Abstract
Engaging in contemplative practice like meditation, yoga, and prayer, is beneficial for psychological and physical well-being. Recent research has identified several underlying psychological and biological pathways that explain these benefits. However, there is not yet consensus on the underlying overlapping physiological mechanisms of contemplative practice benefits. In this article, we integrate divergent scientific literatures on contemplative practice interventions, stress science, and mitochondrial biology, presenting a unified biopsychosocial model of how contemplative practices reduce stress and promote physical health. We argue that engaging in contemplative practice facilitates a restorative state termed “deep rest,” largely through safety signaling, during which energetic resources are directed toward cellular optimization and away from energy-demanding stress states. Our model thus presents a framework for how contemplative practices enhance positive psychological and physiological functioning by optimizing cellular energy consumption.
Keywords: stress, safety signals, resilience, mitochondria, restoration
Stress levels are continuing to rise across the United States (American Psychological Association, 2020). In search of approaches to ameliorate this distress, contemplative practices are being studied as ways to help people better manage distressing emotions and increase well-being (Fricchione, 2023; Van Gordon et al., 2022). Contemplative practices are mind–body exercises that are intentionally practiced to work toward inner well-being, psychological flourishing, and deep connection with self, the world, or a higher power (Davidson & Dahl, 2017). These practices take on many forms, including prayer, visualization, chanting, and meditation, as well as movement centered practices such as yoga, Tai Chi, and Qigong. What unites the various types of contemplative practices is that they alter habitual cognitive processes (e.g., reduced attention to negative stimuli, decreased perseverative cognition) and emotional processes (e.g., increased positive emotions) in ways that enhance well-being.
Research shows that despite the different techniques utilized in these various types of practices, they frequently confer similar beneficial impacts on mental and physical health. Randomized controlled trials provide evidence that contemplative practices improve a wide range of psychosocial and health outcomes with small-to-medium effect sizes, despite differences in the training content and format (Black & Slavich, 2016; Bower & Irwin, 2016; Büssing et al., 2012; Chiesa et al., 2011; Donald et al., 2019; Fredrickson et al., 2019; Goldberg et al., 2018; Hilton et al., 2017; Kemeny et al., 2012; Kuyken et al., 2015; Le Nguyen et al., 2019; Ospina et al., 2007). Reviews and meta-analyses of randomized controlled trials of the most commonly researched contemplative practice, mindfulness-based training programs, conclude that these practices decrease distressing emotions, depressive symptoms, and perceived stress, as well as increase positive psychological and physical well-being markers (Chiesa & Serretti, 2009; Goyal et al., 2014; Janssen et al., 2018; Parsons et al., 2017; Xu et al., 2022).
Because of these positive results, recent research has focused on understanding the biological pathways linking contemplative practices to improved health (e.g., Conklin et al., 2019; Househam et al., 2017; Kaliman, 2019; Pascoe et al., 2021; Poli et al., 2021; Reive, 2019; Venditti et al., 2020). Randomized controlled trials suggest that contemplative practices can improve physical health by lowering basal blood pressure (Pascoe et al., 2017), lowering systemic inflammation (Black & Slavich, 2016; Bower et al., 2014; Morgan et al., 2014), decreasing energy expenditure or improving metabolic efficiency (Tyagi & Cohen, 2013), and improving aging-related biomarkers such as telomere length, telomerase, and insulin-like growth factor-1 (Conklin et al., 2019; Gallegos et al., 2013; Schutte et al., 2020). However, not all studies have found or replicated beneficial effects of contemplative practices on biomarkers of health (Black & Slavich, 2016; Bower & Irwin, 2016). A closer look at existing studies suggests that the impact of contemplative practices is moderated by individual difference factors, such as frequency of practice, and specific personality traits, like conscientiousness (de Vibe et al., 2015; Puhlmann et al., 2019).
There are several core scientific challenges that have thus far limited the ability to create a cohesive cumulative science of contemplative practice and well-being. One of the challenges of research on contemplative practices is that there are a wide range of techniques used that vary in content, style, structure, length, cultural, and sometimes religious underpinning. Thus, much of the field has been siloed into research groups studying particular practices. Adding to the complexity is the variety of outcomes, and more recently mechanisms, that are being examined. To move the field past these barriers, there is a need for models and theories that describe the core underlying components of the practices, as well as the proposed biologically plausible mediators that lead to increased well-being. Such a model would advance measurement and study design by proposing core mechanisms of action that researchers can test in an experimental medicine approach, as recommended by the National Institutes of Health (Aklin et al., 2020; Michie et al., 2018; Onken et al., 2014).
In this article, we answer this need by offering a unifying framework to describe the health benefits of contemplative practices. We link theoretical components of the ways in which contemplative practices are designed and practiced, to the activation of autonomic and cellular-level processes associated with health. We posit that contemplative practices exert their salutary health effects by communicating to the body and mind a sense of safety (safety signaling), which allows a physiological shift away from threat states. We hypothesize that reduced energy expenditure resulting from decreased threat arousal activity is the crux of the beneficial impact of contemplative practices on health. We suggest that contemplative practices facilitate a psychological and physiological state we term “deep rest,” which is characterized by parasympathetic dominance, and encourages cellular restoration. The components of our model and their interactions are shown visually in Figure 1.
Figure 1.
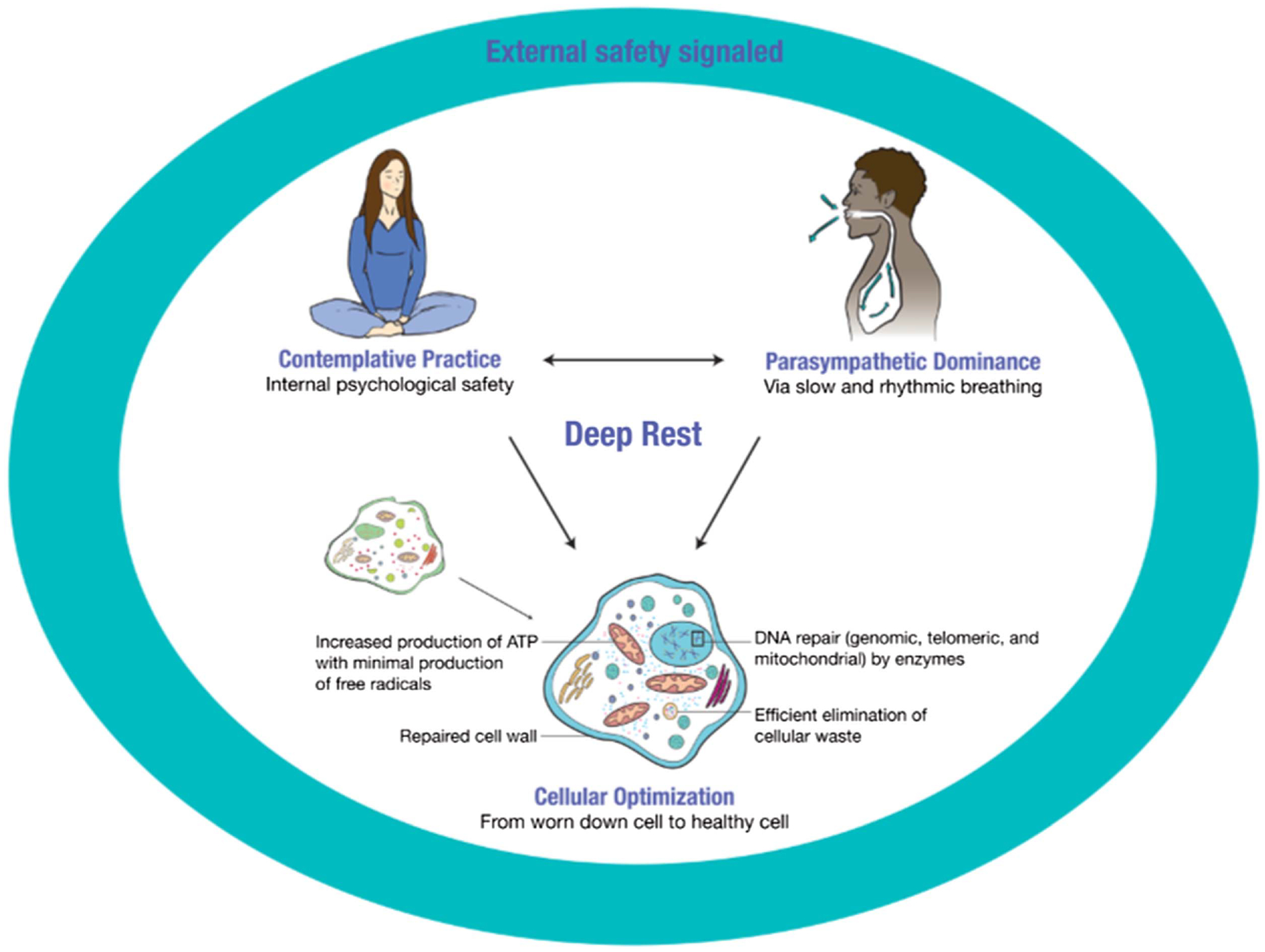
Visual Representation of the State of Deep Rest
Note. The deep rest state necessitates first that external safety is signaled from the physical and social environment. When this need is met, then a person may more easily enter a contemplative practice. During the practice, the mind’s threat-inducing thoughts are quieted, creating a sense of safety, peace, calm, and contentment, a state we label psychological safety. In a reciprocal relationship, the state of psychological safety and the contemplative practices themselves initiate a parasympathetically dominant nervous system state via slowed and rhythmic breathing rate. When safety is perceived at the physical, social, and psychological levels, deep rest follow. Energetic resources shift away from maintaining a threat state of high sympathetic nervous system activity, toward restorative activities within cells, enabling cellular optimization processes. Thus, when the mind and body are in this state of deep rest, cellular optimization ensues. ATP = adenosine triphosphate. See the online article for the color version of this figure.
This article is divided into five sections. Section 1 describes stress physiology, arguing that most of the daily lives of adults living in Western countries are spent in the energetically costly allostatic state of moderate threat arousal. Section 2 describes the concept of signals of safety that allow the body to shift away from high threat arousal during, and as a result of, contemplative practice. Section 3 reviews evidence that contemplative practices directly alter autonomic functioning. Section 4 describes the process by which contemplative practices lead to cellular optimization. Section 5 introduces sleep as the ultimate state of deep rest and highlights how sleep science might inform our understanding of contemplative practice. The Appendix describes testable hypotheses of the model.
Section 1: Stress Processes in Daily Life
Daily physical and psychosocial stressors are primarily managed by the autonomic nervous system. The nervous system’s primary job is to respond to signals from the internal and external environment—appropriately allocating bodily resources to meet the current context. This process is partially described through the concept of predictive regulation—the unconscious process of perceiving environmental demands expected in the imminent future (Sterling, 2004). As the nervous system attunes itself to contextual signals from the environment, it toggles between physiological (allostatic) states of activation throughout the day in a dynamic and highly responsive manner. This process of shifting between physiological states is called allostasis, in which predictive regulation of bodily functions works to meet present and future demands in the most efficient and rewarding way (McEwen & Stellar, 1993; Schulkin & Sterling, 2019; Sterling, 2004). Summarizing from current psychophysiological research, we surmise that during the waking day, healthy functioning means toggling between arousal states of acute stress (e.g., argument with a spouse), moderate threat (e.g., working, caregiving), rest states (e.g., watching TV), and deep rest (e.g., in contemplative states or mind body practices), differentiated by the balance between sympathetic and parasympathetic nervous system activation (see Figure 2). The fourth state, deep rest, is currently the most understudied state. In this article, we primarily focus on the states of moderate threat arousal and deep rest, given their relevance for our model.
Figure 2.
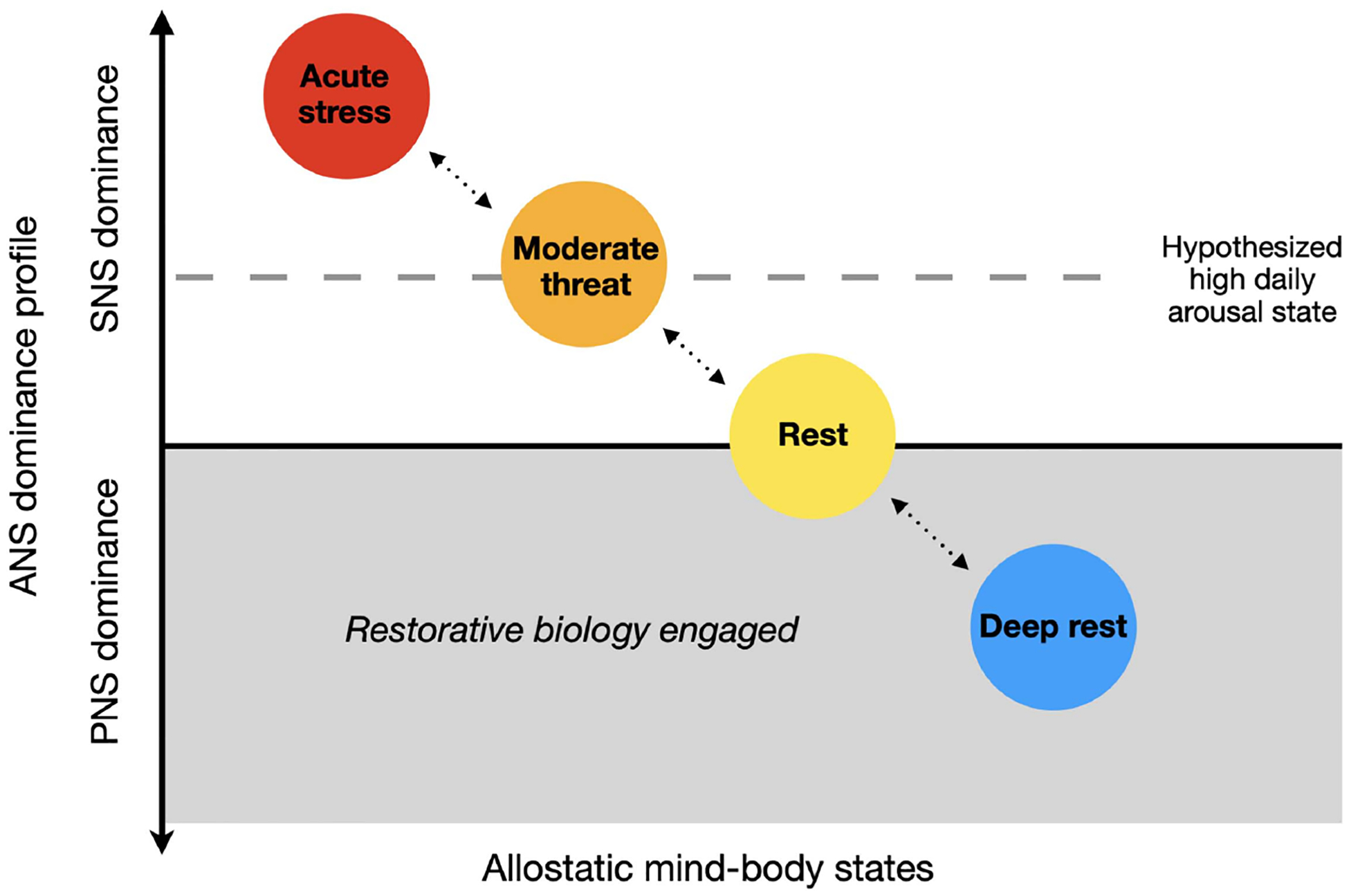
Model of Allostatic Mind Body States
Note. Specific physiological states are plotted on a Y-axis of autonomic nervous system (ANS) dominance, where equal dominance is set at 0 (black line) on the Y-axis. Sympathetic nervous system (SNS) dominant states are represented with circles placed above the X-axis and parasympathetic nervous system (PNS) dominant states are represented with circles below the axis. The X-axis represents the degree to which restorative biological processes are engaged, with allostatic states further to the right indicating greater restoration. Acute stress is shown as the high SNS dominant state (red circle). The moderate threat (orange circle) is SNS dominant though less so than acute stress (orange circle). Rest is when SNS and PNS activity are equally balanced (yellow circle). Deep rest is defined by PNS dominance (blue circle). The grey shaded area indicates that when PNS is dominant, enhanced cellular restoration begins. The dotted line represents our hypothesized average daily arousal levels for typical adults in the Western modernized world of working age. Of note, positive arousal states, such as extreme excitement or joy, can induce subtle short-term arousal that may be similar to an SNS dominant state, and positive physiological stressors (hormetic stress) can create short-term acute stress followed by recovery states that may be restorative (these are not shown in the figure). See the online article for the color version of this figure.
We propose that in the United States, most daytime hours of healthy working-age adults are spent in moderate threat arousal. Two important pieces of empirical data support this hypothesis: high daily-level self-reported perceived stress in survey data and the frequency of minor stressors in daily life in daily-level data. In the American Psychological Association (2022) report, 27% of 3,192 adult respondents reported that most days they were so stressed that they could not function in daily life (American Psychological Association, 2022). Stress levels are even higher for some marginalized groups, for example, over half (56%) of Black adults under 35 agreed with the statement that most days they were so stressed that they could not function in daily life. This may be driven by two core components that drive stress perceptions: uncertainty and lack of control. The report found that levels of uncertainty about the future are significantly increasing year after year, with respondents being primarily distressed by stressors outside of their control. Important to our model, lack of control and uncertainty, are both psychological experiences that are physiologically arousing (Brosschot et al., 2005; Epel et al., 2018; Pieper et al., 2010).
Traditional models of stress have assumed that our baseline state of arousal is a relaxed state that we remain in until a threat is perceived, at which point, we respond with the activation of the acute stress response (Lazarus, 1993; Robinson, 2018). This acute stress response is characterized by the detection of threat and the proceeding activation of neural networks that drive peripheral sympathetic activity and initiate the hypothalamic–pituitary–adrenal axis cascade in order to prioritize survival (Gianaros & Wager, 2015; McEwen & Stellar, 1993; Muscatell & Eisenberger, 2012; Sapolsky et al., 1986). While traditional models suggest that the physiological activation of the acute stress response follows from a specific acute stressor (a specific event), a newer model suggests an alternative. The generalized unsafety theory of stress hypothesizes that physiological stress arousal may actually be the default or baseline state, caused by the general perception of threat that is not due to a singular defined trigger (Brosschot et al., 2017, 2018). As reviewed above, there is evidence that experiencing stress in daily life is common and frequent, and empirical evidence is building to suggest that high stress-related physiological arousal is in fact a common and frequent baseline state in daily life.
Minor daily stressors are commonly experienced in the United States. Data from a population-based study, the midlife in the United States study, found that on average participants experienced 3.26 (SD = 1.76) daily stressors across a week of data collection using the gold standard measurement tool for capturing daily stressors, the Daily Inventory of Stressful Events (Charles et al., 2013). Research also shows that experiencing daily stressors leads to heightened daily physiological arousal. Mak et al. (2023) published results from a large ecological momentary assessment study of 32,169 adults, with nearly 500,000 momentary responses, using both self-report items and physiology collected via optic sensors in a watch or smartphone. Analyses of these data found that participants with more frequent reports of daily stressors had higher than average heart rate and blood pressure across 3 weeks. Furthermore, even on days without a stressor, greater self-reported in-the-moment perceived stress averaged across all momentary assessments was associated with higher than average heart rate and blood pressure using wearable technology. These results suggest that even in the absence of a daily stressor, greater daily perceived stress is associated with greater activation of physiological threat arousal systems as indexed by higher daily blood pressure and heart rate.
In addition to the high number of daily stressors, ecological momentary assessments of adults in the United States show that daily mind states that are commonly experienced are likely also contributing to physiological arousal. Specifically, Killingsworth and Gilbert (2010) found that the majority of U.S. adults spend half of their day mind wandering toward the past or future. Mind wandering includes rumination and worry, thought patterns that have well-established associations with arousal of stress-related physiological systems, including increased blood pressure, heart rate, and cortisol (Ottaviani et al., 2016; Zoccola & Dickerson, 2012). While stress theories have previously argued that perseverative cognitions (worry, anxiety, rumination) in the anticipation or aftermath of an acute stressor is what makes stress toxic for health (Brosschot et al., 2005; Zoccola & Dickerson, 2012), it may be that even in the absence of an acute stressor, the common mind states of worry and anxiety contribute to heightened stress-related physiology in daily life.
Stress States Are Energetically Costly
Spending too much time in the moderate threat arousal state likely harms long-term physical and mental health because threat arousal states are extremely energetically demanding (Bobba-Alves, Juster, & Picard, 2022; Picard et al., 2018). Threat states increase production of neuroendocrine factors—cortisol, sympathetically derived epinephrine and norepinephrine, and cytokines—which then activate gene expression and the production of proteins (Sapolsky et al., 1986). While this is an evolutionarily adaptive response meant to support survival under threat (e.g., prepare the body to fight or flee; McEwen & Sapolsky, 1995; Sapolsky et al., 2000), protein synthesis is one of the most energetically demanding cellular processes (Kafri et al., 2016). Heart rate also speeds up during threat arousal to pump blood to the parts of the body that may need more oxygen and nutrients, like muscles (Sapolsky et al., 2000). While evolutionarily adaptive for survival, each heartbeat is a high-energy demand (Lemieux & Hoppel, 2009). Heart rate is also subject to predictive regulation as it increases in preparation for a required action or potential response to a threat, another example of its energetic burden (Sapolsky et al., 2000). In sum, an incredible amount of cellular energy is spent navigating states of moderate threat arousal, energy that could otherwise be used for other health-promoting biological processes such as cellular restoration, as described later.
In addition to proposing that adults in the United States are spending the majority of their time in moderate threat states, we also hypothesize that the fourth allostatic state of deep rest is rarely achieved. Our assessment that Americans are spending little time in a deep rest state is based on time use data from The U.S. Bureau of Labor Statistic’s American Time Use Survey. The 2021 survey data show that for the average adult living in the United States, 70% of the waking day is consumed by working, household activities, eating meals, purchasing items, calls/emails/mail, and watching TV (Economic News, 2021). Activities we would classify as deep rest-inducing activities (based on the perceptions of safety and slowed breathing inherent to the activity) were not measured directly in this survey; however, some of the measured activities that could broadly fall under this category included religious and spiritual activities, participating in sports, exercise, or recreation, or other activities not elsewhere classified. Combined, these categories made up only .63 hr of the average day (4.2% of the waking day; Appendix of the American Time Use Survey Results), indicating that people are spending very little time in activities that have the potential to activate a state of deep rest. Interestingly, while this study reported that only 8% of people engage in daily religious or spiritual activity, another study of over 35,000 Americans found that 55% report praying everyday (Pew Research Center, 2017). The discrepancy in these numbers is large and perplexing, warranting further research and potentially alternative methodological approaches to uncover how people are describing and experiencing spirituality in daily life.
Section 2: Perceiving Safety to Shift Away From Stress
To shift away from threat states and into rest states, safety must be perceived. Feeling safe is essential to our poisitive and optimal functioning in the world (Porges, 2022). Safety can be perceived through safety signals, which are “learned cues that predict the nonoccurrence of an adverse event … [and] are potent inhibitors of fear and stress responses”; they are the signals of being safe that the physical body interprets either consciously or unconsciously (Christianson et al., 2012). Safety signals can buffer the effects of uncontrollable stress in mouse models of stress by shutting down the fear response during an acute stressor (Christianson et al., 2008, 2011) and preventing the development of new fears (Jovanovic et al., 2005). Recent work on safety signals has begun to uncover the neural bases of safety signal learning (Meyer et al., 2019).
The process by which these safety signals are perceived is termed neuroception—the neural process responsible for the unconscious evaluation and distinguishing between environmental indicators of threat, risk, and safety (Porges, 1995, 2001, 2021). Porges’ Polyvagal theory proposes that once a threat or safety cue is perceived, the autonomic nervous system shifts its allostatic state to match the environmental demands. This idea has been echoed by others, namely, the neurovisceral integration model (Thayer et al., 2012; Thayer & Lane, 2000). We hypothesize that the biological signature of safety would include high parasympathetic arousal and low sympathetic arousal, coupled with cellular activity indicative of cellular restoration (described in Section 4).
The interpretation of safety signals is likely an individual difference factor. For example, research shows that individuals with a history of early life stress are more likely to perceive neutral stimuli as negative compared to those who grew up in safe and loving homes (Chen et al., 2004; Duffy et al., 2018; Luecken et al., 2006; McLaughlin et al., 2015; Repetti et al., 2002). This may mean that there are more challenges for some people (such as those with early adversity) to move away from threat states. We hypothesize that having both access to safety, as well as the ability to perceive safety, is essential to the ability to down regulate the threat state.
Entering Deep Rest via Perceptions of Safety
We suggest that there is a two-step process to entering a state of deep rest. The first step is a felt sense of external safety—physical and social safety. Physical safety means the environment is free of threats to the physical integrity of the person. Social safety means the environment offers experiences of acceptance, belonging, and inclusion and is free of social threats in the form of emotional distress or social status harm such as judging, shaming, or excluding (Slavich, 2020). We hypothesize that when the physical and social environment is perceived to be free of people or stimuli that may cause harm, then the metabolic energy and cognitive attentional capacities that are no longer spent predicting and planning for an upcoming threat or assessing risk, are conserved. We propose that the availability of these cognitive resources creates a second layer of safety—psychological safety. By psychological safety, we mean that the mind is free from distressing thought patterns such as worry, rumination, self-criticism, and shame, that promote threat arousal states (Brosschot et al., 2006; Gruenewald et al., 2004; Nealis et al., 2020). As described in the following sections, we hypothesize that many contemplative practices have overlapping elements that signal physical and social safety to practitioners, and create psychological safety.
Physical Safety in Contemplative Practice
We hypothesize that one of the core ways contemplative practices confer benefit is through safety signals, with safety signals being a natural part of many contemplative environments. The physical environment is a predominate source of safety (and threat) signals, and traditional contemplative practices are often undertaken in intentionally safe spaces. For example, in one of the earliest and most widely used Buddhist texts, there are detailed instructions on how to engage in breath-based meditation. The very first line describes that to prepare one must first find a secluded space (Thanissaro, 2013). A secluded space implies one free of the potential threat of other people. As another example, in a major text of the Tibetan Buddhist tradition written in the sixteenth century, a harmonious environment is listed as the first cause of tranquility (Namgyal, 2006). This historical context highlights the longstanding acknowledgement in Eastern traditions that a perceived safe space is foundational to meditative practice.
There are also sensory-based aspects of contemplative practice environments that may cue schemas of safety. For example, some contemplative teachers and practitioners use scents such as aromas from essential oils, and many religious rituals include the burning of incense (Ackermann, 2022; Episcopal Church, n.d.; Zhao, 2023). Research has shown that specific scents such as lavender, cypress, and cedar, increase well-being and decrease stress-related physiological activity (Atsumi & Tonosaki, 2007; Li et al., 2009; Masuo et al., 2021; Matsubara & Ohira, 2018; Sowndhararajan & Kim, 2016). Some contemplative practices also use tactile props such as prayer beads, which are used by holding and moving a string of beads through one’s hand in a slow rhythmic manner. This movement may activate specific sensory receptors that respond to gentle and slow stroking touch (Eckstein et al., 2020; Kearney & Lanius, 2022). Touch has been shown to regulate amygdala arousal via the insula (Eisenberger et al., 2011), and thus it may be that the slow rhythmic touch of prayer beads calms threat arousal through this pathway.
Finally, many contemplative practices also include nature-based objects and symbols, and in some cases include being physically in or engaging with nature (such as forest bathing). While the current empirical evidence for nature-based objects or symbols (e.g., having a potted plant in a classroom) is weak (van den Bogerd et al., 2020), there is strong evidence for physical and mental health effects of actually being in nature. Being immersed in a natural setting like a forest provides the sensory inputs of sight, smell, and sound that support the restoration of attentional resources and efficient physiological recovery from daily stress (Grahn & Stigsdotter, 2010; Markevych et al., 2017; Schertz & Berman, 2019; Stier-Jarmer et al., 2021; Stoltz & Schaffer, 2018). Future studies should explore the role of sensory stimuli in the ability for people to shift between allostatic states by testing different sensory conditions, while participants enter practice to see if the presence of specific sensory stimuli facilitates the shift to rest state physiology.
Social Safety in Contemplative Practice
A core mechanism of the positive benefits of contemplative practices may be the activation of schemas of secure attachment and social belonging via relationships with trusted teachers and fellow practitioners (Conklin et al., 2019). Social safety, or a sense that you are welcomed and accepted, is transmitted between people through eye contact, movement, and positioning of the head, facial expressions, the action of listening, the intonation of voice, and physical gestures (as extensively studied and described by Porges, 1995, 2001, 2021).
A foundational example of social safety signaling is the soothing voice parents use to calm their babies. Neuroanatomically, it is clear why sound is a profound safety cue; as Porges describes (2021, p. 3), “Mammals uniquely have a detached middle ear bone which expands the frequency band that mammals can hear and provides a ‘safe’ frequency band in which they can socially communicate.” Thus, sound is key to the ways we are alerted to the presence of safety and absence of danger.
We hypothesize that contemplative spaces include safety-inducing sounds such as rhythmic and slow talking from a teacher, soothing background music, or chanting in unison with fellow practitioners. Other social safety cues may include positive social interactions between practitioners (Sandstrom & Dunn, 2014) and visual cues of group belonging (as explored in other research areas; e.g., Cheryan et al., 2009). These elements should be studied more closely in future contemplative research to establish whether indeed they are important or needed for the efficacy of the practice.
Safety Perception Equity
Physical and social safety are not experienced in the daily lives of all people. The American Psychological Association (2022) report found that 10% of respondents reported feeling physically unsafe every day. There are large external factors that shape historical and current experiences of feeling safe (Lynch et al., 2021; Swope et al., 2022). Physical safety is likely hard to access for individuals living in areas of increased violence or vandalism, those that are a target of discrimination, or those living in chaotic home environments. Indeed, these contexts are associated with heightened threat arousal, which is likely adaptive in those contexts as the body prioritizes survival-related activity (e.g., Evans & Kim, 2010; Mezuk et al., 2011; Repetti et al., 2002; Taylor et al., 1997; Thoits, 2010).
The lack of equity in perceptions of safety in the United States is exemplified well in an essay excerpt from a participant of The Urban Yogis nonprofit (https://www.urbanyogis.org/). Raheem Lewis writes about the lack of safety (and freedom) in his neighborhood in his essay What is Freedom and How Do I Experience It?:
Living in the Baisley Housing Project, in South-Side, Jamaica, Queens we are harassed on a daily basis. We are unable to be comfortable in our own community. The people in my neighborhood are treated like prisoners. Surveillance cameras watch us 24/7. We have many restrictions that we have to follow such as no bicycle riding, no skateboarding, no rollerblading, and no barbecuing (except by permit only).
Inequity in safety perceptions should be considered in contemplative group settings. As social mammals, we derive a sense of safety by being in relationships with others that support and share our goals (Coan & Sbarra, 2015). However, not everyone feels safe in contemplative practice groups despite the shared intentions for being there. In his book, Awakening Together, meditation teacher and author, Larry Yang, describes entering the meditation hall during his first retreat at a popular retreat center. He saw that he was the only person of color in the room of over one hundred people, and instead of feeling safe, he felt excluded and isolated, conditioned by a lifetime of discrimination (Yang, 2017). Yang suggests ways to create social safety, such as creating groups of people based on sociocultural demographic similarity, because being with similar people creates a sense of safety. One example is the development of “workplace affinity groups” which are groups for marginalized people to form around a collective social identity in order to promote inclusion and belonging in workplaces (e.g., Blitz & Kohl, 2012). Studies measuring safety might consider measuring aspects of group safety, such as sense of belonging or exclusion.
There are interindividual differences in the perception of safety that can be partially explained by historical factors and current life circumstances. For example, research has shown that adults who experienced childhood adversity (McLaughlin et al., 2015), and adults who report high loneliness (Bangee et al., 2014), are more likely to perceive ambiguous information as threatening. Trauma history is a particularly important factor that influences what a person perceives as safe versus threatening. Trauma-informed trainings for contemplative teachers are increasingly common, with the goal of making more people feel safe and welcome in contemplative settings (Treleaven, 2018). Empirical examination of which practices, techniques, and environments elicit feelings of safety in which individuals, will allow for closing some of these gaps.
Psychological Safety in Contemplative Practice
Contemplative practices increase psychological safety by reducing distressing thought patterns like rumination and self-criticism through a number of psychological mechanisms outlined by others (Dahl & Davidson, 2019; Hölzel et al., 2011; Pascoe et al., 2021; Riley & Park, 2015). In the following section, we describe three of the primary pathways through which contemplative practice may lead to perceived safety and reduce stress-related arousal. These are: (a) interoception, becoming aware of previously unconscious internal bodily sensations, (b) attentional capacity, becoming skilled at shifting attention away from self-referential thoughts toward present-moment awareness, and (c) emotion regulation, effectively navigating distressing situations.
Interoceptive Awareness
Contemplative practices may lead to psychological safety by using techniques that help practitioners become consciously aware of internal bodily sensations (e.g., heart pounding, muscle tension, jaw clamped, stomach fluttering), a phenomena called interoceptive awareness (Craig, 2003). Interoception is an individual difference characteristic that can be quantified via self-report questionnaire (Mehling et al., 2018) or behavioral tasks, such as a heartbeat tracking task (Ludwick-Rosenthal & Neufeld, 1985). A core technique for many contemplative practices is noticing bodily sensations. Mindfulness training in particular helps participants notice subtle bodily cues that they would not otherwise be conscious of. For example, mindfulness training often includes a “body scan” where one directs attention to each part of the body, tuning attention to subtle bodily sensations emanating from that region (Hölzel et al., 2011). Increased awareness of bodily arousal may be beneficial as it allows a person to notice and respond effectively to arousal states (Jamieson et al., 2013).
Attentional Capacity
Another core component of contemplative practices is improving attentional capacity. Increases in attentional capacity allow for increased ability to intentionally turn the mind away from threat-provoking thought patterns, a sign of enhanced emotion regulation (Jha et al., 2015; Morrison et al., 2014). Attentional capacity can be increased by contemplative practices in several ways. In some practices such as Qigong, Tai Chi, specific forms of yoga, and breath-based meditations, attention is directed to physical movements of the body throughout the length of the practice, with encouragement to bring attention back to the body when the mind wanders. Other practices focus attention on a singular set of words or short text, such as reading specific religious texts slowly and mindfully (e.g., Lectio Divina) or repeating specific phrases (e.g., mantra or prayer). These practices of directed attention increase one’s capacity for sustained attention (Jha et al., 2015; Lutz et al., 2008; Morrison et al., 2014; Mrazek et al., 2013) and increases the ability to intentionally turn attention away from threat-inducing thoughts (Hawley et al., 2014; Perestelo-Perez et al., 2017).
Emotion Regulation
Many contemplative practices include expanding one’s toolkit of coping techniques to deal with negative emotional reactivity patterns. These emotion regulation techniques include skills like controlled breathing (Banushm et al., 2023) re-appraisal (Brown et al., 2007; Brown & Ryan, 2003), and acceptance of negative states (Lindsay & Creswell, 2019). Many of these coping techniques enable more thoughtful exploration of one’s emotional life, including the ability to name one’s emotional experience with specificty. Naming emotions with specificty, or emotion labeling, may be a pathway by which contemplative practices increase psychological safety, as the ability to name specific emotions has been shown to decrease the intensity and impact of distressing emotions (see Moyal et al., 2014). Lieberman et al. (2007, for a summary of this literature) found evidence for the neural pathways involved in this phenomenon. Labeling emotions was associated with decreased activity in the amygdala and increased activity in the right ventrolateral prefrontal cortex, and the inverse relationship between these brain regions was mediated by activity in medial prefrontal cortex. This brain imaging evidence suggests that describing or naming emotions as they are experienced activates brain regions that help reduce the stress-related reactivity linked to that emotion. Noticing and identifying distressing thoughts and emotions without judgment may be one of the key techniques taught both directly and indirectly in contemplative practices that allows individuals to learn how to shift from autonomic states of stress to rest. Indeed, a systematic review of 135 studies found that aspects of emotion regulation—efficient negative affect regulation, adaptive emotional coping, and greater affective flexibility—were associated with higher resting cardiac vagal control, which indicates greater resting parasympathetic dominance (Balzarotti et al., 2017). Future studies should directly test the relationships proposed here—that contemplative practices increase one’s ability to nonjudgmentally view and identify specific emotions, which in turn allows individuals to shift away from threat states to rest states.
Section 3: Parasympathetic Dominance as a Key Mechanism of the Benefits of Contemplative Practices
The most empirically supported way that contemplative practices confer their psychological and physiological benefits is by lowering threat arousal through shifting the autonomic nervous system to parasympathetic dominance via slowed and/or regulated breathing (Gerritsen & Band, 2018). The autonomic nervous system regulates the body through its two governing divisions—the sympathetic and the parasympathetic nervous systems. These two systems work antagonistically, synergistically, and independently to coordinate responses to internal and external demands. Both systems work to respond to environmental cues to allow blood and oxygen to reach the brain and muscles at the rate the environmental demands require, as well as to restore and maintain homeostasis.
The sympathetic branch is primarily responsible for energy mobilization (e.g., metabolism, blood oxygenation, etc.) in response to threat or challenge. The primary endocrine outputs of the sympathetic branch—epinephrine and norepinephrine—are released either systemically from the adrenal medulla or locally in most organs through peripheral nerve endings. Both epinephrine and norepinephrine are powerful energy-mobilizing hormones, causing the breakdown of lipids and the mobilization of fatty acids and glucose in the bloodstream (Bachen et al., 2002). The parasympathetic branch has dominion when organisms are at rest. The core component of this process is the vagus nerve, which is the primary nerve of the parasympathetic branch. At rest, respiration is tightly regulated by both sympathetic and parasympathetic activation to ensure proper tissue oxygenation, with the vagus nerve relaying the necessary information about the environmental and internal demands for oxygen (Komisaruk & Frangos, 2022).
Physiology of Slowed Breath
Parasympathetic dominance can occur through slowing and/or controlling breath, as detailed by Gerritsen and Band (2018). Most contemplative practices share the commonality of slowed breathing, either intentionally, or as a consequence of the calming practice. The breathing techniques used in contemplative practices often include slowing down breath, elongating exhales, shifting the breath to the abdomen versus thorax (diaphragmatic breathing), shifting one’s attention toward natural in-and-out breath cycles, and syncing physical movements to breath cycles. These cues engage the baroreceptor reflex and pulmonary mechanoreceptors, which directly increase vagus nerve activation and decrease sympathetic activity (Cernes & Zimlichman, 2017; Gerritsen & Band, 2018). The parasympathetic and sympathetic systems are tonically active, with efferent pathways extending from the brainstem and hypothalamus to all major peripheral organs and afferent nerves from the lungs, airways, and heart, projecting to the brainstem and to the hypothalamus and higher order neural regions (Prescott et al., 2020; Thayer & Lane, 2009). Because of this anatomical connectivity, changes in breathing rate are quickly signaled to the brain, allowing the brain to interpret that the body is in a relaxed, calm state, and safe state.
Body to Brain Communication Is Essential for Deep Rest
The vagus nerve perceives and communicates the internal state of the body to the brain through afferent nerve signals from the vagus to the central nervous system (Porges, 2001). Roughly 75% of the fibers in the vagus nerve are afferents, which project to the brainstem and then to higher order neural regions like the amygdala and prefrontal cortex (Thayer & Lane, 2009). This means that most neural fibers within the vagal nerge send information upward—from the body to the brain. We hypothesize that there is a positive feedback loop generated during contemplative practice via afferent and efferent nerve signals that creates a calm state of being. Specifically, as slow and deep breathing is initiated during a contemplative practice, the internal state of being settles into a relaxed state with a slower heart rate (Gerritsen & Band, 2018). With this slowing of heart rate, a positive feedback loop is initiated: The vagus nerve sends this lowered heart rate information via afferent fibers to the brain, and after it receives these signals that imply safety from the body, the brain then turns off threat arousal activation (Mather & Thayer, 2018). Now, parasympathetic activity is dominant over sympathetic activity, initiating a felt sense of peace, safety, and contentment (in potentially a quadratic, nonlinear relationship; Duarte & Pinto-Gouveia, 2017; Petrocchi et al., 2017). This shift to parasympathetic dominance, coupled with slowed breathing rates and safety signals that reduce threat arousal system activation, and the resulting positive feedback loops these create, is what we identify as a state of deep rest.
Evidence Linking Contemplative Practices to Parasympathetic Dominance
The next step in our model is demonstrating that during contemplative practices, parasympathetic activity increases while sympathetic activity decreases. There is some initial evidence for this, though more high-quality work is needed to confirm this hypothesis.
The strongest empirical link between contemplative practices and autonomic functioning come from long standing evidence that breathing rates slow during contemplative practice, given the known link between slowed breath and parasympathetic activity. In one of the earliest studies of meditation effects on biological activity in novice practitioners, R. K. Wallace (1970) found that practicing Transcendental Meditation significantly slowed breathing rates, oxygen consumption, and heart rate compared to a general resting state. This hypometabolic state induced by transcendental meditation was proposed by R. K. Wallace et al. (1971) to be a fourth state of consciousness, during which metabolic rates drop below those seen even during sleep.
Research over the past several decades have supported R. K. Wallace’s (1971) idea of a hypometabolic state induced by yoga, meditation, and prayer. Studies of yoga have shown that practicing specific forms of yoga decreases oxygen consumption and generally slows metabolic rate (Chaya & Nagendra, 2008; Telles et al., 2000). Another early study in this area documented the breathing and cardiac activity of experienced Zazen practitioners as they performed a core meditative practice of tanden breathing, which is a form of guided breathwork focused on breathing from the abdomen (Lehrer et al., 1999). Results showed that respiration rates significantly decreased from sitting at rest to performing the breathwork, and heart rate variability increased (an index of vagally mediated cardiac activity, signifying an increase in parasympathetic activity; Lehrer et al., 1999). Similarly, Bernardi et al. (2001) demonstrated that repeating prayers of either Ave Maria or a yoga mantra—without directly telling participants to slow their breathing—slowed breathing to a rate of 6 breaths per minute (bpm), a rate significantly slower than spontaneous breathing rate. This breath rate of around 6 bpm has been linked to positive health outcomes (Bernardi et al., 1998) and has been called resonant breathing or vagal breathing, given its effect on increasing heart rate variability and reducing blood pressure (Sevoz-Couche & Laborde, 2022). It has not yet been established in the literature whether the wide range of contemplative practices can induce a breathing rate of 6 bpm or if this slow rate is only related to specific forms.
There is limited, but promising, evidence for contemplative practices influencing pure metrics of parasympathetic and sympathetic activity. Tang et al. (2009) investigated how meditation may be linked to functional changes in the brain that explain the associations between meditation and autonomic control, specifically examining the dorsal and ventral anterior cingulate cortex (ACC), given that functional magnetic resonance imaging studies have linked these regions to autonomic control (Critchley et al., 2003). In a short 5-day randomized controlled trial of meditation compared to a relaxation training control group (both groups practiced for 20 min a day), the meditation group showed more parasympathetic activity during and after practicing meditation (measured via heart rate variability), as well as greater change in regional cerebral blood flow in the right subgenual ACC and adjacent ventral ACC than the relaxation group.
While similar evidence for increases in parasympathetic activity has also been found for yogic breathing (R. P. Brown & Gerbarg, 2005; Kromenacker et al., 2018), Christian prayer recitation (Stanley, 2009), Muslim prayer (Doufesh et al., 2014), Qigong (Lee et al., 2002), and Yoga Nidra (Chhetri et al., 2020), not all studies have found this link (e.g., Peng et al., 2004; Raghuraj et al., 1998). The methodological quality of these studies limits firm conclusions given the small sample sizes (less than 30 participants) and a lack of comparability of autonomic functioning measures (including the absence of sympathetic activity measures). These limitations undermine the reproducibility of the findings and necessitates the results of these initial studies to be considered preliminary. Future lab-based studies should use larger samples, and higher quality measurements of nervous system functioning, including galvanic skin response or preejection period to measure sympathetic activity to test the hypothesis that sympathetic activity lowers during practice.
Furthermore, an open question in contemplative science is whether other aspects of the practice are necessary beyond intentionally slowing or regulating breathing rates to receive the health benefits of the practice. Past work on paced breathing practices have shown benefits on mental and physical health, including down-shifting sympathetic arousal and increasing parasympathetic activity, without any other didactic or psychospiritual components (Blum et al., 2019; Harada et al., 2014; Laborde et al., 2019). It is possible that the didactic aspects of contemplative practice are not necessary to shift physiology. Perhaps instead, there are unique benefits of the diatic or social aspects of the practice that give meaning to the practice, which allows the person to engage with it for longer periods of time or to exert more intentional effort to perform it more deeply. Contemplative practices that come with a set of social ethics, prosocial values, and community, may be more likely to be practiced consistently over longer periods of time compared to types of controlled breathing that do not have a richer psychosocial or cultural context. In addition, the contemplative aspects of the practice may provide other psychological benefits (e.g., insights into self and interdependence, self-compassion, perspective taking, emotion regulation tools, experiences of awe) that increase positive affect and decrease distress, which confers their own health-promoting benefits. These mood-boosting and distress-reducing psychological effects may also motivate the practitioner to come back to the practice day after day, promoting long-term adherence.
Long-Term Changes to Nervous System Functioning
There is growing evidence that long-term engagement in contemplative practices can change resting state autonomic nervous system activity. A meta-analysis of 19 randomized controlled meditation studies concluded that meditation training can lower resting systolic and diastolic blood pressure (Shi et al., 2017). While the evidence is less robust for the impact of contemplative practices on independent measures of sympathetic and parasympathetic activity, the work in this area is accumulating. Several studies have found that contemplative practice training can shift one’s baseline set point toward greater parasympathetic dominance. For example, a four-arm randomized trial with an active control arm that compared 4 weeks of (a) yoga, (b) mindfulness, and (c) yoga combined with mindfulness, and a control condition of playing with a therapy dog, found that resting heart rate variability was significantly higher postintervention in the three contemplative groups compared to the control condition (Hunt et al., 2018). Similarly, practicing Qi Gong for 30 min three times a week for 12 weeks increased resting heart rate variability (Lee et al., 2002). Additionally, a small observational study found that experienced practitioners who had engaged in meditation or prayer for 30 or more minutes a day for 15 or more years had greater cerebral blood flow at rest in brain regions associated with autonomic functioning compared to matched controls (Newberg et al., 2010). However, not all studies have found significant changes in autonomic profiles from pre- to postintervention. For example, Krygier et al. (2013) reported no change in resting heart rate variability after a 10-day intensive Vipassana meditation retreat.
If training in contemplative practices can ultimately shift one’s autonomic nervous system set point so that the “baseline” or usual allostatic state is one of lower arousal, then it may be easier for that person to shift to, and spend time in, deep rest. Training in contemplative practices may also enhance the nervous system’s ability to flexibly shift between states. This concept is supported by another set of studies showing that training in contemplative practices can enhance autonomic functioning in response to acute stress. A meta-analysis of experimental studies found that meditation immediately before undergoing an acute laboratory stress task, like giving an impromptu speech to a panel of judges (the Trier Social Stress Test), dampens the impact of the acute stressor on psychological and physiological stress arousal profiles (M. L. Morton et al., 2020).
Despite the typical assumption that less reactivity is an index of greater well-being, research shows that it is not necessarily beneficial to reduce arousal during the process of coping with challenging situations. Rather, a resilient acute stress response profile actually looks like having high reactivity coupled with fast recovery, and a pattern of “challenge” hemodynamic arousal (high cardiac output or low total peripheral resistance). This pattern is associated with improved performance on social and cognitive tasks (Jamieson et al., 2010). In one study, training in mindfulness led to slightly improved emotional and physiological challenge response to a lab stressor (Daubenmier et al., 2019). In another study, mindfulness training led to faster physiological and emotional recovery after an acute stress task (Crosswell et al., 2017). These findings suggest that contemplative practices may increase flexible coping during demanding emotional circumstances. Examining the impact of contemplative practices on acute stress reactivity is likely a better test of their impact on the autonomic nervous system as compared to examining the baseline set point physiology given that the autonomic nervous system is a dynamic system and it is difficult to measure true baseline activity in a laboratory setting (see Appendix, for research suggestions). Using acute stress tasks enables the direct testing of the individual’s fitness for responding dynamically to a challenge (aka stress resilience).
There are other contemplative practices that likely also increase stress resilience, but not because the practice elicits deep rest. Some contemplative practices are energetic and fast paced such as the yogic practice of breath of fire which increases heart rate and sympathetic activity with reduced vagal activity (Peng et al., 2004; Raghuraj et al., 1998). Another example of an arousing practice is the Wim Hof Method, a protocol that includes cycles of hyperventilation followed by breath retention (intermittent hypoxia), combined with short-term cold exposure, with the goal of activating stress arousal to develop resilience. Healthy volunteers who trained in this technique for 10 days showed strong sympathetic activation (epinephrine release) during the practice and an improved immune response to endotoxin (Kox et al., 2014). These arousing practices that briefly activate sympathetic responses (a form of hormetic stress) may have salutary consequences such as improving the nervous system’s ability to recover quickly from challenges, a ripe area for investigation as described elsewhere (Epel, 2020).
Section 4: Cellular Restoration as a Core Pathway to Health
Our model proposes that in a state of deep rest, energy can be allocated toward cellular restoration processes instead of toward maintaining heightened threat arousal. Reducing threat arousal frees up cellular and organismal energetic resources, allowing energy to go to the optimization of biological and physiological functions. This concept is similar to that previously proposed by Robles and Carroll (2011) and can be conceptualized as a hierarchy of biological needs analogous to the hierarchy of psychological needs classically depicted as Maslow’s pyramid (Maslow, 1943).
As represented in Figure 3, at the bottom of the hierarchy of biological needs are basic survival needs—responding to and recovering from immediate acute threats, directing available energy toward the maintenance of physical integrity, and ensuring immediate survival. When basic survival needs are met, the organism can direct energy toward allostasis and predictive regulation, during which the organism prepares for future threats (Bobba-Alves, Juster, & Picard, 2022; Sterling, 2012). At the top of the pyramid is optimization of biological functioning. When there are no threats to attend to immediately, the cellular and physiological energy resources that are usually devoted to surviving and preparing, are instead devoted to optimizing cellular restoration, which directly contributes to health and longevity.
Figure 3.
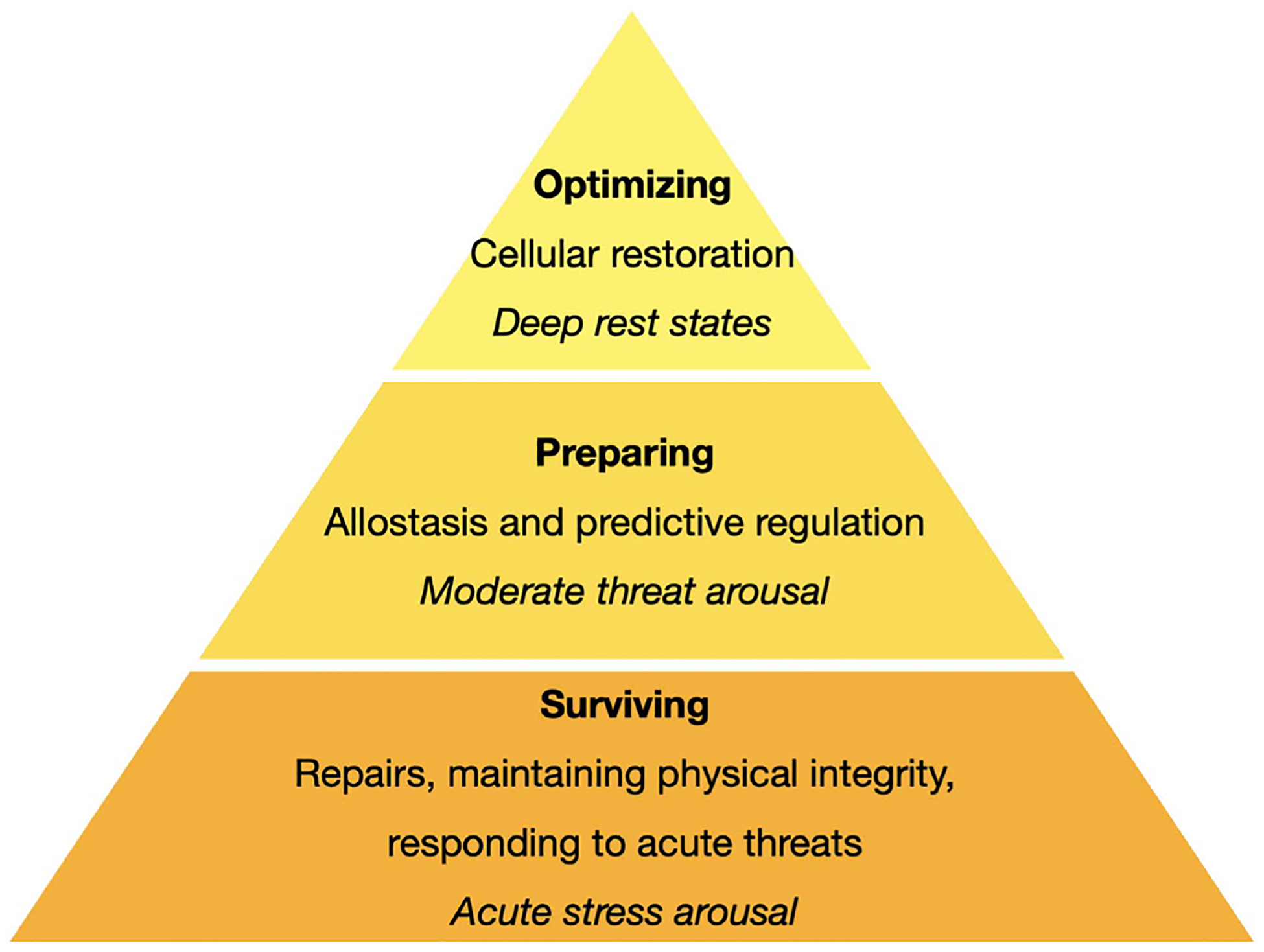
Hierarchy of Biological Functions Based on Contextual Demands and States of Arousal
Note. This figure represents a hierarchy of biological needs that depends on energy resources deployed among cells and across the body. At the bottom of the hierarchy are basic needs for surviving in the present moment, such as repairing acute damage (e.g., proteins) and maintaining physical integrity (e.g., membrane potential), as well as responding to acute threats. Once those needs are met, then the organism can devote energy toward the next level of need—preparing for coming environmental demands; this process is also termed predictive regulation or allostasis (Bobba-Alves, Juster, & Picard, 2022). If this level does not consume all energy resources, then energy can finally be used toward optimizing. In other words, once cellular and physiological systems are working well, and there are no threats to take care of or plan for, then energy is directed toward cellular restoration. Each level of the hierarchy is associated with a different allostatic state, as indicated in the figure with italicized text. See the online article for the color version of this figure.
Cellular restoration is an umbrella term for the processes related to housekeeping and quality control within and surrounding cells. Even without major or acute stressors, there is a biological cost of living that leads to “wear and tear” on cells themselves and their microenvironment. The constant activity of cellular processes creates oxidative stress and causes damage to DNA and other molecules, which are counterbalanced by reparative and protective cellular functions (i.e., maintenance) including but not limited to antioxidant defenses and the activity of DNA repair enzymes (Maynard et al., 2009). Restorative functions include the process of autophagy, which degrades and removes misfolded proteins, damaged organelles, and other waste products (Levine & Kroemer, 2008; Robles & Carroll, 2011). Similarly, mitophagy is key to maintaining cellular health as it allows for the turnover of mitochondria and prevents buildup of dysfunctional mitochondria, which leads to cellular degeneration (Youle & van der Bliek, 2012). A common characteristic and cause of aging is a decline in these “housekeeping functions” or quality control of the cell, which manifests as an impaired ability to protect DNA, an increase in DNA damage and misfolded proteins, and resistance to apoptosis, which can lead to immunosenescence (Salminen et al., 2011; Salminen & Kaarniranta, 2009).
Mitochondria Are Essential to Cellular Restoration
A core component of cellular restoration is energy production capacity. Cellular energy is derived from two main sources—glycolysis and mitochondrial respiration. In breathing animals like humans, all energy is derived aerobically in mitochondria (Nicholls & Ferguson, 2013). Mitochondria are small intracellular organelles with their own DNA—the maternally inherited mitochondrial DNA (mtDNA; Giles et al., 1980). Mitochondria are multifaceted: dozens of different types of mitochondria populate the cells of our brain, immune system, and other organ systems (Monzel et al., 2023). Hundreds to thousands of dynamic mitochondria populate the inside of every nucleated cell in the human body. Mitochondria transform energy from glucose, fatty acids, and amino acids into adenosine triphosphate (ATP) through a process called oxidative phosphorylation, which consumes the oxygen we breathe to extract energy from food substrates (Nicholls & Ferguson, 2013). Through respiration, mitochondria build an internal charge—much like batteries—that can be used for ATP/energy production, and for a number of other life-sustaining and stress-induced functions (Picard, 2022). For example, mitochondria are responsible for producing steroid hormones including cortisol and all biological sex-defining hormones (estrogens, testosterone, etc.; Selvaraj et al., 2018). Thus, mitochondria are critical to the healthy neuroendocrine physiological stress response (Papadopoulos & Miller, 2012; Picard et al., 2015).
The central role of mitochondria in human health likely arose for three main reasons. First, the human body and all other multicellular life forms arose from the endosymbiosis of an aerobic bacterium (the ancestor of mitochondria; Lane & Martin, 2010; Sagan, 1967). Without mitochondria, there would be only single cell organisms—no multicellular life, no thinking, feeling, conscious animals (D. C. Wallace, 2010). Second, energy flow is the ultimate currency of life and the main distinguishing factor between a cadaver and the living body (Bobba-Alves, Juster, & Picard, 2022). We must continuously breathe precisely to bring oxygen to mitochondria to fuel oxidative phosphorylation, to charge our mitochondria, which sustain cellular life, brain activity, and consciousness (Lane, 2002; Shulman et al., 2009). And third, evolutionary evidence suggests that all living creatures and their behaviors have been optimized specifically for energy efficiency (Damasio, 2018; Makarieva et al., 2008), arguing that cellular functions, behaviors, and the mind are secondary to energy flow (Damasio, 2018). Thus, mitochondria have evolved as the energy hub of the human body.
Mitochondria are directly linked to stress-related processes and stress-related diseases. Poor mitochondrial functioning has been found in otherwise healthy individuals who are living in conditions of chronic stress (Picard et al., 2018), and recent evidence tie mitochondrial dysregulation to multiple chronic inflammatory, metabolic, and degenerative diseases (B. G. Hill et al., 2019; Picard et al., 2016; Wu et al., 2019), and aging (Jang et al., 2018). Because chronic stress elicits complex, energy-demanding, multisystem physiological responses, it consumes energy resources (Picard & McEwen, 2018). Therefore, stress can be understood as an environmental demand that either consumes an excessive amount of energetic resources or causes recalibrations that disrupt mitochondria (mitochondrial allostatic load; Bobba-Alves, Sturm, et al., 2022). Mitochondrial allostatic load may arise due to the constant energy demand stimulated by stress hormone signaling as part of the physiological stress response (Du et al., 2009).
Responding to stress greatly taxes mitochondria, which has downstream negative health effects. In a model of cellular allostatic load in vitro, glucocorticoid signaling increased energy expenditure by close to 60% (a state termed hypermetabolism) in parallel with increased cytokine and extracellular DNA release (Bobba-Alves, Sturm, et al., 2022). Over a 10-month period, this state of hypermetabolism was associated with increased mitochondrial DNA damage, accelerated telomere shortening, increased rate of epigenetic aging, and increased cell mortality. Mechanistically, exaggerating excess energy consumption by impairing energy flow in mitochondria further amplified hypermetabolism and exaggerated telomere erosion. These findings support the notion that expending energy on stress-related pathways of surviving and preparing (at the bottom and middle of the pyramid; Figure 3) comes at the expense of restorative and longevity-promoting cellular activities, as posited in the energetic model of allostatic load (Bobba-Alves, Juster, & Picard, 2022).
Additionally, some evidence ties mitochondrial functioning to acute stress reactivity profiles. Impairments in mitochondrial biology can produce hyperreactive physiological and endocrine responses to psychophysiological challenges, including exercise in humans (Taivassalo et al., 2003), and induced stress in rodents (Picard et al., 2015). These results suggest that defective mitochondria impair the ability of the organism to optimally respond to stress, likely because of reduced energy production capacity or alterations in mitochondrial signaling. Conversely, when energetic demands are low, mitochondria can undergo biogenesis, replicate, and increase in number and quality (Neufer et al., 2015).
Contemplative Practices and Cellular Restoration
There is limited work examining whether contemplative practices directly alter mitochondrial energy transformation capacity or other aspects of mitochondrial biology (Fricchione, 2023). There is, however, a series of studies out of one research group demonstrating that “relaxation response training” alters gene expression in a variety of gene sets, including those that regulate mitochondrial biology. The intervention in these studies is an 8-week relaxation training intervention consisting of 20-min of daily guided meditation which includes body scan, focused word, and mindfulness meditation techniques, as well as a weekly hour-long one-on-one health coaching session geared toward problem solving barriers to daily practice (Dusek et al., 2006). In a variety of clinical and nonclinical samples, this intervention was shown to enact vast changes in gene expression related to energy metabolism and mitochondrial functioning, insulin secretion, telomere maintenance, and inflammatory processes (Bhasin et al., 2013, 2018; Dusek et al., 2008). Through network analysis, the authors found nuclear factor kappa B genes downregulated other pathways including isolated components of the mitochondrial ATP synthase. This work is compelling, and we encourage more scientists to explore links between psychosocial interventions and mitochondrial biology.
Basic science and animal studies support a link between mitochondrial functioning and functioning of the parasympathetic branch of the autonomic nervous system. First, receptors for acetylcholine (ACh), a neurotransmitter of parasympathetic nerves, are present on the membranes of mitochondria, thereby enabling mitochondria to sense parasympathetic signals (Lu et al., 2014). When challenged with stressors, mitochondria also can release their DNA (mtDNA) into the inside of the cell (cytoplasm) and even in the blood as circulating cell-free mtDNA (Trumpff et al., 2021). A brief socioevaluative challenge is sufficient to trigger cell-free mtDNA in the bloodstream (Hummel et al., 2018; Trumpff et al., 2019), directly linking psychological threat to one form of mitochondrial signaling. Interestingly, mtDNA release in the cytoplasm appears induced by glucocorticoid signaling (Trumpff et al., 2019), but inhibited by the parasympathetic neurotransmitter ACh (Lu et al., 2014). Additionally, a drug that stimulates vagus nerve activity, donepezil, was shown to prevent adverse changes in mitochondrial fusion proteins and the loss of membrane potential, and to mitigate mitochondrial reactive oxygen species production in rats with cardiac ischemia (Khuanjing et al., 2021). This parasympathetic-mitochondrial connection suggests that the same acetylcholine signaling pathway that links vagal activation to decreased proinflammatory cytokine production (Pavlov et al., 2009) may also link vagal nerve stimulation to other cellular restoration processes, including antioxidant enzymes, antiapoptotic actions, and the upregulation of mitochondrial biogenesis and mitophagy. Evidence for contemplative practices altering both the autonomic nervous system and the functioning of mitochondria is needed as part of rigorous testing of the deep rest model.
Section 5: Sleep as the Ultimate Time for Restoration
While we argue that contemplative practices allow the body to spend time in a state of deep rest, characterized by parasympathetic instead of sympathetic dominance, the other time this state is reached is during sleep. The primary way humans engage restorative processes is during nighttime sleep, and particularly, deep slow wave sleep. By understanding the biology of high-quality restorative sleep, we can gain insight into the biology of deep rest.
Sleep is categorized into two parts: nonrapid eye movement (NREM) sleep, comprising Stages 1–3 that progress from lighter sleep to deeper sleep stages, and rapid eye movement (REM) sleep. During sleep, humans disengage from the external environment and a series of cellular, molecular, and systemic processes ensue to support neural and bodily homeostasis and restoration. High quality sleep is essential for the optimization of biological functioning, including for restorative cleansing processes in the brain. During deep NREM sleep, toxic molecules that are byproducts of normal neuronal function are cleared away; Fultz et al. (2019) demonstrated that the large amplitude slow waves characteristic of deep NREM sleep are coupled with hemodynamic oscillations and cyclic patterns of cerebral spinal fluid movement that clears waste materials from the brain. This clearance system removes toxic metabolites (Xie et al., 2013), like β amyloid, the accumulation of which is associated with the neuronal death common in neurodegenerative disorders like Alzheimer’s disease (McKhann et al., 1984). Despite these active processes during sleep, the body still uses less energy during slow wave sleep than it does during waking hours (Shechter et al., 2013), suggesting that restorative activities are energetically efficient.
Sleep exerts considerable influence over the autonomic nervous system and vice versa. When initially falling asleep, parasympathetic activity increases and sympathetic activity decreases, like what happens during deep rest. Specifically, sleep onset is accompanied by a reduction in heart rate paired with an increase in parasympathetic activity (measured by the high frequency component of heart rate variability, a measure of vagally mediated cardiac activity), and a decrease in sympathetic activity (measured by preejection period, a measure of cardiac sympathetic tone; Tobaldini et al., 2013; Trinder et al., 2012). During NREM sleep, compared to waking and REM sleep, heart rate decreases further and this is coupled with a reduction in overall cardiac output with a relative dominance in vagally mediated parasympathetic activity. These autonomic changes across NREM sleep facilitate an organism’s efficient use of the metabolic resources available while supporting bodily homeostasis, restoration, and rejuvenation (Zoccoli & Amici, 2020). Taken together, mechanistic studies show that coordinated neural activity during sleep supports the conditions necessary for restoration and rejuvenation of the brain and body, and this physiological state is characterized by lowered heart rate, increased parasympathetic activity, and decreased sympathetic activity—a physiological profile similar to what we hypothesize is accomplished when a person enters a wakeful state of deep rest.
Energy Expenditure During Sleep and Deep Rest
At the whole organism level, lower heart rate and reduced physiological arousal reflects lower energy expenditure. Every physiological function—heart beating, breathing, digesting, seeing, hearing, thinking, and moving—requires energy. Because most of our physiological, sensory, and motor functions are reduced or interrupted when we sleep, life can be sustained at a significantly lower energy cost. Measuring energy expenditure via the amount of oxygen consumed during wakefulness and at different stages of sleep among different mammalian species, including humans, shows that sleep decreases energy expenditure by 7%–69% (generally ~30%–50% from waking values, and typically ~10% lower than basal metabolic rate in humans; e.g., Shechter et al., 2013). In fact, energy conservation is one of the proposed functions of sleep (Berger & Phillips, 1995). Consequently, sleep deprivation increases overall energy expenditure (Jung et al., 2011; Shechter et al., 2013), preventing the potential restorative benefits associated with the hypometabolic state of low physiological arousal during sleep. Under ideal circumstances, the body’s need for restoration might be met during sleep, creating less need for deep rest states during the daytime.
Despite the essential role sleep plays in allowing for restoration of core biological functions, most adults do not get enough sleep. Nearly one-third of American adults receive significantly less than the 7 hr of sleep per night that is recommended by the Centers for Disease Control (Liu et al., 2016). Poor sleep quality is now considered a public health crisis given the high rates of poor sleep and beacuse strong experimental and epidemiological studies show that disruptions in sleep profoundly influence mental and physical health, including increasing risk of mortality (Itani et al., 2017).
Safety Signaling and Sleep Equity
One of the reasons quality and quantity of sleep may be disrupted for many Americans is because they lack the physical and psychological safety that is critical for high-quality sleep. Sleep science shows that individuals experiencing discrimination and those living in unsafe neighborhoods have less restorative sleep than those not experiencing chronic stress (Euteneuer et al., 2014; Tomfohr et al., 2010). Hale and colleagues demonstrated that living in neighborhoods with greater crime rates and physical disorder is associated with lower sleep quality and shortened sleep duration (Hale et al., 2010; T. D. Hill et al., 2009). Linking lack of physical safety to autonomic functioning during sleep, Mellman et al. (2018) found that unsafe neighborhood conditions were associated with lower parasympathetic activity during sleep in a sample of Black Americans, and for women, greater exposure to violence was positively associated with sympathetic activity during sleep. Furthermore, Troxel et al. (2017) found that daily stressors, relationship conflict, and perceived discrimination are each associated with less of a dip in blood pressure during sleep.
We hypothesize that individuals living in unsafe environments have difficultly shifting to restorative states during sleep because their bodies are maintaining hypervigilance, even at night, which interferes with the physical recovery that happens during sleep. These studies suggest that we may carry daily stress at an unconscious level while we sleep, interfering with optimal restoration by keeping the sympathetic and cardiovascular systems in heightened activation.
Sleep and Contemplative Practices
Contemplative practices may offer a way to support physiologically restorative activities for those getting too little or low-quality sleep by offering a daytime practice that can supplement and/or support nighttime restoration. Meta-analytic evidence of randomized controlled interventions have supported the use of mindfulness meditation, yoga, and Qi Gong in improving sleep quality (but not sleep quantity) in clinical and nonclinical samples (Rusch et al., 2019; Wang et al., 2020; Wayne et al., 2018). In addition, studies comparing expert Vipassana meditation practitioners to nonmeditating controls have found that expert meditators have greater amounts of slow wave sleep and a higher number of sleep cycles (Pattanashetty et al., 2010; Sulekha et al., 2006). The difference may be due to a shift in autonomic balance from sympathetic to parasympathetic dominance during contemplative practices that decreases perceptions of, and reactivity to, daily stressors and related threat arousal during the day. Decreased daily threat arousal may in turn bleed over into the evening, decreasing presleep arousal, and thus improving sleep quality (Winzeler et al., 2014).
Future Directions
The deep rest model has several important implications for future well-being and contemplative science research. Specific research questions and suggested methodological approaches following our model’s propositions are detailed in the Appendix. There are also broader implications from our model that if adopted would enhance the quality of research in contemplative science—we describe these next.
Contemplative science will be greatly improved when there is a larger focus on the psychophysiological states that the practices induce, and an exporation of how those states mediate the relationship between contemplative practices and health. Throughout this article, we describe several mechanistic components that future work can investigate (i.e., perceptions of psychological, social, and physical safety; parasympathetic and sympathetic activity during the practices, during a true resting baseline while awake, and during sleep; cellular processes related to energy production and restoration). Unpacking mechanistic pathways will allow for the development of more effective and targeted interventions.
Our model also suggests that future research should avoid the reductionist approach of breaking down each contemplative practice in order to dissect the “active ingredient” of a specific practice, and instead focus on testing whether the practice in its wholeness supports people in achieving a desired psychophysiological state. It could be that the specific type or style of practice matters less than whether the participant was in fact able to achieve a state of deep rest for a significant amount of time. Focusing on whether a person reaches a state of deep rest across a variety of practices may help individuals identify which one works best for them.
In bringing together the science of contemplative practices, stress physiology, and cellular functioning, we have made leaps in logic that do not yet have empirical support. The biggest area in need of testing within this model is whether contemplative practices influence the cellular-level optimization processes hypothesized. Specifically, do they impact the functions and behaviors of mitochondria involved in energy transformation and are those effects mediated in part by alterations in parasympathetic activity? Cardiac activity and respiration rate can be measured noninvasively and relatively inexpensively, and thus should be considered in future contemplative science studies. Increased use of these measures as either mechanisms or outcomes is a first step to advancing the mechanistic understanding of these practices. The use of gene expression to identify cellular restorative pathways is now less expensive and can be used in studies that collect blood samples. There is also evidence of other biological pathways not described in this article, including oxytocin (Mascaro et al., 2015), melatonin (Bushell & Theise, 2009; Harinath et al., 2004), antioxidants (Olivo, 2009), and epigenetic clocks (Chaix et al., 2017). Future studies should unpack whether these pathways operate in conjunction with one another or operate as separate biological mechanisms.
Finally, unpacking the relationship between contemplative practices and sleep health is of urgent importance given the strong evidence that sleep is fundamental to health and well-being along with the significant disruptions in sleep in a large percentage of U.S. adults and the emerging work on sleep inequity (Hale et al., 2020). Core research questions include: Does daily contemplative practice engagement increase sleep latency, quantity, and/or quality? Do deep rest interventions facilitate healthier sleep, further permitting rejuvenation processes during sleep? Can contemplative practices practiced during the day enhance toxin clearance during sleep, such as slow oscillations?
It is important to note that contemplative practices are not the only nonpharmacological interventions that increase mental health and decrease disease risk, and thus other practices leading to well-being should not be ignored. Physical activity, leisure activities, and time spent in nature, each have profound and long-lasting mental and physical health benefits (Ekelund et al., 2019; Fancourt et al., 2021; Frumkin et al., 2017; Kondo et al., 2018; Schertz & Berman, 2019). Additionally, there are other techniques for promoting the related concept of rejuvenation, such as through acute hormetic stress (Epel, 2020). There are many areas of well-being and biological optimization that are ripe for rigorous examination where aspects of the deep rest model may be relevant.
Conclusion
In this article, we presented a model of deep rest—a physiological and psychological state that creates a biological environment which allows for cellular restoration, and that we hypothesize can be facilitated through contemplative practices. We built on evidence that our bodies move between allostatic states depending on environmental signals, and propose that modern life demands that we spend much of the day in the energetically costly state of moderate threat arousal. We described how safety signals that are part of contemplative practices allow for movement away from threat arousal, thereby freeing energetic resources for repairing cellular damage and optimizing cellular functioning. We argued that deep rest, as an allostatic state, is one that is achieved through the combination of safety perceptions and parasympathetic dominance. Our core hypothesis is that spending time in a state of deep rest allows for more energetically efficient biological functioning and greater well-being and may have profound positive impacts on health and aging.
In conclusion, contemplative practices are a powerful tool for enhancing health. Routinely practicing a contemplative technique may reduce harmful stress-related threat arousal, promote cellular-level healing and restoration, and ultimately promote positive mental and physical health. Should the deep rest model be empirically substantiated, it would provide the basis to design more targeted, powerful, and equitable mind–body interventions to increase well-being across the lifespan.
Acknowledgments
Research reported in this publication was supported by Grants R24AG048024, U24AG072699 (PI: Elissa S. Epel), K01AG057859 (PI: Alexandra D. Crosswell), R00AG062778 (PI: Stefanie E. Mayer) from the National Institute on Aging, and by Grant T32MH019391 from the National Institute of Mental Health.
The authors thank their colleagues Wendy Berry Mendes, George Slavich and Quinn Conklin for their helpful comments on the article. They also thank Raheem Lewis for giving permission to quote from his essay produced and published as part of the Urban Yogis project (https://www.urbanyogis.org/). Figures for this article were illustrated by James Adams Illustration (https://jamesadamsart.com/).
Appendix
Deep Rest Model Principles and Corresponding Research Questions
To provide further evidence for the model, we lay out the deep rest model principles - our core assumptions and the testable research questions, along with potential scientific approaches for examining each assumption.
Principle 1: Chronic Stress Arousal Is Common
Assumption:
Most working-age adults living in Western urban societies have elevated stress-related physiology across the day, indicating chronic stress arousal.
Research question:
Do working-age adults living in Western urban societies have greater daily cardiovascular and sympathetic activation as compared to groups for whom daily and trait perceived stress is lower and social safety is higher, such as in groups with greater social support or sense of belonging (e.g., collectivist cultural groups, or communities with greater social networks)?
Possible approach:
The primary method for testing the biology of social safety includes wearable technology that continuously monitors how the autonomic nervous system responds to different contexts. It includes minute to minute assessment of stress-related physiology including heart rate, heart rate variability, respiration rate, blood pressure, and ideally sympathetic activity. This method will enable researchers to test deep rest hypotheses across large groups of people throughout the world. For a sociobiological approach, it will be important to examine how this daily-level physiological activity differs based on demographic subgroups (e.g., age, race/ethnicity, geography, cultural groups, intersectionality). Cross-cultural data may be especially useful to examine group level variations. With recent advancements is biomarker assessment (using blood and saliva-based approaches), it will soon be feasible to examine related biomarkers in large global population-based studies such as proteomic and gene expression patterns to examine pathways of arousal, restoration, and aging in large samples.
Principle 2: There Is a Biological Signature of Safety
Assumption:
Objective and subjective measures of safety will be associated with a basal biological signature of high parasympathetic and low sympathetic activity, as well as less cellular damage and active cellular restoration.
Research questions:
Does feeling safe and being in safe environments have a unique biological signature compared to states of moderate threat or acute threat, and if so, what are the core measurable elements of this signature? Which dimension of safety (physical, social, psychological) is more highly correlated with this biological signature? Is there evidence that this biological safety signature is present and measurable during the day and at night, during sleep? How does feeling safe influence sleep-related neural and autonomic nervous system patterns?
Possible approach:
A biological signature of safety could be quantified by comparing profiles of biological indices (i.e., select autonomic, neuroendocrine, immune, and cellular-functioning biomarkers of stress and restoration) using both stable metrics reflecting baseline states and examining change from when individuals move from unsafe to safe conditions. Examples include deployed military members who then return home to safe conditions, unemployed individuals who obtain financially stabilizing jobs, or individuals who move out of conflictual, chaotic, and/or unsafe home environments to safer residences. In addition, alterations in biological signals of safety could be tested to quantify the amount of intraindividual variation in acute changes in safety conditions (workday versus weekend, with strangers versus with close others, noisy urban versus peaceful natural settings). It will be important to test the validity of biological signatures of safety with psychosocial constructs related to safety perceptions (e.g., perceptions of neighborhood safety, marital satisfaction, sense of belonging, workplace support, financial well-being) and threat (e.g. danger, violence, criticism, social rejection, discrimination, financial strain).
Principle 3: The Deep Rest State Is Quantifiable
Assumption:
It is possible to quantifiably measure whether and for how long an individual is spending in a state of deep rest each day.
Research questions:
What are the biological indicators of states of deep rest? Can behvioral metrics from wearable technology quantify whether and for how long an individual is spending in rest each day? What experimental approaches can induce states of deep rest that get close to the autonomic profile seen during NREM slow wave sleep (e.g., intensive meditation practice or yin yoga)?
Possible approach:
Given that NREM slow wave sleep is the deepest rest period during each day, autonomic activity at this time could be used as an individual’s “true” deep rest biological signature. Wearable activity could then track how close an individual gets to reaching that state during the day, and whether spending more time close to the true state of deep rest is associated with greater well-being. This is most easily tested with wearble technology that captures both parasympathetic and sympathetic activity over the entire day. Experimental studies could then test whether all or only specific forms of restorative activities allow individuals to reach their own “true” deep rest biological signature.
Priniple 4: A Felt Sense of Safety Underlies Emotional Well-Being
Assumption:
Contemplative practices are effective in improving emotional well-being to the extent they create perceived psychological safety.
Research questions:
Do perceptions of safety increase after contemplative practice sessions? Is this a core mechanism by which contemplative practices reduce perceived stress? Do those with contemplative practice as part of their daily life show a daily emotion profile indicative of high social safety and connectivity?
Possible approach:
To test these questions, perceptions of felt safety and social safety (trust, belonging, compassion) should be directly measured with self-report scales (e.g., L. Morton et al., 2022) in intervention and experimental studies. In addition, novel measures need to be developed to capture and differentiate between types of safety such as psychological, social, physical, and unconscious safety. Unconscious safety is a concept in need of further development as it may play a crucial role in the processes described in this paper. Ecological momentary assessment technology can capture both perceptions of safety and emotional experiences throughout daily life, ideally coupled with wearable technology capturing continuous autonomic nervous system activity and other physiological measures such as saliva sampling for hormonal patterns.
Principle 5: Spending Time in Deep Rest Increases Basal Parasympathetic Activity
Assumption:
Engaging in contemplative practices consistently over time will alter the resting allostatic state, shifting it toward an autonomic profile of greater parasympathetic dominance, which is ultimately associated with greater well-being.
Research questions:
Do long-term contemplative practitioners have parasympathetic dominance at rest during daily life, when not engaging in a contemplative practice? Do they recover from stress more quickly and spend more time in rest and deep rest states during the day? Do people who spend more time in rest and deep rest report greater well-being?
Possible approach:
To test this, wearable technology capturing continuous daily autonomic activity could be tested as a way to assess an individual’s time spent in various allostatic states. Previous studies have defined “rest state” physiological activity by having research participants engage in “restful” activities such as sitting quietly with nothing to do in the research laboratory setting while waiting for a study protocol to start. However, because waiting in a research setting may in fact be anxiety inducing for some individuals, measurements taken at that time may not be truly capturing “rest” state physiology.
One possible way to capture true rest, is to create opportunities in a lab setting for participants to engage in self-selected leisure activities most susceptible to elicit rest (e.g., setting up a comfortable and welcoming room with a comfortable chair and having participants bring a book to read). This could provide an individual’s rest state physiological baseline which would serve as a comparison point for daily data that is collected with wearable technology and ecological momentary assessment methodology. With this approach, researchers could track how much time an individual spends in a resting physiological state each day naturalistically compared to their own true resting baseline. This same lab-based method to creating a true assessment of rest state physiology could be used to create individualized physiological activity ranges for various allostatic states. Capturing individualized allostatic state physiology would allow for the quantification of how much time individuals are spending in each state, and how that ties to well-being (e.g., if more time is spent in rest or deep rest in a single day, is that person less fatigued?).
Principle 6: Unsafe Environments Prevent Rest States
Assumption:
Environmental contexts with consistent threats (e.g., unsafe neighborhoods, chaotic homes) make it more challenging to experience rest states.
Research questions:
Is it possible to reach states of rest when the context of the environment is psychologically, socially, and/or physically unsafe? Under what conditions can rest states be achieved in environments where real or perceived threat is present?
Possible approach:
To test this, qualitative methods can be deployed to explore how and when rest and deep rest is felt for those living in unsafe or chronically stressful contexts. Quantitative approaches involving experimental induction of rest and deep rest states could also be used in individuals living in chronically stressful contexts to see if and how it is possible to override the persistent threat perception and achieve rest state physiology. Another approach would be to examine autonomic functioning during deep sleep (the ultimate state of deep rest) in people who differ in their level of environmental safety.
Principle 7: Some People May Uniquely Benefit From Deep Rest
Assumption:
Trauma-exposed and certain clinical populations operate in higher threat arousal states in daily life compared to nontrauma exposed or nonclinical populations, and thus may uniquely benefit from spending more time in rest states.
Research questions:
Are contexts such as history of trauma, current clinical disorder (i.e., anxiety), or chronic stress, associated with greater daily physiological arousal and decreased ability to perceive safety? Do these groups uniquely benefit from contemplative practices?
Potential approach:
The benefits of achieving rest states for trauma-exposed and clinical populations should be explored since rest may be particularly beneficial to those whose nervous systems have a high sympathetic baseline. Additionally, considerable research has shown that trauma-exposed and clinical populations lack restorative sleep, and increasing time spent in rest states during the day may reduce presleep arousal and thus improve overall sleep quality. Intervention studies in individuals with a history of trauma and specific clinical samples should examine whether contemplative practice training can alter daily allostatic states, self-reported daily feelings of threat and safety, and sleep metrics in ways that benefit overall health.
Footnotes
There are no relevant data or study materials to make publicly available. A general description of the model was shared by senior author of this article, Elissa S. Epel, in the introduction of the book The Stress Prescription, citing the article under review. A preprint of an earlier draft of the article was posted at https://psyarxiv.com/bkg3j. Elissa S. Epel is an advisor to Meru Health and joined the Oura Medical Advisory Board in July 2023, after this article was submitted.
References
- Ackermann D (2022). The offering of incense make: Jewish mystical perspectives on disease and healing. Dialog: A Journal of Theology, 61(2), 119–124. 10.1111/dial.12730 [DOI] [Google Scholar]
- Aklin WM, Stoeckel LE, Green PA, Keller C, King JW, Nielsen L, & Hunter C (2020). Commentary: National Institutes of Health (NIH) Science of Behavior Change (SOBC). Health Psychology Review, 14(1), 193–198. 10.1080/17437199.2020.1716383 [DOI] [PubMed] [Google Scholar]
- American Psychological Association. (2020). Stress in America™ 2020: A National Mental Health Crisis. https://www.apa.org/news/press/releases/stress/2020/sia-mental-health-crisis.pdf
- American Psychological Association. (2022). Stress in America™ 2022. https://www.apa.org/news/press/releases/stress/2022/october-2022-topline-data.pdf
- Atsumi T, & Tonosaki K (2007). Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Research, 150(1), 89–96. 10.1016/j.psychres.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Bachen EA, Muldoon MF, Matthews KA, & Manuck SB (2002). Effects of hemoconcentration and sympathetic activation on serum lipid responses to brief mental stress. Psychosomatic Medicine, 64(4), 587–594. 10.1097/01.PSY.0000021943.35402.8A [DOI] [PubMed] [Google Scholar]
- Balzarotti S, Biassoni F, Colombo B, & Ciceri MR (2017). Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biological Psychology, 130, 54–66. 10.1016/j.biopsycho.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Bangee M, Harris RA, Bridges N, Rotenberg KJ, & Qualter P (2014). Loneliness and attention to social threat in young adults: Findings from an eye tracker study. Personality and Individual Differences, 63, 16–23. 10.1016/j.paid.2014.01.039 [DOI] [Google Scholar]
- Banushm B, Brendle M, Ragnhildstveit A, Murphy T, Moore C, Egberts J, & Robison R (2023). Breathwork interventions for adults with clinically diagnosed anxiety disorders: A scoping review. Brain Sciences, 13(2), 256. 10.3390/brainsci13020256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RJ, & Phillips NH (1995). Energy conservation and sleep. Behavioural Brain Research, 69(1–2), 65–73. 10.1016/0166-4328(95)00002-B [DOI] [PubMed] [Google Scholar]
- Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, & Lagi A (2001). Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: Comparative study. The BMJ, 323(7327), Article 1446. 10.1136/bmj.323.7327.1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Spadacini G, Bellwon J, Hajric R, Roskamm H, & Frey AW (1998). Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet, 351(9112), 1308–1311. 10.1016/S0140-6736(97)10341-5 [DOI] [PubMed] [Google Scholar]
- Bhasin MK, Denninger JW, Huffman JC, Joseph MG, Niles H, Chad-Friedman E, Goldman R, Buczynski-Kelley B, Mahoney BA, Fricchione GL, Dusek JA, Benson H, Zusman RM, & Libermann TA (2018). Specific transcriptome changes associated with blood pressure reduction in hypertensive patients after relaxation response training. Journal of Alternative and Complementary Medicine, 24(5), 486–504. 10.1089/acm.2017.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin MK, Dusek JA, Chang BH, Joseph MG, Denninger JW, Fricchione GL, Benson H, & Libermann TA (2013). Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLOS ONE, 8(5), Article e62817. 10.1371/journal.pone.0062817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, & Slavich GM (2016). Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Annals of the New York Academy of Sciences, 1373(1), 13–24. 10.1111/nyas.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz LV, & Kohl BG Jr. (2012). Addressing racism in the organization: The role of white racial affinity groups in creating change. Administration in Social Work, 36(5), 479–498. 10.1080/03643107.2011.624261 [DOI] [Google Scholar]
- Blum J, Rockstroh C, & Göritz AS (2019). Heart rate variability biofeedback based on slow-paced breathing with immersive virtual reality nature scenery. Frontiers in Psychology, 10, Article 2172. 10.3389/fpsyg.2019.02172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobba-Alves N, Juster RP, & Picard M (2022). The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology, 146, Article 105951. 10.1016/j.psyneuen.2022.105951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobba-Alves N, Sturm G, Lin J, Ware SA, Karan KR, Monzel A, Bris C, Procaccio V, Lenaers G, Higgins-Chen A, Levine M, Horvath S, Santhanam BS, Kaufman BA, Hirano M, Epel E, & Picard M (2022). Chronic glucocorticoid stress reveals increased energy expenditure and accelerated aging as cellular features of allostatic load. bioRxiv. 10.1101/2022.02.22.481548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Arevalo J, & Cole SW (2014). Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology, 43, 20–29. 10.1016/j.psyneuen.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, & Irwin MR (2016). Mind–body therapies and control of inflammatory biology: A descriptive review. Brain, Behavior, and Immunity, 51, 1–11. 10.1016/j.bbi.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–124. 10.1016/j.jpsychores.2005.06.074 [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, & Thayer JF (2005). Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology, 30(10), 1043–1049. 10.1016/j.psyneuen.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Verkuil B, & Thayer JF (2017). Exposed to events that never happen: Generalized unsafety, the default stress response, and prolonged autonomic activity. Neuroscience and Biobehavioral Reviews, 74(B), 287–296. 10.1016/j.neubiorev.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Verkuil B, & Thayer JF (2018). Generalized unsafety theory of stress: Unsafe environments and conditions, and the default stress response. International Journal of Environmental Research and Public Health, 15(3), Article 464. 10.3390/ijerph15030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, & Ryan RM (2003). The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology, 84(4), 822–848. 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, & Creswell JD (2007). Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry, 18(4), 211–237. 10.1080/10478400701598298 [DOI] [Google Scholar]
- Brown RP, & Gerbarg PL (2005). Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: Part I-neurophysiologic model. Journal of Alternative and Complementary Medicine, 11(1), 189–201. 10.1089/acm.2005.11.189 [DOI] [PubMed] [Google Scholar]
- Bushell WC, & Theise ND (2009). Toward a unified field of study: Longevity, regeneration, and protection of health through meditation and related practices. Annals of the New York Academy of Sciences, 1172(1), 5–19. 10.1111/j.1749-6632.2009.04959.x [DOI] [PubMed] [Google Scholar]
- Büssing A, Michalsen A, Khalsa SBS, Telles S, & Sherman KJ (2012). Effects of yoga on mental and physical health: A short summary of reviews. Evidence-Based Complementary and Alternative Medicine, 2012, Article 165410. 10.1155/2012/165410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernes R, & Zimlichman R (2017). Role of paced breathing for treatment of hypertension. Current Hypertension Reports, 19(6), Article 45. 10.1007/s11906-017-0742-1 [DOI] [PubMed] [Google Scholar]
- Chaix R, Alvarez-López MJ, Fagny M, Lemee L, Regnault B, Davidson RJ, Lutz A, & Kaliman P (2017). Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology, 85, 210–214. 10.1016/j.psyneuen.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Piazza JR, Mogle J, Sliwinski MJ, & Almeida DM (2013). The wear and tear of daily stressors on mental health. Psychological Science, 24(5), 733–741. 10.1177/0956797612462222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaya MS, & Nagendra HR (2008). Long-term effect of yogic practices on diurnal metabolic rates of healthy subjects. International Journal of Yoga, 1(1), 27–32. 10.4103/0973-6131.36761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, & Matthews KA (2004). Socioeconomic status and health in adolescents: The role of stress interpretations. Child Development, 75(4), 1039–1052. 10.1111/j.1467-8624.2004.00724.x [DOI] [PubMed] [Google Scholar]
- Cheryan S, Plaut VC, Davies PG, & Steele CM (2009). Ambient belonging: How stereotypical cues impact gender participation in computer science. Journal of Personality and Social Psychology, 97(6), 1045–1060. 10.1037/a0016239 [DOI] [PubMed] [Google Scholar]
- Chhetri P, Shrestha L, Rana BS, Banstola D, & Mahotra NB (2020). Immediate effects of yoga based relaxation technique yoga nidra on heart rate variability in young and healthy volunteers. Journal of Karnali Academy of Health Sciences, 3(2), 65–72. 10.3126/jkahs.v3i2.30807 [DOI] [Google Scholar]
- Chiesa A, Calati R, & Serretti A (2011). Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review, 31(3), 449–464. 10.1016/j.cpr.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Chiesa A, & Serretti A (2009). Mindfulness-based stress reduction for stress management in healthy people: A review and meta-analysis. Journal of Alternative and Complementary Medicine, 15(5), 593–600. 10.1089/acm.2008.0495 [DOI] [PubMed] [Google Scholar]
- Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Baratta MV, Paul ED, Campeau S, Watkins LR, Barth DS, & Maier SF (2008). The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. The Journal of Neuroscience, 28(50), 13703–13711. 10.1523/JNEUROSCI.4270-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, & Sangha S (2012). Inhibition of fear by learned safety signals: A mini-symposium review. The Journal of Neuroscience, 32(41), 14118–14124. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JGN, Benison AM, Barth DS, Watkins LR, & Maier SF (2011). Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biological Psychiatry, 70(5), 458–464. 10.1016/j.biopsych.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Sbarra DA (2015). Social baseline theory: The social regulation of risk and effort. Current Opinion in Psychology, 1, 87–91. 10.1016/j.copsyc.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin QA, Crosswell AD, Saron CD, & Epel ES (2019). Meditation, stress processes, and telomere biology. Current Opinion in Psychology, 28, 92–101. 10.1016/j.copsyc.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2003). Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology, 13(4), 500–505. 10.1016/S0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, & Dolan RJ (2003). Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain, 126(10), 2139–2152. 10.1093/brain/awg216 [DOI] [PubMed] [Google Scholar]
- Crosswell AD, Moreno PI, Raposa EB, Motivala SJ, Stanton AL, Ganz PA, & Bower JE (2017). Effects of mindfulness training on emotional and physiologic recovery from induced negative affect. Psychoneuroendocrinology, 86, 78–86. 10.1016/j.psyneuen.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl CJ, & Davidson RJ (2019). Mindfulness and the contemplative life: Pathways to connection, insight, and purpose. Current Opinion in Psychology, 28, 60–64. 10.1016/j.copsyc.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Damasio A (2018). The strange order of things: Life, feeling, and the making of cultures. Vintage Books. [Google Scholar]
- Daubenmier J, Epel ES, Moran PJ, Thompson J, Mason AE, Acree M, Goldman V, Kristeller J, Hecht FM, & Mendes WB (2019). A randomized controlled trial of a mindfulness-based weight loss intervention on cardiovascular reactivity to social-evaluative threat among adults with obesity. Mindfulness, 10(12), 2583–2595. 10.1007/s12671-019-01232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, & Dahl CJ (2017). Varieties of contemplative practice. JAMA Psychiatry, 74(2), 121–123. 10.1001/jamapsychiatry.2016.3469 [DOI] [PubMed] [Google Scholar]
- de Vibe M, Solhaug I, Tyssen R, Friborg O, Rosenvinge JH, Sørlie T, Halland E, & Bjørndal A (2015). Does personality moderate the effects of mindfulness training for medical and psychology students? Mindfulness, 6(2), 281–289. 10.1007/s12671-013-0258-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald JN, Sahdra BK, Van Zanden B, Duineveld JJ, Atkins PWB, Marshall SL, & Ciarrochi J (2019). Does your mindfulness benefit others? A systematic review and meta-analysis of the link between mindfulness and prosocial behaviour. British Journal of Psychology, 110(1), 101–125. 10.1111/bjop.12338 [DOI] [PubMed] [Google Scholar]
- Doufesh H, Ibrahim F, Ismail NA, & Wan Ahmad WA (2014). Effect of Muslim prayer (Salat) on α electroencephalography and its relationship with autonomic nervous system activity. Journal of Alternative and Complementary Medicine, 20(7), 558–562. 10.1089/acm.2013.0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, & Manji HK (2009). Dynamic regulation of mitochondrial function by glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3543–3548. 10.1073/pnas.0812671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J, & Pinto-Gouveia J (2017). Positive affect and parasympathetic activity: Evidence for a quadratic relationship between feeling safe and content and heart rate variability. Psychiatry Research, 257, 284–289. 10.1016/j.psychres.2017.07.077 [DOI] [PubMed] [Google Scholar]
- Duffy KA, McLaughlin KA, & Green PA (2018). Early life adversity and health-risk behaviors: Proposed psychological and neural mechanisms. Annals of the New York Academy of Sciences, 1428(1), 151–169. 10.1111/nyas.13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Chang BH, Zaki J, Lazar S, Deykin A, Stefano GB, Wohlhueter AL, Hibberd PL, & Benson H (2006). Association between oxygen consumption and nitric oxide production during the relaxation response. Medical Science Monitor, 12(1), CR1–CR10. 10.1371/journal.pone.0002576 [DOI] [PubMed] [Google Scholar]
- Dusek JA, Otu HH, Wohlhueter AL, Bhasin M, Zerbini LF, Joseph MG, Benson H, & Libermann TA (2008). Genomic counter-stress changes induced by the relaxation response. PLOS ONE, 3(7), Article e2576. 10.1371/journal.pone.0002576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Mamaev I, Ditzen B, & Sailer U (2020). Calming effects of touch in human, animal, and robotic interaction—Scientific state-of-the-art and technical advances. Frontiers in Psychiatry, 11, Article 555058. 10.3389/fpsyt.2020.555058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economic News. (2021). American Time Use Survey Results. https://www.bls.gov/news.release/atus.t12.htm
- Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD, & Naliboff BD (2011). Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11721–11726. 10.1073/pnas.1108239108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn IM, Hagströmer M, Edwardson C, Yates T, Shiroma E, Anderssen SA, & Lee IM (2019). Dose–response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. The BMJ, 366, Article l4570. 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES (2020). The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Research Reviews, 63, Article 101167. 10.1016/j.arr.2020.101167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, & Mendes WB (2018). More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146–169. 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episcopal Church. (n.d.). Incense. In An episcopal dictionary of the church. Retrieved August 14, 2023, from https://www.episcopalchurch.org/glossary/incense/ [Google Scholar]
- Euteneuer F, Mills PJ, Pung MA, Rief W, & Dimsdale JE (2014). Neighborhood problems and nocturnal blood pressure dipping. Health Psychology, 33(11), 1366–1372. 10.1037/hea0000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2010). Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the New York Academy of Sciences, 1186(1), 174–189. 10.1111/j.1749-6632.2009.05336.x [DOI] [PubMed] [Google Scholar]
- Fancourt D, Aughterson H, Finn S, Walker E, & Steptoe A (2021). How leisure activities affect health: A narrative review and multi-level theoretical framework of mechanisms of action. The Lancet Psychiatry, 8(4), 329–339. 10.1016/S2215-0366(20)30384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Arizmendi C, Van Cappellen P, Firestine AM, Brantley MM, Kim SL, Brantley J, & Salzberg S (2019). Do contemplative moments matter? Effects of informal meditation onemotions and perceived social integration. Mindfulness, 10(9), 1915–1925. 10.1007/s12671-019-01154-2 [DOI] [Google Scholar]
- Fricchione G (2023). Mind body medicine: A modern bio-psycho-social model forty-five years after Engel. BioPsychoSocial Medicine, 17(1), Article 12. 10.1186/s13030-023-00268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumkin H, Bratman GN, Breslow SJ, Cochran B, Kahn PH, Lawler JJ, Levin PS, Tandon PS, Varanasi U, Wolf KL, & Wood SA (2017). Nature contact and human health: A research agenda. Environmental Health Perspectives, 125(7), Article 075001. 10.1289/EHP1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, & Lewis LD (2019). Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science, 366(6465), 628–631. 10.1126/science.aax5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Hoerger M, Talbot NL, Krasner MS, Knight JM, Moynihan JA, & Duberstein PR (2013). Toward identifying the effects of the specific components of mindfulness-based stress reduction on biologic and emotional outcomes among older adults. Journal of Alternative and Complementary Medicine, 19(10), 787–792. 10.1089/acm.2012.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen RJS, & Band GPH (2018). Breath of life: The respiratory vagal stimulation model of contemplative activity. Frontiers in Human Neuroscience, 12, Article 397. 10.3389/fnhum.2018.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, & Wager TD (2015). Brain–body pathways linking psychological stress and physical health. Current Directions in Psychological Science, 24(4), 313–321. 10.1177/0963721415581476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles RE, Blanc H, Cann HM, & Wallace DC (1980). Maternal inheritance of human mitochondrial DNA. Proceedings of the National Academy of Sciences of the United States of America, 77(11), 6715–6719. 10.1073/pnas.77.11.6715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, & Simpson TL (2018). Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clinical Psychology Review, 59, 52–60. 10.1016/j.cpr.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EMS, Gould NF, Rowland-Seymour A, Sharma R, Berger Z, Sleicher D, Maron DD, Shihab HM, Ranasinghe PD, Linn S, Saha S, Bass EB, & Haythornthwaite JA (2014). Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Internal Medicine, 174(3), 357–368. 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn P, & Stigsdotter UK (2010). The relation between perceived sensory dimensions of urban green space and stress restoration. Landscape and Urban Planning, 94(3–4), 264–275. 10.1016/j.landurbplan.2009.10.012 [DOI] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, & Fahey JL (2004). Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic Medicine, 66(6), 915–924. 10.1097/01.psy.0000143639.61693.ef [DOI] [PubMed] [Google Scholar]
- Hale L, Hill TD, & Burdette AM (2010). Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Preventive Medicine, 51(3–4), 275–278. 10.1016/j.ypmed.2010.06.017 [DOI] [PubMed] [Google Scholar]
- Hale L, Troxel W, & Buysse DJ (2020). Sleep health: An opportunity for public health to address health equity. Annual Review of Public Health, 41(1), 81–99. 10.1146/annurev-publhealth-040119-094412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada D, Asanoi H, Takagawa J, Ishise H, Ueno H, Oda Y, Goso Y, Joho S, & Inoue H (2014). Slow and deep respiration suppresses steady-state sympathetic nerve activity in patients with chronic heart failure: From modeling to clinical application. American Journal of Physiology-Heart and Circulatory Physiology, 307(8), H1159–H1168. 10.1152/ajpheart.00109.2014 [DOI] [PubMed] [Google Scholar]
- Harinath K, Malhotra AS, Pal K, Prasad R, Kumar R, Kain TC, Rai L, & Sawhney RC (2004). Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. Journal of Alternative and Complementary Medicine, 10(2), 261–268. 10.1089/107555304323062257 [DOI] [PubMed] [Google Scholar]
- Hawley LL, Schwartz D, Bieling PJ, Irving J, Corcoran K, Farb NAS, Anderson AK, & Segal ZV (2014). Mindfulness practice, rumination and clinical outcome in mindfulness-based treatment. Cognitive Therapy and Research, 38(1), 1–9. 10.1007/s10608-013-9586-4 [DOI] [Google Scholar]
- Hill BG, Shiva S, Ballinger S, Zhang J, & Darley-Usmar VM (2019). Bioenergetics and translational metabolism: Implications for genetics, physiology and precision medicine. Biological Chemistry, 401(1), 3–29. 10.1515/hsz-2019-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TD, Burdette AM, & Hale L (2009). Neighborhood disorder, sleep quality, and psychological distress: Testing a model of structural amplification. Health & Place, 15(4), 1006–1013. 10.1016/j.healthplace.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, Colaiaco B, Maher AR, Shanman RM, Sorbero ME, & Maglione MA (2017). Mindfulness meditation for chronic pain: Systematic review and meta-analysis. Annals of Behavioral Medicine, 51(2), 199–213. 10.1007/s12160-016-9844-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, & Ott U (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science, 6(6), 537–559. 10.1177/1745691611419671 [DOI] [PubMed] [Google Scholar]
- Househam AM, Peterson CT, Mills PJ, & Chopra D (2017). The effects of stress and meditation on the immune system, human microbiota, and epigenetics. Advances in Mind–Body Medicine, 31(4), 10–25. [PubMed] [Google Scholar]
- Hummel EM, Hessas E, Müller S, Beiter T, Fisch M, Eibl A, Wolf OT, Giebel B, Platen P, Kumsta R, & Moser DA (2018). Cell-free DNA release under psychosocial and physical stress conditions. Translational Psychiatry, 8(1), Article 236. 10.1038/s41398-018-0264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M, Al-Braiki F, Dailey S, Russell R, & Simon K (2018). Mindfulness training, yoga, or both? Dismantling the active components of a mindfulness-based stress reduction intervention. Mindfulness, 9(2), 512–520. 10.1007/s12671-017-0793-z [DOI] [Google Scholar]
- Itani O, Jike M, Watanabe N, & Kaneita Y (2017). Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Medicine, 32, 246–256. 10.1016/j.sleep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Mendes WB, Blackstock E, & Schmader T (2010). Turning the knots in your stomach into bows: Reappraising arousal improves performance on the GRE. Journal of Experimental Social Psychology, 46(1), 208–212. 10.1016/j.jesp.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Mendes WB, & Nock MK (2013). Improving acute stress responses. Current Directions in Psychological Science, 22(1), 51–56. 10.1177/0963721412461500 [DOI] [Google Scholar]
- Jang JY, Blum A, Liu J, & Finkel T (2018). The role of mitochondria in aging. The Journal of Clinical Investigation, 128(9), 3662–3670. 10.1172/JCI120842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen M, Heerkens Y, Kuijer W, Van Der Heijden B, & Engels J (2018). Effects of mindfulness-based stress reduction on employees’ mental health: A systematic review. PLOS ONE, 13(1), Article e0191332. 10.1371/journal.pone.0191332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AP, Morrison AB, Dainer-Best J, Parker S, Rostrup N, & Stanley EA (2015). Minds “at attention”: Mindfulness training curbs attentional lapses in military cohorts. PLOS ONE, 10(2), Article e0116889. 10.1371/journal.pone.0116889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, & Duncan EJ (2005). Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry, 57(12), 1559–1564. 10.1016/j.biopsych.2005.02.025 [DOI] [PubMed] [Google Scholar]
- Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, & Wright KP (2011). Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. The Journal of Physiology, 589(1), 235–244. 10.1113/jphysiol.2010.197517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri M, Metzl-Raz E, Jona G, & Barkai N (2016). The cost of protein production. Cell Reports, 14(1), 22–31. 10.1016/j.celrep.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman P (2019). Epigenetics and meditation. Current Opinion in Psychology, 28, 76–80. 10.1016/j.copsyc.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Kearney BE, & Lanius RA (2022). The brain–body disconnect: A somatic sensory basis for trauma-related disorders. Frontiers in Neuroscience, 16, Article 1015749. 10.3389/fnins.2022.1015749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME, Foltz C, Cavanagh JF, Cullen M, Giese-Davis J, Jennings P, Rosenberg EL, Gillath O, Shaver PR, Wallace BA, & Ekman P (2012). Contemplative/emotion training reduces negative emotional behavior and promotes prosocial responses. Emotion, 12(2), 338–350. 10.1037/a0026118 [DOI] [PubMed] [Google Scholar]
- Khuanjing T, Palee S, Kerdphoo S, Jaiwongkam T, Anomasiri A, Chattipakorn SC, & Chattipakorn N (2021). Donepezil attenuated cardiac ischemia/reperfusion injury through balancing mitochondrial dynamics, mitophagy, and autophagy. Translational Research, 230, 82–97. 10.1016/j.trsl.2020.10.010 [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, & Gilbert DT (2010). A wandering mind is an unhappy mind. Science, 330(6006), Article 932. 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, & Frangos E (2022). Vagus nerve afferent stimulation: Projection into the brain, reflexive physiological, perceptual, and behavioral responses, and clinical relevance. Autonomic Neuroscience: Basic & Clinical, 237, Article 102908. 10.1016/j.autneu.2021.102908 [DOI] [PubMed] [Google Scholar]
- Kondo MC, Fluehr JM, McKeon T, & Branas CC (2018). Urban green space and its impact on human health. International Journal of Environmental Research and Public Health, 15(3), Article 445. 10.3390/ijerph15030445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FCGJ, van der Hoeven JG, & Pickkers P (2014). Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proceedings of the National Academy of Sciences of the United States of America, 111(20), 7379–7384. 10.1073/pnas.1322174111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromenacker BW, Sanova AA, Marcus FI, Allen JJB, & Lane RD (2018). Vagal mediation of low-frequency heart rate variability during slow yogic breathing. Psychosomatic Medicine, 80(6), 581–587. 10.1097/PSY.0000000000000603 [DOI] [PubMed] [Google Scholar]
- Krygier JR, Heathers JAJ, Shahrestani S, Abbott M, Gross JJ, & Kemp AH (2013). Mindfulness meditation, well-being, and heart rate variability: A preliminary investigation into the impact of intensive Vipassana meditation. International Journal of Psychophysiology, 89(3), 305–313. 10.1016/j.ijpsycho.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, … Byford S (2015). Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antide-pressant treatment in the prevention of depressive relapse or recurrence (PREVENT): A randomised controlled trial. Lancet, 386(9988), 63–73. 10.1016/S0140-6736(14)62222-4 [DOI] [PubMed] [Google Scholar]
- Laborde S, Hosang T, Mosley E, & Dosseville F (2019). Influence of a 30-day slow-paced breathing intervention compared to social media use on subjective sleep quality and cardiac vagal activity. Journal of Clinical Medicine, 8(2), Article 193. 10.3390/jcm8020193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N (2002). Oxygen: The molecule that made the world. Oxford University Press. [Google Scholar]
- Lane N, & Martin W (2010). The energetics of genome complexity. Nature, 467(7318), 929–934. 10.1038/nature09486 [DOI] [PubMed] [Google Scholar]
- Lazarus RS (1993). From psychological stress to the emotions: A history of changing outlooks. Annual Review of Psychology, 44(1), 1–21. 10.1146/annurev.ps.44.020193.000245 [DOI] [PubMed] [Google Scholar]
- Le Nguyen KD, Lin J, Algoe SB, Brantley MM, Kim SL, Brantley J, Salzberg S, & Fredrickson BL (2019). Loving-kindness meditation slows biological aging in novices: Evidence from a 12-week randomized controlled trial. Psychoneuroendocrinology, 108, 20–27. 10.1016/j.psyneuen.2019.05.020 [DOI] [PubMed] [Google Scholar]
- Lee MS, Huh HJ, Kim BG, Ryu H, Lee HS, Kim JM, & Chung HT (2002). Effects of Qi-training on heart rate variability. The American Journal of Chinese Medicine, 30(4), 463–470. 10.1142/S0192415X02000491 [DOI] [PubMed] [Google Scholar]
- Lehrer P, Sasaki Y, & Saito Y (1999). Zazen and cardiac variability. Psychosomatic Medicine, 61(6), 812–821. 10.1097/00006842-199911000-00014 [DOI] [PubMed] [Google Scholar]
- Lemieux H, & Hoppel CL (2009). Mitochondria in the human heart. Journal of Bioenergetics and Biomembranes, 41(2), 99–106. 10.1007/s10863-009-9211-0 [DOI] [PubMed] [Google Scholar]
- Levine B, & Kroemer G (2008). Autophagy in the pathogenesis of disease. Cell, 132(1), 27–42. 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kobayashi M, Wakayama Y, Inagaki H, Katsumata M, Hirata Y, Hirata K, Shimizu T, Kawada T, Park BJ, Ohira T, Kagawa T, & Miyazaki Y (2009). Effect of phytoncide from trees on human natural killer cell function. International Journal of Immunopathology and Pharmacology, 22(4), 951–959. 10.1177/039463200902200410 [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, & Way BM (2007). Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18(5), 421–428. 10.1111/j.1467-9280.2007.01916.x [DOI] [PubMed] [Google Scholar]
- Lindsay EK, & Creswell JD (2019). Mindfulness, acceptance, and emotion regulation: Perspectives from monitor and acceptance theory (MAT). Current Opinion in Psychology, 28, 120–125. 10.1016/j.copsyc.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, & Croft JB (2016). Prevalence of healthy sleep duration among adults—United States, 2014. Morbidity and Mortality Weekly Report, 65(6), 137–141. 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, Joshi S, Wang H, Andersson U, Chavan SS, & Tracey KJ (2014). α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Molecular Medicine, 20(1), 350–358. 10.2119/molmed.2013.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwick-Rosenthal R, & Neufeld RWJ (1985). Heart beat interoception: A study of individual differences. International Journal of Psychophysiology, 3(1), 57–65. 10.1016/0167-8760(85)90020-0 [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Appelhans BM, Kraft A, & Brown A (2006). Never far from home: A cognitive–affective model of the impact of early-life family relationships on physiological stress responses in adulthood. Journal of Social and Personal Relationships, 23(2), 189–203. 10.1177/0265407506062466 [DOI] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, & Davidson RJ (2008). Attention regulation and monitoring in meditation. Trends in Cognitive Sciences, 12(4), 163–169. 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch EE, Malcoe LH, Laurent SE, Richardson J, Mitchell BC, & Meier HCS (2021). The legacy of structural racism: Associations between historic redlining, current mortgage lending, and health. SSM—Population Health, 14, Article 100793. 10.1016/j.ssmph.2021.100793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak HW, Gordon AM, Prather AA, Epel ES, & Mendes WB (2023). Acute and chronic stress associations with blood pressure: An ecological momentary assessment study on an app-based platform. Psychosomatic Medicine, 85(7), 585–595. 10.1097/PSY.0000000000001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarieva AM, Gorshkov VG, Li BL, Chown SL, Reich PB, & Gavrilov VM (2008). Mean mass-specific metabolic rates are strikingly similar across life’s major domains: Evidence for life’s metabolic optimum. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 16994–16999. 10.1073/pnas.0802148105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevych I, Schoierer J, Hartig T, Chudnovsky A, Hystad P, Dzhambov AM, de Vries S, Triguero-Mas M, Brauer M, Nieuwenhuijsen MJ, Lupp G, Richardson EA, Astell-Burt T, Dimitrova D, Feng X, Sadeh M, Standl M, Heinrich J, & Fuertes E (2017). Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environmental Research, 158, 301–317. 10.1016/j.envres.2017.06.028 [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Darcher A, Negi LT, & Raison CL (2015). The neural mediators of kindness-based meditation: A theoretical model. Frontiers in Psychology, 6, Article 109. 10.3389/fpsyg.2015.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow AH (1943). A theory of human motivation. Psychological Review, 50(4), 370–396. 10.1037/h0054346 [DOI] [Google Scholar]
- Masuo Y, Satou T, Takemoto H, & Koike K (2021). Smell and stress response in the brain: Review of the connection between chemistry and neuropharmacology. Molecules, 26(9), Article 2571. 10.3390/molecules26092571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Thayer JF (2018). How heart rate variability affects emotion regulation brain networks. Current Opinion in Behavioral Sciences, 19, 98–104. 10.1016/j.cobeha.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara E, & Ohira T (2018). Inhalation of Japanese cedar (Cryptomeria japonica) wood odor causes psychological relaxation after monotonous work among female participants. Biomedical Research, 39(5), 241–249. 10.2220/biomedres.39.241 [DOI] [PubMed] [Google Scholar]
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, & Bohr VA (2009). Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis, 30(1), 2–10. 10.1093/carcin/bgn250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Sapolsky RM (1995). Stress and cognitive function. Current Opinion in Neurobiology, 5(2), 205–216. 10.1016/0959-4388(95)80028-X [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, & Stadlan EM (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology, 34(7), 939–944. 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Acree M, Stewart A, Silas J, & Jones A (2018). The multidimensional assessment of interoceptive awareness, Version 2 (MAIA-2). PLOS ONE, 13(12), Article e0208034. 10.1371/journal.pone.0208034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Bell KA, Abu-Bader SH, & Kobayashi I (2018). Neighborhood stress and autonomic nervous system activity during sleep. Sleep, 41(6), Article zsy059. 10.1093/sleep/zsy059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, & Gee DG (2019). Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26970–26979. 10.1073/pnas.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Kershaw KN, Hudson D, Lim KA, & Ratliff S (2011). Job strain, workplace discrimination, and hypertension among older workers: The health and retirement study. Race and Social Problems, 3(1), 38–50. 10.1007/s12552-011-9041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, Carey RN, Johnston M, Rothman AJ, de Bruin M, Kelly MP, & Connell LE (2018). From theory-inspired to theory-based interventions: A protocol for developing and testing a methodology for linking behaviour change techniques to theoretical mechanisms of action. Annals of Behavioral Medicine, 52(6), 501–512. 10.1007/s12160-016-9816-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzel AS, Enríquez JA, & Picard M (2023). Multifaceted mitochondria: Moving mitochondrial science beyond function and dysfunction. Nature Metabolism, 5(4), 546–562. 10.1038/s42255-023-00783-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N, Irwin MR, Chung M, & Wang C (2014). The effects of mind–body therapies on the immune system: Meta-analysis. PLOS ONE, 9(7), Article e100903. 10.1371/journal.pone.0100903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AB, Goolsarran M, Rogers SL, & Jha AP (2014). Taming a wandering attention: Short-form mindfulness training in student cohorts. Frontiers in Human Neuroscience, 7, Article 897. 10.3389/fnhum.2013.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton L, Cogan N, Kolacz J, Calderwood C, Nikolic M, Bacon T, Pathe E, Williams D, Porges SW (2022). A new measure of feeling safe: Developing psychometric properties of the neuroception of Psychological Safety Scale (NPSS). Psychological Trauma: Theory, Research, Practice, and Policy. Advance online publication. 10.1037/tra0001313 [DOI] [PubMed] [Google Scholar]
- Morton ML, Helminen EC, & Felver JC (2020). A systematic review of mindfulness interventions on psychophysiological responses to acute stress. Mindfulness, 11(9), 2039–2054. 10.1007/s12671-020-01386-7 [DOI] [Google Scholar]
- Moyal N, Henik A, & Anholt GE (2014). Cognitive strategies to regulate emotions-current evidence and future directions. Frontiers in Psychology, 4, Article 1019. 10.3389/fpsyg.2013.01019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek MD, Phillips DT, Franklin MS, Broadway JM, & Schooler JW (2013). Young and restless: Validation of the Mind-Wandering Questionnaire (MWQ) reveals disruptive impact of mind-wandering for youth. Frontiers in Psychology, 4, Article 560. 10.3389/fpsyg.2013.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, & Eisenberger NI (2012). A social neuroscience perspective on stress and health. Social and Personality Psychology Compass, 6(12), 890–904. 10.1111/j.1751-9004.2012.00467.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgyal T (2006). Mahamudra: The moonlight: Quintessence of mind and meditation. Wisdom Publications. [Google Scholar]
- Nealis LJ, Sherry SB, Perrot T, & Rao S (2020). Self-critical perfectionism, depressive symptoms, and HPA-axis dysregulation: Testing emotional and physiological stress reactivity. Journal of Psychopathology and Behavioral Assessment, 42(3), 570–581. 10.1007/s10862-020-09793-9 [DOI] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, … Laughlin MR (2015). Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabolism, 22(1), 4–11. 10.1016/j.cmet.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wintering N, Waldman MR, Amen D, Khalsa DS, & Alavi A (2010). Cerebral blood flow differences between long-term meditators and non-meditators. Consciousness and Cognition, 19(4), 899–905. 10.1016/j.concog.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Nicholls D, & Ferguson S (2013). Bioenergetics (4th ed.). Academic Press. [Google Scholar]
- Olivo EL (2009). Protection throughout the life span: The psychoneur-oimmunologic impact of Indo-Tibetan meditative and yogic practices. Annals of the New York Academy of Sciences, 1172(1), 163–171. 10.1111/j.1749-6632.2009.04415.x [DOI] [PubMed] [Google Scholar]
- Onken LS, Carroll KM, Shoham V, Cuthbert BN, & Riddle M (2014). Reenvisioning clinical science: Unifying the discipline to improve the public health. Clinical Psychological Science, 2(1), 22–34. 10.1177/2167702613497932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina MB, Bond K, Karkhaneh M, Tjosvold L, Vandermeer B, Liang Y, Bialy L, Hooton N, Buscemi N, Dryden DM, & Klassen TP (2007). Meditation practices for health: State of the research. Evidence Report/Technology Assessment, 155, 1–263. [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, & Brosschot JF (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142(3), 231–259. 10.1037/bul0000036 [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, & Miller WL (2012). Role of mitochondria in steroidogenesis. Best Practice & Research Clinical Endocrinology & Metabolism, 26(6), 771–790. 10.1016/j.beem.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Parsons CE, Crane C, Parsons LJ, Fjorback LO, & Kuyken W (2017). Home practice in mindfulness-based cognitive therapy and mindfulness-based stress reduction: A systematic review and meta-analysis of participants’ mindfulness practice and its association with outcomes. Behaviour Research and Therapy, 95, 29–41. 10.1016/j.brat.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe MC, de Manincor M, Hallgren M, Baldwin PA, Tseberja J, & Parker AG (2021). Psychobiological mechanisms underlying the mental health benefits of yoga-based interventions: A narrative review. Mindfulness, 12(12), 2877–2889. 10.1007/s12671-021-01736-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe MC, Thompson DR, & Ski CF (2017). Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology, 86, 152–168. 10.1016/j.psyneuen.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Pattanashetty R, Sathiamma S, Talakkad SP, Nityananda P, Trichur R, & Kutty BM (2010). Practitioners of vipassana meditation exhibit enhanced slow wave sleep and REM sleep states across different age groups. Sleep and Biological Rhythms, 8(1), 34–41. 10.1111/j.1479-8425.2009.00416.x [DOI] [Google Scholar]
- Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, & Tracey KJ (2009). Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity, 23(1), 41–45. 10.1016/j.bbi.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CK, Henry IC, Mietus JE, Hausdorff JM, Khalsa G, Benson H, & Goldberger AL (2004). Heart rate dynamics during three forms of meditation. International Journal of Cardiology, 95(1), 19–27. 10.1016/j.ijcard.2003.02.006 [DOI] [PubMed] [Google Scholar]
- Perestelo-Perez L, Barraca J, Peñate W, Rivero-Santana A, & Alvarez-Perez Y (2017). Mindfulness-based interventions for the treatment of depressive rumination: Systematic review and meta-analysis. International Journal of Clinical and Health Psychology, 17(3), 282–295. 10.1016/j.ijchp.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocchi N, Piccirillo G, Fiorucci C, Moscucci F, Di Iorio C, Mastropietri F, Parrotta I, Pascucci M, Magrì D, & Ottaviani C (2017). Transcranial direct current stimulation enhances soothing positive affect and vagal tone. Neuropsychologia, 96, 256–261. 10.1016/j.neuropsychologia.2017.01.028 [DOI] [PubMed] [Google Scholar]
- Pew Research Center. (2017). Religious landscape study. https://www.pewresearch.org/religion/religious-landscape-study/
- Picard M (2022). Energy transduction and the mind–mitochondria connection. The Biochemist, 44(4), 14–18. 10.1042/bio_2022_118 [DOI] [Google Scholar]
- Picard M, & McEwen BS (2018). Psychological stress and mitochondria: A systematic review. Psychosomatic Medicine, 80(2), 141–153. 10.1097/PSY.0000000000000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, Epel E, & Sandi C (2018). An energetic view of stress: Focus on mitochondria. Frontiers in Neuroendocrinology, 49, 72–85. 10.1016/j.yfrne.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, & Wallace DC (2015). Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proceedings of the National Academy of Sciences of the United States of America, 112(48), E6614–E6623. 10.1073/pnas.1515733112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Wallace DC, & Burelle Y (2016). The rise of mitochondria in medicine. Mitochondrion, 30, 105–116. 10.1016/j.mito.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper S, Brosschot JF, van der Leeden R, & Thayer JF (2010). Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosomatic Medicine, 72(6), 570–577. 10.1097/PSY.0b013e3181dbc0e9 [DOI] [PubMed] [Google Scholar]
- Poli A, Gemignani A, Soldani F, & Miccoli M (2021). A systematic review of a polyvagal perspective on embodied contemplative practices as promoters of cardiorespiratory coupling and traumatic stress recovery for PTSD and OCD: Research methodologies and state of the art. International Journal of Environmental Research and Public Health, 18(22), Article 11778. 10.3390/ijerph182211778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. 10.1111/j.1469-8986.1995.tb01213.x [DOI] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. 10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Porges SW (2021). Polyvagal theory: A biobehavioral journey to sociality. Comprehensive Psychoneuroendocrinology, 7, Article 100069. 10.1016/j.cpnec.2021.100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2022). Polyvagal theory: A science of safety. Frontiers in Integrative Neuroscience, 16, Article 871227. 10.3389/fnint.2022.871227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Umans BD, Williams EK, Brust RD, & Liberles SD (2020). An airway protection program revealed by sweeping genetic control of vagal afferents. Cell, 181(3), 574–589.e14. 10.1016/j.cell.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann LMC, Engert V, Apostolakou F, Papassotiriou I, Chrousos GP, Vrtička P, & Singer T (2019). Only vulnerable adults show change in chronic low-grade inflammation after contemplative mental training: Evidence from a randomized clinical trial. Scientific Reports, 9(1), Article 19323. 10.1038/s41598-019-55250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraj P, Ramakrishnan AG, Nagendra HR, & Telles S (1998). Effect of two selected yogic breathing techniques of heart rate variability. Indian Journal of Physiology and Pharmacology, 42(4), 467–472. [PubMed] [Google Scholar]
- Reive C (2019). The biological measurements of mindfulness-based stress reduction: A systematic review. Explore, 15(4), 295–307. 10.1016/j.explore.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, & Seeman TE (2002). Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin, 128(2), 330–366. 10.1037/0033-2909.128.2.330 [DOI] [PubMed] [Google Scholar]
- Riley KE, & Park CL (2015). How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry. Health Psychology Review, 9(3), 379–396. 10.1080/17437199.2014.981778 [DOI] [PubMed] [Google Scholar]
- Robinson AM (2018). Let’s talk about stress: History of stress research. Review of General Psychology, 22(3), 334–342. 10.1037/gpr0000137 [DOI] [Google Scholar]
- Robles TF, & Carroll JE (2011). Restorative biological processes and health. Social and Personality Psychology Compass, 5(8), 518–537. 10.1111/j.1751-9004.2011.00368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch HL, Rosario M, Levison LM, Olivera A, Livingston WS, Wu T, & Gill JM (2019). The effect of mindfulness meditation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. Annals of the New York Academy of Sciences, 1445(1), 5–16. 10.1111/nyas.13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagan L (1967). On the origin of mitosing cells. Journal of Theoretical Biology, 14(3), 255–274. 10.1016/0022-5193(67)90079-3 [DOI] [PubMed] [Google Scholar]
- Salminen A, & Kaarniranta K (2009). Regulation of the aging process by autophagy. Trends in Molecular Medicine, 15(5), 217–224. 10.1016/j.molmed.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, & Kaarniranta K (2011). Apoptosis and aging: Increased resistance to apoptosis enhances the aging process. Cellular and Molecular Life Sciences, 68(6), 1021–1031. 10.1007/s00018-010-0597-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom GM, & Dunn EW (2014). Is efficiency overrated?: Minimal social interactions lead to belonging and positive affect. Social Psychological & Personality Science, 5(4), 437–442. 10.1177/1948550613502990 [DOI] [Google Scholar]
- Sapolsky RM, Krey LC, & McEwen BS (1986). The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews, 7(3), 284–301. 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, & Munck AU (2000). How do glucocorticoids influence stress responses? Preparative actions. Endocrine Reviews, 21(1), 55–89. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Schertz KE, & Berman MG (2019). Understanding nature and its cognitive benefits. Current Directions in Psychological Science, 28(5), 496–502. 10.1177/0963721419854100 [DOI] [Google Scholar]
- Schulkin J, & Sterling P (2019). Allostasis: A brain-centered, predictive mode of physiological regulation. Trends in Neurosciences, 42(10), 740–752. 10.1016/j.tins.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, & Keng SL (2020). Meditation and telomere length: A meta-analysis. Psychology & Health, 35(8), 901–915. 10.1080/08870446.2019.1707827 [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM, & Clark BJ (2018). Current knowledge on the acute regulation of steroidogenesis. Biology of Reproduction, 99(1), 13–26. 10.1093/biolre/ioy102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevoz-Couche C, & Laborde S (2022). Heart rate variability and slow-paced breathing: When coherence meets resonance. Neuroscience and Biobehavioral Reviews, 135, Article 104576. 10.1016/j.neubiorev.2022.104576 [DOI] [PubMed] [Google Scholar]
- Shechter A, Rising R, Albu JB, & St-Onge MP (2013). Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. The American Journal of Clinical Nutrition, 98(6), 1433–1439. 10.3945/ajcn.113.069427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang D, Wang L, Zhuang J, Cook R, & Chen L (2017). Meditation and blood pressure: A meta-analysis of randomized clinical trials. Journal of Hypertension, 35(4), 696–706. 10.1097/HJH.0000000000001217 [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, & Rothman DL (2009). Baseline brain energy supports the state of consciousness. Proceedings of the National Academy of Sciences of the United States of America, 106(27), 11096–11101. 10.1073/pnas.0903941106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2020). Social safety theory: A biologically based evolutionary perspective on life stress, health, and behavior. Annual Review of Clinical Psychology, 16, 265–295. 10.1146/annurev-clinpsy-032816-045159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowndhararajan K, & Kim S (2016). Influence of fragrances on human psychophysiological activity: With special reference to human electroen-cephalographic response. Scientia Pharmaceutica, 84(4), 724–751. 10.3390/scipharm84040724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R (2009). Types of prayer, heart rate variability, and innate healing. Zygon, 44(4), 825–846. 10.1111/j.1467-9744.2009.01036.x [DOI] [Google Scholar]
- Sterling P (2004). Principles of allostasis: Optimal design, predictive regulation, pathophysiology, and rational therapeutics. In Schukin J (Ed.), Allostasis, homeostasis, and the costs of physiological adaptation (pp. 17–64). Cambridge University Press. 10.1017/CBO9781316257081.004 [DOI] [Google Scholar]
- Sterling P (2012). Allostasis: A model of predictive regulation. Physiology & Behavior, 106(1), 5–15. 10.1016/j.physbeh.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Stier-Jarmer M, Throner V, Kirschneck M, Immich G, Frisch D, & Schuh A (2021). The psychological and physical effects of forests on human health: A systematic review of systematic reviews and meta-analyses. International Journal of Environmental Research and Public Health, 18(4), Article 1770. 10.3390/ijerph18041770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz J, & Schaffer C (2018). Salutogenic affordances and sustainability: Multiple benefits with edible forest gardens in urban green spaces. Frontiers in Psychology, 9, Article 2344. 10.3389/fpsyg.2018.02344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulekha S, Thennarasu K, Vedamurthachar A, Raju TR, & Kutty BM (2006). Evaluation of sleep architecture in practitioners of Sudarshan Kriya yoga and Vipassana meditation. Sleep and Biological Rhythms, 4(3), 207–214. 10.1111/j.1479-8425.2006.00233.x [DOI] [Google Scholar]
- Swope CB, Hernández D, & Cushing LJ (2022). The relationship of historical redlining with present-day neighborhood environmental and health outcomes: A scoping review and conceptual model. Journal of Urban Health, 99(6), 959–983. 10.1007/s11524-022-00665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, & Haller RG (2003). The spectrum of exercise tolerance in mitochondrial myopathies: A study of 40 patients. Brain, 126(2), 413–423. 10.1093/brain/awg028 [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, & Fan M (2009). Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 8865–8870. 10.1073/pnas.0904031106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, & Seeman T (1997). Health psychology: What is an unhealthy environment and how does it get under the skin? Annual Review of Psychology, 48(1), 411–447. 10.1146/annurev.psych.48.1.411 [DOI] [PubMed] [Google Scholar]
- Telles S, Reddy SK, & Nagendra HR (2000). Oxygen consumption and respiration following two yoga relaxation techniques. Applied Psychophysiology and Biofeedback, 25(4), 221–227. 10.1023/A:1026454804927 [DOI] [PubMed] [Google Scholar]
- Thanissaro B (2013). Ānāpānasati Sutta: Mindfulness of breathing (MN 118, BCBS edition). Insight. http://www.accesstoinsight.org/tipitaka/mn/mn.118.than.html [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33(2), 81–88. 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Thoits P (2010). Stress and health: Major findings and policy implications. Journal of Health and Social Behavior, 51(1_suppl), 41–53. 10.1177/0022146510383499 [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, & Montano N (2013). Heart rate variability in normal and pathological sleep. Frontiers in Physiology, 4, Article 294. 10.3389/fphys.2013.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr L, Cooper DC, Mills PJ, Nelesen RA, & Dimsdale JE (2010). Everyday discrimination and nocturnal blood pressure dipping in Black and White Americans. Psychosomatic Medicine, 72(3), 266–272. 10.1097/PSY.0b013e3181d0d8b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treleaven DA (2018). Trauma-sensitive mindfulness practices for safe and transformative healing. W.W. Norton. [Google Scholar]
- Trinder J, Waloszek J, Woods MJ, & Jordan AS (2012). Sleep and cardiovascular regulation. Pflügers Archiv: European Journal of Physiology, 463(1), 161–168. 10.1007/s00424-011-1041-3 [DOI] [PubMed] [Google Scholar]
- Troxel WM, DeSantis A, Germain A, Buysse DJ, & Matthews KA (2017). Marital conflict and nocturnal blood pressure dipping in military couples. Health Psychology, 36(1), 31–34. 10.1037/hea0000434 [DOI] [PubMed] [Google Scholar]
- Trumpff C, Marsland AL, Basualto-Alarcón C, Martin JL, Carroll JE, Sturm G, Vincent AE, Mosharov EV, Gu Z, Kaufman BA, & Picard M (2019). Acute psychological stress increases serum circulating cell-free mitochondrial DNA. Psychoneuroendocrinology, 106, 268–276. 10.1016/j.psyneuen.2019.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpff C, Michelson J, Lagranha CJ, Taleon V, Karan KR, Sturm G, Lindqvist D, Fernström J, Moser D, Kaufman BA, & Picard M (2021). Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion, 59, 225–245. 10.1016/j.mito.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi A, & Cohen M (2013). Oxygen consumption changes with yoga practices: A systematic review. Journal of Evidence-Based Complementary & Alternative Medicine, 18(4), 290–308. 10.1177/2156587213492770 [DOI] [Google Scholar]
- van den Bogerd N, Coosje Dijkstra S, Koole SL, Seidell JC, de Vries R, & Maas J (2020). Nature in the indoor and outdoor study environment and secondary and tertiary education students’ well-being, academic outcomes, and possible mediating pathways: A systematic review with recommendations for science and practice. Health and Place, 66, Article 102403. 10.1016/j.healthplace.2020.102403 [DOI] [PubMed] [Google Scholar]
- Van Gordon W, Sapthiang S, & Shonin E (2022). Contemplative psychology: History, key assumptions, and future directions. Perspectives on Psychological Science, 17(1), 99–107. 10.1177/1745691620984479 [DOI] [PubMed] [Google Scholar]
- Venditti S, Verdone L, Reale A, Vetriani V, Caserta M, & Zampieri M (2020). Molecules of silence: Effects of meditation on gene expression and epigenetics. Frontiers in Psychology, 11, Article 1767. 10.3389/fpsyg.2020.01767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC (2010). Colloquium paper: Bioenergetics, the origins of complexity, and the ascent of man. Proceedings of the National Academy of Sciences of the United States of America, 107(Suppl. 2), 8947–8953. 10.1073/pnas.0914635107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RK (1970). Physiological effects of transcendental meditation. Science, 167(3926), 1751–1754. 10.1126/science.167.3926.1751 [DOI] [PubMed] [Google Scholar]
- Wallace RK, Benson H, & Wilson AF (1971). A wakeful hypometabolic physiologic state. The American Journal of Physiology, 221(3), 795–799. 10.1152/ajplegacy.1971.221.3.795 [DOI] [PubMed] [Google Scholar]
- Wang WL, Chen KH, Pan YC, Yang SN, & Chan YY (2020). The effect of yoga on sleep quality and insomnia in women with sleep problems: A systematic review and meta-analysis. BMC Psychiatry, 20(1), Article 195. 10.1186/s12888-020-02566-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne PM, Lee MS, Novakowski J, Osypiuk K, Ligibel J, Carlson LE, & Song R (2018). Tai Chi and Qigong for cancer-related symptoms and quality of life: A systematic review and meta-analysis. Journal of Cancer Survivorship: Research and Practice, 12(2), 256–267. 10.1007/s11764-017-0665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler K, Voellmin A, Schäfer V, Meyer AH, Cajochen C, Wilhelm FH, & Bader K (2014). Daily stress, presleep arousal, and sleep in healthy young women: A daily life computerized sleep diary and actigraphy study. Sleep Medicine, 15(3), 359–366. 10.1016/j.sleep.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Wu NN, Zhang Y, & Ren J (2019). Mitophagy, mitochondrial dynamics, and homeostasis in cardiovascular aging. Oxidative Medicine and Cellular Longevity, 2019, Article 9825061. 10.1155/2019/9825061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, & Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Jo H, Noorbhai L, Patel A, & Li A (2022). Virtual mindfulness interventions to promote well-being in adults: A mixed-methods systematic review. Journal of Affective Disorders, 300, 571–585. 10.1016/j.jad.2022.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L (2017). Awakening together: The spiritual practice of inclusivity and community. Wisdom Publications. [Google Scholar]
- Youle RJ, & van der Bliek AM (2012). Mitochondrial fission, fusion, and stress. Science, 337(6098), 1062–1065. 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S (2023). Ritual and space: The therapeutic function of the recitations of the Hexi Baojuan. Religions, 14(8), Article 1025. 10.3390/rel14081025 [DOI] [Google Scholar]
- Zoccola PM, & Dickerson SS (2012). Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research, 73(1), 1–9. 10.1016/j.jpsychores.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Zoccoli G, & Amici R (2020). Sleep and autonomic nervous system. Current Opinion in Physiology, 15, 128–133. 10.1016/j.cophys.2020.01.002 [DOI] [Google Scholar]


