Abstract
Introduction
Before being implemented in daily clinical routine, new production strategies for platelet concentrates (PCs) must be validated for their efficacy. Besides in vitro testing, the establishment of new methods requires the labeling of platelets for in vivo studies of platelets’ survival and recovery. Indocyanine green (ICG) is a Food and Drug Administration-approved near-infrared (NIR) fluorescent dye for diagnostic use in vivo, suitable for non-radioactive direct cell labeling of platelets.
Methods
Platelets from PCs in storage solutions with different plasma concentrations were labeled with ICG up to concentrations of 200 μm. Whole blood (WB) was used as an ex vivo matrix to monitor the labeling stability of ICG-labeled platelets. The impact of labeling processes was assessed by the quantification of CD62P expression and PAC-1 binding as platelet function markers. Platelet aggregation was analyzed by light transmission aggregometry. ICG-labeling efficiency and stability of platelets were determined by flow cytometry.
Results
Platelets from PCs could be successfully labeled with 10 μm ICG after 1 and 4 days of storage. The best labeling efficiency of 99.8% ± 0.1% (immediately after labeling) and 81% ± 6.2% (after 24 h incubation with WB) was achieved by plasma replacement by 100% platelet additive solution for the labeling process. Since the washing process slightly impaired platelet function, ICG labeling itself did not affect platelets. Immediately after the ICG-labeling process, plasma was re-added, resulting in a recovered platelet function.
Conclusion
We developed a Good Manufacturing Practice compatible protocol for ICG fluorescent platelet labeling suitable for survival and recovery studies in vivo as a non-radioactive labeling alternative.
Keywords: Platelet labeling, Cell tracking, Indocyanine green, Platelets, Platelet concentrate
Plain Language Summary
We developed a simple, reproducible method according to Good Manufacturing Practice guidelines for labeling of platelets from platelet concentrates (PCs) with indocyanine green (ICG) as a non-radioactive alternative. PCs are medicinal drugs that are transfused to prevent or treat bleeding. They consist of the blood cells’ platelets which are responsible for clotting processes in the body. Manufacturing procedures of PCs are continuously refined, and for in vivo testing, these platelets have to be labeled to track and to distinguish them from proband’s or patient’s own cells. Radioactive labeling, for a long time the gold standard for cell labeling, is no longer accepted. ICG is a fluorescent dye approved by the drug authorities and already used for diagnostic purposes in humans. We used ICG to label platelets from PCs. With our method, we achieved a labeling efficiency of 99.8%. We used whole blood (WB) as an ex vivo matrix to monitor the labeling stability of ICG-labeled platelets. After the addition of ICG-labeled platelets to WB, the labeling efficiency decreased to 81% after 24 h. However, we were still able to distinguish the ICG-labeled platelets from the WB platelets. We could also show that platelet function was not impaired by the labeling processes. The good tolerance of ICG indicates a short path to clinical application in healthy volunteers and investigations of novel PC-manufacturing procedures. As a read-out system, flow cytometry systems equipped with NIR lasers and filters could offer the possibility of rapid visualization, cell tracking, re-isolation, and ex vivo studies.
Introduction
Human platelets play a key role in hemostasis [1]. Platelet concentrates (PCs) are an essential blood product to treat patients with severe thrombocytopenia, e.g., after surgery, trauma, or hypoproliferative thrombocytopenia after chemotherapy [2]. During preparation and storage of PCs, platelet storage lesions may occur [3, 4]. Quality of PCs is typically assessed by in vitro studies and clinical trials [5–7]. For in vivo assessment of PCs, platelets obtained from healthy volunteers have been used for survival and recovery studies [8]. This requires labeling of transfused platelets to allow their discrimination from endogenous platelets. Radioactive labeling by chromium (51-Cr) and indium (111-In) was considered the gold standard for in vivo assessment of platelets after PC transfusion for a long period [5, 8]. However, exposure of patients or healthy volunteers to radioactive isotopes is no longer accepted in the European Union and strictly regulated [9]. Besides radiation exposure, radioactive cell labeling is limited due to impaired platelet function after labeling and the difficulty of producing radioactive isotopes in a Good Manufacturing Practice (GMP)-compliant manner [8, 10]. Highly required alternative approaches have been developed such as biotinylation of platelets. Biotinylated platelets have already been used in clinical trials [11, 12]. One limitation of this method is the impact on platelet function [7, 13]. Although labeling platelets using magnetic nanoparticles showed promising results, it is currently limited since the particle containing drug preparation Resovist® used as the approved labeling agent has been taken from the market [14]. Determination of different human leukocyte antigen markers or mitochondrial DNA was also used to track platelets [15, 16], but can only be used in an allogeneic setting [8].
A new perspective for platelet labeling is the use of fluorescent dyes [17] as an optical method of direct labeling [18]. Indocyanine green (ICG, Diagnostic Green, IR-125, Verdye®) is the only near-infrared (NIR) fluorescent dye approved by the Food and Drug Administration (FDA) and by national authorities in several European countries for in vivo use as a diagnostic agent (e.g., SPY AGENT Green® [19] or Verdye®). It is used in micro-circulatory evaluation of liver function or retinal angiography [20, 21]. We evaluated a method to label platelets from pooled and apheresis PCs with ICG as a non-radioactive labeling method which could be used for recovery and survival studies.
Methods
Manufacturing of PCs
Pooled PCs from Whole Blood Buffy Coats
Whole blood (WB) was collected from healthy donors according to the German guidelines for hemotherapy with written informed consent. Pooled PCs from WB buffy coats were produced according to GMP standard. In brief, after centrifugation (4,000 g for 10 min, Roto Silenta 630 RS, Hettich GmbH, Germany), WB in citrate phosphate dextrose solution (CPD, Macopharma, France) was separated into red cell concentrate, plasma, and buffy coat (day 0, Macopress Smart, Macopharma). Buffy coats of four blood group identical donations were pooled and 250 mL platelet additive solution (PAS) E (SSP+, Macopharma) was added (day 1). Platelets were separated from residual red blood cells by centrifugation (700 g for 4 min, Roto Silenta RS 630) and leukocyte depleted (LEUCOFLEX® LXT Filter, Macopharma), resulting in a final plasma concentration of 30%. Pooled PCs were stored agitated at room temperature (RT, Linear Platelet Reciprocator, Type LPR1, Melco Engineering Corp., Glendale, CA, USA).
Apheresis PCs
PCs were collected from healthy volunteers using an automated apheresis blood collection system (COM.TEC, Fresenius GmbH, Germany) in 100% plasma. Apheresis PCs were stored agitated at RT before usage.
Platelet Labeling with ICG
In the first study part, platelets from apheresis or pooled PCs in small sample tubes were labeled with ICG dissolved in water for injection (5 mg/mL, according to manufacturer’s instruction of Verdye®) to final ICG concentrations of 5–200 µm. Platelets and ICG were allowed to incubate for 1 h at RT under constant agitation without subsequent removal of ICG.
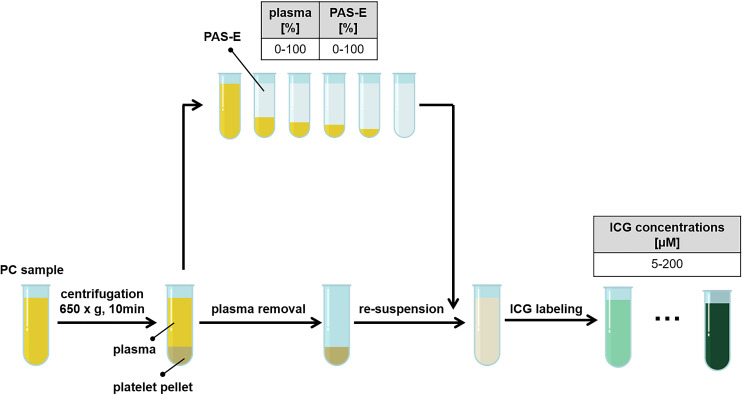
The impact of plasma content and different ICG concentrations on platelet labeling was examined using apheresis PCs (100% plasma) in small samples. After centrifugation (650 g for 10 min, ROTANA 460 R, Hettich, Germany), the supernatant plasma was completely removed. Platelets were resuspended in plasma and PAS-E with plasma concentrations of 0–100% (Fig. 1). ICG was added to the PC samples in increasing concentrations (5–200 µm).
Fig. 1.
Preparation scheme of platelets from PCs with different plasma and PAS-E ratios, followed by labeling with ICG in concentrations of 5–200 µm. ICG, indocyanine green; PAS-E, platelet additive solution E; PC, platelet concentrate.
Labeling Efficiency
All samples were diluted 1:50 in 0.5% paraformaldehyde/phosphate buffered saline (MORPHISTO Ltd., Germany/PAN-Biotech, Germany) and analyzed for the ICG signal determined as mean fluorescence intensity (MFI) by flow cytometry (CytoFlex S, Beckman Coulter, Brea, CA, USA, excitation: 808 nm, filter set: 885/40 nm). For gating, all platelets were stained using a mouse anti-human CD61-Phytoerythrin (PE, Beckman Coulter, USA) monoclonal antibody. In the CD61-PE+ population, the ratio of ICG-positive platelets to ICG-negative platelets was quantified.
Labeling Stability in WB
We determined labeling stability of ICG-labeled platelets in WB by adding these to WB in different ratios. WB was collected from healthy volunteers in 10 mL sample tubes, anticoagulated with 10 μL hirudin (5,400 U/mL, REVASC, Canyon Pharm., USA), and stored at 37 °C (Binder, Germany). To differentiate WB platelets from added ICG-labeled platelets, the WB platelets were stained using a mouse anti-human CD61-AlexaFluor (AF) 647 (Biolegend, USA) monoclonal antibody. In parallel, ICG-labeled platelets were stained with anti-human CD61-PE as described above.
A sample of ICG-labeled platelets from a PC was added to WB in a volume ratio of 1:17 or 1:68 and stored at 37 °C. The 1:17 volume ratio corresponds to the addition of one apheresis PC (300 mL) to an average human blood volume (5 L WB). Samples were taken over 24 h.
A potential leakage of ICG from ICG-labeled platelets and the resulting labeling of WB platelets were identified via flow cytometry. The ICG signal was assessed for the CD61-PE+ (ICG-labeled platelets) and the CD61-AF647+ (WB platelets)-gated platelets fractions (online suppl. Fig. S1; for all online suppl. material, see https://doi.org/10.1159/000533623). Different antibody staining of these two platelet fractions allowed distinguishing a possible ICG loss of the original ICG-labeled platelet population and potential ICG uptake by the WB platelet population.
Impact of ICG-Labeling on Platelet Function
The impact of ICG labeling on platelet function was analyzed by the determination of CD62P expression and PAC-1 binding to glycoprotein IIbIIIa by flow cytometry before and after addition of thrombin receptor-activating peptide 6 (TRAP-6, Haemochrom, Germany), and by the evaluation of platelet aggregation ability after addition of collagen as an inductor by light transmission aggregometry (LTA). CD62P expression and PAC-1 binding was analyzed using 3 × 108/mL platelets, incubated for 10 min at RT with 20 μm TRAP-6 or phosphate buffered saline buffer (PBS, without Ca2+, Mg2+; pH 7.2) as negative control (displayed as fold change increase). The increase of CD62P expression or PAC-1 binding of platelet events was determined by flow cytometry using a mouse anti-human CD62P-AF647 (Biolegend, USA) monoclonal antibody and a mouse anti-human PAC-1 fluorescein isothiocyanate (FITC, BD Pharmingen, USA) monoclonal antibody.
LTA was performed with 180 μL platelet-rich plasma incubated with 20 μL collagen (8 μg/mL, Takeda Pharmaceutical, Japan). Platelet aggregation was monitored over 6 min using a 4-channel aggregometer (LABiTec, Ahrensburg, Germany) and the maximum aggregation was documented.
Upscaling of the ICG-Labeling Method to Pooled Platelet Concentrate Units
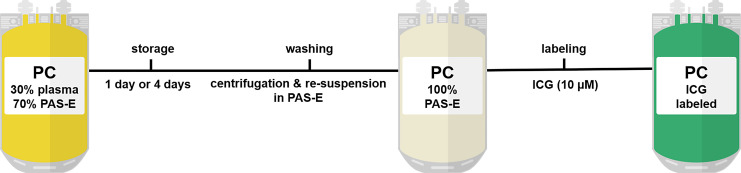
Pooled PCs (30% plasma) were stored for 1 or 4 days. To further reduce the plasma concentration in PCs for optimal ICG uptake, acid citrate dextrose solution A (ACD-A) was added (10% of the PC volume), PCs were then centrifuged (2,000 g for 10 min, Roto Silenta RS 630) and rested for 15 min. Complete supernatant was separated followed by the addition of 250 mL of PAS-E. After 1 h resting, platelets were resuspended and stored under agitation for 15 min at RT. ICG was injected into the PC bags via a sampling coupler (Macopharma), resulting in a final ICG concentration of 10 μm. ICG-labeled PCs were stored under agitation for 1 h at RT (Fig. 2). All procedures were carried out under EU-GMP conditions in a clean room facility.
Fig. 2.
Scheme of manufacturing procedure of ICG-labeled PCs in 100% PAS-E. ICG, indocyanine green; PAS-E, platelet additive solution E; PC, platelet concentrate.
After the labeling process, plasma was re-added to ICG-labeled PC units to a final concentration of 30% to mimic the impact of plasma after transfusion. Samples were taken at each manufacturing step (before and after washing, after labeling) and one and 24 h after final plasma addition. Labeling efficiency and platelet reactivity were determined by flow cytometry and LTA as described above.
Statistical Data Evaluation
All experiments were repeated with n ≥ 3. Sample sizes are indicated in each figure. Values are shown as mean ± standard deviation. Normal distribution was tested by Shapiro-Wilk test. Nonparametric data were analyzed by Friedman test followed by Dunn’s multiple comparisons post hoc test. Parametric data were analyzed by repeated measures one-way ANOVA (one independent variable) or two-way ANOVA (two independent variables) followed by Tukey multiple comparisons post hoc test. p < 0.05 was considered as statistically significant with *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Labeling Procedure: Impact of Plasma and ICG Concentrations
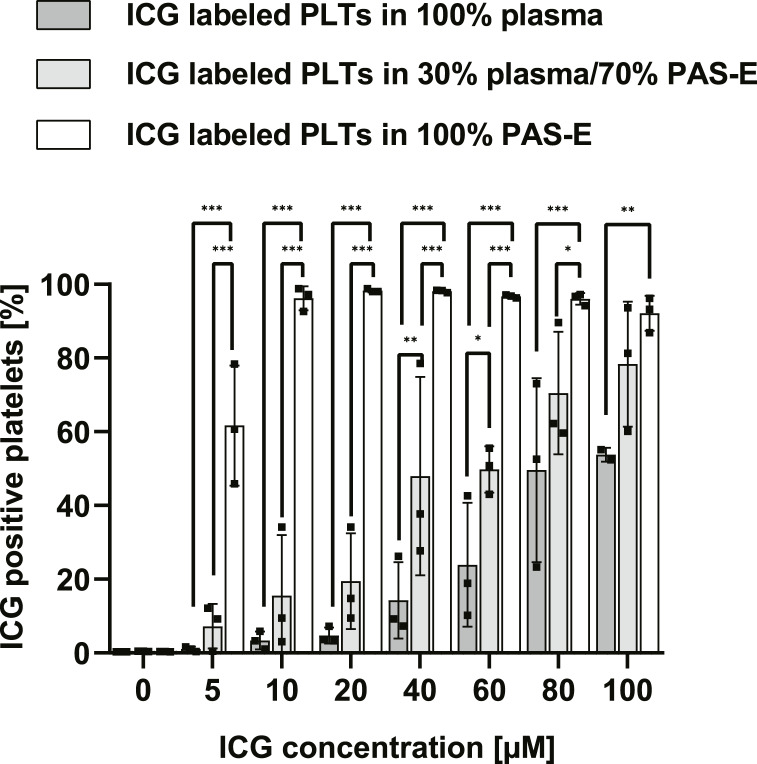
The percentage of ICG-positive platelets correlated positively with increasing ICG concentrations in all preparations and inversely with plasma concentration (Fig. 3). In 100% PAS-E and 10 μm ICG platelet gating for ICG was positive for 96.2% ± 3.2% of the platelets (vs. 30% plasma: 15.5% ± 16.4% vs. 100% plasma: 3.4% ± 2.4%; n = 3, p < 0.001).
Fig. 3.
Labeling efficiency of platelets depends on ICG concentration and plasma concentration in the platelet storage solution (PAS-E). ICG-positive platelets after labeling in PAS-E containing plasma concentrations from 0 to 100% and ICG concentrations from 0 to 100 µm. n = 3, mean ± SD. PLTs, platelets; ICG, indocyanine green; PAS-E, platelet additive solution E; SD, standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. Analyzed by two-way repeated measures ANOVA with multiple comparisons.
ICG-Labeled Platelets Added to WB as an ex vivo Matrix to Assess Labeling Stability
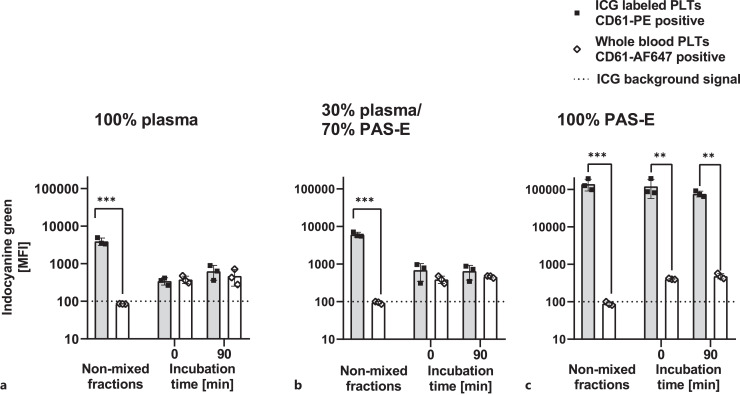
Platelets in PAS-E with increasing plasma concentrations were labeled with 50 μm ICG, stained with a CD61-PE antibody and subsequently added to WB. The WB platelets had been stained before using a CD61-AF647 antibody. Before addition, non-ICG-labeled platelets from WB did not emit infrared ICG signals (cut-off/background signal = 100 MFI). After addition of ICG-labeled platelets to WB, the ICG signal was measured immediately (0 min, t0) and after 90 min. At t0, only platelets labeled with ICG in 100% PAS-E showed distinguishable ICG signals compared to WB platelets (Fig. 4c, MFI ICG: 121,811 ± 64,702 vs. 406 ± 16, n = 3, p < 0.01). However, regardless of whether plasma was present in the buffer during ICG labeling, the ICG signal of WB platelets increased (from average MFIs of 90–400, Fig. 4a–c).
Fig. 4.
ICG signal of labeled platelets added to WB. ICG (50 μm final concentration) labeling of platelets in storage solutions with: (a) 100% plasma, (b) 30% plasma/70% PAS-E, (c) 100% PAS-E; MFI of ICG-labeled platelets (stained by CD61-PE) and WB platelets (stained by CD61-AF647) before and after the addition of WB during 90 min incubation time in a volume ratio of 1:17. (n = 3, mean ± SD). PLTs, platelets; MFI, mean fluorescence intensity; ICG, indocyanine green; PAS-E, platelet additive solution E; SD, standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. Analyzed by one-way repeated measures ANOVA with multiple comparisons.
Impact of ICG-Labeling Procedure on Platelet Function
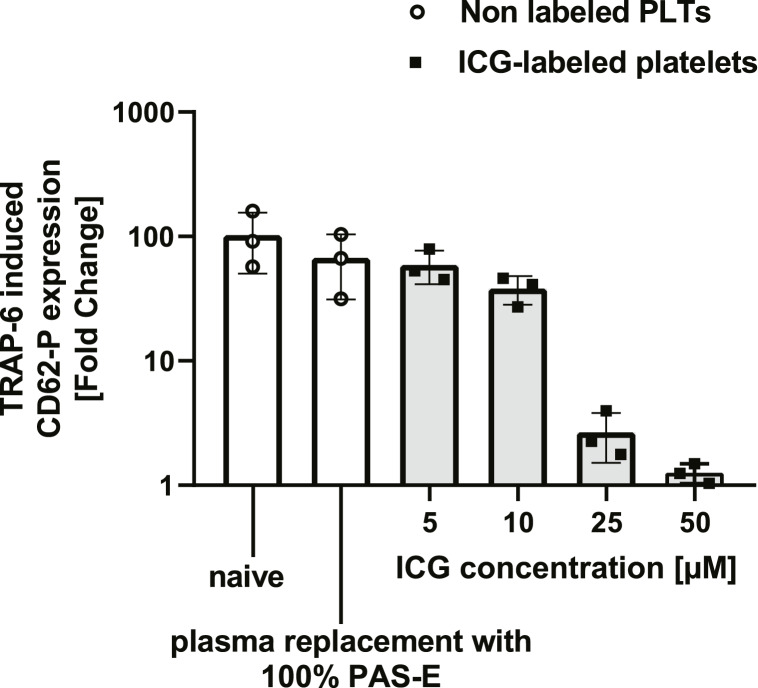
According to Figure 4c, the best labeling efficiency was achieved using 100% PAS-E during labeling procedure with 50 μm ICG. However, TRAP-6 induced platelet reactivity was reduced at ICG concentrations above 10 μm (Fig. 5). For that reason, we decided to use an ICG concentration of 10 μm to label platelets for upscaling the method to entire pooled PCs.
Fig. 5.
Platelet function after ICG labeling in 100% PAS-E is dependent on ICG concentration; fold change of CD62P after TRAP-6 induction (20 μm final concentration). (n = 3, mean ± SD). PLTs, platelets; MFI, mean fluorescence intensity; TRAP-6, thrombin receptor activator peptide 6; ICG, indocyanine green; PAS-E, platelet additive solution E; SD, standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. Analyzed by one-way repeated measures ANOVA with multiple comparisons.
Upscaling of the ICG-Labeling Method to Pooled Platelet Concentrate Units
In the second study part, we upscaled the method to label entire pooled PCs. Plasma was removed and replaced by PAS-E. After the ICG-labeling process, plasma was re-added to a final concentration of 30% to assess reversibility of platelet impairment due to plasma replacement.
We analyzed ICG-labeling efficiency and the impact on platelet function of the following washing and labeling steps: (1) before and (2) after removal of plasma, (3) immediately after labeling with ICG, (4) 1 h, and (5) 24 h after re-addition of 30% plasma.
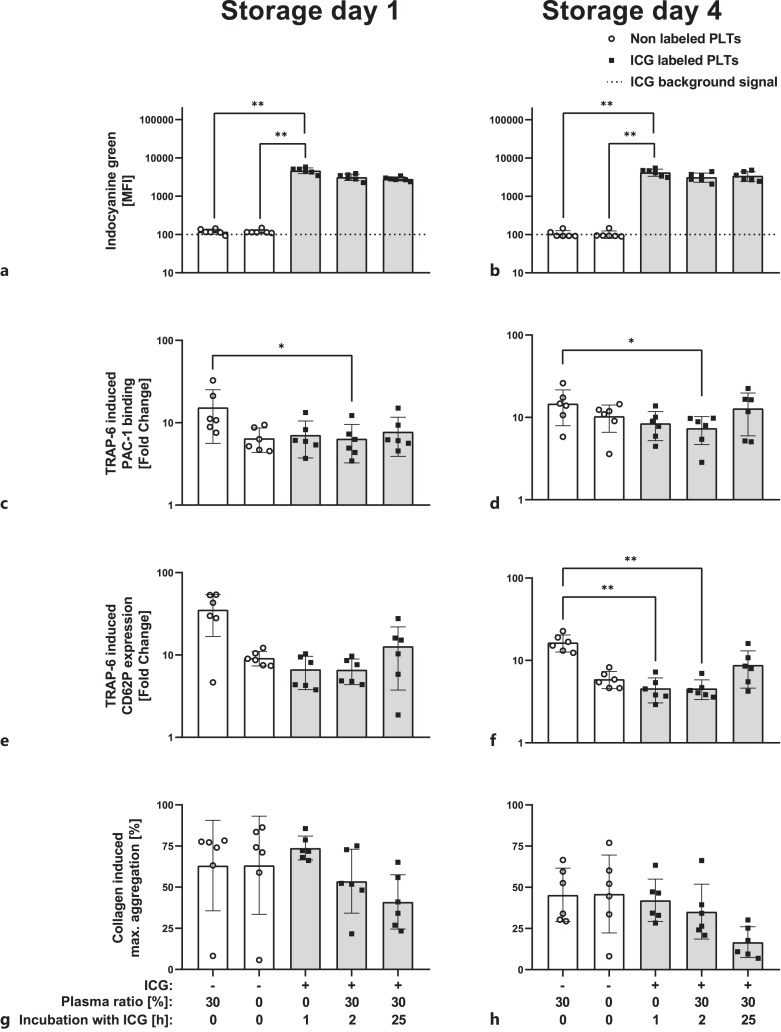
Labeled platelets in PCs showed a sufficient ICG signal that remains stable for at least 24 h (Fig. 6a, b). Platelets from PCs stored for one or 4 days before labeling presented an adequate labeling efficiency. Plasma removal of PCs led to minor impact on PAC-1 binding and CD62P expression after TRAP-6 induction (Fig. 6c–f and had no impact on collagen-induced platelet aggregation (Fig. 6g, h). ICG labeling had no impact on platelet function. Re-addition of 30% plasma to ICG-labeled platelets improved CD62P expression and PAC-1 binding after TRAP-6 addition (Fig. 6e, f).
Fig. 6.
Labeling efficiency and platelet function of platelet in fresh (storage day 1) or stored pooled PC (storage day 4) after ICG labeling (10 µM). Flow cytometric ICG signal in MFI (a, b); platelet function as PAC-1 binding after TRAP-6 (20 μM; c, d); CD62P expression after TRAP-6 (20 µM; e, f); and collagen (8 μg/mL) induced platelet aggregation (g, h). n = 6, mean ± SD. PLTs, platelets; MFI, mean fluorescence intensity; TRAP-6, thrombin receptor activator peptide 6; ICG, indocyanine green; PAS-E, platelet additive solution E; SD, standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. Analyzed by Friedman test with multiple comparisons.
Stability of Labeled Platelets from PCs in WB
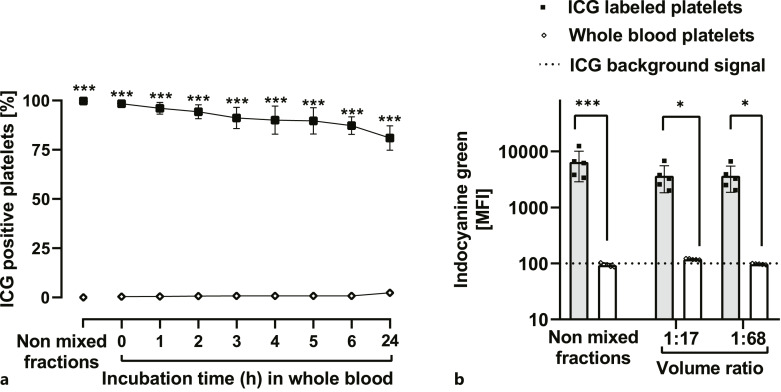
In WB, platelets showed stable ICG labeling with minimal leakage within 24 h (Fig. 7a). WB platelets did not take up ICG. ICG signals of labeled platelets and WB platelets were identical in volume ratios of 1:17 or 1:68 corresponding to 300 mL or 75 mL PC in 5 L WB, respectively (Fig. 7b).
Fig. 7.
Labeling stability of ICG-labeled platelets from pooled PC after addition to WB. a Percentage of ICG positive gated platelets before and after the addition to WB during 24 h incubation time in a volume ratio of 1:17. b ICG signal before and immediately after the addition to WB in a volume ratio of 1:17 and 1:68 (n = 6, mean ± SD). PLTs, platelets; MFI, mean fluorescence intensity; ICG, indocyanine green; PAS-E, platelet additive solution E; SD, standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. Analyzed by repeated measures ANOVA (a) and Friedman test (b) with multiple comparisons.
Discussion
We developed a method for labeling platelets from PCs with ICG, a fluorescent dye approved by FDA and European national authorities for in vivo application in humans. ICG has an excellent safety profile [22–26]. It is described as one of the least toxic agents administered to humans, only known for rare anaphylaxis [27]. ICG has good biodegradability and biocompatibility [13] and is rapidly metabolized by the liver and excreted in the bile [25, 28, 29]. ICG does not undergo extrahepatic elimination or enterohepatic circulation, is rarely uptaken in peripheral tissues like kidney, lungs, or placenta, and is not detectable in the urine [30–32]. It is one of the most commonly used clinical diagnostic NIR dyes. It can be applied without a carcinogenic, mutagenic, or phototoxic risk [33] or ionizing radiation as with radiolabeling. The normative quality parameters and the ICG labeling of PCs under GMP conditions as shown in the present study pave the way for in vivo application.
Dependent on the plasma concentration of the platelet storage solutions (0%, 30%, 100%), increasing labeling rates could be reached with ICG concentrations of 5–100 µm and 1 h of ICG-incubation. The lower the plasma concentration in the storage solution, the lower are the ICG concentrations sufficient for high labeling rates. In 100% PAS-E, a maximum labeling rate of 99.8% platelets with 10 μm ICG was achieved. Further reduction of the ICG concentration resulted in insufficient labeled platelets.
ICG offers high plasma protein binding and remains stable in human serum in vitro for several days. β-Apo-lipoprotein B acts as a main carrier (95%) [34], but ICG also binds strongly to other serum proteins (e.g., albumin, γ-globulin, and α-lipoprotein) [31, 35–37], preventing sufficient ICG uptake by platelets and impair the ICG-labeling stability. In plasma-free storage solutions, ICG is able to bind to hydrophobic structures like phospholipids in the platelet membrane, resulting in stable platelet labeling [38, 39].
Higher ICG concentrations up to 200 μm aimed to saturate binding partners in storage solutions containing plasma showed no advantage in ICG signal but decreased platelet reactivity. Those platelets also lost their ICG signal when incubated with WB (data not shown). This is consistent to previous studies describing aggregate formation [36], overlap of absorption and emission [40], or self-quenching for ICG at higher concentrations [37, 38, 41].
After finding the optimal labeling conditions, we scaled up the method to label platelets in entire PC units. To reduce the plasma concentration in PCs for the labeling process, removal of the plasma was required. ICG labeling was then performed in 100% PAS-E. Since it is well known that platelet function is reduced in storage solution without plasma [42, 43] we re-added 30% plasma (final concentration) after finishing the labeling procedure to mimic the impact of plasma after transfusion.
ICG-labeled PCs showed a labeling rate of 99.8% ± 0.1% platelets. The labeling process had only minor impact on platelet function: Platelet aggregation ability showed no significant differences between native PCs, 100% PAS-E PCs, and ICG-labeled PCs. Platelet reactivity measured by CD62P expression and PAC-1 binding was slightly reduced. Although ICG labeling could be optimized by the removal of plasma, the absence of glucose may lead to lower adenosine triphosphate levels and may result in increased apoptosis [44]. Subsequent addition of plasma was sufficient to restore platelet reactivity, also demonstrating that transfusion in patients of ICG-labeled platelets is most likely to recover platelet function in vivo.
In addition, we assessed the labeling stability of ICG-labeled platelets in WB as an ex vivo matrix. When incubated, we aimed to differentiate both platelet fractions based on their ICG signal. Platelets labeled with ICG in 30% or 100% plasma lost most of their ICG signal, while only platelets labeled in 100% PAS-E retained high labeling stability.
Platelets from ICG-labeled PC units were added to WB in a volume ratio of 1:17, corresponding to the transfusion of 300 mL PC to a mean WB volume of 5 L. After 24 h incubation with WB, we could recover 81% ± 6.2% of ICG-labeled platelets by flow cytometry. The loss of ICG-labeled platelets in WB may have technical reasons: (1) due to storage in WB ex vivo in a tube platelets can be activated and form aggregates that are recognized as a single event in flow cytometry; (2) for the differentiation between ICG-labeled platelets from PCs and WB platelets we had to stain both platelet population by two platelet surface antibodies with different fluorescent dyes. Incubation times of 24 h caused antibody dissociation from the platelet surface, followed by double-staining of platelets due to free CD61 antibodies. We did not consider double-stained platelets for quantification of the ICG signal, although they may belong to the ICG-labeled platelets from PCs. We recognize this as limitation. A subsequent study with healthy volunteers could clarify the signal loss in vivo.
To reduce the volume of ICG-labeled PCs that is required for survival studies, we analyzed the signal of ICG platelets that were added to WB in a ratio of 1:68 (corresponding to 75 mL PC in 5 L WB). This volume produced the same signal as the 1:17 ratio. An additional loss of the ICG signal during volume dilution was not observed, which makes the application of smaller amounts as an investigational medicinal product possible. When ICG-labeled PCs are applied, the ICG dose is much lower than given intravenously for diagnostic purpose: 9% for 300 mL and 2.3% for 75 mL of ICG-PC with regard to i.v. bolus application of Verdye® (37.5 mg for 75 kg body weight), respectively [21].
We developed a simple, reproducible method according to GMP guidelines for ICG labeling of platelets from PCs with ICG as a non-radioactive alternative. An ICG concentration of 10 μm was suitable for sufficient ICG signals with minimal impact in platelet reactivity. Using WB at 37°C simulating the natural environment of platelets as an ex vivo matrix as a medium, ICG labeling of platelets remains stable over a 24-h period. This may indicate successful ICG platelet-labeling stability in survival and recovery studies in vivo. ICG stability in serum and its non-targetability are commonly described challenges and are required to be tested in vivo [45, 46]. Here, we present a successful and stable ICG labeling of platelets in WB ex vivo as a proof of concept for clinical trials. An in vivo study with ICG-labeled platelets for systematic application for efficacy testing of novel PC manufacturing methods is currently being prepared.
The good tolerance of ICG indicates a short path to clinical application in healthy volunteers (autologous PCs from apheresis with subsequent transfusion after ICG labeling) and investigations of novel manufacturing procedures for PCs. As a read-out system, flow cytometry systems equipped with NIR lasers and filters could offer the possibility of rapid visualization, cell tracking, re-isolation, and ex vivo studies [47].
Statement of Ethics
Our research complies with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The Institutional Ethics Review Board of Universitätsmedizin Greifswald approved the study (BB 014/14). All blood donors gave written informed consent before donation according to German hemotherapy guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to declare.
Author Contributions
Johannes-Moritz von Behren, Konstanze Aurich, and Jan Wesche performed the experiments and analyzed the data. Konstanze Aurich and Andreas Greinacher designed the study. All authors wrote the manuscript.
Funding Statement
The authors have no funding sources to declare.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133–48. 10.2147/VHRM.S44469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wandt H, Schäfer-Eckart K, Greinacher A. Platelet transfusion in hematology, oncology and surgery. Deutsches Arzteblatt Int. 2014 Nov 28;111(48):809–15. 10.3238/arztebl.2014.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klinger MH. The storage lesion of platelets: ultrastructural and functional aspects. Ann Hematol. 1996 Sep;73(3):103–12. 10.1007/s002770050210. [DOI] [PubMed] [Google Scholar]
- 4. Ng MSY, Tung JP, Fraser JF. Platelet storage lesions: what more do we know now? Transfus Med Rev. 2018 Apr 17;32(3):144–54. 10.1016/j.tmrv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 5. Murphy S. Radiolabeling of PLTs to assess viability: a proposal for a standard. Transfusion. 2004 Jan;44(1):131–3. 10.1111/j.0041-1132.2003.00607.x. [DOI] [PubMed] [Google Scholar]
- 6. Arnold DM, Heddle NM, Kulczycky M, Carruthers J, Sigouin C, Blajchman MA. In vivo recovery and survival of apheresis and whole blood-derived platelets: a paired comparison in healthy volunteers. Transfusion. 2006 Feb;46(2):257–64. 10.1111/j.1537-2995.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 7. de Bruin S, van de Weerdt EK, Sijbrands D, Vlaar R, Gouwerok E, Biemond BJ, et al. Biotinylation of platelets for transfusion purposes a novel method to label platelets in a closed system. Transfusion. 2019 Sep;59(9):2964–73. 10.1111/trf.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Meer PF, Tomson B, Brand A. In vivo tracking of transfused platelets for recovery and survival studies: an appraisal of labeling methods. Transfus Apher Sci. 2010 Feb;42(1):53–61. 10.1016/j.transci.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9. Decristoforo C, Penuelas I, Patt M, Todde S. European regulations for the introduction of novel radiopharmaceuticals in the clinical setting. Q J Nucl Med Mol Imaging. 2017 Jun;61(2):135–44. 10.23736/S1824-4785.17.02965-X. [DOI] [PubMed] [Google Scholar]
- 10. van Wonderen SF, van Baarle FLF, de Bruin S, Peters AL, de Korte D, van Bruggen R, et al. Biotinylated platelets: a promising labeling technique? Transfus Med Rev. 2023 Apr;37(2):150719. 10.1016/j.tmrv.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 11. Stohlawetz P, Horvath M, Pernerstorfer T, Nguyen H, Vondrovec B, Robisch A, et al. Effects of nitric oxide on platelet activation during plateletpheresis and in vivo tracking of biotinylated platelets in humans. Transfusion. 1999 May;39(5):506–14. 10.1046/j.1537-2995.1999.39050506.x. [DOI] [PubMed] [Google Scholar]
- 12. Ravanat C, Pongérard A, Freund M, Heim V, Rudwill F, Ziessel C, et al. Human platelets labeled at two discrete biotin densities are functional in vitro and are detected in vivo in the murine circulation: a promising approach to monitor platelet survival in vivo in clinical research. Transfusion. 2021 May;61(5):1642–53. 10.1111/trf.16312. [DOI] [PubMed] [Google Scholar]
- 13. Grigoriadis G, Stewart AG. Albumin inhibits platelet-activating factor (PAF)-induced responses in platelets and macrophages: implications for the biologically active form of PAF. Br J Pharmacol. 1992 Sep;107(1):73–7. 10.1111/j.1476-5381.1992.tb14465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aurich K, Wesche J, Palankar R, Schluter R, Bakchoul T, Greinacher A. Magnetic nanoparticle labeling of human platelets from platelet concentrates for recovery and survival studies. ACS Appl Mater Interfaces. 2017 Oct 11;9(40):34666–73. 10.1021/acsami.7b10113. [DOI] [PubMed] [Google Scholar]
- 15. Hughes DL, Evans G, Metcalfe P, Goodall AH, Williamson LM. Tracking and characterisation of transfused platelets by two colour, whole blood flow cytometry. Br J Haematol. 2005 Sep;130(5):791–4. 10.1111/j.1365-2141.2005.05671.x. [DOI] [PubMed] [Google Scholar]
- 16. Doescher A, Petershofen EK, Hertenstein B, Kraemer D, Casper J, Schmidt JP, et al. Platelet recovery and survival measured in patients by quantitative polymerase chain reaction of mitochondrial DNA. Transfusion. 2015 Jan;55(1):55–63. 10.1111/trf.12778. [DOI] [PubMed] [Google Scholar]
- 17. Da Q, Derry PJ, Lam FW, Rumbaut RE. Fluorescent labeling of endogenous platelets for intravital microscopy: effects on platelet function. Microcirculation. 2018 Aug;25(6):e12457. 10.1111/micc.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011 Sep 27;8(11):677–88. 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 19. Food and Drug Administration . Multi-disciplinary review and evaluation SPY AGENT green. Available from: https://www.fda.gov/media/124115/download.11/07/2023. [Google Scholar]
- 20. Kogure K, Choromokos E. Infrared absorption angiography. J Appl Physiol. 1969 Jan;26(1):154–7. 10.1152/jappl.1969.26.1.154. [DOI] [PubMed] [Google Scholar]
- 21. Fachinformation Verdye(R) . Diagnostic green GmbH; 2016. [Google Scholar]
- 22. Benson RC, Kues HA. Absorption and fluorescence properties of cyanine dyes. J Chem Eng Data. 1977 Oct 1;22(4):379–83. 10.1021/je60075a020. [DOI] [Google Scholar]
- 23. Henschen S, Busse MW, Zisowsky S, Panning B. Determination of plasma volume and total blood volume using indocyanine green: a short review. J Med. 1993;24(1):10–27. [PubMed] [Google Scholar]
- 24. Regillo CD. The present role of indocyanine green angiography in ophthalmology. Curr Opin Ophthalmol. 1999 Jun;10(3):189–96. 10.1097/00055735-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 25. Burra P, Masier A. Dynamic tests to study liver function. Eur Rev Med Pharmacol Sci. 2004 Jan–Feb;8(1):19–21. [PubMed] [Google Scholar]
- 26. Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005 Jul–Sep;4(3):172–81. 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 27. Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003 Oct;7(5):626–34. 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 28. Ott P, Keiding S, Bass L. Intrinsic hepatic clearance of indocyanine green in the pig: dependence on plasma protein concentration. Eur J Clin Invest. 1992 May;22(5):347–57. 10.1111/j.1365-2362.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 29. Su MY, Lin DY, Sheen IS, Chu CM, Chiu CT, Liaw YF. Indocyanine green clearance test in non-cirrhotic hepatitis patients: a comparison and analysis between conventional blood sampling method and Finger Piece Monitoring method. Changgeng yi xue za zhi. 1999 Mar;22(1):17–23. [PubMed] [Google Scholar]
- 30. Wheeler HO, Cranston WI, Meltzer JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958 Oct;99(1):11–4. 10.3181/00379727-99-24229. [DOI] [PubMed] [Google Scholar]
- 31. Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39(4):592–600. 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fineman MS, Maguire JI, Fineman SW, Benson WE. Safety of indocyanine green angiography during pregnancy: a survey of the retina, macula, and vitreous societies. Arch Ophthalmol. 2001 Mar;119(3):353–5. 10.1001/archopht.119.3.353. [DOI] [PubMed] [Google Scholar]
- 33. Wei X, Runnels JM, Lin CP. Selective uptake of indocyanine green by reticulocytes in circulation. Invest Ophthalmol Vis Sci. 2003 Oct;44(10):4489–96. 10.1167/iovs.03-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoneya S, Saito T, Komatsu Y, Koyama I, Takahashi K, Duvoll-Young J. Binding properties of indocyanine green in human blood. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1286–90. [PubMed] [Google Scholar]
- 35. Muckle TJ. Plasma proteins binding of indocyanine green. Biochem Med. 1976 Feb;15(1):17–21. 10.1016/0006-2944(76)90069-7. [DOI] [PubMed] [Google Scholar]
- 36. Mordon S, Devoisselle JM, Soulie-Begu S, Desmettre T. Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res. 1998 Mar;55(2):146–52. 10.1006/mvre.1998.2068. [DOI] [PubMed] [Google Scholar]
- 37. Pansare VJ, Faenza WJ, Lu H, Adamson DH, Prud’homme RK. Formulation of long-wavelength indocyanine green nanocarriers. J Biomed Opt. 2017 Sep;22(9):1–11. 10.1117/1.JBO.22.9.096007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kraft JC, Ho RJ. Interactions of indocyanine green and lipid in enhancing near-infrared fluorescence properties: the basis for near-infrared imaging in vivo. Biochemistry. 2014 Mar 4;53(8):1275–83. 10.1021/bi500021j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. An F, Yang Z, Zheng M, Mei T, Deng G, Guo P, et al. Rationally assembled albumin/indocyanine green nanocomplex for enhanced tumor imaging to guide photothermal therapy. J Nanobiotechnology. 2020 Mar 17;18(1):49. 10.1186/s12951-020-00603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sabapathy V, Mentam J, Jacob PM, Kumar S. Noninvasive optical imaging and in vivo cell tracking of indocyanine green labeled human stem cells transplanted at superficial or in-depth tissue of SCID mice. Stem Cells Int. 2015;2015:606415. 10.1155/2015/606415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Filippi M, Garello F, Pasquino C, Arena F, Giustetto P, Antico F, et al. Indocyanine green labeling for optical and photoacoustic imaging of mesenchymal stem cells after in vivo transplantation. J Biophotonics. 2019 May;12(5):e201800035. 10.1002/jbio.201800035. [DOI] [PubMed] [Google Scholar]
- 42. Marini I, Aurich K, Jouni R, Nowak-Harnau S, Hartwich O, Greinacher A, et al. Cold storage of platelets in additive solution: the impact of residual plasma in apheresis platelet concentrates. Haematologica. 2019 Jan;104(1):207–14. 10.3324/haematol.2018.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green SM, Padula MP, Marks DC, Johnson L. The lipid composition of platelets and the impact of storage: an overview. Transfus Med Rev. 2020 Apr;34(2):108–16. 10.1016/j.tmrv.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 44. Saunders C, Rowe G, Wilkins K, Collins P. Impact of glucose and acetate on the characteristics of the platelet storage lesion in platelets suspended in additive solutions with minimal plasma. Vox Sang. 2013 Jul;105(1):1–10. 10.1111/vox.12013. [DOI] [PubMed] [Google Scholar]
- 45. Bell OH, Carreño E, Williams EL, Wu J, Copland DA, Bora M, et al. Intravenous indocyanine green dye is insufficient for robust immune cell labelling in the human retina. PLoS One. 2020;15(2):e0226311. 10.1371/journal.pone.0226311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ji Y, Jones C, Baek Y, Park GK, Kashiwagi S, Choi HS. Near-infrared fluorescence imaging in immunotherapy. Adv Drug Deliv Rev. 2020 Dec;167:121–34. 10.1016/j.addr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Telford WG. Near infrared lasers in flow cytometry. Methods. 2015 Jul 1;82:12–20. 10.1016/j.ymeth.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.