Abstract
Photodynamic therapy (PDT) is an emerging clinical treatment that is expected to become an important adjuvant strategy for the immunotherapeutic cancer treatment. Recently, numerous works have reported combination strategies. However, clinical data showed that the anti-tumor immune response of PDT was not lasting though existing. The immune activation effect will eventually turn to immunosuppressive effect and get aggravated at the late stage post-PDT. So far, the mechanism is still unclear, which limits the design of specific correction strategies and further development of PDT. Several lines of evidence suggest a role for TGF-β1 in the immunosuppression associated with PDT. Herein, this study systematically illustrated the dynamic changes of immune states post-PDT within the tumor microenvironment. The results clearly demonstrated that high-light-dose PDT, as a therapeutic dose, induced early immune activation followed by late immunosuppression, which was mediated by the activated TGF-β1 upregulation. Then, the mechanism of PDT-induced TGF-β1 accumulation and immunosuppression was elucidated, including the ROS/TGF-β1/MMP-9 positive feedback loop and CD44-mediated local amplification, which was further confirmed by spatial transcriptomics, as well as by the extensive immune inhibitory effect of local high concentration of TGF-β1. Finally, a TGF-β blockade treatment strategy was presented as a promising combinational strategy to reverse high-light-dose PDT-associated immunosuppression. The results of this study provide new insights for the biology mechanism and smart improvement approaches to enhance tumor photodynamic immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03479-3.
Keywords: PDT, TGF-β1, Immunosuppression, TGF-β blockade, Digital spatial profiler
Introduction
Photodynamic therapy (PDT) is a Food and Drug Administration (FDA)-approved anti-tumor therapy [1], with advantages of noninvasiveness, spatiotemporally controllability and low therapy resistance, which is applied as an ideal combination or alternative therapy in clinic [2]. Its direct anti-tumor effect depends on the tumor cell killing activity of the reactive oxygen species (ROS), which are generated by photosensitizers excited by laser. Recently, numerous studies have focused on PDT improvement strategies by overcoming unsatisfactory photosensitizers properties [3], tumor hypoxia [4] and low laser penetration of tissue [5]. More importantly, PDT has also been demonstrated to exert indirect tumor suppression effect through activating anti-tumor immune response [6], which is more attractive for designing enhanced combinational photodynamic immunotherapy [7–9].
However, part of clinical cases shows that the immune activation effect induced by PDT does not last long [6, 10, 11]. In fact, the antitumor immune response post-PDT only occurs at the early stage and PDT could induce immunosuppression effect on tumor microenvironment (TME) at the late stage [12–14]. The reasons and mechanisms for this dynamic variation are still unclear and certainly limit the application of PDT. The revelation of its inner cause may precipitate the exploitation of smarter combinational strategies for the photodynamic immunotherapy. By far, previous opinions usually attribute this phenomenon to a normal result of inflammation elimination and tissue repair [12]. What cannot be ignored is that tumor itself has hallmarks of immune escape, with a range of immunosuppressive mechanisms in TME [15]. It reminds us whether these intrinsic immunosuppression mechanisms in TME contribute to such result.
Transforming growth factor-beta 1 (TGF-β1) is a cytokine that mediates tumor immune escape and immunosuppression through an extensive effect [16, 17], attracting our concern. Previously, an early study has preliminarily confirmed that the combination of TGF-β blockade with PDT could markedly augment the cure rates of tumors [18], suggesting that TGF-β1 may reduce PDT-induced immune activation and tumor control. Furthermore, several recent studies reported that PDT on skin diseases could activate the latent TGF-β1 and induced downstream signaling [19–21], which may also probably exist in PDT of tumors. Coincidentally, the mechanism of immunosuppression post-radiotherapy, another classic tumor therapy, has been previously clarified [22, 23], which was mediated by the upregulated TGF-β1 induced by the generated ROS from radiotherapy. Inspired from the above evidence, since PDT also generates large amounts of ROS, it seemed likely that TGF-β1, upregulated by PDT-induced ROS, may be associated with the tumor immunosuppression aggravating at the late stage post-PDT. However, as TGF-β1 also mediates a range of important physiological functions such as wound healing [24] and immune homeostasis [25], simply conducting the long-term systemic TGF-β blockade may lead to adverse effects. How to specifically block the aggravating immunosuppression in the tumor sites post-PDT needs the distinct elaboration of this inner mechanism from the perspective of occurrence and development.
In this work, taking a triple-administrations of high-light-dose PDT as models [26], we firstly investigated the dynamic changes of immune status in TME during PDT and found that PDT could activate antitumor immune response at the early stage, but cause tumor immunosuppression aggravating at the late stage, which could be reversed by TGF-β blockade. On this basis, we confirmed the key role of TGF-β1 in aggravation on tumor immunosuppression and clarified its local accumulation mechanism via a ROS/TGF-β1/Matrix metalloproteins-9 (MMP-9) positive feedback loop and CD44-mediated local amplification using spatial local analysis. This study systemically revealed the mechanism that PDT can aggravate tumor immunosuppression through upregulating TGF-β1 in TME and confirmed the potential application value of TGF-β blockade in reversing the immunosuppression as well as prolonging the duration of immune activation, which provides novel enlightenment for the smart design of organically synergistic tumor photodynamic immunotherapy in clinic.
Materials and methods
Experimental design
The PDT regimen was as follows. In vitro: Chlorin e6 (Ce6, Meilunbio, MB5006) at a concentration of 0.4 μg/mL was incubated with cells. After 2 h of uptake, cells were irradiated by laser (638 nm, 300 mW/cm2) for 2 min (36 J/cm2). In vivo: 2.5 mg/kg Ce6 was intratumorally injected into murine tumors. 2 h after injection, tumors were locally irradiated by laser (638 nm, 300 mW/cm2) for 5 min through a noninvasive surface irradiation approach (a total light dose of 90 J/cm2 as a high-light-dose PDT model). PDT was conducted once every 3 days for a total of three treatments.
LY2109761 (LY, TOPSCIENCE, T2123, Shanghai, China), a novel selective TGF-β receptor type I/II dual inhibitor (Supplementary Fig. 1a), was used for TGF-β blockade. The dose of LY was 0.8 μg/mL in vitro and 5 mg/kg for intratumoral injection in vivo. The administration route and timing during PDT were also consistent with that of Ce6.
Cell culture
4T1 breast cancer cells and RAW264.7 macrophages were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultured in complete RPMI 1640 cell culture medium (Gibco) containing 10% FBS (Gibco) and maintained at 37 °C in 5% CO2 atmosphere. The medium was replaced three times weekly, and the cells were passaged using 0.05% trypsin/EDTA (Corning) and preserved at early passages.
Mice and tumor modeling
Six- to eight-week-old female BALB/c mice were purchased from the Institute of Comparative Medicine Yangzhou University. All animal experiments were performed according to the guidelines for laboratory animals established by China Pharmaceutical University (SYXK 2021-0011). 1 × 106 4T1 cells were injected into the fourth breast fat pad of mice to establish a tumor-bearing model. After 2 weeks, tumor volume was measured and is calculated with the following formula: V = (length × width2)/2.
Immune analysis in vivo
Administration: As described above, the 4T1 orthotopic breast cancer model was established, and continuous PDT was performed. Animals were killed, n = 6, from each group. The groups included: 1 day before PDT (Pre), 12 h after each PDT treatment (1st, 2nd and 3rd), and the 5th, 7th and 10th days after the last PDT (Post5d, Post7d and Post10d). Tumor tissue was removed to prepare cell suspension for flow cytometry. A control group without PDT (control) and a control group with PDT combined with LY (PDT + TGF-β blockade) were also set.
Pretreatment: Lymph nodes were directly added with MACS (500 mL PBS + 2 mL 0.5 M EDTA + 5 mL FBS) and filtrated with a 200-mesh cell sieve. Tumors were removed, minced and digested with 1 mg/mL type IV collagenase (Gibco) at 37℃ for 1 h. Pre-cooled MACS solution was used to stop digestion; then, cells were ground through a 200-mesh filter, suspended in an appropriate amount of 40% Percoll (Sigma), and then gradiently centrifuged (Thermo, 1400 × g, 1/9) for 20 min. Red blood cell lysate (Solarbio) was used to removed red blood cells as needed.
Staining: First, samples were blocked on ice for 15 min with anti-CD16/32 antibody (Biolegend). Then, cells were stained with combinations of conjugated antibodies in sequence (see Supplementary information). For intracellular staining, fixation and permeabilization were performed (Biolegend, Cyto-Fast™ Fix/Perm Buffer Set). Each step should be performed on ice for 20 min in the dark and washed twice with 1 × Wash Buffer (Biolegend). After passing through a 200-mesh sieve, flow cytometry was performed (BD, LSRFortessa) and results were analyzed by Flowjo (VX).
Flow cytometry: Seven tumor-associated immune cell subtypes were assessed: tumor-associated macrophages (TAMs, M1 TAMs: F4/80+CD64+iNOS+, M2 TAMs: F4/80+CD64+Arg-1+), tumor-associated neutrophils (TANs, N1 TANs: CD11b+Ly6G+iNOS+, N2 TANs: CD11b+Ly6G+Arg-1+), myeloid-derived suppressor cells (MDSCs: CD11b+Gr1+), matured dendritic cells (DCs: CD11c+CD80+CD86+), CD8+ T cells and CD4+ T cells (CD3+CD8+ and CD3+CD4+, respectively) and regulatory T (Treg) cells (CD3+CD4+CD25+).
Principal component analysis (PCA)
All data for different time points and groups were imported into SPSS 22.0 analysis software for PCA. Among them, MDSCs and Treg cells were negatively correlated indicators of the immune environment, so opposite numbers were taken before statistics was performed. Principal components were obtained based on the contribution rate of each variable. Cells with the greatest influence were identified within the principal component loading matrix and the rubble diagram. These cells were studied as the main object in the following experiments. Further, using the principal components with a cumulative variance contribution rate over 60%, a comprehensive scoring equation was obtained according to the ratio of each principal component variance contribution rate to the cumulative variance contribution rate. The comprehensive principal component score was calculated to describe the TME immune status comprehensively and quantitatively. Moreover, the time points of immune activation/suppression at the early/late stages of PDT (Postearly, Postlate) were identified, and the correction effect of the TGF-β receptor kinase inhibitor on immunosuppression at the late stage post-PDT was investigated.
Incubation of RAW264.7 cells with conditioned medium to assess isotype transition
Tumors from different groups were homogenized and centrifuged. The groups included: 1 day before PDT (Pre), 1 day and 10 days after the last PDT (Postearly and Postlate), and the control group at the same time points without PDT (CGearly and CGlate). The level of activated TGF-β1 was measured by ELISA (Neobioscience). Supernatants were mixed with RPMI 1640 medium at a ratio of 1:5 and filtered with a 0.22-μm filter as tumor-conditioned medium to culture with RAW264.7 macrophages for 48 h. In addition, a blank control without tumor-conditioned medium was set, and a Postlate conditioned medium with sufficient TGF-β1 neutralizing antibody (Bioxcell, BE0057, 50 μg/mL) was prepared as a negative control (Postlate + TGF-βdown). Flow cytometry was used to detect the levels of iNOS (PE conjugated, eBioscience, 12-5920) and Arg-1 (APC conjugated, eBioscience, 17-3697) in RAW264.7 cells for subtype transition assessment. ELISA was used to assess secreted levels of IL-1β, TNF-α and IL-10 in cell supernatants (Neobioscience).
ROS/TGF-β1/MMP-9 positive feedback loop assessment
In vitro: 4T1 cells in logarithmic growth phase were subjected to non-cytotoxic dose PDT (Ce6 0.4 μg/mL, 36 J/cm2) and a control group without PDT (only Ce6 was added without laser irradiation) was set. After 24 h, cell lysates were assayed for ROS using a DCFH-DA Reactive Oxygen Species Assay Kit (Beyotime). Supernatants were collected for detection of activated TGF-β1 (Neobioscience) and MMP-9 (Bioworld) using ELISA. The results were divided by the cell protein level detected using BCA protein assay kit to correct the cell count differences. Other groups included: 4T1 cells post-PDT were treated with 1 mg/mL ROS scavenger, acetylcysteine N-Acetyl-L-cysteine (NAC, Beyotime), identified as ROSdown, 50 μg/mL TGF-β1 neutralizing antibody (Bioxcell, BE0057), identified as TGF-β1down, 1 μg/mL GM6001 (Selleck, S7157), identified as MMP-9down in sequence. Another 4T1 cells without PDT were treated with 1000 × H2O2, identified as ROSup, 5 ng/mL recombinant TGF-β1 (Proteintech, HZ-1011), identified as TGF-β1up and 10 ng/mL recombinant MMP-9 (Abcam, ab39309), identified as MMP-9up.
In vivo: Mice bearing 4T1 tumors for 2 weeks either received PDT (Postearly, Postlate or did not receive PDT (CGearly, CGlate). Negative control groups of mice received intraperitoneal injections of sufficient TGF-β1 neutralizing antibody (200 ng per mouse), identified as Postlate + TGF-βdown, or GM6001 (200 ng per mouse), identified as Postlate + MMP-9down, during PDT. Immunohistochemistry and immunofluorescence were used to assess levels of ROS, TGF-β1, MMP-9, and P-SMAD2 (to assess TGF-β1 downstream signaling within tumor tissue).
CD44-mediated local amplification effect investigation
RAW264.7 cells in logarithmic growth phase were incubated for 24 h with lipopolysaccharide (LPS, Solarbio), as a positive control group, or with Postearly tumor-conditioned medium for 24 h. A blank control group and a negative control group (conditioned medium with 50 μg/mL CD44 neutralizing antibody, BE0262) were prepared. CD44 expression was assessed by flow cytometry (FITC conjugated CD44 antibody, BD, 561,859). Based on the Postearly tumor-conditioned medium incubation group, a CD44down group was established (the cells were pre-incubated with CD44 neutralizing antibody). Nuclei of RAW264.7 cells for each group were labeled with DAPI and injected at a concentration of 1 × 106 cells into the tumors of mice. After 5 days, the animals were killed, tumor-frozen sections prepared, and the distribution of TGF-β1 and MMP-9, as well as their co-localization with CD44 were assessed with a laser confocal microscope (Zeiss, LSM800).
Tumors from the Postearly group, blank control group, and negative control group (intraperitoneal injection of 200 ng of CD44 neutralizing antibody per mouse during PDT) were obtained. CD44 level of TANs (labeled with Ly-6G, Abcam, ab25377) and TAMs (labeled with F4/80, CST, 71299S), the distribution of TGF-β1 (Proteintech, 21898-1-AP), and MMP-9 (Proteintech, 10375-2-AP), as well as their co-localization with CD44 (R&D, AF6127) were assessed by immunofluorescence.
Digital spatial profiling
Tumors from 4T1 mice that received PDT were formalin-fixed and prepared as paraffin-embedded tissue sections (FFPE) of Post1d, Post5d and Post10d were prepared. Regions of interest (ROIs) were selected according to labeled fluorescence, and the whole transcriptome atlas (WTA) of ROIs containing 1.8 × 104 genes was analyzed using GeoMx DSP technology (FynnBio) to investigate the immune status of the local microenvironment. In detail, FFPEs were subjected to antigen retrieval (citrate buffer pH6) and stained with a cocktail of antibodies labeled with photocleavable DNA-indexing oligos. Fluorescent antibodies were used to identify tumor (PanCK), immune cells (CD45) and TGF-β1 (Bioss, bs-0086R). Tissues were imaged by fluorescence microscopy on the GeoMX platform, and ROIs were chosen for molecular profiling (300 μm). ROIs were exposed to ultraviolet light (365 nm) to release oligos which were captured via microfluidics and stored in individual wells of a microtiter plate. Following collection from all ROIs, oligos were hybridized to unique four-color, six-spot optical barcodes and enumerated on the nCounter platform. Data were normalized to ERCC-sequence specific probes to control for technical variation in hybridization efficiency, followed by area normalization to control for ROI size and control IgG to normalize for background. Data were visualized by unsupervised hierarchical clustering or grouped dot plots.
Anti-tumor and anti-metastasis activity in vitro and in vivo
The antitumor activity in vivo was evaluated in 4T1 tumor-bearing mice. 4T1 tumor-bearing mice were randomly divided into three groups when the tumor volume reached approximately 100 mm3. The mice were treated with saline, PDT or PDT + LY at the LY dose of 5 mg/kg. The weight of the mice and the length and width of the tumors were recorded every three days. The mice were killed on day 18, tumors and lungs were harvested, fixed in 4% paraformaldehyde solution and weighed. H&E staining was used to assess lung metastasis of tumors, which was labeled in dark dots.
Statistical analysis
All experiments were performed at least in triplicate. All data are shown as means ± SD, unless noted otherwise. Statistical analysis was performed with unpaired two-tailed Student’s t test or ordinary one-way ANOVA. For all statistical tests, α was limited to 0.05 and P < 0.05 was considered statistically significant. All semi-quantification of graphs using optical density was performed using Image J or Image Pro Plus.
Results
Immune state post-PDT shows early activation and late suppression
To systematically investigate PDT-induced dynamic immune changes, we used Ce6, a classic type-II photosensitizer, for intratumoral injection into 4T1-bearing mice to exclude the interference from systemic circulation and tumor targeting efficiency. Also, the Ce6 dose was reduced compared with intravenous injection to prevent excess local concentration. 4T1 is a refractory triple-negative breast cancer cell model, prone to metastasis and immune escape [27], as well as a superficial tumor suitable for PDT. Details of administration and detection are shown in Fig. 1a. After killing, tumor-associated immune cells, including TAMs, TANs, DCs, MDSCs, CD4/CD8+ T cells, and Treg cells were assessed by flow cytometric (FCM) analysis. N1 TANs can destroy tumor cells. M1 TAMs can engulf apoptotic cells and present tumor-associated antigens. CD4/CD8+ T cells are activated by matured antigen-presenting DCs. Each of these cell populations contributes to an antitumor immune response [28]. In this process, immunosuppressive factors (like TGF-β1) secreted by tumor or immunosuppressive cells (such as pro-tumor type of N2 TANs, M2 TAMs, Treg cells, MDSCs) in TME would downregulate the function of various immune cells. For TAMs and TANs, as there were different polarization states, we used M1/M2 and N1/N2 to represent the dominant state. The greater the ratio, the greater the immune cell activation, which was similar to the analysis of matured DCs and CD4+ or CD8+ T cells. As shown in Supplementary Fig. 1b–f, compared with the control group, within the PDT course for three times, the PDT group showed an upregulation of most immune cells in different degree. However, a decline of these immune cells was found post-PDT for different days, the amount of some cells was even less than the control group in Post10d, revealing the immunosuppression trend. The opposite change also occurred in MDSC and Treg cells due to their immunosuppression property (Supplementary Fig. 1 g, h). This phenomenon demonstrated that there was an immune activation effect during PDT and an immunosuppression effect 10 days post-PDT. The differences of immune effects between different PDT regimens, such as light doses and treatment times, were also investigated and discussed (Supplementary Fig. 2). The results showed that compared to single low-light-dose PDT, multiple high-light-dose PDT could lead to a better anti-tumor effect, but induce a more aggravated immunosuppression and metastasis trend.
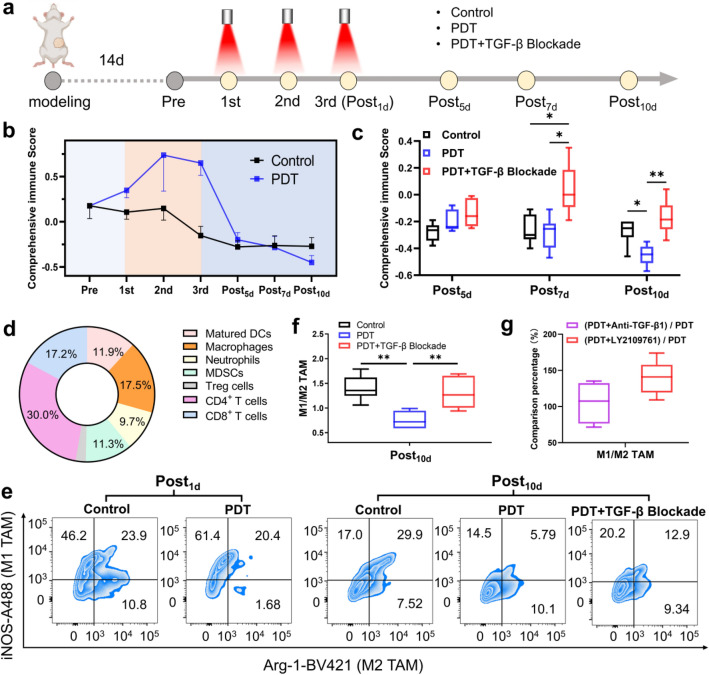
Fig. 1.
Dynamic changes of immune state post-PDT from immune activation early to immunosuppression late. a Experimental schedule of PDT and immune analysis of 4T1-bearing mice. After modeling, the mice received PDT for three times and were killed for tumor flow cytometry analysis at different days post-PDT. The detailed administration schedule is described in “Experimental design” section. b Comprehensive score curves of immune state post-PDT. The comprehensive immune score quotation of Y = 0.24823*X1 + 0.18815*X2 + 0.18321*X3 was calculated. For different experimental points, X1, X2 and X3 derived from PCA of seven detection indicators were imported into the quotation for the comprehensive immune score. c Comprehensive immune score of different groups at different days post-PDT. d Pie diagram of comprehensive contribution of different cells to principal components. Data are calculated according to the comprehensive score quotation described above. e Flow cytometric percentage graphs of TAMs at Post1d and Post10d. f Quantitation analysis of M1/M2 TAMs at Post10d. g Comparison of M1/M2 TAM between PDT and different TGF-β blockade strategies (TGF-β1 antibody or receptor inhibitor). Data are presented as mean ± SD (n = 6). *P < 0.05, **P < 0.01
Since TGF-β1 was one of the most secreted immunosuppressive factors, considering its extensive inhibition effect on TME, we then investigated whether TGF-β1 was associated with the aforementioned phenomenon, we added the group of PDT with TGF-β blockade using LY, a TGF-β receptor kinase inhibitor [29]. Notably, TGF-β blockade reversed the change in immune cell populations (Supplementary Fig. 1). Especially at Post10d, TGF-β blockade corrected the observed immunosuppression within the PDT group for the seven immune cell subtypes, suggesting immune enhancement (Supplementary Fig. 4). The similar reversal effect of TGF-β blockade was also found in different PDT regimens (Supplementary Fig. 3). Therefore, TGF-β1 appears to be involved in PDT-induced immunosuppression and the relative mechanism worth investigating.
In order to comprehensively analyze the dynamic change in multiple immune cell populations and find the susceptible cells for further study, it is necessary to carry out multivariate statistical analysis. Analysis was based on curve change for seven immune cell populations at different time points and groups. For MDSCs and Tregs cells, opposite numbers were used for PCA due to their negative relationship with the immune environment. KMO and Bartlett’s test were used (Supplementary Table 1) and a KMO value of 0.532 was obtained, which was higher than the minimum standard. The p value for the Bartlett sphericity test was lower than 0.001, indicating suitability for factor analysis. Then, three principal components (X1, X2, X3) were obtained from the variance contribution rate (Supplementary Table 2). The scores for the three principal components were multiplied by the cumulative weights of the principal components to obtain the comprehensive score equation: Y = 0.24823 * X1 + 0.18815 * X2 + 0.18321 * X3. In that manner, the comprehensive score for the tumor immune state was calculated, and the comprehensive curve for immune state post-PDT (Fig. 1b) was drawn for further analysis. As a result, the dynamic changes of the immune state post-PDT could be concluded: Between the PDT process, the immune activation state was getting stronger (Fig. 1b); With time going on post-PDT, the immune state became suppressive and lower than normal by the 10th day (Fig. 1c). This process was quite different from the control group, where the immune state was gradually decreased over time due to the immunosuppression progress of tumors. Meanwhile, PDT with TGF-β blockade could reverse the immunosuppression effectively (Fig. 1c). According to the principal component matrix (Supplementary Table 3) and the pie diagram of comprehensive contribution of different cells to the principal components (Fig. 1d), it could be found that CD4+ T cells and macrophages had the greatest impact on the principal components, indicating that it could be used as model cells in subsequent mechanism study. Since macrophages were the main infiltrating immune cells in most solid tumors [30], and RAW264.7 cells were commercially available and a common cell line for in vitro macrophage functional analysis [31], we decided to focus on TAMs. From the dynamic change of immune state of TAMs (Supplementary Fig. 1b), we selected two time points post-PDT for further study, Post1d as the most immune activated stage and Post10d as the most immunosuppressive stage (Fig. 1e, f). Moreover, we also compared the immunosuppression reversal effect of different TGF-β blockade strategies; the results showed that TGF-β1 receptor kinase inhibitor (LY) exhibited stronger reversal effect than TGF-β1 antibody (Fig. 1g), suggesting that transient suppression of antibodies on existing TGF-β1 could not deal with its continuous production post-PDT. Instead, inhibition of TGF-β1 receptor-mediated downstream signaling may be more effective regardless of the amount of TGF-β1.
TGF-β1 is a key factor contributing to the immunosuppression post-PDT
To investigate whether TGF-β1 is a key factor contributing to the immunosuppression post-PDT, we determined the content of activated TGF-β1 in tumor homogenates of each group, including the tumors before PDT (Pre), the early stage (1 day post-PDT, Postearly), the late stage (10 days post-PDT, Postlate) and the correspondent control groups (Fig. 2a). As can be seen in Fig. 2c, for the group without PDT, the activated TGF-β1 level gradually increased, indicating transformation to tumor immunosuppressive microenvironment. Instead, for the groups with PDT, the activated TGF-β1 level increased a little in the Postearly group and sharply increased in the Postlate group. It is probable that the acute inflammatory reaction caused by PDT during the early stage places the TME in a pro-inflammatory and immune enhancement state, overwhelming the weak pro-tumor effect induced by the activated TGF-β1. At the late stage post-PDT, as the inflammation subsided, an anti-inflammatory microenvironment resulted. Dead tumor cells might release more signals to the survived cells, which further exacerbated the immunosuppressive microenvironment. To assess the immunological effect of tumor homogenates on TME, we used them as conditioned medium for cell culture in vitro and prepared one more group adding TGF-β1 neutralizing antibodies based on the homogenates of the Postlate group, where most activated TGF-β1 could be inhibited (Fig. 2c).
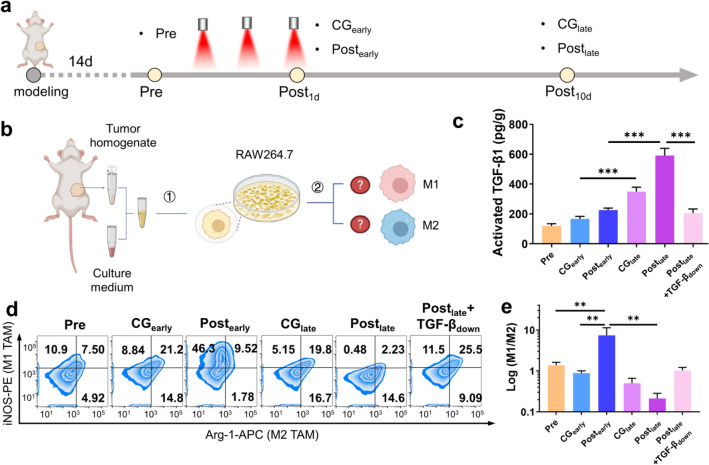
Fig. 2.
TGF-β1 is a key factor contributing to the immunosuppression post-PDT. a The different treatments and acquisition time points of tumor samples. The samples were grouped as: before PDT (Pre), Post1d w/o PDT (CGearly), Post1d w/ PDT (Postearly), Post10d w/o PDT (CGlate) and Post10d w/ PDT (Postlate). b The scheme of study methods using conditioned medium prepared by tumors post-PDT in vitro. (1) The tumor homogenate supernatant was mixed with RPMI1640 medium as tumor-conditioned medium, as well as a negative control using Postlate conditioned medium added with sufficient TGF-β1 neutralizing antibody as Postlate + TGF-βdown. (2) RAW264.7 cells were incubated with different conditioned medium for macrophages polarization analysis. c Activated TGF-β1 Content in different conditioned medium determined by ELISA. d Flow cytometric percentage graph of RAW264.7 cells incubated with different conditioned medium for 48 h. e Logarithm of M1/M2 ratio to observe the degree of RAW264.7 cells polarization incubated with different conditioned medium for 48 h. Data are presented as mean ± SD (n = 3). **P < 0.01, ***P < 0.001
According to the results in the immune analysis, macrophages showed a similar change to the PDT comprehensive score curve (Fig. 1b and Supplementary Fig. 1b) and are therefore suitable as an in vitro model. RAW264.7 cells are a macrophage type classic cell line often used for polarization studies and herein were incubated with the above-conditioned media (Fig. 2b). The phenotypes of these cells were analyzed by flow cytometry. We chose iNOS as a M1 macrophage marker and Arginase-1 (Arg-1) as a M2 macrophage marker. At the same time, we used the M1/M2 value to assess the degree of cell polarization. Results are shown in Fig. 2d,e. Overall, the control group (Pre, CGearly, CGlate) showed a gradually decreased degree of M1 polarization, indicating the tumor malignant progress with time going on. For Postearly group, its M1/M2 value was significantly augmented, which might be due to the acute pro-inflammatory microenvironment generated at the early stage post-PDT, mobilizing macrophages to exert their immune activation function. While the M1/M2 value decreased significantly at the late stage post-PDT, indicating that macrophages could generate obvious M2 polarization and promote the formation of immunosuppressive microenvironment, which will be an adverse effect at the late stage post-PDT. Further, we found the group adding the TGF-β1 antibody could reverse M1/M2 values, which implied that the accumulated activated TGF-β1 promoted the construction of tumor immunosuppressive microenvironment post-PDT. The inflammatory cytokines determination in vitro and the tumor growth and metastasis evaluation in vivo also exhibited a similar trend (Supplementary Figs. 5, 6).
ROS/TGF-β1/MMP-9 positive feedback loop promotes TGF-β1
Then, to clarify the mechanism of PDT-induced TGF-β1 accumulation, we tried to proceed from the relationship of PDT and TGF-β1. As is well known, PDT kills tumors through the much ROS generated by photosensitizers within cells, which can release activated TGF-β1 from the latent TGF-β1, similar to MMP-9. Besides, activated TGF-β1 could initiate the downstream pathway to promote ROS and MMP-9. Therefore, whether there existed a positive feedback loop between ROS/TGF-β1/MMP-9 post-PDT was evaluated (Fig. 3d). 4T1 cells were used as models. PDT was the experimental group. PDT with different inhibitors was the negative control. Cells with recombinant proteins were the positive controls. ROS, activated TGF-β1, and MMP-9 levels were assessed. The PDT used here was at a non-toxic dose of 0.4 μg/mL Ce6 at 36 J/cm2, which was lower than 20% IC50 dose (Supplementary Fig. 7). The detailed PDT procedure could be seen in Materials and methods. According to the results (Fig. 3a–c), the PDT group had an improved effect on the three detection indexes compared with the control group, indicating that the generation of ROS post-PDT could not only activate the latent TGF-β1 but also promote the secretion and MMP-9. Through a series of inhibition and upregulation of each factor, it could be seen that the concentrations of most negative controls were below the control group, and most positive controls were above the control group, indicating the existence of a ROS/MMP-9/TGF-β1 positive feedback loop. The similar positive feedback loop effect was also found and discussed in different PDT regimens (Supplementary Fig. 8).
Fig. 3.
ROS/TGF-β1/MMP-9 positive feedback effect promotes TGF-β1 accumulation. a Content of ROS detected by DCFH-DA fluorescence kit in cell lysis solution generated in 4T1 cells under different treatments for 24 h. b–c Content of MMP-9 and activated TGF-β1 detected by ELISA in cell supernatant secreted by 4T1 cells under different treatments for 24 h. d The scheme of the ROS/TGF-β1/MMP-9 positive feedback loop in tumor sites post-PDT. e Immunohistochemistry of ROS, MMP-9, TGF-β1 and the downstream P-SMAD2 expression in tumors under different treatments post-PDT. Scale bar represents 100 μm. f–i Quantitative analysis of ROS, MMP-9, TGF-β1, and P-SMAD2 in the immunohistochemistry results. The PDT procedure for a–c: 0.4 μg/mL Ce6 was administrated to cells. 2 h after cell uptake, and cells were irradiated by laser (638 nm, 300 mW/cm2) for 2 min. The semi-quantification of graphs for (f-i) was performed using Image J. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001
To evaluate the positive feedback loop in vivo, ROS, MMP-9, TGF-β1 and downstream P-SMAD2 expression were observed by immunohistochemistry (Fig. 3e–i). It could be seen that with progression of the tumor, various factor levels were gradually increased, with levels in the Postlate group greater than those in the control group. These results indicated that excessive ROS generated at the early stage led to the full activation of this positive feedback loop and cause the continuously increasing expression of TGF-β1 and MMP-9, which constructed the immunosuppressive TME at the late stage. By adding TGF-β1 or MMP-9 antibodies, the factors level was downregulated, which in turn proved the existence of the positive feedback loop. The similar results drawn from immunofluorescence of MMP-9 and TGF-β1 expression are shown in Supplementary Fig. 9.
CD44-mediated local amplification effect promotes TGF-β1
During the early stage post-PDT, along with the generated ROS, an acute inflammation would occur caused by ROS-induced local damage, which would recruit more immune cells and upregulate the expression of their CD44 receptors in TME. CD44 receptors could further recruit MMP-9 through their binding affinity, providing more chances to achieve the above positive feedback (Fig. 4a). To investigate the local amplification effect of inflammation, we initially evaluated the expression of CD44 on the RAW264.7 cells incubated with Postearly conditioned medium by flow cytometry (Fig. 4b and Supplementary Fig. 10). Compared with the positive control in which LPS was used to induce inflammation and the negative control using CD44 antibodies, PDT could also induce inflammation and significantly upregulate CD44 expression of macrophages, suggesting the potential for local amplification.
Fig. 4.
CD44-mediated local amplification effect promotes TGF-β1 accumulation. a The scheme of CD44-mediated local amplification effect in tumor sites post-PDT. b Flow cytometry plot of the expression of CD44 receptors on the RAW264.7 cells incubated with different conditioned media for 24 h. c The scheme of study methods using nuclei-labeled RAW264.7 cells incubated with conditioned medium for constructing a tumor model mimicking recruitment of macrophages with different CD44 expression levels and immunofluorescence observation of local amplification of MMP-9 and TGF-β1. d Confocal images of MMP-9, CD44 and RAW264.7 cells in tumors injected with RAW264.7 cells incubated with conditioned medium. RAW264.7 cells were pre-stained by DAPI (blue). Scale bar represents 100 μm. e Immunofluorescence graphs of TAMs (labeled with F4/80 antibodies), CD44, TGF-β1, MMP-9 and DAPI in the tumor sections from 4T1 bearing mice under different treatments. Scale bar represents 100 μm. f Immunofluorescence graphs of TANs (labeled with Ly6G antibodies), CD44, TGF-β1, MMP-9 and DAPI in the tumor sections from 4T1 bearing mice under different treatments. Scale bar represents 100 μm. Due to limited channels of detection, the immunofluorescence of TGF-β1 and MMP-9 in e, f was conducted in separate tests
Then, we further verified the CD44-mediated local amplification in vivo. By injecting nuclei-labeled RAW264.7 cells incubated with conditioned medium around the 4T1 tumor of mice for another five days growth, we constructed a model mimicking the tumors recruiting macrophages with different levels of CD44 expression and observed whether CD44 had the function of local amplification of MMP-9 and TGF-β1 (Fig. 4c). The results are shown in Fig. 4d and Supplementary Fig. 11a. In the control group, the fluorescence of RAW264.7 cells and CD44 did not largely overlap, suggesting that the injected RAW264.7 cells were not completely polarized into M1 type, so the expression of CD44 was not obvious. For the LPS and Postearly groups, the fluorescence of CD44 was stronger, and the colocalization effect was more obvious, indicating that there were more M1 macrophages in the Postearly group and could induce more MMP-9 recruitment. Additionally, the group with CD44 receptors blockade resulted in weak fluorescence and poor MMP-9 localization. Similarly, the analysis of TGF-β1 suggested that MMP-9 promoted the enrichment of TGF-β1 (Supplementary Fig. 11b, c), which promoted the ROS/TGF-β1/MMP-9 positive feedback loop.
Further, to reflect the CD44-induced local amplification in TME post-PDT more authentically, we directly studied the tumor sections from 4T1-bearing mice at the early stage post-PDT. In Fig. 4e and Supplementary Fig. 12a, b, chemotaxis of TAMs (labeled using F4/80 antibody) in the Postearly group was better than that in the group without PDT, and the recruitment ability of the group with CD44 antibody before PDT also weakened, which was speculated that the chemotaxis of immune cells in vivo was inhibited by blockade of CD44 [32]. At the same time, due to the pro-inflammatory effect of PDT, CD44 expression of TAMs was upregulated and more MMP-9 was recruited, which further enriched and activated TGF-β1 through positive feedback loop. Similar results of TANs (labeled using Ly6G antibody) were also obtained (Fig. 4f and Supplementary Fig. 12c, d). So far, the mechanism of immunosuppression post-PDT mediated by TGF-β1 was clarified.
Spatial transcriptomics further depicts immunosuppression process post-PDT
After a series of validation and analysis from various aspects, to depict the immunosuppression process directly in situ, the technique of spatial transcriptomics was carried out [33]. The scheme of digital spatial profiler (DSP) is shown in Fig. 5a. DSP analysis technology first used the traditional immunohistochemical method to prepare paraffin section samples and then combined with the different chromogenic antibodies to distinguish tissue morphology or cell types, and nucleic acid labeled oligos to recognize targets specifically in situ. The nucleic acid chains from different region of interests (ROIs) were dissociated by UV photolysis technology, hybridized with capture probe and fluorescent probe, and quantified by the unique nCounter system to realize the analysis of expression profile in each ROI.
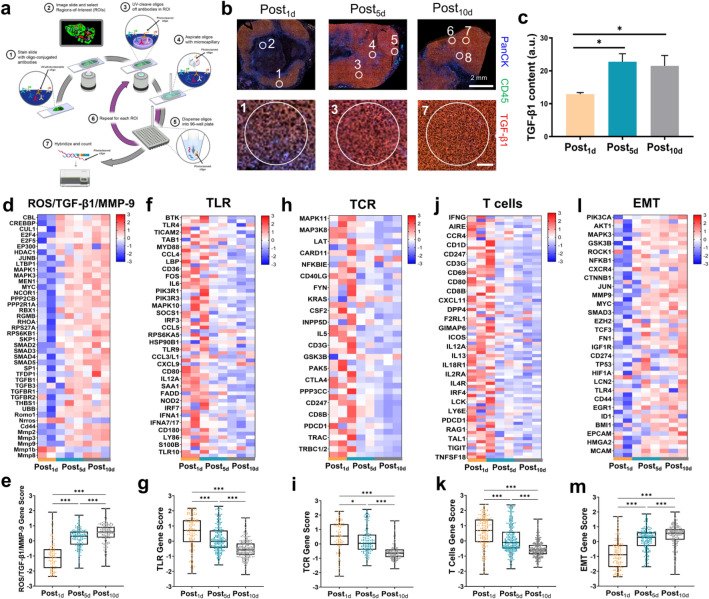
Fig. 5.
Spatial transcriptomics further depicts immunosuppression process post-PDT. a Scheme of DSP protocols. b Immunofluorescence images of tumor sections at different stages post-PDT and ROIs were marked by white circles. Scale bar in the lower row represents 100 μm. Tumor cells were labeled in blue (PanCK antibodies), immune cells were labeled in green (CD45 antibodies). c Quantitative analysis of TGF-β1 expression (labeled in red) in b. d Heat map of transcriptomics analysis of gene expression of ROS/TGF-β1/MMP-9 in ROIs from three groups. e Box plots of total gene expression of ROS/TGF-β1/MMP-9. f Heat map of transcriptomics analysis of gene expression of TLR in ROIs from three groups. g Box plots of total gene expression of TLR. h Heat maps of transcriptomics analysis of gene expression of TCR in ROIs from three groups. i Box plots of total gene expression of TCR. j Heat maps of transcriptomics analysis of gene expression of T cells in ROIs from three groups. k Box plots of total gene expression of T cells. l Heat maps of transcriptomics analysis of gene expression of EMT in ROIs from three groups. m Box plots of total gene expression of EMT. Data are presented as mean ± SD. *P < 0.05, ***P < 0.001. All the gene data had been processed through normalization, logarithmicization and Z-score standardization. The semi-quantification of graphs for c were performed using Image J
To elucidate the relationship between TGF-β1 and immune state of different tumor stages post-PDT, we firstly determined the activated TGF-β1 level by means of ELISA to select the suitable stages for DSP analysis (Supplementary Fig. 13). The results showed that the activated TGF-β1 level was gradually increased and highest at 5 days post-PDT; then, it seemed to reach the plateau, which declared that there would be other negative control mechanism to balance the activated TGF-β1 level for fear of infinite increase. On this basis, we chose tumors at 1, 5 and 10 days post-PDT for spatial transcriptomics analysis and selected 12 ROIs according to the different TGF-β1 levels marked in red. It could be seen that most regions of Post5d and Post10d tumor sections expressed large amounts of TGF-β1 (Fig. 5b, c). The heat map of transcriptomics exhibited that for the same group, ROI selected from different TGF-β1 levels made a big difference to the transcriptomics results, even the opposite trend appeared (such as A and B of Post5d, or C and D of Post10d in Supplementary Fig. 14). The reason for such a great difference could be the low TGF-β1 content in these ROIs (Supplementary Fig. 15). From the PCA, ROIs with high levels of TGF-β1 were distant from those with low levels (Supplementary Fig. 16). Thus, analysis of an entire tumor section would miss the nuance of individual ROIs, with spatial analysis providing for unique insights and advantages over other techniques. Differential gene expression by regions with distinct TGF-β1 levels was presented as a volcano plot (Supplementary Fig. 17).
To explore the effect of TGF-β1 on tumor immune state post-PDT, we selected the ROIs with high levels of TGF-β1 for further analysis and compare the differences in various signaling pathways. Firstly, we focused on the related transcripts of genes of ROS/TGF-β1/MMP-9 positive feedback loop and the CD44-mediated local amplification. From Figure 5d,e, most genes about TGF-β1 were upregulated over time, implying that albeit TGF-β1 levels plateaued, the downstream effect would continuously occur and influence the immune microenvironment. Besides, the ROS-related romo1, CD44, MMP-2, -3 and -9 genes all increased (Supplementary Figure 18), which was consistent with the previous results in protein levels. For immune related pathways, the T-cell receptor (TCR) signaling, Toll-like receptor (TLR) signaling and T cell-related genes exhibited a gradual decrease (Figure 5f–k), such as the gene expression of classical markers: CD4, CD8A, CD80 and CD86 (Supplementary Figure 19), reflecting the progressive immunosuppression state. When it came to the immunosuppressive genes, such as arginine metabolism and Treg cell-related pathways (Supplementary Figure 20), their upregulation provided further evidence of immunosuppression in TME. Last but not least, the rise of epithelial mesenchymal transformation (EMT)-related genes was also observed (Figure 5l, m), reminding us that the close inducement, TGF-β1 [34], was a risk factor for tumor metastasis at the late stage post-PDT. To sum up, the spatial transcriptomics analysis prompted that the risk of immunosuppression and metastasis post-PDT need more attention and developing appropriate strategies to realize effective reversal.
TGF-β1 blockade enhances anti-tumor effect of PDT
From above all, we have demonstrated the potential effect and the mechanism of TGF-β1 induced immunosuppression post-PDT. Faced with this dilemma, TGF-β blockade may be an alternative approach. Here, we conducted in vivo anti-tumor experiments to validate this strategy (Fig. 6a). We set the control, PDT and PDT + TGF-β blockade (LY) groups for a total three times of PDT treatments. Tumor growth and metastasis were evaluated. As can be seen in Fig. 6b, with time going, the tumor volume decreased more in the PDT + LY group, suggesting that TGF-β blockade could inhibit the TGF-β1-mediated protumor effect and control the tumor growth in some degree. What’s more, not only the final tumor weight reduced (Fig. 6c, d), the tumor metastasis in lungs was also obviously improved (Fig. 6e), which could be attributed to the TGF-β blockade as TGF-β1 was also a famous tumor metastatic factor. This explanation could be demonstrated from the result of immunohistochemistry analysis of TGF-β1 in situ (Fig. 6f, g). PDT with LY could significantly reverse the TGF-β1 accumulation post-PDT by blockade of TGF-β downstream signaling and the subsequent ROS/TGF-β1/MMP-9 positive feedback.
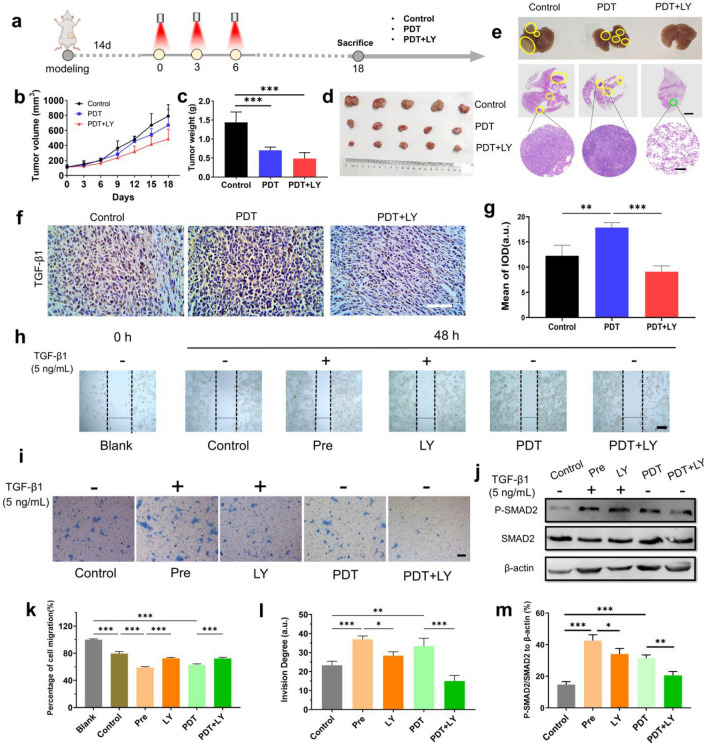
Fig. 6.
TGF-β1 blockade enhances anti-tumor effect of PDT. a Experimental schedule of PDT with or without LY and anti-tumor effect evaluation of 4T1-bearing mice. The detailed administration schedule is described in “Experimental design” section. b Tumor volume curve of 4T1-bearing mice under different treatments at different days during and post-PDT (n = 5). c Tumor weight of 4T1-bearing mice under different treatments at the end of observation day (n = 5). d Graphs of tumors from 4T1 bearing mice under different treatments at the end of observation day. (n = 5). e Graphs of lung metastasis from 4T1-bearing mice under different treatments at the end of observation day. The tumor metastases were marked by yellow circles. H&E stating of lung metastasis was also provided. The tumor metastases were marked by yellow circles. Scale bar represents 2 mm in the full view and 200 μm in the partial enlarged view. f Immunohistochemistry of TGF-β1 expression in tumors under different treatments at the end of observation day post-PDT. Scale bar represents 100 μm. g Quantitative analysis of TGF-β1 in the immunohistochemistry results (n = 3). h Graphs of scratch test to investigate cell migration of 4T1 cells under different treatments for 48 h. Scratches were marked by the dotted lines. Scale bar represents 100 μm. i Graphs of cell invasion test of 4T1 cells under different treatments for 48 h. The invaded cells were stained by 0.1% crystal violet. Scale bar represents 100 μm. j Western blot graphs of SMAD2 phosphorylation of 4T1 cells under different treatments for 48 h. k Quantitative analysis of migration rates of different groups in the scratch test (n = 3). l Quantitative analysis of invasion degree of different groups (n = 3). m Quantitative analysis of P-SMAD2/SMAD2 to β-actin in the Western blot results (n = 3). The PDT procedure for h–m: 0.4 μg/mL Ce6 was administrated to cells. 2 h after cell uptake, cells were irradiated by laser (638 nm, 300 mW/cm2) for 2 min. The semi-quantification of graphs for (g, k, l, m) was performed using Image J. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
In addition, we further evaluated the anti-metastasis effect in vitro. In the scratch test, a classic experiment to investigate cell migration, by exploring the changes of cell mobility in different treatment groups, as shown in Fig. 6h and k, we found that TGF-β1 pretreatment group can significantly promote migration, which was closely related to its functions of promoting EMT [35]. Instead, LY could effectively inhibit the phosphorylation of the TGF-β receptor, block the downstream pathway and alleviate the rapid migration. After the implementation of PDT under non-cytotoxic dose (Supplementary Fig. 7), the cells also migrated significantly, with reversed migration in the PDT + LY group (Supplementary Fig. 21). It was likely that PDT accelerated cell migration through generating more TGF-β1 [21]. By Transwell invasion assay, the number of cells invaded from the upper basement membrane was stained with crystal violet to compare the effects of different treatment groups on cell invasion. The results shown in Fig. 6i and l revealed that cells pretreated with TGF-β1 were more likely to invade, similar to those post-PDT. The PDT + LY group could effectively inhibit cell invasion, which also verified the superiority of this combination strategy.
As an inhibitor of TGF-β receptors, LY could block the downstream signaling pathways of TGF-β1, especially the classic SMAD pathways related to metastasis. Herein, we observed the level of SMAD2 phosphorylation as an index of TGF-β blockade (Fig. 6j and m). The results showed that P-SMAD2 was significantly upregulated in the PDT group after 48 h of treatment, indicating that PDT promoted TGF-β signaling. On the contrary, the PDT + LY group notably downregulated P-SMAD2, which could effectively reverse the potential TGF-β signaling activation effect of PDT alone. In conclusion, the combination of TGF-β blockade could overcome shortcomings of PDT at the late stage, which were local immunosuppression in the TME and potential risk of tumor metastasis induced by activation and regulation of TGF-β1 downstream signaling.
Discussion
As a clinical tumor treatment method, PDT has the advantages of space–time selectivity, noninvasiveness, low resistance to treatment and ease for combination therapy. At present, the limitations of PDT include insufficient penetration of photosensitizers and lasers, as well as tumor hypoxia. A large number of studies have reported specific improvement strategies [4, 5]. Besides, PDT can induce immunogenic cell death (ICD) effect [36], but the immune activation effect cannot last long [6, 10, 11], which promotes the research of PDT combined with other strategies to strengthen immunotherapy [7–9]. However, although enhanced immunotherapy efficacy was achieved through intelligent delivery systems, most of these strategies currently focused on combination of immunoenhancement or immune checkpoint inhibition, which were the lack of specificity. The former may cause adverse immune reactions due to over-activation of the immune system [37]. Although the latter is less toxic, it only targets a single link of tumor immune escape, resulting in limited beneficiary groups. In fact, the antitumor immune response of PDT only occurs at the early stage and PDT could induce immunosuppression effect within TME at the late stage [12]. This phenomenon is more obvious under the circumstances of multiple administrations using high light doses in clinic [13]. The mechanistic basis for this dynamic variation is still unclear, which will certainly limit the application of PDT. The aim of this study is to clarify the mechanism of immunosuppression induced at the late stage post-high-light-dose PDT, which could generate sufficient therapeutic effects, and find the key target or pathway, so as to provide insights for the design of targeted correction strategies to reverse adverse consequences caused by clinical high-light-dose PDT.
An early study showed that the combination of TGF-β antibody and PDT can significantly improve the tumor inhibition rates [18]. TGF-β1 is known to mediate tumor immune escape and immunosuppression through an extensive effect [16, 17], which could induce the pro-tumoral polarity of TAMs and TANs [38, 39], inhibit the maturation of DCs, decrease the anti-tumor activity of CD4+ and CD8+ T cells, recruit and induce the immunosuppressive MDSCs and Treg cells. Meanwhile, tumor cells, fibroblasts and the above immunosuppressive cells could secret large amounts of TGF-β1, to form a positive feedback loop for immunosuppression based on TGF-β1 [40]. Furthermore, several recent studies of skin diseases therapies have reported that PDT could activate TGF-β1 [19–21], and similar effects could also exist in tumor therapies. Combined with the clear mechanism of TGF-β1 in radiotherapy-induced immunosuppression [23], which therapy also generates ROS, these evidences supported our hypothesis and we decide to explore involvement of TGF-β1 in PDT-induced tumor immunosuppression. Firstly, from the dynamic tumor immunological evaluation and comprehensive score analysis of different stages of PDT in vivo, we find that the late stage post-PDT will indeed aggravate immunosuppression in which macrophages were the most influential cells. The important role of TGF-β1 is preliminarily verified by TGF-β blockade treatment group. Then, we find that there are significant differences in TGF-β1 levels within tumors from different treatment groups, with TGF-β1 greatest in the Postlate group. By incubating macrophages with conditioned medium in vitro, we detect the immunophenotype and observe the tumor progress, which is inoculated using tumor cells cocultured with these macrophages. Finally, we confirm the key role of TGF-β1, suggesting that it has the risk of promoting immunosuppression and tumor metastasis.
So how does PDT promote TGF-β1 accumulation and eventually lead to immunosuppression? Commonly, the secreted TGF-β1 is stored in extracellular matrix (ECM) in a latent form and can be appropriately activated based on the specific needs [41]. TME is comprised of a series of activation factors for TGF-β1. ROS is one of the main facilitators, which can not only activate TGF-β1, but also promote the expression and signal transduction, boosting the extensive immunosuppression effect of TGF-β1 within TME [42]. As is well known, PDT can produce large quantities of ROS, except for inducing cell apoptosis, triggering ICD effects and initiating antitumor immune response, ROS itself, like MMP-9 [43], can activate TGF-β1 released by apoptotic tumor cells. In addition, ROS can promote TGF-β1 expression and downstream signal transduction, as well as MMP-9 expression. The activated TGF-β1 can in turn facilitate the upregulation of ROS and MMP-9. So there actually exists a positive feedback loop between ROS, TGF-β1 and MMP-9 within TME, which was demonstrated in our study that ROS/TGF-β1/MMP-9 has positive feedback effect under PDT in vivo and in vitro. Moreover, acute inflammation caused by PDT at the early stage can recruit a large number of immune cells and upregulate the expression level of CD44 receptors [44]. Subsequently, MMP-9 is compartmentalized to the cell surface through docking to CD44 [45]. Hence, the ROS/TGF-β1/MMP-9 positive feedback loop will occur in the proximity of immune cells with upregulated CD44, giving rise to high local concentrations of TGF-β1, which would aggravate immunosuppression.
Acute inflammation caused by PDT at the early stage can remodel TME and activate systemic antitumor immune response. However, with the elimination of inflammation and the initiation of tissue repair at the late stage post-PDT, acute inflammation-caused local high concentration of TGF-β1 will continue to accelerate the immunosuppression within local TME, and then spread to the whole tumor, which is likely to significantly weaken the previously accumulated anti-tumor immune state. Therefore, local analysis has important guiding significance for the prognosis of overall immune microenvironment. Through spatial transcriptomic analysis, we find that the local high concentration of activated TGF-β1 has been formed at the 5th day post-PDT, and there is a significant difference in the expression of immune-related genes in ROIs with high and low concentrations of TGF-β1. The tumors of the Post10d group further develop to immunosuppressive state under TGF-β1 domestication, and we also confirm the existence of ROS/TGF-β1/MMP-9 positive feedback loop and CD44-mediated amplification effect. On these grounds, we reasonably propose that PDT combined with TGF-β blockade can minimize immunosuppression and promote an antitumor effect. The result demonstrates the effectiveness of this strategy in anti-tumor growth and metastasis in vivo and in vitro.
So far, to analyze the advantages of TGF-β blockade strategy over existing immune checkpoint inhibition strategy, we take radiotherapy for example. Similar to PDT, radiotherapy also has two sides of immune activation and suppression [22]. At present, the immunosuppressive mechanism of radiotherapy has been basically clarified, and a series of clinical studies are also exploring relevant correction strategies [46]. Among them, radiotherapy will upregulate PD-L1 in TME and promote the immune escape of tumors [47], which makes PD-1/PD-L1 antibody an important strategy combined with radiotherapy. Similarly, recent reports have discussed the upregulation of immune checkpoints in TME by PDT [2], and immune checkpoint inhibitors can also effectively activate the systemic antitumor immune response of PDT [48]. However, as mentioned above, TGF-β1 can produce extensive and powerful immunosuppressive effect on TME. ROS will further promote the expression, activation and downstream signal transduction functions of TGF-β1 within TME. This will widely inhibit multiple links involved in the systemic antitumor immune response [23], resulting in the limited synergistic immune activation effect of immune checkpoint inhibitors due to influencing only one single link [47]. Therefore, TGF-β blockade strategy may improve the response and benefit rate of patients. It should be noted that the PDT mode discussed in this study is type-II PDT at the high light dose for a repeated administration, depending on oxygen consumption, which may be a prerequisite for using this strategy.
Here, on the ground of the clarified mechanism, to take an outlook of the smart combinational strategies of PDT and TGF-β blockade, we discussed several workable plans to fully exploit the inner mechanism of PDT so as to improve the therapeutic safety and efficacy. First, due to the limited penetration of surface laser irradiation, superficial tumors such as breast cancers, melanoma, oral cancer, nasopharyngeal carcinoma and laryngocarcinoma were still the clinical application priority of PDT. Fortunately, with the development of fiber optic and interventional technology, improved PDT on deep tumors was hopefully accessible [49, 50]. On this basis, since TGF-β inhibitors were not applied for anti-tumor proliferation directly (with low cytotoxicity), but more for regulation of the immune microenvironment and anti-tumor metastasis. To further enhance the tumor control effect of TGF-β blockade, the administration regimens like light dose, route, frequency and timing need more optimizations. For drug delivery systems, the tumor targeting and controlled release abilities are primary considerations. Since PDT upregulates TGF-β1 through ROS/TGF-β1/MMP-9 positive feedback loop and CD44-mediated amplification effect, why not take advantage of these characteristics to achieve above targets. For example, hyaluronic acid (HA)-modified carriers are excellent tumor active targeting units through HA-CD44 binding interaction under the circumstances of increased CD44 expression during PDT. Besides, the generated ROS and MMP-9 at PDT sites are favorable stimulants for specific disassembly of carriers to release drugs controllably through introduction of sensitive linkers. When it comes to the delivery time nodes of TGF-β1 inhibitors, more precise details should be considered. Too early or too late delivery will weaken the treatment efficacy. Sequential delivery designs or an independent in situ drug repository (like hydrogels) can be utilized. Therefore, we believe there are still much valuable work worth further exploring.
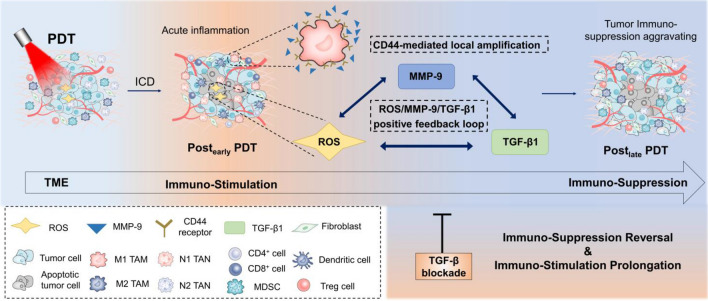
To sum up, this study investigates the occurrence process of immunosuppression post-PDT and TGF-β1 was identified as the crucial risk factor that mediates this phenomenon. By spatial local analysis, we focus on the local accumulation of TGF-β1 and systemically clarify the mechanism of aggravating immunosuppression at the late stage post-PDT, namely ROS/TGF-β1/MMP-9 positive feedback loop and CD44-mediated amplification effect (Fig. 7). Most importantly, we propose a specific and efficient correction strategy of TGF-β1 blockade to reverse this process as well as prolonging the duration of immune activation. Further, several potential targeting and controlled delivery strategies were discussed for further improvement and application of PDT and TGF-β1 inhibitors, which provides novel insights for tumor immunotherapy.
Fig. 7.
Scheme of the process and mechanism of PDT-induced immunosuppression. After PDT, large amounts of ROS are generated and acute inflammation occurs, forming an immune-activation state in TME. Then, on the one hand, ROS will activate the latent TGF-β1 and augment the ROS/MMP-9/TGF-β1 positive feedback effect. On the other hand, acute inflammation causes CD44 receptors upregulation and recruitment of MMP-9, thus promoting the local amplification of the positive feedback loop and TGF-β1 downstream signaling. Finally, under the enough accumulation of TGF-β1, tumor immuno-activation sharply weakens and immunosuppression dominates. Therefore, TGF-β1 mediates tumor immunosuppression aggravating at the late stage post-PDT. Here, a promising strategy is proposed that TGF-β blockade in time will effectively reverse the TGF-β1-induced immunosuppression and prolong the immune-activation time post-PDT
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LH did conceptualization, methodology, investigation, validation, writing—original draft. XH performed methodology, investigation, validation, and formal analysis. BZ done methodology and investigation. HZ was involved in formal analysis and visualization. RW done visualization. SL and HL contributed to methodology. FF supervised the study. XM did funding acquisition. FL was involved in conceptualization and funding acquisition. JX did funding acquisition and supervision. WL performed funding acquisition, supervision, and project administration.
Funding
This study was supported by the National Natural Science Foundation of China (82173954, 81801819, 82204340), China Postdoctoral Science Foundation (2021T140504, 2020M682230, 2022M723508), Natural Science Foundation of Shandong Province of China (ZR2020MH295), Natural Science Foundation of Jiangsu Province of China (BK20221048), Jiangsu Funding Program for Excellent Postdoctoral Talent (2022ZB295) and State Drug Administration-Key Laboratory of Quality Control of Chinese Medicinal Materials and Detection Pieces (2022GSMPA-KL06).
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding authors upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All animal experiments were performed according to the guidelines for laboratory animals established by China Pharmaceutical University (SYXK 2021-0011).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingfei Han and Xiaoxian Huang have contributed equally to this work.
Contributor Information
Fulei Liu, Email: liu_fulei@126.com.
Jingwei Xue, Email: xuejingwei11111@163.com.
Wenyuan Liu, Email: liuwenyuan@cpu.edu.cn.
References
- 1.Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy for the treatment and diagnosis of cancer–a review of the current clinical status. Front Chem. 2021;9:686303. doi: 10.3389/fchem.2021.686303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic therapy and immune checkpoint blockade(dagger) Photochem Photobiol. 2020;96(5):954–961. doi: 10.1111/php.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham TC, Nguyen V, Choi Y, Lee S, Yoon J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem Rev. 2021;121(21):13454–13619. doi: 10.1021/acs.chemrev.1c00381. [DOI] [PubMed] [Google Scholar]
- 4.Han L, Wang Y, Huang X, Liu F, Ma C, Feng F, Zhang J, Liu W, Qu W, Pang H, Xue J. Specific-oxygen-supply functionalized core-shell nanoparticles for smart mutual-promotion between photodynamic therapy and gambogic acid-induced chemotherapy. Biomaterials. 2020;257:120228. doi: 10.1016/j.biomaterials.2020.120228. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Feng K, Wu W, Ruan Y, Liu H, Han X, Shao G, Sun X. Smart (131) I-labeled self-illuminating photosensitizers for deep tumor therapy. Angew Chem Int Ed Engl. 2021;60(40):21884–21889. doi: 10.1002/anie.202107231. [DOI] [PubMed] [Google Scholar]
- 6.Beltran HI, Yu Y, Ossendorp F, Korbelik M, Oliveira S. Preclinical and clinical evidence of immune responses triggered in oncologic photodynamic therapy: clinical recommendations. J Clin Med. 2020;9(2):333. doi: 10.3390/jcm9020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song R, Li T, Ye J, Sun F, Hou B, Saeed M, Gao J, Wang Y, Zhu Q, Xu Z, Yu H. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv Mater. 2021;33(31):2101155. doi: 10.1002/adma.202101155. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Liao Y, Tang Q, Lin J, Huang P. Biomimetic nanoemulsion for synergistic photodynamic-immunotherapy against hypoxic breast tumor. Angew Chem Int Ed. 2021;60(19):10647–10653. doi: 10.1002/anie.202015590. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Deng G, Sun Z, Luo Y, Liu J, Yu X, Zhao Y, Gong P, Liu G, Zhang P, Pan F, Cai L, Tang BZ. A biomimetic aggregation-induced emission photosensitizer with antigen-presenting and hitchhiking function for lipid droplet targeted photodynamic immunotherapy. Adv Mater. 2021;33(33):2102322. doi: 10.1002/adma.202102322. [DOI] [PubMed] [Google Scholar]
- 10.Reginato E, Lindenmann J, Langner C, Schweintzger N, Bambach I, Smolle-Juttner F, Wolf P. Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochem Photobiol Sci. 2014;13(9):1281–1289. doi: 10.1039/c4pp00186a. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini C, Orlandi A, Costanza G, Di Stefani A, Piccioni A, Di Cesare A, Chiricozzi A, Ferlosio A, Peris K, Fargnoli MC. Expression of IL-23/Th17-related cytokines in basal cell carcinoma and in the response to medical treatments. PLoS ONE. 2017;12(8):e183415. doi: 10.1371/journal.pone.0183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korbelik M, Hamblin MR. The impact of macrophage-cancer cell interaction on the efficacy of photodynamic therapy. Photochem Photobiol Sci. 2015;14(8):1403–1409. doi: 10.1039/c4pp00451e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanos SM, Halliday GM, Damian DL. Nicotinamide reduces photodynamic therapy-induced immunosuppression in humans. Br J Dermatol. 2012;167(3):631–636. doi: 10.1111/j.1365-2133.2012.11109.x. [DOI] [PubMed] [Google Scholar]
- 14.Matthews YJ, Damian DL. Topical photodynamic therapy is immunosuppressive in humans. Br J Dermatol. 2010;162(3):637–641. doi: 10.1111/j.1365-2133.2009.09562.x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20(9):1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 16.Larson C, Oronsky B, Carter CA, Oronsky A, Knox SJ, Sher D, Reid TR. TGF-beta: a master immune regulator. Expert Opin Ther Targets. 2020;24(5):427–438. doi: 10.1080/14728222.2020.1744568. [DOI] [PubMed] [Google Scholar]
- 17.Tauriello D, Sancho E, Batlle E. Overcoming TGFbeta-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22(1):25–44. doi: 10.1038/s41568-021-00413-6. [DOI] [PubMed] [Google Scholar]
- 18.Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38(5):500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Tan Y, Zhang W, Yang W, Luo J, Chen L, Liu H, Yang G, Lei X. Effects of ALA-PDT on the healing of mouse skin wounds infected with pseudomonas aeruginosa and its related mechanisms. Front Cell Dev Biol. 2020;8:585132. doi: 10.3389/fcell.2020.585132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Luo J, Tan Y, Wang M, Liu Z, Yang T, Lei X. Effects of low-dose ALA-PDT on fibroblast photoaging induced by UVA irradiation and the underlying mechanisms. Photodiagn Photodyn Ther. 2019;27:79–84. doi: 10.1016/j.pdpdt.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Han J, Wei M, Xu Y, Zhang G, Zhang H, Shi L, Liu X, Hamblin MR, Wang X. Remodeling of dermal collagen in photoaged skin using low-dose 5-aminolevulinic acid photodynamic therapy occurs via the transforming growth factor-beta pathway. J Biophotonics. 2018;11(6):e201700357. doi: 10.1002/jbio.201700357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Citrin DE. Recent developments in radiotherapy. N Engl J Med. 2017;377(11):1065–1075. doi: 10.1056/NEJMra1608986. [DOI] [PubMed] [Google Scholar]
- 23.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFbeta Is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiaojie W, Banda J, Qi H, Chang AK, Bwalya C, Chao L, Li X. Scarless wound healing: current insights from the perspectives of TGF-beta, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022;66:26–37. doi: 10.1016/j.cytogfr.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9(6):a22236. doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidolin K, Ding L, Yan H, Englesakis HM, Chadi S, Quereshy F, Zheng G. Photodynamic therapy for colorectal cancer: a systematic review of clinical research. Surg Innov. 2022;29(6):788–803. doi: 10.1177/15533506221083545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soundararajan R, Fradette JJ, Konen JM, Moulder S, Zhang X, Gibbons DL, Varadarajan N, Wistuba II, Tripathy D, Bernatchez C, Byers LA, Chang JT, Contreras A, Lim B, Parra ER, Roarty EB, Wang J, Yang F, Barton M, Rosen JM, Mani SA. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers. 2019;11(5):714. doi: 10.3390/cancers11050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pena-Romero AC, Orenes-Pinero E. Dual effect of immune cells within tumour microenvironment: pro- and anti-tumour effects and their triggers. Cancers. 2022;14(7):1681. doi: 10.3390/cancers14071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang D, Ramsey JD, Makita N, Sachse C, Jordan R, Sokolsky-Papkov M, Kabanov AV. Novel poly(2-oxazoline) block copolymer with aromatic heterocyclic side chains as a drug delivery platform. J Control Release. 2019;307:261–271. doi: 10.1016/j.jconrel.2019.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Khatua S, Simal-Gandara J, Acharya K. Understanding immune-modulatory efficacy in vitro. Chem Biol Interact. 2022;352:109776. doi: 10.1016/j.cbi.2021.109776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutas G, Bajnok E, Gal I, Finnegan A, Glant TT, Mikecz K. CD44-specific antibody treatment and CD44 deficiency exert distinct effects on leukocyte recruitment in experimental arthritis. Blood. 2008;112(13):4999–5006. doi: 10.1182/blood-2008-04-150383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lövgren K, Warren S, Jirström K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jönsson G. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 34.Hao Y, Baker D, Ten Dijke P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11):2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, Zhu C, Yuan X, Zhang J, Luo Z, Du Y, Li Q, Lou Y, Qiu Y, You J. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10(1):3349. doi: 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Liu R, Su X, Pan Y, Han X, Shao C, Shi Y. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol Cancer. 2019;18(1):177. doi: 10.1186/s12943-019-1102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derynck R, Turley SJ, Akhurst RJ. TGFbeta biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung J, Huda MN, Shin Y, Han S, Akter S, Kang I, Ha J, Choe W, Choi TG, Kim SS. Correlation between oxidative stress and transforming growth factor-beta in cancers. Int J Mol Sci. 2021;22(24):13181. doi: 10.3390/ijms222413181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Sci World J. 2014;2014:1–14. doi: 10.1155/2014/521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol. 2015;6:150. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessenbrock K, Plaks V, Werb Z. Matrix Metalloproteinases: regulators of the Tumor Microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol. 2019;16(12):729–745. doi: 10.1038/s41571-019-0238-9. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Kim SA, Nam GH, Hong Y, Kim GB, Choi Y, Lee S, Cho Y, Kwon M, Jeong C, Kim S, Kim IS. In situ immunogenic clearance induced by a combination of photodynamic therapy and rho-kinase inhibition sensitizes immune checkpoint blockade response to elicit systemic antitumor immunity against intraocular melanoma and its metastasis. J Immunother Cancer. 2021;9(1):e1481. doi: 10.1136/jitc-2020-001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun B, Bte Rahmat JN, Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials. 2022;291:121875. doi: 10.1016/j.biomaterials.2022.121875. [DOI] [PubMed] [Google Scholar]
- 50.Shafirstein G, Oakley E, Hamilton S, Habitzruther M, Chamberlain S, Sexton S, Curtin L, Bellnier DA. In vivo models for studying interstitial photodynamic therapy of locally advanced cancer. Methods Mol Biol. 2022;2451:151–162. doi: 10.1007/978-1-0716-2099-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding authors upon reasonable request.