Key Points
Question
What is the clinical benefit of cancer drugs granted accelerated approval, and on what basis are they converted to regular approval?
Findings
In this cohort study of cancer drugs granted accelerated approval from 2013 to 2017, 41% (19/46) did not improve overall survival or quality of life in confirmatory trials after more than 5 years of follow-up, with results not yet available for another 15% (7/46). Among drugs converted to regular approval, 60% (29/48) of conversions relied on surrogate measures.
Meaning
Although accelerated approval can be useful, some cancer drugs do not end up demonstrating benefit in extending patients’ lives or improving their quality of life.
Abstract
Importance
The US Food and Drug Administration’s (FDA) accelerated approval pathway allows approval of investigational drugs treating unmet medical needs based on changes to surrogate measures considered “reasonably likely” to predict clinical benefit. Postapproval clinical trials are then required to confirm whether these drugs offer clinical benefit.
Objective
To determine whether cancer drugs granted accelerated approval ultimately demonstrate clinical benefit and to evaluate the basis of conversion to regular approval.
Design, Setting, and Participants
In this cohort study, publicly available FDA data were used to identify cancer drugs granted accelerated approval from 2013 to 2023.
Main Outcomes and Measures
Demonstrated improvement in quality of life or overall survival in accelerated approvals with more than 5 years of follow-up, as well as confirmatory trial end points and time to conversion for drug-indication pairs converted to regular approval.
Results
A total of 129 cancer drug–indication pairs were granted accelerated approval from 2013 to 2023. Among 46 indications with more than 5 years of follow-up (approved 2013-2017), approximately two-thirds (29, 63%) were converted to regular approval, 10 (22%) were withdrawn, and 7 (15%) remained ongoing after a median of 6.3 years. Fewer than half (20/46, 43%) demonstrated a clinical benefit in confirmatory trials. Time to withdrawal decreased from 9.9 years to 3.6 years, and time to regular approval increased from 1.6 years to 3.6 years. Among 48 drug-indication pairs converted to regular approval, 19 (40%) were converted based on overall survival, 21 (44%) on progression-free survival, 5 (10%) on response rate plus duration of response, 2 (4%) on response rate, and 1 (2%) despite a negative confirmatory trial. Comparing accelerated and regular approval indications, 18 of 48 (38%) were unchanged, while 30 of 48 (63%) had different indications (eg, earlier line of therapy).
Conclusions and Relevance
Most cancer drugs granted accelerated approval did not demonstrate benefit in overall survival or quality of life within 5 years of accelerated approval. Patients should be clearly informed about the cancer drugs that use the accelerated approval pathway and do not end up showing benefits in patient-centered clinical outcomes.
This study aims to determine whether cancer drugs granted accelerated approval from the US Food and Drug Administration ultimately demonstrate clinical benefit and to evaluate the basis of conversion to regular approval.
Introduction
The US Food and Drug Administration (FDA) approves new drugs “shown to be safe and effective” when their proven benefits outweigh their risks.1 In response to the HIV epidemic of the 1980s, the accelerated approval pathway was developed to provide a pathway for promising drugs treating unmet medical needs to reach the market sooner based on changes to unvalidated surrogate measures considered “reasonably likely” to predict clinical benefit.2,3 After accelerated approval is granted, mandatory postapproval trials are then required to confirm patient benefit and indications are either converted to regular approval or withdrawn. These confirmatory studies are intended to determine whether—and how much—clinical benefit a new therapy may offer (Box).4,5
Box. Definitions of Key Regulatory Terms.
Accelerated approval: an expedited pathway that allows for the approval of certain drugs treating serious conditions, for which there is an unmet medical need, based on changes in a surrogate end point.
Clinical end point: a trial outcome that measures improvement in how a patient feels, functions, or how long they survive (eg, quality of life, overall survival).
Confirmatory trial: for drugs granted accelerated approval, a requirement that drug manufacturers conduct a postapproval clinical trial measuring a clinical end point to confirm a drug’s effectiveness.
Indication: the specific medical context for which a drug has been tested in patients and approved for use by the US Food and Drug Administration (FDA). In the US, an approved medication may be prescribed by an appropriately licensed clinician for any reason—prescriptions for uses other than listed indications are known as “off-label” use.
Pivotal trial: sometimes known as preapproval trials, this is the general term given to any clinical trial that results in FDA approval.
Regular approval: approval granted based on changes to clinical end points. For drugs granted accelerated approval, regular approval occurs after confirmatory trials are conducted.
Surrogate end point: an outcome (eg, tumor size on a scan or a tumor marker blood test) that does not measure how a patient feels, functions, or survives, but is thought to predict clinical benefit.
Despite its origins in HIV treatment, accelerated approval is now most common in oncology, with approximately one-third of all oncology drug approvals using the pathway6 and more than 80% of all accelerated approvals being granted for cancer therapies.7 The surrogate measures typically used for accelerated approvals of cancer treatments include tumor response rate and progression-free survival (PFS),8 based on imaging or laboratory tests.
The record of accelerated approvals for cancer drugs has been mixed. In one review covering 2008 to 2012, only 14% demonstrated improvements in overall survival9 and more than 40% of confirmatory trials used surrogate measures to assess efficacy.5 Because many surrogate measures in oncology correlate poorly with survival,10,11 even after confirmatory trials, substantial uncertainty can remain as to the clinical benefit of accelerated approval drugs.12 Many confirmatory trials have been delayed, with some products being used for more than a decade without confirming clinical benefit.13,14,15 Furthermore, evidence suggests that trials powered to measure clinical outcomes are of similar duration to trials powered to assess surrogate measures, raising questions about whether confirmatory studies should use surrogate measures as their primary end points.16,17
A recent cohort of cancer drugs granted accelerated approval was analyzed to determine clinical benefit and to assess FDA decisions to convert indications to regular approval.
Methods
We evaluated cancer accelerated approval indications from 2013 to 2023 and conducted 2 analyses. For approved indications with more than 5 years of follow-up, we assessed the timing of confirmatory trial completion and whether confirmatory trials ultimately demonstrated improvement in either overall survival or quality of life. For indications converted to regular approval, we analyzed confirmatory trial characteristics informing the conversion decision. This cohort study followed the STROBE reporting guidelines; it was not submitted for institutional review board review because it used publicly available data and was not considered human subjects research.
Cohort Selection and Data Extraction
We used the published FDA Accelerated Approval list to identify accelerated approvals for cancer drug–indication pairs approved between January 2013 and July 2023.18 For our first analysis of quality of life and overall survival, we analyzed accelerated approvals with more than 5 years of follow-up (approved through December 2017). For our second analysis focused on evidence supporting conversion decisions, we analyzed indications converted to regular approval before August 2023. Using the Drugs@FDA database19 and established methods,20 we extracted the date and indication of each accelerated approval, supplemental or original indication status, projected completion date for confirmatory studies, and—where applicable—the conversion or withdrawal date and regular approval indication.
Accelerated Approval Determinations
We used multiple data sources to extract the names, National Clinical Trial (NCT) numbers, and end points of trials used to support accelerated approval from (in descending preference) FDA and manufacturer press releases, Drugs@FDA, and academic publications.
We identified the pivotal trial efficacy end point explicitly relied on by the FDA to support the granting of accelerated approval from the Drugs@FDA database, which sometimes differed from the primary efficacy end point of the pivotal trial (eg, primary end point is PFS, but accelerated approval based on overall response rate). When no end point was explicitly referenced, we assumed accelerated approval was based on the primary efficacy end point of the pivotal trial (eg, “Approval was based on data from [X] trial…”). In the few cases when multiple trials or efficacy end points were equally referenced for any approval determination, we conservatively chose the most clinically relevant end point (in descending preference: overall survival, PFS, response rate) or, when evaluating multiple efficacy trials with the same end point, the end point with greater magnitude of change.
Overall Survival and Quality-of-Life Data
For drugs that received regular approval, we used names and NCT numbers to search Google Scholar and ClinicalTrials.gov and chose the most up-to-date overall survival and quality-of-life data (ie, longest follow-up) from each confirmatory trial supporting regular approval in our first analysis. We assessed overall survival benefit as confirmed if there was a significant increase in overall survival in the trial’s primary intention-to-treat cohort. We characterized quality-of-life benefit as confirmed if there was a significant increase in a validated and prespecified global quality-of-life measure (eg, European Organisation for Research and Treatment of Cancer Core Quality of Life questionnaire)21 at any prespecified time point or an increase in time to deterioration compared with standard care.22 Withdrawn drugs were considered not to have shown clinical benefit.
Time to Regulatory Outcome
We calculated the time between the accelerated approval date and projected completion date of confirmatory studies, as well as between accelerated approval and conversion or withdrawal date.
Conversion to Regular Approval
For the second analysis of evidence underlying conversion decisions to regular approval from 2013 to 2023, we used the above methods to assess confirmatory trials. To investigate indication changes throughout the approval process, 2 of the authors (I.T.T.L. and E.R.S.C.) manually compared accelerated and regular approval indications for each converted drug. Indications were classified as same, earlier line of therapy, broadened, narrowed, or other. Any indication that was both broadened and moved to an earlier line of therapy we placed in the “earlier line of therapy” category; we resolved differences by consensus. For indications converted to regular approval on response rate, we extracted postmarketing requirements from the FDA’s Postmarket Requirements and Commitments database.
Statistical Analysis
Linear trend regression (for timing outcomes) and 1-sided Fisher exact test with a prespecified α of .05 (to compare supplemental and original indications) were used. All statistics were generated using Microsoft Excel version 16.
Results
We identified 129 accelerated approvals for cancer indications from 2013 to 2023 (eFigure 1 in Supplement 1). Following accelerated approval, conversion to regular approval most commonly occurred in 1 to 2 years, withdrawals occurred evenly between 1 and 5 years, and—as of database lock—ongoing approvals had been most commonly waiting 3 to 4 years (eFigure 2 in Supplement 1).
Five-Year Outcomes of Accelerated Approval
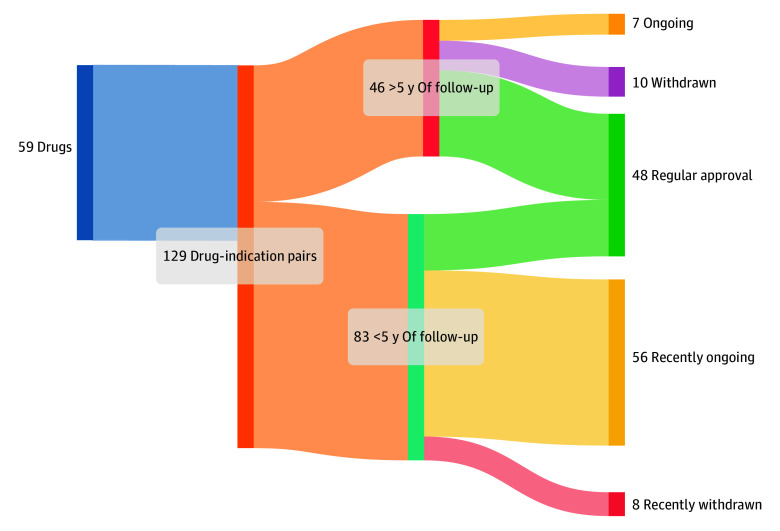
Our first analysis included 46 indications with at least 5 years of follow-up, of which 24 (52%) were original indications and 22 (48%) were supplemental. Among these 46 indications, 29 (63%) were converted to regular approval, 10 (22%) were withdrawn, and 7 (15%) remained without a definitive outcome (Figure 1); these proportions were similar between original and supplemental indications (Table).
Figure 1. Oncology Drugs Granted Accelerated Approval From 2013 to 2023, and Regulatory Outcomes.
Oncology drugs granted accelerated approval and regulatory outcome, based on follow-up time.
Table. Pivotal Trials Supporting Accelerated Approvals and Regulatory Outcomes of Oncology Accelerated Approval Drug-Indication Pairs, 2013 to 2017.
| No. (%) | |||
|---|---|---|---|
| All indications (n = 46) | Original indications (n = 24) | Supplemental indications (n = 22) | |
| Pivotal trial efficacy outcomes | |||
| Response rate | 19 (41) | 9 (38) | 10 (45) |
| Response rate plus duration of response | 21 (46) | 11 (46) | 10 (45) |
| Progression-free survival | 4 (9) | 2 (8) | 2 (9) |
| Overall survival | 1 (2) | 1 (4) | 0 |
| Complete remission rate | 1 (2) | 1 (4) | 0 |
| Regulatory outcome | |||
| Converted to regular approval | 29 (63) | 14 (58) | 15 (68) |
| Ongoing | 7 (15) | 4 (17) | 3 (14) |
| Withdrawn | 10 (22) | 6 (25) | 4 (18) |
| Time to conversion, median (IQR), d | 972 (680-1274) | ||
| Time to withdrawal, median (IQR), d | 1923 (1449-2517) | ||
These 46 drug-indication pairs were tested in 48 corresponding preapproval pivotal trials (Table). Nineteen drugs (41%) used preapproval pivotal trials with response rate as a primary end point, with a mean response rate of 50.6% (range, 16%-81%). Twenty-one drugs (46%) used response rate with duration of response, with a mean response rate of 40.7% (range, 13%-59%) and average median duration of response of 10.1 months (range, 5.3-13.8 months). PFS was used in 4 (9%) and overall survival and complete remission rate were each the primary basis for 1 accelerated approval (2%; eFigure 3 in Supplement 1).
Of the 29 indications converted to regular approval, we identified peer-reviewed articles with confirmatory trial quality-of-life data in 26 (90%) and a reported overall survival analysis in 25 (86%). Of these 29 indications, 20 (69%) demonstrated clinical benefit: 7 (24%) showed improvements in both overall survival and quality of life, 7 (24%) improved overall survival without demonstrating quality-of-life benefit, and 6 (21%) improved quality of life without improving overall survival (Figure 1). The remaining 9 (31%) were converted without showing a benefit in either overall survival or quality of life in their confirmatory trials. Accelerated approvals for original indications reported higher rates of confirmed overall survival or quality-of-life benefit than supplemental indications, although this difference was not statistically significant (original: 11/14 [79%]; supplemental: 9/15 [60%], P = .93; eTable 1 in Supplement 1).
Overall, 26 of 46 drug-indication pairs (57%) failed to demonstrate clinical benefit after at least 5 years of follow-up.
Time to Conversion or Withdrawal
The duration from accelerated approval to projected confirmatory trial completion at the time of initial approval increased from 3.4 years in 2013 to 4.5 years in 2017 (eFigure 4 in Supplement 1). For the 10 withdrawn indications, duration from accelerated approval to withdrawal date decreased over the course of the study period from 9.9 years to 3.6 years. For the 29 converted indications, duration from accelerated approval to conversion to regular approval increased from 1.6 years to 3.6 years (eFigure 5 in Supplement 1). The time between projected trial completion to actual withdrawal or conversion narrowed over time (eFigure 6 in Supplement 1).
Indications Converted to Regular Approval
In our second cohort focused on conversion decisions, we identified 66 accelerated approval drug-indication pairs approved and either converted (48, 73%) or withdrawn (18, 27%) between 2013 and August 2023 (Figure 2 and Figure 3). Eighteen drug-indication pairs (38%) had the same indication for accelerated and regular approval, 18 (38%) included an earlier line of therapy, 8 (17%) were broadened without moving to an earlier line of therapy, 3 (6%) were narrowed, and 1 (2%) was changed in an alternate way (examples in eTable 2 in Supplement 1; full list in eTable 3 in Supplement 1).
Figure 2. Cancer Drugs Granted Accelerated Approval From January 2013 to May 2017.
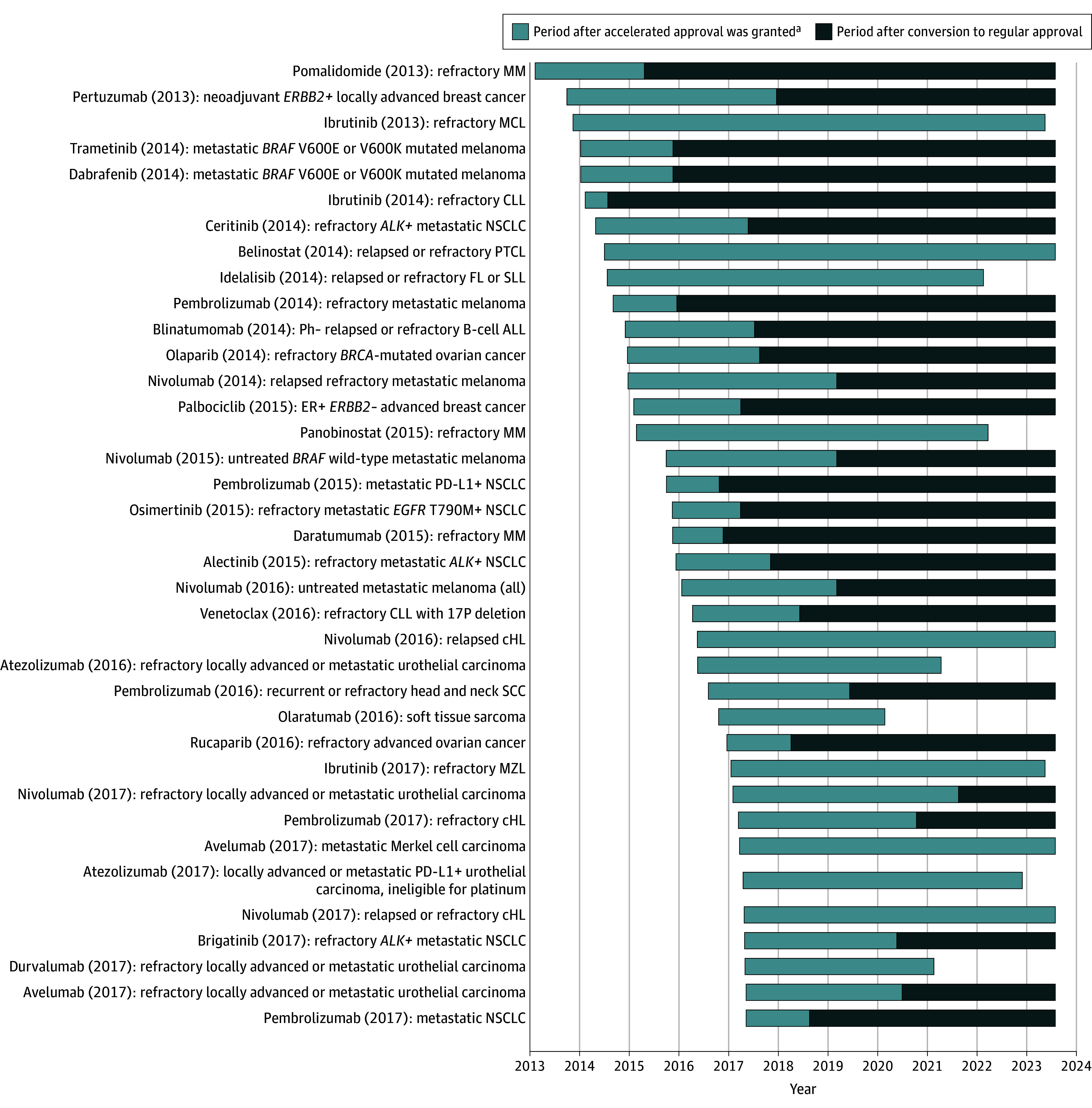
This figure excludes ongoing accelerated approvals with less than 5 years of follow-up (n = 74). Drug (year of accelerated approval) indicates accelerated approval indication. ALL indicates acute lymphoblastic leukemia; cHL, classical Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; ER, estrogen receptor; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MZL, marginal zone lymphoma; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death ligand 1; PTCL, peripheral T-cell lymphoma; SCC, squamous cell carcinoma; and SLL, small lymphocytic lymphoma.
aWithdrawn drugs are represented by the lighter bars ending prior to the cutoff date of August 1, 2023.
Figure 3. Cancer Drugs Granted Accelerated Approval Since 2017 .
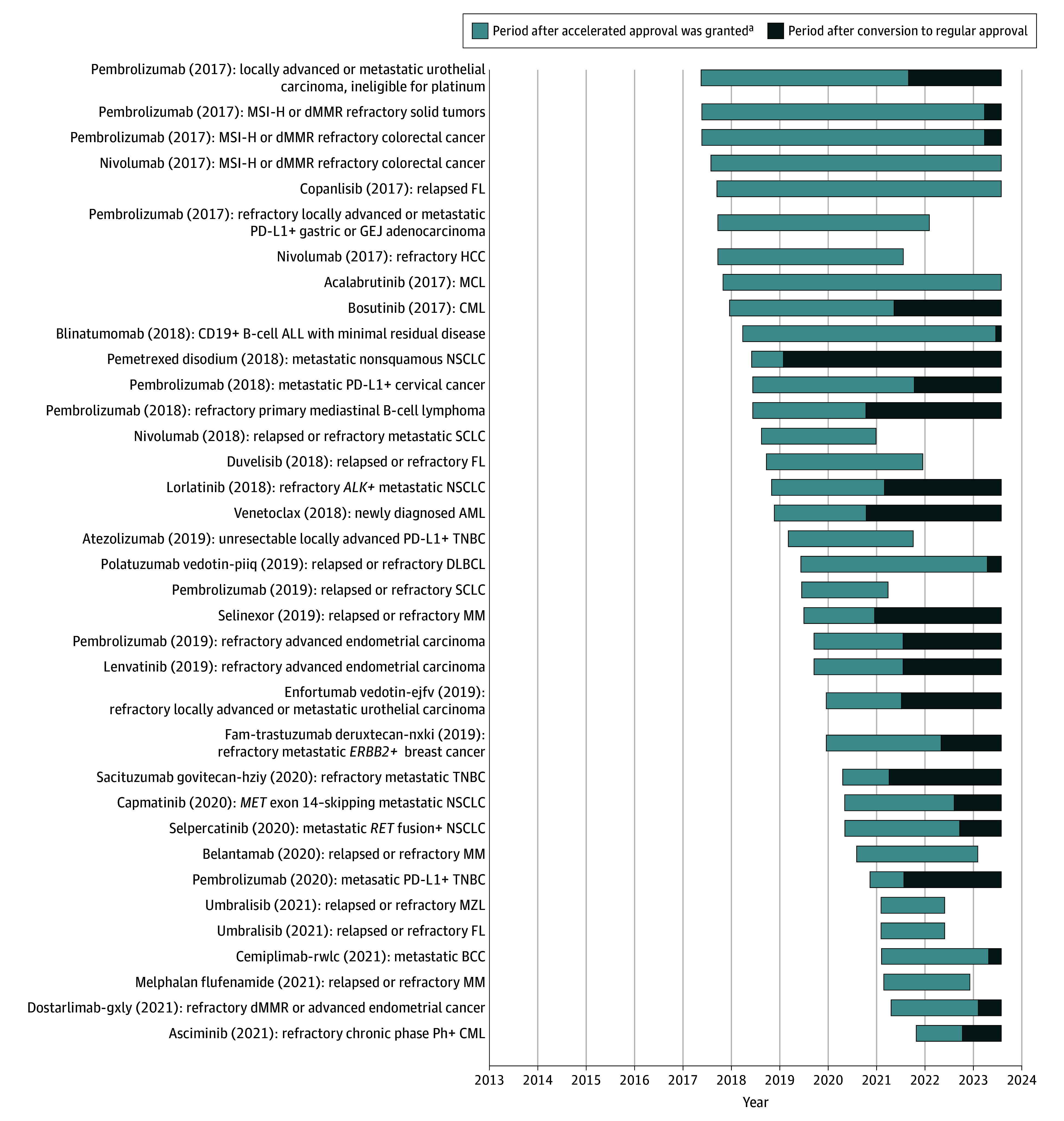
This figure excludes ongoing accelerated approvals with less than 5 years of follow-up (n = 74). Drug (year of accelerated approval) indicates accelerated approval indication. ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCC, basal cell carcinoma; CML, chronic myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; dMMR, mismatch repair deficient; FL, follicular lymphoma; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MSI-H, microsatellite instability high; MZL, marginal zone lymphoma; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death ligand 1; SCLC, small cell lung cancer; and TNBC, triple-negative breast cancer.
aWithdrawn drugs are represented by the lighter bars ending prior to the cutoff date of August 1, 2023.
Of 48 converted indications, 19 (40%) were converted based on a pivotal trial(s) demonstrating improvement in overall survival. Twenty-one (44%) were converted based on improvement in progression-free, event-free, or disease-free survival, 5 (10%) on response rate plus duration of response, 2 (4%) on response rate, and 1 (2%) despite a negative confirmatory trial that showed no improvement in either overall survival or PFS (eFigure 7 in Supplement 1).23 From 2013 to 2020, 0 of 28 conversions were based on response rate, whereas from 2021 to 2023, 7 of 19 conversions (37%) were based on response rate (2 were in chronic myeloid leukemia and used major molecular response rate, while 5 used overall response rate plus duration of response; eTable 4 in Supplement 1). The 7 conversions based on response rate were associated with 14 mandatory postmarketing commitments (eTable 5 in Supplement 1). Of the 48 converted indications, 40 reported PFS data with mean improvement in median PFS of 6.1 months (range, 1.2-23.9; mean hazard ratio, 0.53). Among the 23 converted drugs that reported overall survival data, mean improvement in median overall survival was 10.1 months (range, 3.7-18.5; mean hazard ratio, 0.64).
Discussion
Despite more than 5 years of follow-up, most cancer drug indications granted accelerated approval between 2013 and 2017 did not demonstrate a benefit in overall survival or quality of life to patients in confirmatory trials by mid-2023. Among this cohort of accelerated approvals for cancer drugs, some improved overall survival, several ineffective drugs were withdrawn, multiple drugs had ongoing confirmatory studies, and others were converted to regular approval based on surrogate measures. Among drugs converted to regular approval, only two-thirds showed improvements in overall survival or quality of life, while one-third failed to show significant improvement in these outcomes. In the expanded cohort of conversion decisions from the past 10 years, the FDA has increasingly used surrogate measures such as response rate to support conversion from accelerated to regular approval.
Accelerated approvals in oncology have been the subject of much research. One study analyzed the therapeutic value of cancer drugs granted accelerated approval between 2007 and 2021 using ratings derived from international health technology assessment evaluations, finding about 40% of US accelerated approvals to be of high added therapeutic value.23 However, health technology assessment ratings were only available for 62% of FDA-approved indications. Another study investigated 18 accelerated approvals for cancer drugs that failed to reach their primary end point in confirmatory trials, finding that 11 were voluntarily withdrawn by manufacturers, 6 remained on the market, and 1 was revoked by the FDA.24 That investigation did not focus on the quality of a confirmatory trial’s primary end point and excluded approvals that reached their primary trial end point. By contrast, the current study analyzed 2 commonly reported, patient-centered end points to evaluate recent FDA accelerated approvals, while also reviewing recent trends in conversion decisions.
In an analysis of oncology drugs approved on the basis of surrogate end points from 2008 to 2013, Kim and Prasad9 reported that 14% (5/36) demonstrated overall survival benefit with 5 years of follow-up, while another analysis covering 1992 to 2017 reported an overall survival benefit in 20% of accelerated approvals for cancer drugs.5 Finding overall survival benefit in about one-third of accelerated approval indications in a more recent cohort suggests some progress in this area. An additional 5 drugs that demonstrated quality-of-life benefits are highlighted because these end points can also be meaningful for patients. Of the 29 converted indications in the first analysis, the current data revealed substantial overlap between trials showing quality-of-life (13 indications) and overall survival (14 indications) benefit (20 total), consistent with prior observations.10
Characteristics of pivotal trials used to determine conversion from accelerated to regular approval were also analyzed. While 0 of 28 conversions from 2013 to 2020 were based on response rate (± duration of response), nearly 40% of conversions (7/19) from 2021 to 2023 used this surrogate measure to grant regular approval. Response rate, which measures tumor shrinkage, is a logical surrogate measure to use for accelerated approval because many tumor types are unlikely to spontaneously regress.25 However, it cannot by itself determine whether a drug offers patients a clinical benefit, and—as highlighted by the FDA26—should therefore not be used without additional patient-centered information to establish regular approval.
One example of the potential issues created by the use of response rate as a study end point is the anti-CD19 antibody tafasitamab. Tafasitamab received accelerated approval in 2020 in combination with lenalidomide for the treatment of relapsed-refractory diffuse large B-cell lymphoma based on an objective response rate of 55% and a median duration of response of 21.7 months in the L-MIND trial (NCT02399085).27 Despite these promising markers of sustained tumor shrinkage, however, a recent real-world consortium study of tafasitamab-lenalidomide measured a median PFS of just 1.9 months.28 Another example comes from bosutinib in chronic myeloid leukemia. In the BFORE trial (NCT02130557), which compared bosutinib vs imatinib, bosutinib improved major molecular response rate but also substantially increased toxicity, which is not captured by this end point and therefore leaves uncertainty as to bosutinib’s net clinical benefit. These examples highlight the fallibility of using preliminary end points as the basis for conversion to regular approval.
Determining whether a drug offers a clinical benefit for a given accelerated approval indication before conversion to regular approval is important because it is substantially more difficult for the FDA to seek withdrawal of an indication after regular approval. While the FDA has requested withdrawal of 27 cancer drug indications approved via accelerated approval (including 17 since 2021 alone),29,30 it rarely requests withdrawal of indications that have received regular approval. Furthermore, once a cancer drug is granted accelerated approval by the FDA, it is generally placed on treatment guidelines (though it is less likely to be “preferred” compared with regular approvals)31 and covered by insurance providers. This results in clinical availability,32,33 but is accompanied by patient exposure to the high prices34 and out-of-pocket costs of many cancer therapies.35,36 Because there is little difference in patient access between accelerated and regular approvals, confirmatory trials could routinely be powered to determine whether novel therapies offer patients a clinical benefit without compromising availability.
One goal of confirmatory trials should be to identify the population in which a drug is most effective. Olaparib in prostate cancer,37 ibrutinib in mantle cell lymphoma,38 and romidepsin in T-cell lymphoma39 are recent examples in which subgroup analyses40,41 of phase 3 clinical trials suggested divergent results among different populations. The current analysis of indication changes between accelerated and regular approval suggests, however, that indications are infrequently narrowed (2/48 [4%] of converted indications).
In addition to finding that approximately 40% of accelerated approval indications were moved to an earlier line of treatment at regular approval, indications were found to be broadened in a further 20%. When confirmatory trials are conducted in different populations from accelerated approval trials, uncertainty may persist regarding efficacy for a drug’s initial indication. This occurred in the case of romidepsin, when a negative confirmatory trial in a different indication (first-line treatment of peripheral T-cell lymphoma, in combination with chemotherapy) led to withdrawal of the drug,38 while uncertainty remains around its original indication (as monotherapy in relapsed/refractory disease). Regulatory flexibility is important to appropriately target indications based on the best available clinical evidence; however, agencies should not allow this flexibility to generate confusing and nondefinitive evidence in disparate clinical contexts.
The current analysis of conversion decision timing decisions demonstrated a decrease in time from accelerated approval to withdrawal or conversion. In the current sample, this trend appears to be driven by more rapid withdrawal decisions, which decreased from more than 10 years to fewer than 2 years over the study period. Reducing the time from accelerated approval to completion of confirmatory trials is important. It would be unwise to pursue faster conversion decisions at the expense of gaining superior data with meaningful clinical end points, even if it takes longer to acquire.
Limitations
The limitations of this study include, first, that only confirmatory trial data were looked at to evaluate the clinical benefit of cancer indications for accelerated approvals. Subsequent or larger trials in the same trial population could provide evidence of clinical benefit. Second, although this study was conducted with a minimum of 5 years of follow-up for the clinical benefit cohort, 7 drugs were still awaiting confirmatory trial results, and these drugs may yet show benefit. However, patients receiving these drugs still receive interventions of uncertain clinical efficacy in the meantime.7,42,43 Third, because published overall survival and quality-of-life data were relied on in this study and primary data were not reevaluated, it is possible that clinical benefit may have been overestimated because statistical improvements in end points may or may not be clinically meaningful to patients depending on magnitude and methodologic approach (eg, trial population differs from real-world patients,44 control group offered substandard care,45 differential survey response rates46).
Conclusions
Most cancer drugs granted accelerated approval did not demonstrate benefit in overall survival or quality of life within 5 years of accelerated approval. Patients should be clearly informed about the cancer drugs that use the accelerated approval pathway and do not end up showing benefits in patient-centered clinical outcomes.
eFigure 1A. Cancer Drugs Granted Accelerated Approval Between January 2013 and July 2023 (and eFigure 1B including those with <5 years follow up) and Administrative Status
eFigure 2. Time From Accelerated Approval to Definitive Outcome for Cancer Drugs Granted Accelerated Approval Between 2013 and 2023, by Administrative Status
eFigure 3A. Administrative Outcome of Accelerated Approvals for Cancer Drugs Between 2013-2017
eFigure 3B. Efficacy Outcomes Used in Pivotal Trials to Support Accelerated Approvals for Cancer Indications Between 2013-2017
eFigure 4. Projected Time to Required Study Completion Following Accelerated Approval for Cancer Indications Approved Between 2013-2017
eFigure 5. Time Between Accelerated Approval and Definitive Outcome (Conversion or Withdrawal) for Cancer Indications Approved Between 2013 and 2017
eFigure 6. Difference Between Projected and Actual Definitive Outcome for Accelerated Approval Cancer Indications Approved Between 2013-2017
eFigure 7. Confirmatory Trial Endpoints Used for Conversion to Regular Approval
eTable 1. Clinical Benefit of Drug-Indication Pairs Granted Accelerated Approval Between 2013 and 2017 That Have Been Converted to Regular Approval
eTable 2. Examples of Indication Changes Between Accelerated and Regular Approval
eTable 3. Indications of Oncology Drugs Receiving Accelerated After January 2013 and Converted to Regular Approval by July 2023
eTable 4. Oncology Drugs Converted to Regular Approval Since 2021 on the Basis of Response Rate or Response Rate Plus Duration of Response
eTable 5. FDA-Required Postmarketing Commitments for Oncology Drugs Converted to Regular Approval Since 2021 on the Basis of Response Rate or Response Rate Plus Duration of Response
Data Sharing Statement
References
- 1.US Food and Drug Administration . Applications for FDA approval to market a new drug: subpart A: general provisions: purpose. 21 CFR §314.2 (2023).
- 2.US Food and Drug Administration . Accelerated Approval Program. Accessed August 21, 2023. https://www.fda.gov/drugs/nda-and-bla-approvals/accelerated-approval-program
- 3.Gyawali B, Kesselheim AS. Reinforcing the social compromise of accelerated approval. Nat Rev Clin Oncol. 2018;15(10):596-597. doi: 10.1038/s41571-018-0066-3 [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh AD, Kesselheim AS, Rome BN. Timing of confirmatory trials for drugs granted accelerated approval based on surrogate measures from 2012 to 2021. JAMA Health Forum. 2023;4(3):e230217. doi: 10.1001/jamahealthforum.2023.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott EC, Baines AC, Gong Y, et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat Rev Drug Discov. 2023;22(8):625-640. doi: 10.1038/s41573-023-00723-4 [DOI] [PubMed] [Google Scholar]
- 7.Beakes-Read G, Neisser M, Frey P, Guarducci M. Analysis of FDA’s Accelerated Approval Program performance December 1992-December 2021. Ther Innov Regul Sci. 2022;56(5):698-703. doi: 10.1007/s43441-022-00430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030-1033. doi: 10.1200/JCO.2011.38.7571 [DOI] [PubMed] [Google Scholar]
- 9.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992-1994. doi: 10.1001/jamainternmed.2015.5868 [DOI] [PubMed] [Google Scholar]
- 10.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389-1398. doi: 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
- 11.Gyawali B, Hey SP, Kesselheim AS. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine. 2020;21:100332. doi: 10.1016/j.eclinm.2020.100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyawali B, Hey SP, Kesselheim AS. A comparison of response patterns for progression-free survival and overall survival following treatment for cancer with pd-1 inhibitors: a meta-analysis of correlation and differences in effect sizes. JAMA Netw Open. 2018;1(2):e180416. doi: 10.1001/jamanetworkopen.2018.0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahzad M, Naci H, Wagner AK. Association between preapproval confirmatory trial initiation and conversion to traditional approval or withdrawal in the FDA Accelerated Approval Pathway. JAMA. 2023;329(9):760-761. doi: 10.1001/jama.2023.0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemery S, Pazdur R. Approvals in 2021: dangling accelerated approvals, drug dosing, new approvals and beyond. Nat Rev Clin Oncol. 2022;19(4):217-218. doi: 10.1038/s41571-022-00605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaver JA, Howie LJ, Pelosof L, et al. A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849-856. doi: 10.1001/jamaoncol.2017.5618 [DOI] [PubMed] [Google Scholar]
- 16.Wallach JD, Ramachandran R, Bruckner T, Ross JS. Comparison of duration of postapproval vs pivotal trials for therapeutic agents granted US Food and Drug Administration accelerated approval, 2009-2018. JAMA Netw Open. 2021;4(11):e2133601. doi: 10.1001/jamanetworkopen.2021.33601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen EY, Joshi SK, Tran A, Prasad V. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern Med. 2019;179(5):642-647. doi: 10.1001/jamainternmed.2018.8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration . Resources for information: approved drugs. Accessed October 30, 2023. https://www.fda.gov/drugs/drug-approvals-and-databases/resources-information-approved-drugs
- 19.US Food and Drug Administration . Drugs@FDA: FDA-approved drugs. Accessed August 24, 2023. https://www.accessdata.fda.gov/scripts/cder/daf/
- 20.Naci H, Smalley KR, Kesselheim AS. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA. 2017;318(7):626-636. doi: 10.1001/jama.2017.9415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fayers P, Bottomley A; EORTC Quality of Life Group; Quality of Life Unit; European Organisation for Research and Treatment of Cancer . Quality of life research within the EORTC: the EORTC QLQ-C30. Eur J Cancer. 2002;38(suppl 4):S125-S133. doi: 10.1016/S0959-8049(01)00448-8 [DOI] [PubMed] [Google Scholar]
- 22.Samuel JN, Booth CM, Eisenhauer E, Brundage M, Berry SR, Gyawali B. Association of quality of life outcomes in cancer drug trials with survival outcomes and drug class. JAMA Oncol. 2022;8(6):879-886. doi: 10.1001/jamaoncol.2022.0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vokinger KN, Kesselheim AS, Glaus CEG, Hwang TJ. Therapeutic value of drugs granted accelerated approval or conditional marketing authorization in the US and Europe from 2007 to 2021. JAMA Health Forum. 2022;3(8):e222685. doi: 10.1001/jamahealthforum.2022.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyawali B, Rome BN, Kesselheim AS. Regulatory and clinical consequences of negative confirmatory trials of accelerated approval cancer drugs: retrospective observational study. BMJ. 2021;374:n1959. doi: 10.1136/bmj.n1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachdev A, Sharpe I, Bowman M, Booth CM, Gyawali B. Objective response rate of placebo in randomized controlled trials of anticancer medicines. EClinicalMedicine. 2022;55:101753. doi: 10.1016/j.eclinm.2022.101753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merino M, Kasamon Y, Theoret M, Pazdur R, Kluetz P, Gormley N. Irreconcilable differences: the divorce between response rates, progression-free survival, and overall survival. J Clin Oncol. 2023;41(15):2706-2712. doi: 10.1200/JCO.23.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration . FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. Accessed January 3, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tafasitamab-cxix-diffuse-large-b-cell-lymphoma
- 28.Qualls DA, Lambert N, Caimi PF, et al. Tafasitamab and lenalidomide in large B-cell lymphoma: real-world outcomes in a multicenter retrospective study. Blood. 2023;142(26):2327-2331. doi: 10.1182/blood.2023021274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration . Withdrawn: cancer accelerated approvals. Updated July 25, 2023. Accessed January 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-cancer-accelerated-approvals
- 30.Cramer A, Sørup FKH, Christensen HR, Petersen TS, Karstoft K. Withdrawn accelerated approvals for cancer indications in the USA: what is the marketing authorisation status in the EU? Lancet Oncol. 2023;24(9):e385-e394. doi: 10.1016/S1470-2045(23)00357-1 [DOI] [PubMed] [Google Scholar]
- 31.Cliff ERS, Rome RS, Kesselheim AS, Rome BN. National Comprehensive Cancer Network guideline recommendations of cancer drugs with accelerated approval. JAMA Netw Open. 2023;6(11):e2343285. doi: 10.1001/jamanetworkopen.2023.43285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti RM, Bernstein AC, Villaflor VM, Schilsky RL, Rosenthal MB, Bach PB. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol. 2013;31(9):1134-1139. doi: 10.1200/JCO.2012.42.7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green AK, Wood WA, Basch EM. Time to reassess the cancer compendia for off-label drug coverage in oncology. JAMA. 2016;316(15):1541-1542. doi: 10.1001/jama.2016.12770 [DOI] [PubMed] [Google Scholar]
- 34.Rome BN, Egilman AC, Kesselheim AS. Trends in prescription drug launch prices, 2008-2021. JAMA. 2022;327(21):2145-2147. doi: 10.1001/jama.2022.5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouchveridze E, Banerjee R, Desai A, et al. Financial toxicity in hematological malignancies: a systematic review. Blood Cancer J. 2022;12(4):74. doi: 10.1038/s41408-022-00671-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381-390. doi: 10.1038/nrclinonc.2017.31 [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration . FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. May 31, 2023. Accessed September 12, 2023. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration
- 38.Cliff ERS, Hilal T, Kesselheim AS. Complicated regulatory decision-making following inconsistent trial results: the issue with ibrutinib for mantle cell lymphoma. Nat Rev Clin Oncol. 2024;21(1):1-2. doi: 10.1038/s41571-023-00821-7 [DOI] [PubMed] [Google Scholar]
- 39.Pro B, Horwitz SM, Prince HM, et al. Romidepsin induces durable responses in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma. Hematol Oncol. 2017;35(4):914-917. doi: 10.1002/hon.2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar SK, Harrison SJ, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1630-1642. doi: 10.1016/S1470-2045(20)30525-8 [DOI] [PubMed] [Google Scholar]
- 41.Russler-Germain DA, Cliff ERS, Bartlett NL. Cell-of-origin effect of polatuzumab vedotin in diffuse large B-cell lymphoma: no ordinary subgroup analysis. Blood. 2023;142(25):2216-2219. doi: 10.1182/blood.2023022048 [DOI] [PubMed] [Google Scholar]
- 42.Parikh RB, Hubbard RA, Wang E, et al. Exposure to US cancer drugs with lack of confirmed benefit after US Food and Drug Administration accelerated approval. JAMA Oncol. 2023;9(4):567-569. doi: 10.1001/jamaoncol.2022.7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skydel JJ, Egilman AC, Wallach JD, Ramachandran R, Gupta R, Ross JS. Spending by the Centers for Medicare & Medicaid Services before and after confirmation of benefit for drugs granted US Food and Drug Administration accelerated approval, 2012 to 2017. JAMA Health Forum. 2022;3(5):e221158. doi: 10.1001/jamahealthforum.2022.1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mailankody S, Prasad V. Overall survival in cancer drug trials as a new surrogate end point for overall survival in the real world. JAMA Oncol. 2017;3(7):889-890. doi: 10.1001/jamaoncol.2016.5296 [DOI] [PubMed] [Google Scholar]
- 45.Hilal T, Sonbol MB, Prasad V. Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US Food and Drug Administration. JAMA Oncol. 2019;5(6):887-892. doi: 10.1001/jamaoncol.2019.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakraborty R, Cannella L, Cottone F, Efficace F. Quality of patient-reported outcome reporting in randomised controlled trials of haematological malignancies according to international quality standards: a systematic review. Lancet Haematol. 2020;7(12):e892-e901. doi: 10.1016/S2352-3026(20)30292-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1A. Cancer Drugs Granted Accelerated Approval Between January 2013 and July 2023 (and eFigure 1B including those with <5 years follow up) and Administrative Status
eFigure 2. Time From Accelerated Approval to Definitive Outcome for Cancer Drugs Granted Accelerated Approval Between 2013 and 2023, by Administrative Status
eFigure 3A. Administrative Outcome of Accelerated Approvals for Cancer Drugs Between 2013-2017
eFigure 3B. Efficacy Outcomes Used in Pivotal Trials to Support Accelerated Approvals for Cancer Indications Between 2013-2017
eFigure 4. Projected Time to Required Study Completion Following Accelerated Approval for Cancer Indications Approved Between 2013-2017
eFigure 5. Time Between Accelerated Approval and Definitive Outcome (Conversion or Withdrawal) for Cancer Indications Approved Between 2013 and 2017
eFigure 6. Difference Between Projected and Actual Definitive Outcome for Accelerated Approval Cancer Indications Approved Between 2013-2017
eFigure 7. Confirmatory Trial Endpoints Used for Conversion to Regular Approval
eTable 1. Clinical Benefit of Drug-Indication Pairs Granted Accelerated Approval Between 2013 and 2017 That Have Been Converted to Regular Approval
eTable 2. Examples of Indication Changes Between Accelerated and Regular Approval
eTable 3. Indications of Oncology Drugs Receiving Accelerated After January 2013 and Converted to Regular Approval by July 2023
eTable 4. Oncology Drugs Converted to Regular Approval Since 2021 on the Basis of Response Rate or Response Rate Plus Duration of Response
eTable 5. FDA-Required Postmarketing Commitments for Oncology Drugs Converted to Regular Approval Since 2021 on the Basis of Response Rate or Response Rate Plus Duration of Response
Data Sharing Statement