Abstract
People with HIV (PWH) can now enjoy longer, healthier lives due to safe and highly effective antiretroviral therapy (ART), and improved care and prevention strategies. New drug formulations such as long-acting injectables (LAI) may overcome some limitations and issues with oral antiretroviral therapy and strengthen medication adherence. However, challenges and questions remain regarding their use in aging populations. Here, we review unique considerations for LAI-ART for the treatment of HIV in older PWH, including benefits, risks, pharmacological considerations, implementation challenges, knowledge gaps, and identify factors that may facilitate uptake of LA-ART in this population.
Keywords: aging, antiretroviral therapy, cabotegravir, geriatric, HIV, injectable antiretroviral therapy
Introduction
People with HIV (PWH) are now able to enjoy longer, healthier lives due to safe and highly effective antiretroviral therapy (ART) and prevention strategies [1,2]. By 2030, PWH aged 60 years or older are expected to represent over 40% of all PWH globally [3,4], and nearly 25% of all ART users in the United States may be age 65 or older [5]. However, aging with HIV carries unique risks – a higher incidence of health complications and comorbidities compared to older persons without HIV, with resultant polypharmacy and inappropriate prescribing [6–12]. These issues increase the risk of higher pill-burden, drug–drug interactions (DDIs), and toxicities, which may compromise ART adherence and health outcomes [11].
New approaches to drug delivery, such as long-acting formulations, are being developed to strengthen adherence to ART. Long-acting agents in various stages of development include oral therapies (islatravir), implants (tenofovir), microarray patches (rilpivirine), and vaginal rings (dapivirine), as well as long-acting injectable (LAI) injectables like lenacapavir and cabotegravir-rilpivirine (CAB/RPV; Cabenuva), a recently FDA-approved ART given every one to two months for the treatment of HIV infection [13–15]. Though CAB/RPV’s novel mode of delivery holds promise in circumventing some of the issues with oral therapy, new challenges and unanswered questions remain, particularly around its use in aging populations. Some studies have explored considerations for LAI-ART acceptability, applicability, and implementation for certain subpopulations [16–20]. However, its benefits, risks, and applications for aging populations and their distinct needs remain understudied. Here, we review unique considerations for LAI-ART for the treatment of HIV in older PWH.
The challenges of oral antiretroviral therapy in older persons with HIV
The current landscape of oral ART is layered with complexity – more antiviral medications are approved for treating HIV infection than for any other viral infection. Despite oral ART regimens of optimal antiretroviral efficacy and tolerability, including those containing integrase strand transfer inhibitors (INSTIs), adherence to daily oral ART remains an issue. In 2020, fewer than two-thirds of people with diagnosed HIV in the United States took all of their prescribed ART doses in the past 30 days [21]. This suboptimal adherence to ART is the most common cause of treatment failure and the development of resistance [22].
Although older PWH have relatively high rates of adherence to oral ART, they face distinct pharmacologic challenges (Fig. 1) [13]. These include treatment fatigue, pill burden and size, food and timing requirements surrounding dosing, side effects, and changes in daily routine [23–27]. Older PWH are at also risk for depression and neurocognitive disorders, whether associated with HIV infection itself or other factors, as well as age-related cognitive decline which may impact adherence to ART [28]. Age-related visual, auditory, and dexterity impairments may also affect elders’ adherence to medications [29].
Fig. 1.
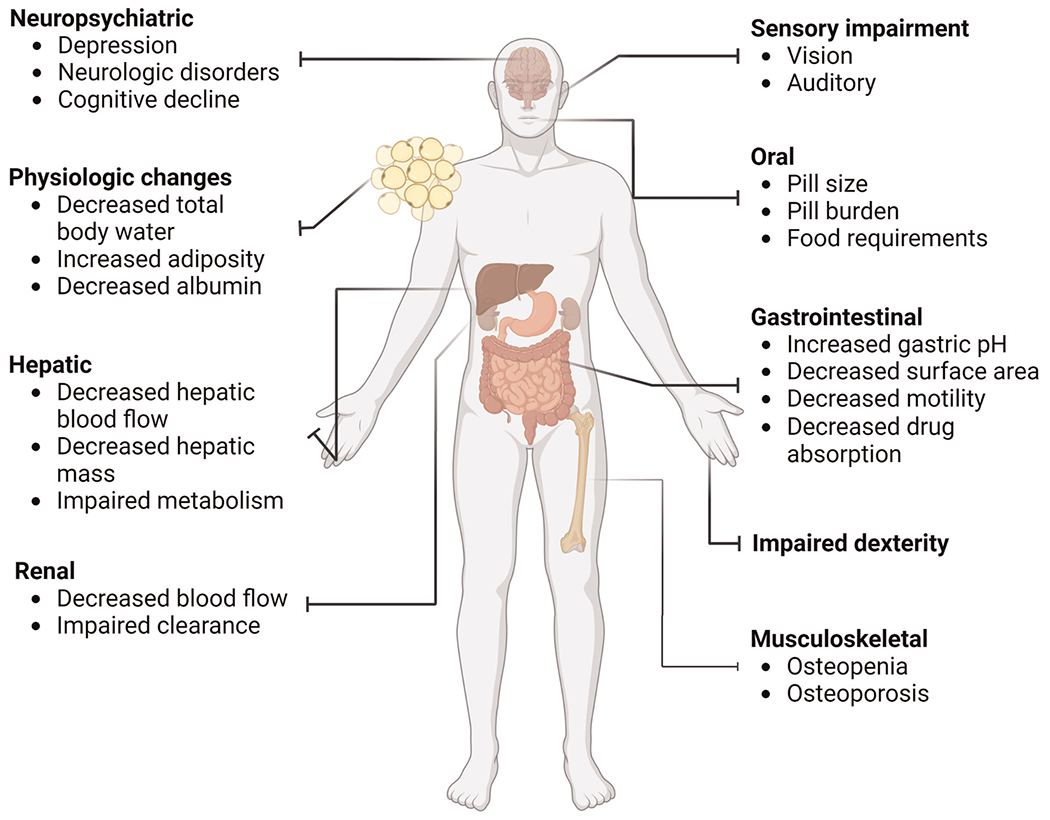
Age-related changes affecting oral ART adherence and pharmacology.
Many adverse effects of oral ART are particularly relevant to aging populations. However, most ART trials have included only a small proportion of persons older than 50 years. As a result, there is less of an understanding and quantification of adverse effects in older patients compared to populations more represented in studies. The Adult and Adolescent ART Guidelines recommend the same regimens for older adults with HIV as for younger persons with HIV, typically with a regimen that includes an integrase strand transfer inhibitor (INSTI) and two nucleoside reverse transcriptase inhibitors (NRTI) [13]. Important toxicities to consider when initiating or changing an ART regimen include bone and renal effects related to tenofovir disoproxil fumarate, weight gain related to INSTIs and tenofovir alafenamide, neurocognitive and neuropsychiatric toxicities related to efavirenz and rarely reported with dolutegravir and cabotegravir, and increased cardiovascular risk associated with abacavir and boosted protease inhibitors [13]. Older PWH, especially postmenopausal cisgender women, have an increased risk of osteopenia, osteoporosis, and fractures, which may be exacerbated by uncontrolled HIV and certain ART regimens that cause loss of bone mineral density (e.g. tenofovir disoproxil fumarate; TDF) [13]. Increasing age is also associated with higher rate of treatment changes due to toxicity with protease inhibitor (PI)-based regimens, albeit use of this drug class is generally less common today [30].
Some oral ART-specific drug toxicity in the older PWH may be related to age-associated physiologic changes that alter pharmacokinetics and pharmacodynamics. These changes include increased adiposity (which affects distribution of fat-soluble drugs), decreased albumin levels, and changes in the cytochrome P450 enzyme system [31–35]. Since both nonnucleoside reverse transcriptase inhibitors (NNRTIs) and PIs are metabolized by cytochrome P450, older PWH may have significantly higher drug exposure. Hepatic metabolism and renal elimination are the major routes of drug clearance, including the clearance of many ART medications. Both liver and kidney function can decrease with age and may result in impaired drug elimination and increased drug exposure [36–38]. Although there are no recommendations for dose adjustments of oral ART based on age alone, renal and hepatic function is recommended for assessment on an ongoing basis, as lower drug clearance can be seen with chronic kidney or liver disease [13].
Potential benefits of long-acting injectable-antiretroviral therapy in older persons with HIV
ART regimens that include less frequent dosing and/or different routes of administration may improve viral suppression by mitigating barriers to adherence [39,40]. There are several different long-acting medications and modes of ART delivery under development [41]. In January 2021, the Federal Drug Administration (FDA) approved the first complete LAI-ART regimen, CAB/RPV, for the treatment of HIV in adults. Multiple clinical trials have demonstrated the efficacy and safety of the CAB/RPV LAI-ART and confirmed that 4-week to 8-week dosing regimen is noninferior to standard daily three-drug oral regimens [42–45]. A pooled analysis of multiple trials comparing outcomes between adults aged at least 50 (albeit with limited number of persons older than 65) and less than 50 years demonstrated similar rates of virologic suppression, adverse events, injection site reactions and severity, and changes in baseline treatment satisfaction compared to oral ART between both groups [46].
Qualitative studies conducted among diverse populations have demonstrated a high level of enthusiasm and interest for LAI-ART (Table 1). These perceived benefits include ‘freedom’ from the logistical and psychosocial demands of daily oral ART and its attached stigma, less risk of missing a dose, convenience, privacy, less risk of treatment failure, and thereby less risk of transmission of HIV to partners. Pilot studies prior to regulatory approval among various outpatient clinical settings and providers have demonstrated LAI-ART to be acceptable, feasible, and sustainable [47].
Table 1.
Acceptability of LAI-ART among diverse populations, including among older persons with HIV.
| Study | Year published | Location(s) | Description | Total n | Participants ≥50 years old | Participants ≥65 years old | Other demographic factors | Findings |
|---|---|---|---|---|---|---|---|---|
| Adachi et al. [87] | 2023 | Japan (1 site) | Prospective, cross-sectional study of 76 PWH eligible for LAI-ART comparing background factors associated with choosing to switch to injection therapy vs. remaining on oral ART | 76 | Yes; mean age of PWH switching to LAI-ART 47 (range 28–64); mean age of PWH continuing prior regimen 53 (26–90) (n unknown) | Yes (n unknown) | Sex/gender: Male (95%) | No statistically significant differences by age between those who switched to LAI-ART and those who did not. Most common reason for switch was to experience life without daily oral medication; most common reason for not switching was concomitant medications or complications. |
| Akinwunmi [88] | 2021 | Germany, Italy, UK, France (web-based survey) | Survey of 688 PWH on treatment (and 120 HIV physicians) exploring interest in trying (and interest in offering) hypothetical LAI-ART cost-neutral to daily oral ART | 688 | Yes; n = 204 | Unknown | Sex/gender: male (66%) | Among PWH aged ≥50 years, 56% expressed interest in trying LAI-ART. Odds of being interested in trying LAI-ART were lower among PWH aged ≥ 50 years than those aged <50 (AOR 0.55, 95% CI 0.39–0.78). |
| Sexual orientation: homosexual (61%) | ||||||||
| Carillon et al. [89] | 2020 | Paris, France (2 sites) | In-depth interviews with 15 PWH well controlled on ART exploring experiences with oral ART, knowledge of novel ART modalities including LAI-ART, and willingness to change mode of administration | 15 | Yes; mean age 54 (range 31–79) (n = 11) | Yes (n = 2) | Sex/gender: Male (73%) | Potential benefits of LAI-ART cited by participants (simplifications of regimen, reduced social stigma) were often counterbalanced by reservations including inconveniences and disruptions from switching from habituation to oral ART and need for repeat medical appointments. PWH with negative prior experiences with injections had higher reluctance for LAI-ART. Though some cited concerns about a ‘new’ or ‘experimental’ treatment, the majority reported trusting their HIV provider if LAI-ART was felt to not be harmful. |
| Sexual orientation: 6 of 15 men who have sex with men (MSM) | ||||||||
| Fletcher et al. [90] | 2023 | Boston, USA (single site) | Qualitative interviews with PWH, at an alternative administration site for people with substance use disorder, housing instability | 26 | Yes (n = 6, 23% of study participants) (range 18–64) | No | Race: White (42%), Black or African American (42%), Hispanic/Latino (38%) | Preference for LAI-ART over oral ART noted |
| Sex/gender: male (69%) | ||||||||
| Northeastern US (100%) | ||||||||
| Garris et al. [91] | 2019 | USA, Canada (online survey) | Cross-sectional survey of 51 PWH on ART ≥ 6 months and self-reported viral suppression as well as HIV providers utilizing a discrete choice experiment to explore treatment preferences for oral vs. LAI-ART based on treatment attributes | 202 | Yes; mean age 54 (n aged ≥50 years unknown) | Unknown | Sex/gender: male (80%) | Across discrete choice experiment, 47% of respondents preferred to continue current oral ART regimen; 43% preferred switching to LAI-ART; 7% preferred switching to different oral ART. |
| Duration of ART: 63% ≥10 years | ||||||||
| Garris et al. [92] https://academic.oup.com/ofid/article/6/Supplement_2/S866/5604105 | 2022 | USA (8 sites) | Quantitative surveys and interviews among participants (n = 115) of the CUSTOMIZE phase IIIb LAI-ART implementation-effectiveness study to assess LAI-ART acceptability and appropriateness at different time points before and during LAI-ART implementation | 202 | Yes; surveys: n = 26 aged ≥50 years (24% of total n = 109); interviews: n = 7 aged ≥ 50 years (21% of total n = 34) | Yes (upper range: age 65; n ≥ 65 not specified) | Race/ethnicity: White (59% survey respondents; 53% interviewees), Black (35%; 41%) | High mean LAI-ART acceptability and appropriateness among participants at baseline, month 4, and month 12; more participants preferred LAI-ART compared to oral ART over time (84% at 4 months; 92% at 12 months). At month 12, 26% of interviewees (n = 8) indicated transportation barriers. |
| Sex/gender: Male (87%; 82%) | ||||||||
| Koren et al. [93] | 2020 | USA (1 site) | Cross-sectional survey assessing perceptions and preferences of LAI-ART among a convenience sample of PWH at an urban academic medical center | 202 | Yes; median age 49 (range 36–58) (n not specified) | No | Race/ethnicity: Black (82%), Hispanic (18%), White (9%) | A majority of respondents (57%) were categorized as ’likely acceptors’ of LAI-ART; Likert scores of LAI-ART acceptability among ‘likely acceptors’ and ‘unlikely acceptors’ both increased (favoring LAI-ART) with decreasing frequency of injections and decreased with increasing duration of injection site reaction. A majority preferred clinician offices as preferred site for injection (57%). |
| Sex/gender: 60% male | ||||||||
| Sexual orientation: 33% LGBTQ-identifying | ||||||||
| Margolis et al. [63] | 2017 | USA, Canada, Spain, France, Germany (50 sites) | Measured treatment satisfaction using the HIV Treatment Satisfaction Questionnaire prior to oral induction period and at multiple timepoints of LAI-ART maintenance period | 309 | Yes (range 19–64) (n not specified) | No | Race: White (79%) Sex/gender: male (92%) |
LAI-ART CAB-RPV was associated with high satisfaction for continuing the regimen (≥99%), more so than oral ART (78%). |
| Massaroni et al. [94] | 2022 | Italy (1 site) | Mixed methods study using anonymous phone surveys of 202 Italian-speaking adult PWH on combined oral ART to explore knowledge, perceptions, and preferences of use of LAI-ART | 202 | Yes; median age 53 (range 22–72) (n ≥ 50 not specified) | Yes (n ≥65 not specified) | Sex/gender: male (50%) | Majority of respondents viewed taking oral ART as an undemanding commitment (61%), however a majority were also willing to stop daily oral ART (78%) (particularly among PWH >10 years since diagnosis or since start of oral ART). More than half (51%) reported need for hospital visits for injections to be an obstacle, a challenge cited particularly by women, employed PWH, and PWH with undetectable viremia. Employed and younger PWH perceived LAI-ART to have less limitations than unemployed/retired and older PWH. |
| Other: 60% employed; 69% on oral ART >10 years | ||||||||
| Palacios et al. [95] | 2022 | Paris, France (single site) | Survey of PWH in Paris, France exploring patient expectations and acceptability of LAI-ART options | Yes (median age 52, range 30–76) (n not specified) | Yes | Sex/gender: male 67% | Majority (65%) in favor of changing to injectable ARTs; reasons for LAI-ART refusal included frequency of clinic/hospital visits (1–2 months vs. 6 months) (58%); used to current ARTs (40%); already on a daily treatment for other chronic diseases (29%) | |
| Philbin et al. [16] | 2022 | USA (6 urban sites) | Qualitative study exploring LAI-ART preferences in women with and at risk for HIV (Women’s Interagency HIV Study cohort) in the United States | 89 | Yes (n = 53, 60% of study participants) | Yes; 15 participants (17%) ≥60 years old (n ≥ 65 not specified) | Race: Black (76%) | 58% of women interviewed would prefer LAI ART |
| Gender: female (100%) | ||||||||
| Geography: six urban centers | ||||||||
| Other: public insurance (82%) | ||||||||
| Saberi et al. [96] | 2020 | USA (national survey) | Perceptions of HIV virologic control strategies among younger and older age groups of PWH in the United States | 281 | Yes (n = 134, 48% of study participants) | Yes; 44 participants (33%) ≥60 years old (n > 65 not specified) | Race: White (65%) | PWH ≥50 years old significantly more likely to prefer a version of a currently available ART in an injectable or implantable form that would last 1 or 2 months compared with PWH ≤50 (P < .05). |
| Gender: cisgender (64%) | ||||||||
| Minimum 4-yr college degree (50%) | ||||||||
| Southern U.S. (45%) | ||||||||
| Simoni et al. [97] | 2020 | USA (3 sites) | Qualitative study (conjoint analysis) examining acceptability of hypothetical LAI-ART regimen across different scenarios (e.g., administration at home vs. clinic; number of injections per dose; dosing frequency; injection pain; efficacy compared to oral ART) | 56 | Yes; median age 52 (range 20–64) (n not specified) | Not specified | Race: Black (36%), White (34%); Hispanic (20%) | Overall acceptability ‘neutral’ to ‘somewhat likely,’ with no significant differences across demographics. Greater acceptability with lower dosing frequency (2 weeks vs. 1w eek) and better effectiveness vs. oral ART. |
| Sex/gender: male (71%) | ||||||||
| Geography: West Coast (100%) | ||||||||
| Other: Included PWH who had ever self-injected drugs | ||||||||
| Simoni et al. [98] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6442271/ | 2019 | USA (3 sites) | Qualitative study involving focus group discussions or interviews with English-speaking PWH including heterosexual men, MSM, women, individuals who struggle with adherence, young adults, and parents of children with HIV; as well as HIV providers | 36 | Yes; median age 52 (range 20–64) (n not specified) | No | Race: Black (50%), White (25%), Other (25%) | Enthusiasm for LAI-ART, particularly among young adults; more ambivalence among PWH accustomed to oral regimen or taking multiple oral medications for other chronic conditions; HIV providers enthusiastic but wary of implications for consistent engagement in care. |
| Other: included survey of HIV providers | ||||||||
| Slama et al. [99] | 2022 | Paris and Melun, France (3 sites) | Survey of PWH and PrEP users in France eliciting perspectives on LAI-ART and to determine which subset of individuals would be preferred targets regarding expectations, tolerability, adherence, and quality of life | 100 | Yes (median age of PWH 50, range 42–56) (n not specified) | No | Sex/gender: male 76% (of PWH) | 74% of PWH expressed interest in every other month LAI-ART; 11% did not see any benefit in LAI-ART. No significant differences in age, sex, sexual orientation, substance use, other medical or psychiatric comorbidities, or use of additional non-HIV treatments between PWH who would accept LAI-ART and those who would not |
| Sexual orientation: MSM 50% (of PWH) | ||||||||
| Time since HIV diagnosis: mean 15 years | ||||||||
| Time on ART at least 10 years: 58% All PWH well controlled | ||||||||
| Toska et al. [100] | 2023 | Eastern Cape Province, South Africa (53 health facilities) | Adolescents living with HIV in South Africa | 900 | No | No | Sex/gender: 54.1% female | Preference for LAI-ART among recent ART initiators, stigma experiencers, side effects from oral ART, medication changes <1 year, polypharmacy, stockouts from care facilities |
| Geography: ~25% in rural communities | ||||||||
| Williams et al. [101] | 2013 | USA (2 sites) | A survey of 400 adults with HIV to gauge interest in nanoformulated injectable ART and other factors including current ART regimen and adherence, reasons for interest or concerns about nanoformulated ART. |
399 | Yes; median age 47 (range 18–71) (n ≥ 50 not specified) | Yes (n ≥ 65 not specified) | Race: Black (53%), White (40%) | Majority of respondents indicated interest in parenteral ART, with a statistically significant increase in interest among PWH who reported missed ART doses and who used injection drugs. |
| Other: 75% with perfect self-reported adherence in last 4 days; 27% with history of intravenous drug use |
ART, antiretroviral therapy; LAI, long-acting injectables.
The advantages of LAI-ART include a reduction in dosing frequency, total pill burden, stigma associated with daily oral regimens, drug–food interactions, and potential gastrointestinal (GI) side effects, as well as direct provider monitoring of adherence via in office injections. Pharmacologically, the benefits of LAI-ART for older populations potentially include that the pharmacokinetic variability after intramuscular administration may be reduced compared to oral formulations. Intramuscular administration avoids the complexity of the absorption process including avoidance of intestinal transporters and first pass metabolism, which is usually reduced in older patients [48,49]. With LAI CAB/RPV specifically, many cumbersome absorption related drug–drug interactions can be avoided. It is estimated that a third of older adults take a proton pump inhibitor which significantly impacts the absorption of oral rilpivirine [50]. Additionally, 70% of older adults report taking dietary supplements – management of timing of polyvalent cation-containing dietary supplements with INSTI-based ART can increase medication administration complexity [51,52]. Circumventing gastrointestinal tract with intra-muscular administration of CAB/RPV prevents these drug–drug interactions.
As related to quality of life, long-acting antiretroviral therapy can simultaneously improve patient privacy, confidentiality, reduce social stigmas associated with HIV and reduce constant reminders of one’s HIV status through daily pill use. From the clinic and provider perspective, characteristics appropriate for consideration of LAI-ART include treatment fatigue, history of side effects, and stress or anxiety related to daily oral ART adherence [47,53].
Lessons learned from other injectables in older persons with HIV
Long-acting injectable therapies have helped facilitate treatment adherence for other stigmatized conditions like mental illness and substance use disorders. LAI antipsychotic (LAI-APS) medications can prevent psychiatric relapse in noncompliant or forgetful patients and lower rates of relapse and re-hospitalization, resulting in cost savings compared with oral antipsychotics [54]. The decision to treat patients with LAI-APS agents involves similar considerations as LAI-ART, including a patients’ history of response and personal preference, clinician’s previous experience, and the medication’s pharmacokinetic properties [55]. Providers often consider LAI-APS in older patients who have difficulties with compliance, irregularly take their medications, or have other characteristics such as memory, vision, or auditory impairment that contribute to partial compliance [55].
Despite being frequently viewed as ‘treatment of last resort’ for those with nonadherence or treatment failure, LAI-APS can benefit patients in all phases of psychiatric illness [56]. Similarly, it is critical to present LAI-ART to the aging population not as a solution to nonadherence but instead as an option to ensure all PWH receive patient-centered treatment. LAI-ART should be broadly accessible such that goals like U=U (undetectable viral load equals untransmissible virus) are achievable. Furthermore, while involuntary administration of LAI-APS are uncommon, LAI-ART must only be administered with consent of the patient or an appropriate surrogate, based on substituted judgment or the patient’s best interest [56].
As data are limited evaluating older patient preferences towards LAI-ART, studies from other long-acting injectables, both in the general population and among PWH, may be useful to inform patient satisfaction and preference. Denosumab (Prolia), an injectable human monoclonal antibody used for the treatment of osteoporosis, has been compared with oral bisphosphonates. Studies have found that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with subcutaneous denosumab injections every 6 months than with once-weekly alendronate tablets and reported increased treatment preference, convenience, and satisfaction with injectable denosumab over oral alendronate [57,58]. A randomized placebo-controlled clinical trial demonstrated that 6-monthly injections of extended-release naltrexone were acceptable and more than doubled the odds of viral suppression among incarcerated individuals with HIV and alcohol use disorders transitioning to the community [59].
Long-acting injectable- antiretroviral therapy biologic challenges and considerations in older persons with HIV
Knowledge is still incomplete regarding LAI-ART’s benefits, risks, efficacy, and implications for older PWH. Table 2 contains a summary of knowledge gaps relevant to LAI-ART in older PWH and agenda items for further needed research. Most studies on LAI-ART have been carried out with younger PWH, with older age strata poorly represented. It is therefore difficult to determine differences in response between younger and older (including elderly) individuals – a factor which could potentially limit the generalizability of LAI-ART’s efficacy and rate of failure to older populations [60]. The median age of participants in noninferiority randomized control trials (ATLAS, FLAIR, LATTE-2) has ranged from 34–42 years (Table 3) [42,43,1–63]. Two trials (ATLAS-2 M, LATTE-2) did not report the number of enrolled participants aged 50 years or older; of other trials that did, only 228 patients enrolled were 50 years old or older, and of these, 99 were randomized to receive LAI-ART [42–45,61–63]. In each of the treatment studies, a small number of participants (1%) experienced virologic failure with both new NNRTI resistance and integrase inhibitor resistance. Pharmacokinetic data of CAB (oral or injectable) are scarce in people aged 65 years or older. Given these limitations, the FDA has advised caution when considering CAB-RPV for older patients, given increased burden of chronic comorbidity and potential for polypharmacy in this population [60]. Lastly, many older PWH may have mutations conferring HIV resistance, making them ineligible for CAB-RPV.
Table 2.
LAI-ART knowledge gaps and future research for older persons with HIV.
| Knowledge Gap | Research Agenda Item |
|---|---|
| Unknown long-term efficacy in older PWH | Longitudinal prospective or retrospective studies assessing rates of LAI-ART treatment failure or discontinuation/reversion to oral regimens in older patients |
| Unclear impact of age on pharmacokinetic parameters (e.g. absorption, distribution, metabolism) | Pharmacokinetic studies in patients >50 years old to determine optimal dosing regimens |
| Acceptability of LAI-ART in older PWH | Qualitative assessment of LAI-ART interest and acceptability among older PWH, highlighting perceived benefits and factors of concern which may limit acceptability |
| Implementation challenges of LAI-ART for older PWH | Implementation studies across different clinical contexts (e.g. Veteran’s Administration, private, public, academic, and combined HIV-geriatric care model clinics) highlighting specific needs and challenges in acquiring and delivering injectable therapy for older patients |
| Risk factors and optimal mitigation strategies for missed LAI-ART doses for older PWH | Sub-analysis of characteristics of patients with missed doses in implementation studies, and success of strategies to prescribe and deliver bridge regimens; qualitative studies with older PWH and caregivers exploring perceived or anticipated challenges with regular LAI-ART visits, preferences for protocols to receive bridge regimens in-hand |
ART, antiretroviral therapy; LAI, long-acting injectables; PWH, people with HIV.
Table 3.
Randomized control trials demonstrating CAB-RPV efficacy, including subsets of older persons with HIV.
| Authors | Study name | Year published | Location(s) | Description | Median age (years) | Participants ≥50 years old | Subgroup analysis for older patients |
|---|---|---|---|---|---|---|---|
| ATLAS | Swindells et al. [42,62] | 2020, 2022 | Argentina, Australia, Canada, France, Germany, Italy, Mexico, Russia, South Africa, South Korea, Spain, Sweden, USA (number of sites not specified) | Assessed noninferiority of long-acting CAB-RPV to oral ART in adults with treated, virologically suppressed HIV | 42.4 (range 18–82) | 162 (of 616 total) LAI-ART: 66 Oral ART: 96 | See Rizzardini et al. [102]; Benn et al. [46] |
| ATLAS-2M | Overton et al. [44] | 2021 | Compared efficacy and safety of 4weeks vs. 8 weeks dosing of CAB-RPV LAI-ART | 42 (IQR 34–50) | >25% of enrolled participants (of 1045 total) | See Rizzardini et al. [102]; Benn et al. [46] | |
| Chounta et al. [103] | 2021 | Analyzed patient-reported outcomes comparing participants’ experiences with monthly or every two-month LAI-ART dosing regimens. | Adjusted for age as a covariate | ||||
| FLAIR | Orkin et al. [43,61] | 2021, 2020 | Canada, France, Germany, Italy, Japan, the Netherlands, Russia, South Africa, Spain, UK, USA (108 sites) | Assessed noninferiority of long-acting CAB-RPV to daily oral ABC-DTG-3TC after oral lead-in for previously treatment-naïve patients | 34 (range 19–68) | 62 (of 566 total) LAI-ART: 33 Oral ART: 29 | See Rizzardini et al. [102]; Benn et al. [46] |
| LATTE-2 | Margolis et al. [63] | 2017 | USA, Canada, Spain, France, Germany (50 sites) | Comparing oral CAB plus abacavirlamivudine to CAB-RPV at 4-week dosing and 8-week dosing intervals for virologically unsuppressed adults | 35 (range 19–64) | Not specified (of 309 total) | No |
| Pooled analysis of ATLAS, FLAIR | Rizzardini et al. [102] | 2020 | Per ATLAS, FLAIR | Pooled analysis to determine LAI-ART efficacy at week 48, as well as safety, tolerability, and confirmed virologic failure. | LAI-ART: 38 (range 19–74); oral ART: 38 (range 18–82) | 224 (of 1184) LAI-ART: 99 Oral ART: 125 | No significant differences in viral suppression between PWH aged ≥ 50 years and <50 years at week 48 (supplemental Figure S3) |
| Pooled analysis of ATLAS, ATLAS-2M, FLAIR, | Benn et al. [46] | 2021 | Per ATLAS, FLAIR | Pooled analysis stratified by age ≥50 years and age <50 years to determine the efficacy, safety, adherence, and treatment satisfaction outcomes | NA | 399 (of 1850) LAI-ART: 274 Oral ART: 125 | PWH aged ≥50 years and <50 years had comparable rates of virologic suppression, adverse events, injection site reactions and severity, and changes in baseline treatment satisfaction compared to oral ART. |
| SOLAR | Ramgopal et al. [45] | 2023 | Not specified | Noninferiority study comparing switch from oral BIC/FTC/TAF to CAB-RPV LAI-ART every 8 weeks vs. continuing oral BIC/FTC/TAF in adults with virologically suppressed PWH | 37 (range: 18–74) | 128 (of 670 total) | No |
ART, antiretroviral therapy; LAI, long-acting injectables; PWH, people with HIV.
By design, LAI-ART has a long half-life and cannot be withdrawn once administered. Although oral lead-in is now optional based on the FLAIR extension study, the oral lead-in period might be considered in aging populations to assess safety, side effects, and tolerability. Older populations are more at risk of experiencing side effects due to coexisting medical illnesses and concomitant medications, prompting close monitoring for development of known side effects [55]. Nearly nine in ten older adults take at least one prescription medication daily with more than half taking four or more prescription drugs [64]. Given the higher frequency of concomitant medications for comorbidities in older patients, the utility of LAI-ART in terms of diminishing pill burden when most ART regimens are only once daily single tablet regimens must also be considered [65].
Although intramuscular administered pharmacokinetics bypass age-related changes in GI absorption and metabolism, they may be affected by other factors such as body mass index (BMI), decreased muscle mass, gender, exercise, local blood flow, and age [48,53,55]. Changes in fat distribution at gluteal injection sites in older populations may impact pharmacokinetics and dynamics. With significantly decreased muscle mass plus potential for decreased blood perfusion to the muscle, absorption from intramuscular injections could be impaired. Differences in CAB and RPV exposure after intramuscular administration have been observed according to BMI and gender with lower (early-time point) CAB concentrations in participants with BMI ≥30 kg/m2 and in female participants [66–68]. Although population pharmacokinetic (popPK) modeling has also shown a possible role for covariates as gender and BMI, it could not analyze age impact due to insufficient number of older PWH [48,69]. Further study is needed on the impact of aging on the pharmacokinetics of intramuscular administration, particularly drug absorption in people with frailty, to ensure optimal dosing and administration and drug level monitoring.
Age-related changes in body habitus and muscle volume risk improper injection technique with subsequent injection site pain or reactions requiring close monitoring. In a pooled analysis across ATLAS and FLAIR studies over 48 weeks, up to 25% of all injections had injection site reactions. Most were mild-moderate, with less than 1% being grade 3 severity or greater. Incidence was highest for the first dose but attenuated over time. The most frequently reported reaction was injection site pain (21%), with nodules, induration, and swelling noted less frequently – but only leading to a 1% discontinuation rate. To address injection site reactions in older patients as a potential barrier to uptake, providers might consider not only counseling on the possible discomfort of receiving ART injections (particularly with the initial dose), but also the potential impacts that gluteal pain, induration, and swelling in injection sites may have on mobility and function – even if mild or temporary. For patients with impaired mobility for whom injection-site pain or reactions may further affect function, shared decision-making to explore potential risks and benefits might ensure local side effects do not impede independence or diminish quality of life. Strategies to ameliorate symptoms include the application of cold or warm packs, the use of over-the-counter pain relievers, or massage of the affected area [70].
Although two-drug LAI-ART has less potential for drug-drug interactions compared with multidrug oral ART regimens, from the perspective of polypharmacy and medication reactions, certain drugs that induce UGT1A1 or cytochrome P450 3A4 have been found to potentially lead to LAI-ART treatment failure by decreasing concentrations of both CAB and RPV [60]. These drugs include certain anticonvulsants (carbamazepine, oxcarbazepine, phenobarbital, phenytoin), antimycobacterials (rifabutin, rifampin, rifapentine), systemic steroids (more than a single dose of dexamethasone), and St. John’s Wort. Conversely, macrolide antibiotics (specifically azithromycin, clarithromycin, and erythromycin) can increase rilpivirine concentrations and the risk of QT interval prolongation and Torsades de Pointes [60]. Even short courses routinely prescribed for some comorbidities (e.g. systemic steroids for gout flares) might transiently reduce ART efficacy and thereby increase risk of HIV resistance with LA-ART [60]. Because older patients may see multiple specialists, care coordination with other care team members might aid all providers being aware of potential drug–drug interactions and contraindications if LAI-ART is being considered. Patients (and caregivers) may also need to be counseled on interacting medications, to avoid errant prescribing from other care settings, such as urgent care clinics, acute inpatient settings, or rehabilitation or long-term care facilities. Lastly, CAB/RPV can also reduce levels of methadone in some patients, an important consideration for older PWH on methadone for medication-assisted treatment or, less commonly, chronic pain management [60].
Aside from drug–drug interactions, comorbidity profiles in older PWH are important to consider prior to initiating CAB/RPV. CAB/RPV has been associated with depressive disorders, which have a high prevalence and can present atypically in older patients [60,71,72]. Additionally, hepatotoxicity has been reported in patients with and without known hepatic disease who received cabotegravir or rilpivirine [60]. There has been increasing prevalence of chronic liver disease in older age groups [73]. For instance, conditions more common in older patients such as dyslipidemia, diabetes mellitus, and hypertension increase risk for nonalcoholic fatty liver disease [73]. Alcohol-related liver disease is often under-recognized in older adults, and elderly patients tend to have more histologically advanced alcohol-related liver disease compared to younger patients despite a lack of difference in routine laboratory liver function tests between both demographics [73,74]. Therefore, history and lab-based screening for behavioral and hepatic comorbidities in older PWH before and during treatment is important when considering appropriateness of LAI-ART.
Long-acting injectable- antiretroviral therapy implementation challenges and considerations in older persons with HIV
Despite the potential advantages of LAI-ART, structural challenges limit implementation in clinical practice [75–77]. Challenges range from limited clinical capacity, need for new clinical processes, and insurance- or patient-related factors, and may vary based on each clinic’s resources and populations served [47]. For clinics interested in implementing LAI-ART for older patients, Table 4 highlights a proposed checklist of key considerations to facilitate the safe and equitable implementation of LAI-ART for older PWH.
Table 4.
Checklist for consideration and implementation of LAI-ART in older persons with HIV.
| Domains | Considerations | Checklist Items | Next Steps |
|---|---|---|---|
| 1. Does the patient meet criteria for LAI-ART?* | |||
| Department of Health and Human Services guidelines [13] | Does patient meet baseline treatment qualifications? | □ Sustained virologic suppression (viral load <50 HIV-RNA copies/mL) for 3–6 months □ On a stable antiretroviral regimen □ No history of treatment failure □ No known or suspected resistance to CAB or RPV (may consider genotype resistance testing; |
Proceed if all met (Step 2) |
| 2. Determine potential benefits of LAI-ART | |||
| Patient and provider preferences | Perceived benefits | □ Convenience, increased freedom; reduced risk of inadvertent disclosure; pill aversion, fatigue, or intolerance; deprescribing (polypharmacy, concerns about inappropriate dosing); poor adherence* | Consider LAI-ART appropriateness and feasibility (Step 3) |
| Perceived risks/harms | □ Patient preference for oral ART, hesitations about novel therapy, needle aversion, concerns about adverse effects; unreliable follow-up or transportation | LAI-ART likely not appropriate | |
| 3. LAI-ART appropriateness and feasibility | |||
| LAI-ART eligibility screening | Risk of CAB-RPV treatment failure and need for mitigating factors | □ Body mass index (BMI) < 30 kg/m2 | Proceed if all met (Step 4) If any are not met, reevaluate appropriateness of initiating CAB-RPV LAI-ART |
| Medical history, comorbidity, and functional status screening | Medical/surgical history | □ Willingness to adhere to monthly or every other month injections □ No history of chronic hepatitis B infection □ No increased baseline risk for hepatotoxicity □ No history of buttock implants or fillers |
|
| Pharmacologic considerations | □ Contraindicated drugs: Certain anticonvulsantsa, antimycobacterialsb, systemic steroids, St. John’s Wort | Explore feasibility and potential need to change or deprescribe medications with respective providers; otherwise reevaluate appropriateness of initiating CAB-RPV LAI-ART | |
| □ Engage pharmacists to screen for risk of drug–drug interactions with other concomitant long-term medications: macrolide antibioticsd, methadonee | |||
| Past and potential future needs for interacting medications (ex. steroids, antimycobacterials, methadone) | □ Chronic reactive or inflammatory conditions (ex. chronic obstructive pulmonary disease, multiple sclerosis, gout, other autoimmune or rheumatologic disease) or chronic pain (ex. lumbago, sciatica)? | Coordinate with PCP, other specialists to ensure other providers are aware of potential drug–drug interactions and contraindications in setting of LAI-ART | |
| □ Risk for tuberculosis (TB) or TB exposure (ex. travel and work exposure history)? | |||
| Risk for LAI-ART adverse effects | □ Baseline impaired gait and/or ambulation? | Shared decision-making and anticipatory guidance on adverse effects including injection site pain, swelling, and potential impacts on function | |
| □ Past or current typical or atypical depression? | Psychobehavioral history, behavioral health screening (ex. PHQ9) | ||
| □ Consider oral lead-in period (4 weeks) to ensure tolerability | Monitor for drug–drug interactions and adverse effects;if any suspected, reconsider appropriateness of LAI-ART | ||
| Financial feasibility | Potential for increased out-of-pocket costs for copays/coinsurance for both clinic visits and injections on a monthly or every other month schedule | □ Engage pharmacists and case managers | Determine if LAI-ART falls under a medical benefit, pharmacy benefit, or both |
| Determine anticipated out-of-pocket costs to patient | |||
| Determine if and for how long patient may qualify for manufacturer or RWHAP assistance programs | |||
| Shared decision-making with patient on anticipated costs of regular visits and medication | |||
| 4. Implementation considerations for older patients | |||
| Patient education | Cognitive decline or sensory impairment | □ Develop accessible printed and digital materials about treatment regimen, drug–drug interactions, side effects, patient expectations, and plan for missed doses □ Focused, educational clinic visits on LAI-ART process leading up to and with initial doses □ Involve caregivers in education and planning discussions, if appropriate |
|
| Patient scheduling and reminders | Lower technological literacy | □ Identify best means of communication (phone, letter, online portal) to ensure reliable contact for clinical updates and scheduling | Develop a communication and clinic scheduling plan with the patient (and/or caregiver); discuss back-up means of communication if primary method is unsuccessful |
| Patient transportation | Transportation barriers (ex. vision impairment, limited access, dependence on others for rides) | □ Screen for transportation challenges | If positive, engage social work if to explore transportation options |
| Plan for missed doses | Structural and socioeconomic barriers may increase risk for missed doses among older PWH | □ Anticipatory planning with patient (and caregivers, if appropriate) on best backup plan for oral bridge therapy (ex. prescribing a preemptive bridge dose; in-person vs. mail-order pharmacy) | |
| Plan if LAI-ART resistance develops | Certain oral regimens have toxicities of particular relevance to older populations, resulting in higher rates of treatment changes (e.g., greater loss in bone mineral density) | □ Patient-provider discussion of risks of resistance to CAB-RPV and implications of toxicities of next-line therapies | |
| Integrating with chronic disease management | Multiple comorbid conditions, preventive health | □ Consider coordinating injection visits with other health or lab monitoring (ex. blood pressure monitoring, A1c, mental health screening) through discussions with PCP and specialists | |
ART, antiretroviral therapy; CAB, cabotegravir; LAI, long-acting injectables; PWH, people with HIV; RPV, rilpivirine.
Limited studies have demonstrated benefits of CAB-RPV injectable therapy if paired with comprehensive wraparound services for virally unsuppressed or ART-nonadherent patients, such as those with psycho-behavioral or social barriers to adherence.
Anticonvulsants: carbamazepine, oxcarbazepine, phenobarbital, phenytoin.
Antimycobacterials: rifabutin, rifampin, rifapentine.
Systemic steroids: more than a single dose of dexamethasone.
Increases risk for QT prolongation, Torsades de Pointes
CAB-RPV can reduce levels of methadone in some patients.
Capacity and process-related challenges faced by clinics have included additional time needed to assess patient eligibility for LAI-ART, the need for additional skilled clinicians to administer injections, private space requirements for injections, cold-chain storage, increased scheduling demands for injections, navigating missed injections or loss to follow up, and ensuring equitable access and distribution of LAI-ARTs in a cost-effective manner [78].
In the United States, insurance-related challenges encountered by pilot LAI-ART implementation programs have included difficulties with complex drug procurement including lack of drugs on payor formularies, the need for prior authorizations and to appeal insurance denials, reimbursement, and lack of clarity of whether LAI-ART is covered under a pharmacy benefit or a medical benefit [47,79].
Patient access and affordability issues, including socioeconomic status, transportation, living conditions, and time-off-work, have been frequently cited as barriers for LAI-ART implementation [47]. Some older PWH may be less independent or have less access to reliable transportation, potentially raising concerns about returning to clinic for scheduled injections [79,80]. Further, monthly or bimonthly LAI-ART injections may require more frequent clinic visits than oral ART creating an increased transportation burden. Some of these challenges may be overcome by expanded use of community pharmacies, mobile clinics, as well as home-delivered or self-administered services.
LAI-ART prices and healthcare costs can affect acceptance of therapies for older PWH, who may already face higher overall non-HIV related healthcare costs for other chronic comorbidities than younger counterparts. Cabotegravir-rilpivirine may be cost-prohibitive for some older underinsured or uninsured PWH [19]. In the United States, 9% of people aged 65 and older were living in poverty in 2020, and in 2016, 50% of Medicare beneficiaries had incomes below $26 200 with a quarter living on less than $15 250 [81,82]. A majority of PWH on Medicare are dual-eligible for Medicaid and qualify for low-income subsidies under Medicare Part D [83]. Out-of-pocket costs for patients on Medicare receiving LAI-ART would consist of co-pays or co-insurance for both the medication and an office visit. While Medicare Part D would at least partly cover the lead-in oral ARTwhen it is indicated (with patients bearing costs for co-pay or co-insurance), patients on Medicare would be responsible for 20% of the insurance costs for injectable formulations under Medicare Part B, as well as 20% of their visit costs. Dual-eligible PWH living under 150% of the federal poverty level may face less of an out-of-pocket cost burden as both oral lead-in and LAI-ART would each cost no more than $8, with costs for office visits similarly reduced.
Although LAI-ART manufacturers and the Ryan White HIV AIDS Program’s AIDS Drug Assistance Program (ADAP) have assistance programs to help offset costs for certain patients, the long-term feasibility of some options is uncertain [75,78]. Given the complex financial and social factors which may affect the feasibility of LAI-ART for older PWH, multidisciplinary care teams consisting not only of physicians, nurses, and pharmacists, but also case managers and social workers, can assist older adults potentially eligible for LAI-ART [79].
If LAI-ART is deemed appropriate for an older patient, there are additional logistical considerations to be explored. LAI-ART pilot programs have included toolkits with digital and hard-copy media for patients including educational flashcards, patient-reminder systems, and what-to-expect videos [47]. For older PWH, such materials could ideally be accessible to the vision- or hearing-impaired, and clear expectations should be discussed with patients (and caregivers, if appropriate). While technological literacy among older individuals is increasing, gaps still persist [84]. Therefore, patient scheduling and reminder system pathways could be adaptable and account for the lower digital literacy and access faced by many older individuals. In the event of missed injection doses, ‘bridging,’ or a rapid but temporary transition to oral dosing, has not been evaluated for older PWH feasibility . Older PWH (and caregivers) must be educated on the risks of missed doses, and plan accordingly for missed injections by obtaining the oral bridge regimen at time of missed dose or already having it in-hand (i.e. advising to keep one month of their prior oral ART regimen for this purpose) [85]. In addition, hospitals may not have LAI-ART available, so patients will need contingency plans for prolonged hospitalizations such as through oral bridging or by providing a home supply of medication.
Lastly, LAI-ART implementation may present additional opportunities to comprehensively address unique HIV care and treatment needs of older PWH, as highlighted by other comprehensive HIV care programs [86]. For instance, the need for more frequent clinic visits for injections may be leveraged to facilitate other preventive healthcare or chronic disease monitoring.
Conclusion
LAI-ART is a promising therapeutic option for older PWH, which can address issues relating to oral ART adherence and polypharmacy that are common amongst older patients. With the growing number of people aging with HIV, LAI-ART should not just be viewed as a tool for adherence but as an option to ensure all PWH receive patient-centered treatment. To end the HIV epidemic, treatment such as LAI-ART should be broadly and equitably accessible. However, much remains unknown concerning the applicability of LAI-ART among older PWH, as well as unique pharmacological considerations and implementation challenges for this population as few studies have captured older PWH. As the options for LA-ART regimens are expanding, more studies including older PWH are essential to further assess LA-ART effectiveness and safety, and clarify the needs of, and advance health equity for, this growing population.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative reanalysis. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet 2000;355:1131. [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017;4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000–2020. PLoS One 2018; 13:e0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althoff KN, Stewart CN, Humes E, Zhang J, Gerace L, Boyd CM, et al. The shifting age distribution of people with HIV using antiretroviral therapy in the United States. AIDS 2022; 36:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS 2016; 11:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong C, Gange SJ, Moore RD, Justice AC, Buchacz K, Abraham AG, et al. Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill S, Valadez R. Growing older with HIV/AIDS: new public health challenges. Am J Public Health 2013; 103:e7–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008; 47:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg RS, Eyawo O, Collins AB, Zhang W, Jabbari S, Hull MW, et al. Health-adjusted life expectancy in HIV-positive and HIV-negative men and women in British Columbia, Canada: a population-based observational cohort study. Lancet HIV 2017; 4:e270–e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allavena C, Hanf M, Rey D, Duvivier C, BaniSadr F, Poizot-Martin I, et al. Antiretroviral exposure and comorbidities in an aging HIV-infected population: the challenge of geriatric patients. PLoS One 2018; 13:e0203895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug–drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc 2014; 62:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. July 24th, 2023. Available at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/whats-new [Accessed July 27, 2023].
- 14.Kim YS. Long-acting injectable antiretroviral agents for HIV treatment and prevention. Infect Chemother 2021;53:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoueille P, Choong E, Cavassini M, Buclin T, Decosterd LA. Long-acting antiretrovirals: a new era for the management and prevention of HIV infection. J Antimicrob Chemother 2022; 77:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philbin MM, Bergen S, Parish C, Kerrigan D, Kinnard EN, Reed S, et al. Long-Acting injectable ART and PrEP among women in six cities across the United States: a qualitative analysis of who would benefit the most. AIDS Behav 2022; 26:1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philbin MM, Parish CL, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, et al. Multisite study of women living with HIV’s perceived barriers to, and interest in, long-acting injectable antiretroviral therapy. J Acquir Immune Defic Syndr 2020; 84:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philbin MM, McCrimmon T, Shaffer VA, Kerrigan D, Pereyra M, Cohen MH, et al. A patient decision aid (I.ARTS) to facilitate women’s choice between oral and long-acting injectable antiretroviral treatment for HIV: protocols for its development and randomized controlled pilot trial. JMIR Res Protoc 2022; 11:e35646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sued O, Nardi N, Spadaccini L. Key population perceptions and opinions about long-acting antiretrovirals for prevention and treatment: a scoping review. Curr Opin HIV AIDS 2022; 17:145–161. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa JT, Saberi P, Sauceda JA, Dube K. The LAIs are coming! Implementation science considerations for long-acting injectable antiretroviral therapy in the United States: a scoping review. AIDS Res Hum Retroviruses 2021; 37:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasgupta S, Tie Y, Beer L, Crim SM, McManus T, Basu M, et al. Behavioral and clinical characteristics of persons with diagnosed HIV infection—Medical Monitoring Project, United States, 2020 Cycle (June 2020–May 2021). July 12th, 2022. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance-special-reports/no-29/index.html [Accessed August 6, 2023].
- 22.Gardner EM, Burman WJ, Steiner JF, Anderson PL, Bangsberg DR. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS 2009; 23:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branas F, Berenguer J, Sanchez-Conde M, Lopez-Bernaldo de Quiros JC, Miralles P, Cosin J, et al. The eldest of older adults living with HIV: response and adherence to highly active antiretroviral therapy. Am J Med 2008; 121:820–824. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, Quesenberry CP Jr. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med 2007; 167:684–691. [DOI] [PubMed] [Google Scholar]
- 25.Catz SL, Heckman T, Kochman A, DiMarco M. Rates and correlates of HIV treatment adherence among late middle-aged and older adults living with HIV disease. Psychol Health Med 2001; 6:47–58. [Google Scholar]
- 26.TT C, Rhee SY, Hare CB, Shafer RW, Sainani FK. Adherence to contemporary antiretroviral treatment regimens and impact on immunological and virologic outcomes in a US healthcare system. PLoS One 2022; 17:e0263742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess MJ, Zeuli JD, Kasten MJ. Management of HIV/AIDS in older patients-drug/drug interactions and adherence to antiretroviral therapy. HIV AIDS (Auckl) 2015; 7:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 2004; 18 (Suppl 1):S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yap AF, Thirumoorthy T, Kwan YH. Systematic review of the barriers affecting medication adherence in older adults. Geriatr Gerontol Int 2016; 16:1093–1101. [DOI] [PubMed] [Google Scholar]
- 30.Marin RC, Behl T, Negrut N, Bungau S. Management of antiretroviral therapy with boosted protease inhibitors-darunavir/ritonavir or darunavir/cobicistat. Biomedicines 2021; 9:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.My Ou, Zhang H, Tan PC, Zhou SB, QF Li. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis 2022; 13:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer AK, Jensen MD. Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest 2022; 132:e158451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnheim K. Drug therapy in the elderly. Exp Gerontol 2004; 39:1731–1738. [DOI] [PubMed] [Google Scholar]
- 34.Gom I, Fukushima H, Shiraki M, Miwa Y, Ando T, Takai K, et al. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J Nutr Sci Vitaminol (Tokyo) 2007; 53:37–42. [DOI] [PubMed] [Google Scholar]
- 35.Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab 2011; 12:601–610. [DOI] [PubMed] [Google Scholar]
- 36.Cieslak KP, Baur O, Verheij J, Bennink RJ, van Gulik TM. Liver function declines with increased age. HPB (Oxford) 2016; 18:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gekle M. Kidney and aging – a narrative review. Exp Gerontol 2017; 87 (Pt B):153–155. [DOI] [PubMed] [Google Scholar]
- 38.Alexander-Magalee MA. Addressing pharmacology challenges in older adults. Nursing 2013; 43:58–60. [DOI] [PubMed] [Google Scholar]
- 39.Sutton SS, Hardin JW, Bramley TJ, D’Souza AO, Bennett CL. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care 2016; 22:242–248. [PubMed] [Google Scholar]
- 40.Ross EL, Weinstein MC, Schackman BR, Sax PE, Paltiel AD, Walensky RP, et al. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis 2015; 60:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flexner C, Owen A, Siccardi M, Swindells S. Long-acting drugs and formulations for the treatment and prevention of HIV infection. Int J Antimicrob Agents 2021; 57:106220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masia M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–1123. [DOI] [PubMed] [Google Scholar]
- 43.Orkin C, Oka S, Philibert P, Brinson C, Bassa A, Gusev D, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8:e185–e196. [DOI] [PubMed] [Google Scholar]
- 44.Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 48-week results: a randomised, multicentre, open-label, phase 3b, noninferiority study. Lancet 2021; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 45.Ramgopal MN, Castagna A, Cazanave C, Diaz-Brito V, Dretler R, Oka S, et al. Solar 12-month results: randomized switch trial of CAB+RPV LA vs oral B/FTC/TAF. Conference on Retroviruses and Opportunistic Infections 2023. Seattle, US; 2023. [Google Scholar]
- 46.Benn P, Dakhia S, Wu S, Hudson K,Wang Y, D’Amico R, et al. Long-acting cabotegravir+rilpivirine in older adults: pooled phase 3 week-48 results In: Conference on Retroviruses and Opportunistic Infections 2021. Boston, US; 2021. [Google Scholar]
- 47.Czarnogorski M, Garris CP, Dalessandro M, D’Amico R, Nwafor T, Williams W, et al. Perspectives of healthcare providers on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings from a Hybrid III Implementation-effectiveness study (CUSTOMIZE). J Int AIDS Soc 2022; 25:e26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calcagno A, Trunfio M, D’Avolio A, Di Perri G, Bonora S. The impact of age on antiretroviral drug pharmacokinetics in the treatment of adults living with HIV. Expert Opin Drug Metab Toxicol 2021; 17:665–676. [DOI] [PubMed] [Google Scholar]
- 49.Rajoli RK, Back DJ, Rannard S, Freel Meyers CL, Flexner C, Owen A, et al. Physiologically based pharmacokinetic modelling to inform development of intramuscular long-acting nanoformulations for HIV. Clin Pharmacokinet 2015; 54:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toth JM, Jadhav S, Holmes HM, Sharma M. Prescribing trends of proton pump inhibitors, antipsychotics and benzodiazepines of Medicare Part D providers. BMC Geriatr 2022; 22:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use was very high among older adults in the United States in 2011–2014. J Nutr 2017; 147:1968–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu CH, Bednarczyk EM, Catanzaro LM, Shon A, Xu JC, Ma Q. Pharmacokinetic drug interactions of integrase strand transfer inhibitors. Curr Res Pharmacol Drug Discov 2021; 2:100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray M, Kerrigan D, Hudson KJ, Walters N, Karver TS, Mantsios A, et al. Identifying appropriate candidates for long-acting antiretroviral therapy: findings from a survey of healthcare providers in the ATLAS-2M trial. HIV Res Clin Pract 2020; 21:105–113. [DOI] [PubMed] [Google Scholar]
- 54.Lin CH, Chen FC, Chan HY, Hsu CC. A comparison of long-acting injectable antipsychotics with oral antipsychotics on time to rehospitalization within 1 year of discharge in elderly patients with schizophrenia. Am J Geriatr Psychiatry 2020; 28:23–30. [DOI] [PubMed] [Google Scholar]
- 55.Masand PS, Gupta S. Long-acting injectable antipsychotics in the elderly: guidelines for effective use. Drugs Aging 2003; 20:1099–1110. [DOI] [PubMed] [Google Scholar]
- 56.Kates OS. What we know about long-acting injectable antipsychotics can help innovate HIV care. AMA J Ethics 2021; 23:E405–409. [DOI] [PubMed] [Google Scholar]
- 57.Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiligsmann M, Dellaert BG, Dirksen CD, van der Weijden T, Goemaere S, Reginster J-Y, et al. Patients’ preferences for osteoporosis drug treatment: a discrete-choice experiment. Arthritis Res Ther 2014; 16:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Springer SA, Di Paola A, Barbour R, Azar MM, Altice FL. Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV and alcohol use disorders transitioning to the community: results from a double-blind, placebo-controlled trial. J Acquir Immune Defic Syndr 2018; 79:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.FDA. Cabenuva [package insert]. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. [Google Scholar]
- 61.Orkin C, Arasteh K, Gorgolas Hernandez-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–1135. [DOI] [PubMed] [Google Scholar]
- 62.Swindells S, Lutz T, Van Zyl L, Porteiro N, Stoll M, Mitha E, et al. Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS 2022; 36:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, noninferiority trial. Lancet 2017; 390:1499–1510. [DOI] [PubMed] [Google Scholar]
- 64.Kirzinger A, Neuman T, Cubanski J, Brodie M. Data note: prescription drugs and older adults. Kaiser Family Foundation. August 9th, 2019. Available at: https://www.kff.org/health-reform/issue-brief/data-note-prescription-drugs-and-older-adults/ [Accessed August 6, 2023]. [Google Scholar]
- 65.Kim SJ, Kwon OD, Han EB, Lee CM, Oh SW, Joh HK, et al. Impact of number of medications and age on adherence to antihypertensive medications: A nationwide population-based study. Medicine (Baltimore) 2019; 98:e17825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landovitz RJ, Li S, Eron JJ Jr, Grinsztejn B, Dawood H, Liu AY, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 2020; 7:e472–e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cutrell AG, Schapiro JM, Perno CF, Kuritzkes DR, Quercia R, Patel P, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS 2021; 35:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliot E, Polli JW, Patel P, Garside L, Grove R, Barnett V, et al. Efficacy and safety outcomes by BMI category over 48 weeks in phase 3/3b cabotegravir and rilpivirine long-acting trials. 18th European AIDS Conference. London, UK; 2021. [Google Scholar]
- 69.Han K, Baker M, Lovern M, Paul P, Xiong Y, Patel P, et al. Population pharmacokinetics of cabotegravir following administration of oral tablet and long-acting intramuscular injection in adult HIV-1-infected and uninfected subjects. Br J Clin Pharmacol 2022; 88:4607–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper SE, Rosenblatt J, Gulick RM. Barriers to uptake of long-acting antiretroviral products for treatment and prevention of Human Immunodeficiency Virus (HIV) in high-income countries. Clin Infect Dis 2022; 75 (Suppl 4):S541–S548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zenebe Y, Akele B, M. WS, Necho M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry 2021; 20:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCullough PK. Geriatric depression: atypical presentations, hidden meanings. Geriatrics 1991; 46:72–76. [PubMed] [Google Scholar]
- 73.Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing 2009; 38:11–18. [DOI] [PubMed] [Google Scholar]
- 74.DiBartolo MC, Jarosinski JM. Alcohol use disorder in older adults: challenges in assessment and treatment. Issues Ment Health Nurs 2017; 38:25–32. [DOI] [PubMed] [Google Scholar]
- 75.Johnson K, Sawkin MT. Experiences to date with the logistical management of long-acting cabotegravir and rilpivirine. Drugs Context 2022; 11:2021-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mantsios A, Murray M, Karver TS, Davis W, Galai N, Kumar P, et al. Multilevel considerations for optimal implementation of long-acting injectable antiretroviral therapy to treat people living with HIV: perspectives of healthcare providers participating in phase 3 trials. BMC Health Serv Res 2021; 21:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waters L, Sparrowhawk A. Clinical implementation of long-acting antiretroviral treatment in high-income countries: challenges and advantages. Curr Opin HIV AIDS 2022; 17:121–126. [DOI] [PubMed] [Google Scholar]
- 78.HIV Medicine Association. Preparing for long-acting antiretroviral treatment. 2021. Available at: https://www.hivma.org/globalassets/hivma/long-acting-arvs-_final.pdf [Accessed August 6, 2023].
- 79.Collins LF, Corbin-Johnson D, Asrat M, Morton ZP, Dance K, Condra A, et al. Early experience implementing long-acting injectable cabotegravir/rilpivirine for Human Immunodeficiency Virus-1 treatment at a Ryan White-funded clinic in the US South. Open Forum Infect Dis 2022; 9:ofac455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dickerson AE, Molnar L, Bedard M, Eby DW, Classen S, Polgar J. Transportation and aging: an updated research agenda for advancing safe mobility. J Appl Gerontol 2019; 38:1643–1660. [DOI] [PubMed] [Google Scholar]
- 81.Shrider EA, Kollar M, Chen F, Semega J. Income and poverty in the United States: 2020. US Census Bureau, Current Population Reports; 2021; P60–273. Available at: https://www.census.gov/library/publications/2021/demo/p60-273.html [Accessed August 6, 2023]. [Google Scholar]
- 82.Jacobson G, Griffin S, Neuman T, Smith K. Income and Assets of Medicare Beneficiaries, 2016–2035. Washington, DC: Henry J Kaiser Family Foundation. April 21st, 2017. Available at: https://www.kff.org/medicare/issue-brief/income-and-assets-of-medicare-beneficiaries-2016–2035/ [Accessed August 6, 2023]. [Google Scholar]
- 83.Kaiser Family Foundation. Medicare and HIV. Washington, DC: Henry J Kaiser Family Foundation. March 27th, 2023. Available at: https://www.kff.org/hivaids/fact-sheet/medicare-and-hiv/ [Accessed August 6, 2023]. [Google Scholar]
- 84.Anderson M, Perrin A. Tech adoption climbs among older adults. Pew Research Center. May 17th, 2017. Available at: http://www.pewinternet.org/2017/05/17/tech-adoption-climbs-among-older-adults/ [Accessed August 6, 2023].
- 85.Rusconi S, Santoro MM, Capetti AF, Gianotti N, Zazzi M. The future of long-acting cabotegravir plus rilpivirine therapy: deeds and misconceptions. Int J Antimicrob Agents 2022; 60:106627. [DOI] [PubMed] [Google Scholar]
- 86.Tan JY, Greene M, Blat C, Albers A, Grochowski J, Oskarsson J, et al. Examining the impact of the golden compass clinical care program for older people with HIV: a qualitative study. AIDS Behav 2022; 26:1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adachi E, Ikeuchi K, Koga M, Yotsuyanagi H. Background factors in people living with HIV in Japan who switch to cabotegravir plus rilpivirine: a pilot study. J Infect Chemother 2023; 29:109–111. [DOI] [PubMed] [Google Scholar]
- 88.Akinwunmi B, Buchenberger D, Scherzer J, Bode M, Rizzini P, Vecchio F, et al. Factors associated with interest in a long-acting HIV regimen: perspectives of people living with HIV and healthcare providers in four European countries. Sex Transm Infect 2021; 97:566–573. [DOI] [PubMed] [Google Scholar]
- 89.Carillon S, Gallardo L, Linard F, Chakvetadze C, Viard JP, Cros A, et al. Perspectives of injectable long acting antiretroviral therapies for HIV treatment or prevention: understanding potential users’ ambivalences. AIDS Care 2020; 32 (Suppl 2):155–161. [DOI] [PubMed] [Google Scholar]
- 90.Fletcher L, Burrowes SAB, Khan GK, Sabin L, Johnson S, Kimmel SD, et al. Perspectives on long-acting injectable HIV antiretroviral therapy at an alternative care site: a qualitative study of people with HIV experiencing substance use and/or housing instability. Harm Reduct J 2023; 20:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garris C, Heidenreich S, Arthurs E, Spinelli F, Cutts K, Lowman E, et al. 2499. Perceptions of and preferences for oral or long-acting injectable antiretroviral treatment regimens in the United States and Canada. Open Forum Infect Dis 2019; 6 (Suppl 2):S866–867. [Google Scholar]
- 92.Garris CP, Czarnogorski M, Dalessandro M, D’Amico R, Nwafor T, Williams W, et al. Perspectives of people living with HIV-1 on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings: results from the CUSTOMIZE hybrid III implementation-effectiveness study. J Int AIDS Soc 2022; 25:e26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koren DE, Fedkiv V, Zhao H, Kludjian G, Bettiker RL, Tedaldi E, et al. Perceptions of long-acting injectable antiretroviral treatment regimens in a United States urban academic medical center. J Int Assoc Provid AIDS Care 2020; 19:2325958220981265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Massaroni V, Delle Donne V, Borghetti A, Ciccullo A, Lombardi F, Giuliano G, et al. Use of long-acting therapies for HIV care in Italy: are people living with HIV prepared for change? A cross-sectional study. AIDS Patient Care STDS 2022; 36:178–185. [DOI] [PubMed] [Google Scholar]
- 95.Palacios C, Wilpotte C, Adda A, Allaf S, Thibaut P, Chas J, et al. Expectations and acceptability of long-acting injectable antiretrovirals by patients living with HIV/AIDS. Infect Dis Now 2022; 52:238–239. [DOI] [PubMed] [Google Scholar]
- 96.Saberi P, Eskaf S, Sauceda J, Evans D, Dube K. Perceptions of hiv virologic control strategies among younger and older age groups of people living with HIV in the United States: a cross-sectional survey. AIDS Res Hum Retroviruses 2020; 36:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simoni JM, Tapia K, Lee SJ,Graham SM, Beima-Sofie K, Mohamed ZH, et al. A conjoint analysis of the acceptability of targeted long-acting injectable antiretroviral therapy among persons living with HIV in the US. AIDS Behav 2020; 24:1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simoni JM, Beima-Sofie K, Mohamed ZH, Christodoulou J, Tapia K, Graham SM, et al. Long-acting injectable antiretroviral treatment acceptability and preferences: a qualitative study among US providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDS 2019; 33:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slama L, Porcher R, Linard F, Chakvetadze C, Cros A, Carillon S, et al. Injectable long acting antiretroviral for HIV treatment and prevention: perspectives of potential users. BMC Infect Dis 2023; 23:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toska E, Zhou S, Chen-Charles J, Gittings L, Operario D, Cluver L. Factors associated with preferences for long-acting injectable antiretroviral therapy among adolescents and young people living with HIV in South Africa. AIDS Behav 2023; 27:2163–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams J, Sayles HR, Meza JL, Sayre P, Sandkovsky U, Gendelman HE, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013; 8:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rizzardini G, Overton ET, Orkin C, Swindells S, Arasteh K, Gorgolas Hernandez-Mora M, et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J Acquir Immune Defic Syndr 2020; 85:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chounta V, Overton ET, Mills A, Swindells S, Benn PD, Vanveggel S, et al. Patient-reported outcomes through 1 year of an HIV-1 clinical trial evaluating long-acting cabotegravir and rilpivirine administered every 4 or 8 weeks (ATLAS-2 M). Patient 2021; 14:849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]


