Abstract
Over the last several decades, periprosthetic joint infection has been increasing in incidence and is occurring in more complex patients. While there have been advances in both surgical and medical treatment strategies, there remain important gaps in our understanding. Here, we share our current approaches to the diagnosis and management of periprosthetic joint infection, focusing on frequent clinical challenges and collaborative interdisciplinary care.
Keywords: periprosthetic joint infection, total joint arthroplasty, revision arthroplasty, antimicrobial treatment, rifampin
This review describes the current state of care for periprosthetic joint infection, focusing on multidisciplinary collaboration, common diagnostic and management conundrums, and patient-focused decision-making.
Since the advent of modern arthroplasty in the 1970s, joint replacement has become one of the most common surgical procedures and has afforded significant quality-of-life gains. Improvements in surgical strategies and infection prevention protocols may reduce infectious complications; however, these have been offset by the increasing medical complexity of patients who undergo arthroplasty. Overall, periprosthetic joint infection (PJI) impacts more than 2% of arthroplasty patients, a risk that has not substantially changed over time [1–4]. Given the growth in arthroplasty procedures, the incidence of PJI continues to rise [4, 5]. Over the last decade, there has been a new focus on optimal PJI care; however, many management questions evade treatment guidelines, and outcomes remain suboptimal. We aim to provide an approach to the diagnosis and management of PJI, focusing on clinical challenges, collaborative multidisciplinary care, and management of uncertainty.
CLINICAL PRESENTATION
The clinical presentation of PJI differs based on the timing of infection. Acute infections present within the first few weeks to months after the index procedure [6–8], usually with classic signs of infection: pain, redness, warmth, and swelling at the surgical site. Some patients with acute PJI present with wound complications or persistent drainage. Patients with hematogenous infection may also present with pain, redness, warmth, and swelling, though the onset of infection is often much later, often years after the index procedure. These delayed presentations occur in previously well-functioning devices due to bacteremic seeding, such as from a remote infection or mucosal breach. Typically, the bacteremic event itself is unrecognized.
Chronic PJI, caused by indolent organisms inoculating the surgical site, usually present within 2 years after the index procedure. The most common presenting symptom of chronic PJI is pain, which overlaps with many noninfectious diagnoses, including polyethylene wear, aseptic loosening, and adverse local tissue reaction to metal (ALTR). Associated symptoms and specific pain localization may help to differentiate PJI from aseptic causes. For example, patients with polyethylene wear may also complain of instability, and patients with aseptic femoral component loosening after hip arthroplasty may describe thigh pain when initiating ambulation.
DIAGNOSTIC APPROACHES
The diagnosis of acute PJI is often straightforward, as it is typically accompanied by classic signs and symptoms of infection, including fever, purulent drainage, and synovial inflammation. Likewise, the diagnosis of hematogenous infection is usually clearcut, though rarely crystalline disease or acute flare of inflammatory arthritis may mimic infection. However, the diagnosis of chronic PJI remains challenging, in part, because these present more slowly, with less inflammation and with symptoms that overlap those of aseptic complications. Multiple guidelines have been developed for the diagnosis of PJI in hips and knees, including from the Musculoskeletal Infection Society [9], the International Consensus Meeting on Musculoskeletal Infection [10], and the European Bone and Joint Infection Society [11]. In these guidelines, the presence of sinus tract that communicates to the joint or prosthesis and/or the recovery of the same organism in at least 2 separate synovial fluid and/or periprosthetic tissue cultures confirms PJI. However, these criteria are often not met preoperatively in chronic PJI. The guidelines differ with respect to the thresholds of and weight afforded other factors, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), synovial fluid white blood cell (WBC) count and neutrophil percentage, synovial fluid alpha-defensin, and histology. Algorithms have been developed to guide evaluation, but the presence of PJI is not always confirmed or refuted, especially preoperatively. Further, the impact of race and gender on these diagnostic protocols has not been well explored and may be important; for example, ESR is less accurate in the identification of PJI among females and Blacks [12]. Yet, surgical decision-making, including whether revision surgery is offered and what type of surgery to perform, often depends on the preoperative assessment of PJI likelihood.
Diagnostic Challenges
Infectious diseases (ID) physicians may be asked to evaluate the significance of a single positive culture when other factors do not confirm PJI. A single positive culture for pathogenic organisms, such as Staphylococcus aureus, is highly likely to represent a true infection, while positive cultures for organisms such as Cutibacterium acnes and/or coagulase-negative Staphylococci (CoNS), may be true positives or represent contamination [13]. Understanding surgical plans may assist with the diagnostic approach. If surgery would only be offered if PJI was confirmed preoperatively or if the type of surgery to be performed would differ if PJI were confirmed, then repeat synovial fluid sampling could be performed with attention to optimized culture techniques, molecular pathogen detection if available, and the use of additional synovial fluid biomarkers, as reviewed below.
This scenario is particularly challenging when Cutibacterium species are recovered in suspected shoulder PJI. Shoulder arthroplasty infection is typically less inflammatory than hip and knee PJI, and serum inflammatory markers and synovial fluid studies may be normal. Further, Cutibacterium species evade topical antisepsis and may contaminate surgical cultures, even with optimal surgical skin preparation [14]. A consensus definition of shoulder PJI was recently developed and includes weighting of 13 factors to develop a probability score [15]. The microbial factors include different weights based on organism virulence and the number of same-organism positive cultures. This definition requires validation but may provide a framework to assess the likelihood that positive cultures represent true infection.
ID physicians may be asked to consider the diagnosis of infection in the setting of ALTR, which results from metal corrosion in metal-on-metal implants or involving the trunnion between the femoral head and neck in total hip arthroplasty. Metal corrosion leads to substantial periprosthetic inflammation. Patients with ALTR in the absence of infection may have elevated inflammatory markers and synovial fluid cell counts but are also at higher risk of PJI [16]. When suspected, preoperative measurement of serum chromium and cobalt levels and magnetic resonance imaging (MRI) using a metal-suppression technique (metal artifact reduction sequence [MARS]) are often sufficient to diagnose ALTR but may not enable exclusion of concurrent PJI. In this setting, surgery is often indicated to revise components, at which time additional tissue cultures may be collected to enable a definitive diagnosis.
Additional Testing Options
When confirmation of infection is not achieved through initial synovial fluid testing, additional synovial fluid biomarkers, such as alpha-defensin, synovial CRP, and calprotectin, may be used [17]. Of these, alpha-defensin has been the most widely adopted. Alpha-defensin is an antimicrobial peptide released by neutrophils activated in the presence of pathogens and has a high reported sensitivity (96%) and specificity (95%) when measured in synovial fluid for PJI [18]. It remains useful even with prior antibiotic exposure, less virulent organisms, and in inflammatory arthritides, though it may be falsely positive in ALTR [19]. Alpha-defensin is an expensive test and adds little to diagnostic certainty in straightforward cases [20]. It is often used when initial test results are equivocal [21], though its role in this setting has not yet been effectively scrutinized. The alpha-defensin test is also available as a point-of-care lateral flow assay [22] and may be a helpful intraoperative adjunct for decision-making when PJI is suspected but not confirmed preoperatively.
In general, imaging studies have limited utility in confirming PJI diagnosis, though they are still useful in evaluating noninfectious causes of pain and in informing surgical decision-making. Plain films are an important tool to assess loosening, subsidence, and periprosthetic fracture but are neither sensitive nor specific for PJI. MARS-MRI is often performed when ALTR is suspected, and both MRI and computed tomography (CT) may demonstrate associated soft tissue abscesses when PJI is suspected. A 3-phase bone scan may be useful to exclude infection when negative [11] but has limited specificity and is not diagnostic when positive. The role of other imaging modalities, including WBC scintigraphy and fluorodeoxyglucose positron emission tomography/computed tomography, are not as well established [23].
Optimizing Microbial Identification
While confirmation of infection informs the need for surgery, preoperative microbial identification may influence the type of surgery offered. PJI due to organisms such as methicillin-resistant S. aureus, Pseudomonas aeruginosa, and Candida species is more difficult to eradicate, and their preoperative identification should be weighed among other factors (see below) in surgical decision-making. Preoperative identification of organisms also enables crafting of optimal local antimicrobial delivery, including in antimicrobial cement (polymethyl methacrylate [PMMA]) or calcium sulfate beads. Culture yield is improved when antibiotics are withheld at least 14 days prior to sampling, by including incubation in blood culture bottles and via prolonged (14 days) culture incubation [24].
Culture-negative PJI, reported in 5%–42% of cases [25], frustrates physicians, challenges antimicrobial treatment, and may, in some cases, represent conditions other than infection. When organisms do not grow in conventional culture, molecular methods, including 16S ribosomal RNA polymerase chain reaction (PCR) and sequencing (both shotgun and targeted metagenomic sequencing), may be considered. These techniques have demonstrated improved microbial identification in culture-negative PJI [26, 27] in some studies but with disappointing utility in others [28, 29]. While these technologies offer promise, they remain expensive and are not widely available, which functionally limits their role in challenging culture-negative infections. An exception is the newly US Food and Drug Administration–approved synovial fluid multiplex PCR panel (BioFire) [30], which returns results rapidly and may therefore inform surgical decision-making when used preoperatively. Importantly, the BioFire PJI panel does not include C. acnes or any CoNS other than Staphylococcus lugdunensis, therefore, limiting its utility in chronic infections.
SURGICAL MANAGEMENT
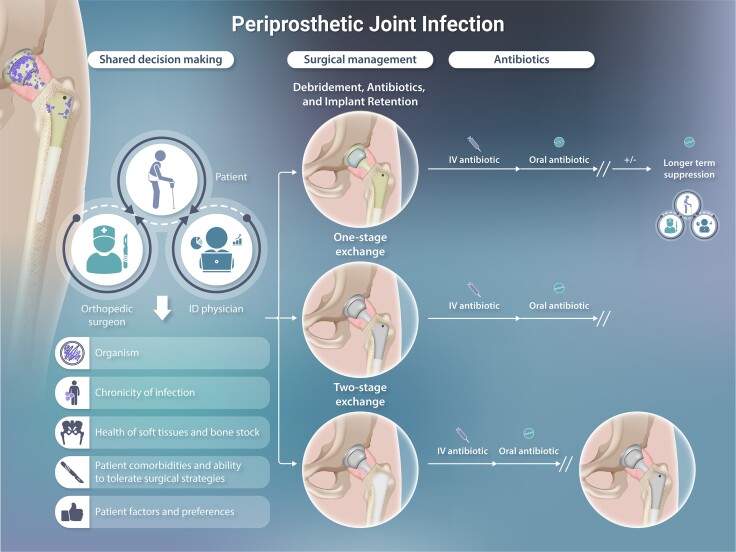
Once the diagnosis of PJI is suspected or confirmed, surgical plans are made. The most commonly used surgical procedures include debridement, antibiotics, and implant retention (DAIR) and 1-stage and 2-stage exchange procedures. Other alternatives may be considered in refractory PJI or when later reconstruction is not feasible; these include amputation, device removal without reimplantation (resection arthroplasty), and arthrodesis (fusion). The choice of surgical procedure hinges on the duration of symptoms, the offending microorganism, and patient comorbidities and considers trade-offs between surgical morbidity and the likelihood of successful infection control (Figure 1). While decisions around management of acute infections should be made expeditiously, those for chronic PJI can be made carefully with input from multiple care providers. Ideally, such decisions are made by informed patients after counsel from an experienced orthopedic surgeon and ID specialist, who consider patient goals, surgical factors, medical risks, the organism involved, and the anticipated ability to tolerate antimicrobial treatment.
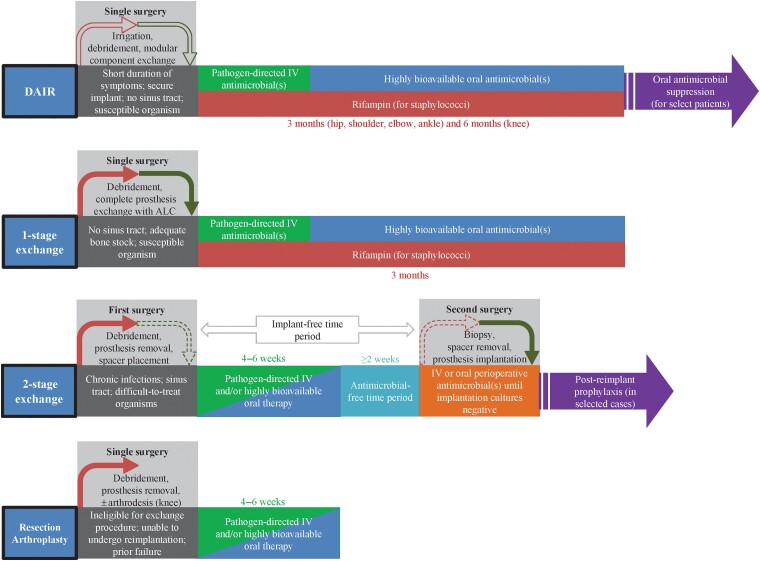
Figure 1.
Interdependence of surgical and antimicrobial management, with suggested antimicrobial treatment protocols.Figure 1 has been reprinted with permission from the American Society of Microbiology in a modified format [Tande AJ and Patel R. Prosthetic joint infection. Clinical Microbiology Reviews 2014; 27(2): 302–345]. Abbreviations: ALC, antimicrobial-loaded cement; DAIR, debridement, antibiotics, and implant retention; IV, intravenous.
Debridement, Antibiotics, and Implant Retention
For acute and hematogenous infections, the first line of treatment is typically a DAIR procedure. In these surgeries, the modular parts (eg, acetabular liner and femoral head in hip arthroplasty; polyethylene insert in knee arthroplasty) are exchanged to reduce organism bioburden and to enable thorough surgical debridement. The prosthesis and periprosthetic tissues are debrided of necrotic tissue, and multiple irrigations are performed to liberate biofilm. New modular parts are placed after debridement and creation of a new surgical field. Double DAIR describes sequential debridement procedures performed approximately 1 week apart, with placement and later removal of antibiotic beads and exchange of modular components during both procedures [31].
In the surgical literature, a successful PJI outcome is defined by clinical infection eradication, no need for further surgery for infection, no PJI-related mortality, and absence of long-term antimicrobial suppression [32]. With this definition, DAIR procedures are less successful compared with exchange procedures, estimated at 60%–67% in several recent meta-analyses [33–35]. Factors that contribute to the failure of DAIR include prolonged duration of symptoms, sinus tract presence, inability to close the surgical wound, older age and medical comorbidities, certain pathogens (including S. aureus, Enterococci, and Candida), and prior history of DAIR in the index joint. However, even when the risk of failure by this definition with DAIR is higher, it may still be reasonable in the setting of medical frailty or when exchange procedures would be poorly tolerated. In these cases, DAIR may reduce the bioburden of organisms, enabling long-term antibiotic suppression to achieve infection control even when cure is not expected. Patients selected for DAIR should have a reasonable likelihood of tolerating a longer antimicrobial course, potentially including the addition of rifampin, as discussed below.
Exchange Arthroplasty
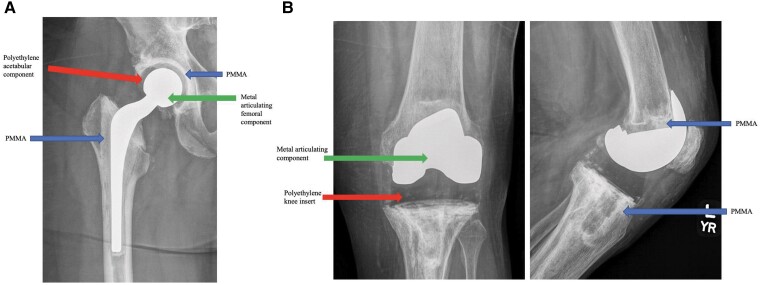
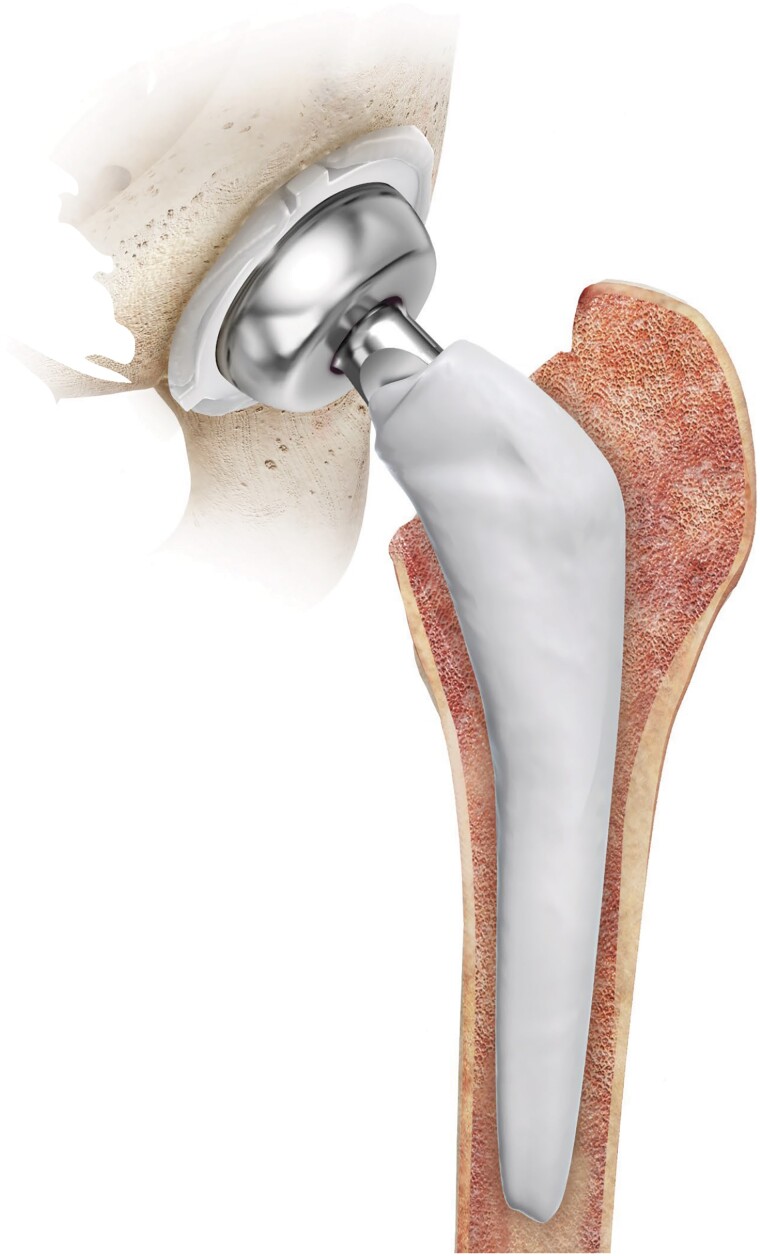
In chronic PJI and when there is a heightened risk of failure after DAIR, patients are often offered a 1- or 2-stage exchange arthroplasty. In 1-stage exchange arthroplasty, all device components are removed, and new revision components are inserted after debridement as part of the same surgical procedure. In 2-stage exchange arthroplasty, all components are removed in the first stage, and a temporary antibiotic-laden spacer device is placed. The second stage of definitive reimplantation is performed months later following the administration of systemic antibiotic therapy. Historically, spacers were composed entirely of antibiotic cement (PMMA) and served both as a delivery mechanism for antibiotics and to enable easier reoperation. Increasingly, spacers are being comprised not only of antibiotic-eluting PMMA but also of metal and polyethylene components that provide an articulating surface much like permanent components (Figures 2 and 3). Some patients elect to maintain their spacer devices. Studies have reported good infection control, functionality, and survivorship of components while still allowing safe component removal for those who undergo second-stage reimplantation [36]. While 2-stage revision in the United States has traditionally been favored for chronic PJI, this paradigm is being challenged. Several randomized, controlled studies comparing 1- vs 2-stage exchange for PJI are ongoing [37, 38].
Figure 2.
Articulating spacers for use following the first stage of a 2-stage exchange arthroplasty. A, Articulating hip spacer. B, Articulating knee spacer. Abbreviation: PMMA, polymethylmethacrylate.
Figure 3.
Schematic image of the Prostalac Hip System. Image reprinted with permission from DePuy Synthes.
Nonsurgical Management
For patients with limited life expectancy and for those who might not survive surgery, treatment with antibiotics alone may be the only option. Without surgery, eradication of infection is not expected, and long-term antibiotic suppression is planned. This approach is not always successful; however, in select cases, it may still be appropriate [39, 40]. In all cases, multidisciplinary discussions to inform patients and their caregivers of the consequences of different treatment approaches is critical.
OVERVIEW OF ANTIMICROBIAL THERAPY
Nearly all approaches to PJI involve antimicrobial therapy. The selection and duration of antimicrobial therapy are inextricably linked to the surgical strategy (Figure 1). A clear understanding of any residual undebrided infection and/or retained implants is important in constructing an optimal antimicrobial plan. Further, successful provision of antimicrobial therapy is contingent on optimal antimicrobial dosing and administration, review of drug interactions, management of side effects, and ongoing safety surveillance. Accordingly, a close working relationship between the ID specialist and orthopedic surgeon is critical to ensure a successful antimicrobial course.
Duration of Antimicrobial Therapy
Antimicrobial therapy for PJI is often considered in stages: treatment, which may consist of a first parenteral phase and a second oral phase, and suppression. Commonly used antimicrobials for PJI are listed in Tables 1 and 2. Decisions about antibiotic duration hinge on whether all components of the arthroplasty are resected or retained. The Infectious Diseases Society of America (IDSA) guidelines [41], now 10 years old, provide guidance on duration of therapy but were based on limited evidence. A treatment duration of 4–6 weeks of antimicrobial therapy following resection arthroplasty (either as part of a 2-stage exchange or as definitive management), 1-stage exchange, or DAIR was advised. For patients undergoing DAIR or 1-stage exchange with staphylococcal infection, recommendations differed according to the joint involved. For hip PJI, 3 months of rifampin-based combination therapy was recommended. For knee PJI, 3 months of such therapy was recommended when 1-stage exchange was performed, and 6 months when the patient underwent DAIR. For both surgical strategies, there was a lack of consensus about the need for chronic suppression thereafter.
Table 1.
Intravenous Antimicrobials Used for Periprosthetic Joint Infection
| Antimicrobial | Recommended Dosea | Targeted Organism |
|---|---|---|
| Ampicillin | 12 g over 24 h in continuous infusion or divided every 4–6 h | Sensitive streptococci and enterococci |
| Cefazolin | 2 g every 8 h | MSSA; methicillin-sensitive CoNS; penicillin-sensitive streptococci |
| Cefepime | 2 g every 8–12 h | Pseudomonas aeruginosa |
| Ceftriaxone | 2 g every 24 h | Streptococci; Cutibacteria; sensitive Enterobacterales; some clinicians use for non-bacteremic MSSA |
| Ceftazidime | 2 g every 8 h | Pseudomonas aeruginosa |
| Daptomycin | 6–10 mg/kg every 24 hb | MRSA; enterococci (including vancomycin-resistant enterococcus) |
| Ertapenem | 1 g every 24 h | Enterobacterales, including ESBL strains; polymicrobial infections, including anaerobes |
| Imipenem | 500 mg every 6 h | Pseudomonas aeruginosa; ESBL-producing Enterobacterales; polymicrobial infections including anaerobes |
| Meropenem | 1 g every 8 h | Pseudomonas aeruginosa; ESBL-producing Enterobacterales; polymicrobial infections including anaerobes |
| Nafcillin | 1.5–2 g every 4–6 h | MSSA; methicillin-sensitive CoNS |
| Oxacillin | 1.5–2 g every 4–6 h | MSSA; methicillin-sensitive CoNS |
| Penicillin G | 20 million units over 24 h in continuous infusion or divided every 4 h | Penicillin-sensitive streptococci and enterococci; Cutibacteria |
| Piperacillin-tazobactam | 3.375–4.5 g every 6 hc | Pseudomonas aeruginosa; Enterobacterales; polymicrobial infections including anaerobes |
| Vancomycin | 15 mg/kg every 12 hd | MRSA; methicillin-resistant CoNS; also second-line for MSSA, methicillin-sensitive CoNS, streptococci and enterococci, Cutibacteria |
Adapted with permission from Tande AJ, Steckelberg JM, Osmon DR, Berbari EF. Osteomyelitis in: Bennett, J. E. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Netherlands: Elsevier Health Sciences. 2020: 1418–1429.
Antimicrobial selection should be based on in vitro sensitivity, allergies and intolerances, drug interactions, and renal and hepatic function.
Abbreviations: CoNS, coagulase-negative staphylococci; ESBL, extended-spectrum beta-lactamase; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Doses provided for normal renal function; adjustment may be needed with reduced renal function.
Higher doses recommended for MRSA and Enterococcus.
Higher doses recommended for Pseudomonas aeruginosa.
Dosing to be adjusted based on therapeutic drug monitoring.
Table 2.
Oral Antimicrobials Used for Treatment of Periprosthetic Joint Infection
| Antimicrobial | Recommended Dosea | Targeted Organism |
|---|---|---|
| Amoxicillin | 1000 mg 3 times daily | Sensitive streptococci and enterococci |
| Cefadroxilb | 1000 mg twice daily | MSSA; methicillin-sensitive CoNS; penicillin-sensitive streptococci |
| Ciprofloxacin | 500–750 mg twice daily | Enterobacterales, Pseudomonas aeruginosa |
| Clindamycinb | 600 mg 3 times daily | MSSA; CoNS |
| Doxycyclineb | 100 mg twice daily | MSSA; CoNS |
| Levofloxacinb | 750 mg daily | MSSA or MRSA; Enterobacterales; Pseudomonas aeruginosa |
| Linezolidb | 600 mg twice daily | Enterococci; MRSA |
| Minocyclineb | 100 mg twice daily | MSSA; CoNS |
| Rifabutinc | 300 mg daily | Combination therapy for staphylococci when rifampin is not feasible |
| Rifampinc | 600–900 mg daily (or in 2 divided doses) | Combination therapy for staphylococci |
| Trimethoprim-sulfamethoxazoleb | 8–10 mg/kg (of trimethoprim component) in 2–3 divided doses daily | MSSA; CoNS; Enterobacterales |
Antimicrobial selection should be based on many factors, including in vitro sensitivity, allergies and intolerances, drug interactions, adverse event risk, renal and hepatic function, and cost. Please note that these doses are not informed by outcomes data. Lower doses may be selected for long-term suppression.
Abbreviations: CoNS, coagulase-negative staphylococci; MRSA, methicillin-resistant staphylococcus aureus; MSSA, methicillin-sensitive staphylococcus aureus.
Doses provided for normal renal function; adjustment may be needed with reduced renal function.
With rifampin when used for treatment of staphylococci. Rifampin has been used for staphylococcal infection in most of the studies evaluating oral therapy. In the case of rifampin resistance or intolerance, each of the listed agents (except levofloxacin) may be used alone, although the data supporting this approach are less robust.
In combination with another antimicrobial, such as cefadroxil, clindamycin, doxycycline, levofloxacin, linezolid, minocycline, or trimethoprim-sulfamethoxazole. Rifampin and rifabutin are not used for suppressive therapy, and drug–drug interactions should be reviewed.
The recent Duration of Antibiotic Treatment in Prosthetic Joint Infection (DATIPO) trial was conducted to provide clarity around duration of PJI therapy. Patients who underwent surgical treatment of PJI were randomized to receive either 6 or 12 weeks of antimicrobial therapy [42]. The primary outcome, persistent infection within 2 years, occurred in 18.1% of the 6-week group and 9.4% of the 12-week group, failing to meet the prespecified noninferiority level. However, most failure events occurred among those who underwent DAIR, supporting current clinical practice of extending antibiotic treatment beyond 6 weeks for PJI treated with DAIR. Findings among those with knee PJI treated with DAIR were particularly notable (38.2% failure, 6-week arm vs 13.5%, 12-week arm). This emphasizes the high failure rates for knee PJI treated with DAIR and suggests that such patients should receive courses of at least 12 weeks and/or be considered for oral suppression.
Among patients undergoing 1-stage exchange, 12 weeks of therapy is supported by guideline documents [43] and large studies [44]. In the DATIPO study, among those undergoing 1-stage exchange, 6 weeks was noninferior to 12 weeks, although it was underpowered to detect a difference within this subgroup [42]. For patients undergoing a 2-stage procedure, the historical standard has been a 6-week treatment course following resection, then an antibiotic-free period prior to reimplantation. In the DATIPO trial, among those who underwent 2-stage exchange, a 10.1% risk difference between the 2 treatment groups favored the 12-week arm [42]. However, in the centers where this study was performed, 1-stage exchange is the standard for most patients with chronic PJI, and 2-stage exchange is reserved for those at a higher risk of failure. In the United States, 2-stage exchange procedures are used more commonly, which limits the direct applicability of these data. Based on these findings and accumulated experience, we support a 6-week antimicrobial duration for most patients who undergo 2-stage exchange. However, there may be a subgroup of patients at higher risk of failure who would benefit from a longer duration of antibiotic therapy.
While 2-stage exchange is associated with a higher likelihood of a successful outcome by surgical definitions, patients who develop recurrent infection are at higher risk of chronic pain, functional limitation, or amputation. There has been increasing interest in the use of oral antibiotics after 2-stage exchange arthroplasty, even when there is no evidence of infection at reimplantation. Secondary prophylaxis given for 3 months following reimplant led to a significant decrease in failure at 2 years in an unblinded, multicenter, randomized, controlled trial [45]. The optimal duration of therapy is not known, and retrospective data suggest that similar benefit may be achieved with 2 weeks of antibiotic prophylaxis [46]. Any benefit must be balanced against an increased risk of resistant pathogens if PJI occurs [47].
Oral Therapy
Historically, PJI was treated with parenteral antimicrobial therapy, based largely on expert opinion [48]. In the last 2 decades, there has been growing practical experience and evidence for oral therapy in the management of bone and joint infection [49–53]. In the United States, initial parenteral therapy is often given for at least 2 weeks, though recent data support transition to oral therapy after 7–10 days [42]. The decision to use oral antimicrobials involves several factors. First, the organism must be susceptible to highly bioavailable oral agents, and ideally the planned regimen should be one studied for use in bone and joint infection (Table 2). Notably, the oral regimens used in the Oral versus Intravenous Antibiotics for Bone and Joint Infection and DATIPO trials differed from those used more routinely in the United States [42, 51]. Most oral therapy studies for bone and joint infection, even in the absence of orthopedic implants and in the setting of 2-stage exchange, have used rifampin-containing combination therapy for staphylococcal infection. Second, patients who receive oral therapy should not have conditions that might impair absorption from the gastrointestinal tract. For example, absorption of certain antimicrobials following bariatric surgery may be decreased [54]. Third, the treating provider must be able to follow the patient closely to ensure adherence and optimize tolerability. This is particularly important for patients with lower health literacy or when language or cultural barriers exist. While parenteral therapy does not guarantee adherence, outpatient parenteral antimicrobial therapy team structures provide a mechanism for essential follow-up. Finally, there are little data on the use of oral therapy for patients with obesity, which may impact the achievement of sufficient drug levels at the site of infection compared with patients of normal weight.
Rifampin
Biofilm, a complex community of microorganisms embedded within an extracellular matrix of polysaccharides, proteins, and nucleic acids, serves as a fundamental mechanism of organism survival and persistence in PJI. Biofilm alters local pH and impacts microbial metabolic activity and replication, impairing the activity of many antimicrobials. The in vitro and in vivo efficacy of rifampin in biofilm-associated staphylococcal infections has been consistently demonstrated [55]. Most clinical studies suggest significant benefit of rifampin-based combination therapy in staphylococcal PJI [55–58]. However, a recent small, open-label, randomized trial demonstrated no benefit of 6 weeks of rifampin vs placebo (combined with vancomycin or cloxacillin) for staphylococcal PJI treated with DAIR [59]. This study included primarily hip arthroplasties, limited rifampin treatment to 6 weeks, and did not include quinolone as the companion medication. As other studies have shown greater benefit with a longer duration of rifampin, for knee vs hip PJI, and when rifampin is paired with a fluoroquinolone [56, 57], the conclusions of this study may not be applicable. Given the consistent association with improved outcomes and the magnitude of benefit in other studies, the authors of this review use rifampin for staphylococcal PJI following DAIR or 1-stage exchange, barring contraindications. While not frequently done, we sometimes also use rifampin combination therapy when treating orally in the setting of 2-stage exchange, in alignment with published data [42, 51].
Given its low barrier to development of resistance, rifampin must always be given with a companion medication to which the staphylococcal isolate is susceptible. Initial combination therapy with rifampin is typically with intravenous vancomycin or daptomycin for methicillin-resistant staphylococci or with intravenous cefazolin or an anti-Staphylococcal penicillin (oxacillin, nafcillin, or flucloxacillin) for methicillin-sensitive strains. Historically, levofloxacin or ciprofloxacin was used most commonly with rifampin during the oral phase. Data supports the effectiveness of this combination compared with other companion medications [56, 60]; however, an increasing focus on fluoroquinolone toxicity has led some to move away from fluoroquinolones as the companion drug [61]. While fluoroquinolones remain appropriate as a companion drug for many patients, shared decision-making is important, and before prescribing, particular attention should be paid to preexisting QTc prolongation and history of aortic aneurysm or prior quinolone tendinopathy. Other appropriate companions include cefadroxil, cephalexin, dicloxacillin, trimethoprim-sulfamethoxazole, doxycycline, minocycline, clindamycin, and linezolid. Rifampin leads to decreased concentrations of doxycycline [62] and clindamycin [63] when administered concurrently, though the clinical relevance is uncertain, and both remain appropriate companion medications. In the setting of drug–drug interactions, there is in vitro and in vivo data as well as a small case series to support rifabutin as an alternative [64–66].
The timing of rifampin initiation is impacted by both theoretical and practical concerns. Theoretically, the risk of rifampin resistance is greatest when the burden of bacteria is high and/or the concentration of the companion antimicrobial is low. Accordingly, rifampin should be started only after debridement and exchange of modular components; after removal of drains, which may support biofilm; and after the companion antimicrobial is at a therapeutic level. Practically, there are additional considerations. Nausea may accompany rifampin treatment; therefore, any adverse effect from anesthesia should be resolved prior to its initiation. When rifampin drug interactions are relevant (eg, with anticoagulants and opiates), rifampin should be started once the background regimen has stabilized following surgery. Based on these considerations, the authors typically wait until at least the third or fourth postoperative day to initiate rifampin; notably, one recent study suggested a benefit if rifampin was not started until at least postoperative day 5 [56].
Rifampin dosing strategies vary, with most clinicians using a total daily dose of 600 to 900 mg, either once daily or divided twice daily. The optimal dose and frequency are not known [67], though higher doses may not necessarily be associated with improved outcomes [68]. Likewise, the duration of rifampin necessary to optimize its benefit is unknown. An early randomized study used a 6-month rifampin combination regimen for knee PJI and a 3-month regimen for hip PJI following DAIR; this duration was also incorporated into IDSA treatment guidelines [41, 60]. As evidenced by the subsequent DATIPO and other studies, the poor outcomes of knee arthroplasty infection treated with DAIR do support a longer course of rifampin therapy [42, 56, 57]. When treating staphylococcal PJI, the authors recommend a 6-month course of rifampin for knee infections and a 3-month course of rifampin for other arthroplasty infections following DAIR, with careful monitoring for adverse reactions requiring early discontinuation in all patients.
Rifampin has also been investigated for PJI due to organisms other than staphylococci, including streptococci, enterococci, and C. acnes. Retrospective clinical studies on the use of rifampin for streptococcal and Cutibacterium PJI are mixed, though a recent meta-analysis based on few studies suggests the possibility of benefit [67]. Several retrospective studies suggest a better outcome with rifampin for enterococcal PJI, but event size and lack of adjustment for confounders limit the ability to draw firm conclusions [69, 70]. Based on the lack of consistent, high-quality clinical data, the authors do not routinely use rifampin for non-staphylococcal PJI, though they may consider it in selected high-risk cases due to these other pathogens.
Long-Term Suppression
There remains considerable debate regarding the need for long-term suppression following antibiotic treatment in patients who undergo DAIR. Practically, these decisions are challenging for both clinicians and patients, as there is no test that confirms cure prior to antibiotic completion, and recurrent infection is often significantly morbid. When long-term suppression is used, the goal of therapy changes from cure of infection to control of infection, maintenance of function, and freedom from pain. Some clinicians use long-term suppression in nearly all cases after DAIR [57], while others rarely or never do [56]. We believe that neither approach is optimal, as the former exposes some already cured patients to the unnecessary risk of antimicrobials, while the latter withholds potentially effective treatment from selected high-risk patients. Long-term suppression should be targeted to those at highest risk for failure and/or those for whom recurrence would be most devastating (Table 3).
Table 3.
Considerations for Long-Term Suppression When Debridement, Antibiotics, and Implant Retention or No Surgery Performed
| Risk Factor for Treatment Failure | |
| Host factors | Medical frailty |
| Advanced age | |
| Limited ability to tolerate additional surgery in the setting of relapse | |
| Surgical and anatomic factors | Delay of surgery in acute infection |
| Surgery performed less likely to lead to cure (eg, DAIR performed for chronic infection) | |
| Inability to exchange modular components during DAIR | |
| Need for additional DAIR procedure during initial course | |
| No surgical procedure | |
| Knee (vs hip) | |
| Microbial and infection factors | Late hematogenous infection (vs early postoperative infection) |
| Resistant or difficult-to-treat organisms (methicillin-resistant Staphylococcus aureus, enterococci, candida, Pseudomonas) | |
| Lack of rifampin (for Staphylococcal infection treated with DAIR) | |
| Consequences of recurrence | |
| If recurrence would be life-threatening or limb-threatening | |
Abbreviations: DAIR, debridement, antibiotics, and implant retention.
At this point, there are no clear data to inform who does and does not need long-term suppression. Studies indicate that patients with early postoperative PJI, those who receive DAIR promptly after symptom onset, those with hip arthroplasty infection (versus knee infection), those who require only a single debridement and undergo exchange of modular components, and those with staphylococcal infection who received an adequate duration of rifampin therapy all have lower likelihood of failure [56, 71] and may be less likely to benefit from suppression. Several risk scores have been developed to predict failure after DAIR, including the KLIC (Kidney, Liver, Indication, Cemented prosthesis and C-reactive protein value) score for early acute (post-surgical) PJI [72] and the CRIME80 (COPD and C-reactive protein value, Rheumatoid arthritis, Indication, Male, Exchange of mobile components, Age >80 years) score for late acute (hematogenous) PJI [73]. Artificial intelligence may also hold future advances for predicting failure after DAIR [74].
Shared decision-making between patient and providers is critical in deciding whether to use suppression and, correspondingly, whether and when to stop. In these situations, the ID physician and orthopedic surgeon should estimate the likelihood of relapse and consider the resulting treatment if failure were to occur. Among patients who stop suppression, the timing of discontinuation and need for active vs passive monitoring should be carefully planned a priori. We carefully consider each patient’s goals along with their medical risks and, at times, their event calendar to choose a time during which potential relapse would be least disruptive. Typically, we obtain ESR and CRP when suppression is discontinued to serve as a baseline in the setting of later concern for recurrence.
PATIENT COUNSELING
The diagnosis of PJI is intensely stressful for both patients [75] and providers [76]. Ensuring that patients have access to both optimal infection care and the information needed to inform choices that align with their goals is paramount. When facing infection after arthroplasty, many patients do not initially have a full understanding of the functional consequences, the prognosis of recovery from pain, and the potential need for longer courses of antibiotics than are typically used for other, more common infections. ID physicians can play an important role in ensuring that patients are provided this information as soon as is feasible.
Patient education and counseling may be especially important for groups that have historically been marginalized within the healthcare system. Individuals from some minority groups are more likely to have obesity, diabetes, and poor dental health, all of which increase the risk of developing PJI, and may be more likely to suffer poor outcomes after arthroplasty [77–81]. Black patients who sustain knee PJI are more likely to receive an above-knee amputation compared with White patients [82]. The extent of difference and reasons for adverse outcomes in PJI is unknown, but physicians involved in PJI care should work to establish open and trusting relationships with all impacted patients. In response to historic injustices and present-day barriers to healthcare, patients from racial and ethnic minority groups often have high levels of medical mistrust [83–85], which may lead to delays in seeking medical care, missed appointments, and nonadherence to medical advice [86]. These effects may be amplified among individuals with limited English proficiency [87, 88]. ID and orthopedic physicians who care for patients with PJI should strive to provide empathic care and may consider dedicated appointments to build trust. For patients with limited English proficiency, access to language-concordant physicians and competent language/interpreter services can also build trust [87, 89]. Community engagement in PJI research, advocacy for improved insurance coverage, and access to multidisciplinary centers of PJI care may also help to improve outcomes [90, 91].
CONCLUSIONS
Despite improvements in infection prevention, more individuals are being diagnosed with PJI each year. While the last several decades have seen important advances in diagnostic approaches and surgical and antimicrobial treatments, significant gaps in our understanding remain. Treatment has become more nuanced over time, and decisions traditionally made by surgeons and those traditionally made by ID physicians can no longer be made in isolation. A collaborative, patient-centered approach with frequent communication and joint decision-making is more essential than ever. Patients diagnosed with PJI face significant physical and emotional stress. Ensuring access to timely, informed, equitable, and culturally centered care can go a long way toward mitigating the stress of this devastating condition.
Contributor Information
Sandra B Nelson, Division of Infectious Diseases, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Jodian A Pinkney, Division of Infectious Diseases, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Antonia F Chen, Department of Orthopedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Aaron J Tande, Division of Public Health, Infectious Diseases, and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Notes
Author Contributions . S. B. N. conceived of the manuscript, edited and compiled the manuscript sections, and prepared the tables and Figure 2. A. J. T. provided the framework for Tables 1 and 2 and Figure 1. All authors performed individual literature review and contributed essential portions of the manuscript. All authors reviewed and approved the final manuscript.
Financial support . J. A. P. was supported by funding provided by the National Institute of Allergy and Infectious Disease.
References
- 1. Kurtz SM, Lau EC, Son M-S, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty 2018; 33:3238–45. [DOI] [PubMed] [Google Scholar]
- 2. McMaster Arthroplasty Collaborative . Incidence and predictors of prosthetic joint infection following primary total knee arthroplasty: a 15-year population-based cohort study. J Arthroplasty 2022; 37:367–372.e1. [DOI] [PubMed] [Google Scholar]
- 3. McMaster Arthroplasty Collaborative . Risk factors for periprosthetic joint infection following primary total hip arthroplasty: a 15-year, population-based cohort study. J Bone Joint Surg Am 2020; 102:503–9. [DOI] [PubMed] [Google Scholar]
- 4. Premkumar A, Kolin DA, Farley KX, et al. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J Arthroplasty 2021; 36:1484–1489.e3. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz AM, Farley KX, Guild GN, Bradbury TL. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty 2020; 35:S79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McPherson E, Tontz W Jr, Patzakis M, et al. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop (Belle Mead NJ) 1999; 28:161–5. [PubMed] [Google Scholar]
- 7. Zimmerli W, Ochsner P. Management of infection associated with prosthetic joints. Infection 2003; 31:99–108. [DOI] [PubMed] [Google Scholar]
- 8. Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am 2003; 85-A Suppl 1:S75–80 [DOI] [PubMed] [Google Scholar]
- 9. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011; 469:2992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shohat N, Bauer T, Buttaro M, et al. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints?: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019; 34:S325–7. [DOI] [PubMed] [Google Scholar]
- 11. McNally M, Sousa R, Wouthuyzen-Bakker M, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021; 103-B:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padua FG, Yayac M, Parvizi J. Variation in inflammatory biomarkers among demographic groups significantly affects their accuracy in diagnosing periprosthetic joint infection. J Arthroplasty 2021; 36:1420–8. [DOI] [PubMed] [Google Scholar]
- 13. Boyle KK, Kapadia M, Chiu Y-F, et al. The James A. Rand Young Investigator’s Award: are intraoperative cultures necessary if the aspiration culture is positive? A concordance study in periprosthetic joint infection. J Arthroplasty 2021; 36:S4–S10. [DOI] [PubMed] [Google Scholar]
- 14. Falconer TM, Baba M, Kruse LM, et al. Contamination of the surgical field with Propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am 2016; 98:1722–8. [DOI] [PubMed] [Google Scholar]
- 15. Garrigues GE, Zmistowski B, Cooper AM, Green A; ICM Shoulder Group . Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: the definition of periprosthetic shoulder infection. J Shoulder Elbow Surg 2019; 28:S8–S12. [DOI] [PubMed] [Google Scholar]
- 16. Prieto HA, Berbari EF, Sierra RJ. Acute delayed infection: increased risk in failed metal on metal total hip arthroplasty. J Arthroplasty 2014; 29:1808–12. [DOI] [PubMed] [Google Scholar]
- 17. Tang H, Xu J, Yuan W, Wang Y, Yue B, Qu X. Reliable diagnostic tests and thresholds for preoperative diagnosis of non-inflammatory arthritis periprosthetic joint infection: a meta-analysis and systematic review. Orthop Surg 2022; 14:2822–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan J, Yan Y, Zhang J, Wang B, Feng J. Diagnostic accuracy of alpha-defensin in periprosthetic joint infection: a systematic review and meta-analysis. Int Orthop 2017; 41:2447–55. [DOI] [PubMed] [Google Scholar]
- 19. Okroj KT, Calkins TE, Kayupov E, et al. The alpha-defensin test for diagnosing periprosthetic joint infection in the setting of an adverse local tissue reaction secondary to a failed metal-on-metal bearing or corrosion at the head-neck junction. J Arthroplasty 2018; 33:1896–8. [DOI] [PubMed] [Google Scholar]
- 20. Ivy MI, Sharma K, Greenwood-Quaintance KE, et al. Synovial fluid α defensin has comparable accuracy to synovial fluid white blood cell count and polymorphonuclear percentage for periprosthetic joint infection diagnosis. Bone Joint J 2021; 103-B:1119–26. [DOI] [PubMed] [Google Scholar]
- 21. Kleeman-Forsthuber LT, Johnson RM, Brady AC, Pollet AK, Dennis DA, Jennings JM. Alpha-defensin offers limited utility in routine workup of periprosthetic joint infection. J Arthroplasty 2021; 36:1746–52. [DOI] [PubMed] [Google Scholar]
- 22. Zeng Y-Q, Deng S, Zhu X-Y, et al. Diagnostic accuracy of the synovial fluid α-defensin lateral flow test in periprosthetic joint infection: a meta-analysis. Orthop Surg 2021; 13:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romanò CL, Petrosillo N, Argento G, et al. The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: multidisciplinary consensus statements. J Clin Med 2020; 9:2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel R. Periprosthetic joint infection. N Engl J Med 2023; 388:251–62. [DOI] [PubMed] [Google Scholar]
- 25. Abdel Karim M, Andrawis J, Bengoa F, et al. Hip and knee section, diagnosis, algorithm: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019; 34:S339–50. [DOI] [PubMed] [Google Scholar]
- 26. Tarabichi M, Shohat N, Goswami K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am 2018; 100:147–54. [DOI] [PubMed] [Google Scholar]
- 27. Kullar R, Chisari E, Snyder J, Cooper C, Parvizi J, Sniffen J. Next-generation sequencing supports targeted antibiotic treatment for culture negative orthopedic infections. Clin Infect Dis 2023; 76:359–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bémer P, Plouzeau C, Tande D, et al. Evaluation of 16S rRNA gene PCR sensitivity and specificity for diagnosis of prosthetic joint infection: a prospective multicenter cross-sectional study. J Clin Microbiol 2014; 52:3583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kildow BJ, Ryan SP, Danilkowicz R, et al. Next-generation sequencing not superior to culture in periprosthetic joint infection diagnosis. Bone Joint J 2021; 103-B:26–31. [DOI] [PubMed] [Google Scholar]
- 30. Saeed K, Ahmad-Saeed N, Annett R, et al. A multicentre evaluation and expert recommendations of use of the newly developed BioFire joint infection polymerase chain reaction panel. Eur J Clin Microbiol Infect Dis 2023; 42:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McQuivey KS, Bingham J, Chung A, et al. The double DAIR: a 2-stage debridement with prosthesis-retention protocol for acute periprosthetic joint infections. JBJS Essent Surg Tech 2021; 11:e19.00071–e19.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res 2013; 471:2374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gerritsen M, Khawar A, Scheper H, et al. Modular component exchange and outcome of DAIR for hip and knee periprosthetic joint infection: a systematic review and meta-regression analysis. Bone Jt Open 2021; 2:806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheper H, Gerritsen LM, Pijls BG, van Asten SA, Visser LG, de Boer MGJ. Outcome of debridement, antibiotics, and implant retention for staphylococcal hip and knee prosthetic joint infections, focused on rifampicin use: a systematic review and meta-analysis. Open Forum Infect Dis 2021; 8:ofab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kunutsor SK, Beswick AD, Whitehouse MR, Wylde V, Blom AW. Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. J Infect 2018; 77:479–88. [DOI] [PubMed] [Google Scholar]
- 36. Hernandez NM, Buchanan MW, Seyler TM, Wellman SS, Seidelman J, Jiranek WA. 1.5-stage exchange arthroplasty for total knee arthroplasty periprosthetic joint infections. J Arthroplasty 2021; 36:1114–9. [DOI] [PubMed] [Google Scholar]
- 37. Strange S, Whitehouse MR, Beswick AD, et al. One-stage or two-stage revision surgery for prosthetic hip joint infection—the INFORM trial: a study protocol for a randomised controlled trial. Trials 2016; 17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. One stage versus two stage for periprosthetic hip and knee infection. 2022. Available at: clinical trials.gov. Accessed 1 February 2023.
- 39. Prendki V, Ferry T, Sergent P, et al. Prolonged suppressive antibiotic therapy for prosthetic joint infection in the elderly: a national multicentre cohort study. Eur J Clin Microbiol Infect Dis 2017; 36:1577–85. [DOI] [PubMed] [Google Scholar]
- 40. Sandiford NA, Hutt JR, Kendoff DO, Mitchell PA, Citak M, Granger L. Prolonged suppressive antibiotic therapy is successful in the management of prosthetic joint infection. Eur J Orthop Surg Traumatol 2020; 30:313–21. [DOI] [PubMed] [Google Scholar]
- 41. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25 [DOI] [PubMed] [Google Scholar]
- 42. Bernard L, Arvieux C, Brunschweiler B, et al. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N Engl J Med 2021; 384:1991–2001. [DOI] [PubMed] [Google Scholar]
- 43. Zijlstra WP, Ploegmakers JJW, Kampinga GA, et al. A protocol for periprosthetic joint infections from the Northern Infection Network for Joint Arthroplasty (NINJA) in The Netherlands. Arthroplasty 2022; 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeller V, Lhotellier L, Marmor S, et al. One-stage exchange arthroplasty for chronic periprosthetic hip infection: results of a large prospective cohort study. J Bone Joint Surg Am 2014; 96:e1. [DOI] [PubMed] [Google Scholar]
- 45. Yang J, Parvizi J, Hansen EN, et al. 2020 Mark Coventry Award: microorganism-directed oral antibiotics reduce the rate of failure due to further infection after two-stage revision hip or knee arthroplasty for chronic infection: a multicentre randomized controlled trial at a minimum of two years. Bone Joint J 2020; 102-B:3–9. [DOI] [PubMed] [Google Scholar]
- 46. Ryan SP, Warne CN, Osmon DR, et al. Short course of oral antibiotic treatment after two-stage exchange arthroplasty appears to decrease early reinfection. J Arthroplasty 2023; 38:909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kelly MP, Gililland JM, Blackburn BE, Anderson LA, Pelt CE, Certain LK. Extended oral antibiotics increase bacterial resistance in patients who fail 2-stage exchange for periprosthetic joint infection. J Arthroplasty 2022; 37:S989–96. [DOI] [PubMed] [Google Scholar]
- 48. Cortés-Penfield NW, Kulkarni PA. The history of antibiotic treatment of osteomyelitis. Open Forum Infect Dis 2019; 6:ofz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Euba G, Murillo O, Fernández-Sabé N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother 2009; 53:2672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Azamgarhi T, Shah A, Warren S. Clinical experience of implementing oral versus intravenous antibiotics (OVIVA) in a specialist orthopedic hospital. Clin Infect Dis 2021; 73:e2582–8. [DOI] [PubMed] [Google Scholar]
- 51. Li H-K, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marconi L, Tedeschi S, Zamparini E, et al. Oral versus standard antimicrobial treatment for pyogenic native vertebral osteomyelitis: a single-center, retrospective, propensity score-balanced analysis. Open Forum Infect Dis 2022; 9:ofac366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wald-Dickler N, Holtom PD, Phillips MC, et al. Oral is the new IV. Challenging decades of blood and bone infection dogma: a systematic review. Am J Med 2022; 135:369–379.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anvari S, Lee Y, Lam M, Doumouras AG, Hong D. The effect of bariatric surgery on oral antibiotic absorption: a systematic review. Obes Surg 2020; 30:2883–92. [DOI] [PubMed] [Google Scholar]
- 55. Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother 2019; 63:e01746-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beldman M, Löwik C, Soriano A, et al. If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin Infect Dis 2021; 73:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tai DBG, Berbari EF, Suh GA, Lahr BD, Abdel MP, Tande AJ. Truth in DAIR: duration of therapy and the use of quinolone/rifampin-based regimens after debridement and implant retention for periprosthetic joint infections. Open Forum Infect Dis 2022; 9:ofac363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Espíndola R, Vella V, Benito N, et al. Rates and predictors of treatment failure in Staphylococcus aureus prosthetic joint infections according to different management strategies: a multinational cohort study—the ARTHR-IS Study Group. Infect Dis Ther 2022; 11:2177–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karlsen ØE, Borgen P, Bragnes B, et al. Rifampin combination therapy in staphylococcal prosthetic joint infections: a randomized controlled trial. J Orthop Surg Res 2020; 15:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 61. Vollmer NJ, Rivera CG, Stevens RW, et al. Safety and tolerability of fluoroquinolones in patients with staphylococcal periprosthetic joint infections. Clin Infect Dis 2021; 73:850–6. [DOI] [PubMed] [Google Scholar]
- 62. Colmenero JD, Fernández-Gallardo LC, Agúndez JA, Sedeño J, Benítez J, Valverde E. Possible implications of doxycycline-rifampin interaction for treatment of brucellosis. Antimicrob Agents Chemother 1994; 38:2798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernard A, Kermarrec G, Parize P, et al. Dramatic reduction of clindamycin serum concentration in staphylococcal osteoarticular infection patients treated with the oral clindamycin-rifampicin combination. J Infect 2015; 71:200–6. [DOI] [PubMed] [Google Scholar]
- 64. Karau MJ, Schmidt-Malan SM, Albano M, et al. Novel use of rifabutin and rifapentine to treat methicillin-resistant Staphylococcus aureus in a rat model of foreign body osteomyelitis. J Infect Dis 2020; 222:1498–504. [DOI] [PubMed] [Google Scholar]
- 65. Thill P, Robineau O, Roosen G, et al. Rifabutin versus rifampicin bactericidal and antibiofilm activities against clinical strains of Staphylococcus spp. isolated from bone and joint infections. J Antimicrob Chemother 2022; 77:1036–40. [DOI] [PubMed] [Google Scholar]
- 66. Monk M, Elshaboury R, Tatara A, Nelson S, Bidell MR. A case series of rifabutin use in staphylococcal prosthetic infections. Microbiol Spectr 2022; 10:e0038422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kruse CC, Ekhtiari S, Oral I, et al. The use of rifampin in total joint arthroplasty: a systematic review and meta-analysis of comparative studies. J Arthroplasty 2022; 37:1650–7. [DOI] [PubMed] [Google Scholar]
- 68. Tonnelier M, Bouras A, Joseph C, et al. Impact of rifampicin dose in bone and joint prosthetic device infections due to Staphylococcus spp.: a retrospective single-center study in France. BMC Infect Dis 2021; 21:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tornero E, Senneville E, Euba G, et al. Characteristics of prosthetic joint infections due to Enterococcus sp. and predictors of failure: a multi-national study. Clin Microbiol Infect 2014; 20:1219–24. [DOI] [PubMed] [Google Scholar]
- 70. Thompson O, Rasmussen M, Stefánsdóttir A, Christensson B, Åkesson P. A population-based study on the treatment and outcome of enterococcal prosthetic joint infections. A consecutive series of 55 cases. J Bone Jt Infect 2019; 4:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56:182–94. [DOI] [PubMed] [Google Scholar]
- 72. Tornero E, Morata L, Martínez-Pastor JC, et al. KLIC-score for predicting early failure in prosthetic joint infections treated with debridement, implant retention and antibiotics. Clin Microbiol Infect 2015; 21:e9–786.e17. [DOI] [PubMed] [Google Scholar]
- 73. Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J Infect 2019; 78:40–7. [DOI] [PubMed] [Google Scholar]
- 74. Shohat N, Goswami K, Tan TL, et al. 2020 Frank Stinchfield Award: identifying who will fail following irrigation and debridement for prosthetic joint infection. Bone Joint J 2020; 102-B:11–9. [DOI] [PubMed] [Google Scholar]
- 75. Knebel C, Menzemer J, Pohlig F, et al. Peri-prosthetic joint infection of the knee causes high levels of psychosocial distress: a prospective cohort study. Surg Infect (Larchmt) 2020; 21:877–83. [DOI] [PubMed] [Google Scholar]
- 76. Mallon C, Gooberman-Hill R, Blom A, Whitehouse M, Moore A. Surgeons are deeply affected when patients are diagnosed with prosthetic joint infection. PLoS One 2018; 13:e0207260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sabesan VJ, Rankin KA, Nelson C. Movement is life-optimizing patient access to total joint arthroplasty: obesity disparities. J Am Acad Orthop Surg 2022; 30:1028–35. [DOI] [PubMed] [Google Scholar]
- 78. Wiznia DH, Jimenez R, Harrington M. Movement is life-optimizing patient access to total joint arthroplasty: diabetes mellitus disparities. J Am Acad Orthop Surg 2022; 30:1017–22. [DOI] [PubMed] [Google Scholar]
- 79. Jones LC, Green TM, Burney DW. Movement is life-optimizing patient access to total joint arthroplasty: dental health disparities. J Am Acad Orthop Surg 2022; 30:1036–8. [DOI] [PubMed] [Google Scholar]
- 80. Aseltine RH, Wang W, Benthien RA, et al. Reductions in race and ethnic disparities in hospital readmissions following total joint arthroplasty from 2005 to 2015. J Bone Joint Surg Am 2019; 101:2044–50. [DOI] [PubMed] [Google Scholar]
- 81. Okewunmi J, Mihalopoulos M, Huang H-H, et al. Racial differences in care and outcomes after total hip and knee arthroplasties: did the comprehensive care for joint replacement program make a difference? J Bone Joint Surg Am 2022; 104:949–58. [DOI] [PubMed] [Google Scholar]
- 82. Lieber AM, Kirchner GJ, Kerbel YE, Moretti VM, Vakil JJ, Brahmabhatt S. Socioeconomic status is associated with risk of above-knee amputation after periprosthetic joint infection of the knee. Clin Orthop Relat Res 2019; 477:1531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hamel L, Lopes L, Munana C, Artiga S, Brodie M. KFF/The undefeated survey on race and health. San Francisco, CA: KFF, 2020. [Google Scholar]
- 84. Jaiswal J, Halkitis PN. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav Med 2019; 45:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ojikutu BO, Bogart LM, Dong L. Mistrust, empowerment, and structural change: lessons we should be learning from COVID-19. Am J Public Health 2022; 112:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res 2009; 44:2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilson E, Chen AHM, Grumbach K, Wang F, Fernandez A. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med 2005; 20:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sewell AA. Disaggregating ethnoracial disparities in physician trust. Soc Sci Res 2015; 54:1–20. [DOI] [PubMed] [Google Scholar]
- 89. Divi C, Koss RG, Schmaltz SP, Loeb JM. Language proficiency and adverse events in US hospitals: a pilot study. Int J Qual Health Care 2007; 19:60–7. [DOI] [PubMed] [Google Scholar]
- 90. Hostetter M, Klein S. Understanding and ameliorating medical mistrust among black Americans. Transforming Care (newsletter), Commonwealth Fund. 2021. Available at: 10.26099/9grt-2b21. Accessed 1 February 2023. [DOI]
- 91. Ojikutu BO, Stephenson KE, Mayer KH, Emmons KM. Building trust in COVID-19 vaccines and beyond through authentic community investment. Am J Public Health 2021; 111:366–8. [DOI] [PMC free article] [PubMed] [Google Scholar]