Summary
Photoacoustic imaging (PAI) with co-registered ultrasound (US) is a hybrid non-invasive imaging modality that enables visualization and quantification of tumor hypoxia in live animals. Here, using a breast tumor xenograft model as an example, we present a stepwise protocol describing animal preparation, positioning, instrument setup, and US-PAI image acquisition procedures. This protocol also guides through detailed data analysis, explains functional readouts obtained from PAI, and discusses the potential application of the technology to study the hypoxic tumor microenvironment.
For complete details on the use and execution of this protocol, please refer to Dai et al.1
Subject areas: Biophysics, Cell Biology, Cancer, Model Organisms
Graphical abstract

Highlights
-
•
Guidance on quantifying tumor hemoglobin content and oxygen saturation in live animals
-
•
Steps for instrument setup and image acquisition for breast cancer xenograft mouse model
-
•
Instructions for US-PAI data analysis and interpretation
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Photoacoustic imaging (PAI) with co-registered ultrasound (US) is a hybrid non-invasive imaging modality that enables visualization and quantification of tumor hypoxia in live animals. Here, using a breast tumor xenograft model as an example, we present a stepwise protocol describing animal preparation, positioning, instrument setup, and US-PAI image acquisition procedures. This protocol also guides through detailed data analysis, explains functional readouts obtained from PAI, and discusses the potential application of the technology to study the hypoxic tumor microenvironment.
Before you begin
Experimental design considerations
Understanding the principles of US-PAI imaging is essential for precise data interpretation. Here, we include a brief introduction about the basics of US-PAI and highlight the advantages over other imaging techniques. We also discuss the tumor models and mouse strains that can be used for US-PAI based tumor imaging. Researchers with knowledge in US-PAI can directly proceed to the step-by-step method details.
Principles of US-PAI
US-PAI systems typically includes two components: a pulsed (often tunable) laser covering wavelengths in the near-infrared range, and an ultrasound transducer for photoacoustic signal detection. The pulsed laser provides the initial excitation of tissue chromophores, with the absorption and thermal relaxation process leading to a localized thermoelastic expansion producing acoustic waves that are captured by the ultrasonic detection system.2,3,4,5 These signals are then reconstructed to provide information on the concentration and spatial distribution of chromophores within tissues. Hemoglobin is one of the primary endogenous chromophores in the near-infrared range that can be detected by US-PAI. Interestingly, hemoglobin has a different absorption spectrum based on whether or not it is bound to oxygen, allowing US-PAI to distinguish between oxy-hemoglobin (HbO2) from deoxyhemoglobin (Hb), thereby enabling quantification of total hemoglobin concentration (HbT) and oxygen saturation (%sO2) in tumors.3 Co-registered ultrasound, which is compatible with PAI as they share the same detection system, provides anatomically useful information of the tumor to PAI.
Advantages of US-PAI
Unlike Computed Tomography (CT), Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET), US-PAI is relatively inexpensive and does not require the administration of radioactive isotopes or exogenous imaging agents, making it a safe and desirable approach for longitudinal imaging.3 While oxygen-sensitive electrodes have also been commonly used to measure oxygen partial pressure (pO2) in tumors in vivo; these probes are invasive and suffer from limited sampling of the tumors. In contrast, the three dimensional nature of PAI allows for assessment of temporal and spatial mapping of hypoxia within the entire tumor.6 Given these advantages, US-PAI is currently being evaluated for applications in oncology, including detection of breast cancer7 (ClinicalTrials.gov ID NCT03897270 and NCT04437030).
US-PAI can be used to quantify tumor hemoglobin content and oxygen saturation
Hypoxia commonly exists in solid tumors and has been associated with rewired tumor metabolism, dysfunctional immune response, increased metastatic potential and resistance to therapies.8,9,10 Therefore, besides its substantial value in cancer detection and diagnosis, US-PAI can potentially be used to assess tumor hypoxia in patients as a readout for treatment guidance and therapy monitoring. US-PAI derived measurements of tumor oxygenation and hypoxia have been validated against gold standard imaging modalities and histological approaches in pre-clinical models.6,11,12 Mouse tumors that show low tumor oxygenation in PAI have lower density of large vessels (CD31+) and more hypoxic regions (CA9+ or pimonidazole+) than those with high tumor oxygenation.11,12 US-PAI was also found able to detect Oxi-4503 induced tumor ischemia in breast cancer mouse models.11 Hyperoxic challenge increases tumor oxygen saturation levels on PAI in HNSCC mouse model, which spatially aligned with oxygen-enhanced magnetic resonance imaging.6 Similarly, US-PAI %sO2 measurements were validated against direct pO2 measurements in vitro, as well as with blood oxygenation level dependent (BOLD) MRI in a mouse HNSCC tumor model.13 Together, these studies strongly support US-PAI as a reliable approach to measure tumor hypoxia.
Application of US-PAI in cancer research
Since US-PAI is a non-invasive, quantitative and inexpensive approach, it can be used to monitor changes in tumor oxygenation status and the development of tumor hypoxia over the course of tumor progression. This allows a broad application of US-PAI in research that aims to study tumor oxygenation and tumor. First, US-PAI can be used to measure tumor perfusion and oxygen delivery, which provides quantitative readout to assess the efficacy of various anti-cancer regimens, such as anti-angiogenesis or vessel normalization therapy. Second, since tumor hypoxia has been found associated with disease progression and therapy resistance, the level of tumor oxygenation and hypoxia measured by US-PAI could be explored as potent prognostic and predictive biomarkers of disease aggression. Lastly, for research aiming to study tumor hypoxia ex vivo, monitoring the development of tumor hypoxia can ensure the establishment of tumor hypoxia before the tumor is harvested for downstream histologic or molecular analyses.
Choice of tumor model
When determining if US-PAI is suitable for your tumor model, the primary consideration is tumor size and depth. For optimal PA signal generation and spectral coloring, adequate light fluence must be delivered to the desired target depth. However, light fluence decreases with increased imaging depth due to scattering and absorption of light in tissue. Therefore, in the deepest portion of excessively large mouse tumors (>1.5 cm), PAI performance can be limited. The depth performance of PAI can be improved through the use of user-controlled time-gain-compensation settings that allows deeper signals to be amplified, and through advanced fluence correction methods. Furthermore, the selection of lower frequency ultrasound transducers can provide a boost to sensitivity for detecting PA signals and as a result are recommended for large tumors. Imaging depth will also be dependent on whether the tumor model is subcutaneous or orthotopic. Fortunately, most mouse tumor models are well within these depth constraints and should be acceptable for US-PAI studies.
Choice of mouse strain
Black pigmentation and black fur can generate significant imaging artifacts on US-PAI. When working with black haired mouse strains, exceptional care must be made to remove all hair around the imaging site. Albino B6 mouse strains are available and provide an excellent alternative if suitable for the tumor model of choice. These considerations aren’t necessary for nude and white-haired mice strains.
Equipment for US-PAI
The workflow established in this protocol was developed using the Vevo LAZR system sold by Fujifilm VisualSonics (Toronto, Canada). The other systems, such as MSOT from iThera Medical and TRITOM from PhotoSound Technologies, are functionally distinct from the Vevo LAZR system. Therefore, this protocol does not apply to any other instruments. However, the application of photoacoustic imaging as a non-invasive approach in general to detect and quantify tumor oxygenation and hypoxia in live animals remains valid despite the usage of different imaging systems.
Institutional permissions
This protocol includes the use of cancer mouse models, so ethical approval is required before the experiment. Animal experiments in this protocol were approved by Institutional Animal Care and Use Committee (IACUC) at Roswell Park Comprehensive Cancer Center.
Establish tumor models
Timing: 3–4 weeks
As an example, this protocol below describes the steps to establish human LM3.3 breast cancer xenograft model; we have also used multiple oral cancer xenograft models to examine tumor oxygenation by US-PAI imaging.6,14,15
-
1.
Order 6–8 weeks old female CB-17 SCID mice.
Note: Athymic nude mice, NOD-SCID, NSG, or other immune-deficient mice can also be used for this human xenograft model. For murine cancer cells, immune-competent mouse strains with an identical genetic background can be used to establish syngeneic models.
-
2.
One day prior to implantation, shave the hair off the injection area around animal’s #4 mammary gland and perform ear-punching or ear-tagging for animal identification.
-
3.
On the day of implantation, prepare LM3.3 tumor cell suspension (PBS: Matrigel = 1:1) on ice. We typically implant 5 × 105 LM3.3 tumor cells in a volume of 50–100 μL per mouse.
Note: The detailed protocol for cell culture is not the main focus of this paper and has been illustrated in other publications.16,17
CRITICAL: Matrigel solidifies at room temperature (25°C), so all procedures with Matrigel should be handled on ice to avoid solidification. Keeping the tumor cells on ice for more than 30 min before tumor implantation will compromise tumor cell viability.
-
4.
Anesthetize animals with 2%–3% isoflurane in 1 L/min O2.
-
5.
Pinch up #4 mammary fat pad using sterile fine tweezer. Inject cell suspension into the mammary fat pad (50–100 μL) using insulin syringe with 28-gauge needle. Detailed protocol can be found elsewhere.18
Note: Isoflurane should be stored in a cabinet in a ventilated room at a controlled room temperature of 15°C–30°C, as indicated in the SDS. Bottles should be tightly closed and returned to the storage cabinet immediately after use. Anesthetic gas scavenging systems should be used to control waste anesthetic.
CRITICAL: Avoid having needle penetrate through the peritoneum, or the tumors may grow in the abdomen. Also avoid injecting the tumor cells right below the skin (subcutaneous injection)—this easily causes ulceration, necrosis, or bleeding on the surface of the tumor, which may introduce artifacts in US-PAI imaging.
-
6.
Depending on the purpose of experiments, the tumors can be imaged as soon as they are palpable and can be imaged routinely during tumor progression or drug treatment to track dynamic changes in tumor oxygenation.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Matrigel membrane matrix | Corning | Cat# 354234 |
| PBS, pH 7.4 | Thermo Fisher Scientific | Cat# 10010049 |
| Aquasonic clear ultrasound transmission gel | Aquasonic Gel | Cat# PLI 03-50 |
| Protex disinfectant spray | Parker Laboratories, Inc. | Cat# 42-32 |
| Experimental models: Cell lines | ||
| Human: LM3.3 cells, a metastatic clone of MDA-MB-231 | (Minn et al., 2005) | |
| Experimental models: Organisms/strains | ||
| Mouse: 6‒10-week-old female C.B-Igh-1b/IcrTac-Prkdcscid | RPCCC in-house | N/A |
| Software and algorithms | ||
| Vevo LAZR | VisualSonics Inc. | https://www.visualsonics.com/product/imaging-systems/vevo-lazr |
| GraphPad Prism v9.3.1 | GraphPad Software | https://www.graphpad.com/ |
| Other | ||
| BD Micro-Fine IV insulin syringes 28G | BD | Cat# 329424 |
| Extra fine Graefe forceps | Fine Science Tools | Cat# 11150-10 |
| Hair removal cream | Veet | NA |
| Puralube ophthalmic ointment | Dechra | NA |
| Vevo LAZR LZ250 transducer | VisualSonics Inc. | Part of Vevo LAZR |
| Nd:YAG laser with optical parametric oscillator (OPO) | VisualSonics Inc. | Part of Vevo LAZR |
| Puritan 6″ sterile standard rayon swab w/wooden handle | Puritan | Cat# 25-806 1WR |
Step-by-step method details
Hair removal before US-PAI
Timing: 10 min
If possible, remove the hair above imaging area 24 h prior to imaging. This will allow the skin to recover from the depilatory creams induced irritation and saves time during the imaging exam.
-
1.
Anesthetize animals in an isoflurane induction chamber with 2%–3% flow rate.
-
2.
Apply a thin but solid layer of depilatory cream to the area to be imaged, i.e., the skin over and around the mammary tumor, using cotton swab. (troubleshooting 1).
-
3.
Use the cotton swab to gently rub the cream into the fur.
-
4.
Wait for around 1 min.
-
5.
Gently wipe off depilatory creams with water-moistened gauze.
Note: Carefully remove all residual depilatory creams to avoid skin irritation.
-
6.
Place the animals back to the cage and monitor their recovery from anesthesia.
Laser energy calibration
Timing: 15 min
Prior to the imaging exam, it is recommended to fully warm up the laser and perform an energy calibration to ensure optimal energy output. The Vevo LAZR comes with an external energy sensor (referred to as a calibration puck) that is used for this purpose.
-
7.
Turn on the laser cart at least 2 h before the imaging session.
-
8.
Make sure the calibration puck is connected to the laser and remove the lid from top of the puck.
-
9.
Place the puck on the imaging platform directly underneath the transducer, and lower the transducer so that it’s about 5 mm off the surface (Figure 1).
-
10.
Close the enclosure doors and then activate PA Mode on the system to begin firing the laser.
-
11.
Allow the system to fire for 5 min before moving to the next step.
-
12.
After 5 min, navigate to the laser control on the system and click “Optimize” (Figure 2).
-
13.
After optimization is complete, then select “Calibrate”.
-
14.
The laser is now ready to be used for imaging.
Figure 1.
Setting up the energy calibration sensor
Figure 2.
Calibrating the laser system
Animal positioning and transducer set-up
Timing: 15 min
The below steps include anesthesia and animal positioning to prepare animals for US-PAI imaging.
-
15.
Anesthetize animals in an isoflurane induction chamber with 2%–3% flow rate.
-
16.
Apply eye ointment to eyes to prevent from drying during imaging.
-
17.Position the animals on the imaging station (Figure 3).
-
a.Secure the animals on the imaging platform using tapes.
-
b.Apply nose cone with isoflurane flow to the animal’s head to maintain anesthesia during imaging.
-
c.Elevate the position to be imaged by placing gauze underneath the animal.
-
a.
Note: For example, if you are to image a mammary tumor implanted in the right-side #4 fat pad, place gauze underneath the right hind limb to elevate and expose the imaging area.
-
18.Set up ultrasound transducers on the animal. (troubleshooting 2).
-
a.Overfill transducer cavity with ultrasound gel. Avoid introducing bubbles.
 CRITICAL: Ultrasound gel is needed for the transmission of ultrasound and effective coupling of the light to the animals. Inadequate gel contact may result in a suboptimal image. Avoid introducing bubbles in the gel, which can cause artifacts and shadowing in the image. It is recommended to centrifuge gel or purchase bubble-free one-time-use gel packets to prevent these artifacts.
CRITICAL: Ultrasound gel is needed for the transmission of ultrasound and effective coupling of the light to the animals. Inadequate gel contact may result in a suboptimal image. Avoid introducing bubbles in the gel, which can cause artifacts and shadowing in the image. It is recommended to centrifuge gel or purchase bubble-free one-time-use gel packets to prevent these artifacts. -
b.Apply ultrasound gel to the imaging area of the animal. Avoid introducing bubbles.Note: Bigger tumors will require more gel to provide a larger standoff for positioning of the transducer.
-
c.Lower the transducer onto the gel on the imaging area until contact is made. You will see the image shown in ultrasound mode.
-
d.Gel should completely cover the area between the skin and the transducer. No air space should exist between the transducer and the gel.
-
a.
-
19.Position target organ (e.g., mammary tumor) in the image. (troubleshooting 3).
-
a.In B-mode, position the target such that the skin line appears horizontal, i.e., parallel to the transducer.
-
b.Lower the transducer until the skin line appears around 8–9 mm in the image (Figure 4; look at depth scale on right side of screen).
 CRITICAL: The light delivered from the integrated laser fibers intersect between 9‒11 mm beneath the transducer. Keeping the skin line around 8–9 mm usually yields the best penetration depth and reduces artifacts observed with PAI. For larger tumors, the skin line can be placed lower to reduce the reverb artifact, which occurs at twice the depth as the skin line.
CRITICAL: The light delivered from the integrated laser fibers intersect between 9‒11 mm beneath the transducer. Keeping the skin line around 8–9 mm usually yields the best penetration depth and reduces artifacts observed with PAI. For larger tumors, the skin line can be placed lower to reduce the reverb artifact, which occurs at twice the depth as the skin line. -
c.Lock and secure the transducer. Manipulate the imaging stage to locate the tissue of interest.
-
a.
Figure 3.
Vevo LAZR US-PAI instrument with enclosure (left and middle) and its imaging station (right)
Figure 4.
Position of the target organ in B-mode ultrasound
US-PAI image acquisition
Timing: 10 min
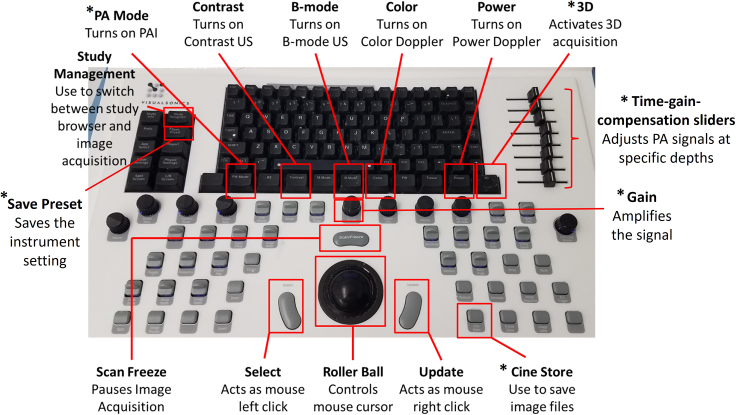
Note: The design of the Vevo LAZR allows the user to switch seamlessly between imaging modes directly on the system. This is controlled through the keyboard (Figure 5), with specific buttons for each imaging mode. Upon selecting “PA Mode”, the user is prompted to choose which type of US-PAI they intend to perform. The user can then acquire US-PAI data for a single 2D slice of interest or can select the “3D” button to collect images for the entire tumor.
-
20.
Once the transducer is in position, press the “PA Mode” button on the system and select “Oxy-Hemo” mode.
Note: During oxy-hemo mode imaging, the system alternates between two wavelengths, 750 nm and 850 nm, with the 750 nm wavelength associated with the deoxyhemoglobin signal and the 850 wavelength associated with the oxyhemoglobin signal. The system then uses the PA signal measured at these wavelengths to automatically generate sO2 and HbT maps.
-
21.
Use the “Gain” knob to increase or decrease the PAI signal until a satisfactory level is achieved. The time-gain-compensation sliders to the right of the keyboard can be adjusted to tune PA signals at specific depths. (troubleshooting 4).
CRITICAL: Adjustments to gain or the time-gain-compensation settings will impact PA measurements. These changes must be saved and applied to future imaging sessions using the “Save Preset” button in the top left section of the keyboard.
-
22.
Acquire 2D scans for 20–30 s, then press the “Cine Store” button to save the images.
-
23.
To acquire images in 3D mode, manipulate the imaging stage (instead of moving the transducer) to manually scan the whole tissue of interest to locate the central slice in B-mode, where you can see the maximum tumor area in B-mode ultrasound. This is where the automatic 3D-mode scanning will start from.
Note: It is important to acquire US and PAI images in 3D mode if you need to quantify whole tumor oxygenation level and tumor volume.
-
24.
Press the “3D” button on the system and enter the scan distance to cover the entire tumor, then define the desired step size.
Note: If you want higher spatial resolution in the imaging direction, a smaller step size should be used. If you want to cover a large area and reduce the imaging time, a larger step size should be used.
-
25.
Start image acquisition in 3D mode.
Note: The transducer will automatically scan from one side to the other side of the tumor based on the distance that you set and acquire the images at every step. If the setting and the image acquisition is successful, the images will cover from one end of the tumor to the center of the tumor and all the way to the other end of the tumor. (troubleshooting 5).
-
26.
After the completion of the scan, press “Cine Store” on the system and review the 3D dataset to ensure the quality is satisfactory.
-
27.
After imaging, remove animal from the imaging platform back to the cage and monitor its recovery from anesthesia.
-
28.
Clean the transducer, imaging stage and anesthesia induction chamber and any other surface that may be soiled. Protex spray (Parker Laboratories, Inc.) should be used to clean in the imaging area.
-
29.
Turn off any sensors or lasers that were used during image acquisition.
-
30.
Proceed to data analysis.
Figure 5.
Vevo LAZR US-PAI button layout with brief instructions (∗mentioned in the protocol)
Expected outcomes
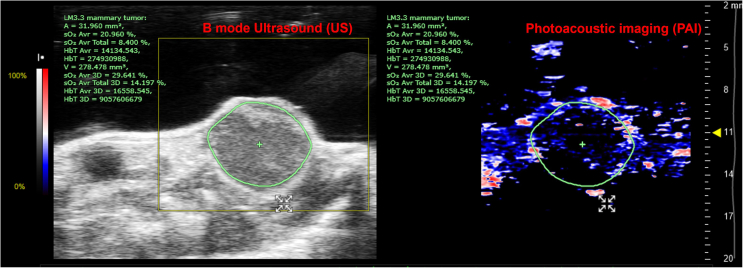
With the successful acquisition of 3D-mode US-PAI datasets for the tumors of interest, the US and PAI images are now available for analysis as shown in Figure 6. We have detailed major steps for data processing and quantification of tumor volume, total hemoglobin level in the tumor and overall oxygenation of the tumor in the next section.
Figure 6.
US-PAI images in Vevo Lab interface
Quantification and statistical analysis
Analysis can be performed on-board the Vevo LAZR imaging system or through Vevo LAB, VisualSonics offline analysis software package. For this protocol, we will demonstrate data processing and analysis using Vevo LAB with the example below. We will cover a few key steps for data analysis, but more advanced US-PAI features and functions of the software can be found on VisualSonics website (https://www.visualsonics.com/learning-hub-online-video-training-our-users). The principles behind this data analysis should apply to other imaging systems and software.
-
1.
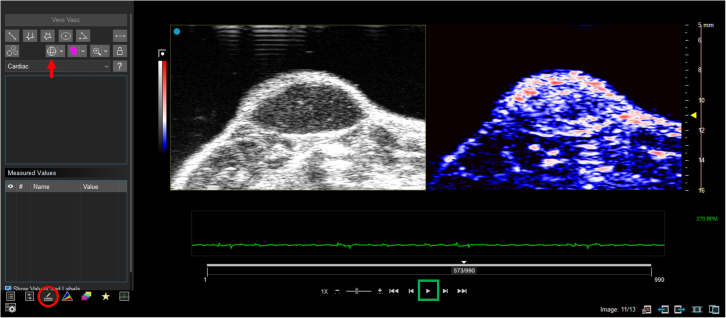
Open the data in Vevo Lab and double left click on the dataset of interest to load it into viewing mode. If 3D mode is used for imaging, you will see all the US and PAI images or frames covering the whole tumor. The video can be toggled to start and stop by clicking on the play button (Figure 7, green box).
-
2.
Navigate to the measurement tab (Figure 7, red circle) and select the 3D region of interest tool (Figure 7, red arrow) to contour the entire tumor for the ultrasound image in each frame. Trace the region of interest over the whole tumor. The solid tumor should look darker than the skin due to it difference in cellular density/composition compared to surrounding tissues. This region of interest will be applied to corresponding PAI image automatically. A HbT threshold can be set up and adjusted to eliminate the background noise; however, the same threshold should be applied to all other groups that are intended to analyze together.
-
3.
Once the tumor area is defined, the software will calculate the volume of the tumor, tumor oxygenation level (sO2, oxygen saturation) and total hemoglobin level (HbT). More detailed interpretation of these parameters and their biological meaning can be found below in Table 1. ‘sO2 Average Total 3D’ and ‘HbT Average 3D’ should be most informative in assessing overall tumor hypoxia level. Higher ‘sO2 Average Total 3D’ means that the tumor has overall more oxygen supply; while higher ‘HbT Average 3D’ means that the tumor has more blood flow. ‘sO2 Average 3D’ only takes into account the area above HbT threshold, i.e., intratumoral blood vessel; thereby high value would mean that the tumor vasculature is carrying more oxygen and more efficient in delivering oxygen.
-
4.
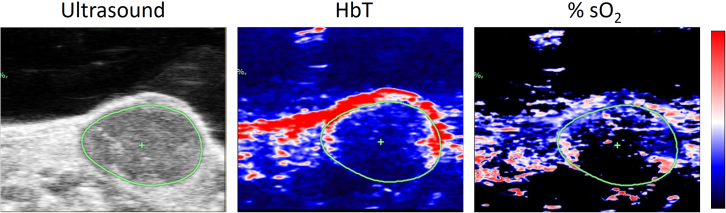
Representative images typically include an ultrasound image, from which the tumor area is defined, and the color maps representing corresponding HbT and %sO2 using a color lookup table (Figure 8). If the tumor oxygenation level (%sO2) is quantified frame by frame from one edge of the tumor all the way to the other edge of the tumor, the tumor spatial oxygenation level can be visualized as shown graph plot in Figure 9. If hypoxia develops at the tumor core, the middle frame where the tumor core is will show decreased %sO2 compared to either edge of the tumor.
Figure 7.
Vevo LAB interface for tumor contouring
Table 1.
Interpretation of different parameters obtained from 3D-mode US-PAI
| Parameter | Units | Data interpretation | Biological meaning |
|---|---|---|---|
| Volume | mm3 | Tumor volume | Tumor volume |
| sO2 Average 3D | % | Average ratio of HbO2 to Hb only including voxels above the HbT threshold | Oxygenation level in the tumor vasculature |
| sO2 Average Total 3D | % | Average ratio of HbO2 to Hb without applying HbT threshold | Oxygenation level in the whole tumor mass |
| HbT Average 3D | arbitrary units | Average HbT level per voxel in the tumor | Blood flow normalized to tumor size |
| HbT 3D | arbitrary units | Total HbT level in the tumor | Total blood flow in the tumor |
Figure 8.
Representative US-PAI images from a LM3.3 mammary tumor
Figure 9.

Representative spatial tumor oxygenation level from a LM3.3 mammary tumor
Limitations
The limitation of tumor depth and PA signal quality was discussed under the experimental design consideration section above. Another important consideration that can become a limitation is the consistency for imaging setup and parameters from one animal to the next. Care must be given for every animal to properly position the tumor and transducer so that the tumor is at the center of the image and the skin line is at 8–9 mm. If any imaging parameters have been adjusted for previous sessions, they should be saved as a preset and applied going forward. The laser system should be allowed to warm up for several hours prior to US-PAI and calibrated to ensure the output energy levels are in the correct range.
Troubleshooting
Problem 1
Artifacts in US or PAI due to animal fur and black pigmentation (step 2).
Potential solution
Exceptional care must be made to remove all hair around the imaging site preferably using depilatory cream, especially when working with black hair mouse strains. Hair clippers cannot completely remove the hair, thus resulting in artifacts in US or PAI.
Problem 2
Bubbles exist in the ultrasound gel between the transducer and the animal resulting in artifacts in US or PAI (step 18).
Potential solution
To avoid introducing bubbles while applying the gel to the animal and the transducer cavity, transfer the gel to a 50 mL centrifuge tube and spin down the gel in a centrifuge to remove the bubbles from the gel. Always apply generous amount of gel. If bubbles are found between the animal and the transducer and they cause artifacts in US or PAI, remove the gel with the bubbles and reapply new gel. Bubble free single-use gel packets can also be purchased if a centrifuge is not available.
Problem 3
Fail to position the target organ at the center of the screen to reach best penetration depth (usually 9–11 mm depth) under US mode, thus compromising the imaging quality (step 19).
Potential solution
Reposition the animal to make the target organ accessible to the transducer. For mammary tumor, place gauze pad underneath the right hind limb to elevate and expose the tumor.
Problem 4
Apply different gain or time-gain-compensation settings to animals in the same experiment (step 21).
Potential solution
Animals with PAI measured under different gain or time-gain-compensation settings cannot be used for comparisons or group together for analysis. PAI needs to be performed with the same settings for all the animals throughout the experiment.
Problem 5
In 3D mode image acquisition, the target organ does not stay at the center of the screen in all the frames (step 25).
Potential solution
Check and make sure that the animal is secured on the imaging platform and the transducer is locked tightly before 3D image acquisition. Manually scan the target organ over via manipulating the imaging stage to make sure that the target organ always stays at the center of the screen. For large subcutaneous tumor, apply more gel on and around the tumor to provide a larger standoff for positioning of the transducer, which could help secure the tumor position during the scan.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Subhamoy Dasgupta (subhamoy.dasgupta@roswellpark.org).
Technical contact
Questions about the technical specifics of performing the protocol should be directed to and will be answered by the technical contact, Laurie J. Rich (laurie.rich@fujifilm.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any datasets or code.
Acknowledgments
We would like to thank the Translational Imaging Shared Resource at Roswell Park Comprehensive Cancer Center supported by NCIP30CA16056. This work was primarily supported by NIH Director’s Award DP2CA260421, R01CA252092, the Roswell Park Alliance Foundation, and the Susan G. Komen Career Catalyst Research Award (CCR18547413) to S.D.; a Predoctoral Fellowship from the Breast Cancer Coalition of Rochester to T.D.; and NIH S10OD010393–01 and S10OD016450-01 to M.S. The funding sponsors had no role in the design of the study; collection, analyses, or interpretation of the data; writing of the manuscript; and the decision to publish the results.
Graphical abstract was created using Biorender.com.
Author contributions
T.D. and L.J.R. were responsible for the composition of the manuscript. L.J.R., M.S., and S.D. edited the manuscript. T.D. and L.J.R. conducted the experiments. M.S. and S.D. were responsible for the study design.
Declaration of interests
L.J.R. is currently an employee of Fujifilm-VisualSonics Corporation. S.D. holds equity in CoRegen Inc.
Contributor Information
Mukund Seshadri, Email: mukund.seshadri@roswellpark.org.
Subhamoy Dasgupta, Email: subhamoy.dasgupta@roswellpark.org.
References
- 1.Dai T., Rosario S.R., Katsuta E., Sawant Dessai A., Paterson E.J., Novickis A.T., Cortes Gomez E., Zhu B., Liu S., Wang H., et al. Hypoxic activation of PFKFB4 in breast tumor microenvironment shapes metabolic and cellular plasticity to accentuate metastatic competence. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zackrisson S., van de Ven S.M.W.Y., Gambhir S.S. Light In and Sound Out: Emerging Translational Strategies for Photoacoustic Imaging. Cancer Res. 2014;74:979–1004. doi: 10.1158/0008-5472.can-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallidi S., Luke G.P., Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011;29:213–221. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laufer J., Delpy D., Elwell C., Beard P. Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: application to the measurement of blood oxygenation and haemoglobin concentration. Phys. Med. Biol. 2007;52:141–168. doi: 10.1088/0031-9155/52/1/010. [DOI] [PubMed] [Google Scholar]
- 5.Xu M., Wang L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006;77 doi: 10.1063/1.2195024. [DOI] [Google Scholar]
- 6.Rich L.J., Seshadri M. Photoacoustic monitoring of tumor and normal tissue response to radiation. Sci. Rep. 2016;6 doi: 10.1038/srep21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre T.L., Brown E., Hacker L., Else T., Oraiopoulou M.E., Tomaszewski M.R., Jena R., Bohndiek S.E. The Potential of Photoacoustic Imaging in Radiation Oncology. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.803777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godet I., Doctorman S., Wu F., Gilkes D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells. 2022;11 doi: 10.3390/cells11040686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rankin E.B., Giaccia A.J. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicks E.E., Semenza G.L. Hypoxia-inducible factors: cancer progression and clinical translation. J. Clin. Invest. 2022;132 doi: 10.1172/JCI159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Zion A., Yin M., Adam D., Foster F.S. Functional Flow Patterns and Static Blood Pooling in Tumors Revealed by Combined Contrast-Enhanced Ultrasound and Photoacoustic Imaging. Cancer Res. 2016;76:4320–4331. doi: 10.1158/0008-5472.can-16-0376. [DOI] [PubMed] [Google Scholar]
- 12.Gerling M., Zhao Y., Nania S., Norberg K.J., Verbeke C.S., Englert B., Kuiper R.V., Bergström A., Hassan M., Neesse A., et al. Real-time assessment of tissue hypoxia in vivo with combined photoacoustics and high-frequency ultrasound. Theranostics. 2014;4:604–613. doi: 10.7150/thno.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich L.J., Seshadri M. Photoacoustic Imaging of Vascular Hemodynamics: Validation with Blood Oxygenation Level–Dependent MR Imaging. Radiology. 2015;275:110–118. doi: 10.1148/radiol.14140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma A., Rich L.J., Vincent-Chong V.K., Seshadri M. Visualizing the effects of metformin on tumor growth, vascularity, and metabolism in head and neck cancer. J. Oral Pathol. Med. 2018;47:484–491. doi: 10.1111/jop.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rich L.J., Damasco J.A., Bulmahn J.C., Kutscher H.L., Prasad P.N., Seshadri M. Photoacoustic and Magnetic Resonance Imaging of Hybrid Manganese Dioxide-Coated Ultra-Small NaGdF4 Nanoparticles for Spatiotemporal Modulation of Hypoxia in Head and Neck Cancer. Cancers. 2020;12:3294. doi: 10.3390/cancers12113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J.E., MacDonald C.R., Gandhi N., Das G., Repasky E.A., Mohammadpour H. Isolation of human and mouse myeloid-derived suppressor cells for metabolic analysis. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2022.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocatürk B., Versteeg H.H. Orthotopic injection of breast cancer cells into the mammary fat pad of mice to study tumor growth. J. Vis. Exp. 2015;96:51967. doi: 10.3791/51967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G.L., Zhang Y., Cao K.X., Wang X.M. Orthotopic Injection of Breast Cancer Cells into the Mice Mammary Fat Pad. J. Vis. Exp. 2019;143:58604. doi: 10.3791/58604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any datasets or code.