Abstract
Objectives:
White fat contributes to body weight (BW) but accumulates very little [18F]fluorodeoxyglucose ([18F]FDG) in the fasting state. As a result, higher standardised uptake values normalised to BW (SUV) are observed in non-fatty tissue in obese patients compared to those in non-obese patients. Therefore, SUV normalised to lean body mass (SUL) that makes tumour uptake values less dependent on patients’ body habitus is considered more appropriate. This study aimed to assess ten mathematical equations to predict lean body mass (LBM) by comparison with dual-energy X-ray absorptiometry (DXA) as the reference method.
Methods:
DXA-based LBM was compared with ten equation-based estimates of LBM in terms of the slope, bias and 95% limits of agreement (LOA) of Bland-Altman plots, and Pearson correlation coefficients (r). Data from 747 men and 811 women aged 60–65 years were included.
Results:
Gallagher’s equation was optimal in males (slope = 0.13, bias = −2.4 kg, LOA = 12.8 kg and r = 0.900) while Janmahasatian’s equation was optimal in females (slope = 0.14, bias = −0.9 kg, LOA = 10.7 kg and r = 0.876). Janmahasatian’s equation performed slightly better than Gallagher’s in the pooled male and female data (slope = 0.00, bias = −1.6 kg, LOA = 12.3 kg and r = 0.959).
Conclusions:
The Gallagher and Janmahasatian equations were optimal and almost indistinguishable in predicting LBM in subjects aged 60–65 years.
Advances in knowledge:
Determination of the optimum equation for predicting lean body mass to improve the calculation of SUL for [18F]FDG PET quantification.
Introduction
[18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET) scans are commonly used for imaging cancer patients. 1 PET images are interpreted quantitatively using standardised uptake values (SUV). 2 SUV provides a measure of tracer uptake per unit volume within a region of interest (ROI) over a small interval at a particular time t, calculated as follows:
It is used to express the level of tracer uptake in tumours for various clinical and research purposes. 3 Assumptions associated with SUV, their advantages and disadvantages have been discussed previously. 4–6
Tumour activity normalisation using lean body mass (LBM) or body surface area (BSA) has been suggested to be better than using body weight (BW) in various [18F]FDG PET oncology studies. 7–13 The main rationale for using LBM for normalising tumour activity is that very low uptake of [18F]FDG is observed in white fat in the fasting state. 7,8 The observation that fat contributes to BW but accumulates very little [18F]FDG in the fasting state has been considered to be a reason for higher SUV in non-fatty tissue in obese patients compared to non-obese patients. Therefore, the use of a normalisation factor such as LBM or BSA may be more appropriate.
Many different methods exist to measure LBM in humans including whole-body dual-energy X-ray absorptiometry (DXA), air displacement plethysmography, skinfold thickness and bioimpedance. 14 Measuring LBM using imaging in clinical trials is challenging in terms of standardisation, cost, scan time and radiation dose to patients. Therefore, the use of mathematical equations to predict LBM based on patients’ height, weight, age, ethnicity and sex is a practical and cost-effective alternative. For this reason, predictive equations for assessing LBM have long been a topic of interest to researchers. 15,16
Many different equations to predict LBM have been used in the PET literature. However, a thorough investigation of predictive equations to assess LBM in a large British population in greater detail is missing. Therefore, this study aimed to assess ten predictive equations for calculating LBM, some of which are commonly used in the PET literature, to find the most accurate equation by comparison with DXA as the reference method.
Methods and materials
Whole-body DXA scans are two-dimensional projection scans in which X-ray attenuation through the body is measured at two different photon energies enabling a pixel-by-pixel estimate of the areal densities (units g/cm2) of two different types of tissue. 17 After first flagging those pixels that include bone, the remaining pixels are used to measure the areal densities of lean and fat tissue. In bone pixels, bone mineral density and the areal densities of lean and fat are measured on the assumption that the percentage body fat (%BF) can be interpolated from the adjacent non-bone pixels. DXA manufacturers fine-tune their algorithms for deriving lean and fat mass by calibrating them against a suite of more sophisticated methods. 14,17 Although DXA is not a gold standard method for measuring LBM, the low radiation dose, low cost and widespread availability make it an attractive method for many studies.
Data demographics
Whole-body DXA measurements of LBM in 1,558 subjects (747 men, 811 women) acquired with Hologic Discovery densitometers (Hologic Inc., Malborough, MA) were obtained from the Medical Research Council (MRC) National Survey of Health and Development (NSHD). 18 All were acquired in the period 2006–2011 using the same DXA scan mode. Demographic details of this cohort are described in Table 1. These data were retrospectively analysed to compare equation-based predicted LBM and DXA-based LBM to find the optimum predictive equation. Because the brain is enclosed by bone, there are no appropriate non-bone pixels for inferring its composition and the manufacturer’s software makes assumptions about its fat content. For this reason, the results of DXA scans are sometimes reported excluding the head. However, since [18F]FDG is taken up by the brain, for the present study we choose to compare the predictive equations with DXA data that included the head.
Table 1.
Demographic details of the MRC National Survey of Health and Development DXA body composition study cohort 17 expressed as mean and range
| Sex | N | Mean Age (range) in yrs | Mean Weight (range) in kg | Mean Height (range) in cm |
|---|---|---|---|---|
| Males | 747 | 63 (60–65) | 85.3 (50.6–128.5) | 175.3 (156.7–195.7) |
| Females | 811 | 63 (60–65) | 72.3 (38–136.5) | 162.2 (144.3–179.0) |
| Both sexes | 1558 | 63 (60–65) | 78.5 (38–136.5) | 168.5 (144.3–195.7) |
DXA, dual energy X-ray absorptiometry; N, number of subjects; cm, centimeters; kg, kilograms; yrs, years.
Predictive equations
The equations examined were those of Hume, 19 Hume & Weyers, 20 James & Waterlow, 21 Hallynck et al., 22 Boer, 15 Deurenberg et al., 23 Zasadny & Wahl, 7 Morgan & Bray, 16 Gallagher et al. 24 and Janmahasatian et al. 25 A brief description of each equation is provided in Table 2 and a detailed description is given in the Supplementary Material 1. These ten equations were selected as ones commonly found in the PET literature for normalising SUV to LBM.
Table 2.
Demographic details of the ten study cohorts used to develop the predictive equations expressed as mean and range
| Eq. No. | Ref | Sex | N | Mean Age (range) - y | Mean Weight (range) - kg | Mean Height (range) - cm | Predictive Equations |
|---|---|---|---|---|---|---|---|
| Eq1 | 19 | M | 29 | 61.0 (40–77) | 75.7 (43–133) | 169.6 (151–188) | LBM = 0.32810*W(kg)+0.33929*H(cm) – 29.53 |
| W | 27 | 60.0 (37–80) | 68.4 (45–116) | 156.3 (139–182) | LBM = 0.29569*W(kg)+0.41813*H(cm) – 43.29 | ||
| Eq2 | 20 | M | 30 | 54.5 (35–71) | 71.8 (36–122) | 170.2 (132–185) | LBM= [0.296785*W(kg)+0.194786*H(cm) – 14.01]*100/73 |
| W | 30 | 53.7 (33–84) | 64.7 (32–108) | 156.3 (144–170) | LBM = [0.183809*W(kg)+0.344547*H(cm) – 35.27]*100/73 | ||
| Eq3 | 21 | M | - | - | - | - | %BF = 1.281*[W(kg)/H(m)^2] – 10.13 |
| W | %BF = 1.48*[W(kg)/H(m)^2] – 7.0 | ||||||
| Eq4 | 22 | M | - | - | - | - | LBM = 1.10*W(kg) – 128*[W(kg)/H(cm)]^2 |
| W | LBM = 1.07*W(kg) – 148*[W(kg)/H(cm)]^2 | ||||||
| Eq5 | 15 | M | 47 | Not provided (21–72) | - | - | LBM = 0.407*W(kg)+26.7*H(m) – 19.2 |
| W | 40 | Not provided (19–68) | - | - | LBM = 0.252*W(kg)+47.3*H(m) – 48.3 | ||
| Eq6 | 23 | M | 521 | - | - | - | %BF = 1.2*BMI + 0.23*AGE(yrs) – 16.2 |
| W | 708 | %BF = 1.2*BMI + 0.23*AGE(yrs) – 5.4 | |||||
| Eq7 | 7 | M | - | - | - | - | LBM = 48+1.06*[H(cm) – 152] |
| W | 28 | 54 (29–75) | 72 (45–107) | LBM = 45.5+0.91*[H(cm) – 152] | |||
| Eq8 | 16 | M | - | - | - | - | LBM = 1.10*W(kg) – 120*[W(kg/H(cm)]^2 |
| W | LBM = 1.07*W(kg) – 148*[W(kg)/H(cm)]^2 | ||||||
| Eq9 | 24 | M | 291 | 49.2 (Not provided) | 80.2 (Not provided) | 176 (Not provided) | %BF = 48.1–848/BMI + 0.084*AGE(yrs)+39/BMI |
| W | 380 | 51.8 (Not provided) | 66.3 (Not provided) | 163 (Not provided) | %BF = 64.5–848/BMI + 0.079*AGE(yrs) | ||
| Eq10 | 25 | M | 168 | 42.1 (18–82) | 106.0 (60–217) | 177.4 (159–208) | FFM = 9270*W(kg)/(6680 + 216*BMI) |
| W | 205 | 41.2 (19–79) | 88.3 (41–196) | 164.2 (137–187) | FFM = 9270*W(kg)/(8780 + 244*BMI) |
AGE(yrs), Age in years; %BF, Percentage body fat; BMI, Body mass index; Eq, Equation; H(cm), height in cemtimeters; H(m), height in meters; LBM, Lean body masss; M, males; N, number of subjects; Ref, reference number in text; W, females; W(kg), weight in kilograms; cm, centimeters; kg, kilograms; yrs, years.
Statistical analysis
R software 26 was used to analyse combined data of males and females, as well as males and females separately. To assess the appropriateness of parametric statistical tests, DXA-based LBM, the results of the predictive equations and their differences were tested for normality using the Shapiro-Wilk test. The differences between the DXA-based LBM and equation-based LBM were then assessed in terms of four parameters: (i) the slope, (ii) bias and (iii) 95% limits of agreement (LOA) of their respective Bland-Altman (BA) plots, 27 and (iv) the Pearson correlation coefficient.
We first chose the optimum equation in terms of each of these four parameters and eliminated other equations which had values outside the 95% confidence intervals (95% CI). The remaining equations were considered statistically indistinguishable from each other. (i) Based on the slope of the regression line fitted to the BA plots, equations were eliminated if the 95% CI of the slope did not include zero. (ii) Based on the bias, equations were eliminated if the 95% CI of the bias did not include zero. (iii) Based on the LOA, the standard deviation (SD) of the Y-axis values in the BA plot was obtained by dividing LOA by 3.92 (i.e., 2 × 1.96). The SD of each equation was then compared with the lowest value using the F-test and equations were excluded for those with p < 0.05. (iv) Based on the Pearson correlation coefficient, the equation with the highest value was taken and compared with 95% CI of the r-values for the other equations to check if they included or excluded this highest value.
Results
The results of slope, bias and LOA of the differences of the BA plots and correlation coefficients for all equations for males and females and their pooled data are summarised in Table 3. For the pooled data, in terms of slope, Eqs 7, 8, 9 and 10 all showed values with 95% CI consistent with zero. For bias, Eqs 1, 6 and 10 were all consistent with zero. For LOA, Eq 9 was best with an SD of 2.93 kg. The next best was Eq 10 (SD: 3.08 kg, p = 0.029). For the correlation coefficient, Eq 9 gave the largest value (r = 0.962), but Eqs 2, 5, 6 and 10 were within the 95% CI. When males and females were analysed separately, Eq 9 was optimal in males and Eq 10 in females (Table 3).
Table 3.
The slope, bias and limit of agreement (LOA) obtained from the Bland-Altman plots of the differences between the LBM from DXA and each of the predictive equations. Pearson correlations are also reported between LBM from DXA and each of the predictive equations. Results are shown for pooled males and females (M + W), males only (M) and females only (W). *highlights the optimum fit. +indicates other fits that are statistically consistent with the optimum
| M + W | M + W | M + W | M + W | M | M | M | M | W | W | W | W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Bias | LOA | Pearson | Slope | Bias | LOA | Pearson | Slope | Bias | LOA | Pearson | |
| Eq1: Hume 19 | 0.32 | −1.1* | 16.9 | 0.945 | 0.32 | 1.8* | 14.2+ | 0.890+ | 0.08 | −3.8 | 11.3 | 0.861+ |
| Eq2: Hume & Weyers 20 | 0.13 | −3.5 | 13.0 | 0.957+ | 0.20 | −2.5+ | 13.0+ | 0.899+ | 0.15 | −4.3 | 12.1 | 0.840 |
| Eq3: James & Waterlow 21 | 0.15 | −4.1 | 14.2 | 0.948 | 0.21 | −3.3 | 13.6+ | 0.889+ | 0.33 | −4.9 | 14.0 | 0.776 |
| Eq4: Hallynck 22 | 0.15 | −4.1 | 14.2 | 0.948 | 0.21 | −3.2 | 13.6+ | 0.882 | 0.33 | −4.9 | 14.0 | 0.776 |
| Eq5: Boer 15 | 0.14 | −3.6 | 13.1 | 0.957+ | 0.20 | −2.6+ | 13.0+ | 0.899+ | 0.15 | −4.5 | 12.1 | 0.840 |
| Eq6: Deurenberg 23 | 0.14 | 1.5+ | 13.9 | 0.950+ | 0.31 | 2.0+ | 14.2 | 0.887+ | 0.42 | 1.0+ | 13.3 | 0.810 |
| Eq7: Zasadny & Wahl 7 | 0.02+ | −12.8 | 25.0 | 0.830 | 0.12 | −12.9 | 27.5 | 0.525 | 0.10 | −12.7 | 22.4 | 0.451 |
| Eq8: Morgan & Bray 16 | 0.05+ | −5.0 | 13.6 | 0.949 | 0.13 | −5.1 | 13.1+ | 0.896+ | 0.33 | −4.9 | 14.0 | 0.776 |
| Eq9: Gallagher 24 | 0.04+ | −2.4 | 11.8* | 0.962* | 0.13 | −2.4+ | 12.8* | 0.900* | 0.17 | −2.3 | 10.7* | 0.877* |
| Eq10: Janmahasatian 25 | 0.00* | −1.6+ | 12.3 | 0.959+ | 0.21 | −2.3+ | 13.3+ | 0.895+ | 0.14 | −0.9* | 10.7* | 0.876+ |
DXA, Dual-energy X-ray ansorptiometry; Eq, Equation; LBM, Lean body mass; LOA, Limit of agreement.
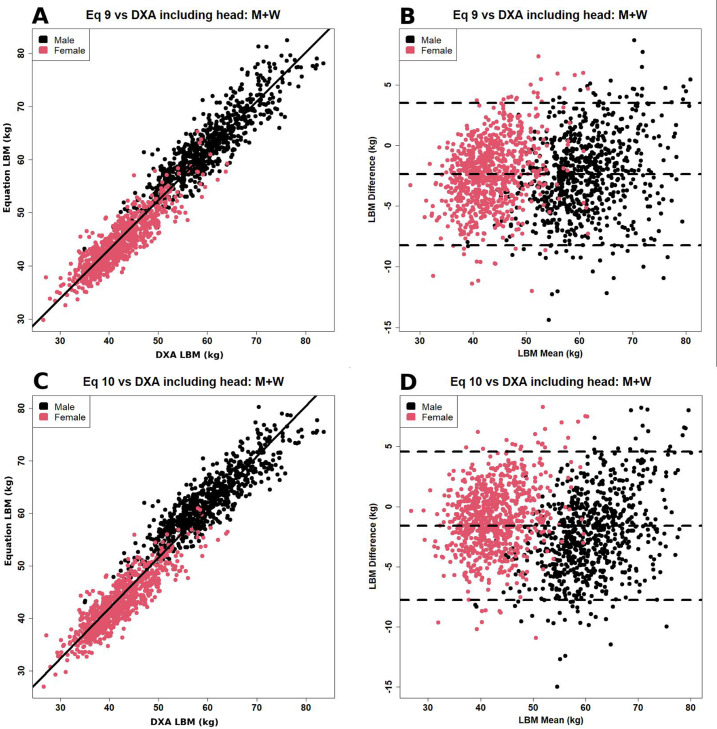
Overall, for the male, female and pooled data Eqs 9 and 10 were optimal and were not statistically distinguishable. Figure 1 shows the scatter and Bland-Altman plots for these two equations. Similar plots for the other eight equations are shown in Figures S1 to S4 in the Supplementary Material 1.
Figure 1.
(A) Scatter and (B) Bland-Altman plot of the relationship between lean body mass measured by dual-energy X-ray absorptiometry (DXA) and predicted by the Gallagher equation (equation 9 in Table 2). 24 (C) and (D): Similar graphs for the Janmahasatian equation (equation 10 in Table 2). 25
Discussion
In this study, we have used DXA data to compare ten mathematical equations that predict LBM and to choose an optimal equation for use in PET oncology studies.
It is known that body composition varies with race, gender, age and body size. In adults, weight gain mostly represents the accumulation of body fat. For this reason, many researchers have aimed to develop an age-specific formula to predict %BF. However, Eqs 1-5, 7, 8 and 10 in Table 2 do not include age as an independent variable, and these equations may be too simple to model LBM over a wide age range, which might explain their poorer predictions. On the other hand, Eq 9 provided better predictions as it included age, sex and body mass index (BMI) as the independent predictors of LBM. Interestingly, Eq 10 agreed well with DXA although it does not include age as an independent predictor of LBM despite including data from subjects with a wide age range of 18–82 years for modelling the equation. Although our study shows that Eq 10 works well in the 60–65 year age group, we cannot comment on how well it works for other age ranges.
There are technical reasons why some of the equations under consideration might perform poorly. Hume & Weyers 20 modelled Eq 2 on the assumption that total body water is 73% of fat-free mass (FFM). Nyman 28 noticed that Eq 3 was not appropriate for predicting LBM in obese population as the model plateaued at a BMI of 37 kg/m2 in females and 43 kg/m2 in males before decreasing at higher BMI. Eqs 1-5 and Eqs 7-8 were regressed based on small datasets which may contribute to some degree of error in these models. In contrast, Eq 6 is based on 1,229 subjects, Eq 9 on 671 subjects, and Eq 10 on 373 subjects, which may have contributed to making them more reliable than others. The better agreement of Eq 9 and 10 may also be because these two equations were partly derived using DXA measurements while others were not.
Carnevale et al. 29 compared Eq 1 in 100 subjects against the DXA-based LBM. Their results (r = 0.83, BA plot bias: 1.36 kg, LOA: 14.78 kg) were similar to ours (r = 0.95, BA plot bias: −1.10 kg, LOA: 16.89 kg). More importantly, a large slope was visible in the BA plots between Eq 1 and DXA values in their study, consistent with our results (slope = 0.32). This was the primary reason why we dropped Eq 1 as a method for predicting LBM in this analysis. Another study analysed data for 1,655 older males over the age of 65 and reported Eq 10 to be optimal when compared with DXA data. 30 However, these authors did not consider Eq 9.
Many studies have suggested that measurement of SUV normalised to lean body mass (SUL) may have advantages over the conventional normalisation to BW for [18F]FDG PET imaging of solid tumours and lymphoma. 31 32 Hence, there is interest in equations using factors such as BW, height, age and sex to predict LBM. It is important to note that cancer applications using PET tend to include older patients. That was the reason why we evaluated the predictive equations with a population of age between 60 and 65 years, which is representative of the ages of patients at our centre.
Other factors also require consideration. The fact that [18F]FDG does not significantly accumulate in white fat in the fasting state suggests that changes in fat and muscle composition in the early stages of cancer and during chemotherapy may be relevant. Cachexia has been reported in patients with breast, colorectal, urological and pancreatic cancers, lymphoma and pediatric patients. Therefore, the use of DXA or air displacement plethysmography may be more accurate in assessing body composition than predictive equations in these patients.
Limitations of the study include: (1) we assessed only a small number of equations to predict LBM as it was not practical to include all equations in the literature; (2) Our analysis presumes that DXA total body scanning is a suitable reference method for measuring LBM; (3) Most of the equations were developed for white populations and may not be appropriate for other ethic groups in the United Kingdom; (4) This study examines the differences between predictive equations for LBM but not their application to the correction of clinical PET measurements where other factors such as calibration, injected activity and uptake time 33 may be important.
Conclusion
In conclusion, the Gallagher and Janmahasatian equations performed optimally and were almost indistinguishable when compared to the DXA-based LBM in subjects with ages between 60 and 65 years. Application of these equations to a large [18F]FDG dataset is required to test the hypothesis that normalisation of tracer uptake to LBM may perform better for PET quantification than normalisation to body weight.
Supplementary Material
Footnotes
Acknowledgment: This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas' NHS Foundation Trust and King’s College London. The authors would like to thank staff at the MRC National Survey of Health and Development, MRC Unit for Lifelong Health and Ageing at University College London for their administrative support in sharing the DXA data (DOI: 10.5522/NSHD/S102A) at no cost. We also thank Dr Andrew Wong for his generous support.
Contributor Information
Tanuj Puri, Email: tanujpuri82@gmail.com, School of Biomedical Engineering and Imaging Sciences, King’s College London, St. Thomas’ Hospital, London, United Kingdom .
Glen M Blake, Email: glen.blake@kcl.ac.uk, School of Biomedical Engineering and Imaging Sciences, King’s College London, St. Thomas’ Hospital, London, United Kingdom .
REFERENCES
- 1. Kuwabara Y, Ichiya Y, Otsuka M, Miyake Y, Gunasekera R, et al. . High [18f]fdg uptake in primary cerebral lymphoma: a pet study . J Comput Assist Tomogr 1988. ; 12: 47 – 48 . doi: 10.1097/00004728-198801000-00008 [DOI] [PubMed] [Google Scholar]
- 2. Leskinen-Kallio S, Ruotsalainen U, Någren K, Teräs M, Joensuu H . Uptake of carbon-11-methionine and fluorodeoxyglucose in non-hodgkin’s lymphoma: a pet study . J Nucl Med 1991. ; 32: 1211 – 18 . [PubMed] [Google Scholar]
- 3. Weber WA, Schwaiger M, Avril N . Quantitative assessment of tumor metabolism using fdg-pet imaging . Nucl Med Biol 2000. ; 27: 683 – 87 . doi: 10.1016/s0969-8051(00)00141-4 [DOI] [PubMed] [Google Scholar]
- 4. Keyes JW . SUV: standard uptake or silly useless value? J Nucl Med 1995. ; 36: 1836 – 39 . [PubMed] [Google Scholar]
- 5. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, et al. . Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18f] 2-deoxy-2-fluoro-d-glucose . J Nucl Med 1978. ; 19: 1154 – 61 . [PubMed] [Google Scholar]
- 6. Wahl RL, Kaminski MS, Ethier SP, Hutchins GD . The potential of 2-deoxy-2[18f]fluoro-d-glucose (fdg) for the detection of tumor involvement in lymph nodes . J Nucl Med 1990. ; 31: 1831 – 35 . [PubMed] [Google Scholar]
- 7. Zasadny KR, Wahl RL . Standardized uptake values of normal tissues at pet with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction . Radiology 1993. ; 189: 847 – 50 . doi: 10.1148/radiology.189.3.8234714 [DOI] [PubMed] [Google Scholar]
- 8. Sugawara Y, Zasadny KR, Neuhoff AW, Wahl RL . Reevaluation of the standardized uptake value for fdg: variations with body weight and methods for correction . Radiology 1999; 213: 521–25. 10.1148/radiology.213.2.r99nv37521 [DOI] [PubMed] [Google Scholar]
- 9. Graham MM, Peterson LM, Hayward RM . Comparison of simplified quantitative analyses of fdg uptake . Nucl Med Biol 2000. ; 27: 647 – 55 . doi: 10.1016/s0969-8051(00)00143-8 [DOI] [PubMed] [Google Scholar]
- 10. Paquet N, Albert A, Foidart J, Hustinx R . Within-patient variability of (18)f-fdg: standardized uptake values in normal tissues . J Nucl Med 2004. ; 45: 784 – 88 . [PubMed] [Google Scholar]
- 11. Keramida G, Hunter J, Dizdarevic S, Peters AM . The appropriate whole-body index on which to base standardized uptake value in 2-deoxy-2-[(18)f]fludeoxyglucose pet . Br J Radiol 2015. ; 88( 1052 ): 20140520 . doi: 10.1259/bjr.20140520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tatcı E, Uslu Biner İ, Emir S, Tanyıldız HG, Özmen Ö, et al. . The correlation between pre-treatment fluorodeoxyglucose positron emission tomography/computed tomography parameters and clinical prognostic factors in pediatric hodgkin lymphoma . Mol Imaging Radionucl Ther 2017. ; 26: 9 – 16 . doi: 10.4274/mirt.94914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarikaya I, Albatineh AN, Sarikaya A . Revisiting weight-normalized suv and lean-body-mass-normalized suv in pet studies . J Nucl Med Technol 2020. ; 48: 163 – 67 . doi: 10.2967/jnmt.119.233353 [DOI] [PubMed] [Google Scholar]
- 14. Kuriyan R . Body composition techniques . Indian J Med Res 2018. ; 148: 648 – 58 . doi: 10.4103/ijmr.IJMR_1777_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boer P . Estimated lean body mass as an index for normalization of body fluid volumes in humans . Am J Physiol 1984. ; 247: F632 - 6 . doi: 10.1152/ajprenal.1984.247.4.F632 [DOI] [PubMed] [Google Scholar]
- 16. Morgan DJ, Bray KM . Lean body mass as a predictor of drug dosage. implications for drug therapy . Clin Pharmacokinet 1994. ; 26: 292 – 307 . doi: 10.2165/00003088-199426040-00005 [DOI] [PubMed] [Google Scholar]
- 17. Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB . Body composition by dxa . Bone 2017. ; 104: 101 – 5 . doi: 10.1016/j.bone.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, et al. . Cohort profile: updating the cohort profile for the mrc national survey of health and development: a new clinic-based data collection for ageing research . Int J Epidemiol 2011. ; 40: e1 - 9 . doi: 10.1093/ije/dyq231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hume R . Prediction of lean body mass from height and weight . J Clin Pathol 1966; 19: 389–91. 10.1136/jcp.19.4.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hume R, Weyers E . Relationship between total body water and surface area in normal and obese subjects . J Clin Pathol 1971. ; 24: 234 – 38 . doi: 10.1136/jcp.24.3.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. James WPT, Waterlow JC . Research on obesity: a report of the DHSS/MRC group . HM Stationery Office; ; 1976. . [Google Scholar]
- 22. Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, et al. . Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol 1981. ; 11: 523 – 26 . doi: 10.1111/j.1365-2125.1981.tb01163.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deurenberg P, Weststrate JA, Seidell JC . Body mass index as a measure of body fatness: age- and sex-specific prediction formulas . Br J Nutr 1991. ; 65: 105 – 14 . doi: 10.1079/bjn19910073 [DOI] [PubMed] [Google Scholar]
- 24. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, et al. . Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index . Am J Clin Nutr 2000. ; 72: 694 – 701 . doi: 10.1093/ajcn/72.3.694 [DOI] [PubMed] [Google Scholar]
- 25. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, et al. . Quantification of lean bodyweight . Clin Pharmacokinet 2005. ; 44: 1051 – 65 . doi: 10.2165/00003088-200544100-00004 [DOI] [PubMed] [Google Scholar]
- 26. R: A Language and Environment for Statistical Computing, {R Core Team}, R Foundation for Statistical Computing . Vienna, Austria: ; 2021. . Available from : https://www.R-project.org/ [Google Scholar]
- 27. Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement . Lancet 1986. ; 1: 307 – 10 . [PubMed] [Google Scholar]
- 28. Nyman U . James lean body weight formula is not appropriate for determining ct contrast media dose in patients with high body mass index . Radiology 2016. ; 278: 956 – 57 . doi: 10.1148/radiol.2016152031 [DOI] [PubMed] [Google Scholar]
- 29. Carnevale V, Piscitelli PA, Minonne R, Castriotta V, Cipriani C, et al. . Estimate of body composition by hume’s equation: validation with dxa . Endocrine 2015. ; 49: 65 – 69 . doi: 10.1007/s12020-014-0419-3 [DOI] [PubMed] [Google Scholar]
- 30. Mitchell SJ, Kirkpatrick CMJ, Le Couteur DG, Naganathan V, Sambrook PN, et al. . Estimation of lean body weight in older community-dwelling men . Br J Clin Pharmacol 2010; 69: 118–27. 10.1111/j.1365-2125.2009.03586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wahl RL, Jacene H, Kasamon Y, Lodge MA . From recist to percist: evolving considerations for pet response criteria in solid tumors . J Nucl Med 2009. ; 50 Suppl 1: 122S - 50S . doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narita A, Shiomi S, Katayama Y, Yamanaga T, Daisaki H, et al. . Usefulness of standardized uptake value normalized by individual ct-based lean body mass in application of pet response criteria in solid tumors (percist) . Radiol Phys Technol 2016. ; 9: 170 – 77 . doi: 10.1007/s12194-016-0346-5 [DOI] [PubMed] [Google Scholar]
- 33. Dunn JT, Mikhaeel NG, Puri T, Marsden PK, O’Doherty MJ, et al. . The importance of uptake time in fdg imaging of patients with lymphoma eanm’16 . Eur J Nucl Med Mol Imaging 2016. ; 43: 1 – 734 . doi: 10.1007/s00259-016-3484-4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.