One group was retained without any treatment as controls (NT). Tumor measurements and further studies were carried out as indicated above.
Fig. 6.
SB431542 reduced tumor burden and rescued T cell responses in tumor bearing mice. Mice were inoculated i. m with tumor cells on right flank. SB431542 (10 mg/Kg body weight) or DMSO (50 µL) were administered i.p from days 3-7 and 10-14. a Scheme of experiment. b WEHI-164 cells were culture for 72 h with increasing concentrations of SB431542 and cell viability was assessed used MTT assay. c Tumor volume measurements on alternate days from day 10 of inoculation, d Image of dissected tumors from the different treatment groups. e Percentage of CD4+ cells, f CD8+ cells, g CD19+ cells and h CD4+FOXP3+ cells in spleens from treatment groups on day 20. Spleen cells were labelled with CFSE stimulated with i 1 µg/mL con A and j stimulator spleen cells from C57BL/6 mice (MHC H-2b) exposed to 30 Gy of γ-radiation (MLR) for 72 h. Then, cells were labelled with anti CD4+ antibody and percentage of daughter cells that have undergone proliferation was assessed in CD4+ gated cells. Data shown are mean ± SEM from two independent experiments (n = 10). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 where treated is greater than control and #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.001 where treated is lesser than control
Statistical analysis
All experiments were repeated three times. The values are represented as mean ± SEM of three experiments. The statistical significance of the differences between groups was calculated by two-tailed Student’s t test. Results were considered statistically significant at p < 0.05.
Results
Tumor-conditioned medium promoted total spleen cell proliferation but inhibited T cell responses to mitogen and allo-antigen
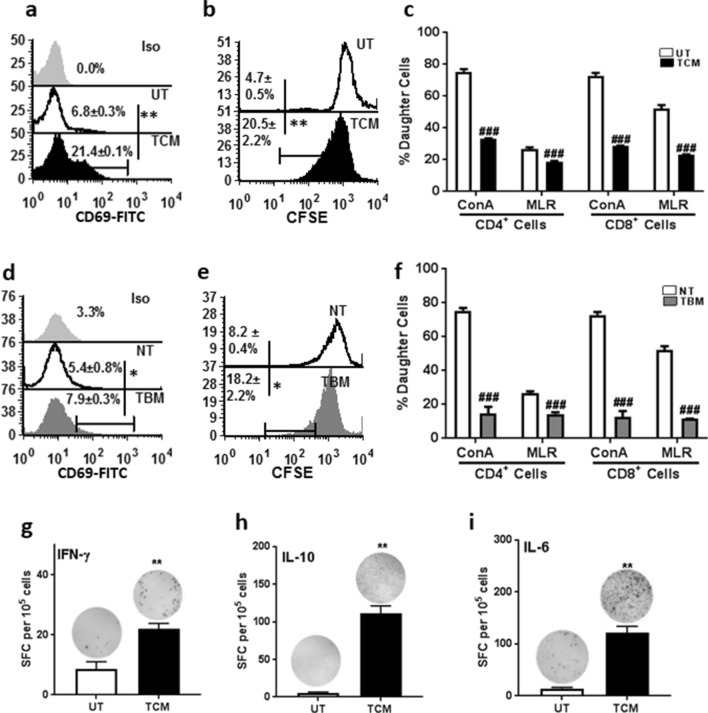
TCM increased the proliferation of spleen cells and this effect was concentration dependent and increased up to 50% of TCM after which there was a decline (Fig. S2a). Hence, 20% TCM was used for further experiments. Treatment of spleen cells with 20% TCM did not induce apoptosis (Fig. S2b), but a three-fold increase in proliferation as assessed by expression of early activation marker CD69, 24 h after treatment (Fig. 1a). Presence of lymphoblast cell clusters (Fig. S2c) as well as increase in percent divided cells by means of CFSE dye dilution confirmed (Fig. 1b) that TCM increased proliferation of total spleen cells. The same responses were replicated in vivo also as evidenced by significant increase in CD69 expression as well as CFSE dye dilution in TBM as compared to NT mice (Fig. 1d, e).
Fig. 1.
Tumor-conditioned medium promoted total spleen cell proliferation but inhibited T cell responses to mitogen and allo-antigen. Spleen cells from BALB/c mice were treated with TCM and assessed for its proliferation efficiency by a CD69 labelling and b CFSE dye dilution in untreated and TCM-treated cells. c CFSE labelled spleen cells were stimulated with 1 µg/mL con A or allo antigen (irradiated stimulator spleen cells from C57BL/6 mice (MHC H-2b) (mixed lymphocyte reaction MLR) along with TCM and proliferation of CD4+ and CD8+ cells was assessed. Proliferation was assessed in spleen cells from no tumor (NT) or tumor-bearing mice (TBM) by d CD69 labeling and e CFSE dilution. f Proliferation of CD4+ cells and CD8+ cells in NT and TBM upon stimulation with 1 µg/mL con A and allo antigen. Spleen cells were cultured with TCM in ELISpot assay, number of spot forming colonies (SFC) secreting g IFN-γ, h IL-10 and i IL-6. Data represented are mean ± SEM from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 where treated is greater than control and #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.001 where treated is lesser than control
In addition, when spleen cells from TBM were treated with TCM, proliferation increased further in terms of CFSE dilution (Fig. S2d). Time kinetics of CFSE dilution revealed that proliferation of spleen cells from TBM increased on days 1–3 after treatment but did not increase further (Fig. S2d). On the other hand, spleen cells from NT and TBM treated with TCM exhibited increased proliferation from days 1 to 6 (Fig. S2d).
When the response of spleen cells treated with TCM to mitogen con A and allo-antigen (irradiated spleen cells from C57BL/6 mice in a mixed lymphocyte reaction, MLR) was examined, there was significant suppression of both CD4+ and CD8+ T cell responses (Fig. 1c). Similarly, CD4+ and CD8+ T cell responses from TBM to con A and allo-antigen were also suppressed (Fig. 1f).
TCM by itself did not contain detectable amount of the cytokines IL-6, IL-10, IFN- γ, IL-2, IL-4 and TNF-α (data not shown). Hence, the ability of TCM to elicit a cytokine response in spleen cells was assessed by ELISpot assay. Amongst the cytokines tested, there was no significant difference in IL-2, IL-4 and TNF-α producing cells between the control and TCM-treated groups (Fig. S2e, S2f and S2g). However, there was a significant increase in IFN- γ (2.5 fold), IL-6 (8.5 fold) and IL-10 (22 fold) producing cells in the TCM-treated group as compared to the control (Fig. 1g–i).
Tumor increased differentiation of T cells to regulatory phenotype both in vitro and in vivo
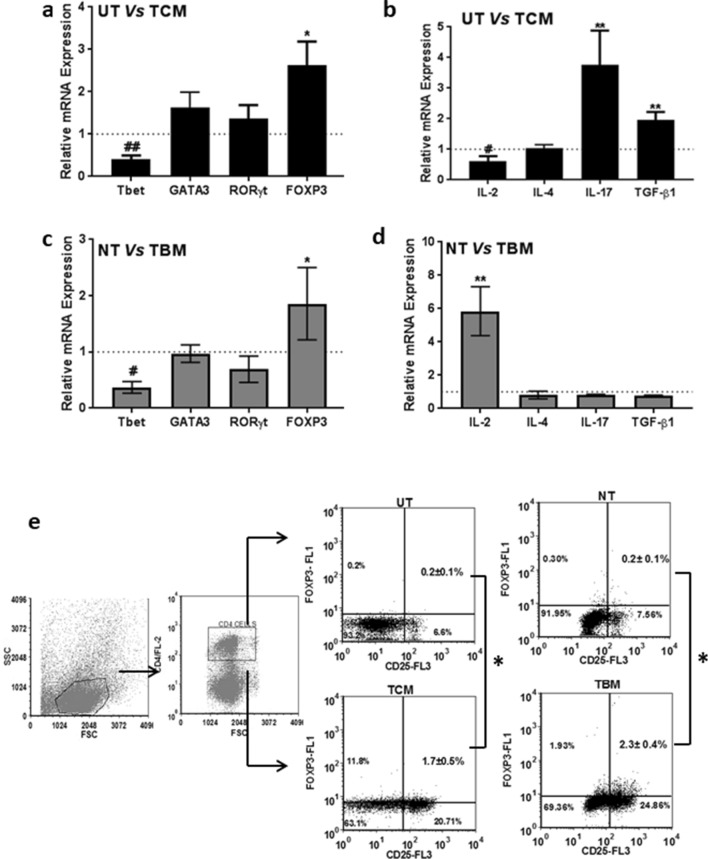
Since TCM treatment increased proliferation of spleen cells and inhibited response to antigens, its effect on T cell differentiation was evaluated by analysing the mRNA level expression of the classical transcription factors, T-bet, GATA-3, ROR-γ and FOXP3 of TH1, TH2, TH17 and Treg subtypes and the signature cytokines for these four cell types, IL-2, IL-4, IL-17 and TGF-β1. The TH1 phenotype-specific transcription factor T-bet was down-regulated significantly in TCM-treated cells (Fig. 2a) with a concomitant down-regulation of IL-2 mRNA (Fig. 2b). The TH2-specific transcription factor GATA-3 and its major cytokine IL-4 was found to be unaffected by the TCM treatment (Fig. 2a and 2b). Even though IL-17 was significantly up-regulated in TCM, the transcription factor, ROR-γ for TH17 cells remained unchanged (Fig. 2a, b). Both FOXP3 the master transcription factor and TGF-β1, the signature cytokine, of T regulatory cell phenotype was significantly up-regulated in TCM-treated splenic lymphocytes (Fig. 2a, b).
Fig. 2.
Tumor increased differentiation of T cells to regulatory phenotype both in vitro and in vivo. Expression of TH cell subtype markers was assessed in spleen cells treated with TCM or isolated from TBM by real-time PCR. a Transcription factors specific to TH1, TH2, TH17, Treg cells in TCM-treated spleen cells and b cytokines representative of TH1, TH2, TH17, Treg in TCM-treated spleen cells. c Transcription factors specific to TH1, TH2, TH17, Treg cells in TBM spleen cells and d cytokines specific to TH1, TH2, TH17, Treg cells in TBM spleen cells. Data are represented as fold change in expression as compared to control. e Percentage of CD4+CD25+FOXP3+ cells obtained in TCM-treated spleen cells and those obtained from TCM from flow cytometric analysis along with the gating strategy. All data represented are mean ± SEM from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 where treated is greater than control and #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.001 where treated is lesser than control
The expression of these genes was evaluated in spleen cells of tumor-bearing mice also. With respect to master transcription factors, a similar pattern to that of TCM treatment was observed in lymphocytes obtained from TBM (Fig. 2c). However, unlike TCM treatment, the cytokine expression followed a different pattern, with a significant up-regulation of IL-2 and down-regulation of IL-17 and TGF-β1 (Fig. 2d).
Since the transcription factor analysis showed an increase in FOXP3 in spleen cells treated with TCM or those from TBM, they were further analysed for the phenotype of T regulatory cells. The abundance of CD4+CD25+FOXP3+ T regulatory cells increased up to 7 fold in TCM-treated cells as compared to UT cells (Fig. 2e). In TBM, T regulatory cells increased up to 11 fold in TBM as compared to NT (Fig. 2e). These results confirmed that there were increased T regulatory cells under in vitro and in vivo conditions in fibrosarcoma.
TCM decreased splenic CD4+ and CD8+ cells but increased proliferation of CD19+ cells
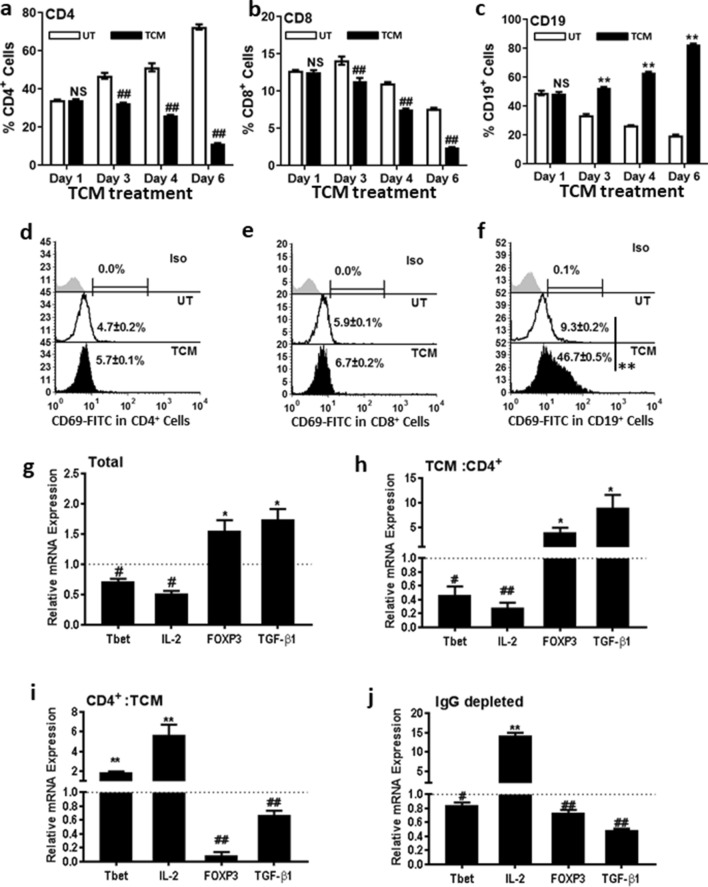
To assess the effect of TCM on T cell and B cell status, spleen cells were treated with TCM for 6 days. Cells were labelled with anti-CD4, CD8 and CD19 antibodies on days 1, 3, 4 and 6, and assessed by flow cytometry. TCM treatment for 24 h did not result in any change in the proportion of CD4+, CD8+ and CD19+ cells (Fig. 3a–c). However, on day 3, there was a decrease in CD4+ and CD8+ cells to 30% and 20%, respectively (Fig. 3a, b) and on day 6, to 80% and 65% in TCM-treated cells as compared to UT (Fig. 3a, b). In contrast, the ratio of CD19+ cells increased following TCM treatment, with a 35% increase on day 3 and 75% on day 6 (Fig. 3c). There was no change in proportion of CD4+ and CD8+ cells expressing early activation marker CD69 indicating that TCM did not activate TH (Fig. 3d) or TC cells (Fig. 3e). In contrast, there was an increase in CD19+ cells expressing the activation marker CD69 indicating that increased proliferation could be the reason for the increase in CD19+ cells. The percentages of CD69+ CD19+ cells increased from 3.07 ± 0.09% in UT to 17.32 ± 0.87% in TCM-treated cells (Fig. 3f). Increased proliferation of CD19+ cells and not in CD4+ or CD8+ cells was also confirmed with CFSE analysis (Fig. S3a–c). These results suggest that TCM-induced proliferation was in B cells.
Fig. 3.
TCM decreased splenic CD4+ and CD8+ T cells but increased proliferation of CD19+ B cells. Spleen cells were treated with TCM for 6 days and evaluated for the proportion of a CD4+ cells, b CD8+ cells and c CD19+ cells on days 1- 6 after treatment. Percentages of d CD4+, e CD8+ and f CD19+ cells expressing CD69 was evaluated in spleen cells 24 h after treatment with TCM. Expression of Tbet, IL-2, FOXP3 and TGF-β1 in g total spleen cells treated with TCM for 24 h, h TCM: CD4+ cells (incubation of spleen cells with TCM for 24 h followed by purification of CD4 + cells), i CD4+:TCM (incubation of purified CD4+ cells with TCM for 24 h), j IgG depleted cells treated with TCM for 24 h. Data represented are mean ± SEM from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 where treated is greater than control and #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.001 where treated is lesser than control
To confirm that TCM induces proliferation only in B cells, TH and B cells were purified using anti-CD4 and IgG conjugated magnetic beads from mouse spleen cells and incubated with TCM for 24 h and assessed for CD69 expression (Fig. S3d, e). There was no change in CD69 expression in CD4+ cells (Fig. S3d) and IgG depleted cells (Fig. S3f) due to TCM treatment. In accordance with the previous results, CD69 expression increased 4 fold in TCM-treated B (IgG+) cells (Fig. S3e) and 7.5 fold in CD4 depleted cells (Fig. S3g). These results confirm that TCM induced proliferation only in B cells.
To evaluate whether the TCM-induced up-regulation of Tregs is through B cells or not, TH1 (T-bet and IL-2) and Treg (FOXP3 and TGF-β1) markers were assessed in CD4+ and IgG depleted cells before and after TCM treatment. Briefly, spleen cells were categorized into four groups, namely (1) total spleen cells treated with TCM, (2) total spleen cells treated with TCM followed by purification of CD4+ cells (TCM:CD4+), (3) purification of CD4+ cells followed by TCM treatment (CD4+:TCM) and (4) IgG depleted spleen cells incubated with TCM for 24 h. After incubation with TCM for 24 h, mRNA levels of T-bet, IL-2, FOXP3 and TGF-β1 were analysed by real-time PCR.
There was a significant down-regulation of both the TH1 markers (T-bet and IL-2) and up-regulation of Treg markers (FOXP3 and TGF-β1) in total spleen cells treated with TCM (Fig. 3g). Similar pattern of expression was observed for the transcription factors and cytokines in the CD4+ cells purified after TCM treatment (TCM: CD4+) (Fig. 3h). However, if CD4+ cells were purified before TCM treatment (CD4+: TCM), this pattern was reverted (Fig. 3i) with up-regulation of T-bet and IL-2 and down-regulation of FOXP3 and TGF-β1. These results indicated that TCM-induced Treg phenotype required the involvement of accessory cells. In accordance to that, if B cells were first depleted (IgG depleted), followed by TCM treatment, there was no increase in Treg markers, and transcription factors’ profile showed similar pattern as of CD4+:TCM (Fig. 3j). Together, these results confirm that the TCM-induced Treg up-regulation was mediated by the B cells.
TCM-induced Treg phenotype is through tumor-evoked Breg
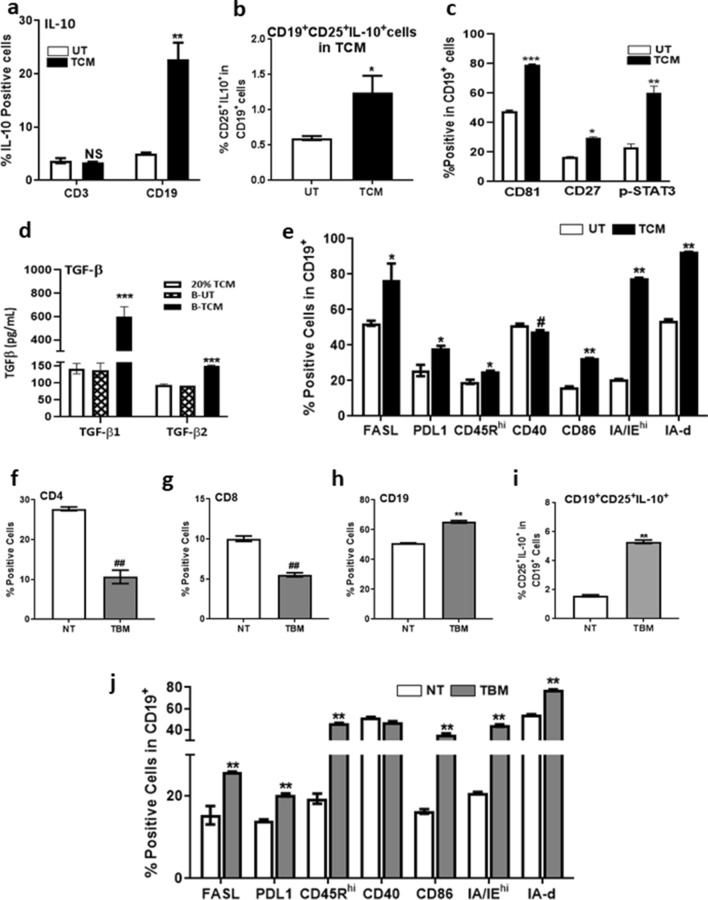
Though there was an increase in both pro-inflammatory cytokines such as IL-6, IFN-γ, IL-17 and anti-inflammatory cytokines such as IL-10, TGF-β1, B cells in tumor microenvironment are reported to secrete IL-10 and TGF-β1 [13–16] and hence, we focussed on these cytokine for further studies. Tumor-conditioned T and B cells were evaluated for secretion of IL-10 and TGF-β1. Spleen cells cultured with TCM for 24 h showed increased proportion of intracellular IL-10 in CD19+ cells (Fig. 4a) and not in CD3+ cells (Fig. 4a), confirming our hypothesis that TCM-induced B cells secrete IL-10. B cells treated with TCM also secreted significantly higher levels of TGF- β1 and TGF- β2 (Fig. 4d).
Fig. 4.
TCM induced T reg phenotype is through Breg. Spleen cells were treated with TCM for 24 h and assessed for percentages of a CD3+IL-10+ and CD19+IL-10+ cells. b CD19+CD25+IL-10+ cells c CD19+CD81+ cells, CD19+CD27+ cells, CD19+pSTAT3+ cells. d Estimation of TGF-β1 and TGF-β2 by ELISA in conditioned media of B cells treated with TCM. e Cell surface expression of different markers in TCM-treated CD19+ cells. Percentages of f CD4+, g CD8+ and h CD19+ cells, i percentage of CD19+CD25+IL-10+ cells and j cell surface expression of different markers in CD19+ cells of NT and TBM. Data represented are mean ± SEM from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 where treated is greater than control and #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.001 where treated is lesser than control
A new subset of immune-regulatory B cells has been identified recently, with active roles in controlling inflammation and autoimmune diseases [17]. These B regulatory cells, reported to have a CD1dhiCD5+CD19+ phenotype, have the ability to suppress immune responses during cancer immune surveillance, through the release of anti-inflammatory mediators, such as interleukin-10 (IL-10) and the expression of inhibitory molecules, such as PD-L1 [18]. There was no significant difference in expression of CD1d and CD5 in B cells from UT and TCM-treated cells with a decrease in CD1dhiCD5+ cells following TCM (Fig. S4a) treatment indicating that, TCM-evoked Breg cells are not B10 cells.
Since we did not find the enhancement of B10 cells in TCM, we analyzed the possibility of other types of Breg cells. Given that multiple types of Bregs do exist and there is no conventional marker available for Breg identification as in Treg, we evaluated markers like CD25 that are up-regulated in tumor-associated B cells [16]. Percent CD25+IL-10+ B cells were significantly higher in TCM-treated cells than in UT group (Fig. 4b). Expression of B cell co-receptor marker CD81 was higher in TCM-treated B cells (Fig. 4c). CD27, a surface marker which is tightly regulated by IL-10 [19] and phospho STAT3, a downstream transcription factor of IL-10 [20] was also found to be increased in CD19+ cells treated with TCM (Fig. 4c). In addition to this, CD19+ cells from TCM had significantly increased levels of CD86, IA/IE and IA-d, cell surface inhibitory molecules like FASL and PDL-1 than those in UT (Fig. 4e). They were also found to be CD45Rhi with significantly lower expression of CD40.
Similar to in vitro results, there was a 50% decrease in percentage of CD4+ cells, 40% decrease in CD8+ cells and 20% increase in CD19+ cells in spleens of TBM as compared to NT mice (Fig. 4f–h). There was a significant decrease in CD4+ and CD8+ cells with an increase in CD19+ cells in tumor draining lymph node (TDLN) from TBM than the LN from NT mice (Fig. S4b S4c and S4d). A three-fold increase in CD19+CD25+IL-10+ cells with increased expression of the markers CD86, IA/IE, IA-d, FASL and PD-L1 was observed in splenocytes of TBM as compared to NT mice (Fig. 4i, j).
TGF-βR inhibitor SB431542 restored TCM-evoked Breg-mediated suppression of T cell responses
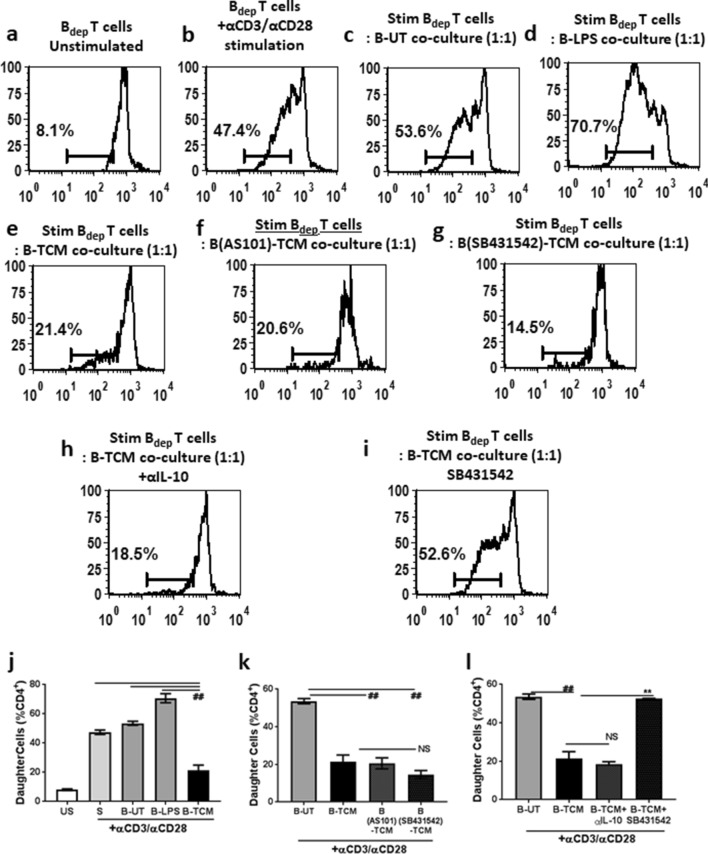
To verify whether tumor-evoked Breg cells suppressed T cell responses, a modified T cell suppression assay was performed [15]. Purified B cells were cultured for 72 h in three groups, namely (a) in complete medium (B-UT), (b) in the presence of LPS, a classical activator of B cells, (B-LPS) and (c) in the presence of 20% TCM (B-TCM). After 72 h, these cells were harvested, washed twice with medium and co-cultured with B depleted T cells in ratio of 1:1 and stimulated with anti CD3/CD28. B depleted (Bdep) T cells cultured alone served as the control (US) and had 8% daughter cells in CD4+ gated T cells as evaluated by CFSE (Fig. 5a). When Bdep cells were stimulated with α-CD3/CD28 beads, there was a six-fold increase in CD4+ T cell proliferation (Fig. 5b). Co-culture, with B-UT cells, (Fig. 5c) resulted in comparable proliferation (5.9 fold in UT and 6.7 fold with B-UT). Co-culture with B-LPS enhanced (30% increase) and B-TCM suppressed (60% decrease) the proliferation of CD4+ T cells (Fig. 5d, e). These results confirm that the inhibition of T cell responses to mitogen or allo-antigen was mediated through tumor evoked Breg cells.
Fig. 5.
TGF-βR inhibitor SB431542 restored TCM-induced Breg-mediated T cell proliferation responses. CFSE labeled B depleted Tcells (Bdep) were co-cultured with B cells generated under different conditions and stimulated with anti-CD3/CD28 coated magnetic dynabeads. Representative images of CFSE dilution are shown, a un-stimulated Bdep cells, b Bdep cells stimulated with anti CD3/CD28 beads. Stimulated Bdep cells were co-cultured with either c untreated (UT) B cells, d B cell generated with 1 µg/mL LPS (B-LPS) or e B cells generated with TCM (B-TCM) f B-TCM generated with TCM and 10 µM AS101, g B-TCM generated with TCM and 10 µM SB431542. Stimulated Bdep and B-TCM were co-cultured in presence of h neutralizing anti IL-10 antibody or i SB431542. j–l Graphical representation of the data. Each bar represents mean ± SEM from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 where treated is greater than control and #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.001 where treated is lesser than control
We evaluated the role of IL-10 in B-TCM-mediated suppression of CD4+ T cell proliferation. This was done by either (1) generating B-TCM in the presence of AS-101, a small molecule inhibitor of IL-10 synthesis (B AS101-TCM) or (2) treatment of Bdep T cells with neutralizing anti-IL-10 antibody during the co-culture with B-TCM. Both conditions did not abrogate the B-TCM-induced suppression (Fig. 5f, j, h, k). These results suggest that IL-10 is not the primary mediator of the suppressive function.
Since B cells treated with TCM secreted high levels of TGF-β1 and TGF-β2, we hypothesized a possible role of TGF-β in the regulatory properties of B-TCM. To confirm the role of TGF-β, the effect of TGF-βR inhibitor SB431542 was assessed. This was done by (1) generating B-TCM in presence of SB 431542 ((B SB431542-TCM) as well as (2) treatment of Bdep T cells with SB431542 during co-culture with B-TCM. Presence of SB431542 at the time of B-TCM generation did not affect the regulatory function of B-TCM (Fig. 5g, k), suggesting, tumor-derived TGF-β signaling is not necessary for Breg generation upon treatment with TCM. Interestingly, when Bdep cells were treated with SB431542, the suppressive effect of B-TCM was completely abrogated (Fig. 5h, k) indicating that B-TCM-derived TGF-β is responsible for inhibition of T cell responses. These results indicate that the B-TCM-mediated suppression of T cell responses in vitro is through TGF-β signalling.
SB431542 reduced tumor burden and rescued T cell responses in tumor-bearing mice
Since SB431542 could abrogate the suppressive effect of B-TCM and rescue T cell responses in vitro, we investigated whether this effect can be recapitulated in vivo.
The experimental scheme for this experiment along with the treatment protocol is given in Fig. 6a. Though SB431542 did not have any direct toxicity to tumor cells in vitro (Fig. 6b), SB431542 administration significantly decreased tumor burden in mice (Fig. 6c–d). To study if SB431542 had any immunomodulatory effects that could possibly have an indirect effect on tumor burden, we evaluated the effect of SB431542 administration on splenic T cell responses. SB431542 treatment significantly increased the percentages of splenic CD4+ and CD8+ cells with no change in CD19+ cells (Fig. 6e–g). This observation corroborates with our in vitro data that Breg generation is independent of TGF-β signalling. The percentage of CD4+FOXP3+ Treg cells was significantly reduced in spleen cells of SB431542-treated mice (Fig. 6h), indicating the possible abrogation of Breg–Treg axis in vivo also. SB431542 administration also rescued T cell responses to mitogen con A (Fig. 6i) and allo-antigen (Fig. 6j) resulting in increased T cell immunogenicity.
Collectively, our data suggest that WEHI-164-induced suppression of splenic T cell responses is through a TGF-β-mediated pathway, at least partly due to the generation of CD19+ CD81+, CD27+, IL-10+, pSTAT3 + TGF-β secreting regulatory B cells. Though these cells also express activation marker CD69, whether it is essential for its suppressive function or these are independent functions needs to be ascertained.
Discussion
B regulatory cells (Breg), an immunosuppressive subset of B cells, play an important role in inflammatory and autoimmune conditions and exert their suppressive function by acting on dendritic cells [21], macrophages [22] and Treg cells. The immune regulatory functions of Bregs, in cancer have become a recent focus area. In this study, we report that, tumor evoked CD19+ CD81+, CD27+, IL-10+, pSTAT3+, TGF-β secreting regulatory B cells can suppress T cell responses in spleen and possibly favour tumor growth through a TGF-β-mediated pathway. Administration of SB431542, TGFβRI inhibitor, significantly reduced tumor burden, along with restoration of T cell responses.
There are two well-elucidated examples of Bregs promoting cancer. B10 cells are the first type initially identified by Yanaba et al. [23] and are most studied. They are naturally occurring in lymphoid organs; characterized by CD1dhiCD5+CD19+ phenotype [18, 24, 25] and signature cytokine IL-10 [26]. In our studies, the percentage of CD19+CD1dhiCD5+ B cells did not increase after TCM treatment as compared to control cells suggesting that TCM evoked IL-10 producing B cells were not B10 cells.
A second, well-studied group of Bregs in murine tumor are a unique subset of tBregs [16, 27–29] that phenotypically resemble B2 cells and constitutively expressed STAT3, poorly proliferative and do not express CD27, CD5 or CD1d. These tBregs were termed as CD19+ B cells that are pSTAT3+CD81hiCD25+ Breg cells that suppress the activity of T cells. These tBregs could convert CD4+ naïve cells to T regulatory cell, inactivate anti-tumor NK cells and protect metastasizing cancer cells in the lungs [16]. WEHI-164 fibrosarcoma evoked Breg cells from naïve B cells were found to be pSTAT3hi, CD81hi, CD25hi PDL1hi CD45Rhi CD85hi and MHCIIhi. Though these Bregs share similarities with those identified by Biragyn et al. in 4T1 tumor-bearing mice, they differ with them in that WEHI-164 tBregs are highly proliferative, while the latter are poorly proliferative even though both are characterized as CD69hi [16]. WEHI-164-induced Breg cells also expressed CD27 and secreted IL-10 in contrary to those identified by Biragyn et al. [16]. In addition, these Bregs were also associated with increased FOXP3 expression and effectively suppressed the proliferation of CD4+ and CD8+ cells upon αCD3/αCD28 stimulation.
Cytokines are the major soluble mediators through which the tumor and immune cells in the microenvironment interact with each other. In spleen cells, TCM treatment induced both pro- and anti-inflammatory cytokines. Elevated levels of IL-17, without increase of ROR-γt expression, suggests that the cytokine may not be indicative of the classical TH17 cells [30]. The source of IL-17, therefore, could be either tumor-associated macrophages [31–33], B cells [34], γδT17 cells, a variant of γδT cells that secrete IL-17 [35] or IL-17+ Treg cells [36]. Though it has been reported that IL-17, secreted by non-TH17 cells, may be associated with increased tumor growth and poorer survival rate [37], we have not explored this in detail.
Spleen cells from tumor-bearing mice did not show increase in TGF-β mRNA levels even though there was increased FOXP3 levels and CD4+CD25+FOXP3+ cells. A major reason for this discordance may be because the gene expression studies were carried out in total spleen cells, and there is a decrease of T lymphocytes in the spleens of tumor-bearing mice as we and others have reported [38]. In addition, T regulatory cells employ activation of membrane bound TGF-β along with latency associated peptide for their suppressive functions that need not necessarily correlate with TGF-β mRNA [39].
Regulatory B cells are known to exert their regulatory functions through two of the prominent anti-inflammatory cytokines, IL-10 and TGF-β. Hence, we focussed on elucidating the effect of these two cytokines in the suppressive capacity of Breg cells. Though the paradoxical role of these pro-inflammatory cytokines IL-6, IFN-γ, IL-17 on inducing Breg cells in autoimmunity has been reported, their effect on tumor evoked B regulatory cells is not known and will be worthwhile to study in the future [40, 41]. Blocking IL-10 synthesis or signalling did not affect the suppressive activity of tBregs even though these cells secrete high levels of IL-10. Most of the Bregs reported in autoimmunity employ IL-10 as the key player for immune suppression and recent studies suggest the presence of IL-10 producing subsets of Bregs in skin squamous carcinoma [42], chronic lymphocytic leukemia [43] and murine mammary carcinoma [28].
In addition to IL-10, there was an increased expression of FOXP3 and elevated levels of TGF-β in cells treated with TCM. Increased secretion of both TGF-β1 and TGF-β2 were observed in Breg cells treated with TCM. Inhibition of TGF-β signalling by treatment with SB-431542, completely abrogated Breg-induced suppression of proliferation responses in T cells in the co-culture. SB431542 is a novel small molecule that potentially inhibits TGF-β signalling through the inhibition of TGFβRI activity [44]. Interestingly, this abrogation was seen only when SB431542 was present during the co-culture of B–T cells and not if it was present during the generation of tBregs. The cytokines IL-10 and TGF- β act synergistically to bring about immune suppression [45]. or block the TH2 signalling and establish tolerance [46]. IL-10, on the other hand, can also increase the TGF-β responsiveness in activated T cell [46]. Interestingly, IL-10 does not seem to have any role in TGF-β-mediated suppression in Breg cells, since blocking its synthesis did not affect the function of Breg or affect the tumor burden in vivo (data not shown). The signal transducer and activator of transcription (STAT) proteins mediate the integration of extrinsic signals provided by cytokines to the regulation of the intracellular processes allowing the cells to adapt to their surroundings. STAT-3 is critical in B cell development and germinal centre maintenance [47, 48] and is also a major player in generation of regulatory B cells [49]. Both IL-10 and TGF-β regulate STAT-3 [50, 51]. Breg cells producing IL-10 or TGF-β can induce Treg and suppression of T cells [52, 53]. However, the regulation of Breg secreting both IL-10 and TGF-β in tumor-induced immune suppression and the dominance of one signalling pathway over the other is not clear and should be delineated.
Next, we wanted to assess the effect of SB431542 in vivo. The questions raised were (1) would SB431542, which was non toxic to tumor cells in vitro have any effect on tumor burden? (2) What would the effect of SB431542 on T cell responses and T-B interactions? Though SB431542 did not induce any direct cytotoxic effects on the tumor cells as evidenced by no loss of viability under in vitro conditions, in vivo administration resulted in a significant decrease in tumor burden. Depending upon the cell lines used, SB431542 has been shown to either inhibit the proliferation of tumor cells in vitro [54–56], induced growth arrest and apoptosis [57], or inhibit TGF-β-induced epithelial–mesenchymal transition (EMT) and invasiveness [57, 58]. In murine model of mammary adenocarcinoma, this drug reduced the lung metastasis but did not affect the primary tumor growth [59]. Even though SB431542 did not reduce the viability of WEHI-164 cells in vitro, the possibility of any cytostatic effect in vivo cannot be completely ruled out.
The next question was whether this effect of SB431542 was immune mediated since the drug was able to restore TCM-induced inhibition of T cell responses. The fact that TBM treated with SB431542 demonstrated decreased CD4+FOXP3+ Tregs and restoration of splenic T cell responses to mitogen and allo-antigen validated our in vitro observations regarding the importance of TGF-β signalling. Though these results indicate SB431542-mediated anti-tumor effects are probably due to increased immunocompetent T cells, whether this is due to abrogation of Bregs, Tregs or their interaction is not known. A decrease in Tregs and the fact that WEHI-164 TCM-induced Tregs was through Bregs would point towards an abrogation of B–T interactions in vivo also as the mechanism of SB43542 action. However, this can be confirmed only in tumor-bearing mice depleted of B cells and treated with SB431542. This is a limitation of our current study and will be addressed in the future studies. However, to address the effect of B cells in tumor, timing of B cell depletion seems to be crucial and can either increase or decrease growth of the inoculated tumor cells [60–62].
Taken together, our results demonstrate that the immunosuppressive effects of tBregs can be abrogated by small molecule inhibitor of TGF-βRI, SB431542 and can be useful as an immunotherapeutic agent in soft tissue sarcomas. Though immune checkpoint therapy has been very successful in a subset of patients, there is a substantial population of non-responders necessitating the use of combination therapies to overcome resistance [63]. In this context, some recent papers have shown promising results of combining TGFβ inhibition with immune checkpoint blockade to induce complete and durable responses in otherwise unresponsive tumors [64, 65]. The association of Breg with cancer is a new wave of research in immuno-oncology. Since more mechanisms are being elucidated that actively convert naïve B cells to Breg cells, modulating B cells have considerable clinical implications. However, the timing seems to be very crucial for achieving desired results [62]. As long as the cancer persists, it can induce the generation of Bregs that can suppress the function of T cells and NK cells directly or educate other cells like myeloid-derived suppressor cells to be immune suppressive [66, 67]. This necessitates the identification and development of strategies that can abrogate the process of tumor-evoked Breg generation or its function which will effectively contribute to inhibit immune suppression and cancer escape. Inhibition of TGF-β signalling is capable of blocking the action of Breg cells and therefore can be a very useful immunotherapeutic strategy in cancer treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Mr Narendra Sidnalkar for his technical assistance.
Abbreviations
- FASL
Fas ligand
- GATA3
GATA-binding protein 3
- NT
Non-tumor
- RORγ
RAR-related orphan receptor gamma
- STS
Soft tissue sarcoma
- Tbet
T-box transcription factor
- TBM
Tumor-bearing mouse
- TCM
Tumor-conditioned medium
- TDLN
Tumor draining lymph node
- UT
Untreated
Author contributions
Ms. Kavitha Premkumar designed and performed the experiments, acquired the samples and analysed the data and wrote the manuscript. Dr Bhavani Shankar conceptualized and designed the study, analysed and interpreted the data, wrote and revised the manuscript. Both the co-authors approved the final version to be submitted.
Funding
The study was funded by Bhabha Atomic Research Centre, Government of India.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
All animal studies were approved and licenced by the Institutional Animal Ethics Committee (BARC/animalhouse/106/RBi/S/99/CPSEA), Bhabha Atomic Research Centre, Government of India, under the project no. BAEC/06/17 (dt 03.04.2017) and carried out in strict accordance with the guidelines issued by the institutional animal ethics committee regarding the maintenance and dissection of small animals.
Cell line authentication
The authenticated mouse fibrosarcoma cell line WEHI-164 was purchased from European Collection of Authenticated Cell Cultures (ECACC).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fletcher CD. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology. 2014;64:2–11. doi: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence W, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349–359. doi: 10.1097/00000658-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie-Large M, James SL, Tiessen L, Davies AM, Grimer RJ. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur J Cancer. 2008;44:1841–1845. doi: 10.1016/j.ejca.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg RD, Martin RG, Romsdahl MM, Barkley HT. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47:2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs AJ, Michels R, Stein J, Levin AS. Improvement in Overall Survival from Extremity Soft Tissue Sarcoma over Twenty Years. Sarcoma. 2015;2015:279601. doi: 10.1155/2015/279601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crunkhorn S. Cancer immunotherapy: targeting regulatory T cells. Nat Rev Drug Discov. 2017;16:754. doi: 10.1038/nrd.2017.206. [DOI] [PubMed] [Google Scholar]
- 8.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017;222:11–20. doi: 10.1016/j.imbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. J Immunol. 1978;121:359–362. [PubMed] [Google Scholar]
- 11.Nelson BH. CD20 + B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 12.Gass JD. Comparison of uveal melanoma growth rates with mitotic index and mortality. Arch Ophthalmol. 1985;103:924–931. doi: 10.1001/archopht.1985.01050070050028. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35. doi: 10.1016/j.oraloncology.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Wen Z, Cheng F, Ma J, Li W, Ren H, et al. Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-κB signal pathway. Oncoimmunology. 2016;5:e1180485. doi: 10.1080/2162402X.2016.1180485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11:670–677. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray D, Gray M. What are regulatory B cells? Eur J Immunol. 2010;40:2677–2679. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- 18.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 19.Jung J, Choe J, Li L, Choi YS. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur J Immunol. 2000;30:2437–2443. doi: 10.1002/1521-4141(2000)30:8<2437::AID-IMMU2437>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 23.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, Adegnika AA, et al. Interleukin 10 (IL-10)-producing CD1dhi regulatory B cells from Schistosoma haematobium-infected individuals induce IL-10-positive T cells and suppress effector T-cell cytokines. J Infect Dis. 2014;210:1207–1216. doi: 10.1093/infdis/jiu257. [DOI] [PubMed] [Google Scholar]
- 25.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 26.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 27.Biragyn A, Lee-Chang C, Bodogai M. Generation and identification of tumor-evoked regulatory B cells. Methods Mol Biol. 2014;1190:271–289. doi: 10.1007/978-1-4939-1161-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013;73:2127–2138. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wejksza K, Lee-Chang C, Bodogai M, Bonzo J, Gonzalez FJ, Lehrmann E, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor α. J Immunol. 2013;190:2575–2584. doi: 10.4049/jimmunol.1201920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo B, Li L, Guo J, Liu A, Wu J, Wang H, et al. M2 tumor-associated macrophages produce interleukin-17 to suppress oxaliplatin-induced apoptosis in hepatocellular carcinoma. Oncotarget. 2017;8:44465–44476. doi: 10.18632/oncotarget.17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vykhovanets EV, Maclennan GT, Vykhovanets OV, Gupta S. IL-17 Expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patients. Int J Clin Exp Pathol. 2011;4:552–565. [PMC free article] [PubMed] [Google Scholar]
- 34.Schlegel PM, Steiert I, Kötter I, Müller CA. B cells contribute to heterogeneity of IL-17 producing cells in rheumatoid arthritis and healthy controls. PLoS ONE. 2013;8:e82580. doi: 10.1371/journal.pone.0082580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- 36.Jung MK, Kwak JE, Shin EC. IL-17A-Producing Foxp3. Immune Netw. 2017;17:276–286. doi: 10.4110/in.2017.17.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, et al. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology. 2015;4:e984539. doi: 10.4161/2162402X.2014.984539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid RM, Achille NJ, Lee JM, Lathers DM, Young MR. Decreased T-cell proliferation and skewed immune responses in LLC-bearing mice. J Environ Pathol Toxicol Oncol. 2005;24:175–192. doi: 10.1615/jenvpathtoxoncol.v24.i3.40. [DOI] [PubMed] [Google Scholar]
- 39.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4 + CD25 + regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med. 2014;20:1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- 41.Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity. 2016;44:683–697. doi: 10.1016/j.immuni.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci USA. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 45.Komai T, Inoue M, Okamura T, Morita K, Iwasaki Y, Sumitomo S, et al. Transforming growth factor-β and interleukin-10 synergistically regulate humoral immunity. Front Immunol. 2018;9:1364. doi: 10.3389/fimmu.2018.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palomares O, Martín-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun. 2014;15:511–520. doi: 10.1038/gene.2014.45. [DOI] [PubMed] [Google Scholar]
- 47.Chou WC, Levy DE, Lee CK. STAT3 positively regulates an early step in B-cell development. Blood. 2006;108:3005–3011. doi: 10.1182/blood-2006-05-024430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding C, Chen X, Dascani P, Hu X, Bolli R, Zhang HG, et al. STAT3 signaling in B cells is critical for germinal center maintenance and contributes to the pathogenesis of murine models of lupus. J Immunol. 2016;196:4477–4486. doi: 10.4049/jimmunol.1502043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, et al. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS ONE. 2013;8:e62855. doi: 10.1371/journal.pone.0062855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmetterer KG, Pickl WF. The IL-10/STAT3 axis: contributions to immune tolerance by thymus and peripherally derived regulatory T-cells. Eur J Immunol. 2017;47:1256–1265. doi: 10.1002/eji.201646710. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mielle J, Audo R, Hahne M, Macia L, Combe B, Morel J, et al. IL-10 producing B cells ability to induce regulatory T cells is maintained in rheumatoid arthritis. Front Immunol. 2018;9:961. doi: 10.3389/fimmu.2018.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, et al. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol. 2014;44:1728–1736. doi: 10.1002/eji.201344062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi J, Feng J, Xie J, Mei Z, Shi T, Wang S, et al. Targeted blockade of TGF-β and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts. Sci Rep. 2017;7:8660. doi: 10.1038/s41598-017-09020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hjelmeland MD, Hjelmeland AB, Sathornsumetee S, Reese ED, Herbstreith MH, Laping NJ, et al. SB-431542, a small molecule transforming growth factor-beta-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol Cancer Ther. 2004;3:737–745. [PubMed] [Google Scholar]
- 56.Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K, et al. SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells. Cancer Res. 2003;63:7791–7798. [PubMed] [Google Scholar]
- 57.Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou HQ, Liu MS, Deng TB, Xie PB, Wang W, Shao T, et al. The TGF-β/Smad pathway inhibitor SB431542 enhances the antitumor effect of radiofrequency ablation on bladder cancer cells. Onco Targets Ther. 2019;12:7809–7821. doi: 10.2147/OTT.S212596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato M, Matsubara T, Adachi J, Hashimoto Y, Fukamizu K, Kishida M, et al. Differential proteome analysis identifies TGF-β-related pro-metastatic proteins in a 4T1 murine breast cancer model. PLoS ONE. 2015;10:e0126483. doi: 10.1371/journal.pone.0126483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4 + and CD8 + T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S, Fridlender ZG, Dunn R, Kehry MR, Kapoor V, Blouin A, et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31:446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- 62.Maglioco A, Machuca DG, Badano MN, Nannini P, Camerano GV, Costa H, et al. B cells inhibit the antitumor immunity against an established murine fibrosarcoma. Oncol Lett. 2017;13:3225–3232. doi: 10.3892/ol.2017.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 66.Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biragyn A, Lee-Chang C. A new paradigm for an old story: the role of regulatory B cells in cancer. Front Immunol. 2012;3:206. doi: 10.3389/fimmu.2012.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.