Abstract
The microbiome is an integral part of the human gut, and it plays a crucial role in the development of the immune system and homeostasis. Apart from the gut microbiome, the airway microbial community also forms a distinct and crucial part of the human microbiota. Furthermore, several studies indicate the existence of communication between the gut microbiome and their metabolites with the lung airways, called “gut–lung axis”. Perturbations in gut microbiota composition, termed dysbiosis, can have acute and chronic effects on the pathophysiology of lung diseases. Microbes and their metabolites in lung stimulate various innate immune pathways, which modulate the expression of the inflammatory genes in pulmonary leukocytes. For instance, gut microbiota-derived metabolites such as short-chain fatty acids can suppress lung inflammation through the activation of G protein-coupled receptors (free fatty acid receptors) and can also inhibit histone deacetylase, which in turn influences the severity of acute and chronic respiratory diseases. Thus, modulation of the gut microbiome composition through probiotic/prebiotic usage and fecal microbiota transplantation can lead to alterations in lung homeostasis and immunity. The resulting manipulation of immune cells function through microbiota and their key metabolites paves the way for the development of novel therapeutic strategies in improving the lung health of individuals affected with various lung diseases including SARS-CoV-2. This review will shed light upon the mechanistic aspect of immune system programming through gut and lung microbiota and exploration of the relationship between gut–lung microbiome and also highlight the therapeutic potential of gut microbiota-derived metabolites in the management of respiratory diseases.
1. Introduction
The human intestine harbors a dense and complex community of microorganisms comprising of viruses, bacteria, archaea, and eukaryotes which constitute the gut microbiota (GM). The total genetic makeup of these microbes defines the gut microbiome.1 The number of microbes in a standard adult human male gut reaches 3.8 × 1013 microbes which outnumbers the total number of host cells (3.0 × 1013).2
A large fraction of GM is composed of facultative and obligate anaerobic bacteria from these five phyla: Firmicutes, Bacteroidetes, Proteobacteria, Verrucomicrobia, and Actinobacteria.3 The rich ecosystem of microbes shares various symbiotic relationships among themselves and with the host. They supply the host with numerous physiological functions such as fermentation of indigestible dietary constituents, synthesis of crucial vitamins, protection against various pathogens, maturation of immune system and assist in maintenance of gut barrier function.4,5 The prenatal human gut is pioneered by microbes in the uterus.6 The mode of child delivery also influences the microbial diversity and composition of an infant gut. The diversity of GM in infants delivered through cesarean section is less compared to those born through normal delivery. Also, the number of beneficial microbes is reduced in the former.7 Furthermore, the milk formula fed versus breastfed infants have differences in GM composition. Hence, the infant diet has a notable role in shaping the GM which achieves its maximum stability by the age of 3–5 years.8,9 However, there are various factors such as diet, lifestyle, and drug usage that can lead to changes in taxonomic composition and function of GM throughout life.10
Most of the non-nutritive components of food like dietary fibers, choline, and polyphenols that are present in an average omnivorous human diet remain undigested by host digestive enzymes and hence pass without or partial digestion to the colon.11 Bacteria residing in the human gut possess a giant pool of degradative enzymes that are usually absent in their host and hence are capable of generating plenty of downstream metabolites. Primarily, the metabolism of macronutrients—carbohydrates, proteins, and lipids—make up the gut metabolic pool.12 The key metabolites synthesized are the branched chain amino acids (BCAAs), short and branched chain fatty acids (SCFAs and BCFAs) and gases including carbon dioxide, methane, ammonia, and hydrogen sulfide. A few metabolites are also derived from other compounds like secondary plant products, bile acids, vitamins, polyamines, polyphenols, choline, volatile organic compounds, and cell wall of bacteria.13,14
Understanding the type and percentages of different metabolites in the colon serve to be helpful in comprehending the potential metabolic effect that microorganisms can have on their host.15−17 These metabolites not only provide energy to intestinal cells but also control the downstream signaling pathways.18 This finding implies that metabolites produced in the gut are connecting links between the GM and general disease-free well-being of its host. Furthermore, GM dysbiosis may not be the only factor affecting the physiology of host because GM-derived metabolites may even have more extensive systemic impacts, such as host energy metabolism and immune system regulation.19
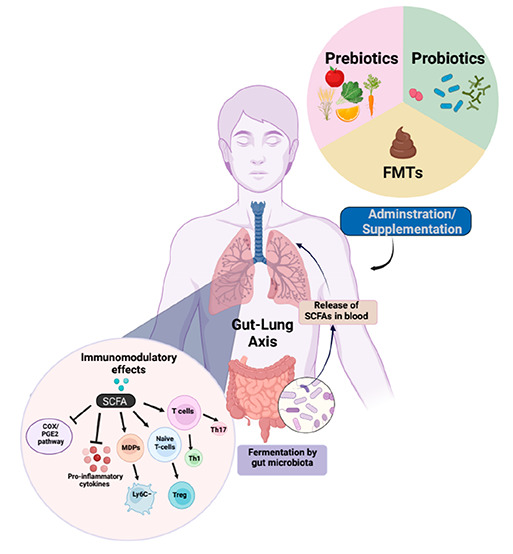
Although the underlying idea that the local microbiota affects health and disease has been around since the beginning of the 20th century, the breakthrough in microbiota studies was made possible by the widespread availability of next-generation sequencing (NGS) techniques. Disapproval of lung sterility and emergence of studies emphasizing the significance of the lung microbiota in pulmonary health represented a remarkable advancement in respiratory science. Additionally, the idea of gut–lung axis was strengthened and the pathways interaction between these bodily parts began to be clarified. These developments offered insights into the ways by which the gut microbiota may impact lung immunology and health.20 One way of interaction between the gut microbiota and the lungs is through soluble bacterial components and their metabolites that are delivered to the bloodstream. SCFAs, mainly acetate, propionate, and butyrate, form an excellent example of this. The bacteria in the cecum and colon produce SCFAs that modify local immunological responses inside the gut after being discharged into the lumen and give colonocytes an energy source (particularly butyrate).21−24
Manipulation of immune cells function through gut microbiota and their key metabolites can be achieved by the usage of probiotics, prebiotics, and fecal microbiota transplantation (FMT). Thus, it paves the way for the formulation of novel therapeutic approaches in improving the lung health of individuals affected with lung diseases such as SARS-CoV-2. This review will shed light upon the mechanistic aspect of immune system programming through lung microbiota, explore the link between gut–lung microbiome, and also highlight the therapeutic role of gut microbiota-derived metabolites in the management of respiratory diseases.
2. Gut Metabolite—SCFA
SCFAs are saturated fatty acids containing aliphatic chains of 2 to 6 carbons.24 The major types of SCFAs produced in the colon are acetate (C2), propionate (C3) and butyrate (C4). Lower amounts of other SCFAs like formate, valerate, and caproate are also formed.25,26 Proximal colon has highly diverse and stable bacterial colony with greatest substrate availability hence the fermentation of dietary fibers occurs here predominantly. Maximum concentration of SCFAs is observed in the ascending colon (70–140 mM), thereafter in transverse colon (20–70 mM) and lowest in the descending colon (20–40 mM).26 SCFA acetate is produced in highest amounts and forms more than 50% of the total concentration of SCFAs detected in the feces.27 The SCFAs, acetate, propionate, and butyrate, exist in the molar ratio of 60:25:15 in the colonic lumen, respectively.25 This ratio however can vary with the composition of gut microbiota, diet, fermentation site and the genotype of the host.28
2.1. Substrates for Production of SCFAs
Nonstarch polysaccharides (NSP) or dietary fibers, as well as resistant starch (RS) are the common carbohydrates that are fermented by specific colonic bacteria under anaerobic conditions.29 The insoluble fibers such as pectin and gum are the major substrates for SCFAs production, whereas the soluble forms such as cellulose and lignin mainly contribute to fecal bulking and lower the colon transit time. To a certain extent, oligosaccharides (e.g., fructooligosaccharides and xylooligosaccharides), branched chain amino acids (e.g., isobutyrate and isovalerate), intermediary metabolites of fermentation (e.g., lactate and ethanol) and host glycoprotein (e.g., mucin) can also function as substrates for the production of SCFAs.30,31
2.2. Pathways for SCFAs Production
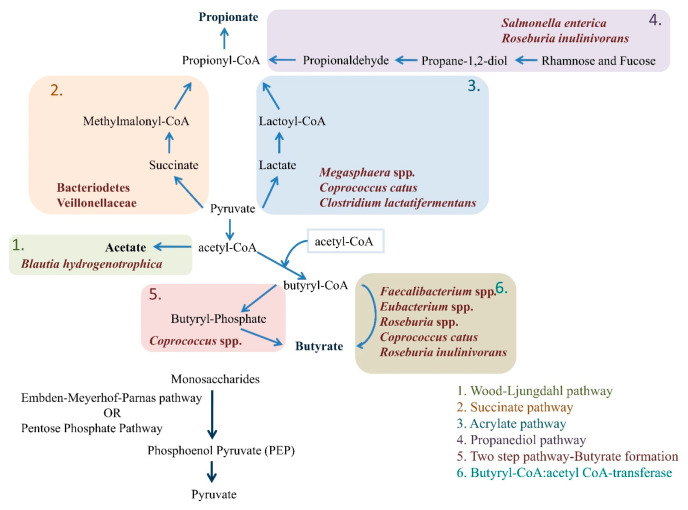
Microbial enzymes create cellulosome complexes to break down undigested polysaccharides and convert complex carbohydrate fibers into simple sugars.29Table 1 mentions all the major gut bacteria producing acetate, propionate and butyrate. The two main processes used by enteric bacteria to produce phosphoenol pyruvate (PEP) from monosaccharides are the Embden–Meyerhof–Parnas pathway (for 6-carbon substrates) and the pentose phosphate pathway (for 5-carbon substrates). Pyruvate is the typical metabolite involved in the synthesis of various SCFAs in a majority of enzymatic reactions. The very first step is the conversion of pyruvate to acetyl-CoA, which also releases CO2 and H2. The majority of the acetate produced during fermentation is an enteric byproduct. Furthermore, roughly one-third of acetate in the gut is produced by acetogenic bacteria such as Blautia hydrogenotrophica via the Wood–Ljungdahl pathway (reductive acetyl-CoA pathway), which convert H2 plus CO2, i.e., formic acid (HCOOH) to acetate.32,33 Propionate can be produced by three different metabolic pathways: the succinate pathway, the acrylate pathway, and the propanediol pathway.34 PEP is converted to succinate in the succinate pathway, which is utilized to produce propionate from propionyl-CoA produced by decarboxylation of methylmalonyl-CoA. This is found in Bacteroidetes and a few members of the Veillonellaceae family within the Firmicutes phylum. In the acrylate pathway, the function of lactoyl-CoA dehydratase and subsequent enzymatic activities is to transform lactate to propionate. Many bacteriae from the Veillonellaceae (e.g., Megasphaera spp.) and Lachnospiraceae families (e.g., Coprococcuscatus) have taken this route.29 In the propanediol pathway, propionate is generated from deoxy sugar substrates such as rhamnose and fucose through conversion of propionaldehyde to propionyl-CoA in the presence of CoA-dependent enzyme propionaldehyde dehydrogenase. Proteobacteria Salmonella enterica and Roseburia inulinivorans (Lachnospiraceae) use this route.33−35
Table 1. Major Producers of SCFAs in Human Gut33,35,57,79,232.
| Substrate | Metabolite (Number of carbon atoms) | Major Producers |
|---|---|---|
| Polysaccharides/Glycan | Butyrate (C4) | Eubacterium ruminantium, Roseburia inulinivorans, Faecalibacterium prausnitzii, Ruminococcus bromii, Anaerostipes spp., Butyrivibrio fibrisolvens, Clostridium leptum, Ruminococcus gnavus, Eubacterium hallii, Eubacterium rectale, Faecalibacterium prausnitzii, Coprococcus comes, Coprococcus catus, Clostridium acetobutylicum, Roseburia intestinalis, Eubacterium cylindroides, Coprococcus eutactus |
| Propionate (C3) | Eubacterium dolichum, Akkermansia muciniphila, Roseburia inulinivorans, Ruminococcus obeum, Dialister succinatiphilus, Bacteroides fragilis, Bacteroides eggerthii, Megasphaera elsdenii, Salmonella enterica, Blautia wexleri, Coprococcus catus, Phascolarctobacterium succinatutens, Eubacterium hallii, Bacteroides spp., Anaerostipes spp., Veillonella parvula, Ruminococcus bromii | |
| Acetate (C2) | Akkermansia muciniphila, Faecalibacterium prausnitzii, Eubacterium cylindroides, Blautia hydrogenotrophica, Prevotella spp., Clostridium spp., Streptococcus spp., Ruminococcus gnavus, Coprococcus spp., Bacteroides spp., Bifidobacterium spp., Methanobrevibacter smithii, Eubacterium hallii, |
Several Firmicute genera are involved in butyrate production. Faecalibacterium Prausnitzii, Eubacterium rectale, Eubacterium hallii (Anaerobutyricum hallii), and Ruminicoccus bromii are the primary producers of butyrate. Clostridium leptum and Coprococcus species also contribute. Initially, two molecules of acetyl-CoA are converted into butyryl-CoA, which can be metabolized to butyrate via two pathways. In the first pathway, butyryl-CoA can be transformed to butyrate and CoASH by phosphotransbutyrylase and butyrate kinase enzymes.36 Only a few Coprococcus species use this pathway.29 In the second pathway, butyryl-CoA can be transformed into butyrate by the enzyme butyryl-CoA:acetyl CoA-transferase in a single step reaction.37 It is the more prevalent pathway and is found in some of the most abundant bacterial genera such as Faecalibacterium, Eubacterium, and Roseburia. Few anaerobic bacteria like Coprococcus catus and R. inulinivorans are the only ones that can generate both propionate and butyrate.33 The various pathways involved in the formation of SCFAs by gut microbiota are summarized in Figure 1.
Figure 1.
Structural outline of different pathways employed by enteric bacteria for production of SCFAs mainly acetate, propionate, and butyrate from monosaccharides originating from undigested carbohydrates.
2.3. Catabolism of Amino Acids for SCFAs and BCFAs Production
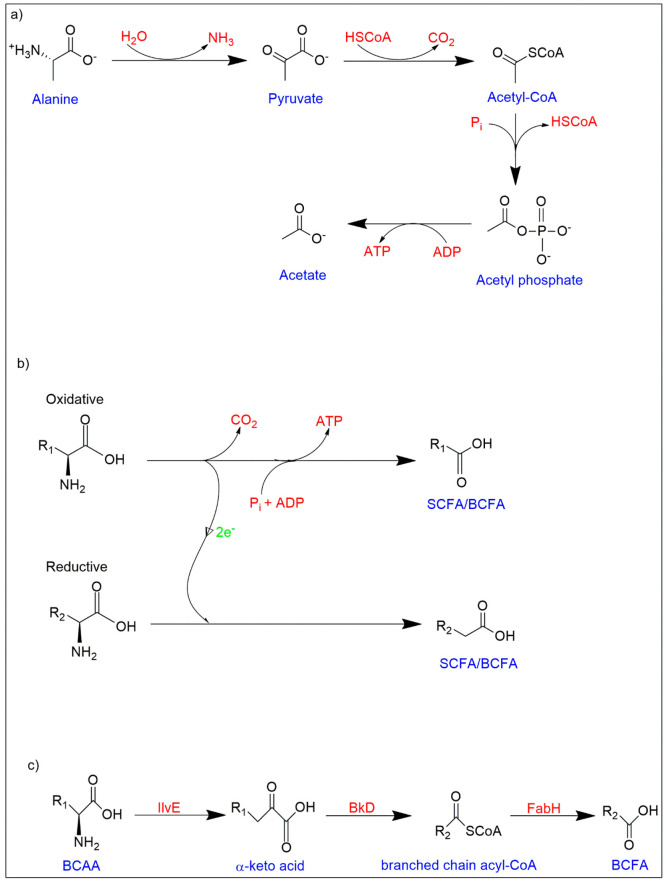
The mechanism of protein digestion by the host shows more variability than carbohydrate and fat digestion, and is influenced by factors such as source of food (plant or animal based), processing, macronutrient ratios and transit time, resulting in variable amino acid constitutions accessible to the gut microbes.38−41 The additional interconversion processes during the fermentation of amino acids result in a wide range of byproducts. A microbe can employ one of two techniques for the initial stage of amino acid catabolism: First is the deamination reaction to produce carboxylic acid and ammonia; and second is the decarboxylation reaction to produce an amine group and carbon dioxide. Because substantial amounts of SCFAs are created from the breakdown of amino acids via deamination pathway, it seems to be the more typical approach used by gut microbiota for the amino acid catabolism.42,43 The subsequent steps are determined by the kind of amino acid substrate available, with the majority finally culminating into pyruvate, tricarboxylic acid cycle (TCA) intermediates, and coenzyme A-linked SCFAs precursors (Figure 2a).44,45 However, Stickland reaction observed in certain bacteria (for instance, Clostridia), in which a linked oxidation and reduction of two amino acids occurs concurrently, is an exception.46,47 In this situation, the addition of phosphate to the reduced amino acid results in oxidative phosphorylation for the synthesis of ATP to occur from the acyl phosphate. As a result, BCFA (for e.g., isovalerate and isobutyrate) might be formed as a byproduct (Figure 2b).44 Furthermore, some members from the Bacilli class, have a specific branched-chain keto acid dehydrogenase complex (Bkd) that directly generates energy from oxidized forms of BCAAs, resulting in BCFA generation.43 In summary, as observed in Staphylococcus aureus, several BCAA transporters transfer extracellular BCAA into the bacterial cell followed by the conversion of isoleucine and leucine (preferentially) to their respective α-ketoacids by BCAA transaminase (IlvE). These α-ketoacids are converted to branched-chain acyl-CoAs by Bkd. These acyl-CoAs are employed by 3-ketoacyl-ACP synthase III (FabH) to activate the bacterial fatty acid biosynthesis cycle to form BCFAs (Figure 2c).48
Figure 2.
Catabolism of amino acids for SCFAs and BCFAs production. (a) Formation of SCFAs from amino acid substrates via TCA cycle intermediates. (b) The Stickland reaction demonstrating the cofermentation of two amino acids, one of which serves as an electron donor and the other as an electron acceptor leading to formation of SCFAs or BCFAs. (c) Extracellular BCAA consumption pathway in S. aureus for BCFA production. (BCFAs - Branched-chain fatty acids, BCAAs - Branched-chain amino acids, BkD - Branched-chain keto acid dehydrogenase complex, IlvE - BCAA transaminase, FabH - 3-ketoacyl-ACP synthase III).
2.4. Cross-Feeding Mechanisms
Cross-feeding in bacteria significantly influences the diversity and quantity of each SCFA generated. Even though lactate is not a SCFA, it is used to prevent metabolic acidosis in the host by certain bacteria that produce butyrate and propionate.49 Numerous in vitro investigations show that bacteria from the genera Roseburia, Eubacterium, and Anaeroestipes utilize the lactate and/or acetate that Bifidobacterium produces when cultured in the presence of oligofructose.50−53 Furthermore, propionate is converted to lactate in vitro by members of Veillonella and Propionibacterium.(54) Acetogenic bacteria, such as Acetobacterium, Acetogenium, Eubacterium, and Clostridium species, can use butyrate and propionate to generate acetate which can be utilized by F. prausnitzii and Roseburia species and converted to butyrate and propionate.31 Sulfate or nitrate reducing acetogenic bacteria like Acetobacterium, Acetogenium, Eubacterium, and Clostridium species may break down butyrate and propionate into acetate.55 However, this process can be reversed by species with higher butyrate production or consumption rates, including F. prausnitzii and Roseburia species.51 Furthermore, acetate generated by B. thetaiotaomicron can act as a substrate for butyrate synthesis by E. rectale. Such relationships exemplify the mutualistic creation of SCFAs.56R. inulinivorans produces butyrate when glucose is available, but when fucose is used as a substrate, propionate may be produced.57 As a result, different forms of SCFAs can be created, depending on the availability of the substrate. The quantity and rate of different biosynthetic pathways are largely regulated not only by enzymes but also by various transporters. Bacteroidetes cannot transport enough substrates for fermentation because they lack enough ATP-binding cassette (ABC) transporters and phosphotransferase system (PTS) enzymes, while Firmicutes can transport acetate and produce butyrate or propionate in large amounts because of the high number of ABC transporters that they possess. Therefore, the ratio and concentration of SCFAs in the gut lumen may also be controlled by intricate and delicate interactions among the microbiota. Prebiotics, probiotics, or synbiotics which modify this equilibrium, may thus influence the synthesis of SCFAs.31,56
3. Transport of SCFAs Across Intestinal Epithelium
The concentration of SCFAs detected in feces is just a small portion of the total production as approximately 90–95% of SCFAs formed are absorbed by the intestinal epithelial cells (IECs).58 Passive diffusion allows for the direct passage of some of the undissociated SCFAs to IECs via the apical membranes, but a larger proportion that remains in ionic form under lumen pH requires the aid of transporters. These transporters belong to two categories, the sodium-coupled monocarboxylate transporters (SMCTs)—1 and 2—and the proton-coupled monocarboxylate transporters (MCTs): MCT1 and MCT4.59 SMCT1, SMCT2, and MCT1 are expressed on the apical membrane of the IECs found in the small intestine and the distal part of the colon. Furthermore, MCT1 and MCT4 are expressed on basolateral membranes.60 This facilitates the transfer of SCFAs for intestinal absorption, as well as into systemic circulation. While SMCT2 exclusively transports butyric acid and iso-butyric acid, the transporters MCT1, MCT4, and SMCT1 may bind and deliver acetate, propionate, and butyrate.61 The expression of the transporters and hence the uptake of various SCFAs varies with the change in bacterial relative abundance, concentration of individual SCFAs and gut inflammation, as in case of several diseases.62−64
The amount of SCFAs in blood circulation and tissues reported in the literature vary, most likely due to changes in diet, illness status, model used, methods of tissue/fluid collection, processing, and assay.65 The molar concentrations of acetate, propionate, and butyrate in the gastrointestinal (GI) tract and hepatic portal vein of sudden death subjects were roughly 57:22:21 and 69:23:8, respectively.24,66 Therefore, colonocytes use the majority of the butyrate generated in the intestinal lumen.67 By undergoing beta oxidation in the mitochondria of the cell, butyrate supplies 60–70% of the energy requirements of colonocytes.24,61 Due to a lack of butyrate, IECs from germ-free (GF) mice exhibit decreased beta oxidation. Such cells experience a lack of mitochondrial respiration and enter autophagy. But supplementing isolated colonocytes with butyrate or administering GF mice with butyrate-producing bacteria like Butyrivibrio fibrisolvens lowers autophagy.68 Only a very small proportion of butyrate that enters the liver through the hepatic portal vein makes it to the peripheral circulation. Acetate is transferred from colonocytes to the liver where almost 70% of it is utilized as energy substrate and for the cholesterol and fatty acids synthesis.69 Acetate enters the portal vein and travels to nearby tissues (mostly muscles).70 Hepatocytes consume the majority of the propionate as a substrate for gluconeogenesis and then enter the TCA cycle. The enzyme propionyl-CoA synthetase converts the propionate to propionyl-CoA, which is then transformed further through a series of steps to succinyl-CoA. Oxaloacetate (OAA), produced from succinyl-CoA, is then metabolized into glucose.71−73 A significant amount of propionate is used by the hepatocytes since studies showed lower concentrations of propionate in the hepatic vein than the hepatic portal vein.74 Only acetate is found in peripheral circulation at significant concentrations as evidenced by experimentations on sudden death victims and isotope flux studies.72,75,76 Therefore, the plasma concentrations of acetate (25–250 μmol/L) is the highest, followed by propionate (1.4–13.4 μmol/L) and least for butyrate (0.5–14.2 μmol/L).60,66
There have been several studies demonstrating the concentrations of SCFAs in respiratory airways. Ghorbani et al. measured the levels of SCFAs in sputum (0.158 −4.570 mM) from cystic fibrosis patients, indicating that they reach the respiratory airways.77 Another study found that, excluding acetate, the SCFA concentrations in exhaled breath condensate (EBC) and bronchoalveolar lavage (BAL) biofluids were 104 μM (22–138 μM) and 28 M (23–133 μM), respectively. In another study, authors used high-performance liquid chromatography (HPLC) to analyze cecal and serum samples from mice by feeding various combinations of diets and discovered that the levels of SCFAs in both cecal and serum samples increased proportionally with larger amounts of soluble dietary fiber. However, these SCFAs were not detected in lung tissue.78
4. SCFA Sensing and Signal Transduction
SCFAs can operate as energy substrates, signaling molecules via G protein coupled receptors (GPCRs), and epigenetic regulators of gene expression by blocking histone deacetylases (HDACs). These three characteristics determine how SCFAs control the activities of the host cells. Propionate and butyrate in particular, which function as an endogenous HDAC inhibitor, can alter gene transcription and allosterically modulate chromatin while also promoting the activity of histone acetyltransferase (HAT) in living cells.60,79−81
4.1. G-Protein Coupled Receptors (GPCRs)
GPCRs are the primary receptors involved in regulating almost all the cellular functions in mammals.82 Following ligand activation, GPCRs can connect to a variety of heterotrimeric G proteins including the Gs, Gi/o, Gq/11, and G12/13 domains. This attachment may affect the activity of one or more effectors, such as enzymes associated with second messengers and ion channels.82 SCFAs are capable of activating signaling processes via a variety of GPCRs. The most significant receptors for SCFAs are GPR41, GPR43, and GPR109A, also referred to as free fatty acid receptor (FFAR) 2, FFAR3, and hydroxycarboxylic acid receptor 2 (HCAR2) respectively. It has been demonstrated that GRP41 has a preference for butyrate and propionate over acetate, whereas GPR43 prefers acetate over propionate and butyrate. GPR109A could be activated mainly by butyrate.82,83
4.2. Inhibition of HDACs by SCFAs
The interconversion of permissive (through acetylation) and restrictive chromatin configurations is made possible by histone acetylation and deacetylation, respectively. HATs add acetyl groups to histone tails, whereas HDACs remove them. Transcriptional factors have decreased access to the DNA binding sites on gene promoters as a result of chromatin condensation caused by HDACs, which function by removing ε-N-acetyl lysine groups from histones. HDAC inhibitors are frequently utilized in the treatment of cancer. Furthermore, it has also been noted that they have anti-inflammatory or immune-suppressive properties. Both butyrate and, to a lesser degree, propionate are known to function as HDAC inhibitors.84,85
5. Healthy Lung Microbiota
Studies on various microbial communities of healthy subjects at several body sites, including the skin, GI tract, urogenital tract, nasal passages, and oral cavity resulted in better understanding of the microbial flora existing in humans during healthy and diseased condition. In comparison to the gut microbiome, pulmonary microbiome studies are still in their early days. However, the significance of direct link between host and microbes in the respiratory tract is progressively becoming clearer as a result of enormous research being done recently.86 Earlier, the distal part of the respiratory tract was considered “sterile”, mostly because lung bacteria from healthy subjects did not grow in regular microbiological cultures. Later with the advances in sequencing technologies, microbial DNA were confirmed in people’s lungs even under healthy conditions.87 However, the discrimination between a permanent/resident microbiome with that of the momentarily present microbial communities in the lower airways possess a challenge owing to several technical constraints including sample collection methodology, oropharyngeal cross-contamination during collection, and low abundance of microbial populations.
The development of microbial flora in the gut of an infant is a continuous and intricate process which begins at delivery and progresses through several phases while being influenced by both internal and external stimuli.88−90 Bacteria of maternal origin inhabit the new-born’s mouth cavity during vaginal birth.91 Therefore, microorganisms residing in the oral cavity contribute to and influence the microbial makeup of lung.92
Different anatomical regions are colonized by niche-specific microbes as distinct species are prevalent at different sites.93 However, some bacterial communities are present in both the mouth cavity and lung, albeit in different concentrations. This suggests that the oral microbiome may have contributed to seeding of the lung microbial community in some way.87,94−96 However, it has been debated whether specimens from the upper part of the respiratory tract can accurately represent the microbiome of the distal portion since the microbiota varies greatly across the upper and lower respiratory tracts as observed in the healthy subjects.94
A study based on investigation of microbial composition in the distal respiratory tract and mouth of nonsmokers and smokers helped to identify a “healthy” microbiome of lower respiratory tract. The most prevalent genera inhabiting the lung are Streptococcus, Prevotella, and Veillonella, and the overall bacterial ecosystems residing there mimic those in the oral cavity due to topological continuity.96,97 Furthermore, Pseudomonas and Fusobacteria are also found along with less prominent genera like Haemophilus and Niesseria.(87,98)Firmicutes, Proteobacteria, and Bacteriodetes are the most common phyla in the oropharynx. Furthermore, Firmicutes and Bacteroidetes are commonly found in the lungs of healthy people.87,93,96,99 These genera can easily survive and flourish in ciliated, oxygen-rich larynxes and the tracheobronchial tree that runs parallel to the mouth cavity. The mucus in nasopharynx, and the upper and lower respiratory tract are first being exposed to a range of air borne particles, thus considered as first line of defense against any microbial pathogen.100 Mucus layer has immunoglobulin A (IgA) released by the B cells that prevent the pathogen to reside at the mucosal surface and prevent interaction with the epithelial surface101,102
6. GI Tract v/s Lung Environment
Despite being mucosa-lined luminal organs with similar embryological ancestry, the GI tract and the lungs have quite diverse microanatomical properties. If vomiting or esophageal reflux is excluded, the movement of microbes through the GI tract is unidirectional from the mouth to the anus. Orally administered microbes must endure both the stomach’s acidic pH and alkaline duodenum in order to reach the cecum. The flow of air, mucus, and microorganisms in the lungs, however, is bidirectional rather than one-way.20,103,104
The GI tract usually maintains a constant temperature of 37 °C throughout its length. However, the respiratory tract’s epithelial surfaces show a gradation in temperature from ambient (proximal to pharynx) to core body temperature (inside the alveoli).103,105 Furthermore, in contrast to the GI tract, the lungs are oxygen-rich (aerobic) under healthy conditions. Although the alveolar surface of trachea and bronchi are covered with a mucus coating just like the GI tract, the alveolar membrane on the other hand, is coated with a lipid-rich surfactant that possess bacteriostatic properties against bacterial species.106 Thus, the microbial communities found at both sites (GI tract and lung) are very distinct and have variable relationships with the host body.
Furthermore, the host–bacteria interactions in the GI tract and lungs are dissimilar. Although the GI tract has substantially greater luminal IgA levels, extraluminal encounters between microbes and host alveolar macrophages are much more common in the lungs.107,108 Therefore, the divergent communities of bacteria found in the gut and lungs are the outcome of these environmental changes.
7. Role of SCFAs in Gut–Lung Axis
The microbiota of all compartments of gut and the respiratory tract is crucial for developing and priming immune cells and maintaining homeostasis of the body’s immune system.109 The imbalance existing between the gut microbiota and the airways is linked to inflammatory conditions responsible for respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).110,111 In neonate studies, it is evident that a decrease in the abundance of intestinal bacteria including Bifidobacteria, Akkermansia, and Faecalibacteria is associated with an increase in risk of asthma development and atopy.112,113 Additionally, one in vivo study suggested that changing the gut microbial composition could increase the level of SCFAs that also correlated with the reduction in lung inflammation.114
Various immunological pathways get activated by the metabolites such as butyrate which activates the NF-κβ pathway in the colonic cell line and ex-vivo mouse model through GPR109A. In addition, the activation of NLRP-3 inflammasome pathway occurs in HT-29 and NMC460 colonic cell lines via sensing of acetate by GPR43.115 Thus, evidence suggest that GPCRs receptor activation by SCFAs has a major role in modulating colonic inflammation.61 The one way of interaction between the lung and gut microbiota is through soluble microbial components and metabolites such as peptidoglycans and lipopolysaccharides (LPS). These microbe-associated molecular patterns (MAMPs) are recognized by receptors expressed by the innate immune cells of the host body, described as pattern-recognition receptors (PRR), namely, Toll-like receptors (TLR), Nod-like receptors (NLR), etc.116 Various shreds of evidence show that the effective immune response can be restored against lung diseases such as influenza by metabolites released by gut microbiota.117
8. Immunomodulatory Effects of SCFAs on Immune Cells
In the case of immune cells such as human monocytes, SCFAs promote the induction of the release of the prostaglandin E2 as well as expression of the IL-10 cytokine via PTX-sensitive GPCR, thus contributing to the blocking of the inflammatory response.118 In addition, these SCFAs act as HDAC inhibitors and are thus able to lower the pro-inflammatory cytokines.119,120 HDACs perform crucial roles in innate immune pathways and regulate the myeloid cell differentiation and inflammation via TLR and interferon-inducible gene expression.121 Therefore, the inhibition of GPR43 and HDAC by propionate mediates the direct stimulation of Treg proliferation.122 From the past studies, it is quite evident that propionate and butyrate stimulate the differentiation of the Treg cells from CD4+ T cells by upregulation of the Foxp3 gene transcription which in turn gets activated by the histone acetylation.21,100 Furthermore, Park et al. showed that SCFAs can directly promote the differentiation of T cells into the IL-17, interferon-γ and IL-10 expressing T cells that is dependent on the concentration of the SCFAs. Basically, the overall effect of SCFAs on the T cells are independent of the GPCR receptors such as GPR41, SMCT1, or GPR43, but it depends on the inhibition of histone deacetylase (HDAC).123,124
9. Immunomodulatory Effects of the SCFAs in Lungs
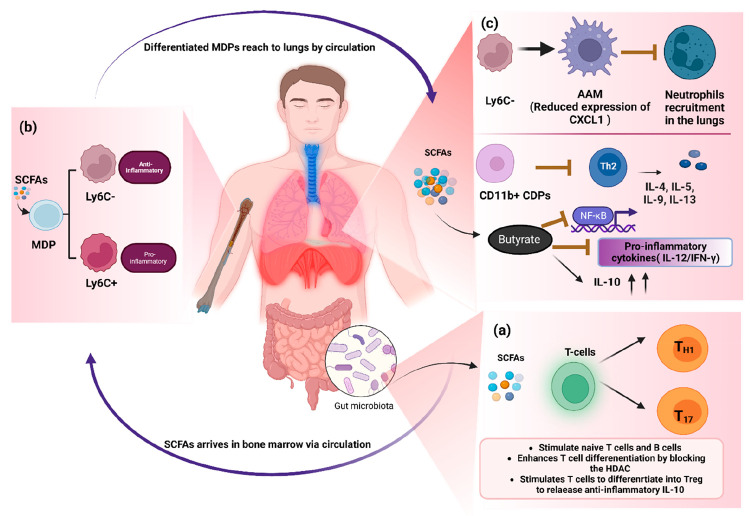
SCFAs can modulate the function of the various immune cells such as in vancomycin-treated mice, the administration of a mixture of SCFAs modulated the DCs functions and found to be effective against allergic response in the lungs.125 Dietary administration of acetate in the asthmatic mice model lead to the epigenetic changes in the Th cells that favored the Treg cells differentiation.126 SCFAs can also establish an extrathymic peripheral Treg cell pool similar to as in gut, which has been associated with reducing allergic airway disorders via HDAC suppression.21 SCFAs also enable the T cells to differentiate into Th1 and Th17 effector cells, as well as Treg cells expressing IL-10, which is independent of GPR41 and GPR43 signaling. SCFAs also allow the differentiation of T cells into the Th1 and Th17 effector cell along with Treg cell expressing IL-10 which is independent of the GPR41 and GPR43 signaling. Furthermore, SCFA is known to inhibit the HDAC in allergic airway disease, thus it can activate the mTOR-S6K pathway that is needed for the T cell differentiation and expression of cytokines by them (Figure 3a).123 In addition to aiding in the maturation of macrophage and DCs during hematopoiesis in the bone marrow, SCFAs perform a significant part in the differentiation of immune cells that reside in the airways. Although there are inadequate traces of evidence that SCFAs are deposited in the lungs, one of the study clearly demonstrated that SCFAs have a direct function in the recruitment and hematopoiesis of immune cells in airways and lungs.127 During Th2 cell-mediated allergic airway inflammation (AAI), acetate and propionate circulate from the gut to the bone marrow to modulate DC hematopoiesis. Furthermore, SCFAs can increase the formation of macrophages and DC progenitors (MDPs) as well as the differentiation of common DC progenitors (CDPs) in bone marrow. Thereafter, these precursor DCs enter in the lungs and differentiate into the CD11b+ DC subtype, which are ineffective at presenting allergens and activating effector Th2 cells.78,128,129 Thus, AAI gets resolved quickly as immune response is not sustained.78 Moreover, SCFAs have a context-specific impact on hematopoiesis and the progenitor cell commitment in the bone marrow. It was found that SCFAs can only affect the MDPs subsets, not other hematopoietic progenitor cells as seen during influenza virus infection.130 Further, MDPs differentiate into either monocytes or CDPs. Monocytes can further differentiate into two subsets: (1) Ly6C+ monocytes that differentiate into inflammatory macrophages or DCs that provide immunopathology and (2) LyC6– monocytes that differentiate into anti-inflammatory alternatively activated macrophages (AAM) possessing anti-inflammatory activity (Figure 3b).130−132 SCFAs enhance the differentiation of monocytes to anti-inflammatory macrophages, thus, providing immunity against viral infections. For instance, during influenza virus infection, butyrate or propionate were found to be involved in promoting the pathway for LyC6– monocytes and AAM formation via GPR41 signaling. These AAM cells in the lungs are unable to release enough neutrophil chemoattractant CXCL1. As a result, AAMs alleviate immunopathology caused due to viral infections by reducing neutrophil influx into the respiratory tract.130,133 As a result, it is clear that SCFAs have an anti-inflammatory action in the lung via priming of myeloid cells of the bone marrow, which then move to lungs to show their effect.78,134 Additionally, SCFAs like butyrate block NF-κβ due to their anti-inflammatory characteristics and elevate the level of cytokines like IL-10 (anti-inflammatory), and inhibit pro-inflammatory cytokines, such as IL-12 and IFN-γ by DCs (Figure 3c).135
Figure 3.
Schematic diagram showing the major communications between the gut–lung axis via circulating SCFAs that are released by gut microbiota and their subsequent effect on the immune system. (a) Formation of SCFAs (such as butyrate, propionate, and acetate) from the gut microbiota metabolism in the cecum and colon, and the general role of SCFAs on the differentiation of T-cells. (b) SCFAs can reach bone marrow via circulation in the blood, where SCFAs have a prominent role in the hematopoiesis of MDPs and their differentiation into the CDPs, and (c) subsequently, precursors DC inhabit the lungs and transforms into the CD11b+ DCs which blocks the Th2 cell activation, and enhance the polarization of the CDPs into the LyC6-monocytes that increase the activation of anti-inflammatory macrophages in a GPR41-dependent manner (thus, AAM has an important role in anti-inflammation by reducing the infiltration of the neutrophils in the lungs), and butyrate may also block the NF-κβ transcription factor and pro-inflammatory cytokines (IL-2, IFN-γ) and elevate the level of anti-inflammatory cytokines (IL-10). (GM – Gut microbiota, MDPs – Macrophage and dendritic progenitors, CDPs – Common DC progenitors, AAM – Alternatively activated macrophages) (Created with https://biorender.com/).
Recently, numerous studies have demonstrated the role of various SCFAs in lung health and disease therapeutics. Administration of the exogenous butyrate to adult BALB/c mice before induction of the disease decreases the development of ovalbumin (OVA)-induced asthma. In addition, same metabolite attenuates the allergic inflammation in the pregnant BALB/c mice and inhibits the elevated frequency of CD25+FoxP3+ Tregs.136 The survival of other immune cells, such as eosinophils, is also modulated by butyrate and propionate SCFAs through epigenetic regulation of HDAC gene expression in eosinophils. Therefore, one can conclude that SCFAs can alter allergic eosinophilia and type 2 allergic response during asthma. For instance, butyrate attenuates the expression of certain chemotactic receptors (such as CD44, CCR3, and CD49d) in allergic donor eosinophilia.137 In the case of human rhinovirus infection in mice, treatment with acetate ameliorated the virus-induced pro-inflammatory responses and reduced the IL-6 expression. In addition, treatment of butyrate modulates the expression of the IFN-β and IFN-ϒ gene in human bronchial and alveolar epithelial cell lines.138 SCFAs impart the anti-inflammatory function due to its inhibitory effects on the immune cell chemotaxis and adhesion, activation of anti-inflammatory cytokines’ levels and acceleration of programmed cell death.139 Besides, SCFAs have tremendous role in enhancing the systemic immunity of the host, preventing the bacterial development and alleviating the integrity of the epithelial cells.22 Furthermore, SCFAs may benefit the host’s health by activating the FFARs 2 and 3 on neutrophils and macrophages, which results in reduced expression of IL-8. Furthermore, SCFAs may improve the health of the host by stimulating the FFARs 2 and 3 present on neutrophils and macrophages, which would decline the expression of IL-8 during airway inflammation.140 Moreover, activation of the FFAR3/GPR41 receptors plays a role in reduction of expression of the pro-inflammatory cytokines (including IL-6, MCP-1, TNF, and inducible NOS) in macrophages.141 Lung integrity and tight junction can be substantially lost due to excessive smoking as in case of CPD patients.142 It has been demonstrated that butyrate has antiasthmatic abilities because it can restrict innate lymphoid cells (ILCs) from generating pro-inflammatory cytokines and GATA binding protein 3.143 Furthermore, T-reg cells can block the progression of asthma by enhancing the tolerogenic immune profile as previously shown in a study, which suggested that the introduction of Treg cells in a mouse model with excessive inflammation in the airways improved the health condition of mice.
Several studies have demonstrated that increasing the number of SCFAs attenuates lung damage or injury. Wu et al. showed that SCAFs reduce lung damage by modulating macrophage immune responses during Klebsiella pneumoniae infection. Furthermore, Baicalin protects against avian pathogenic Escherichia coli (APEC)-induced inflammation by increasing the synthesis of SCFAs in the gut.144,145 However, another study demonstrated antibiotic-induced microbiome depletion; hence, the lower levels of SCFAs could enervate LPS-induced acute lung injury (ALI). In this investigation, antibiotic cocktail (ABX) significantly reduced the amounts of SCFAs such as acetate and propionate in the stool samples.146 The lung SCFAs may have been significantly reduced following the ABX treatment; however, their exact concentration in the lungs was not tested in this study. Considering the importance of SCFAs in inflammatory pathways, it appears that their decreased levels may contribute to ABX’s therapeutic effects in LPS-induced ALI, albeit more research is needed.
10. SCFAs and Infectious Respiratory Diseases
Several studies support that SCFAs have the ability to function as potent therapeutic agents in averting and treating various lung disorders including lung cancer, COVID-19, chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis, tuberculosis, and other infectious diseases. The prominent role played by the major SCFAs in the progression and therapeutics of various lung related disorders has been summarized in Table 2. The role of SCFAs in the therapeutics of COVID-19 will be dealt with in detail in the following sections. The three main SCFAs (butyrate, propionate, and acetate) are demonstrated to have important roles in therapeutics of infectious respiratory diseases. A recent study demonstrated that the patients acquiring allogeneic hematopoietic stem cell transplantation faced a decreased risk of developing virus infection in the lower respiratory tract if their feces had a higher abundance of butyrate-producing bacteria.147 Furthermore, butyrate’s antiviral effects have shown to increase the life expectancy of mice suffering with Klebsiella pneumoniae infection.148 In addition, acetate has been found to prevent respiratory syncytial virus infection in mice and to reduce viral load and inflammation in the lungs. Another study found that the SCFA acetate subsides bacterial load and the resulting inflammation by acting on FFAR2 receptor, hence providing resistance against the Klebsiella pneumoniae infection.149 Furthermore, it was demonstrated that acetate provides protection from the recurrent infections by improving the adequacy of alveolar macrophages in the lungs to combat germs, therefore increasing the survival period of mice.150 These studies show that gut microbial metabolites, particularly SCFAs have the potential to provide protection against common respiratory diseases and infections.
Table 2. Role of SCFAs (Acetate, Propionate, and Butyrate) in Progression and Therapeutics of Various Lung Diseases.
| Disease | Role played by SCFAs via gut–lung axis | References |
|---|---|---|
| COPD | Emphysema attenuation was linked to higher cecal concentrations of SCFAs brought on by whey protein-based diet. | (233−235) |
| A reduction in lung inflammation and enhanced pulmonary function were both associated with higher SCFA levels. | ||
| Patients with Stage III–IV COPD showed elevated levels of SCFAs compared to Stage I–II COPD patients and the healthy volunteers. | ||
| In comparison to healthy participants, COPD patients had more SCFAs in their breath condensate. | ||
| Asthma | Butyrate reduced gut inflammation by restricting NF-B-mediated B-cell stimulation in the colon or by inducing expression regulation of peroxisome proliferator-activated receptor-gamma (PPAR-gamma). | (236, 237) |
| In Group 2 innate lymphoid cells (ILCs) inhabiting lungs, butyrate deciphers antiasthmatic effect via averting the synthesis of GATA binding protein 3 (GATA3) as well as other pro-inflammatory cytokines. | ||
| Lung Cancer | The treatment with sodium butyrate elevated the expression of miR-3935, leading to reduced ability of the A549 lung adenocarcinoma epithelial cells to multiply and migrate. | (236, 238) |
| Propionate surges p21 and reduces survivin expression in the H1299 and H1703 lung cancer cell lines, causing cell cycle arrest and apoptosis. | ||
| Influenza | SCFAs enhance CD8+ T cell functioning via GPR41 activation hence regulating Ly6c-negative patrolling monocyte hematopoiesis. | (130, 188, 239) |
| Butyrate activates GPR109A promoting the development of Treg cells and secretion of IL-10 and IL-18 from cells. | ||
| Decrease in acetate production positively correlated with the ability of alveolar macrophages to eradicate the bacterial superinfection that occurs when influenza strikes. | ||
| FFAR2 receptor was activated by acetate supplementation, which led to a decrease in the local and systemic bacterial loads. | ||
| Cystic Fibrosis | IL-8/CXCL8 (major inflammatory mediator) is triggered in response to SCFAs in the lungs of CF patients. | (130, 195, 240) |
| Blocking of GPR41 in bronchial epithelial cells significantly reduces the generation of IL-8 triggered by SCFA. | ||
| Bronchial epithelial cells are induced by SCFAs to generate pro-inflammatory cytokines (like IL-6), granulocyte colony stimulating factor, and granulocyte-macrophage colony stimulating factor (GM-CSF). | ||
| SCFA-induced IL-8 production was found to be responsible for increased neutrophil recruitment into CF lungs. | ||
| Tuberculosis | Propionate and butyrate may decrease the production of IL-17, suppress Th1 immunity, and increase the number of T regulatory cells, all of which might slow the progression of M. tuberculosis infection. | (241, 242) |
| Indole propionic acid may interfere with M. tuberculosis’ potential to manufacture tryptophan, hence obstruct its growth directly. |
11. Role of SCFAs in SARS-CoV-2 Therapeutics
Recently, the role of SCFAs in Covid-19 therapeutics has been explored by various researchers. During Covid-19 infection, cytokine storms and multiorgan failures that need acute care are the most often seen problems. Age, sex, immunological status, and comorbidities all affect the rate of mortality in critically ill patients. Intestinal dysbiosis has a vital role in COVID-19 pathogenesis as demonstrated by various studies. For an example, SCFAs producers are comparatively reduced in gut flora during COVID-19 infection which could be one of the major cause of adverse clinical consequences.130,151−154 Furthermore, COVID-19 patients have been reported to have poor synthesis of SCFAs and l-isoleucine which results in higher disease severity and increased inflammatory indicators like CRP and CXCL-10, along with increased production of urea. Additionally, even when the disease has gone into remission, the gut microbiota of COVID-19 patients still has a compromised ability to manufacture l-isoleucine and SCFAs.155
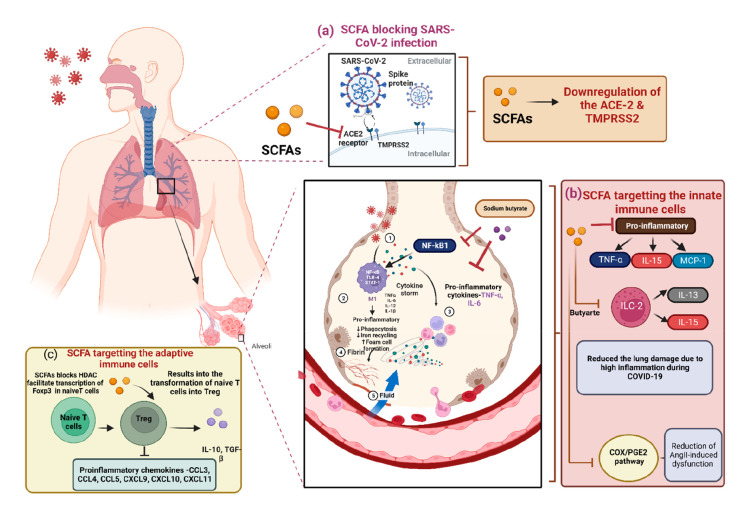
Treatment methods include usage of dexamethasone, a wide range immunosuppressant corticosteroid, used to treat COVID-19 patients’ cytokine storm and hyperinflammation.156,157 However, frequent use of corticosteroids can have significantly adverse outcomes and disturbance of gut–lung axis.158 Recently, Brown et al. showed that angiotensin-converting enzyme 2 (ACE2), a crucial receptor for SARS-CoV-2 entry inside human cells, is downregulated by SCFAs (Figure 4a). Furthermore, SCFAs boost adaptive immunity through GPCR (GPR41 and GPR43) signaling. Together, these results have shown unexpected roles for SCFAs in preventing hypercoagulation and viral entry while enhancing adaptive antiviral immunity.159
Figure 4.
Schematic diagram showing the role of SCFAs in SARS-CoV-2 therapeutics and the various ways to target the SARS-CoV-2 infection in host cells. (a) SCFAs promote the downregulation of the ACE-2 and TMPRSS-2, thus reduce the chance of the SARS-CoV-2 infection in host cell. (b) By targeting the ILCs such as ILC2 and inhibiting the expression of the pro-inflammatory cytokines, SCFAs mainly reduce the excessive inflammation in the lungs. Further, butyrate can also block the COX2/PGE2 pathway leading to reduction in Angiopoietin II-induced dysfunctions and (c) by targeting the adaptive immune cells, where SCFAs can transforms the T-Naïve cells into the Treg cells that exhibit the anti-inflammatory activity, SCFAs have role in inhibiting the proinflammatory chemokines. (ILC – Innate lymphoid cells) (Created with https://biorender.com/).
Out of all of the SCFAs, butyrate has been the most extensively studied. It has been documented that butyrate has a role in suppressing SARS-CoV-2 infection through different mechanisms: (1) decreasing the ACE2 receptor expression and (2) transmembrane protease serine 2 (TMPRSS2) genes, (3) increasing the ADAM17 levels (a metallopeptidase implicated in ACE2 shedding), (4) upregulating various antiviral pathways such as TLR, and (5) butyric acid has also been proven to lessen oxidative stress and hyperinflammation in a number of diseases, including viral respiratory infections.160,161
Patients who are unable to consume liquids or food supplements or who exhibit signs of stomach distress or dysbiosis may be candidates for rectal delivery of butyrate by enema or nasogastric gavage of butyrate formulation. It is important to promote more studies into these prospective adjunct medicines. Furthermore, anti-SARS-CoV-2 activity of a novel butyrate releaser, N-(1-carbamoyl-2-phenyl-ethyl) butyramide (FBA) has been explored in a human host. In biopsy reports of small intestine and human enterocytes, FBA could reduce the infectivity of SARS-CoV-2 virus by downregulating the expression of several receptors, such as ACE2, TMPRSS2, and NRP1, as well as the pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-15 (IL-15), and monocyte chemoattractant protein-1 (MCP-1) (Figure 4b). These findings collectively highlight the importance of a balanced gut microbiota that produces the adequate level of butyrate to guard against the SARS-CoV-2 infection, and also the efficacy of FBA as a treatment for individuals with Covid-19 disease.162,163 Additionally, valproic acid (a short branched-chain fatty acid) which is a long-used medicine for the treatment of epilepsy also lowers the production of ACE2 and TMPRSS2. Valproic acid may be a viable therapeutic target for COVID-19 due to its antithrombotic, antiplatelet, and anti-inflammatory properties.164
In a recent study, authors have mentioned that people at risk of severe COVID-19 infection can be identified early by looking at the polarization of macrophages in order to counteract the process of cytokine storm. The SCFA profiles, cytokine dose, and M1/M2 macrophage ratio will all be taken into consideration when deciding whether to add butyrate to the diet. Due to their well-defined function in the control of the immune response, SCFAs, particularly butyrate, might be thought of as pro-resolving mediators. It is crucial to remember that pro-resolving mediators have the primary benefit of not suppressing but instead downregulating the initial immune response and reducing inflammation.165
With sodium butyrate administered as a preventative measure, myeloperoxidase activity and entry of inflammatory cells into the lungs are markedly reduced, and these effects are linked to the suppression of proinflammatory cytokines (TNF-alpha and IL-6), HMGB1 expression, and NF-κB activation (Figure 4).166 Sodium butyrate and propionate target the lipopolysaccharide (LPS)-related TLR-4/NF-κB pathway and reduce lung damage caused by LPS stimulated cytokines (including IL-6 and IL-12p40.167−169 After being exposed to oxidative stress, hyaluronan ester plus butyric acid therapy promotes apoptosis in mesangial cells, inhibiting cell proliferation via the p38 MAPK pathway.170 The production of IL-13 and IL-15 by type 2 ILCs is inhibited by butyrate. Furthermore, butyrate suppresses the expression of inflammatory genes post-transcriptionally by downregulating a number of RNA binding proteins.171 Sodium butyrate can also block the COX2/PGE2 pathway in an HDAC5/HDAC6 dependent manner resulting in reduction of Angiopoietin II-induced disorders including hypertension, cardiac hypertrophy, endothelial dysfunction and inflammation.172,173 Butyrate also lessens the movement of proinflammatory cells (eosinophils and Th9 cells) into the lungs.174 Treatment with butyrate also resulted in significantly reduced tissue and vascular disruption, inflammatory infiltrates, and hemorrhaging in the airways brought on by influenza infection in the mice. Additionally, sodium butyrate also has the ability to reduce ACE2 expression in gut epithelial cells, which may assist to alleviate COVID-19-related gastrointestinal symptoms.175 By reducing oxidative stress, NF-κB activation and leukocyte infiltration, butyric acid reduces bleomycin-induced lung fibrosis.176
Butyrate increases M2-like macrophage polarization and also exhibits anti-inflammatory effect by stimulating arginase 1 (ARG1) and consequently inhibiting TNF, IL-6, IL-1b, and Nos2.177 Foxp3, CD25, and CD4 are the markers that can be expressed by regulatory T cells (Treg cells), a subpopulation of T cells that exhibit strong immunosuppressive properties. Treg cells also have a role in regulating autoinflammatory reactions and averting pathogenic immune responses from damaging tissues by secreting a range of anti-inflammatory cytokines.178 Several proinflammatory chemokines (CCL3, CCL4, CCL5, CXCL9, CXCL10, and CXCL11) were found to reduce significantly by SCFAs via regulatory T cells.168 Inflammatory illness may be brought on by Treg cell dysfunction or absence.179,180 By blocking the action of histone deacetylase or facilitating the Foxp3 promoter transcription in the naïve T cells, butyrate can aid in transforming naive T cells into Treg cells.21,23 Propionate, another SCFA stimulates the proliferation of Treg population via GPR43 signaling (Figure 4c).181
Interestingly, a recent study demonstrated that the SARS-CoV-2 entrance or replication in intestinal cells was unaffected by SCFAs. These metabolites exhibited an insignificant impact on the synthesis of antiviral and inflammatory mediators and no influence on the permeability of intestinal cells. This data demonstrate that modifications in the microbial community composition and the metabolites of COVID-19 patients, notably SCFAs, do not intervene with the SARS-CoV-2. However, it is important to note that SCFAs can exert systemic effects, that may be of immense significance for SARS-CoV-2 infection in diverse situations,182 as demonstrated by the examples mentioned above.
Recently, many studies have shown the association between Long COVID and microbiome dysbiosis and, hence, the altered levels of SCFAs. According to the World Health Organization, Long COVID or post-COVID is described as a syndrome that typically appear 3 months after the primary infection of SARS-CoV-2 and persist for a minimum period of 2 months.183 Several recent research on the gut microbiome has focused on the function of gut microbiota derived metabolites such as SCFAs in Long COVID. For instance, lower amounts of butyrate were observed in the stool samples of patients even after 30 days following recovery from severe COVID-19. Furthermore, a fecal metabolite study revealed considerably decreased fecal amounts of SCFAs and l-isoleucine in COVID-19 patients before and after disease remission. Moreover, the scarcity of SCFA and l-isoleucine production was associated with elevated plasma concentrations of CXCL-10, NT-proB-type natriuretic peptide, and CRP.65,184 In another study, the SCFA-producing bacteria were found to be significantly reduced in the recovered patients one year after discharge.185 It has also been shown that butyrate can impact hippocampal function and enhance the expression of brain-derived neurotrophic factor, which have been demonstrated to possess antidepressant-like effects in animal models.186,187 Hence, significantly lower levels of SCFAs and microbiota that contribute to the production of SCFAs may delay rehabilitation of pulmonary and mental symptoms and induce long COVID-19.
12. Intestinal Dysbiosis Influences Lung Health
Perturbation in the gut microbial composition and function, termed as intestinal dysbiosis, can greatly affect the susceptibility of the lungs toward respiratory infections. There are various pieces of evidence which prove that gut microbiota plays a distinct function in enhancing lung health by imparting mucosal immunity. Maintaining a balanced and healthy gut microbiota has proven to be prerequisite for lowering the risk of lung inflammation and respiratory conditions including asthma, COPD, Cystic Fibrosis, etc.188 Several factors including both genetic as well as environmental factors like smoking habits, western lifestyle, diet, nutrient supplements, etc. have been related to influencing the intestinal and lung microbial environment. Studies have shown that microbial dysbiosis was found to be connected with inflammatory bowel disease (IBD) and COPD, the inflammatory conditions in the GI tract and respiratory tract respectively. In IBD and COPD, dysbiosis is characterized by decreased Firmicutes species diversity and increased Proteobacteria species diversity respectively. Interestingly, F. prausnitzii and Roseburia intestinalis, two prominent SCFAs-producing bacteria, were found to have lower abundance in the intestinal mucosa and feces of IBD patients than that of healthy individuals.61 Thus, the loss of microbial diversity and the associated alterations in SCFAs levels are quite prominent in IBD patients and need to be restored via novel therapeutic strategies. Furthermore, the aspect of gut–lung axis is more distinctive in those individuals who have encountered respiratory illnesses along with gut dysbiosis induced by severe irritable bowel syndrome (IBS) or IBD.189,190 For instance, in one of the observational studies conducted by Labarca et al. the risk of IBD was found to be higher in all COPD populations compared to population not having this condition.191 However, further evaluation is still desirable to strengthen the findings by taking other variables, such as cigarette smoking, into consideration. Furthermore, a study involving administration of antibiotics in mice model to deplete gut bacteria renders it more susceptible to pneumonia and increased inflammation of the lung.192 Further, a mice model artificially lacking a gut microbiota has been demonstrated to have altered transcriptome upon assessment of their alveolar macrophages present in the alveoli, thereby resulting in reduced phagocytic activity and bacterial lysis. This shows unequivocally the critical role that gut microbiota plays in efficient functioning of macrophages residing in the lungs. Similar to how respiratory infections caused by a diverse variety of viruses or bacterial pathogens can induce dysbiosis in the intestinal microbiota, it is vital to understand that respiratory infections can influence the intestinal health of an individual in a variety of ways.193,194 Although it is uncertain whether gut microbial dysbiosis is the cause or a result of infectious respiratory disease, gut microbial species and metabolites like SCFAs have been related to significant alterations in immune response of the GI tract as well as the distal organs, including the lungs.195 The occurrence or severity of airway infections and the onset of lung illness have both been shown to be significantly influenced by the commensal microbiota inhabiting the gut.
There have been several mechanisms reported so far by means of which the gut microbiome has an influence on the host immune system and modulates lung inflammation and vice versa with respect to the gut–lung axis. The regulation of extraintestinal lymphocytic T cell populations, systemic inflammation, and production of SCFAs are few of the well-reported mechanisms.196 The intestinal microbiome and its associated metabolites, such as SCFAs, can induce immune cells and inflammatory cytokines that can travel through the bloodstream and enter the systemic circulation, where they can exert immunomodulatory effects in the lung and thereby further affect the respiratory health of an individual. For instance, an intriguing study conducted using a mouse model found a link between lung IgE and Th2 cytokine (IL-4 and IL-5) and allergic airway inflammation.197 Another study revealed that gut microbiota can boost pulmonary granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling and provide respiratory protection from bacterial infections by modifying IL-17A levels.198 Therefore, these results imply that any change in the microbiome composition can pose a significant impact on a person’s intestinal and respiratory health.
13. Role of Probiotics, Prebiotics, and FMTs in Improving Lung Health
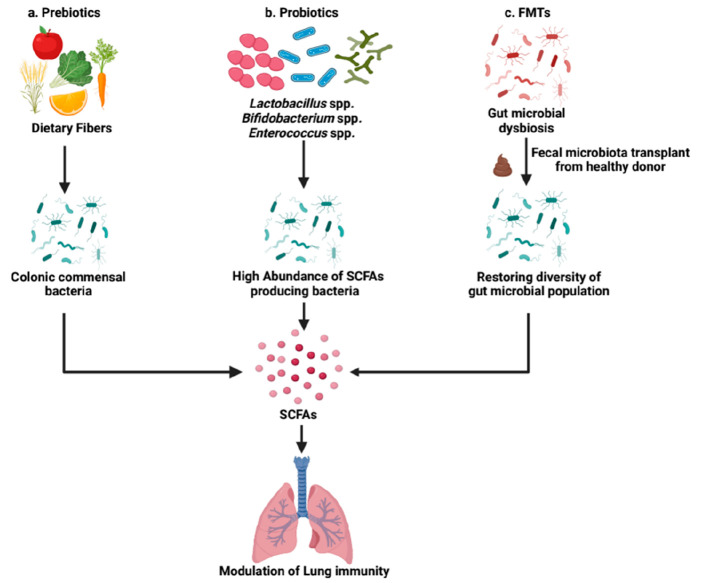
Modulation of microbiome composition directly or indirectly via SCFAs has been explored as a novel treatment strategy to improve the lung health of an individual. In the present scenario of the clinical management of lung disorders, SCFAs are regarded as a promising supplementary treatment approach. It will aid in regaining intestinal balance and offering relief and remission to patients with illness. As far as the infectious and chronic lung diseases are concerned, different treatment strategies can include indirect approaches like supplementation with dietary fibers to produce SCFAs (prebiotics), direct approaches like oral administration of live bacteria which are the key producers of SCFAs [probiotics, FMT] through which they can regulate the resident gut microbiome via diverse mode of action (Figure 5).
Figure 5.
Microbiome-based intervention strategies to improve the lung health of an individual. Modulation of microbiome composition either directly or indirectly via SCFAs has been explored as a novel treatment strategy to modulate lung immunity. (a) Uptake of fiber-rich diet through meals can facilitate the generation of SCFA metabolites upon metabolism by the commensal microbes residing in the gut. (b) The supplementation with probiotics is directed toward increasing the abundance of beneficial bacteria to restore SCFAs levels in dysbiotic individuals. (c) There is an increasing potential of FMTs in managing respiratory tract related disorders through fecal transplant from healthy donor to restore the microbial homeostasis in the gut (FMT – Fecal microbiota transplantation) (Created with https://biorender.com/).
13.1. Prebiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”.199 Prebiotics are usually undigestible dietary substances which improve the host health by facilitating the growth of one or a few specific bacteria in the colon.200 They are well recognized by the names of fructo-oligosaccharides, oligofructose, galacto-oligosaccharides, chicory fiber, pectin, and inulin. These are usually complex carbohydrates which cannot be digested by our body, hence they enter the lower digestive tract and are being metabolized by commensal bacteria in the colon through anaerobic fermentation.200 Prebiotics after fermentation by intestinal bacteria release a variety of metabolites, primarily SCFAs like lactic acid, propionic acid, and butyric acid. These molecules can easily permeate through gut enterocytes and enter blood circulation because of their smaller size. Therefore, prebiotics can exert their protective effect not only on the GI tract but also other distant organs including lungs. They can positively impact the gut environment and also possess several immunomodulatory properties as witnessed in the previous sections of the review, thus conferring an overall health benefit.
Numerous epidemiological and preclinical investigations conducted in the past have related high dietary fiber intake with enhanced lung function and reductions in incidence of COPD and respiratory mortality. There are studies that have highlighted its role in dampening the innate immune-mediated systemic and pulmonary inflammation. There are a number of mechanistic aspects regarding these observed beneficial effects conferred by prebiotics. One of the possible mechanism by which it executes its function is via the inhibitory effect of SCFAs (byproduct of dietary fiber fermentation) on innate immune response by decreasing neutrophils associated inflammation, and macrophage-driven matrix remodeling.201 It does so by interacting with GPCRs or blocking the mevalonate pathway through its rate-limiting enzyme, β-Hydroxy β-methylglutaryl-CoA (HMGCoA) reductase.201 There are numerous studies which suggested that the effect of fiber-rich diet on lung health is mediated via changes in SCFA levels and its associated alteration in microbial composition. Dietary fiber has been shown to directly influence the immune response in the lungs following exposure to allergens or pathogenic substances in murine models. It has been found to be associated with reduction in emphysema development in cigarette smoking-exposed subjects.202 In animal models, such as mice, feeding with a fiber-free diet has made the animal more susceptible to bacterial infections as compared to mice fed with fiber owing to the excessive increase of mucus-degrading bacteria in the gut.78 Fiber-rich diet and subsequent increase in SCFAs levels in the systemic circulation has led to shifts in bacterial abundance (elevated ratio of Bacteroidetes to Firmicutes) in the intestine and the airways.78,203 It conferred protection to mice against allergic inflammation in the lung. Several other studies involving mouse models demonstrated the role of SCFAs in inducing the development and differentiation of extrathymic Treg cells in the colon21,204 and restrict airway inflammation by impeding the DCs’ ability to drive the Th2 effector role and further inhibiting the group 2 innate lymphoid cell (ILC2) activity.78,125 In addition, SCFAs have the ability to improve the immunity of the lungs via alteration of the hematopoiesis of immune cells located in the bone marrow along with a reduction in neutrophils recruitment in the airway and enhance functionality of CD8+ T cells.78,130
Moreover, a cross-sectional research of asthmatic patients in Australia found a negative relationship between dietary fiber consumption and airway inflammation.205 Thus, SCFAs supplementation can alleviate airway inflammation via activation of FFARs during asthma.206 By controlling the activity of T cells and DCs, SCFAs were found to reduce excessive lung inflammation in animal models of allergic airway inflammation.125 Comparable results were observed in COPD animal models as well as patients suffering from COPD. Dietary fiber consumption was associated with improved lung health and decreased incidence of COPD.207 Other studies conducted on infectious disease models like influenza and bacterial pneumonia, administration of SCFAs was found to exhibit a protective role in ameliorating airway inflammation by restoring virus-specific adaptive immune responses and reducing the pro-inflammatory cytokines levels respectively.148,208 There are clinical trials undergoing to unravel the effect of inulin in COPD patients209 and effect of administration of prebiotics in asthmatic subjects to facilitate the growth and proliferation of Bifidobacteria in the gut.210 Together, these studies indicate that dietary fiber intake has significantly aided in the improvement of lung function. We can therefore conclude that high intake of fiber-rich diet encourages the expansion and growth of beneficial SCFA-producing bacteria in the gut, remotely modulating lung immunity. It is regarded as a propitious therapeutic approach for the reduction of the exacerbation during chronic airway inflammation and provides protection against a variety of respiratory bacterial and viral infections via different molecular mechanisms.
13.2. Probiotics
According to ISAAP probiotics is usually defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”.211 They can be found in yogurt and other fermented foods and dietary supplements. Probiotics are composed of a variety of microorganisms. Some of the most commonly used microorganisms include the SCFAs producers that are members of Firmicutes phylum (such as F. prausnitzii and Clostridium leptum), Bacteroidetes, Proteobacteria, Fusobacteria, Spirochaetes, Lactobacillus genera (such as L. rhamnosus GG, L. casei, L. plantarum, L. paracasei), Bifidobacterium genera (such as B. breve, B. animalis, B. longum), and other organisms like Streptococcus thermophilus and Enterococcus faecium.(212,213) Additionally, the phylum Verrucomicrobia comprises of the Akkermansia muciniphila, which degrades mucin, generates both propionate and acetate.
The impact of probiotics on pulmonary diseases has attracted enormous attention. These are often administered as dietary supplements to shoot up the abundance of beneficial bacteria so as to restore the SCFAs levels during dysbiosis in individuals suffering from GI tract and/or respiratory tract related disorders.214 Furthermore, over the past few decades, the potential role of yeast, such as S. cerevisiae and S. boulardii, as probiotic treatments has been demonstrated. Studies conducted on animal models have revealed that S. cerevisiae and S. boulardii were linked to an increase in the abundance of Bacteroidetes and a decrease in the relative abundance of Firmicutes and Proteobacteria of the gut microbiota composition. Additionally, in an experimental model of IBS, mice fed with S. boulardii experienced a marked decrease in gastrointestinal dysfunction.215,216 Hence, yeast can play a potential role in promoting the synthesis of SCFAs in the gut.
Till date, none of the probiotics has been approved as a live biotherapeutic agent, but there are studies which have demonstrated its protective and therapeutic effects with respect to the infections involving the upper respiratory tract in humans.213 For instance, in a randomized double-blind study conducted on Malaysian children belonging to age groups 2–6 years, administration of Bifidobacterium longum BB536 has shown protective effects against upper respiratory diseases. It has significantly increased the abundance of the genus Faecalibacterium, which was found to be correlated with the markers of anti-inflammation and immuno-modulation in the treatment group as compared to that of control.217 In addition, various studies have focused on the protective effects of probiotics in animal models of allergic asthma. They can improve the lung health of an individual by regulating the immune function. In asthmatic murine model, oral intake of Lactobacillus reuteri, Lactobacillus rhamnosus GG, and Bifidobacterium breve decreased the number of inflammatory cells in bronchoalveolar lavage (BAL) fluid, airway hyperresponsiveness, and inflammation of the lung tissue.214 It was accompanied by a reduction in the expression of pulmonary type 2 cytokines (IL-4, IL-13, and IL-5) and an activation of regulatory T cells and anti-inflammatory cytokines like TGF-β.218 Interestingly, in one of the studies using the mice model, the association between probiotics and development of Th17 cells has been critically examined.
The oral supplementation of Enterococcus faecalis FK-23 has yielded favorable outcomes whereby it has successfully suppressed the asthmatic response and dampen the Th17 cell development.219 Although a huge number of studies have been conducted to investigate the beneficial effects of probiotics, studies involving human trials to examine its efficiency in order to cure asthma remain inconclusive. Numerous reviews have highlighted the intriguing interaction between the intestinal and pulmonary mucosa in chronic inflammatory diseases such as COPD. The natural killer (NK) cells were found to be involved in regulating the inflammatory response during COPD exacerbations. The NK cell activity was found to be extensively lower in smokers in comparison to nonsmokers. However, oral intake of Lactobacillus casei strain Shirota (LcS) increased its activity in smokers.220 Hence, this evidence is suggestive of the beneficial effects of probiotics in COPD patients, but it needs to be validated clinically prior any affirmation.
Furthermore, the benefits of consuming probiotics have been explored in animal models during viral and bacterial infections of the lower respiratory tract, as well. Emerging evidence suggested that oral feeding of probiotics Lactobacilli and Bifidobacteria have shown favorable outcomes by clearance of influenza virus, pneumonia virus infection, and RSV and modulation of inflammatory responses and immune cell signaling in mice models of infection.221−223 Moreover, the oral or intranasal administration of Lactobacillus rhamnosus GG and Lactobacillus casei strain Shirota (LcS) and DN114001 was found to confer protective benefits in treating viral and bacterial infections associated with the digestive and respiratory tract including influenza.214,224 Probiotic dietary supplements have been the subject of numerous studies to examine whether they aid in decreasing the frequency and duration of acute lung infections. According to one study, probiotics are significantly more efficient than placebo controls at shortening the duration and frequency of upper respiratory tract infections.225
There are currently a limited number of studies examining probiotics’ effectiveness in treating lower respiratory tract infections. Interestingly, a study on COVID-19 patients who received a combination of bacterial strain composition demonstrated a significant decrease in the probability of respiratory failure in comparison to the untreated group.226 Even if the pandemic is waning due to vaccination, it would be very interesting to see if the beneficial properties of probiotics can assist in the faster recovery of COVID-19 patients. As these studies yielded promising results, they urge additional research into further exploring the clinical evidence to support the therapeutic effect of probiotics in the setting of lower respiratory tract diseases.
13.3. FMT
The FMT mainly involves injecting liquid or encapsulated preprocessed feces from a healthy donor into the colon of a recipient who is thought to have gut dysbiosis in order to restore the normal gut function.227 It has become very popular and rapidly accepted by medical professionals as an efficient biotherapeutic intervention for the treatment of various bacterial infections, mainly recurring Clostridium difficile infection (CDI)228 and even recommended for other metabolic disorders like obesity and diabetes.229 FMT has been proposed as a treatment choice for IBD as well, however the preliminary data concerning the effectiveness of FMT for IBD was quite conflicting and warrants in-depth investigations.230 Such preliminary examinations have sparked interest to dig deeper and explore the potential application of FMTs in managing respiratory tract related disorders by restoring microbial homeostasis in the gut.
Nowadays, there is a rising interest in treating SARS-CoV-2 patients by administering them with FMTs. A very first clinical trial has been proposed in the year 2021, to assess the efficacy of FMTs in COVID patients to dampen the exacerbated cytokine storm and associated lung inflammation.231 As far as respiratory infections are concerned, apart from this, there are no such clinical studies on deciphering the therapeutic potential of FMTs for the management of respiratory diseases. Furthermore, there are other issues related to administration of FMTs that have raised serious concerns over its safety and applicability that have come into existence. However, there is always a scope for improvement as far as the pathophysiological, methodological, mechanistic factors are concerned.230 This has shifted the focus of researchers on preparing formulations that are well-characterized in terms of their physiological characteristics. This may consist of either a mixture of live microbial population derived from feces or the usage of different strains of probiotic so as to minimize the clinical risk for the recipient.
14. Conclusions
Numerous studies have emerged over the past 20 years concerning the functions of the local microbiota in health and illness. The myth of airway sterility has been disproved, and research into the mechanisms of lung colonization has begun recently. The significance of metabolites such as SCFAs originating from the microbiota has been highlighted by these studies, which encourage researchers to investigate the underlying mechanisms behind the gut–lung axis.
In this review, we have emphasized dissecting the effect of gut produced SCFAs in modulating lung immunity. Moreover, we have highlighted the role of SCFAs in the management of lung disease progression and therapeutics including COVID-19. Hence, microbial metabolites could potentially be applied in the clinic to promote healthy and balanced conditions in addition to nutrition. It is apparent that the gut–lung axis is bidirectional, and the mechanisms by which the lung influences the intestinal environment should also be equally explored to gain better mechanistic insight into the pathways and mediator molecules associated with the lung. It is now widely accepted that alterations in the gut microbiome composition and physiology are brought on by both chronic and acute lung diseases. It is now recognized that once-thought-to-be-sterile lungs have a distinctive microbiota. Thus, future research is required to determine the effect of colonization of the lung on host physiology by investigating the lung ecosystem. Supplementation with probiotics, prebiotics, and FMT are considered as novel intervention strategies to modify the gut microbiota, and their protective effects have been investigated in numerous lung diseases over a period of time as evident through various clinical and experimental studies. Although these approaches have yielded promising results in most cases, there are certain challenges that have adhered to them when it comes to their application in the clinical setting. Since probiotics, prebiotics, and FMT fall under the strict regulatory umbrella of foods or nutritional supplements, they are typically out of reach for the general public. Furthermore, it is very difficult to provide experimental evidence for probiotics’ labeled health claims because there are so many variables to take into account, including the species, strain, and dosage of the probiotics, the duration of the treatment and its effects, the type of host response, etc. Similarly, the administration of FMT requires further clinical evaluation to mitigate the serious concerns raised over its safety and applicability.
Deeper investigations may open an avenue to fresh approaches to treat a variety of respiratory disorders. Furthermore, some people tend to be more resistant to colonization than others; it has been proposed that usage of standardized dosage of purified microbial components demonstrating beneficial effects may be able to answer the question of whether living probiotic strains can colonize and function in the human tract. In recent years, various studies on the bacterial microbiota components has dominated; however, less is known about the role of other microbes: fungi, protozoa, helminths, viruses, and phages, which may prove to be of equal significance. The potential therapeutic application could be ascertained by a deeper understanding of the molecular and cellular mechanisms underpinning SCFAs effects and their role in establishing complex cross-talk between the gut–lung axis.
Acknowledgments
Authors would like to acknowledge Indian Council of Medical Research (ICMR), India (61/06/2020/IMM/BMS), and Council of Scientific and Industrial Research (CSIR), India, for research support. U.K.S. acknowledges the support of Fogarty International Center (FIC) of the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grant #3D43TW009345-08S1 awarded to the Northern Pacific Global Health Fellows Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.T. acknowledges the support of UGC-Faculty Recharge Programme (UGC-FRP).
Glossary
Abbreviations
- AAI
Allergic airway inflammation
- AAM
Alternatively activated macrophages
- ABC
ATP-binding cassette
- ABX
Antibiotic cocktail
- ACE2
Angiotensin-converting enzyme 2
- ALI
Acute lung injury
- APEC
Avian pathogenic Escherichia coli
- ARG1
Arginase 1
- BAL
Bronchoalveolar lavage
- BCAAs
Branched-chain amino acids
- BCFAs
Branched-chain fatty acids
- Bkd
Branched-chain keto acid dehydrogenase complex
- CDI
Clostridium difficile infection (CDI)
- CDPs
Common DC progenitors
- COPD
Chronic obstructive pulmonary disease
- DCs
Dendritic cells
- EBC
Exhaled breath condensate
- EMP pathway
Embden–Meyerhof–Parnas pathway
- FabH
3-Ketoacyl-ACP synthase III
- FBA
N-(1-Carbamoyl-2-phenyl-ethyl) butyramide
- FFAR
Free fatty acid receptor
- FMT
Fecal microbiota transplantation
- GF
Germ-free
- GM
Gut microbiota
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GPCRs
G protein coupled receptors
- HAT
Histone acetyltransferase
- HCAR2
Hydroxycarboxylic acid receptor 2
- HDACs
Histone deacetylases
- HMGCoA reductase
β-Hydroxy β-methylglutaryl-CoA reductase
- HPLC
High-performance liquid chromatography
- IBD
Irritable bowel disease
- IBS
Irritable bowel syndrome
- IECs
Intestinal epithelial cells
- IL-15
Interleukin-15
- ILCs
Innate lymphoid cells
- IlvE
BCAA transaminase
- ISAPP
International Scientific Association for Probiotics and Prebiotics
- LcS
Lactobacillus casei strain Shirota
- LPS
Lipopolysaccharides
- MAMPs
Microbe associated molecular patterns
- MCP-1
Monocyte chemoattractant protein-1
- MCTs
Monocarboxylate transporters
- MDPs
Macrophage and DC progenitors
- NGS
Next-generation sequencing
- NK cells
Natural killer cells
- NLR
Nod-like receptors
- NSP
Nonstarch polysaccharides
- OAA
Oxaloacetate
- OVA
Ovalbumin
- PRR
Pattern-recognition receptors
- PTS
Phosphotransferase system
- RS
Resistant starch
- SCFAs
Short chain fatty acids
- SMCTs
Sodium-coupled monocarboxylate transporters
- TCA cycle
Tricarboxylic acid cycle
- Th-2 cells
T helper 2 cells
- TLR
Toll-like receptors
- TNF-α
Tumor necrosis factor-α
Author Present Address
# Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA
The authors declare no competing financial interest.
References
- Thursby E.; Juge N. Introduction to the Human Gut Microbiota. Biochemical J. 2017, 474, 1823–1836. 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R.; Fuchs S.; Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biology 2016, 14, e1002533 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson G. P.; Lee S. M.; Mazmanian S. K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14 (1), 20. 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A.; Neish A. Role of Gut Microbiota in Intestinal Wound Healing and Barrier Function. Tissue Barriers 2018, 6, 1539595. 10.1080/21688370.2018.1539595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.-Y.; Ning M.-X.; Chen D.-K.; Ma W.-T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. 10.3389/fimmu.2019.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Keeler J.; Weitkamp J.-H. Maternal Influences on Fetal Microbial Colonization and Immune Development. Pediatr. Res. 2015, 77, 189. 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello M. G.; Costello E. K.; Contreras M.; Magris M.; Hidalgo G.; Fierer N.; Knight R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (26), 11971–11975. 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi F.; Salvatori G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Inf. Microbiol. 2012, 94. 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J. M.; Murphy K.; Stanton C.; Ross R. P.; Kober O. I.; Juge N.; Avershina E.; Rudi K.; Narbad A.; Jenmalm M. C.; Marchesi J. R.; Collado M. C. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C. A.; Stombaugh J. I.; Gordon J. I.; Jansson J. K.; Knight R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S. Metabolomics: Applications to Food Science and Nutrition Research. Trends Food Sci. Technol. 2008, 19 (9), 482–493. 10.1016/j.tifs.2008.03.003. [DOI] [Google Scholar]
- Scott K. P.; Gratz S. W.; Sheridan P. O.; Flint H. J.; Duncan S. H. The Influence of Diet on the Gut Microbiota. Pharmacol. Res. 2013, 69 (1), 52–60. 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Verbeke K. A.; Boobis A. R.; Chiodini A.; Edwards C. A.; Franck A.; Kleerebezem M.; Nauta A.; Raes J.; Van Tol E. A. F.; Tuohy K. M. Towards Microbial Fermentation Metabolites as Markers for Health Benefits of Prebiotics. Nutr. Res. Rev. 2015, 28 (1), 42–66. 10.1017/S0954422415000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. S.; Davies S. S. Microbial Metabolism of Dietary Components to Bioactive Metabolites: Opportunities for New Therapeutic Interventions. Genome Medicine 2016, 8, 46. 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F.-P. J.; Collino S.; Rezzi S.; Kochhar S. Metabolomic Applications to Decipher Gut Microbial Metabolic Influence in Health and Disease. Front. Physiol. 2012, 3 (1), 113. 10.3389/fphys.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrema H.; IJzerman R. G.; Nieuwdorp M. Emerging Role of Intestinal Microbiota and Microbial Metabolites in Metabolic Control. Diabetologia 2017, 60 (4), 613–617. 10.1007/s00125-016-4192-0. [DOI] [PubMed] [Google Scholar]
- Sonnenburg J. L.; Bäckhed F. Diet-Microbiota Interactions as Moderators of Human Metabolism. Nature 2016, 535 (7610), 56–64. 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H. J. Gut Microbial Metabolites in Health and Disease. Gut Microbes 2016, 7 (3), 187–188. 10.1080/19490976.2016.1182295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane S.; Sen P.; Dickens A. M.; Orešič M.; Bertram H. C. Gut Metabolome Meets Microbiome: A Methodological Perspective to Understand the Relationship between Host and Microbe. Methods 2018, 149, 3–12. 10.1016/j.ymeth.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Wypych T. P.; Wickramasinghe L. C.; Marsland B. J. The Influence of the Microbiome on Respiratory Health. Nat. Immunol. 2019, 20 (10), 1279–1290. 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- Arpaia N.; Campbell C.; Fan X.; Dikiy S.; Van Der Veeken J.; Deroos P.; Liu H.; Cross J. R.; Pfeffer K.; Coffer P. J.; Rudensky A. Y. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504 (7480), 451–455. 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S.; Toh H.; Hase K.; Oshima K.; Nakanishi Y.; Yoshimura K.; Tobe T.; Clarke J. M.; Topping D. L.; Suzuki T.; Taylor T. D.; Itoh K.; Kikuchi J.; Morita H.; Hattori M.; Ohno H. Bifidobacteria Can Protect from Enteropathogenic Infection through Production of Acetate. Nature 2011, 469 (7331), 543–547. 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Furusawa Y.; Obata Y.; Fukuda S.; Endo T. A.; Nakato G.; Takahashi D.; Nakanishi Y.; Uetake C.; Kato K.; Kato T.; Takahashi M.; Fukuda N. N.; Murakami S.; Miyauchi E.; Hino S.; Atarashi K.; Onawa S.; Fujimura Y.; Lockett T.; Clarke J. M.; Topping D. L.; Tomita M.; Hori S.; Ohara O.; Morita T.; Koseki H.; Kikuchi J.; Honda K.; Hase K.; Ohno H. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504 (7480), 446–450. 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Den Besten G.; Van Eunen K.; Groen A. K.; Venema K.; Reijngoud D. J.; Bakker B. M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54 (9), 2325–2340. 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Short Chain Fatty Acids in the Human Colon. Gut. 1981, 22, 763–779. 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L.; Clifton P. M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81 (3), 1031–1064. 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Louis P.; Scott K. P.; Duncan S. H.; Flint H. J. Understanding the Effects of Diet on Bacterial Metabolism in the Large Intestine. J. Appl. Microbiol. 2007, 102 (5), 1197–1208. 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- Fredstrom S. B.; Lampe J. W.; Jung H. J. G.; Slavin J. L. Apparent Fiber Digestibility and Fecal Short-Chain Fatty Acid Concentrations With Ingestion of Two Types of Dietary Fiber. J. Parenter. Enter. Nutr. 1994, 18 (1), 14–19. 10.1177/014860719401800114. [DOI] [PubMed] [Google Scholar]
- Flint H. J.; Scott K. P.; Duncan S. H.; Louis P.; Forano E. Microbial Degradation of Complex Carbohydrates in the Gut. Gut Microbes 2012, 3, 289–306. 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant K.; Allen-Vercoe E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019 71 2019, 7 (1), 1–15. 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.; McKenzie C.; Potamitis M.; Thorburn A. N.; Mackay C. R.; Macia L.. The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Academic Press, 2014; Vol. 121, pp 91–119. 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Miller T. L.; Wolin M. J. Pathways of Acetate, Propionate, and Butyrate Formation by the Human Fecal Microbial Flora. Appl. Environ. Microbiol. 1996, 62 (5), 1589–1592. 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P.; Hold G. L.; Flint H. J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12 (10), 661–672. 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Reichardt N.; Duncan S. H.; Young P.; Belenguer A.; McWilliam Leitch C.; Scott K. P.; Flint H. J.; Louis P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8 (6), 1323–1335. 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Huang S.; Wang Y.; Cai S.; Yu H.; Liu H.; Zeng X.; Zhang G.; Qiao S. Bridging Intestinal Immunity and Gut Microbiota by Metabolites. Cell. Mol. Life Sci. 2019, 76 (20), 3917. 10.1007/s00018-019-03190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P.; Duncan S. H.; McCrae S. I.; Millar J.; Jackson M. S.; Flint H. J. Restricted Distribution of the Butyrate Kinase Pathway among Butyrate-Producing Bacteria from the Human Colon. J. Bacteriol. 2004, 186 (7), 2099–2106. 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P.; Young P.; Holtrop G.; Flint H. J. Diversity of Human Colonic Butyrate-Producing Bacteria Revealed by Analysis of the Butyryl-CoA:Acetate CoA-Transferase Gene. Environ. Microbiol. 2010, 12 (2), 304–314. 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- Roager H. M.; Hansen L. B. S.; Bahl M. I.; Frandsen H. L.; Carvalho V.; Gøbel R. J.; Dalgaard M. D.; Plichta D. R.; Sparholt M. H.; Vestergaard H.; Hansen T.; Sicheritz-Pontén T.; Nielsen H. B.; Pedersen O.; Lauritzen L.; Kristensen M.; Gupta R.; Licht T. R. Colonic Transit Time Is Related to Bacterial Metabolism and Mucosal Turnover in the Gut. Nat. Microbiol. 2016, 1 (9), 16093. 10.1038/nmicrobiol.2016.93. [DOI] [PubMed] [Google Scholar]
- Yao C. K.; Muir J. G.; Gibson P. R. Review Article: Insights into Colonic Protein Fermentation, Its Modulation and Potential Health Implications. Aliment. Pharmacol. Ther. 2016, 43 (2), 181–196. 10.1111/apt.13456. [DOI] [PubMed] [Google Scholar]
- Madsen L.; Myrmel L. S.; Fjære E.; Liaset B.; Kristiansen K. Links between Dietary Protein Sources, the Gut Microbiota, and Obesity. Front. Physiol. 2017, 8, 1047. 10.3389/fphys.2017.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant K.; Allen-Vercoe E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7 (1), 91. 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P.; Li L.; Rezaei A.; Eslamfam S.; Che D.; Ma X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and Their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16 (7), 646–654. 10.2174/1389203716666150630133657. [DOI] [PubMed] [Google Scholar]
- Portune K. J.; Beaumont M.; Davila A.-M.; Tome D.; Blachier F.; Sanz Y. Gut Microbiota Role in Dietary Protein Metabolism and Health-Related Outcomes: The Two Sides of the Coin. Trends in Food Science & Technology 2016, 57, 213–232. 10.1016/j.tifs.2016.08.011. [DOI] [Google Scholar]
- Kanehisa M.; Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28 (1), 27–30. 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P.; Flint H. J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19 (1), 29–41. 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- Fischbach M. A.; Sonnenburg J. L. Eating for Two: How Metabolism Establishes Interspecies Interactions in the Gut. Cell Host Microbe 2011, 10 (4), 336–347. 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Chen H.; Van Treuren W.; Hou B.-H.; Higginbottom S. K.; Dodd D. Clostridium Sporogenes Uses Reductive Stickland Metabolism in the Gut to Generate ATP and Produce Circulating Metabolites. Nat. Microbiol. 2022, 7 (5), 695–706. 10.1038/s41564-022-01109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley S. G.; Frank M. W.; Rock C. O. A Short-Chain Acyl-CoA Synthetase That Supports Branched-Chain Fatty Acid Synthesis in Staphylococcus Aureus. J. Biol. Chem. 2023, 299 (4), 103036 10.1016/j.jbc.2023.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidy S.; Van Den Abbeele P.; Van De Wiele T.; Louis P.; Kleerebezem M. Intestinal Colonization: How Key Microbial Players Become Established in This Dynamic Process: Microbial Metabolic Activities and the Interplay between the Host and Microbes. Bioessays 2013, 35 (10), 913–923. 10.1002/bies.201300073. [DOI] [PubMed] [Google Scholar]
- Duncan S. H.; Louis P.; Flint H. J. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl. Environ. Microbiol. 2004, 70 (10), 5810–5817. 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. H.; Holtrop G.; Lobley G. E.; Calder A. G.; Stewart C. S.; Flint H. J. Contribution of Acetate to Butyrate Formation by Human Faecal Bacteria. Br. J. Nutr. 2004, 91 (6), 915–923. 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- Belenguer A.; Duncan S. H.; Calder A. G.; Holtrop G.; Louis P.; Lobley G. E.; Flint H. J. Two Routes of Metabolic Cross-Feeding between Bifidobacterium Adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Environ. Microbiol. 2006, 72 (5), 3593–3599. 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G.; Vlachou A.; Verbrugghe K.; De Vuyst L. Cross-Feeding between Bifidobacterium Longum BB536 and Acetate-Converting, Butyrate-Producing Colon Bacteria during Growth on Oligofructose. Appl. Environ. Microbiol. 2006, 72 (12), 7835. 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte G. H. M.; Prins R. A.; Janssen R. H. A. M.; De Bie M. J. A. Role of Megasphaera Elsdenii in the Fermentation of Dl-[2-C]Lactate in the Rumen of Dairy Cattle. Appl. Environ. Microbiol. 1981, 42 (4), 649–655. 10.1128/aem.42.4.649-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann P.; Ahring B. K.; Mah R. A. Acetate Production by Methanogenic Bacteria. Appl. Environ. Microbiol. 1989, 55 (9), 2257–2261. 10.1128/aem.55.9.2257-2261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald M. A.; Rey F. E.; Seedorf H.; Turnbaugh P. J.; Fulton R. S.; Wollam A.; Shah N.; Wang C.; Magrini V.; Wilson R. K.; Cantarel B. L.; Coutinho P. M.; Henrissat B.; Crock L. W.; Russell A.; Verberkmoes N. C.; Hettich R. L.; Gordon J. I. Characterizing a Model Human Gut Microbiota Composed of Members of Its Two Dominant Bacterial Phyla. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (14), 5859–5864. 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Covián D.; Ruas-Madiedo P.; Margolles A.; Gueimonde M.; De los Reyes-Gavilán C. G.; Salazar N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7 (FEB), 185. 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziętek M.; Celewicz Z.; Szczuko M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13 (4), 1244. 10.3390/nu13041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby H. B.; Ferguson J. F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross Talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23 (2), 8 10.1007/s11906-020-01125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile B.; Van Oudenhove L.; Vervliet B.; Verbeke K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nature Reviews Gastroenterology and Hepatology 2019, 16, 461–478. 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D.; De la Fuente M. K.; Landskron G.; Gonzalez M. J.; Quera R.; Dijkstra G.; Harmsen H. J. M.; Faber K. N.; Hermoso M. A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cuesta-Zuluaga J.; Mueller N. T.; Álvarez-Quintero R.; Velásquez-Mejía E. P.; Sierra J. A.; Corrales-Agudelo V.; Carmona J. A.; Abad J. M.; Escobar J. S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2019, 11 (1), 51 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villodre Tudela C.; Boudry C.; Stumpff F.; Aschenbach J. R.; Vahjen W.; Zentek J.; Pieper R. Down-Regulation of Monocarboxylate Transporter 1 (MCT1) Gene Expression in the Colon of Piglets Is Linked to Bacterial Protein Fermentation and pro-Inflammatory Cytokine-Mediated Signalling. Br. J. Nutr. 2015, 113 (4), 610–617. 10.1017/S0007114514004231. [DOI] [PubMed] [Google Scholar]
- Ferrer-Picón E.; Dotti I.; Corraliza A. M.; Mayorgas A.; Esteller M.; Perales J. C.; Ricart E.; Masamunt M. C.; Carrasco A.; Tristán E.; Esteve M.; Salas A. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26 (1), 43–55. 10.1093/ibd/izz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Detrimental Effects of COVID-19 in the Brain and Therapeutic Options for Long COVID: The Role of Epstein-Barr Virus and the Gut-Brain Axis. Mol. Psychiatry 2023, 10.1038/s41380-023-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H.; Pomare E. W.; Branch H. W. J.; Naylor E.; Macfarlane G. T. Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood. Gut 1987, 28, 1221. 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasubuchi M.; Hasegawa S.; Hiramatsu T.; Ichimura A.; Kimura I. Dietary Gut Microbial Metabolites, Short-Chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7 (4), 2839–2849. 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D. R.; Garge N.; Zhang X.; Sun W.; O’Connell T. M.; Bunger M. K.; Bultman S. J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13 (5), 517–526. 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G.; Sleeth M. L.; Sahuri-Arisoylu M.; Lizarbe B.; Cerdan S.; Brody L.; Anastasovska J.; Ghourab S.; Hankir M.; Zhang S.; Carling D.; Swann J. R.; Gibson G.; Viardot A.; Morrison D.; Thomas E. L.; Bell J. D. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S.; Bouley C.; Boutron M.-C.; Cummings J. H.; Franck A.; Gibson G. R.; Isolauri E.; Moreau M.-C.; Roberfroid M.; Rowland I. Functional Food Science and Gastrointestinal Physiology and Function. Br. J. Nutr. 1998, 80 (S1), S147–S171. 10.1079/BJN19980108. [DOI] [PubMed] [Google Scholar]
- Wiltrout D. W.; Satter L. D. Contribution of Propionate to Glucose Synthesis in the Lactating and Nonlactating Cow. J. Dairy Sci. 1972, 55 (3), 307–317. 10.3168/jds.S0022-0302(72)85487-0. [DOI] [PubMed] [Google Scholar]
- van der Beek C. M.; Bloemen J. G.; van den Broek M. A.; Lenaerts K.; Venema K.; Buurman W. A.; Dejong C. H. Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans. J. Nutr. 2015, 145 (9), 2019–2024. 10.3945/jn.115.211193. [DOI] [PubMed] [Google Scholar]
- Bloemen J. G.; Olde Damink S. W. M.; Venema K.; Buurman W. A.; Jalan R.; Dejong C. H. C. Short Chain Fatty Acids Exchange: Is the Cirrhotic, Dysfunctional Liver Still Able to Clear Them?. Clin. Nutr. 2010, 29 (3), 365–369. 10.1016/j.clnu.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T.; Gibson G. R.; Cummings J. H. Comparison of Fermentation Reactions in Different Regions of the Human Colon. J. Appl. Bacteriol. 1992, 72 (1), 57–64. 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Bloemen J. G.; Venema K.; van de Poll M. C.; Olde Damink S. W.; Buurman W. A.; Dejong C. H. Short Chain Fatty Acids Exchange across the Gut and Liver in Humans Measured at Surgery. Clin. Nutr. 2009, 28 (6), 657–661. 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Morrison D. J.; Preston T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani P.; Santhakumar P.; Hu Q.; Djiadeu P.; Wolever T. M. S.; Palaniyar N.; Grasemann H. Short-Chain Fatty Acids Affect Cystic Fibrosis Airway Inflammation and Bacterial Growth. Eur. Respir. J. 2015, 46 (4), 1033–1045. 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- Trompette A.; Gollwitzer E. S.; Yadava K.; Sichelstiel A. K.; Sprenger N.; Ngom-Bru C.; Blanchard C.; Junt T.; Nicod L. P.; Harris N. L.; Marsland B. J. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat. Med. 2014, 20 (2), 159–166. 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- Mirzaei R.; Dehkhodaie E.; Bouzari B.; Rahimi M.; Gholestani A.; Hosseini-Fard S. R.; Keyvani H.; Teimoori A.; Karampoor S. Dual Role of Microbiota-Derived Short-Chain Fatty Acids on Host and Pathogen. Biomed. Pharmacother. 2022, 145, 112352 10.1016/j.biopha.2021.112352. [DOI] [PubMed] [Google Scholar]
- Woo V.; Alenghat T. Epigenetic Regulation by Gut Microbiota. Gut Microbes 2022, 14 (1), 2022407 10.1080/19490976.2021.2022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay I.; Gundamaraju R.; Jha N. K.; Gupta P. K.; Dey A.; Mandal C. C.; Ford B. M. Interplay between Dysbiosis of Gut Microbiome, Lipid Metabolism, and Tumorigenesis: Can Gut Dysbiosis Stand as a Prognostic Marker in Cancer?. Dis. Markers 2022, 2022, 2941248 10.1155/2022/2941248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.; Zhang P.; Shen L.; Niu L.; Tan Y.; Chen L.; Zhao Y.; Bai L.; Hao X.; Li X.; Zhang S.; Zhu L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21 (17), 6356. 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretta M. D.; Quiroga J.; López R.; Hidalgo M. A.; Burgos R. A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739 10.3389/fphys.2021.662739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A.; De Vadder F.; Kovatcheva-Datchary P.; Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165 (6), 1332–1345. 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Johnstone R. W. Histone-Deacetylase Inhibitors: Novel Drugs for the Treatment of Cancer. Nat. Rev. Drug Discovery 2002, 1 (4), 287–299. 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- Marsland B. J.; Gollwitzer E. S. Host-Microorganism Interactions in Lung Diseases. Nat. Rev. Immunol. 2014, 14 (12), 827–835. 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
- Morris A.; Beck J. M.; Schloss P. D.; Campbell T. B.; Crothers K.; Curtis J. L.; Flores S. C.; Fontenot A. P.; Ghedin E.; Huang L.; Jablonski K.; Kleerup E.; Lynch S. V.; Sodergren E.; Twigg H.; Young V. B.; Bassis C. M.; Venkataraman A.; Schmidt T. M.; Weinstock G. M. Comparison of the Respiratory Microbiome in Healthy Nonsmokers and Smokers. Am. J. Respir Crit Care Med. 2013, 187 (10), 1067–1075. 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlerberth I.; Wold A. E. Establishment of the Gut Microbiota in Western Infants. Acta Paediatr. 2009, 98 (2), 229–238. 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- Bezirtzoglou E.; Stavropoulou E. Immunology and Probiotic Impact of the Newborn and Young Children Intestinal Microflora. Anaerobe 2011, 17 (6), 369–374. 10.1016/j.anaerobe.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Arumugam M.; Raes J.; Pelletier E.; Paslier D. Le; Yamada T.; Mende D. R.; Fernandes G. R.; Tap J.; Bruls T.; Batto J. M.; Bertalan M.; Borruel N.; Casellas F.; Fernandez L.; Gautier L.; Hansen T.; Hattori M.; Hayashi T.; Kleerebezem M.; Kurokawa K.; Leclerc M.; Levenez F.; Manichanh C.; Nielsen H. B.; Nielsen T.; Pons N.; Poulain J.; Qin J.; Sicheritz-Ponten T.; Tims S.; Torrents D.; Ugarte E.; Zoetendal E. G.; Wang J.; Guarner F.; Pedersen O.; de Vos W. M.; Brunak S.; Doré J.; Weissenbach J.; Ehrlich S. D.; Bork P.; Antolín M.; Artiguenave F.; Blottiere H. M.; Almeida M.; Brechot C.; Cara C.; Chervaux C.; Cultrone A.; Delorme C.; Denariaz G.; Dervyn R.; Foerstner K. U.; Friss C.; van de Guchte M.; Guedon E.; Haimet F.; Huber W.; van Hylckama-Vlieg J.; Jamet A.; Juste C.; Kaci G.; Knol J.; Kristiansen K.; Lakhdari O.; Layec S.; Roux K. Le; Maguin E.; Mérieux A.; Minardi R. M.; M’rini C.; Muller J.; Oozeer R.; Parkhill J.; Renault P.; Rescigno M.; Sanchez N.; Sunagawa S.; Torrejon A.; Turner K.; Vandemeulebrouck G.; Varela E.; Winogradsky Y.; Zeller G. Enterotypes of the Human Gut Microbiome. Nature 2011, 473 (7346), 174–180. 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lif Holgerson P.; Harnevik L.; Hernell O.; Tanner A. C. R.; Johansson I. Mode of Birth Delivery Affects Oral Microbiota in Infants. J. Dent. Res. 2011, 90 (10), 1183–1188. 10.1177/0022034511418973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal L. N.; Clemente J. C.; Tsay J. C. J.; Koralov S. B.; Keller B. C.; Wu B. G.; Li Y.; Shen N.; Ghedin E.; Morris A.; Diaz P.; Huang L.; Wikoff W. R.; Ubeda C.; Artacho A.; Rom W. N.; Sterman D. H.; Collman R. G.; Blaser M. J.; Weiden M. D. Enrichment of the Lung Microbiome with Oral Taxa Is Associated with Lung Inflammation of a Th17 Phenotype. Nat. Microbiol. 2016, 1 (5), 16031 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon K. P.; Klepac-Ceraj V.; Schiffer H. K.; Brodie E. L.; Lynch S. V.; Kolter R. Comparative Analyses of the Bacterial Microbiota of the Human Nostril and Oropharynx. MBio 2010, 1 (3), e00129-10 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard A. F.; Staudinger B. J.; Dowd S. E.; Joshi-Datar A.; Wolcott R. D.; Aitken M. L.; Fligner C. L.; Singh P. K. Direct Sampling of Cystic Fibrosis Lungs Indicates That DNA-Based Analyses of Upper-Airway Specimens Can Misrepresent Lung Microbiota. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (34), 13769–13774. 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassis C. M.; Erb-Downward J. R.; Dickson R. P.; Freeman C. M.; Schmidt T. M.; Young V. B.; Beck J. M.; Curtis J. L.; Huffnagle G. B. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. MBio 2015, 6 (2), e00037-15 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. P.; Erb-Downward J. R.; Martinez F. J.; Huffnagle G. B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson E. S.; Bittinger K.; Haas A. R.; Fitzgerald A. S.; Frank I.; Yadav A.; Bushman F. D.; Collman R. G. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am. J. Respir. Crit. Care Med. 2011, 184 (8), 957–963. 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. M.; Young V. B.; Huffnagle G. B. The Microbiome of the Lung. Transl. Res. 2012, 160 (4), 258–266. 10.1016/j.trsl.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F. E.; Chen T.; Izard J.; Paster B. J.; Tanner A. C. R.; Yu W. H.; Lakshmanan A.; Wade W. G. The Human Oral Microbiome. J. Bacteriol. 2010, 192 (19), 5002–5017. 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-kumar M.; Aitken J. D.; Carvalho F. A.; Cullender T. C.; Mwangi S.; Srinivasan S.; Sitaraman S. V; Knight R.; Ley R. E.; Gewirtz A. T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 2010, 328, 228–231. 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C.; Lipuma L.; Gobourne A.; Viale A.; Leiner I.; Equinda M.; Khanin R.; Pamer E. G. Familial Transmission Rather than Defective Innate Immunity Shapes the Distinct Intestinal Microbiota of TLR-Deficient Mice. J. Exp. Med. 2012, 209 (8), 1445–1456. 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamantopoulos M.; Ronchi F.; Van Hauwermeiren F.; Vieira-Silva S.; Yilmaz B.; Martens L.; Saeys Y.; Drexler S. K.; Yazdi A. S.; Raes J.; Lamkanfi M.; McCoy K. D.; Wullaert A. Nlrp6- and ASC-Dependent Inflammasomes Do Not Shape the Commensal Gut Microbiota Composition. Immunity 2017, 47 (2), 339–348.e4. 10.1016/j.immuni.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Huffnagle G. B.; Dickson R. P.; Lukacs N. W. The Respiratory Tract Microbiome and Lung Inflammation: A Two-Way Street. Mucosal Immunol. 2017, 10 (2), 299–306. 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. P.; Huffnagle G. B. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015, 11 (7), e1004923 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingenito E. P.; Solway J.; McFadden E. R.; Pichurko B.; Bowman H. F.; Michaels D.; Drazen J. M. Indirect Assessment of Mucosal Surface Temperatures in the Airways: Theory and Tests. J. Appl. Physiol. 1987, 63 (5), 2075–2083. 10.1152/jappl.1987.63.5.2075. [DOI] [PubMed] [Google Scholar]
- Wu H.; Kuzmenko A.; Wan S.; Schaffer L.; Weiss A.; Fisher J. H.; Kim K. S.; McCormack F. X. Surfactant Proteins A and D Inhibit the Growth of Gram-Negative Bacteria by Increasing Membrane Permeability. J. Clin. Invest. 2003, 111 (10), 1589–1602. 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusuardi M.; Capelli A.; Di Stefano A.; Donner C. F.; et al. Lung Mucosal Immunity: Immunoglobulin-A Revisited. Eur. Respir. J. 2002, 19 (4), 785–786. 10.1183/09031936.02.00303902. [DOI] [PubMed] [Google Scholar]
- Allard B.; Panariti A.; Martin J. G. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front. Immunol. 2018, 9, 01777 10.3389/fimmu.2018.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budden K. F.; Gellatly S. L.; Wood D. L. A.; Cooper M. A.; Morrison M.; Hugenholtz P.; Hansbro P. M. Emerging Pathogenic Links between Microbiota and the Gut-Lung Axis. Nat. Rev. Microbiol. 2017, 15 (1), 55–63. 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- Rapozo D. C M; Bernardazzi C.; de Souza H. S. P. Diet and Microbiota in Inflammatory Bowel Disease: The Gut in Disharmony. World Journal of Gastroenterology 2017, 23, 2124–2140. 10.3748/wjg.v23.i12.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten E. P. A.; Lenaerts K.; Buurman W. A.; Wouters E. F. M. Disturbed Intestinal Integrity in Patients With COPD: Effects of Activities of Daily Living. Chest 2014, 145 (2), 245–252. 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- Fujimura K. E.; Sitarik A. R.; Havstad S.; Lin D. L.; Levan S.; Fadrosh D.; Panzer A. R.; Lamere B.; Rackaityte E.; Lukacs N. W.; Wegienka G.; Boushey H. A.; Ownby D. R.; Zoratti E. M.; Levin A. M.; Johnson C. C.; Lynch S. V. Neonatal Gut Microbiota Associates with Childhood Multisensitized Atopy and T Cell Differentiation. Nat. Med. 2016, 22 (10), 1187–1191. 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki M.; Kirjavainen P.; Eerola E.; Kero P.; Salminen S.; Isolauri E. Distinct Patterns of Neonatal Gut Microflora in Infants in Whom Atopy Was and Was Not Developing. J. Allergy Clin. Immunol. 2001, 107 (1), 129–134. 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- Mao J.; Li Y.; Feng S.; Liu X.; Tian Y.; Bian Q.; Li J.; Hu Y.; Zhang L.; Ji H.; Li S. Bufei Jianpi Formula Improves Mitochondrial Function and Suppresses Mitophagy in Skeletal Muscle via the Adenosine Monophosphate-Activated Protein Kinase Pathway in Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 2020, 11, 587176. 10.3389/fphar.2020.587176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L.; Tan J.; Vieira A. T.; Leach K.; Stanley D.; Luong S.; Maruya M.; Ian McKenzie C.; Hijikata A.; Wong C.; Binge L.; Thorburn A. N.; Chevalier N.; Ang C.; Marino E.; Robert R.; Offermanns S.; Teixeira M. M.; Moore R. J.; Flavell R. A.; Fagarasan S.; Mackay C. R. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- Wypych T. P.; Wickramasinghe L. C.; Marsland B. J. The Influence of the Microbiome on Respiratory Health. Nat. Immunol. 2019, 20 (10), 1279–1290. 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- Ichinohe T.; Pang I. K.; Kumamoto Y.; Peaper D. R.; Ho J . H.; Murray T. S.; Iwasaki A. Microbiota Regulates Immune Defense against Respiratory Tract Influenza a Virus Infection. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (13), 5354–5359. 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. A.; Jackson J.; Stanton M.; Rojas-Triana A.; Bober L.; Laverty M.; Yang X.; Zhu F.; Liu J.; Wang S.; Monsma F.; Vassileva G.; Maguire M.; Gustafson E.; Bayne M.; Chou C. C.; Lundell D.; Jenh C. H. Short-Chain Fatty Acids Act as Antiinflammatory Mediators by Regulating Prostaglandin E2 and Cytokines. World J. Gastroenterol. 2009, 15 (44), 5549–5557. 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. V.; Hao L.; Offermanns S.; Medzhitov R. The Microbial Metabolite Butyrate Regulates Intestinal Macrophage Function via Histone Deacetylase Inhibition. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (6), 2247–2252. 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. G.; Whittaker R. G.; Neumann J. R.; Ingram V. M. N-Butyrate Causes Histone Modification in HeLa and Friend Erythroleukaemia Cells. Nature 1977, 268 (5619), 462–464. 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Shakespear M. R.; Halili M. A.; Irvine K. M.; Fairlie D. P.; Sweet M. J. Histone Deacetylases as Regulators of Inflammation and Immunity. Trends Immunol. 2011, 32 (7), 335–343. 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Smith P. M.; Howitt M. R.; Panikov N.; Michaud M.; Gallini C. A.; Bohlooly-Y M.; Glickman J. N.; Garrett W. S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T Reg Cell Homeostasis. Science (80-.) 2013, 341 (6145), 569–573. 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.; Kim M.; Kang S. G.; Jannasch A. H.; Cooper B.; Patterson J.; Kim C. H. Short-Chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the MTOR-S6K Pathway. Mucosal Immunol. 2015, 8 (1), 80–93. 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kespohl M.; Vachharajani N.; Luu M.; Harb H.; Pautz S.; Wolff S.; Sillner N.; Walker A.; Schmitt-Kopplin P.; Boettger T.; Renz H.; Offermanns S.; Steinhoff U.; Visekruna A. The Microbial Metabolite Butyrate Induces Expression of Th1- Associated Factors in CD4+ T Cells. Front. Immunol. 2017, 8 (AUG), 1–12. 10.3389/fimmu.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cait A.; Hughes M. R.; Antignano F.; Cait J.; Dimitriu P. A.; Maas K. R.; Reynolds L. A.; Hacker L.; Mohr J.; Finlay B. B.; Zaph C.; McNagny K. M.; Mohn W. W. Microbiome-Driven Allergic Lung Inflammation Is Ameliorated by Short-Chain Fatty Acids. Mucosal Immunol. 2018, 11 (3), 785–795. 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- Thorburn A. N.; McKenzie C. I.; Shen S.; Stanley D.; MacIa L.; Mason L. J.; Roberts L. K.; Wong C. H. Y.; Shim R.; Robert R.; Chevalier N.; Tan J. K.; Marinõ E.; Moore R. J.; Wong L.; McConville M. J.; Tull D. L.; Wood L. G.; Murphy V. E.; Mattes J.; Gibson P. G.; MacKay C. R. Evidence That Asthma Is a Developmental Origin Disease Influenced by Maternal Diet and Bacterial Metabolites. Nat. Commun. 2015, 6, 7320 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- Dang A. T.; Marsland B. J. Microbes, Metabolites, and the Gut-Lung Axis. Mucosal Immunol. 2019, 12 (4), 843–850. 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- Liu K.; Victora G. D.; Schwickert T. A.; Guermonprez P.; Meredith M. M.; Yao K.; Chu F. F.; Randolph G. J.; Rudensky A. Y.; Nussenzweig M. In Vivo Analysis of Dendritic Cell. Science 2009, 324, 392–397. 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M.; Schneider C.; Nobs S. P. The Development and Function of Lung-Resident Macrophages and Dendritic Cells. Nat. Immunol. 2015, 16 (1), 36–44. 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- Trompette A.; Gollwitzer E. S.; Pattaroni C.; Lopez-Mejia I. C.; Riva E.; Pernot J.; Ubags N.; Fajas L.; Nicod L. P.; Marsland B. J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c - Patrolling Monocyte Hematopoiesis and CD8 + T Cell Metabolism. Immunity 2018, 48 (5), 992–1005.e8. 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Landsman L.; Jung S. Lung Macrophages Serve as Obligatory Intermediate between Blood Monocytes and Alveolar Macrophages. J. Immunol. 2007, 179 (6), 3488–3494. 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- Landsman L.; Varol C.; Jung S. Distinct Differentiation Potential of Blood Monocyte Subsets in the Lung. J. Immunol. 2007, 178 (4), 2000–2007. 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- Shirey K. A.; Pletneva L. M.; Puche A. C.; Keegan A. D.; Prince G. A.; Blanco J. C. G.; Vogel S. N. Control of RSV-Induced Lung Injury by Alternatively Activated Macrophages Is IL-4Rα-, TLR4-, and IFN-Β-Dependent. Mucosal Immunol. 2010, 3 (3), 291–300. 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. L.; Gold M. J.; Hartmann M.; Willing B. P.; Thorson L.; Wlodarska M.; Gill N.; Blanchet M. R.; Mohn W. W.; McNagny K. M.; Finlay B. B. Early Life Antibiotic-Driven Changes in Microbiota Enhance Susceptibility to Allergic Asthma. EMBO Rep. 2012, 13 (5), 440–447. 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyarov S. Role of Short-Chain Fatty Acids Produced by Gut Microbiota in Innate Lung Immunity and Pathogenesis of the Heterogeneous Course of Chronic Obstructive Pulmonary Disease. International Journal of Molecular Sciences 2022, 23, 4768 10.3390/ijms23094768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roduit C.; Frei R.; Ferstl R.; Loeliger S.; Westermann P.; Rhyner C.; Schiavi E.; Barcik W.; Rodriguez-Perez N.; Wawrzyniak M.; Chassard C.; Lacroix C.; Schmausser-Hechfellner E.; Depner M.; von Mutius E.; Braun-Fahrlander C.; Karvonen A. M.; Kirjavainen P. V.; Pekkanen J.; Dalphin J.-C.; Riedler J.; Akdis C.; Lauener R.; O'Mahony L. High Levels of Butyrate and Propionate in Early Life Are Associated with Protection against Atopy. Allergy 2019, 74 (4), 799–809. 10.1111/all.13660. [DOI] [PubMed] [Google Scholar]
- Theiler A.; Bärnthaler T.; Platzer W.; Richtig G.; Peinhaupt M.; Rittchen S.; Kargl J.; Ulven T.; Marsh L. M.; Marsche G.; Schuligoi R.; Sturm E. M.; Heinemann A. Butyrate Ameliorates Allergic Airway Inflammation by Limiting Eosinophil Trafficking and Survival. J. Allergy Clin. Immunol. 2019, 144 (3), 764–776. 10.1016/j.jaci.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Antunes K. H.; Singanayagam A.; Williams L.; Faiez T. S.; Farias A.; Jackson M. M.; Faizi F. K.; Aniscenko J.; Kebadze T.; Chander Veerati P.; Wood L.; Bartlett N. W.; Duarte de Souza A. P.; Johnston S. L. Airway-Delivered Short-Chain Fatty Acid Acetate Boosts Antiviral Immunity during Rhinovirus Infection. J. Allergy Clin. Immunol. 2023, 151, 447. 10.1016/j.jaci.2022.09.026. [DOI] [PubMed] [Google Scholar]
- Ratajczak W.; Rył A.; Mizerski A.; Walczakiewicz K.; Sipak O.; Laszczyńska M. Immunomodulatory Potential of Gut Microbiome-Derived Short-Chain Fatty Acids (SCFAs). Acta Biochim. Polym. 2018, 66 (1), 1–12. 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- Brown A. J.; Goldsworthy S. M.; Barnes A. A.; Eilert M. M.; Tcheang L.; Daniels D.; Muir A. I.; Wigglesworth M. J.; Kinghorn I.; Fraser N. J.; Pike N. B.; Strum J. C.; Steplewski K. M.; Murdock P. R.; Holder J. C.; Marshall F. H.; Szekeres P. G.; Wilson S.; Ignar D. M.; Foord S. M.; Wise A.; Dowell S. J. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278 (13), 11312–11319. 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Ohira H.; Fujioka Y.; Katagiri C.; Mamoto R.; Aoyama-Ishikawa M.; Amako K.; Izumi Y.; Nishiumi S.; Yoshida M.; Usami M.; Ikeda M. Butyrate Attenuates Inflammation and Lipolysis Generated by the Interaction of Adipocytes and Macrophages. J. Atheroscler. Thromb. 2013, 20 (5), 425–442. 10.5551/jat.15065. [DOI] [PubMed] [Google Scholar]
- Schamberger A. C.; Mise N.; Jia J.; Genoyer E.; Yildirim A. Ö.; Meiners S.; Eickelberg O. Cigarette Smoke-Induced Disruption of Bronchial Epithelial Tight Junctions Is Prevented by Transforming Growth Factor-β. Am. J. Respir. Cell Mol. Biol. 2014, 50 (6), 1040–1052. 10.1165/rcmb.2013-0090OC. [DOI] [PubMed] [Google Scholar]
- Lewis G.; Wang B.; Shafiei Jahani P.; Hurrell B. P.; Banie H.; Aleman Muench G. R.; Maazi H.; Helou D. G.; Howard E.; Galle-Treger L.; Lo R.; Santosh S.; Baltus A.; Bongers G.; San-Mateo L.; Gilliland F. D.; Rehan V. K.; Soroosh P.; Akbari O. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front. Immunol. 2019, 10, 2051 10.3389/fimmu.2019.02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.; Li H.; Su C.; Xu F.; Yang G.; Sun K.; Xu M.; Lv N.; Meng B.; Liu Y.; Hu L.; Liu Y.; Gao Y.; Wang H.; Lan Y.; Xu D.; Li J. Microbiota-Derived Short-Chain Fatty Acids Promote LAMTOR2-Mediated Immune Responses in Macrophages. mSystems 2020, 10.1128/mSystems.00587-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.-Y.; Shi H.-T.; Tan Y.-R.; Shen S.-Y.; Yi P.-F.; Shen H.-Q.; Fu B.-D. Baicalin Inhibits APEC-Induced Lung Injury by Regulating Gut Microbiota and SCFA Production. Food Funct. 2021, 12 (24), 12621–12633. 10.1039/D1FO02407H. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y.; Eguchi A.; Wei Y.; Shinno-Hashimoto H.; Fujita Y.; Ishima T.; Chang L.; Mori C.; Suzuki T.; Hashimoto K. Antibiotic-Induced Microbiome Depletion Improves LPS-Induced Acute Lung Injury via Gut-Lung Axis. Life Sci. 2022, 307, 120885 10.1016/j.lfs.2022.120885. [DOI] [PubMed] [Google Scholar]
- Haak B. W.; Littmann E. R.; Chaubard J.-L.; Pickard A. J.; Fontana E.; Adhi F.; Gyaltshen Y.; Ling L.; Morjaria S. M.; Peled J. U.; van den Brink M. R.; Geyer A. I.; Cross J. R.; Pamer E. G.; Taur Y. Impact of Gut Colonization with Butyrate-Producing Microbiota on Respiratory Viral Infection Following Allo-HCT. Blood 2018, 131 (26), 2978–2986. 10.1182/blood-2018-01-828996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K.; Raundhal M.; Chen B. B.; Morse C.; Tyurina Y. Y.; Khare A.; Oriss T. B.; Huff R.; Lee J. S.; St. Croix C. M.; Watkins S.; Mallampalli R. K.; Kagan V. E.; Ray A.; Ray P. The Mito-DAMP Cardiolipin Blocks IL-10 Production Causing Persistent Inflammation during Bacterial Pneumonia. Nat. Commun. 2017, 8, 13944 10.1038/ncomms13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes K. H.; Fachi J. L.; de Paula R.; da Silva E. F.; Pral L. P.; Dos Santos A. Á.; Dias G. B. M.; Vargas J. E.; Puga R.; Mayer F. Q.; Maito F.; Zárate-Bladés C. R.; Ajami N. J.; Sant’Ana M. R.; Candreva T.; Rodrigues H. G.; Schmiele M.; Silva Clerici M. T. P.; Proença-Modena J. L.; Vieira A. T.; Mackay C. R.; Mansur D.; Caballero M. T.; Marzec J.; Li J.; Wang X.; Bell D.; Polack F. P.; Kleeberger S. R.; Stein R. T.; Vinolo M. A. R.; de Souza A. P. D. Microbiota-Derived Acetate Protects against Respiratory Syncytial Virus Infection through a GPR43-Type 1 Interferon Response. Nat. Commun. 2019, 10 (1), 3273. 10.1038/s41467-019-11152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão I.; Tavares L. P.; Corrêa R. O.; Fachi J. L.; Rocha V. M.; Rungue M.; Garcia C. C.; Cassali G.; Ferreira C. M.; Martins F. S.; Oliveira S. C.; Mackay C. R.; Teixeira M. M.; Vinolo M. A. R.; Vieira A. T. The Metabolic Sensor GPR43 Receptor Plays a Role in the Control of Klebsiella Pneumoniae Infection in the Lung. Front. Immunol. 2018, 9, 142. 10.3389/fimmu.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S.; Chen Y.; Wu Z.; Chen Y.; Gao H.; Lv L.; Guo F.; Zhang X.; Luo R.; Huang C.; Lu H.; Zheng B.; Zhang J.; Yan R.; Zhang H.; Jiang H.; Xu Q.; Guo J.; Gong Y.; Tang L.; Li L. Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71 (10), 2669–2678. 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T.; Liu Q.; Zhang F.; Lui G. C. Y.; Tso E. Y. K.; Yeoh Y. K.; Chen Z.; Boon S. S.; Chan F. K. L.; Chan P. K. S.; Ng S. C. Depicting SARS-CoV-2 Faecal Viral Activity in Association with Gut Microbiota Composition in Patients with COVID-19. Gut 2020, 70 (2), 276–284. 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.; Gu S.; Gong Y.; Li B.; Lu H.; Li Q.; Zhang R.; Gao X.; Wu Z.; Zhang J.; Zhang Y.; Li L. Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Eng. (Beijing, China) 2020, 6 (10), 1178–1184. 10.1016/j.eng.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T.; Ishizaka A.; Koga M.; Ikeuchi K.; Saito M.; Adachi E.; Yamayoshi S.; Iwatsuki-Horimoto K.; Yasuhara A.; Kiyono H.; Matano T.; Suzuki Y.; Tsutsumi T.; Kawaoka Y.; Yotsuyanagi H. Correlation Analysis between Gut Microbiota Alterations and the Cytokine Response in Patients with Coronavirus Disease during Hospitalization. Microbiol. Spectr. 2022, 10.1128/spectrum.01689-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Wan Y.; Zuo T.; Yeoh Y. K.; Liu Q.; Zhang L.; Zhan H.; Lu W.; Xu W.; Lui G. C. Y.; Li A. Y. L.; Cheung C. P.; Wong C. K.; Chan P. K. S.; Chan F. K. L.; Ng S. C. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology 2022, 162 (2), 548–561.e4. 10.1053/j.gastro.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M. E.; Hasan H. M.; Sridharan K.; Elkady A.; ElSeirafi M. M. Dexamethasone in Severe COVID-19 Infection: A Case Series. Respir. Med. Case Rep. 2020, 31, 101205 10.1016/j.rmcr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Zuniga M. A.; Moreno-Moral A.; Ocana-Granados A.; Padilla-Moreno F. A.; Castillo-Fernandez A. M.; Guillamon-Fernandez D.; Ramirez-Sanchez C.; Sanchez-Palop M.; Martinez-Colmenero J.; Pimentel-Villar M. A.; Blazquez-Rosello S.; Moreno-Sanchez J. J.; Lopez-Vilchez M.; Prior-Sanchez I.; Jodar-Moreno R.; Lopez Ruz M. A. High-Dose Corticosteroid Pulse Therapy Increases the Survival Rate in COVID-19 Patients at Risk of Hyper-Inflammatory Response. PLoS One 2021, 16 (1), e0243964 10.1371/journal.pone.0243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M. A. A Response to the Recommendations for Using Dexamethasone for the Treatment of COVID-19: The Dark Side of Dexamethasone. J. Pharm. Pract. 2021, 34 (2), 179–180. 10.1177/0897190020979608. [DOI] [PubMed] [Google Scholar]
- Brown J. A.; Sanidad K. Z.; Lucotti S.; Lieber C. M.; Cox R. M.; Ananthanarayanan A.; Basu S.; Chen J.; Shan M.; Amir M.; Schmidt F.; Weisblum Y.; Cioffi M.; Li T.; Rowdo F. M.; Martin M. L.; Guo C.-J.; Lyssiotis C.; Layden B. T.; Dannenberg A. J.; Bieniasz P. D.; Lee B.; Inohara N.; Matei I.; Plemper R. K.; Zeng M. Y. Gut Microbiota-Derived Metabolites Confer Protection against SARS-CoV-2 Infection. Gut Microbes 2022, 14 (1), 2105609 10.1080/19490976.2022.2105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty P.; K N. K.; Patil P.; Bhandary S. K.; Haridas V.; N S. K.; E S. Is Butyrate a Natural Alternative to Dexamethasone in the Management of CoVID-19?. F1000Research 2021, 10, 273. 10.12688/f1000research.51786.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Richards E. M.; Handberg E. M.; Pepine C. J.; Raizada M. K. Butyrate Regulates COVID-19-Relevant Genes in Gut Epithelial Organoids From Normotensive Rats. Hypertension. 2021, 77, E13–E16. 10.1161/HYPERTENSIONAHA.120.16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paparo L.; Maglio M. A.; Cortese M.; Bruno C.; Capasso M.; Punzo E.; Ferrucci V.; Lasorsa V. A.; Viscardi M.; Fusco G.; Cerino P.; Romano A.; Troncone R.; Zollo M. A New Butyrate Releaser Exerts a Protective Action against SARS-CoV-2 Infection in Human Intestine. Molecules 2022, 27, 862 10.3390/molecules27030862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer D. L.; Kramer D. C. The Use of Microbial Accessible and Fermentable Carbohydrates and/or Butyrate as Supportive Treatment for Patients With Coronavirus SARS-CoV-2 Infection. Front. Med. 2020, 7, 00292 10.3389/fmed.2020.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B.; Sutton N. R.; Wang Z.; Goonewardena S. N.; Holinstat M. Potential Repurposing of the HDAC Inhibitor Valproic Acid for Patients with COVID-19. Eur. J. Pharmacol. 2021, 898, 173988 10.1016/j.ejphar.2021.173988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardou M.; Lawson R. Supportive Therapy during COVID-19: The Proposed Mechanism of Short-Chain Fatty Acids to Prevent Cytokine Storm and Multi-Organ Failure. Med. Hypotheses 2021, 154, 110661 10.1016/j.mehy.2021.110661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.; Liu X.-X.; Hong M.; Huang X.-Z.; Chen H.; Xu J.-H.; Wang C.; Zhang Y.-X.; Zhong J.-X.; Nie H.; Gong Q. Sodium Butyrate Alleviates LPS-Induced Acute Lung Injury in Mice via Inhibiting HMGB1 Release. Int. Immunopharmacol. 2018, 56, 242–248. 10.1016/j.intimp.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Liu J.; Chang G.; Huang J.; Wang Y.; Ma N.; Roy A. C.; Shen X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor ΚB Signaling Pathway. J. Agric. Food Chem. 2019, 67 (6), 1674. 10.1021/acs.jafc.8b06359. [DOI] [PubMed] [Google Scholar]
- Nastasi C.; Candela M.; Bonefeld C. M.; Geisler C.; Hansen M.; Krejsgaard T.; Biagi E.; Andersen M. H.; Brigidi P.; Ødum N.; Litman T.; Woetmann A. The Effect of Short-Chain Fatty Acids on Human Monocyte-Derived Dendritic Cells. Sci. Rep. 2015, 5, 16148 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. G.; Lee J.; Lee A.-R.; Jo S. V.; Park C. H.; Han D. S.; Eun C. S. Impact of Short-Chain Fatty Acid Supplementation on Gut Inflammation and Microbiota Composition in a Murine Colitis Model. J. Nutr. Biochem. 2022, 101, 108926 10.1016/j.jnutbio.2021.108926. [DOI] [PubMed] [Google Scholar]
- Baraldi O.; Bianchi F.; Menghi V.; Angeletti A.; Croci Chiocchini A. L.; Cappuccilli M.; Aiello V.; Comai G.; La Manna G. An in Vitro Model of Renal Inflammation after Ischemic Oxidative Stress Injury: Nephroprotective Effects of a Hyaluronan Ester with Butyric Acid on Mesangial Cells. J. Inflamm. Res. 2017, 10, 135–142. 10.2147/JIR.S138431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torun A.; Enayat S.; Sheraj I.; Tunçer S.; Ülgen D. H.; Banerjee S. Butyrate Mediated Regulation of RNA Binding Proteins in the Post-Transcriptional Regulation of Inflammatory Gene Expression. Cell. Signal. 2019, 64, 109410 10.1016/j.cellsig.2019.109410. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Deng M.; Lu A.; Chen Y.; Chen Y.; Wu C.; Tan Z.; Boini K. M.; Yang T.; Zhu Q.; Wang L. Sodium Butyrate Attenuates Angiotensin II-Induced Cardiac Hypertrophy by Inhibiting COX2/PGE2 Pathway via a HDAC5/HDAC6-Dependent Mechanism. J. Cell. Mol. Med. 2019, 23 (12), 8139–8150. 10.1111/jcmm.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Vera I.; Toral M.; de la Visitación N.; Aguilera-Sánchez N.; Redondo J. M.; Duarte J. Protective Effects of Short-Chain Fatty Acids on Endothelial Dysfunction Induced by Angiotensin II. Front. Physiol. 2020, 11, 00277 10.3389/fphys.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira R. d. S.; Castoldi A.; Basso P. J.; Hiyane M. I.; Camara N. O. S.; Almeida R. R. Butyrate Attenuates Lung Inflammation by Negatively Modulating Th9 Cells. Front. Immunol. 2019, 10, 67 10.3389/fimmu.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y.; Hayakawa A.; Sano R.; Fukuda H.; Harada M.; Kubo R.; Okawa T.; Kominato Y. Histone Deacetylase Inhibitors Suppress ACE2 and ABO Simultaneously, Suggesting a Preventive Potential against COVID-19. Sci. Rep. 2021, 11 (1), 3379 10.1038/s41598-021-82970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel A. M.; Omar M. S.; Elmaaboud M. A. A. Amelioration of Bleomycin-Induced Lung Fibrosis in Rats by Valproic Acid and Butyrate: Role of Nuclear Factor Kappa-B, Proinflammatory Cytokines and Oxidative Stress. Int. Immunopharmacol. 2016, 39, 335–342. 10.1016/j.intimp.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Scott N. A.; Andrusaite A.; Andersen P.; Lawson M.; Alcon-Giner C.; Leclaire C.; Caim S.; Le Gall G.; Shaw T.; Connolly J. P. R.; Roe A. J.; Wessel H.; Bravo-Blas A.; Thomson C. A.; Kastele V.; Wang P.; Peterson D. A.; Bancroft A.; Li X.; Grencis R.; Mowat A. M.; Hall L. J.; Travis M. A.; Milling S. W. F.; Mann E. R. Antibiotics Induce Sustained Dysregulation of Intestinal T Cell Immunity by Perturbing Macrophage Homeostasis. Sci. Transl. Med. 2018, 10 (464), eaao4755 10.1126/scitranslmed.aao4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esensten J. H.; Muller Y. D.; Bluestone J. A.; Tang Q. Regulatory T-Cell Therapy for Autoimmune and Autoinflammatory Diseases: The next Frontier. J. Allergy Clin. Immunol. 2018, 142 (6), 1710–1718. 10.1016/j.jaci.2018.10.015. [DOI] [PubMed] [Google Scholar]
- Hull C. M.; Peakman M.; Tree T. I. M. Regulatory T Cell Dysfunction in Type 1 Diabetes: What’s Broken and How Can We Fix It?. Diabetologia 2017, 60 (10), 1839–1850. 10.1007/s00125-017-4377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alissafi T.; Kalafati L.; Lazari M.; Filia A.; Kloukina I.; Manifava M.; Lim J. H.; Alexaki V. I.; Ktistakis N. T.; Doskas T.; Garinis G. A.; Chavakis T.; Boumpas D. T.; Verginis P. Mitochondrial Oxidative Damage Underlies Regulatory T Cell Defects in Autoimmunity. Cell Metab. 2020, 32 (4), 591–604.e7. 10.1016/j.cmet.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski K. M.; Vieira A. T.; Ng A.; Kranich J.; Sierro F.; Yu D.; Schilter H. C.; Rolph M. S.; MacKay F.; Artis D.; Xavier R. J.; Teixeira M. M.; MacKay C. R. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461 (7268), 1282–1286. 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoal L. B.; Rodrigues P. B.; Genaro L. M.; Gomes A. B. d. S. P.; Toledo-Teixeira D. A.; Parise P. L.; Bispo-Dos-Santos K.; Simeoni C. L.; Guimaraes P. V.; Buscaratti L. I.; Elston J. G. D. A.; Marques-Souza H.; Martins-de-Souza D.; Ayrizono M. D. L. S.; Velloso L. A.; Proenca-Modena J. L.; Moraes-Vieira P. M. M.; Mori M. A. S.; Farias A. S.; Vinolo M. A. R.; Leal R. F. Microbiota-Derived Short-Chain Fatty Acids Do Not Interfere with SARS-CoV-2 Infection of Human Colonic Samples. Gut Microbes 2021, 13, 1–9. 10.1080/19490976.2021.1874740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J. B.; Murthy S.; Marshall J. C.; Relan P.; Diaz J. V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet. Infect. Dis. 2022, 22 (4), e102–e107. 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Wan Y.; Zuo T.; Yeoh Y. K.; Liu Q.; Zhang L.; Zhan H.; Lu W.; Xu W.; Lui G. C. Y.; Li A. Y. L.; Cheung C. P.; Wong C. K.; Chan P. K. S.; Chan F. K. L.; Ng S. C. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology 2022, 162 (2), 548–561.e4. 10.1053/j.gastro.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Zhou Y.; Ma Y.; Chen P.; Tang J.; Yang B.; Li H.; Liang M.; Xue Y.; Liu Y.; Zhang J.; Wang X. Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge. J. Korean Med. Sci. 2023, 38 (15), e120 10.3346/jkms.2023.38.e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Melas P. A.; Wegener G.; Mathé A. A.; Lavebratt C. Antidepressant-like Effect of Sodium Butyrate Is Associated with an Increase in TET1 and in 5-Hydroxymethylation Levels in the Bdnf Gene. Int. J. Neuropsychopharmacol. 2015, 18 (2), pyu032 10.1093/ijnp/pyu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki Y.; Fuchikami M.; Morinobu S.; Segawa M.; Matsumoto T.; Yamawaki S. Antidepressant-like Effect of Sodium Butyrate (HDAC Inhibitor) and Its Molecular Mechanism of Action in the Rat Hippocampus.. world J. Biol. psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2012, 13 (6), 458–467. 10.3109/15622975.2011.585663. [DOI] [PubMed] [Google Scholar]
- Dang A. T.; Marsland B. J. Microbes, Metabolites, and the Gut–Lung Axis. Mucosal Immunol. 2019, 12 (4), 843–850. 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- Wang H.; Liu J. S.; Peng S. H.; Deng X. Y.; Zhu D. M.; Javidiparsijani S.; Wang G. R.; Li D. Q.; Li L. X.; Wang Y. C.; Luo J. M. Gut-Lung Crosstalk in Pulmonary Involvement with Inflammatory Bowel Diseases. World J. Gastroenterol. 2013, 19 (40), 6794–6804. 10.3748/wjg.v19.i40.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar A.; Atis S.; Konca K.; Pata C.; Akbay E.; Calikoglu M.; Hafta A. Respiratory Symptoms and Pulmonary Functional Changes in Patients with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2001, 96 (5), 1511–1516. 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- Labarca G.; Drake L.; Horta G.; Jantz M. A.; Mehta H. J.; Fernandez-Bussy S.; Folch E.; Majid A.; Picco M. Association between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2019, 19 (1), 1–9. 10.1186/s12890-019-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt T. J.; Lankelma J. M.; Scicluna B. P.; De Sousa E Melo F.; Roelofs J. J. T. H.; De Boer J. D.; Hoogendijk A. J.; De Beer R.; De Vos A.; Belzer C.; De Vos W. M.; Van Der Poll T.; Wiersinga W. J. The Gut Microbiota Plays a Protective Role in the Host Defence against Pneumococcal Pneumonia. Gut 2016, 65 (4), 575–583. 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson D. R.; Charles T. P.; de la Rua N. M.; Taylor C. M.; Blanchard E. E.; Luo M.; Shellito J. E.; Welsh D. A. Analysis of the Intestinal Microbial Community and Inferred Functional Capacities during the Host Response to Pneumocystis Pneumonia. Exp. Lung Res. 2016, 42 (8–10), 425–439. 10.1080/01902148.2016.1258442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze M. A.; Tsuruta M.; Yang S. W. J.; Oh Y.; Man S. F. P.; Hogg J. C.; Sin D. D. Changes in the Bacterial Microbiota in Gut, Blood, and Lungs Following Acute LPS Instillation into Mice Lungs. PLoS One 2014, 9 (10), e111228 10.1371/journal.pone.0111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Li S.; Wang N.; Tan H. Y.; Zhang Z.; Feng Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson D. R.; Welsh D. A.; Shellito J. E. Regulation of Lung Immunity and Host Defense by the Intestinal Microbiota. Front. Microbiol. 2015, 6 (OCT), 1–14. 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst T.; Sichelstiel A.; Schär C.; Yadava K.; Bürki K.; Cahenzli J.; McCoy K.; Marsland B. J.; Harris N. L. Dysregulation of Allergic Airway Inflammation in the Absence of Microbial Colonization. Am. J. Respir. Crit. Care Med. 2011, 184 (2), 198–205. 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- Brown R. L.; Sequeira R. P.; Clarke T. B. The Microbiota Protects against Respiratory Infection via GM-CSF Signaling. Nat. Commun. 2017, 8 (1), 1512 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R.; Hutkins R.; Sanders M. E.; Prescott S. L.; Reimer R. A.; Salminen S. J.; Scott K.; Stanton C.; Swanson K. S.; Cani P. D.; Verbeke K.; Reid G. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14 (8), 491–502. 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Davani-Davari D.; Negahdaripour M.; Karimzadeh I.; Seifan M.; Mohkam M.; Masoumi S. J.; Berenjian A.; Ghasemi Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8 (3), 92 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. P.; Hopkins R. J.; Marsland B. The Gut-Liver-Lung Axis: Modulation of the Innate Immune Response and Its Possible Role in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54 (2), 161–169. 10.1165/rcmb.2015-0250PS. [DOI] [PubMed] [Google Scholar]
- Jang Y. O.; Kim O. H.; Kim S. J.; Lee S. H.; Yun S.; Lim S. E.; Yoo H. J.; Shin Y.; Lee S. W. High-Fiber Diets Attenuate Emphysema Development via Modulation of Gut Microbiota and Metabolism. Sci. Rep. 2021, 11 (1), 1–11. 10.1038/s41598-021-86404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych T. P.; Marsland B. J.; Ubags N. D. J. The Impact of Diet on Immunity and Respiratory Diseases. Ann. Am. Thorac. Soc. 2017, 14, S339–S347. 10.1513/AnnalsATS.201703-255AW. [DOI] [PubMed] [Google Scholar]
- Smith P. M.; Howitt M. R.; Panikov N.; Michaud M.; Gallini C. A.; Bohlooly-Y M.; Glickman J. N.; Garrett W. S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T Reg Cell Homeostasis. Science (80-.) 2013, 341 (6145), 569–573. 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B. S.; MacDonald-Wicks L. K.; Gibson P. G.; Wood L. G. Investigation of the Association between Dietary Intake, Disease Severity and Airway Inflammation in Asthma. Respirology 2013, 18 (3), 447–454. 10.1111/resp.12015. [DOI] [PubMed] [Google Scholar]
- Halnes I.; Baines K. J.; Berthon B. S.; MacDonald-Wicks L. K.; Gibson P. G.; Wood L. G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9 (1), 57 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H.; Stevens J.; Heiss G.; Rose K. M.; London S. J.; et al. Dietary Fiber, Lung Function, and Chronic Obstructive Pulmonary Disease in the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2007, 167 (5), 570–578. 10.1093/aje/kwm343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M.; Ichinohe T. High Ambient Temperature Dampens Adaptive Immune Responses to Influenza A Virus Infection. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (8), 3118–3125. 10.1073/pnas.1815029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen M. P. K. J.Fiber Metabolism in Chronic Obstructive Pulmonary Disease. ClinicalTrials.gov, 2020, NCT04459156. https://clinicaltrials.gov/study/NCT04459156.
- Lester P.The Effects of Prebiotics on Gut Bacterial Parameters, Immune Function & Exercise-Induced Airway Inflammation. ClinicalTrials.gov, 2016, NCT02872675. https://clinicaltrials.gov/show/NCT02872675.
- Hill C.; Guarner F.; Reid G.; Gibson G. R.; Merenstein D. J.; Pot B.; Morelli L.; Canani R. B.; Flint H. J.; Salminen S.; Calder P. C.; Sanders M. E. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11 (8), 506–514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Azad M. A. K.; Sarker M.; Li T.; Yin J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed Res. Int. 2018, 2018, 9478630 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt B. C.; Digiandomenico A. Microbiome Modulation as a Novel Strategy to Treat and Prevent Respiratory Infections. Antibiotics 2022, 11 (4), 474 10.3390/antibiotics11040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E.; Adcock I. M.; Folkerts G.; Barnes P. J.; Paul Vos A.; Garssen J. Probiotics in the Management of Lung Diseases. Mediators Inflamm. 2013, 2013, 751068 10.1155/2013/751068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.; Zhao X. K.; Cheng M. L.; Yang G. Z.; Wang B.; Liu H. J.; Hu Y. X.; Zhu L. L.; Zhang S.; Xiao Z. W.; Liu Y. M.; Zhang B. F.; Mu M. Saccharomyces Boulardii Administration Changes Gut Microbiota and Attenuates D-Galactosamine-Induced Liver Injury. Sci. Rep. 2017, 7 (1), 1–7. 10.1038/s41598-017-01271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun P.; Scarpa M.; Marchiori C.; Sarasin G.; Caputi V.; Porzionato A.; Giron M. C.; Palù G.; Castagliuolo I. Saccharomyces Boulardii CNCM I-745 Supplementation Reduces Gastrointestinal Dysfunction in an Animal Model of IBS. PLoS One 2017, 12 (7), e0181863 10.1371/journal.pone.0181863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. S.-Y.; Yanagisawa N.; Hor Y.-Y.; Lew L.-C.; Ong J.-S.; Chuah L.-O.; Lee Y.-Y.; Choi S.-B.; Rashid F.; Wahid N.; Sugahara H.; Xiao J.-Z.; Liong M.-T. Bifidobacterium Longum BB536 Alleviated Upper Respiratory Illnesses and Modulated Gut Microbiota Profiles in Malaysian Pre-School Children. Benef. Microbes 2018, 9 (1), 61–70. 10.3920/BM2017.0063. [DOI] [PubMed] [Google Scholar]
- Feleszko W.; Jaworska J.; Rha R.-D.; Steinhausen S.; Avagyan A.; Jaudszus A.; Ahrens B.; Groneberg D. A.; Wahn U.; Hamelmann E. Probiotic-Induced Suppression of Allergic Sensitization and Airway Inflammation Is Associated with an Increase of T Regulatory-Dependent Mechanisms in a Murine Model of Asthma. Clin. Exp. Allergy 2007, 37 (4), 498–505. 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- Zhang B.; An J.; Shimada T.; Liu S.; Maeyama K. Oral Administration of Enterococcus Faecalis FK-23 Suppresses Th17 Cell Development and Attenuates Allergic Airway Responses in Mice. Int. J. Mol. Med. 2012, 30 (2), 248–254. 10.3892/ijmm.2012.1010. [DOI] [PubMed] [Google Scholar]
- Morimoto K.; Takeshita T.; Nanno M.; Tokudome S.; Nakayama K. Modulation of Natural Killer Cell Activity by Supplementation of Fermented Milk Containing Lactobacillus Casei in Habitual Smokers. Prev. Med. (Baltim). 2005, 40 (5), 589–594. 10.1016/j.ypmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Zelaya H.; Alvarez S.; Kitazawa H.; Villena J. Respiratory Antiviral Immunity and Immunobiotics: Beneficial Effects on Inflammation-Coagulation Interaction during Influenza Virus Infection. Frontiers in Immunology 2016, 7, 633 10.3389/fimmu.2016.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca W.; Lucey K.; Jang S.; Fujimura K. E.; Rasky A.; Ting H. A.; Petersen J.; Johnson C. C.; Boushey H. A.; Zoratti E.; Ownby D. R.; Levine A. M.; Bobbit K. R.; Lynch S. V.; Lukacs N. W. Lactobacillus Johnsonii Supplementation Attenuates Respiratory Viral Infection via Metabolic Reprogramming and Immune Cell Modulation. Mucosal Immunol. 2017, 10 (6), 1569–1580. 10.1038/mi.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice T. A.; Brenner T. A.; Percopo C. M.; Ma M.; Keicher J. D.; Domachowske J. B.; Rosenberg H. F. Signaling via Pattern Recognition Receptors NOD2 and TLR2 Contributes to Immunomodulatory Control of Lethal Pneumovirus Infection. Antiviral Res. 2016, 132, 131–140. 10.1016/j.antiviral.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T.; Kiyoshima J.; Shida K.; Yasui H. Effect of Intranasal Administration of Lactobacillus Casei Shirota on Influenza Virus Infection of Upper Respiratory Tract in Mice. Clin. Diagn. Lab. Immunol. 2001, 8 (3), 593–597. 10.1128/CDLI.8.3.593-597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q.; Dong B. R.; Wu T. Probiotics for Preventing Acute Upper Respiratory Tract Infections. Cochrane Database Syst. Rev. 2015, (2), CD006895 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- d’Ettorre G.; Ceccarelli G.; Marazzato M.; Campagna G.; Pinacchio C.; Alessandri F.; Ruberto F.; Rossi G.; Celani L.; Scagnolari C.; Mastropietro C.; Trinchieri V.; Recchia G. E.; Mauro V.; Antonelli G.; Pugliese F.; Mastroianni C. M. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7 (July), 1–7. 10.3389/fmed.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrayel P.; Nicco C.; Al Khodor S.; Bilinski J.; Caselli E.; Comelli E. M.; Egert M.; Giaroni C.; Karpinski T. M.; Loniewski I.; Mulak A.; Reygner J.; Samczuk P.; Serino M.; Sikora M.; Terranegra A.; Ufnal M.; Villeger R.; Pichon C.; Konturek P.; Edeas M. Microbiota Medicine: Towards Clinical Revolution. J. Transl. Med. 2022, 20 (1), 1–20. 10.1186/s12967-022-03296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. O.; Gluck M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52 (2), 137–143. 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano M.; Covasa M. Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front. Microbiol. 2020, 11, 590370 10.3389/fmicb.2020.590370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P.; Li X.; Shen J.; Feng Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 2020, 11, 574533 10.3389/fphar.2020.574533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical University of Warsaw. The Impact of Fecal Microbiota Transplantation as an Immunomodulation on the Risk Reduction of COVID-19 Disease Progression With Escalating Cytokine Storm and Inflammatory Parameters (FeMToCOVID). ClinicalTrials.gov, 2021, NCT04824222. https://clinicaltrials.gov/study/NCT04824222.
- Akhtar M.; Chen Y.; Ma Z.; Zhang X.; Shi D.; Khan J. A.; Liu H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Animal Nutrition 2022, 8, 350–360. 10.1016/j.aninu.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Li Y.; Bian Q.; Xuan Y.; Li J.; Wang Z.; Feng S.; Liu X.; Tian Y.; Li S. The Bufei Jianpi Formula Improves Mucosal Immune Function by Remodeling Gut Microbiota through the SCFAs/GPR43/NLRP3 Pathway in Chronic Obstructive Pulmonary Disease Rats. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 1285–1298. 10.2147/COPD.S359428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavaeva T. M.; Maksimenya M. V.; Tereshkov P. P.; Gaimolenko I. N.; Medvedeva T. A.; Parshina A. A. Long-chain fatty acids and short-chain fatty acids in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Biomed. Khim. 2021, 67 (2), 169–174. 10.18097/pbmc20216702169. [DOI] [PubMed] [Google Scholar]
- Kotlyarov S. Role of Short-Chain Fatty Acids Produced by Gut Microbiota in Innate Lung Immunity and Pathogenesis of the Heterogeneous Course of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2022, 23, 4768 10.3390/ijms23094768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrio F.; Martini S.; Francavilla R.; Corvaglia L.; Cristofori F.; Mastrolia S. A.; Neu J.; Rautava S.; Russo Spena G.; Raimondi F.; Loverro G. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-Term Health Development. Front. Pediatr. 2017, 5, 178. 10.3389/fped.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G.; Wang B.; Shafiei Jahani P.; Hurrell B. P.; Banie H.; Aleman Muench G. R.; Maazi H.; Helou D. G.; Howard E.; Galle-Treger L.; Lo R.; Santosh S.; Baltus A.; Bongers G.; San-Mateo L.; Gilliland F. D.; Rehan V. K.; Soroosh P.; Akbari O. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front. Immunol. 2019, 10, 2051. 10.3389/fimmu.2019.02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.; Kwon O.; Ryu T. Y.; Jung C. R.; Kim J.; Min J. K.; Kim D. S.; Son M. Y.; Cho H. S. Propionate of a Microbiota Metabolite Induces Cell Apoptosis and Cell Cycle Arrest in Lung Cancer. Mol. Med. Rep. 2019, 20 (2), 1569–1574. 10.3892/mmr.2019.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sencio V.; Barthelemy A.; Tavares L. P.; Machado M. G.; Soulard D.; Cuinat C.; Queiroz-Junior C. M.; Noordine M.-L.; Salomé-Desnoulez S.; Deryuter L.; Foligné B.; Wahl C.; Frisch B.; Vieira A. T.; Paget C.; Milligan G.; Ulven T.; Wolowczuk I.; Faveeuw C.; Le Goffic R.; Thomas M.; Ferreira S.; Teixeira M. M.; Trottein F. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30 (9), 2934–2947.e6. 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Mirković B.; Murray M. A.; Lavelle G. M.; Molloy K.; Azim A. A.; Gunaratnam C.; Healy F.; Slattery D.; McNally P.; Hatch J.; Wolfgang M.; Tunney M. M.; Muhlebach M. S.; Devery R.; Greene C. M.; McElvaney N. G. The Role of Short-Chain Fatty Acids, Produced by Anaerobic Bacteria, in the Cystic Fibrosis Airway. Am. J. Respir. Crit. Care Med. 2015, 192 (11), 1314–1324. 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negatu D. A.; Yamada Y.; Xi Y.; Go M. L.; Zimmerman M.; Ganapathy U.; Dartois V.; Gengenbacher M.; Dick T. Gut Microbiota Metabolite Indole Propionic Acid Targets Tryptophan Biosynthesis in Mycobacterium Tuberculosis. MBio 2019, 10.1128/mBio.02781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D.; Tarran R.; Ulrich M.; Schwab U.; Cekici A.; Meyer K. C.; Birrer P.; Bellon G.; Berger J.; Weiss T.; Botzenhart K.; Yankaskas J. R.; Randell S.; Boucher R. C.; Döring G. Effects of Reduced Mucus Oxygen Concentration in Airway Pseudomonas Infections of Cystic Fibrosis Patients. J. Clin. Invest. 2002, 109 (3), 317–325. 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]