Abstract
Billions of apoptotic cells are swiftly removed from the human body daily. This clearance process is regulated by efferocytosis, an active anti-inflammatory process during which phagocytes engulf and remove apoptotic cells. However, impaired clearance of apoptotic cells is associated with the development of various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease. In this review, we conducted a comprehensive search of relevant studies published from January 1, 2000, to the present, focusing on efferocytosis, autoimmune disease pathogenesis, regulatory mechanisms governing efferocytosis, and potential treatments targeting this process. Our review highlights the key molecules involved in different stages of efferocytosis—namely, the “find me,” “eat me,” and “engulf and digest” phases—while elucidating their relevance to autoimmune disease pathology. Furthermore, we explore the therapeutic potential of modulating efferocytosis to restore immune homeostasis and mitigate autoimmune responses. By providing theoretical underpinnings for the targeting of efferocytosis in the treatment of autoimmune diseases, this review contributes to the advancement of therapeutic strategies in this field.
Keywords: Apoptotic cell, Macrophage, Efferocytosis, Autoimmune disease, Pathology
Abbreviations
- AC
apoptotic cell
- DC
dendritic cell
- SLE
systemic lupus erythematosus
- IBD
inflammatory bowel disease
- RA
rheumatoid arthritis
- PANX1
pannexin 1
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- PtdSer
phosphatidylserine
- BAI1
brain-specific angiogenesis inhibitor 1
- TIM
T-cell immunoglobulin mucin
- MFGE8
milk fat globule-EFG factor 8
- GAS6
growth arrest-specific 6
- ELMO1
engulfment and cell motility protein 1
- DOCK180
dedicator of cytokinesis protein 1
- CD47
cluster of differentiation 47
- SIRPα
signal-regulatory protein alpha
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ARG1
arginase 1
- ODC
ornithine decarboxylase
- AAM
alternatively activated macrophage
- IL
interleukin
- ERK1/2
extracellular signal-regulated kinase 1/2
- DUSP4
dual-specificity phosphatase 4
- SAM
S-adenosine methionine
- DNMT3A
DNA methyltransferase-3A
- FAO
fatty acid oxidation
- DRP1
dynamin-related protein 1
- SPM
specialized pro-resolving mediator
- ALOX15
arachidonic acid 15-lipoxygenase
- Rv
resolvin
- Th
T helper
- Treg
T regulatory cell
- STAT6
signal transducer and activator of transcription-6
- TSG6
TNF-α stimulated gene-6
- sTAM
soluble TAM
- ADAM
a disintegrin and metalloproteinase
- LXA4
lipoxin A4
- BCA
biochanin A
- Ang
angiotensin
- SCARF1
scavenger receptor class F member 1
- Ig
immunoglobulin
- BMDM
bone marrow-derived macrophage
- UC
ulcerative colitis
- CD
Crohn's disease
- IEC
intestinal epithelial cell
- CHEF
chimeric efferocytosis receptor
- SS
Sjögren's syndrome
- DAMP
damage-associated molecular pattern
- TLR
Toll-like receptor
- T1D
type 1 diabetes
- NOD
non-obese diabetic
- iDC
immature DC
- SSc
systemic sclerosis
- MDM
monocyte-derived macrophage
- SR
scavenger receptor
- ITGβ5
integrin beta 5
- TGF-β
transforming growth factor β
- GPA
granulomatosis with polyangiitis
- PR3
proteinase 3
- CRT
calreticulin
- G-CSF
granulocyte colony-stimulating factor
- MS
multiple sclerosis
1. Introduction
The term “efferocytosis” is derived from the Greek word “effere,” meaning the taking of a corpse to the grave. The cellular process of efferocytosis comprises the removal of apoptotic cells (ACs) by efferocytes [1]. Billions of cells die and are renewed daily, and their timely removal is an important process. Efferocytosis involves various stages, including the “find me,” “eat me,” and “engulf and digest” steps, and unique receptors and ligands can be categorized according to the stage of the process in which they participate [2].
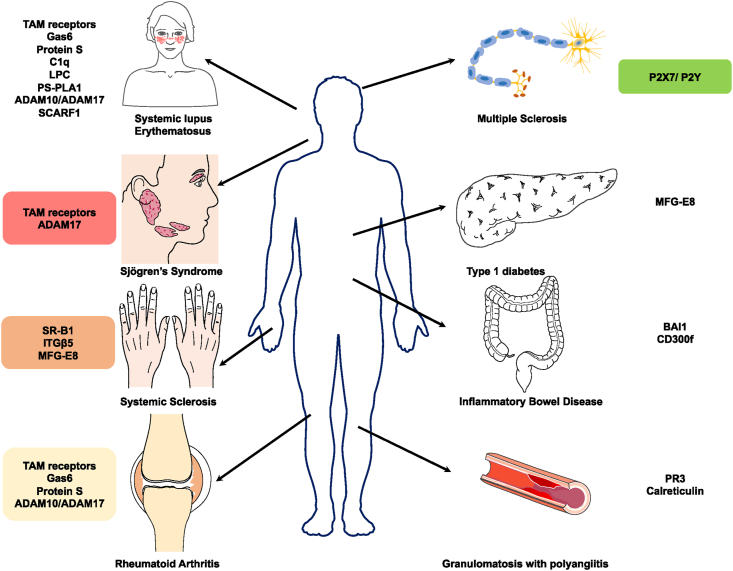
Efferocytosis is typically performed by professional phagocytes (macrophages and dendritic cells [DCs]); however, non-professional phagocytes (epithelial cells and fibroblasts) can also contribute to this process. The rapid removal of ACs prevents secondary necrosis, whereas phagocytes that have engulfed ACs undergo further reprogramming to accelerate inflammation resolution and promote tissue repair [3,4]. However, the dysregulation or dysfunction of efferocytosis can result in the secondary necrosis of accumulated ACs and the corresponding release of inflammatory mediators. In addition, undegraded ACs release autoantigens, which can stimulate autoantibody production [5]. Consequently, impaired AC clearance is associated with various autoimmune diseases (Fig. 1), including systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), and rheumatoid arthritis (RA).
Fig. 1.
The molecules involved in efferocytosis in different diseases and their disease association.
Herein, we provide an overview of the general process of efferocytosis and associated molecular components, with a focus on their potential roles in autoimmune disease pathology. Finally, we explore the potential of efferocytosis as a novel therapeutic target for autoimmune diseases.
2. Stages of efferocytosis
2.1. “Find me” signals
Efferocytosis is initiated following the release of chemoattractants by ACs, including nucleotides (ATP and UTP), sphingosine-1-phosphate (S1P), fractalkine (CX3CL1), and lysophosphatidylcholine (LPC), which recruit phagocytes, constituting the “find me” signal [[6], [7], [8], [9]]. This release precedes the destruction of cell membrane integrity. Most of these chemoattractants are caspase-dependent. Caspases—cysteinyl aspartate-specific proteinases—are a family of proteases intricately associated with apoptosis. Nucleotide release is mediated through the plasma membrane channel pannexin 1 (PANX1). More specifically, the C terminus of PANX1 serves as the shear site of effector caspases (caspase 3/7). Cleavage by caspase 3/7 releases nucleotides from the cytoplasm to the extracellular matrix [10]. The released ATP can induce phagocyte migration through the combination with the purinergic receptor P2Y [6]. Following cleavage by caspase 3, Ca2+-independent phospholipase A2 (iPLA2) hydrolyzes membranous phosphatidylcholine to LPC and arachidonic acid [7]. The release of CX3CL1 by apoptotic lymphocytes is also partially dependent on caspases [9]. Additionally, S1P secreted by ACs regulates erythropoietin (EPO) signaling in vivo and in vitro. Erythropoietin receptor (EPOR)-deficient macrophages exhibit a decreased capacity to phagocytose ACs. The S1P–EPO–PPARγ pathway is necessary for AC clearance, and murine macrophages deficient in Epor or Ppar-γ are associated with an increased risk of developing lupus-like disease [8]. In other words, self-antigens result from the failed clearance of ACs, resulting in the production of autoantibodies and, thus, the development of autoimmune diseases.
2.2. “Eat me” signals
Following the identification of ACs, further signaling initiates the endocytic pathway in phagocytes, i.e., the “eat me” signal. The most common signaling receptor associated with this process is phosphatidylserine (PtdSer), which is typically distributed along the cytoplasmic side of the plasma membrane. Under the action of flippase (P4 ATPases) and scramblase (TMEM16F and Xkr8), PtdSer is rapidly relocated to the outer layer of the plasma membrane during apoptosis to further activate related receptors [1]. Subsequently, ACs expressing PtdSer are recognized by engulfment receptors, including stabilin-2, brain-specific angiogenesis inhibitor 1 (BAI1), T-cell immunoglobulin mucin-1 (TIM1), and TIM4 [[11], [12], [13]]. Alternatively, bridging molecules, including protein S (PROS1), milk fat globule-EFG factor 8 (MFGE8), and growth arrest-specific 6 (GAS6), can create physical connections between the TAM (TYRO3, AXL and MERTK) family receptor tyrosine kinases (RTKs) on the phagocytes and PtdSer on the AC. In TAM receptor-expressing macrophages, TIM4 can strongly enhance PROS- or GAS6-mediated efferocytosis [14]. Following activation of the “eat me” signal in phagocytes, engulfment and cell motility protein 1 (ELMO1) and dedicator of cytokinesis protein 1 (DOCK180) cooperatively function as guanine nucleotide exchange factors of the small GTPase RAC. Subsequently, ELMO-Dock180-Rac triggers related signaling pathways, leading to actin polymerization and cytoskeletal rearrangement [15]. Simultaneously, cluster of differentiation 47 (CD47) expression on the surface of healthy cells conveys the “do not eat me signal.” Thus, combining CD47 with the signal-regulatory protein alpha (SIRPα) receptor suppresses myosin rearrangement-mediated phagocytosis via SIRPα phosphorylation of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) [16]. In this way, phagocytes can differentiate normal cells from apoptotic neighbors. Additionally, it is worth noting that lysophosphatidylserine (lysoPS) produced by neutrophils can serve as an enhancer of efferocytosis. On one hand, as a cone-shaped lipid, lysoPS itself may facilitate the flip-flop across the membrane, potentially enhancing its own exposure as well as promoting the activation of downstream signaling pathways associated with PtdSer [17]. Moreover, lysoPS can bind to the G-protein coupled receptor G2A, ultimately leading to a PKA-dependent augmentation of Rac1 activity. However, it is important to emphasize that lysoPS alone, such as when added to live cells, does not induce efferocytosis [18].
2.3. Post-engulfment
Following engulfment, phagocytes, primarily macrophages, upregulate pro-resolving mediators and actin rearrangement/cell motility genes while downregulating pro-inflammatory genes [19]. To achieve this, macrophages utilize AC-derived metabolites for metabolic immune reprogramming and phenotypic transformation.
2.3.1. AC-derived metabolites
AC-derived amino acids, including arginine and ornithine, increase the amino acid content in macrophages after engulfment [20]. Indeed, arginine and ornithine serve as important precursors for polyamine synthesis. Polyamines, including putrescine, spermidine, and spermine, are aliphatic cations in all living cells. Their positive charge facilitates the binding of polyamines to DNA, RNA, and nuclear proteins, which supports cell proliferation. Polyamines also function as immunomodulators to suppress inflammation [21].
AC-derived arginine is converted by arginase 1 (ARG1) and ornithine decarboxylase (ODC) into putrescine. The ARG1–ODC–putrescine pathway primarily occurs within alternatively activated macrophages (AAMs, i.e., M2 macrophages). Putrescine promotes HuR-mediated Mcf2 mRNA stability, which further upregulates Dbl, resulting in RAC1 activation and continual efferocytosis. Persistent efferocytosis, mediated by the ARG1–ODC–putrescine pathway in AAMs, is particularly important in a high-AC burden environment. A lack of ARG1 or ODC can impair the resolution of atherosclerosis [22]. ODC-dependent putrescine synthesis in macrophages sustains MerTK expression to resolve inflammation via the downstream ERK–AP1–IL-10 pathway [23]. Spermidine and spermine are also elevated in naïve and inflammatory macrophages during efferocytosis but not in response to the AC-derived arginine synthetic pathway. In fact, naïve and inflammatory macrophages import polyamines from the extracellular space via RAC1 activation during efferocytosis. Notably, elevated spermidine and spermine import can also suppress interleukin (IL)-1β and IL-6 production by inflammatory macrophages [20].
AC-derived methionine also plays an important role in maintaining tissue resolution. That is, the initial interaction between ACs and macrophages triggers the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, which induces Ptgs2 expression, thereby activating the downstream COX2–PGE2–TGFβ1 pathway. However, ERK1/2 must also evade the inhibitory effects of dual-specificity phosphatase 4 (DUSP4). To achieve this, AC-derived methionine is converted to S-adenosine methionine (SAM), which methylates Dusp4 under the catalyzation of DNA methyltransferase-3A (DNMT3A), achieving the epigenetic repression of Dusp4 and maintaining p-ERK1/2 activity. Moreover, phagolysosomal DNASE2A hydrolyzes AC-derived DNA into oligonucleotides, which further activates the DNA–PKC–mTORC2/Rictor pathway. The mTORC2–Akt and MerTK–ERK1/2 pathways then function to enhance Myc expression. MYC drives macrophage proliferation by elevating BHLHE40 and downregulating c-MAF. These proliferative macrophages have a pro-resolving phenotype, characterized by the secretion of IL-10 and TGF-β and enhanced efferocytosis ability. However, efferocytosis does not trigger the proliferation of inflammatory macrophages, as this pro-proliferative process can only occur in naïve and resolving macrophages [4,24].
Increases in fatty acid metabolism and oxygen consumption in macrophages ultimately lead to increased IL-10 production. Mitochondrial fatty acid oxidation (FAO) is related to the electron transport chain. Sufficient NAD+ ensures the activity of the NAD+-dependent deacetylase, SIRT1, which facilitates binding of the transcription factor PBX-1 to the IL10 promoter [25]. Meanwhile, AC-derived cholesterol accumulation in macrophages activates LXR, which further induces MerTK expression, simultaneously providing positive feedback to promote further AC uptake and the production of pro-repair factors, such as IL-10 and TGF-β [26].
Although there is a dearth of data regarding the regulatory effects of AC-derived glucose on efferocytosis, SLC2A1 (GLUT1)—a transporter responsible for regulating extracellular glucose uptake—is reportedly upregulated during efferocytosis. Moreover, glucose intake promotes actin polymerization and cytoskeletal rearrangement in pro-resolving macrophages. Nevertheless, additional investigation is warranted to characterize the precise mechanisms by which AC-derived glucose enhances glycolysis in macrophages [19].
2.3.2. Phenotypic reprogramming
Efferocytosis causes the macrophage phenotype to shift in a favorable direction. This includes a progressive increase in the ability to internalize subsequent ACs, i.e., continual efferocytosis [22]. When the number of ACs exceeds that of macrophages markedly, continual efferocytosis ensures efficient clearance. Recent studies have partially characterized the mechanism by which efferocytosis-induced glycolysis regulates continual efferocytosis in pro-resolving macrophages. AC endocytosis activates the downstream TXNIP-PFKFB2-mediated glycolytic pathway. A product of transiently enhanced glycolysis, lactate, promotes the expression of the “eat me” receptors MerTK and LRP1 in a calcium-dependent manner, culminating in continual efferocytosis [27]. Furthermore, AC engulfment activates dynamin-related protein 1 (DRP1); DRP1-mediated mitochondrial fission also participates in continual efferocytosis. Mitochondrial fission increases the cytoplasmic calcium content by preventing excessive MCU-mediated calcium chelation, ensuring rapid AC degradation and subsequent calcium-dependent vesicular transport, both necessary for high-load efferocytosis [28]. Moreover, putrescine production from AC-derived arginine and ornithine enhances continual efferocytosis by increasing RAC1 activation [22].
Efferocytosis promotes the biosynthesis of specialized pro-resolving mediators (SPMs), including lipoxins and resolvin [29], which suppress and/or resolve inflammation. Sterol intermediates in macrophages activate LXR to regulate SPM expression in pro-resolving macrophages. More specifically, following AC engulfment, LXR increases the expression of arachidonic acid 15-lipoxygenase (ALOX15), which is required for SPM synthesis [30]. Newly generated SPMs stimulate the transformation of macrophages into AAMs, contributing to inflammation resolution [31]. Similarly, phagocytes increase resolvin D1 (RvD1), D2 (RvD2), and E2 (RvE2) biosynthesis via efferocytosis [32]. In fact, RvD1 partially enhances the efferocytosis effect by stabilizing MerTK expression in macrophages [33]. RvD1 also activates CDC42, a GTPase involved in the “eat me” signal and engulfment stages. That is, CDC42 stimulates macrophage calreticulin release, effectively tagging necrotic cells and activating actin. The labeled necrotic cells are then rapidly engulfed. During this RvD1-mediated clearance of dead cells, the release of the macrophage pro-inflammatory factor CXCL1 is inhibited [34]. The disruption of SPM synthesis represents a central mechanism in the pathogenesis of many chronic diseases.
AAMs, also known as M2 macrophages, are polarized by the T helper 2 (Th2) cytokines IL-4 and IL-13, among other factors, including apoptotic neutrophils, IL-10, and glucocorticoids. Efferocytosis could also direct the polarization of macrophages toward the M2 phenotype (AAMs) [4,24]. Efferocytosis is considered a distinct feature of M2 macrophages. Human monocyte-derived macrophages undergo enhanced efferocytosis in the presence of IL-10 [22,35]. This process is initiated via IL-13 secretion by T regulatory cells (Tregs), which stimulates IL-10 production in macrophages. Then, IL-10 induces the production of the guanine nucleotide exchange factor VAV1 by macrophages in an autocrine/paracrine manner, activating RAC1 to achieve optimal AC internalization [36]. Indeed, Treg activation is required for macrophages to promote inflammatory resolution and atherosclerotic plaque regression [37]. Conversely, M2 macrophages secrete anti-inflammatory cytokines like TGF-β and IL-10, which further activate Tregs [38]. Tregs are critical for maintaining immune tolerance, and their dysregulation is a characteristic feature of autoimmune disorders, often associated with deficiencies in both the number and function of Treg cells [39,40]. The synergy between efferocytosis and Treg activation underscores their interconnected roles in immune response regulation and tissue homeostasis maintenance. Impaired efferocytosis perpetuates inflammation, contributing to the development of the chronic inflammatory milieu typical of autoimmune disorders.
Additionally, IL-4 produced by eosinophils and basophils participates in M2 polarization, the upregulation of efferocytosis, and inflammatory resolution [41,42]. By activating downstream transcription factor signal transducer and activator of transcription-6 (STAT6), IL-4 and TNF-α stimulated gene-6 (TSG6) induce the polarization of alveolar macrophages toward an anti-inflammatory phenotype, leading to GAS6 upregulation and the rapid clearance of apoptotic neutrophils from the lungs in inflammatory lung injury [43]. In this way, ACs and Th2 cytokines act synergistically to enhance the anti-inflammatory and pro-resolving phenotypes of macrophages, further illustrating the complementary relationship between M2 macrophage polarization and efferocytosis [44]. The impairment of continual efferocytosis causes sustained inflammation, creating a chronic inflammatory environment, characteristic of autoimmune disorders.
3. Associations between efferocytosis and autoimmune diseases
3.1. Rheumatoid arthritis
RA is a chronic and systemic disease of unknown etiology, mainly presenting with inflammatory synovitis. It is characterized by multi-articular, symmetrical, and invasive inflammation of the small joints of the hand and foot, often accompanied by involvement of extra-articular organs and positive serum rheumatoid factors, which can ultimately lead to joint deformity and loss of function [45]. Although impaired efferocytosis and elevated AC levels have been observed in the synovial tissue of patients with osteoarthritis [46], comprehensive evaluations of efferocytosis within RA synovial tissue are lacking. Inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, have important roles in the pathogenesis of RA. Defects in intracellular DNaseII can cause RA-like symptoms, including joint swelling and pannus formation, in animal models. Moreover, a loss of DNaseII impairs the ability of macrophages to digest AC-derived DNA, thereby activating TNFA, which increases the expression of myriad inflammatory cytokines (IL-1β, IL-6, TNF-α, etc.) in synovial fibroblasts [47].
Membrane-bound TAM receptors (Tyro3, Axl, and MerTK) can be modified to generate soluble TAM (sTAM) receptors (sTyro3, sAxl, and sMerTK). More specifically, “a disintegrin and metalloproteinases” (ADAMs), including ADAM10 and ADAM17, cleave the extracellular domain of transmembrane proteins through proteolysis, enabling them to take on a soluble form. These ADAMs are the major enzymes associated with sAxl and sMer production and are significantly elevated in the serum, synovial tissue, and synovial fluid of patients with RA [48,49]. Additionally, sTyro3 and sMer are significantly elevated in the synovial fluid of patients with RA. sTyro3 levels are positively correlated with systemic disease activity [50]. Notably, synovial sTyro3 is a more reliable measure of the severity of RA joint inflammation than plasma sTyro3 [51], supporting the essential role of synovial sTyro3 in the pathology of RA. Additionally, the restriction of MerTK cleavage and exfoliation promotes SPM biosynthesis and inflammation resolution [52]. sMer can act as a decoy receptor for GAS6 to inhibit the GAS6-mediated membrane-binding Mer signaling pathway, further inhibiting AC clearance by macrophages [53]. However, although sMer can bind GAS6, it does not fully antagonize GAS6 activity; hence, the role of decoy receptors is very limited [54].
Bone marrow edema—an early marker of RA—occurs in Tyro3/Axl/Mertk-deficient mice [55]. In inflammatory arthritis, K/BxN mice with Mertk knockdown exhibit progressive arthritis pathologies. The administration of MER-specific agonistic antibodies to CIA mice contributes to the exacerbation of arthritis accompanied by increased ACs in joints and elevated serum IL-16C levels [56]. This suggests that the administration of MER-specific agonistic antibodies inhibits MerTK-mediated efferocytosis, resulting in the impaired uptake of apoptotic neutrophils in the joints. Consequently, ACs accumulate, undergo secondary necrosis, and release pro-inflammatory factors, including IL-16C and TNF-α. Indeed, MerTK-mediated efferocytosis plays a crucial role during arthritis, suggesting that targeting this pathway is a possible therapeutic strategy. Activation of MER via the upregulation of its ligand, PROS1, alleviates arthritic symptoms. However, considering that PROS1 and C4b combine to form a non-covalent complex in vivo and in vitro, PROS1 is not advised for the treatment of patients with RA [57]. The synthesis of analogs may overcome the disadvantages of PROS1, i.e., its strong anticoagulant activity and short half-life. Considering that TAM receptors regulate myriad signaling pathways in vivo, including downregulating pyroptosis, inhibiting inflammatory cytokine expression, and enhancing efferocytosis and necroptosis [58,59], identifying the role of the TAM/efferocytosis axis in RA development and its upstream regulatory factors will inform the development of improved targeted therapeutic strategies.
Efferocytosis-released cytokines exhibit pro-resolutive and pro-efferocytosis properties. In fact, in CIA arthritis, treatment with the efferocytosis-derived cell supernatant (SuperMApo) effectively alleviates symptoms and prevents disease progression. SuperMApo induces plasmacytoid DC and macrophage immune reprogramming, thereby promoting Treg auto-antigen-specific induction [60]. Likewise, a cocktail of apoptotic metabolites reduces K/BxN arthritis symptoms [61], while an AC infusion significantly reduces the clinical arthritis score in CIA mice. AC administration effectively reprograms ACs and inhibits T-cell responses to autoantigens by selectively inducing collagen-specific Tregs. Moreover, anti-TNF-α antibodies function synergistically with apoptosis-based therapies [62]. Collectively, these therapies induce the production of specific Tregs to regulate the overall immune response. However, while the infusion of ACs during the induction phase of CIA significantly alleviates disease severity, AC administration after CIA onset is not protective [63].
In a model of inflammatory arthritis (K/BxN mice), the intestinal concentrations of several SPMs, including N-3 docosapentaenoic acid-derived resolvin D5 (RvD5n-3 DPA), are decreased. Exogenous supplementation with RvD5n-3 DPA exhibits a therapeutic effect in inflammatory arthritis [64], mediated by the orphan receptor GPR101 expressed on the macrophage surface. RvD5n-3 DPA binding to GPR101 enhances the efferocytosis ability of macrophages [65]. In contrast, Gpr101 knockout polarizes macrophages toward an M1 pro-inflammatory phenotype [66].
Several novel mediators have been designed to promote inflammation resolution in animal models of RA by enhancing macrophage efferocytosis. For example, a lipid Lipoxin A4 (LXA4) analog, AT-01-KG, functions as an activator of N-formyl peptide receptor 2 (FPR2/ALX). AT-01-KG reduces neutrophil accumulation in joints by increasing their apoptosis and subsequent efferocytosis, thereby effectively alleviating inflammation [67]. Biochanin A (BCA), a natural organic compound, can modulate inflammatory regression in an antigen-induced arthritis rodent model via the GPR30/PKA pathway, leading to reduced neutrophil accumulation and enhanced neutrophil apoptosis and macrophage efferocytosis [68]. Similarly, angiotensin-(1–7) (Ang- [[1], [2], [3], [4], [5], [6], [7]]) promotes the resolution of neutrophil inflammation in an antigen-induced arthritis model by enhancing neutrophil apoptosis. Boosted efferocytosis accelerates the clearance of apoptotic neutrophils, accompanied by macrophage reprogramming away from proinflammatory phenotypes [69]. An MC1 agonist, PL8177, improves K/BxN arthritis by enhancing macrophage efferocytosis and reducing M1 macrophage polarization and inflammatory cytokine release [70]. Additionally, pulsed electromagnetic fields effectively inhibit synovitis by enhancing macrophage efferocytosis, which may involve the downregulation of p38 phosphorylation [71].
3.2. Systemic lupus erythematosus
SLE is a diffuse connective tissue disease mediated by autoimmune mechanisms, characterized by the presence of multiple autoantibodies in the serum and involving multiple organs and organ systems. AC accumulation, caused by impaired non-inflammatory phagocytosis, is a pathological feature of SLE [72,73]. Various autoantibodies, represented by antinuclear antibodies, are present in the serum of patients with SLE. Many knockout mice in which the efferocytosis process is targeted exhibit lupus-like symptoms (Table 1). Additionally, the complement component C1q is closely associated with the pathogenesis of SLE. The globular head of C1q can bind to ACs, acting as a bridging molecule in the “eat me” signal to promote efferocytosis [74]. C1q mutations are a susceptibility factor for SLE. C1qa−/− mice exhibit the accumulation of ACs and lupus-like glomerulonephritis [75]. Indeed, the impaired uptake of ACs in patients with SLE is associated with C1q, consistent with the reported reduction in serum C1q levels in these patients [76]. In addition, anti-C1q antibodies are highly associated with the pathological progression of lupus nephritis. Hence, although anti-C1q antibodies can bind C1q on the surface of early ACs, they also enhance activation of the classical complement pathway, triggered by immune complexes [77]. Given that multiple ligands and receptors transmit “eat me” signals, it is necessary to definitively ascertain the extent to which C1q-mediated efferocytosis contributes to the pathological mechanisms of SLE (see Table 2).
Table 1.
Autoimmune animal model gene mutations during efferocytosis.
| Molecule/protein | Efferocytosis phase | Genetically-modified murine model | Phenotype |
|---|---|---|---|
| EPOR [8] | “Find me” | Epor−/− mice | Age-dependent lupus-like symptoms |
| G2A [78] | “Find me” | G2A−/− mice | Lymphocytic infiltration into various tissues |
| Lupus-like glomerulonephritis | |||
| Anti-nuclear autoantibodies | |||
| Mertk [79,80] | “Eat me” | Mertk−/− mice | Lupus-like glomerulonephritis |
| Anti-nuclear autoantibodies | |||
| Sjögren's syndrome-like symptoms | |||
| Tyro3/Axl/Mertk [55] | “Eat me” | Tyro3/Axl/Mertk-deficient mice | Systemic lupus erythematosus, pemphigus vulgaris, and rheumatoid arthritis |
| Anti-nuclear autoantibodies | |||
| Xkr8 [81] | “Eat me” | MR-Xkr8−/− mice | Rising effector CD4+ T cells |
| Lupus-like glomerulonephritis | |||
| C1q [75] | “Eat me” | C1qa−/− mice | Uncleared ACs |
| Lupus-like glomerulonephritis | |||
| MFG-E8 [82] | “Eat me” | MFG-E8−/− mice | Splenomegaly |
| Anti-nuclear autoantibodies | |||
| Lupus-like glomerulonephritis | |||
| DNase II [83] | Post-engulfment | DNaseII−/−IFNaR−/− double knockout (DKO) mice | Inflammatory polyarthritis |
| PPARγ, RXRα [84] | Post-engulfment | mice lacking macrophage PPARγ or RXRα | Lupus-like glomerulonephritis |
| Anti-nuclear autoantibodies | |||
| LXR [26] | Post-engulfment | Lxr αβ−/− mice | Age-dependent splenomegaly |
| Lupus-like glomerulonephritis | |||
| Immunoglobulin deposition in other organs including lungs and skin |
Table 2.
Potential efferocytosis therapeutic targets in various autoimmune diseases.
| Disease | Treatment | Efferocytosis association | Reference |
|---|---|---|---|
| Rheumatoid arthritis | SuperMApo | Efferocytosis-released cytokines | [60] |
| AC infusion | Directly promote efferocytosis | [62] | |
| SPMs | Stimulant effect on efferocytosis | [60] | |
| AT-01-KG | Reduce neutrophil accumulation, enhance neutrophil apoptosis and macrophage efferocytosis | [67] | |
| Biochanin A | [68] | ||
| Angiotensin-(1–7) | [69] | ||
| PL8177 | Reduce M1-like macrophage polarization and enhance macrophage efferocytosis | [70] | |
| Pulsed electromagnetic field | Enhance macrophage efferocytosis | [71] | |
| Systemic erythematosus | Combined inhibition of ADAM10/ADAM17 | Reduce sAXL production | [85] |
| IgG deletion | Reduce autoantibodies against the “eat me” receptor SCARF1 | [86] | |
| Exosomes from bone marrow mesenchymal stem cells | miR-16 and miR-21 enhance ACa clearance | [87] | |
| PS-lipos-AuNC@T0901317 | Targeted enhancement of macrophage LXR activation leading to rapid AC clearance | [88] | |
| Idiopathic inflammatory bowel | BELMO TELMO | Enhance efferocytosis efficiency and ensure AC utilization after efferocytosis | [89] |
| Single infusion of AC | Directly promote efferocytosis | [90] | |
| SuperMApo | Efferocytosis-released cytokines | [91] | |
| ChemR23 | Reduce neutrophil accumulation, enhance neutrophil apoptosis and macrophage efferocytosis | [92] | |
| AON | [93] | ||
| Type 1 diabetes | Dendritic cells pulsed with antigen-specific apoptotic bodies | Rehabilitate specific immune tolerance | [94] |
| rMFG-E8 | Accelerate dead cell removal to promote wound healing | [95] | |
| liposomes containing PtdSer, β-cell autoantigens, and human insulin peptide | Rehabilitate specific immune tolerance | [96] | |
| Systemic sclerosis | rMFG-E8 | Significantly improve lung and skin fibrosis in bleomycin-induced fibrosis mouse models | [97] |
| ROCK inhibitor Y27632 | Reverse efferocytosis damaged by SiO2 | [98] | |
| Ruxolitinib | Significantly improve lung and skin fibrosis in bleomycin-induced fibrosis mouse models | [99] |
AC, apoptotic cell.
LPC—a chemoattractant—functions in the “find me” stage of efferocytosis. Serum levels of LPC are significantly elevated in patients with SLE, particularly in those with vascular or renal involvement. The first established step of efferocytosis is the release of LPC from ACs, creating a local LPC gradient to attract macrophages. The current hypothesis is that high serum levels of LPC directly neutralize the local LPC gradient in patients with SLE, preventing macrophages from homing to ACs and, ultimately, contributing to impaired efferocytosis [100].
The phosphatidylserine-specific phospholipase A1 (PS-PLA1) catalyzing lysoPC generation has been found to be elevated in the serum of patients with SLE and is associated with SLE disease activity, suggesting its potential as a biomarker for monitoring SLE disease activity [101]。Additionally, upregulation of the PLA1A gene has been observed in patients with discoid lupus erythematosus [102]。PLA1A can mask PtdSer by hydrolyzing it on apoptotic cells, thereby interfering with the resolution of inflammation [103]。While lysoPC generated can enhance efferocytosis, it does not directly induce efferocytosis.
Scavenger receptor class F member 1 (SCARF1), expressed by phagocytes, is responsible for binding and engulfing ACs. In the serum of patients with SLE, the level of anti-SCARF1 autoantibodies is correlated with the ability to clear ACs. These autoantibodies can block the progression of efferocytosis-related pathways, leading to impaired AC clearance and further promoting the accumulation of secondary necrotic cells and inflammation [86]. Immunoglobulin (Ig)G depletion to ensure the blockade of autoantibodies against SCARF1 enhances efferocytosis in SLE serum [86]. Similarly, purified anti-Tyro3 IgG suppresses efferocytosis in macrophages. Different from other autoimmune diseases (RA and pSS), in SLE, the levels of IgG-type autoantibodies against the Tyro3 receptor increase markedly as disease activity increases. As such, serum anti-Tyro3 IgG titers might represent an effective biomarker for SLE [104].
Serum levels of sTAM receptors (sTyro3, sAxl, and sMerTK) are also increased in patients with SLE [[105], [106], [107]]. The production of sAxl in the macrophages of mice with lupus and the peripheral blood mononuclear cells of patients with SLE is mediated by ADAM10 and ADAM17. Accordingly, combined ADAM10/ADAM17 inhibition might represent an effective treatment option [85].
Exosomes from bone marrow mesenchymal stem cells promote macrophage polarization toward the M2 phenotype by delivering miR-16 and miR-21, contributing to enhanced AC clearance and the alleviation of SLE nephritis in a mouse model of lupus [87]. LXR promotes efferocytosis. The inclusion of an LXR agonist in an AC-mimetic gold nanocage (PS-lipos-AuNC@T0901317) effectively targeted macrophages in mice with SLE, significantly enhancing MerTK expression and efferocytosis in bone marrow-derived macrophages (BMDMs) and splenic macrophages. Moreover, compared to the direct delivery of a conventional LXR agonist (T0901317), PS-lipos-AuNC@T0901317 significantly prevented SLE progression by rapidly clearing ACs and reducing autoantibody titers and pro-inflammatory cytokine production. Hence, targeting LXR enhancement in macrophages, rather than pan-LXR enhancement, to improve efferocytosis represents a potential therapeutic direction for SLE [88].
3.3. Inflammatory bowel disease
IBD is an idiopathic disease that includes ulcerative colitis (UC) and Crohn's disease (CD). The primary treatment strategy includes inhibiting the production of inflammatory cytokines (IL-1 or TNF-α). However, accumulated ACs are also present within the intestinal tissues in IBD, the primary cause of which is posited to be impaired efferocytosis. Under normal physiological conditions, CD300f positively regulates the efficient clearance of ACs by macrophages and negatively regulates DC-mediated efferocytosis, which involves TNF-α production. A CD300f deficiency limits the macrophage-mediated clearance of ACs. Elevated DC-mediated efferocytosis continuously generates TNF-α, which inhibits macrophage efferocytosis, while promoting IFN-γ production in Cd300f−/− mouse intestinal mucosal cells. Thus, a CD300f deficiency exacerbates colitis and impedes inflammation resolution [108].
In mice with DSS-induced colitis, BAI1 knockout resulted in enhanced colonic inflammation accompanied by increased AC accumulation and inflammatory cytokines. By contrast, BAI1 overexpression attenuated DSS-induced colitis via the BAI1-induced activation of the ELMO-DOCK1 complex downstream of efferocytosis and promotion of AC internalization. However, this efferocytosis activity may rely primarily on non-professional phagocytes, in particular, colonic epithelial cells [109]. Indeed, the therapeutic potential of targeting enhanced non-professional phagocyte intestinal epithelial cell (IEC) efferocytosis in enterocolitis warrants further investigation.
A chimeric efferocytosis receptor (CHEF) was recently designed as a truncated cytoplasmic tail of PtdSer receptors (BAI1 and TIM4) fused to ELMO1, a signaling intermediate downstream of efferocytosis. These two CHEFs were designated BELMO and TELMO. BELMO improves the efferocytosis efficiency substantially via the ELMO–DOCK180–RAC pathway and improves proteostasis, thus ensuring the utilization of ACs following endocytosis. The targeting of BELMO to IECs effectively alleviates DSS-induced colonic pathology and decreases the expression of pro-inflammatory factors in the intestine accompanied by IL-10 upregulation. Although adeno-associated virus vector-mediated gene delivery is a successful strategy for BELMO targeted therapy, additional research is necessary to optimize its delivery [89].
Additionally, a single AC infusion can inhibit macrophage NF-κB and NLRP3-inflammasome activation, thereby alleviating the clinical symptoms and histopathology of DSS-induced colitis. The downregulation of the NLRP3–IL-1β pathway involves a reduction in the production of reactive oxygen species, stabilization of lysosomes, and negative regulation of K+ efflux [90]. The administration of efferocytosis-released cytokines (SuperMApo) controls IBD progression and reduces inflammatory cell infiltration. SuperMApo also induces IEC proliferation and activates local fibroblasts in vivo to restore intestinal barrier permeability [91]. ChemR23, a GPCR targeted by resolvin E1, is overexpressed in inflamed colonic tissues of patients with severe IBD. Administration of an anti-ChemR23 agonist reduces neutrophil migration, enhances neutrophil apoptosis, and continuously enhances macrophage efferocytosis. Thus, ChemR23 monoclonal antibody treatment rapidly resolves neutrophil-associated enteritis, including in DSS-induced colitis and trinitro-benzene-sulfonic acid-induced mouse models [92]. Finally, an oxidation-responsive nanoparticle containing a mimetic peptide of the pro-resolving annexin A1 active terminal peptide Ac2-26 (AON) significantly downregulates pro-inflammatory mediators and inflammatory cell infiltration, thus promoting apoptotic neutrophil clearance and switching to the pro-resolving macrophage phenotype, effectively alleviating DSS-induced colitis [93].
3.4. Sjögren's syndrome
Sjögren's syndrome (SS) is an autoimmune disease characterized by reduced secretion by the lacrimal and salivary glands. Increased apoptosis of salivary gland epithelial cells and impaired efferocytosis have been detected in patients with SS and a murine model [110,111]. This reduction in efferocytosis involves a decrease in the phagocytosis of peripheral blood mononuclear cells and macrophages in patients, likely due to the presence of serum IgG anti-AC antibodies, which interfere with the phagocytic clearance of ACs [112]. ACs that are not phagocytosed release DNA and other self-antigens, which act as damage-associated molecular patterns (DAMPS) and trigger Toll-like receptors (TLRs), further activating the inflammatory immune response. Moreover, SS mouse-derived BMDMs produce inflammatory cytokines upon AC stimulation, primarily due to the decreased ability of BMDMs to clear ACs, leading to intracellular content overflow and TLR activation in macrophages. This abnormal inflammatory response can be reversed via TLR7 and TLR9 inhibition [113]. However, characterizing the origin of IgG anti-AC antibodies may inform the design of improved strategies to target and improve impaired efferocytosis in SS. Moreover, damaged efferocytosis in SS is thought to be associated with elevated serum activity of ADAM17, which cleaves MerTK to form sMer. Indeed, patients with SS exhibit elevated levels of sMer in the plasma. In a murine model, MerTK knockdown resulted in submandibular gland pathology similar to SS, along with a decrease in salivary flow and the presence of positive antinuclear antibodies [80]. In general, AC accumulation is not only a consequence of autoimmune responses but also a driver of inflammatory signaling, further exacerbating disease progression.
3.5. Type 1 diabetes
Type 1 diabetes (T1D) is caused by autoreactive T cell-mediated pancreatic islet β cell destruction and a consequent deficiency in insulin production. In particular, a lack of T cell tolerance to autoantigens is an important contributor to the pathogenesis of T1D. However, macrophages of a T1D animal model, non-obese diabetic (NOD) mice, exhibit defective efferocytosis in vivo and in vitro, accompanied by a significant increase in apoptotic β cells in the pancreas of newborns [114,115]. Apoptotic pancreatic cell accumulation may lead to increased necrosis and inflammation as well as the release of autoantigens. A common feature of T1D and T2D is slowed wound healing. Efficient efferocytosis may promote inflammation regression and wound healing through the rapid clearance of dead cells. However, high levels of pro-inflammatory factors and low expression of anti-inflammatory factors in diabetic wounds are responsible for the inefficient phagocytosis of dead cells by local macrophages [116]. Furthermore, a hyperglycemic environment and late glycation end products may inactivate MFG-E8, a bridging molecule during efferocytosis, further delaying diabetic wound healing. In fact, topical rMFG-E8 has demonstrated significant potential in the treatment of diabetic wounds [95]. However, the ideal therapy for T1D lies in the re-establishment of immune tolerance to islet β cells, which can be achieved by exploiting ACs to reprogram DCs for specific immune tolerance [117]. Immature DCs (iDCs) derived from the bone marrow of NOD mice and pulsed with antigen-specific β-cell apoptotic bodies express lower levels of the co-stimulatory molecules CD40 and CD86 on their surface and produce reduced pro-inflammatory cytokine levels. The administration of tolerogenic DCs to NOD mice significantly reduced the incidence of T1D and decreased the occurrence of islet inflammation [94]. Another approach involves the synthesis of liposomes containing PtdSer, β-cell autoantigens, and human insulin peptide. Liposomes inhibit DC antigen presentation and T-cell proliferation in samples from patients with T1D. This process is accompanied by the upregulation of genes associated with tolerogenic/anti-inflammatory pathways [96]. Although these studies provide possible avenues for targeting efferocytosis in T1D treatment, further research is needed.
3.6. Systemic sclerosis
Systemic sclerosis (SSc), also known as scleroderma, is a systemic autoimmune disease characterized by limited or diffuse skin thickening and fibrosis. Hyperplasia of skin fibers and onion skin changes in blood vessels ultimately lead to skin sclerosis and vascular ischemia. Moreover, the efferocytosis of monocyte-derived macrophages (MDMs) from the blood of patients with SSc is impaired and characterized by the downregulation of scavenger receptor (SR)-B1, SR-A1, and integrin beta 5 (ITGβ5). Moreover, ITGβ5 knockdown exerts a greater inhibitory effect on efferocytosis in MDMs from healthy controls than that of the knockdown of either SR [118]. ITGβ5-dependent efferocytosis is mediated by the bridging molecule MFG-E8, which is downregulated in the skin and serum of patients with SSc. Combined, MFG-E8 and integrin can inhibit potential transforming growth factor β (TGF-β)-induced fibrosis, while rMFG-E8 administration to a bleomycin-induced fibrosis mouse model significantly improves lung and skin fibrosis [97]. Hence, further research is warranted to elucidate the role of MFG-E8 and integrin binding in efferocytosis within the context of the pathogenesis of SSc.
Crystalline silicon (SiO2) inhalation has also been associated with the pathogenesis of SSc. It significantly impairs the efferocytosis index of human MDMs and mouse alveolar macrophages in vitro accompanied by an SR-B1-dependent increase in expression. The ROCK inhibitor Y27632 reverses the SiO2-induced reduction in efferocytosis. Y27632 also promotes efferocytosis in SSc MDMs [98]. Hence, Silica/RhoA/ROCK could represent a potential therapeutic target for SSc.
Necrosis of accumulated AC triggers antibody production. Immune complexes containing SSc-specific autoantibodies can induce profibrotic and proinflammatory phenotypes in skin fibroblasts, and this transformation is mediated by the interaction of TLRs with nucleic acid fragments embedded in SSc-ICs [119]. Furthermore, the JAK-STAT inhibitor ruxolitinib attenuates skin and lung fibrosis in a BLM-SSc mouse model and decreases ACs [99].
3.7. Granulomatosis with polyangiitis
Granulomatosis with polyangiitis (GPA), also known as Wegener's granulomatosis, is characterized by necrotizing inflammation and granuloma formation in small vessels. Anti-neutrophil cytoplasmic antibodies can be detected in the serum of patients. Proteinase 3 (PR3), a serine protease in neutrophil azurophil granules, is expressed on the cell membrane of apoptotic neutrophils and functions as a primary target antigen of anti-neutrophil cytoplasmic antibodies [120]. Increased expression of membrane PR3 on neutrophils in patients with GPA can impede macrophage efferocytosis [121,122]. Neutrophilic apoptosis is unregulated in GPA, as evidenced by a decrease in the proportion of ACs and delayed apoptosis [123]. Consequently, the overabundance of neutrophils expressing membrane PR3 is a risk factor for autoimmune vasculitis. That is, PR3 directly binds calreticulin (CRT), thereby blocking the CRT/LRP “eat me” signal and delaying the clearance of apoptotic neutrophils. Following their phagocytosis, apoptotic neutrophils expressing PR3 induce the increased secretion of inflammatory cytokines by macrophages, including granulocyte colony-stimulating factor (G-CSF) and various chemokines and cytokines [124,125]. These inflammatory cytokines further enhance the recruitment and infiltration of local inflammatory cells, including macrophages, plasmacytoid DCs, and neutrophils. Furthermore, PR3 disrupts immune silencing associated with apoptotic neutrophil clearance, resulting in increased inflammation [125]. It can also impair C1q-enhanced AC engulfment [126]. It is important to determine whether other proteins expressed on apoptotic neutrophils regulate impaired efferocytosis in GPA.
3.8. Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system; the immune system is involved in its onset and progression. Nerve fibers are enveloped by myelin sheaths, which are mistakenly attacked by the immune system, leading to axonal injury, demyelination, and the death of oligodendrocytes. Purinergic receptors, including the P2X7 and P2Y receptors, are associated with the pathogenesis of MS [127]. However, these receptors are capable of transmitting “find me” and “eat me” signals [128,129]. Therefore, establishing a pathological link between efferocytosis and MS is an important future research goal.
4. Conclusion and perspectives
Given the direct associations between dysregulated efferocytosis and autoimmune diseases, more in-depth investigations are needed to elucidate the underlying mechanisms and develop novel targeted therapies. However, as efferocytosis is an emerging field of research, several difficulties and challenges need to be overcome. Firstly, the subdivision of the functions of different types of phagocytes in autoimmune diseases has proven challenging. Secondly, research on the relationship between efferocytosis and autoimmune diseases remains at the cellular and organismal levels. Yet, the intricate and heterogeneous nature of autoimmune disorders suggests that impaired efferocytosis represents just one facet of the condition. Consequently, focusing solely on macrophage efferocytosis might not suffice for effective treatment strategies. Therefore, it is imperative to conduct clinical trials to validate preliminary findings. A comprehensive understanding of efferocytosis's role in autoimmune disorders is crucial for informing the development of innovative therapeutics in the future.
Funding
This study was supported by the Clinical Research Incubation Project and 1.3.5 Project for Disciplines of Excellence of West China Hospital, Sichuan University [grant numbers 2019HXFH038, 2021HXFH018, and ZYJC21024].
Data availability
Data included in article/supplementary material/referenced in article.
CRediT authorship contribution statement
Qianwei Li: Writing – original draft, Visualization, Conceptualization. Huan Liu: Writing – review & editing, Supervision, Conceptualization. Geng Yin: Writing – review & editing, Supervision, Funding acquisition. Qibing Xie: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Geng Yin reports financial support was provided by West China Hospital, Sichuan University. Qibing Xie reports financial support was provided by West China Hospital, Sichuan University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Geng Yin, Email: yingeng1975@163.com.
Qibing Xie, Email: xieqibing1971@163.com.
References
- 1.Segawa K., Nagata S. An apoptotic 'Eat Me' signal: phosphatidylserine exposure. Trends Cell Biol. 2015;25(11):639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Doran A.C., Yurdagul A., Jr., Tabas I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020;20(4):254–267. doi: 10.1038/s41577-019-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latour Y.L., Gobert A.P., Wilson K.T. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids. 2020;52(2):151–160. doi: 10.1007/s00726-019-02719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlach B.D., Ampomah P.B., Yurdagul A., Jr., Liu C., Lauring M.C., Wang X., Kasikara C., Kong N., Shi J., Tao W., Tabas I. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metabol. 2021;33(12):2445. doi: 10.1016/j.cmet.2021.10.015. 2463.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimani S.G., Geng K., Kasikara C., Kumar S., Sriram G., Wu Y., Birge R.B. Contribution of defective PS recognition and efferocytosis to chronic inflammation and autoimmunity. Front. Immunol. 2014;5:566. doi: 10.3389/fimmu.2014.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott M.R., Chekeni F.B., Trampont P.C., Lazarowski E.R., Kadl A., Walk S.F., Park D., Woodson R.I., Ostankovich M., Sharma P., Lysiak J.J., Harden T.K., Leitinger N., Ravichandran K.S. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauber K., Bohn E., Kröber S.M., Xiao Y.J., Blumenthal S.G., Lindemann R.K., Marini P., Wiedig C., Zobywalski A., Baksh S., Xu Y., Autenrieth I.B., Schulze-Osthoff K., Belka C., Stuhler G., Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 8.Luo B., Gan W., Liu Z., Shen Z., Wang J., Shi R., Liu Y., Liu Y., Jiang M., Zhang Z., Wu Y. Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity. 2016;44(2):287–302. doi: 10.1016/j.immuni.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Truman L.A., Ford C.A., Pasikowska M., Pound J.D., Wilkinson S.J., Dumitriu I.E., Melville L., Melrose L.A., Ogden C.A., Nibbs R., Graham G., Combadiere C., Gregory C.D. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 10.Chekeni F.B., Elliott M.R., Sandilos J.K., Walk S.F., Kinchen J.M., Lazarowski E.R., Armstrong A.J., Penuela S., Laird D.W., Salvesen G.S., Isakson B.E., Bayliss D.A., Ravichandran K.S. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S., Owen K.A., Ly K.T., Park D., Black S.G., Wilson J.M., Sifri C.D., Ravichandran K.S., Ernst P.B., Casanova J.E. Brain angiogenesis inhibitor 1 (Bai1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc. Natl. Acad. Sci. U. S. A. 2011;108(5):2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyanishi M., Tada K., Koike M., Uchiyama Y., Kitamura T., Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 13.Park S.Y., Jung M.Y., Kim H.J., Lee S.J., Kim S.Y., Lee B.H., Kwon T.H., Park R.W., Kim I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15(1):192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 14.Yanagihashi Y., Segawa K., Maeda R., Nabeshima Y.I., Nagata S. Mouse macrophages show different requirements for phosphatidylserine receptor Tim4 in efferocytosis. Proc. Natl. Acad. Sci. U. S. A. 2017;114(33):8800–8805. doi: 10.1073/pnas.1705365114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park D., Tosello-Trampont A.C., Elliott M.R., Lu M., Haney L.B., Ma Z., Klibanov A.L., Mandell J.W., Ravichandran K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450(7168):430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 16.Tsai R.K., Discher D.E. Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 2008;180(5):989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frasch S.C., Bratton D.L. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog. Lipid Res. 2012;51(3):199–207. doi: 10.1016/j.plipres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasch S.C., Fernandez-Boyanapalli R.F., Berry K.Z., Leslie C.C., Bonventre J.V., Murphy R.C., Henson P.M., Bratton D.L. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J. Biol. Chem. 2011;286(14):12108–12122. doi: 10.1074/jbc.M110.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morioka S., Perry J.S.A., Raymond M.H., Medina C.B., Zhu Y., Zhao L., Serbulea V., Onengut-Gumuscu S., Leitinger N., Kucenas S., Rathmell J.C., Makowski L., Ravichandran K.S. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563(7733):714–718. doi: 10.1038/s41586-018-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCubbrey A.L., McManus S.A., McClendon J.D., Thomas S.M., Chatwin H.B., Reisz J.A., D'Alessandro A., Mould K.J., Bratton D.L., Henson P.M., Janssen W.J. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep. 2022;38(2) doi: 10.1016/j.celrep.2021.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia T.Y., Zolp A., Miska J. Polyamine Immunometabolism: central regulators of inflammation, cancer and autoimmunity. Cells. 2022;11(5) doi: 10.3390/cells11050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yurdagul A., Jr., Subramanian M., Wang X., Crown S.B., Ilkayeva O.R., Darville L., Kolluru G.K., Rymond C.C., Gerlach B.D., Zheng Z., Kuriakose G., Kevil C.G., Koomen J.M., Cleveland J.L., Muoio D.M., Tabas I. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metabol. 2020;31(3):518. doi: 10.1016/j.cmet.2020.01.001. 533.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yurdagul A., Jr., Kong N., Gerlach B.D., Wang X., Ampomah P., Kuriakose G., Tao W., Shi J., Tabas I. ODC (ornithine decarboxylase)-dependent putrescine synthesis maintains MerTK (MER tyrosine-protein kinase) expression to drive resolution. Arterioscler. Thromb. Vasc. Biol. 2021;41(3):e144–e159. doi: 10.1161/ATVBAHA.120.315622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ampomah P.B., Cai B., Sukka S.R., Gerlach B.D., Yurdagul A., Jr., Wang X., Kuriakose G., Darville L.N.F., Sun Y., Sidoli S., Koomen J.M., Tall A.R., Tabas I. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat. Metab. 2022;4(4):444–457. doi: 10.1038/s42255-022-00551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S., Weinberg S., DeBerge M., Gainullina A., Schipma M., Kinchen J.M., Ben-Sahra I., Gius D.R., Yvan-Charvet L., Chandel N.S., Schumacker P.T., Thorp E.B. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metabol. 2019;29(2):443. doi: 10.1016/j.cmet.2018.12.004. 456.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.N A.G., Bensinger S.J., Hong C., Beceiro S., Bradley M.N., Zelcer N., Deniz J., Ramirez C., Díaz M., Gallardo G., de Galarreta C.R., Salazar J., Lopez F., Edwards P., Parks J., Andujar M., Tontonoz P., Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schilperoort M., Ngai D., Katerelos M., Power D.A., Tabas I. PFKFB2-mediated glycolysis promotes lactate-driven continual efferocytosis by macrophages. Nat. Metab. 2023;5(3):431–444. doi: 10.1038/s42255-023-00736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Subramanian M., Yurdagul A., Jr., Barbosa-Lorenzi V.C., Cai B., de Juan-Sanz J., Ryan T.A., Nomura M., Maxfield F.R., Tabas I. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171(2):331. doi: 10.1016/j.cell.2017.08.041. 345.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schebb N.H., Kühn H., Kahnt A.S., Rund K.M., O'Donnell V.B., Flamand N., Peters-Golden M., Jakobsson P.J., Weylandt K.H., Rohwer N., Murphy R.C., Geisslinger G., FitzGerald G.A., Hanson J., Dahlgren C., Alnouri M.W., Offermanns S., Steinhilber D. Formation, signaling and occurrence of specialized pro-resolving lipid mediators-what is the evidence so far? Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.838782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snodgrass R.G., Benatzy Y., Schmid T., Namgaladze D., Mainka M., Schebb N.H., Lütjohann D., Brüne B. Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation. Cell Death Differ. 2021;28(4):1301–1316. doi: 10.1038/s41418-020-00652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalli J., Serhan C.N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rymut N., Heinz J., Sadhu S., Hosseini Z., Riley C.O., Marinello M., Maloney J., MacNamara K.C., Spite M., Fredman G. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. Faseb. J. 2020;34(1):597–609. doi: 10.1096/fj.201902126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerlach B.D., Marinello M., Heinz J., Rymut N., Sansbury B.E., Riley C.O., Sadhu S., Hosseini Z., Kojima Y., Tang D.D., Leeper N.J., Spite M., Barroso M., Rayner K.J., Fredman G. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27(2):525–539. doi: 10.1038/s41418-019-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gharib S.A., McMahan R.S., Eddy W.E., Long M.E., Parks W.C., Aitken M.L., Manicone A.M. Transcriptional and functional diversity of human macrophage repolarization. J. Allergy Clin. Immunol. 2019;143(4):1536–1548. doi: 10.1016/j.jaci.2018.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proto J.D., Doran A.C., Gusarova G., Yurdagul A., Jr., Sozen E., Subramanian M., Islam M.N., Rymond C.C., Du J., Hook J., Kuriakose G., Bhattacharya J., Tabas I. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018;49(4):666. doi: 10.1016/j.immuni.2018.07.015. 677.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma M., Schlegel M.P., Afonso M.S., Brown E.J., Rahman K., Weinstock A., Sansbury B.E., Corr E.M., van Solingen C., Koelwyn G.J., Shanley L.C., Beckett L., Peled D., Lafaille J.J., Spite M., Loke P., Fisher E.A., Moore K.J. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ. Res. 2020;127(3):335–353. doi: 10.1161/CIRCRESAHA.119.316461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K., Ohkura N. Regulatory T cells and human disease. Annu. Rev. Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 39.Scheinecker C., Göschl L., Bonelli M. Treg cells in health and autoimmune diseases: new insights from single cell analysis. J. Autoimmun. 2020;110 doi: 10.1016/j.jaut.2019.102376. [DOI] [PubMed] [Google Scholar]
- 40.Dominguez-Villar M., Hafler D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018;19(7):665–673. doi: 10.1038/s41590-018-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolbinger A., Schäufele T.J., Steigerwald H., Friedel J., Pierre S., Geisslinger G., Scholich K. Eosinophil-derived IL-4 is necessary to establish the inflammatory structure in innate inflammation. EMBO Mol. Med. 2022 doi: 10.15252/emmm.202216796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellefigues C., Naidoo K., Mehta P., Schmidt A.J., Jagot F., Roussel E., Cait A., Yumnam B., Chappell S., Meijlink K., Camberis M., Jiang J.X., Painter G., Filbey K., Uluçkan Ö., Gasser O., Le Gros G. Basophils promote barrier dysfunction and resolution in the atopic skin. J. Allergy Clin. Immunol. 2021;148(3):799. doi: 10.1016/j.jaci.2021.02.018. 812.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nepal S., Tiruppathi C., Tsukasaki Y., Farahany J., Mittal M., Rehman J., Prockop D.J., Malik A.B. STAT6 induces expression of Gas6 in macrophages to clear apoptotic neutrophils and resolve inflammation. Proc. Natl. Acad. Sci. U. S. A. 2019;116(33):16513–16518. doi: 10.1073/pnas.1821601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosurgi L., Cao Y.G., Cabeza-Cabrerizo M., Tucci A., Hughes L.D., Kong Y., Weinstein J.S., Licona-Limon P., Schmid E.T., Pelorosso F., Gagliani N., Craft J.E., Flavell R.A., Ghosh S., Rothlin C.V. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342):1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 46.Del Sordo L., Blackler G.B., Philpott H.T., Riviere J., Gunaratnam L., Heit B., Appleton C.T. Impaired efferocytosis by synovial macrophages in patients with knee osteoarthritis. Arthritis Rheumatol. 2022;75(5):685–696. doi: 10.1002/art.42412. [DOI] [PubMed] [Google Scholar]
- 47.Nagata S. Rheumatoid polyarthritis caused by a defect in DNA degradation. Cytokine Growth Factor Rev. 2008;19(3–4):295–302. doi: 10.1016/j.cytogfr.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Ishii S., Isozaki T., Furuya H., Takeuchi H., Tsubokura Y., Inagaki K., Kasama T. ADAM-17 is expressed on rheumatoid arthritis fibroblast-like synoviocytes and regulates proinflammatory mediator expression and monocyte adhesion. Arthritis Res. Ther. 2018;20(1):159. doi: 10.1186/s13075-018-1657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charbonneau M., Harper K., Grondin F., Pelmus M., McDonald P.P., Dubois C.M. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor alpha-induced increases in tumor necrosis factor-alpha converting enzyme/ADAM17 expression by synovial cells. J. Biol. Chem. 2007;282(46):33714–33724. doi: 10.1074/jbc.M704041200. [DOI] [PubMed] [Google Scholar]
- 50.Vullings J., Vago J.P., Waterborg C.E.J., Thurlings R.M., Koenders M.I., van Lent P., van der Kraan P.M., Amaral F.A., van de Loo F.A.J. Selective increment of synovial soluble TYRO3 correlates with disease severity and joint inflammation in patients with rheumatoid arthritis. J Immunol Res. 2020;2020 doi: 10.1155/2020/9690832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L., Hu F., Zhu H., Liu X., Shi L., Li Y., Zhong H., Su Y. Soluble TAM receptor tyrosine kinases in rheumatoid arthritis: correlation with disease activity and bone destruction. Clin. Exp. Immunol. 2018;192(1):95–103. doi: 10.1111/cei.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai B., Thorp E.B., Doran A.C., Subramanian M., Sansbury B.E., Lin C.S., Spite M., Fredman G., Tabas I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. U. S. A. 2016;113(23):6526–6531. doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sather S., Kenyon K.D., Lefkowitz J.B., Liang X., Varnum B.C., Henson P.M., Graham D.K. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109(3):1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsou W.I., Nguyen K.Q., Calarese D.A., Garforth S.J., Antes A.L., Smirnov S.V., Almo S.C., Birge R.B., Kotenko S.V. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J. Biol. Chem. 2014;289(37):25750–25763. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waterborg C.E.J., Koenders M.I., van Lent P., van der Kraan P.M., van de Loo F.A.J. Tyro3/Axl/Mertk-deficient mice develop bone marrow edema which is an early pathological marker in rheumatoid arthritis. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waterborg C.E.J., Beermann S., Broeren M.G.A., Bennink M.B., Koenders M.I., van Lent P., van den Berg W.B., van der Kraan P.M., van de Loo F.A.J. Protective role of the MER tyrosine kinase via efferocytosis in rheumatoid arthritis models. Front. Immunol. 2018;9:742. doi: 10.3389/fimmu.2018.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahlbäck B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb. Haemostasis. 1991;66(1):49–61. [PubMed] [Google Scholar]
- 58.Lee C.H., Chun T. Anti-inflammatory role of TAM family of receptor tyrosine kinases via modulating macrophage function. Mol. Cell. 2019;42(1):1–7. doi: 10.14348/molcells.2018.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Najafov A., Mookhtiar A.K., Luu H.S., Ordureau A., Pan H., Amin P.P., Li Y., Lu Q., Yuan J. TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol. Cell. 2019;75(3):457. doi: 10.1016/j.molcel.2019.05.022. 468.e4. [DOI] [PubMed] [Google Scholar]
- 60.Bonnefoy F., Gauthier T., Vallion R., Martin-Rodriguez O., Missey A., Daoui A., Valmary-Degano S., Saas P., Couturier M., Perruche S. Factors produced by macrophages eliminating apoptotic cells demonstrate pro-resolutive properties and terminate ongoing inflammation. Front. Immunol. 2018;9:2586. doi: 10.3389/fimmu.2018.02586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medina C.B., Mehrotra P., Arandjelovic S., Perry J.S.A., Guo Y., Morioka S., Barron B., Walk S.F., Ghesquière B., Krupnick A.S., Lorenz U., Ravichandran K.S. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580(7801):130–135. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnefoy F., Daoui A., Valmary-Degano S., Toussirot E., Saas P., Perruche S. Apoptotic cell infusion treats ongoing collagen-induced arthritis, even in the presence of methotrexate, and is synergic with anti-TNF therapy. Arthritis Res. Ther. 2016;18(1):184. doi: 10.1186/s13075-016-1084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray M., Miles K., Salter D., Gray D., Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104(35):14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flak M.B., Colas R.A., Muñoz-Atienza E., Curtis M.A., Dalli J., Pitzalis C. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight. 2019;4(13) doi: 10.1172/jci.insight.125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flak M.B., Koenis D.S., Sobrino A., Smith J., Pistorius K., Palmas F., Dalli J. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J. Clin. Invest. 2020;130(1):359–373. doi: 10.1172/JCI131609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flak M.B., Koenis D.S., Gonzalez-Nunez M., Chopo-Pizarro A., Dalli J. Deletion of macrophage Gpr101 disrupts their phenotype and function dysregulating host immune responses in sterile and infectious inflammation. Biochem. Pharmacol. 2023;207 doi: 10.1016/j.bcp.2022.115348. [DOI] [PubMed] [Google Scholar]
- 67.Galvão I., Melo E.M., de Oliveira V.L.S., Vago J.P., Queiroz-Junior C., de Gaetano M., Brennan E., Gahan K., Guiry P.J., Godson C., Teixeira M.M. Therapeutic potential of the FPR2/ALX agonist AT-01-KG in the resolution of articular inflammation. Pharmacol. Res. 2021;165 doi: 10.1016/j.phrs.2021.105445. [DOI] [PubMed] [Google Scholar]
- 68.Felix F.B., Vago J.P., Fernandes D.O., Martins D.G., Moreira I.Z., Gonçalves W.A., Costa W.C., Araújo J.M.D., Queiroz-Junior C.M., Campolina-Silva G.H., Soriani F.M., Sousa L.P., Grespan R., Teixeira M.M., Pinho V. Biochanin A regulates key steps of inflammation resolution in a model of antigen-induced arthritis via GPR30/PKA-dependent mechanism. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barroso L.C., Magalhaes G.S., Galvão I., Reis A.C., Souza D.G., Sousa L.P., Santos R.A.S., Campagnole-Santos M.J., Pinho V., Teixeira M.M. Angiotensin-(1-7) promotes resolution of neutrophilic inflammation in a model of antigen-induced arthritis in mice. Front. Immunol. 2017;8:1596. doi: 10.3389/fimmu.2017.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garrido-Mesa J., Thomas B.L., Dodd J., Spana C., Perretti M., Montero-Melendez T. Pro-resolving and anti-arthritic properties of the MC(1) selective agonist PL8177. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1078678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouyang J., Zhang B., Kuang L., Yang P., Du X., Qi H., Su N., Jin M., Yang J., Xie Y., Tan Q., Chen H., Chen S., Jiang W., Liu M., Luo X., He M., Ni Z., Chen L. Pulsed electromagnetic field inhibits synovitis via enhancing the efferocytosis of macrophages. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/4307385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muñoz L.E., Janko C., Schulze C., Schorn C., Sarter K., Schett G., Herrmann M. Autoimmunity and chronic inflammation - two clearance-related steps in the etiopathogenesis of SLE. Autoimmun. Rev. 2010;10(1):38–42. doi: 10.1016/j.autrev.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Herrmann M., Voll R.E., Zoller O.M., Hagenhofer M., Ponner B.B., Kalden J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41(7):1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 74.Trendelenburg M. Autoantibodies against complement component C1q in systemic lupus erythematosus. Clin Transl Immunology. 2021;10(4) doi: 10.1002/cti2.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19(1):56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 76.Bijl M., Reefman E., Horst G., Limburg P.C., Kallenberg C.G. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Ann. Rheum. Dis. 2006;65(1):57–63. doi: 10.1136/ard.2005.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pickering M.C., Botto M. Are anti-C1q antibodies different from other SLE autoantibodies? Nat. Rev. Rheumatol. 2010;6(8):490–493. doi: 10.1038/nrrheum.2010.56. [DOI] [PubMed] [Google Scholar]
- 78.Le L.Q., Kabarowski J.H., Weng Z., Satterthwaite A.B., Harvill E.T., Jensen E.R., Miller J.F., Witte O.N. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity. 2001;14(5):561–571. doi: 10.1016/s1074-7613(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 79.Cohen P.L., Caricchio R., Abraham V., Camenisch T.D., Jennette J.C., Roubey R.A., Earp H.S., Matsushima G., Reap E.A. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 2002;196(1):135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witas R., Rasmussen A., Scofield R.H., Radfar L., Stone D.U., Grundahl K., Lewis D., Sivils K.L., Lessard C.J., Farris A.D., Nguyen C.Q. Defective efferocytosis in a murine model of sjögren's syndrome is mediated by dysfunctional mer tyrosine kinase receptor. Int. J. Mol. Sci. 2021;22(18) doi: 10.3390/ijms22189711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawano M., Nagata S. Lupus-like autoimmune disease caused by a lack of Xkr8, a caspase-dependent phospholipid scramblase. Proc. Natl. Acad. Sci. U. S. A. 2018;115(9):2132–2137. doi: 10.1073/pnas.1720732115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 83.Kawane K., Tanaka H., Kitahara Y., Shimaoka S., Nagata S. Cytokine-dependent but acquired immunity-independent arthritis caused by DNA escaped from degradation. Proc. Natl. Acad. Sci. U. S. A. 2010;107(45):19432–19437. doi: 10.1073/pnas.1010603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roszer T., Menéndez-Gutiérrez M.P., Lefterova M.I., Alameda D., Núñez V., Lazar M.A., Fischer T., Ricote M. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J. Immunol. 2011;186(1):621–631. doi: 10.4049/jimmunol.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orme J.J., Du Y., Vanarsa K., Mayeux J., Li L., Mutwally A., Arriens C., Min S., Hutcheson J., Davis L.S., Chong B.F., Satterthwaite A.B., Wu T., Mohan C. Heightened cleavage of Axl receptor tyrosine kinase by ADAM metalloproteases may contribute to disease pathogenesis in SLE. Clin. Immunol. 2016;169:58–68. doi: 10.1016/j.clim.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jorge A.M., Lao T., Kim R., Licciardi S., El Khoury J., Luster A.D., Means T.K., Ramirez-Ortiz Z.G. SCARF1-Induced efferocytosis plays an immunomodulatory role in humans, and autoantibodies targeting SCARF1 are produced in patients with systemic lupus erythematosus. J. Immunol. 2022;208(4):955–967. doi: 10.4049/jimmunol.2100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang M., Johnson-Stephenson T.K., Wang W., Wang Y., Li J., Li L., Zen K., Chen X., Zhu D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res. Ther. 2022;13(1):484. doi: 10.1186/s13287-022-03174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu N., Li J., Gao Y., Zhou N., Ma Q., Wu M., Zhang Y., Sun X., Xie J., Shen G., Yang M., Tu Q., Xu X., Zhu J., Tao J. Apoptotic cell-mimicking gold nanocages loaded with LXR agonist for attenuating the progression of murine systemic lupus erythematosus. Biomaterials. 2019;197:380–392. doi: 10.1016/j.biomaterials.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 89.Morioka S., Kajioka D., Yamaoka Y., Ellison R.M., Tufan T., Werkman I.L., Tanaka S., Barron B., Ito S.T., Kucenas S., Okusa M.D., Ravichandran K.S. Chimeric efferocytic receptors improve apoptotic cell clearance and alleviate inflammation. Cell. 2022;185(26):4887. doi: 10.1016/j.cell.2022.11.029. 4903.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grau A., Tabib A., Grau I., Reiner I., Mevorach D. Apoptotic cells induce NF-κB and inflammasome negative signaling. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin-Rodriguez O., Gauthier T., Bonnefoy F., Couturier M., Daoui A., Chagué C., Valmary-Degano S., Gay C., Saas P., Perruche S. Pro-resolving factors released by macrophages after efferocytosis promote mucosal wound healing in inflammatory bowel disease. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.754475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trilleaud C., Gauttier V., Biteau K., Girault I., Belarif L., Mary C., Pengam S., Teppaz G., Thepenier V., Danger R., Robert-Siegwald G., Néel M., Bruneau S., Glémain A., Néel A., Poupon A., Mosnier J.F., Chêne G., Dubourdeau M., Blancho G., Vanhove B., Poirier N. Agonist anti-ChemR23 mAb reduces tissue neutrophil accumulation and triggers chronic inflammation resolution. Sci. Adv. 2021;7(14) doi: 10.1126/sciadv.abd1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li C., Zhao Y., Cheng J., Guo J., Zhang Q., Zhang X., Ren J., Wang F., Huang J., Hu H., Wang R., Zhang J. A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv. Sci. 2019;6(18) doi: 10.1002/advs.201900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marin-Gallen S., Clemente-Casares X., Planas R., Pujol-Autonell I., Carrascal J., Carrillo J., Ampudia R., Verdaguer J., Pujol-Borrell R., Borràs F.E., Vives-Pi M. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin. Exp. Immunol. 2010;160(2):207–214. doi: 10.1111/j.1365-2249.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Das A., Ghatak S., Sinha M., Chaffee S., Ahmed N.S., Parinandi N.L., Wohleb E.S., Sheridan J.F., Sen C.K., Roy S. Correction of MFG-E8 resolves inflammation and promotes cutaneous wound healing in diabetes. J. Immunol. 2016;196(12):5089–5100. doi: 10.4049/jimmunol.1502270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodriguez-Fernandez S., Pujol-Autonell I., Brianso F., Perna-Barrull D., Cano-Sarabia M., Garcia-Jimeno S., Villalba A., Sanchez A., Aguilera E., Vazquez F., Verdaguer J., Maspoch D., Vives-Pi M. Phosphatidylserine-liposomes promote tolerogenic features on dendritic cells in human type 1 diabetes by apoptotic mimicry. Front. Immunol. 2018;9:253. doi: 10.3389/fimmu.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujiwara C., Uehara A., Sekiguchi A., Uchiyama A., Yamazaki S., Ogino S., Yokoyama Y., Torii R., Hosoi M., Suto C., Tsunekawa K., Murakami M., Ishikawa O., Motegi S.I. Suppressive regulation by MFG-E8 of latent transforming growth factor β-induced fibrosis via binding to αv integrin: significance in the pathogenesis of fibrosis in systemic sclerosis. Arthritis Rheumatol. 2019;71(2):302–314. doi: 10.1002/art.40701. [DOI] [PubMed] [Google Scholar]
- 98.Lescoat A., Ballerie A., Lelong M., Augagneur Y., Morzadec C., Jouneau S., Jégo P., Fardel O., Vernhet L., Lecureur V. Crystalline Silica impairs efferocytosis abilities of human and mouse macrophages: implication for silica-associated systemic sclerosis. Front. Immunol. 2020;11:219. doi: 10.3389/fimmu.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bellamri N., Lelong M., Joannes A., Le Tallec E., Jouneau S., Vernhet L., Lescoat A., Lecureur V. Effects of Ruxolitinib on fibrosis in preclinical models of systemic sclerosis. Int. Immunopharm. 2023;116 doi: 10.1016/j.intimp.2023.109723. [DOI] [PubMed] [Google Scholar]
- 100.Grossmayer G.E., Keppeler H., Boeltz S., Janko C., Rech J., Herrmann M., Lauber K., Muñoz L.E. Elevated serum lysophosphatidylcholine in patients with systemic lupus erythematosus impairs phagocytosis of necrotic cells in vitro. Front. Immunol. 2017;8:1876. doi: 10.3389/fimmu.2017.01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sawada T., Kurano M., Shirai H., Iwasaki Y., Tahara K., Hayashi H., Igarashi K., Fujio K., Aoki J., Yatomi Y. Serum phosphatidylserine-specific phospholipase A(1) as a novel biomarker for monitoring systemic lupus erythematosus disease activity. Int J Rheum Dis. 2019;22(11):2059–2066. doi: 10.1111/1756-185X.13689. [DOI] [PubMed] [Google Scholar]
- 102.Jabbari A., Suárez-Fariñas M., Fuentes-Duculan J., Gonzalez J., Cueto I., Franks A.G., Jr., Krueger J.G. Dominant Th1 and minimal Th17 skewing in discoid lupus revealed by transcriptomic comparison with psoriasis. J. Invest. Dermatol. 2014;134(1):87–95. doi: 10.1038/jid.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aoki J., Nagai Y., Hosono H., Inoue K., Arai H. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim. Biophys. Acta. 2002;1582(1–3):26–32. doi: 10.1016/s1388-1981(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Z., Xu A., Teng J., Wang F., Tan Y., Liu H., Cheng X., Su Y., Shi H., Hu Q., Chi H., Li J., Hou J., Sun Y., Yang C., Ye J. Anti-Tyro3 IgG associates with disease activity and reduces efferocytosis of macrophages in new-onset systemic lupus erythematosus. J Immunol Res. 2020;2020 doi: 10.1155/2020/2180708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Recarte-Pelz P., Tàssies D., Espinosa G., Hurtado B., Sala N., Cervera R., Reverter J.C., de Frutos P.G. Vitamin K-dependent proteins GAS6 and Protein S and TAM receptors in patients of systemic lupus erythematosus: correlation with common genetic variants and disease activity. Arthritis Res. Ther. 2013;15(2):R41. doi: 10.1186/ar4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liphaus B.L., Lima L., Palmeira P., Silva C.A., Goldenstein-Schainberg C., Carneiro-Sampaio M. Increased sMer, but not sAxl, sTyro3, and Gas6 relate with active disease in juvenile systemic lupus erythematosus. Clin. Rheumatol. 2020;39(2):509–514. doi: 10.1007/s10067-019-04799-5. [DOI] [PubMed] [Google Scholar]
- 107.Bellan M., Quaglia M., Nerviani A., Mauro D., Lewis M., Goegan F., Gibbin A., Pagani S., Salmi L., Molinari L., Castello L.M., Avanzi G.C., Cantaluppi V., Pirisi M., Sainaghi P.P., Pitzalis C. Increased plasma levels of Gas6 and its soluble tyrosine kinase receptors Mer and Axl are associated with immunological activity and severity of lupus nephritis. Clin. Exp. Rheumatol. 2021;39(1):132–138. doi: 10.55563/clinexprheumatol/xyylza. [DOI] [PubMed] [Google Scholar]
- 108.Lee H.N., Tian L., Bouladoux N., Davis J., Quinones M., Belkaid Y., Coligan J.E., Krzewski K. Dendritic cells expressing immunoreceptor CD300f are critical for controlling chronic gut inflammation. J. Clin. Invest. 2017;127(5):1905–1917. doi: 10.1172/JCI89531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee C.S., Penberthy K.K., Wheeler K.M., Juncadella I.J., Vandenabeele P., Lysiak J.J., Ravichandran K.S. Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity. 2016;44(4):807–820. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong L., Robinson C.P., Peck A.B., Vela-Roch N., Sakata K.M., Dang H., Talal N., Humphreys-Beher M.G. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin. Exp. Rheumatol. 1998;16(6):675–681. [PubMed] [Google Scholar]
- 111.Humphreys-Beher M.G., Peck A.B., Dang H., Talal N. The role of apoptosis in the initiation of the autoimmune response in Sjögren's syndrome. Clin. Exp. Immunol. 1999;116(3):383–387. doi: 10.1046/j.1365-2249.1999.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manoussakis M.N., Fragoulis G.E., Vakrakou A.G., Moutsopoulos H.M. Impaired clearance of early apoptotic cells mediated by inhibitory IgG antibodies in patients with primary Sjögren's syndrome. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Witas R., Shen Y., Nguyen C.Q. Bone marrow-derived macrophages from a murine model of Sjögren's syndrome demonstrate an aberrant, inflammatory response to apoptotic cells. Sci. Rep. 2022;12(1):8593. doi: 10.1038/s41598-022-12608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O'Brien B.A., Geng X., Orteu C.H., Huang Y., Ghoreishi M., Zhang Y., Bush J.A., Li G., Finegood D.T., Dutz J.P. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J. Autoimmun. 2006;26(2):104–115. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 115.O'Brien B.A., Huang Y., Geng X., Dutz J.P., Finegood D.T. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51(8):2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- 116.Khanna S., Biswas S., Shang Y., Collard E., Azad A., Kauh C., Bhasker V., Gordillo G.M., Sen C.K., Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pujol-Autonell I., Ampudia R.M., Planas R., Marin-Gallen S., Carrascal J., Sanchez A., Marin A., Puig-Domingo M., Pujol-Borrell R., Verdaguer J., Vives-Pi M. Efferocytosis promotes suppressive effects on dendritic cells through prostaglandin E2 production in the context of autoimmunity. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ballerie A., Lescoat A., Augagneur Y., Lelong M., Morzadec C., Cazalets C., Jouneau S., Fardel O., Vernhet L., Jégo P., Lecureur V. Efferocytosis capacities of blood monocyte-derived macrophages in systemic sclerosis. Immunol. Cell Biol. 2019;97(3):340–347. doi: 10.1111/imcb.12217. [DOI] [PubMed] [Google Scholar]
- 119.Raschi E., Chighizola C.B., Cesana L., Privitera D., Ingegnoli F., Mastaglio C., Meroni P.L., Borghi M.O. Immune complexes containing scleroderma-specific autoantibodies induce a profibrotic and proinflammatory phenotype in skin fibroblasts. Arthritis Res. Ther. 2018;20(1):187. doi: 10.1186/s13075-018-1689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brieske C., Lamprecht P., Kerstein-Staehle A. Immunogenic cell death as driver of autoimmunity in granulomatosis with polyangiitis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henderson S.R., Horsley H., Frankel P., Khosravi M., Goble T., Carter S., Antonelou M., Evans R.D.R., Zhang X., Chu T.Y., Lin H.H., Gordon S., Salama A.D., Proteinase 3 promotes formation of multinucleated giant cells and granuloma-like structures in patients with granulomatosis with polyangiitis, Ann. Rheum. Dis. (2023)82:848–856. [DOI] [PMC free article] [PubMed]
- 122.Kantari C., Pederzoli-Ribeil M., Amir-Moazami O., Gausson-Dorey V., Moura I.C., Lecomte M.C., Benhamou M., Witko-Sarsat V. Proteinase 3, the Wegener autoantigen, is externalized during neutrophil apoptosis: evidence for a functional association with phospholipid scramblase 1 and interference with macrophage phagocytosis. Blood. 2007;110(12):4086–4095. doi: 10.1182/blood-2007-03-080457. [DOI] [PubMed] [Google Scholar]
- 123.Surmiak M., Hubalewska-Mazgaj M., Wawrzycka-Adamczyk K., Musiał J., Sanak M. Delayed neutrophil apoptosis in granulomatosis with polyangiitis: dysregulation of neutrophil gene signature and circulating apoptosis-related proteins. Scand. J. Rheumatol. 2020;49(1):57–67. doi: 10.1080/03009742.2019.1634219. [DOI] [PubMed] [Google Scholar]
- 124.Gabillet J., Millet A., Pederzoli-Ribeil M., Tacnet-Delorme P., Guillevin L., Mouthon L., Frachet P., Witko-Sarsat V. Proteinase 3, the autoantigen in granulomatosis with polyangiitis, associates with calreticulin on apoptotic neutrophils, impairs macrophage phagocytosis, and promotes inflammation. J. Immunol. 2012;189(5):2574–2583. doi: 10.4049/jimmunol.1200600. [DOI] [PubMed] [Google Scholar]
- 125.Millet A., Martin K.R., Bonnefoy F., Saas P., Mocek J., Alkan M., Terrier B., Kerstein A., Tamassia N., Satyanarayanan S.K., Ariel A., Ribeil J.A., Guillevin L., Cassatella M.A., Mueller A., Thieblemont N., Lamprecht P., Mouthon L., Perruche S., Witko-Sarsat V. Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J. Clin. Invest. 2015;125(11):4107–4121. doi: 10.1172/JCI78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tacnet-Delorme P., Gabillet J., Chatfield S., Thieblemont N., Frachet P., Witko-Sarsat V. Proteinase 3 interferes with C1q-mediated clearance of apoptotic cells. Front. Immunol. 2018;9:818. doi: 10.3389/fimmu.2018.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burnstock G., Krügel U., Abbracchio M.P., Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog. Neurobiol. 2011;95(2):229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 128.Lovászi M., Branco Haas C., Antonioli L., Pacher P., Haskó G. The role of P2Y receptors in regulating immunity and metabolism. Biochem. Pharmacol. 2021;187 doi: 10.1016/j.bcp.2021.114419. [DOI] [PubMed] [Google Scholar]
- 129.Zhou Y., Fei M., Zhang G., Liang W.C., Lin W., Wu Y., Piskol R., Ridgway J., McNamara E., Huang H., Zhang J., Oh J., Patel J.M., Jakubiak D., Lau J., Blackwood B., Bravo D.D., Shi Y., Wang J., Hu H.M., Lee W.P., Jesudason R., Sangaraju D., Modrusan Z., Anderson K.R., Warming S., Roose-Girma M., Yan M. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity. 2020;52(2):357. doi: 10.1016/j.immuni.2020.01.014. 373.e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.