Abstract
Background and Aim:
Campylobacter infections in sheep may be asymptomatic or cause enteritis, ileitis, infertility, and abortion. Thus, this study aimed to estimate the prevalence of Campylobacter spp. in farming sheep and to detect risk factors, molecular patterns, and antimicrobial susceptibility status of these pathogens.
Materials and Methods:
Four hundred and eight fecal samples were collected from 12 flocks in the Mymensingh and Sherpur districts. Samples were tested by both basic (culture and biochemical tests) and molecular (initially 16S rRNA and later hipO gene-based polymerase chain reaction). Furthermore, the antimicrobial susceptibility status of Campylobacter jejuni was confirmed using disk diffusion. Flock- and animal-level data were captured using semi-structured interviews with farm owners under bivariate and multivariate logistic regression analyses to confirm the risk factors for Campylobacter-positive status.
Results:
The prevalence of C. jejuni staining at the animal and flock levels was 8.82% (36/408) and 66.70% (8/12), respectively. The age of sheep was identified as an important risk factor. Up to 1 year of age, sheep were 3.78 times more likely to be infected with C. jejuni (95% confidence interval: 1.0736–13.3146, p = 0.038). Of the 36 isolates of C. jejuni, all were found to be fully susceptible (100%) to gentamicin and ciprofloxacin. In this study, three antimicrobial agents, oxytetracycline, azithromycin, and ceftriaxone, were fully resistant (100%). The majority of isolates were resistant to a combination of 4–6 antimicrobial agents.
Conclusion:
The present study highlights the predominant maintenance of zoonotic Campylobacter species in sheep, and their burden on human health is enormous. Therefore, environmental, animal, and human health needs to be focused under a One Health lens to mitigate the occurrence of Campylobacter in farm settings and to prevent further introduction to animals and humans.
Keywords: antimicrobial resistance, campylobacter jejuni, identification, isolation, prevalence, risk factors, sheep
Introduction
Since the last decade, Campylobacter has been considered one of the main causal agents of gastrointestinal infection worldwide in both developed and developing countries [1]. Campylobacter jejuni and Campylobacter coli account for most of the reported cases of Campylobacter infection in humans, whereas C. coli has a minor contribution to the overall burden. Food-producing animals such as poultry, cattle, sheep, pigs, and pets such as dogs and cats are considered to be associated with human Campylobacter infection [2]. However, a large number of animals have been shown to be reservoirs of Campylobacter, and there is no significant evidence of infection [2, 3]. C. jejuni is the major cause of human campylobacteriosis [4].
In general, poultry meat is accounted the pivotal cause of infection for human Campylobacter. The pathogens usually do live in the gastrointestinal tract (GIT) as commensalism of poultry species, especially commercial chickens and turkeys [5]. At the present time, ruminant species such as cattle and sheep contribute to the ecology of Campylobacter, which has been widely demonstrated in different geographical locations [6–10]. Ruminant-related Campylobacters could be spread to humans through food chains such as milk and water, contaminated environments, or even direct contact with source animals [11]. Notwithstanding, a significant source of human infection worldwide is the consumption of undercooked Campylobacter-contaminated poultry or lack of cleaning and sanitation during raw poultry-product handling [12]. However, source confirmation studies have elucidated that ruminant Campylobacter is the primary source of human infection [13–15].
Classically, Campylobacter inhabits the GIT tract in animals; however, it can also transfer through the epithelial barrier, resulting in bacteremia by systemic infection, abortion in ruminant animals, and sporadically causing infections in humans [16, 17].
Campylobacteriosis is primarily associated with C. jejuni, including Campylobacter fetus subsp. fetus, is known to cause abortion and stillbirth in reproductive sheep. Transmission occurs through oral or genital contact with contaminated feces, water, or aborted fetuses [18]. Although it is a common cause of abortion in the United Kingdom, it has been confirmed in Western Australia that it is rare. Formerly recognized as vibriosis, this disease is now referred to as campylobacteriosis.
Bangladesh has 1.34 million sheep, and the number of this species has been steadily increasing since the last decade [19], which would increase the public health burden as most of the sheep keepers of Bangladesh are landless marginalized communities [20] and are less aware of their health burden.
To the best of our knowledge, there have been no reports of sheep Campylobacter in Bangladesh. To understand the overall burden of the zoonotic pathogen in source animals, several reports have been published on the occurrence of Campylobacter in poultry [21–24] and dairy cows [25, 26] in different geographical locations in Bangladesh.
Thus, this study aimed to estimate the prevalence of Campylobacter spp. in farming sheep and to detect risk factors, molecular patterns, and antimicrobial susceptibility status of these pathogens.
Materials and Methods
Ethical approval and Informed consent
The study was approved by the Animal Welfare and Experimentation Ethics Committee (AWEEC) of Bangladesh Agricultural University [AWEEC/BAU/2021(11)]. All participants (farmers) included in this study were aged ≥18 years. All respondents were informed about the aims of the research. Verbal permissions were obtained as a considerable number of participants are illiterate, as they cannot read and write. The participants had the right to withdraw or not to participate in animal sampling from his/her farm and subsequent animal-level data collection.
Study period and location
The study was conducted from April to December 2021 in the Mymensigh and Sherpur districts of the Mymemsingh division of Bangladesh. Mymensingh (24°74’N, 90°40’E) and Sherpur (25° 1’ 9.8580’’ N, 90° 0’ 49.4388’’ E) are situated in the northeastern part of Bangladesh (Figure-1). These districts are located in the Jamuna Basin and are promising for profitable sheep production that could meet the meat requirement, improve livelihoods, and provide sustainable income [27].
Figure-1.
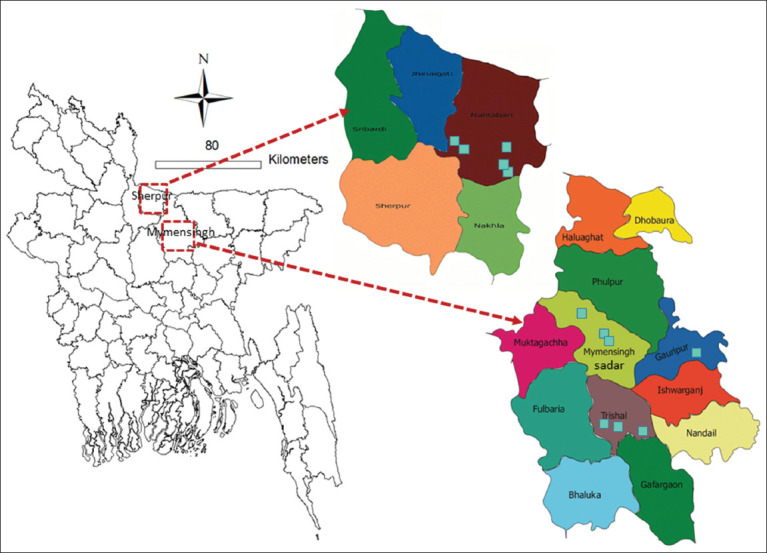
Study districts (Mymensingh and Sherpur). A total of 12 farms (five from Sherpur district and seven from Mymensingh district) were included under this study (Source: The map was generated using ArcGIS version 10.3).
Selection of sheep farm and sheep
A list of sheep farms was collected from the respective upazila livestock offices located in each district. Sheep flocks with a flock size of ≥15 sheep were randomly selected.
Sample size and sampling method
The sample size used in this study was calculated using the following equation [28].

Where, n represents the requisite sample size, Z2 is the Z-score at a 95% confidence interval (CI) of 1.96, p is the anticipated prevalence of Campylobacter likely at the animal-level (53.3% = 0.53) [25], and d is the desired absolute precession (5% = 0.05); therefore, a sample size of 385 was obtained. However, we included 408 sheep from 12 farms from four upazilas (subdistricts) of two districts for animal-level sampling.
Data collection
A data collection team consisting of a veterinarian and one veterinary field staff collected data and samples from each flock. A semi-structured questionnaire was developed and used to collect data from farmers through semi-structured interviews. The same team collected samples from each sheep flock. A questionnaire template containing determinants related to (i) flock-level characteristics (15 questions) and (ii) animal-level characteristics (nine questions). Questionnaire responses were recorded in hard copies and then stored in Excel data sheets for descriptive and inferential statistical analyses.
Sample collection from the animals
A single fecal sample was collected from each sheep and 408 samples were collected from 12 flocks in total. Flock-level positivity status was established on the face sample evaluation status (either positive or negative). Aseptic measures were followed during sample collection. For each animal, 1–5 mL or g of swab material of feces was sampled. Each collected sample (swab material) was placed in an Eppendorf tube containing normal saline and a unique identification number was given. The sample was transported through an ice box to the designated laboratory of the Department of Microbiology and Hygiene, BAU, Mymensingh, for evaluation, maintaining a cool chain at 4°C–6°C.
Laboratory evaluation
Culture and biochemical tests
Samples were independently assessed using a cellulose filter with a porosity of 0.45 m (Biotech, Göttingen, Germany) and filtration. This filter paper size is excellent for retaining 90% of the cells [29] and high flow rates could enable optimal colony growth. Campylobacter culture was accomplished in selective media using the standard method described by Bolton et al. [29] with minor adjustments. Briefly, 100 μL of each collected sample was blow-out on filters that were kept onto the surface of blood agar base no. 2 (HiMedia, Mumbai, India) (supplemented with 5% sheep blood) with Skirrow supplement for both C. jejuni and C. coli (HiMedia) and maintained at 42°C for 30 min. After removing the filter from Skirrow and/or growth-supplemented blood agar, the plates were incubated at 37°C for approximately 48 h in a microaerophilic environment using AnaeroPouch®-MicroAero (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) for enrichment.
After 48 h, the incubated media were evaluated for the growth of bacteria. Grey, flat, and intermittently spreading colonies were observed on the surface of the media. The colony was stained using Gram’s staining method and observed under a light microscope to confirm the presence of Gram-negative curve structures. A few selected colonies from the agar media were then subcultured on the supplemented Blood agar base no. 2. Based on growth characteristics, different biochemical tests were performed according to standard methods to confirm Campylobacter spp. [30–32]. C. jejuni tested positive in biochemical tests, such as catalase, oxidase, hippurate hydrolysis, nitrate reduction, indoxyl acetate, and 1% glycerin. However, the triple sugar iron test showed a negative result.
Molecular detection through polymerase chain reaction (PCR)
Culture-positive isolates were provisionally confirmed as Campylobacter spp. by biochemical tests and PCR assays, respectively. The boiling method was used to extract DNA from a pure culture of Campylobacter spp. [33].
Detection of Campylobacter spp.
In this procedure, the genus Campylobacter was confirmed through 16S rRNA gene amplification using oligonucleotide primers in accordance with the standard procedure [34]. For the detection of Campylobacter spp., primers 16S9F-16S1540R and sequences (5´-3´): GAGTTTGATCCTGGCTC/AAGGAGGTGATCCAGCC with an amplicon size of 1530 bp were used. In this assay, the PCR conditions for 30 cycles were as follows: (a) Denaturation at 94°C for 30 s, (b) annealing at 47°C for 30 s, and (c) extension at 72°C for 90 s [34].
Confirmation of C. jejuni
In the present study, C. jejuni was identified using a molecular-based assay after confirmation of Campylobacter spp., hippuricase (hipO) gene-based PCR was performed using all isolates to discriminate C. jejuni according to a standard protocol [35]. For the detection of C. jejuni primers HIP400F–HIP1134R, sequences (5´-3´): GAAGAGGGTTTGGGTGGTG/AGCTAGCTTCGCATAATAACTTG with an amplicon size of 735 bp were utilized. In this assay, the PCR conditions for 30 cycles were as follows: (a) denaturation at 94°C for 30 s, (b) annealing at 55°C for 30 s, and (c) extension at 72°C for 45 s.
DNA templates of C. jejuni ATCC 33560, C. coli ATCC 33559, and C. fetus ATCC 27374 strains were used as positive controls in all PCR assays. Escherichia coli ATCC 25922 was used as a negative control. PCR products were visualized using gel electrophoresis (1.5% agarose, Invitrogen, Carlsbad, CA, USA). After staining with ethidium bromide (0.5 μg/mL) and recoloring with distilled water for 10 min, further gel images were captured using an ultraviolet transilluminator (Biometra, Göttingen, Germany).
Antimicrobial susceptibility testing
The antimicrobial susceptibility pattern of isolated strains of C. jujuni was evaluated using the disk diffusion method [36] with eight commonly used antimicrobial agents, namely, amoxicillin (AMX) (30 μg), oxytetracycline (OTE) (30 μg), gentamicin (GEN) (10 μg), streptomycin (10 μg), erythromycin (ERY) (30 μg), azithromycin (AZM) (30 μg), and ciprofloxacin (CIP) (5 μg) and ceftriaxone (CRO) (30 μg) (HiMedia). As per the protocol of the Clinical and Laboratory Standard Institute [37], we compared the growth zone of inhibition with the zone diameter recommended as resistant (R), intermediate resistant (I), or susceptible (S) to the assigned antimicrobial agents. The E. coli strain ATCC 25922 was employed in this evaluation as a quality measure organism. The results of antimicrobial susceptibility patterns were accomplished by performing at least double the disk diffusion method.
Statistical analysis
Data obtained through the semi-structured interviews (SSI) and laboratory interpretation was recorded in a Microsoft Excel 2017 sheet (Microsoft Office, Washington, USA). Data quality was checked for completeness and uniformity and exported to STATA 13 (USA, StataCrop, 4905, Lakeway Drive, College Station, Texas, 77845, USA) for analysis.
Data on flock and animal risk factors were summarized using descriptive statistics. In this evaluation, frequency and proportion were estimated for categorical variables. In the logistic regression analysis, all continuous determinants, such as sheep age and body weight, were classified according to the analysis prerequisite. The herd level data were not suitable for logistic regression analysis due to the small size. Therefore, a Chi-square test was performed to evaluate the association of flock-level positive status with the risk factors. Therefore, p ≤ 0.05 was used to determine statistical significance.
Both bivariate and multivariate logistic analyses were performed to identify determinants associated with the occurrence of C. jejuni at the animal-level. p = 0.2 was considered as a screening standard for the inclusion of variables in the multivariable regression analysis in this study. p < 0.05 was considered to indicate statistical significance in this analysis. Determinants were entered into the multivariate model using the forward stepwise regression method.
Results
Descriptive epidemiology
A total of 408 fecal samples from 12 flocks of two districts were collected for bacteriological evaluations (one sample per animal). Of the surveyed farms, 58.3% (n = 7) from Mymensingh district and 41.7% (n = 5) from Sherpur district were included in this study. The majority of farms (67.7%, n = 8) were 5 years old, and 75% (n = 9) farms fed their sheep through a conventional feeding system (free-ranging/scavenging). All farms (n = 12) practiced peste des petits ruminants vaccination for immunization and did not raise any other animals (Table-1).
Table-1.
Characteristics of flock composition, management practices, and flock level prevalence (n = 12 sheep farm).
| Variables | Positive | Prevalence (%) | 95% CI | p-value |
|---|---|---|---|---|
| Number of flocks/farms (n = 12) | 8 | 66.70 | 34.9–90.1 | - |
| District | ||||
| Mymensingh (n = 7) | 5 | 71.40 | 29.0–96.3 | 0.67 |
| Sherpur (n = 5) | 3 | 60.00 | 14.7–94.7 | |
| Age of the farm | ||||
| >5 years (n = 8) | 7 | 87.50 | 47.3–99.7 | 0.0303 |
| 1–5 years (n = 4) | 1 | 25.00 | 0.6–80.6 | |
| Feeding practice | ||||
| Conventional (free ranging) (n = 9) | 7 | 77.80 | 40.0–97.2 | 0.1572 |
| Stall feeding (n = 3) | 1 | 33.30 | 0.8–90.6 | |
| Use vaccine (Peste des petits ruminants) | ||||
| Yes (n = 12) | 8 | 66.70 | 34.9–90.1 | - |
| Raise other animals | ||||
| No (n = 12) | 8 | 66.70 | 34.9–90.1 | - |
| Sheep shed type | ||||
| Newly build (within a year) (n = 4) | 3 | 75.00 | 19.4–99.4 | 0.6650 |
| Old (more than 1 year) (n = 8) | 5 | 62.50 | 24.5–91.5 | |
| Floor condition | ||||
| Dry (n = 5) | 3 | 60.00 | 14.7–94.7 | 0.6788 |
| Wet (n = 7) | 5 | 71.40 | 29.0–96.3 | |
| Access of sunlight | ||||
| No (n = 7) | 5 | 71.40 | 29.0–96.3 | 0.6788 |
| Yes (n = 5) | 3 | 60.00 | 14.7–94.7 | |
| Air ventilation | ||||
| Bad (n = 7) | 5 | 71.40 | 29.0–96.3 | 0.6788 |
| Good (n = 5) | 3 | 60.00 | 14.7–94.7 | |
| Veterinary health-care facilities | ||||
| No (n = 7) | 4 | 57.10 | 18.4–90.1 | 0.4076 |
| Yes (n = 5) | 4 | 80.00 | 28.4–99.5 | |
| Deworming | ||||
| No (n = 5) | 2 | 40.00 | 5.3–85.3 | 0.0976 |
| Yes (n = 7) | 6 | 85.70 | 42.1–99.6 | |
| Cleaning and disinfection | ||||
| Good practices (n = 5) | 3 | 60.00 | 14.67–94.72 | 0.6788 |
| Poor/no practice (n = 7) | 5 | 71.40 | 29.04–96.33 | |
| Faces use | ||||
| Aquaculture (n = 3) | 1 | 33.30 | 0.8–90.6 | 0.1535 |
| Fertilizer (n = 9) | 7 | 77.80 | 40.0–97.2 | |
| History of diarrhea | ||||
| Yes (n = 8) | 7 | 87.50 | 47.3–99.7 | 0.0002 |
| No (n = 4) | 1 | 25.00 | 0.6–80.6 | |
CI=Confidence interval
Prevalence and risk factors of flock-level
In this study, the flock-level prevalence was 66.70% (95% CI: 34.9–90.1). Among the districts, Mymensingh has a higher prevalence (71.40%) than Sherpur district. However, it was found to be non-significant (Table-1). Older farms (>5 years) and those with a history of diarrhea demonstrated high levels of contamination with C. jejuni at farm-level (p < 0.05). Other factors of flock levels were found to be non-significant (Table-1).
Animal-level prevalence and risk factors
Of the 408 fecal samples, 36 were found to be positive through culture, biochemical tests, and molecular-based assays (Figures-2 and 3); thus, an animal-level prevalence of 8.8% (95% CI: 6.3%–12.0%) was confirmed (Table-2).
Figure-2.
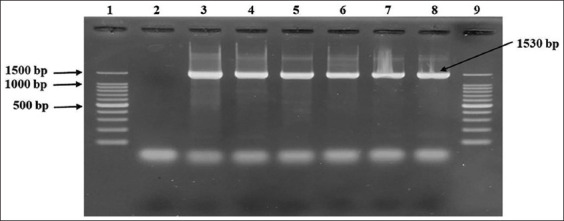
Detection of Campylobacter spp. by 16S rRNA gene-based polymerase chain reaction. Here, 1 and 9: 100 bp DNA ladder (Takara, Japan); Lane 4–8: Representative Campylobacter isolates of sheep origin; 3: Positive control (Campylobacter jejuni ATCC 33560); 2= Negative control.
Figure-3.
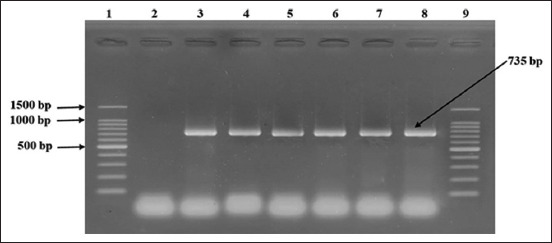
Detection of Campylobacter jejuni by hipO gene-based polymerase chain reaction. Here, 1 and 9: 100 bp DNA ladder (Takara, Japan); 2: Negative control; 3: Positive control (C. jejuni ATCC 33560); Lane 4–8: Representative C. jejuni isolates of sheep origin.
Table-2.
Bivariable analysis of animal-level risk factors analysis (n = 408).
| Risk factor | Category | Positive (%) | ORR | (95% CI) | p-value |
|---|---|---|---|---|---|
| Sex | Male (n = 172) | 20 (11.6) | 0.55 | 0.27–1.10 | 0.05 |
| Female (236) | 16 (6.8) | Ref | |||
| Breed | Local (270) | 26 (9.6) | Ref | ||
| Crossbred/Garole (n = 138) | 10 (7.2) | 0.733 | 0.34–1.56 | 0.21 | |
| Age of the sheep | Up to 1 year (n = 36) | 33 (91.7) | 3.88 | 1.16–12.93 | 0.006 |
| >1 year (n = 372) | 3 (0.81) | Ref | |||
| Source | Bought (182) | 17 (9.3) | 1.122 | 0.56–2.22 | 0.37 |
| Farm (226) | 19 (8.4) | Ref | |||
| Body weight | ≤10 kg (n = 300) | 29 (9.7) | 1.54 | 0.65–3.63 | 0.163 |
| >10 kg (n = 108) | 7 (6.5) | Ref | |||
| Pregnancy status (n = 232) | Pregnant (n = 69) | 3 (4.4) | 0.48 | 0.13–1.74 | 0.25 |
| Non-pregnant (n = 163) | 14 (8.6) | Ref | |||
| Parity (n = 68) | 1–2 (n = 35) | 3 (8.6%) | - | - | - |
| 3–5 (n = 33) | 0 (0%) | ||||
| Body condition score | Bad/medium (n = 369) | 35 (9.5) | 3.98 | 0.53–29.89 | 0.07 |
| Good (n = 39) | 1 (2.6) | Ref | |||
| Season | Summer/rainy season (n = 373) | 35 (9.4) | 3.52 | 0.46–26.5 | 0.09 |
| Winter (n = 35) | 1 (2.9) | Ref |
CI=Confidence interval, ORR=Objective response rate
A total of nine animal-level determinates were included in the bivariable analysis, of which two, sex and age, were found to be statistically significant (Table-2). Among the variables, four (sex, age, body weight, and body condition score) were considered as candidate variables for further multivariable model analysis (Table-3). The most important risk factor identified by this model was the age of the sheep. A 3.78-fold (95% CI: 1.0736–13.3146, p = 0.038) higher likelihood of infection with C. jejuni was observed in sheep up to 1 year of age (Table-3).
Table-3.
Multivariable logistic regression analysis of Campylobacter jejuni infection in sheep (n = 408).
| Risk factors | Adjusted odds ratio | 95% C.I. | p-value |
|---|---|---|---|
| Sex | |||
| Male | 0.5379 | 0.2653–1.0905 | 0.0855 |
| Female | Ref | ||
| Body weight | |||
| ≤10 kg | 0.7259 | 0.3277–1.6078 | 0.4298 |
| >10 kg | Ref | ||
| Age of the sheep | |||
| Up to 1 year | 3.7809 | 1.0736–13.3146 | 0.0384 |
| >1 year | Ref | ||
| Body condition score | |||
| Bad/medium | 1.5464 | 0.1836–13.0274 | 0.6885 |
| Good | Ref | ||
CI=Confidence interval
Antimicrobial profile
Antimicrobial susceptibility status
Of the 36 isolates of C. jejuni, all were found to be fully susceptible (100%) to GEN and CIP. Three antimicrobial agents, OTE, AZM, and CRO, were fully R (100%) in this study. However, some antimicrobial agents, such as AMX (11.11%, n = 4), streptomycin (5.56%, n = 2), and ERY (2.78%, n = 1), were mildly susceptible in the present study. Four antimicrobials, (AMX; 8.33%) streptomycin (S; 11.11%), (ERY; 11.11%), and AZM (AZM; 38.89%) were documented as intermediate outcomes of resistance/susceptibility (Table-4).
Table-4.
Antimicrobial susceptibility status of C. jejuni isolates.
| Antimicrobial agents | No. (%) of C. jejuni isolates | ||
|---|---|---|---|
|
| |||
| Resistant | Intermediate | Susceptible | |
| Amoxicillin | 29 (80.56) | 3 (8.33) | 4 (11.11) |
| Oxytetracycline | 36 (100.00) | 0 (0.00) | 0 (0.00) |
| Gentamicin | 0 (0.00) | 0 (0.00) | 36 (100.00) |
| Streptomycin | 30 (83.33) | 4 (11.11) | 2 (5.56) |
| Erythromycin | 31 (86.11) | 4 (11.11) | 1 (2.78) |
| Azithromycin | 22 (61.11) | 14 (38.89) | 0 (0.00) |
| Ciprofloxacin | 0 (0.00) | 0 (0.00) | 36 (100.00) |
| Ceftriaxone | 36 (100.00) | 0 (0.00) | 0 (0.00) |
C. jejuni=Campylobacter jejuni
Antimicrobial resistance (AMR) status
In this study, four antimicrobial agents (ERY-CRO-OTE-AZM and CRO-OTE-AZM-S) were R to 11.1% (n = 4) and 8.3% (n = 3) isolates of 36 isolates through two different antimicrobial combinations. However, three different antimicrobial combinations of five antimicrobial agents (ERY-AMX-CRO-OTE-S, AMX-CRO-OTE-S-AZM, ERY-AMX-CRO-OTE-AZM) were found to be R to 38.9% (n = 14), 5.6% (n = 2), and 30.6% (n = 11) isolates, respectively. Alternatively, 30.6% (n = 11) isolates were R to six antimicrobial agents (ERY-AMX-CRO-OTE-S-AZM) (Figure-4).
Figure-4.
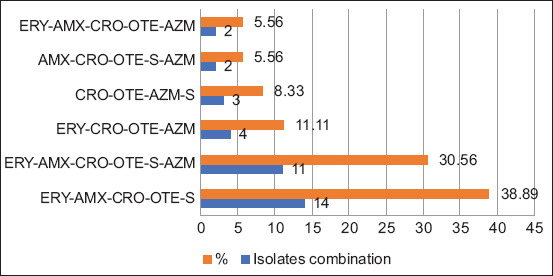
Distribution of antimicrobial resistance pattern in Campylobacter jejuni isolates (n = 36) from sheep. ERY=Erythromycin, AMX=Amoxicillin, CRO=Ciprofloxacin, OTE=Oxytetracycline, AZM=Azithromycin, S=Streptomycin.
Discussion
This is a maiden study in Bangladesh that confirms the distribution of C. jejuni in semi-scavenging sheep in the Mymensingh division of Bangladesh and confirms the prevalence, molecular pattern, antimicrobial susceptibility status, and risk factors of Campylobacter infection.
The estimated farm-level occurrence of C. jejuni was 66.70% (95% CI: 34.9–91.1), with an estimated animal/sample-level prevalence of 8.8% (95% CI: 6.3%–12.0%). Younger animals (1 year old) were more likely to be associated with C. jejuni infection in semi-scavenging sheep from the farms studied. The results of this study have highlighted the overall risk of zoonotic pathogens in the source animal species. In addition, the study suggests plausible risk reduction options such as cleaning and sanitation, including hygienic practices at the farm-level, which could impede the pathogen transmission cycle at the animal-human interface.
This study documented an animal-level prevalence of 8.8% (36/408) through fecal sample evaluation. There are no published reports on Campylobacter occurrence in Bangladesh; therefore, we were unable to compare this data with previous records in Bangladesh. A study in Bangladesh confirmed that the overall prevalence of Campylobacter spp. and C. jejuni in farmed dairy cattle was 18% and 12.6%, respectively [25]. However, another study reported a prevalence of 25% (20/80) in different samples collected from crossbred high-yield dairy cattle [26]. However, in Bangladesh, several studies using poultry and environmental samples from live bird markets have confirmed that the prevalence rate of Campylobacter varies from 26.4% to 75% [21–24]. The prevalence of Campylobacter spp. was 9.33% in sheep in the coastal region of Odisha, India [38] and 12.5% in Kashmir, India, according to vaginal swabs and aborted material examination [39]. The findings of these studies corroborated with our study findings.
However, several studies in different geographical locations have confirmed the prevalence of Campylobacter in sheep, namely, 13% in Algeria [40] and 18.6% in Ghana [41]. The findings of these studies are narrowly consistent with those of our study.
In flock-level risk factor evaluation, older farms (>5 years) were more likely to be infected with C. jejuni (p = 0.0303). The findings of this study are consistent with those of another study conducted on dairy cattle in Bangladesh, in which >5-year-old cattle farms were found to be >10 times more risky with a Campylobacter-positive outcome [25]. In the case of poultry, older farms in European countries have also been reported to be Campylobacter-positive [42]. A history of diarrhea (p = 0.0002) was also found to be associated with the Campylobacter-positive status of the flock in this study.
In multivariable logistic regression analysis, four factors (sex, body weight, age of the sheep, and body condition score) were included in the animal-level risk factor evaluation: The odds of Campylobacter-positive status were 3.78 (95% CI: 1.0736–11.3246) times higher in sheep aged >1 year compared to sheep aged >1 year. This organism can grow in the rumen and live as a commensal organism. However, in young animals with immature rumen, a favorable condition for infection in the lower part of the GIT could be observed [11]. Thus, in this study, young animals were estimated to have a higher risk of Campylobacter occurrence.
The emergence of Campylobacter-related AMR is a persistent public health problem. Considering a vital public health risk, the World Health Organization has recorded multidrug-R (MDR) bacteria [43–45]. C. jejuni, C. coli, and C. fetus have been confirmed in dairy cattle, bulls, and poultry, including live bird market environmental samples in Bangladesh [21–26, 46]. However, systematic screening focusing on the antimicrobial susceptibility pattern of Campylobacter isolates in semi-scavenging sheep has been less considered. Thus, the data generated in this study can be used as helpful reference information.
In this study, several antimicrobial agents, such as OTE (100%), CRO (100%), streptomycin (83.33%), ERY (86.11%), AMX (80.56%), and AZM (61.11%), were documented to be R to C. jejuni isolates, which is an immense public health concern. The same pattern of AMR to these antibiotics has also been reported in C. jejuni, C. coli, and C. fetus in poultry and livestock from Bangladesh [21–26, 46]. The antimicrobial susceptibility rate of CIP and gentamycin was confirmed to be 100% for both antibiotics. The reported susceptibility rate of CIP has been confirmed in different geographical locations [47–49]. In this study, very few antimicrobial agents were documented as I/susceptible with variable proportions, such as AZM, streptomycin, ERY, and AMX in 38.89%, 11.11%, 11.11%, and 8.33%, respectively. This phenomenon has developed due to the inappropriate use of antimicrobial agents in animal production in Bangladesh [50].
A notable finding of this study was the high prevalence of MDR pathogens in sheep samples showing registrants against four to six antimicrobial agents with different combinations, such as ERY-CRO-OTE-AZM and CRO-OTE-AZM-S, AMX-CRO-OTE-AZM and AMX-CRO-OTE-S-AZM, and ERY-AMX-CRO-OTE-S-AZM. This study observed a variable distribution (5.5%–38.9%) of MDR C. jejuni isolates, sanitation. This distribution is sparsely consistent with other studies in poultry and livestock in Bangladesh [21, 23, 26]. In a previous report, C. jejuni isolates were documented to be MDR against tetracycline, ampicillin, norfloxacin, and nalidixic acid; however, the MDR status of C. coli was reported to be R against tetracycline, ampicillin, ERY, norfloxacin, and nalidixic acid [51].
The data obtained in this study would be helpful for the systematic assessment of zoonotic Campylobacter infection in sheep and its subsequent transmission in humans and the environment. The outcomes of this research will assist in making evidence-based decisions and ranking control options, such as farm cleaning, sanitation, and personal hygiene of the animal attendants.
In Bangladesh, C. jejuni is the primary causative agent of diarrhea in young children (25.5%) [52]. Campylobacter infection causes acute flaccid paralysis associated with Guillain-Barré syndrome (GBS) and has been confirmed in Bangladesh with an expected incidence of 3.25 cases/100,000 children aged 15 years [53, 54]. Many measures have been taken to minimize the burden of Campylobacter infection, including associated GBS threats, without considering the sources of infection in low-resource settings. Therefore, consideration should be given to the significant hazard of Campylobacter present in source animals such as sheep. A comprehensive understanding of the forms of release of Campylobacter by animals on a farm and the relationship between host animals and the environment, including pathogen genotypes, is crucial for the application of appropriate intervention approaches.
Limitations
Identification of Campylobacter spp. in farmed sheep relied on fecal samples. It should be noted; however, that the survey did not collect data on the history of abortion, premature parturition, or other pathological lesions commonly associated with Campylobacter infection. Collecting these data would strengthen the validity of our findings. Therefore, we recommend conducting a future survey with a broader range of sample sizes to address these issues.
Conclusion
This study highlighted the widespread presence of zoonotic Campylobacter in sheep, emphasizing its role in environmental contamination and posing a public health burden. To minimize the increasing burden of Campylobacter transmission from animals to humans, it is essential to develop innovative approaches, therapies, and interventions. To mitigate the occurrence of Campylobacter in farms and prevent its further transmission between animals and humans, it is essential to adopt a “one health” approach focusing on environmental, animal, and human health.
Authors’ Contributions
MAN, AKMZH, MRI: Contributed to the field survey and data and sample collection from sheep. MAN, MA, and MRI: Performed laboratory assessment and drafted the manuscript. SSI: Performed statistical analysis of the data and reviewed the manuscript. MHS and SMLK: Supervised the study and reviewed the manuscript. All authors have read, reviewed, and approved the final manuscript.
Acknowledgment
The authors would also like to extend their appreciation to sheep farmers, managers, and farm workers for their assistance during data/sample collection. Furthermore, we acknowledge the Bangladesh Agricultural University Research System (BAURES), Mymensingh, Bangladesh, for providing the funding that supported this research under Project No. 2021/99/BAU.
Footnotes
The authors would also like to extend their appreciation to sheep farmers, managers, and farm workers for their assistance during data/sample collection. Furthermore, we acknowledge the Bangladesh Agricultural University Research System (BAURES), Mymensingh, Bangladesh, for providing the funding that supported this research under Project No. 2021/99/BAU.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Costa D, Iraola G. Pathogenomics of emerging Campylobacter species. Clin. Microbiol. Rev. 2019;32(4):e00072–18. doi: 10.1128/CMR.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäesaar M, Tedersoo T, Meremäe K, Roasto M. The source attribution analysis revealed the prevalent role of poultry over cattle and wild birds in human campylobacteriosis cases in the Baltic States. PLoS One. 2020;15(7):e0235841. doi: 10.1371/journal.pone.0235841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson T.J, Shank J.M, Johnson J.G. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front. Microbiol. 2017;8:487. doi: 10.3389/fmicb.2017.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coker A.O, Isokpehi R.D, Thomas B.N, Amisu K.O, Obi C.L. Human campylobacteriosis in developing countries-synopsis-statistical data included. Emerg. Infect. Dis. 2002;8(3):237–243. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin O, Kassem I.I, Shen Z, Lin J, Rajashekara G, Zhang Q. Campylobacter in poultry:Ecology and potential interventions. Avian Dis. 2015;59(2):185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- 6.Anderson R.C, Harvey R.B, Wickersham T.A, MacDonald J.C, Ponce C.H, Brown M, Pinchak W.E, Osterstock J.B, Krueger N.A, Nisbet D.J. Effect of distillers feedstuffs and lasalocid on Campylobacter carriage in feedlot cattle. J. Food Prot. 2014;77(1):1968–1975. doi: 10.4315/0362-028X.JFP-14-169. [DOI] [PubMed] [Google Scholar]
- 7.Bianchini V, Luini M, Borella L, Parisi A, Jonas R, Kittl S, Kuhnert P. Genotypes and antibiotic resistances of Campylobacter jejuni isolates from cattle and pigeons in dairy farms. Int. J. Environ. Res. Public Health. 2014;11(7):7154–7162. doi: 10.3390/ijerph110707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y, Sahin O, Pavlovic N, LeJeune J, Carlson J, Wu Z, Dai L, Zhang Q. Rising fluoroquinolone resistance in Campylobacter isolated from feedlot cattle in the United States. Sci. Rep. 2017;7(1):494. doi: 10.1038/s41598-017-00584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott L, Menzies P, Reid-Smith R.J, Avery B.P, McEwen S.A, Moon C.S, Berke O. Antimicrobial resistance in Campylobacter spp. isolated from Ontario sheep flocks and associations between antimicrobial use and antimicrobial resistance. Zoonoses Public Health. 2012;59(4):294–301. doi: 10.1111/j.1863-2378.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Sippy R, Sahin O, Plummer P, Vidal A, Newell D, Zhang Q. Genetic diversity and antimicrobial susceptibility of Campylobacter jejuni isolates associated with sheep abortion in the United States and Great Britain. J. Clin. Microbiol. 2014;52(6):1853–1861. doi: 10.1128/JCM.00355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley K, Jones K. Cattle and sheep farms as reservoirs of Campylobacter. J. Appl. Microbiol. 2003;94(Suppl):104S–113S. doi: 10.1046/j.1365-2672.94.s1.12.x. [DOI] [PubMed] [Google Scholar]
- 12.Mullner P, Shadbolt T, Collins-Emerson J.M, Midwinter A.C, Spencer S.E.F, Marshall J, Carter P.E, Campbell D.M, Wilson D.J, Hathaway S. Molecular and spatial epidemiology of human campylobacteriosis:Source association and genotype-related risk factors. Epidemiol. Infect. 2010;138(10):1372–1383. doi: 10.1017/S0950268809991579. [DOI] [PubMed] [Google Scholar]
- 13.Thépault A, Rose V, Quesne S, Poezevara T, Béven V, Hirchaud E, Touzain F. Ruminant and chicken:important sources of campylobacteriosis in France despite a variation of source attribution in 2009 and 2015. Sci. Rep. 2018;8:9305. doi: 10.1038/s41598-018-27558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyson G.H, Tate H.P, Abbott J, Tran T.T, Kabera C, Crarey E, Young S, McDermott P.F, Sprague G, Campbell M, Adeyemo O, Browne-Silva J, Myers M, Thitaram S, Zhao S. Molecular subtyping and source attribution of Campylobacter isolated from food animals. J. Food Prot. 2016;79(11):1891–1897. doi: 10.4315/0362-028X.JFP-16-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Haan C.P, Kivistö R.I, Hakkinen M, Corander J, Hänninen M.L. Multilocus sequence types of Finnish bovine Campylobacter jejuni isolates and their attribution to human infections. BMC Microbiol. 2010;10:200. doi: 10.1186/1471-2180-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin O, Yaeger M, Wu Z, Zhang Q. Campylobacter-associated diseases in animals. Annu. Rev. Anim. Biosci. 2017;5:21–42. doi: 10.1146/annurev-animal-022516-022826. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.L. Campylobacter jejuni infection during pregnancy:Long-term consequences of associated bacteremia, Guillain-Barrésyndrome, and reactive arthritis. J. Food Prot. 2002;65(4):696–708. doi: 10.4315/0362-028x-65.4.696. [DOI] [PubMed] [Google Scholar]
- 18.Xia J, Pang J, Tang Y, Wu Z, Dai L, Singh K, Xu C, Ruddell B, Kreuder A, Xia L, Ma X, Brooks K.S, Ocal M.M, Sahin O, Plummer P.J, Griffith R.W, Zhang Q. High prevalence of fluoroquinolone-resistant Campylobacter bacteria in sheep and increased Campylobacter counts in the bile and gallbladders of sheep medicated with tetracycline in feed. Appl. Environ. Microbiol. 2019;85(11):e00008–19. doi: 10.1128/AEM.00008-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BBS. Preminiary Findings on Agriculture Sample Census-2020. Bangladesh Bureau of Statistics, Bangladesh. 2022 [Google Scholar]
- 20.Rahman M.M. Sheep Production and Development in Bangladesh. In:Proceedings of the Workshop on Sheep Production in Asia. PACCARD, Los Banos, Philippines. 1989:81–95. [Google Scholar]
- 21.Alam B, Uddin M.N, Mridha D, Akhter A.H.M.T, Islam S.K.S, Haque A.K.M.Z, Kabir S.M.L. Occurrence of Campylobacter spp. in selected small scale commercial broiler farms of Bangladesh related to good farm practices. Microorganisms. 2020;8(11):1778. doi: 10.3390/microorganisms8111778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan M.M, Talukder S, Mandal A.K, Tasmim S.T, Parvin M.S, Ali M.Y, Sikder M.H, Islam M.T. Prevalence and risk factors of Campylobacter infection in broiler and cockerel flocks in Mymensingh and Gazipur districts of Bangladesh. Prev. Vet. Med. 2020;180:105034. doi: 10.1016/j.prevetmed.2020.105034. [DOI] [PubMed] [Google Scholar]
- 23.Neogi S.B, Islam M.M, Islam S.K.S, Akhter A.H.M.T, Sikder M.M.H, Yamasaki S, Kabir S.M.L. Risk of multi-drug resistant Campylobacter spp. and residual antimicrobials at poultry farms and live bird markets in Bangladesh. BMC Infect. Dis. 2020;20(1):278. doi: 10.1186/s12879-020-05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam M.K, Kabir S.M.L, Haque A.K.M.Z, Sarker Y.A, Sikder M.H. Molecular detection and characterization of Escherichia coli, Salmonella spp. and Campylobacter spp. isolated from broiler meat in Jamalpur, Tangail, Netrokona and Kishoreganj districts of Bangladesh. Afr. J. Microbiol. Res. 2018;12:761–770. [Google Scholar]
- 25.Hoque N, Islam S.K.S, Uddin M.N, Arif M, Haque A.K.M.Z, Neogi S.B, Hossain M.M, Yamasaki S, Kabir S.M.L. Prevalence, risk factors, and molecular detection of Campylobacter in farmed cattle of selected districts in Bangladesh. Pathogens. 2021;10(3):313. doi: 10.3390/pathogens10030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabir S.M.L, Lubna M.M, Islam M, Haque A.K.M.Z, Neogi S.B, Yamasaki S. Isolation, molecular identification and antimicrobial resistance patterns of Campylobacter species of dairy origin:First report from Bangladesh. Vet. Sci. Dev. 2018;8:7838. [Google Scholar]
- 27.Hossain M, Sun M, Islam T, Rahman M, Rahman M, Hashem M. Socio-economic characteristics and present scenario of sheep farmers at Sherpur district in Bangladesh. SAARC J. Agric. 2021;19(1):185–199. [Google Scholar]
- 28.Thrusfield M. Veterinary Epidemiology. John Wiley &Sons Ltd., United States. 2018 [Google Scholar]
- 29.Bolton F.J, Hutchinson D.N, Parker G. Reassessment of selective agars and filtration techniques for isolation of Campylobacter species from faeces. Eur. J. Clin. Microbiol. Infect. Dis. 1988;7(2):155–160. doi: 10.1007/BF01963069. [DOI] [PubMed] [Google Scholar]
- 30.Nachamkin I. Campylobacter and Arcobacter. In: Murray P.R, Baron E.J, Jorgensen J.H, Pfaller M.A, Yolken R.H, editors. Manual of Clinical Microbiology. Washington, D.C: American Society for Microbiology; 2003. pp. 902–914. [Google Scholar]
- 31.Foster G, Holmes B, Steigerwalt A.G, Lawson P.A, Thorne P, Byrer D.E, Ross H.M, Xerry J, Thompson P.M, Collins M.D. Campylobacter insulaenigrae sp. nov., isolated from marine mammals. Int. J. Syst. Evol. Microbiol. 2004;54(Pt 6):2369–2373. doi: 10.1099/ijs.0.63147-0. [DOI] [PubMed] [Google Scholar]
- 32.Swai E.S, Hulsebosch J, Van der Heijden W. Prevalence of genital campylobacteriosis and trichomonosis in crossbred breeding bulls kept on zero-grazed smallholder dairy farms in the Tanga region of Tanzania. J. S. Afr. Vet. Assoc. 2005;76(4):224–227. doi: 10.4102/jsava.v76i4.431. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino K, Yamasaki S, Mukhopadhyay A.K, Chakraborty S, Basu A, Bhattacharya S.K, Nair G.B, Shimada T, Takeda Y. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 1998;20(3):201–207. doi: 10.1111/j.1574-695X.1998.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 34.Samosornsuk W, Asakura M, Yoshida E, Taguchi T, Nishimura K, Eampokalap B, Phongsisay V, Chaicumpa W, Yamasaki S. Evaluation of a cytolethal distending toxin (cdt) gene?based species?specific multiplex PCR assay for the identification of Campylobacter strains isolated from poultry in Thailand. Microbiol. Immunol. 2007;51(9):909–917. doi: 10.1111/j.1348-0421.2007.tb03974.x. [DOI] [PubMed] [Google Scholar]
- 35.Linton D, Lawson A.J, Owen R.J, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 1997;35(10):2568–2572. doi: 10.1128/jcm.35.10.2568-2572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luangtongkum T, Morishita T.Y, El-Tayeb A.B, Ison A.J, Zhang Q. Comparison of antimicrobial susceptibility testing of Campylobacter spp. by the agar dilution and the agar disk diffusion methods. J. Clin. Microbiol. 2007;45(2):590–594. doi: 10.1128/JCM.00986-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CLSI. Clinical and Laboratory Standards Institute (CLSI) (2016). Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI Supplement M100S Clinical and Laboratory Standards Institute Wayne, Wayne, PA. 2016:1–256. [Google Scholar]
- 38.Mohakud N.K, Patra S.D, Kumar S, Sahu P.S, Misra N, Shrivastava A.K. Detection and molecular typing of Campylobacter isolates from human and animal faeces in coastal belt of Odisha, India. Indian J. Med. Microbiol. 2019;37(3):345–350. doi: 10.4103/ijmm.IJMM_19_394. [DOI] [PubMed] [Google Scholar]
- 39.Bisma G, Sabia Q, Amin K.Z, Ahmad W.S, Nabi M.S, Ahmad M.F, Isfaqul H.M, Aasim H, Ali R.M, Majeed K.S, Rafia M, Shafi S.M. RFLP analysis of flagellin (Fla) gene of Campylobacter jejuni from ovines of Kashmir, India. J. Food Saf. 2018;38(5):e12509. [Google Scholar]
- 40.Meryem G, Zehor G, Fares A, Sadjia M, Amina H. Campylobacter in sheep, calves and broiler chickens in the central region of Algeria:Phenotypic and antimicrobial resistance profiles. Afr. J. Microbiol. Res. 2016;10(39):1662–1667. [Google Scholar]
- 41.Karikari A.B, Obiri-Danso K, Frimpong E.H, Krogfelt K.A. Antibiotic resistance of Campylobacter recovered from faeces and carcasses of healthy livestock. Biomed. Res. Int. 2017;2017:4091856. doi: 10.1155/2017/4091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommer H.M, Høg B.B, Larsen L.S, Sørensen A.I.V, Williams N, Merga J.Y, Cerdà-Cuéllar M, Urdaneta S, Dolz R, Wieczorek K, Osek J, David B, Hofshagen M, Jonsson M, Wagenaar J.A, Bolder N, Rosenquist H. Analysis of farm specific risk factors for Campylobacter colonization of broilers in six European countries. Microb. Risk Anal. 2016;2–3:16–26. [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC). Preliminary Food Net data on the incidence of infection with pathogens transmitted commonly through food–10 states, 2009. MMWR Morb. Mortal. Wkly. Rep. 2010;59(14):418–422. [PubMed] [Google Scholar]
- 44.WHO. The Increasing Incidence of Human Campylobacteriosis Report and Proceedings of a WHO Consultation of Experts, Copenhagen, Denmark, World Health Organization. 2001 [Google Scholar]
- 45.Ethelberg S, Simonsen J, Gerner-Smidt P, Olsen K.E.P, Mølbak K. Spatial distribution and registry-based case-control analysis of Campylobacter infections in Denmark, 1991–2001. Am. J. Epidemiol. 2005;162(10):1008–1015. doi: 10.1093/aje/kwi316. [DOI] [PubMed] [Google Scholar]
- 46.Hoque N, Islam S.S, Saddam M.J.I, Rafikuzzaman M, Sikder M.H, Castellan D.M, Kabir S.M.L. Investigation of Campylobacter fetus in breeding bulls of private farms in Bangladesh. Vet. Med. Sci. 2022;9(1):417–428. doi: 10.1002/vms3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang Y.S, Cho Y.S, Yoon S.K, Yu M.A, Kim C.M, Lee J.O, Pyun Y.R. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from raw chicken meat and human stools in Korea. J. Food Prot. 2006;69(12):2915–2923. doi: 10.4315/0362-028x-69.12.2915. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Naren G.W, Wu C.M, Wang Y, Dai L, Xia L.N, Luo P.J, Zhang Q, Shen J.Z. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet. Microbiol. 2010;144(1–2):133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 49.Kovaļenko K, Roasto M, Šantare S, Bērziņš A, Hörman A. Campylobacter species and their antimicrobial resistance in Latvian broiler chicken production. Food Control. 2014;46:86–90. [Google Scholar]
- 50.Chowdhury R, Haque M.N, Islam K.M.S, Khaleduzzaman A. A review on antibiotics in an animal feed. Bangladesh J. Anim. Sci. 2009;38(1–2):22–32. [Google Scholar]
- 51.Karmaker S, Kabir S.M.L, Haque A.K.M.Z, Khan M.F.R, Sarker Y.A. Screening of human diarrhoeal samples in Mymensingh city of Bangladesh for the isolation, identification and antimicrobial resistance profiles of Campylobacter spp. Afr. J. Microbiol. Res. 2018;12(32):771–778. [Google Scholar]
- 52.Haq J.A, Rahman K.M. Campylobacter jejuni as a cause of acute diarrhoea in children:A study at an urban hospital in Bangladesh. J. Trop. Med. Hyg. 1991;94(1):50–54. [PubMed] [Google Scholar]
- 53.Islam Z, Jacobs B.C, Islam M.B, Mohammad Q.D, Diorditsa S, Endtz H.P. High incidence of Guillain-Barre syndrome in children, Bangladesh. Emerg. Infect. Dis. 2011;17(7):1317–1318. doi: 10.3201/eid1707.101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islam Z, Gilbert M, Mohammad Q.D, Klaij K, Li J, Van Rijs W, Tio-Gillen A.P, Talukder K.A, Willison H.J, Van Belkum A. Guillain-Barrésyndrome-related Campylobacter jejuni in Bangladesh:Ganglioside mimicry and cross-reactive antibodies. PLoS One. 2012;7(8):e43976. doi: 10.1371/journal.pone.0043976. [DOI] [PMC free article] [PubMed] [Google Scholar]


