Abstract
We have found that the replicative helicase E1 of bovine papillomavirus type 1 (BPV-1) interacts with a key cell cycle regulator of S phase, the cyclin E-Cdk2 kinase. The E1 helicase, which interacts with cyclin E and not with Cdk2, presents the highest affinity for catalytically active kinase complexes. In addition, E1, cyclin E, and Cdk2 expressed in Xenopus egg extracts are quantitatively coimmunoprecipitated from crude extracts by either anti-Cdk2 or anti-E1 antibodies. E1 protein is also a substrate of the cyclin E-Cdk2 kinase in vitro. Using the viral components required for in vitro BPV-1 replication and free-membrane cytosol from Xenopus eggs, we show that efficient replication of BPV plasmids is dependent on the addition of E1-cyclin E-Cdk2 complexes. Thus, the BPV initiator of replication and cyclin E-Cdk2 are likely to function together as a protein complex which may be the key to the cell cycle regulation of papillomavirus replication.
Papillomaviruses are small DNA tumor viruses, widely distributed in nature, that are at least in part responsible for many human cancers (1, 17, 23, 25, 62). Bovine papillomavirus type 1 (BPV-1), which induces fibropapillomas in the skin of cattle, can also transform somatic cells in culture. Cells transformed by this virus retain a low copy number of BPV genome within the cell nucleus without drastically compromising the interaction between cellular growth control signals and the DNA replication machinery, since its replication occurs exclusively during S phase (16, 26, 36). Because of its ability to replicate as a regulated episome in dividing cells, this small virus provides an interesting model with which to investigate cell cycle control of DNA replication. As most enzymatic activities are carried out by host cell proteins, it is likely that this virus exploits not only the host replication machinery but also the cellular mechanisms that regulate host cell DNA replication, so as to ensure the stable maintenance of its own genome in infected cells.
The viral components required for transient BPV-1 replication are two proteins, the products of the E1 and E2 open reading frames and a cis sequence termed the origin of replication (54–56). This sequence is 60 nucleotides (nt) long and contains binding sites for both E1 and E2 and an AT-rich region. E1 is the major viral replication protein, similar to another well-studied viral initiator protein, simian virus 40 (SV40)/polyomavirus large T antigen: it is a nuclear phosphoprotein with ATPase activity (32, 45, 46, 51) which binds to the BPV origin specifically, unwinding a 18-bp palindromic sequence, and acts as a helicase that translocates in the 3′-to-5′ direction (47, 58). Thus, E1 provides the functions of (i) origin recognition and melting of the DNA template within the viral replication origin and (ii) acting as the DNA helicase at the replication fork. Indeed, in a study on cis and trans factors required for replication of BPV origin-containing DNA in a cell-free system, we previously demonstrated that E1 alone can efficiently drive multiple rounds of DNA synthesis from a single BPV plasmid, as do the initiators of lytic viruses such as SV40 large T antigen (6). However, in latently infected cells, BPV replication does not continue after reaching a threshold number of copies per cell, which suggests that E1-mediated DNA replication may be negatively controlled in dividing cells. We have also shown that in the presence of the BPV transcriptional regulator E2, normally required in vivo, the level of E1-dependent DNA synthesis and the frequency of reinitiation events were not affected in the cell-free system consisting of crude cytosolic extracts from human 293 cells supplemented with baculovirus-produced viral proteins (8). Indeed, E2 appeared to suppress replication only from nonspecific origin-like sequences, suggesting that by interacting with E1, E2 helps to restrict the initiation events to the BPV origin. Active control elements restraining viral DNA runaway replication in proliferating infected cells are therefore absent in this cell-free system. Our aim being to understand the molecular mechanisms involved in the control of BPV replication in latently infected cells, this cell-free system was clearly inadequate. We and others have shown that BPV DNA replication can occur in vitro in cell extracts from human, simian, or murine cells, indicating that BPV replication is not cell type specific (7, 37, 38). Therefore, as an alternative approach for characterizing the cellular factors involved in the cell cycle formation and activation of initiation complexes at the BPV origin, we chose to use the only cell-free system known to contain all of the factors required for replication under the same cell cycle control as in vivo: extracts derived from activated eggs of the frog Xenopus laevis (3, 21, 39, 40). In this system, initiation of S phase is dependent on cyclin E-Cdk2 kinase activity. Xenopus extracts, which normally replicate exogenously added chromatin templates efficiently, fail to do so after removal of either cyclin E or Cdk2 (4, 14, 15, 22). We have begun to investigate cell cycle control of BPV replication by questioning whether these key cell cycle-regulatory elements can interact with the BPV initiator of replication in vitro. We report here that Xenopus cyclin E-Cdk2 interacts with E1 specifically and show that E1-cyclin E-Cdk2 complexes can be efficiently reconstituted in Xenopus egg extracts. Functional analysis of the E1-cyclin E-Cdk2 complex, performed with an in vitro replication system derived from Xenopus egg extracts, demonstrates that the association of E1 with this S-phase kinase regulator complex is required to obtain efficient E1-dependent replication of plasmids containing the BPV origin of replication.
MATERIALS AND METHODS
Purification of E1 protein.
Glutathione S-transferase (GST) fusion protein was expressed in bacteria and purified essentially as previously described (7). Specific proteolysis to release E1 protein was at 4°C in buffer containing 50 mM Tris-HCl (pH 8.0), 10 mM MgSO4, 200 mM NaCl, 10% glycerol, 5 mM dithiothreitol, 1 mM CaCl2, and factor Xa (Boehringer).
Pull-down assays and immunoblotting.
The binding reactions were carried out by incubating 1 to 10 μg of GST-E1 fusion protein with 10 to 20 μl of Xenopus high-speed egg extracts or 2 μl of reticulocyte lysate in 100 μl of buffer A (20 mM K-HEPES [pH 7.7], 100 mM potassium acetate, 1 mM MgCl2, 2 mM dithiothreitol, 10% glycerol, 0.1% Triton X-100) for 1 h at 4°C on a rotating wheel. Proteins associated with GST or GST-E1 were pulled down with glutathione-Sepharose beads. The beads were washed four times with 0.5 ml of binding buffer, and bound proteins were eluted by boiling in Laemmli’s sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, separated by SDS-PAGE, and detected either by Western blotting using appropriate antibodies or by autoradiography for radiolabeled proteins expressed in rabbit reticulocyte lysates. The antibodies used in this study were anti-BPV E1 antibodies raised against the C-terminal part of E1 (amino acids 314 to 605) (45). Additional rabbit anti-E1 serum was generated with a GST-E1 fusion protein. Anti-Xenopus cyclin E serum was previously described (9).
Anti-Cdk2 antibodies were raised against a COOH-terminal peptide (sequence N-CTHPFFRDVSRPTPHLI) coupled to thyroglobulin and used crude or affinity purified with peptide coupled to bovine serum albumin. Additional anti-Cdk2 antibodies and suc1-coupled beads (suc1-beads) were generously provided by M. Dorée (Centre de Recherches de Biochimie Macromoléculaire Montpellier, France).
Preparation of Xenopus extracts and depletion procedure.
RNase-treated translation extracts from Xenopus egg were prepared essentially as detailed by Matthews (33). Crude Xenopus egg extracts were prepared as described by Murray and Kirschner (39) except that dejellied eggs were disrupted by direct centrifugation in a modified XB buffer (20 mM K-HEPES [pH 7.7], 50 mM potassium acetate, 5 mM MgCl2, 0.1 mM CaCl2, 50 mM sucrose, 10 mg of aprotinin per ml) supplemented with 80 μg of cytochalasin B per ml. High-speed supernatants (HSS) were prepared by further fractionation of the crude extract for 1 h at 45,000 rpm in the SW50.1 rotor of a Beckman centrifuge. The cytosol was collected and frozen in liquid nitrogen. suc1-depleted extracts were prepared by mixing 0.2 volume of beads (10 mg of suc1 protein coupled per ml of beads) per volume of freshly prepared extract for 30 min at 4°C. Beads were spun down, and the supernatant was again incubated with 0.2 volume suc1-beads for 30 min at 4°C. Beads were separated by centrifugation for 10 min at 10,000 rpm at 4°C. Immunodepletion with crude sera was performed by mixing rabbit sera to protein A-Sepharose CL4B beads (1.5 μl of serum per μl of protein A beads) in XB buffer, followed by extensive washing of the beads with XB buffer. Fresh extracts were immunodepleted by two successive incubations with 0.2 volume of beads per volume of extract for 60 min at 4°C. Control depletions used preimmune serum from the same rabbit. Depleted HSS extracts were frozen and stored in liquid nitrogen; depleted translation extracts were used immediately for experiments.
In vitro labeling of E1 protein and phosphoamino acid analysis.
Immunoprecipitates of either cyclin E or Cdk2 were prepared by mixing 10 μl of anti-cyclin E or anti-Cdk2 serum with 10 μl of HSS extract for 2 h at 4°C. Immune complexes were precipitated by addition of 10 μl of protein A-Sepharose CL4B beads, diluted to 100 μl of XB buffer for 1 h at 4°C on a rotating wheel. Pellets were washed and incubated at 30°C in the presence of a mixture containing 20 mM K-HEPES (pH 7.7), 10 mM MgCl2, 0.8 mM ATP, 3 μCi of [γ-32P]ATP, and 1 μg of purified Escherichia coli E1 or histone H1 (Boehringer) for 30 min. Reactions were stopped by addition of 2× Laemmli sample buffer and then boiled for 5 min. The products were analyzed by SDS-PAGE, and phosphorylation of the substrates was quantified by phosphorimager analysis. For phosphoamino acid analysis, resolved phosphorylated E1 protein was transferred to polyvinylidene difluoride membranes. E1 protein was visualized by autoradiography, and the band containing E1 was excised from the membrane. Hydrolysis of labeled E1 protein to amino acids was done directly on the membrane, using 6 N HCl at 110°C for 2 h. Labeled amino acids were dried and separated by two-dimensional electrophoresis on thin-layer plates.
Preparation of synthetic mRNA and translation reaction.
The recombinant vector used for preparation of synthetic E1 mRNA was constructed by inserting the BPV NruI-StuI restriction fragment (nt 838 to 3351) ligated to BamHI linkers, within the unique BglII site of the pBluescript RN3 vector (28). Pepex-Cdk2 was from J. Gautier and J. Paris (41). Capped transcripts prepared as described by Matthews and Coleman (34) were adjusted to 1 mg/ml after sequential precipitation with LiCl and ethanol. Translation in RNase-treated Xenopus egg extracts were carried out as detailed by Matthews (33) except that only fresh extracts without addition of reticulocyte lysate were used. Five microliters of capped transcripts was usually mixed with 50 μl of extract in the presence of [35S]methionine (Amersham) for 120 min at 21°C. Pepex-Xenopus cyclin E and Pepex-Xenopus Cdk2 were also transcribed and translated in a nuclease-treated rabbit reticulocyte lysate system containing [35S]methionine according to the manufacturer’s instructions (Promega).
Immunoprecipitation and isolation of E1-cyclin E-Cdk2 complexes.
Xenopus translation extracts containing radiolabeled BPV E1 or Xenopus cyclin E or Cdk2 were combined and incubated at 23°C for 30 min. Immunoprecipitation experiments were performed by mixing 10 μl of extracts with 10 μl of either anti-Cdk2 or anti-E1 antiserum bound to protein A-Sepharose beads diluted in 40 μl of XB for 60 min at 4°C on a rotating wheel. Beads were separated by centrifugation for 10 min at 10,000 rpm at 4°C. Isolation of E1 complexes by ion-exchange chromatography was usually performed by diluting 20 μl of translation extracts in 100 μl of buffer B (20 mM K-HEPES [pH 8], 10 mM MgSO4, 2 mM dithiothreitol, 150 mM potassium acetate, 10% glycerol), loaded onto a 0.1-ml column containing Q-Sepharose (fast flow; Pharmacia) equilibrated in buffer B, and kept on the column for 15 min at 4°C with gentle stirring. The flowthrough was collected, and the column was washed successively with 5 volumes of buffer B containing 150 mM potassium acetate and 20 volumes of buffer B containing 500 mM potassium acetate prior to elution with buffer B containing 1 M potassium acetate. The eluate was then dialyzed against buffer C (20 mM K-HEPES [pH 7.7], 100 mM potassium acetate, 5 mM MgSO4, 2 mM dithiothreitol, 10% glycerol) and used immediately for in vitro replication assays.
In vitro DNA replication assay.
The Xenopus HSS samples were thawed immediately before use, and an ATP-regenerating system was added to a final concentration of 10 mM creatine phosphate, 7.5 μg of creatine phosphokinase per ml, and 2 mM ATP. Reactions were typically carried out by first mixing 15 μl of HSS with appropriate amounts of E1 fraction for 15 min at 23°C before the addition of 150 ng of plasmid DNA. Reaction mixtures were incubated for 180 min at 23°C (little additional incorporation into DNA was seen after this time), and DNA synthesis was followed by addition of 10 μCi of [α-32P]dCTP to the reaction. Following incubation, the reactions were quenched by addition of 20 mM EDTA. Samples to be analyzed for DNA synthesis were deproteinized by using proteinase K (500 μg/ml) and 0.5% SDS for 60 min at 37°C, followed by two phenol-chloroform extractions. DNA synthesis was monitored on aliquots spotted onto GF-C glass fiber filters, precipitated in ice-cold 5% trichloroacetic acid (TCA)–10% sodium pyrophosphate for 10 min, and then washed three times in ice-cold 1 M HCl for 15 min and twice in 95% ethanol. Dried filters were counted by liquid scintillation and normalized to equivalent samples that were not TCA precipitated. For agarose gel electrophoresis analysis, aliquots of purified DNA (usually 10 μl of the reaction mixture) were digested with two successive additions of 5 U of DpnI in the presence of 200 mM NaCl for 5 h at 37°C, in order to distinguish replicated plasmid molecules from DNA molecules submitted to reparation events. The DpnI assay relies on the fact that the restriction enzyme cleaves DNA at specific sites that are methylated at adenine residues on both strands. Semiconservative replication of such templates, prepared in E. coli dam+ cells, results in the production in vitro of hemimethylated DNA molecules which are resistant to DpnI digestion. The plasmid DNA template used for BPV replication, pSKori, containing a 160-bp origin-bearing BPV-1 DNA fragment (nt 7855 to 81) in pBluescript SK+ vector, was previously described (8). The SV40 plasmid DNA substrate, pSVori, contains the 203-bp origin-bearing fragment of the SV40 genome (nt 5172 to 132) in the pBR322 derivative pML2. Both plasmids were found to be fully sensitive to DpnI.
RESULTS
BPV E1 protein and Xenopus cyclin E-Cdk2 interact.
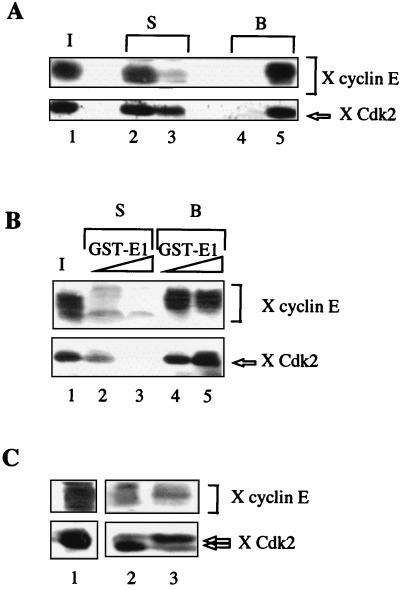
To study the mechanisms by which BPV-1 replication is connected to S phase of the cell cycle, we first examined whether the viral helicase E1 and a positive regulator of the initiation of DNA replication such as the cyclin E-Cdk2 kinase interacted physically. We have previously shown that several cellular proteins can be selectively retained on affinity columns containing a GST fusion protein (GST-E1). Among these proteins, DNA polymerase α-primase was identified (7). We decided to use a similar approach to determine whether the viral helicase E1 could bind cyclin E and/or Cdk2 present in interphase Xenopus egg extracts. Antibodies to cyclin E recognized multiple cyclin E species which have been previously shown to represent different phosphorylated states of the cyclin E protein (9, 44). As shown in Fig. 1A, both cyclin E and Cdk2 were retained on GST-E1 beads, as demonstrated by the recovery of both cyclin E and Cdk2 in the proteins eluted from GST-E1 beads (lane 5) and their concomitant decrease in the Xenopus extract (lane 3), but neither cyclin E nor Cdk2 was bound to control beads (lane 4). Thus, cyclin E and Cdk2 appear to interact specifically with E1. In experiments performed with higher amounts of GST-E1 beads, almost all of the cyclin E species and all Cdk2 from Xenopus extracts were depleted (Fig. 1B). The slowest-migrating phosphorylated forms of cyclin E were previously shown to coincide with the associated H1 kinase activity of Xenopus cyclin E immunocomplexes (44). To determine whether E1 associates preferentially catalytically active kinase complexes, GST-E1 beads loaded with Xenopus cyclin E and Cdk2 were submitted to washes with increasing salt concentrations. In the blot shown in Fig. 1C, two forms of Cdk2 were resolved. The slower-migrating form, which correlates with the kinase-inactive form, was the major form recovered in the salt eluates (lane 3), while the faster-migrating form of Cdk2, corresponding to the kinase-active form, phosphorylated on threonine 160 (12, 18), remained bound to GST-E1 beads (lane 2). Consistent with the correlation between the electrophoretic mobility of cyclin E and kinase activity, the major cyclin E species coeluting with inactive kinase subunit was a rapidly migrating form of cyclin E. A salt concentration of 1.5 M was, however, required to dissociate the inactive kinase complexes from E1 beads. Therefore, the strength of the interaction between E1 and cyclin E-Cdk2 is highest for catalytically active kinase complexes.
FIG. 1.
BPV E1 and Xenopus cyclin E and Cdk2 interact. (A) Cyclin E and Cdk2 are quantitatively retained on GST-E1 beads. Proteins bound to GST or GST-E1 after incubation with 20 μl of Xenopus (X) HSS were visualized by immunoblotting with anti-Xenopus cyclin E serum (upper panel) and with polyclonal anti-Xenopus Cdk2 antibody (lower panel). Lane 1, input (I), 20 μl; lanes 2 and 4, supernatant (S) and bound proteins (B) from GST beads; lanes 3 and 5, supernatant (S) and bound proteins (B) from GST-E1 beads. (B) GST-E1 beads deplete Xenopus cyclin E-Cdk2 from HSS. Ten microliters of HSS was incubated with either 5 or 10 μg of GST-E1. Cyclin E and Cdk2 were visualized as in panel A. Lane 1, input, 10 μl; lanes 2 and 3, supernatant (S) after incubation with 5 and 10 μg of GST-E1, respectively; lanes 4 and 5, bound proteins (B) released from 5 and 10 μg of GST-E1 beads, respectively. (C) Strength of interaction between E1 and active or inactive cyclin E-Cdk2 kinase complexes. Twenty microliters of HSS was mixed with 10 μg of GST-E1. The beads were first eluted with 1.5 M NaCl (lane 3), and remaining proteins were released with SDS sample buffer (lane 2). Lane 1, input, 20 μl. Cyclin E and Cdk2 were visualized as in panel A.
E1 associates with cyclin E and not with Cdk2.
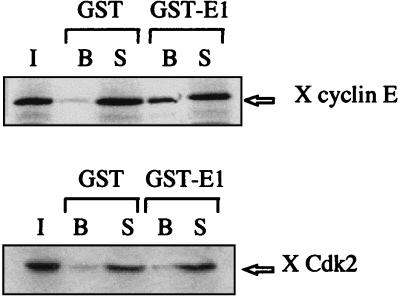
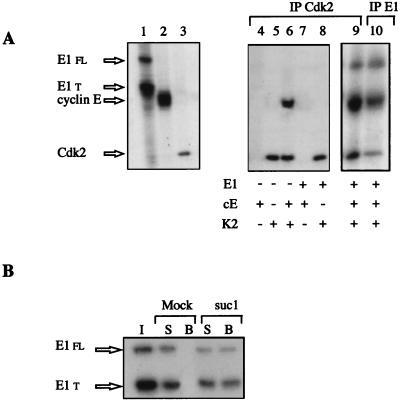
To characterize the molecular mechanisms of interaction between the viral helicase and the cyclin E-Cdk2 complex, we next tested the independent association of E1 with cyclin E and with Cdk2. Cyclin E and Cdk2 were produced separately in rabbit reticulocyte lysates by in vitro transcription-translation, and the radiolabeled proteins were mixed with GST or GST-E1 beads. While cyclin E associated with GST-E1 beads specifically, Cdk2 alone did not interact with E1 (Fig. 2), indicating that the binding between E1 and the cyclin E-Cdk2 complex is mediated by the cyclin subunit. To further substantiate this observation, mRNAs coding for E1, cyclin E, or Cdk2 were translated separately in RNase-treated Xenopus egg extracts lacking most of endogenous cyclin E and Cdk2 prepared by immunodepletion with anti-cyclin E and anti-Cdk2 antibodies coupled to beads. As shown in Fig. 3A, each translation generates strong radioactive bands that were detected autoradiographically. In the case of E1 translation, two types of E1 species were obtained (Fig. 3A, lane 1), the 70-kDa full-length E1 protein and shorter peptides. The radiolabeled proteins were tested for association with each other in immunoprecipitation experiments performed with antibodies against Cdk2. Coimmunoprecipitation of E1 with Cdk2 was observed only upon addition of the translation extract that contained cyclin E (lane 9). Immunoprecipitations performed with antibodies directed against the C-terminal part of E1 also coimmunoprecipitated significant amounts of cyclin E and of Cdk2 (lane 10). These immunoprecipitation experiments confirm that E1 interact with cyclin E and not with Cdk2. Because endogenous Xenopus cyclin E-Cdk2 has previously been shown to be part of a multiprotein complex (22), we next examined whether E1, translated at a low concentration in Xenopus interphase egg extracts, could associate with endogenous cyclin E-Cdk2 complexes by competing with their cellular partners. As shown in Fig. 3B, a significant amount of E1 molecules were bound in complexes with endogenous cyclin E-Cdk2, as indicated by the recovery of E1 on a Cdk-binding suc1-agarose matrix. All of these results demonstrate that the binding of cyclin E-Cdk2 to E1 is not an artifact of the high concentration of E1 on affinity beads, as E1, translated in the extract from exogenously added mRNAs, and cyclin E-Cdk2, either preexisting or newly translated, also interact under conditions found in crude egg extracts.
FIG. 2.
BPV E1 associates with free cyclin E and not with free Cdk2. GST or GST-E1 proteins were incubated with Xenopus (X) cyclin E or Cdk2 produced by in vitro transcription and translation using reticulocyte lysate containing [35S]methionine. I, B, and S designate the input radiolabeled protein, bound proteins, and supernatant, respectively.
FIG. 3.
Anti-Cdk2 antibodies coprecipitate E1 only in the presence of cyclin E. (A) BPV E1 (lane 1), Xenopus cyclin E (lane 2), and Xenopus Cdk2 (lane 3) were translated separately in Cdk2-depleted Xenopus egg extracts in the presence of [35S]methionine. E1, cyclin E, and Cdk2 contain 9, 13, and 5 methionine residues, respectively. Extracts were mixed differently, and immunoprecipitations (IP) were performed with anti-Cdk2 antibodies (lanes 4 to 9) or with anti-E1 antibodies (lane 10). E1 fl and E1 t correspond to full-length E1 protein and truncated E1 protein, respectively. In vitro-translated proteins and products of immunoprecipitation were analyzed by SDS-PAGE (10% gel) and autoradiographed. (B) E1 synthesized in Xenopus egg extracts is retained on suc1-beads. A sample of Xenopus extract containing radiolabeled translated E1 products (1 μl) was incubated either with beads coupled to the cyclin-Cdk-associated protein suc1 or with agarose beads (Mock). The behavior of E1 was analyzed by SDS-PAGE and autoradiography. I, input extract (1 μl); B, beads; S, supernatant after incubation with beads.
E1 is phosphorylated by cyclin E-Cdk2 in vitro and in interphase Xenopus egg extracts.
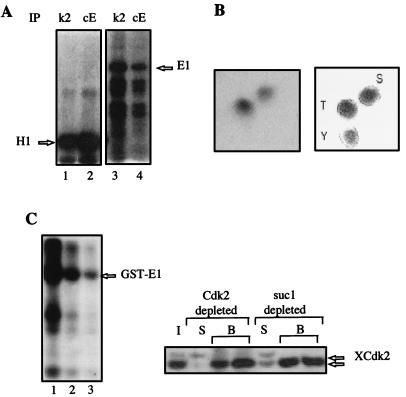
The protein expressed from the E1 open reading frame in different heterologous systems was shown to be phosphorylated (45, 51). The stable association of E1 with cyclin E-Cdk2 suggested that E1 might be phosphorylated by this kinase, which controls the entry into S phase. A Ser/Thr-Pro dipeptide is the only absolutely conserved phosphorylated sequence in all of the known cyclin-dependent kinase substrates. Within the E1 coding sequence, there are two threonine-proline dipeptides, at amino acids 102 to 103 and 126 to 127, and one serine-proline at dipeptide 283 to 284. One of these sites (threonine 102) was previously shown to be phosphorylated by a mitotic Cdk, Cdk1, in vitro (29). As shown in Fig. 4A, when purified E. coli E1 protein was incubated with immunoprecipitates of cyclin E or Cdk2 prepared from Xenopus egg extracts and [γ-32P]ATP, the associated kinase activity phosphorylated E1 as well as histone H1. While the Cdk1 kinase was reported to phosphorylate E1 only on threonine 102 (29), E1 treated with cyclin E-Cdk2 appears to be phosphorylated on both threonine and serine residues (Fig. 4B). E1 was also efficiently phosphorylated in Xenopus egg extracts (Fig. 4C). To further demonstrate that E1 was a substrate of cyclin E-Cdk2 in egg extracts, GST-E1 beads were added to egg extracts either depleted of all Cdk-associated kinase by suc1-beads or depleted of only Cdk2-associated kinase activity by anti-Cdk2 beads. Consistent with the remaining Cdk2 after suc1 or Cdk2 depletion (detected by immunoblotting with anti-Cdk2 [Fig. 4C]), the level of 32P incorporation was decreased to 50% in suc1-depleted extracts (lane 2) and to more than 60% in Cdk2-depleted extracts (lane 3). Thus, E1 is clearly a substrate of cyclin E-Cdk2 in interphase egg extracts. Although it is not the only kinase that phosphorylates E1 in interphase egg extract, our results indicate that it is at least the only cyclin-dependent kinase.
FIG. 4.
The viral helicase E1 is a substrate of cyclin E-Cdk2. (A) E1 is phosphorylated by cyclin E-Cdk2 in vitro. Xenopus HSS were immunoprecipitated (IP) with cyclin E antiserum (cE) or Cdk2 antiserum (k2), and the associated protein kinase activity was tested by using histone H1 (lanes 1 and 2) or E1 (lanes 3 and 4) as substrate in the presence of [γ-32P]ATP. Phosphorylated proteins were resolved by SDS-PAGE and visualized by autoradiography. (B) Cyclin E-Cdk2 phosphorylates E1 on serine and threonine. Purified E1 protein was phosphorylated by Cdk2-associated kinase as in panel A, and radiolabeled E1 protein was hydrolyzed to amino acids that were separated by two-dimensional electrophoresis on thin-layer plates (left); the mobilities of phosphoserine (S), phosphothreonine (T), and phosphotyrosine (Y) standards are shown on the right. (C) E1 is a substrate of cyclin E-Cdk2 in Xenopus egg extracts. GST-E1 beads were incubated in undepleted Xenopus egg extract (lane 1), in suc1-depleted extract (lane 2), or in Cdk2-depleted extract (lane 3) in the presence of [γ-32P]ATP. Xenopus Cdk2 (XCdk2) depletion was monitored by Western blotting (right). I, input extract (half amount); B, beads; S, supernatant after incubation with beads.
E1-dependent DNA replication in Xenopus egg extracts.
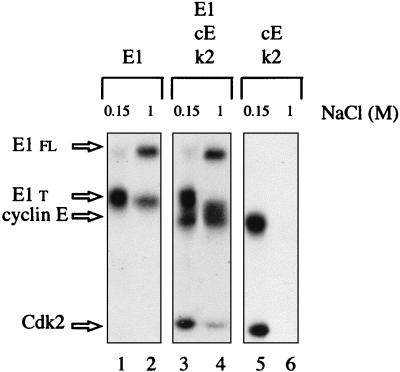
Since the viral helicase forms a stable complex with cyclin E-Cdk2 and is phosphorylated by this kinase in Xenopus egg extracts, we decided to develop an E1-dependent DNA replication assay with Xenopus egg extracts to further investigate the role of cyclin E-Cdk2 in E1 function. The replication of either chromosomal DNA or of purified double-stranded DNA in Xenopus egg extracts has been reported to occur only after the assembly of DNA molecules into pseudonuclei (5, 40). When nuclear assembly is prevented by removing vesicular material from extracts by high-speed centrifugation, replication of plasmid DNA does not take place, although the extract is competent for replication of single-stranded DNA into double-stranded DNA (31, 40, 48). Given that E1-dependent initiation of replication can be observed in vitro without nuclear formation (6), we thus used free-membrane cytosol to prevent cellular initiation events. As a source of BPV E1, Xenopus cyclin E, and Xenopus Cdk2, the proteins were expressed by translation of their mRNAs in Xenopus extracts. However, as translation experiments are carried out in low-speed egg extracts, one purification step was necessary to eliminate the membrane components from the extract containing E1, cyclin E, or Cdk2. As depicted in Fig. 5, isolation of the radiolabeled E1 translation products by ion-exchange chromatography also allowed for partial separation of the E1 full-length protein from a majority of shorter E1 products. As already found with the baculovirus-produced E1 protein (7), full-length E1 protein expressed in Xenopus egg extracts remained bound to a Q-Sepharose column when 0.5 M salt was applied and was eluted with a buffer containing 1 M salt. In contrast, when the translation extracts containing radioactive cyclin E and Cdk2 were mixed and applied to a Q-Sepharose column, neither one could be detected in a 1 M salt eluate. However, combination of E1, cyclin E, and Cdk2 translation extracts allowed the reconstitution of E1-cyclin E-Cdk2 complexes that could be eluted at 1 M salt, as expected (Fig. 5, lane 4).
FIG. 5.
Cyclin E and Cdk2 copurify with E1. E1 protein complexes used for replication assays were isolated by ion-exchange chromatography on Q-Sepharose columns. Xenopus translation extracts containing either radiolabeled E1 alone, E1, cyclin E (cE), and Cdk2 (k2), or cyclin E and Cdk2 were loaded onto small Q-Sepharose columns. The low-salt flowthrough (0.15 M NaCl; lanes 1, 3, and 5) and the 1 M salt eluate (lanes 2, 4, and 6) from each column were analyzed by SDS-PAGE, and the proteins were detected by autoradiography. E1 fl and E1 t, full-length and truncated E1 protein, respectively.
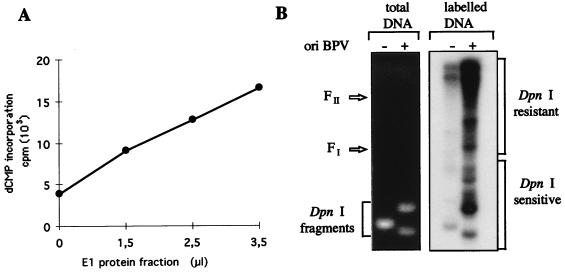
We next attempted to replicate BPV origin-containing DNA by using free-membrane interphase egg extracts (also referred to as HSS) supplemented with partially purified E1–Q-Sepharose fractions. The capacity of E1 to cooperate with the Xenopus replication machinery is demonstrated in Fig. 6A, which shows that HSS catalyzes extensive deoxynucleotide incorporation only upon addition of the E1 fraction. To test the specificity of DNA replication with HSS for a BPV origin of replication, we carried out reactions with a double-stranded plasmid template that contained either a BPV origin of replication or an SV40 origin of replication as a control. Products formed during the reactions were analyzed by agarose gel electrophoresis following a DpnI digestion (see Materials and Methods). Analysis of the bulk of DNA by agarose gel electrophoresis and ethidium bromide staining revealed cleavage of the majority of both the BPV and SV40 origin DNAs into small fragments (Fig. 6B). In contrast, newly replicated DNA, radiolabeled by incorporation of [α-32P]dCTP and resistant to DpnI digestion, was observed only with the template containing the BPV origin of replication (Fig. 6B), demonstrating the origin specificity of the E1-dependent initiation of replication in egg extracts. These results indicate also that the incorporation of dCMP observed with Xenopus HSS supplemented with partially purified E1 protein reflected bona fide DNA replication and not an increase in repair DNA synthesis due to, for example, E1 protein-associated nicking activity. The bulk of the synthesized DNA migrated between the origin of the gel and the position of the form II marker DNA. These DpnI-resistant DNA products are most likely replication termination intermediates similar to the forms observed in SV40-infected cells (52, 53). Of the monomer plasmid molecules formed, highly negative supercoiled DNA molecules (form I) were detected, which indicated assembly of the newly synthesized DNA into chromatin. Despite the fact that the experimental conditions of our replication assay do not favor the termination of DNA replication, these experiments show that replication of double-stranded DNA templates catalyzed by Xenopus HSS is dependent on the presence of the viral helicase and a BPV origin of replication.
FIG. 6.
BPV DNA synthesis in membrane-depleted egg extracts. (A) Replication of double-stranded DNA in Xenopus HSS required E1 and a BPV origin of replication. E1 protein translated in Xenopus egg extracts was partially purified on a Q-Sepharose column, and increasing volumes of dialyzed E1 fraction, as indicated, were directly added to 15 μl of Xenopus HSS. Reaction mixtures were incubated for 3 h at 23°C after the addition of 150 ng of pSKori, containing the BPV minimal origin of replication (ori). The incorporation of [α-32P]dCMP into an acid-insoluble form was measured by scintillation counting. (B) DpnI assay of reaction products obtained by incubating 150 ng of pSKori or pSVori DNA in 20-μl reaction mixtures containing 15 μl of HSS and 3.5 μl of E1 fraction for 3 h at 23°C. The reaction products were digested with DpnI and subjected to agarose gel electrophoresis. Both the ethidium bromide-stained gel and the autoradiogram are shown. FI and FII designate the migration positions of supercoiled monomer circle and nicked monomer circle, respectively.
Replication of BPV plasmids in Xenopus egg extracts is dependent on E1-cyclin E-Cdk2 complex.
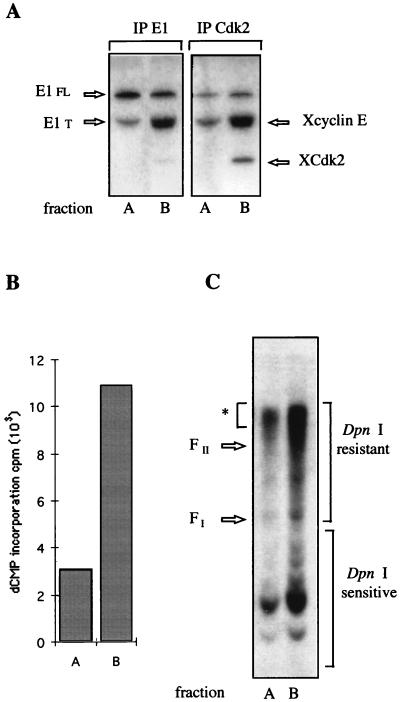
As a first step toward understanding the role of the interaction between E1 and cyclin E-Cdk2, translation reactions were carried out in Xenopus egg extracts immunodepleted in cyclin E-Cdk2. Extracts containing either E1 alone or E1, cyclin E, and Cdk2 were then subjected to Q Sepharose chromatography as described for Fig. 5. Given the difficulty of totally immunodepleting endogenous cyclin E-Cdk2 without affecting the efficiency of the translation extracts, immunoprecipitation experiments with anti-E1 or anti-Cdk2 antibodies were used to detect the global level of E1 compared to the level of reconstituted E1-cyclin E-Cdk2 complexes in each fraction (Fig. 7A). The Q Sepharose fractions were analyzed for the ability to initiate the replication of BPV plasmids in reactions carried out with HSS depleted of cyclin E-Cdk2. As shown in Fig. 7B, addition of the cyclin E-Cdk2-complemented E1 fraction (fraction B) to the depleted extract increased the overall extent of dCMP incorporation by a factor of 3.5. Analysis of the replication products on agarose gel after DpnI treatment again showed an accumulation of replicating intermediates resistant to DpnI, indicative of complete semiconservative synthesis, and confirmed the stimulatory effect of cyclin E/Cdk2 on E1-dependent DNA replication. We quantified a twofold increase in DNA synthesis of the major replication products in the presence of cyclin E-Cdk2-complemented E1 fraction (Fig. 7C). In addition, the level of replicated DpnI resistant products clearly correlated with the level of E1 associated with cyclin E-Cdk2 and not with the total level of E1 present in each fraction. These results strongly support the conclusion that initiation of replication at the BPV origin depends on the association of E1 with cyclin E-Cdk2.
FIG. 7.
Requirement of E1-cyclin E-Cdk2 complex for E1-dependent DNA replication in vitro. (A) Relative amount of 35S-radiolabeled E1 protein associated with Xenopus cyclin E -Xenopus Cdk2 (XCdk2) in partially purified E1 fraction of Q-Sepharose columns that were loaded with translation extracts containing either E1 (fraction A) or E1, cyclin E, and Cdk2 (fraction B). Protein synthesis was performed in Cdk2-depleted translation extracts from Xenopus eggs. Aliquots (5 μl) of fractions A and B were immunoprecipitated (IP) with either anti-E1 antibody or anti-Cdk2 antibody (the anti-E1 antibody, generated with a GST-E1 fusion protein, was shown to induce significant dissociation of the E1-cyclin E-Cdk2 complexes). (B) E1-cyclin E-Cdk2 complexes stimulate DNA synthesis. Fraction A or B (7 μl) or no E1 protein (7 μl of buffer C) was added to 15 μl of Cdk2-depleted HSS. After 15 min of incubation, pSKori (150 ng) was added, and DNA synthesis was carried out for 3 h at 23°C. DNA synthesis was evaluated by measuring the incorporation of radioactive dCMP into TCA-precipitable material. Background incorporation measured in the control assay with no E1 addition was subtracted from the values obtained with fraction A or B. (C) Aliquots of reaction mixtures performed with fraction A and B were digested with DpnI and subjected to agarose gel electrophoresis. The amount of replicated DNA was quantified from the DpnI-resistant DNA products marked with an asterisk and bracket on the right. FI and FII designate the migration positions of supercoiled monomer circle and nicked monomer circle markers, respectively.
DISCUSSION
The results presented in this report demonstrate that the cyclin E-Cdk2 kinase is most likely a cellular partner of the BPV replicative helicase E1. In support of this view, we have shown that E1, cyclin E, and Cdk2 added to Xenopus egg extracts through translation of their respective mRNAs re-form an extremely tight complex that can be immunoprecipitated from extracts. E1 translated in egg extract is also retained on the Cdk-binding suc1-agarose matrix; conversely, endogenous Xenopus cyclin E and Cdk2 can be totally depleted from Xenopus extracts by beads charged with bacterially produced E1 protein. While the critical cellular targets of cyclin E-Cdk2 are not known, it has been reported that Xenopus cyclin E-Cdk2 are part of a multiprotein complex (22). E1 added in excess is therefore able to compete for the binding of cyclin E-Cdk2. Like Xenopus cyclin E-Cdk2, their homologs from human or mouse cells were also found to bind E1 with a similar high affinity (not shown), but a total depletion of these complexes on GST-E1 beads was never observed. In mammalian cells, cyclin E-Cdk2 has been shown to interact with a number of proteins, including E2F, p107, p130, p27, p21, and PCNA (10, 19, 27, 30, 49, 57, 61). A p21-like protein, Xic1, which might couple PCNA to cyclin E/Cdk2, was recently identified in egg extracts (50); however, proteins such as p107, p130, and E2F, involved in transcriptional regulation, do not interact with Cdk2 in the early embryo (42). Since no transcription occurs before the midblastula transition, it is likely that most of the cyclin E-Cdk2 complexes stored in oocytes will contribute directly to DNA replication. This would explain the quantitative difference that we have observed when analyzing the retention of cyclin E-Cdk2 from Xenopus or human extracts on E1 beads. The biological significance of the interaction of E1 with cyclin E-Cdk2 was difficult to study with the previously used in vitro replication assays carried out with cytosolic extracts from human or mouse cells. Indeed, the only E1 protein active in these replication assays was a baculovirus-produced E1 protein, which was already phosphorylated and furthermore tightly associated with a kinase activity from insect cells, possibly an insect cyclin-dependent kinase. None of our preparations of E. coli E1 protein exhibited a significant replication activity. In addition, as we previously reported, the baculovirus-produced E1 helicase, once purified, exhibits a highly unstable replicative activity (7). We have therefore developed another in vitro replication system that is E1 dependent, using Xenopus egg extracts, which has the strong advantage of providing a homologous in vitro system to both express E1 and analyze its activity.
The level of E1 protein translated from synthetic E1 mRNAs in Xenopus egg extracts is less than 1 ng/μl, and we can estimate that 30 to 50% of the synthesized E1 molecules are usually engaged in complexes with endogenous cyclin E-Cdk2. By immunodepleting most of cyclin E-Cdk2 complexes from translation and replication egg extracts and complementing them with reconstituted E1-cyclin E-Cdk2 complexes, we have shown that the association of E1 with cyclin E-Cdk2 is essential for the E1-dependent replication of double-stranded plasmids containing the BPV origin of replication. We show that the viral helicase E1 interacts with cyclin E-Cdk2 with very high affinity since E1 cannot be dissociated from active cyclin E-Cdk2 kinase complex or require high salt concentrations to dissociate from an inactive kinase complex. The strongest binding occurs with active kinase complex, which accumulates precisely at the G1/S transition during the somatic cell cycle (13, 24). Thus, the protection or sequestration of E1, before the onset of DNA replication, might be one of the functions ensured by the cyclin E-Cdk2 complex. Our preliminary data support this hypothesis, as the only radiolabled E1 molecules recovered after incubation in a fresh Xenopus egg extract are those associated with cyclin E-Cdk2. This would explain the good correlation between the level of E1-cyclin E-Cdk2 complexes and the level of in vitro replication. Interestingly, in a stable E1-expressing cell line, intracellular E1 levels were found to be minimal in G0 and G1 phases and to further increase as cells progressed through G1 to G2 phase (2). Such a cell cycle-dependent fluctuation of E1 levels could thus be correlated with the appearance of cyclin E-Cdk2 in late G1 and the association of stable E1-cyclin E-Cdk2 complexes.
In addition to its tight association with cyclin E-Cdk2, E1 is also specifically phosphorylated by the kinase in Xenopus egg extracts. A number of studies carried out with a cell-free system from Xenopus egg extracts have shown that activation of DNA replication at S phase is dependent on Cdk2 kinase activity (4, 14, 15, 20, 22). Thus, one possible speculation is that phosphorylation by cyclin E-Cdk2 might directly activate the viral initiator. A precedent for activation of a viral initiator of replication by a Cdk is SV40 T antigen (35). T-antigen phosphorylation at threonine 124 is absolutely required for the replication of SV40 DNA both in vitro and in vivo (reviewed in reference 43). Among the different regions of sequence similarity between E1 and SV40 T antigen is the region including this Cdk phosphorylation site (11). The corresponding threonine 102 in E1 has been shown to be also phosphorylated by a cell cycle-dependent kinase, Cdk1, both in vitro and in insect cells. However, a BPV genome with a mutation of this threonine to isoleucine still replicated in transient replication assays, suggesting that phosphorylation at this site is not critical (29). E1 contains two additional putative Cdk phosphorylation sites, threonine 126 and serine 283, which were not phosphorylated by the mitotic kinase. Indeed, when an E1 protein is mutated at threonine 102, it is no longer a substrate for Cdk1 in vitro. We have shown that cyclin E-Cdk2 phosphorylates E1 both on threonine and serine in vitro. This difference is of particular interest as it might reflect the specificity of these two Cdks which regulate different phases of the cell cycle. It is thus still possible that phosphorylation of E1 at threonine 126 or/and serine 283 by cyclin E-Cdk2 directly regulates E1’s function in replication. Another possibility is that the highly stable E1-cyclin E-Cdk2 association functions to recruit cyclin E-Cdk2 to the viral origin of replication, ensuring localized phosphorylation of other replication proteins. Functional analysis of E1 Cdk phosphorylation site mutants should help to resolve this issue. E1 has been shown to be phosphorylated at numerous sites in vivo (29), and our data show also that cyclin E-Cdk2 is not the only kinase that phosphorylates E1 in interphase Xenopus egg extract. Serine 109 of E1 was recently identified as a target for phosphate addition in vivo and was shown to be phosphorylated by protein kinase A and protein kinase C in vitro. Interestingly, phosphorylation at serine 109 appears to regulate negatively BPV DNA replication in vivo in a transient replication assay (60). It is therefore likely that the replication functions of E1 are both positively and negatively regulated by phosphorylation.
It is now clearly established that the initiation of replication in Xenopus egg extracts is dependent on template DNA being assembled into a nuclear structure. For this reason, the implication of cyclin E-Cdk2 in E1-dependent DNA replication was carried out with free-membrane cytosol in which the specificity of DNA replication could be unambiguously ascribed to the added viral helicase and not to Xenopus factors involved in the initiation of replication. The demonstration that the BPV E1 helicase can efficiently associate and cooperate with the Xenopus factors provides the first opportunity to further investigate the E1-dependent replication of BPV plasmids in reconstituted nuclei rather than in cytosolic extracts. This should allow us to address the question of the mechanism of replication control in a more relevant in vitro system, since regulation mechanisms active in controlling DNA replication in egg extracts require a nuclear envelope (3, 21). The recent study showing that degradation of Xic1, an inhibitor of cyclin E-Cdk2, depends on its nuclear localization is a good example of such a requirement (59).
In conclusion, the present work identifies BPV helicase E1 as a highly potential replicative target of cyclin E-Cdk2 kinase. Whatever its precise effect, the association of E1 with cyclin E-Cdk2 provides the first insight into the mechanism leading to the activation of the BPV origin at a specific time in the cell cycle. The interaction between viral replicative helicase and this cell cycle-regulatory protein kinase may represent a key event in the control of papillomavirus replication.
ACKNOWLEDGMENTS
We thank all members of the Dorée laboratory for scientific advice and discussions, especially D. Fesquet for helpful suggestions concerning the use of the translation system from Xenopus eggs and N. Morin for assistance in the preparation of extracts from Xenopus eggs. We acknowledge the contribution of J. P. Capony, who kindly performed the phosphoamino acid analysis for us. We are particularly grateful to M. C. Morris and M. Dorée for critical reading of the manuscript.
This work was supported by an ATIPE from the CNRS and by a grant from the Association pour la Recherche sur le Cancer (grant 5042 to C.B.-A.).
Footnotes
Dedicated to the memory of Jean-Claude Cavadore.
REFERENCES
- 1.Androphy E J. Molecular biology of human papillomavirus infection and oncogenesis. J Invest Dermatol. 1994;103:248–256. doi: 10.1111/1523-1747.ep12393230. [DOI] [PubMed] [Google Scholar]
- 2.Belyavskyi M, Miller J, Wilson V G. The bovine papillomavirus E1 protein alters the host cell cycle and growth properties. Virology. 1994;204:132–143. doi: 10.1006/viro.1994.1517. [DOI] [PubMed] [Google Scholar]
- 3.Blow J J, Laskey R A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- 4.Blow J J, Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990;62:855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- 5.Blow J J, Sleeman A M. Replication of purified DNA in Xenopus egg extracts is dependent on nuclear assembly. J Cell Sci. 1990;95:383–391. doi: 10.1242/jcs.95.3.383. [DOI] [PubMed] [Google Scholar]
- 6.Bonne-Andrea C, Santucci S, Clertant P. Bovine papillomavirus E1 protein can, by itself, efficiently drive multiple rounds of DNA synthesis in vitro. J Virol. 1995;69:3201–3205. doi: 10.1128/jvi.69.5.3201-3205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonne-Andrea C, Santucci S, Clertant P, Tillier F. Bovine papillomavirus E1 binds specifically DNA polymerase α but not replication protein A. J Virol. 1995;69:2341–2350. doi: 10.1128/jvi.69.4.2341-2350.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonne-Andrea C, Tillier F, McShan G D, Wilson V G, Clertant P. Bovine papillomavirus type 1 DNA replication: the transcriptional activator E2 acts in vitro as a specificity factor. J Virol. 1997;71:6805–6815. doi: 10.1128/jvi.71.9.6805-6815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevalier S, Couturier A, Chartrain I, Le Guellec R, Beckhelling C, Le Guellec K, Philippe M, Ford C C. Xenopus cyclin E, a nuclear phosphoprotein, accumulates when oocytes gain the ability to initiate DNA replication. J Cell Sci. 1996;109:1173–1184. doi: 10.1242/jcs.109.6.1173. [DOI] [PubMed] [Google Scholar]
- 10.Claudio P P, De Luca A, Howard C M, Baldi A, Firpo E J, Koff A, Paggi M G, Giordano A. Functional analysis of pRb2/p130 interaction with cyclins. Cancer Res. 1996;56:2003–2008. [PubMed] [Google Scholar]
- 11.Clertant P, Seif I. A common function for polyoma virus large T and papillomavirus E1 proteins? Nature. 1984;311:276–279. doi: 10.1038/311276a0. [DOI] [PubMed] [Google Scholar]
- 12.Connell-Crowley L, Solomon M J, Wei N, Harper J W. Phosphorylation independent activation of human cyclin-dependent kinase 2 by cyclin A in vitro. Mol Biol Cell. 1993;4:79–92. doi: 10.1091/mbc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulic V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 14.Fang F, Newport J. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991;66:731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- 15.Fang F, Newport J. Distinct roles of cdk2 and cdc2 in RPA phosphorylation during the cell cycle. J Cell Sci. 1993;106:983–994. doi: 10.1242/jcs.106.3.983. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert D M, Cohen S M. Bovine papillomavirus plasmids replicate randomly in mouse fibroblasts throughout S phase of the cell cycle. Cell. 1987;50:59–68. doi: 10.1016/0092-8674(87)90662-3. [DOI] [PubMed] [Google Scholar]
- 17.Griep A E, Lambert P F. Role of papillomavirus oncogenes in human cervical cancer: transgenic animal studies. Proc Soc Exp Biol Med. 1994;206:24–34. doi: 10.3181/00379727-206-43720a. [DOI] [PubMed] [Google Scholar]
- 18.Gu V, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannon G J, Demetrick D, Beach D. Isolation of Rb-related p130 through its interaction with Cdk2 and cyclins. Genes Dev. 1993;7:2378–2391. doi: 10.1101/gad.7.12a.2378. [DOI] [PubMed] [Google Scholar]
- 20.Hua X H, Newport J. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J Cell Biol. 1998;140:271–281. doi: 10.1083/jcb.140.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchison C J, Cox R, Ford C C. The control of DNA replication in a cell-free extract of Xenopus eggs that recapitulates a basic cell cycle in vitro. Development. 1988;103:553–566. doi: 10.1242/dev.103.3.553. [DOI] [PubMed] [Google Scholar]
- 22.Jackson P K, Chevalier S, Philippe M, Kirschner M W. Early events in DNA replication require cyclin E and are blocked by p21cip1. J Cell Biol. 1995;130:755–769. doi: 10.1083/jcb.130.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitchener H C. The role of human papillomavirus in the genesis of cervical cancer. Cancer Treat Res. 1994;70:29–41. doi: 10.1007/978-1-4615-2598-1_3. [DOI] [PubMed] [Google Scholar]
- 24.Koff A, Giordano A, Desai D, Yamashita K, Harper J W, Elledge S, Nishimoto T, Morgan D O, Franza R B, Roberts J M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 25.Laimins L A. The biology of human papillomavirus: from warts to cancer. Infect Agents Dis. 1993;2:74–86. [PubMed] [Google Scholar]
- 26.Law M F, Lowy D R, Dvoretzky I, Howley P M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci USA. 1981;78:2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees E, Faha B, Dulic V, Reed S I, Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct maner. Genes Dev. 1992;6:1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- 28.Lemaire P, Garret N, Gurdon J B. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 29.Lentz M R, Pak D, Mohr I, Botchan M R. The E1 replication protein of bovine papillomavirus type 1 contains an extended nuclear localization signal that includes a p34cdc2 phosphorylation site. J Virol. 1993;67:1414–1423. doi: 10.1128/jvi.67.3.1414-1423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Graham C, Lacy S, Duncan A M V, Whyte P. The adenovirus E1A-associated p130-kD protein is encoded by a member of retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993;7:2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- 31.Lohka M J, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic contents. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- 32.MacPherson P, Thorner L, Parker L M, Botchan M. The bovine papillomavirus E1 protein has ATPase activity essential to viral DNA replication and efficient transformation in cells. Virology. 1994;204:403–408. doi: 10.1006/viro.1994.1544. [DOI] [PubMed] [Google Scholar]
- 33.Matthews G. Cell biology: a laboratory handbook. New York, N.Y: Academic Press, Inc.; 1994. pp. 131–139. [Google Scholar]
- 34.Matthews G, Coleman A. A highly efficient, cell-free translation/translocation system prepared from Xenopus eggs. Nucleic Acids Res. 1991;19:6405–6412. doi: 10.1093/nar/19.23.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McVey D, Brizuela L, Mohr I, Marshak D R, Gluzman Y, Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989;341:503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- 36.Mecsas J, Sugden B. Replication of plasmids derived from bovine papillomavirus type 1 and Epstein-Barr virus in cells in culture. Annu Rev Cell Biol. 1987;3:87–108. doi: 10.1146/annurev.cb.03.110187.000511. [DOI] [PubMed] [Google Scholar]
- 37.Melendy T, Sedman J, Stenlund A. Cellular factors required for papillomavirus DNA replication. J Virol. 1995;69:7857–7867. doi: 10.1128/jvi.69.12.7857-7867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller F, Seo Y S, Hurwitz J. Replication of bovine papillomavirus type 1 origin-containing DNA in crude extracts and with purified proteins. J Biol Chem. 1994;269:17086–17094. [PubMed] [Google Scholar]
- 39.Murray A, Kirschner M. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 40.Newport J. Nuclear reconstitution in vitro: stages of assembly around protein free DNA. Cell. 1987;48:205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- 41.Paris J, Leplatois P, Nurse P. Study of the higher eukaryotic gene function CDK2 using fission yeast. J Cell Sci. 1994;107:615–623. doi: 10.1242/jcs.107.3.615. [DOI] [PubMed] [Google Scholar]
- 42.Philpott A, Friend S H. E2F and its developmental regulation in Xenopus laevis. Mol Cell Biol. 1994;14:5000–5009. doi: 10.1128/mcb.14.7.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prives C. The replication functions of SV40 T antigen are regulated by phosphorylation. Cell. 1990;61:735–738. doi: 10.1016/0092-8674(90)90179-i. [DOI] [PubMed] [Google Scholar]
- 44.Rempel R E, Sleight S B, Maller J L. Maternal Xenopus Cdk2-cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- 45.Santucci S, Androphy E J, Bonne-Andrea C, Clertant P. Proteins encoded by the bovine papillomavirus E1 open reading frame: expression in heterologous systems and in virally transformed cells. J Virol. 1990;64:6027–6039. doi: 10.1128/jvi.64.12.6027-6039.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santucci S, Bonne-Andrea C, Clertant P. Bovine papillomavirus type 1 E1 ATPase activity does not depend on binding to DNA nor to viral E2 protein. J Gen Virol. 1995;76:1129–1140. doi: 10.1099/0022-1317-76-5-1129. [DOI] [PubMed] [Google Scholar]
- 47.Seo Y S, Müller F, Lusky M, Hurwitz J. Bovine papillomavirus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc Natl Acad Sci USA. 1993;90:702–706. doi: 10.1073/pnas.90.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheehan M A, Mills A D, Sleeman A M, Laskey R A, Blow J J. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988;106:1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 50.Su J Y, Rempel R E, Erikson E, Maller J L. Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor p27 Xic1. Proc Natl Acad Sci USA. 1995;92:10187–10191. doi: 10.1073/pnas.92.22.10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun S, Thorner L, Lentz M, MacPherson P, Botchan M. Identification of 68-kilodalton nuclear ATP-binding phosphoprotein encoded by papillomavirus type 1. J Virol. 1990;64:5093–5105. doi: 10.1128/jvi.64.10.5093-5105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundin O, Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980;21:103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- 53.Sundin O, Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981;25:659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- 54.Ustav E, Ustav M, Szymanski P, Stenlund A. The bovine papillomavirus origin of replication requires a binding site for the E2 transcriptional activator. Proc Natl Acad Sci USA. 1993;90:898–902. doi: 10.1073/pnas.90.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ustav M, Ustav E, Szymanski P, Stenlund A. Identification of the origin of replication of bovine papillomavirus and characterization of the viral origin recognition factor E1. EMBO J. 1991;10:4321–4329. doi: 10.1002/j.1460-2075.1991.tb05010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao Z X, Ginsberg D, Ewen M, Livingston D M. Regulation of the retinoblastoma protein-related protein p107 by G1 cyclin-associated kinases. Proc Natl Acad Sci USA. 1996;93:4633–4637. doi: 10.1073/pnas.93.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang L, Mohr I, Fouts E, Lim D A, Nohaile M, Botchan M. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 1993;90:5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yew P R, Kirschner M W. Proteolysis and DNA replication: the CDC34 requirement in the Xenopus egg cell cycle. Science. 1997;277:1672–1675. doi: 10.1126/science.277.5332.1672. [DOI] [PubMed] [Google Scholar]
- 60.Zanardi T A, Stanley C M, Saville B M, Spacek S M, Lentz M R. Modulation of bovine papillomavirus DNA replication by phosphorylation of the viral E1 protein. Virology. 1997;228:1–10. doi: 10.1006/viro.1996.8375. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]