Abstract
Background
Since the onset of COVID-19, oncology practices across the US have integrated telemedicine (TM) and remote patient monitoring (RPM) into routine care and clinical trials. The extent of provider experience and comfort with TM/RPM in treatment trials, however, is unknown. We surveyed oncology researchers to assess experience and comfort with TM/RPM.
Methods
Between April 10 and June 1, 2022, we distributed email surveys to US-based members of the American Society of Clinical Oncology (ASCO) whose member records indicated interest or specialization in clinical research. We collected respondent demographic data, clinical trial experience, workplace characteristics, and comfort and experience with TM/RPM use across trial components in phase I and phase II/III trials. TM/RPM was defined as clinical trial-related healthcare and monitoring for patients geographically separated from trial site.
Results
There were 141 surveys analyzed (5.1% response rate). Ninety percent of respondents had been Principal Investigators, 98% practiced in a norural site. Most respondents had enrolled patients in phase I (82%) and phase II/III trials (99%). Across all phases and trial components, there was a higher frequency of researcher comfort compared to experience. Regarding remote care in treatment trials, 75% reported using TM, RPM, or both. Among these individuals, 62% had never provided remote care to trial patients before the pandemic.
Conclusion
COVID-19 spurred the rise of TM/RPM in cancer treatment trials, and some TM/RPM use continues in this context. Among oncology researchers, higher levels of comfort compared with real-world experience with TM/RPM reveal opportunities for expanding TM/RPM policies and guidelines in oncology research.
Keywords: telemedicine, remote patient monitoring, trials, COVID-19
COVID-19 spurred the rise of telemedicine and remote patient monitoring in cancer treatment trials. This article assesses oncology researchers’ experience and comfort level with such methods of treatment in the context of treatment trials.
Implications for Practice.
In this study, we surveyed oncology researchers to assess the frequency of researcher experience and comfort with telemedicine (TM) and remote patient monitoring (RPM) across trial components in phase I and phases II and III clinical trials. Our results show that more researchers reported comfort compared to experience across all phases and trial components. Furthermore, the gaps between comfort and experience highlight specific areas for expanding TM/RPM use in clinical research. This work supports a targeted approach for incorporating TM/RPM into clinical trials based on researcher comfort level, which has the potential to improve the patient clinical trial experience and expand patient access to novel medications and interventions.
Background
Since the onset of the Coronavirus Disease 2019 (COVID-19) global pandemic, oncology practices across the US have integrated telemedicine (TM) and remote patient monitoring (RPM) into routine care and clinical trials. Providers and researchers have written about opportunities and challenges associated with TM in cancer care,1-6 and professional societies including the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) have offered recommendations for incorporating telemedicine into routine oncology practice and clinical trials. Both research and expert opinion have identified the need for additional research on the use of TM/RPM in clinical research to inform and improve current practices.7 In addition, health regulatory authorities including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency have issued guidance on the use of measures such as TM/RPM to facilitate continued conduct and access to clinical trials.8,9
Studies have shown that incorporating TM/RPM into oncology clinical trials is feasible and provides a satisfactory option for patients to communicate with members of their healthcare team, including clinical investigators.10-13 Adams et al showed that TM/RPM has the potential to expand clinical trial access to patients who live far from clinical trial sites.14 Although barriers to universal technology access remain a concern, a number of studies have demonstrated that the majority of underserved patients living in urban areas of the US have access to mobile phones and internet and are interested in using telemedicine for routine cancer care and clinical trials.15-18 Studies have also shown that internet and electronic communication are feasible and acceptable among oncology patients in rural and underserved regions in the US and improve access to healthcare despite barriers to providing these populations with remote clinical trial care.19,20 In general, TM/RPM has the potential to meet the needs of research participants and expand patient access to cancer clinical trials.
Less is known about the perspective of clinical investigators on the use of TM/RPM in cancer clinical trials. Previous research has primarily focused on provider satisfaction with TM/RPM, or the extent to which providers are content with the quality of care they are able to provide patients when using TM/RPM.21-26 Although satisfaction is important, the prevalence of clinical researcher experience (direct participation) and comfort (sense of ease and confidence) using TM/RPM in treatment trials remains unknown, as does clinical researcher perceptions about the benefits and detriments of TM/RPM in treatment trials. In this study, we surveyed oncology researchers to assess the frequencies of researcher experience and comfort with TM/RPM across discrete components of the treatment trial process for phase I and phase II/III trials. We also collected data on clinical researcher perceptions regarding the advantages and disadvantages of integrating TM/RPM into cancer clinical trials.
Methods
Between April 10 and June 1, 2022, we distributed the survey instrument via REDCap email to a total of 2786 US-based ASCO members. Members who were engaged in a patient-care role (determined by member type) and had indicated an interest or cancer specialization in “clinical research” in their member records were eligible. Members working in government, industry, and pharma/biotech were excluded. The survey was classified as “exempt” research by WCG Institutional Review Board on March 18, 2022.
The survey was a questionnaire developed by a multidisciplinary study team following literature review. The study team included representatives from academic and community oncology practices (including clinical investigators and research staff), a patient advocate group, the FDA, and the U.S. Department of Veterans’ Affairs. Survey domains included practice and trial characteristics; self-reported experience employing various TM/RPM methods across treatment trial phases; comfort with employing various TM/RPM methods across treatment trial phases (regardless of experience); perceptions of advantages, disadvantages, and limiting factors associated with the use of TM/RPM; and respondent demographics (age, gender, race/ethnicity, clinical role, and trial experience) (Supplementary Material). Rural and urban characterization of respondent practice setting was estimated using 2010 Rural-Urban Commuting Area Code zip code data provided by the U.S. Department of Agriculture.27,28 TM/RPM was defined as clinical trial-related healthcare (TM) and monitoring for patients (RPM) geographically separated from the site administering the clinical trial.
The survey consisted of 21 question stems with accompanying “Yes/No,” multiple choice, Likert-based, and rank choice responses. Respondent “experience” and “comfort” with TM/RPM activities was recorded as binary Yes/No responses. The survey took approximately 10 minutes to complete and did not solicit any identifiable information from participants, except for an optional question that asked respondents to provide their primary email address if they were willing to be contacted by ASCO staff to provide further input on the topic. Prior to circulating, the survey was pilot tested by 4 ASCO staff with cancer research experience. Feedback from the pilot phase was incorporated into the final version of the survey.
One thousand four hundred and seventy-seven eligible ASCO members received the survey instrument via REDCap email link between April 10 and May 9, 2022. Members received up to 2 reminders to complete the survey. A second, nonintersecting sample of 1309 eligible ASCO members received the survey instrument via Adobe Campaign email link between May 24 and June 1, 2022 and received one reminder to complete the survey. The survey was closed on June 8, 2022.
Descriptive statistics were used to summarize responses, including tabulations with proportions for categorical variables, and means, medians, and SDs for continuous variables. P-values were calculated using Chi-square tests. Statistical analyses were performed using R version 4.1.2.
Results
A total of 141 surveys were analyzed (5.1% response rate). Among respondents, 53% identified as female, 39% were under 45 years of age, and 25% were over 60 years of age. Seventy percent identified as White, 90% had been site or study Principal Investigators, and 98% practiced in a nonrural setting. Most respondents conducted phase I (82%) and phase II/III (99%) trials, enrolling patients on industry-sponsored trials (97%), NCI-sponsored trials (84%), and investigator-initiated trials (83%). Fifty-eight (41%) of respondents had ≥20 years of trial experience (Table 1). Given the small sample size, subgroups were too small to allow for statistical comparisons in most cases.
Table 1.
Participant characteristics (N = 141).
| n | % | |
|---|---|---|
| Clinical role | ||
| Clinical investigator | 122 | 86.5 |
| Research director, administrator, or manager | 15 | 10.6 |
| Research staff | 2 | 1.4 |
| Other clinical role | 2 | 1.4 |
| Practice type | ||
| Hospital or health system owned | 125 | 88.7 |
| Physician owned | 16 | 11.3 |
| Academic site (ie, has a fellowship program) | ||
| Yes | 111 | 78.7 |
| No | 30 | 21.3 |
| Experience as a PI (investigators only) | ||
| Study or site PI | 110 | 90.2 |
| Sub-investigator only | 10 | 8.2 |
| None | 2 | 1.6 |
| Trial participation, sponsors | ||
| Industry-sponsored trials | 137 | 97.2 |
| NCI-sponsored trials (ETCTN or NCTN) | 119 | 84.4 |
| Investigator-initiated trials | 117 | 83 |
| Trial participation, phases | ||
| Phase I trials | 116 | 82.3 |
| Phase II/III trials | 140 | 99.3 |
| Years of clinical trial experience | ||
| <10 years | 31 | 22.1 |
| 10-19 years | 52 | 37.1 |
| 20 or more years | 57 | 40.7 |
| Age | ||
| Under 45 | 54 | 38.6 |
| 45-59 | 51 | 36.4 |
| 60 or older | 35 | 25 |
| Gender | ||
| Female | 74 | 52.5 |
| Male | 65 | 46.1 |
| Nonbinary | 0 | 0 |
| Self-identify | 0 | 0 |
| Prefer not to answer | 2 | 1.4 |
| Race/ethnicity (select all that apply) | ||
| Black or African American | 1 | 0.7 |
| American Indian or Alaska Native | 0 | 0 |
| Native Hawaiian or other Pacific Islander | 0 | 0 |
| Asian | 27 | 19.1 |
| White | 98 | 69.5 |
| Latinx | 6 | 4.3 |
| Other | 3 | 2.1 |
| Prefer not to answer | 7 | 5 |
| Percentage of clinical trial patients that belong to a minority or underserved group | ||
| None (0%-5%) | 10 | 7.1 |
| Few (6%-25%) | 97 | 68.8 |
| Some (26%-50%) | 31 | 22 |
| Most (>50%) | 3 | 2.1 |
Abbreviations: PI: Principal Investigator.
Impact of COVID-19 on Uptake of TM/RPM
Most respondents (75%, n = 105) reported experience using TM, RPM, or both in cancer treatment trials. Among these individuals, 59% (n = 62) had never provided remote care to trial patients before the COVID-19 pandemic. Fifty-one percent (n = 54) of those with TM/RPM experience reported using it “often” or “usually” (greater than 25% of interactions) at the peak of the pandemic, but only 12% (n = 13) of them were currently using TM/RPM with that frequency. Fourteen respondents decreased from intensive use (>25% of interactions) at the peak of the pandemic to rare or no use (<10%) at the time of the survey. At the time of the survey, respondents with any prior TM/RPM experience were most likely to use TM/RPM “rarely” (less than 10% of interactions) as part of trial care (44%, n = 46). There was not a significant association between years of clinical trials experience and experience with TM/RPM: 52% of respondents with 10-19 years of clinical trials experience (n = 27) reported experience with TM/RPM compared with 40% of respondents with ≥20 years of clinical trials experience (n = 23) (β = .825, P = .308).
Areas of High/Low Comfort and Experience With TM/RPM
Researcher comfort with incorporating TM/RMP was more prevalent within the context of prescreening for eligibility (phase I: 73%; phase II/III: 91%), pre-enrollment recruitment, education, and counseling (phase I: 78%; phase II/III: 94%), and patient-reported outcomes as a study endpoint (phase I: 73%; phase II/III: 89%). Respondents were least comfortable incorporating TM/RPM with IV administration of investigational treatment in patients’ homes (phase I: 11%; phase II/III: 24%) and performance of study-required biopsies (phase I: 16%; phase II/III: 26%). The highest prevalence of researcher experience was with the following trial components: prescreening for eligibility (phase I: 41%; phase II/III: 57%), pre-enrollment recruitment, education, and counseling (phase I: 45%; phase II/III: 61%), and symptom monitoring for adverse events (phase I: 41%; phase II/III: 52%). Researchers had the least experience with collecting patient-reported outcomes (PROs) data in real time (phase I: 12%; phase II/III: 21%), performing study-required biopsies (phase I: 6%; phase II/III: 9%), and with IV administration of investigational treatment at patients’ homes (phase I: 2%; phase II/III: 6%).
Gaps Between Frequency of Comfort and Experience With TM/RPM
The frequency of comfort with a TM/RPM trial component exceeded the frequency of experience using that TM/RPM component across all 13 trial components assessed regardless of phase (Fig. 1). For phases I and II/III trials, we observed the biggest gaps between comfort and experience within the following 3 trial components: PROs in real time (comfort outweighed experience in in phase I [70% vs. 12%] and in phase II/III [86% vs. 21%]), PROs as a study endpoint (comfort outweighed in phase I [73% vs. 18%] and in phase II/III [89% vs. 26%]), and routine lab testing (comfort outweighed experience in phase I [70% vs. 25%] and in phase II/III [88% vs. 41%]).
Figure 1.
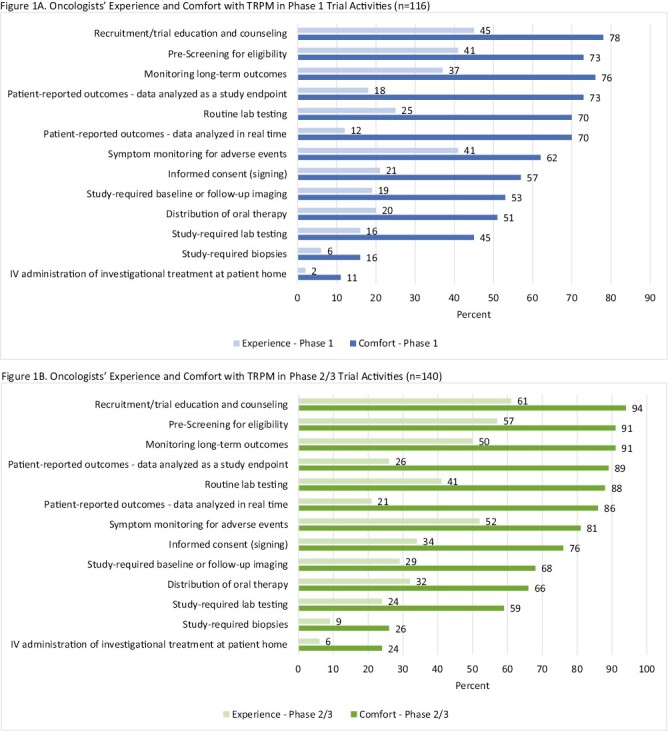
Researcher experience and comfort with TM/RPM in phase I and phase II/III trials. (A) represents the frequencies of clinical researcher experience (light blue) and comfort (dark blue) with TM/RPM in phase I clinical trials. (B) represents the frequencies of clinical researcher experience (light green) and comfort (dark green) with TM/RPM in phase II/III clinical trials.
Advantages, Disadvantages, and Barriers to TM/RPM
A majority of respondents (88%, n = 124) identified patient access to trials as one of their top 3 advantages to TM/RPM, with 63% (n = 89) choosing it at the foremost advantage (Fig. 2A). The next most cited advantages were reducing patient and caregiver burden related to trial participation (eg, cost, time, emotional; 74%, n = 105) and reducing patient and caregiver burden related to prescreening for trial eligibility, recruitment, and consent (68%; n = 94). The most commonly reported disadvantages were limited ability to monitor patients (70%, n = 99), disparities in patient technology access (69%, n = 97), and logistical inconveniences of obtaining lab and imaging results (53%, n = 75; Fig. 2B).
Figure 2.
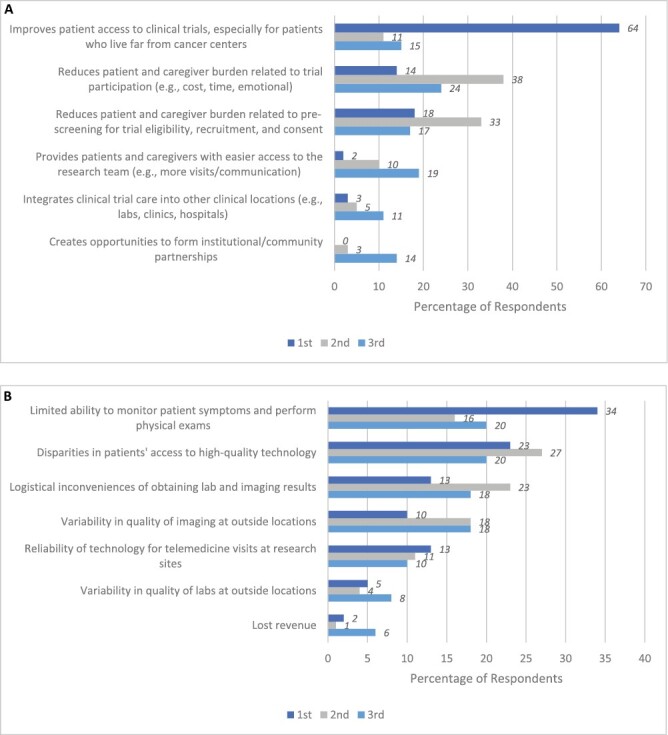
Top advantages and disadvantages of utilizing TM/RPM in clinical trials. (A) Respondents’ top 3 advantages of utilizing TM/RPM in clinical trials, ranked (n = 141). (B) Respondents’ top 3 disadvantages of utilizing TM/RPM in clinical trials, ranked (n = 141). (A) shows the percent frequencies that respondents chose advantages of using TM/RPM in clinical trials when listing their top 3 advantages. (B) shows the percent frequencies that respondents chose disadvantages of using TM/RPM in clinical trials when listing their top 3 disadvantages.
The 101 respondents with any experience in TM were asked about the top 3 barriers they encountered. A majority of those respondents cited sponsors not designing trials that permit use of TM (n = 71, 70%), cross-state licensure challenges (n = 65, 64%), or patient access to high-quality technology and broadband internet (n = 58, 57%) as top factors limiting TM use (Supplementary Fig. S3).
Discussion
Most survey respondents have used TM and/or RPM in cancer treatment trials, and the frequency of researcher comfort is higher than that of experience with TM/RPM in each of the 13 trial components our survey examined. In particular, the 3 most prevalent areas of comfort across trial phases were (1) prescreening for eligibility, (2) patient education and counseling, and (3) PROs as a study endpoint. The 3 biggest gaps between comfort and experience were (1) PRO collection in real time, (2) PRO collection as a study endpoint, and (3) routine lab testing. High prevalence of researcher comfort and broad gaps between the frequencies of comfort and experience highlight potential areas for expanding the use of TM/RPM in oncology research.
Our findings reinforce existing work in this area and prominent cancer association guidelines in favor of continuing TM/RPM in clinical trials to increase access and diversity in enrollment.21-26,29-32 Our analysis is distinct from prior literature, however, in looking at comfort and experience across discrete components of clinical trial care in phase I and phase II/III trials. We found a majority of researchers felt comfortable using TM/RPM across a wide range of trial components and that there is a considerable gap between comfort and experience across trial components. These findings can be used to identify opportunities for training and standardization aimed at expanding the use of TM/RPM in future cancer clinical trials.
In addition to assessing investigator comfort and experience, our study looked at perceived advantages and disadvantages of using TM/RPM in cancer treatment trials. The 3 most commonly selected advantages were all related to patient access and experience: (1) improves patient access to clinical trials; (2) reduces patient and caregiver burden related to prescreening for trial eligibility, recruitment, and consent; and (3) reduces patient and caregiver burden related to trial participation. These findings reveal an awareness that participation in cancer treatment trials is often associated with logistical, financial, and emotional burdens for patients and their caregivers. Ultimately, these burdens may reduce patient interest and ability to participate in trials.33 The top 3 advantages selected by survey respondents highlight that oncology researchers believe TM/RPM has the potential to increase access to trials and to decrease some of the patient and caregiver burdens that accompany trial participation. These potential advantages to TM/RPM merit further exploration in future research.
In our study, the top 3 ranked disadvantages of using TM/RPM in clinical trials include: (1) limited ability to monitor patients, (2) disparities in patient technology access, and (3) reliability of technology for telemedicine visits at research sites. Each of these potential disadvantages require distinct attention. The limited ability to monitor patients remotely may raise safety concerns in certain trial settings. This reinforces the concept of a hybrid model that allows for TM/RPM and in-person care. For example, lower levels of researcher comfort with study-required labs/biopsies, and the administration of investigational therapies implies that these components may be best completed in-person. Notably, for these trial components, the smaller gaps between comfort and experience suggest that researchers may view specific trial elements as less amenable to TM/RPM independent of trial phase. Regarding TM/RPM access, disparities in patient technology literacy and access are limitations to address in future studies. Finally, the inconsistent reliability of TM/RPM at sites raises the importance of supporting infrastructure and developing explicit standards about the use of TM/RPM as part of treatment trials, like other aspects of clinical research.
In addition to advantages and disadvantages, our survey asked each researcher to define the top factor limiting the use of telemedicine in clinical trials, and our results highlight 2 primary limiting factors: (1) trial designs that do not permit telemedicine use and (2) cross-state licensure challenges. Addressing these barriers will require advocacy as well as guidelines and regulations to support its use. With regard to regulatory policy, critical uncertainties persist around the future of telemedicine reimbursement, licensure, and liability concerns in the US after the end of the COVID-19 Public Health Emergency (PHE).34-36 In May 2021, ASCO published a position statement recommending that the Centers for Medicare and Medicaid Services maintain support for TM/RPM in cancer care beyond the ongoing PHE, promoting cross-state licensure agreements, and encouraging medical liability providers to include telemedicine in their policies.37 In addition, Congress recently passed legislation (H.R. 2617) that would extend Medicare telemedicine coverage until 2024, although this bill does not address coverage by Medicaid or private insurance. Currently, there are no centralized regulations or policies regarding the use of TM/RPM in clinical trials.
Our findings further support current literature suggesting that TM/RPM in routine cancer care and clinical research holds potential value to increase access to clinical trials and expand cancer research opportunities. Our results showed a significant decline in the use of TM/RPM, since the onset of the pandemic, and although the barriers mentioned above are likely contributors, the reasons for this change are not entirely clear. In spite of this finding, existing literature demonstrates that oncology researchers favor the continuation of TM/RPM use in trials beyond the pandemic.21,23,26 In 2020, ASCO conducted a survey of U.S. research staff examining the conduct of oncology clinical trials early in the pandemic with a focus on TM/RPM.26 Asked to identify future opportunities for trial conduct, the majority of respondents (n = 32) chose telehealth visits for trial participants (90.3%) and remote patient review of symptoms (77.4%). In 2022, the International Association for the Study of Lung Cancer (IASLC) reported results from a survey focused on international lung cancer clinical trials.23 When asked about the future, respondents most frequently expressed interest in continuing telehealth visits (52%), remote monitoring (49%), electronic signature (47%), and remote patient-reported symptom collection (35%) as part of clinical trials.
In addition to surveys, the oncology researcher perspective on the use of TM/RPM in clinical trials is captured by consensus guidelines and expert opinion published during the pandemic. Guidelines from ASCO, ESMO, and other cancer-focused organizations have identified common priorities for integrating TM/RPM into clinical trial care beyond the pandemic to make participation less burdensome and potentially expand trial access to a broader population. In general, expert recommendations support a hybrid model of care, with remote and in person components tailored to the requirements and goals of each treatment trial. More specifically, these guidelines highlight trial components potentially more amenable to TM/RPM such as the consent process, lab testing and imaging, shipping of oral drugs, and patient-reported outcomes.22,25,30,31 The 2020 ASCO Road to Recovery Report describes remote clinical trial care options as “patient-centric” with the potential to reduce financial toxicity and increase convenience for trial participants.31 In particular, this report recommends continuing remote or virtual consent with e-signatures, administration of study-related treatment at local facilities, conduct of patient assessments, laboratory testing and imaging by local centers, and limiting collection of research-only biospecimens beyond the COVID-19 PHE. These organization-based recommendations reflect institutional and researcher interest in incorporating TM/RPM into cancer treatment trials and provided a springboard for our more specific inquiries. Given that clinical researchers appear interested in using TM/RPM in clinical trials and expert consensus supports the integration of telemedicine into cancer clinical trials, more research is needed to understand the reason for decline in TM/RPM use since the onset of the pandemic.
This study has several limitations. First, the response rate to the survey was low, and the demographics of the respondents suggest that they are not representative of all qualifying ASCO members. In addition, the sample size was too small to conduct subset analyses, which would be valuable in future studies. Second, those who responded may have had more interest or experience using telehealth in the clinical trial space, potentially introducing bias, although experienced researchers may have the most informed insight on this topic. Finally, the survey asks about researcher comfort and experience but does not explicitly address interest and willingness to participate in TM/RPM as part of clinical trial care.
Conclusion
The COVID-19 pandemic spurred the rise of TM/RPM in cancer treatment trials, and TM/RPM use continues in this context, although to a lesser degree than at the height of the pandemic. Prior research shows that clinical investigators favor continuing this approach in specific elements of clinical trial care. Our work confirms that clinical researchers feel comfortable using TM/RPM in multiple clinical trial components across phase I and II/III trials. Furthermore, our findings identify a gap between frequencies of researcher comfort and experience, which highlights specific areas for training and expanding the use of TM/RPM in oncology research. To this end, future work should evaluate patient technology access in this context to understand how using TM/RPM may impact disparities in clinical trials. Studying the use of TM/RPM in cancer clinical trials has the potential not only to revolutionize the way we conduct research but also to expand patient access to novel medications and interventions that patients may not otherwise be able to obtain.
Supplementary Material
Supplementary material is available at The Oncologist online.
Contributor Information
Morgan R L Lichtenstein, Division of Medical Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Laura A Levit, Center for Research and Analytics, American Society of Clinical Oncology, Alexandria, VA, USA.
Caroline Schenkel, Center for Research and Analytics, American Society of Clinical Oncology, Alexandria, VA, USA.
Kelsey Kirkwood, Center for Research and Analytics, American Society of Clinical Oncology, Alexandria, VA, USA.
Lola A Fashoyin-Aje, Oncology Center of Excellence, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Suanna S Bruinooge, Center for Research and Analytics, American Society of Clinical Oncology, Alexandria, VA, USA.
Michael J Kelley, Department of Medicine, Duke Cancer Institute and Medical Oncology, Duke University Medical Center, and Hematology-Oncology, Durham VA Medical Center, Durham, NC, USA.
Josh A Mailman, NorCal CarciNET Community, San Francisco, CA, USA.
Allison Magnuson, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA.
Daniel P Mirda, Providence Medical Group Northern California Napa, CA, USA.
Divya Natesan, University of North Carolina, Chapel Hill, NC, USA.
Dawn L Hershman, Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center, New York, NY, USA.
Funding
This research was partially funded by the National Cancer Institute (T32CA094061, M.R.L.L.), Breast Cancer Research Foundation (D.L.H.), and the American Cancer Society (D.L.H.).
Conflict of Interest
Michael J. Kelley received research funding from EQRx, Mirati Therapeutics, Novartis, Bristol-Myers Squibb, Regeneron, and Genentech. Josh A. Mailman reported a consulting/advisory relationship with Crinetics, Rayzbio, Camurus, and ITM Oncologics; honoraria from Ipsen and Novartis; ownership interests in Sanofi, Danaher, Abbott Laboratories, ThermoFisher Scientific, LabCorp, Anthem, Veeva Systems, AstraZeneca, Dexcom, Medtronic, and Novo Nordisk; and Scientific Advisory Board for Ipsen, Novartis, Camurus, ITM Oncologics. Daniel P. Mirda reported a consulting/advisory relationship with Gilead Sciences and ER Squib Research; honoraria from Pfizer; and speaker’s bureau fees from Sanofi/Aventis. Dawn L. Hershman reported a consulting/advisory relationship with AIM Specialty Health. The other authors indicated no financial relationships.
Author Contributions
Conception/design: All authors; Provision of study material or patients: M.R.L.L., L.A.L., C.S., K.K., D.L.H. Collection and/or assembly of data: M.R.L.L., L.A.L., C.S., K.K., D.L.H. Data analysis and interpretation: All authors. Manuscript writing and final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author, per ASCO restrictions.
Previous Presentation
This study was previously presented as an abstract at ASCO Quality Care Symposium, 2022
References
- 1. Royce TJ, Sanoff HK, Rewari A.. Telemedicine for cancer care in the Time of COVID-19. JAMA Oncol. 2020;6(11):1698–1699. 10.1001/jamaoncol.2020.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greene JA. As telemedicine surges, will community health suffer?. Boston Review. 2020. https://www.bostonreview.net/articles/jeremy-greene-telemedicine-and-new-infrastructure-care/s [Google Scholar]
- 3. Doherty GJ, Goksu M, de Paula BHR.. Rethinking cancer clinical trials for COVID-19 and beyond. Nat Cancer. 2020;1(6):568–572. 10.1038/s43018-020-0083-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patt D, Wilfong L, Kanipe K, Paulson RS.. Telemedicine for cancer care: implementation across a multicenter community oncology practice. Am J Manag Care. 2020;26(10 Spec No.):SP330–SP332. 10.37765/ajmc.2020.88560 [DOI] [PubMed] [Google Scholar]
- 5. Patt DA, Wilfong L, Toth S, et al. Telemedicine in community cancer care: how technology helps patients with cancer navigate a pandemic. JCO Oncol Pract. 2021;17(1):e11–e15. 10.1200/OP.20.00815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knudsen KE, Willman C, Winn R.. Optimizing the use of telemedicine in oncology care: postpandemic opportunities. Clin Cancer Res. 2021;27(4):933–936. 10.1158/1078-0432.CCR-20-3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sirintrapun SJ, Lopez AM.. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book. 2018;38:540–545. 10.1200/EDBK_200141 [DOI] [PubMed] [Google Scholar]
- 8. U.S. Department of Health and Human Services FDA, (CDER) CfDEaR, (CBER) CfBEaR, et al. Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health: Emergency Guidance for Industry, Investigators, and Institutional Review Boards, March 2020. [Google Scholar]
- 9. The European Medicines Agency, (GCP) GCP, IWG) IWGG, et al. Guidance on the Management of Clinical Trials during the COVID-19 (Coronavirus) Pandemic 2022. [Google Scholar]
- 10. Granberg RE, Heyer A, Rising KL, et al. Medical oncology patient perceptions of telehealth video visits. JCO Oncol Pract. 2021;17(9):e1333–e1343. 10.1200/OP.21.00086 [DOI] [PubMed] [Google Scholar]
- 11. Meghiref Y, Parnot C, Duverger C, et al. The use of telemedicine in cancer clinical trials: connect-patient-to-doctor prospective study. JMIR Cancer. 2022;8(1):e31255. 10.2196/31255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JJ, Burbury K, Underhill C, et al. Exploring Australian regional cancer patients’ experiences of clinical trial participation via telehealth. J Telemed Telecare. 2022;28(7):508–516. 10.1177/1357633X20950180 [DOI] [PubMed] [Google Scholar]
- 13. Andrew OP, Grimison P, Boyer M, et al. Patient satisfaction with telehealth consultations in medical oncology clinics: a cross-sectional study at a metropolitan centre during the COVID-19 pandemic. J Telemed Telecare. 2021;0(0):1357633X211045586. [DOI] [PubMed] [Google Scholar]
- 14. Adams DV, Long S, Fleury ME.. Association of remote technology use and other decentralization tools with patient likelihood to enroll in cancer clinical trials. JAMA Netw Open. 2022;5(7):e2220053. 10.1001/jamanetworkopen.2022.20053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbott DE, Voils CL, Fisher DA, Greenberg CC, Safdar N.. Socioeconomic disparities, financial toxicity, and opportunities for enhanced system efficiencies for patients with cancer. J Surg Oncol. 2017;115(3):250–256. 10.1002/jso.24528 [DOI] [PubMed] [Google Scholar]
- 16. McInnes DK, Sawh L, Petrakis BA, et al. The potential for health-related uses of mobile phones and internet with homeless veterans: results from a multisite survey. Telemed J E Health. 2014;20(9):801–809. 10.1089/tmj.2013.0329 [DOI] [PubMed] [Google Scholar]
- 17. Mitchell SJ, Godoy L, Shabazz K, Horn IB.. Internet and mobile technology use among urban African American parents: survey study of a clinical population. J Med Internet. Res. 2014;16(1):e9. 10.2196/jmir.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryan MH, Yoder J, Flores SK, Soh J, Vanderbilt AA.. Using Health Information Technology to reach patients in underserved communities: a pilot study to help close the gap with health disparities. Glob J Health Sci. 2015;8(6):86–94. 10.5539/gjhs.v8n6p86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butzner M, Cuffee Y.. Telehealth interventions and outcomes across rural communities in the United States: narrative review. J Med Internet Res. 2021;23(8):e29575. 10.2196/29575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma JJ, Gross G, Sharma P.. Extending oncology clinical services to rural areas of Texas via teleoncology. J Oncol Pract. 2012;8(2):68. 10.1200/JOP.2011.000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lara Gongora AB, Werutsky G, Jardim DL, et al. Impact of the COVID-19 pandemic on oncology clinical research in Latin America (LACOG 0420). JCO Glob Oncol. 2021;7:649–658. 10.1200/GO.20.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31(10):1320–1335. 10.1016/j.annonc.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smeltzer MP, Scagliotti GV, Wakelee HA, et al. International Association for the Study of Lung Cancer Study of the Impact of Coronavirus Disease 2019 on International Lung Cancer Clinical Trials. J Thorac Oncol. 2022;17(5):651–660. 10.1016/j.jtho.2022.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ndumele A, Park KU.. The impact of COVID-19 on National Clinical Trials Network Breast Cancer Trials. Curr Breast Cancer Rep. 2021;13(3):103–109. 10.1007/s12609-021-00417-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sessa C, Cortes J, Conte P, et al. The impact of COVID-19 on cancer care and oncology clinical research: an experts’ perspective. ESMO Open. 2022;7(1):100339. 10.1016/j.esmoop.2021.100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waterhouse DM, Harvey RD, Hurley P, et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology Survey. JCO Oncol Pract. 2020;16(7):417–421. 10.1200/OP.20.00275 [DOI] [PubMed] [Google Scholar]
- 27. Service ER. AGRICULTURE USDO: Rural-Urban Commuting Area Codes. 2020. USDA Economic Research Service. Rural Urban Commuting Area codes: USDA ERS - Rural-Urban Commuting Area Codes. [Google Scholar]
- 28. Center RHR. Rural Health Research Center Data. 2000. Rural Health Research Center. Rural Urban Commuting Area Code Maps: Rural Urban Commuting Area Codes Maps. [Google Scholar]
- 29. Sabesan S, Zalcberg J.. Telehealth models could be extended to conducting clinical trials-a teletrial approach. Eur J Cancer Care (Engl). 2018;27(2):e12587. 10.1111/ecc.12587 [DOI] [PubMed] [Google Scholar]
- 30. Zon RT, Kennedy EB, Adelson K, et al. Telehealth in oncology: ASCO Standards and Practice Recommendations. JCO Oncol Pract. 2021;17(9):546–564. 10.1200/OP.21.00438 [DOI] [PubMed] [Google Scholar]
- 31. Pennell NA, Dillmon M, Levit LA, et al. American Society of Clinical Oncology Road to Recovery Report: learning from the COVID-19 experience to improve clinical research and cancer care. J Clin Oncol. 2021;39(2):155–169. 10.1200/JCO.20.02953 [DOI] [PubMed] [Google Scholar]
- 32. Lorusso D, Ray-Coquard I, Oaknin A, Banerjee S.. Clinical research disruption in the post-COVID-19 era: will the pandemic lead to change?. ESMO Open. 2020;5(5):e000924. 10.1136/esmoopen-2020-000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME.. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245–255. 10.1093/jnci/djy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. West HJ, Barzi A, Wong D.. Telemedicine in cancer care beyond the COVID-19 pandemic: oncology 2.0?. Curr Oncol Rep. 2022;24(12):1843–1850. 10.1007/s11912-022-01332-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nabhan C, Choueiri TK, Mato AR.. Rethinking clinical trials reform during the COVID-19 pandemic. JAMA Oncol. 2020;6(9):1327–1329. 10.1001/jamaoncol.2020.3142 [DOI] [PubMed] [Google Scholar]
- 36. Nekhlyudov L, Fleisher LA, Jacobsen PB.. Telemedicine across the cancer care continuum: evidence and opportunities for clinical care, research, and policy. Cancer J. 2022;28(2):121–124. 10.1097/PPO.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 37. American Society of Clinical Oncology. ASCO Position Statement: Telemedicine Cross-State Licensure. https://old-prod.asco.org/sites/new-www.asco.org/files/content-files/advocacy-and-policy/documents/2021-ASCO-Telemedicine-Licensure.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author, per ASCO restrictions.


