Abstract
Regulatory B cells (Bregs) are an immunosuppressive cell phenotype that affects the immune system by limiting the inflammatory cascade. Dysregulation of Bregs can interestingly play a dichotomous role in the pathophysiology of many diseases and is especially highlighted when examining cancer pathology compared to allergic disease. This study reviews the existing literature on Bregs and compares their role in allergic disease in contrast to cancer development. Upregulation of Bregs in cancer states has been associated with poor prognostic outcomes across various cancer types, and Breg proliferation was associated with chronic interferon signaling, activation of the BCR–BTK (B cell receptor-Bruton’s tyrosine kinase) pathway, and release of C–X–C motif ligand 13. In contrast, Breg dysfunction has been identified as a key mechanism in many allergic diseases, such as allergic asthma, allergic rhinitis, atopic dermatitis, and contact dermatitis. Development of Breg-targeted immunotherapies is currently at the preclinical level, but strategies differentially focus on Breg depletion in cancer versus Breg stimulation in allergy. Our review highlights the divergent functions that Bregs play in cancer compared to allergy. We conclude that natural homeostasis hinges on a fine balance between the dichotomous role of Bregs—over or underactivation can result in a pathological state.
Keywords: allergy and immunology, cancer immunotherapy, regulatory B cells, tumor microenvironment
1 |. INTRODUCTION
The immune system is a complex entity that can paradoxically play an important role in both disease prevention and acquisition. An important arm of the immune system is the adaptive immune system, which is trained to identify specific antigen motifs.1 Once antigen specificity is recognized through a T- or B-cell receptor, the signalizing cascade either signals the immune system to ignore this antigen (tolerance), to clear it (in the case of a pathogen), or pathologically react (in the case of food allergy, hypersensitivity, and autoimmunity).2 After the insulting antigen has been eradicated, the inflammatory response is programmed to be self-limiting, and resolution is achieved through the release of anti-inflammatory mediators and cytokines.3 In particular, regulatory immune cells play an essential role in the subsequent downregulation of the involved inflammatory cascade.
Regulatory B cells (Bregs) have been found to play an important role in the natural physiology of the immune system. Bregs work in a multitude of functions, including both cell-mediated and cytokine approaches, to downregulate the immune system.4 However, as with all biological processes, there is always a fine line to maintaining homeostasis. Both the overactivity and underactivity of Bregs result in separate but distinct pathological conditions.
In recent years, the regulatory immune cell phenotype has been implicated in the development of both cancer and allergic disease. While these appear to be seemingly different disease processes, our review will underscore the important role that Bregs play in the etiology of both. The dichotomy of Bregs lies in the following: overactivation of Bregs in cancer prevents the immune response from appropriately recognizing and eradicating cancer, whereas underactivation of Bregs in allergy allows for a chronic hyperinflammatory state. In this review, we will focus on understanding the pathophysiology of Bregs and comparing their differing roles in cancer and allergic disease.
2 |. PHYSIOLOGY OF BREGS
B cells originate in the bone marrow where they undergo a period of maturation before they enter the systemic circulation.5 Major B-cell effector functions include antigen presentation, cytokine production, and antibody production (Figure 1). More specifically, Breg cells are a subset of B cells that make up approximately 0.5% of human B cells in the periphery.6 The physiological goal of this suppressive phenotype is to dampen the immune system once the insulting antigen has been eliminated through both cytokine- and cell-mediated approaches (Figure 2).
FIGURE 1.
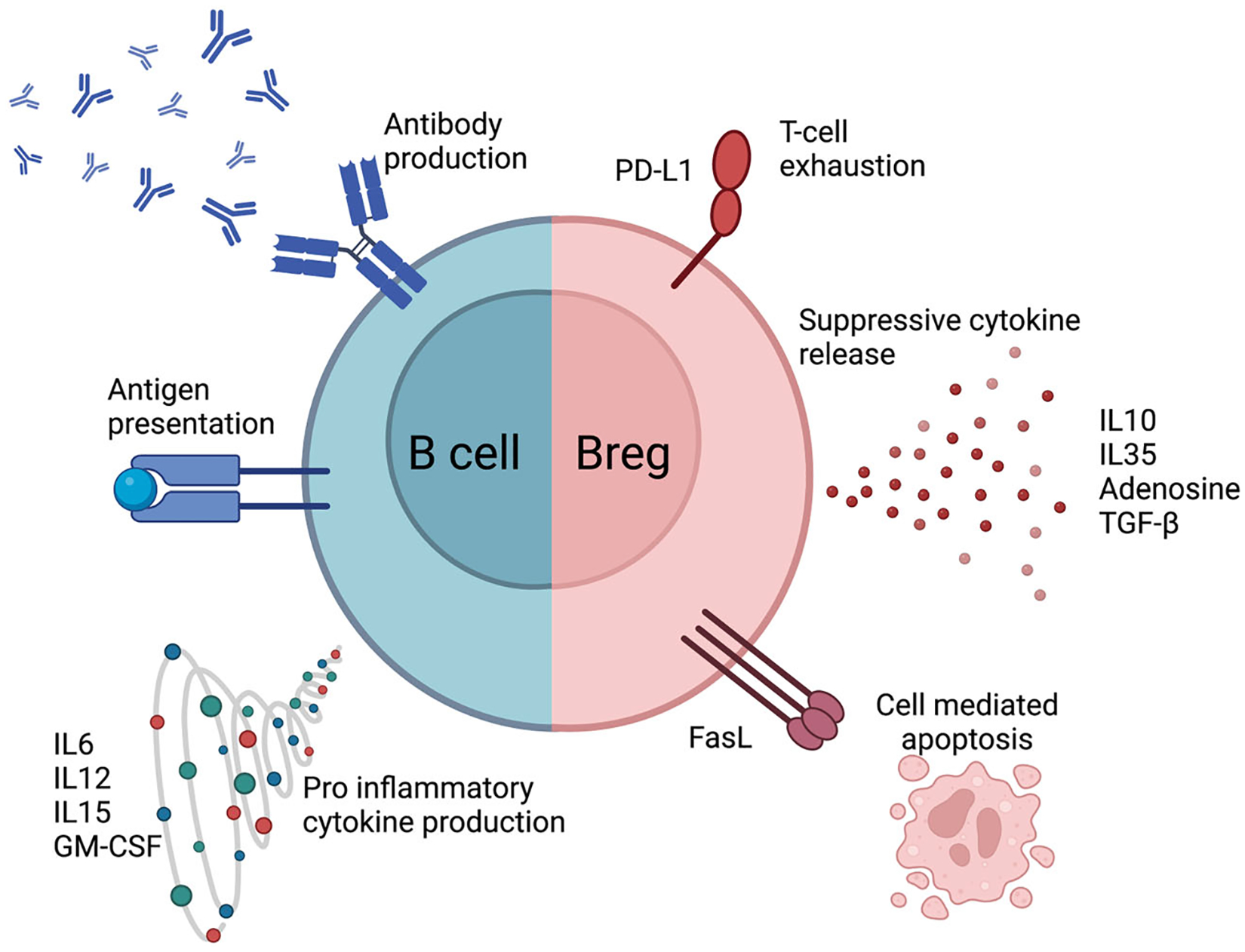
Comparison of physiology of an effector B cell with regulatory B cell. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; PD-L1, programmed death-ligand 1; TGF-β, tumor growth factor-β.
FIGURE 2.
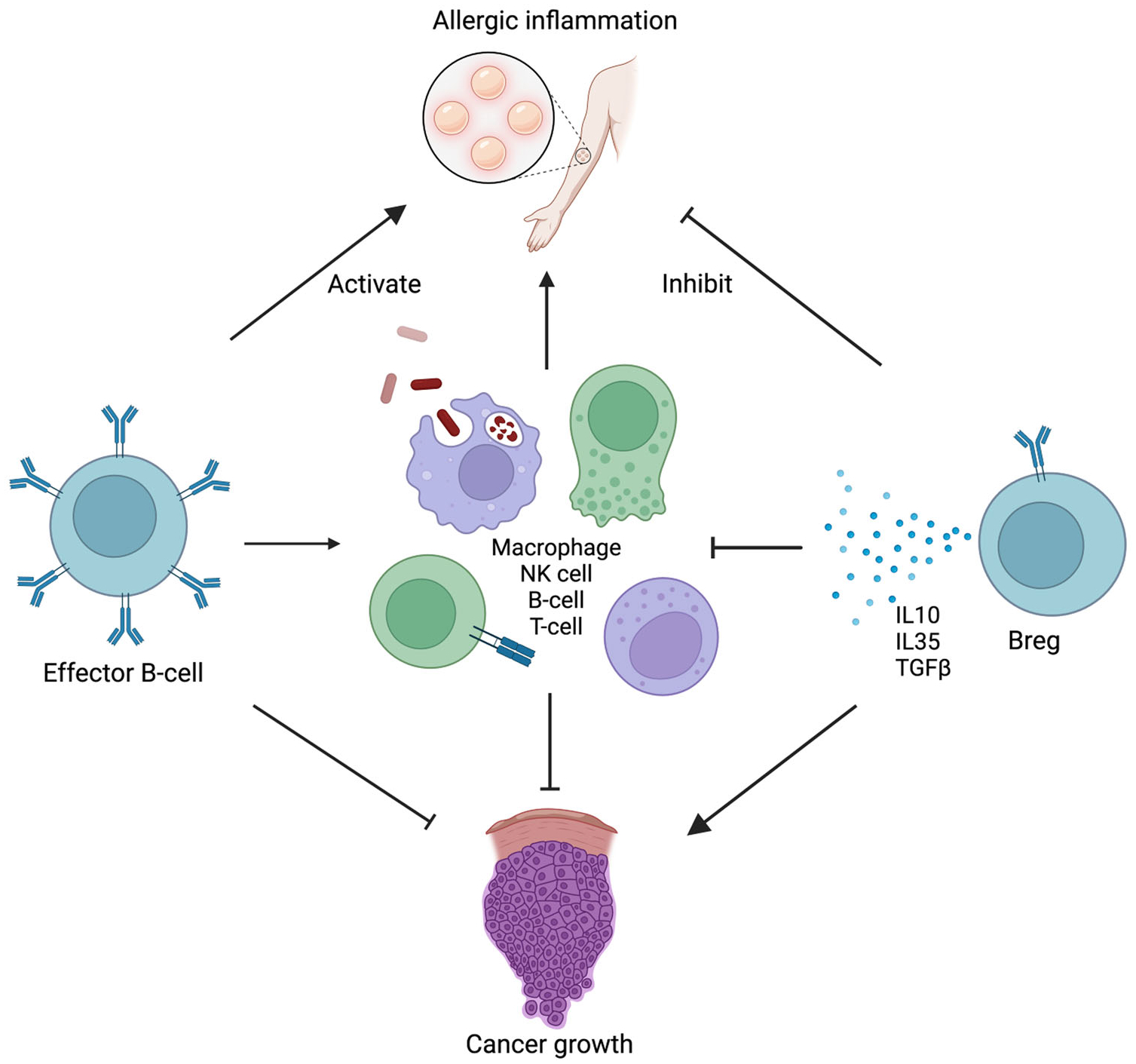
Pathways through which regulatory B cells (Bregs) can inhibit allergic response compared to promote tumor growth. IL, interleukin; NK, natural killer; TGF-β, tumor growth factor-β.
Immunosuppressive cytokine release is a staple of Breg function.7 Two defining suppressive cytokine markers secreted by Bregs are interleukin-10 (IL-10) and IL-35. These two ILs operate in similar manners by suppressing CD4 T-cell proliferation, reducing the T-helper type 1 (Th1) inflammatory response, and inducing regulatory T-cell (Treg) formation.8–11 Other common cytokines secreted by Bregs are tumor growth factor-β (TGF-β), which similarly works to promote Treg formation, and granzyme B (GZMB), which is used as a cytotoxin against effector T cells.12–14
Bregs also modulate the immune response through cell-surface ligand interaction. Bregs are known to express programmed death-ligand 1 (PD-L1), which binds to PD-1 on effector T cells to activate downstream signaling pathways that inhibit T-cell activation.7 In cancer, high PD-L1 expression allows the tumor to “hide” from the immune system, and thus PD-1/PD-L1 antibodies have been used in cancer immunotherapy.15 In addition, Bregs express FasL belonging to the tumor necrosis factor protein family, which binds to Fas on both CD4 and CD8 T cells to induce apoptosis.16–18
3 |. ROLE OF BREG IN CANCER
3.1 |. Protumorigenic function of Bregs in human cancer
Modern advancements in biologic testing, especially in the field of cytometry, have allowed investigators to probe the numerous cell types present in circulating blood and the tumor microenvironment (TME). The identification of an active immunosuppressive B-cell phenotype has been crucial to understanding immune-related mechanisms of cancer growth. Downregulation of the immune system, while crucial for preventing the development of many allergic diseases, allows for cancer to grow unchecked. Data from human studies have shown that the prevalence of Bregs in multiple different lines of human cancer is associated with increased tumor burden and worse prognostic outcomes19–25 (Table 1).
TABLE 1.
Human studies implicating Bregs dysregulation in cancer.
| Cancer | Phenotype | Location | Breg function | Reference |
|---|---|---|---|---|
| HNSCC | CD19+CD39+CD73+ | Tumor, blood | Breg population producing ADO. ADO inhibited BTK phosphorylation and calcium influx in effector B cells | [26] |
| CD19+IL-10+ | Tumor, lymph node | Bregs in tongue squamous cell carcinoma carried poor prognostic outcomes, and when co-cultured with T cells in vitro stimulated Treg formation | [27] | |
| Hepatocellular carcinoma | CD19+CD24+CD38+ | Tumor, blood | Increased circulating Bregs correlated with disease progression. Bregs promote HCC progression through CD40–CD154 interaction | [20] |
| CD19+CD24+CD38+ | Blood | Increased circulating Bregs in HCV-induced HCC is linked to poor prognosis and increased Tregs | [28] | |
| CD19+IL-10+TIM-1+ | Tumor, blood | Increased IL-10 activity by Bregs in HCC. TIM-1+ Bregs downregulated CD4 cell production of granzyme A, B, and perforin | [29] | |
| Gastric cancer | CD19+CD24hiCD27+ | Tumor, blood | Bregs suppress CD4 proliferation and IFN-γ production | [30] |
| Ovarian cancer | CD19+IL-10+ | Ascites | Ascites from ovarian tumor pts collected and enriched in Bregs. Co-cultured with CD8s and inhibited IFN-γ production | [21] |
| AML | CD19+IL-10+ | Bone marrow, blood | Worse outcomes in AML associated with increased Bregs | [22] |
| Breast cancer | CD19+CD25+IL-10+ | Tumor | Poor prognosis outcome. High coexistence of Bregs and Tregs | [24] |
| Cervical cancer | CD19+CD5+CD1d+ | Tumor, blood | Bregs increased in cervical cancer, inhibit effector CD8 T cells from secreting perforin and granzyme B | [25] |
| Esophageal cancer | CD19+CD24hiCD27+ | Blood | Circulating tumor exomes mediate Breg development | [31] |
Abbreviations: ADO, adenosine; AML, acute myeloid leukemia; Breg, regulatory B cell; BTK, Bruton’s tyrosine kinase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HNSCC, head and neck squamous cell cancer; IFN-γ, interferon-γ; Treg, regulatory T cell.
In cancer biology, Bregs have been shown to downregulate the body’s immune response by decreasing T-cell antitumor activity. This can be done indirectly by stimulating the differentiation and development of Tregs, which are the analogous T-cell suppressive phenotype known to inhibit the immune response.24,27,28 In addition, Bregs are able to directly inhibit effector T-cell cytokine production and function by suppressing CD8 T-cell production of GZMB, perforin, and interferon-gamma (IFN-γ). Similar downregulation of CD4 T-cell proliferation, IFN-γ, and GZMB production has also been linked to Breg activity.29,30 Downregulation of the T-cell response resultantly leads to unmonitored tumor growth.
Moreover, Bregs also function to inhibit the antitumor response of other effector B cells. Jeske et al. found a population of adenosine (ADO)-secreting Bregs in head and neck squamous cell carcinoma (HNSCC) that impairs effector B-cell development. In particular, these ADO Bregs prevent phosphorylation of Bruton’s tyrosine kinase (BTK), a crucial enzyme in the B-cell development pathway,32 and prevent the maturation of effector B cells. There is also emerging evidence that certain tumors may promote Breg differentiation to avoid detection by the immune system. Exosomes are membrane-sized vesicles released by various cells that may contain proteins or RNAs.33 Mao et al. detected specific exosomes (CD9+ and CD81+) released by esophageal squamous cell carcinoma in the peripheral blood and showed that these exosomes can induce the formation of IL-10-secreting Bregs in vitro.31
3.2 |. Mechanisms of Breg development under investigation in preclinical models
While Bregs have been shown to play an important part in the immune response to cancer, the circumstances that promote Breg development and formation are still actively under investigation. Understanding Breg development will be crucial toward developing targeted depletion of Bregs as a novel cancer therapy, and on the flip-side Breg activation will play an important role in allergen tolerance therapy. Specific factors involved in Breg development have been studied using various preclinical models predominately in murine models of cancer.
Classically, type I interferon signaling (IFN-1) was thought to mediate immune activation through invigoration of T and B cells.34 The stimulator of interferon genes (STING) protein is an important upstream regulatory protein that works to activate the IFN-1 response. However, a recent study showed that administration of STING agonists, including cGAMP, actually triggered the development of IL-35-producing Bregs in a pancreatic mouse model.35 Mechanistically, the STING-IL-35 pathway was shown to reduce natural killer (NK) cell proliferation and attenuate the NK-driven antitumor response. This fits into the emerging theory that chronic interferon stimulation may result in immunosuppression.36
Another stimulator of Breg activity under investigation is the C–X–C motif ligand 13 (CXCL13). CXCL13 is a known chemoattractant that aids in B-cell trafficking.37 This chemokine has been theorized to play an important role in the formation of tertiary lymphoid structures, which are intratumoral hubs of T- and B-cell activity.38 However, CXCL13 may also play an important role in regulating Breg formation. CXCL13 genetic knockout in a murine B16 melanoma model was associated with decreased pulmonary metastases and a better response to chemo- (cyclophosphamide) and immunotherapy (PD-1 inhibition).39 Investigation of the pulmonary metastatic TME showed a decreased number of Bregs compared to wild-type control. Pharmacological blockade using a novel CXCL13 sequestering nanoparticle (a “nano trap”) developed by Shen and colleagues analogously showed reduced intratumoral Bregs in murine models of pancreatic cancer, BRAF-mutant melanoma, and triple-negative breast cancer.39
4 |. ROLE OF BREGS IN ALLERGIC DISEASES
Allergic diseases result from uncontrolled inflammation and manifest in a number of ways to include asthma, allergic rhinitis (AR), food allergy, atopic dermatitis (AD), and contact dermatitis. Many cell types are involved in the allergic response with mast cells, with eosinophils and basophils being the primary players. Mechanisms of inflammation are attributable to type 2 immune dysregulation and IgE elevation.40 Tregs have been extensively studied for their role in allergen tolerance, meaning sustained nonresponsiveness to an antigen, but more recently Bregs have been identified to play an important role in this process. Initial studies in the 1970s investigated the role of Bregs in delayed hypersensitivity reactions in animal models using cyclophosphamide and B-cell-depleted splenocytes to diminish B cells.41 This study helped set the stage in understanding the important suppressive role of Bregs in allergic disease. In the following paragraphs, we will synthesize literature about different allergic diseases and the involvement of Bregs.
4.1 |. Allergic asthma (AA)
Asthma can be defined as a disease of chronic airway inflammation with airflow obstruction and bronchial hyperresponsiveness with involvement of cytokines and chemical mediators.42 Different endotypes of asthma have been identified and separated into two broad categories—type 2-high asthma (AA and eosinophilic asthma) and type 2-low asthma (paucigranulocytic and neutrophilic). More specifically, AA is a subtype of asthma that creates a T-helper type 2 (Th2) response to inhaled allergens.43 Bregs have been shown to be dysfunctional in AA patients.44 In particular, patients with AA have Bregs with an impairment in IL-10 production and subsequently difficulty in inducing Treg formation. Biologics have also been studied as potential treatment strategies for AA. Dupilumab, a human monoclonal antibody that inhibits the IL-4 receptor alpha signaling induced by IL-4 and IL-13, is a commonly used biologic in AA. In one study, the levels of immunosuppressive B cells in response to dupilumab were increased in patients with severe asthma.45
4.2 |. AR
AR is an IgE-mediated clinical syndrome with symptoms of nasal pruritus, sneezing, rhinorrhea, and nasal congestion in response to aeroallergen exposure.46 Breg dysfunction has been similarly implicated in the pathophysiology of AR. Patients with AR have decreased Breg numbers compared to healthy controls.47,48 Bregs in AR had decreased expression of cell-surface marker CD25 and the ⍺-chain of the IL-2 receptor, which is critical for Treg development and survival. Patients with AR and asthma had markedly decreased Breg numbers compared to AR alone. Changes in Breg activity across the upper and lower airways may play a role in the clinical transition that is seen from AR to AR with asthma.49,50
4.3 |. Food allergies
The prevalence of food allergies has risen dramatically with some reports suggesting that more than 11% of adults in the United States are food allergic, while in children this number is closer to 8%.51,52 Food allergies can be subdivided into Ig-E-mediated, non-IgE-mediated, or mixed-type.53 Compared to Ig-E-mediated food allergy reactions, which can result in anaphylaxis, non-IgE-mediated food allergies are delayed and primarily affect the gut.54 In humans with cow milk allergy, there is significantly decreased IL-10 and TGF-β production by circulating Bregs.55–57 When intestinal inflammation was further investigated in a mouse model, Liu et al. were able to identify a subpopulation of Bregs in a healthy mouse intestinal mucosa that secreted TGF-β. These cells were found to induce Treg differentiation in vitro, and when adoptively transferred into a mouse model of food allergy, there was decreased Th2 intestinal inflammation.58
4.4 |. AD
AD can be defined as a systemic inflammatory skin condition with chronic, relapsing, pruritic, dry lesions with a predilection for atopy.59 This disease impacts approximately 20% of children and 3% of adults worldwide.60 Cytokines in AD development have been found to be variable based on disease stage, race, and age of patients. The etiology of immune dysregulation has not been well elucidated as the heterogeneous nature of the innate and adaptive immune system plays a role in AD.61 However, recent research in B cells helps uncover some of the underlying mechanisms of AD.62
A mouse model observed Breg frequency and IgE expression in AD by using a 2,4-dinitrofluorobenzene solution applied to the skin of mice to assess dryness, excoriation, erosion, and hemorrhaging.63 The study found that a subpopulation of Bregs had decreased IL-10 production resulting in defective IgE regulation. The mainstay of treatment for AD includes a combination of topical corticosteroids and in severe cases can progress to the initiation of biologics such as dupilumab or omalizumab. A study by Čelakovská et al. investigated immunophenotyping B cells in patients with AD with and without dupilumab treatment.64 The study found that patients treated with dupilumab had higher CD200 expression on switched B lymphocytes compared to the control group. Further studies need to be conducted to investigate Bregs as a marker of biologic response to guide therapy.
4.5 |. Allergic contact dermatitis
Contact hypersensitivity (CHS), known clinically as allergic contact dermatitis, is an immune reaction that occurs after exposure to exogenous haptens, leading to a delayed hypersensitivity reaction.65 Common contact allergens include nickel, fragrance, and preservatives. CHS has been known as a T-cell-dependent process through Th1 cell-mediated inflammation.66 However, there is emerging evidence that B cells play an important role in CHS. CD19-deficient mice have been shown to have more intense and prolonged CHS reactions.67
Murine models have been utilized to elucidate the underlying pathophysiology of CHS, with Breg dysfunction being exposed as a major hit. Several major pathways in Bregs are likely disrupted in CHS development. Blockade of the PI3k–AKT pathway results in decreased IL-10 synthesis by Bregs and worsened disease outcomes.68 In addition, peroxisome proliferator-activated receptor-α (PPAR-α) knockout in B cells also disrupts IL-10 synthesis, though does not impact FasL expression, and results in exaggerated CHS.69 Conversely, ultraviolet B (UVB) irradiation is known to suppress contact dermatitis.70,71 UVB irradiation showed increased Breg population expansion that was hypothesized to be mediated via upregulation of toll-like receptor 4 (Table 2).72
TABLE 2.
Various studies highlighting Bregs with allergic disease.
| Disease | Role of Bregs in select studies | Study population |
|---|---|---|
| AA | Decreased influx of Bregs with impaired IL-10 secretion in allergic asthma44 | AA patients with house dust mite allergy versus controls |
| Bregs could suppress or reverse known airway inflammation via CD1d, functioning from an IL-10-mediated pathway103 | B10 cells from spleens of helminth-infected mice that were transferred to ovalbumin-sensitized mice | |
| AR | Reduced Breg expression in AR patients with Bregs suppressing Th2 responses and lower levels of Tfh-like cells and IL-21 production47 | PBMCs isolated from AR individuals versus controls |
| Peripheral B-cell subsets of AR patients found a decrease in Bregs48 | AR patients versus controls B-cell subsets in peripheral blood | |
| Percentage of Bregs in total B cells were decreased in AR and even more so with disease progression to AR associated with asthma50 | AR and AR+ asthma peripheral blood subset of Tfh cells and Breg cells | |
| Food allergy | B10 responses decreased in the milk allergy group and increased in the milk-tolerant group55 | Milk allergy patients versus milk-tolerant patients |
| Adoptive transfer of tolerant B cells into a mouse with food allergy demonstrated deceased Th2 intestinal inflammation58 | Food allergy mouse with Th2 inflammation of the intestine | |
| AD | B10 in CD19+ B cells were downregulated in the AD63 | AD mouse model |
| Allergic contact dermatitis | PI3K–Akt pathway is vital for B10 cells and CHS responses68 | B-cell-specific PTEN-deficient mice |
| Bregs played a role in UVB-induced immunosuppression in CHS72 | CHS mouse model |
Abbreviations: AA, allergic asthma; AD, atopic dermatitis; AR, allergic rhinitis; Breg, regulatory B cell; CHS, contact hypersensitivity; IL, interleukin; PBMC, peripheral blood mononuclear cell; PTEN, phosphatase and tensin homolog; Tfh, T follicular helper; Th2, T-helper type 2; UVB, ultraviolet B.
5 |. DEVELOPING BREG-TARGETED IMMUNOTHERAPIES
5.1 |. Cancer immunotherapy
While no therapies targeting Bregs to date have gone into clinical trials, there are several proposed therapies targeted toward modulating the Breg axis to stunt tumor growth. In contrast with allergen immunotherapy (AIT), cancer immunotherapies focus on depleting or inhibiting Breg functionality to activate the immune system against cancer antigens.
Breg depletion has been a proposed strategy for targeted immunotherapy. Depletion using anti-CD20 antibodies against all B cells has been tried in mouse models of pancreatic ductal carcinoma and HNSCC, with results showing improved outcomes and response to platinum- and taxol-based chemotherapies.73,74 Inhibition of the BTK enzyme using tirabrutinib has also been shown to result in reduction of Bregs in the pancreatic TME, which is a favorable response.75 However, these systemic B-cell targeting strategies also deplete normal effector B cells that would otherwise be important to mounting a healthy immune response.
Selective depletion of Bregs has been technically difficult due to the lack of specific cell-surface receptors that define the phenotype.76 Matsushita et al. describe using a monoclonal mouse antibody against CD22 that was shown to selectively deplete splenic B10 cells in an autoimmune encephalitis model,77 but this has not been replicated in cancer immunotherapy to date. Weißenborn et al. created a cre-flox mouse model for inducible deletion of IL-10 on CD19 cells. However, Breg depletion did not show as significant of a tumor growth reduction in murine neuroblastoma as compared to Treg depletion.78 Future work will be important to identify targetable cell-surface markers to allow for selective Breg depletion.
The mitogen-activated protein kinase (MAPK) cascade is an important pathway that regulates cell proliferation, and mutations in the upstream RAS or RAF proteins causing constitutive activation of the MAPK cascade have been shown to induce a variety of cancers.79 The MAPK pathway is also located downstream of the BCR signaling pathway and is thought to play a role in the B-cell response.80 Pharmacological blockage of mitogen/extracellular signal-regulated kinase (MEK), an intermediary kinase downstream of RAS/RAF, has shown promising results in human melanoma clinical trials.81–83 MEK inhibition using cobimetinib has also been found to improve antitumor immunity in colorectal cancer (CRC) mouse model by decreasing the number of Bregs found in the tumor-draining lymph node and disrupting chronic BCR signaling.84 Similarly, direct inhibition of downstream extracellular signal-regulated kinase (ERK) and STAT3 has been shown to decrease Bregs’ development and improve cancer immunity.85,86 This promising preclinical data is suggestive that targeting the MAPK–MEK–ERK signaling pathway may translate to future anti-Breg therapies in the clinic.
Aside from pharmacological therapies, emerging research demonstrates that Breg development may be associated with metabolic signaling. PPAR-α is a transcription factor used to promote fatty acid oxidation.87 Murine breast cancer cells produce metabolites including leukotriene B4 that also activate the PPAR-α pathway. Wejksza et al. found that PPAR-α stimulation by these leukotriene molecules in B cells could induce the formation of Bregs and result in increased lung metastases.88 Inactivation of leukotriene B4 and/or PPAR-α abrogated the metastatic process. In addition, Wang et al. found a subpopulation of Bregs expressing TGF-β associated with worse outcomes for CRC in both mice and humans. These Bregs expressed leucine-tRNA-synthetase-2 (LARS2) (LARS B) and exhibited a nutritional preference for leucine. A high leucine diet was found to induce LARS B-cell generation, whereas a low diet was able to repress LARS B-cell generation.89 These novel metabolic findings provide additional avenues for Breg modulation and underscore the importance of the intersection between immune function and metabolism.
5.2 |. AIT
AIT was first investigated by Leonard Noon in 1911 in models for grass pollen seasonal AR.90 AIT works to reduce nasal and ocular symptoms by desensitizing mast cells and basophils as well as skewing away from Th2-directed immune response.91 Administered subcutaneously or sublingually, it has been shown to have long-lasting disease-modifying effects after discontinuation, though induction of sustained tolerance varies widely across patients and antigens. Mechanistically, this can likely be explained in part by the lasting increase in Bregs that are induced by AIT, with one study showing increased Bregs at the 2-year posttreatment mark.92 In contrast to cancer immunotherapy, AIT strategies focus on upregulating Breg infiltration to suppress inflammation and promote tolerance.
While currently there are no clinically approved AITs specifically for Bregs modulation, there is promising preclinical research.93 Abatacept, a fusion CTLA-4 protein approved for rheumatoid and psoriatic arthritis, has been shown to influence the IL-10 axis. Intranasal administration of abatacept in asthmatic mice showed increased IL-10- and IL-35-producing Bregs and LAG3+ Tregs.94 Administration of the protein Alt a 1, a major allergen found in fungi (specifically Alternaria alternata), was shown to decrease asthmatic symptoms and increase Breg levels in a mouse model.95 Last, IL expression is known to be regulated by epigenetic shifts in histone acetylation/deacetylation.96 Treatment of CHS using entinostat, a histone deacetylase inhibitor, was found to increase the population of in vivo B10 cells and suppress the allergic phenotype.
A novel area of AIT research has focused on Breg antibody characterization. IgG4 has been identified as an important antibody for immune tolerance due to several reasons such as low affinity for activating Fc receptors, less likelihood to fix complement, and competing against IgE for antigen binding.97,98 It was recently discovered that Bregs can produce IgG4 antibodies. In particular, Bregs with IgG4 expression specific for the major bee venom allergen phospholipase A2 were isolated from healthy nonallergic beekeepers, who displayed tolerance to bee venom antigens.99 IgG4 has also been found to increase after administration of subcutaneous AIT against dust mites after a brief rise in specific IgE.92 This identifies IgG4 as a mechanism for AIT-induced tolerance and a possible therapeutic target that could be studied in intravenous Immunoglobulin treatments in the future (Figure 3).
FIGURE 3.
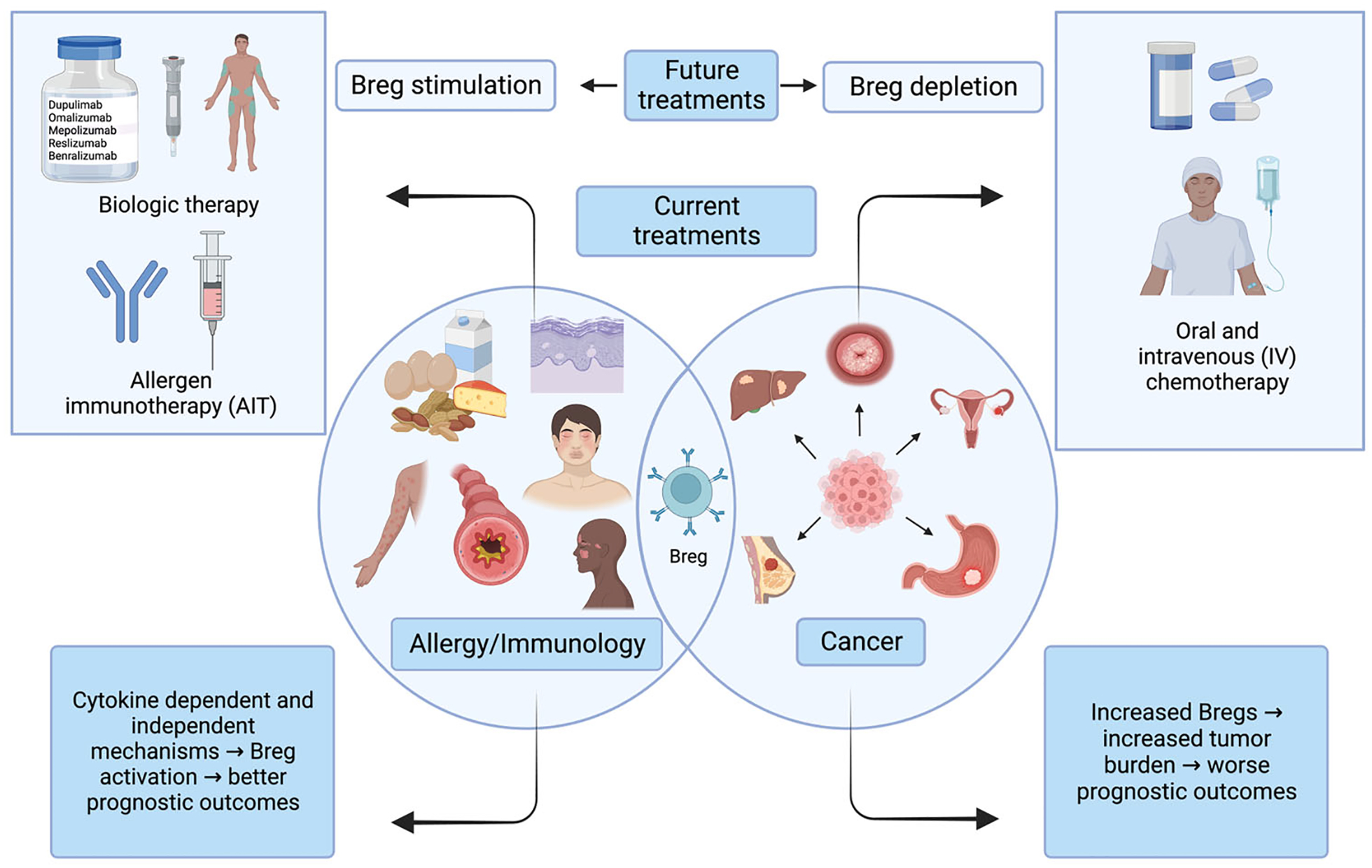
Common and divergent roles of regulatory B cells (Bregs) in allergy/immunology versus cancer.
5.3 |. Connection between allergy immunotherapy and cancer risk
Given the opposing actions of AIT compared to cancer immunotherapy, there is a risk that administering Breg activating AIT could theoretically increase or worsen a patient’s cancer. One of the more studied examples of this phenomenon is the biologic drug dupilumab, an IL-4 receptor inhibitor. Dupilumab has been approved for atopic diseases like asthma, AD, chronic rhinosinusitis, and eosinophilic esophagitis,100 and as discussed earlier has been observed to increase immunosuppressive B cells in severe asthma.45
While the role of IL-4 on cancer development is still actively under investigation, there is some preclinical data suggesting that IL-4 receptor blockade may lead to a loss of protection against certain malignancies.101 The clinical data is similarly difficult to parse through with somewhat conflicting results. Owji et al. examined patients with AD using dupilumab for at least 2 months over a period of 5 years and did not find an association with the development of primary or recurrent malignancy.100 However, there still have been case reports of patients on dupilumab who subsequently developed cancers while on treatment.102 Whether other risk factors or dupilumab played a larger role in these outcomes is questionable; however, at this point, it would be prudent to suggest that patients with active cancer should likely refrain from undergoing these immunomodulatory therapies.
6 |. CONCLUSION
Our review of the literature indicates that while Bregs present with similar functions in cancer compared to allergy, they have opposing results on disease progression. The underlying differences in cancer and allergy demonstrate different treatment goals—in cancer, immune cell activation, and in allergy, immune cell suppression to promote tolerance and reduce inflammation. The presence of Bregs in cancer is associated with tumor escape from the immune system and therefore increased tumor growth. Increasing prevalence of Bregs in human cancer studies is linked to poor prognostic outcomes. Induction of Breg development in cancer is still under investigation, but has been linked to chronic interferon signaling, BCR–BTK pathway, and CXCL13.
On the other hand, Bregs’ activation has been associated with improved control of allergic disease by both cytokine- and cell-mediated mechanisms. More studies are needed to assess the possibility of antigen-specific Bregs as a biomarker of response to therapy. As discussed, the primary function of Bregs is to downregulate the immune response and ensure homeostasis by preventing hyperactivation of the inflammatory system. Bregs studied in both mouse and human models have demonstrated decreased numbers in allergic conditions.
Ongoing research has been directed toward development of Breg-targeted immunotherapies in both fields. The crux of targeted Breg cancer immunotherapy is focused on disrupting Breg function either through direct depletion or downstream inhibition. This is in contrast to AIT, where therapy is conversely targeted toward Breg activation. Emergence of immunotherapy targets specific to Bregs that include IL-10-mediated pathways and IgG4 production are still being investigated but show promising potential in reducing the burden of allergic disease. There remains a theoretical risk of cancer development while on immunosuppressive therapy, and for patients with active cancer, it would be prudent to try to avoid these treatments.
The dichotomy of Bregs perpetuates a theme common among immune cell subtypes: natural homeostasis often hinges on a thin margin. Both over and underactivity result in pathological states that initially may appear vastly different, but on closer inspection actually represent two sides of the same coin.
ACKNOWLEDGMENTS
J. Y. is supported by an NIH T32 training grant (5T32DC012280-09).S. D. K. receives funding from the NIDCR/NCI (R01 DE028528-01; R01 DE028282-01; R01 CA284561-01; 1 P50 CA261605-01) and clinical trial funding from AstraZeneca, Genentech, and Ionis, all of which are unrelated to this work. S. D. K. reports grant funding from Roche and Amgen unrelated to this work.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The information used in the findings of this review article is available in the PubMed online database.
REFERENCES
- 1.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357(9270):1777–1789. doi: 10.1016/S0140-6736(00)04904-7 [DOI] [PubMed] [Google Scholar]
- 2.Yatim KM, Lakkis FG. A brief journey through the immune system. Clin J Am Soc Nephrol. 2015;10(7):1274–1281. doi: 10.2215/CJN.10031014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 4.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- 6.Chekol Abebe E, Asmamaw Dejenie T, Mengie Ayele T, Dagnew Baye N, Agegnehu Teshome A, Tilahun Muche Z. The role of regulatory B cells in health and diseases: a systemic review. J Inflamm Res. 2021;14:75–84. doi: 10.2147/JIR.S286426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalán D, Mansilla MA, Ferrier A, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. 2021;12:611795. doi: 10.3389/fimmu.2021.611795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rincón-Arévalo H, Villa-Pulgarín J, Tabares J, et al. Interleukin-10 production and T cell-suppressive capacity in B cell subsets from atherosclerotic apoE −/− mice. Immunol Res. 2017;65(5):995–1008. doi: 10.1007/s12026-017-8939-6 [DOI] [PubMed] [Google Scholar]
- 9.Nova-Lamperti E, Fanelli G, Becker PD, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4+ T-cell responses. Sci Rep. 2016;6:20044. doi: 10.1038/srep20044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mielle J, Audo R, Hahne M, et al. IL-10 producing B cells ability to induce regulatory T cells is maintained in rheumatoid arthritis. Front Immunol. 2018;9:961. doi: 10.3389/fimmu.2018.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R-X, Yu C-R, Dambuza IM, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–641. doi: 10.1038/nm.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah S, Qiao L. Resting B cells expand a CD4+CD25+Foxp3+ Treg population via TGF-β3. Eur J Immunol. 2008;38(9):2488–2498.doi: 10.1002/eji.200838201 [DOI] [PubMed] [Google Scholar]
- 13.Nouël A, Pochard P, Simon Q, et al. B-Cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J Autoimmun. 2015;59:53–60. doi: 10.1016/j.jaut.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Lindner S, Dahlke K, Sontheimer K, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulateT cells. Cancer Res. 2013;73(8):2468–2479. doi: 10.1158/0008-5472.CAN-12-3450 [DOI] [PubMed] [Google Scholar]
- 15.Filippone A, Lanza M, Mannino D, et al. PD1/PD-L1 immune checkpoint as a potential target for preventing brain tumor progression. Cancer Immunol Immunother. 2022;71(9):2067–2075. doi: 10.1007/s00262-021-03130-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundy SK, Lerman SP, Boros DL. Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL(+) T and B cells during murine Schistosoma mansoni infection. Infect Immun. 2001;69(1):271–280. doi: 10.1128/IAI.69.1.271-280.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich RF, Cook WJ, Green WR. Spontaneous in vivo retrovirus-infected T and B cells, but not dendritic cells, mediate antigen-specific Fas ligand/Fas-dependent apoptosis of anti-retroviral CTL. Virology. 2006;346(2):287–300. doi: 10.1016/j.virol.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 18.Eiza N, Zuckerman E, Carlebach M, Rainis T, Goldberg Y, Vadasz Z. Increased killer B cells in chronic HCV infection may lead to autoimmunity and increased viral load. Clin Exp Immunol. 2018;193(2):183–193. doi: 10.1111/cei.13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud D, Steward CR, Mirlekar B, Pylayeva-Gupta Y. Regulatory B cells in cancer. Immunol Rev. 2021;299(1):74–92. doi: 10.1111/imr.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y, Lo CM, Ling CC, et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355(2):264–272. doi: 10.1016/j.canlet.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 21.Wei X, Jin Y, Tian Y, et al. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biol. 2016;37(5):6581–6588. doi: 10.1007/s13277-015-4538-0 [DOI] [PubMed] [Google Scholar]
- 22.Lv Y, Wang H, Liu Z. The role of regulatory B cells in patients with acute myeloid leukemia. Med Sci Monit. 2019;25:3026–3031. doi: 10.12659/MSM.915556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhao Y, Liu L, et al. MicroRNA-19b promotes nasopharyngeal carcinoma more sensitive to cisplatin by suppressing KRAS. Technol Cancer Res Treat. 2018;17:153303381879365. doi: 10.1177/1533033818793652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishigami E, Sakakibara M, Sakakibara J, et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer. 2019;26(2):180–189. doi: 10.1007/s12282-018-0910-4 [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Zhu Y, Du R, et al. Role of regulatory B cells in the progression of cervical cancer. Mediators Inflamm. 2019;2019:1–8. doi: 10.1155/2019/6519427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeske SS, Brand M, Ziebart A, et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol Immunother. 2020;69(7): 1205–1216. doi: 10.1007/s00262-020-02535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Su Y-X, Lao X-M, Liang Y-J, Liao G-Q. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+) Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35. doi: 10.1016/j.oraloncology.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 28.Hetta HF, Mekky MA, Zahran AM, et al. Regulatory B cells and their cytokine profile in HCV-related hepatocellular carcinoma: association with regulatory T cells and disease progression. Vaccines. 2020;8(3):380. doi: 10.3390/vaccines8030380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue H, Lin F, Tan H, Zhu Z-Q, Zhang Z-Y, Zhao L. Over-representation of IL-10-expressing B cells suppresses cytotoxic CD4+ T cell activity in HBV-induced hepatocellular carcinoma. PLoS One. 2016;11(5):e0154815. doi: 10.1371/journal.pone.0154815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami Y, Saito H, Shimizu S, et al. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep. 2019;9(1):13083. doi: 10.1038/s41598-019-49581-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao Y, Wang Y, Dong L, et al. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and PD-1high Breg cells. Cancer Sci. 2019;110(9):2700–2710. doi: 10.1111/cas.14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131(4):959–971. doi: 10.1016/j.jaci.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 33.Gutzeit C, Nagy N, Gentile M, et al. Exosomes derived from Burkitt’s lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells. J Immunol. 2014;192(12):5852–5862. doi: 10.4049/jimmunol.1302068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–414. doi: 10.1038/nri3845 [DOI] [PubMed] [Google Scholar]
- 35.Li S, Mirlekar B, Johnson BM, et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610(7931):373–380. doi: 10.1038/s41586-022-05254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017;38(8):542–557. doi: 10.1016/j.it.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansel KM, Harris RBS, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16(1):67–76. doi: 10.1016/s1074-7613(01)00257-6 [DOI] [PubMed] [Google Scholar]
- 38.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375(6576):eabf9419. doi: 10.1126/science.abf9419 [DOI] [PubMed] [Google Scholar]
- 39.Ren J, Lan T, Liu T, et al. CXCL13 as a novel immune checkpoint for regulatory B cells and its role in tumor metastasis. J Immunol. 2022;208(10):2425–2435. doi: 10.4049/jimmunol.2100341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braza F, Chesne J, Castagnet S, Magnan A, Brouard S. Regulatory functions of B cells in allergic diseases. Allergy. 2014;69(11):1454–1463. doi: 10.1111/all.12490 [DOI] [PubMed] [Google Scholar]
- 41.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251(5475):550–551. doi: 10.1038/251550a0 [DOI] [PubMed] [Google Scholar]
- 42.Busse WW, Lemanske RF. Asthma. N Engl J Med. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507 [DOI] [PubMed] [Google Scholar]
- 43.Fan K, Jin L, Yu S. Roles of regulatory B cells in the pathogenesis of allergic rhinitis. Allergol Immunopathol. 2022;50(5):7–15. doi: 10.15586/aei.v50i5.615 [DOI] [PubMed] [Google Scholar]
- 44.van der Vlugt LEPM, Mlejnek E, Ozir-Fazalalikhan A, et al. CD24(hi) CD27(+) B cells from patients with allergic asthma have impaired regulatory activity in response to lipopolysaccharide. Clin Exp Allergy. 2014;44(4):517–528. doi: 10.1111/cea.12238 [DOI] [PubMed] [Google Scholar]
- 45.Lommatzsch M, Dost M, Jaishankar N, et al. Dupilumab treatment increases transitional B cells in severe asthma. Allergy. 2023;78(7):2055–2057. doi: 10.1111/all.15703 [DOI] [PubMed] [Google Scholar]
- 46.Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(1 suppl):S2–S8. doi: 10.1067/mai.2001.115569 [DOI] [PubMed] [Google Scholar]
- 47.Kim AS, Doherty TA, Karta MR, et al. Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J Allergy Clin Immunol. 2016;138(4):1192–1195. doi: 10.1016/j.jaci.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo J, Guo H, Liu Z, et al. Analysis of peripheral B cell subsets in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2018;10(3):236–243. doi: 10.4168/aair.2018.10.3.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miglani A, Brar TK, Lal D. Unified airway disease. Otolaryngol Clin North Am. 2023;56(1):169–179. doi: 10.1016/j.otc.2022.09.013 [DOI] [PubMed] [Google Scholar]
- 50.Kamekura R, Shigehara K, Miyajima S, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015;158(2):204–211. doi: 10.1016/j.clim.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 51.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17. doi: 10.1542/peds.2011-0204 [DOI] [PubMed] [Google Scholar]
- 52.Gupta RS, Warren CM, Smith BM, et al. Prevalence and severity of food allergies among US adults. JAMA Network Open. 2019;2(1):e185630. doi: 10.1001/jamanetworkopen.2018.5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramesh S Food allergy overview in children. Clin Rev Allergy Immunol. 2008;34(2):217–230. doi: 10.1007/s12016-007-8034-1 [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Sicherer S, Berin MC, Agyemang A. Pathophysiology of mon-IgE-mediated food allergy. Immunotargets Ther. 2021;10: 431–446. doi: 10.2147/ITT.S284821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noh J, Lee JH, Noh G, et al. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in cow milk allergy. Cell Immunol. 2010;264(2):143–149. doi: 10.1016/j.cellimm.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. Allergen-specific transforming growth factor-β-producing CD19+CD5+ regulatory B-cell (Br3) responses in human late eczematous allergic reactions to cow’s milk. J Interferon Cytokine Res. 2011;31(5):441–449. doi: 10.1089/jir.2010.0020 [DOI] [PubMed] [Google Scholar]
- 57.Noh J, Noh G, Kim HS, Kim A-R, Choi WS. Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell Immunol. 2012;274(1–2):109–114. doi: 10.1016/j.cellimm.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 58.Liu ZQ, Wu Y, Song JP, et al. Tolerogenic CX3CR1+ B cells suppress food allergy-induced intestinal inflammation in mice. Allergy. 2013;68(10):1241–1248. doi: 10.1111/all.12218 [DOI] [PubMed] [Google Scholar]
- 59.Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35(4):354–359. doi: 10.1016/j.clindermatol.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 60.Mei-Yen Yong A, Tay Y-K. Atopic dermatitis. Dermatol Clin. 2017;35(3):395–402. doi: 10.1016/j.det.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 61.Zysk W, Gleń J, Trzeciak M. Current insight into the role of IL-35 and its potential involvement in the pathogenesis and therapy of atopic dermatitis. Int J Mol Sci. 2022;23(24):15709. doi: 10.3390/ijms232415709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W-M, Guo L, Jin H-Z. Role of B cells in immune-mediated dermatoses. Mol Immunol. 2020;126:95–100. doi: 10.1016/j.molimm.2020.07.016 [DOI] [PubMed] [Google Scholar]
- 63.Li J, Shen C, Liu Y, et al. Impaired function of CD5+CD19+CD1dhi B10 cells on IgE secretion in an atopic dermatitis-like mouse model. PLoS One. 2015;10(8):e0132173. doi: 10.1371/journal.pone.0132173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Čelakovská J, Čermáková E, Boudková P, Andrýs C, Krejsek J. Evaluation of leukocytes, B and T lymphocytes, and expression of CD200 and CD23 on B lymphocytes in patients with atopic dermatitis on dupilumab therapy—pilot study. Dermatol Ther. 2023;13(5):1171–1192. doi: 10.1007/s13555-023-00918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaspari AA, Katz SI, Martin SF. Contact hypersensitivity. Curr Protoc Immunol. 2016;113:4.2.1–4.2.7. doi: 10.1002/0471142735.im0402s113 [DOI] [PubMed] [Google Scholar]
- 66.Samuni A, Min A, Krishna CM, Mitchell JB, Russo A. SOD-like activity of 5-membered ring nitroxide spin labels. Adv Exp Med Biol. 1990;264:85–92. doi: 10.1007/978-1-4684-5730-8_12 [DOI] [PubMed] [Google Scholar]
- 67.Watanabe R, Fujimoto M, Ishiura N, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171(2):560–570. doi: 10.2353/ajpath.2007.061279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsushita T, Le Huu D, Kobayashi T, et al. A novel splenic B1 regulatory cell subset suppresses allergic disease through phosphatidylinositol 3-kinase-Akt pathway activation. J Allergy Clin Immunol. 2016;138(4):1170–1182. doi: 10.1016/j.jaci.2015.12.1319 [DOI] [PubMed] [Google Scholar]
- 69.Su J, Wang K, Zhou X, et al. B-cell-specific-peroxisome proliferator-activated receptor γ deficiency augments contact hypersensitivity with impaired regulatory B cells. Immunology. 2019;156(3):282–296. doi: 10.1111/imm.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarz A, Maeda A, Wild MK, et al. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172(2):1036–1043. doi: 10.4049/jimmunol.172.2.1036 [DOI] [PubMed] [Google Scholar]
- 71.Rana S, Byrne SN, MacDonald LJ, Chan CY-Y, Halliday GM. Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am J Pathol. 2008;172(4):993–1004. doi: 10.2353/ajpath.2008.070517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X, Huang H, Gao H, et al. Regulatory B cells induced by ultraviolet B through toll-like receptor 4 signalling contribute to the suppression of contact hypersensitivity responses in mice. Contact Dermatitis. 2018;78(2):117–130. doi: 10.1111/cod.12913 [DOI] [PubMed] [Google Scholar]
- 73.Lee KE, Spata M, Bayne LJ, et al. Hif1a deletion reveals pro- neoplastic function of B cells in pancreatic neoplasia. Cancer Discov. 2016;6(3):256–269. doi: 10.1158/2159-8290.CD-15-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Affara NI, Ruffell B, Medler TR, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–821. doi: 10.1016/j.ccr.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das S, Bar-Sagi D. BTK signaling drives CD1dhiCD5+ regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene. 2019;38(17):3316–3324. doi: 10.1038/s41388-018-0668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and antitumor immunity. Cell Mol Immunol. 2017;14(8):662–674. doi: 10.1038/cmi.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185(4):2240–2252. doi: 10.4049/jimmunol.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weißenborn C, von Lenthe S, Hinz N, et al. Depletion of Foxp3+ regulatory T cells but not the absence of CD19+IL-10+ regulatory B cells hinders tumor growth in a para-orthotopic neuroblastoma mouse model. Int J Cancer. 2022;151(11):2031–2042. doi: 10.1002/ijc.34262 [DOI] [PubMed] [Google Scholar]
- 79.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richards JD, Davé SH, Chou CHG, Mamchak AA, DeFranco AL. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J Immunol. 2001;166(6):3855–3864. doi: 10.4049/jimmunol.166.6.3855 [DOI] [PubMed] [Google Scholar]
- 81.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92(17):7686–7689. doi: 10.1073/pnas.92.17.7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caunt CJ, Sale MJ, Smith PD, Cook SJ. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat Rev Cancer. 2015;15(10):577–592. doi: 10.1038/nrc4000 [DOI] [PubMed] [Google Scholar]
- 83.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 84.Yarchoan M, Mohan AA, Dennison L, et al. MEK inhibition suppresses B regulatory cells and augments anti-tumor immunity. PLoS One. 2019;14(10):e0224600. doi: 10.1371/journal.pone.0224600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, Cheng Q, Tang K, et al. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Lett. 2015;364(2):118–124. doi: 10.1016/j.canlet.2015.04.030 [DOI] [PubMed] [Google Scholar]
- 86.Lee-Chang C, Bodogai M, Martin-Montalvo A, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191(8):4141–4151. doi: 10.4049/jimmunol.1300606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peeters A, Baes M. Role of PPARα in hepatic carbohydrate metabolism. PPAR Res. 2010;2010:1–12. doi: 10.1155/2010/572405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wejksza K, Lee-Chang C, Bodogai M, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor α. J Immunol. 2013;190(6):2575–2584. doi: 10.4049/jimmunol.1201920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z, Lu Z, Lin S, et al. Leucine-tRNA-synthetase-2-expressing B cells contribute to colorectal cancer immunoevasion. Immunity. 2022;55(6):1067–1081. doi: 10.1016/j.immuni.2022.04.017 [DOI] [PubMed] [Google Scholar]
- 90.Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016;8(3):191–197. doi: 10.4168/aair.2016.8.3.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.James C, Bernstein DI. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017;17(1):55–59. doi: 10.1097/ACI.0000000000000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boonpiyathad T, van de Veen W, Wirz O, et al. Role of Der p 1-specific B cells in immune tolerance during 2 years of house dust mite-specific immunotherapy. J Allergy Clin Immunol. 2019;143(3):1077–1086. doi: 10.1016/j.jaci.2018.10.061 [DOI] [PubMed] [Google Scholar]
- 93.van de Veen W The role of regulatory B cells in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2017;17(6):447–452. doi: 10.1097/ACI.0000000000000400 [DOI] [PubMed] [Google Scholar]
- 94.Alenazy MF, Sharif-Askari FS, El-Wetidy MS, et al. Intranasal administration of abatacept enhances IL-35+ and IL-10+ producing Bregs in lung tissues of ovalbumin-sensitized asthmatic mice model. PLoS One. 2022;17(9):e0271689. doi: 10.1371/journal.pone.0271689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, Yin J. Immunotherapy with recombinant Alt a 1 suppresses allergic asthma and influences T follicular cells and regulatory B cells in mice. Front Immunol. 2021;12:747730. doi: 10.3389/fimmu.2021.747730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cierzniak A, Kaliszewski K, Małodobra-Mazur M. The preliminary evaluation of epigenetic modifications regulating the expression of IL10 in insulin-resistant adipocytes. Genes. 2022;13(2):294. doi: 10.3390/genes13020294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van de Veen W, Akdis M. Role of IgG4 in IgE-mediated allergic responses. J Allergy Clin Immunol. 2016;138(5):1434–1435. doi: 10.1016/j.jaci.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 98.Bindon CI, Hale G, Brüggemann M, Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988;168(1):127–142. doi: 10.1084/jem.168.1.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–1212. doi: 10.1016/j.jaci.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 100.Owji S, Ungar B, Dubin DP, et al. No association between dupilumab use and short-term cancer development in atopic dermatitis patients. J Allergy Clin Immunol. 2023;11(5):1548–1551. doi: 10.1016/j.jaip.2022.12.018 [DOI] [PubMed] [Google Scholar]
- 101.Ingram N, Northwood EL, Perry SL, et al. Reduced type II interleukin-4 receptor signalling drives initiation, but not progression, of colorectal carcinogenesis: evidence from transgenic mouse models and human case-control epidemiological observations. Carcinogenesis. 2013;34(10):2341–2349. doi: 10.1093/carcin/bgt222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siliquini N, Giura MT, Viola R, et al. Atopic dermatitis, dupilumab and cancers: a case series. J Eur Acad Dermatol Venereol. 2021;35(10):e651–e652. doi: 10.1111/jdv.17264 [DOI] [PubMed] [Google Scholar]
- 103.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(5):1114–1124. doi: 10.1016/j.jaci.2010.01.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The information used in the findings of this review article is available in the PubMed online database.


