Abstract
Background
Osteoarthritis is the most common form of joint disease and the leading cause of pain and physical disability in older people. Opioids may be a viable treatment option if people have severe pain or if other analgesics are contraindicated. However, the evidence about their effectiveness and safety is contradictory. This is an update of a Cochrane review first published in 2009.
Objectives
To determine the effects on pain, function, safety, and addiction of oral or transdermal opioids compared with placebo or no intervention in people with knee or hip osteoarthritis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL (up to 28 July 2008, with an update performed on 15 August 2012), checked conference proceedings, reference lists, and contacted authors.
Selection criteria
We included randomised or quasi‐randomised controlled trials that compared oral or transdermal opioids with placebo or no treatment in people with knee or hip osteoarthritis. We excluded studies of tramadol. We applied no language restrictions.
Data collection and analysis
We extracted data in duplicate. We calculated standardised mean differences (SMDs) and 95% confidence intervals (CI) for pain and function, and risk ratios for safety outcomes. We combined trials using an inverse‐variance random‐effects meta‐analysis.
Main results
We identified 12 additional trials and included 22 trials with 8275 participants in this update. Oral oxycodone was studied in 10 trials, transdermal buprenorphine and oral tapentadol in four, oral codeine in three, oral morphine and oral oxymorphone in two, and transdermal fentanyl and oral hydromorphone in one trial each. All trials were described as double‐blind, but the risk of bias for other domains was unclear in several trials due to incomplete reporting. Opioids were more beneficial in pain reduction than control interventions (SMD ‐0.28, 95% CI ‐0.35 to ‐0.20), which corresponds to a difference in pain scores of 0.7 cm on a 10‐cm visual analogue scale (VAS) between opioids and placebo. This corresponds to a difference in improvement of 12% (95% CI 9% to 15%) between opioids (41% mean improvement from baseline) and placebo (29% mean improvement from baseline), which translates into a number needed to treat (NNTB) to cause one additional treatment response on pain of 10 (95% CI 8 to 14). Improvement of function was larger in opioid‐treated participants compared with control groups (SMD ‐0.26, 95% CI ‐0.35 to ‐0.17), which corresponds to a difference in function scores of 0.6 units between opioids and placebo on a standardised Western Ontario and McMaster Universities Arthritis Index (WOMAC) disability scale ranging from 0 to 10. This corresponds to a difference in improvement of 11% (95% CI 7% to 14%) between opioids (32% mean improvement from baseline) and placebo (21% mean improvement from baseline), which translates into an NNTB to cause one additional treatment response on function of 11 (95% CI 7 to 14). We did not find substantial differences in effects according to type of opioid, analgesic potency, route of administration, daily dose, methodological quality of trials, and type of funding. Trials with treatment durations of four weeks or less showed larger pain relief than trials with longer treatment duration (P value for interaction = 0.001) and there was evidence for funnel plot asymmetry (P value = 0.054 for pain and P value = 0.011 for function). Adverse events were more frequent in participants receiving opioids compared with control. The pooled risk ratio was 1.49 (95% CI 1.35 to 1.63) for any adverse event (9 trials; 22% of participants in opioid and 15% of participants in control treatment experienced side effects), 3.76 (95% CI 2.93 to 4.82) for drop‐outs due to adverse events (19 trials; 6.4% of participants in opioid and 1.7% of participants in control treatment dropped out due to adverse events), and 3.35 (95% CI 0.83 to 13.56) for serious adverse events (2 trials; 1.3% of participants in opioid and 0.4% of participants in control treatment experienced serious adverse events). Withdrawal symptoms occurred more often in opioid compared with control treatment (odds ratio (OR) 2.76, 95% CI 2.02 to 3.77; 3 trials; 2.4% of participants in opioid and 0.9% of participants control treatment experienced withdrawal symptoms).
Authors' conclusions
The small mean benefit of non‐tramadol opioids are contrasted by significant increases in the risk of adverse events. For the pain outcome in particular, observed effects were of questionable clinical relevance since the 95% CI did not include the minimal clinically important difference of 0.37 SMDs, which corresponds to 0.9 cm on a 10‐cm VAS.
Keywords: Humans; Administration, Cutaneous; Administration, Oral; Analgesics, Opioid; Analgesics, Opioid/administration & dosage; Analgesics, Opioid/adverse effects; Osteoarthritis, Hip; Osteoarthritis, Hip/drug therapy; Osteoarthritis, Knee; Osteoarthritis, Knee/drug therapy; Pain Measurement; Randomized Controlled Trials as Topic
Plain language summary
Opioids for osteoarthritis
This summary of a Cochrane review of 22 studies with 8275 participants (search update: 15 August 2012) presents what we know from research about the effect of opioids on osteoarthritis (OA). We searched scientific databases for clinical trials looking at pain, function, safety, and addiction of oral or transdermal opioids compared with placebo or no intervention in people with knee or hip osteoarthritis.
The review shows that in people with osteoarthritis:
‐ Opioids have a small effect on pain or physical function. ‐ Opioids probably cause side effects. However, we do not have precise information about rare but serious side effects.
What is osteoarthritis and what are opioids?
OA is a disease of the joints, such as your knee or hip. When the joint loses cartilage, the bone grows to try to repair the damage. Instead of making things better, however, the bone grows abnormally and makes things worse. For example, the bone can become misshapen and make the joint painful and unstable. This can affect your physical function or ability to use your knee.
Opioids are generally conceived as powerful pain‐relieving substances that are used for the pain of cancer or osteoarthritis. Some examples of opioids are codeine‐containing Tylenol® (1, 2, 3, and 4), hydromorphone (Dilaudid), oxycodone (Percocet, Percodan), morphine, and others. They can be taken in a pill form, as an injection, or as a patch placed on the painful area.
Best estimate of what happens to people with osteoarthritis who take opioids
Pain
‐ People who took opioids rated improvement in their pain to be about 3 points on a scale of 0 (no pain) to 10 (extreme pain) after one month. ‐ People who took a placebo rated improvement in their pain to be about 2 points on a scale of 0 (no pain) to 10 (extreme pain) after one month.
Another way of saying this is: ‐ 41 people out of 100 who used opioids responded to treatment (41%). ‐ 31 people out of 100 who used placebo responded to treatment (31%). ‐ 10 more people responded to treatment with opioids than with placebo (difference of 10%). (High‐quality evidence)
Physical function
‐ People who took opioids rated improvement in their physical function to be about 2 points on a scale of 0 (no disability) to 10 (extreme disability) after one month. ‐ People who took a placebo rated improvement in their physical function to be about 1 point on a scale of 0 (no disability) to 10 (extreme disability) after one month.
Another way of saying this is:
‐ 34 people out of 100 who used opioids responded to treatment (34%). ‐ 26 people out of 100 who used placebo responded to treatment (26%). ‐ 8 more people responded to treatment with opioids than with placebo (difference of 8%). (High‐quality evidence)
Side effects
‐ 22 people out of 100 who used opioids experienced side effects (22%). ‐ 15 people out of 100 who used a placebo experienced side effects (15%). ‐ 7 more people experienced side effects with opioids than with placebo (difference of 7%). (Moderate‐quality evidence)
Drop‐outs because of side effects
‐ 64 people out of 1000 who used opioids dropped out because of side effects (6.4%). ‐ 17 people out of 1000 who used a placebo dropped out because of side effects (1.7%). ‐ 47 more people dropped out because of side effects with opioids than with placebo (difference of 4.7%). (High‐quality evidence)
Side effects resulting in hospitalisation, persistent disability, or death
‐ 13 people out of 1000 who used opioids experienced side effects resulting in hospitalisation, persistent disability, or death (1.3%). ‐ 4 people out of 1000 who used a placebo experienced side effects resulting in hospitalisation, persistent disability, or deaths (0.4%). ‐ 9 more people experienced side effects resulting in hospitalisation, persistent disability, or death with opioids than with placebo (difference of 0.9%). (Low‐quality evidence)
Withdrawal symptoms
‐ 24 people out of 1000 who used opioids experienced withdrawal symptoms (2.4%). ‐ 9 people out of 1000 who used a placebo experienced withdrawal symptoms (0.9%). ‐ 15 more people experienced withdrawal symptoms with opioids than with placebo (difference of 1.5%). (Moderate‐quality evidence)
Summary of findings
Summary of findings for the main comparison. Oral or transdermal opioids compared with placebo for osteoarthritis of the knee or hip.
| Oral or transdermal opioids compared with placebo for osteoarthritis of the knee or hip | ||||||
|
Patient or population: participants with osteoarthritis of the knee or hip Settings: various orthopaedic or rheumatology clinics Intervention: oral or transdermal opioids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Opioids | |||||
|
Pain intensity Various pain scales. (median follow‐up: 4 weeks) |
‐1.8 cm change
on 10‐cm VAS1 29% improvement |
‐2.5 cm change
(Δ ‐0.7 cm, ‐0.9 to ‐0.5)2 41% improvement (Δ 12%, 9% to 15%)3 |
SMD ‐0.28 (‐0.35 to ‐0.20) | 8275 (22) | ++++ high | NNTB 10 (95% CI 8 to 14)4 |
|
Function Various validated function scales. (median follow‐up: 5 weeks) |
‐1.2 units
on WOMAC (range 0 to 10)1 21% improvement |
‐1.8 units on WOMAC
(Δ ‐0.6, ‐0.8 to ‐0.4)5 32% improvement (Δ 11%, 7% to 14%)6 |
SMD ‐0.26 (‐0.35 to ‐0.17) | 3553 (12) | ++++ high | NNTB 12 (95% CI 10 to 18)7 |
|
Number of participants experiencing any adverse event (median follow‐up: 8 weeks) |
150 per 1000 participant‐years8 | 224 per 1000 participant‐years (203 to 245) | RR 1.49 (1.35 to 1.63) | 4898 (9) | +++O moderate9 | NNTH 14 (95% CI 11 to 19) |
|
Number of participants who withdrew because of adverse events (median follow‐up: 6 weeks) |
17 per 1000 participant‐years8 | 64 per 1000 participant‐years (50 to 82) | RR 3.76 (2.93 to 4.82) | 7712 (19) | ++++ high | NNTH 21 (95% CI 15 to 30) |
|
Number of participants experiencing any serious adverse event (median follow‐up: 8 weeks) |
4 per 1000 participant‐years8 | 13 per 1000 participant‐years (3 to 54) | RR 3.35 (0.83 to 13.56) | 681 (3) | ++OO low10 | Little evidence of harmful effect (NNTH not statistically significant) |
|
Withdrawal symptoms (median follow‐up: 16 weeks) |
9 per 1000 participant‐years11 | 24 per 100 participant‐years (18 to 33) | OR 2.67 (2.02 to 3.77) | 1151 (3) | +++O moderate12 | NNTH 65 (95% CI 42 to 110) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: GRADE Working Group grades of evidence (see explanations); NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio; RR: risk ratio; SMD: standardised mean difference; WOMAC: Western Ontario and McMaster Universities Arthritis Index. | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
1 Median reduction as observed across placebo groups in large osteoarthritis trials (see methods section, Nüesch 2009). 2 SMDs were back‐transformed onto a 10‐cm visual analogue scale (VAS) on the basis of a typical pooled standard deviation (SD) of 2.5 cm in large trials that assessed pain using a VAS and expressed as change based on an assumed standardised reduction of 0.72 |SD units in the control group. 3 Percentage of improvement was calculated based on median observed pain at baseline across control groups of large osteoarthritis trials of 6.1 cm on 10‐cm VAS (Nüesch 2009). 4 Absolute response risks for pain in the control groups were assumed 31% (see methods section). 5 SMDs were back‐transformed onto a standardised WOMAC disability score ranging from 0 to 10 on the basis of a typical pooled SD of 2.1 in trials that assessed function using WOMAC disability scores and expressed as change based on an assumed standardised reduction of 0.58 standard deviation units in the control group. 6 Percentage of improvement was calculated based on median observed WOMAC function scores at baseline across control groups of large osteoarthritis trials of 5.6 units (Nüesch 2009). 7 Absolute response risks for function in the control groups were assumed 26% (see methods section). 8 Median control risk across placebo groups in large osteoarthritis trials (see methods section, Nüesch 2009). 9 Downgraded (1 level) because: 9 out of 19 studies reported this outcome, possibly leading to selective outcome reporting bias. 10 Downgraded (2 levels) because: 3 out of 19 studies reported this outcome, possibly leading to selective outcome reporting bias, the CI of the pooled estimate is wide and crossed no difference. 11 Median risk across control groups in included trials. 12 Downgraded (1 level) because 3 out of 22 studies reported this outcome, possible leading to selective outcome reporting bias.
Background
Description of the condition
Osteoarthritis is the most common form of joint disease and the leading cause of pain, functional limitations, and loss of independence in older adults (Altman 1986). It is a progressive disease of synovial joints resulting from biomechanical and systemic effects, and is characterised by a breakdown of the joint cartilage accompanied by subchondral bone changes, deterioration of tendons and ligaments, and various degrees of inflammation of the synovium (Hochberg 2012).
Description of the intervention
Pharmacological therapy for osteoarthritis, as an alternative or in addition to other therapeutic options, consists mainly of analgesics and non‐steroidal anti‐inflammatory drugs (NSAIDs). However, paracetamol may be inadequate to treat more severe, long‐term pain in osteoarthritis and chronic NSAID use may cause serious gastrointestinal and cardiovascular adverse events. Opioids could be a viable alternative if people have severe pain with insufficient response to conventional treatment or if other analgesics are contraindicated (Avouac 2007).
How the intervention might work
Opioids are potent analgesics that work by targeting mainly spinal and supraspinal opioid receptors. In addition, cellular studies suggest that there are peripheral opioid receptors in inflamed osteoarthritic synovial tissue, which may mediate analgesic effects (Stein 1996).
Why it is important to do this review
The American College of Rheumatology guidelines on management of osteoarthritis, updated in 2012, suggest that opioids can be used in people with osteoarthritis after having failed medical therapy who were not willing or had contraindications for total joint replacement (Hochberg 2012). British guidelines propose opioids as an alternative if inadequate pain relief is achieved with topical NSAIDs or paracetamol (Eccles 1998; NICE 2008). However, the use of strong opioids for the treatment of non‐cancer pain remains controversial. Concerns have been expressed about long‐term use of opioids for chronic non‐cancer pain mainly due to the risks of addiction (Von Korff 2004; Zhang 2008).
Objectives
To determine the effects on pain, function, safety, and addiction of oral or transdermal opioids compared with placebo or no intervention in people with knee or hip osteoarthritis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials with a control group receiving placebo or no intervention.
Types of participants
At least 75% of participants with clinically or radiologically confirmed osteoarthritis of the knee or hip. We did not consider trials exclusively including people with inflammatory arthritis, such as rheumatoid arthritis.
Types of interventions
Any type of opioid except tramadol, which is covered in a separate Cochrane Review (Cepeda 2006).
Types of outcome measures
Primary outcomes
The main outcomes were pain and function, as currently recommended for osteoarthritis trials (Altman 1996; Pham 2004). If data on more than one pain scale were provided for a trial, we referred to a previously described hierarchy of pain‐related outcomes (Jüni 2006; Reichenbach 2007), and extracted data on the pain scale that was highest on this list:
global pain;
pain on walking;
Western Ontario and McMaster Universities Arthritis Index (WOMAC) osteoarthritis index pain subscore;
composite pain scores other than WOMAC;
pain on activities other than walking;
rest pain or pain during the night;
WOMAC global algofunctional score;
Lequesne osteoarthritis index global score;
other algofunctional scale;
participant's global assessment;
physician's global assessment.
If data on more than one function scale were provided for a trial, we extracted data according to the hierarchy:
global disability score;
walking disability;
WOMAC disability subscore;
composite disability scores other than WOMAC;
disability other than walking;
WOMAC global scale;
Lequesne osteoarthritis index global score;
other algofunctional scale;
participant's global assessment;
physician's global assessment.
If pain or function outcomes were reported at several time points, we extracted the measure at the end of the treatment period.
Secondary outcomes
Secondary outcomes were the number of participants who experienced any adverse event, withdrew because of adverse events, experienced any serious adverse events, and experienced symptoms of opioid dependence such as craving or physical withdrawal symptoms. We defined serious adverse events as events resulting in hospitalisation, prolongation of hospitalisation, persistent or significant disability, congenital abnormality or birth defect of offspring, life‐threatening events, or death.
Search methods for identification of studies
Electronic searches
We searched the electronic databases the Cochrane Central Register of Controlled Trials (CENTRAL) (mrw.interscience.wiley.com/cochrane/), MEDLINE and EMBASE through the Ovid platform (www.ovid.com), and CINAHL through EBSCOhost (all from implementation to July 28 2008) using truncated variations of preparation names including brand names combined with truncated variations of terms related to osteoarthritis, all as text words. We applied a validated methodological filter for controlled clinical trials (Dickersin 1994). The specific search algorithms are displayed in Appendix 1 and Appendix 2. We updated the search using CENTRAL, MEDLINE, and EMBASE up to 15 August 2012.
Searching other resources
We manually searched conference proceedings, used Science Citation Index to retrieve reports citing relevant articles, contacted content experts and trialists, and screened reference lists of all obtained articles. Finally, we searched several clinical trial registries (clinicaltrials.gov, metaRegister of Controlled Trials, Australian New Zealand Clinical Trials Registry, UMIN Clinical Trials Registry) to identify ongoing trials. We performed the last update of the search on 20 September 2012. We did not search OARSI conference proceedings for the update, as we no longer had access to this database.
Data collection and analysis
We used a generic protocol with instructions for data extraction, quality assessment, and statistical analyses, which was approved by the editorial board of the Cochrane Musculoskeletal Group. We applied the same protocol in our previous reviews (Rutjes 2009a; Rutjes 2009b; Reichenbach 2010; Rutjes 2010; da Costa 2012b).
Selection of studies
Two review authors independently evaluated all titles and abstracts for eligibility (originally EN and AR, BdC and RK for the update) (see Figure 1). We resolved disagreements by discussion. We applied no language restrictions. If multiple reports described the same trial, we considered all.
1.
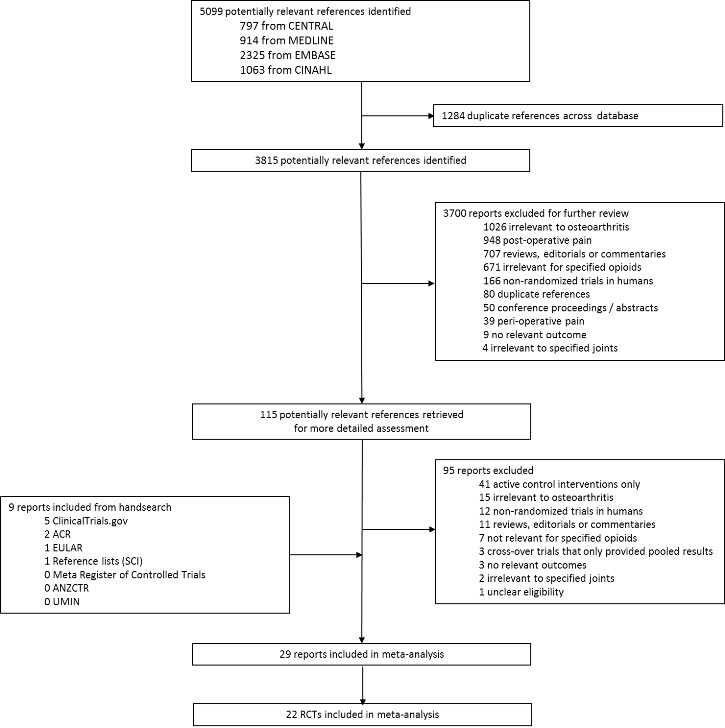
Study flow chart.
Data extraction and management
Two review authors (originally EN and AR, BdC and RK for the update) extracted trial information independently using a standardised, piloted extraction form accompanied by a codebook. We resolved disagreements by discussion. We extracted both the generic and trade name of the experimental intervention, the type of control used, dosage, frequency, route of administration, duration of treatment, participant characteristics (gender, mean age and duration of symptoms, types of joints affected), types of measures used and pain‐ and function‐related outcomes, trial design, trial size, duration of follow‐up, type and source of financial support, and publication status. When necessary, we approximated means and measures of dispersion from figures in the reports. For cross‐over trials, we extracted data from the first period only. Whenever possible, we used results from an intention‐to‐treat analysis. If effect sizes could not be calculated, we contacted the authors for additional data.
Assessment of risk of bias in included studies
Two review authors (originally EN and AR, BdC and RK for the update) independently assessed randomisation, blinding, and adequacy of analyses (Jüni 2001). We resolved disagreements by consensus. We assessed two components of randomisation: generation of allocation sequences and concealment of allocation. We considered generation of sequences to be adequate if it resulted in an unpredictable allocation schedule; mechanisms considered adequate included random‐number tables, computer‐generated random numbers, minimisation, coin tossing, shuffling cards, and drawing lots. We considered trials using an unpredictable allocation sequence to be randomised; we considered trials using potentially predictable allocation mechanisms, such as alternation or the allocation of participants according to date of birth, to be quasi‐randomised. We considered concealment of allocation to be adequate if participants and investigators responsible for participant selection were unable to suspect before allocation which treatment was next. Methods considered adequate include central randomisation; pharmacy‐controlled randomisation using identical pre‐numbered containers; and sequentially numbered, sealed, opaque envelopes. We considered blinding of participants to be adequate if experimental and control preparations were explicitly described as indistinguishable or if a double‐dummy technique was used. We considered analyses to be adequate if all randomised participants were included in the analysis according to the intention‐to‐treat principle. We further assessed the reporting of primary outcomes, sample size calculations, and funding source. Finally, we used GRADE to describe the quality of the overall body of evidence (Guyatt 2008; Higgins 2011), defined as the extent of confidence into the estimates of treatment benefits and harms.
Measures of treatment effect
We summarised continuous outcomes using standardised mean differences (SMD) with 95% confidence intervals (CI), with the differences in mean values at the end of treatment across treatment groups divided by the pooled standard deviation (SD). If differences in mean values at the end of the treatment were unavailable, we used differences in mean changes. If some of the required data were unavailable, we used approximations, as previously described (Reichenbach 2007). An SMD of ‐0.20 SD units can be considered a small difference between the experimental and control groups, an SMD of ‐0.50 a moderate difference, and ‐0.80 a large difference (Cohen 1988; Jüni 2006). SMDs can also be interpreted in terms of the per cent of overlap of the experimental group's scores with scores of the control group. An SMD of ‐0.20 indicates an overlap in the distribution of pain or function scores in about 85% of cases, an SMD of ‐0.50 in about 67%, and an SMD of ‐0.80 in about 53% of cases (Cohen 1988; Jüni 2006). On the basis of a median pooled SD of 2.5 cm, found in large‐scale osteoarthritis trials that assessed pain using a 10‐cm visual analogue scale (VAS) (Nüesch 2009), SMDs of ‐0.20 correspond to approximate differences in pain scores between experimental and control groups of 0.5 on a 10‐cm VAS, ‐0.50 of 1.25 on a 10‐cm VAS, and ‐0.80 of 2 on a 10‐cm VAS. We back transformed SMDs for function to a standardised WOMAC disability score (Bellamy 1995), ranging from 0 to 10 on the basis of a median pooled SD of 2.1 units observed in large‐scale osteoarthritis trials (Nüesch 2009). We expressed binary outcomes as risk ratios (RR) with 95% CI.
Data synthesis
We used a standard inverse‐variance random‐effects meta‐analysis to combine the trials (DerSimonian 1986). We quantified heterogeneity between trials using the I2 statistic (Higgins 2003), which describes the percentage of variation across trials that is attributable to heterogeneity rather than to chance. I2 values of 25% may be interpreted as low, 50% as moderate, and 75% as high between‐trial heterogeneity, although its interpretation depends on the size and number of trials included (Rücker 2008). The association between trial size and treatment effects was investigated in funnel plots, plotting effect sizes on the vertical axis against their standard errors on the horizontal axis (Sterne 2011). We assessed asymmetry by the asymmetry coefficient, the difference in effect size per unit increase in standard error (Sterne 2001), which is mainly a surrogate for sample size, and used univariable, meta‐regression analysis to predict treatment effects in trials as large as the largest trials included in the meta‐analysis using the standard error as the explanatory variable (Shang 2005). We then performed analyses of the primary outcomes, pain and function, stratified by the following trial characteristics: type of opioid, analgesic potency (strong versus weak), route of administration (oral versus transdermal), type of control (placebo versus no intervention), concealment of allocation (adequate versus inadequate or unclear), blinding of participants (adequate versus inadequate or unclear), analysis in accordance with the intention‐to‐treat principle (yes versus no or unclear), trial size, funding (funding by pharmaceutical industry or unclear versus no funding by pharmaceutical industry), duration of treatment, and type of osteoarthritis (hip only versus knee only versus mixed). We classified buprenorphine, fentanyl, morphine, oxycodone, oxymorphone, and tapentadol as strong opioids, and codeine and dextropropoxyphene as weak opioids. We used a cut‐off of 200 allocated participants to distinguish between small‐scale and large‐scale trials. A sample size of 2 x 100 participants will yield more than 80% power to detect a small‐to‐moderate SMD of ‐0.40 at a two‐sided P value of 0.05, which corresponds to a difference of 1 cm on a 10‐cm VAS between the experimental and control intervention (Nüesch 2010). We used a cut‐off of one month to distinguish between short‐term and long‐term trials. We used univariable, random‐effects meta‐regression models to determine whether treatment effects were affected by these factors (Thompson 1999). In addition, we included the following two continuous variables at trial level in univariable meta‐regression: daily morphine equivalence dosage and treatment duration. We calculated morphine equivalence doses as previously described: oral morphine 10 mg was considered equivalent to oral codeine 65 mg, oral hydromorphone 2 mg, oral oxycodone 7.5 mg, and oral oxymorphone 10 mg and oral tapentadol 25 mg (Loeser 2001; Schug 2006). Patches of fentanyl 25 μg/hour was considered equivalent to oral morphine 90 mg per day and patches of buprenorphine 5, 10, and 20 μg/hour equivalent to 10, 15, and 30 mg oral morphine per day (British Pain Society 2010).
We converted SMDs of pain intensity and function to odds ratios (OR) (Chinn 2000; da Costa 2012a) to derive numbers needed to treat to cause one additional treatment response on pain or function as compared with placebo (NNTB), and numbers needed to treat to cause one additional adverse outcome (NNTH). We defined treatment response as a 50% improvement in scores (Clegg 2006). With a median standardised pain intensity at baseline of 2.4 SD units, observed in large osteoarthritis trials (Nüesch 2009), this corresponds to a mean decrease in scores of 1.2 SD units. Based on the median standardised decrease in pain scores of 0.72 SD units (Nüesch 2009), we calculated that a median of 31% of participants in the placebo group would achieve an improvement of pain scores of 50% or more. This percentage was used as the control group response rate to calculate NNTBs for treatment response on pain. Based on the median standardised WOMAC function score at baseline of 2.7 SD units and the median standardised decrease in function scores of 0.58 SD units (Nüesch 2009), 26% of participants in the placebo group would achieve a reduction in function of 50% or more. Again, this percentage was used as the control group response rate to calculate NNTBs for treatment response on function. We used the median risks of 150 participants with adverse events per 1000 participant‐years, four participants with serious adverse events per 1000 participant‐years, and 17 drop‐outs due to adverse events per 1000 participant‐years as observed in placebo groups in large osteoarthritis trials (Nüesch 2009), to calculate NNTHs for safety outcomes. All P values were two‐sided. We performed analyses using Review Manager 5 (RevMan 2012), and STATA version 11.2 (StataCorp, College Station, Texas).
Results
Description of studies
We identified 5099 potentially relevant references through our electronic searches (Figure 1); we excluded 4984 references after screening titles and abstracts and retrieved 115 potentially relevant references for full‐text assessment. We included 22 randomised controlled trials in the review. Checking reference lists, trial registers, and handsearching of conference proceedings yielded five additional trials.
Three trials evaluated weak opioids. All three compared codeine with placebo (Kjaersgaard‐Andersen 1990; Quiding 1992; Peloso 2000), one of these with paracetamol 3000 mg daily as analgesic co‐intervention administered in both the experimental and control groups (Kjaersgaard‐Andersen 1990), and another with ibuprofen 1200 mg daily administered in both groups (Quiding 1992). Strong opioids were compared with placebo in 19 trials. Hydromorphone was used in one trial (NCT00980798), morphine in two trials (Caldwell 2002; Katz 2010), oxymorphone in two trials (Matsumoto 2005; Kivitz 2006), oxycodone in 10 trials (Chindalore 2005; Markenson 2005; Matsumoto 2005; Zautra 2005; Hartrick 2009; Afilalo 2010; Etropolski 2011; Fidelholtz 2011; Friedmann 2011; NCT00486811), and tapentadol in four trials (Hartrick 2009; Afilalo 2010; Etropolski 2011; NCT00486811). Transdermal opioids were studied in five trials: buprenorphine in four trials (Shannon 2005; Breivik 2010; Munera 2010; NCT00531427), and fentanyl in one trial (Langford 2006). Opioids were administered at a median daily dose of 59‐mg morphine equivalents (range 13 to 160 mg).
The median treatment duration was four weeks (range three days to six months). Trials randomised a median of 344 participants (range 27 to 10301 participants). Twenty trials (90%) were multicentre trials, 21 were parallel group, and one was a cross‐over trial (Quiding 1992). Two trials exclusively included participants with hip osteoarthritis (Kjaersgaard‐Andersen 1990; Quiding 1992), four trials included only participants with knee osteoarthritis (Zautra 2005; NCT0048681; Afilalo 20101; NCT00531427), and 16 trials included a mixed population of both knee and hip osteoarthritis (Peloso 2000; Caldwell 2002; Chindalore 2005; Markenson 2005; Matsumoto 2005; Shannon 2005; Kivitz 2006; Langford 2006; Hartrick 2009; Breivik 2010; Katz 2010; Munera 2010; Etropolski 2011; Fidelholtz 2011; Friedmann 2011; NCT00980798). In 17 studies, only participants with insufficient analgesic response to paracetamol, NSAIDs, or previous opioid treatment were included (NCT00980798; NCT00531427; Caldwell 2002; Chindalore 2005; Markenson 2005; Matsumoto 2005; Shannon 2005; Kivitz 2006; Langford 2006; Hartrick 2009; Afilalo 2010; Breivik 2010; Katz 2010; Munera 2010; Etropolski 2011; Friedmann 2011; NCT00486811). None of these trials provided detailed information about the dosage of the analgesic treatments before entering the trial. The three trials assessing codeine included participants with a need for analgesic treatment but without any requirement of previous insufficient treatment response (Kjaersgaard‐Andersen 1990; Quiding 1992; Peloso 2000); two trials did not provide information about eligibility criteria concerning the previous analgesic therapy (Zautra 2005; Fidelholtz 2011).
The Characteristics of excluded studies table displays the reasons why we did not consider trials in this systematic review. Typical reasons were more than 25% of participants with rheumatoid arthritis in the sample, the use of active control interventions, or the use of cross‐over designs without providing sufficient information on the first phase.
Risk of bias in included studies
Figure 2 summarises the methodological characteristics and sources of funding of included trials. Six trials (27%) reported both adequate sequence generation and adequate allocation concealment (Markenson 2005; Kivitz 2006; Langford 2006; Afilalo 2010; Breivik 2010; Etropolski 2011), two trials reported only adequate sequence generation (Matsumoto 2005; Hartrick 2009), and two trials reported adequate concealment but remained unclear about the generation of allocation sequence (Zautra 2005; Katz 2010). In the remaining 12 trials, low quality of reporting hampered any judgement regarding sequence generation and concealment of allocation. All 22 trials were described as double blind. Eleven trials reported the use of indistinguishable interventions to blind participants whereas another four trials used double‐dummy techniques (Quiding 1992; Caldwell 2002; Kivitz 2006; Afilalo 2010). Fourteen trials explicitly reported adequate blinding of physicians. Seventeen trials described their analysis to be according to the intention‐to‐treat principle, but only one trial was considered to have an intention‐to‐treat analysis of pain (NCT00531427), and one trial of function outcomes at end of treatment (Katz 2010), according to our criteria. Exclusion of participants from the analysis of pain outcomes ranged from 0.6% to 52% in the experimental groups and from 0% to 33% in the control groups. For eight trials, no information was available on the proportion of excluded participants (NCT00980798; Quiding 1992; Caldwell 2002; Markenson 2005; Langford 2006; Hartrick 2009; Fidelholtz 2011; NCT00486811). For the analysis of function outcomes, exclusion of participants ranged from 1% to 73% in the experimental groups and from 0.6% to 53% in the control groups; in four trials, no information was available on the proportion of excluded patients (Caldwell 2002; Markenson 2005; Langford 2006; NCT00486811).
2.
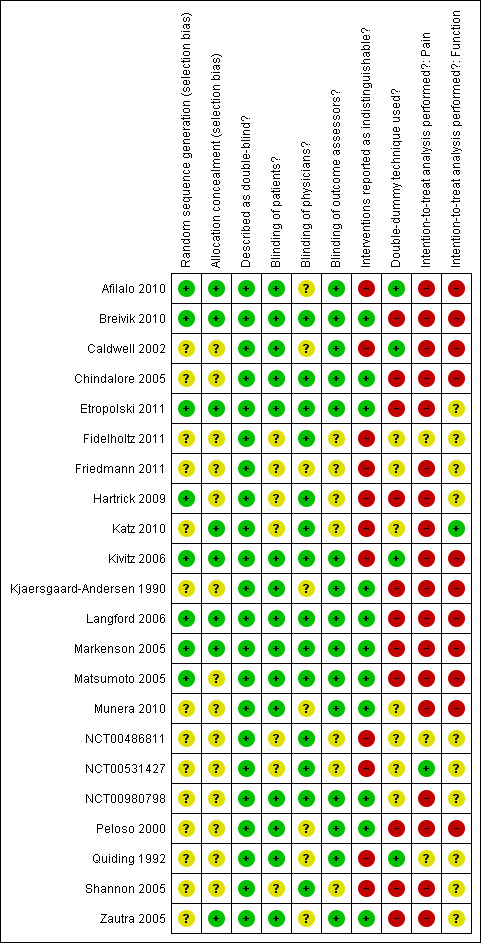
Methodological characteristics of included trials. (+) indicates low risk of bias, (?) unclear, and (‐) a high risk of bias on a specific item.
All trials (95%) except for one (Quiding 1992) reported a primary outcome of which eight explicitly reported it to be pre‐specified in the protocol (Peloso 2000; Caldwell 2002; Markenson 2005; Matsumoto 2005; Langford 2006; Katz 2010; NCT00486811; NCT00531427), and 13 trials reported a sample size calculation for this primary outcome. Twenty trials received financial support from a commercial organisation, two were unclear about their source of funding (Kjaersgaard‐Andersen 1990; Quiding 1992), whereas no trial was explicitly supported by a non‐profit organisation. For the effectiveness outcomes pain and function, the quality of the evidence (Guyatt 2008) was classified as high in view of the low risk of bias in the included trials and the low heterogeneity between trials (Table 1). For adverse event and serious adverse event outcomes, the quality of the evidence (Guyatt 2008) was classified as moderate to low because of the small number of trials reporting the outcomes and the small number of serious adverse events, which resulted in imprecise estimates (Table 1).
Effects of interventions
See: Table 1
Primary outcomes
Knee or hip pain
Twenty‐two trials including 5180 participants in experimental groups and 3095 participants in control groups contributed to the analyses of knee or hip pain. Figure 3 presents results of the analysis, overall and stratified according to type of opioid. In the overall analysis, combined oral and transdermal opioids were more effective in pain reduction than control interventions (SMD ‐0.28, 95% CI ‐0.35 to ‐0.20), which corresponds to a difference in pain scores of 0.7 cm on a 10‐cm VAS between opioids and placebo. This corresponds to a difference in improvement of 12% (95% CI 9% to 15%) between opioids and placebo (Table 1), which translates into an NNTB to cause one additional treatment response on pain of 10 (95% CI 8 to 14) (Table 1). An I2 statistic of 58% indicated a moderate degree of between‐trial heterogeneity (P for heterogeneity < 0.001). A visual inspection of the funnel plot suggested asymmetry (asymmetry coefficient ‐1.86, 95% CI ‐3.50 to ‐0.21) and the test for asymmetry indicated some evidence for asymmetry (P value = 0.054) (Figure 4). Benefits were moderate for codeine (SMD ‐0.51, 95% CI ‐1.01 to ‐0.01; 3 trials); small to moderate for oxycodone (SMD ‐0.31, 95% CI ‐0.47 to ‐0.15; 10 trials), oxymorphone (SMD ‐0.39, 95% CI ‐0.58 to ‐0.21; 2 trials), and tapentadol (SMD ‐0.31, 95% CI ‐0.46 to ‐0.16, 4 trials); and small for morphine (SMD ‐0.25, 95% CI ‐0.42 to ‐0.09; 2 trials) and transdermal opioids such as buprenorphine (SMD ‐0.19, 95% CI ‐0.30 to ‐0.09, 4 trials) and fentanyl (SMD ‐0.22, 95% CI ‐0.42 to ‐0.03; 1 trial). No benefit was observed for hydromorphone (SMD 0.04, 95% CI ‐0.19 to 0.28, 1 trial). The CIs were wide and a test for interaction between benefit and type of opioid was non‐significant (P value = 0.66). Table 2 presents the results of stratified analyses. We found little evidence for an association of SMDs with analgesic potency, route of administration, type of control intervention, use of analgesic co‐interventions, type of osteoarthritis, concealment of allocation, adequate blinding of participants, or intention‐to‐treat analysis. Effects were similar in studies including participants with only knee osteoarthritis (SMD ‐0.22, 95% CI ‐0.41 to ‐0.04, 4 trials), with only hip osteoarthritis (SMD ‐0.33, 95% CI ‐0.93 to 0.28, 2 trials), and with knee or hip osteoarthritis (SMD ‐0.29, 95% CI ‐0.38 to ‐0.20, 16 trials, P value for interaction = 0.77). We found larger benefits in trials with 200 or fewer randomised participants (difference in SMD ‐0.23, 95% CI ‐0.49 to 0.02, P for interaction = 0.08) and in trials with a short treatment duration of one month or less (difference in SMD ‐0.25, 95% CI ‐0.37 to ‐0.13, P value for interaction = 0.001). The effect of treatment duration on treatment benefits was similar, when we restricted the analyses to large trials only (P value for interaction 0.001). Thirty‐three comparisons from 22 trials contributed to the analysis of a linear association between equivalence dose and treatment benefit (Figure 5). We found little evidence for a linear association between daily equivalence doses and pain reduction (P value = 0.49).
3.
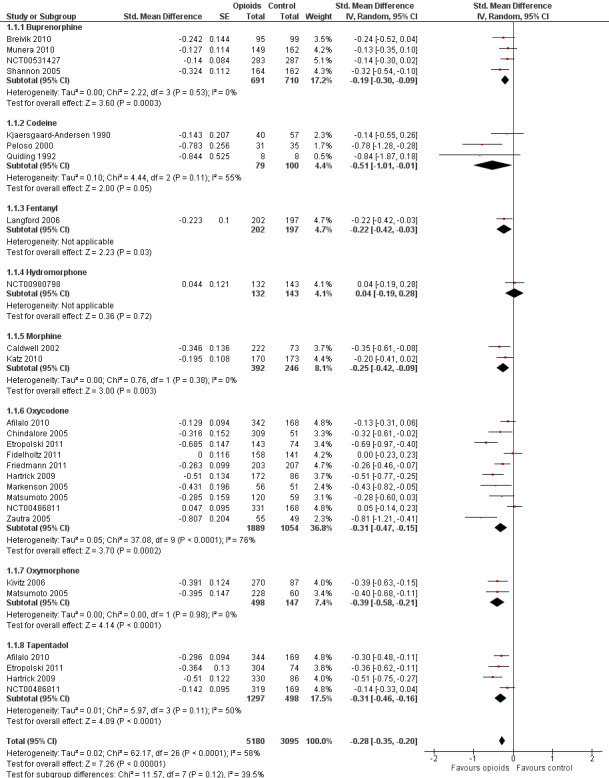
Forest plot of 22 trials comparing the effects of any type of opioids and control (placebo or no intervention) on knee or hip pain. Values on x‐axis denote standardised mean differences. The plot is stratified according to type of opioids. Matsumoto 2005, Hartrick 2009, Afilalo 2010, Etropolski 2011, and NCT00486811 contributed with two comparisons and the standard error was inflated and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis. Data relating to the 3, 3, 3, 2, 2, and 2 active intervention arms in Caldwell 2002, Chindalore 2005, Kivitz 2006, Matsumoto 2005, Hartrick 2009, and Etropolski 2011, respectively, were pooled.
4.
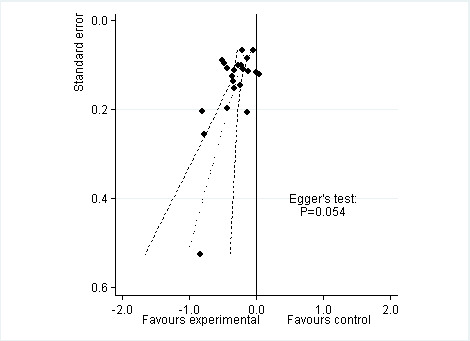
Funnel plot for effects on knee or hip pain. Numbers on x axis refer to standardised mean differences (SMDs), on y axis to standard errors of SMDs.
1. Stratified analyses: pain.
| Variable | Number of studies | N of participants opioids | N of participants control | Pain intensity SMD (95% CI) | Heterogeneity I2 (%) | P value* |
| All trials | 22 | 5180 | 3095 | ‐0.28 (‐0.35 to ‐0.20) | 58% | |
| Analgesic potency | 0.32 | |||||
| Weak | 3 | 79 | 100 | ‐0.51 (‐1.01 to ‐0.01) | 55% | |
| Strong | 19 | 5101 | 2995 | ‐0.26 (‐0.35 to ‐0.18) | 64% | |
| Route of administration | 0.36 | |||||
| Oral | 17 | 4287 | 2188 | ‐0.30 (‐0.41 to ‐0.20) | 70% | |
| Transdermal | 5 | 893 | 907 | ‐0.20 (‐0.29 to ‐0.11) | 0% | |
| Allocation concealment | 0.31 | |||||
| Adequate | 8 | 1981 | 1141 | ‐0.32 (‐0.44 to ‐0.21) | 48% | |
| Inadequate or unclear | 14 | 3199 | 1954 | ‐0.24 (‐0.35 to ‐0.13) | 67% | |
| Blinding of participants | 0.23 | |||||
| Adequate | 15 | 3050 | 1616 | ‐0.32 (‐0.42 to ‐0.22) | 53% | |
| Inadequate or unclear | 7 | 2130 | 1479 | ‐0.21 (‐0.34 to ‐0.08) | 73% | |
| Intention‐to‐treat analysis | 0.43 | |||||
| Yes | 1 | 283 | 287 | ‐0.14 (‐0.30 to 0.02) | N/A | |
| No or unclear | 21 | 4897 | 2808 | ‐0.29 (‐0.37 to ‐0.20) | 63% | |
| Type of control intervention | 0.97 | |||||
| Placebo | 20 | 5132 | 3030 | ‐0.28 (‐0.36 to ‐0.19) | 65% | |
| No intervention | 2 | 48 | 65 | ‐0.33 (‐0.93 to 0.28) | 35% | |
| Number of participants randomised | 0.08 | |||||
| > 200 | 16 | 4895 | 2796 | ‐0.24 (‐0.33 to ‐0.16) | 64% | |
| ≤ 200 | 6 | 285 | 299 | ‐0.47 (‐0.71 to ‐0.23) | 48% | |
| Duration of treatment | 0.001 | |||||
| > 1 month | 10 | 2635 | 1972 | ‐0.15 (‐0.22 to ‐0.08) | 25% | |
| ≤ 1 month | 12 | 2545 | 1123 | ‐0.40 (‐0.50 to ‐0.30) | 37% | |
| Use of analgesic co‐interventions | 0.59 | |||||
| Similar between groups | 6 | 1189 | 891 | ‐0.31 (‐0.46 to ‐0.16) | 60% | |
| Unclear | 16 | 3991 | 2204 | ‐0.26 (‐0.36 to ‐0.16) | 65% | |
| Type of osteoarthritis | 0.77 | |||||
| Hip only | 2 | 48 | 65 | ‐0.33 (‐0.93 to 0.28) | 35% | |
| Knee only | 4 | 1674 | 1010 | ‐0.22 (‐0.41 to ‐0.04) | 78% | |
| Knee and hip | 16 | 3458 | 2020 | ‐0.29 (‐0.38 to ‐0.20) | 56% | |
*P value for interaction. N/A: not available.
5.
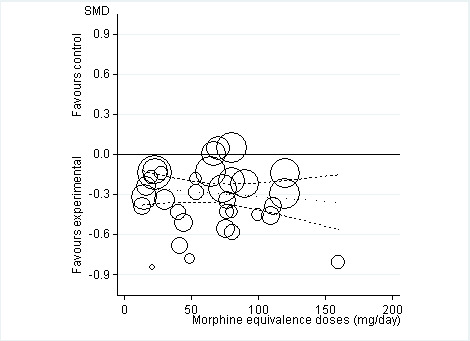
Standardised mean differences of knee or hip pain (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
Function
Twelve studies including 2124 participants in experimental groups and 1429 participants in control groups contributed to the analysis of function. Improvement of function was larger in opioid‐treated participants compared with control groups (SMD ‐0.26, 95% CI ‐0.35 to ‐0.17) (Figure 6), which corresponds to a difference in function scores of 0.6 units between opioids and placebo on a standardised WOMAC disability scale ranging from 0 to 10. This corresponds to a difference in improvement of 11% (95% CI 7% to 14%) between opioids and placebo (Table 1), which translates into an NNTB to cause one additional treatment response on function of 11 (95% CI 7 to 14) (Table 1). An I2 statistic of 32% indicated a low degree of between‐trial heterogeneity (P value for heterogeneity = 0.12). We found a moderate benefit for codeine (SMD ‐0.42, 95% CI ‐0.74 to ‐0.10; 2 trials) and oxymorphone (SMD ‐0.38, 95% CI ‐0.56 to ‐0.19, 2 trials) and small benefits for morphine (SMD ‐0.20, 95% CI ‐0.38 to ‐0.02, 2 trials), oxycodone (SMD ‐0.30, 95% CI ‐0.58 to ‐0.01, 4 trials), tapentadol (SMD ‐0.15, 95% CI ‐0.45 to 0.16, 2 trials), and for transdermal opioids such as buprenorphine (SMD ‐0.23, 95% CI ‐0.40 to ‐0.05, 2 trials) and fentanyl (SMD ‐0.28, 95% CI ‐0.48 to ‐0.09; 1 trial). As was the case for pain, CIs of estimates were wide and a test for interaction between benefit and type of opioid was non‐significant (P value = 0.87). Heterogeneity between trials was low with an I2 statistic estimate of 32% (P value for heterogeneity = 0.12). The funnel plot (Figure 7) appeared asymmetrical (asymmetry coefficient ‐3.33, 95% CI ‐5.76 to ‐0.89, P value for asymmetry = 0.011). Table 3 presents the results of the stratified analyses. We found little evidence for an association of SMDs with analgesic potency, route of administration, type of control intervention, treatment duration, use of analgesic co‐interventions, type of osteoarthritis, allocation concealment, and intention‐to‐treat analysis. Effects were similar in studies including participants with only knee osteoarthritis (SMD ‐0.16, 95% CI ‐0.43 to 0.11, 2 trials), only hip OA (SMD ‐0.29, 95% CI ‐0.68 to 0.11, 1 trial), and knee or hip OA (SMD ‐0.31, 95% CI ‐0.41 to ‐0.20, 9 trials, P value for interaction 0.45). Adequately powered trials with 200 or more randomised participants tended to show smaller improvements of function (difference in SMD 0.23, 95% CI ‐0.06 to 0.52, P value for interaction = 0.11) and trials with adequate participant blinding larger benefits of function (difference in SMD ‐0.25, 95% CI ‐0.41 to ‐0.09, P value for interaction = 0.008). Eighteen comparisons from 12 trials contributed to the analysis of a linear association between equivalence dose and treatment benefit for function (Figure 8). We found no evidence for an association between daily equivalence doses and improvement of function (P value = 0.48).
6.
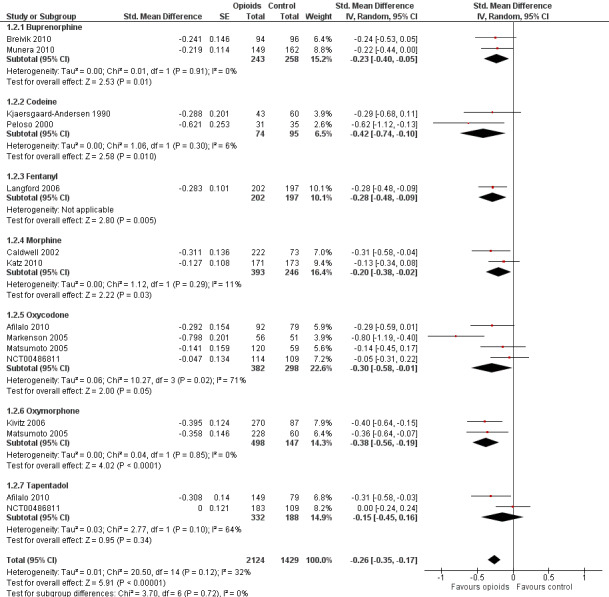
Forest plot of 12 trials comparing the effects of any type of opioids and control (placebo or no intervention) on function. Values on x axis denote standardised mean differences. The plot is stratified according to type of opioids. Matsumoto 2005 contributed with two comparisons and the standard error was inflated and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis. Data relating to the 3, 3, and 2 active intervention arms in Caldwell 2002, Kivitz 2006, and Matsumoto 2005, respectively, were pooled.
7.
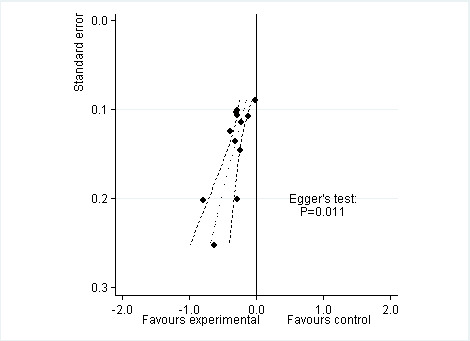
Funnel plot for effects on functioning of the knee or hip. Numbers on x axis refer to standardised mean differences (SMDs), on y axis to standard errors of SMDs
2. Stratified analyses: function.
| Variable | Number of studies | N of participants opioids | N of participants control | Function SMD (95% CI) | Heterogeneity I2 (%) | P value* |
| All trials | 12 | 2124 | 1429 | ‐0.26 (‐0.35 to ‐0.17) | 32% | |
| Analgesic potency | 0.42 | |||||
| Weak | 2 | 74 | 95 | ‐0.42 (‐0.74 to ‐0.10) | 6% | |
| Strong | 10 | 2050 | 1334 | ‐0.26 (‐0.36 to ‐0.16) | 48% | |
| Route of administration | 0.76 | |||||
| Oral | 9 | 1679 | 974 | ‐0.30 (‐0.43 to ‐0.16) | 58% | |
| Transdermal | 3 | 445 | 455 | ‐0.25 (‐0.38 to ‐0.12) | 0% | |
| Allocation concealment | 0.43 | |||||
| Adequate | 6 | 1034 | 762 | ‐0.32 (‐0.45 to ‐0.18) | 47% | |
| Inadequate or unclear | 6 | 1090 | 667 | ‐0.23 (‐0.37 to ‐0.09) | 39% | |
| Blinding of participants | 0.008 | |||||
| Adequate | 10 | 1656 | 1038 | ‐0.32 (‐0.40 to ‐0.24) | 0% | |
| Inadequate or unclear | 2 | 468 | 391 | ‐0.07 (‐0.20 to 0.07) | 0% | |
| Intention‐to‐treat analysis | 0.34 | |||||
| Yes | 1 | 171 | 173 | ‐0.13 (‐0.34 to 0.08) | N/A | |
| No or unclear | 11 | 1953 | 1256 | ‐0.29 (‐0.40 to ‐0.19) | 44% | |
| Type of control intervention | 0.96 | |||||
| Placebo | 11 | 2081 | 1369 | ‐0.28 (‐0.38 to ‐0.18) | 49% | |
| No intervention | 1 | 43 | 60 | ‐0.29 (‐0.68 to 0.11) | N/A | |
| Number of participants randomised | 0.11 | |||||
| > 200 | 8 | 1900 | 1187 | ‐0.23 (‐0.32 to ‐0.14) | 26% | |
| ≤ 200 | 4 | 224 | 242 | ‐0.46 (‐0.73 to ‐0.19) | 51% | |
| Duration of treatment | 0.41 | |||||
| > 1 month | 6 | 1061 | 893 | ‐0.25 (‐0.41 to ‐0.09) | 66% | |
| ≤ 1 month | 6 | 1063 | 536 | ‐0.31 (‐0.42 to ‐0.20) | 0% | |
| Use of analgesic co‐interventions | 0.38 | |||||
| Similar between groups | 4 | 460 | 456 | ‐0.40 (‐0.67 to ‐0.13) | 71% | |
| Unclear | 8 | 1664 | 973 | ‐0.24 (‐0.33 to ‐0.15) | 16% | |
| Type of osteoarthritis | 0.45 | |||||
| Hip only | 1 | 43 | 60 | ‐0.29 (‐0.68 to 0.11) | N/A | |
| Knee only | 2 | 538 | 376 | ‐0.16 (‐0.43 to 0.11) | 76% | |
| Knee and hip | 9 | 1543 | 993 | ‐0.31 (‐0.41 to ‐0.20) | 31% | |
*P value for interaction. N/A: not available.
8.
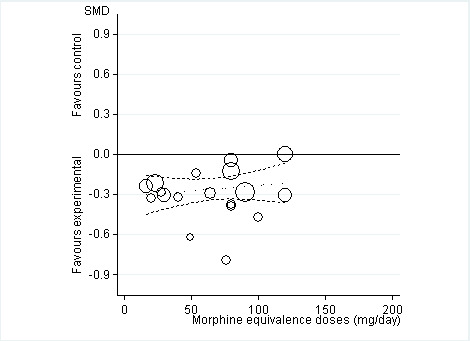
Standardised mean differences of function (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
Secondary outcomes
Ten trials reported the occurrence of any adverse event in 2490 out of 3222 participants in experimental groups and 891 of 1676 participants in control groups (Figure 9). Participants were 49% more likely to experience adverse events in experimental groups compared with placebo (RR 1.49, 95% CI 1.35 to 1.63). The NNTH to cause one additional participant to experience an adverse event, as compared to placebo, was 14 (95% CI 11 to 19) (Table 1). We found high heterogeneity between different studies (I2 = 71%, P value for heterogeneity < 0.001), but no evidence that RRs differed between different types of opioids (P value for interaction = 0.47) or length of treatment duration (P value = 0.09). Eighteen comparisons in nine trials contributed to the analysis of the association between equivalence dose and log relative risk (Figure 10). We found little evidence for a relationship (P value = 0.24).
9.
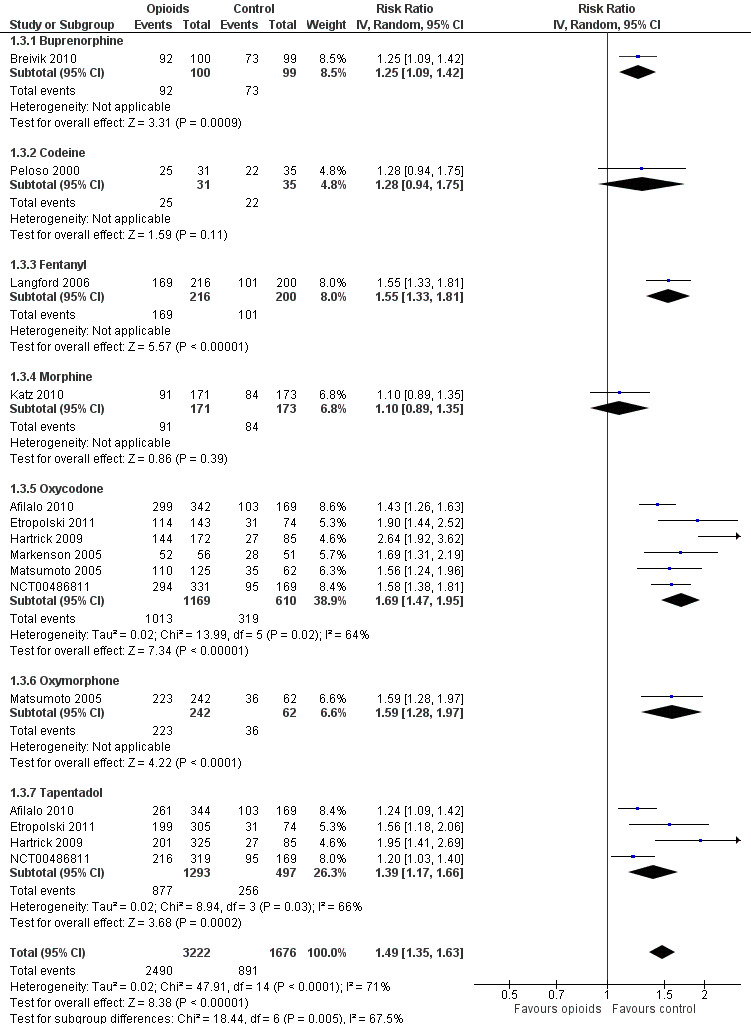
Forest plot of 10 trials comparing participants experiencing any adverse event between any opioid and control (placebo or no intervention). Values on x axis denote risks ratios. The plot is stratified according to type of opioid. Matsumoto 2005, Hartrick 2009, Afilalo 2010, Etropolski 2011, and NCT00486811 contributed with two comparisons and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis.
10.
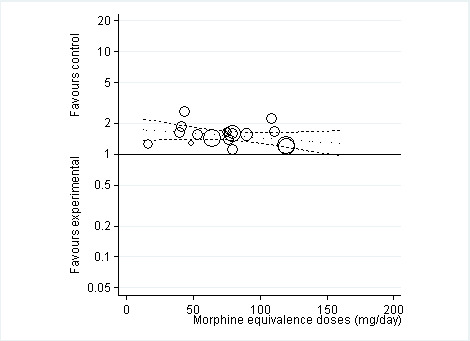
Risk ratios of participants experiencing any adverse event between opioids and control groups (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
Twenty‐one trials with 8128 participants contributed to the meta‐analysis of participants withdrawn or dropped out because of adverse events (Figure 11). Participants receiving opioid therapy were 3.8 times as likely as participants receiving placebo to be withdrawn or drop‐out due to adverse events (RR 3.76, 95% CI 2.93 to 4.82), with moderate between‐trial heterogeneity (I2 = 59%, P value for heterogeneity < 0.001). The NNTH to cause one additional drop‐out or withdrawal due to adverse events compared with placebo was 21 (95% CI 15 to 30) (Table 1). We found the highest pooled RR for oxycodone versus placebo (RR 5.55, 95% CI 3.47 to 8.87, 9 trials) and the lowest pooled RR for morphine versus placebo (RR 2.12, 95% CI 0.87 to 5.15, 2 trials) but CIs were wide and a test for interaction between type of opioids and relative risk of being withdrawn or dropping out because of adverse events negative gave a P value for interaction of 0.41. We found no evidence for an association between treatment duration and risk of withdrawals or drop‐outs due to adverse events (P value for interaction 0.78). Thirty‐two comparisons in 22 trials contributed to the analysis of the association between equivalence dose and log relative risk (Figure 12). We found little evidence for a relationship (P value = 0.94).
11.
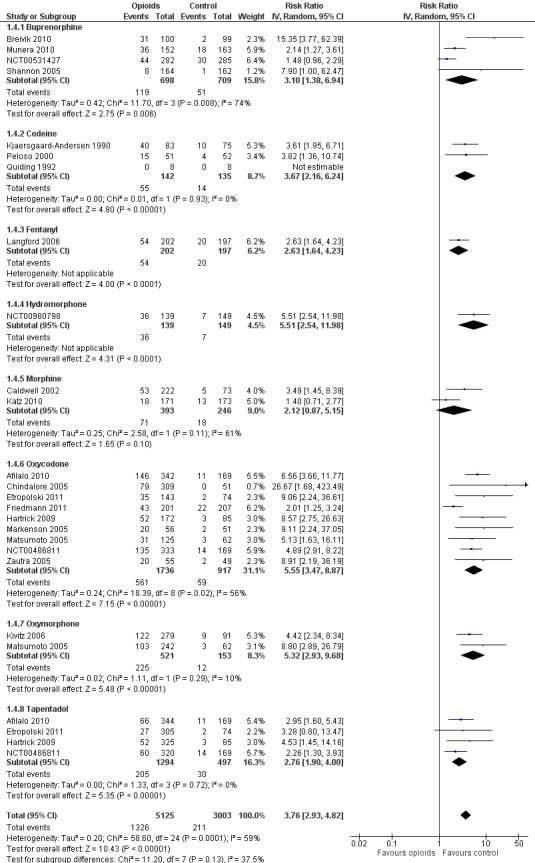
Forest plot of 21 trials comparing participants withdrawn or dropped out because of adverse events between any opioid and control (placebo or no intervention). Values on x axis denote risks ratios. The plot is stratified according to type of opioid. Matsumoto 2005, Hartrick 2009, Afilalo 2010, Etropolski 2011, and NCT00486811 contributed with two comparisons and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis. The risk ratio in one trial could not be estimated because no withdrawals or drop‐outs because of adverse events occurred in either group.
12.
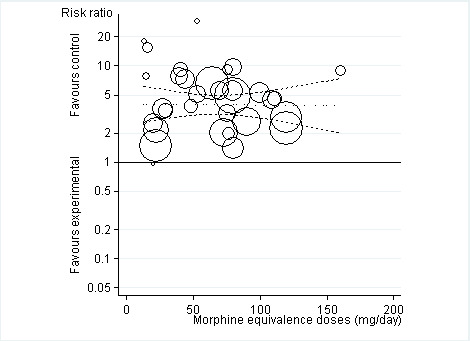
Risk ratios of participants withdrawn or dropped out because of adverse events between opioids and control groups (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
Three trials with 681 participants contributed to the analysis of participants experiencing any serious adverse event (Figure 13). One trial reported one death in the oxycodone group, but no other serious adverse events and was not included in the analysis (Afilalo 2010). Of the three trials included, one trial reported that no participant experienced a serious adverse event (Kjaersgaard‐Andersen 1990). Overall data from the remaining two trials indicated that participants receiving opioids tended be more likely to experience a serious adverse event (RR 3.35, 95% CI 0.83 to 13.56). Due to the low number of trials and events, we neither performed an analysis of the association between treatment duration or equivalence dose and log relative risk for this outcome, nor a calculation of NNTH to cause one additional participant to experience a serious adverse event compared with placebo.
13.
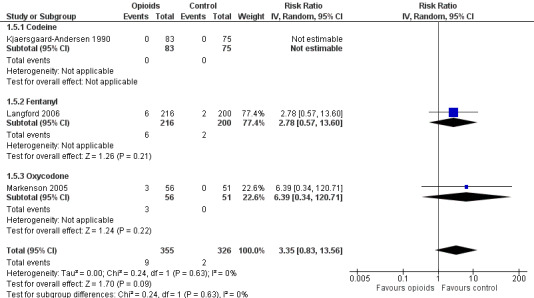
Forest plot of three trials comparing participants experiencing any serious adverse event between any opioid and control (placebo or no intervention). Values on x axis denote risks ratios. The plot is stratified according to type of opioid. The risk ratio in one trial could not be estimated because no serious adverse event occurred in either group.
Three trials reported symptoms of opioid dependency (Langford 2006; Afilalo 2010; Katz 2010). Two studies reported 25 of 397 participants with withdrawal symptoms in oral opioids and five of 255 in control groups (Afilalo 2010; Katz 2010). One study assessed opiate withdrawal symptoms after eight weeks of transdermal fentanyl therapy, using the Short Opiate Withdrawal Scale questionnaire (Gossop 1990; Langford 2006). On average, participants in the opioids groups had a 2.8‐fold increased risk of withdrawal symptoms compared with control groups with a pooled OR of 2.76 (95% CI 2.02 to 3.77) (Figure 14). The NNTH to cause one additional participant to experience withdrawal symptoms, as compared with control, was 65 (95% CI 42 to 110).
14.
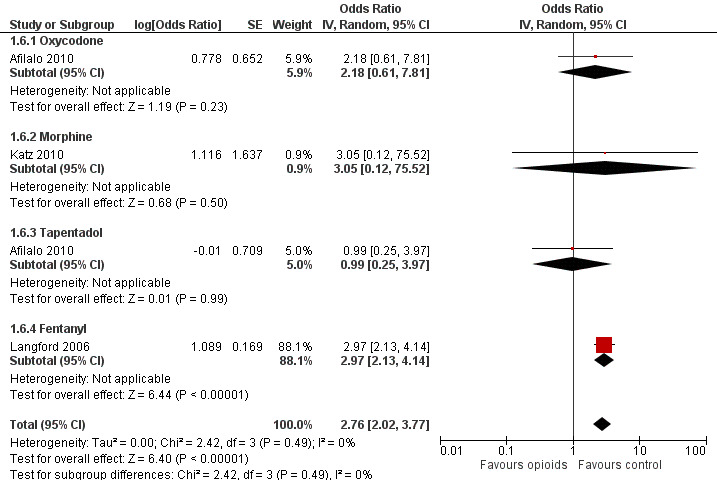
Forest plot of 4 comparisons in three trials comparing participants experiencing withdrawal symptoms between any opioid and control (placebo or no intervention). Values on x axis denote odds ratios. The plot is stratified according to type of opioid. Afilalo 2010 contributed with two comparisons and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis.
Discussion
Summary of main results
In this update of our systematic review and meta‐analysis, we found only small benefits of oral or transdermal opioids being more effective compared with placebo in terms of pain relief and improvement of function in people with osteoarthritis. If participants received opioids for more than four weeks, benefits on pain relief were even further reduced. The occurrence of adverse events often caused participants to stop taking the opioids, which is likely to limit the usefulness of opioids in the long term. The potentially higher risk of serious adverse events and substance addiction might further limit their use. The reporting of safety outcomes was incomplete and adverse events were reported in only about half of the trials, and serious adverse events in three trials only. Trials that did report safety outcomes consistently observed a significant increase in the risk of adverse events with opioid use.
Quality of the evidence
Most of the trials were funded by the pharmaceutical industry and we did not have enough data to explore whether the type of funding was associated with the estimated treatment effects. We found larger benefits on pain relief in studies with opioid use for less than four weeks compared with longer treatments, but not dependence of benefits on function or safety outcomes according to treatment duration. Thus, the effectiveness of opioids may drop during chronic use as the analgesic effects of opioids are mediated through opioids receptors, but safety concerns were not affected by this. The relatively low dose of morphine equivalents (median daily dose 67 mg) administered in the included trials might provide an explanation of the small benefits observed as compared with other studies (Maier 2002). Our ability to provide a reliable assessment of dose dependency might have been hampered by the generally low morphine equivalent doses used and the lack of individual participant data. The generally used distinction between weak and strong opioids can be misleading, because the analgesic potency depends also on the dosage. Thus, we calculated morphine equivalence doses to be able to compare different opioids, but found no evidence for dose‐dependent effects. We found little evidence that stronger opioid agents or higher doses of these agents will result in larger treatment effects. However, it is possible that type of opioids interacts with dosage. For instance, higher doses could have larger treatment effects for stronger but not for weaker opioids. The characteristics of the trials included in our review did not allow us to explore such interaction properly.
Data on risks of addiction due to opioid therapy is scarce, and currently available trials are not designed to evaluate these issues. There is a clear need for additional randomised trials and observational studies using longer follow‐up times to address the risks of substance dependence associated with different opioids. In this systematic review, only three out of 22 trials reported measures of withdrawal symptoms (Langford 2006; Afilalo 2010; Katz 2010). Similar to previous systematic reviews of randomised trials on opioids therapy for non‐cancer pain (Kalso 2004; Furlan 2006), we found that most of the trials included in our review had a treatment duration of several days or a few weeks only. Although some of the newer trials in the update had slightly longer treatment durations (Afilalo 2010; Breivik 2010; NCT00486811; NCT00980798), in none of the trials did participants receive opioids for longer than six months. This is still too short to address the impact of opioid treatment on routine clinical practice in the treatment of a chronic condition such as osteoarthritis. While no evidence of long‐term effects is available from randomised trials, observational studies indicate that long‐term treatment with opioids of chronic conditions such as osteoarthritis may have deleterious effects and do not seem to improve pain relief (Eriksen 2006).
Potential biases in the review process
We based our review on a broad literature search. Even though we cannot exclude potential publication bias, it seems rather unlikely that we missed relevant trials (Egger 2003). Two review authors independently performed selection of trials and data extraction to minimise bias and transcription errors (Egger 2001; Gøtzsche 2007). The most recent systematic review on opioids for osteoarthritis (Avouac 2007), updated in October 2006, considered 18 studies that compared opioids with placebo. We included data from six of these in our meta‐analysis and data from four additional trials (Kjaersgaard‐Andersen 1990; Quiding 1992; Matsumoto 2005; Kivitz 2006). We excluded six trials with tramadol as the experimental intervention and one trial that was likely to have included only a minority of people with osteoarthritis. In our update, we identified 12 additional trials, of which three are unpublished. In conclusion, we are likely to have included all relevant trials in our systematic review.
Agreements and disagreements with other studies or reviews
We excluded tramadol from our review to avoid overlap with another Cochrane review that focused on this specific opioid in osteoarthritis (Cepeda 2006). Extracted pain and function outcomes and follow‐up time in the previous systematic review about opioids for osteoarthritis (Avouac 2007) were similar to our systematic review. Comparing opioids with placebo controls, Avouac 2007 found a large pooled effect for pain intensity (SMD ‐0.79, 95% CI ‐0.98 to ‐0.59) and a moderate pooled effect for function (SMD ‐0.31, 95% CI ‐0.39 to ‐0.24). These effects are consistent with our results for function but are substantially larger for pain reduction. This discrepancy might be due to the exclusion of some trials in our systematic review and to inclusion of newer trials in our update in 2012. Avouac 2007 reported moderate‐to‐large effects of tramadol for pain, between ‐0.36 to ‐0.93 SD units, in several large trials and unrealistically large beneficial effects on pain intensity in an oxycodone trial that was excluded from our review due to the likely very low percentage of participants with knee or hip osteoarthritis (Roth 2000). These trials often did not report function outcomes and could not, therefore, contribute to the pooled analysis, or they reported considerably smaller effects for function than for pain (Avouac 2007). In line with other studies, we found that adverse events occurring in participants treated with opioids often caused withdrawals and drop‐outs (Kalso 2004; Furlan 2006; Avouac 2007; Gehling 2011). Tramadol may be similar to, or even more effective than, the opioids evaluated in our review in reducing pain and improving function, but safety concerns have to be addressed further (Cepeda 2006).
Authors' conclusions
Implications for practice.
Opioids decrease pain intensity and improve function but the benefits observed are small. Dose increases do not appear to result in further pain reduction, while prolongation of treatment duration resulted in even smaller pain reduction. Observed effects for pain were of questionable clinical relevance since the 95% confidence intervals did not include the minimal clinically important difference of 0.37 standardised mean differences (SMDs), which corresponds to 0.9 cm on a 10‐cm visual analogue scale (VAS) (Wandel 2010; Rutjes 2012). The occurrence of adverse events caused one in 20 participants to stop taking the preparations, which is likely to limit their usefulness in the long‐term treatment of osteoarthritis of the hip or knee. The higher risk of serious adverse events and the occurrence of addiction to opioid therapy might further limit their clinical use, although evidence is limited by the short duration of follow‐up of the studies assessing these outcomes. Nevertheless, use of opioids might be warranted in special situations, such as for short‐term treatment of later stage osteoarthritis awaiting surgery. However, clinicians should inform participants about the substantial risks and only small benefits of opioid treatment and therapeutic alternatives.
Implications for research.
The effectiveness and safety of opioid and non‐opioid analgesics in participants with inadequate pain relief should be directly compared in appropriately powered randomised controlled trials accompanied by separate Cochrane reviews or reviews of reviews including network meta‐analyses, which integrate direct and indirect evidence in one single analysis while maintaining randomisation (Caldwell 2005). The evidence of the effectiveness and safety of opioid therapy is mainly from a few short‐term trials, despite the fact that the underlying condition is chronic and requires safe, long‐term treatments (Kalso 2004; Furlan 2006). Further long‐term observational studies would increase our understanding of their long‐term effectiveness, safety, and the potential for addiction. In addition, future trials might be performed in participants with clear failures of previous analgesic therapies with non‐steroidal anti‐inflammatory drugs or opioids and might target special subgroups, such as separately study and report participants with knee or hip osteoarthritis to acknowledge the different mechanisms resulting in pain in these two phenotypes, or participants with and without pain sensitisation.
What's new
| Date | Event | Description |
|---|---|---|
| 22 March 2013 | New search has been performed | Search updated with 12 additional trials included. |
| 22 August 2012 | New citation required but conclusions have not changed | Change in authorship |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 8 October 2009 | Amended | NNTs for pain and function were corrected |
| 13 May 2008 | Amended | Change in authorship |
| 1 May 2008 | Amended | CMSG ID C141‐R |
Acknowledgements
We thank the Cochrane Musculoskeletal editorial team for valuable comments and Malcolm Sturdy for database support. The authors are grateful to Hans Quiding for providing us with additional information concerning design and outcome data.
Appendices
Appendix 1. MEDLINE, EMBASE, and CINAHL search strategy
| Ovid MEDLINE | Ovid EMBASE | CINAHL through EBSCOhost |
|
Search terms for design 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trial.sh. 4. random allocation.sh. 5. double blind method.sh. 6. single blind method.sh. 7. clinical trial.pt. 8. exp clinical trial/ 9. (clin$ adj25 trial$).ti,ab. 10. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 11. placebos.sh. 12. placebo$.ti,ab. 13. random$.ti,ab. 14. research design.sh. 15. comparative study.sh. 16. exp evaluation studies/ 17. follow up studies.sh. 18. prospective studies.sh. 19. (control$ or prospectiv$ or volunteer$).ti,ab. |
Search terms for design 1. randomized controlled trial.sh. 2. randomization.sh. 3. double blind procedure.sh. 4. single blind procedure.sh. 5. exp clinical trials/ 6. (clin$ adj25 trial$).ti,ab. 7. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 8. placebo.sh. 9. placebo$.ti,ab. 10. random$.ti,ab. 11. methodology.sh. 12. comparative study.sh. 13. exp evaluation studies/ 14. follow up.sh. 15. prospective study.sh. 16. (control$ or prospectiv$ or volunteer$).ti,ab. |
Search terms for design 1. (MH "Clinical Trials+") 2. (MH "Random Assignment") 3. (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") 4. TX (clin$ n25 trial$) 5. TX (sing$ n25 blind$) 6. TX (sing$ n25 mask$) 7. TX (doubl$ n25 blind$) 8. TX (doubl$ n25 mask$) 9. TX (trebl$ n25 blind$) 10. TX (trebl$ n25 mask$) 11. TX (tripl$ n25 blind$) 12. TX (tripl$ n25 mask$) 13. (MH "Placebos") 14. TX placebo$ 15. TX random$ 16. (MH "Study Design+") 17. (MH "Comparative Studies") 18. (MH "Evaluation Research") 19. (MH "Prospective Studies+") 20. TX (control$ or prospectiv$ or volunteer$) 21. S1 or S2 or (…….) or S20 |
|
Search terms for Osteoarthritis 20. exp osteoarthritis/ 21. osteoarthriti$.ti,ab,sh. 22. osteoarthro$.ti,ab,sh. 23. gonarthriti$.ti,ab,sh. 24. gonarthro$.ti,ab,sh. 25. coxarthriti$.ti,ab,sh. 26. coxarthro$.ti,ab,sh. 27. arthros$.ti,ab. 28. arthrot$.ti,ab. 29. ((knee$ or hip$ or joint$) adj3 (pain$ or ach$ or discomfort$)).ti,ab. 30. ((knee$ or hip$ or joint$) adj3 stiff$).ti,ab. |
Search terms for Osteoarthritis 17. exp osteoarthritis/ 18. osteoarthriti$.ti,ab,sh. 19. osteoarthro$.ti,ab,sh. 20. gonarthriti$.ti,ab,sh. 21. gonarthro$.ti,ab,sh. 22. coxarthriti$.ti,ab,sh. 23. coxarthro$.ti,ab,sh. 24. arthros$.ti,ab. 25. arthrot$.ti,ab. 26. ((knee$ or hip$ or joint$) adj3 (pain$ or ach$ or discomfort$)).ti,ab. 27. ((knee$ or hip$ or joint$) adj3 stiff$).ti,ab. |
Search terms for Osteoarthritis 22. osteoarthriti$ 23. (MH "Osteoarthritis") 24. TX osteoarthro$ 25. TX gonarthriti$ 26. TX gonarthro$ 27. TX coxarthriti$ 28. TX coxarthro$ 29. TX arthros$ 30. TX arthrot$ 31. TX knee$ n3 pain$ 32. TX hip$ n3 pain$ 33. TX joint$ n3 pain$ 34. TX knee$ n3 ach$ 35. TX hip$ n3 ach$ 36. TX joint$ n3 ach$ 37. TX knee$ n3 discomfort$ 38. TX hip$ n3 discomfort$ 39. TX joint$ n3 discomfort$ 40. TX knee$ n3 stiff$ 41. TX hip$ n3 stiff$ 42. TX joint$ n3 stiff$ 43. S22 or S23 or S24….or S42 |
|
Search terms for Opioids 31. exp Analgesics, Opioid/ 32. exp Narcotics/ 33. acetyldihydrocodeine.tw. 34. alfentanil.tw. 35. allylprodine.tw. 36. alphamethylfentanyl.tw. 37. alphaprodine.tw. 38. benzylmorphine.tw. 39. betaprodine.tw. 40. bezitriamide.tw. 41. buprenorphine.tw. 42. butorphanol.tw. 43. bremazocine.tw. 44. carfentan$.tw. 45. codeine.tw. 46. contin.tw. 47. dextromoramide.tw. 48. dextropropoxyphene.tw. 49. dezocine.tw. 50. diacetylmorphine.tw. 51. diamorphine.tw. 52. dihydrocodeine.tw. 53. dihydromorphine.tw. 54. dihydromorphone.tw. 55. diphenoxylate.tw. 56. dipipanone.tw. 57. enadoline.tw. 58. ethylketazocine.tw. 59. ethylmorphine.tw. 60. etonitazene.tw. 61. etorphine.tw. 62. fentanyl.tw. 63. heroin.tw. 64. hydrocodone.tw. 65. hydromorphin$.tw. 66. hydromorphone.tw. 67. ketazocine.tw. 68. ketobemidone.tw. 69. lefetamine.tw. 70. levomethadon.tw. 71. levomethadyl.tw. 72. levomethorphan$.tw. 73. levorphanol.tw. 74. loperamide.tw. 75. meperidine.tw. 76. meptazinol.tw. 77. methadone.tw. 78. methadyl.tw. 79. methylmorphine.tw. 80. morphin$.tw. 81. nalbuphine.tw. 82. narcotic$.tw. 83. nicocodeine.tw. 84. nicomorphine.tw. 85. normorphine.tw. 86. noscapin$.tw. 87. ohmefentanyl.tw. 88. opiate$.tw. 89. opioid$.tw. 90. opium.tw. 91. oripavine.tw. 92. oxycodone.tw. 93. oxycontin.tw. 94. oxymorphone.tw. 95. papaveretum.tw. 96. papaverin.tw. 97. pentazocine.tw. 98. percocet.tw. 99. peronine.tw. 100. pethidine.tw. 101. phenazocine.tw. 102. phencyclidine.tw. 103. pholcodine.tw. 104. piritramid$.tw. 105. prodine.tw. 106. promedol.tw. 107. propoxyphene.tw. 108. remifentanil.tw. 109. sufentanil.tw. 110. tapentadol.tw. 111. thebaine.tw. 112. tilidine.tw. |
Search terms for Opioids 28. exp Analgesics, Opioid/ 29. exp Narcotic Analgesic Agent/ 30. acetyldihydrocodeine.tw. 31. alfentanil.tw. 32. allylprodine.tw. 33. alphamethylfentanyl.tw. 34. alphaprodine.tw. 35. benzylmorphine.tw. 36. betaprodine.tw. 37. bezitriamide.tw. 38. buprenorphine.tw. 39. butorphanol.tw. 40. bremazocine.tw. 41. carfentan$.tw. 42. codeine.tw. 43. contin.tw. 44. dextromoramide.tw. 45. dextropropoxyphene.tw. 46. dezocine.tw. 47. diacetylmorphine.tw. 48. diamorphine.tw. 49. dihydrocodeine.tw. 50. dihydromorphine.tw. 51. dihydromorphone.tw. 52. diphenoxylate.tw. 53. dipipanone.tw. 54. enadoline.tw. 55. ethylketazocine.tw. 56. ethylmorphine.tw. 57. etonitazene.tw. 58. etorphine.tw. 59. fentanyl.tw. 60. heroin.tw. 61. hydrocodone.tw. 62. hydromorphin$.tw. 63. hydromorphone.tw. 64. ketazocine.tw. 65. ketobemidone.tw. 66. lefetamine.tw. 67. levomethadon.tw. 68. levomethadyl.tw. 69. levomethorphan$.tw. 70. levorphanol.tw. 71. loperamide.tw. 72. meperidine.tw. 73. meptazinol.tw. 74. methadone.tw. 75. methadyl.tw. 76. methylmorphine.tw. 77. morphin$.tw. 78. nalbuphine.tw. 79. narcotic$.tw. 80. nicocodeine.tw. 81. nicomorphine.tw. 82. normorphine.tw. 83. noscapin$.tw. 84. ohmefentanyl.tw. 85. opiate$.tw. 86. opioid$.tw. 87. opium.tw. 88. oripavine.tw. 89. oxycodone.tw. 90. oxycontin.tw. 91. oxymorphone.tw. 92. papaveretum.tw. 93. papaverin.tw. 94. pentazocine.tw. 95. percocet.tw. 96. peronine.tw. 97. pethidine.tw. 98. phenazocine.tw. 99. phencyclidine.tw. 100. pholcodine.tw. 101. piritramid$.tw. 102. prodine.tw. 103. promedol.tw. 104. propoxyphene.tw. 105. remifentanil.tw. 106. sufentanil.tw. 107. tapentadol.tw. 108. thebaine.tw. 109. tilidine.tw. |
Search terms for Opioids 44. MH " Analgesics, Opioid" 45. MH "Narcotics" 46. TX acetyldihydrocodeine 47. TX alfentanil 48. TX allylprodine 49. TX alphamethylfentanyl 50. TX alphaprodine 51. TX benzylmorphine 52. TX betaprodine 53. TX bezitriamide 54. TX buprenorphine 55. TX butorphanol 56. TX bremazocine 57. TX carfentan$ 58. TX codeine 58. TX contin 60. TX dextromoramide 61. TX dextropropoxyphene 62. TX dezocine 63. TX diacetylmorphine 64. TX diamorphine 65. TX dihydrocodeine 66. TX dihydromorphine 67. TX dihydromorphone 68. TX diphenoxylate 69. TX dipipanone 70. TX enadoline 71. TX ethylketazocine 72. TX ethylmorphine 73. TX etonitazene 74. TX etorphine 75. TX fentanyl 76. TX heroin 77. TX hydrocodone 78. TX hydromorphin$ 79. TX hydromorphone 80. TX ketazocine 81. TX ketobemidone 82. TX lefetamine 83. TX levomethadon 84. TX levomethadyl 85. TX levomethorphan$ 86. TX levorphanol 87. TX loperamide 88. TX meperidine 89. TX meptazinol 90. TX methadone 91. TX methadyl 92. TX methylmorphine 93. TX morphin$ 94. TX nalbuphine 95. TX narcotic$ 96. TX nicocodeine 97. TX nicomorphine 98. TX normorphine 99. TX noscapin$ 100. TX ohmefentanyl 101. TX opiate$ 102. TX opioid$ 103. TX opium 104. TX oripavine 105. TX oxycodone 106. TX oxycontin 107. TX oxymorphone 108. TX papaveretum 109. TX papaverin 110. TX pentazocine 111. TX percocet 112. TX peronine 113. TX pethidine 114. TX phenazocine 115. TX phencyclidine 116. TX pholcodine 117. TX piritramid$ 118. TX prodine 119. TX promedol 120. TX propoxyphene 121. TX remifentanil 122. TX sufentanil 123. TX tapentadol 124. TX thebaine 125. TX tilidine 126. S44 or S45 or S125 |
|
Combining terms 113. or/31‐112 114. or/1‐19 115. or/20‐30 116. and/113‐115 117. animal/ 118. animal/ and human/ 119. 117 not 118 120. 116 not 119 121. remove duplicates from 120 |
Combining terms 110. or/28‐109 111. or/1‐16 112. or/17‐27 113. and/110‐112 114. animal/ 115. animal/ and human/ 116. 114 not 115 117. 113 not 116 118. remove duplicates from 117 |
Combining terms 127. S21 and S43 and S126 |
Appendix 2. CENTRAL search strategy
| CENTRAL |
|
Search terms for Osteoarthritis #1. MeSH descriptor Osteoarthritis explode all trees #2. (osteoarthritis* OR osteoarthro* OR gonarthriti* OR gonarthro* OR coxarthriti* OR coxarthro* OR arthros* OR arthrot* OR ((knee* OR hip* OR joint*) near/3 (pain* OR ach* OR discomfort*)) OR ((knee* OR hip* OR joint*) near/3 stiff*)) in Clinical Trials Search terms for Opioids #3. MeSH descriptor Analgesics, Opioid explode all trees #4. MeSH descriptor Narcotics explode all trees #5. (acetyldihydrocodeine OR alfentanil OR allylprodine OR alphamethylfentanyl OR alphaprodine OR benzylmorphine OR betaprodine OR bezitriamide OR buprenorphine OR butorphanol OR bremazocine OR carfentan* OR codeine OR contin OR dextromoramide OR dextropropoxyphene OR dezocine OR diacetylmorphine OR diamorphine OR dihydrocodeine OR dihydromorphine OR dihydromorphone OR diphenoxylate OR dipipanone OR enadoline OR ethylketazocine OR ethylmorphine OR etonitazene OR etorphine OR fentanyl OR heroin OR hydrocodone OR hydromorphin* OR hydromorphone OR ketazocine OR ketobemidone OR lefetamine OR levomethadon OR levomethadyl OR levomethorphan* OR levorphanol OR loperamide OR meperidine OR meptazinol OR methadone OR methadyl OR methylmorphine OR morphin* OR nalbuphine OR narcotic* OR nicocodeine OR nicomorphine OR normorphine OR noscapin* OR ohmefentanyl OR opiate* OR opioid* OR opium OR oripavine OR oxycodone OR oxycontin OR oxymorphone OR papaveretum OR papaverin OR pentazocine OR percocet OR peronine OR pethidine OR phenazocine OR phencyclidine OR pholcodine OR piritramid* OR prodine OR promedol OR propoxyphene OR remifentanil OR sufentanil OR tapentadol OR thebaine OR tilidine) in Clinical Trials Combining terms #6. (#1 OR #2) #7. (#3 OR #4 OR #5) #8. (#6 AND #7) in Clinical Trials |
Data and analyses
Comparison 1. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 22 | 8275 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.35, ‐0.20] |
| 1.1 Buprenorphine | 4 | 1401 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.30, ‐0.09] |
| 1.2 Codeine | 3 | 179 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐1.01, ‐0.01] |
| 1.3 Fentanyl | 1 | 399 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.42, ‐0.03] |
| 1.4 Hydromorphone | 1 | 275 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.19, 0.28] |
| 1.5 Morphine | 2 | 638 | Std. Mean Difference (Random, 95% CI) | ‐0.25 [‐0.42, ‐0.09] |
| 1.6 Oxycodone | 10 | 2943 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.47, ‐0.15] |
| 1.7 Oxymorphone | 2 | 645 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.58, ‐0.21] |
| 1.8 Tapentadol | 4 | 1795 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.46, ‐0.16] |
| 2 Function | 12 | 3553 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.35, ‐0.17] |
| 2.1 Buprenorphine | 2 | 501 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.40, ‐0.05] |
| 2.2 Codeine | 2 | 169 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.74, ‐0.10] |
| 2.3 Fentanyl | 1 | 399 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.48, ‐0.09] |
| 2.4 Morphine | 2 | 639 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 2.5 Oxycodone | 4 | 680 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.58, ‐0.01] |
| 2.6 Oxymorphone | 2 | 645 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.56, ‐0.19] |
| 2.7 Tapentadol | 2 | 520 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.45, 0.16] |
| 3 Number of participants experiencing any adverse event | 10 | 4898 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.35, 1.63] |
| 3.1 Buprenorphine | 1 | 199 | Risk Ratio (IV, Random, 95% CI) | 1.25 [1.09, 1.42] |
| 3.2 Codeine | 1 | 66 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.94, 1.75] |
| 3.3 Fentanyl | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.33, 1.81] |
| 3.4 Morphine | 1 | 344 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.89, 1.35] |
| 3.5 Oxycodone | 6 | 1779 | Risk Ratio (IV, Random, 95% CI) | 1.69 [1.47, 1.95] |
| 3.6 Oxymorphone | 1 | 304 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.28, 1.97] |
| 3.7 Tapentadol | 4 | 1790 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.17, 1.66] |
| 4 Number of participants who withdrew because of adverse events | 21 | 8128 | Risk Ratio (IV, Random, 95% CI) | 3.76 [2.93, 4.82] |
| 4.1 Buprenorphine | 4 | 1407 | Risk Ratio (IV, Random, 95% CI) | 3.10 [1.38, 6.94] |
| 4.2 Codeine | 3 | 277 | Risk Ratio (IV, Random, 95% CI) | 3.67 [2.16, 6.24] |
| 4.3 Fentanyl | 1 | 399 | Risk Ratio (IV, Random, 95% CI) | 2.63 [1.64, 4.23] |
| 4.4 Hydromorphone | 1 | 288 | Risk Ratio (IV, Random, 95% CI) | 5.51 [2.54, 11.98] |
| 4.5 Morphine | 2 | 639 | Risk Ratio (IV, Random, 95% CI) | 2.12 [0.87, 5.15] |
| 4.6 Oxycodone | 9 | 2653 | Risk Ratio (IV, Random, 95% CI) | 5.55 [3.47, 8.87] |
| 4.7 Oxymorphone | 2 | 674 | Risk Ratio (IV, Random, 95% CI) | 5.32 [2.93, 9.68] |
| 4.8 Tapentadol | 4 | 1791 | Risk Ratio (IV, Random, 95% CI) | 2.76 [1.90, 4.00] |
| 5 Number of participants experiencing any serious adverse event | 3 | 681 | Risk Ratio (IV, Random, 95% CI) | 3.35 [0.83, 13.56] |
| 5.1 Codeine | 1 | 158 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Fentanyl | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 2.78 [0.57, 13.60] |
| 5.3 Oxycodone | 1 | 107 | Risk Ratio (IV, Random, 95% CI) | 6.39 [0.34, 120.71] |
| 6 Withdrawal symptoms | 3 | Odds Ratio (Random, 95% CI) | 2.76 [2.02, 3.77] | |
| 6.1 Oxycodone | 1 | Odds Ratio (Random, 95% CI) | 2.18 [0.61, 7.81] | |
| 6.2 Morphine | 1 | Odds Ratio (Random, 95% CI) | 3.05 [0.12, 75.52] | |
| 6.3 Tapentadol | 1 | Odds Ratio (Random, 95% CI) | 0.99 [0.25, 3.97] | |
| 6.4 Fentanyl | 1 | Odds Ratio (Random, 95% CI) | 2.97 [2.13, 4.14] |
1.1. Analysis.
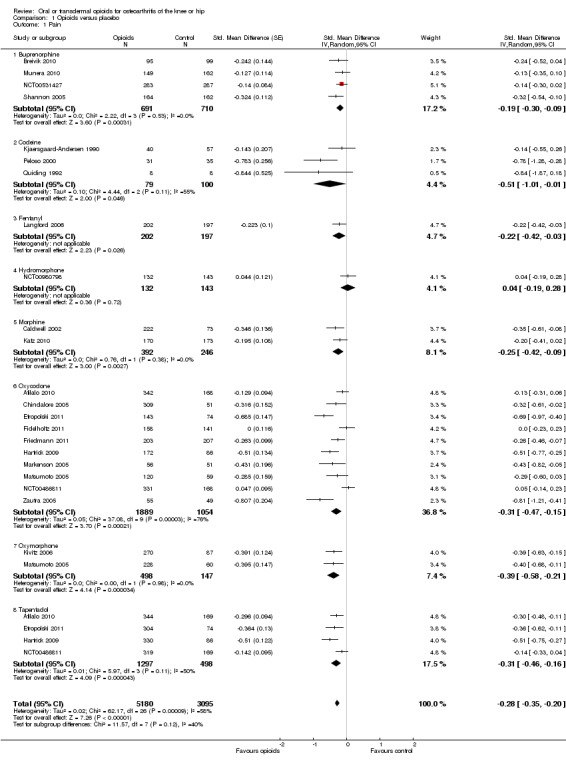
Comparison 1 Opioids versus placebo, Outcome 1 Pain.
1.2. Analysis.
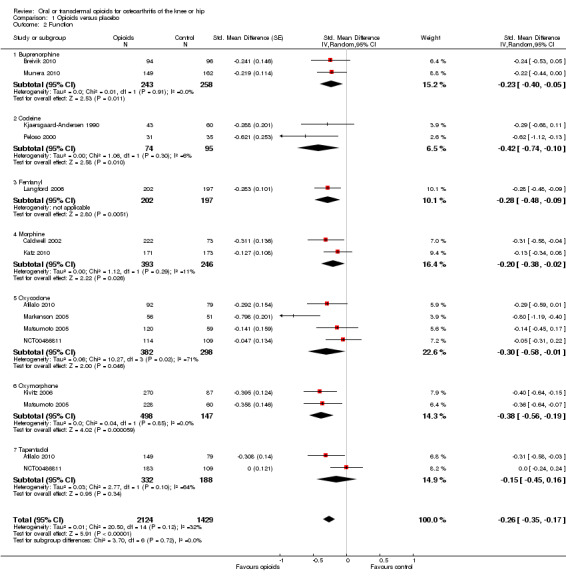
Comparison 1 Opioids versus placebo, Outcome 2 Function.
1.3. Analysis.
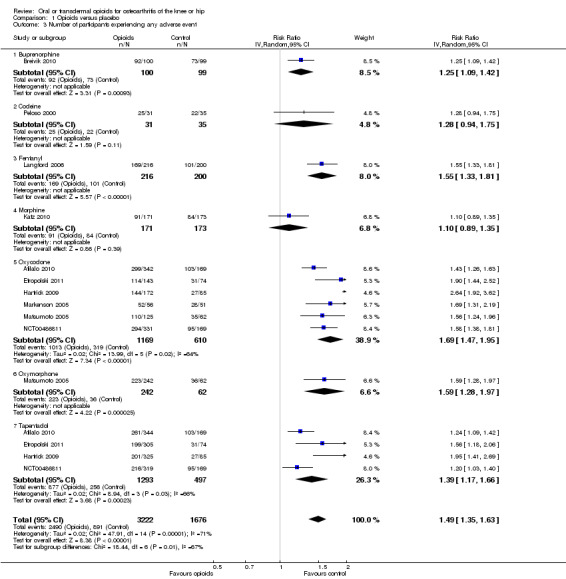
Comparison 1 Opioids versus placebo, Outcome 3 Number of participants experiencing any adverse event.
1.4. Analysis.
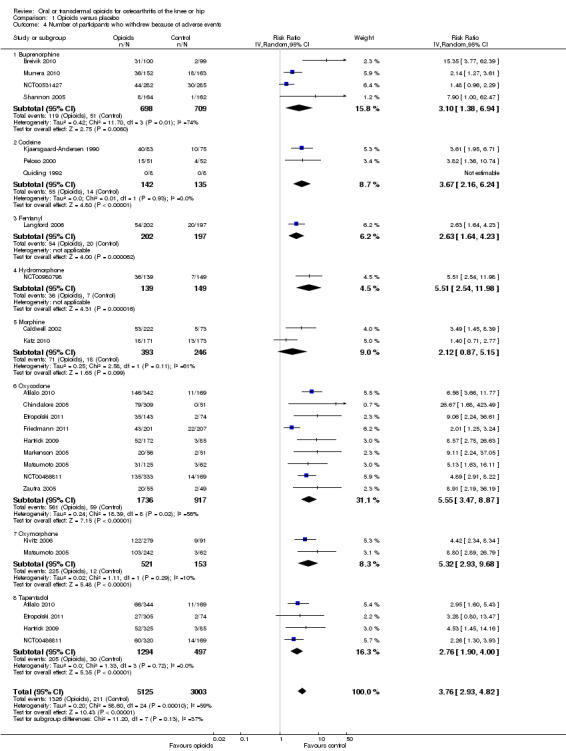
Comparison 1 Opioids versus placebo, Outcome 4 Number of participants who withdrew because of adverse events.
1.5. Analysis.
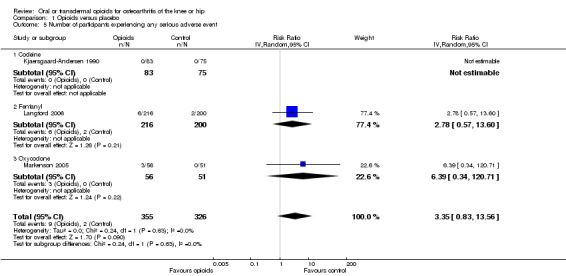
Comparison 1 Opioids versus placebo, Outcome 5 Number of participants experiencing any serious adverse event.
1.6. Analysis.
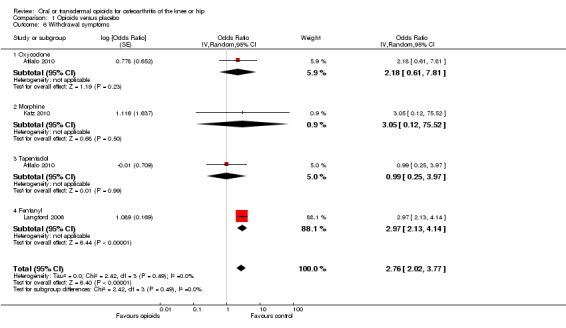
Comparison 1 Opioids versus placebo, Outcome 6 Withdrawal symptoms.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afilalo 2010.
| Methods | Randomised controlled trial 3‐arm parallel group design Trial duration: 17 weeks Randomisation stratified according by centre Multicentre trial with 112 centres Power calculation reported | |
| Participants | Participants with moderate‐to‐severe joint pain who needed analgesics for at least 3 months and were dissatisfied with their current treatment were eligible 1030 participants were randomised 1023 participants with knee osteoarthritis were reported at baseline Affected joints: 1023 knees Number of females: 618 of 1023 (60%) Mean age: 58 years Mean BMI: 34 kg/m2 | |
| Interventions |
Experimental interventions Oral extended‐release tapentadol, 100‐250 mg twice daily Oral controlled‐release oxycodone, 20‐50 mg twice daily Control intervention Placebo, twice daily Treatment duration: 15 weeks Analgesics other than study drugs allowed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 17 weeks Extracted function outcome: WOMAC disability subscore after 17 weeks Primary outcome: change in mean pain intensity | |
| Notes | Sponsor: Johnson & Johnson, Grünenthal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer‐generated randomization list, balanced using permuted blocks, and stratified by study site" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was implemented through an interactive voice response system (IVRS) to dispense blinded study medication. Placebo tablets and capsules (one for each active treatment) were used to maintain blinded treatments. Investigators were not provided with the randomization codes, and the schedule was maintained with the IVRS. The blinding was not broken until all participants had completed the trial, except in the case of a suspected unexpected serious adverse reaction or if emergency treatment required knowledge of a patient's treatment status" |
| Described as double‐blind? | Low risk | Quote: "This was a randomized, double‐blind, active‐ and placebo controlled, parallel‐arm, multicentre, phase III study..." |
| Blinding of patients? | Low risk | Because the study was described as a double‐dummy, we considered participants to be blinded Quote: "Placebo tablets and capsules (one for each active treatment) were used to maintain blinded treatments" |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | High risk | Quote: "Placebo tablets and capsules (one for each active treatment)…" |
| Double‐dummy technique used? | Low risk | Quote: "Placebo tablets and capsules (one for each active treatment) were used to maintain blinded treatments" |
| Intention‐to‐treat analysis performed? Pain | High risk | 2 of 346 participants excluded in experimental group. 171 of 339 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 197 of 346 participants excluded in experimental group, 260 of 339 participants excluded in control group |
Breivik 2010.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 28 weeks Multicentre trial with 19 centres Power calculation reported | |
| Participants | Participants with insufficient relief of moderate‐to‐severe osteoarthritis pain using NSAIDs or COXIBs and without previous exposure to opioids were eligible. 199 participants were randomised 199 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 126 knees, 73 hips Number of females: 136 of 199 (68%) Mean age: 63 years |
|
| Interventions |
Experimental intervention
Transdermal buprenorphine (Norspan; BuTrans), 5‐20 μg/hour Control intervention Placebo, change of patch every 7 days Treatment duration: 24 weeks No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: WOMAC pain subscore after 28 weeks Extracted function outcome: WOMAC disability subscore after 28 weeks Primary outcome: WOMAC pain | |
| Notes | Sponsor: Norpharma, Mundipharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed using a validated computer system that automates the random assignment of subjects to randomisation numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: Coded‐drugs of identical appearance: drugs "were identical in appearance, packed in a labelled foil pouch, containing coded treatment group identification. The medication codes were not available until the completion of the study and clinical database lock, except in case of emergency." Also: "The randomisation schedule was filed in a secure location in a manner such that blinding was properly maintained throughout the study" |
| Described as double‐blind? | Low risk | Quote: "This was a 6 months (24 weeks; 168 days), randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre study" |
| Blinding of patients? | Low risk | Because medication was described as identical and participants were explicitly described as blinded, we considered participants to be blinded Quote: "All patients, investigators, and study centre and Sponsor personnel were blinded to the medication codes" |
| Blinding of physicians? | Low risk | Quote: "All patients, investigators, and study centre and Sponsor personnel were blinded to the medication codes" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: drugs "were identical in appearance, packed in a labelled foil pouch, containing coded treatment group identification. The medication codes were not available until the completion of the study and clinical database lock, except in case of emergency" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 5 of 100 participants excluded in experimental group, 0 of 99 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 6 of 100 participants excluded in experimental group, 3 of 99 participants excluded in control group |
Caldwell 2002.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 4 weeks Multicentre trial No power calculation reported | |
| Participants | Participants with prior suboptimal analgesic response to NSAIDs/paracetamol or previous intermittent opioid therapy were eligible 295 participants with knee or hip (or both) osteoarthritis were reported at baseline Number of females: 184 of 295 (62%) Mean age: 62 years | |
| Interventions |
Experimental interventions
Oral morphine (Avinza), 30 mg once daily in the morning
Oral morphine (Avinza), 30 mg once daily in the evening
Oral morphine sulphate (Contin), 15 mg twice daily Control intervention Placebo, twice daily Treatment duration: 4 weeks No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks Extracted function outcome: WOMAC disability subscore after 4 weeks Primary outcome: WOMAC OA index | |
| Notes | Sponsor: Elan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors' description of the randomisation process does not explain how they generated the random sequence of allocation Quote: "Eligible participants entered a washout period of up to seven days and were subsequently randomized to one of four treatments" |
| Allocation concealment (selection bias) | Unclear risk | The authors' description of the randomisation process does not explain whether the random sequence of allocation was concealed from study personnel responsible for participant recruitment Quote: "Eligible participants entered a washout period of up to seven days and were subsequently randomized to one of four treatments" |
| Described as double‐blind? | Low risk | Quote: "The double‐blind trial was a 4‐week, multicenter, randomized, double‐blind, double‐dummy, placebo controlled, parallel trial" |
| Blinding of patients? | Low risk | Because the study was described as a double‐dummy, we considered participants to be blinded Quote: "Placebo Avinza and placebo MSC [morphine sulphate controlled‐release] matched the appearance of the respective active treatments. Avinza capsules and encapsulated MSC tablets did not look identical; therefore, to maintain the study blind, all participants consumed two capsules (one each representing Avinza and MSC) every morning and evening (Table 1)" |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | High risk | Quote: "Placebo Avinza and placebo MSC matched the appearance of the respective active treatments. Avinza capsules and encapsulated MSC tablets did not look identical; therefore, to maintain the study blind, all participants consumed two capsules (one each representing Avinza and MSC) every morning and evening" |
| Double‐dummy technique used? | Low risk | Quote: "Placebo Avinza and placebo MSC matched the appearance of the respective active treatments. Avinza capsules and encapsulated MSC tablets did not look identical; therefore, to maintain the study blind, all participants consumed two capsules (one each representing Avinza and MSC) every morning and evening" |
| Intention‐to‐treat analysis performed? Pain | High risk | Not all participants randomised were analysed Quote: "Efficacy and safety analyses for both trials were performed on all patients who received at least one dose of study medication" |
| Intention‐to‐treat analysis performed? Function | High risk | Not all participants randomised were analysed Quote: "Efficacy and safety analyses for both trials were performed on all patients who received at least one dose of study medication" |
Chindalore 2005.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 4 weeks Randomisation stratified according to gender Multicentre trial with 37 centres No power calculation reported | |
| Participants | Participants with moderate to severe hip or knee pain while taking ≥1 oral analgesic medication were eligible 362 participants were randomised 360 participants with hip or knee osteoarthritis were reported at baseline Number of females: 249 of 360 (69%) Average age: 54 years | |
| Interventions |
Experimental interventions
Oral oxycodone, 10 mg 4 times daily
Oral oxycodone, 2.5 mg 4 times daily, plus naltrexone 0.001 mg 4 times daily (Oxytrex)
Oral oxycodone, 2.5 mg 4 times daily, plus natronex 0.001 mg twice daily (Oxytrex) Control intervention Placebo, twice daily Treatment duration: 3 weeks Analgesics other than study drugs allowed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks Extracted function outcome: WOMAC disability subscore after 4 weeks Primary outcome: pain intensity during the past 24 hours | |
| Notes | Sponsor: Pain Therapeutics For WOMAC disability, insufficient data were reported to calculate standardised mean differences and it was, therefore, not included in the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided Quote: "Qualifying patients were randomly assigned and stratified by sex to 1 of 4 treatments for 3 weeks" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Described as double‐blind? | Low risk | Quote: "This study was a randomized, double‐blind, placebo and active‐controlled dose escalation trial" |
| Blinding of patients? | Low risk | Because the interventions were described as indistinguishable, we considered participants to be blinded Quote: "All study medications were identical in appearance, and patients, site personnel, and study monitors were blinded to treatment assignments" |
| Blinding of physicians? | Low risk | Quote: "All study medications were identical in appearance, and patients, site personnel, and study monitors were blinded to treatment assignments" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "All study medications were identical in appearance, and patients, site personnel, and study monitors were blinded to treatment assignments" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 1 of 310 participants (0.3%) excluded in experimental groups, 1 of 52 participants (1.9%) excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 1 of 310 participants (0.3%) excluded in experimental groups, 1 of 52 participants (1.9%) excluded in control group |
Etropolski 2011.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 8 weeks Randomisation stratified according to study centre Multicentre trial with 84 centres No power calculation reported | |
| Participants | Participants with joint disease requiring surgery and insufficient pain relief by stable analgesic regimens were eligible 598 participants were randomised 598 participants with knee or hip osteoarthritis reported at baseline Number of females: 349 of 596 (59%) Mean age: 59 years | |
| Interventions |
Experimental interventions
Oral immediate‐release tapentadol, 50 mg 3‐6 times daily
Oral immediate‐release tapentadol, 75 mg 3‐6 times daily
Oral immediate‐release oxycodone, 10 mg 3‐6 times daily Control intervention Placebo, 3‐6 times daily Treatment duration: 2 weeks Analgesics other than study drugs allowed and intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 8 weeks No function outcome reported Primary outcome: change in pain intensity | |
| Notes | Sponsor: Johnson & Johnson | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer‐generated randomization schedule, stratified by study center, and implemented using an interactive voice response system" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was based on a computer‐generated randomization schedule, stratified by study center, and implemented using an interactive voice response system" |
| Described as double‐blind? | Low risk | Quote: "In this double‐blind study, patients with end‐stage joint disease were randomized to tapentadol IR (50 mg or 75 mg), oxycodone HCL IR 10 mg, or placebo" |
| Blinding of patients? | Low risk | Quote: "All study drugs were provided as overencapsulated tablets or capsules and were identical in shape, color, and size" |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "All study drugs were provided as overencapsulated tablets or capsules and were identical in shape, color, and size" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 2 of 306 participants excluded in experimental group, 74 of 148 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Fidelholtz 2011.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration unclear Multicentre trial with 99 centres No power calculation reported | |
| Participants | Participants with moderate‐to‐severe osteoarthritis pain of knees or hips were eligible | |
| Interventions |
Experimental intervention
Oral oxycodone, 10‐40 mg twice daily Control intervention Placebo Treatment duration: not reported Unclear whether analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: WOMAC pain subscore after 8 weeks No function outcome reported Primary outcome: WOMAC pain | |
| Notes | Sponsor: Pfizer, Pain Solutions 2 trial arms excluded from review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "A randomized, double‐blind, placebo (PBO)‐ & active‐controlled study..." |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Low risk | Quote from ClinicalTrial.gov: "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessors? | Unclear risk | Because outcomes were self reported, and because it was unclear whether participants were properly blinded, it was unclear whether outcome assessors were blinded |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | Unclear risk | Description of intervention is not detailed enough to assess whether double‐dummy technique was used |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear how many participants were randomised in this study, so it was not possible to assess whether all participants randomised were included in the analysis |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Friedmann 2011.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 14 weeks Multicentre trial with 61 centres Power calculation reported | |
| Participants | Participants with moderate‐to‐severe osteoarthritis pain using NSAIDs or opioids were eligible 412 participants were randomised 412 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 323 knees and 89 hips Number of females: 288 of 412 (70%) Mean age: 58 years | |
| Interventions |
Experimental intervention
Oral extended‐release oxycodone (Remoxy), 5‐20 mg twice daily Control intervention Placebo, twice daily Treatment duration: 12 weeks Unclear whether analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 14 weeks No function outcome reported Primary outcome: change in pain intensity score | |
| Notes | Sponsor: Pain Therapeutics, King, Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "...a double‐blind, multicenter, placebo‐controlled trial..." |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Unclear risk | Because outcomes were self reported, and because it was unclear whether participants were properly blinded, it was unclear whether outcome assessors were blinded |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | Unclear risk | Description of intervention is not detailed enough to assess whether double‐dummy technique was used |
| Intention‐to‐treat analysis performed? Pain | High risk | 2 of 205 participants excluded in experimental group, 0 of 207 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Hartrick 2009.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 2 weeks Randomisation stratified according to study centre Multicentre trial Power calculation reported | |
| Participants | Participants with insufficient relief of moderate‐to‐severe osteoarthritis pain who were candidates for joint replacement surgery were eligible 674 participants were randomised 659 participants with knee or hip osteoarthritis were reported at baseline Number of females: 324 of 659 (49%) Mean age: 61 years Mean BMI: 33 kg/m2 | |
| Interventions |
Experimental interventions
Oral immediate‐release tapentadol, 50 mg every 4‐6 hours
Oral immediate‐release tapentadol, 75 mg every 4‐6 hours
Oral oxycodone, 10 mg every 4‐6 hours Control intervention Placebo, every 4‐6 hours Treatment duration: 1 week Analgesics other than study drugs allowed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 2 weeks No function outcome reported Primary outcome: sum of pain intensity difference | |
| Notes | Sponsor: Johnson & Johnson, Grünenthal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks were used to balance the number of participants across groups, so generation of sequence of random allocation was likely computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment of allocation was provided, so risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "...randomized, double‐blind, active‐ and placebo‐controlled study..." |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "This is a double‐blind study, i.e., neither patients nor investigators will know what treatment is given" |
| Blinding of outcome assessors? | Unclear risk | Because outcomes were self reported, and because it was unclear whether participants were properly blinded, it was unclear whether outcome assessors were blinded |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 0 of 330 participants excluded in experimental group, 86 of 172 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Katz 2010.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 14 weeks Randomisation stratified according to joint (hip/knee), daily dosage at end of titration, and study site Multicentre trial with 81 centres Power calculation reported | |
| Participants | Participants with insufficient pain relief with non‐opioids analgesics, tramadol, or other opioids at ≤ 40‐mg morphine equivalent per day were eligible 344 participants were randomised 344 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 267 knees and 77 hips Number of females: 201 of 344 (58%) Mean age: 54 years Mean BMI: 32 kg/m2 | |
| Interventions |
Experimental intervention
Oral morphine sulphate and naltrexone hydrochloride (EMBEDA), 20‐80 mg twice daily Control intervention Placebo, twice daily Treatment duration: 12 weeks Analgesics other than study drugs allowed and intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 14 weeks Extracted function outcome: WOMAC disability subscore after 14 weeks Primary outcome: change in average pain intensity | |
| Notes | Sponsor: King, Quintiles Medical Communications, Alphapharm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Low risk | Quote: "The outpatient site contacted the Interactive Web Response System to receive a randomization number and treatment assignment" |
| Described as double‐blind? | Low risk | Quote: "This randomized, double‐blind, placebo‐controlled, multicenter outpatient study" |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Investigator)" |
| Blinding of outcome assessors? | Unclear risk | Because outcomes were self reported, and because it was unclear whether participants were properly blinded, it was unclear whether outcome assessors were blinded |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | Unclear risk | Unclear whether double‐dummy technique was used |
| Intention‐to‐treat analysis performed? Pain | High risk | 1 of 171 participants excluded in experimental group, 0 of 173 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Low risk | All randomised participants included in the analysis |
Kivitz 2006.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 2 weeks Multicentre trial Power calculation reported | |
| Participants | Participants with suboptimal analgesic response to NSAIDs/paracetamol or previous opioid therapy were eligible 370 participants were randomised 370 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 297 knees and 73 hips Number of females: 224 of 370 (61%) | |
| Interventions |
Experimental interventions
Oral extended‐release oxymorphone, 10 mg twice daily
Oral extended‐release oxymorphone, 40 mg twice daily
Oral extended‐release oxymorphone, 50 mg twice daily Control intervention Placebo, twice daily Treatment duration: 2 weeks No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 2 weeks Extracted function outcome: WOMAC disability subscore after 2 weeks Primary outcome: change in pain intensity | |
| Notes | Sponsor: Endo Pharmaceuticals Inc, Penwest Pharmaceuticals Co | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization schedule was used to assign them to 1 of 4 groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study medications had computer‐generated 2‐part labels. One part of the label, which contained study and patient information, was attached to the box that contained all 4 bottles of study medication. The other part of the label was a tear‐off section containing the same information. This tear‐off section was removed at the time of dispensing and was attached to the appropriate page of the case report form; a copy of this page was made and retained in the investigator's study file. The treatment to which a patient had been assigned was concealed by an alcohol‐removable‐ink overlay on the tear‐off part of the label" |
| Described as double‐blind? | Low risk | Quote: "This was a 2‐week, multicenter, randomized, double‐blind, parallel‐group, dose‐ranging, Phase lll trial" |
| Blinding of patients? | Low risk | Because the study was described as a double‐dummy, we considered participants to be blinded Quote: "Study medications were overencapsulated in gelatin capsules so they were visually indistinguishable, and they were administered in a double‐dummy fashion to maintain blinding" |
| Blinding of physicians? | Low risk | Quote: "The study patients, study personnel, and investigators were blinded to the identity of the study treatments" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | High risk | The authors reported that interventions were only visually indistinguishable, which is probably the reason why double‐dummy was implemented Quote: "Study medications were overencapsulated in gelatin capsules so they were visually indistinguishable, and they were administered in a double‐dummy fashion to maintain blinding" |
| Double‐dummy technique used? | Low risk | Quote: "Study medications were overencapsulated in gelatin capsules so they were visually indistinguishable, and they were administered in a double‐dummy fashion to maintain blinding" |
| Intention‐to‐treat analysis performed? Pain | High risk | 9 of 279 participants (0.7%) excluded in experimental groups, 4 of 91 participants (4.4%) excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 9 of 279 participants (0.7%) excluded in experimental groups, 4 of 91 participants (4.4%) excluded in control group |
Kjaersgaard‐Andersen 1990.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 4 weeks Multicentre trial with 7 centres Power calculation reported | |
| Participants | Participants with chronic pain requiring analgesic treatment were eligible 158 participants with hip osteoarthritis were reported at baseline Affected joints: 158 hips Number of females: 72 of 158 (46%) Mean age: 66 years Mean BMI: 26 kg/m2 | |
| Interventions |
Experimental intervention
Oral codeine 60 mg plus paracetamol 1000 mg, 3 times daily Control intervention Paracetamol 1000 mg, 3 times daily Treatment duration: 4 weeks No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks Extracted function outcome: participant's global assessment after 4 weeks | |
| Notes | No information about source of funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "The study was designed as a randomised, double‐blind and parallel investigation" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "The tablets were identical in weight, appearance and taste" |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "The tablets were identical in weight, appearance and taste" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 43 of 83 participants (52%) excluded in experimental group, 18 of 75 participants (24%) excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 40 of 83 participants (48%) excluded in experimental group, 15 of 75 participants (20%) excluded in control group |
Langford 2006.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 8 weeks Randomisation stratified according to target joint (knee/hip) Multicentre trial Power calculation reported | |
| Participants | Participants without adequate pain control under weak opioid treatment (with and without paracetamol) were eligible 416 participants were randomised 399 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 211 knees and 188 hips Number of females: 265 of 399 (66%) | |
| Interventions |
Experimental intervention
Transdermal fentanyl (Durogesic), median dosage 25 μg/hour Control intervention Placebo Treatment duration: 6 weeks Analgesics other than study drugs allowed and intake assessed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 8 weeks Extracted function outcome: WOMAC disability subscore after 8 weeks Primary outcome: pain relief on VAS | |
| Notes | Sponsor: Janssen‐Cilag | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were assigned consecutive treatment codes, and investigators were unaware of the treatment allocation" |
| Described as double‐blind? | Low risk | Quote: "The aim of the present trial was therefore to assess pain relief from treatment with TDF [transdermal fentanyl] as compared with placebo in a double‐blind study" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "TDF and placebo patches were identical" |
| Blinding of physicians? | Low risk | Quote: "investigators were unaware of the treatment allocation" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "TDF and placebo patches were identical" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | No information on exclusions available |
| Intention‐to‐treat analysis performed? Function | High risk | No information on exclusions available |
Markenson 2005.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 13 weeks Multicentre trial with 9 centres Power calculation reported | |
| Participants | Participants with moderate‐to‐severe pain while taking NSAIDs/paracetamol, with contraindications to NSAID therapy or with previous oral opioid therapy were eligible 109 participants were randomised 107 participants with osteoarthritis were reported at baseline Affected joints: 33 knees, 19 hips, and 57 other joints Number of females: 78 of 107 (73%) Mean age: 63 years | |
| Interventions |
Experimental intervention
Oral oxycodone (OxyContin), 10 mg twice daily Control intervention Placebo, twice daily Treatment duration: 13 weeks Analgesics other than study drugs allowed and intake assessed, but it was unclear whether intake was similar |
|
| Outcomes | Extracted pain outcome: global pain after 13 weeks Extracted function outcome: WOMAC global scale after 13 weeks | |
| Notes | Sponsor: Purdue Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer‐generated randomization code and study drug bottles labeled with randomization numbers were supplied by the sponsor" |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer‐generated randomization code and study drug bottles labeled with randomization numbers were supplied by the sponsor" |
| Described as double‐blind? | Low risk | Quote: "This was a double blind, randomized, placebo‐controlled, parallel‐group study" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "Patients who met the entry criteria were randomly assigned in double blind fashion to receive either 10‐mg tablets of CR oxycodone or matching placebo every 12 hours" |
| Blinding of physicians? | Low risk | Because coded labelled bottles were provided by sponsor and drug tables were matching the placebo tablets, physicians were considered blinded as well |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "Patients who met the entry criteria were randomly assigned in double blind fashion to receive either 10‐mg tablets of CR [controlled release] oxycodone or matching placebo every 12 hours" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 2 randomised participants who withdrew before receiving treatment were excluded from the analyses |
| Intention‐to‐treat analysis performed? Function | High risk | 2 randomised participants who withdrew before receiving treatment were excluded from the analyses |
Matsumoto 2005.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 4 weeks Simple randomisation Multicentre trial Power calculation reported | |
| Participants | Participants with suboptimal analgesic response to NSAIDs, paracetamol, or opioids were eligible 491 participants were randomised 489 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 373 knees and 116 hips Number of females: 297 of 489 (61%) Mean age: 62 years Mean BMI: 34 kg/m2 | |
| Interventions |
Experimental interventions
Oral extended‐release oxymorphone, 20 mg twice daily
Oral extended‐release oxymorphone, 40 mg twice daily
Oral controlled‐release oxycodone, 20 mg twice daily Control intervention Placebo, twice daily Treatment duration: 4 weeks No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: WOMAC pain subscore after 4 weeks Extracted function outcome: WOMAC disability subscore after 4 weeks Primary outcome: change in arthritis pain intensity | |
| Notes | Sponsors: TheraQuest Biosciences, Endo Pharmaceuticals, Penwest Pharmaceuticals Co | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The list of randomization numbers was based on a computer generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "The study was a multicenter, 4‐week, randomized, double‐blind, parallel‐group study" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "Active study medication tablets were overencapsulated and visually indistinguishable from each other and from the placebo tablets" |
| Blinding of physicians? | Low risk | Quote: "Study enrollees, study personnel, and investigators were blinded to the identity of the treatments" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "Active study medication tablets were overencapsulated and visually indistinguishable from each other and from the placebo tablets" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 19 of 367 participants (5.2%) excluded in experimental groups, 5 of 124 (4.0%) participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 19 of 367 participants (5.2%) excluded in experimental groups, 5 of 124 (4.0%) participants excluded in control group |
Munera 2010.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 4 weeks Multicentre trial with 25 centres Power calculation reported | |
| Participants | Participants with inadequate pain control using NSAIDs were eligible 315 participants were randomised 315 participants with knee or hip osteoarthritis were reported at baseline Affected joints: 173 knees and 142 hips Number of females: 212 of 315 (67%) Mean age: 61 years | |
| Interventions |
Experimental intervention
Transdermal buprenorphine, 5, 10, or 20 μg/hour Control intervention Placebo Treatment duration: 4 weeks No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks Extracted function outcome: participant's global assessment after 4 weeks Primary outcome: percentage of participants considered to have achieved treatment success | |
| Notes | Sponsor: Purdue | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "…randomized, placebo‐controlled, double‐blind, parallel‐group investigation" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "Placebo TDS [transdermal buprenorphine]‐treated patients received identical‐looking patches for each strength level" |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "Placebo TDS‐treated patients received identical‐looking patches for each strength level" |
| Double‐dummy technique used? | Unclear risk | No information provided |
| Intention‐to‐treat analysis performed? Pain | High risk | 3 of 152 participants excluded in experimental group, 1 of 163 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 3 of 152 participants excluded in experimental group, 1 of 163 participants excluded in control group |
NCT00486811.
| Methods | Randomised controlled trial 3‐arm parallel group design Trial duration unclear Multicentre trial with 101 centres No power calculation reported | |
| Participants | Participants who were dissatisfied with their prior analgesic therapy were eligible 987 participants with knee osteoarthritis were reported at baseline Number of females: 707 of 987 (72%) Mean age: 62 years | |
| Interventions |
Experimental interventions
Oral extended‐release tapentadol, 100‐250 mg twice daily
Oral controlled‐release oxycodone, 20‐50 mg twice daily Control intervention Placebo, twice daily Treatment duration: 15 weeks Unclear whether analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 15 weeks Extracted function outcome: WOMAC global scale after 15 weeks Primary outcome: change in mean pain intensity |
|
| Notes | Sponsor: Grünenthal GmbH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Investigator)" |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Investigator)" |
| Blinding of outcome assessors? | Unclear risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | Unclear risk | No information provided |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | It was unclear whether all participants randomised were also analysed |
NCT00531427.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration unclear Multicentre trial with 83 centres No power calculation reported | |
| Participants | Participants with suboptimal analgesic response to opioids were eligible 570 participants were randomised 570 participants with knee osteoarthritis were reported at baseline Affected joints: 567 knees Number of females: 356 of 567 (63%) Mean age: 59 years | |
| Interventions |
Experimental intervention
Transdermal buprenorphine, 10 or 20 μg/hour Control intervention Placebo Treatment duration: 12 weeks Analgesics other than study drugs allowed and intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 12 weeks. No function outcome reported Primary outcome: mean pain over the last 24 hours | |
| Notes | Sponsor: Purdue Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Investigator)" |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Investigator)" |
| Blinding of outcome assessors? | Unclear risk | Because it was unclear whether participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered unclear |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | Unclear risk | No information provided |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
NCT00980798.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration unclear Power calculation reported | |
| Participants | Participants with insufficient pain relief using NSAIDs, paracetamol, or a weak opioid were eligible 88 participants with knee or hip osteoarthritis were reported at baseline Number of females: 208 of 288 (72%) Mean age: 65 years | |
| Interventions |
Experimental intervention
Oral hydromorphone (OROS), 4‐32 mg once daily Control intervention Placebo, once daily Treatment duration: 16 weeks Analgesics other than study drugs allowed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 16 weeks No function outcome reported Primary outcome: mean pain (Item 5 of Brief Pain Inventory) | |
| Notes | Sponsor: Janssen‐Cilag | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote from ClinicalTrials.gov: "the control group receives an optically identical tablet with no active ingredient, a so‐called placebo." |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote from ClinicalTrials.gov: "the control group receives an optically identical tablet with no active ingredient, a so‐called placebo" |
| Double‐dummy technique used? | Unclear risk | No information available |
| Intention‐to‐treat analysis performed? Pain | High risk | 13 randomised participants were excluded from the analyses |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Peloso 2000.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 4 weeks Multicentre trial with 4 centres Power calculation reported | |
| Participants | Participants with osteoarthritis symptoms requiring therapy with paracetamol, anti‐inflammatory agents or opioids were eligible 103 participants were randomised 103 participants with osteoarthritis were reported at baseline Affected joints: 94 knees and 49 hips Number of females: 64 of 103 (62%) Mean age: 62 years Mean BMI: 34 kg/m2 Mean disease duration: 10.3 years | |
| Interventions |
Experimental intervention
Oral codeine (Contin), 100 mg twice daily Control intervention Placebo, twice daily Treatment duration: 4 weeks Analgesics other than study drugs allowed and intake assessed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks Extracted function outcome: WOMAC disability subscore after 4 weeks Primary outcome: WOMAC pain and overall pain intensity | |
| Notes | Sponsor: Purdue Frederick | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "Randomized, balanced, double blind parallel group assignment" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "identical appearing placebo" |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "identical appearing placebo" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 20 of 51 participants (39%) excluded in experimental group, 17 of 52 participants (33%) excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 20 of 51 participants (39%) excluded in experimental group, 17 of 52 participants (33%) excluded in control group |
Quiding 1992.
| Methods | Randomised controlled trial 3‐arm cross‐over design Trial duration: 1 week No power calculation reported | |
| Participants | Participants in need of analgesic medication for hip osteoarthritis were eligible 27 participants were randomised 26 participants with hip osteoarthritis were reported at baseline Affected joints: 26 hips Number of females: 22 of 26 (85%) Mean age: 53 years | |
| Interventions |
Experimental intervention
Oral codeine 30 mg plus ibuprofen 200 mg, 6 times in 32 hours Control intervention Ibuprofen 200 mg, 6 times in 32 hours Treatment duration: 32 hours No analgesics other than study drugs allowed |
|
| Outcomes | Extracted pain outcome: global pain after 1 week No function outcome reported No primary outcome reported | |
| Notes | No information about source of funding provided 1 trial arm excluded from review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "double‐blind, placebo‐controlled cross‐over design" |
| Blinding of patients? | Low risk | Because the study was described as a double‐dummy, we considered participants to be blinded Quote: "a double‐dummy technique was used to ensure blindness of the study" |
| Blinding of physicians? | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | Low risk | Quote: "a double‐dummy technique was used to ensure blindness of the study" |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | No information on exclusions available |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Shannon 2005.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 30 weeks Multicentre trial with 41 centres No power calculation reported | |
| Participants | Participants with moderate‐to‐severe pain while taking paracetamol, non‐steroidal anti‐inflammatory agents or opioids were eligible 327 participants were randomised 327 participants with knee or hip osteoarthritis were reported at baseline Number of females: 219 of 326 (67%) Mean age: 61 years | |
| Interventions |
Experimental intervention
Transdermal buprenorphine (Butrans), 5, 10 or 20 μg/hour Control intervention Placebo Treatment duration: 4 weeks Analgesics other than study drugs allowed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 30 weeks Extracted function outcome: after 30 weeks Primary outcome: time to development of inadequate analgesia | |
| Notes | Sponsor: Purdue Pharma L.P | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Described as double‐blind? | Low risk | Quote: "Randomized, double‐blind, placebo‐controlled" |
| Blinding of patients? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of physicians? | Low risk | Quote from ClinicalTrials.gov: "Double Blind (Subject, Investigator)" |
| Blinding of outcome assessors? | Unclear risk | Because it was unclear whether participants were blinded and the outcomes are participant‐reported, the risk of bias was unclear |
| Interventions reported as indistinguishable? | High risk | Interventions were not reported as indistinguishable |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 1 of 165 participants excluded in experimental group, 0 of 162 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
Zautra 2005.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 13 weeks Multicentre trial with 9 centres No power calculation reported | |
| Participants | 107 participants were randomised 104 participants with knee osteoarthritis were reported at baseline Number of females: 76 of 104 (73%) Mean age: 63 years | |
| Interventions |
Experimental intervention
Oral oxycodone (Oxycontin), 10 mg twice daily Control intervention Placebo, twice daily Treatment duration: 13 weeks Analgesics other than study drugs allowed, but it was unclear whether intake was similar between groups |
|
| Outcomes | Extracted pain outcome: global pain after 13 weeks No function outcome reported Primary outcome: coping efficacy and arthritis helplessness | |
| Notes | Sponsor: Purdue Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "The bottles of medication were labeled with a randomization number and dispensed by the investigators" |
| Described as double‐blind? | Low risk | Quote: "Double‐blind, randomized, placebo‐controlled, parallel‐group study" |
| Blinding of patients? | Low risk | Because the study used indistinguishable interventions, we considered participants to be blinded Quote: "Patients were randomized at each of the 9 participating clinics to receive either oral CR oxycodone (10 mg) or matching placebo" |
| Blinding of physicians? | Unclear risk | No information provided |
| Blinding of outcome assessors? | Low risk | Because participants were blinded and outcomes were participant‐reported, the risk of detection bias was considered low |
| Interventions reported as indistinguishable? | Low risk | Quote: "Patients were randomized at each of the 9 participating clinics to receive either oral CR oxycodone (10 mg) or matching placebo" |
| Double‐dummy technique used? | High risk | No double‐dummy technique used |
| Intention‐to‐treat analysis performed? Pain | High risk | 1 of 56 participants (1.8%) excluded in experimental group, 2 of 51 participants (3.9%) excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
BMI: body mass index; COXIB: cyclo‐oxygenase inhibitor; NSAID: non‐steroidal anti‐inflammatory drug; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Arthritis Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2006 | Only active control interventions |
| Andrei 1984 | Percentage of participants with knee or hip osteoarthritis 17% (5/30) |
| Boureau 1990 | Only active control interventions |
| Boyer 2012 | Cross‐over trial providing pooled results only |
| Brooks 1982 | Percentage of participants with osteoarthritis 50%, no information about joints involved |
| Burch 2004 | Not a randomised controlled trial |
| Caldwell 1999 | Percentage of participants with knee or hip osteoarthritis likely to be below 50% |
| Choquette 2008 | Not a randomised controlled trial |
| Conaghan 2011 | Only active control interventions |
| Corsinovi 2009 | Only active control interventions |
| Doak 1992 | Cross‐over trial providing pooled results only |
| Fancourt 1984 | Mixed population of rheumatoid arthritis and osteoarthritis, no information about number of participants with osteoarthritis |
| Friedmann 2011b | Percentage of participants with knee or hip osteoarthritis 15% (123/827) |
| Gazi 2005 | Only active control interventions |
| Hale 2007 | Only active control interventions |
| James 2010 | Only active control interventions |
| Katz 2010b | Only active control interventions |
| Le Loet 2005 | Not a randomised controlled trial |
| McIlwain 2005 | Not a randomised controlled trial |
| Mitchell 1984 | Mixed population of rheumatoid arthritis and osteoarthritis, no information about number of participants with osteoarthritis |
| Neubauer 1983 | Percentage of participants with osteoarthritis 15% (5/33) |
| Rosenthal 2007 | Not a randomised controlled trial |
| Roth 2000 | Percentage of participants with knee or hip osteoarthritis likely to be below 50% |
| Salzman 1983 | Only active control interventions |
| Tassain 2003 | Percentage of participants with osteoarthritis 7% (2/28) |
| Torres 2001 | Not a randomised controlled trial |
| Vignon 1999 | Comparison of combination of dextropropoxyphene, paracetamol, and caffeine with placebo |
| Vlok 1987 | Cross‐over trial providing pooled results only |
| Vorsanger 2011 | Only active control interventions |
| Wallace 1994 | Cross‐over trial providing pooled results only |
| Wang 1965 | Percentage of participants with osteoarthritis 6% (2/34) |
| Wild 2010 | Only active control interventions |
Characteristics of studies awaiting assessment [ordered by study ID]
Kroner 1991.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 3 weeks Multicentre trial |
| Participants | 131 participants with hip osteoarthritis were reported at baseline Number of females: 70 of 131 (53%) |
| Interventions |
Experimental intervention
Codeine 30 mg plus paracetamol 500 mg Control intervention Paracetamol 500 mg Treatment duration: 3 weeks |
| Outcomes | Assessed efficacy outcomes: pain intensity, pain relief, participant's evaluation of the effect of treatment Assessed safety outcomes: number of participants withdrawn due to adverse events, serious adverse events |
| Notes | Insufficient data provided in published abstract, no full‐text article available. Awaiting author response |
Differences between protocol and review
The cut‐off to distinguish between short‐term and long‐term trials was changed from 26 weeks to one month. Six months was considered to be rather long as the cut‐off for an agent that is not considered to be a structure‐modifying drug. In the absence of definitions for short‐term treatment in osteoarthritis treatment guidelines, we used the median follow‐up duration in the trials included in the first review (four weeks) as a cut‐off to discriminate between trials of shorter and longer duration. We did not include the electronic database CINAHL in our search update since, in our previous search, this database did not identify any additional hits. Finally, we did not include the OARSI database in our search update, as we no longer had access to this database. We added analyses stratified by type of osteoarthritis (hip only versus knee only versus mixed) upon request of peer reviewers.
Contributions of authors
Protocol completion: Nüesch, Rutjes, Husni, Jüni. Acquisition of data: Nüesch, da Costa, Kasteler, Rutjes. Analysis and interpretation of data: Nüesch, da Costa, Kasteler, Husni, Welch, Rutjes, Jüni. Manuscript preparation: Nüesch, da Costa, Kasteler, Husni, Welch, Rutjes, Jüni. Statistical analysis: Nüesch, da Costa, Rutjes, Jüni.
Drs. Nüesch and da Costa contributed equally to this review.
Sources of support
Internal sources
-
Institute of Social and Preventive Medicine, University or Bern, Switzerland.
Intramural grants
External sources
-
Swiss National Science Foundation, Switzerland.
National Research Program 53 on musculoskeletal health (grant numbers 4053‐40‐104762/3 and 3200‐066378)
-
Marie Curie Intra‐European Fellowship, Other.
Dr Nüesch was recipient of a Marie Curie Intra‐European Fellowship for Career Development (grant number FP7‐PEOPLE‐2010‐IEF‐273673)
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Afilalo 2010 {published data only}
- Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Hove I, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double‐blind, placebo‐ and active‐controlled phase III study. Clinical Drug Investigation 2010;30(8):489‐505. [DOI] [PubMed] [Google Scholar]
- Grünenthal GmbH. A study to evaluate the efficacy and safety of cg5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. clinicaltrials.gov/show/NCT00486811 (accessed 16 August 2014).
- Kelly K, Etropolski M, Kuperwasser B, Okamoto A, Steup A, vanHove I, et al. Similar analgesic effect and improved tolerability of tapentadol extended release versus oxycodone controlled release for the management of chronic osteoarthritis knee pain: results from a randomized, double‐blind, phase3 trial. Proceedings of the Annual EULAR Conference; 2009 Jun 10‐13; Copenhagen, Denmark.
- Kelly K, Lange B, Etropolski M, Kuperwasser B, Okamoto A, vanHove I, et al. Dose stability of tapentadol extended release (ER) for the relief of moderate‐to‐severe chronic osteoarthritic knee pain. Proceedings of the 26th Annual Meeting of the American Academy of Pain Medicine; 2010 Feb 3‐6; San Antonio, TX.
- Lange B, Kuperwasser B, Okamoto A, Häufel T, Ashworth J. Efficacy and safety of tapentadol prolonged release (PR) based on prior experience in patients with chronic osteoarthritis knee pain. Annals of Rheumatic Diseases 2010;69:287. [Google Scholar]
- Lange R, Lange B, Greene A, Okamoto A, Etropolski M, Ashworth J. Short Form‐36 (SF‐36) and EUROQOL‐5 Dimension (EQ‐5D) results from randomized, double‐blind phase 3 studies of tapentadol prolonged release (PR) in patients with moderate to severe chronic nociceptive and neuropathic pain. Osteoarthritis and Cartilage 2010;18:S147‐8. [Google Scholar]
Breivik 2010 {published data only}
- Breivik H, Ljosaa TM, Stengaard‐Pedersen K, Persson J, Aro H, Villumsen J, et al. A 6‐months, randomised, placebo‐controlled evaluation of efficacy and tolerability of a low‐dose 7‐day buprenorphine transdermal patch in osteoarthritis patients naive to potent opioids. Scandinavian Journal of Pain 2010;1:122‐41. [DOI] [PubMed] [Google Scholar]
Caldwell 2002 {published data only}
- Caldwell JR, Rapoport RJ, Davis JC, Offenberg HL, Marker HW, Roth SH, et al. Efficacy and safety of a once‐daily morphine formulation in chronic, moderate‐to‐severe osteoarthritis pain: results from a randomized, placebo‐controlled, double‐blind trial and an open‐label extension trial. Journal of Pain and Symptom Management 2002;23(4):278‐91. [DOI] [PubMed] [Google Scholar]
Chindalore 2005 {published data only}
- Chindalore VL, Craven RA, Yu KP, Butera PG, Burns LH, Friedmann N. Adding ultra low‐dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of oxytrex. Journal of Pain 2005;6(6):392‐9. [DOI] [PubMed] [Google Scholar]
Etropolski 2011 {published data only}
- Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Advances in Therapy 2011;28(5):401‐17. [DOI] [PubMed] [Google Scholar]
- Johnson, Johnson. A study to compare the frequency of constipation symptoms with tapentadol immediate release (IR) treatment versus oxycodone IR treatment in patients with end‐stage joint disease. clinicaltrials.gov/show/NCT00784277 (accessed 16 August 2014).
Fidelholtz 2011 {published data only}
- Fidelholtz J, Tark M, Spierings E, Wolfram G, Annis K, Smith MD, et al. A phase 3 placebo‐ and oxycodone‐controlled study of tanezumab in adults with osteoarthritis. Proceedings of the ACR/ARHP Scientific Meeting; 2011 Nov 9‐11; Chicago.
- Pfizer. Tanezumab in osteoarthritis of the hip or knee. clinicaltrials.gov/show/NCT00985621 (accessed 16 August 2014).
Friedmann 2011 {published data only}
- Friedmann N, Klutzaritz V, Webster L. Efficacy and safety of an extended‐release oxycodone (Remoxy®) formulation in patients with moderate to severe osteoarthritic pain. Journal of Opioid Management 2011;7(3):193‐202. [DOI] [PubMed] [Google Scholar]
Hartrick 2009 {published data only}
- Hartrick C, Hove I, Stegmann JU, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end‐stage joint disease: a 10‐day, phase III, randomized, double‐blind, active‐ and placebo‐controlled study. Clinical Therapeutics 2009;31(2):260‐71. [DOI] [PubMed] [Google Scholar]
- Johnson, Johnson. A study to evaluate the effectiveness and safety of multiple doses of tapentadol (CG5503) in patients awaiting joint replacement surgery. clinicaltrials.gov/show/NCT00361582 (accessed 16 August 2014).
Katz 2010 {published data only}
- Alpharma. A study of embeda (Kadian NT, ALO‐01) in subjects with pain due to osteoarthritis of the hip or knee. clinicaltrials.gov/show/NCT00420992 (accessed 16 August 2014).
- Jones JB, Wagner G, Morris D, Stauffer J. Efficacy of morphine sulfate extended‐release with sequestered naltrexone hydrochloride (ALO‐01) in patients with chronic, moderate to severe pain of osteoarthritis of the hip or knee. Proceedings of the American College of Rheumatology Annual Scientific Meeting; 2008 Oct 24‐29; San Francisco.
- Katz N, Hale M, Morris D, Stauffer J. Morphine sulfate and naltrexone hydrochloride extended release capsules in patients with chronic osteoarthritis pain. Postgraduate Medicine 2010;122(4):112‐28. [DOI] [PubMed] [Google Scholar]
- Katz N, Setnik B, Webster L. Safety profile of EMBEDA™ (morphine sulfate and naltrexone hydrochloride) Extended Release Capsules in older patients. Proceedings of the 29th Annual Scientific Meeting of the American Pain Society; 2010 May 6‐8; Baltimore, MD.
Kivitz 2006 {published data only}
- Kivitz A, Ma C, Ahdieh H, Galer BS. A 2‐week, multicenter, randomized, double‐blind, placebo‐controlled, dose‐ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee. Clinical Therapeutics 2006;28(3):352‐64. [DOI] [PubMed] [Google Scholar]
Kjaersgaard‐Andersen 1990 {published data only}
- Kjaersgaard‐Andersen P, Nafei A, Skov O, Madsen F, Andersen HM, Kroner K, et al. Codeine plus paracetamol versus paracetamol in longer‐term treatment of chronic pain due to osteoarthritis of the hip. A randomised, double‐blind, multi‐centre study. Pain 1990;43(3):309‐18. [DOI] [PubMed] [Google Scholar]
Langford 2006 {published data only}
- Langford R, McKenna F, Ratcliffe S, Vojtassak J, Richarz U. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis: a randomized, placebo‐controlled trial. Arthritis and Rheumatism 2006;54(6):1829‐37. [DOI] [PubMed] [Google Scholar]
Markenson 2005 {published data only}
- Markenson JA, Croft J, Zhang PG, Richards P. Treatment of persistent pain associated with osteoarthritis with controlled‐release oxycodone tablets in a randomized controlled clinical trial. Clinical Journal of Pain 2005;21(6):524‐35. [DOI] [PubMed] [Google Scholar]
Matsumoto 2005 {published data only}
- Matsumoto AK, Babul N, Ahdieh H. Oxymorphone extended‐release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double‐blind, placebo‐ and active‐controlled phase III trial. Pain Medicine 2005;6(5):357‐66. [DOI] [PubMed] [Google Scholar]
Munera 2010 {published data only}
- Munera C, Drehobl M, Sessler NE, Landau C. A randomized, placebo‐controlled, double‐blinded, parallel‐group, 5‐week study of buprenorphine transdermal system in adults with osteoarthritis. Journal of Opioid Management 2010;6(3):193‐202. [DOI] [PubMed] [Google Scholar]
- Purdue. Safety and efficacy of the buprenorphine transdermal delivery system in subjects with osteoarthritis pain. clinicaltrials.gov/show/NCT00314652 (accessed 16 August 2014).
NCT00486811 {published data only}
- Grünenthal. A study to evaluate the efficacy and safety of CG5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. clinicaltrials.gov/show/NCT00486811 (accessed 16 August 2014).
NCT00531427 {published data only}
- Purdue. A study to evaluate the efficacy and safety of CG5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. clinicaltrials.gov/show/NCT00531427 (accessed 16 August 2014).
NCT00980798 {published data only}
- Janssen‐Cilag. Placebo‐controlled trial with OROS hydromorphone hydrochloride to treat patients with moderate to severe pain induced by osteoarthritis of the hip or the knee. clinicaltrials.gov/show/NCT00980798 (accessed 16 August 2014).
Peloso 2000 {published data only}
- Peloso PM, Bellamy N, Bensen W, Thomson GTD, Harsanyi Z, Babul N, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. Journal of Rheumatology 2000;27(3):764‐71. [PubMed] [Google Scholar]
Quiding 1992 {published data only}
- Quiding H, Grimstad J, Rusten K, Stubhaug A, Bremnes J, Breivik H. Ibuprofen plus codeine, ibuprofen, and placebo in a single‐ and multidose cross‐over comparison for coxarthrosis pain. Pain 1992;50(3):303‐7. [DOI] [PubMed] [Google Scholar]
- Öhrvik, J. Nonparametric methods in crossover trials. Biometrical Journal 1998;7:771‐89. [Google Scholar]
Shannon 2005 {published data only}
- Landau CJ, Shannon M, Kivitz AJ, Sessler NE, Xia Y, Ripa SR. Buprenorphine transdermal system in chronic pain due to osteoarthritis. Arthritis and Rheumatism 2008;58(Suppl 9):866. [Google Scholar]
- Purdue. Safety and efficacy of buprenorphine transdermal system in subjects with moderate to severe osteoarthritis of hip or knee. clinicaltrials.gov/show/NCT00313846 (accessed 16 August 2014).
- Shannon MJ, Kivitz AJ, Landau CJ, Sessler NE, Xia Y, Ripa SR. Buprenorphine transdermal system in chronic pain due to osteoarthritis. Archives of Physical Medicine and Rehabilitation 2005;86:E32. [Google Scholar]
Zautra 2005 {published data only}
- Zautra AJ, Smith BW. Impact of controlled‐release oxycodone on efficacy beliefs and coping efforts among osteoarthritis patients with moderate to severe pain. Clinical Journal of Pain 2005;21(6):471‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2006 {published data only}
- Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. Journal of Pain and Symptom Management 2006;31(5):465‐76. [DOI] [PubMed] [Google Scholar]
Andrei 1984 {published data only}
- Andrei A, Schiaroli G, Algeri R, Valentini P. Recent data on antiinflammatory and analgesic treatment of degenerative arthropathies. Double‐blind controlled clinical trial. [Italian]. Archivio di Medicina Interna 1984;36(4):245‐56. [Google Scholar]
Boureau 1990 {published data only}
- Boureau F, Delecoeuillerie G, Orvain J. Comparative study of the efficacy and tolerance of 2 dosages of the paracetamol 400 mg codeine 25 mg association versus paracetamol 1000 mg in non‐inflammatory rheumatic pain. Rhumatologie Revue International de Rhumatologie 1990;20(1):41‐7. [Google Scholar]
Boyer 2012 {published data only}
- Boyer KA, Angst MS, Asay J, Giori NJ, Andriacchi TP. Sensitivity of gait parameters to the effects of anti‐inflammatory and opioid treatments in knee osteoarthritis patients. Journal of Orthopaedic Research 2012;30(7):1118‐24. [DOI] [PubMed] [Google Scholar]
Brooks 1982 {published data only}
- Brooks PM, Dougan MA, Mugford S, Meffin E. Comparative effectiveness of 5 analgesics in patients with rheumatoid arthritis and osteoarthritis. Journal of Rheumatology 1982;9(5):723‐6. [PubMed] [Google Scholar]
Burch 2004 {published data only}
- Burch F, Codding C, Patel N, Sheldon E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open‐label effectiveness trial. Osteoarthritis and Cartilage 2004;12(3):253‐5. [DOI] [PubMed] [Google Scholar]
Caldwell 1999 {published data only}
- Caldwell JR, Hale ME, Boyd RE, Hague JM, Iwan T, Shi M, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial. Journal of Rheumatology 1999;26(4):862‐9. [PubMed] [Google Scholar]
Choquette 2008 {published data only}
- Choquette D, McCarthy TG, Rodrigues JFN, Kelly AJ, Camacho F, Horbay GLA, et al. Transdermal fentanyl improves pain control and functionality in patients with osteoarthritis: an open‐label Canadian trial. Clinical Rheumatology 2008;27(5):587‐95. [DOI] [PubMed] [Google Scholar]
Conaghan 2011 {published data only}
- Conaghan PG, O'Brien CM, Wilson M, Schofield, JP. Transdermal buprenorphine plus oral paracetamol vs an oral codeine‐paracetamol combination for osteoarthritis of hip and/or knee: a randomised trial. Osteoarthritis & Cartilage 2011;19(8):930‐8. [DOI] [PubMed] [Google Scholar]
Corsinovi 2009 {published data only}
- Corsinovi L, Martinelli E, Fonte G, Astengo M, Sona, A, Gatti A, et al. Efficacy of oxycodone/acetaminophen and codeine/acetaminophen vs. conventional therapy in elderly women with persistent, moderate to severe osteoarthritis‐related pain. Archives of Gerontology & Geriatrics 2009;49(3):378‐82. [DOI] [PubMed] [Google Scholar]
Doak 1992 {published data only}
- Doak W, Hosie J, Hossain M, James IGV, Reid I, Miller AJ. A novel combination of ibuprofen and codeine phosphate in the treatment of osteoarthritis: a double‐blind placebo controlled study. Journal of Drug Development 1992;4(4):179‐87. [Google Scholar]
Fancourt 1984 {published data only}
- Fancourt GJ, Flavell Matts SG. A double‐blind comparison of meptazinol versus placebo in chronic rheumatoid arthritis and osteoarthritis. Current Medical Research and Opinion 1984;9(3):184‐91. [DOI] [PubMed] [Google Scholar]
Friedmann 2011b {published data only}
- Friedmann N, Klutzaritz V, Webster L. Long‐term safety of Remoxy® (extended‐release oxycodone) in patients with moderate to severe chronic osteoarthritis or low back pain. Pain Medicine 2011;12(5):755‐60. [DOI] [PubMed] [Google Scholar]
Gazi 2005 {published data only}
- Gazi MCB, Machado Issy A, Kimiko Sakata R. Intra‐articular bupivacaine and morphine for knee osteoarthritis analgesia. Comparative study. Revista Brasileira de Anestesiologia 2005;55(5):491‐9. [DOI] [PubMed] [Google Scholar]
Hale 2007 {published data only}
- Hale M, Tudor IC, Khanna S, Thipphawong J. Efficacy and tolerability of once‐daily OROS hydromorphone and twice‐daily extended‐release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: results of a 6‐week, randomized, open‐label, noninferiority analysis. Clinical Therapeutics 2007;29(5):874‐88. [DOI] [PubMed] [Google Scholar]
James 2010 {published data only}
- James IGV, O'Brien CM, McDonald CJ. A randomized, double‐blind, double‐dummy comparison of the efficacy and tolerability of low‐dose transdermal buprenorphine (BuTrans seven‐day patches) with buprenorphine sublingual tablets (Temgesic) in patients with osteoarthritis pain. Journal of Pain & Symptom Management 2010;40(2):266‐78. [DOI] [PubMed] [Google Scholar]
Katz 2010b {published data only}
- Katz N, Sun S, Johnson F, Stauffer J. ALO‐01 (morphine sulfate and naltrexone hydrochloride) extended‐release capsules in the treatment of chronic pain of osteoarthritis of the hip or knee: pharmacokinetics, efficacy, and safety. Journal of Pain 2010;11(4):303‐11. [DOI] [PubMed] [Google Scholar]
Le Loet 2005 {published data only}
- Loet X, Pavelka K, Richarz U. Transdermal fentanyl for the treatment of pain caused by osteoarthritis of the knee or hip: an open, multicentre study. BMC Musculoskeletal Disorders 2005;6(31):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIlwain 2005 {published data only}
- McIlwain H, Ahdieh H. Safety, tolerability, and effectiveness of oxymorphone extended release for moderate to severe osteoarthritis pain: a one‐year study. American Journal of Therapeutics 2005;12(2):106‐12. [DOI] [PubMed] [Google Scholar]
Mitchell 1984 {published data only}
- Mitchell H, Cunningham TJ, Mathews JD, Muirden KD. Further look at dextropropoxyphene with or without paracetamol in the treatment of arthritis. Medical Journal of Australia 1984;140(4):224‐5. [DOI] [PubMed] [Google Scholar]
Neubauer 1983 {published data only}
- Neubauer M, Bach GL. Short‐term therapy of painful muscular disorders. Results of a multicenter double‐blind test of 2 new suppository preparations with and without codeine. Fortschritte der Medizin 1983;101(21):1009‐13. [PubMed] [Google Scholar]
Rosenthal 2007 {published data only}
- Rosenthal M, Moore P, Groves E, Iwan T, Greenberg Schlosser L, Dziewanowska Z, et al. Sleep improves when patients with chronic OA pain are managed with morning dosing of once a day extend‐release morphine sulfate (AVINZA): findings from a pilot study. Journal of Opioid Management 2007;3(3):145‐53. [DOI] [PubMed] [Google Scholar]
Roth 2000 {published data only}
- Roth SH, Fleischmann RM, Burch FX, Dietz F, Bockow B, Rapoport RJ, et al. Around‐the‐clock, controlled‐release oxycodone therapy for osteoarthritis‐related pain: placebo‐controlled trial and long‐term evaluation. Archives of Internal Medicine 2000;160(6):853‐60. [DOI] [PubMed] [Google Scholar]
Salzman 1983 {published data only}
- Salzman RT, Brobyn RD. Long‐term comparison of suprofen and propoxyphene in patients with osteoarthritis. Pharmacology 1983;27 Suppl 1:55‐64. [DOI] [PubMed] [Google Scholar]
Tassain 2003 {published data only}
- Tassain V, Attal N, Fletcher D, Brasseur L, Degieux P, Chauvin M, et al. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non‐cancer pain. Pain 2003;104(1‐2):389‐400. [DOI] [PubMed] [Google Scholar]
Torres 2001 {published data only}
- Torres Huerta JC, Hernandez Santos JR, Tenopala Villegas S. Transdermal fentanyl in patients with nononcological chronic pain [Spanish]. Revista Mexicana de Anestesiologia 2001;24(2):65‐8. [Google Scholar]
Vignon 1999 {published data only}
- Vignon E, Bannwarth B, Conrozier T, Derobert E, Verdoncq B. Multicenter, double‐blind, clinical trial comparing two tablets bid to one tablet qid of the same acetaminophen‐dextropropoxyphen‐caffeine combination in patients with osteoarthritis [French]. Semaine des Hopitaux 1999;75(13‐14):419‐25. [Google Scholar]
Vlok 1987 {published data only}
- Vlok GJ, Vuren JP. Comparison of a standard ibuprofen treatment regimen with a new ibuprofen/paracetamol/codeine combination in chronic osteo‐arthritis. South African Medical Journal [Suid Afrikaanse Tydskrif vir Geneeskunde] 1987;Suppl:1, 4‐6. [PubMed] [Google Scholar]
Vorsanger 2011 {published data only}
- Vorsanger G, Xiang J, Biondi D, Upmalis D, Delfgaauw J, Allard R, et al. Post hoc analyses of data from a 90‐day clinical trial evaluating the tolerability and efficacy of tapentadol immediate release and oxycodone immediate release for the relief of moderate to severe pain in elderly and nonelderly patients. Pain Research & Management 2011;16(4):245‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 1994 {published data only}
- Wallace WA, Elliott CA, Price VH. A combination of ibuprofen and codeine phosphate provides superior analgesia to ibuprofen alone in osteoarthritis. British Journal of Clinical Research 1994;5:33‐46. [Google Scholar]
Wang 1965 {published data only}
- Wang RI. Analgesic effectiveness of new propoxyphene preparations. Journal of New Drugs 1965;5(3):171‐6. [DOI] [PubMed] [Google Scholar]
Wild 2010 {published data only}
- Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, Lange B, et al. Long‐term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Practice 2010;10(5):416‐27. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kroner 1991 {published data only}
- Kroner K, Hansen TB, Harving S, Hvass I, Madsen F, Nafei A, et al. Individually dosed codeine plus paracetamol versus paracetamol in long‐term treatment of chronic pain due to arthrosis of the hip ‐ a randomised, double blind, multicenter study. Acta Orthopaedica Scandinavica, Supplement 1991;62(246):43. [Google Scholar]
Additional references
Altman 1986
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis and Rheumatism 1986;29:1039‐49. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis and Cartilage 1996;4(4):217‐43. [DOI] [PubMed] [Google Scholar]
Avouac 2007
- Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta‐analysis of randomized controlled trials. Osteoarthritis and Cartilage 2007;15(8):957‐65. [DOI] [PubMed] [Google Scholar]
Bellamy 1995
- Bellamy N. Outcome measurement in osteoarthritis clinical trials. Journal of Rheumatology Supplement 1995;43:49‐51. [PubMed] [Google Scholar]
British Pain Society 2010
- British Pain Society. Opioids for persistent pain: good practice, 2010. www.britishpainsociety.org/book_opioid_main.pdf (accessed 16 August 2014).
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cepeda 2006
- Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005522.pub2] [DOI] [PubMed] [Google Scholar]
Chinn 2000
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐31. [DOI] [PubMed] [Google Scholar]
Clegg 2006
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. New England Journal of Medicine 2006;354(8):795‐808. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
da Costa 2012a
- Costa BR, Rutjes AWS, Johnston BC, Reichenbach S, Nüesch E, Tonia T, et al. Methods to convert continuous outcomes into odds ratios of treatment response and numbers needed to treat: meta‐epidemiological study. International Journal of Epidemiology 2012;41(5):1445‐59. [DOI] [PubMed] [Google Scholar]
da Costa 2012b
- Costa BR, Nüesch E, Reichenbach S, Jüni P, Rutjes AWS. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007323.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 1998
- Eccles M, Freemantle N, Mason J. North of England Evidence Based Guidelines Development Project: summary guideline for non‐steroidal anti‐inflammatory drugs versus basic analgesics in treating of pain of degenerative arthritis. BMJ 1998;317:526‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Smith GD. Principles of and procedures for systematic reviews. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. London: BMJ Books, 2001:23‐42. [Google Scholar]
Egger 2003
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment (Winchester, England) 2003;7(1):1‐76. [PubMed] [Google Scholar]
Eriksen 2006
- Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non‐cancer pain: an epidemiological study. Pain 2006;125(1‐2):172‐9. [DOI] [PubMed] [Google Scholar]
Furlan 2006
- Furlan AD, Sandoval JA, Mailis‐Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. Canadian Medical Association Journal 2006;1(11):1589‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehling 2011
- Gehling M, Hermann B, Tryba M. Meta‐analysis of dropout rates in randomized controlled clinical trials: opioid analgesia for osteoarthritis pain. Schmerz 2011;25(3):296‐305. [DOI] [PubMed] [Google Scholar]
Gossop 1990
- Gossop M. The development of a Short Opiate Withdrawal Scale (SOWS). Addictive Behaviors 1990;15(5):487‐90. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gøtzsche 2007
- Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta‐analyses that use standardized mean differences. JAMA 2007;298(4):430‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hochberg 2012
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care and Research 2012;64(4):465‐74. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2006
- Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Practice & Research. Clinical Rheumatology 2006;20(4):721‐40. [DOI] [PubMed] [Google Scholar]
Kalso 2004
- Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain 2004;112(3):372‐80. [DOI] [PubMed] [Google Scholar]
Loeser 2001
- Loeser JD, Butler SH, Chapman CR, Turk DC. Bonica's Management of Pain. 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2001. [Google Scholar]
Maier 2002
- Maier C, Hildebrandt J, Klinger R, Henrich‐Eberl C, Lindena G, MONTAS Study Group. Morphine responsiveness, efficacy and tolerability in patients with chronic non‐tumor associated pain ‐ results of a double‐blind placebo‐controlled trial (MONTAS). Pain 2002;97(3):223‐33. [DOI] [PubMed] [Google Scholar]
NICE 2008
- NICE clinical guideline 59. Osteoarthritis ‐ the care and management of osteoarthritis in adults. www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf (accessed 16 August 2014).
Nüesch 2009
- Nüesch E, Trelle S, Reichenbach S, Rutjes AWS, Bürgi E, Scherer M, et al. The effects of the exclusion of patients from the analysis in randomised controlled trials: meta‐epidemiological study. BMJ 2009;339:b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pham 2004
- Pham T, Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT‐OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis and Cartilage 2004;12(5):389‐99. [DOI] [PubMed] [Google Scholar]
Reichenbach 2007
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, et al. Meta‐analysis: chondroitin for osteoarthritis of the knee or hip. Annals of Internal Medicine 2007;146(8):580‐90. [DOI] [PubMed] [Google Scholar]
Reichenbach 2010
- Reichenbach S, Rutjes AW, Nüesch E, Trelle S, Jüni P. Joint lavage for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007320.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rutjes 2009a
- Rutjes AW, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2009b
- Rutjes AW, Nuesch E, Reichenbach S, Juni P. S‐Adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007321.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2010
- Rutjes AW, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2] [DOI] [PubMed] [Google Scholar]
Rutjes 2012
- Rutjes AW, Jüni P, Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(3):180‐91. [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schug 2006
- Schug SA, Gandham N. Opioids: clinical use. In: McMahon S, Klotzenburg M editor(s). Wall and Melzack's Textbook of Pain. 5th Edition. Oxford: Elsevier Limited, 2006:443‐57. [Google Scholar]
Shang 2005
- Shang A, Huwiler‐Muntener K, Nartey L, Juni P, Dorig S, Sterne JA, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo‐controlled trials of homoeopathy and allopathy. Lancet 2005;366(9487):726‐32. [DOI] [PubMed] [Google Scholar]
Stein 1996
- Stein C, Pfluger M, Yassouridis A, Hoelzl J, Lehrberger K, Welte C, et al. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. Journal of Clinical Investigation 1996;98:793‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Von Korff 2004
- Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind?. Pain 2004;109(3):207‐9. [DOI] [PubMed] [Google Scholar]
Wandel 2010
- Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta‐analysis. BMJ 2010;341(16):c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2008
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence‐based, expert consensus guidelines. Osteoarthritis and Cartilage 2008;16(2):137‐62. [DOI] [PubMed] [Google Scholar]


