Abstract
Currently, Nuclear Medicine has a clearly defined role in clinical practice due to its usefulness in many medical disciplines. It provides relevant diagnostic and therapeutic options leading to patients' healthcare and quality of life improvement. During the first two decades of the 21stt century, the number of Nuclear Medicine procedures increased considerably.
Clinical and research advances in Nuclear Medicine and Molecular Imaging have been based on developments in radiopharmaceuticals and equipment, namely, the introduction of multimodality imaging. In addition, new therapeutic applications of radiopharmaceuticals, mainly in oncology, are underway.
This review will focus on radiopharmaceuticals for positron emission tomography (PET), in particular, those labeled with Fluorine-18 and Gallium-68. Multimodality as a key player in clinical practice led to the development of new detector technology and combined efforts to improve resolution. The concept of dual probe (a single molecule labeled with a radionuclide for single photon emission computed tomography)/positron emission tomography and a light emitter for optical imaging) is gaining increasing acceptance, especially in minimally invasive radioguided surgery. The expansion of theranostics, using the same molecule for diagnosis (γ or positron emitter) and therapy (β minus or α emitter) is reshaping personalized medicine.
Upcoming research and development efforts will lead to an even wider array of indications for Nuclear Medicine both in diagnosis and treatment.
Introduction
Nuclear Medicine has established itself in clinical practice in a large variety of indications. Recent innovation in radiopharmaceuticals and equipment has led to a significant increase in diagnostic and therapeutic applications. According to the World Nuclear Association 1 more than 40 million nuclear medicine procedures are performed each year worldwide. In developed countries, the frequency of diagnostic nuclear medicine is 1.9% per year, and the frequency of therapy with radioisotopes is about 1/10 of this. 99mTc is the most common radioisotope used in diagnosis, representing about 80% of all nuclear medicine procedures.
In the 21st century, several radiopharmaceuticals have been developed. In the last two decades, the number of PET radiopharmaceuticals has increased almost exponentially. However, 18F-fludeoxyglucose (FDG) is still the radiopharmaceutical with the widest applicability. In this paper, we focus on positron emission tomography (PET) radiopharmaceuticals due to their increased availability and importance in current clinical practice. The use of 18F-FDG was initially approved by the Food and Drug Administration (FDA) in 1994 only for epileptic foci identification. In 2000, applications in oncology (mapping of tumor metabolism) were added. In 2005, the FDA approved its use for the differential diagnosis of dementias, mainly between Alzheimer’s disease and frontotemporal dementia. 18F-FDG was approved by the European Medicines Agency (EMA) in 2012 for clinical use in oncology and neuropsychiatry predominantly. 18F-FDG represents the most important PET radiopharmaceutical used nowadays, due to its wide range of clinical applications, ranging from oncology to neuropsychiatry, and also including cardiology, inflammatory/infectious diseases, orthopedics and rheumatology as other areas of clinical interest.
Nuclear Medicine has been widening its clinical applications due to technology innovation in hardware and software. In this century, the wider availability of PET scanners is demonstrated in a report by www.globalinforeaserch.com (https://www.globalinforesearch.com/global- positron-emission-tomography ). It reports a global revenue of PET of nearly 782 million USD in 2019, expecting to reach 819 million USD in 2025. Meanwhile, in current state-of-the-art scanners, spatial resolution has already improved by a factor of 10 and sensitivity by a factor of 40 in comparison to the scanners from the early 1970’s. Multimodality configurations emerged (e.g. single photon emission CT/CT (SPECT/CT), PET/CT, PET/MRI and now it is possible to complete whole body PET/CT scans in less than 10 min. 2
The concept of dual probe (the same molecule labeled with a γ or positron emitter for SPECT/PET and a light emitter for optical imaging) is gaining wider acceptability for guidance of high precision and less invasive robotic surgery. Sentinel lymph node detection and improved lymphadenectomy procedures are prominent examples of dual probe applications in clinical practice. 3,4
At present, it is possible to label the pharmaceutical compound with either a γ or positron emitting radionuclide for diagnostic applications (SPECT or PET), or a β-minus or α radionuclide emitter for therapeutic purposes. This concept is the fundamental basis of theranostics. The 21st century has been seeing the development of new radiopharmaceuticals for theranostics to reshape personalized medicine with expanding indications mainly in neuroendocrine tumor and prostate cancer patients. Pharmaceutical companies are investing in radiopharmaceuticals with therapeutic purposes, which may reach an estimated revenue of 13 billion USD in 2025. 5
Finally, artificial intelligence, using machine learning algorithms based on features extracted from images, is also emerging in the Nuclear Medicine and promising developments to use functional imaging as a biomarker are expected. 6
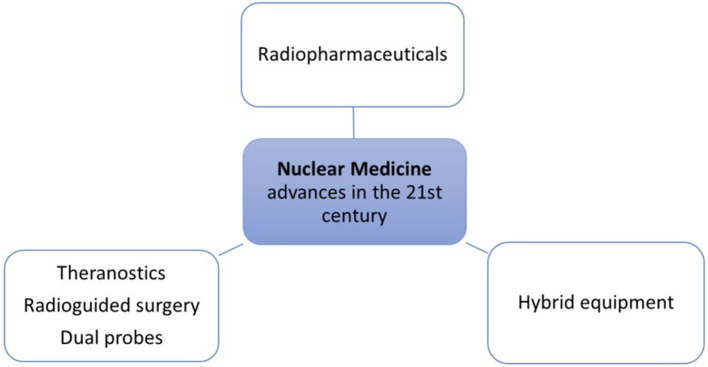
For the sake of simplification, this review of the 21st century advances in Nuclear Medicine and Molecular Imaging is subdivided into three sections: (1) Advances in PET radiopharmaceuticals; (2) Developments in equipment; (3) Radiopharmaceutical applications in therapy and theranostics (Figure 1).
Figure 1.
Main fields of advances in Nuclear Medicine in the 21st century.
Advances in PET radiopharmaceuticals
PET radiopharmaceuticals enable the functional study of several pathologies within the majority of medical specialties (Table 1 and Figure 2). During the 20th century, 99mTc labeled radiopharmaceuticals were the cornerstone of Nuclear Medicine clinical practice and examinations. In the 21st century, PET has proved its important clinical role through the use of established Flourine-18 labelled radiopharmaceuticals. More recently, Gallium-68 labelled radiopharmaceuticals are increasingly used due to the availability of DOTA chelators, Germanium-68/Gallium-68 generators, and their pharmacological properties for theranostics.
Table 1.
Radiopharmaceuticals used in clinical practice in different medical fields
| Medical Field | Radiopharmaceuticals | FDA approval | EMA approval |
|---|---|---|---|
| Oncology |
18F-FDG
(Fludeoxyglucose) |
2000 – cancer applications | 2012 |
|
68Ga/18F-PSMA
(prostate specific membrane antigen) |
2012 | ||
| 18F-Fluciclovine | 2016 | 2017 | |
|
68Ga-DOTA-conjugated peptides
(DOTA-NOC, DOTA-TOC and DOTA-TATE) |
2016 | ||
|
18F-FDOPA
(Dihydroxyphenylalanine) |
NA | 2016 | |
|
Hypoxia tracers
18F-FMISO (fluoromisonidazole), 18F-FAZA (fluoroazomycin-arabinozide) and 7 Cu-ATSM (diacetyl-bis-methylthiosemicarbazone) |
FMISO-1986 ATSM-1997 FAZA −1999 |
||
|
18F-FLT
(Fluorothymidine) |
2009 | NA | |
|
68Ga-FAPI
(fibroblast activation protein inhibitors) |
2018 | ||
| 18F-FES (Fluoroestradiol) | NA | ||
| 89Zr-trastuzumab | NA | ||
| Iodine-124 | NA | ||
|
Neurology
And Psychiatry |
18F-FDG
(Fludeoxyglucose) |
1994 –
epilepsy application 2005 – dementia application |
2012 |
|
β-Amyloid tracers
(Florbetaben, Florbetapir and Flutemetamol) |
2015 | ||
|
Perfusion tracers
(Rubidium-82 chloride, 13N-Ammonia, 15O-Water and 18F-Flurpiridaz) |
Rb-1989 Ammonia-2000 Flurpiridaz-2020 |
Rb-2009 | |
|
Amino-acids tracers
11C-MET (methionine) and 18F-FET (Fluoro-ethyl-Tyrosine) |
NA | ||
|
Tau-tracers
(18F-Flortaucipir, 18F-THK5317, and 11C-PBB3) |
NA | ||
|
18F-DOPA
(Dihydroxyphenylalanine) |
NA | 2016 | |
| Cardiology |
18F-FDG
(Fludeoxyglucose) |
1994 –
epilepsy application 2000 – cancer applications 2005 – dementia application |
2012 |
| Perfusion tracers | Rb-1989 Ammonia-2000 Flurpiridaz-2020 |
Rb-2009 | |
| Hypoxia tracers | FMISO-1986 ATSM-1997 FAZA-1999 |
||
| Musculoskeletal |
18F-FDG
(Fludeoxyglucose) |
1994 –
epilepsy application 2000 – cancer applications 2005 – dementia application |
2012 |
|
18F-NaF
(Sodium-fluoride) |
1972 | 2015 | |
NA, not approved yet.
Radiopharmaceuticals without FDA/EMA approval are displayed in gray.
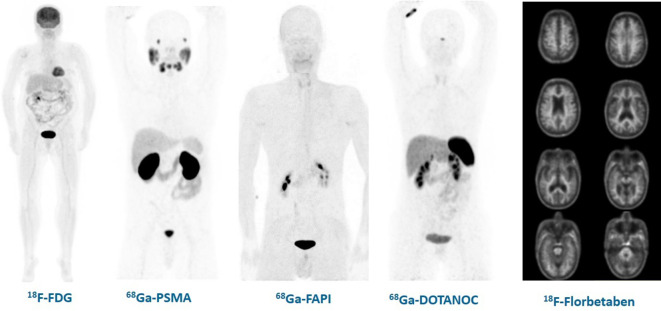
Figure 2.
Images of the physiologic distribution of PET radiopharmaceuticals used in clinical practice: 18F-FDG, 68Ga-PSMA, 68Ga-FAPI, 68Ga-DOTANOC and 18F-Florbetaben. 18F-FDG, fludeoxyglucose; FAPI, fibroblast-activation-protein inhibitor; PET, positron emmision tomogrpahy.
In the following section, PET radiopharmaceuticals bearing a major impact in oncology, neuropsychiatry, cardiology and musculoskeletal medical fields will be discussed.
Oncology
18F-FDG (Fludeoxyglucose) is a glucose analog, which enters the cell via glucose transporters and then is subsequently phosphorylated by a hexokinase. 18F-FDG-6-phosphate is not a substrate for glucose-6-phosphate isomerase and does not undergo further metabolism. Therefore, 18F-FDG is trapped within the cells as 18F-FDG-6-phosphate. It quickly became the most important PET radiopharmaceutical of the 21st century. 18F-FDG PET/CT is now recommended 8 in a wide variety of both solid and hematological cancers for staging, restaging and assessment of response to therapies. In addition, it is useful in the detection of primary tumors in patients with paraneoplastic syndromes and occult cancers, and it may help to distinguish between benign and malignant lesions. In some cases, it will be able to differentiate tumor recurrence/persistence from post-treatment fibrosis/necrosis, for example in colorectal and head and neck cancers following post chemoradiotherapy. 9,10 Last but not the least, 18F-FDG is more often used for metabolic-based radiotherapy planning of a wide variety of primary and/or secondary tumors as well as guide location for biopsies.
PSMA (prostate specific membrane antigen) is a transmembrane protein expressed in prostatic tissues. Significant advances have occurred in prostate cancer staging and monitoring, with the development of PSMA ligands for PET in 2012. 11 PSMA expression is increased in both benign and malignant lesions. 12 According to the “Joint Guideline of the European Association of Nuclear Medicine and Molecular Imaging and the American Society of Nuclear Medicine and Molecular Imaging” 13 and the “Consensus on molecular imaging and theranostics in prostate cancer,” 14 PSMA-ligand PET is recommended in the following situations: initial staging of high-risk disease, targeted biopsy after previous negative biopsy, biochemical recurrence, and monitoring of systemic treatment in metastatic prostate cancer.
Currently, it is accepted that PSMA is expressed by a variety of malignant and benign non-prostate entities, due to its presence on the neovasculature of endothelial cells. 15 Although many different PSMA-ligands are available, fluorinated compounds seem to offer advantages, such as the 18F-PSMA-1007 that shows a unique distribution, with hepatobiliary rather than urinary excretion. 16,17
18F-Fluciclovine (Axumin®) is a leucine analog (FACBC amino-acid) with high cancer uptake due to increased protein turnover and nucleotide synthesis. It was approved by the FDA in 2016 and by the EMA in 2017. According to the “European Association of Nuclear Medicine (EANM) Guideline/ Society of Nuclear Medicine and Molecular Imaging (SNMMI) Procedure Standard on 18F-Fluciclovine PET/CT for Prostate Cancer Imaging” 18 its clinical indications are similar to those of PSMA-ligands PET/CT. However, it seems to have low specificity (32%–40%) for recurrent disease and low sensitivity (21%–39%) for nodal disease if PSA values are less than 1 ng ml−1. 18
DOTA-conjugated peptides/ somatostatin analogs (SSA) (i.e. DOTA-NOC, DOTA-TOC and DOTA-TATE) have high affinity for somatostatin receptors, which are expressed by most neuroendocrine tumors. 19 Studies with somatostatin analogs started in the early 21st century with 111In-Octreotide (e.g. OctreoScan®) for scintigraphy, but the use of DOTA-conjugated peptides for PET starting in 2016 contributed to the popularity and widespread use of this examination. Despite all these radiopharmaceuticals bind to somatostatin receptor 2, they have different affinity profiles for other somatostatin receptor subtypes. 19 The “Guidelines of the European Association of Nuclear Medicine” 19 and “2016 ENETS Consensus Guidelines for the Diagnosis and Treatment of Neuroendocrine Tumours” (https://www.enets.org/basics.119.html) recommend using 20 Ga-DOTA-conjugated peptides PET in well (Ki-67 <2%) and moderately differentiated (Ki-67 <20%) neuroendocrine tumors to detect the primary tumor site, to stage and restage, as well as to assess prognosis and select patients for somatostatin receptor radionuclide therapy with 177Lu-DOTA-peptides SSA.
There are some other solid and hematological malignancies capable of expressing somatostatin receptors. 21 There is also new literature in molecular imaging with 20 Ga-DOTA-conjugated peptides in cardiovascular research (e.g. cardiac sarcoidosis – NCT01729169, 22 residual post-infarction myocardial inflammation 23 and imaging arterial wall inflammation in atherosclerosis – VISION study, NCT02021188. 24
18F-DOPA is a marker of the dopa-decarboxylase activity within the pre-synaptic vesicles, and therefore capable of mapping endogenous catecholamine availability. According to “EANM Practice Guideline/SNMMI Procedure Standard 2019 for radionuclide imaging of pheochromocytoma and paraganglioma,” 25 18F-FDOPA is indicated to detect small and sporadic pheochromocytomas, as well as, paragangliomas, including head and neck glomus tumors. 25
Hypoxia tracers, most commonly, 18F-FMISO (18F-fluoromisonidazole), 18F-FAZA (18F-fluoroazomycin-arabinozide) and 7 Cu-ATSM (64Cu-diacetyl-bis-methylthiosemicarbazone) 26 passively diffuse through the cell membrane due to their high lipophilicity. Hypoxia imaging has been investigated in several solid tumors (gliomas, head and neck carcinoma, non-small cell lung cancer, breast cancer and renal carcinoma) and seems to be useful in radiotherapy metabolic planning. 26,27
18F-FLT (Fluorothymidine) is monophosphorylated by thymidine kinase 1 (increased during the S phase of the cell division cycle), trapped intracellularly and therefore reflects tumor cell proliferation. FLT has been reported as a good predictor of early response to chemo- and radiotherapy, both in solid and hematological neoplasms. FLT uptake correlates with progression-free survival and disease-free survival. 28,29
68Ga-FAPI (Fibroblast activation protein inhibitor) is a compound based on a fibroblast activation protein (FAP), a specific enzyme inhibitor that targets cancer associated fibroblasts. The first clinical studies on 20 Ga-FAPI PET were published in 2018. 30 Advantages of this tracer include is its low background signal, nearly complete cellular internalization, quick renal clearance and no significant brain, heart or liver uptake. 30 FAPI is not approved for clinical practice so far. However, breast, esophagus, lung, pancreatic, head and neck and colorectal cancers have a remarkably high uptake, suggesting that FAPI might be useful in these settings. As FAPI contains the DOTA-chelator, a theranostic approach may be possible. 30,31 A broader application of FAPI imaging in non‐oncological diseases may also be possible. 30
18F-FES (Fluoroestradiol) targets estrogen receptors and is useful for breast cancer detection (e.g. staging and restaging) and molecular characterization. 32,33
89Zr-trastuzumab targets human epidermal growth factor receptor two and supports clinical decisions about breast cancer patients, even when HER2 status cannot be determined by standard work-up. 34
Iodine-124 is useful in cases of differentiated thyroid cancer and biochemical recurrence with no detectable site of neoplastic tissue by conventional diagnostic techniques. 35 18F-FDG also plays a role in differentiated thyroid cancer recurrence and it is even more useful in poorly differentiated thyroid carcinoma. 36,37 18F-FDG, 18F-FDOPA and 20 Ga-DOTA-conjugated peptides are useful in medullary thyroid carcinoma management. 38
Neurology and psychiatry
During the 21st century, significant developments occurred in the management of movement disorders and other central nervous system diseases.
One of the radiopharmaceuticals with higher impact on neurology practice is 123I-Ioflupane (DaTscan™), 39 a ligand for the pre-synaptic dopamine transporter (DAT). The reduction of its uptake in the striatum is directly correlated to pre-synaptic dopaminergic degeneration. Its main indication is therefore the “in vivo” confirmation of the characteristic nigrostriatal degeneration in patients with idiopathic Parkinson’s disease and its distinction from essential tremor, medication-induced Parkinsonism and confirmation of Parkinsonian plus syndromes.
The corresponding pre-synaptic PET radioligand is 18F-FDOPA (e.g. 18F-PE2I). 40 However, this is a marker of the intraneuronal vesicular transport and the dopa-decarboxylase activity. Despite its different mechanism of action, 18F-FDOPA and 123I-Ioflupane may have the same indications according to the “EANM Practice Guideline/SNMMI Procedure Standard for Dopaminergic Imaging in Parkinsonian Syndromes 1.0.” 41
To map the distribution and availability of D2 post-synaptic dopamine receptors, 123I-IBZM (Iodobenzamide) for SPECT and 11C-raclopride, 18F-fallypride or 18F-desmethoxyfallypride for PET have been used. At present, their main clinical indication is differential diagnosis between idiopathic Parkinson’s disease and other parkinsonian plus syndromes (i.e. supranuclear palsy and multisystem atrophy). Whilst in the Parkinsonian plus syndromes the D2 post-synaptic receptors availability is markedly reduced, in idiopathic Parkinson’s disease it is normal or upregulated.
18F-FDG is useful in the diagnosis and differential diagnosis of dementias. Its brain distribution in Alzheimer’s disease is different from other types of dementia, in particular Lewy Body dementia, frontotemporal lobar degeneration, vascular dementia and from cases of pseudodementia. 42
β-Amyloid tracers are used in the identification of one of the neuropathological hallmarks of Alzheimer’s disease, i.e.abnormal deposition of β-amyloid in the brain. The first radiopharmaceutical to map β-amyloid in the brain was 18F-FDDNP. 43 Later, the introduction of 11C–labeled Pittsburgh compound B (11C-PIB) 44 in 2004 enabled detection of brain β-amyloid deposition in vivo. In 2015, the FDA and EMA approved three fluoride-18-labelled amyloid PET radiopharmaceuticals for clinical use: florbetaben (Neuraceq®), florbetapir (Amyvid®) and flutemetamol (Vizamyl®). The “SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0” 45 suggests amyloid PET in cases of persistent or progressive and unexplained mild cognitive impairment, when core clinical criteria for possible Alzheimer’s disease are satisfied but there is a doubtful clinical presentation or dementia is progressive and early onset. Both positive and negative results significantly influence diagnosis and treatment of patients. 46 The potential use of amyloid PET tracer accumulation in cerebral white matter as a marker of myelin is also being investigated. 47
Tau-tracers for PET imaging include several first- and second-generation compounds used to detect mild cognitive impairment patients that are actively converting to Alzheimer disease, thus improving its early diagnosis. Tau-tracers have high correlation with neurodegenerative markers and clinical severity of dementia. 48,49
Amino-acids tracers include 11C-MET (methionine), 18F-FET (tyrosine), 18F-FACBC (fluciclovine) and 18F-FDOPA. Amino-acids are essential nutrients, involved in metabolism, cell growth, protein synthesis and gene expression. 50 Besides glucose, certain amino-acids are also energy sources and anabolic precursors for tumors. Amino-acid PET tracers can overcome limitations of 18F-FDG because there is low amino-acid uptake in normal brain and in inflammatory cells, enabling identification of primary and metastatic brain tumors. According to the “Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and FDG,” 51 amino-acids tracers are also useful for therapy monitoring, tumor recurrence detection (differentiating true recurrent disease from pseudoprogression and radionecrosis), biopsy guidance, as well as surgery and radiotherapy planning. 51,52
Perfusion tracers may be used to evaluate brain perfusion in the context of cerebral ischemia, through the determination of cerebral blood flow and cerebral metabolic rate of oxygen consumption. 53
Cardiology
18F-FDG is helpful to evaluate myocardial viability (in case of myocardial infarction, stunned and hibernating myocardium), to detect endocarditis, infection of cardiac devices, metastatic or infectious foci and endothelial inflammation due to atherosclerosis plaque formation.
Perfusion tracers include Thallium-201 chloride in use since the 70’s and 99mTc labelled radiopharmaceuticals (Sestamibi and Tetrofosmin) in use since the 90’s for SPECT myocardial perfusion. Recently (Table 1), PET perfusion radiopharmaceuticals became available including Rubidium-82 chloride, 13N-Ammonia, 15O-Water and 18F-Flurpiridaz. 54,55 According to “ASNC imaging guidelines/SNMMI procedure standard for PET nuclear cardiology procedures,” 55 non-invasive imaging of myocardial perfusion is useful for diagnosis and management of patients with known or suspected coronary artery disease, enabling absolute quantitative PET measurements of myocardial blood flow (millilitres per gram per minute). Moreover, it allows identification of early atherosclerosis or microvascular dysfunction and assessment of balanced reduction of myocardial blood flow in the major coronary arteries territories. 54,55
Musculoskeletal
18F-FDG is useful when arthritis, synovitis and vasculitis (e.g. polymyalgia rheumatic and large vessel vasculitis) are suspected, mainly to locate inflammatory active sites and to monitor response to therapy. 56
18F-NaF is a bone seeking agent that marks bone remodelling activity. Its main clinical indications are identification and anatomical extension of bone metastases and primary bone malignancies. Additionally, benign bone disease and orthopaedic lesions can be also evaluated. 57 The clinical use of this PET radiopharmaceutical may increase due to the ongoing global Molybdenum-99 shortage and possible reduction of bone scan procedures. 58
Developments in equipment
Advances in PET systems
Commercial preclinical PET systems can provide unprecedented high spatial resolution reaching 0.55 mm full width at half-maximum (FWHM) values. Common preclinical PET systems are based on photomultiplier tubes (analog or digital) attached to a single or multiple scintillation crystal. A different approach based on resistive plate chamber has been proposed with a preclinical prototype reaching a 0.4 mm FWHM spatial resolution. 59 Current commercial preclinical PET systems allow combined imaging with CT, MRI or SPECT.
In terms of commercial clinical PET-imaging systems, there is a range of different PET/CT systems available from different vendors with the top-of-the-line systems now being equipped with digital detector technology. In some of them, the spatial resolution is slightly better than 4 mm. 60,61 Digital systems are now using silicon photomultipliers (SiPM) attached to the crystal instead of analog photomultiplier tubes (PMT). The majority of PET systems use lutetium oxyorthosilicate (LSO) or lutetium yttrium oxyorthosilicate (LYSO) as scintillator materials. 62 They provide grossly similar compromise between energy resolution, stopping power and dead time.
Clinical PET/MRI is less available than clinical PET/CT, but significant developments have recently occurred. Currently, there are two clinical human whole-body PET/MRI commercially available: the Biograph mMR (Siemens Healthineers) and the Signa PET/MRI (GE Healthcare). Considering the advantages of MRI relatively to CT, PET/MRI systems might have potentially higher diagnostic accuracy than PET/CT especially in organs such as liver, prostate and head neck region among others. 63,64 Furthermore, problems with co-registration of PET and MRI images performed at different moments and in different equipment settings have practically disappeared. Initial issues related to attenuation correction based on MRI are solved for the majority of tissues (bone being the exception), achieving SUV deviations less than 10%, similar to PET/CT. 7,65
There are already clinical systems available which combine three modalities (e.g. AnyScan SPECT–CT–PET from Mediso Medical Imaging Systems). These may be a suitable and attractive option for small nuclear medicine departments.
Time of flight
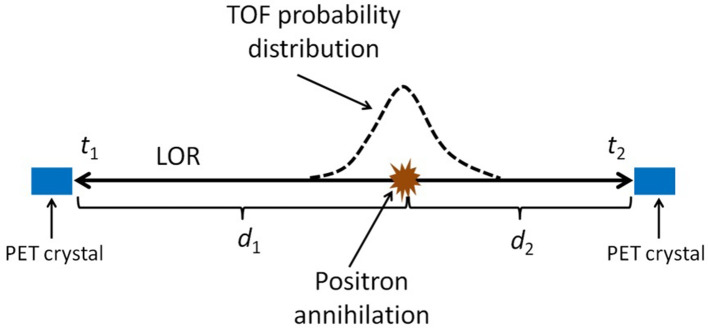
Time of flight (TOF) technology has improved significantly over the last decade thanks to the development of SiPM and introduction of scintillators with better performance, like LSO and LYSO. 66 Recent clinical PET systems have very low coincidence time resolution (CTR), e.g. currently stated as low as 210 ps FWHM. 61 Experimental set-ups have achieved CTR inferior to 100 ps FWHM. 66 TOF information spatially narrows down the event location. A positron emission precise location of around 1.5 cm is achieved by these experimental setups based only on the time difference between the detection of both photons emitted from the positron annihilation (Figure 3). In the future, with the continuous CTR reduction, PET reconstruction algorithms may no longer be necessary, since the line of response (LOR) identification and the time difference of the detection will give an accurate positron annihilation location. So far, TOF technology has improved the reconstructed image signal to noise ratio and reconstruction algorithm convergence speed.
Figure 3.
Illustration of the TOF principle. Annihilation of the positron originates two 511 kev photon that are detected by PET detectors. Taking the time difference between detection and speed of light, annihilation distance difference to both detectors can be easily computed ( , where is the speed of light and and are the times of the detection). If the time measurements had no error, the exact position of the annihilation in the LOR could be computed, assuming the two photons describe exactly a 180° angle. Uncertainty of the time measurements is modulated by the TOF probability distribution. LOR, line of response; PET, positron emmision tomography; TOF,
Total-body PET
Recently, the first total-body PET, the EXPLORER PET/CT, 67,68 has been introduced. It has an axial field of view of 194 cm, allowing it to cover the entire adult human body in a single acquisition in more than 99% of the cases. 68 Since it covers the entire body in a single acquisition, it is possible to perform total body dynamic acquisitions simultaneously, facilitating application of kinetic models to estimate the transfer rates between compartments. Therefore, total body radiopharmaceutical distribution studies are now possible. Despite its developmental stage, it already reaches a spatial resolution of approximately 2.9 mm, 68 which is an improvement compared to the commercially available state-of-the-art PET/CT scanners. Another advantage is the higher sensitivity, allowing a reduction in acquisition time by 1–2 min, even with lower injected activity than in current clinical practice. This improves patient comfort, reduces movement artifacts and radiation exposure. Financial constraints and space requirements are barriers towards widespread use of these scanners.
Advances in SPECT systems
Recently, commercial preclinical SPECT systems have reached very high spatial resolution, partly even higher than PET. For instance, the U-SPECT from the MILabs has a 0.12 mm FWHM (ex vivo examinations) and 0.25 mm (in vivo examinations).
Solid-state detectors based on cadmium zinc telluride (CZT) have better energy and spatial resolutions compared to conventional systems (crystals coupled to PMT). These new detectors have already been incorporated into SPECT systems. An example is the NM/CT 870 CZT SPECT/CT (GE Healthcare) with planar arrays of detectors. Other example are the VERITON and VERITON-CT (Spectrum Dynamics) with 12 independent detector arms providing 360° coverage to perform whole-body SPECT scans. The remarkably improved CZT’s energy resolution is more suitable for dual radionuclide acquisition studies than sodium iodide scintillators used in conventional γ cameras. 20 It is also often used for dedicated cardiac SPECT cameras.
An approach similar to the VERITON, but not based on CZT technology, is proposed by MILabs G-SPECT with a 3 mm FWHM spatial resolution. 69 This was developed for large animals and may be suitable for human brain SPECT.
In this paper, we focused mainly on whole-body imaging solutions, but there are several clinical SPECT and PET systems designed for specific applications. 20
Quantification
PET/CT is a quantitative technique and, frequently, semi-quantitative techniques rather than absolute quantification (real radiopharmaceutical concentration) have been used. The standardized uptake value, mostly used in PET/CT, has now been implemented to SPECT/CT. Ratios between a region of interest and a reference region uptake have also been frequently used. 70,71 In 2006, the leadership of the EANM launched EANM Research Ltd (EARL©http://earl.eanm.org/cms/website.php) to promote multicentre nuclear medicine and research. The main aim was to harmonize quantification among different equipment in a wide range of tumor types, with focus on therapy assessment using either the European Organization for Research and Treatment of Cancer (EORTC) criteria, PET Evaluation Response Criteria in Solid Tumors (PERCIST) and Deauville score for lymphoma. The accreditation program is available for 18F-FDG PET/CT, 72 Zr-PET/CT and PET/MRI. 73,74 Nevertheless, some authors have evaluated the impact these reconstructions algorithms may have on image interpretation and advised that new reconstruction algorithms should not be used alone for response assessment, but rather complemented with a second data set reconstruction with another algorithm (e.g. ordered subset expectation maximization (OSEM) reconstruction for Deauville score). 75
Dynamic acquisitions allow kinetic parameters quantification, such as transference rates between tissue compartments. They have been used only in a research setting due to practical reasons, because long acquisition time and arterial cannulation are needed. In some specific applications, arterial input function obtained from arterial cannulation may be replaced by the concentration over time in a reference region, 76 usually the heart, a vessel or a region without specific uptake. This has been shown to be comparable to quantification with arterial sampling.
Radiopharmaceutical applications in therapy and theranostics
Dual probes and optical imaging
Radio-guided surgery actively encompasses non-invasive molecular imaging and minimally invasive surgery. Handheld detectors are mobile devices used intraoperatively to detect radiopharmaceutical distribution during surgery after diagnostic localization. These are mostly γ handheld detectors, depending on the type of radioisotopes administered. Initially used to locate parathyroid adenomas early in 21st century, at present, the most common image-guided surgery applications are for sentinel node procedures 3 using 99mTc-labelled radio-nanocolloids (melanoma, breast, 77 cervical, 78 vulvar, penile and head and neck cancers 79 and radioguided occult lesion localization procedures using 99mTc-labelled macroaggregates (breast, lung and thyroid cancers). Additionally, clinical reports on metabolic targeted surgical resections using different radiopharmaceuticals (e.g. 18F-FDG 68Ga-PSMA and 68Ga-DOTATATE) for other tumors have been published. 3,80–82
During the last decade, there has been a significant development of bimodal imaging probes applicable in PET as well as in optical imaging (OI). 4,83 The main advantages are related to the combination of higher tissue penetration of γ radiation resulting from positron emitter radionuclides (allowing non-invasive quantitative imaging and tumor detection) and light emitted by the fluorescent probe for optical imaging during surgery, in particular, robotic surgery.
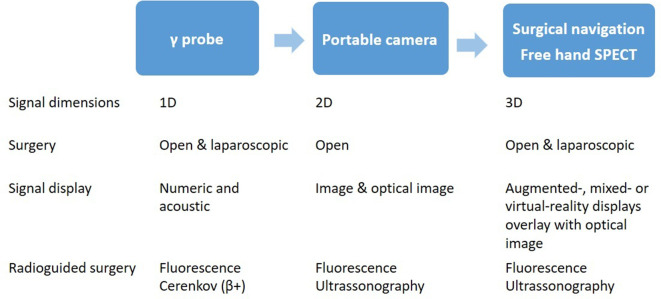
Different types of optical imaging can be used with hybrid PET/OI procedures 83 : (i) fluorescent protein molecules, (ii) Cherenkov luminescence imaging, (iii) near-infrared light and (iv) quantum dots from Cd/Te or Cd/Se materials. Substances emitting light within the near-infrared and infrared spectrum (700–900 nm) are most useful, as light of these wavelengths exhibit “in vivo” the highest tissue permeability from several millimetres to slightly more than 1 cm. 83 This feature offers the possibility to use hybrid probes during both open and laparoscopic surgery. Recently, it has been shown that an integration of hybrid detection probes with surgical navigation systems is able to produce three-dimensional fluorescence tomography reconstructions (e.g. freehand fluorescence scans) 83,84 (Figure 4). It is likely that in the near future the increasing trend to combine radioguidance with other imaging signatures (fluorescence, Cerenkov, optical coherence tomography, and ultrasonography) and software-based guidance solutions (surgical navigation and augmented mixed/virtual reality displays) will lead to significant changes in surgical procedures. 3,80
Figure 4.
Evolution sequence of radioguided surgery (adapted from Van Oosterom et al). 3 Both γ and positron emitting radiopharmaceuticals can be used.
Theranostics
There is a significant growth in number of therapeutic applications of radiopharmaceuticals. This opens new avenues of clinical interest to the Nuclear Medicine specialty. Theranostics is its most important concept. It combines diagnostic and therapeutic capabilities with radiopharmaceuticals derived from binding to a specific molecular target. Theranostic applications use the same molecule labeled with a γ or positron emitter radionuclide for diagnosis and a β minus or α emitter radioisotope for therapy. 85,86
For almost 80 years, the best-known theranostic paradigm is represented by radioactive iodine, namely the pair Iodine-123 (γ emitter) – Iodine-131 (β minus emitter) for thyroid disease diagnosis and therapy, respectively. The first Iodine-131 treatments for hyperthyroidism were in 1941 87 and 1946 for thyroid cancer. 88
Another example is metaiodobenzylguanidine (MIBG) labeled with Iodine-123 or Iodine-131 for advanced pheochromocytoma and neuroblastoma diagnosis and treatment, respectively. Bone imaging and palliation with radiolabeled bisphosphonates (e.g. 99mTc-HDP and 188Re-HEDP) is another longstanding example.
Recently, several studies demonstrated the efficacy of systemic therapy with peptide receptor radionuclide therapy in patients with advanced metastatic neuroendocrine neoplasms, using somatostatin-analogs (e.g. DOTA-TATE and DOTA-TOC) labeled with either Yttrium-90 or Lutetium-177. The first randomized controlled Phase III study, NETTER-1, 89 showed a significant clinical benefit from this therapy, achieving a prolonged progression-free survival of around 40 months, an overall response rate of 18% and presumably, a longer overall survival (median not reached) than standard treatment. This treatment, 177Lu-DOTA-TATE (Lutathera®), was approved by EMA in 2017 and FDA in 2018.
During the last 5 years, several studies have demonstrated treatment efficacy of 177Lu-PSMA ligands, reaching biochemical response in more than 50% of the patients. Partial response, measured by imaging, was achieved in more than one-third of the total number of patients. 72 Several clinical trials are ongoing. The VISION Phase III trial aims for approval of 177Lu-PSMA-617 (NCT03511664).
Despite the utility of β-minus emitters (Yttrium-90 and Lutetium-177), α-emitters (Actinium-225 and Astatine-211) have the major advantage of lower penetration power, resulting in a more potent absorbed dose to the tumoral lesions. Good overall treatment response and reduced adverse effects have led to increasing research and development in this field and new therapeutic agents will soon be available. 90–95
Conclusion
Nuclear Medicine and Molecular Imaging are versatile specialties with significant impact on both diagnosis and therapy. Their success and future depends on further innovation of radiopharmaceuticals, hardware equipment, regulatory acceptance and approval.
The development and success of new radiopharmaceuticals is expected. Onsite labelling using generators will positively affect clinical practice within Nuclear Medicine departments.
Both PET and SPECT technologies are rapidly evolving, embedded in hybrid devices, and the adjunction of MRI makes the field even more versatile. Multimodality imaging will continue to enhance PET use in the coming years. The evolution of PET systems will further improve significantly in terms of sensitivity and spatial/timing resolution with the continuous improvement of scintillators and electronics. Also, total-body scanners, dual-probes and optical imaging may bring significant benefits to diagnostic accuracy, radiation exposure and cost-efficiency, ultimately leading to highly personalized imaging and subsequent treatment.
Finally, theranostics encompasses two worlds, introducing the concept of personalized and targeted-treatment into state-of-the-art clinical routine. Further accelerated developments and wider use of radiolabeled nuclide therapies is likely to happen in the years to come within the 21st century.
Footnotes
Funding: Patrick Veit-Haibach: personal fees, travel support and IIS grants from GE Healthcare; personal fees, travel support, IIS grants and non-financial support from Siemens Healthineers, IIS grants and non-financial support from Bayer, ISS grants from Roche Pharmaceuticals. All support outside the submitted work.
Conflicts of Interest: Ken Herrmann: personal fees from Bayer, other from Sofie Biosciences, personal fees from SIRTEX, other from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees and nonfinancial support from Siemens Healthineers, and nonfinancial support from GE Healthcare, all outside the submitted work.
Contributor Information
Sofia C. Vaz, Email: sofiacarrilhovaz@gmail.com, Nuclear Medicine - Radiopharmacology, Champalimaud Centre for the Unknown, Champalimaud Foundation, Lisbon, Portugal .
Francisco Oliveira, Email: francisco.oliveira@fundacaochampalimaud.pt, Nuclear Medicine - Radiopharmacology, Champalimaud Centre for the Unknown, Champalimaud Foundation, Lisbon, Portugal .
Ken Herrmann, Email: Ken.Herrmann@uk-essen.de, Department of Nuclear Medicine, University Hospital Essen, University of Duisburg-Essen, Essen, Germany .
Patrick Veit-Haibach, Email: patrick.veit-haibach@uhn.ca.
REFERENCES
- 1.https://www.world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx
- 2. Jones T, Townsend D . History and future technical innovation in positron emission tomography . J Med Imaging 2017. ; 4: 011013 . doi: 10.1117/1.JMI.4.1.011013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Oosterom MN, Rietbergen DDD, Welling MM, Van Der Poel HG, Maurer T, Van Leeuwen FWB . Recent advances in nuclear and hybrid detection modalities for image-guided surgery . Expert Rev Med Devices 2019. ; 16: 711-734 . doi: 10.1080/17434440.2019.1642104 [DOI] [PubMed] [Google Scholar]
- 4. van Leeuwen FWBde Jong MCommittee E , . EANM Translational Molecular Imaging Committee . Molecular imaging: the emerging role of optical imaging in nuclear medicine . Eur J Nucl Med Mol Imaging 2014. ; 41: 2150-3 . doi: 10.1007/s00259-014-2845-0 [DOI] [PubMed] [Google Scholar]
- 5. Herrmann K, Schwaiger M, Lewis JS, Solomon SB, McNeil BJ, Baumann M, et al. . Radiotheranostics: a roadmap for future development . Lancet Oncol 2020. ; 21: e146-e156 . doi: 10.1016/S1470-2045(19)30821-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hustinx R . Physician centred imaging interpretation is dying out - why should I be a nuclear medicine physician? Eur J Nucl Med Mol Imaging 2019. ; 46: 2708-2714 . doi: 10.1007/s00259-019-04371-y [DOI] [PubMed] [Google Scholar]
- 7. Liu G, Cao T, Hu L, Zheng J, Pang L, Hu P, et al. . Validation of MR-Based attenuation correction of a newly released whole-body simultaneous PET/MR system . Biomed Res Int 2019. ; 2019: 8213215 . doi: 10.1155/2019/8213215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. . Fdg PET/CT: EANM procedure guidelines for tumour imaging: version 2.0 . Eur J Nucl Med Mol Imaging 2015. ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehanna H, Wong WL, McConkey CC, Rahman JK, Robinson M, Hartley AG, et al. . Pet-Ct surveillance versus neck dissection in advanced head and neck cancer . N Engl J Med 2016. ;. [DOI] [PubMed] [Google Scholar]
- 10. Jadvar H, Colletti PM, Delgado-Bolton R, Esposito G, Krause BJ, Iagaru AH, et al. . Appropriate Use Criteria for (18)F-FDG PET/CT in Restaging and Treatment Response Assessment of Malignant Disease . J Nucl Med 2017. ;. [DOI] [PubMed] [Google Scholar]
- 11. Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, et al. . 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging . Bioconjug Chem 2012. ; 23: 688-97 . doi: 10.1021/bc200279b [DOI] [PubMed] [Google Scholar]
- 12. Backhaus P, Noto B, Avramovic N, Grubert LS, Huss S, Bögemann M, et al. . Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives . Eur J Nucl Med Mol Imaging 2018. ; 45: 860-877 . doi: 10.1007/s00259-017-3922-y [DOI] [PubMed] [Google Scholar]
- 13. Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. . 68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0 . Eur J Nucl Med Mol Imaging 2017. ;. [DOI] [PubMed] [Google Scholar]
- 14. Fanti S, Minozzi S, Antoch G, Banks I, Briganti A, Carrio I, et al. . Consensus on molecular imaging and theranostics in prostate cancer . Lancet Oncol 2018. ; 19: e696-e708 . doi: 10.1016/S1470-2045(18)30604-1 [DOI] [PubMed] [Google Scholar]
- 15. Salas Fragomeni RA, Amir T, Sheikhbahaei S, Harvey SC, Javadi MS, Solnes LB, et al. . Imaging of Nonprostate cancers using PSMA-Targeted radiotracers: rationale, current state of the field, and a call to arms . J Nucl Med 2018. ; 59: 871-877 . doi: 10.2967/jnumed.117.203570 [DOI] [PubMed] [Google Scholar]
- 16. Kesch C, Kratochwil C, Mier W, Kopka K, Giesel FL . 68)Ga or (18)F for Prostate Cancer Imaging? J Nucl Med 2017. ;. [DOI] [PubMed] [Google Scholar]
- 17. Czarniecki M, Mena E, Lindenberg L, Cacko M, Harmon S, Radtke JP, et al. . Keeping up with the prostate-specific membrane antigens (PSMAs): an introduction to a new class of positron emission tomography (PET) imaging agents . Transl Androl Urol 2018. ; 7: 831-843 . doi: 10.21037/tau.2018.08.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nanni C, Zanoni L, Bach-Gansmo T, Minn H, Willoch F, Bogsrud TV, et al. . 18)F]Fluciclovine PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging-version 1.0 . Eur J Nucl Med Mol Imaging 2019. ;. [DOI] [PubMed] [Google Scholar]
- 19. Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. . Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE . Eur J Nucl Med Mol Imaging 2010. ; 37: 2004-10 . doi: 10.1007/s00259-010-1512-3 [DOI] [PubMed] [Google Scholar]
- 20. Hutton BF, Erlandsson K, Thielemans K . Advances in clinical molecular imaging instrumentation . Clin Transl Imaging 2018. ;. [Google Scholar]
- 21. Pauwels E, Cleeren F, Bormans G, Deroose CM . Somatostatin receptor PET ligands - the next generation for clinical practice . Am J Nucl Med Mol Imaging 2018. ; 8: 311-331 . [PMC free article] [PubMed] [Google Scholar]
- 22. Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P . A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis . EJNMMI Res 2016. ; 6: 52 . doi: 10.1186/s13550-016-0207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarkin JM, Calcagno C, Dweck MR, Evans NR, Chowdhury MM, Gopalan D, et al. . 68Ga-DOTATATE PET Identifies Residual Myocardial Inflammation and Bone Marrow Activation After Myocardial Infarction . J Am Coll Cardiol 2019. ; 73: 2489-2491 . doi: 10.1016/j.jacc.2019.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. . Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging . J Am Coll Cardiol 2017. ; 69: 1774-1791 . doi: 10.1016/j.jacc.2017.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taieb D, Hicks RJ, Hindie E, Guillet BA, Avram A, Ghedini P, et al. . European association of nuclear medicine practice Guideline/Society of nuclear medicine and molecular imaging procedure standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma . Eur J Nucl Med Mol Imaging 2019. ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopci E, Grassi I, Chiti A, Nanni C, Cicoria G, Toschi L, et al. . Pet radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence . Am J Nucl Med Mol Imaging 2014. ; 4: 365-84 . [PMC free article] [PubMed] [Google Scholar]
- 27. Arabi M, Piert M . Hypoxia PET/CT imaging: implications for radiation oncology . Q J Nucl Med Mol Imaging 2010. ; 54: 500-9 . [PubMed] [Google Scholar]
- 28. Bollineni VR, Kramer GM, Jansma EP, Liu Y, Oyen WJG . A systematic review on [(18)F]FLT-PET uptake as a measure of treatment response in cancer patients . Eur J Cancer 2016. ; 55: 81-97 . doi: 10.1016/j.ejca.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 29. Been LB, Suurmeijer AJH, Cobben DCP, Jager PL, Hoekstra HJ, Elsinga PH . 18F]FLT-PET in oncology: current status and opportunities . Eur J Nucl Med Mol Imaging 2004. ; 31: 1659-72 . doi: 10.1007/s00259-004-1687-6 [DOI] [PubMed] [Google Scholar]
- 30. Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. . A tumor-imaging method targeting cancer-associated fibroblasts . J Nucl Med 2018. ; 59: 1423-1429 . doi: 10.2967/jnumed.118.210435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. . 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer . J Nucl Med 2019. ; 60: 801-805 . doi: 10.2967/jnumed.119.227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chae SY, Ahn SH, Kim S-B, Han S, Lee SH, Oh SJ, et al. . Diagnostic accuracy and safety of 16α-[18 F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study . Lancet Oncol 2019. ; 20: 546 – 55 . doi: 10.1016/S1470-2045(18)30936-7 [DOI] [PubMed] [Google Scholar]
- 33. van Kruchten M, de Vries EGE, Brown M, de Vries EFJ, Glaudemans AWJM, Dierckx RAJO, et al. . Pet imaging of oestrogen receptors in patients with breast cancer . Lancet Oncol 2013. ; 14: e465 – 75 . doi: 10.1016/S1470-2045(13)70292-4 [DOI] [PubMed] [Google Scholar]
- 34. Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, et al. . 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up . Eur J Nucl Med Mol Imaging 2018. ; 45: 2300-2306 . doi: 10.1007/s00259-018-4099-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santhanam P, Taieb D, Solnes L, Marashdeh W, Ladenson PW . Utility of I-124 PET/CT in identifying radioiodine avid lesions in differentiated thyroid cancer: a systematic review and meta-analysis . Clin Endocrinol 2017. ; 86: 645-651 . doi: 10.1111/cen.13306 [DOI] [PubMed] [Google Scholar]
- 36. Marcus C, Whitworth PW, Surasi DS, Pai SI, Subramaniam RM . Pet/Ct in the management of thyroid cancers . AJR Am J Roentgenol 2014. ; 202: 1316-29 . doi: 10.2214/AJR.13.11673 [DOI] [PubMed] [Google Scholar]
- 37. Liu M, Cheng L, Jin Y, Ruan M, Sheng S, Chen L . Predicting 131I-avidity of metastases from differentiated thyroid cancer using 18F-FDG PET/CT in postoperative patients with elevated thyroglobulin . Sci Rep 2018. ; 8: 4352 . doi: 10.1038/s41598-018-22656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Treglia G, Castaldi P, Villani MF, Perotti G, de Waure C, Filice A, et al. . Comparison of 18F-dopa, 18F-FDG and 68Ga-somatostatin analogue PET/CT in patients with recurrent medullary thyroid carcinoma . Eur J Nucl Med Mol Imaging 2012. ; 39: 569-80 . doi: 10.1007/s00259-011-2031-6 [DOI] [PubMed] [Google Scholar]
- 39. Van Laere K, Varrone A, Booij J, Vander Borght T, Nobili F, Kapucu OL, et al. . EANM procedure guidelines for brain neurotransmission SPECT/PET using dopamine D2 receptor ligands, version 2 . Eur J Nucl Med Mol Imaging 2010. ; 37: 434-42 . doi: 10.1007/s00259-009-1265-z [DOI] [PubMed] [Google Scholar]
- 40. Delva A, Van Weehaeghe D, van Aalst J, Ceccarini J, Koole M, Baete K, et al. . Quantification and discriminative power of 18F-FE-PE2I PET in patients with Parkinson's disease . Eur J Nucl Med Mol Imaging 2019. ;27 Nov 2019. doi: 10.1007/s00259-019-04587-y [DOI] [PubMed] [Google Scholar]
- 41. Morbelli SEG, Arbizu J, Barthel H, Boellaard R, Bohnen NI, Brooks DJ, et al. . EANM practice Guideline/SNMMI procedure standard for dopaminergic imaging in parkinsonian syndromes 1.0 . Eur J Nucl Med Mol Imaging 2019. ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nestor PJ, Altomare D, Festari C, Drzezga A, Rivolta J, Walker Z, et al. . Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia . Eur J Nucl Med Mol Imaging 2018. ; 45: 1509-1525 . doi: 10.1007/s00259-018-4035-y [DOI] [PubMed] [Google Scholar]
- 43. Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. . Pet of brain amyloid and tau in mild cognitive impairment . N Engl J Med 2006. ; 355: 2652-63 . doi: 10.1056/NEJMoa054625 [DOI] [PubMed] [Google Scholar]
- 44. Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. . Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B . Ann Neurol 2004. ; 55: 306-19 . doi: 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- 45. Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, et al. . SNMMI procedure Standard/EANM practice guideline for amyloid PET imaging of the brain 1.0 . J Nucl Med 2016. ;. [DOI] [PubMed] [Google Scholar]
- 46. de Wilde A, van der Flier WM, Pelkmans W, Bouwman F, Verwer J, Groot C, et al. . Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project . JAMA Neurol 2018. ; 75: 1062-1070 . doi: 10.1001/jamaneurol.2018.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Catafau AM, Bullich S . Amyloid PET imaging: applications beyond Alzheimer's disease . Clin Transl Imaging 2015. ; 3: 39-55 . doi: 10.1007/s40336-014-0098-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Okamura N, Harada R, Ishiki A, Kikuchi A, Nakamura T, Kudo Y . The development and validation of tau PET tracers: current status and future directions . Clin Transl Imaging 2018. ; 6: 305-316 . doi: 10.1007/s40336-018-0290-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ossenkoppele R, Rabinovici GD, Smith R, Cho H, Schöll M, Strandberg O, et al. . Discriminative Accuracy of [18F]flortaucipir Positron Emission Tomography for Alzheimer Disease vs Other Neurodegenerative Disorders . JAMA 2018. ; 320: 1151-1162 . doi: 10.1001/jama.2018.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun A, Liu X, Tang G . Carbon-11 and fluorine-18 labeled amino acid tracers for positron emission tomography imaging of tumors . Front Chem 2017. ; 5: 124 10.3389/fchem.2017.00124 . doi: 10.3389/fchem.2017.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. . Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0 . Eur J Nucl Med Mol Imaging 2019. ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Najjar AM, Johnson JM, Schellingerhout D . The emerging role of amino acid PET in neuro-oncology . Bioengineering 2018. ; 5: E10428 11 2018. doi: 10.3390/bioengineering5040104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evans NR, Tarkin JM, Buscombe JR, Markus HS, Rudd JHF, Warburton EA . Pet imaging of the neurovascular interface in cerebrovascular disease . Nat Rev Neurol 2017. ; 13: 676-688 . doi: 10.1038/nrneurol.2017.129 [DOI] [PubMed] [Google Scholar]
- 54. Davidson CQ, Phenix CP, Tai TC, Khaper N, Lees SJ . Searching for novel PET radiotracers: imaging cardiac perfusion, metabolism and inflammation . Am J Nucl Med Mol Imaging 2018. ; 8: 200-227 . [PMC free article] [PubMed] [Google Scholar]
- 55. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. . ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures . J Nucl Cardiol 2016. ; 23: 1187-1226 . doi: 10.1007/s12350-016-0522-3 [DOI] [PubMed] [Google Scholar]
- 56. Slart R, Writing g, Reviewer g . Members of EC, Members of EI, Inflammation, et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC . Eur J Nucl Med Mol Imaging 2018. ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beheshti M, Mottaghy FM, Paycha F, Behrendt FFF, Van den Wyngaert T, Fogelman I, et al. . Correction to: 18F-NaF PET/CT: EANM procedure guidelines for bone imaging . Eur J Nucl Med Mol Imaging 2018. ; 45: 322 . doi: 10.1007/s00259-017-3874-2 [DOI] [PubMed] [Google Scholar]
- 58. Hockley BG, Scott PJH . An automated method for preparation of [(18)F]sodium fluoride for injection, USP to address the technetium-99m isotope shortage . Appl Radiat Isot 2010. ; 68: 117-9 . doi: 10.1016/j.apradiso.2009.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martins P, Blanco A, Crespo P, Marques MFF, Marques RF, Gordo PM, et al. . Towards very high resolution RPC-PET for small animals . Journal of Instrumentation 2014. ;. [Google Scholar]
- 60. Vandenberghe S, Mikhaylova E, D'Hoe E, Mollet P, Karp JS . Recent developments in time-of-flight PET . EJNMMI Phys 2016. ; 3: 3 . doi: 10.1186/s40658-016-0138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Sluis J, de Jong J, Schaar J, Noordzij W, van Snick P, Dierckx R, Jv S, Jd J, Pv S, et al. . Performance characteristics of the digital Biograph vision PET/CT system . J Nucl Med 2019. ; 60: 1031-1036 . doi: 10.2967/jnumed.118.215418 [DOI] [PubMed] [Google Scholar]
- 62. Alva-Sánchez H, Zepeda-Barrios A, Díaz-Martínez VD, Murrieta-Rodríguez T, Martínez-Dávalos A, Rodríguez-Villafuerte M . Understanding the intrinsic radioactivity energy spectrum from 176Lu in LYSO/LSO scintillation crystals . Sci Rep 2018. ; 8: 17310 . doi: 10.1038/s41598-018-35684-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mayerhoefer ME, Prosch H, Beer L, Tamandl D, Beyer T, Hoeller C, et al. . Pet/Mri versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations . Eur J Nucl Med Mol Imaging 2020. ; 47: 51-60 . doi: 10.1007/s00259-019-04452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Catalano OA, Rosen BR, Sahani DV, Hahn PF, Guimaraes AR, Vangel MG, et al. . Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients--a hypothesis-generating exploratory study . Radiology 2013. ; 269: 857-69 . doi: 10.1148/radiol.13131306 [DOI] [PubMed] [Google Scholar]
- 65. Bailey DL, Pichler BJ, Guckel B, Antoch G, Barthel H, Bhujwalla ZM, et al. . Combined PET/MRI: global Warming-Summary report of the 6th International workshop on PET/MRI . . Mol Imaging Biol 2018March 27-29, 2017, Tubingen, Germany . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gundacker S, Acerbi F, Auffray E, Ferri A, Gola A, Nemallapudi MV, et al. . State of the art timing in TOF-PET detectors with LuAG, GAGG and L(Y)SO scintillators of various sizes coupled to FBK-SiPMs . Journal of Instrumentation 2016. ;. [Google Scholar]
- 67. Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD . Total-Body PET: maximizing sensitivity to create new opportunities for clinical research and patient care . J Nucl Med 2018. ; 59: 3-12 . doi: 10.2967/jnumed.116.184028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, et al. . First human imaging studies with the explorer total-body PET scanner . J Nucl Med 2019. ; 60: 299-303 . doi: 10.2967/jnumed.119.226498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beekman FJ, Fvd H, Goorden MC, Vaissier PEB, Jv R, During H, et al. . G -SPECT-I: a full ring high sensitivity and ultra-fast clinical molecular imaging system with . Eur J Nucl Med Mol Imaging 2015. ;. [Google Scholar]
- 70. Oliveira FPM, Faria DB, Costa DC, Castelo-Branco M, Tavares JMRS . Extraction, selection and comparison of features for an effective automated computer-aided diagnosis of Parkinson's disease based on [123I]FP-CIT SPECT images . Eur J Nucl Med Mol Imaging 2018. ; 45: 1052-1062 . doi: 10.1007/s00259-017-3918-7 [DOI] [PubMed] [Google Scholar]
- 71. Oliveira F, Leuzy A, Castelhano J, Chiotis K, Hasselbalch SG, Rinne J, et al. . Data driven diagnostic classification in Alzheimer's disease based on different reference regions for normalization of PIB-PET images and correlation with CSF concentrations of Aβ species . Neuroimage Clin 2018. ; 20: 603-610 . doi: 10.1016/j.nicl.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kratochwil C, Fendler WP, Eiber M, Baum R, Bozkurt MF, Czernin J, et al. . EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT . Eur J Nucl Med Mol Imaging 2019. ;. [DOI] [PubMed] [Google Scholar]
- 73. Kaalep A, Sera T, Oyen W, Krause BJ, Chiti A, Liu Y, et al. . EANM/EARL FDG-PET/CT accreditation - summary results from the first 200 accredited imaging systems . Eur J Nucl Med Mol Imaging 2018. ; 45: 412-422 . doi: 10.1007/s00259-017-3853-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R . EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies . Eur J Nucl Med Mol Imaging 2017. ; 44( Suppl 1 ): 17-31 . doi: 10.1007/s00259-017-3740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barrington SF, Sulkin T, Forbes A, Johnson PWM . All that glitters is not gold - new reconstruction methods using Deauville criteria for patient reporting . Eur J Nucl Med Mol Imaging 2018. ;. [DOI] [PubMed] [Google Scholar]
- 76. Oliveira FPM, Moreira AP, Ad M, Verdelho A, Xavier C, Barroca D, et al. . Can 11C-PiB-PET relative delivery R1 or 11C-PiB-PET perfusion replace 18F-FDG-PET in the assessment of brain neurodegeneration? J Alzheimers Dis 2018. ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, et al. . The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer . Eur J Nucl Med Mol Imaging 2013. ; 40: 1932-47 . doi: 10.1007/s00259-013-2544-2 [DOI] [PubMed] [Google Scholar]
- 78. Giammarile F, Bozkurt MF, Cibula D, Pahisa J, Oyen WJ, Paredes P, et al. . The EANM clinical and technical guidelines for lymphoscintigraphy and sentinel node localization in gynaecological cancers . Eur J Nucl Med Mol Imaging 2014. ; 41: 1463-77 . doi: 10.1007/s00259-014-2732-8 [DOI] [PubMed] [Google Scholar]
- 79. Giammarile F, Schilling C, Gnanasegaran G, Bal C, Oyen WJG, Rubello D, et al. . The EANM practical guidelines for sentinel lymph node localisation in oral cavity squamous cell carcinoma . Eur J Nucl Med Mol Imaging 2019. ; 46: 623-637 . doi: 10.1007/s00259-018-4235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Leeuwen FWB, van Oosterom MN, Meershoek P, van Leeuwen PJ, Berliner C, van der Poel HG, et al. . Minimal-Invasive robot-assisted image-guided resection of prostate-specific membrane antigen-positive lymph nodes in recurrent prostate cancer . Clin Nucl Med 2019. ; 44: 580-581 . doi: 10.1097/RLU.0000000000002600 [DOI] [PubMed] [Google Scholar]
- 81. Ambrosini V, Fanti S . Radioguided surgery with 68Ga-DOTATATE for patients with neuroendocrine tumors . Hepatobiliary Surg Nutr 2020. ; 9: 67-69 . doi: 10.21037/hbsn.2019.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bugby SL, Lees JE, Perkins AC . Hybrid intraoperative imaging techniques in radioguided surgery: present clinical applications and future outlook . Clin Transl Imaging 2017. ; 5: 323-341 . doi: 10.1007/s40336-017-0235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Seibold U, Wängler B, Schirrmacher R, Wängler C . Bimodal imaging probes for combined PET and OI: recent developments and future directions for hybrid agent development . Biomed Res Int 2014. ; 2014: 153741 . doi: 10.1155/2014/153741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wendler T, Traub J, Ziegler SI, Navab N . Navigated three dimensional beta probe for optimal cancer resection . Med Image Comput Comput Assist Interv 2006. ; 9( Pt 1 ): 561-9 . doi: 10.1007/11866565_69 [DOI] [PubMed] [Google Scholar]
- 85. Yordanova A, Eppard E, Kürpig S, Bundschuh RA, Schönberger S, Gonzalez-Carmona M, et al. . Theranostics in nuclear medicine practice . Onco Targets Ther 2017. ; 10: 4821-4828 . doi: 10.2147/OTT.S140671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Langbein T, Weber WA, Eiber M . Future of theranostics: an outlook on precision oncology in nuclear medicine . J Nucl Med 2019. ; 60( Suppl 2 ): 13S-19S . doi: 10.2967/jnumed.118.220566 [DOI] [PubMed] [Google Scholar]
- 87. Hertz S, Roberts A . Radioactive iodine as an indicator in thyroid physiology. V. the use of radioactive iodine in the differential diagnosis of two types of Graves' disease . J Clin Invest 1942. ; 21: 31-2 . doi: 10.1172/JCI101276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Seidlin SM, Rossman I . Radioiodine therapy of metastases from carcinoma of the thyroid; a 6-year progress report . J Clin Endocrinol Metab 1949. ; 9: 1122-37, illust . doi: 10.1210/jcem-9-11-1122 [DOI] [PubMed] [Google Scholar]
- 89. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. . Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors . N Engl J Med 2017. ; 376: 125-135 . doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kratochwil C, Giesel F, Bruchertseifer F, Rius-Montraveta M, Apostolidis C, Haberkorn U, et al. . Ac-225-PSMA617 - a single center experience of 40 patients receiving PSMA-targeted Alpha therapy . J Nucl Med 2016. ;. [Google Scholar]
- 91. Kiess AP, Minn I, Vaidyanathan G, Hobbs RF, Josefsson A, Shen C, et al. . 2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido)Pentyl)Ureido)-Pentanedioic Acid for PSMA-Targeted α-Particle Radiopharmaceutical Therapy . J Nucl Med 2016. ; 57: 1569-1575 . doi: 10.2967/jnumed.116.174300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ruigrok EAM, van Weerden WM, Nonnekens J, de Jong M . The future of PSMA-Targeted radionuclide therapy: an overview of recent preclinical research . Pharmaceutics 2019. ; 11: E56029 10 2019. doi: 10.3390/pharmaceutics11110560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Navalkissoor S, Grossman A . Targeted alpha particle therapy for neuroendocrine tumours: the next generation of peptide receptor radionuclide therapy . Neuroendocrinology 2019. ; 108: 256-264 . doi: 10.1159/000494760 [DOI] [PubMed] [Google Scholar]
- 94. Zalutsky MR, Vaidyanathan G . Astatine-211-labeled radiotherapeutics: an emerging approach to targeted alpha-particle radiotherapy . Curr Pharm Des 2000. ; 6: 1433-55 . doi: 10.2174/1381612003399275 [DOI] [PubMed] [Google Scholar]
- 95. Zustovich F, Barsanti R . Targeted α therapies for the treatment of bone metastases . Int J Mol Sci 2017. ; 19: E7428 Dec 2017. doi: 10.3390/ijms19010074 [DOI] [PMC free article] [PubMed] [Google Scholar]