Abstract
The induced membrane technique (IMT) is among the most innovative reconstructive methods for clavicle defects after fracture-related infection (FRI). Herein, we report a case in which a clavicle bone defect after FRI was reconstructed with an autogenous cancellous bone graft mixed with β-tricalcium phosphate (β-TCP) in the second stage of the IMT. A 62-year-old male patient with left clavicle fracture underwent open reduction and internal fixation. Refracture occurred immediately after the implant was removed. The patient was diagnosed with FRI after reopen reduction and internal fixation and was then referred to our hospital. The surgery was performed using the IMT. In the second stage of the IMT, the bone defect was filled with an autogenous cancellous bone mixed with wool-type β-TCP. At 8 months after surgery, the nonunion area had fused, and the patient had no restrictions in activities of daily living. The IMT with β-TCP can be a reconstructive method for bone defects after clavicular nonunion.
Keywords: Clavicle fracture, Nonunion, Bone defect, Induced membrane technique
Introduction
Fracture-related infection (FRI) is a major complication of fracture. Bone transport using the Ilizarov technique, free vascularized bone flaps, and induced membrane technique (IMT) are utilized for reconstructing bone defects after debridement [1]. Masquelet et al. have reported the use of the IMT, which is one of the most innovative reconstructive methods for bone defects after FRI [2,3]. This technique comprised two steps. In the first stage, the necrotic area is debrided comprehensively, and the bone defect is filled with a polymethylmethacrylate (PMMA) spacer. In the second stage, the PMMA spacer is removed, and the autogenous cancellous bone is filled into the membrane tissue, which is referred to as the induced membrane. If the bone defect is large, an autogenous cancellous bone with a considerable size should be harvested in the second stage. Sasaki et al. have reported the use of autogenous cancellous bone grafts mixed with beta-tricalcium phosphate (β-TCP) in the second stage of the IMT [4].
Clavicular fractures are common, and the incidence rate of nonunion ranges from 0.1 % to 24 % and that of FRI from 0.4 % to 7.8 % [5]. The corticocancellous bone graft harvested from the iliac crest is the gold standard treatment for clavicular nonunion [1]. Although the number of reports on the application of IMT for the reconstructing clavicle defects is increasing [6,7], there are no case reports on IMT using β-TCP.
Herein, we report a case in which the autogenous cancellous bone graft was mixed with β-TCP at the second stage of the IMT for reconstructing clavicle bone defects after FRI.
Case report
A 62-year-old-male patient crashed from a 2 m height while pruning his garden. He was rushed to a nearby hospital and was diagnosed with left clavicle shaft fracture, left scapula fracture, multiple left rib fractures, left distal radius fracture, and traumatic hemopneumothorax. After improvement in traumatic hemopneumothorax, the left clavicle shaft fracture was treated with open reduction and internal fixation (ORIF). At 1 year after ORIF, the implants were removed. However, refracture occurred a few days after surgery. Osteosynthesis was performed again. However, a cutaneous fistula formed 3 weeks after surgery. The patient was diagnosed with FRI, and the second implant was removed. The culture tests detected coagulase-negative Staphylococcus. Local antibiotic perfusion was continuously administered for 4 weeks. The cutaneous fistula closed. However, nonunion did not improve. The patient was referred to our hospital 1 year and 2 months after the injury (Fig. 1a).
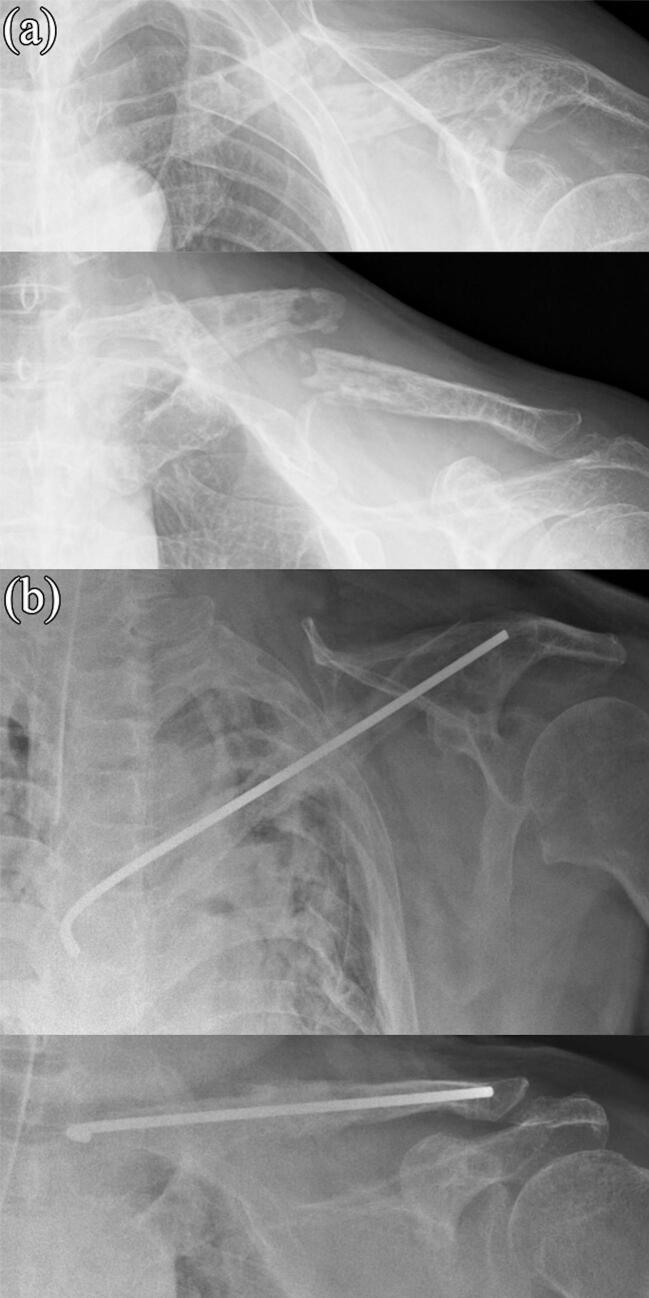
Fig. 1.
Radiographic image obtained (a) before and (b) after the first stage of the induced membrane technique (IMT). The clavicle was fixed with a φ3.0-mm Kirschner wire. The bone defect was filled with a polymethylmethacrylate (PMMA) spacer mixed with vancomycin.
On physical examination, his range of motion in the left shoulder was restricted (flexion: 75°, abduction: 75°, and external rotation: 0°). The wound had no signs of infection and fistula. The white blood cell count was 5800 /μL, and the C-reactive protein level was 0.07 mg/dL. Bone continuity in the nonunion area was completely disrupted. The patient presented with comorbidities including type 2 diabetes (HbA1c of 7.3 % with oral medication) and arterial hypertension.
The surgery was performed using the IMT. In the first stage, atrophic and/or avascular bone segments were excised aggressively. The nonunion area was fixed with a φ3.0-mm Kirschner wire. The bone defect was filled with 40 g of PMMA spacer mixed with 2 g of vancomycin. A 2.5-cm bone defect was created (Fig. 1b). The second stage was performed after 5 weeks of surgical waiting. During the waiting period, the laboratory data and skin condition of the patient did not worsen. Further, the PMMA spacer was removed cautiously to prevent damaging the induced membrane. A rapid culture test was performed, and the results did not show any bacteria and neutrophil infiltration. The bone defects were expanded with a spreader (Fig. 2a) and filled with the autogenous cancellous bone taken from the left ilium crest and mixed with 0.2 g of wool-like β-TCP material (ReBOSSIS®) (Fig. 2b). The locking plate was used for definitive internal fixation (Fig. 2c, Fig. 3).
Fig. 2.
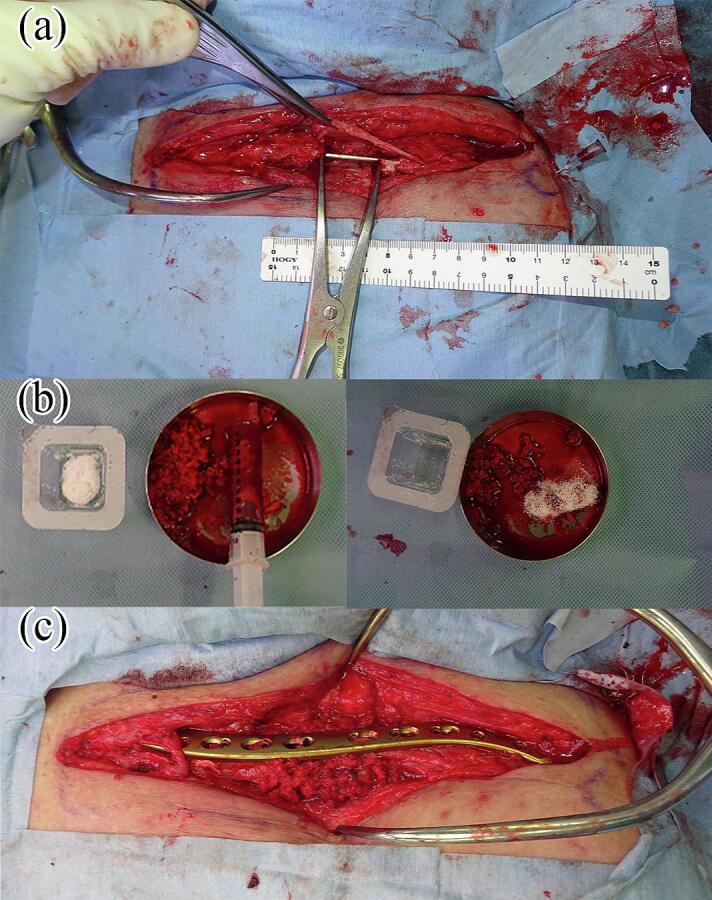
The second stage of the induced membrane technique (IMT). (a) After removing the polymethylmethacrylate (PMMA) spacer. The induced membrane was observed around the PMMA spacer. The bone defect was opened with a spreader to prevent clavicle shortening. (b) The wool-like β-tricalcium phosphate (β-TCP) material (ReBOSSIS®) was mixed with an autogenous cancellous bone. (c) The clavicle was fixed with a locking plate. Then, the bone defect was filled with an autogenous cancellous bone mixed with β-TCP.
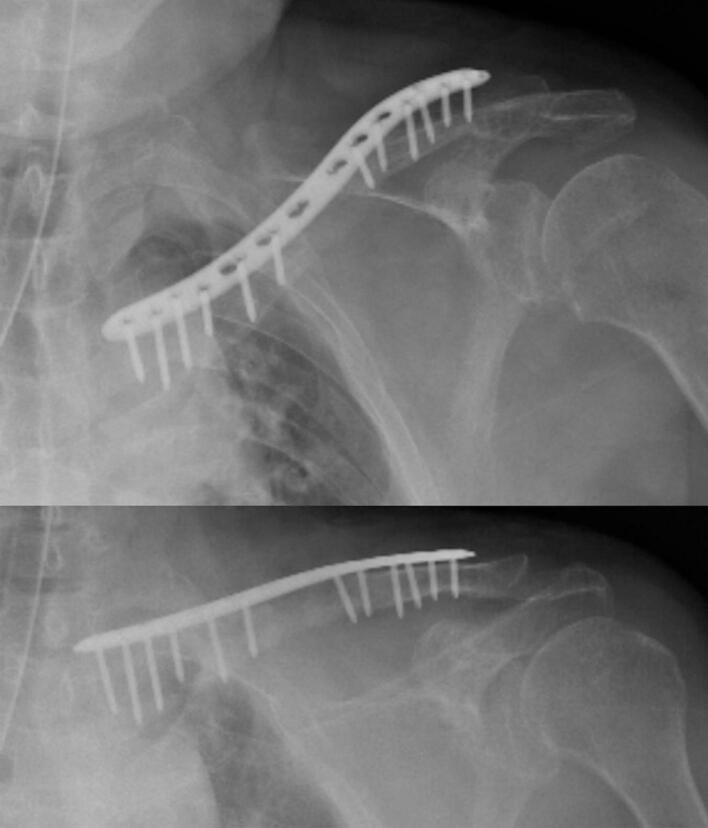
Fig. 3.
Radiographic image obtained after the second stage of the induced membrane technique (IMT).
The patient performed active range of motion exercise of the left shoulder immediately after surgery. There was no wound or neurovascular complications. At 8 months after surgery, the nonunion area had fused (Fig. 4), and the patient had no restriction in activities of daily living. The patient provided informed consent for the publication of data concerning his case.
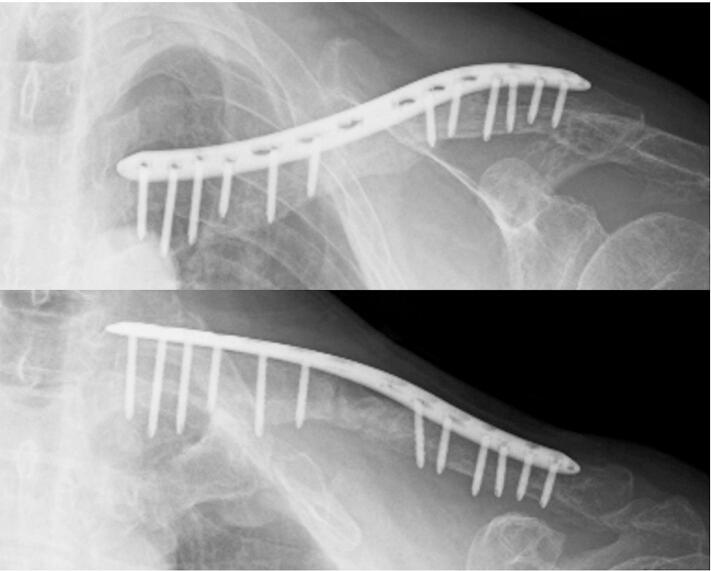
Fig. 4.
Radiographic image obtained at 8 months after the second stage of the induced membrane technique (IMT). The nonunion area was completely fused.
Discussion
We found out two important clinical issues. The IMT with β-TCP can be applied as a reconstruction method for bone defects after infectious nonunion of clavicle, and the wool-like β-TCP may decrease the migration of β-TCP.
First, the IMT with β-TCP can be applied for bone defects after infectious nonunion of the clavicle. Although the IMT was originally designed for long bones of the extremities, it was also useful for the clavicle, which has a different embryology. Traditionally, the corticocancellous bone graft, cancellous bone graft, and vascularized bone graft have been used to reconstruct clavicle bone defects [1]. In addition, the development of the IMT method, as reported by Masquelet et al., has been successful in achieving good bone formation via autologous cancellous bone grafting and in reducing the risk of complications at the donor site [2,3]. However, postoperative complications at the donor site (pain, prolonged surgical time, donor site fracture, and nonunion) could not be completely prevented [8,9]. In addition, a sufficient autogenous cancellous bone may not be harvested from the iliac bone. Meanwhile, if the bone defect is extremely large, the cancellous bone may be relatively insufficient. Therefore, by adding β-TCP to the autogenous cancellous bone, the IMT technique can be applied to cases of massive bone defects and cases in which a sufficient cancellous bone cannot be obtained.
Secondly, the wool-like β-TCP may decrease the migration of β-TCP. Generally, the granule-type β-TCP is used. However, osteogenesis was only observed in the dorsal aspect of the femoral diaphysis after transplanting the autogenous cancellous bone mixed with the granule-type β-TCP into a defect in the femoral shaft [10]. In bones covered with muscles and other mobile tissues, due to gravity, the β-TCP and cancellous bone can move within the gap between the bone defects during daily life activities. The same phenomenon could occur in the clavicle. That is, the periphery is covered with sparse connective tissues. Therefore, in this case, the wool-like β-TCP was used [11]. Moreover, a full circumferential bone formation was achieved. The wool-like β-TCP may decrease the migration of β-TCP and autogenous cancellous bone within the defect in bone reconstruction where the surrounding area is covered with sparse connective tissues.
In conclusion, the IMT with β-TCP can be a reconstructive method for bone defects after clavicular nonunion.
Ethical statement
Informed consent for publication was obtained from the patient, and this report was conducted in compliance with the tenets of the Declaration of Helsinki.
Funding
This research received no external funding.
Informed consent
Written informed consent was obtained from the patient for publication of this case report, including the images.
CRediT authorship contribution statement
Kunihiko Arakawa: Writing – original draft. Yoshinobu Watanabe: Supervision, Conceptualization. Gen Sasaki: Data curation. Mari Nishizawa: Data curation. Akifumi Honda: Data curation. Natsumi Saka: Data curation. Hirotaka Kawano: Writing – review & editing.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
No acknowledgement.
References
- 1.Calori G.M., Mazza E.L., Colombo A., Mazzola S., Colombo M. Treatment of an atrophic clavicle non-union with the chamber induction technique: a case report. Injury. Oct 2017;48(3):S71–S75. doi: 10.1016/S0020-1383(17)30662-9. [DOI] [PubMed] [Google Scholar]
- 2.Masquelet A.C., Fitoussi F., Begue T., Muller G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. Jun 2000;45(3):346–353. [PubMed] [Google Scholar]
- 3.Masquelet A.C., Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. North Am. Jan 2010;41(1):27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki G., Watanabe Y., Miyamoto W., Yasui Y., Morimoto S., Kawano H. Induced membrane technique using beta-tricalcium phosphate for reconstruction of femoral and tibial segmental bone loss due to infection: technical tips and preliminary clinical results. Int. Orthop. Jan 2018;42(1):17–24. doi: 10.1007/s00264-017-3503-5. [DOI] [PubMed] [Google Scholar]
- 5.Sliepen J., Hoekstra H., Onsea J., et al. Treatment and outcome of fracture-related infection of the clavicle. Injury. Aug 2023;54(8) doi: 10.1016/j.injury.2023.110910. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor C.M., Perloff E., Drinane J., Cole K., Marinello P.G. An analysis of complications and bone defect length with the use of induced membrane technique in the upper limb: a systematic review. Hand (N Y) May 2022;17(3):572–577. doi: 10.1177/1558944720918368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdellaoui H., Atarraf K., Chater L., Afifi M.A. Congenital pseudarthrosis of the clavicle treated by Masquelet technique. BMJ Case Rep. Nov 08 2017:2017. doi: 10.1136/bcr-2017-221557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calori G.M., Colombo M., Mazza E.L., et al. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. Dec 2014;45(Sup 6):S116–S120. doi: 10.1016/j.injury.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.C., Alford J., Willson T., et al. Improved success rate with corticocancellous block compared to cancellous-only trephine technique in alveolar bone grafting from the iliac crest. Plast. Reconstr. Surg. Aug 2022;150(2):387e–395e. doi: 10.1097/PRS.0000000000009352. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki G., Watanabe Y., Yasui Y., et al. Clinical and radiological assessment of the induced membrane technique using beta-tricalcium phosphate in reconstructive surgery for lower extremity long bone defects. Bone Joint J. Mar 2021;103-B(3):456–461. doi: 10.1302/0301-620X.103B3.BJJ-2020-1542.R1. [DOI] [PubMed] [Google Scholar]
- 11.Nepola J.C., Petersen E.B., DeVries-Watson N., et al. Electrospun PLGA and β-TCP(Rebossis-85) in a Lapine posterolateral fusion model. Iowa Orthop. J. 2019;39(2):9–19. [PMC free article] [PubMed] [Google Scholar]