Abstract
Ultraviolet radiation (UVR) is primarily recognized for its detrimental effects such as cancerogenesis, skin aging, eye damage, and autoimmune disorders. With exception of ultraviolet B (UVB) requirement in the production of vitamin D3, the positive role of UVR in modulation of homeostasis is underappreciated. Skin exposure to UVR triggers local responses secondary to the induction of chemical, hormonal, immune, and neural signals that are defined by the chromophores and extent of UVR penetration into skin compartments. These responses are not random and are coordinated by the cutaneous neuro-immuno-endocrine system, which counteracts the action of external stressors and accommodates local homeostasis to the changing environment. The UVR induces electrical, chemical, and biological signals to be sent to the brain, endocrine and immune systems, as well as other central organs, which in concert regulate body homeostasis. To achieve its central homeostatic goal, the UVR-induced signals are precisely computed locally with transmission through nerves or humoral signals release into the circulation to activate and/or modulate coordinating central centers or organs. Such modulatory effects will be dependent on UVA and UVB wavelengths. This leads to immunosuppression, the activation of brain and endocrine coordinating centers, and the modification of different organ functions. Therefore, it is imperative to understand the underlying mechanisms of UVR electromagnetic energy penetration deep into the body, with its impact on the brain and internal organs. Photo-neuro-immuno-endocrinology can offer novel therapeutic approaches in addiction and mood disorders; autoimmune, neurodegenerative, and chronic pain-generating disorders; or pathologies involving endocrine, cardiovascular, gastrointestinal, or reproductive systems.
Keywords: ultraviolet radiation, skin, body homeostasis, neuroimmunoendocrinology, stress
The skin is continuously exposed to different wavelengths of solar radiation during the daytime. The biologically relevant wavelengths of ultraviolet radiation (UVR) are UVB (ultraviolet B) (280 to 315 nm) and UVA (UVA2 = 315 to 340 nm and UVA1 = 340 to 400 nm), representing 3% of solar energy reaching Earth’s surface (1–4). Any wavelength shorter than 290 nm including short UVB spectrum and UVC (200 to 280 nm) is filtered by the atmosphere’s ozone layer. Artificially generated UVC is highly mutagenic and can be lethal. UVC from artificial sources is absorbed by the stratum corneum, the outermost nonviable epidermal layer, causing limited damage to the viable regions of the skin in comparison to UVB (5, 6). Representing only 5% of the total UV energy, UVB is approximately 1,000 more effective than UVA in inducing cutaneous biological effects (7, 8), and is absorbed by the human epidermis, and the upper portion of the papillary dermis (4, 9). On the other hand, UVA has better cutaneous penetration up to the deep levels of the reticular dermis (7, 8).
UVA and UVB differ in their mechanisms of action. UVB phenotypic effects are predominantly secondary to its absorption by cellular chromophores such as 7-dehydrocholesterol (7DHC), DNA, aromatic amino acids and the products of their transformation, purines, pyrimidines, trans-urocanic acid, indoles, quinones, different types of melanin and their precursors, unsaturated lipids to name few, and to lower degree mediated by the generation of reactive oxygen species (ROS) (1–4, 9–19). The UVA effects are predominately mediated by ROS with contribution from limited numbers of cellular chromophores such as porphyrins, riboflavin, NADPH and NADH and weak absorption by DNA (4, 11). In addition, UVA generates nitric oxide (NO) from nitrosoglutathione, and nitroxyl NO− also leading to oxidative/nitrosative stress (20–22). Thus, there are differential and in some cases overlapping mechanisms of action of different UVR spectra.
The negative effects of UVR on the skin are widely documented. These are exemplified by skin cancers including most frequent human malignancy such as basal cell and squamous cell carcinomas secondary to the action of UVB (3, 9). Cutaneous melanoma is the deadliest UVR-induced skin tumor to which both UVB and UVA can contribute (23). UVA plays an important role in skin photoaging, and UVB contributes to the exacerbation of autoimmune disorders such as systemic lupus erythematosus (4, 9).
Traditionally, the positive effect of UVR is solely assigned to the UVB-induced transformation of 7DHC to vitamin D3 that serves as a prohormone to its active form 1,25(OH)2D3 (2, 17, 24, 25). However, it is now recognized that vitamin D can be transformed to several biologically active hydroxyderivatives through alternative activation pathways (26–29) and that other photoproducts such as lumisterol and tachysterol can be activated by enzymatic hydroxylation (30–32), opening a wide range of possibilities. In addition, it has been proposed that UVB played an important role in the origin of life and its evolution according to the laws of thermodynamics, eventually contributing to a diversity of organisms on Earth (1).
The epidemiological observations document that UVB has beneficial health effects independent from classical vitamin D actions. For example, the probability of developing autoimmune disorders such as rheumatoid arthritis (RA) or multiple sclerosis (MS) increases significantly as one moves further away from the Equator (1, 33). In addition, the positive effects of UVR on the cardiovascular system, body metabolism, or behavioral functions are indicated (9, 16, 34–38). Unfortunately, the positive role of UVA on body homeostasis is largely ignored, except for some combined phototherapy approaches (39). The above considerations will be analyzed in the context of precise activation of neuroendocrine and immune pathways at the local and systemic levels.
Local Neuro-Immuno-Endocrine Pathways Induced by UVR
For more than 20 y, the skin has been recognized as the peripheral neuroendocrine organ (40, 41), that is endowed with neuroendocrine signaling systems being equivalents or precursors (42, 43) to the central neuroendocrine signaling involved in the regulation of body homeostasis (44). Both epidermal and dermal compartments composed of resident and circulating cells can produce hormones, neurotransmitters, biogenic amines, and cytokines with neuroendocrine capabilities (Table 1). Classical examples of these mediators include corticotropin-releasing hormone (CRH) or CRH-related urocortins, proopiomelanocortin (POMC)-derived peptides, including ACTH, α-MSH, β-endorphin, and cytokines regulating their expression; proenkephalin (PENK)-derived peptides; and other “hypothalamic” and “pituitary” neurohormones; leptin, adipokinines other diverse neuropeptides (reviewed in refs. (41–43, 45–50). The resident skin cells can produce secosteroids, glucocorticoids, sex hormones, histamine, catecholamines, L-dihydrohyphelyalanine (L-DOPA), serotonin, melatonin, acetylcholine, thyroid hormones, endocannabinoids (reviewed in refs. (2, 10, 25, 41, 51–59), and biologically active lipids or their derivatives (60). The local production of these various mediators acting in concert with corresponding receptors would regulate skin responses to environmental factors, with some following precise central regulatory algorithms such as hypothalamic–pituitary (HP), HP–adrenal (HPA), hypothalamic–thyroid–pituitary, or other axes (reviewed in refs. (10, 42, 46, 48, 57, 61–63).
Table 1.
Production of mediators in the skin after exposure to UVR
| Category of mediators | Molecules affected by UVR | Predominant wavelength of UVR | Phenotypic effects |
|---|---|---|---|
| Hypothalamic hormones (43, 62) | CRH | Stimulated by UVC, UVB, and UVA | 1) Stimulations of POMC production and processing in the pituitary or peripheral organs to produce and release of POMC peptides through action of CRH-R1; 2) direct action on peripheral organs and immune cells via corresponding membrane-bound CRH-R1 and CRH-R2 receptors; and 3) actions on the brain after crossing the blood brain barrier (BBB) in circumventricular organs or when the BBB is disrupted |
| CRH-related peptides (1) | Urocortin 1 | UVB | 1) Stimulation of POMC peptides at the central or peripheral levels; 2) action on peripheral and immune organs/cells via CRH-R1 and CRH-R2 receptors; and 3) actions on the brain after crossing the BBB |
| Pituitary hormones (42, 62, 64) | POMC and derived peptides including ACTH, α-MSH, β-MSH, γ-MSH, and β-endorphin. | Stimulated by UVC, UVB, and by UVA only for β-endorphin | 1) Activation of cortisol/corticosterone production in the adrenal cortex or peripheral organs via the ACTH-MC2 axis; 2) regulation of the skin phenotype, immune system, and other peripheral organs through action on MC or opioid receptors; 3) possible receptor-mediated actions in the brain if crossed the BBB; and 4) ACTH may disrupt circadian rhythm; |
| Opioids (45, 65) | PENK-derived met- and leu-enkephalins | 1) Regulation of immune system and peripheral tissue functions through action on opioid receptors and 2) regulation of brain functions after crossing the BBB | |
| Neuropeptides (46, 50) | CGRP and SP | Predominantly UVB with some effects by UVA | 1) Regulation of skin functions through corresponding membrane-bound receptors and 2) nociceptive effects |
| Cytokines (47, 66) | TNFα, IL-1, IL-4 IL-6, IL-10, TGF-β, and IL-33 | Predominantly UVB with some effects by UVA | 1) TNFα, IL-1, and IL-6 activate the central HPA axis or its equivalents in the periphery with downstream regulation of homeostasis and indirect immunosuppressive effects; 2) IL-4, IL-10, and TGF-β act as direct immunosuppressors; and 3) IL-33 has both pro- and anti-inflammatory functions that are context dependent and protective effect on the cardiovascular system |
| Immune cells (47, 66) | T and B regulatory cells, LC, and DC | Predominantly UVB with some effects by UVA | Circulating immune cells will affect the systemic immune response and function of other organs |
| Antimicrobial peptides (47) | Β-defensins, cathelicidin, and LL-37 | UVB |
Antimicrobial, immunoregulatory activities and regulation of the epidermal barrier |
| Biogenic amines and precursors (58, 67) | L-DOPA and dopamine | L-DOPA production is enhanced by UVB and UVA; dopamine is inhibited in the skin, while enhanced in the brain | 1) Action in the brain when L-DOPA crosses the BBB; 2) pleiotropic actions in the periphery through activation of dopaminergic and adrenergic receptors or supplying catecholamine-producing cells/organs with L-DOPA; and 3) actions as neurotransmitters in the sympathetic (SNS) system |
| Biogenic amines (51, 68) |
Serotonin |
Stimulated by UVB and UVA | 1) Action in the brain after active transport through the BBB and 2) pleiotropic actions in the periphery and on the immune system through activation of membrane-bound serotonin receptors. |
| Steroids (62, 69) | Cortisol, corticosterone, and pregnenolone | UVC and UVB | 1) Glucocorticoids will inhibit local and systemic immune system and other organ functions through action on corresponding nuclear receptors; 2) they will affect brain functions if crossed the BBB; 3) cortisol/corticosterone will inhibit the HPA axis; and 4) pregnenolone will serve as precursor for steroid production |
| Secosteroids (17, 70) | Vitamin D3 with full or short side chain; lumisterol3 and tachysterol3 with full or short side chain | UVB acting on the B-ring of 7DHC or 7DHP (7-dehydropregnenolone) or their hydroxymetabolites | Action on VDR (vitamin D receptor) and alternative nuclear receptors to regulate 1) body calcium metabolism, 2) musculoskeletal systems, and 3) functions of immune and other systems or organs including the skin, neuroendocrine system, gastrointestinal tract (GI), brain, and reproductive system |
| Imidazole derivative (71–73) | cis-UCA (urocanic acid) | UVB | Local and systemic immunosuppressive effects through action on 5-HT2A or other receptors. |
| Tryptophan derivatives (74, 75) | FITC (6-formylindolo[3,2-b]carbazole) and other photoproducts | UVB | Local phenotypic effects action through activation of the AhR (aryl-hydrocarbon receptor) with implications for the systemic immune system |
| Melatonin metabolites (19, 76) | N-Acetyl-N-formyl-5-methoxykynurenamine (AFMK), N1-Acetyl-5-Methoxykynuramine (AMK), and 2-and 4-hydroxymelatonin | UVB and UVA | Cytoprotective activities; receptor candidate is represented by the AhR (77) |
| Membrane lipids (60, 78, 79) | PAF (platelet-activating factor) and PAF-like molecules | UVB | Local and systemic immunoregulatory functions by interactions with the PAF receptor |
| Gases (1, 22) | NO and NO− | UVA | 1) Regulation of local vascular, immune, antimicrobial, and barrier functions and 2) systemic vasodilatory effects |
| DNA (3, 80) | CPD (cyclobutane pyrimidine dimer) and 6–4PP | Predominantly UVB with some effects by UVA | Immunosuppression and stimulation of melanogenesis and local POMC activity |
The table lists hormones, neurohormones/neurotransmitters, and immune signals activated by UVB or/and UVA or biologically active chemicals formed in the integument with predictable local or systemic phenotypic effects. These molecules would target specific receptors or regulatory proteins triggering local or systemic signal transduction pathways in response to different wavelengths of UVR. Citations are in parentheses.
The production of neuroendocrine and immune factors stated above is affected by UVR in a wavelength and context-dependent fashion, with some entering circulation and others activating sensory nerve endings in the skin (Fig. 1). For example, it is well documented that UVB can stimulate the production of POMC-derived ACTH, α-MSH, β-endorphin, CRH and related peptides, enkephalins, and cytokines (reviewed in refs. (1, 42, 47, 50, 81), some of the latter being involved in the modulation of the central or local HPA axis (61). Furthermore, it regulates the expression of G protein–coupled receptor targets with upregulation of the MC1 or CRHR1α with changes of isoform pattern as it relates to the latter receptor (43, 82). UVB can stimulate cutaneous corticosteroid production with inhibition of glucocorticoid receptors expression on keratinocytes (62, 69) as a part of an epidermal protective mechanism from UVR damage to maintain the barrier function while inhibiting immune attack against UVB exposed keratinocytic antigens. Interestingly, the shorter the wavelength, the stronger is induction of stress hormone production, with UVC being most effective followed by UVB and with UVA being the least effective (62, 69). UVA stimulates production of only β-endorphin and CRH and is ineffective in stimulation of ACTH or corticosteroidogenesis.
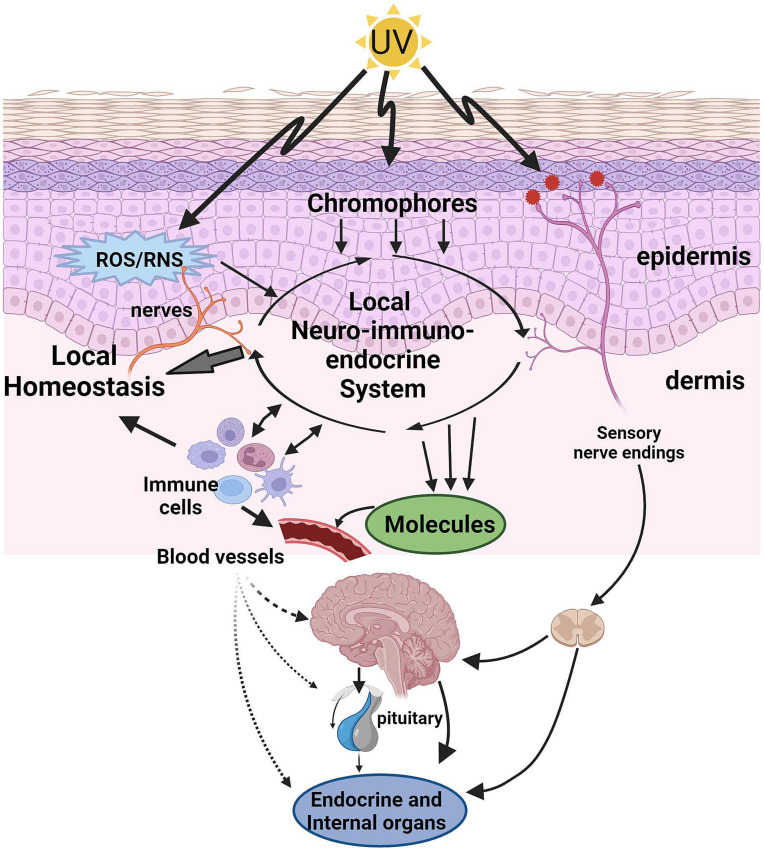
Fig. 1.
UVR activates neuro-immuno-endocrine pathways leading to the regulation of the local and global homeostasis. UVR energy absorbed by the skin induces functional changes directly (chromophores, ROS/RNS, and physicochemical alterations) or indirectly (cellular production and release of biomolecules) and is coordinated by the local neuro-immuno-endocrine system to regulate homeostasis. Locally released molecules or activated immune cells (cellular messengers) can enter systemic circulation and act on brain, endocrine and internal organs. Furthermore, nerve ending in the skin is stimulated directly or indirectly with further neurotransmission to the brain and central organs. All of these elements act in a coordinated manner to regulate systemic homeostasis. UV: ultraviolet radiation, ROS: reactive oxygen species, RNS: reactive nitrogen species.
Locally induced neuroendocrine or immune factors with endocrine capabilities can enter the circulation depending on the solar energy absorbed by the skin and modulate body homeostasis through action at the central coordinating elements, including the hypothalamus, pituitary, adrenal, and thyroid glands as well as the immune system (Fig. 1). Examples of these factors include CRH, urocortins, POMC-derived peptides, enkephalins (42, 45, 63), IL-1, IL-6, and TNFα (47), with latter also acting on the hypothalamus or pituitary (44, 61, 83). These are in addition to classical UVB cutaneous messenger, vitamin D3 (2, 25), and the recently defined prohormones, lumisterol (31) and tachysterol (30). Concerning other peptides produced by skin, including hypothalamic (TRH, GHRH), pituitary factors (TSH, oxytocin) or leptin, it remains to be determined whether UVR stimulates their release to the systemic circulation, to exert homeostatic effects as discussed recently for advanced cancers (61).
Concerning biogenic amines, stimulation of systemic serotonin in defined subgroups of patients exposed to UVR was reported (reviewed in ref. (68)). In support of this hypothesis, we note that UVA can induce nonenzymatic transformation of L-tryptophan to L-5-hydroxytryptophan, a direct precursor of serotonin (52). In addition, our studies using human immortalized keratinocytes and cocultured keratinocytes and melanocytes have shown that low doses of UVB (10 mJ/cm2) stimulate production of serotonin and inhibition at 10 times higher cytotoxic doses of 100 mJ//cm2 (51). These cell cultures have shown that UVB inhibited production of dopamine, an inhibitor of serotonin synthesis, indicating the potential for increased accumulation of serotonin in vivo. With respect to catecholamines, UVB and to certain degree UVA stimulate cutaneous melanogenesis (3), with increased production of L-DOPA, a precursor to melanin and catecholamines (58). This may lead to an indirect effect of UVR on catecholamine production in pigmented skin, as suggested by observation of rescuing catecholamine production in tyrosine hydroxylase knock-out mice by rate-limiting melanogenic enzyme tyrosinase (84). Finally, UVR can affect local production of endocannabinoids leading to changes in their concentrations in the human serum (85).
The current challenge is to dissect, which systemic effects are secondary to entry of UVR induced biomolecules to the circulation, entry of UVR primed immune cells or direct activation of sensory nerves in the skin following transmission to corresponding brain centers (Fig. 1) (1). These could include different reflex effects bypassing the brain as proposed previously (40). The skin is heavily innervated with somatosensory and autonomic nerves supplying not only epidermal compartment but sensory nerve fiber reaching the level of stratum corneum (reviewed in refs. (42, 46, 48, 86–88). Therefore, neurotransmitters, opioids, endocannabinoids, and other biologically active molecules induced by UVR including neuropeptides can activate corresponding receptors on sensory nerves with further rapid transmission to the central levels (Table 1). Anatomic specificity is defined by transmission through either cranial or spinal nerves, with latter effects being dermatome specific. Furthermore, changes in physicochemical composition within nonviable or differentiating layers of the epidermis such as stratum granulosum or stratum corneum induced by UVR can be sensed by nerve endings supplying these compartments. The expected mechanism of activation could be similar or overlapping with nociception, itching and temperature sensation described in the skin or other barrier organs such gastrointestinal system that also shows neuroendocrine capabilities (89). The UVR-induced physicochemical changes sensed by nerve ending would include pH, ion, and ROS concentrations, representing conserved mechanisms of animal nociception (1, 90).
UVR-Dependent Formation of Biologically Active Chemical Structures
Absorption of UVR energy in the skin would lead to the nonenzymatic formation of biologically active chemical structures in a wavelength-defined fashion and its ability to penetrate epidermal and dermal compartments (Table 1). They would act locally (directly or after enzymatic modification) through interaction with specific receptors or regulatory proteins to trigger optimal transduction pathways aimed at the preservation of local homeostasis best suited for environmental changes driven by UVR. They would also enter systemic circulation or act on nerve endings to affect global homeostasis.
An example of this phenomenon is vitamin D3 (D3) formation, a recognized prohormone, after absorption by the B-ring of 7DHC of the UVB electromagnetic energy, with a peak at 295 nm [reviewed in refs. (2, 24)]. Conventionally, it is activated by C25 and C1α hydroxylases to produce 1,25(OH)2D3, which interacts with VDR to exert a variety of phenotypic effects by heterodimerizing with cognate retinoid X receptors in the genomic route (2, 24, 25, 91). However, alternative pathways of vitamin D activation by CYP11A1 leading to the production of several hydroxymetabolites (27, 28, 59) with biological activities defined by activation of several nuclear receptors are now recognized (92–94). They are produced in the skin and detectable on the systemic level in the human serum, skin, and placenta (26, 28, 95). Some of these hydroxymetabolites are detectable in natural products such as honey (96). Also, the photoproducts of pre-D3 such as lumisterol and tachysterol are activated by CYP enzymes in vivo and detectable in the human serum (30–32), breaking the conventional opinion that they are biologically inactive. Notably, in the skin, D3-hydroxymetabolites can stimulate the expression of different elements of CRH and POMC signaling (97). It can also affect brain functions through stimulation of the central serotoninergic system (98–100). Other Δ5,7 sterols that are intermediates of cholesterol synthesis, and 7DHP, a product of the cleavage of the site chain of 7DHC and its hydroxyderivatives, represent educated UVB-generated photoderivatives with biological activities (27, 92, 101). In this context, 7DHC could serve as a chromophore for UVB receptors, a protein to which this molecule can bound including retinoic acid orphans receptors, liver X receptors or AhR (30, 93) since pre-D3 is thermodynamically unstable undergoing isomerization to D3 or lumisterol and tachysterol that are reversible and depend on temperature and UVB energy (17).
DNA is another classical chromophore, which after absorption of UVB energy has systemic immunosuppressive effects related to production of CPD (80, 102). The UVB-induced DNA damage with fragments excised during its repair can stimulate melanin pigmentation and local POMC activity (103, 104). As relates to small molecules present in the skin, an absorption of the UVB energy by L-tryptophan can generate photoproducts including FITC which would activate AhR with downstream phenotypic effects (74, 75). Similarly, UCA after absorption of UVB transforms into cis-UCA, which is a potent systemic immunosuppressor with a capability of interacting with serotonin receptors (71, 72, 105). Furthermore, melatonin which is produced in the skin from tryptophan (56) after absorption of UVB energy will undergo nonenzymatic transformation to kynuric metabolites including AFMK and AMK (76), which are biologically active (106, 107). Another example of UVB-induced molecule is PAF (1-alkyl-2-acetyl-glycero-3-phosphocholine), derived from glycerophosphocholines, PAF is a potent activator of many cell types through membrane-bound receptors (60, 78). These include release of microvesicle particles, causing a variety of local and systemic immunological effects(60). The above systems transforming electromagnetic energy of UVB into biological effects would fulfill definition of UVB photoreceptors as suggested previously (108, 109).
For UVR with longer wavelength, recent evidence demonstrates that UVA photoreception in epidermal melanocytes involves mechanisms similar to phototransduction in the eye (110) or the activation of transient receptor potential A1 ion channels (110–112).
With respect to the former, it has been reported that different opsins such as melanopsin, rhodopsin, as well as circadian clock proteins are expressed in the epidermal and dermal skin cells, and their activation can induce local phenotypic effects, which include changes in melanin pigmentation (1, 113–123).
Such diverse cutaneous photoreception, which is dependent on the wavelength and energy of the UVR and use of different chromophores, target regulatory proteins and signal transduction pathways set the background for an educated analysis of links between photoproduced molecules and regulation of local neuro-immuno-endocrine pathways. The current challenge is how to connect such cutaneous regulatory systems with central coordinating centers and immune system.
UVR Activation of the Central Neuro-Endocrine System
There are several examples of the UVB activating central neuro-endocrine system through the skin (1), which are linked or are aside of the systemic immunosuppressive effects (1, 47). UVB can activate the central HPA axis in which adrenocortical stimulation of corticosterone production required an intact pituitary (63), as is expected for the classical HPA (44). These effects were also associated with increased production of CRH, urocortin, β-endorphin, and ACTH in the brain (63, 124, 125). UVB also stimulates POMC signaling in the arcuate nucleus of the hypothalamus (125). Interestingly, cutaneous exposure to UVB rapidly (60 to 90 min after exposure) stimulates central systemic CRH, ACTH, β-endorphin, and CORT production accompanied by induction of immunosuppressive effects in spleen lymphocytes independent of the pituitary (124). Studies also show that exposure of the eye to the UVB (126) or UVA (127) could stimulate the central HP or HPA axis with downstream positive phenotypic effects not only in the skin but also in ameliorating inflammatory responses in the GI.
Other important examples of the UVR effects on the central neuroendocrine system include the activation of the brain–gonadal axis with regulation of sexual behavior (128) or regulation of feeding behavior (1) and body metabolism (35, 129, 130). Additionally, moderate UV exposure enhances learning and memory by stimulating glutamate signaling in the brain (36). However, UVB depending on the dose and frequency of exposure can have negative systemic effects such as induction of fatigue-like behavior connected with increased serum levels of β-endorphin (131). In addition, UVB and UVA can lead to the addictive behavior through different mechanisms (1, 50, 64, 67, 81, 132, 133). However, UVR has positive effects on mood and depressive disorders and enhances feeling of well-being (134).
Thus, UVR can activate different neuroendocrine mechanisms regulating functions of the brain, endocrine and immune systems, and by inference peripheral organs. Many of these mechanisms are independent from the UVB-induced vitamin D production. The best example is beneficial effect of UVB on development and/or progression of MS, which in big part appears to be independent from the classical vitamin D signaling (9, 135) as measured by production of 25(OH)D3, expression of the VDR, or action of an activating enzyme CYP27B1 (135–137). Thus, these beneficial effects could be secondary to activation of central neuroendocrine axes of which HP or HPA are examples. It must be noted that POMC-derived peptides are not only anti-inflammatory but also have cytoprotective and neuroprotective functions (42, 82, 138). However, since vitamin D3 play a protective role in MS, novel CYP11A1 initiated pathways of vitamin D or related lumisterol and tachysterol activations must be taken into consideration since these can act on alternative to the VDR nuclear receptors and are independent of CYP27B1 and 25(OH)D3 (30, 92, 93).
A better understanding of the complex regulatory mechanisms induced by UVR would require use of computational biology tools and AI. However, there is lack of computational resources for neuroendocrine signaling in gene set databases. The Pathway Annotated List and Gene Signature Electronic Repository (139, 140) shows that a query of “neuroendocrine” returned only 37 gene sets out of 113,830 PAGs from 37 data sources. Although PAGs (for example: WEX000069 and TAX028307) identify differentially regulated genes related to neuroendocrine function, there are no data on various conditions where UVR affects neuroendocrine systems yet. Therefore, such studies need to be performed and analyzed by rigorous bioinformatics network and systems biology analyses.
Concluding Remarks
In this perspective, we present overwhelming evidence supporting the complex regulation of the central and global homeostasis by UVA and UVB in particular, with predictable general mechanisms of action. Many of these mechanisms are independent or only partially dependent on the UVB-induced production of vitamin D in the skin. These include rapid or slow activation of the local and central neuro-immuno-endocrine responses involving brain, endocrine and immune systems with secondary effects on peripheral organs. Proposed mechanisms by which precision of such responses is regulated include the following: 1) the interaction with classical axes such as HP, HPA, SNS, or autonomous (ANS) nervous systems or key regulatory brain centers indirectly activated by UVR-generated “messengers” in the periphery, which would precisely regulate body homeostasis according to the need; 2) they are defined by the nature of the produced molecules that activate corresponding receptors at the local or systemic effects; 3) they include activation of cutaneous UVA and UVB photoreceptors, recently identified in the skin; 4) physicochemical changes induced by UVR in the skin (e.g., pH, ions, ROS) can lead to the activation or can affect the sensory nerve endings activities with attendant neural transmission to the brain or activation of the SNS or ANS leading to “reflex-driven” responses; and 5) UVR would activate skin immune cells that after leaving the skin would serve as cellular “photomessengers” to regulate systemic immune activity or neuroendocrine responses (40).
It is also worth mentioning about the recent advances in alternative pathways activating vitamin D and related lumisterol and tachysterol, defined as pro-hormones, leading to production of large number of molecules (70), which can activate not only VDR but also several alternative nuclear receptors. This offers a new Copernican point of view on vitamin D biology versus the old Ptolemaic opinion that is still being held by many in the scientific community: one active molecule 1,25(OH)2D3 and one receptor—VDR as the reflection of the vitamin D action. These new findings not only open a “Pandora’s Box” of scientific possibilities but also show that UVB can generate a myriad of molecules that could eventually regulate local and global homeostasis in a manner defined by the nature of the activated receptor.
There are multiple therapeutic implications of the above models extending beyond phototherapy of skin inflammatory disorders. These include systemic UVR therapy of autoimmune disorders such as RA, inflammatory bowel disorders (IBD), scleroderma, or neurodegenerative disorders with autoimmune component of which MS is an example. In addition, UVR therapy can be applied to treatment of addiction and mood disorders. However, precise selection of an optimal narrowband wavelength for a specific disorder with minimal site effects on the skin remains to be defined both experimentally and clinically.
Although there is overwhelming evidence that UVR in humans regulates the central neuro-endocrine system (1) with profound systemic homeostatic effects, we recognize certain limitations. The precise mechanisms of action are unknown or are based predominantly on laboratory animal models, ex vivo experiments on human skin, and limited human data. However, as discussed, epidemiological data and natural history of certain disorders such as MS, RA, mood disorders, or IBD point to the need for clinical trials on educated use of UVR therapy. State-of-the-art noninvasive use of sophisticated high-resolution imaging of the brain and body technologies combined with omics approaches promise to advance this new field of photo-neuro-immuno-endocrinology with broad clinical implications. Using systems biology approaches, we expect three levels of data to be collected and models, from UVR damage at the organ level and cross-organ endocrine signaling, at the cell population level and the cross cell-type communications via cytokines and neurohormones, and at the intracellular signaling level at the single-cell type via multiomics measurements. Furthermore, use of free running animals exposed daily to the sun should lead to understanding how UVR can affect their well-being and body homeostasis that would be dependent on the animal species and their ecosystem.
In summary, we have gathered experimental and observational evidence that UVR spectra of solar radiation have profound effects on systemic body homeostasis, which are defined by their wavelength and energy, nature of chromophores and of molecules produced in the barrier organ. Local physicochemical changes induced by solar radiation can lead to activation of local neuro-immuno-endocrine sensing and regulatory mechanisms with their projection to the central or systemic levels. While the precise mechanisms of these interactions remain to be explored, their health benefits and therapeutic implications can outweigh the negative effects if responsibly applied in an educated manner.
Acknowledgments
We acknowledge the current support of NIH grants 1R01AR073004-01A1 and R01AR071189-01A1, VA merit award (no. I01BX00 4293-01A1), and DOD grant #W81XWH2210689 to A.T.S.; R21 AI149267-01A1 to C.R. and A.T.S.; R21 MD015319 to C.R.; and previous support by NSF grants IOS-0918934, IBN-9604364, 9896030, and 049087 and NIH grants RO1AR052190, 1R01AR056666, R21AR0665051, and AR-047079 to A.T.S., which contributed to the development of the presented concept. Fig. 1 was created with BioRender.com.
Author contributions
R.M.S., C.R., and A.T.S. analyzed data; and R.M.S., J.Y.C., C.R., and A.T.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
There are no data underlying this work.
References
- 1.Slominski A. T., Zmijewski M. A., Plonka P. M., Szaflarski J. P., Paus R., How UV light touches the brain and endocrine system through skin, and why. Endocrinology 159, 1992–2007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wacker M., Holick M. F., Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 5, 51–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Orazio J., Jarrett S., Amaro-Ortiz A., Scott T., UV radiation and the skin. Int. J. Mol. Sci. 14, 12222–12248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorn L. O., Photobiology: The Sience of Life and Light (Springer, New York, NY, ed. 2, 2008). [Google Scholar]
- 5.Fukui T., et al. , Exploratory clinical trial on the safety and bactericidal effect of 222-nm ultraviolet C irradiation in healthy humans. PLoS One 15, e0235948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch L., et al. , Far-UVC- and UVB-induced DNA damage depending on skin type. Exp. Dermatol. 32, 1582–1587 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Brenner M., Hearing V. J., The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 84, 539–549 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish J. A., Jaenicke K. F., Anderson R. R., Erythema and melanogenesis action spectra of normal human skin. Photochem. Photobiol. 36, 187–191 (1982). [DOI] [PubMed] [Google Scholar]
- 9.Hart P. H., Norval M., Byrne S. N., Rhodes L. E., Exposure to ultraviolet radiation in the modulation of human diseases. Ann. Rev. Pathol. Mech. Dis. 14, 55–81 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Slominski A. T., et al. , Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 212, v, vii 1–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young A. R., Chromophores in human skin. Phys. Med. Biol. 42, 789 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Cockell C. S., Carbon biochemistry and the ultraviolet radiation environments of F, G, and K main sequence stars. Icarus 141, 399–407 (1999). [Google Scholar]
- 13.Dworkin J., Deamer D., Sandford S., Allamandola L., Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. U.S.A. 98, 815–819 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulkidjanian A. Y., Cherepanov D. A., Galperin M. Y., Survival of the fittest before the beginning of life: Selection of the first oligonucleotide-like polymers by UV light. BMC Evol. Biol. 3, 12 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raven J. A., Cockell C. S., De La Rocha C. L., The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos. Trans. R Soc. Lond. B Biol. Sci. 363, 2641–2650 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juzeniene A., et al. , Solar radiation and human health. Rep. Progress Phys. 74, 066701 (2011). [Google Scholar]
- 17.Holick M. F., Vitamin D: A millenium perspective. J. Cell Biochem. 88, 296–307 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Bikle D. D., Vitamin D: An ancient hormone. Exp. Dermatol. 20, 7–13 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Slominski A. T., et al. , Melatonin, mitochondria, and the skin. Cell Mol. Life Sci. 74, 3913–3925 (2017), 10.1007/s00018-017-2617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sexton D. J., Muruganandam A., McKenney D. J., Mutus B., Visible light photochemical release of nitric oxide from S-nitrosoglutathione: Potential photochemotherapeutic applications. Photochem. Photobiol. 59, 463–467 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Addison C. C., Gamlen G. A., Thompson R., The ultra-violet absorption spectra of sodium hyponitrite and sodium α-oxyhyponitrite: The analysis of mixtures with sodium nitrite and nitrate. J. Chem. Soc. 1952, 338–345 (1952). [Google Scholar]
- 22.Serafim R. A., Primi M. C., Trossini G. H., Ferreira E. I., Nitric oxide: State of the art in drug design. Curr. Med. Chem. 19, 386–405 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Lo J. A., Fisher D. E., The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 346, 945–949 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewison M., et al. , Feldman and Pike’s Vitamin D (Academic Press, ed. 5, 2024). [Google Scholar]
- 25.Bikle D., Christakos S., New aspects of vitamin D metabolism and action–Addressing the skin as source and target. Nat. Rev. Endocrinol. 16, 234–252 (2020), 10.1038/s41574-019-0312-5. [DOI] [PubMed] [Google Scholar]
- 26.Slominski A. T., et al. , Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 5, 14875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski A. T., et al. , Novel activities of CYP11A1 and their potential physiological significance. J. Steroid. Biochem. Mol. Biol. 151, 25–37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuckey R. C., Cheng C. Y. S., Slominski A. T., The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid. Biochem. Mol. Biol. 186, 4–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slominski A. T., et al. , In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 26, 3901–3915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski A. T., et al. , Metabolic activation of tachysterol3 to biologically active hydroxyderivatives that act on VDR, AhR, LXRs, and PPARgamma receptors. FASEB J. 36, e22451 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slominski A. T., et al. , Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci. Rep. 7, 11434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuckey R. C., et al. , CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites. J. Steroid. Biochem. Mol. Biol. 181, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostkamp P., et al. , Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc. Natl. Acad. Sci. U.S.A. 118, e2018457118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller R. B., et al. , Does incident solar ultraviolet radiation lower blood pressure? J. Am. Heart Assoc. 9, e013837 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorman S., Sun exposure: An environmental preventer of metabolic dysfunction? Curr. Opin. Endocrine and Metab. Res. 11, 1–8 (2020). [Google Scholar]
- 36.Zhu H., et al. , Moderate UV exposure enhances learning and memory by promoting a novel glutamate biosynthetic pathway in the brain. Cell 173, 1716–1727.e17 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Parikh S., et al. , Food-seeking behavior is triggered by skin ultraviolet exposure in males. Nat. Metab. 4, 883–900 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae J. M., et al. , Both cardiovascular and cerebrovascular events are decreased following long-term narrowband ultraviolet B phototherapy in patients with vitiligo: A propensity score matching analysis. J. Eur. Acad. Dermatol. Venereol. 35, 222–229 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Archier E., et al. , Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 26 (suppl. 3), 11–21 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Slominski A., Wortsman J., Neuroendocrinology of the skin. Endocr. Rev. 21, 457–487 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Tobin D. J., Biochemistry of human skin–our brain on the outside. Chem. Soc. Rev. 35, 52–67 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Slominski A. T., et al. , Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol. Cell Physiol. 323, C1757–C1776 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slominski A. T., et al. , Key role of CRF in the skin stress response system. Endocr. Rev. 34, 827–884 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrousos G. P., Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Slominski A. T., et al. , Regulated proenkephalin expression in human skin and cultured skin cells. J. Invest. Dermatol. 131, 613–622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramot Y., Bohm M., Paus R., Translational neuroendocrinology of human skin: Concepts and perspectives. Trends Mol. Med. 27, 60–74 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Bernard J. J., Gallo R. L., Krutmann J., Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 19, 688–701 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Marek-Jozefowicz L., et al. , Molecular mechanisms of neurogenic inflammation of the skin. Int. J. Mol. Sci. 24, 5001 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson Z. T., Dawson A. D., Slominski A. T., Harris M. L., Current insights into the role of neuropeptide Y in skin physiology and pathology. Front. Endocrinol. (Lausanne) 13, 838434 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moattari C. R., Granstein R. D., Neuropeptides and neurohormones in immune, inflammatory and cellular responses to ultraviolet radiation. Acta Physiol. 232, e13644 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Slominski A. T., et al. , Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal. Res. 68, e12626 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schallreuter K. U., et al. , Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: Epidermal H2O2/ONOO(-)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J. 26, 2457–2470 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Grando S. A., Pittelkow M. R., Schallreuter K. U., Adrenergic and cholinergic control in the biology of epidermis: Physiological and clinical significance. J. Invest. Dermatol. 126, 1948–1965 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Schallreuter K. U., Epidermal adrenergic signal transduction as part of the neuronal network in the human epidermis. J. Investig Dermatol. Symp. Proc. 2, 37–40 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Grando S. A., Kawashima K., Wessler I., A historic perspective on the current progress in elucidation of the biologic significance of non-neuronal acetylcholine. Int. Immunopharmacol. 81, 106289 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slominski A. T., et al. , Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 138, 490–499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bíró T., Tóth B. I., Haskó G., Paus R., Pacher P., The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 30, 411–420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slominski R. M., et al. , Melanoma, melanin, and melanogenesis: The Yin and Yang relationship. Front. Oncol. 12, 842496 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski R. M., et al. , The significance of CYP11A1 expression in skin physiology and pathology. Mol. Cell Endocrinol. 530, 111238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frommeyer T. C., et al. , UVB-induced microvesicle particle release and its effects on the cutaneous microenvironment. Front. Immunol. 13, 880850 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slominski R. M., Raman C., Chen J. Y., Slominski A. T., How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 46, 263–275 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skobowiat C., Dowdy J. C., Sayre R. M., Tuckey R. C., Slominski A., Cutaneous hypothalamic-pituitary-adrenal axis homolog: Regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 301, E484–493 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skobowiat C., Slominski A. T., UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J. Invest. Dermatol. 135, 1638–1648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fell G. L., Robinson K. C., Mao J., Woolf C. J., Fisher D. E., Skin beta-endorphin mediates addiction to UV light. Cell 157, 1527–1534 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shime H., et al. , Proenkephalin(+) regulatory T cells expanded by ultraviolet B exposure maintain skin homeostasis with a healing function. Proc. Natl. Acad. Sci. U.S.A. 117, 20696–20705 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hart P. H., Norval M., More than effects in skin: Ultraviolet radiation-induced changes in immune cells in human blood. Front. Immunol. 12, 694086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aubert P. M., et al. , Dopamine efflux in response to ultraviolet radiation in addicted sunbed users. Psychiatry Res. Neuroimag. 251, 7–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slominski A., Wortsman J., Tobin D. J., The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 19, 176–194 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Skobowiat C., Sayre R. M., Dowdy J. C., Slominski A. T., Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br. J. Dermatol. 168, 595–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slominski A. T., Tuckey R. C., Jetten A. M., Holick M. F., Recent advances in vitamin D biology: Something new under the sun. J. Invest. Dermatol. 143, 2340–2342 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walterscheid J. P., et al. , Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 17420–17425 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ullrich S. E., Sunlight and skin cancer: Lessons from the immune system. Mol. Carcinog. 46, 629–633 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noonan F. P., De Fabo E. C., Immunosuppression by ultraviolet B radiation: Initiation by urocanic acid. Immunol. Today 13, 250–254 (1992). [DOI] [PubMed] [Google Scholar]
- 74.Rannug A., Fritsche E., The aryl hydrocarbon receptor and light. Biol. Chem. 387, 1149–1157 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Fritsche E., et al. , Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. U.S.A. 104, 8851–8856 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischer T. W., et al. , Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 20, 1564–1566 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Slominski A. T., et al. , Melatonin and its metabolites can serve as agonists on the aryl hydrocarbon receptor and peroxisome proliferator-activated receptor gamma. Int. J. Mol. Sci. 24, 15496 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barber L. A., et al. , Expression of the platelet-activating factor receptor results in enhanced ultraviolet B radiation-induced apoptosis in a human epidermal cell line. J. Biol. Chem. 273, 18891–18897 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Liu L., et al. , Keratinocyte-derived microvesicle particles mediate ultraviolet B radiation-induced systemic immunosuppression. J. Clin. Invest. 131, e144963 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kripke M. L., Cox P. A., Alas L. G., Yarosh D. B., Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc. Natl. Acad. Sci. U.S.A. 89, 7516–7520 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen N. T., Fisher D. E., MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 32, 224–236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swope V. B., Abdel-Malek Z. A., MC1R: Front and center in the bright side of dark eumelanin and DNA repair. Int. J. Mol. Sci. 19, 2667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turnbull A. V., Rivier C. L., Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 79, 1–71 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Eisenhofer G., et al. , Tyrosinase: A developmentally specific major determinant of peripheral dopamine. FASEB J. 17, 1248–1255 (2003). [DOI] [PubMed] [Google Scholar]
- 85.Felton S. J., et al. , Serum endocannabinoids and N-acyl ethanolamines and the influence of simulated solar UVR exposure in humans in vivo. Photochem. Photobiol. Sci. 16, 564–574 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Misery L., Loser K., Stander S., Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 30 (suppl 1), 2–8 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Choi J. E., Di Nardo A., Skin neurogenic inflammation. Semin. Immunopathol. 40, 249–259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roosterman D., Goerge T., Schneider S. W., Bunnett N. W., Steinhoff M., Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 86, 1309–1379 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Mayer E. A., Nance K., Chen S., The gut-brain axis. Annu. Rev. Med. 73, 439–453 (2022). [DOI] [PubMed] [Google Scholar]
- 90.Arenas O. M., et al. , Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci. 20, 1686–1693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouillon R., et al. , Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 40, 1109–1151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slominski A. T., et al. , Photoprotective properties of vitamin D and lumisterol hydroxyderivatives. Cell Biochem. Biophys. 78, 165–180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slominski A. T., et al. , Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Sci. Rep. 11, 8002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song Y., et al. , Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 209, 1111–1123 (2022). [DOI] [PubMed] [Google Scholar]
- 95.Jenkinson C., et al. , Simultaneous measurement of 13 circulating vitamin D3 and D2 mono and dihydroxy metabolites using liquid chromatography mass spectrometry. Clin. Chem. Lab Med. 59, 1642–1652 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim T. K., et al. , Detection of 7-dehydrocholesterol and vitamin D3 derivatives in honey. Molecules 25, 2583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wierzbicka J. M., et al. , Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol. Cell Endocrinol. 437, 312–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.R. P. Patrick, B. N. Ames, Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J 28, 2398–2413 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Kaneko I., et al. , 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 29, 4023–4035 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Patrick R. P., Ames B. N., Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 29, 2207–2222 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Slominski A. T., et al. , Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One 4, e4309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nishigori C., et al. , Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 93, 10354–10359 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eller M. S., Ostrom K., Gilchrest B. A., DNA damage enhances melanogenesis. Proc. Natl. Acad. Sci. U.S.A. 93, 1087–1092 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gilchrest B. A., Park H. Y., Eller M. S., Yaar M., Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 63, 1–10 (1996). [DOI] [PubMed] [Google Scholar]
- 105.Sreevidya C. S., Khaskhely N. M., Fukunaga A., Khaskina P., Ullrich S. E., Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 68, 3978–3984 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Janjetovic Z., et al. , Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. 7, 1274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim T. K., et al. , Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 27, 2742–2755 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pawelek J. M., et al. , Molecular cascades in UV-induced melanogenesis: A central role for melanotropins? Pigment Cell Res. 5, 348–356 (1992). [DOI] [PubMed] [Google Scholar]
- 109.Slominski A., Pawelek J., Animals under the sun: Effects of ultraviolet radiation on mammalian skin. Clin. Dermatol. 16, 503–515 (1998). [DOI] [PubMed] [Google Scholar]
- 110.Bellono N. W., Najera J. A., Oancea E., UV light activates a Galphaq/11-coupled phototransduction pathway in human melanocytes. J. Gen. Physiol. 143, 203–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bellono N. W., Kammel L. G., Zimmerman A. L., Oancea E., UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. U.S.A. 110, 2383–2388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Q. M., et al. , Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif. 50, e12372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haltaufderhyde K., Ozdeslik R. N., Wicks N. L., Najera J. A., Oancea E., Opsin expression in human epidermal skin. Photochem. Photobiol. 91, 117–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim H. J., et al. , Violet light down-regulates the expression of specific differentiation markers through Rhodopsin in normal human epidermal keratinocytes. PLoS One 8, e73678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsutsumi M., et al. , Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp. Dermatol. 18, 567–570 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Al-Nuaimi Y., et al. , A meeting of two chronobiological systems: Circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J. Invest. Dermatol. 134, 610–619 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Zanello S. B., Jackson D. M., Holick M. F., Expression of the circadian clock genes clock and period1 in human skin. J. Invest. Dermatol. 115, 757–760 (2000). [DOI] [PubMed] [Google Scholar]
- 118.Lan Y., Wang Y., Lu H., Opsin 3 is a key regulator of ultraviolet A-induced photoageing in human dermal fibroblast cells. Br. J. Dermatol. 182, 1228–1244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karthikeyan R., Davies W. I. L., Gunhaga L., Non-image-forming functional roles of OPN3, OPN4 and OPN5 photopigments. J. Photochem. Photobiol. 15, 100177 (2023). [Google Scholar]
- 120.Olinski L. E., Lin E. M., Oancea E., Illuminating insights into opsin 3 function in the skin. Adv. Biol. Regul. 75, 100668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Assis L. V., Moraes M. N., da Silveira Cruz-Machado S., Castrucci A. M., The effect of white light on normal and malignant murine melanocytes: A link between opsins, clock genes, and melanogenesis. Biochim. Biophys. Acta 1863, 1119–1133 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Wicks N. L., Chan J. W., Najera J. A., Ciriello J. M., Oancea E., UVA phototransduction drives early melanin synthesis in human melanocytes. Curr. Biol. 21, 1906–1911 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Randhawa M., et al. , Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One 10, e0130949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skobowiat C., Postlethwaite A. E., Slominski A. T., Skin exposure to ultraviolet B rapidly activates systemic neuroendocrine and immunosuppressive responses. Photochem. Photobiol. 93, 1008–1015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Skobowiat C., Slominski A. T., Ultraviolet B stimulates proopiomelanocortin signalling in the arcuate nucleus of the hypothalamus in mice. Exp. Dermatol. 25, 120–123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hiramoto K., Yanagihara N., Sato E. F., Inoue M., Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of alpha-melanocyte-stimulating hormone-responsive cells. J. Invest. Dermatol. 120, 123–127 (2003). [DOI] [PubMed] [Google Scholar]
- 127.Hiramoto K., Jikumaru M., Yamate Y., Sato E. F., Inoue M., Ultraviolet A irradiation of the eye induces immunomodulation of skin and intestine in mice via hypothalomo-pituitary-adrenal pathways. Arch. Dermatol. Res. 301, 239–244 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Parikh R., et al. , Skin exposure to UVB light induces a skin-brain-gonad axis and sexual behavior. Cell Rep. 36, 109579 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dieguez C., Nogueiras R., Sun exposure stimulates appetite in males. Nat. Metab. 4, 796–797 (2022). [DOI] [PubMed] [Google Scholar]
- 130.Ferguson A. L., et al. , Exposure to solar ultraviolet radiation limits diet-induced weight gain, increases liver triglycerides and prevents the early signs of cardiovascular disease in mice. Nutr. Metab. Cardiovasc. Dis. 29, 633–638 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Hermann A. L., et al. , beta-Endorphin mediates radiation therapy fatigue. Sci. Adv. 8, eabn6025 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kemeny L. V., et al. , Vitamin D deficiency exacerbates UV/endorphin and opioid addiction. Sci. Adv. 7, eabe4577 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iacopetta K., Collins-Praino L. E., Buisman-Pijlman F. T., Hutchinson M. R., Can neuroimmune mechanisms explain the link between ultraviolet light (UV) exposure and addictive behavior? Brain Behav. Immunity 73, 125–132 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Veleva B. I., van Bezooijen R. L., Chel V. G. M., Numans M. E., Caljouw M. A. A., Effect of ultraviolet light on mood, depressive disorders and well-being. Photodermatol. Photoimmunol. Photomed. 34, 288–297 (2018). [DOI] [PubMed] [Google Scholar]
- 135.Irving A. A., Marling S. J., Seeman J., Plum L. A., DeLuca H. F., UV light suppression of EAE (a mouse model of multiple sclerosis) is independent of vitamin D and its receptor. Proc. Natl. Acad. Sci. U.S.A. 116, 22552–22555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y., Marling S. J., Martino V. M., Prahl J. M., Deluca H. F., The absence of 25-hydroxyvitamin D3–1alpha-hydroxylase potentiates the suppression of EAE in mice by ultraviolet light. J. Steroid. Biochem. Mol. Biol. 163, 98–102 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Wang Y., Marling S. J., Zhu J. G., Severson K. S., DeLuca H. F., Development of experimental autoimmune encephalomyelitis (EAE) in mice requires vitamin D and the vitamin D receptor. Proc. Natl. Acad. Sci. U.S.A. 109, 8501–8504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Slominski A., Wortsman J., Luger T., Paus R., Solomon S., Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 80, 979–1020 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Yue Z., et al. , PAGER-CoV: A comprehensive collection of pathways, annotated gene-lists and gene signatures for coronavirus disease studies. Nucleic Acids Res. 49, D589–D599 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yue Z., Slominski R., Bharti S., Chen J. Y., PAGER web APP: An interactive, online gene set and network interpretation tool for functional genomics. Front. Genet. 13, 820361 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.