Abstract
Background:
Phthalates have been linked to adverse male reproductive health including poor sperm quality and embryo quality as well as longer time to pregnancy (months of unprotected intercourse before conception occurs). The present study was aimed to evaluate the effect of preconception exposure to two ubiquitous phthalate chemicals di(2-ethylhexyl) phthalate (DEHP), di-n-butyl phthalate (DBP) and their mixture on sperm function, fertilization and embryo development in mice.
Materials and methods:
Adult male C57BL/6J mice aged 8–9 weeks were exposed to DEHP, DBP or its mixture (DBP+DEHP) at 2.5 mg/kg/day or vehicle for 40 days (equivalent to one spermatogenic cycle), via surgically implanted osmotic pumps. Caudal epididymal sperm were extracted and analyzed for motility using computer assisted sperm analyses (CASA). Sperm phosphorylation of PKA substrates and tyrosine phosphorylation, markers of early and late capacitation events, respectively, were analyzed by Western blots. In vitro fertilization was used to evaluate the sperm fertilizing capacity.
Results:
While the study did not reveal any significant differences in sperm motility and fertilization potential, abnormal sperm morphology was observed in all phthalate exposures, particularly in the phthalate mixture group. In addition, the study revealed significant differences in sperm concentration between control and exposed groups. Moreover, protein phosphorylation of protein kinase A (PKA) substrates was decreased in the DEHP and mixture exposure groups while no significant changes in protein tyrosine phosphorylation were observed in any of the groups. Assessment of the reproductive functionality did not reveal significant effects in IVF and early embryo development rates but showed wide variability in the phthalate mixture group.
Conclusion:
Our findings suggest that preconception phthalate exposure affects sperm numbers and phosphorylation of PKA substrates involved in capacitation. Future research is warranted to examine the associations between phthalate exposures and capacitation in human sperm.
Keywords: Phthalates, sperm quality, capacitation, infertility, reproductive outcomes
Introduction
Infertility and subfertility are critical health problems affecting about 8 to 12 % of couples worldwide. Among these couples, between 40 and 50 % are due to male infertility issues; moreover, it is estimated that 2 % of all men have suboptimal sperm parameters 1. A decline in human male reproductive potential has been a matter of growing concern over the last four decades. While recent meta-analyses have reported a nearly 50% decline in sperm quality from 1938–2011 2, epidemiological studies reveal an increasing trend of testicular cancer and malformations at birth associated with reduced sperm count 1,3–5. Decline in sperm counts have been detected within a short time span and suggest changes in environmental factors and lifestyle that are detrimental to sperm quality rather than genetic factors 6. Humans are exposed to a variety of exogenic chemicals during their lifetime that have endocrine disrupting capabilities which may in turn adversely impact the male reproductive development 7. Although the mode of action of these toxicants are still uncertain, we have previously shown that preconception urinary phthalate concentrations are associated with sperm DNA methylation in humans suggesting that these compounds may influence epigenetic reprogramming during spermatogenesis 8. However, the extent by which phthalates affect sperm physiological processes such as capacitation, motility and the acrosome reaction has not yet been investigated.
It is known that males contribute to nearly 25–50% of all fertility related problems worldwide 9,10. This observation encouraged analysis of reproductive effects of environmental xenobiotics in the general population. Phthalates are a group of manmade endocrine-disrupting chemicals that are used as industrial plasticizers and are common components of various household items 10. Phthalates make their entry into the human body through different routes and are rapidly metabolized to their monoesters while some get conjugated to glucuronic acid before being excreted through the urinary tract 9. Despite of its capacity to be rapidly metabolized and excreted, it is assumed that a steady state may be reached through its chronic low levels of exposure 11. Phthalates encompass a group of compounds that differ in their structural properties; these compounds have been widely documented for their anti-androgenic activity in rodents and human studies resulting in male reproductive abnormalities 12–14. However, the health effects of phthalates exposure in adults on the male reproductive system have not yet been fully understood 15. Importantly, phthalate exposure studies lack a broader understanding of mode of action of these compounds in causing male reproductive anomalies during different stages of lifetime from foetal development to adulthood 10. Although and important first step, testing the effects of these individual toxic compounds on the male reproductive potential is not representative of the actual life situation in which, individuals are exposed to a combination of phthalates. Therefore, the relevance of understanding their mixture effects for an overall assessment of the potential of their toxicity has been highlighted previously 3.
To this end, in the present work we aimed to evaluate the reproductive toxicity effect of chronic exposure in adult male mice to two omnipresent phthalates, di(2-ethylhexyl) phthalate (DEHP), dibutylphthalate (DBP), and their mixture. These phthalates are considered to be the most potent for their anti-androgenic activity and, in addition, are detected in human urine 11,16. As part of these studies, we investigated the effect of phthalates on mature sperm functional parameters including sperm concentration, motility, capacitation-induced phosphorylation pathways, the sperm ability to fertilize in vitro and, upon fertilization, the extent that those fertilized embryos develop to blastocysts.
Materials and Methods
Materials:
Chemicals and other lab reagents were purchased from the following companies follows (catalogue # between parenthesis): di (2-ethylhexyl) phthalate (DEHP, 36735), dibutyl phthalate (DBP, 524980), tetraglycol (T3396), polyethylene glycol (PHR3336), kolliphor EL (C5135), 1,2-propanediol (398039), for exposure treatment, bovine serum albumin (BSA, fatty acid-free, A0281) for media, pregnant mare serum gonadotropin (PMSG) (G4877), and human chorionic gonadotropin (hCG) (CG5), light mineral oil (ES-005-C), EmbryoMax R KSOM Medium (1X) with 1/2 Amino Acids (MR-106-D) for fertilization assays, Trizma base, sodium chloride (NaCl), magnesium chloride (MgCl2), potassium chloride (KCl), β-mercaptoethanol (βME), sodium bicarbonate (NaHCO3) from Sigma-Aldrich (St. Louis, MO). HEPES (cat #BP310) and glycerol were obtained from Fisher Scientific; Sodium dodecyl sulfate (SDS) from Bio Rad (BioRad Laboratories, Hercules, CA); anti - phospho PKA substrate monoclonal antibody, and anti- alpha tubulin polyclonal antibody (cat #9624 and #2144, respectively) from Cell Signaling Technology (Danvers, MA); anti-pY antibody (clone 4G10) (cat #05–321) from Millipore (Burlington, MA); horseradish peroxidase-conjugated anti-mouse and horseradish peroxidase-conjugated anti-rabbit IgG from Jackson ImmunoResearch laboratories (West Grove, PA). Finally, ECL Prime Western blotting detection reagents were from GE Healthcare (Pittsburgh, PA).
Animals and Treatment
Adult C57BL/6J males aged (8–9 weeks old) with an approximate weight 30–35 g were purchased from Jackson Laboratories. The animals were single-housed in an optimum environment with temperature-controlled at (23°C ± 2°C) and humidity-controlled (40 % ±10 %) environment, with a 12-hour light/dark cycle and food and water available ad libitum. The experiment described below was reproduced four times. In each experiment 16 mice were distributed to four experimental groups (n=4 per group) and were exposed to 2.5 mg/kg/day of DBP or DEHP, or a combination of both compounds, each at 2.5 mg/kg/day. Given that the “no observed adverse effect level (NOAEL)” for DEHP with male reproductive endpoint in rat is 4.8 mg/kg/day 17, we considered our DEHP exposure of 2.5 mg/kg/day as approximately 50% below the NOAEL. This dose was also found to result in significant epigenetic changes in F0 sperm as well as epigenetic and gene expression changes in F1 embryos 18. For DBP, we chose to keep exposure levels consistent with DEHP to allow comparison. Control group was exposed to vehicle only: 25 % v/v tetraglycol, 25 % v/v polyethylene glycol 300, 25 % v/v kolliphor EL, 15 % v/v ethanol, and 10 % v/v 1,2-propanediol. Exposure groups were denoted as DBP, DEHP, Mix and C respectively. Exposures were conducted for 40 days via surgically implanted subcutaneous osmotic pumps (Mini-osmotic pump, model 2006, Alzet, Cupertino, CA). All animals including the control group were kept in same microclimatic conditions and uniformity was maintained throughout the exposure period. Animal care and use of experimental animals were conducted in accordance with specific guidelines and standards dictated by the Office of Laboratory Animal Welfare (OLAW) and approved by the Institutional Animal Use and Care Committee (IACUC), University of Massachusetts-Amherst (Protocol #2019–0008).
Sperm Sample Collection and Media Preparation
Spermatozoa from the caudal epididymal region from exposed and control mice were collected using a swim-out approach. Briefly, for each male mouse, caudal epididymides collected from both sides were incised longitudinally and incubated for an approximate 10 minutes in 1 ml of m-TYH medium at 37°C. Epididymides were then discarded, leaving the sperm suspended in the m-TYH medium. The m-TYH medium termed as non-capacitating (NCAP) is a modified Toyoda-Yokoyama-Hosi (m-TYH) medium 19 that comprises the following ingredients: 119.37mM NaCl, 4.78mM KCl, 1.19mM KH2PO4, 1.19mM MgSO4, 5.56mM glucose, 1.71mM CaCl2, 20mM HEPES, 0.51mM Na-pyruvate, 10μg/mL gentamicin, 0.0006 % phenol red with a pH maintained at 7.3. In mouse sperm, absence of HCO3− and BSA is sufficient to prevent capacitation 20. To prepare the capacitating m-TYH (CAP) media, the non-capacitating media was supplemented with 15 mM NaHCO3 and 5 mg/mL of bovine serum albumin (BSA, Sigma cat # A0281, St. Louis, MO) with pH maintained between 7.2 and 7.4.
Sperm Concentration
Sperm suspension was diluted at 1:100 with 1X PBS with gentle mixing and 10 μL of the suspension was placed on the Neubauer hemocytometer. The cells were examined under 20X phase contrast objective lens and were counted. Each sample was examined three times using a hemocytometer and quantified and the concentration was determined as number of sperm cells per milliliter.
Sperm Motility and Hyperactivation
The sperm motility and hyperactivation measurements were conducted using computer assisted sperm analysis (CASA). Briefly, spermatozoa from the sperm suspension were diluted to contain an approximate concentration of 1 × 106 spermatozoa and incubated in NCAP or CAP media for 60 minutes. Following incubation, 30 μL of the sperm suspension was loaded onto the pre-warmed chamber slide (depth, 100 μm) (Leja slide, Spectrum Technologies) and placed on a microscope stage at 37°C. Sperm motility was examined using CASA (Hamilton Thorne Research, Beverly, MA, United States) as previously described 21 with default settings (frames acquired: 90; frame rate: 60 Hz; minimum cell size: 4 pixels; static head size: 0.13–2.43; static head intensity: 0.10–1.52; and static head elongation: 5–100). Sperm with hyperactivated motility, defined as motility with high amplitude thrashing patterns and short distance of travel, were sorted and analyzed using CASA software. Sperm with curvilinear velocity (VCL) > 271.00 μm/sec, linearity (LIN) < 50.00 % and amplitude of lateral head (ALH) > 3.50 μm were considered hyperactive. At least five microscopy fields corresponding to a minimum of 200 sperm were analyzed for each treatment in each experiment.
Sperm Morphology
For evaluation of sperm morphology, a thin smear of the sperm suspension was made on clean grease-free glass slide, air dried and covered by coverslip using vectashield (Vectashield, H-1000) as mounting agent. A maximum of hundred cells were counted per slide (n=6) for each exposure group under difference interference contrast (DIC) light microscopy using a 60X magnification oil immersion objective lens. All abnormal spermatozoa were counted and recorded and expressed as percentage abnormal morphology per total spermatozoa counted.
Sperm Capacitation and Protein Extraction
Caudal spermatozoa obtained post swim-out were counted and adjusted to an approximate 1 × 106 spermatozoa per condition. Spermatozoa from each group were incubated in media to promote capacitation (CAP) or not (NCAP) as described above for 60 minutes at 37 °C. After this incubation period, sperm were centrifuged at 12,100 × g for 5 minutes and washed twice in 600 μl of ice-cold PBS. Pellets were stored at −80°C until further use.
Gel electrophoresis and Western blotting
On the day of the experiment, sperm pellets were thawed and resuspended in 50 μl of the Laemmli buffer (without β-mercapthoethanol) containing SDS and boiled for 5 minutes at 100°C followed by centrifugation at 12,100 × g for 5 minutes. Following centrifugation, the pellets were discarded, and the supernatants were supplemented with 5 % β-mercaptoethanol, boiled at 100 °C for 5 minutes and centrifuged as mentioned above. The samples were then loaded onto 8% SDS-PAGE gels for protein separation through electrophoresis using a BioRAD (Hercules, CA, USA) electrophoretic chamber following recommended protocol. Proteins were then transferred subsequently onto the PVDF membranes, and protein blocking and incubation with specific antibodies were performed as follows: PVDF membranes were incubated in 5% bovine serum albumin (BSA, Sigma cat # A7906, St. Louis, MO) in Tris-buffered saline with 0.01% Tween-20 (TBS-T) for one hour at room temperature.
For the detection of the phosphorylated PKA substrates, rabbit generated anti-pPKA substrates monoclonal antibody (Cell Signaling, cat# 9624, Danvers, MA) was used at a dilution of 1:10,000 in 1% BSA prepared in TBS-T and kept at 4°C with slow rocking. The following day membranes were washed with TBS-T (3 times) at an interval of 5 minute each and incubated with the corresponding HRP conjugated secondary anti- rabbit antibody (Jackson Immunoresearch Laboratories, West Grover, PA) at a dilution of 1:10,000 prepared in TBS-T for one hour at room temperature. The same blots were stripped as described 22 by incubating in the presence of 2% SDS, 0.74% β-mercaptoethanol, 62.6mM Tris, pH-6.5 at 60 °C for 15 minutes and washed three times for 5 minutes in TBS-T. After stripping the PVDF membranes were blocked once more with 20 % fish gelatin (Sigma cat#G7765, St. Louis, MO) prepared in TBS-T for one hour at room temperature. Protein detection was done using mouse-generated anti-pY antibody (clone 4G10, Millipore, cat#05–321, Burlington, MA) at a dilution of 1:10,000 kept overnight at 4°C with slow rocking, washed and developed with anti-mouse HRPx conjugated secondary antibody. For loading controls, PVDF membranes were stripped once more using the same conditions as described above and used with rabbit-generated anti-alpha tubulin antibody (Cell Signaling, cat# 2144S) diluted at 1:1,000. Membranes were developed by using an enhanced chemiluminescence detection kit (ECLprime, GE Healthcare, Pittsburg, PA).
In-vitro Fertilization and Embryo Development
Animals and Treatment
Mouse cumulus-oocyte-complexes (COCs) were collected from 8–10-week-old super ovulated CD1 females (Charles River Laboratories, Wilmington, MA, USA) with 7.5 IU pregnant mare serum gonadotropin [PMSG] (Lee BioSolutions, cat # 493–10, Maryland Heights, MO) and 7.5 IU human chorionic gonadotrophin [hCG] (Sigma, cat # CG5, St. Louis, MO) 48 hours later.
Media
The medium used for COCs collection was TL-HEPES, consisting of 114 mM NaCl, 3.22 mM KCl, 2.04 mM CaCl2.2 H2O, 0.35 mM NaH2PO4.2H2O, 0.49 mM MgCl2.6H2O, 2.02 mM NaHCO3, 10 mM Lactic acid (sodium salt), and 10.1 mM HEPES at pH 7.4. Medium used for sperm fertilization assay and fusion assay was Toyoda-Yokoyama-Hosi (IVF-TYH) medium, consisting of 119.37 NaCl, 4.78 KCl, 1.19 KH2PO4, 1.19 MgSO4. 7 H2O, 5.56 glucose, 1.71 CaCl2.2 H2O, 25.1 mM NaHCO3−, 0.51 Na-pyruvate, 4 mg/mL bovine serum albumin (BSA), 10 μg/mL gentamicin, 0.0006 % phenol red at pH 7.4 equilibrated overnight with 5% CO2.
In vitro fertilization (IVF) assay and embryo development.
Caudal sperm samples were collected from each treatment group in IVF-TYH medium previously equilibrated in a CO2 incubator (37°C, 5% CO2). Samples were capacitated for 60 minutes in an incubator at 37°C, 5% CO2. Females were sacrificed 13-hours post hCG and COCs were collected in TL-HEPES medium. COCs were then washed through IVF-TYH prior to being placed in the insemination droplets, previously equilibrated in a CO2 incubator (37°C, 5% CO2). After 60 minutes of capacitation, the insemination droplet containing COCs was inseminated with approximately 100,000 sperm per 90 μL droplet. After 4 hours, oocytes were removed from the insemination droplet, moved to a clean droplet of IVF-TYH and allowed to culture for 20 hours. The following day, fertilization was investigated by visualization of 2-cell cleavage. 2-cell embryos were then moved to KSOM (Fisher Scientific, cat MR-106-D, Hampton, NH) plates (previously equilibrated in a CO2 incubator at 37°C, 5% CO2) for embryo development until embryonic day 3.75 or blastocyst stage. At embryonic day 3.75, successful embryo development was assessed by formation of blastocysts.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism software version 9.3.1, San Diego, California (www.graphpad.com). Data generated from all experiments were expressed as mean ± standard error of mean (SEM). The normality tests for the results obtained were performed using Kolmogorov-Smirnov test. The data that met assumptions of normal distribution and homogeneity of variance were subjected to one-way analysis of variance (ANOVA) followed by either Tukey’s or Dunnett post-hoc test. The data that neither showed normal distribution or met homogeneity of variance assumptions were analyzed using the Kruskal-Wallis non-parametric test. Statistical significances are indicated as asterisks (*). One asterisk (*) indicates p < 0.05, two asterisks (**) indicate p < 0.01, three asterisks (***) indicates p < 0.001.
Results
Effect of phthalates on sperm parameters.
Exposures to DEHP and DBP decreased the total number of mature cauda epididymal sperm; not surprising, the phthalate mixture exposure had a more dramatic effect in sperm numbers reaching about 30 % of the average amount obtained with control-treated mice (Fig. 1 A). Despite the reduced number of sperm, motility parameters (Curvilinear velocity (VCL), straight linear velocity (VSL), average path velocity (VAP), and progressive motility) measured with CASA (which considers only motile sperm) in media not supporting capacitation were similar across all treatments (Fig. 1B to E), while sperm morphology was altered in exposed groups relative to the control (Fig. 2A). Quantitative assessment showed significant increase in abnormal morphology (%) in the exposed groups (Fig. 2 B, C and D). While the control groups showed minimal morphological damage (Fig. 2 A), sperm obtained from mice treated with DBP (Fig. 2 B) and DEHP (Fig. 2 C) depicted a higher degree of defective spermatozoa. These effects were greater in sperm obtained from the phthalate mixture group (Fig. 2 D). The overall percentage of morphological defects were quantified (Fig. 2 E). These data show a two-fold increase in morphological defects from exposures to either DBP or DEHP (42 % (for DBP) 43 % (for DEHP) as compared to 22 % (for control treatment). When individual morphological defects were analyzed in more detail, defects were seen in the head, neck as well as in the tail (Fig. 3); in particular, the number of decapitated sperm is considerably higher in the DEHP and in the mixture group (Fig. 3 A, B). In addition, we have also found higher percentages of folded, bent, and dislodged necks (Fig. 3 C, D) as well as other tail defects (Fig. 3 E, F) in all phthalate exposures.
Figure-1: Sperm functional parameters.
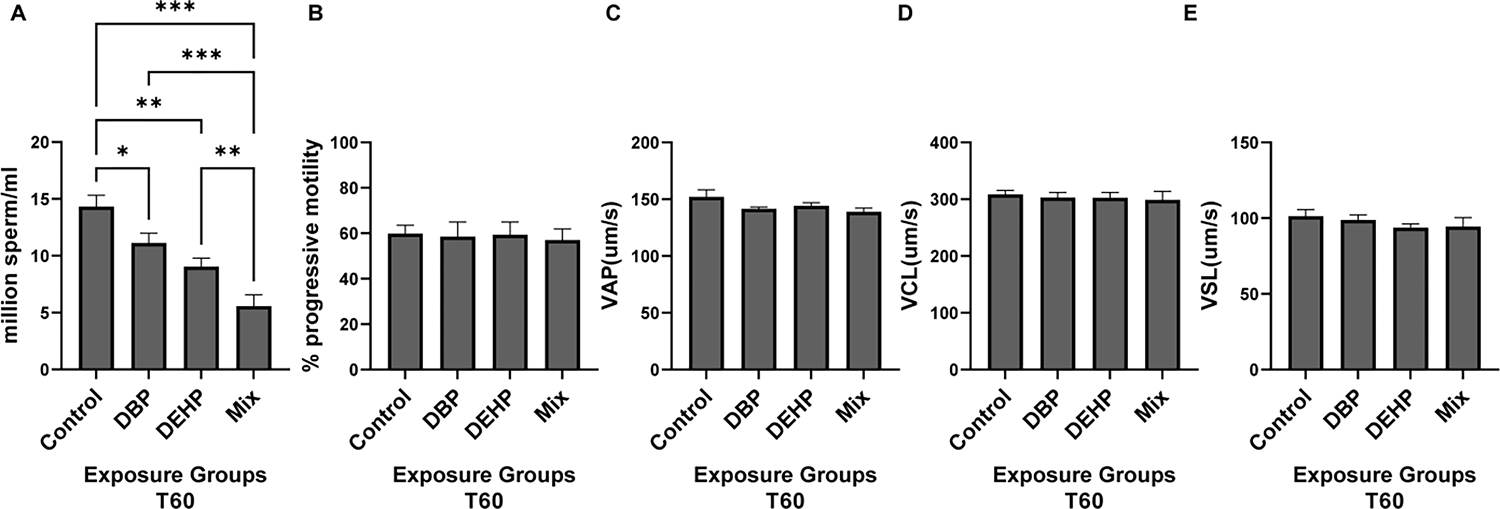
Cauda epididymal sperm were obtained by swim-out from mice previously exposed to control, DBP, DEHP or mixed conditions. A: Upon discarding the epididymis, the total number of sperm was count with an improved Neubauer hemocytometer. B, C, D, E: Sperm suspensions from each different conditions obtained in A were incubated in complete capacitating TYH media for 60 min and their kinematic parameters analyzed by CASA. Progressive motility (B), average path velocity (VAP) (C), curvilinear velocity (VCL) (D) and, straight linear velocity (VSL) (E) were evaluated between the different exposure groups. For VCL, VSL and VAP values indicate mean ± SEM and expressed as μ m/sec. Progressive motility is expressed as percentage out of the total motile sperm. Sperm numbers, progressive motility and VAP were analyzed using one-way ANOVA followed by Tukey’s post-hoc test. VCL and VSL data were analyzed using non-parametric Kruskal-Wallis test followed by Dunn’s post-hoc test. In all cases, these analyses were done using Graph Pad Prism software for statistical analysis with p<0.05(*), p<0.01 (**), p< 0.001 (***) (N=12).
Figure-2: Sperm morphology. Aliquots obtained as described in Fig. 1 were used to assess sperm morphology.
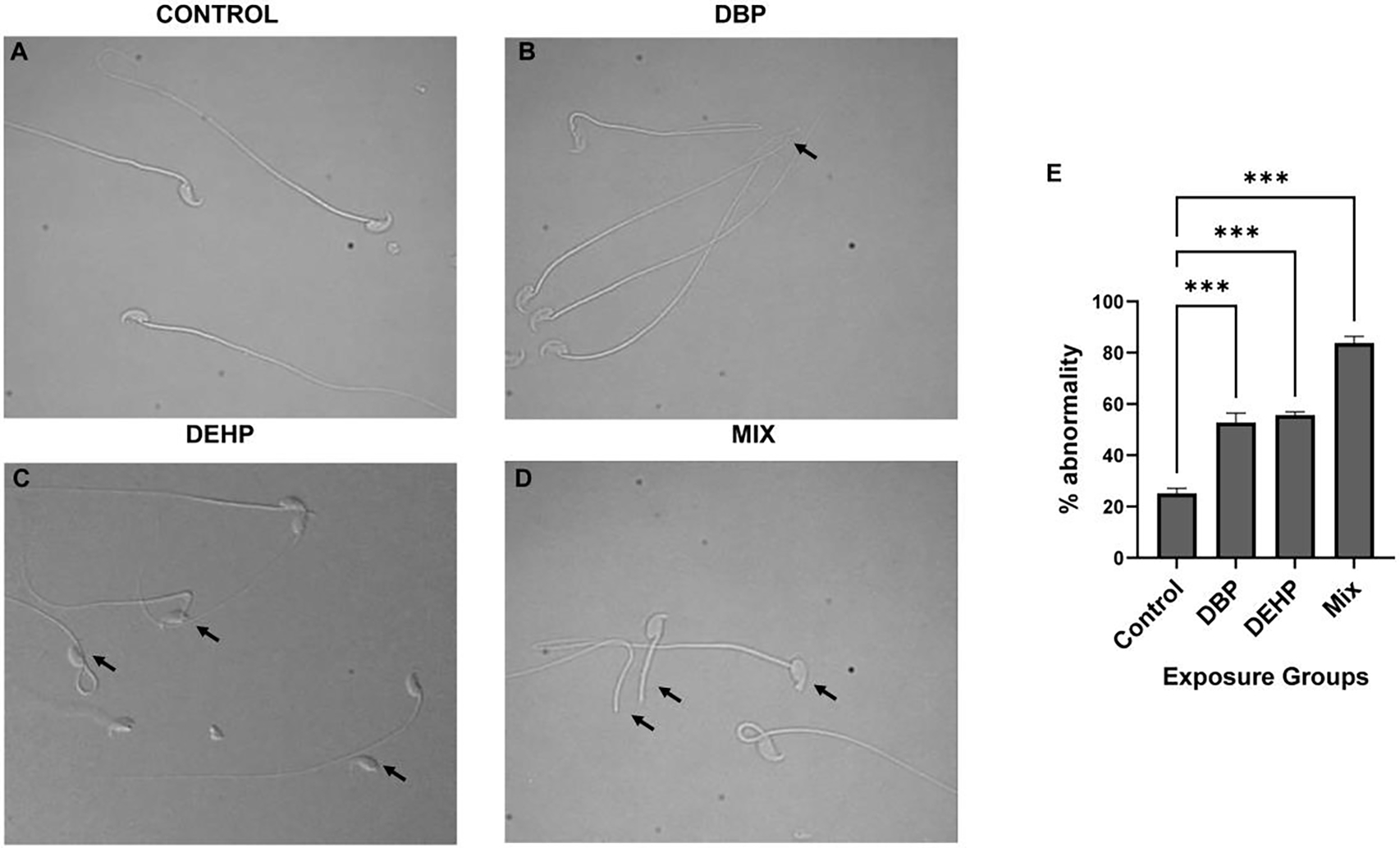
using differential interference contrast (DIC) microscopy. A-D: Representative images of abnormal morphology in the different exposure groups. Arrows indicate some of the examples of abnormal morphology. For all exposure conditions a minimum of 100 spermatozoa per sample was counted for morphology evaluation. Data are represented as mean percentage of abnormal sperm ± SEM (N=6). A one-way ANOVA was performed followed by Dunnett test for multiple comparisons p< 0.001 (***), considered as statistically significant. Data were compared to the Control group only.
Figure-3: Compartmentalization of sperm morphological defects in the different exposure groups.
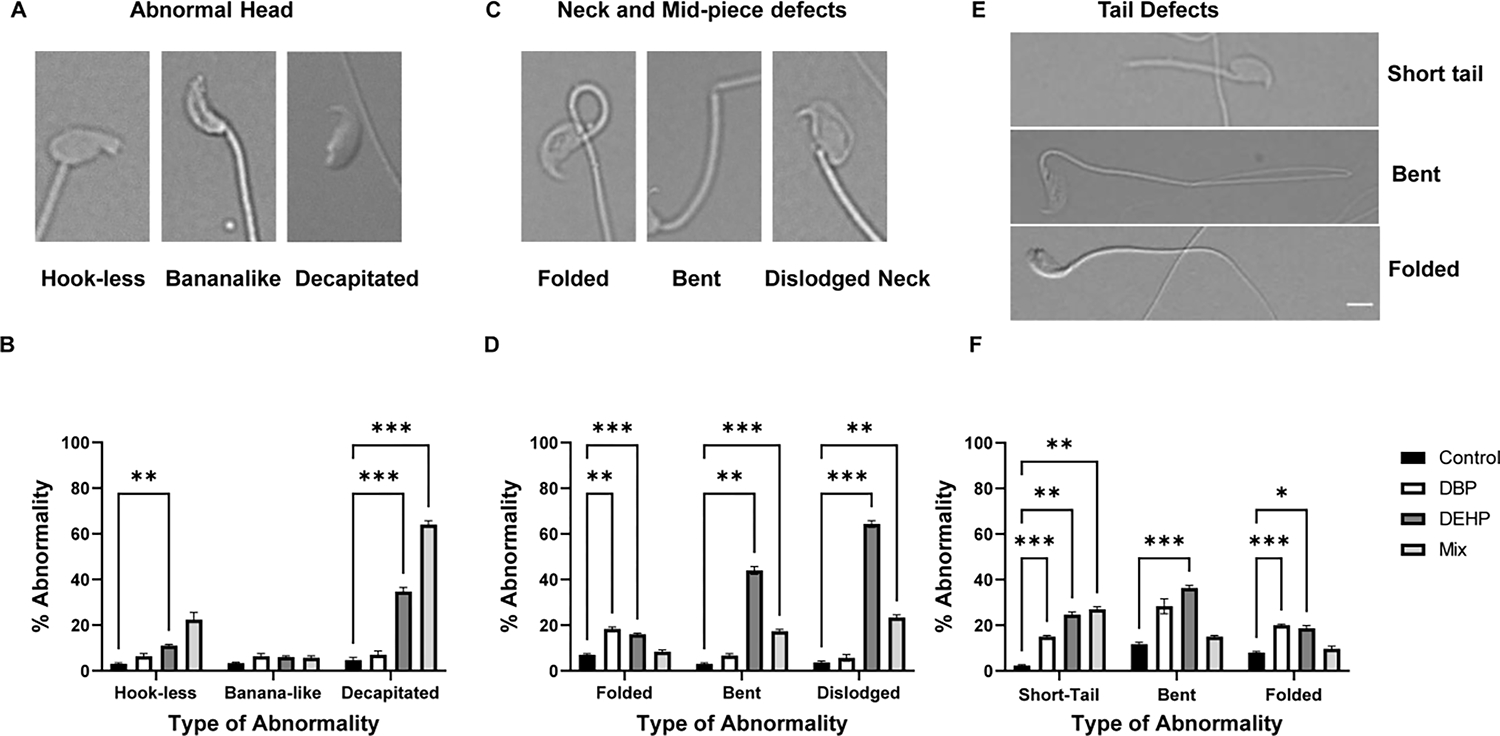
Sperm used for the analyses described in figure 2 were further analyzed regarding the type of morphological defects in the head (A and B), mid-piece (C and D) and tail (E and F). Notice that the tail defects do not include double tails, in some of the insets in E, there is superposition of a second spermatozoon’s tail. Spermatozoa with multiple abnormalities were included based on the defects exhibited for each compartment. Data are represented as mean percentage of abnormal sperm ± SEM (N=6). A two-way Anova followed by Dunnett post-hoc test was performed for each compartment of the exposure group with p<0.05 (*), p<0.01 (**), p< 0.001 (***). Data were compared to the control group only.
Effect of phthalates exposure on capacitation-associated phosphorylation pathways.
Upon ejaculation, mammalian sperm require to undergo capacitation. Capacitation associated changes can be mimicked in vitro, and it is associated with a fast (< 1 min) HCO3−-dependent activation of PKA followed by a slower (~ 30 min) increase in tyrosine phosphorylation. Up-regulation of the cAMP-dependent pathways can be studied by Western blots using anti phospho PKA substrates antibodies (anti-PKAS) (Fig. 4 A and B)). Similarly, the capacitation-associated increase in tyrosine phosphorylation can be followed using anti phospho tyrosine antibodies (anti PY)(Fig. 4 C and D). Sperm obtained from controls as well as from DBP-treated mice depicted normal levels of PKA substrates phosphorylation; however, PKA-dependent phosphorylation was significantly reduced in sperm from DEHP exposure as well as those obtained from mice exposed to phthalate mixtures. Surprisingly, the increase in tyrosine phosphorylation was not affected by any of the exposures.
Figure-4: Analysis of sperm capacitation phosphorylation pathways in sperm derived from mice exposed to different phthalate conditions.
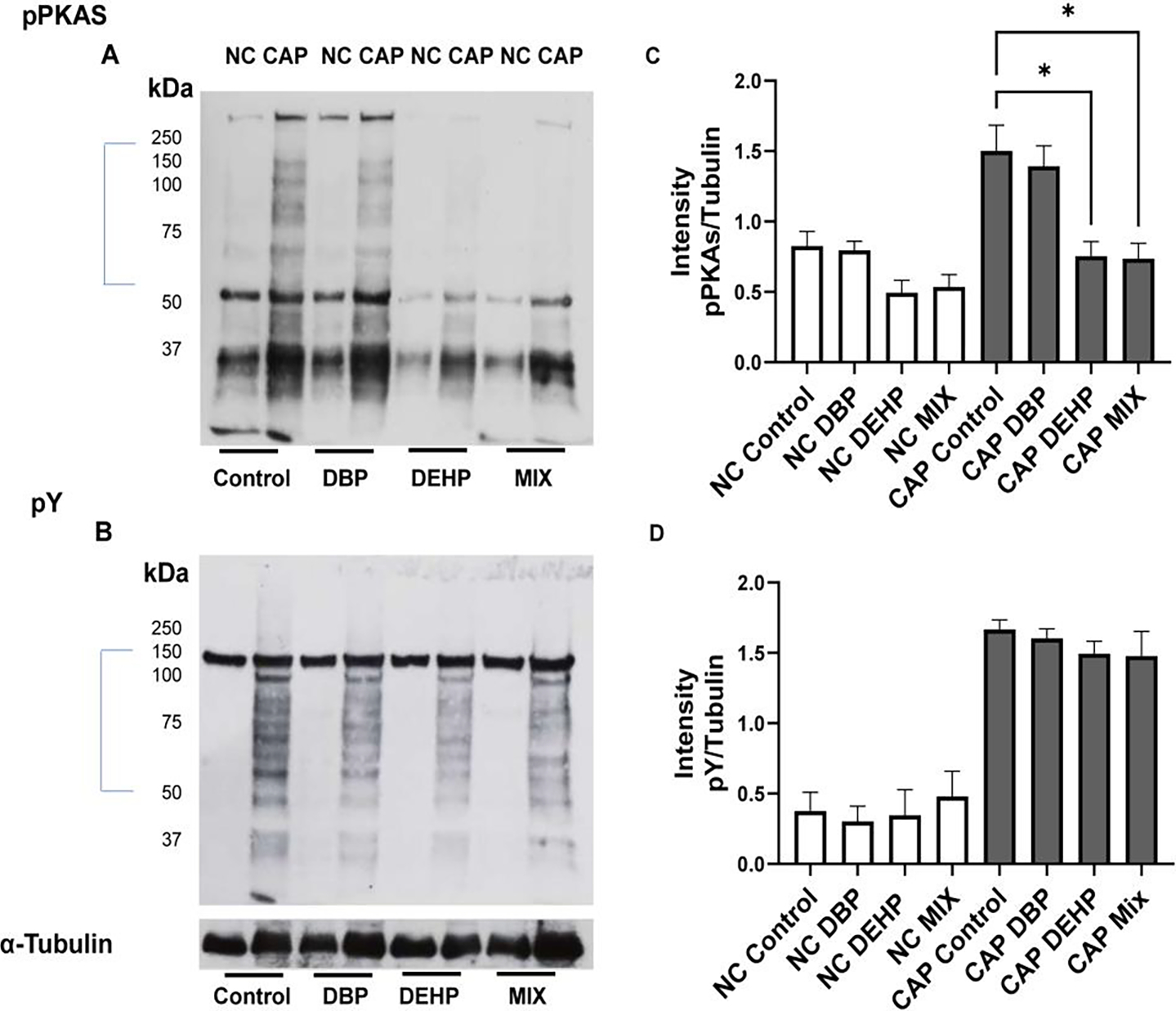
A: Phosphorylation of PKA substrates were evaluated by Western blots using anti phospho PKA substrate antibody (pPKAs). Each lane contain protein extracts from ~106 sperm as described in Methods. B: Changes in protein tyrosine phosphorylation were assessed using anti PY antibodies in the same blots used for A after stripping the immobilon membrane as described in Methods. The same blots were stripped once more and probed with anti-tubulin antibody. C and D: Densitometric analyses of pPKAs and PY were done with IMAGE J software. For this analysis, only the section marked with a bar in A and B were used for the evaluation. Pixels were normalized with the values obtained from the anti-tubulin Western blots. Kruskal Wallis non-parametric tests followed by Dunn’s test were performed using GraphPad prism. Data are represented as mean percentage of abnormal sperm ± SEM with p<0.05(*) (N=12),. NC denotes non-capacitated while CAP denotes capacitated condition
Effect of phthalates on fertilization and embryonic development
Capacitation is also associated with a change in motility pattern, known as hyperactivation. Hyperactivation results in an asymmetric movement of the sperm flagellum and it is correlated with the sperm ability to fertilize. Levels of hyperactivation upon 60 min capacitation were similar in all treatments (Fig. 5 A). Finally, sperm derived from each of the treated mice were used to evaluate their ability to fertilize in vitro, and subsequent embryo developmental potential. Fertilization rates were unaffected with the DEHP and DBP exposures; however, sperm derived from mice treated with the phthalate mixture had a greater variability; one mouse had fertilization close to zero, while others have normal levels of fertilization (Fig. 5 B). Once fertilization occurred, in all treatments, two-cell embryos were equally capable to develop to blastocysts (Fig. 5 C).
Figure-5: Sperm fertilization parameters.
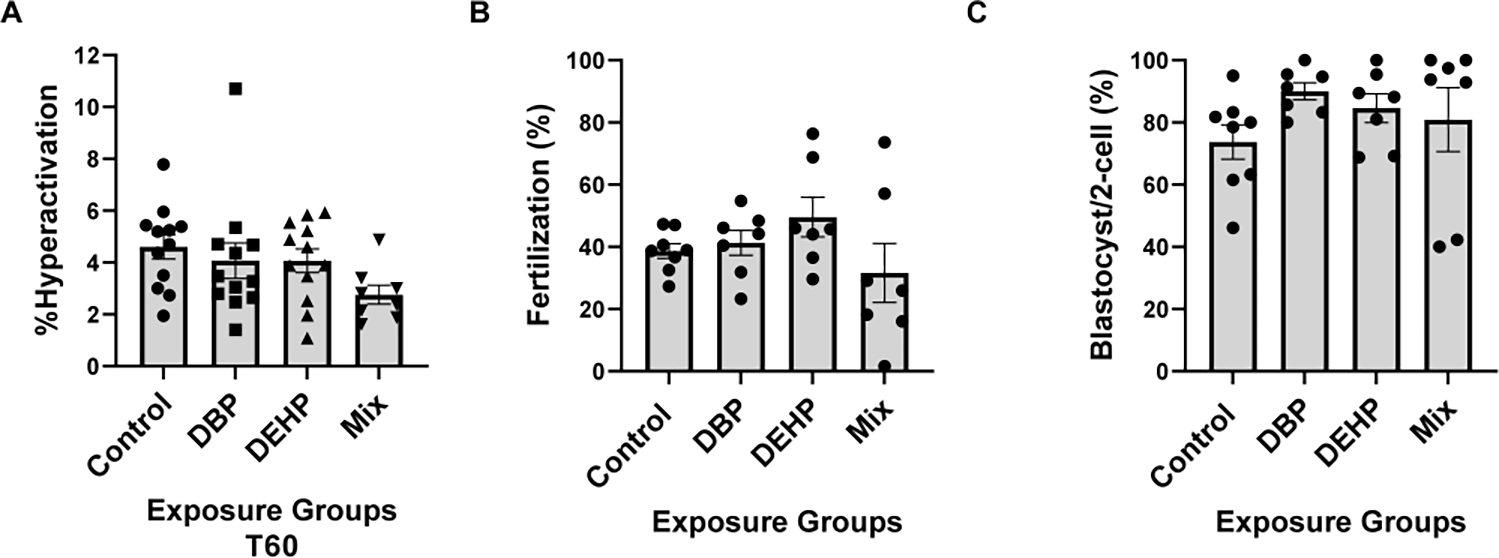
A. Sperm hyperactivation was measured using CASA with the same samples obtained for the analyses depicted in Fig. 1; those spermatozoa with VCL > 271.00 μm/sec, LIN<50.00% and ALH>3.50μm were considered to be hyperactive. A one-way Anova was performed followed by Dunnett test for multiple comparisons using GraphPad prism with a sample size of N=12. Data are represented as mean percentage of abnormal sperm ± SEM (N=12). B. Epididymal sperm were obtained from mice exposed to control or to phthalates and capacitated for 1 hour in complete TYH media. Approximately 1 × 105 sperm were added to the inseminaton droplet. Fertilization was calculated as percentage of oocytes capable of reaching two-cell embryo stage after heterologous fertilization between CD-1 female oocytes and phthalate exposed C57BL/6J sperm from each group (control, DBP, DEHP, Mix), respectively. Kruskal-Wallis non-parametric test was performed followed by Dunn’s test using Graph Pad Prism software to compare within groups with a sample size of N=7. C. Embryo development rates. Two-cell embryos were incubated in KSOM media for 3.75 days up-to-blastocyst stage. Data are expressed as percentage of two-cell embryos from B that reached blastocyst stage. For each of the fertilization assays Kruskal-Wallis non-parametric test was performed followed by Dunn’s test for multiple comparisons using GraphPad Prism software to compare within groups (N=7) at p<0.05.
Discussion
Mammalian fertilization is a culmination of a series of steps that primarily comprises the fusion of the capacitated spermatozoon with that of the metaphase II-arrested oocyte resulting in the development of an embryo and finally a new individual 23. The spermatozoon is further entrusted with a compact haploid genome that needs to be delivered to the oocyte in a way that can be easily accessed by the developing embryo. Despite of the essential role played by the male factor in zygote formation, the extent of sperm contribution to the epigenetics of embryonic development is still not well understood 24. Recent work on epigenetic transgenerational inheritance 25,26 has shown that sperm from males fed with high fat diets can transmit environmental information to offspring. Most interesting, this transmission can occur even when the offspring is generated by intracellular sperm injection (ICSI). The authors hypothesized that information is transmitted through sperm RNA (most likely small noncoding RNAs such as miRNA or tRNA fragments). As an alternative hypothesis, other groups have shown that contrary to what was previously thought, sperm chromatin has several open regions. In general, these studies using ATACseq 27,28, HiCseq 29, and precipitation of modified histones 27,28,30 evaluated chromatin changes occurring upon exposure of mice to special diets 30. Considering these reports and others it has been proposed that epigenetic modifications in gene expression without change in sequence or copy number through adverse environmental challenges, such as exposure to toxicants, may also result in the acquisition of congenital malformations in the offspring 21. In this regard, our group has recently shown that, in humans and mice, differentially methylated regions (DMRs) in the sperm DNA associated with DEHP are within or near genes related to growth and development 16,18. In the absence of any maternal effect, several studies have revealed that abnormalities in the paternal factor negatively impacts embryo quality 31–34. However, it is not clear if these effects are correlated with poor sperm function.
In the present work, we examined the effect of phthalates on sperm function, particularly DBP and DEHP, which have been proposed to present great risk to male fertility. Phthalates are a group of endocrine disrupting chemicals and have been shown to directly affect sperm suggesting the presence of receptors in mature spermatozoa 35–37. In addition, these compounds are known for their anti-androgenic properties affecting reproductive and developmental processes 38. Several studies have been undertaken to understand their mode of action and assess the adverse impacts of these compounds on human health. However, there is a deficit in the understanding of their mechanism of action on paternal effects at the molecular level. Individual assessment of these compounds is not a representative of the actual life challenges that human faces. Thus, we focused on the independent and mixture effect of DEHP and DBP on sperm quality, capacitation, fertilization, and embryo development.
Although many groups have used phthalate exposure, as far as we know this is the first study investigating the effect in mature mouse sperm. An important challenge for these studies was to choose phthalate doses that can be compared with human daily exposures. The daily intake in humans is estimated from phthalate metabolic products in the urine and has been reported to have a median exposure of 5.6 μg/ kg body weight and a 95th percentile of 21 μg/kg body weight 39. Having these starting values, the next step was to scale them to mice studies. Scaling doses between humans and other animals is not trivial. For toxicological studies, it is not appropriate to consider only body weight because: 1) larger animals have lower metabolic rates, physiological process are slower and, they required smaller drug doses on weight basis 39. Therefore, to estimate the doses, we applied the factor method which, in addition to body weight, also considers body surface area to calculate the estimate “human equivalent dose (HED)”. Considering the published factor for mice is 0.081, we divided 21 μg/kg by this factor to obtain a 95th percentile equivalent of 0.308 mg/kg of body weigh in mouse. However, contrary to the limited exposure period of our studies (i.e. 40 days), values for humans represent chronic exposure of only one of many phthalates 40. Importantly, the “no observed adverse effect” (NOAEL) in rats is 4.8 mg/kg/day in rats 17. Finally, our previous work 16 showed that male preconception DEHP exposure at both 2.5 and 25 mg/kg/day resulted in significant epigenetic and gene expression changes in F1 embryos. Thus, moving forward, we only considered the lower 2.5 mg/kg/day exposure level, which is lower than the reported DEHP NOAEL.
Here, we observed reduced numbers and morphological abnormalities in mature sperm from individual DEHP and DBP exposures, and more so in the mixture exposure compared to controls. This last finding is important in the context to previous reports which have suggested a close association between abnormal sperm morphology and embryo quality 31. At a more fundamental level, the relationship between sperm morphology and specific signaling pathways is a difficult topic to study. Most of what is known is related to knock-out mice models resulting in sperm morphology defects 41,42. However, these observations are phenomenological and there is no evidence on the mechanisms involved in phthalate-induced abnormal sperm morphology. To continue our investigation, we examined the effects of DEHP, DPB and their mixture on capacitation associated events.
To gain fertilizing capacity, mammalian sperm undergo a series of biochemical and physiological processes known as capacitation 43. Capacitation can be mimicked in vitro, and it is initiated by a HCO3−-dependent increase in cAMP levels with the consequent activation of PKA. Although the PKA substrates have not yet been identified, loss-of-function experiments using knock-out mice models revealed that this pathway is essential for the sperm to gain fertilizing capacity. Mice lacking either the atypical adenylyl cyclase Adcy10, responsible for cAMP synthesis in sperm 44, or the sperm specific PKA catalytic variant Cα2 45 are sterile. On the other hand, it is important to consider that another sterile mouse model, lacking CatSper Ca2+ channel complex has increased PKA-dependent phosphorylation 46 suggesting that although PKA-dependent phosphorylation is necessary for capacitation, it is not sufficient to complete the process. Our study revealed reduced phosphorylation of PKA substrates in sperm from mice treated with DEHP and from the phthalate mixture but not with DBP alone. This decrease suggests that sperm cAMP-dependent pathways are sensitive to phthalates exposure. Because PKA is a central regulator of sperm physiology, this is a significant finding; nevertheless, the data suggest a modulation of the pathway and not a binary response. In this regard, several endocrine disruptors including DBP were shown to activate the sperm specific Ca2+ channel complex CatSper 47. As mentioned above, CatSper KO sperm depict an enhanced phosphorylation of PKA substrates 46 likely due to the fact that Ca2+ has a biphasic effect in PKA activation 36. Therefore, we cannot discard that the effect of phthalate exposure is due to CatSper activation and that the subsequent increase in intracellular Ca2+ might reduce phosphorylation of PKA substrates.
Despite the lower numbers, the morphology defects, and the decrease in phosphorylation of PKA substrates, other capacitation-associated parameters such as hyperactivation and the increase in tyrosine phosphorylation were not affected. Considering that the main endpoint of capacitation is the sperm ability to fertilize, we next evaluated fertilization and developmental potential in vitro. Neither fertilization nor embryo development were altered due to exposure to individual phthalates (DBP or DEHP). On the other hand, mice treated with the phthalate mixture resulted in two subpopulations: one of which had very low fertility, while the other one had normal fertilization rates. Once fertilized, two-cell embryos were incubated up-to blastocyst stage. Similarly, two-cell embryos derived from individual phthalate exposures, reached the blastocyst state with similar success rate. However, as observed for fertilization, exposure to the phthalate mixture resulted in two sub-populations, one of them with lower developmental rates.
Overall, our findings have some alternative interpretations. On one hand, we found that the effect of phthalate exposure on sperm potential has no drastic effects neither on their fertilization capacity nor on the obtained zygote development potential. On the other hand, our results indicate that sub-chronic exposure to DBP, DEHP and the mixture of both compounds for 40 days have deleterious effects in mature sperm numbers, morphology and at least, one capacitation-associated pathway. Although IVF was not severely affected, taken together, these results suggest that phthalate exposure negatively influence some sperm quality parameters as has been reported for human populations 48. These findings warrant more studies that might include longer phthalate exposures with lower dose that are more common in humans as well as to investigate the molecular mechanisms of phthalate action in the male reproductive system using system biology approaches.
Acknowledgements:
The work was supported by the following funding agencies: National Institute of Health; National Institute of Environmental Health Sciences (R01ES030942: P.E.V and to J.R.P). National Institute of Health; Biotechnology Training Program (BTP) supported by National Research Service Award T32 GM108556 to DAT. We also thank the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, University of Massachusetts-Amherst, which is supported by the Massachusetts Life Sciences Center.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
References
- (1).Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. Journal of human reproductive sciences. 2015;8 (4):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Jolles M, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update. 2022. [DOI] [PubMed] [Google Scholar]
- (3).Sumner RN, Tomlinson M, Craigon J, England GCW, Lea RG. Independent and combined effects of diethylhexyl phthalate and polychlorinated biphenyl 153 on sperm quality in the human and dog. Scientific reports. 2019;9(1):3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Sengupta P, Dutta S, Krajewska-Kulak E. The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. Am J Mens Health. 2017; 11 (4):1279–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017; 23 (6): 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Giwercman A, Rylander L, Lundberg Giwercman Y. Influence of endocrine disruptors on human male fertility. Reprod Biomed Online. 2007;15(6); 633–642. [DOI] [PubMed] [Google Scholar]
- (7).Lea RG, Byers AS, Sumner RN, Rhind SM, Zhang Z, Freeman SL, Moxon R, Richardson HM, Green M, Craigon J, et al. Environmental chemicals impact dog semen quality in vitro and may be associated with a temporal decline in sperm motility and increased cryptorchidism. Scientific reports. 2016; 6:31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wu H, Estill MS, Shershebnev A, Suvorov A, Krawetz SA, Whitcomb BW, Dinnie H, Rahil T, Sites CK, Pilsner JR. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: a cross-sectional study. Hum Reprod. 2017; 32 (11):2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Caporossi L, Alteri A, Campo G, Paci E, Tranfo G, Capanna S, Papaleo E, Pigini D, Vigano P, Papaleo B. Cross Sectional Study on Exposure to BPA and Phthalates and Semen Parameters in Men Attending a Fertility Center. International journal of environmental research and public health. 2020;17 (2):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Radke EG, Braun JM, Meeker JD, Cooper G S Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ Int. 2018; 121 (Pt 1):764–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Genuis SJ, Beesoon S, Lobo RA, Birkholz D. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. ScientificWorldJournal 2012; 2012:615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Huffman AM, Wu H, Rosati A, Rahil T, Sites CK, Whitcomb BW, Pilsner JR. Associations of urinary phthalate metabolites and lipid peroxidation with sperm mitochondrial DNA copy number and deletions. Environ Res. 2018; 163:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Thurston SW, Mendiola J, Bellamy AR, Levine H, Wang C, Sparks A, Redmon JB, Drobnis EZ, Swan SH. Phthalate exposure and semen quality in fertile US men. Andrology. 2016;4 (4):632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22 (3):688–695. [DOI] [PubMed] [Google Scholar]
- (15).Estill M, Hauser R, Nassan FL, Moss A, Krawetz SA. The effects of di-butyl phthalate exposure from medications on human sperm RNA among men. Scientific reports. 2019;9 (1):12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Oluwayiose OA, Wu H, Saddiki H, Whitcomb BW, Balzer LB, Brandon N, Suvorov A, Tayyab R, Sites CK, Hill L, et al. Sperm DNA methylation mediates the association of male age on reproductive outcomes among couples undergoing infertility treatment. Scientific reports. 2021; 11 (1):3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Blystone CR, Kissling GE, Bishop JB, Chapin RE, Wolfe GW, Foster P M Determination of the di-(2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: importance of the retention of extra animals to adulthood. Toxicol Sci. 2010; 116 (2):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Oluwayiose OA, Marcho C, Wu H, Houle E, Krawetz SA, Suvorov A, Mager J, Pilsner JR. Paternal preconception phthalate exposure alters sperm methylome and embryonic programming. Environ Int. 2021;155:106693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Tourzani DA, Paudel B, Miranda PV, Visconti PE, Gervasi MG. Changes in Protein O-GlcNAcylation During Mouse Epididymal Sperm Maturation. Frontiers in cell and developmental biology. 2018; 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Escoffier J, Navarrete F, Haddad D, Santi CM, Darszon A, Visconti PE. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol Reprod. 2015; 92 (5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sanchez-Cardenas C, Montoya F, Navarrete FA, Hernandez-Cruz A, Corkidi G, Visconti PE, Darszon A. Intracellular Ca2+ threshold reversibly switches flagellar beat off and on. Biol Reprod. 2018;99 (5):1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of Ser/Thr Phosphatases Induces Capacitation-associated Signaling in the Presence of Src Kinase Inhibitors. J Biol Chem. 2010;285 (11):7977–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Xin A, Qu R, Chen G, Zhang L, Chen J, Tao C, Fu J, Tang J, Ru Y, Chen Y, et al. Disruption in ACTL7A causes acrosomal ultrastructural defects in human and mouse sperm as a novel male factor inducing early embryonic arrest. Sci Adv. 2020; 6 (35):eaaz4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Loutradi KE, Tarlatzis BC, Goulis DG, Zepiridis L, Pagou T, Chatziioannou E, Grimbizis GF, Papadimas I, Bontis I. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. Journal of assisted reproduction and genetics. 2006;23 (2):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Conine CC, Sun F, Song L, Rivera-Perez JA, Rando OJ. Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Developmental cell. 2018; 46 (4):470–480 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Developmental cell. 2018; 46 (4):481–494 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Jung YH, Sauria MEG, Lyu X, Cheema MS, Ausio J, Taylor J, Corces VG. Chromatin States in Mouse Sperm Correlate with Embryonic and Adult Regulatory Landscapes. Cell reports. 2017; 18 (6):1366–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jung YH, Kremsky I, Gold HB, Rowley MJ, Punyawai K, Buonanotte A, Lyu X, Bixler BJ, Chan AWS, Corces VG. Maintenance of CTCF- and Transcription Factor-Mediated Interactions from the Gametes to the Early Mouse Embryo. Molecular cell. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ke Y, Xu Y, Chen X, Feng S, Liu Z, Sun Y, Yao X, Li F, Zhu W, Gao L, et al. 3D Chromatin Structures of Mature Gametes and Structural Reprogramming during Mammalian Embryogenesis. Cell. 2017; 170 (2):367–381 e20. [DOI] [PubMed] [Google Scholar]
- (30).Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nature communications. 2013;4:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Virro MR, Larson-Cook KL, Evenson D P Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004; 81 (5):1289–1295. [DOI] [PubMed] [Google Scholar]
- (32).Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Francois Guerin J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007; 87 (1):93–100. [DOI] [PubMed] [Google Scholar]
- (33).Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SE. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod. 2010; 25 (7):1594–1608. [DOI] [PubMed] [Google Scholar]
- (34).Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004; 19 (3): 611–615. [DOI] [PubMed] [Google Scholar]
- (35).Amjad S, Rahman MS, Pang WK, Ryu DY, Adegoke EO, Park YJ, Pang MG. Effects of phthalates on the functions and fertility of mouse spermatozoa. Toxicology. 2021; 454: 152746. [DOI] [PubMed] [Google Scholar]
- (36).Mohamed el S A, Park YJ, Song WH, Shin DH, You YA, Ryu BY, Pang MG. Xenoestrogenic compounds promote capacitation and an acrosome reaction in porcine sperm. Theriogenology. 2011;75 (6):1161–1169. [DOI] [PubMed] [Google Scholar]
- (37).Park YJ, Mohamed el S A, Kwon WS, You YA, Ryu BY, Pang MG. Xenoestrogenic chemicals effectively alter sperm functional behavior in mice. Reprod Toxicol. 2011; 32 (4):418–424. [DOI] [PubMed] [Google Scholar]
- (38).Grady R, Sathyanarayana S. An update on phthalates and male reproductive development and function. Curr Urol Rep. 2012;13 (4):307–310. [DOI] [PubMed] [Google Scholar]
- (39).Nair AB, Jacob S A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016, 7 (2), 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Efsa Panel on Food Contact Materials E, Processing A, Silano V, Barat Baviera J M, Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R, Gott DM, Grob K, et al. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019;17 (12):e05838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Li W, Tang W, Teves ME, Zhang Z, Zhang L, Li H, Archer KJ, Peterson DL, Williams DC Jr, Strauss JF 3, et al. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development. 2015;142 (5):921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL Moss SB, et al. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod. 2006;74 (4):751–759. [DOI] [PubMed] [Google Scholar]
- (43).Gervasi MG, Visconti P E Chang’s meaning of capacitation: A molecular perspective. Mol Reprod Dev. 2016; 83 (10): 860–874. [DOI] [PubMed] [Google Scholar]
- (44).Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental cell. 2005;9 (2):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A. 2004;101 (37):13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell. 2014; 157 (4):808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Schiffer C, Muller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A, Frederiksen H, Waschle B, Kaupp UB, Balbach M, et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 2014; 15 (7):758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Oluwayiose OA, Houle E, Wu H, Whitcomb BW, Mumford SL, Schisterman EF, Suvorov A, Balzer LB, Pilsner JR. Urinary phthalate metabolites and their mixtures are associated with advanced sperm epigenetic aging in a general population. Environ Res. 2022; 214 (Pt 4):114115. [DOI] [PubMed] [Google Scholar]


