Abstract
Background:
One in five individuals live with chronic pain globally, which often co-occurs with sleep problems, anxiety, depression, and substance use disorders. Although these conditions are commonly managed with cannabinoid-based medicines (CBM), health care providers report lack of information on the risks, benefits, and appropriate use of CBM for therapeutic purposes.
Aims:
We present these clinical practice guidelines to help clinicians and patients navigate appropriate CBM use in the management of chronic pain and co-occurring conditions.
Materials and Methods:
We conducted a systematic review of studies investigating the use of CBM for the treatment of chronic pain. Articles were dually reviewed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Clinical recommendations were developed based on available evidence from the review. Values and preferences and practical tips have also been provided to support clinical application. The GRADE system was used to rate the strength of recommendations and quality of evidence.
Results:
From our literature search, 70 articles met inclusion criteria and were utilized in guideline development, including 19 systematic reviews and 51 original research studies. Research typically demonstrates moderate benefit of CBM in chronic pain management. There is also evidence for efficacy of CBM in the management of comorbidities, including sleep problems, anxiety, appetite suppression, and for managing symptoms in some chronic conditions associated with pain including HIV, multiple sclerosis, fibromyalgia, and arthritis.
Conclusions:
All patients considering CBM should be educated on risks and adverse events. Patients and clinicians should work collaboratively to identify appropriate dosing, titration, and administration routes for each individual.
Systematic Review Registration:
PROSPERO no. 135886.
Keywords: cannabinoids, cannabinoid-based medicines, cannabis, marijuana, chronic pain, sleep disorders
Introduction
Across the globe, one in five individuals live with chronic pain,1 and estimates of chronic pain prevalence may be 20–25% in some countries and regions.2 Two thirds of individuals report it as moderate to severe.3–5 Approximately 50% of these individuals have lived with chronic pain for more than 10 years.3 Chronic pain often co-occurs with insomnia, anxiety, depression, post-traumatic stress disorder (PTSD), and substance use disorders such as opioid and alcohol use disorder.6–12 Chronic pain and these co-occurring conditions are also among the most common conditions for which cannabinoid-based medicines (CBM), derived from the cannabis plant, are used therapeutically.13–16
Recently, there has been a proliferation of systematic reviews on CBM and chronic pain and co-occurring conditions. Given new legal regimes globally regarding recreational cannabis and CBM derived from the cannabis plant, health care providers need to be aware of the potential efficacy and harms. Here, we present Clinical Practice Guidelines for the use of Cannabis and CBM in the Management of Chronic Pain and Co-occurring Conditions. These guidelines examine literature focused on cannabis and CBM derived from the cannabis plant rather than synthetic, pharmaceutical-grade cannabinoids in an effort to fill an important knowledge gap.
Materials and Methods
Our previously published protocol17 outlined the inclusion/exclusion criteria for studies (PICOS breakdown), process for data extraction (using Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] conventions) strategy for data synthesis, assessment of evidence and recommendations (GRADE system), risk of bias assessment, and procedures for data analysis/synthesis. Conclusions relate to availability of evidence, and the evidence available does not support doing this for every cannabinoid and cannabinoid combination.
When we report on adverse effects for specific cannabinoids (i.e., tetrahydrocannabinol [THC] vs. cannabidiol [CBD] vs. THC/CBD products), the reason is that evidence exists for these specific cannabinoids or combinations. Note that we cannot extrapolate from one cannabinoid to another, nor from a combination product to one cannabinoid, since the evidence for doing so does not exist.
Practical tips were formulated qualitatively based on a discussion of panel members from their cumulative clinical experience, rather than a formal algorithm.
Search strategy and study eligibility
In May 2019, an electronic search was conducted for peer-reviewed articles (2001–2019), in English, in the following electronic databases: Academic Search Complete, Cochrane Database of Systematic Reviews, Evidence Based Medicine Reviews, OVID Medline, PsychINFO, PubMed, CINAHL, and Web of Science. The search strategy was as previously described.17 Studies focusing exclusively on efficacy of synthetic pharmaceutical cannabinoids (such as nabilone or dronabinol) were excluded.
In addition, studies reporting on results of a single patient (n=1) were also excluded. As nabiximols contain plant-derived cannabinoids, they were included. Complete inclusion and exclusion criteria are listed in Supplementary Table S1 in the protocol.17 Study types including systematic reviews, meta-analyses, controlled trials, uncontrolled trials, observational studies, and cross-sectional designs were included, whereas systematic reviews and original research articles were summarized separately. Guideline development was primarily based on original research given the heterogeneity of previous systematic reviews.
Study screening and selection
Using the PRISMA conventions,18 a Data Synthesis Committee determined study eligibility by reading identified abstracts. Each abstract was independently dually reviewed. Full-text screening occurred in cases of uncertainty. Disagreements on inclusion were resolved through a discussion by two reviewers. Full-text screening was also independently dually reviewed. Reference lists of included studies were searched for potential additions. A PRISMA flowchart outlines this process (Supplementary Appendix SA1).
Data extraction
Data were extracted independently using a standardized Data Extraction Form to create evidence tables. For each study, data were extracted related to study identification (author, year published, number and location of centres, funding, journal name), number of participants, form of CBM, dose and route, study design and setting, inclusion/exclusion criteria, aggregate demographic (age, sex, type of pain, co-occurring conditions) and clinical characteristics (co-morbidities), outcome measures, and results. We also identified types and frequencies of adverse events. Final evaluation of study quality (very low, low, moderate or high) included considerations of limitations, inconsistencies, indirectness, and imprecision.
Data synthesis
Data were extracted using standard data extraction tools. There is significant variability in cannabis research due to heterogeneity of sample populations, study types and lengths, and CBM interventions (e.g., CBM type, dosing, administration route, etc.). Patterns related to efficacy, safety, and tolerability were explored through narrative synthesis.19,20 Data were compiled based on availability of quality evidence. Consistent findings and discrepancies were discussed. Evidence for CBM in the management of chronic pain and co-occurring conditions related to efficacy, tolerability, safety, indications, dosing, drug interactions, adverse events, negative effects, and contraindications.
Assessment of evidence and recommendations
Clinical recommendations and indications were developed and presented in relation to CBM use for chronic pain and comorbidities. The Task Force used the GRADE system to rate quality of evidence and strength of recommendations.21–26 Values and preferences and practical tips have also been provided to support clinical application.
Strength of recommendations
Strength of recommendations was specified as strong or weak based on risk and benefit of CBM in the specific condition. Patient values and preferences, magnitude of effect, and confidence in the evidence were considered. A recommendation was deemed to be strong if the committee considered the benefit to clearly outweigh the risk for most individuals. All other recommendations were specified as weak, indicating a need to consider individual clinical circumstances, values, and preferences.
Risk-of-bias assessment
Two reviewers (M.S.P. and P.W.) assessed potential bias and discrepancies were adjudicated by the Data Synthesis Committee (C.C., Z.W., S.M.). The National Institutes of Health risk-of-bias assessment tools27 were used to assess study quality. These tools were developed specifically for different study design types, and therefore heterogeneity of the designs of included studies will not affect ability to assess quality appropriately. Study bias was graded as either “good quality” (score of 3), implying low risk of bias, “fair quality” (score of 2) implying some risk of bias, or “poor quality” (score of 1), implying high risk of bias. Assessments of bias were performed at the overall study level.
Results
We identified 4989 records following the removal of duplicates (Supplementary Appendix SA1). Following abstract review, 4824 records were excluded, resulting in full-text review of 165 articles. Reasons for exclusion are presented in Supplementary Appendix SA1. Seventy studies were included in the final review, including 19 systematic reviews (Supplementary Appendix SA2) and 51 original research studies (Supplementary Appendix SA3). To avoid redundancy, systematic reviews were considered independent of primary literature.
Characteristics of systematic reviews
Details of the 19 included systematic reviews are presented in Supplementary Appendix SA2, along with each review's quality rating as an assessment of risk of bias. Most reviews were rated as either good, or fair, representing low or moderate risk of bias, respectively. Included reviews were published between 2007 and 2018, with 16 of 19 reviews published in 2015 or more recently. The majority of reviews (12) included only randomized controlled trials (RCTs).
Three reviews included only systematic reviews,28,29,30 three contained primary research,31–33 and one included other systematic reviews, in addition to primary research.34 People with chronic pain were the most commonly studied population (14). One review focused on rheumatic disease-associated pain,35 one included studies of multiple sclerosis (MS) or comparable neuropathic pain,36 one included systematic reviews that analyzed cannabinoids for pain, spasticity, or nausea and vomiting,29 and two included people using medical cannabinoids regardless of indication.32,37
Most reviews explored both pharmaceutical synthetic and plant-based cannabinoids (15/19). Three reviews focused exclusively on plant-based cannabis and plant-derived cannabinoid extracts.34,38,39 Park and Wu focused exclusively on non-synthetic cannabis, as their review solely included people using medical cannabis. Reviews that focused exclusively on pharmaceutical synthetic cannabinoids were excluded.
In terms of efficacy for chronic pain reduction, most reviews (14/19) reported that cannabinoids provided analgesia in at least some contexts. Nugent et al found non-synthetic cannabinoids beneficial for neuropathic pain, but they found insufficient evidence in other types of pain. In addition, Stockings et al reported nabiximols to be effective for MS-related pain; however, generally there was insufficient evidence for cannabinoids to be used as a pain treatment. Five reviews found inadequate evidence to support cannabinoids as an effective pain treatment.28,29–31,35
Of the eight reviews that obtained a “good” quality rating, representing the lowest risk of bias, seven found cannabinoids to be beneficial for pain relief. However, in some instances, the analgesic effect of cannabinoids was described as “moderate”40 or “small.”41
Most reviews analyzed prevalence of adverse events associated with cannabinoids. Although common, adverse events were typically mild to moderate in severity.34,39–42 Adverse events were similar between reviews and included drowsiness, dizziness, and dry mouth. Aviram and Samuelly-Leichtag suggested that adverse events could not entirely be attributed to cannabinoids as “the participating patients in the included trials had pre-existing diagnoses and in many of the trials, they used concomitant medications.”43
A few reviews included analysis of pain comorbidities. Of the reviews that studied sleep and sleep problems within the context of chronic pain, analyses fairly consistently supported that cannabinoids provided at least partial benefit.32,33,35,37,40–42,44,45 Findings were more heterogenous regarding the efficacy of cannabinoids for mood disorders and related issues. Four reviews reported that cannabinoids improved anxiety in pain populations.35,37,41,44
Stockings et al did not find any significant difference between cannabinoid and comparator groups in overall emotional functioning or depressive or anxiety symptoms. Whiting et al reported significant improvement in anxiety with cannabinoids over placebo; however, in studies where anxiety was studied as a secondary outcome, they found no significant difference. Martin-Sanchez et al analyzed mood disturbances only within the context of adverse events but found euphoria to be a common occurrence with a number needed to harm (NNH) of 8, and dysphoria to be less common with an NNH of 29.
Finally, in a review focused on people living with chronic neuropathic pain, Mucke et al reported cannabinoids to be more efficacious than placebo for treating psychological distress.
Evidence summaries and clinical guideline recommendations
1. CBM use for people with chronic pain
Forty-seven studies relevant to pain management were reviewed, including 22 RCT,46–67 11 pre-post studies or uncontrolled trials,68–78 11 cross-sectional or observational cohort studies,79–89 and 3 case series.90–92 Most studies (38/47) reported at least moderate benefits of CBM for chronic pain,46–48,50,51,54,55,57,59–70,73–80,82–84,86–92 seven were inconclusive or found insufficient evidence,49,53,56,58,71,72,85 and two reported mixed results.52,81
Associated improvements in secondary outcomes, including QoL, functionality, and mood, have also been observed with the use of medical cannabis in addition to reductions in pain severity, intensity, and interference. For details of the individual studies, see Appendix A in Supplementary Data.
Recommendations
1. We recommend the use of CBM as monotherapy, replacement, or adjunct treatment, in people living with chronic pain, for the management of chronic pain including central and/or peripheral neuropathic pain to improve pain outcomes.
Strong Recommendation, Moderate-Quality Evidence
2. We recommend the use of CBM as monotherapy, replacement or adjunct treatment, in people living with chronic pain, for mobility in those not achieving adequate response to other modalities.
Weak Recommendation, Low-Quality Evidence
Values and preferences
The recommendations place high value on the improvement in chronic pain, functionality, and secondary outcomes, including time to sleep, quality of sleep, anxiety, and depression, in those living with chronic pain and using CBM compared with placebo. The recommendations also outweigh the risks of non-serious adverse events with CBM (dizziness, disturbance in attention, somnolence, dry mouth, nausea, diarrhea) as compared with adverse events from standard analgesia (opioids and serotonin-norepinephrine reuptake inhibitors [SNRIs] or opioids monotherapy), including constipation, loss of appetite, unclear mentation, reduced affect, hemorrhoids, and substance use disorder.
Practical tip
A wide variety of formulations and routes including smoking, vaping, oral capsule, oral oil, and oromucosal sprays showed benefits in chronic pain, mood disorders, mobility, and sleep. Adverse events due to combustion, and exposure to second-hand smoke, make smoked CBM less favorable. The inhalation route of CBM is restricted to the vaporization of dried flower over combustion for the management of breakthrough pain due to rapid onset and shorter duration of action.
Oral products, as a route of administration may be preferred for the longer duration of action (6–8 h) compared with inhaled cannabis, particularly in the evening (due to somnolence). This will allow for greater symptom control in patients who experience chronic persistent daily symptoms and/or disease. Oral oil and capsule formulations can be easier to dose accurately (mg) and provide consistent and reproducible dosing.
Practical tip
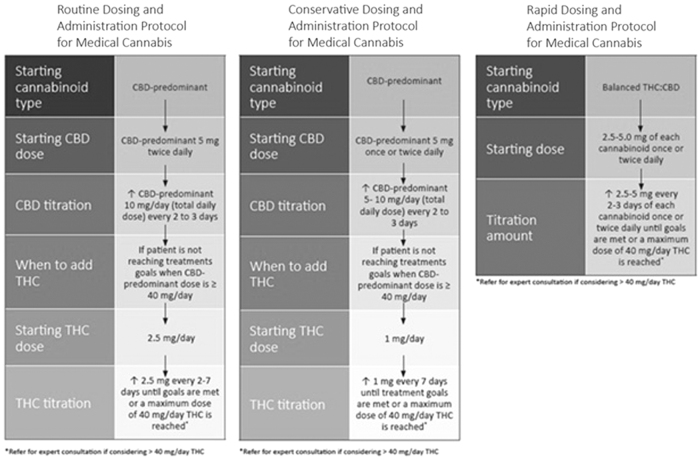
The strongest evidence for reduction of chronic pain symptoms is for THC formulations, not CBD. The majority of adverse events are associated with THC. Adverse Events due to CBM are mild and may be better tolerated than other centrally acting prescription medications. Patients report that adverse effects generally subside within 48 h or when the titration phase is stopped. The best way to reduce the potential adverse effects with THC is with safe, low-dose initiation and titration (Fig. 2). In addition, a CBD dominant product can be used in combination with THC to attenuate side effects. The initiation of CBD during daytime with the addition of THC at bedtime can also further mitigate these effects.
FIG. 2.
Example of oral CBM Dosing and Titration Protocols. Reproduced with permission of Bhaskar et al.133 CBM, cannabinoid-based medicines.
2. CBM use for people with HIV and chronic pain
Three studies examined CBM to treat pain in people with HIV, including two RCTs46,51 and one cross-sectional study.88 All three studies reported significant improvements in HIV-related pain management associated with CBM use. For details of the individual studies, see Appendix B in Supplementary Data.
Recommendations
1. We recommend the use of CBM for the management of muscular and neuropathic pain in people living with HIV who are not achieving adequate response, or those experiencing adverse effects to other treatment modalities.
Strong Recommendation, Moderate-Quality Evidence
2. We recommend CBM use for the management of HIV-related symptoms, including nausea, anxiety, depression, lack of appetite, and weight loss in people living with HIV. CBM use is for symptom management only and should not replace the use of antiretroviral therapies.
Strong Recommendation, Low-Quality Evidence
Values and preferences
The recommendations place high value on the benefit of neuropathic and muscular pain relief in people with HIV over the risks of adverse events of a mainly non-serious nature such as dizziness, disturbance in attention, balance disorder, somnolence, dry mouth, nausea, diarrhea, fatigue, or confused state and those of a more serious nature such as pulmonary and cardiovascular effects of inhaled substances.
Practical tip
A wide variation is seen in the dose and administration routes of cannabinoids used to optimize the treatment effect and adverse event ratio. A slow dose titration, initiated with CBD-predominant cannabinoids, should be used to individualize treatment (Fig. 2).
Practical tip
Although benefit was observed in HIV nerve and muscle pain mostly with smoked formulations, oils and capsules have been shown to have the strongest evidence in MS. Oils and capsules provide the greatest consistency for dosage and titration and are not associated with potential adverse events associated with inhalation of CBM.
3. CBM use for people living with multiple sclerosis and chronic pain
Twelve studies examined CBM for pain in people with MS, including nine RCTs,50,52,53,55,58,65,66,67,77 two open-label studies,77,78 and one cross-sectional study.82 Seven studies involved nabiximols,49,52,53,55,58,77,78 three involved extracts delivered through capsule,65–67 and two involved whole plant cannabis.50,82 Study length ranged from 3 days to 2 years. Most studies (9/12) reported improvements in pain associated with CBM use, including both studies involving whole plant cannabis and all three involving extracts delivered through capsules.
The majority of studies were limited by small numbers of participants, short duration of treatment, and some crossover and blinding deficiencies. For details of the individual studies, see Appendix C in Supplementary Data.
Recommendations
1. We recommend the use of CBM, as adjunct treatment, for pain management in people with MS not achieving adequate response to other modalities.
Strong Recommendation, Moderate-Quality Evidence
2. We recommend the use of CBM, as adjunct treatment, for the management of muscle spasm in people living with MS in those not achieving adequate response to other modalities.
Strong Recommendation, Moderate-Quality Evidence
3. We recommend the use of CBM, as adjunct treatment, for the management of sleep disorder in people living with MS in those not achieving adequate response to other modalities.
Strong Recommendation, Low-Quality Evidence
Values and preferences
The recommendations place high value on the benefit of pain, spasticity, and sleep disturbance relief seen in people with MS over the risks of adverse events, of a mainly non-serious nature, including dizziness, disturbance in attention, balance disorder, somnolence, dry mouth, nausea, diarrhea, fatigue, or confused state.
Practical tip
A wide dose variation of cannabinoids was used in studies involving MS patients. Total daily THC oral dose of 10–15 mg, as a divided dose twice daily, was most commonly used. A slow dose titration should be used to individualize treatment (Fig. 2).
Practical tip
Although benefit was observed in pain, spasticity, and sleep with oral oil, capsule, smoked and oromucosal formulations, the strongest evidence is for the use of oils and capsules. These formulations also provide the greatest consistency for dosage and titration and are not associated with potential adverse events associated with inhalation of CBM.
4. CBM use for people living with an arthritic condition experiencing chronic pain
One RCT,48 one pre-post survey,70 and one published abstract93 have been identified in the literature search. Both the RCT and published abstract demonstrated improvement in pain in patients with an arthritic condition. For details of the individual studies, see Appendix D in Supplementary Data.
Recommendation
1. We recommend the use of CBM, as adjunct treatment, for the management of chronic pain in people living with arthritic conditions in those not achieving adequate response to other modalities.
Strong Recommendation, Low-Quality Evidence
Values and preferences
The recommendation places high value on the benefit of improvement in pain, sleep, and other co-morbid conditions over the risks of adverse events of a mainly non-serious nature such as dizziness, disturbance in attention, balance disorder, somnolence, dry mouth, nausea, diarrhea, fatigue, or confused state.
Practical tip
Best evidence of benefit is in participants with rheumatoid arthritis for improvement in pain, sleep, other co-morbid conditions, and markers of inflammation. A balanced THC/CBD oromucosal product titrated to ∼15 mg of each component may be tried. If an oral formulation is used, a slow titration of THC as shown in Figure 2 should be employed.
Practical tip
As dizziness and falls have been identified as potential adverse events associated with CBM use, a clear understanding of risks should be achieved before CBM initiation, especially for populations with an increased risk of bone loss/osteoporosis. Consider a lower THC starting dose, slower titration period, and consistent monitoring.
Practical tip
A single abstract publication has suggested benefit from topical CBD 125 mg bid for localized pain management of knee osteoarthritis. This format can be applied to the affected joints as a cream, oil, or spray. This approach can be expected to be associated with a very low risk of any adverse events. More research is needed regarding the efficacy and safety of topical CBM.
5. CBM use for people living with fibromyalgia and chronic pain
There was a total of six studies that included participants with fibromyalgia and pain. Four of these studies included people with fibromyalgia as part of the study participants but the authors did not produce a separate analysis for pain management in fibromyalgia and therefore these studies are not heavily considered in this section.72,76,91,92 Each of these studies found improvements in pain across their wider study sample. In an open-label study, two thirds of study participants living with fibromyalgia responded well to sublingual THC treatment.72 For details of the individual studies, see Appendix E in Supplementary Data.
Recommendation
1. We recommend the use of CBM, as adjunct treatment, for management of back pain, fibromyalgia pain, or other chronic pain in people with fibromyalgia who are not achieving an adequate response to standard analgesics.
Strong Recommendation, Low-Quality Evidence
Values and preferences
This recommendation places high value on the improvement in back pain, chronic pain, fibromyalgia pain, and improvement in function, quality of life (QOL), and secondary outcomes, including anxiety, among patients living with fibromyalgia from CBM use, over the low risk of non-serious adverse events (reddening of the eyes, increased appetite, and sore throat) as compared with adverse events with standard analgesics (constipation, loss of appetite, lack of mental clarity, reduced affect, and hemorrhoids).
Practical tip
Fibromyalgia is a heterogeneous complex pain syndrome, which frequently occurs with other pain syndromes and symptom clusters. This needs to be considered when extrapolating this study information to other patients living with fibromyalgia.
Practical tip
In clinical practice, SNRI are used off-label as a multimodal treatment for symptoms such as insomnia, depression, and anxiety in patients suffering from fibromyalgia; likewise, CBM can treat these symptom clusters.
Practical tip
All patients using CBM should be educated on proper vaporization (to avoid harms of combustion/smoking) and/or cannabis oil/capsule dosing and titration regimens. To limit adverse effects while still achieving pain outcomes, THC dosing should be started low and titrated slowly to an individual response. For elderly and frail patients, consider a lower THC starting dose, slower titration period, and consistent monitoring.
6. CBM use for people experiencing chronic headache and migraine
Four included studies specifically measured associations between CBM and chronic headache or migraine.70,88,94,95 One study was a conference abstract and included as gray literature.94 Of these four studies, two utilized pre/post designs,70,94 and two were cross-sectional.88,95 Each study reported at least some improvement from cannabis in participants experiencing headaches. For details of the individual studies, see Appendix F in Supplementary Data.
Recommendation
1. We recommend the use of CBM, as an adjunct treatment, for the management of chronic migraine or chronic headache, in those not achieving adequate response to other modalities.
Weak Recommendation, Low-Quality evidence
Values and preferences
The recommendations place high value on the benefit of migraine and headache relief over the risk of adverse events, which are mainly non-serious in nature and include somnolence and dizziness.
Practical tip
Some people also experience headache due to cannabis, therefore it will be important to assess the individual treatment response. Dosage form will be important to consider if CBM is used for prophylaxis versus treatment of migraine or headache, given different timing of onset.
Practical tip
To limit exposure to adverse events, individuals should start low, go slow, and follow a structured initiation and titration plan (Fig. 2). Patients and physicians should work collaboratively to identify appropriate administration route(s) that meet the needs of the individual.
7. CBM use for people living with chronic pain and nausea
Five studies analyzed participant perceptions of cannabis as a potential treatment for nausea as a comorbidity of pain.79,85,88,91,92 Each study involved whole plant cannabis in inhaled or oral formats, with patients reporting moderate relief. Although these five studies found improvements in nausea with cannabis use, other studies found nausea to be an adverse event associated with cannabis use among some participants.46–50,54,57–59,71,72,74,77,78
These studies were limited by study design (case series or cross-sectional surveys), small numbers of patients, unknown duration or dosing of cannabis, and the possibility of selection and recall bias. It is also unclear which cannabis formulation or route of administration is optimal (smoked vs. oral, THC vs. CBD vs. THC/CBD products) for nausea. For details of the individual studies, see Appendix G in Supplementary Data.
Recommendation
1. We recommend considering the use of CBM to reduce nausea in people living with chronic pain as monotherapy or adjunct treatment for those not achieving adequate response to other treatment modalities.
Weak Recommendation, Low-Quality Evidence
Values and preferences
This recommendation does not refer to the use of CBM in cancer-related pain or emetogenic therapy, and places high value on the benefit of CBM for nausea relief, over the risk of adverse events, which are mainly non-serious in nature.
Practical tip
All published data on nausea benefit in chronic pain have come from survey or cross-sectional studies. As such, no practical tips on cannabis type, mode of administration, or concentration of THC or CBD can be made.
8. CBM use for people with sleep problems and symptoms of sleep deprivation experiencing chronic pain
Sleep issues are often a target symptom for cannabis use and 25 of the studies assessed impacts of CBMs on sleep, including 16 RCTs,47–49,52–55,57–60,65–67,74,75 four cross-sectional survey studies,79,80,82,87 three pre-post style studies,70,73,85 and two case series studies.91,92 Of these studies, 10 included consumption of whole plant cannabis by participants,60,70,73,79,80,82,85,87,91,92 12 involved cannabis extracts (CEs) consumed by oromucosal spray,47–49,52–55,57–59,74,75 and 3 involved participants treated with cannabis extract capsules.65–67 Almost all studies found benefit for sleep in some or most participants. For details of the individual studies, see Appendix H in Supplementary Data.
Recommendation
1. We recommend the use of CBM as monotherapy, replacement or adjunct treatment, to improve sleep and symptoms of sleep deprivation in people living with chronic pain not responsive to, or intolerant of, other modalities or pharmacologic treatment.
Strong Recommendation, Moderate-Quality Evidence
Values and preferences
The recommendations place high value on the benefit of CBM for disturbances in sleep and poor sleep quality. Adverse events such as somnolence, drowsiness, and sleepiness rarely caused withdrawal from studies and point to the potential benefits for patients with chronic pain states.
Practical tip
Many studies used CBM in the form of standardized-dose CE (nabiximols and others), which allowed for easier titration and effective dose-finding. The presence of products, including CBD, may reduce the incidence of psychiatric or euphoric effects. Oral formulations may also be considered and are not associated with potential adverse events associated with inhalation of CBM.
Practical tip
Sleep disturbances in patients with MS showed the most improvement with CBM used for pain and/or spasticity. This is a group who should be routinely assessed for the suitability of treatment with CBM.
Practical tip
To limit exposure to adverse events, individuals should start low, go slow, and follow a structured initiation and titration plan (Fig. 2).
9. CBM use for people living with chronic pain experiencing appetite loss
Seven studies assessed CBM use on appetite in participants experiencing chronic pain, including two RCTs,59,63 two cross-sectional studies,79,88 two case series,91,92 and one pre-post study.70 The RCT assessed did not demonstrate a significant difference between CBM and placebo. The two case series and one cross-sectional study reported some improvement in appetite, whereas another case series did not find significant benefit. For details of the individual studies, see Appendix I in Supplementary Data.
Recommendation
1. We recommend the use of THC-dominant cannabis for people with problematic loss of appetite in association with chronic pain, over no treatment.
Strong Recommendation, Low-Quality evidence
Values and preferences
The recommendation places high value on the benefit of improved appetite, and presumably nutrition, over the risks of adverse events of a mainly non-serious nature such as dizziness, disturbance in attention, balance disorder, somnolence, dry mouth, nausea, diarrhea, fatigue, or confused state.
Practical tip
All published data on appetite improvement in chronic pain have been associated with the use of THC dominant products. A single RCT failed to demonstrate benefit from CBD; however, this cannabinoid may provide other benefits, including a reduction in pain and anxiety.
Practical tip
One cross-sectional study noted an increased risk of problematic cannabinoid use in patients using CBM for appetite improvement. All patients using CBM should be monitored for cannabis use disorder. The Cannabis Use Identification Test- Revised may be used for this purpose. To limit exposure to adverse events, individuals should follow a structured initiation and titration plan (Fig. 2). Patients and physicians should work collaboratively to identify appropriate administration route(s) that meet individual needs.
10. CBM use for people with chronic pain experiencing PTSD
Two studies included analysis of cannabis as a treatment for PTSD symptoms.70,79 One study evaluating cannabis in patients with PTSD as the primary condition rated the mean helpfulness of CBM in the moderately helpful range79 Among the larger sample (n=186), higher levels of traumatic intrusions were associated with higher perceived helpfulness of cannabis.79 The second study found that the numbers of participants with PTSD reporting severe pain at baseline dropped from 56% at baseline to 11% at 4 months of CBM use. Improvements were also reported in ability to cope with pain and overall QOL, mood, sleep, and concentration.70 For details of the individual studies, see Appendix J in Supplementary Data.
Recommendation
1. We recommend the use of CBM to improve PTSD symptoms in people living with chronic pain not responsive to, or intolerant of, non-pharmacologic treatment.
Weak Recommendation, Low-Quality Evidence
Values and preferences
The recommendations place high value on the benefit of CBM for pain, intrusion symptoms, sleep disturbance, and improved mood and QOL seen in people living with PTSD over the risks of adverse events of a mainly non-serious nature such as dry mouth, disturbance in attention and memory, as well as the potential for the development of non-medical use.
Practical tip
The single study that reported dose suggested that 1–1.5 g/day of herbal cannabis was typical. However, other modes of administration were not discussed but might nonetheless be advantageous; oils and capsules provide the greatest consistency for dosage and titration, are not associated with potential adverse events associated with inhalation of CBM, and may also offer advantages in the context of sleep disturbance and nightmares that are prominent among some individuals with PTSD.
11. CBM use for people living with chronic pain experiencing anxiety
Eight studies within this review examined the treatment of anxiety with CBM in people living with chronic pain.55,61,70,76,96–99 Although these studies utilized a variety of CBM approaches, most evidence—including the relatively higher quality studies—reported anxiolytic effects of cannabis. For details of the individual studies, see Appendix K in Supplementary Data.
Recommendation
1. We recommend the use of CBM as adjunct therapy to improve symptoms of anxiety in people living with chronic pain not responsive to, or intolerant of, non-pharmacologic treatment.
Strong Recommendation, Moderate-Quality Evidence
Values and preferences
The recommendations place high value on the benefit of CBM for anxiety symptoms over the risks of adverse events of a mainly non-serious nature such as dry mouth, disturbance in attention and memory, as well as the potential for acute transient increases in anxiety and panic.
Practical tip
The only RCT to compare doses of herbal cannabis suggested that chemovars (strains) with 9% THC—which would be generally considered a moderate to low strength herbal cannabis—are more effective than herbal cannabis with low to very low levels of THC, regardless of CBD content. To limit exposure to adverse events, individuals should follow a structured initiation and titration plan (Fig. 2).
Practical tip
Other modes of administration might also be advantageous; specifically, orally ingested CBMs provide the greatest consistency for dosage and titration, are not associated with potential adverse events associated with inhalation of CBM, and may also offer advantages in the context of sleep disturbance that may be prominent among some individuals with problematic anxiety.
Practical tip
It is well recognized that THC has the potential to trigger acute transient increases in anxiety and panic. Individuals should be warned of this adverse effect and closely followed for it.
12. CBM use for people living with chronic pain experiencing depression
Findings for depressive symptom outcomes appeared to be contingent on the types of CBMs used, with herbal cannabis appearing to be more effective than extracts. An RCT of smoked cannabis found that it improved depressive symptoms significantly with a medium-sized effect over placebo.60 Three cross-sectional studies also report antidepressant effects of CBM.82,97,98 Studies involving the use of cannabis extracts are generally less positive regarding the benefits of CBM for treating depression in people with chronic pain. In contrast to these positive results, three RCTs found no significant difference between nabiximols and placebo in depression scores.53,55,58 For details of the individual studies, see Appendix L in Supplementary Data.
Recommendation
1. We recommend the use of CBM as adjunct therapy to improve symptoms of depression in people living with chronic pain experiencing unsatisfactory results from standard treatment.
Weak Recommendation, Moderate-Quality Evidence
Values and preferences
The recommendations place high value on the benefit of CBM for depressive symptoms over the risks of adverse events of a mainly non-serious nature such as dry mouth, disorientation, and disturbance in attention and memory. The CBM should not take the place of other anti-depressant treatments, including pharmacological and non-pharmacological treatments, such as psychotherapeutic intervention.
Practical tip
The only RCT to compare doses of herbal cannabis suggested that chemovars with 9% THC—which would be generally considered a moderate to low strength herbal cannabis—are more effective than herbal cannabis with low to very low levels of THC. To limit exposure to adverse events, individuals should follow a structured initiation and titration plan (Fig. 2).
Practical tip
Other modes of administration might also be advantageous; specifically, orally ingested CBMs provide the greatest consistency for dosage and titration, are not associated with potential adverse events associated with inhalation of CBM, and may also offer advantages in the context of sleep disturbance that may be prominent among some individuals with depression.
13. Adjunctive CBM use for people living with chronic pain experiencing unsatisfactory analgesia from opioid treatment
Four studies specifically addressed the efficacy of combined opioid analgesics with cannabis therapy for chronic pain. One study evaluated the addition of vaporized cannabis to patients taking sustained-release opioids for a variety of chronic pain conditions.68 These studies demonstrated a reduction in pain with the addition of CBM to their opioid regimen. Several additional studies found improvements in chronic pain associated with CBM use within samples that included participants concurrently using opioids to treat pain.52,54,60,100,101 For details of the individual studies, see Appendix M in Supplementary Data.
Recommendation
1. We recommend the use of CBM, as adjunctive treatment to opioids, for the management of chronic pain in those experiencing unsatisfactory analgesia from opioid treatment.
Strong Recommendation, Moderate-Quality Evidence
Values and preferences
The recommendations place high value on the improvement in chronic pain, fibromyalgia pain, functionality, spasms, and secondary outcomes of depression and anxiety, in those with chronic pain using CBM adjunctly over the low risk of non serious adverse events (fatigue, sedation, impairment in concentration, reduced salivation) as compared with adverse events with standard opioid analgesic therapy (constipation, loss of appetite, feeling of reduced mental acuity and flat affect, hemorrhoids, dependency, respiratory depression).
Practical tip
Evidence included in this review found efficacy of inhaled CBM as adjunct treatment for chronic pain. Inhalation as an administration route is advantageous for managing break-through pain due to rapid onset and shorter duration of action. Other routes of administration, including oils and capsules, may be preferred due to dosage consistency and lack of adverse events associated with inhalation, though further research with these routes is needed. Pain management can be individualized above baseline and can be managed on demand and titrated to desired effect.
Practical tip
Cannabis is rarely used as a first-line agent. It is important to assess and document the response to currently approved medications. This includes medication name, dose, duration, response, and tolerability. Some physicians will use cannabis in conditions where other treatment options have failed or are not tolerated.
Practical tip
Concomitant analgesics can be tapered or discontinued once a stable CBM dose is established. This may lead to a reduction in polypharmacy, side effects, drug interactions, as well as an improvement in adherence and cost saving.
Practical tip
As there was no change in plasma opioid levels after exposure to cannabis, improved analgesic response in patients using cannabis and opioids is likely due to synergy or additive effect. More research is required especially regarding whether cannabis should be considered as a treatment option before opioid initiation based on the lower risk of adverse effects versus opioids.
Practical tip
Long-term pain control was observed through a reduction in pain scores. Tolerance to adverse effects occurs within 48 h of a THC dose increase; however, tolerance to benefits did not develop over time. Starting at a low dose and gradually titrating to the lowest effective dose without adverse effects is suggested (Fig. 2).
14. CBM use and opioid sparing for people using opioids as treatment for chronic pain
Opioid sparing, or the reduction of opioid use, resulting from the use of CBM for pain management was the primary outcome in one study86 and a secondary outcome in six studies.69,73,81,84,87,91
Three studies found positive associations between medical cannabis use and opioid sparing.69,73,102 Two other studies reported that the majority of participants reduced their routine pain medications by 60–70%; however, the extent to which opioid medications were specifically reduced was unclear.87,99 For details of the individual studies, see Appendix N in Supplementary Data.
Recommendations
1. We recommend the use of CBM as adjunct treatment among people using moderate/high doses of opioids (>50 morphine equivalent) for the management of chronic pain and/or to increase opioid sparing.
Strong Recommendation, Moderate-Quality Evidence
2. We recommend the use of CBM as adjunct treatment for chronic pain among people using any dose of opioids who are not reaching chronic pain goals, are experiencing opioid-related adverse events, or display risk factors for opioid-related harm.
Strong Recommendation, Low-Quality Evidence
Values and preferences
The recommendations place high value on the reduction in the reliance on opioids with secondary outcomes improvements, including sleep, anxiety, and mood, in those living with chronic pain and using CBM as adjunct/concurrent to opioids over the low risk of non-serious adverse events (dry mouth, dizziness, increased appetite, sedation, concentration difficulties) as compared with adverse events with opioids/standard of care (constipation, loss of appetite, feeling of reduced mental acuity and flat affect, hemorrhoids, dependency, respiratory depression).
Practical tip
There is a physiologic rationale for coadministration of cannabis and opioids, which prevents opioid-tolerance and the need for dose escalation. In addition, cannabis can treat the symptoms of opioid withdrawal, reduce or replace opioids. Thus, cannabis is safer than opioids and makes opioid consumption safer. More research is required in this area.
Practical tip
Clinicians should re-evaluate any patient on >50 mg morphine equivalent dose (MED) as their risk of fatal overdose is doubled compared to 20 mg MED; the risk increases 10-fold with >90 mg MED. Both the United States and Canadian opioid guidelines advise clinicians to carefully reassess risk-benefit ratio when >50 mg MED and to avoid >90 mg MED as there is low evidence for improvements in pain, but a significant increase in the risk of harm.
Practical tip
Individuals should keep a daily log, including dosing and monitoring for efficacy, effects on mood and function, and possible side effects. This will encourage individuals to slowly titrate cannabis to symptom control, while minimizing adverse events. Once individuals using medical cannabis are stabilized, generally they do not require dose escalation over time.
Practical tip
Health care providers are encouraged to implement standardized self-administered questionnaires such as Patient Health Questionnaire-9, General Anxiety Disorder-7, or Brief Pain Inventory starting with the initial intake, and to continue in all subsequent follow ups to reassess risk benefit.
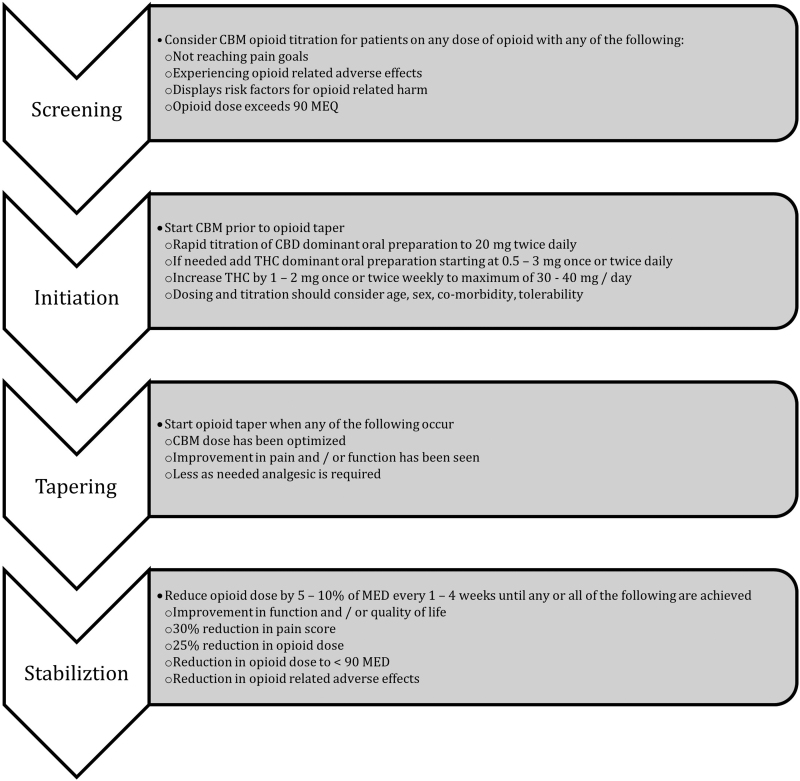
A Suggested Approach for Adjunct Cannabinoid Use for Opioid Sparing. Adapted from Sihota et al103 is shown in Figure 1.
FIG. 1.
Suggested approach for adjunct cannabinoid use for opioid sparing. Adapted from Sihota et al.103
Additional Practical Considerations
Drug interactions
Both THC and CBD are predominantly metabolized, by the liver, through the action of the cytochrome P450 system.104,105 A paucity of clinical studies are available regarding the effect of cannabinoids on this enzyme system, but in vitro studies suggest that THC inhibits CYP3A4, CYP3A5, CYP2C9, and CYP2C19, whereas CBD inhibits CYP2C19, CYP3A4, and CYP3A5. Due to the weak inhibitory effect of these cannabinoids, higher concentrations than those seen clinically are likely to be required for clinical inhibitory effect.106
This, however, may be of concern when cannabinoids are co-administered with drugs having a narrow therapeutic window and also metabolized by these enzymes, such as direct acting oral anticoagulants metabolized through CYP3A4107,108 and clopidogrel requiring conversion to its active metabolite by CYP2C19.109 Significantly elevated levels of the antiepileptic clobazam and its metabolite, n-desmethylclobazam, have been observed when co-administered with very high doses of CBD, likely due to co-metabolism through CYP2C19 and CYP3A4.110
Preclinical studies have reported that cannabinoids may also bind membrane transporters including breast cancer resistance protein111 and P-gp,112,113 which can, theoretically, impact the effect of other medications. A comprehensive overview of the pharmacokinetic interactions of cannabinoids has been reported by Alsherbiny and Li114 Pharmacodynamic interactions also need to be considered with CBM, particularly THC, administration. Additive effects can occur when cannabinoids are combined with sympathomimetics (e.g., tachycardia, hypertension), central nervous system depressants such as alcohol and opioids (e.g., drowsiness, ataxia), and anticholinergics (e.g., tachycardia, confusion).115
Adverse effects
▸ A concern from patients and clinicians are the adverse effects associated with cannabis use. The adverse effects that are the most commonly associated with cannabis are related to THC-dose and the route of administration.116 The THC-related adverse effects include dizziness, cognitive impairment, dry mouth, tachycardia, anxiety, drowsiness, and fatigue.116 There is evidence to suggest a positive association between cannabis use and development of psychosis, in people susceptible to psychotic disorders.117–119 Although no definitive causal effects have been established, there are case reports of stroke, acute coronary syndrome, and cardiac arrhythmias associated with use of cannabis.120,121 Maternal exposure to cannabis can adversely affect conception and/or maintenance of pregnancy.122–124 Significant decline in sperm count, concentration, and motility, as well as an increase in abnormal sperm morphology have been reported.
Smoking cannabis is associated with respiratory adverse effects such as cough, an increase in phlegm, and bronchitis.116 Long-term use is associated with risk of cannabis use disorder, hyperemesis syndrome, as well as withdrawal symptoms including insomnia, anxiety, depression, and tremulousness.125,126
Adverse effects can be mitigated when initiating CBM through low-dose initiation, slow titration and avoiding smoked cannabis.116 In people experiencing adverse effects, clinicians can consider adjustment in the strain (chemovar) with higher CBD and lower THC, reduce the dose, or alter the route of administration to minimize these effects.116 The CBD can be associated with transaminitis, which is typically dose-related and more common at very high doses (800 mg/day). Transaminitis typically improves by lowering the dose of CBD. Patients using CBD regularly should undergo periodic monitoring of liver enzymes and should avoid excessive alcohol use.127,128
Additional safety concerns
The CBM use is typically not recommended for children and youth.129 In addition, parents and those living with children should use caution to avoid exposure to second-hand smoke. People living with chronic pain and prescribing clinicians should always have a clear understanding of risks and adverse events before CBM initiation, including legal ramifications such as the inability to drive after THC consumption. It is recommended that CBM use follow a structured initiation process and have clinical supervision throughout.
Dosing
Two publications and one poster addressing dosing of CBM have been identified.130–132 All are concordant with the concept of low starting dose to be titrated slowly to achieve optimal target symptom improvement with minimal off-target effects, including euphoria. The optimal therapeutic dose is the dose that allows the patient to reach treatment goals, including pain and symptom reduction and improvement in function, with minimal or no side effects. Patients do not need to feel “high” or impaired to have symptom improvement.
Bhaskar et al propose three dosing regimens based on the clinical situation (Fig. 2): a routine protocol appropriate for most patients, a rapid protocol for those with severe pain, terminal illness or those already taking higher dose CBM and a conservative protocol for those with frailty, severe comorbidities, and polypharmacy. PRN dosing and micro-dosing may also be acceptable for breakthrough symptom management.
Authorization
Wide variation in legal status of medical and recreational cannabis exists globally. Clinicians authorizing or prescribing CBM should understand and comply with local laws and other regulations regarding its use. Individuals with conditions where CBM may be useful are urged to consult and be guided by regulated heath care professionals familiar with its use.
Strengths and limitations
These guidelines fill an important gap in the literature by providing guidance for clinicians and patients during a period of cannabis regulation changes. They include a thorough systematic review with rigorous study selection and methods for data extraction, quality assessment, and data synthesis. Although CBMs may be used for other purposes, these guidelines present recommendations for people living with chronic pain and for co-occurring conditions within the context of chronic pain and may not be transferable.
From the perspectives of people with lived experience, differences between sativa-predominant and indica-predominant chemovars of cannabis are important. For example, anecdotally, THC-dominant sativa tends to be stimulating and impede sleep, can augment anxiety and increase heart rate. However, the science behind the differences between sativa-predominant and indica-predominant chemovars is not well defined and the published research is undeveloped in this area. For these reasons, they were not included in the search results that are the foundation of the article.
There exist several challenges within CBM and chronic pain research. With respect to some co-occurring conditions, there still exist relatively few controlled trials. The data related to co-morbid conditions were typically not the primary focus of included studies and, subsequently, may be underpowered. The lack of comparative studies where the safety and efficacy of CBM is compared with typical pain treatments is also problematic. In addition, challenges commonly exist with unmasking in placebo-controlled trials, representing potential risk of bias, especially as pain and many comorbidities are measured with Visual Analog Scale or other subjective measures.
Supplementary Material
Acknowledgment
The authors thank Nancy Chow, Dr. Rob Sealey, and Dr. Lynda Balneaves for serving as external reviewers for these guidelines.
Abbreviations Used
- CBD
cannabidiol
- CBM
cannabinoid-based medicines
- CEs
cannabis extracts
- MED
morphine equivalent dose
- MS
multiple sclerosis
- NNH
number needed to harm
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PTSD
post-traumatic stress disorder
- QOL
quality of life
- RCTs
randomized controlled trials
- THC
tetrahydrocannabinol
Contributor Information
Collaborators: External Review Panel
Patient and Public Involvement
Chronic pain and CBM patients were included on the guidelines task force and were involved at every stage of development, including project inception, data synthesis, guideline development, and manuscript preparation.
Authors' Contributions
P.W. and L.B.-I. drafted the protocol. The Data Synthesis Committee (C.C., Z.W., S.M.) led and facilitated the systematic review and were consulted on guideline development. T.S. and S.A. conducted the literature search. M.S.P. and P.W. performed data extraction and data interpretation. A.D.B., C.M., C.C., E.M., Z.W., P.J.D., L.F., and P.J.D. drafted guideline sections and recommendations. All authors provided revisions to guidelines. P.W. and C.C. prepared the manuscript. All authors conceived and designed the review, and they read and approved the final manuscript. PW and CC are the guarantors of the review.
Author Disclosure Statement
T.S., S.A., L.B.-I., M.S.P., S.B., M.M.E., L.F., J.K.D., and J.O. do not have any conflicts of interest. P.W. and G.L.'s employer, the Canadian AIDS Society, has received a grant from Canopy Growth Corporation to support the development of the clinical practice guidelines. Z.W. is an advisory board member of Multidisciplinary Association for Psychedelic Studies—Canada. He has received research support from DOJA and Tilray licensed producers of cannabis and is a former Director of Clinical Research for Indigenous Bloom cannabis company. S.M. is the co-owner of a start-up company (“Cannabiscotti, Inc.”) that will be applying for a cannabis processing license. She holds shares in Canopy Growth Corporation, Emblem Corp, and Aphria, Inc. She has received honorarium for research projects funded by Canopy Growth Corporation and Tilray. A.D.B. has received funding for consulting, speaking, and/or research from the following commercial organizations: Amgen, Bristol Myers Squibb, Janssen, AstraZeneca, Novartis, Pfizer, Bayer, Lilly, Boehringer Ingelheim, HLS Therapeutics, Spectrum Therapeutics, Sanofi, Bausch Health, Akcea, and Eisai. He holds shares in a wide variety of pharmaceutical companies and cannabis licensed producers. He has contributed, pro bono, to publications, position statements, and/or clinical practice guidelines from the following non-commercial organizations: Thrombosis Canada, Hypertension Canada, Heart and Stroke Foundation, Canadian Cardiovascular Society, and the Canadian Aids Society.
P.J.D. is a member of the Medical Advisory Board for Shoppers Drug Mart, a consultant for Reformulary Group, a member of the Speakers Bureau for Medical Cannabis Education for Shoppers Drug Mart and Spectrum Therapeutics, and participates in clinical trials for CancerCare Manitoba as per contract requirements. M.G. is the president and co-founder of the Harm Reduction Nurses Association. She was a board member of The Canadian HIV/AIDS Legal Network. She has received an honorarium payment from Merck for a presentation on HIV medication side-effects. C.M. is the Medical Director of Greenleaf Medical Clinic and Translational Life Sciences. She is on the Board of Directors for the Green Organic Dutchman. She is an advisor to Andira, and previously Emerald Health Therapeutics, Vitality Biopharma, Shoppers Drug Mart, True Leaf, Resolve Digital Health, Doja, and Compass Cannabis Clinics. She teaches medical cannabis to medical residents and pharmacy students at the University of British Columbia. She has provided medical consultation and/or received support for industry-sponsored continuing medical education from: Canopy/Spectrum, Tilray, Strainprint, Scientus Pharma, Aurora, MedReleaf & MD Briefcase.
E.M. is the co-owner of a start-up company (“Cannabiscotti, Inc.”) that will be applying for a cannabis processing license, and is employed by MJardin Canada. C.C. has received cannabinoids from Tilray, Inc., for use in a clinical trial but has not received any grant support nor honoraria from the company. She has received research funding from Merck and Gilead, speaker honorarium from Gilead, and consultant fees from Viiv Healthcare. She has received funding to attend conferences from Gilead and Viiv Healthcare.
Funding Information
This work was supported by the Canadian Institutes for Health Research, grant no.: 402773; Arthritis Society of Canada, grant no.: NA; Canopy Growth Corporation, grant no.: NA. Neither the funders nor authors' affiliated institutions played any role in the development of the guidelines.
Supplementary Material
Cite this article as: Bell AD, MacCallum C, Margolese S, Walsh Z, Wright P, Daeninck PJ, Mandarino E, Lacasse G, Deol JK, Freitas L, St. Pierre M, Belle-Isle L, Gagnon M, Bevan S, Sanchez T, Arlt S, Monahan-Ellison M, O'Hara J, Boivin M, Costiniuk C; and External Review Panel (2024) Clinical practice guidelines for cannabis and cannabinoid-based medicines in the management of chronic pain and co-occurring conditions, Cannabis and Cannabinoid Research 9:2, 669–687, DOI: 10.1089/can.2021.0156.
References
- 1. Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770; doi: 10.1186/1471-2458-11-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson TP, Stabile VS, McQueen KAK. The global burden of chronic pain. ASA Newsl 2014;78(6):24–27. [Google Scholar]
- 3. Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag 2011;16(6):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reitsma ML, Tranmer JE, Buchanan DM, et al. . The prevalence of chronic pain and pain-related interference in the Canadian population from 1994 to 2008. Chronic Dis Inj Can 2011;31(4):157–164. [PubMed] [Google Scholar]
- 5. Steingrímsdóttir ÓA, Landmark T, Macfarlane GJ, et al. . Defining chronic pain in epidemiological studies: A systematic review and meta-analysis. Pain 2017;158(11):2092–2107; doi: 10.1097/j.pain.0000000000001009 [DOI] [PubMed] [Google Scholar]
- 6. Asmundson GJG, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 2009;26(10):888–901; doi: 10.1002/da.20600 [DOI] [PubMed] [Google Scholar]
- 7. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: Evolving views on their comorbidity. Perspect Psychiatr Care 2015;51(4):295–304; doi: 10.1111/ppc.12093 [DOI] [PubMed] [Google Scholar]
- 8. Ilgen MA, Perron B, Czyz EK, et al. . The timing of onset of pain and substance use disorders. Am J Addict 2010;19(5):409–415; doi: 10.1111/j.1521-0391.2010.00065.x [DOI] [PubMed] [Google Scholar]
- 9. Lerman SF, Rudich Z, Brill S, et al. . Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom Med 2015;77(3):333–341; doi: 10.1097/PSY.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 10. Morasco BJ, Gritzner S, Lewis L, et al. . Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. Pain 2011;152(3):488–497; doi: 10.1016/j.pain.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem Biodivers 2007;4(8):1729–1743; doi: 10.1002/cbdv.200790150 [DOI] [PubMed] [Google Scholar]
- 12. Yalcin I, Barrot M. The anxiodepressive comorbidity in chronic pain. Curr Opin Anaesthesiol 2014;27(5):520–527; doi: 10.1097/ACO.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 13. Belle-Isle L, Hathaway A. Barriers to access to medical cannabis for Canadians living with HIV/AIDS. AIDS Care 2007;19(4):500–506; doi: 10.1080/09540120701207833 [DOI] [PubMed] [Google Scholar]
- 14. Belle-Isle L, Walsh Z, Callaway R, et al. . Barriers to access for Canadians who use cannabis for therapeutic purposes. Int J Drug Policy 2014;25(4):691–699; doi: 10.1016/j.drugpo.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 15. Hill KP, Palastro MD, Johnson B, et al. . Cannabis and pain: A clinical review. Cannabis Cannabinoid Res 2017;2(1):96–104; doi: 10.1089/can.2017.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walsh Z, Callaway R, Belle-Isle L, et al. . Cannabis for therapeutic purposes: Patient characteristics, access, and reasons for use. Int J Drug Policy 2013;24(6):511–516; doi: 10.1016/j.drugpo.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 17. Wright P, Walsh Z, Margolese S, et al. . Canadian clinical practice guidelines for the use of plant-based cannabis and cannabinoid-based products in the management of chronic non-cancer pain and co-occurring conditions: Protocol for a systematic literature review. BMJ Open 2020;10(5):e036114; doi: 10.1136/bmjopen-2019-036114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Shamseer L, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1; doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roen K, Arai L, Roberts H, et al. . Extending systematic reviews to include evidence on implementation: Methodological work on a review of community-based initiatives to prevent injuries. Soc Sci Med 2006;63(4):1060–1071; doi: 10.1016/j.socscimed.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 20. Armstrong R, Waters E, Roberts H, et al. . The role and theoretical evolution of knowledge translation and exchange in public health. J Public Health (Oxf) 2006;28(4):384–389; doi: 10.1093/pubmed/fdl072 [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–926; doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyatt GH, Oxman AD, Kunz R, et al. . What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336(7651):995–998; doi: 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Kunz R, et al. . Going from evidence to recommendations. BMJ 2008;336(7652):1049–1051; doi: 10.1136/bmj.39493.646875.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaeschke R, Guyatt GH, Dellinger P, et al. . Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744; doi: 10.1136/bmj.a744 [DOI] [PubMed] [Google Scholar]
- 25. Schünemann HJ, Schünemann AHJ, Oxman AD, et al. . Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106–1110; doi: 10.1136/bmj.39500.677199.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guyatt GH, Oxman AD, Kunz R, et al. . Incorporating considerations of resources use into grading recommendations. BMJ 2008;336(7654):1170–1173; doi: 10.1136/bmj.39504.506319.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Heart Lung and Blood Institute. Study Quality Assessment Tools. NHLBI, NIH. 2020. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Last accessed: August 7, 2022].
- 28. Häuser W, Petzke F, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management—An overview of systematic reviews. Eur J Pain 2018;22(3):455–470; doi: 10.1002/ejp.1118 [DOI] [PubMed] [Google Scholar]
- 29. Allan GM, Finley CR, Ton J, et al. . Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018;64(2):e78–e94. [PMC free article] [PubMed] [Google Scholar]
- 30. Häuser W, Fitzcharles MA, Radbruch L, et al. . Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int 2017;114(38):627–634; doi: 10.3238/arztebl.2017.0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madden K, van der Hoek N, Chona S, et al. . Cannabinoids in the management of musculoskeletal pain: A critical review of the evidence. JBJS Rev 2018;6(5):e7; doi: 10.2106/JBJS.RVW.17.00153 [DOI] [PubMed] [Google Scholar]
- 32. Park JY, Wu LT. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: A review. Drug Alcohol Depend 2017;177:1–13; doi: 10.1016/j.drugalcdep.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stockings E, Campbell G, Hall WD, et al. . Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018;159(10):1932–1954; doi: 10.1097/j.pain.0000000000001293 [DOI] [PubMed] [Google Scholar]
- 34. Nugent SM, Morasco BJ, O'Neil ME, et al. . The effects of cannabis among adults with chronic pain and an overview of general harms: A systematic review. Ann Intern Med 2017;167(5):319–331; doi: 10.7326/M17-0155 [DOI] [PubMed] [Google Scholar]
- 35. Fitzcharles MA, Baerwald C, Ablin J, et al. . Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz 2016;30(1):47–61; doi: 10.1007/s00482-015-0084-3 [DOI] [PubMed] [Google Scholar]
- 36. Iskedjian M, Bereza B, Gordon A, et al. . Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin 2007;23(1):17–24; doi: 10.1185/030079906x158066 [DOI] [PubMed] [Google Scholar]
- 37. Whiting PF, Wolff RF, Deshpande S, et al. . Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015;313(24):2456–2473; doi: 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 38. Andreae MH, Carter GM, Shaparin N, et al. . Inhaled cannabis for chronic neuropathic pain: A meta-analysis of individual patient data. J Pain 2015;16(12):1221–1232; doi: 10.1016/j.jpain.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deshpande A, Mailis-Gagnon A, Zoheiry N, et al. . Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can Fam Physician 2015;61(8):e372–e381. [PMC free article] [PubMed] [Google Scholar]
- 40. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol 2011;72(5):735–744; doi: 10.1111/j.1365-2125.2011.03970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meng H, Johnston B, Englesakis M, et al. . Selective cannabinoids for chronic neuropathic pain: A systematic review and meta-analysis. Anesth Analg 2017;125(5):1638–1652; doi: 10.1213/ANE.0000000000002110 [DOI] [PubMed] [Google Scholar]
- 42. Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol 2015;10(2):293–301; doi: 10.1007/s11481-015-9600-6 [DOI] [PubMed] [Google Scholar]
- 43. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017;20(6):E755–E796. [PubMed] [Google Scholar]
- 44. Boychuk DG, Goddard G, Mauro G, et al. . The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: A systematic review. J Oral Facial Pain Headache 2015;29(1):7–14; doi: 10.11607/ofph.1274 [DOI] [PubMed] [Google Scholar]
- 45. Mücke M, Phillips T, Radbruch L, et al. . Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018;3:CD012182; doi: 10.1002/14651858.CD012182.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abrams DI, Jay CA, Shade SB, et al. . Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007;68(7):515–521; doi: 10.1212/01.wnl.0000253187.66183.9c [DOI] [PubMed] [Google Scholar]
- 47. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: Results of a randomised controlled trial. Pain 2004;112(3):299–306; doi: 10.1016/j.pain.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 48. Blake DR, Robson P, Ho M, et al. . Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45(1):50–52; doi: 10.1093/rheumatology/kei183 [DOI] [PubMed] [Google Scholar]
- 49. Collin C, Ehler E, Waberzinek G, et al. . A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 2010;32(5):451–459; doi: 10.1179/016164109X12590518685660 [DOI] [PubMed] [Google Scholar]
- 50. Corey-Bloom J, Wolfson T, Gamst A, et al. . Smoked cannabis for spasticity in multiple sclerosis: A randomized, placebo-controlled trial. CMAJ 2012;184(10):1143–1150; doi: 10.1503/cmaj.110837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ellis RJ, Toperoff W, Vaida F, et al. . Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology 2009;34(3):672–680; doi: 10.1038/npp.2008.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Langford RM, Mares J, Novotna A, et al. . A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol 2013;260(4):984–997; doi: 10.1007/s00415-012-6739-4 [DOI] [PubMed] [Google Scholar]
- 53. Novotna A, Mares J, Ratcliffe S, et al. . A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011;18(9):1122–1131; doi: 10.1111/j.1468-1331.2010.03328.x [DOI] [PubMed] [Google Scholar]
- 54. Nurmikko TJ, Serpell MG, Hoggart B, et al. . Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133(1–3):210–220; doi: 10.1016/j.pain.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 55. Rog DJ, Nurmikko TJ, Friede T, et al. . Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65(6):812–819; doi: 10.1212/01.wnl.0000176753.45410.8b [DOI] [PubMed] [Google Scholar]
- 56. Selvarajah D, Gandhi R, Emery CJ, et al. . Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: Depression is a major confounding factor. Diabetes Care 2010;33(1):128–130; doi: 10.2337/dc09-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Serpell M, Ratcliffe S, Hovorka J, et al. . A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014;18(7):999–1012; doi: 10.1002/j.1532-2149.2013.00445.x [DOI] [PubMed] [Google Scholar]
- 58. Wade DT, Makela P, Robson P, et al. . Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 2004;10(4):434–441; doi: 10.1191/1352458504ms1082oa [DOI] [PubMed] [Google Scholar]
- 59. Wade DT, Robson P, House H, et al. . A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 2003;17(1):21–29; doi: 10.1191/0269215503cr581oa [DOI] [PubMed] [Google Scholar]
- 60. Ware MA, Wang T, Shapiro S, et al. . Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. CMAJ 2010;182(14):E694–E701; doi: 10.1503/cmaj.091414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weizman L, Dayan L, Brill S, et al. . Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology 2018;91(14):e1285–e1294; doi: 10.1212/WNL.0000000000006293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilsey B, Marcotte T, Deutsch R, et al. . Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013;14(2):136–148; doi: 10.1016/j.jpain.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilsey B, Marcotte T, Tsodikov A, et al. . A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008;9(6):506–521; doi: 10.1016/j.jpain.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilsey B, Marcotte TD, Deutsch R, et al. . An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain 2016;17(9):982–1000; doi: 10.1016/j.jpain.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zajicek J, Fox P, Sanders H, et al. . Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003;362(9395):1517–1526; doi: 10.1016/S0140-6736(03)14738-1 [DOI] [PubMed] [Google Scholar]
- 66. Zajicek JP, Hobart JC, Slade A, et al, MUSEC Research Group. Multiple sclerosis and extract of cannabis: Results of the MUSEC trial. J Neurol Neurosurg Psychiatry 2012;83(11):1125–1132; doi: 10.1136/jnnp-2012-302468 [DOI] [PubMed] [Google Scholar]
- 67. Zajicek JP, Sanders HP, Wright DE, et al. . Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 2005;76(12):1664–1669; doi: 10.1136/jnnp.2005.070136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abrams DI, Couey P, Shade SB, et al. . Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 2011;90(6):844–851; doi: 10.1038/clpt.2011.188 [DOI] [PubMed] [Google Scholar]
- 69. Bellnier T, Brown GW, Ortega TR. Preliminary evaluation of the efficacy, safety, and costs associated with the treatment of chronic pain with medical cannabis. Ment Health Clin 2018;8(3):110–115; doi: 10.9740/mhc.2018.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chan S, Blake A, Wolt A, et al. . Medical cannabis use for patients with post-traumatic stress disorder (PTSD). J Pain Manage 2017;10(4):385–396. [Google Scholar]
- 71. Cuñetti L, Manzo L, Peyraube R, et al. . Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc 2018;50(2):461–464; doi: 10.1016/j.transproceed.2017.12.042 [DOI] [PubMed] [Google Scholar]
- 72. Haroutiunian S, Rosen G, Shouval R, et al. . Open-label, add-on study of tetrahydrocannabinol for chronic nonmalignant pain. J Pain Palliat Care Pharmacother 2008;22(3):213–217; doi: 10.1080/15360280802251215 [DOI] [PubMed] [Google Scholar]
- 73. Haroutounian S, Ratz Y, Ginosar Y, et al. . The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: A prospective open-label study. Clin J Pain 2016;32(12):1036–1043; doi: 10.1097/AJP.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 74. Hoggart B, Ratcliffe S, Ehler E, et al. . A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol 2015;262(1):27–40; doi: 10.1007/s00415-014-7502-9 [DOI] [PubMed] [Google Scholar]
- 75. Notcutt W, Price M, Miller R, et al. . Initial experiences with medicinal extracts of cannabis for chronic pain: Results from 34 “N of 1” studies. Anaesthesia 2004;59(5):440–452; doi: 10.1111/j.1365-2044.2004.03674.x [DOI] [PubMed] [Google Scholar]
- 76. Poli P, Crestani F, Salvadori C, et al. . Medical cannabis in patients with chronic pain: Effect on pain relief, pain disability, and psychological aspects. A prospective non randomized single arm clinical trial. Clin Ter 2018;169(3):e102–e107; doi: 10.7417/T.2018.2062 [DOI] [PubMed] [Google Scholar]
- 77. Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: An uncontrolled, open-label, 2-year extension trial. Clin Ther 2007;29(9):2068–2079; doi: 10.1016/j.clinthera.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 78. Russo M, Naro A, Leo A, et al. . Evaluating Sativex® in neuropathic pain management: A clinical and neurophysiological assessment in multiple sclerosis. Pain Med 2016;17(6):1145–1154; doi: 10.1093/pm/pnv080 [DOI] [PubMed] [Google Scholar]
- 79. Bonn-Miller MO, Boden MT, Bucossi MM, et al. . Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2014;40(1):23–30; doi: 10.3109/00952990.2013.821477 [DOI] [PubMed] [Google Scholar]
- 80. Brunt TM, van Genugten M, Höner-Snoeken K, et al. . Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J Clin Psychopharmacol 2014;34(3):344–349; doi: 10.1097/JCP.0000000000000129 [DOI] [PubMed] [Google Scholar]
- 81. Campbell G, Hall WD, Peacock A, et al. . Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: Findings from a 4-year prospective cohort study. Lancet Public Health 2018;3(7):e341–e350; doi: 10.1016/S2468-S2667(18)30110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clark AJ, Ware MA, Yazer E, et al. . Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004;62(11):2098–2100; doi: 10.1212/01.wnl.0000127707.07621.72 [DOI] [PubMed] [Google Scholar]
- 83. Degenhardt L, Lintzeris N, Campbell G, et al. . Experience of adjunctive cannabis use for chronic non-cancer pain: Findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 2015;147:144–150; doi: 10.1016/j.drugalcdep.2014.11.031 [DOI] [PubMed] [Google Scholar]
- 84. Nugent SM, Yarborough BJ, Smith NX, et al. . Patterns and correlates of medical cannabis use for pain among patients prescribed long-term opioid therapy. Gen Hosp Psychiatry 2018;50:104–110; doi: 10.1016/j.genhosppsych.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tripp DA, Nickel JC, Katz L, et al. . A survey of cannabis (marijuana) use and self-reported benefit in men with chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J 2014;8(11–12):E901–E905; doi: 10.5489/cuaj.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vigil JM, Stith SS, Adams IM, et al. . Associations between medical cannabis and prescription opioid use in chronic pain patients: A preliminary cohort study. PLoS One 2017;12(11):e0187795; doi: 10.1371/journal.pone.0187795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ware MA, Doyle CR, Woods R, et al. . Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain 2003;102(1–2):211–216; doi: 10.1016/s0304-3959(02)00400-1 [DOI] [PubMed] [Google Scholar]
- 88. Woolridge E, Barton S, Samuel J, et al. . Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage 2005;29(4):358–367; doi: 10.1016/j.jpainsymman.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 89. Yassin M, Oron A, Robinson D. Effect of adding medical cannabis to analgesic treatment in patients with low back pain related to fibromyalgia: An observational cross-over single centre study. Clin Exp Rheumatol 2019;37 Suppl 116(1):13–20. [PubMed] [Google Scholar]
- 90. Fanelli G, De Carolis G, Leonardi C, et al. . Cannabis and intractable chronic pain: An explorative retrospective analysis of Italian cohort of 614 patients. J Pain Res 2017;10:1217–1224; doi: 10.2147/JPR.S132814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lynch ME, Young J, Clark AJ. A case series of patients using medicinal marihuana for management of chronic pain under the Canadian Marihuana Medical Access Regulations. J Pain Symptom Manage 2006;32(5):497–501; doi: 10.1016/j.jpainsymman.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 92. Ware MA, Gamsa A, Persson J, et al. . Cannabis for chronic pain: Case series and implications for clinicians. Pain Res Manag 2002;7(2):95–99; doi: 10.1155/2002/380509 [DOI] [PubMed] [Google Scholar]
- 93. Hunter D, Oldfield G, Tich N, et al. . Synthetic transdermal cannabidiol for the treatment of knee pain due to osteoarthritis. Osteoarthritis Cartilage 2018;26:S26; doi: 10.1016/j.joca.2018.02.067 [DOI] [Google Scholar]
- 94. Nicolodi M, Sandoval V, Terrine A.. Therapeutic use of cannabinoids—Dose finding, effects, and pilot data of effects in chronic migraine and cluster headache. Abstract presented at 3rd congress of the European Academy of Neurology, June 24–27, 2017, Amsterdam, the Netherlands. [Google Scholar]
- 95. Rhyne DN, Anderson SL, Gedde M, et al. . Effects of medical marijuana on migraine headache frequency in an adult population. Pharmacotherapy 2016;36(5):505–510; doi: 10.1002/phar.1673 [DOI] [PubMed] [Google Scholar]
- 96. Wilsey B, Marcotte TD, Deutsch R, et al. . Low dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013;14(2):136–148; doi: 10.1016/j.jpain.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Woolridge E, Barton S, Samuel J, et al. . Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage 2005;29(4):358–367; doi: 10.1016/j.jpainsymman.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 98. Bonn-Miller MO, Boden MT, Bucossi MM, et al. . Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2014;40(1):23–30; doi: 10.3109/00952990.2013.821477 [DOI] [PubMed] [Google Scholar]
- 99. Lynch ME, Young J, Clark AJ. A case series of patients using medicinal marihuana for management of chronic pain under the Canadian marihuana medical access regulations. J Pain Symptom Manage 2006;32(5):497–501; doi: 10.1016/j.jpainsymman.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 100. Nugent SM, Yarborough BJ, Smith NX, et al. . Patterns and correlates of medical cannabis use for pain among patients prescribed long-term opioid therapy. Gen Hosp Psychiatry 2018;50:104–110; doi: 10.1016/j.genhosppsych.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Campbell G, Hall WD, Peacock A, et al. . Cannabis use, pain and prescription opioid use in people living with chronic non-cancer pain: Findings from a four-year prospective cohort. Lancet Public Health 2018;3(7):e341–e350; doi: 10.1016/S2468-S2667(18)30110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vigil JM, Stith SS, Adams IM, et al. . Associations between medical cannabis and prescription opioid use in chronic pain patients: A preliminary cohort study. PLoS One 2017;12(11):e0187795; doi: 10.1371/journal.pone.0187795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sihota A, Smith BK, Ahmed SA, et al. . Consensus-based recommendations for titrating cannabinoids and tapering opioids for chronic pain control. Int J Clin Pract 2021;75(8):e13871; doi: 10.1111/ijcp.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007;4(8):1770–1804; doi: 10.1002/cbdv.200790152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Agurell S, Halldin M, Lindgren JE, et al. . Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 1986;38(1):21–43. [PubMed] [Google Scholar]
- 106. Yamaori S, Kushihara M, Yamamoto I, et al. . Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem Pharmacol 2010;79(11):1691–1698; doi: 10.1016/j.bcp.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 107. Bayer, Inc. Xarelto Product Monograph. Bayer Inc. 2018. Available from: https://pdf.hres.ca/dpd_pm/00043960.PDF [Last accessed: April 13, 2018].
- 108. Pfizer Canada. Eliquis Product Monograph. Pfizer Canada: Quebec, Canada; 2018. [Google Scholar]
- 109. Sanofi-Aventis. Plavix Product Monograph. Sanofi Aventis: Quebec, Canada; 2022. [Google Scholar]
- 110. Geffrey AL, Pollack SF, Bruno PL, et al. . Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015;56(8):1246–1251; doi: 10.1111/epi.13060 [DOI] [PubMed] [Google Scholar]
- 111. Holland ML, Lau DTT, Allen JD, et al. . The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br J Pharmacol 2007;152(5):815–824; doi: 10.1038/sj.bjp.0707467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Holland ML, Panetta JA, Hoskins JM, et al. . The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol 2006;71(8):1146–1154; doi: 10.1016/j.bcp.2005.12.033 [DOI] [PubMed] [Google Scholar]
- 113. Zhu HJ, Wang JS, Markowitz JS, et al. . Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther 2006;317(2):850–857; doi: 10.1124/jpet.105.098541 [DOI] [PubMed] [Google Scholar]
- 114. Alsherbiny MA, Li CG. Medicinal cannabis-potential drug interactions. Medicines (Basel) 2018;6(1):E3; doi: 10.3390/medicines6010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018;84(11):2477–2482; doi: 10.1111/bcp.13710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. MacCallum CA, Lo LA, Boivin M. “Is medical cannabis safe for my patients?” A practical review of cannabis safety considerations. Eur J Intern Med 2021;89:10–18; doi: 10.1016/j.ejim.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 117. van Os J, Bak M, Hanssen M, et al. . Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol 2002;156(4):319–327; doi: 10.1093/aje/kwf043 [DOI] [PubMed] [Google Scholar]
- 118. D'Souza DC, Abi-Saab WM, Madonick S, et al. . Delta-9-tetrahydrocannabinol effects in schizophrenia: Implications for cognition, psychosis, and addiction. Biol Psychiatry 2005;57(6):594–608; doi: 10.1016/j.biopsych.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 119. D'Souza DC, Perry E, MacDougall L, et al. . The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004;29(8):1558–1572; doi: 10.1038/sj.npp.1300496 [DOI] [PubMed] [Google Scholar]
- 120. Health Canada. Information for Health Care Professionals: Cannabis (Marihuana, Marijuana) and the Cannabinoids. aem. 2018. Available from: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids.html [Last accessed: June 20, 2020].
- 121. Ravi D, Ghasemiesfe M, Korenstein D, et al. . Associations between marijuana use and cardiovascular risk factors and outcomes: A systematic review. Ann Intern Med 2018;168(3):187–194; doi: 10.7326/M17-1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Battista N, Pasquariello N, Di Tommaso M, et al. . Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J Neuroendocrinol 2008;20 Suppl 1:82–89; doi: 10.1111/j.1365-2826.2008.01684.x [DOI] [PubMed] [Google Scholar]
- 123. Fried PA. Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J Child Psychol Psychiatry 2002;43(1):81–102; doi: 10.1111/1469-7610.00005 [DOI] [PubMed] [Google Scholar]
- 124. Hembree WC, Nahas GG, Zeidenberg P, et al. . Changes in human spermatozoa associated with high dose marihuana smoking. Adv Biosci 1978;22–23:429–439; doi: 10.1016/b978-0-08-023759-6.50038-x [DOI] [PubMed] [Google Scholar]
- 125. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: Current insights. Subst Abuse Rehabil 2017;8:9–37; doi: 10.2147/SAR.S109576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Russo EB, Spooner C, May L, et al. . Cannabinoid hyperemesis syndrome survey and genomic investigation. Cannabis Cannabinoid Res 2022;7(3):336–344; doi: 10.1089/can.2021.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goyal H, Rahman MR, Perisetti A, et al. . Cannabis in liver disorders: A friend or a foe? Eur J Gastroenterol Hepatol 2018;30(11):1283–1290; doi: 10.1097/MEG.0000000000001256 [DOI] [PubMed] [Google Scholar]
- 128. Andrews CN, Devlin SM, Le Foll B, et al. . Canadian association of gastroenterology position statement: Use of cannabis in gastroenterological and hepatic disorders. J Can Assoc Gastroenterol 2019;2(1):37–43; doi: 10.1093/jcag/gwy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rieder MJ, Canadian Paediatric Society, Drug Therapy and Hazardous Substances Committee. Is the medical use of cannabis a therapeutic option for children? Paediatr Child Health 2016;21(1):31–34; doi: 10.1093/pch/21.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 2018;49:12–19; doi: 10.1016/j.ejim.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 131. MacCallum CA, de Freitas L, Lo LA, et al. . Cannabinoid-based medicines: dosing, titration & monitoring. In: Cannabinoids and Pain (Narouze SN. ed.) Springer International Publishing: Cham, Switzerland; 2021; pp. 167–178. [Google Scholar]
- 132. Boehnke KF, Clauw DJ. Brief commentary: Cannabinoid dosing for chronic pain management. Ann Intern Med 2019;170(2):118; doi: 10.7326/M18-2972 [DOI] [PubMed] [Google Scholar]
- 133. Bhaskar A, Bell A, Boivin M, et al. . Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: results of a modified Delphi process. J Cannabis Res 2021;3(1):22; doi: 10.1186/s42238-021-00073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.