Abstract
Objectives
Enfortumab vedotin (EV) is a novel antibody–drug conjugate approved for metastatic urothelial carcinoma (UC) refractory to prior treatment with immune checkpoint inhibitors (ICIs). However, the difference in efficacy of EV after each ICIs and prognostic factors are not well known. We aimed to compare the efficacy of EV in patients with metastatic UC who were treated with avelumab or pembrolizumab and to identify the prognostic factors.
Methods
The records of 100 patients with advanced metastatic UC who received EV after the administration of either avelumab or pembrolizumab were retrospectively collected from five academic hospitals in Japan.
Results
The median follow-up period was 6.7 months. The median overall survival (OS) and progression-free survival (PFS) in the EV after avelumab/pembrolizumab group were not reached/14.7 months (p = 0.17) and 10.4/5.2 months (p = 0.039), respectively. The objective response rates (ORR) were 66.6% and 46.8% in EV after avelumab and EV after pembrolizumab groups, respectively (p = 0.14). Multivariate analysis identified histological variants, liver metastasis, low serum albumin levels, and high serum CRP level as significant poor prognostic factors. The median OS and PFS of cachexia patients with both low serum albumin levels and high serum CRP levels were 6.0 months and 0.93 months, respectively.
Conclusion
PFS was superior in patients treated with EV after avelumab to EV after pembrolizumab. However, OS showed no significant difference between the two groups. Because the prognosis of patients with cachexia is extremely poor, the initiation of EV should be discussed in these patients.
Keywords: Enfortumab vedotin, Pembrolizumab, Avelumab, Urothelial carcinoma, Bladder cancer
Introduction
According to the latest global cancer statistics, bladder cancer was the 10th most common cancer worldwide in 2020 (Estimated Number of New Cases 2020). Pure urothelial carcinoma (UC) is the most common histological form of bladder cancer, accounting for approximately 75% of all bladder cancer cases (Rogers et al. 2006). Non-UC tumors, such as squamous, neuroendocrine, micropapillary, and sarcomatoid cancers, are rare and mostly aggressive (Ismaili 2011). UC has a poor prognosis, and metastatic UC (mUC), which occurs in 5% of patients with UC, shows an extremely poor prognosis, with a reported 5-year survival rate of 4.6% (Saginala et al. 2020). Systemic therapy for mUC has undergone a significant transformation in recent years. First-line chemotherapy with methotrexate, vinblastine, adriamycin, and cisplatin (M-VAC) has been established as the standard of systemic therapy for mUC since 1988 (Nishio et al. 1988). In 2000, gemcitabine and cisplatin (GC) therapy was introduced and has demonstrated overall survival (OS) and objective response rates (ORRs) comparable to M-VAC therapy (Maase et al. 2000). GC therapy is also better tolerated with fewer severe adverse events, such as febrile neutropenia, making it more favored and widely used as the first-line systemic therapy for mUC (Maase et al. 2000). For a long time, vinflunine was the only approved second-line therapeutic agent for mUC with tumor progression (Oing et al. 2016); however, it showed only a 2.6-month improvement in OS in comparison with best supportive care (BSC) (p = 0.04) (Bellmunt et al. 2013, 2009). Other second-line chemotherapeutic agents, including docetaxel, taxanes, or cyclophosphamide, are yet to be established. By 2016, the treatment landscape for mUC transitioned to the next stage with the advent of immune checkpoint inhibitors (ICIs). Atezolizumab was approved by the U.S. FDA as the first PD-L1 inhibitor for second-line treatment in patients with mUC following chemotherapy with regimens such as GC or M-VAC. Subsequently, the PD-1 antibody pembrolizumab was approved by the U.S. FDA after showing significant OS benefit in a phase III randomized control trial (RCT) (Bellmunt et al. 2017). At present, five FDA-approved ICIs, namely, pembrolizumab, atezolizumab, duralumab, nivolumab, and avelumab, are available for second-line therapy after platinum-based chemotherapy for mUC. However, ICIs have some limitations; only approximately one-quarter to one-fifth of the patients respond to ICIs. Moreover, the ORR was approximately 20% in the second-line setting after progression under platinum-based chemotherapy (Bellmunt et al. 2017).
Enfortumab vedotin (EV) is an ADC conjugated to monomethyl auristatin E, a microtubule-disrupting agent that targets nectin-4, a cell adhesion molecule highly expressed in several solid tumors, including breast, lung, gastric, and UC (Bouleftour et al. 2022). The confirmatory phase III trial EV-301 demonstrated a median OS of 12.88 months, with a median progression-free survival (PFS) period of 5.55 months versus 3.71 months after prior chemotherapy and ICI therapy (Powles et al. 2021). Patients who respond with stable disease (SD) or show improvement after chemotherapy transition to avelumab treatment, while those who experience disease progression (PD) switch to pembrolizumab treatment. Furthermore, if PD occurs after these treatments, transition to EV treatment is considered. However, few trials have compared the efficacy of EV in these treatment arms. The superior efficacy of EV in patients who were previously treated with either avelumab or pembrolizumab has not been sufficiently verified. Therefore, this study aimed to compare the efficacy of EV in patients with mUC who had previously received pembrolizumab or avelumab.
Materials and methods
This multi-institutional study was approved by the Institutional Review Board and complied with the 1964 Declaration of Helsinki and its later amendments. We identified 109 consecutive patients with advanced UC who received EV at five institutions (Jikei University School of Medicine, Osaka Medical and Pharmaceutical University, Fujita Health University School of Medicine, Kindai University Faculty of Medicine, and Tokyo Medical University) between December 2022 and June 2023. Patients who did not receive any ICIs before the initiation of EV therapy (n = 3) and those who did not undergo radiographic examinations after the initiation of EV therapy (n = 6) were excluded. Thus, 100 patients were enrolled in this study. All patients were histopathologically diagnosed with UC and radiologically confirmed to have PD after receiving ICIs as second-line therapy. Radiological evaluations were generally performed using computed tomography before and after every three–six cycles of EV; however, evaluations were also performed as needed when clinicians thought it necessary, for example, when the clinical symptoms worsened. Radiological analysis was based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (Eisenhauer et al. 2009). For each metastatic lesion, the best response was classified as follows: (1) complete response (CR; disappearance or reduction to a short-axis diameter < 10 mm for all LN metastases), (2) partial response (PR; > 30% reduction), (3) SD (neither CR, PR, nor PD), and (4) PD (> 20% growth). Laboratory data were collected within 1 month of the initial administration of EV therapy. EV was administered at a dose of 1.25 mg per kg of body weight as a single intravenous infusion over 30 min on days 1, 8, and 15 of a 4-week cycle.
We defined the “EV after avelumab group” as patients who received avelumab followed by EV, and the “EV after pembrolizumab group” as those who received pembrolizumab followed by EV. The primary endpoint was OS, and the secondary endpoints were PFS, ORR, and disease control rate (DCR).
The significance tests used to compare the two groups were the Student’s t test for continuous variables and the χ2 test for categorical variables. Kaplan–Meier analysis and the log-rank test were used to obtain and compare the OS and PFS between the two groups, respectively. Multivariate analysis was performed to identify independent prognostic variables affecting the OS of all patients by using a stepwise Cox proportional-hazards regression model. Receiver operating characteristic curves and the Youden index were used to determine the optimum cut-off values of the continuous variables. All statistical analyses were performed using R 3.1.0 for Windows (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). All parameters in this study with a p < 0.05 were considered statistically significant.
Results
One hundred patients from five academic institutions in Japan who had been treated with EV after avelumab or pembrolizumab were enrolled in the present study. Baseline patient characteristics are shown in Table 1. The mean patient age was 73.6 years, and most (67%) of the patients were male; 54% of the patients had a history of smoking or were currently smoking. Visceral metastasis was observed in 52.3% of the patients in the EV after avelumab group and 67% of those in the EV after pembrolizumab group (p = 0.30). Liver metastasis was present in 19% and 16.4% of the patients in the two groups (p = 0.75), respectively. The two groups showed no significant differences in patient characteristics, except for sex (Table 1). The median follow-up period was 7.6 months (range: 0.97–15.5 months) in the EV after avelumab group and 6.4 months (range: 0.4–16.6 months) in the EV after pembrolizumab group. A total of 31 deaths (31%) occurred, including four in the EV after avelumab group (19%) and 27 in the EV after pembrolizumab group (34.1%). The median PFS of all 100 patients was 6.5 months (Fig. 1A). The median PFS was 10.4 months (95% CI: 0.19–0.72) in the EV after avelumab group and 5.2 months (95% CI: 0.36–0.59) in the EV after pembrolizumab group, and was significantly better in the EV after avelumab group (p = 0.039; Fig. 2). The median OS of all 100 patients was 14.7 months. (Fig. 1B). The median OS was not determinable in the EV after avelumab group, but was 14.7 months (95% CI: 0.21–0.63) in the EV after pembrolizumab group. Nonetheless, it did not differ significantly between the two groups (p = 0.17; Fig. 3). CR/PR was achieved in 14.2%/52.3% of the patients in the EV after avelumab group and 2.5%/44.3% of those in the EV after pembrolizumab group (Table 2). The ORR was 66.6% in the EV after avelumab group and 46.8% in the EV after pembrolizumab group (p = 0.14) (Table 2). The DCR was 85.7% in the EV after avelumab group and 70.8% in the EV after pembrolizumab group (p = 0.26) (Table 2). Multivariate analysis using the Cox proportional-hazards model identified histological variant (HR: 21.4, p = 0.000017), liver metastasis (HR: 4.5, p = 0.0018), low serum albumin (Alb) level (Alb < 3.3 g/dL; (HR: 4.2, p = 0.0031), and high serum C-reactive protein (CRP) level (CRP > 1.9 mg/dL; HR: 3.3, p = 0.011) as significant independent poor prognostic factors in the EV after ICI groups (Table 3). Thirty-two patients (32%) with cachexia met the criteria of both low serum Alb levels (Alb < 3.3 g/dL) and high serum CRP levels (CRP > 1.9 mg/dL) in all patients. The median OS and PFS of these patients were 6.0 months (95% CI, 0.27–0.64) (Fig. 4) and 0.93 months (95% CI, 0.30–0.65) (Fig. 5), respectively.
Table 1.
Baseline patient characteristics
| All | Prior Anti-PD-(L)1 therapy; Avelumab group | Prior Anti-PD-(L)1 therapy; Pembrolizumab group | p-value | |
|---|---|---|---|---|
| Number of patients | 100 | 21 | 79 | |
| Age, years (Mean, SD) | 73.6 ± 8.6 | 74.3 ± 6.7 | 73.5 ± 9.1 | 0.69 |
| Age ≥ 75 years, no. (%) | 49 (49%) | 10 (47.6%) | 39 (49.3%) | 1.0 |
| Male sex | 67 (67%) | 9 (42.8%) | 58 (73.4%) | 0.016 |
| ECOG | 0.34 | |||
| 0–1 | 82 (82%) | 19 (90.4%) | 63 (79.7%) | |
| ≥ 2 | 18 (18%) | 2 (9.5%) | 16 (20.2%) | |
| Tobacco use, no. (%) | 0.23 | |||
| Former user | 50 (50%) | 8 (38%) | 42 (53.1%) | |
| Current user | 4 (4%) | 0 | 4 (5%) | |
| Never user | 46 (46%) | 13 (61.9%) | 33 (41.7%) | |
| Primary tumor site | 0.62 | |||
| Bladder | 57 (57%) | 11 (52.3%) | 46 (58.2%) | |
| Upper urinary tract | 42 (42%) | 10 (47.6%) | 32 (40.5%) | |
| Missing | 1 (1%) | 0 | 1 (1.2%) | |
| Histologic type | 0.83 | |||
| Urothelial carcinoma | 78 (78%) | 17 (76.1%) | 61 (77.2%) | |
| Urothelial carcinoma with variant histology | 5 (5%) | 0 | 5 (6.3%) | |
| Other | 9 (9%) | 2 (9.5%) | 7 (8.8%) | |
| Missing | 8 (8%) | 2 (9.5%) | 6 (7.5%) | |
| Symptomatic disease | 60 (60%) | 11 (52.3%) | 49 (62%) | 0.61 |
| Missing | 1 (1%) | 1 (4.7%) | 0 | |
| Prior surgery or radiation | 0.52 | |||
| Surgery | 58 (58%) | 10 (47.6%) | 48 (60.7%) | |
| Radiation | 8 (8%) | 2 (9.5%) | 6 (7.5%) | |
| Surgery + Radiation | 3 (3%) | 0 | 3 (3.7%) | |
| None | 31 (31%) | 9 (42.8%) | 22 (27.8%) | |
| Previous chemotherapy | 0.26 | |||
| Cisplatin | 67 (67%) | 17 (80.9%) | 50 (63.2%) | |
| Carboplatin | 23 (23%) | 4 (19%) | 19 (24%) | |
| Other | 8 (8%) | 0 | 8 (10.1%) | |
| NA | 2 (2%) | 0 | 2 (2.5%) | |
| Metastatic disease | ||||
| Visceral metastasis | 64 (64%) | 11 (52.3%) | 53 (67%) | 0.30 |
| Liver | 17 (17%) | 4 (19%) | 13 (16.4%) | 0.75 |
| Lung | 49 (49%) | 6 (28.5%) | 43 (54.4%) | 0.049 |
| Bone | 28 (28%) | 6 (28.5%) | 22 (27.8%) | 1.0 |
| Lymph node | 65 (65%) | 13 (61.9%) | 52 (65.8%) | 0.79 |
| Number of metastasis sites (mean, standard deviation) | 1.9 ± 1.0 | 1.7 ± 1.3 | 2.0 ± 0.91 | 0.31 |
| Number of visceral metastasis sites (mean, standard deviation) | 0.8 ± 0.68 | 0.61 ± 0.66 | 0.84 ± 0.68 | 0.17 |
| Perioperative therapy | 0.84 | |||
| Neoadjuvant chemotherapy | 6 (6%) | 1 (4.7%) | 5 (6.3%) | |
| Adjuvant chemotherapy | 6 (6%) | 2 (9.5%) | 4 (5%) | |
| Salvage chemotherapy | 86 (86%) | 18 (85.7%) | 68 (86%) | |
| Best response among patients who previously received chemotherapy | 0.0035 | |||
| CR | 6 (6%) | 1 (4.7%) | 5 (6.3%) | |
| PR | 26 (26%) | 10 (47.6%) | 16 20.2%) | |
| SD | 30 (30%) | 9 (42.8%) | 21 (26.5%) | |
| PD | 31 (31%) | 1 (4.7%) | 30 (37.9%) | |
| NA | 7 (7%) | 0 | 7 (8.8%) | |
| Best response among patients | 0.97 | |||
| who previously received ICIs | ||||
| CR | 4 (4%) | 0 | 4 (5%) | |
| PR | 15 (15%) | 3 (14.2%) | 12 (15.1%) | |
| SD | 19 (19%) | 4 (19%) | 15 (18.9%) | |
| PD | 60 (60%) | 13 (61.9%) | 47 (59.4%) | |
| Follow-up period, months (Median, range) | 6.7 (0.4–16.6) | 7.6 (0.97–15.5) | 6.4 (0.4–16.6) | 0.35 |
PD-(L)1 programmed death-1 receptor/programmed death ligand-1; ECOG Eastern Cooperative Oncology Group; ICIs immune checkpoint inhibitors; CR complete response; PR partial response; SD stable disease; PD progressive disease; NA not applicable
Fig. 1.
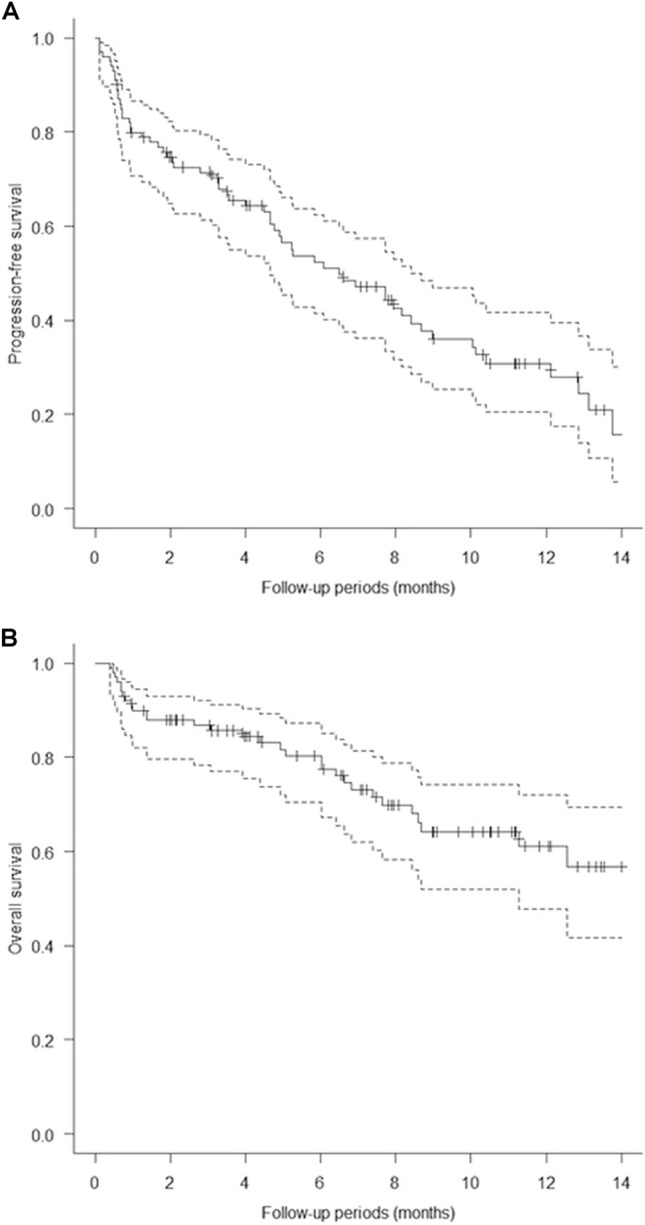
A Kaplan–Meier curves of progression-free survival of all patients (n = 100). B Kaplan–Meier curves of overall survival of all patients (n = 100)
Fig. 2.
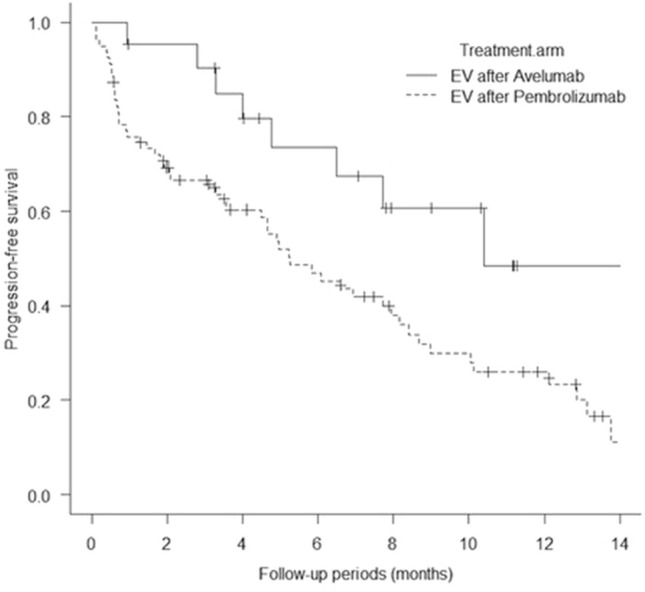
Kaplan–Meier curves of progression-free survival according to treatment group. (log-rank test, p = 0.0392)
Fig. 3.
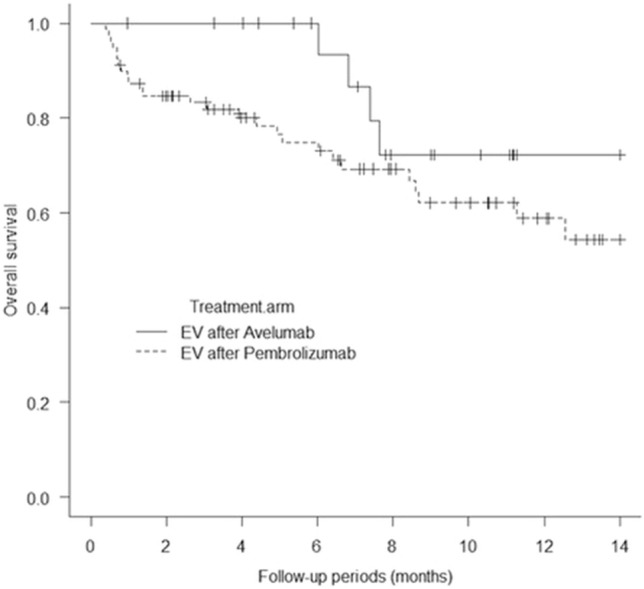
Kaplan–Meier curves of overall survival according to treatment group. (log-rank test, p = 0.176)
Table 2.
Objective response rate
| Variable | All | EV after avelumab group | EV after pembrolizumab group | p-value |
|---|---|---|---|---|
| No. of patients | 100 | 21 | 79 | |
| Best response assessed using RECIST criteria | 0.11 | |||
| CR | 5 (5%) | 3 (14.2%) | 2 (2.5%) | |
| PR | 46 (46%) | 11 (52.3%) | 35 (44.3%) | |
| SD | 23 (23%) | 4 (19%) | 19 (24%) | |
| PD | 26 (26%) | 3 (14.2%) | 23 (29.1%) | |
| ORR | 51 (51%) | 14 (66.6%) | 37 (46.8%) | 0.14 |
| DCR | 74 (74%) | 18 (85.7%) | 56 (70.8%) | 0.26 |
DCR = CR + PR + SD
ORR = CR + PR
EV enfortumab vedotin; CR complete response; PR partial response; SD, table disease; PD progressive disease; ORR objective response rate; DCR disease control rate
Table 3.
Univariate and multivariate Cox regression models for overall survival in patients with metastatic urothelial carcinoma who were treated with enfortumab vedotin after ICI therapy
| Variable | Univariate analysis Hazard ratio (95% confidence interval) | p-value | Multivariate analysis Hazard ratio (95% confidence interval) | p-value |
|---|---|---|---|---|
| Male sex (vs. female) | 0.95 (0.44–2.0) | 0.90 | ||
| ECOG > 2 (vs. < 0–1) | 2.6 (1.2–5.8) | 0.013 | ||
| Smoking (vs. never) | ||||
| Former | 1.4 (0.67–2.9) | 0.36 | ||
| Current | 0.99 (0.12–7.6) | 0.99 | ||
| Symptom (vs. none) | 2.3 (1.0–5.5) | 0.044 | ||
| Pembrolizumab as prior treatment (vs Avelumab) | 2.0 (0.71–5.8) | 0.18 | ||
| Histological type (vs. urothelial carcinoma) | ||||
| Other | 3.8 (1.5–9.6) | 0.0048 | 5.4 (1.9–14.9) | 0.00093 |
| UC with variant | 6.7 (2.1–21.1) | 0.00097 | 21.4 (5.2–86.6) | 0.000017 |
| Missing | 0.50 (0.067–3.8) | 0.51 | 0.32 (0.04–2.6) | 0.29 |
| BOR of chemotherapy PR-CR (vs. PD-SD) | 0.40 (0.16–0.98) | 0.046 | ||
| BOR of immunotherapy PR-CR (vs. PD-SD) | 0.37 (0.11–1.2) | 0.10 | ||
| Number of visceral metastatic site (vs 0) | ||||
| 1 | 0.81 (0.35–1.8) | 0.62 | ||
| 2 | 2.5 (1.0–6.4) | 0.045 | ||
| Liver metastasis | 2.1 (0.95–4.8) | 0.064 | 4.5 (1.7–11.9) | 0.0018 |
| Alb < 3.3 g/dL (vs. > 3.3 g/dL) | 3.6 (1.7–7.6) | 0.00058 | 4.2 (1.6–10.9) | 0.0031 |
| CRP > 1.9 mg/dL (vs. < 1.9 mg/dL) | 6.0 (2.6–13.5) | 0.000015 | 3.3 (1.3–8.3) | 0.011 |
| Hb < 11 g/dL (vs. > 11 g/dL) | 2.1 (1.0–4.4) | 0.049 | ||
| NLR > 3.8 (vs. < 3.8) | 3.8 (1.5–9.4) | 0.0028 |
ICIs Immune checkpoint inhibitors; ECOG Eastern Cooperative Oncology Group; UC Urothelial carcinoma; CR complete response; PR partial response; SD stable disease; PD progressive disease; Alb albumin level; CRP C-reactive protein level; NLR neutrophil-to-lymphocyte ratio
Fig. 4.
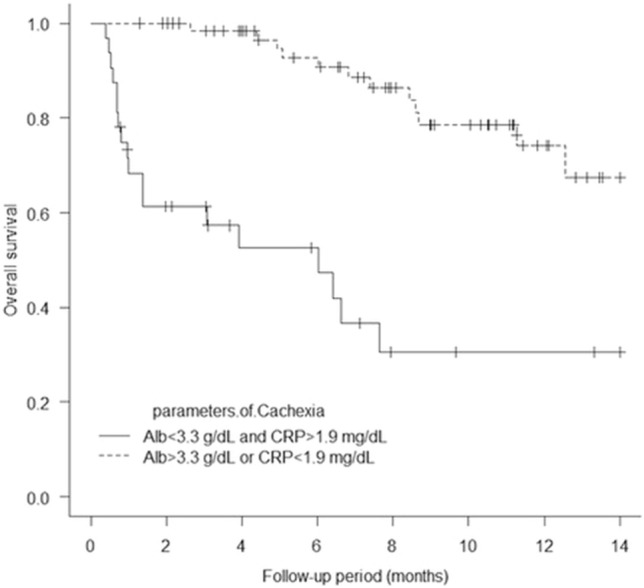
Kaplan–Meier curves of overall survival in patients with and without cachexia who were treated with enfortumab vedotin after ICIs. (log-rank test, p = 0.000000011)
Fig. 5.
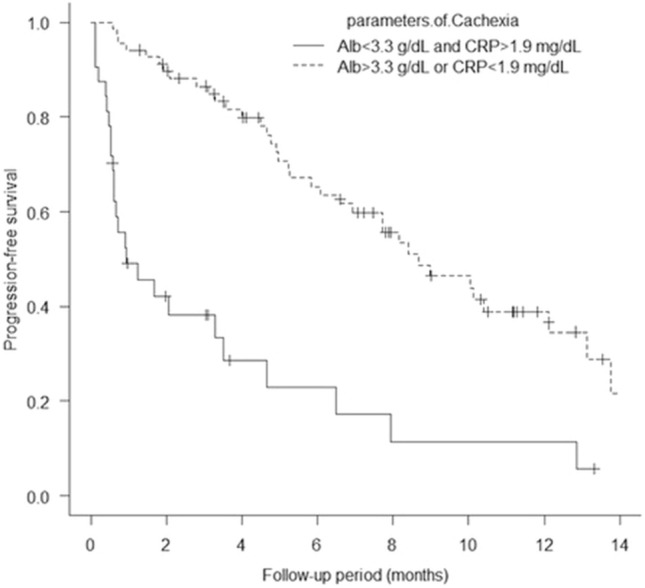
Kaplan–Meier curves of progression-free survival in patients with and without cachexia who were treated with enfortumab vedotin after ICIs. (log-rank test, p = 0.000000070)
Discussion
To the best of our knowledge, this is the first multi-institutional retrospective study to compare the efficacy of EV between patients with mUC who had been previously treated with avelumab or pembrolizumab.
Herein, we report the data of 100 patients from five academic hospitals in Japan. The median OS in our study was 14.7 months (Fig. 1B), which is comparable to the OS in the UNITE study (14.4 months) (Koshkin et al. 2022), and slightly longer than those reported in the EV-201 (11.7 months) (Rosenberg et al. 2019) and EV-301 (12.8 months) (Bouleftour et al. 2022) studies. When analyzed by each arm, the median OS in the EV after avelumab group was not applicable, and that in the EV after pembrolizumab group was 14.7 months. The 12-month survival rates in the EV after avelumab and pembrolizumab groups were 72.2% and 58.8%, respectively. The two groups showed no significant difference in OS (p = 0.17) (Fig. 3). Furthermore, based on the multivariate analysis, ICI therapy prior to EV was not a significant factor affecting OS (Table 3). In the UNITE study, Koshkin et al. reported that the expression of PD-L1 was not significant in affecting OS in patients with advanced UC who were treated using EV (Koshkin et al. 2022). The current study is the first to report that the difference in using PD-1 and PD-L1 inhibitors in prior treatment does not affect OS in patients with advanced UC who were treated using EV. Our results showed a slightly better OS than those of the EV-201 and EV-301 studies. However, our patient population had a lower percentage of individuals with visceral (64%) and liver (17%) metastases than the EV-301 group (77.7% and 30.9%, respectively). Conversely, our study included a higher percentage of patients with lymph node metastasis (22%) than the EV-301 group (11.3%). Thus, our study may have included a relatively larger population of patients with a better prognosis. In terms of PFS, the median PFS in our study was 6.5 months (Fig. 1A), which is comparable to the PFS in the UNITE study (6.8 months) (Koshkin et al. 2022), and was slightly longer than those in the EV-201 study (5.8 months) (Rosenberg et al. 2019) and the EV-301 study (5.55 months) (Bouleftour et al. 2022). When analyzed by each arm, the median PFS in the EV after avelumab group was 10.4 months and that in the EV after pembrolizumab group was 5.2 months. The PFS in the EV after avelumab group was significantly superior to that in the EV after pembrolizumab group (p = 0.039) (Fig. 2). These findings might be attributed to the fact the avelumab arm included patients who responded to first-line chemotherapy (SD or better), whereas the pembrolizumab arm included patients who experienced disease progression (PD). Thus, the two groups may have shown differences in tumor progression rate, tumor burden, and tumor quantity at the initiation of EV treatment. In the present study, the actual number of metastases, number of metastases, and visceral metastases tended to be lower in the EV after avelumab group than in the EV after pembrolizumab group, although no statistically significant differences were observed (Table 1). Moreover, the number of patients with lung metastases in addition to liver or bone metastases was higher in the EV after pembrolizumab group (Table 1). However, despite the superior PFS in the avelumab arm, the two groups showed no significant difference in OS. Notably, approximately 6 months after initiating EV treatment, the two groups showed no substantial difference in OS in the present study (Fig. 3). The pembrolizumab group included more patients with advanced tumors, resulting in a subgroup of patients who quickly succumbed to cancer in the early stages, leading to a poorer OS trend. However, the avelumab group also showed gradual worsening of disease progression, which may ultimately result in a less favorable long-term OS outcome. More recently, Nizam et al. reported at the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) meeting that patients with advanced UC treated with EV following maintenance therapy using Avelumab had outcomes consistent with data for EV in chemotherapy- and ICI-refractory advanced UC. (Nizam et al. 2024) Both their study and ours support the use of EV as third-line therapy after progression on maintenance therapy using Avelumab. However, validation in larger cohorts is required.
Multivariate analysis indicated that histological variants, liver metastasis, low serum Alb levels (Alb < 3.3 g/dL), and high serum CRP levels (CRP > 1.9 mg/dL) were significant independent poor prognostic factors (Table 3). All of these are known poor prognostic factors for UC; however, we focused on low Alb and high CRP levels. Low Alb (Cong et al. 2022) (Liu et al. 2021) (Liu et al. 2022) and high CRP levels (Cong et al. 2022) (Fearon et al. 2006) (McMillan 2008) (Marsik et al. 2008) (Hilmy et al. 2005) are typical indicators of cachexia. In the present study, 32 patients (32%) met the criteria of low Alb (Alb < 3.3 g/dL) and high CRP (CRP > 1.9 mg/dL) levels, and approximately half of them experienced cancer-related death within 3 months of starting treatment, with 70% succumbing to cancer within 7 months (Fig. 4). The median OS and PFS of the patients with cachexia were 6.0 months (95% CI, 0.27–0.64; Fig. 4) and 0.93 months (95% CI, 0.30–0.65; Fig. 5), respectively. This can be explained by the fact that the proportion of patients with cachexia due to advanced UC is higher among patients who are starting EV as a third-line treatment. As the prognosis of patients with cachexia is extremely poor, the initiation of EV treatment in these patients should be well discussed, especially within the context of payer restrictions and limited access in emerging markets. In addition, although the Bellmunt Risk Score has been reported as a prognostic factor in patients with UC who were treated with chemotherapy or immunotherapy (Bellmunt et al. 2010) (Abuhelwa et al. 2022), in our multivariate analysis, Hb and ECOG PS were not significant prognostic factors (Table 3). Thus, there is a need to develop new risk models to predict prognosis at the time of administering the EV.
The current study is limited by its retrospective nature and variable follow-up protocols resulting from its multi-institution design. In addition, it lacked data regarding the tumor burden in the two groups. Further research is needed to investigate the intergroup differences in the tumor burden and patient backgrounds. Moreover, while we intended to analyze the prognostic factors for each group, we could not analyze the prognostic factors of patients treated with avelumab followed by EV because of the small sample size in that group. Large-scale and long-term studies are needed to clarify these points.
Conclusions
The efficacy of EV in patients with mUC in this study was comparable to that of previous studies. The PFS was superior in patients treated with EV after avelumab to those treated with EV after pembrolizumab. However, OS showed no significant difference between the two groups. Because the prognosis of patients with cachexia is extremely poor, the initiation of EV should be well discussed in these patients.
Acknowledgements
This work was supported in part by research support from the Center for Diversity at Tokyo Medical University (TMUCD-202302).
Author contributions
Yosuke Hirasawa: conceptualization, data curation, analysis, investigation, methodology, software, visualization, writing original draft, supervision. Takahiro Adachi: data curation, investigation, methodology. Takeshi Hashimoto: data curation, investigation, investigation, review, editing, supervision. Wataru Fukuokaya: investigation, data curation, validation. Yuhei Koike: investigation, data curation, validation. Yuji Yata: investigation, data curation, validation. Kazuki Nishimura: data curation. Kazumasa Komura: validation, methodology, review, editing, supervision. Taizo Uchimoto: data curation, investigation, validation, methodology. Takuya Tsujino: methodology, validation. Masanobu Saruta: data curation. Mamoru Hashimoto: analysis. Kiyoshi Takahara: review, editing, supervision. Kazutoshi Fujita: review and editing, supervision. Ryoichi Shiroki: supervision. Hirotsugu Uemura: supervision. Takashi Azuma: supervision. Takahiro Kimura: review, editing, investigation, validation, supervision. Yoshio Ohno: review, editing, investigation, validation, supervision. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately and resolved.
Funding
Yosuke Hirasawa was partially supported by Grant-in-Aid No. 22K09512 (Japan Society for the Promotion of Science: JSPS), the Cancer Research Grant afforded by the Tokyo Medical University Cancer Research Foundation, the grant to the Department of Urology, Tokyo Medical University and Yosuke Hirasawa from BioPhenoMA Inc.
Data availability
The data from this study can be accessed by academic and commercial partners upon a reasonable request, subject to Institutional Review Board (IRB) approval and a data use agreement. For further details or to reanalyze the study's data, contact the Lead Contact at kazumasa.komura@ompu.ac.jp.
Declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Ethical approval
This study was approved by the Institutional Review Board (IRB) from Osaka Medical and Pharmaceutical University (approval number: RIN-750–2571, approved on 24th January 2020). This observational study adhered to ethical principles aligned with the Declaration of Helsinki, the International Conference on Harmonisation's Good Clinical Practice guidelines, Good Pharmacoepidemiology Practices, and relevant laws for noninterventional and observational studies.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abuhelwa A, Bellmunt J, Kichenadasse G, McKinnon R, Rowland A, Sorich MJ, et al. Enhanced bellmunt risk score for survival prediction in urothelial carcinoma treated with immunotherapy. Clin Genitourin Cancer. 2022;20(2):132–138. doi: 10.1016/j.clgc.2021.11.010. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, Choueiri TK, Fougeray R, Schutz F, Salhi Y, Winquist E, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, Fougeray R, Rosenberg JE, von der Maase H, Schutz FA, Salhi Y, et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol. 2013;24:1466–1472. doi: 10.1093/annonc/mdt007. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouleftour W, Guillot A, Magné N. The anti-nectin 4: a promising tumor cells target. A systematic review. Mol Cancer Ther. 2022;21:493–501. doi: 10.1158/1535-7163.MCT-21-0846. [DOI] [PubMed] [Google Scholar]
- Cong M, Song C, Xu H, Song C, Wang C, Fu Z, et al. The patient-generated subjective global assessment is a promising screening tool for cancer cachexia. BMJ Support Palliat Care. 2022;12(e1):e39–e46. doi: 10.1136/bmjspcare-2020-002296. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Estimated Number of New Cases in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/ online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr& sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D= 17&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (Accessed on 25 May 2021).
- Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study Group Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- Hilmy M, Bartlett JM, Underwood MA, McMillan D. The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2005;92:625–627. doi: 10.1038/sj.bjc.6602406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili N. A rare bladder cancer-small cell carcinoma: review and update. Orphanet J Rare Dis. 2011;6:75. doi: 10.1186/1750-1172-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshkin VS, Henderson N, James M, Natesan D, Freeman D, Nizam A, et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: analysis from the urothelial cancer network to investigate therapeutic experiences (UNITE) study. Cancer. 2022;128(6):1194–1205. doi: 10.1002/cncr.34057. [DOI] [PubMed] [Google Scholar]
- Liu XY, Zhang X, Ruan GT, Zhang KP, Tang M, Zhang Q, et al. One-year mortality in patients with cancer cachexia: association with albumin and total protein. Cancer Manag Res. 2021;29(13):6775–6783. doi: 10.2147/CMAR.S318728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CA, Zhang Q, Ruan GT, Shen LY, Xie HL, Liu T, et al. Novel diagnostic and prognostic tools for lung cancer cachexia: based on nutritional and inflammatory status. Front Oncol. 2022;11(12):890745. doi: 10.3389/fonc.2022.890745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsik C, Kazemi-Shirazi L, Schickbauer T, Winkler S, Joukhadar C, Wagner O, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54:343–349. doi: 10.1373/clinchem.2007.091959. [DOI] [PubMed] [Google Scholar]
- McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Shirahase T, Shichiri Y, Habuchi T, Matsuda T, Nishimura K, et al. Experience with combination chemotherapy consisting of methotrexate, vinblastine, adriamycin and cisplatin (M-VAC) in advanced urothelial cancer. Hinyokika Kiyo Acta Urol Jpn. 1988;34:1371–1375. [PubMed] [Google Scholar]
- Nizam A, Jindal T, Jiang CY, Alhalabi O, Bakaloudi DR, Talukder R, et al. Outcomes in patients (pts) with advanced urothelial carcinoma (aUC) treated with enfortumab vedotin (EV) after switch maintenance avelumab (MAv) in the UNITE study. J Clin Oncol. 2024;42(4_suppl):537–537. doi: 10.1200/JCO.2024.42.4_suppl.537. [DOI] [Google Scholar]
- Oing C, Rink M, Oechsle K, Seidel C, von Amsberg G, Bokemeyer C. Second line chemotherapy for advanced and metastatic urothelial carcinoma: vinflunine and beyond—a comprehensive review of the current literature. J Urol. 2016;195:254–263. doi: 10.1016/j.juro.2015.06.115. [DOI] [PubMed] [Google Scholar]
- Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Duran I, Lee J, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CG, Palapattu GS, Shariat SF, Karakiewicz PI, Bastian PJ, Lotan Y, et al. Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. J Urol. 2006;175:2048–2053. doi: 10.1016/S0022-5347(06)00317-X. [DOI] [PubMed] [Google Scholar]
- Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592–2600. doi: 10.1200/JCO.19.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci. 2020;8:15. doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study can be accessed by academic and commercial partners upon a reasonable request, subject to Institutional Review Board (IRB) approval and a data use agreement. For further details or to reanalyze the study's data, contact the Lead Contact at kazumasa.komura@ompu.ac.jp.


