Abstract
The Togaviridae family, genus, Alphavirus, includes several mosquito-borne human pathogens with the potential to spread to near pandemic proportions. Most of these are zoonotic, with spillover infections of humans and domestic animals, but a few such as chikungunya virus (CHIKV) have the ability to use humans as amplification hosts for transmission in urban settings and explosive outbreaks. Most alphaviruses cause nonspecific acute febrile illness, with pathogenesis sometimes leading to either encephalitis or arthralgic manifestations with severe and chronic morbidity and occasional mortality. The development of countermeasures, especially against CHIKV and Venezuelan equine encephalitis virus that are major threats, has included vaccines and antibody-based therapeutics that are likely to also be successful for rapid responses with other members of the family. However, further work with these prototypes and other alphavirus pathogens should target better understanding of human tropism and pathogenesis, more comprehensive identification of cellular receptors and entry, and better understanding of structural mechanisms of neutralization.
Keywords: Togaviridae, Alphavirus, prototype pathogen
INTRODUCTION TO THE TOGAVIRIDAE FAMILY
Taxonomy
The Togaviridae family consists of positive-sense, single-stranded ribonucleic acid (RNA) viruses with only 1 genus, Alphavirus. The International Committee on the Taxonomy of Viruses (https://talk.ictvonline.org/ictv-reports/ictv_9th_report/positive-sense-rna-viruses-2011/w/posrna_viruses/275/togaviridae) recognizes 32 species of alphaviruses; the majority are mosquito-borne and cause disease in humans and/or domesticated animals, whereas a few are important pathogens of fish. One species, Eilat virus, is considered an insect-specific alphavirus that is completely defective for replication in vertebrates and appears to only infect mosquitoes in nature [1]. Although most alphaviruses cause acute febrile disease in humans, infection with the Old World members is often accompanied by severe arthralgia, whereas the New World viruses sometimes cause central nervous system disease, which can be fatal [2]. An important exception is Mayaro virus (MAYV), a New World arthritogenic alphavirus that is genetically related to the Old World viruses.
Ecology and Epidemiology
The mosquito-borne alphaviruses are zoonotic and use a wide range of amplifying hosts during enzootic transmission cycles, including rodents, birds, and nonhuman primates [2]. Human infection generally occurs via spillover, where enzootic or bridge vectors with an appropriate host range feed first on an infected zoonotic host, then later a human. Only 1 alphavirus, chikungunya virus (CHIKV), has shown sustained amplification in humans after emergence from nonhuman primate (NHP)-amplified enzootic cycles in sub-Saharan Africa. Sustained human-human transmission is mediated by peridomestic Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus mosquitoes, leading to major, explosive epidemics that travel globally via infected people [3]. Several other alphaviruses, including MAYV [4] and Venezuelan equine encephalitis virus (VEEV) [5, 6], are also capable of generating human viremia levels sufficient to infect A aegypti, suggesting their potential for emergence to near-pandemic proportions such as CHIKV. Ross River virus (RRV) is probably also transmitted through human amplification in sustained cycles, although the vectors in this case are likely Aedes vigilax, Aedes camptorhynchus, and Culex annulirostris, which are not highly peridomestic like A aegypti and have much narrower geographic distributions [7]. Other alphaviruses, including Eastern equine encephalitis (EEEV), Western equine encephalitis (WEEV), and Madariaga virus generate little human viremia despite being among the most virulent members of the family. This tendency for humans to be “dead-end” hosts (insufficient viremia to serve as amplifying hosts) is a major factor in limiting the pandemic potential for many alphaviruses.
Replication
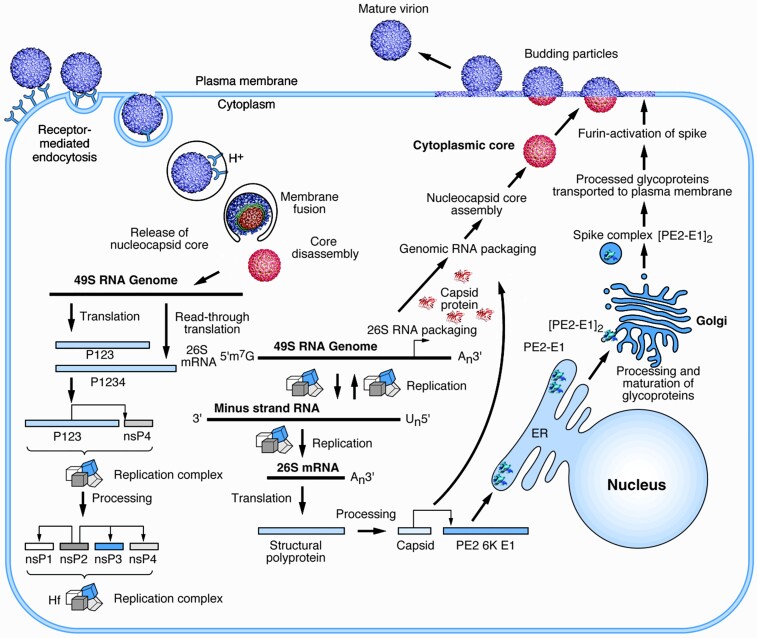
The human-pathogenic alphaviruses generally enter cells via receptor-mediated endocytosis, with the receptors recently identified for several (Figure 1) [8–10]. These receptors bind to the E2 glycoprotein that, along with E1, forms heterotrimeric spikes on the surface of enveloped virions [11]. Upon E1-mediated endosomal fusion, the nucleocapsid disassembles to release the genomic RNA, leading to translation of the nonstructural polyprotein open reading frame (ORF1). The nonstructural proteins, along with host components, form replication complexes on the surface of cytoplasmic vesicles, leading to the production of minus-strand genomic and plus-strand subgenomic (SG) RNAs; the latter encodes an ORF for the structural polyprotein (ORF2). Minus strands are then copied into plus-strand genomic and SG RNAs for further translation, and encapsidation signals near the 5′ end of the genomic RNA [12] combine with capsid proteins to form cytoplasmic nucleocapsids. The envelope glycoproteins are inserted into the endoplasmic reticulum and processed through the secretory pathway to be embedded as E2/E1 trimers in the plasma membrane. These combine with nucleocapsids via a capsid-cytoplasmic E2-tail interaction to initiate budding of virions from the cell surface.
Figure 1.
Replication cycle of an alphavirus. The start of the cycle is shown on the left with the attachment of a virion to a cellular receptor. After fusion of the viral envelope, disassembly of the core, and release of the genomic ribonucleic acid (RNA), replication proteins are translated and processed (bottom left). These proteins enable the replication of the input genomic RNA (bottom center) and translation of the subgenomic messenger RNA (mRNA) into structural proteins. Cytoplasmic assembly of genomic RNA and capsid proteins produces the nucleocapsid core that associates with processed envelope glycoproteins (right) at the plasma membrane resulting in budding of infectious virions. Scale varies. Courtesy of Richard Kuhn with permission from the publisher [9]. ER, endoplasmic reticulum.
Pathogenesis
Alphavirus infections are frequently asymptomatic, or they manifest as general flu-like illness with rash [2]. However, alphaviruses are often broadly categorized into 2 groups based on their associated pathologies, which manifest in severe infections. The arthritogenic (predominantly Old World) alphaviruses cause systemic infection characterized by joint pain with swelling and myalgia, whereas the encephalitic (New World) alphaviruses are associated with infection of the central nervous system (CNS) and encephalitic disease. Although alphaviruses typically cause acute infections that resolve within weeks of symptoms, long-term joint (arthritogenic viruses) and neurological (encephalitic viruses) sequelae have been described for many of these viruses. Details for individual alphaviruses and pathogenic categories are found below.
GAPS IN THE KNOWLEDGE BASE
To develop countermeasures for prototype alphaviruses, which could also be rapidly adapted for any member of the family, a few important gaps in basic virology remain to be addressed. These include sampling the genetic and antigenic diversity of key members including CHIKV and the VEE complex viruses, as well as viruses yet to be discovered. Additional gaps include (1) the lack of receptor identification or confirmation for many human-pathogenic members and (2) high-resolution imaging of receptor-E2 interactions for most. The role of receptor interactions in determining the tropism and pathogenesis of these viruses is still far from understood. Structural intermediates that occur between receptor binding, endosomal fusion, and budding are also lacking. Much progress has been made in understanding epitopes involved in attachment of antibodies (including those that neutralize), the mechanisms of neutralization, and to a lesser extent identification of T cell epitopes; however, most of this work has been performed on only a small number of alphaviruses.
Although several antiviral host factors and their mechanisms of action for controlling alphavirus replication have been elucidated (eg, PKR, IFIT1, ZAP, ISG20), there is still much to be discovered regarding the role of innate immune factors in alphavirus restriction [2]. In particular, variability in the resistance or susceptibility of different family members to antiviral factors, and the molecular mechanisms that underlie these differences, is lacking for many of the host factors described. As with virus-receptor interactions described above, understanding of how intracellular host factors (both antiviral and non-antiviral genes) contribute to cellular tropism is not well understood, and it has only been explored for a limited number of viruses. Likewise, host factors and responses that determine viral tropism and pathogenesis for distinct niches in the host have been explored in more detail for some (eg, the brain and mechanisms of neuroinvasion and blood-brain barrier disruption) but less so for others (eg, the liver).
MODELS OF DISEASE
Cell culture and animal models are critical (1) for the interrogation of disease mechanisms driven by viral infection and (2) for testing safety and efficacy of therapeutics before their approval for use by the US Food and Drug Administration (FDA). The evaluation of the preclinical efficacy in a model depends on well defined end points such as (1) species or cell line selection, (2) challenge strain and dose, (3) route of exposure, (4) clinical endpoints that mimic human disease, and (5) route and timing of countermeasure administration.
Animal models for the study of arthritogenic and encephalitic alphavirus infection include mice, hamsters, guinea pigs, birds, and/or NHPs [13–18]. For alphaviruses, mice and NHP stand as the 2 most widely accepted models for proof-of-concept or preclinical evaluation of the efficacy of therapeutics and vaccines. For diseases with low incidence in the human population, such as the neurotropic alphaviruses, the path to FDA approval for licensure may require validated animal models for Phase II and III clinical trials. Hence, although several elements are known about the progression of the host response and disease in animal models of alphaviruses, well characterized, validated models are not available for most of these viruses and represents an important gap in the field. Successful implementation of animal models in preclinical or clinical trials will also require validated in vitro assays of immune and clinical correlates to measure outcomes. More importantly, the correlates of protection must bridge the animal model to the human experience. These assays should be readily transferable across different organizations engaged in the efforts.
In general, validation of an animal model requires investigation and justification of the viral dose administered, the viral strain, the route of virus administration, the animal, and the clinical signs and optimal endpoints in the animal model chosen. Validation of viruses and cells demands historical tracking of origin and passage history (ie, authenticating the origin of the cells and viruses used). The evaluation of each viral seed stock for its 50% infectious or lethal dose and validation of the viral genome by sequencing are critical for validated animal studies. Current recommendations in the field are to use viral seed stocks amplified from infectious clones to minimize seed stock variation and avoid selection of genotypes that impact phenotype (eg, glycosylation, receptor binding, and virulence). Cells used in preclinical and clinical studies require routine testing for contamination (eg, cell, virus, and mycoplasma), morphology, and functionality.
Arthritogenic Alphavirus Models
The global distribution of arthritogenic alphaviruses present a continued threat to public health [19, 20]. The most notable of these include CHIKV, o’nyong-nyong virus, MAYV, and RRVs. To be able to generate relevant animal models, it is critical to understand the patterns of infection and pathogenesis of each type of alphavirus. Although the timeline of incubation (3–13 days) and illness vary after transmission to a human, most infections present with fever and have a short viremia of a few days followed by acute and subacute phases that may lead to chronic illness. The chronic phase, defined as ongoing pain longer than 12 weeks, can last for years and can include inflammatory rheumatism, musculoskeletal pain, asthenia, and headache. Chronic conditions are generally associated with illness caused by CHIKV, MAYV, and RRV infections [20–22]. Chronic illness is associated with the inflammatory responses elicited from the persistence of viral replication in synovial tissues. Human illness caused by arthritogenic alphaviruses is nonlethal and typically self-limiting, albeit in some cases, symptoms may last for years.
After transmission via mosquito bite, arthritogenic alphaviruses replicate in tissue-resident myeloid cells and fibroblasts, then they traffic to the proximal draining lymph node [2]. Here, virus replicates and further disseminates via the blood to other peripheral organs, including the liver, spleen, and joints. In the joints, CHIKV replicates in fibroblasts (connective tissue), myofibers (muscle cells), and macrophages. Joint pathology is driven by immune cell infiltration (mononuclear cells) into the site of infection (synovia) with robust proinflammatory responses in the joint. Infection of the joints also leads to bone destruction, resulting from perturbed osteoclast/osteoblast homeostasis. Mechanistically, this process results from production of interleukin 6 that stimulates production of receptor activator of nuclear factor-κB ligand (RANKL) from osteoblasts, which inhibit osteoprotegerin, leading to increased osteoclastogenesis and bone resorption [23]. Aside from acute infection, arthritogenic alphaviruses, CHIKV in particular, has been associated with recurring and chronic arthralgia that can last from months to years. Although the precise mechanism of chronic arthralgia is unknown (persistent viral replication vs immunopathology in the absence of virus), studies suggest that prolonged inflammatory and antibody (AB) responses likely contribute.
As would be expected, animal models for the arthritogenic alphaviruses are not lethal; however, the virulence varies across strains. Although mouse models are not ideal for preclinical efficacy due to potential lethality, lack or involvement of neurological symptoms, and limitations in arthritis at sites of infection, they provide useful tools for proof-of-concept studies [24–27]. A key endpoint in evaluation of therapeutics and vaccines for the arthritogenic alphaviruses is joint swelling, which is evaluated and measured in ankles, wrists, and gastrocnemius muscles. Clinical signs for CHIKV and MAYV include acute biphasic swelling response in the ipsilateral foot and ankle that peaks on days 6–8 postinfection. In addition, severe inflammatory synovitis and myositis occur in the joints and skeletal muscle around the foot and are evaluated by histopathological scoring of hematoxylin and eosin-stained hind limb tissues [25, 28]. Immune-deficient mouse models of arthritogenic alphaviruses would not be appropriate for preclinical or clinical testing for obvious challenges of translation of outcomes to healthy individuals.
The most advanced NHP model for vaccine testing is for CHIKV and has been in development since the 1950s [14]. The pathogenesis of CHIKV in both rhesus and cynomolgus macaques mirrors human disease, although how the route of viral infection impacts pathogenesis is less understood. Disease severity correlates with viral infection dose. Nonhuman primates show viremia, fever, rash, lymphopenia, and immunoglobulin (Ig)M antibody response during the first week of infection. Of these clinical signs, viremia, fever, and lymphopenia provide excellent endpoints for efficacy testing [29, 30]. In addition, CHIKV persists in the spleen in rhesus and cynomolgus macaques with the later having more severe disease and greater duration of viral persistence [25, 31]. Limitations of NHP models include the lack of neurological signs observed in humans.
Encephalitic Alphavirus Models
VEEV, WEEV, and EEEV are significant pathogens of both medical and veterinary importance. Human disease is highlighted by fatal encephalitis and permanent neurological sequelae in survivors. Of the 3 viruses, EEEV causes the most severe disease with human case-fatality rates of 30%–90% in those with neurological disease [32]. The survivors suffer from debilitating and permanent long-term neurological sequelae at rates of 35%–80% [32, 33]. Despite the discovery of these viruses more than 80 years ago, the mechanism(s) that underlie the pathogenesis are not well understood. The vast majority of infections are diagnosed at late stages, and the virus-induced pathology and/or host inflammatory response are presumably responsible for the fatal outcome.
Similar to the arthritogenic alphaviruses during the acute phase of infection, the encephalitic alphaviruses replicate in tissue resident cells in the periphery that traffic to the draining lymph node where virus replicates further and disseminates to peripheral organs including the liver, spleen, and CNS. Differences in cellular tropism among encephalitic alphaviruses relate to the distinct pathogenic mechanisms of these related viruses [34]. Although VEEV predominantly infects myeloid cells in the periphery and lymph node leading to robust production of interferon (IFN), EEEV replicates poorly in myeloid cells, thus circumventing robust activation of innate immunity [35]. Indeed, robust activation of innate immunity and production of IFN is thought to cause significant prodrome observed after VEEV infection, but which is absent in EEEV infections. Although several routes of CNS infection may be involved for different encephalitic alphaviruses, neuroinvasion after natural routes of infection appears to predominantly involve the circumventricular organs of the brain (eg, pineal body) and the nerves innervating the olfactory neuroepithelium [36]. Long-term neurological sequelae are particularly prevalent in EEEV and WEEV cases, and this may be an underappreciated consequence of VEEV infection due to the higher prevalence of asymptomatic and undiagnosed VEEV infections relative to EEEV and WEEV [37].
Interferons play a key role in early restriction of alphavirus replication, and as with many other viruses, deficiencies in IFN signaling results in greater disease severity [38]. Natural infection and immunization typically produce robust long-term protective humoral immunity including neutralizing antibodies (NAbs) that are important for resolution of acute infection. T cells have also been shown to play both protective and pathogenic roles during alphavirus infections, although different T cell subsets seem to be protective in different models [39, 40]. Protective T cell responses, in particular, are important during infection with encephalitic alphaviruses, which invade the CNS before the onset of robust IgG responses.
Two recent studies with EEEV in cynomolgus macaques provide insights into the potential underlying mechanism(s) of pathogenesis [41, 42]. After introduction into the brain via the aerosol route, many critical physiological parameters under the control of the autonomic nervous system (ANS) such as respiration, activity, temperature, heart rate, blood pressure, food/fluid intake, circadian rhythm, sleep, and electrical activity of the heart and the brain were rapidly and profoundly changed leading to the NHPs meeting the euthanasia criteria. We were surprised to find that one of the NHPs met the euthanasia criteria by exhibiting a sudden cardiac event. A follow-up pathology study on the organs and tissues of the NHPs at the time of euthanasia demonstrated rapid virus dissemination throughout the brain and spinal cord including the ANS control centers [43]. The virus likely spread by hijacking the axonal transport system, which is an essential neuronal homeostatic process responsible for movement of RNA, proteins, and organelles within the neuron. Thirty-five virions were observed in a single axon of a neuron in a 160 nm section [43]. Consequently, this mechanism has the potential to rapidly transport a tremendous amount of virus throughout the CNS. However, despite the extensive dissemination, most brain and spinal cord tissues exhibited minimal or no microscopic lesions with the cellular architecture remaining intact. In addition, minimal or no host inflammatory infiltrate was observed in majority of the tissues. This strongly suggests that EEEV infection causes local and global neuronal dysfunction leading to dysregulation of critical physiological parameters. This neuronal dysfunction likely contributes to or exacerbates viral and host-induced pathology to produce the fatal outcome. Whether these mechanisms also underlie VEEV and/or WEEV pathogenesis remains to be determined.
LANDSCAPE OF MEDICAL COUNTERMEASURES
There is a lack of approved human vaccines and antiviral drugs for public use against alphavirus infections [44]. Further research is needed to expand current knowledge of alphavirus immunity to identify safe, immunogenic, and protective medical countermeasures for alphavirus outbreaks including vaccines and antibodies.
Vaccination Strategies to Prevent Alphavirus Infection or Disease
Numerous approaches to identify vaccine candidates have been tried or are currently being tested in ongoing clinical trials (Table 1). Strategies used include the use of live-attenuated viruses, generation of chimeric viruses, and formalin inactivation of virus particles [44–50]. These vaccine candidates have been shown in some experiments and trials to be immunogenic and protective for several alphaviruses [48, 51–54]. However, some vaccine candidates are reactogenic, require frequent boosting, or their immunogenicity is disrupted by the inactivation methods used [44, 47, 55–57].
Table 1.
Clinical Trials of Alphavirus Vaccine Candidatesa
| NCT Number | Title of Clinical Trial | Type | Biologicals | Phases | Study Design | Sponsor/ Collaborators |
|---|---|---|---|---|---|---|
| NCT03879603 | VRC 313: A Trivalent Virus-like Particle (VLP) Encephalitis Vaccine (WEVEE) in Healthy Adults | VLP | VRC-WEVVLP073-00-VP | Phase 1 | Prevention | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT03776994 | Venezuelan Equine Encephalitis Monovalent Virus-Like Particle Vaccine | VLP | VEE VLP | SRI International; US Army Medical Research Institute of Infectious Diseases | ||
| NCT03829384 | Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of mRNA-1944 in Healthy Adults | mRNA | mRNA-1944 | ModernaTX, Inc. | ||
| NCT04603131 | Clinical Trial to Evaluate the Immunogenicity of Chikungunya Vaccine | Formalin-inactivated | BBV87 | Bharat Biotech International Limited | ||
| NCT03382964 | Study to Assess the Safety and Immunogenicity of a Chikungunya Virus Vaccine Candidate (VLA1553) in Healthy Volunteers | Live-attenuated | VLA1553 | Valneva Austria GmbH | ||
| NCT03325075 | Safety, Tolerability, and Immunogenicity of VAL-181388 in Healthy Subjects | mRNA | VAL-181388 | ModernaTX, Inc.; Defense Advanced Research Projects Agency | ||
| NCT01489358 | Chikungunya Virus Vaccine Trial in Healthy Adults | VLP | VRC-CHKVLP059-00-VP | National Institute of Allergy and Infectious Diseases (NIAID); National Institutes of Health Clinical Center (CC) | ||
| NCT03028441 | Phase I Trial of Measles Vectored Chikungunya Vaccine | VLP | VRC-CHKVLP059-00-VP | National Institute of Allergy and Infectious Diseases (NIAID) | ||
| NCT01984983 | Study of a DNA-based Venezuelan Equine Encephalitis Virus DNA Vaccine Administered by Electroporation in Healthy Volunteers | DNA | VEE DNA Vaccine Candidate | Ichor Medical Systems Incorporated; US Army Medical Research Institute of Infectious Diseases | ||
| NCT04440774 | Research Study to Assess New Chikungunya and Zika Vaccines in Healthy Adults in Mexico | Adenovirus | ChAdOx1 Chik | University of Oxford | ||
| NCT04131595 | Vaccination Trial of a Recombinant MVA-BN-WEV Vaccine in Healthy Adult Subjects | Vaccinia virus | MVA-BN-WEV | Bavarian Nordic; JPM CBRN Medical | ||
| NCT01159561 | Western Equine Encephalitis Vaccine, Inactivated | Inactivated | WEE: TSI-GSD 210 | US Army Medical Research and Development Command | ||
| NCT00582088 | Safety and Immunogenicity of Venezuelan Equine Encephalomyelitis Vaccine (VEE C-84) as a Booster to VEE TC-83 | Formalin-inactivated | VEE C-84 | Phase 2 | US Army Medical Research and Development Command | |
| NCT03992872 | Phase 2 Open-label Study of Alum-adjuvanted Chikungunya Virus-like Particle Vaccine (PXVX0317) | VLP | WRAIR | Emergent BioSolutions; Walter Reed Army Institute of Research (WRAIR) | ||
| NCT03807843 | Chikungunya Vaccine Study in Previously Exposed Adults (V184-006) | VLP | MV-CHIK | Themis Bioscience GmbH; Walter Reed Army Institute of Research (WRAIR) | ||
| NCT02861586 | Phase II Study to Evaluate Safety and Immunogenicity of a Chikungunya Vaccine | VLP | MV-CHIK | Themis Bioscience GmbH | ||
| NCT03635086 | Safety, Tolerability and Long-term Immunogenicity of Different Formulations of a Chikungunya Vaccine (V184-005) | VLP | MV-CHIK | Themis Bioscience GmbH | ||
| NCT03483961 | Trial of a Chikungunya Vaccine, PXVX0317 CHIKV-VLP, in Healthy Adults | VLP | CHIKV VLP | Emergent BioSolutions | ||
| NCT03101111 | Study of a Live Attenuated Chikungunya Vaccine in a Previously Epidemic Area | VLP | MV-CHIK | Themis Bioscience GmbH; Walter Reed Army Institute of Research (WRAIR) | ||
| NCT02562482 | Trial for Safety and Immunogenicity of a Chikungunya Vaccine, VRC-CHKVLP059-00-VP, in Healthy Adults | VLP | VRC-CHKVLP059-00-VP | National Institute of Allergy and Infectious Diseases (NIAID) | ||
| NCT05065983 | A Study to Assess the Safety and Immunogenicity of PXVX0317 Chikungunya Virus-Like Particle Vaccine | VLP | CHIKV VLP | Emergent BioSolutions | ||
| NCT03531242 | Safety and Immunogenicity Study of Venezuelan Equine Encephalomyelitis (VEE) Vaccine as Booster Vaccine in Adults | Inactivated, Dried | VEE: C-84, TSI-GSD 205 | US Army Medical Research and Development Command | ||
| NCT03051386 | Safety and Immunogenicity of Venezuelan Equine Encephalomyelitis Vaccine in Healthy Adults | Live-attenuated | VEE TC-83 | US Army Medical Research and Development Command; US Army Medical Research Institute of Infectious Diseases | ||
| NCT02654509 | Safety and Immunogenicity Study of the Eastern Equine Encephalitis (EEE) Vaccine | Formalin-inactivated | EEE: TSI-GSD 104 | US Army Medical Research and Development Command | ||
| NCT02466750 | Safety and Immunogenicity Study of the Western Equine Encephalitis (WEE) Vaccine | Inactivated | WEE: TSI-GSD 210 | US Army Medical Research and Development Command | ||
| NCT00582504 | Safety and Immunogenicity Study of the Venezuelan Equine Encephalomyelitis Vaccine | Live-attenuated | VEE TC-83 | US Army Medical Research and Development Command | ||
| NCT00584805 | Safety and Immunogenicity Study of Eastern Equine Encephalitis (EEE) Vaccine | Formalin-inactivated | EEE: TSI-GSD 104 | US Army Medical Research and Development Command | ||
| NCT04566484 | Seamless Controlled Trial To Evaluate Safety And Immunogenicity of Chikungunya Vaccine in Latin America and Asia | Formalin-inactivated | BBV87 | Phases 2 and 3 | International Vaccine Institute | |
| NCT01604746 | Additional 6-Month Safety Follow-up After Completion of Precursor Study 880801 | Inactivated | Ross River Virus (RRV) Vaccine | Phase 3 | Ology Bioservices | |
| NCT01242670 | Ross River Virus (RRV) Vaccine Study | Inactivated | Ross River Virus (RRV) Vaccine | Ology Bioservices | ||
| NCT04786444 | Study to Demonstrate Consistency of Three Lots of a Live-attenuated Chikungunya Virus Vaccine Candidate in Healthy Adults | Live-attenuated | VLA1553 | Valneva Austria GmbH | ||
| NCT04546724 | Pivotal Study to Evaluate Safety and Immunogenicity of a Live-Attenuated Chikungunya Virus Vaccine Candidate in Adults | Live-attenuated | VLA1553 | Valneva Austria GmbH | ||
| NCT05072080 | A Phase 3 Trial of the VLP-Based Chikungunya Vaccine PXVX0317 | VLP | CHIKV VLP/adjuvant | Emergent BioSolutions | ||
| NCT04838444 | Antibody Persistence And Long Term Safety Of A Chikungunya Virus Vaccine Candidate (VLA1553) | Live-attenuated | VLA1553 | Valneva Austria GmbH | ||
| NCT04650399 | A Multicenter Study to Evaluate Safety and Immunogenicity of a Live-attenuated Chikungunya Vaccine in Adolescents | Live-attenuated | VLA1553 | Butantan Institute; Valneva Austria GmbH | ||
| NCT04441905 | Phase 1 Study of SAR440894 vs Placebo | mAb | SAR440894 | Phase 1 | Treatment | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT03590392 | Safety and Immunogenicity of a Candidate CHIKV Vaccine (CHIK001) | Adenovirus | ChAdOx1 Chik | University of Oxford | ||
| NCT02230163 | Clinical Evaluation of Anti-CHIKV Hyperimmune Intravenous Immunoglobulins | Ig | anti-CHIKV hyperimmune immunoglobulins | Phases 1 and 2 | Centre Hospitalier Universitaire de Pointe-a-Pitre |
Abbreviations: CHIKV, chikungunya virus; DNA, deoxyribonucleic acid; Ig, immunoglobulin; mRNA, messenger ribonucleic acid; NCT, ClinicalTrials.gov Identifier;
Adapted from ClinicalTrials.gov.
Another candidate vaccination approach involves using virus-like particles (VLPs), which are noninfectious molecules that structurally resemble intact virions [58, 59]. Monovalent or trivalent VLP vaccines elicited immunogenic responses in nonhuman primates for protection and were safe and tolerable in Phase I clinical trials [59–61]. Yet another strategy is to use deoxyribonucleic acid (DNA)- or messenger RNA (mRNA)-based antigen delivery methods, which may enhance the speed of candidate vaccine generation. The DNA and mRNA vaccines encoding some alphavirus structural proteins are immunogenic in animals [62–64].
Antibody-Mediated Mechanisms of Action Against Alphaviruses
In addition to vaccines, antibodies (Abs) provide an alternate route to medical countermeasures. Furthermore, understanding the Ab response can help inform rational vaccine design. Antibody responses are important in the protection, treatment, clearance, and maintenance of alphaviruses [65–68]. Passive transfer studies of immune animal serum or purified IgG from plasma samples of immune individuals highlight the ability of Abs to protect mice against alphavirus infection [69, 70]. In addition, mRNA vectors discussed above also can express Abs in recipients. Expression of a potent CHIKV monoclonal Ab (mAb) as a lipid-encapsulated mRNA protected against infection in mice, expressed well in nonhuman primates [71], and was safe, tolerable, and expressed in Phase I clinical trials [72].
Neutralizing E2-Specific Antibody Response
The E2 glycoprotein is a target for many neutralizing anti-alphavirus mAbs. In general, neutralization activity corresponds with protection [73], and virus-specific humoral responses from immunized mice or immune individuals are well characterized for several alphaviruses. Numerous Ab-binding epitopes have been identified within the E2 glycoprotein [74, 75], and cross-neutralizing E2-specific mAbs have been identified against the arthritogenic alphaviruses [76–78]. In contrast, a cross-neutralizing E2-specific mAb against the encephalitic alphaviruses has yet to be identified [79, 80]. Potently neutralizing E2-targeting Abs can interfere with different steps in the virus replication cycle, including virus entry, viral egress, and cell-to-cell spread. Blockade of virus entry can occur through multiple mechanisms, including virus aggregation [81], direct blockade of attachment to host receptors (such as Mxra8 or LDLRAD3), or indirect blockade through steric hindrance [8, 9, 82]. After attachment, mAbs can inhibit viral entry by blocking structural transitions [83] or inhibit viral fusion by structurally stabilizing the E2 glycoprotein [78, 84–86].
Protective E1-Specific Antibody Response
The E1 glycoprotein is another target for protective anti-alphavirus mAbs [73, 87–91]. In contrast to E2-specific mAbs, E1-specific mAbs are generally nonneutralizing or weakly neutralize virus in standard focus-forming assays [82, 90, 91]. This may be due to obstruction by the E2 glycoprotein, because exposure of cryptic E1 epitopes requires presentation of different conformational states [92–94] or pretreatment with altered conditions [88, 95–99]. Weakly neutralizing antibodies (NAbs) target Domain III, likely due to its greater exposure on mature virions [82, 100].
Several mAbs recognize the highly conserved fusion loop region and exhibit broad binding to alphaviruses. The ability of nonneutralizing mAbs to inhibit virus egress corresponds with protective in vivo efficacy against homologous and heterologous alphaviruses [90, 91]. During the diagnostic assessment of infection, cross-protective anti-alphavirus mAbs could serve as pan-alphavirus medical countermeasure candidates to limit viral replication and increase the therapeutic window for potent virus-specific treatments. Understanding the conserved epitopes recognized by these Abs can also aid in rational, structure-based, pan-alphavirus vaccine design.
Fc-Mediated Antibody Functions
Because protective capability does not necessarily correlate with neutralization potency of anti-alphavirus mAbs, Fc-mediated effector functions likely play a substantial role in protection against alphaviruses [66, 73, 88]. In mouse models, optimal clearance of infection and reduction of joint swelling for CHIKV- or MAYV-induced musculoskeletal disease required Fc-FcγR interactions, primarily on monocytes [77, 101]. In some cases, reduced efficacy in FcγR−/− mice was observed, and protection depended on mAb isotype and N297 glycosylation, which modulates effector function [77, 91]. Further assessment is needed to identify non-NAb-based medical countermeasures that are efficacious against alphaviruses.
PROTOTYPE PATHOGENS
Considerations for prototype pathogen assignments included importance as human pathogens, representative pathogenesis patterns, the availability of animal models that recapitulate human disease, current knowledge of replication and pathogenesis, and the status of countermeasure development. Chikungunya virus is by far the most important cause of human disease, with recent outbreaks spreading to near-pandemic proportions due to its propensity for human amplification and peridomestic vector transmission [3]. It is also one of the more heavily studied alphaviruses, has good murine and excellent NHP models, and has vaccines in late stages of clinical trials [102, 103] as well as promising monoclonal antibody therapies [72, 101]. Among the other arthritogenic alphaviruses, RRV is also well studied with some vaccine development reported but has not shown the potential for widespread epidemics beyond Australia and some Oceanic islands.
The second prototype selected was VEEV, for many of the same reasons as CHIKV. It is also relatively well studied for structure and replication, and it is well understood epidemiologically with extensive human disease and some potential for widespread outbreaks (equine-amplified to date, but with potential for human amplification), a long history of vaccine development, but with limited clinical trials due in part to an underappreciated disease burden [104], and some therapeutic monoclonal antibody development [91]. Compared to the arthritogenic alphaviruses, EEEV and WEEV are more virulent but cause less human disease and seem to have less pandemic potential, due to their lack of equine or human amplification [2]. They also have limited vaccine or therapeutic antibody development.
There are important disadvantages in selecting CHIKV and VEEV as prototypes, most obviously their recommended biosafety level 3 (BSL3) containment. However, reliable methods for alphavirus attenuation including chimerization [105–107], genomic deletions [108], and rearrangements that alter levels of gene expression [109] have facilitated generation of viruses that are structurally identical to these and other BSL3 alphaviruses [107] but that can be safely handled at BSL2.
SUMMARY AND FUTURE DIRECTIONS
Preparing for an alphaviral pandemic requires a focus on 2 primary pathogen types: arthritogenic and encephalitic. Although the developmental algorithm is similar for both, there are specific elements that must be considered for each. Key among these elements are the need for appropriate models, an understanding of the various routes of pathogenesis and host immune response, and data regarding the modes of action for the wide array of vaccines and therapeutics.
Critical to future development of any countermeasures is the need for better and appropriate testing models. For alphaviruses, although many distinct in vitro and animal models exist, they are not well standardized and must be refined to incorporate variables such as age, microbiomes, and long-term sequelae or chronic conditions that are not currently considered. Cell culture models are extremely limited in that they do not simulate entire systems with complex interactions such as synovial joint tissues or brain parenchyma, minimizing the understanding of specific cell types involved in infection. In addition, cell culture models of neuroinvasion do not provide information on delivery across the blood-brain barrier. Thus, until appropriate cell models can be developed, relevant animal systems are critical.
Although animal models do give the most complete profile of pathogenesis, there remains a general lack of knowledge regarding both early infection events and the chronic conditions that exist for many alphaviruses. Receptors are not typically identified, but there is hope that CRISPR technology could facilitate this process. In addition, for the encephalitic alphaviruses, particularly VEEV, animal models also need to address the immunodeficiency that follows infection. Finally, because most alphavirus countermeasure development has focused on the bioweapon property of being infectious by aerosol, there is a strong need for re-evaluation of models to focus on natural route of infection (via mosquito bite).
A final challenge that limits extensive research on the alphavirus infection processes is that many key human pathogens are Risk Group 3 (RG3) and require BSL3 laboratory practices. Because these facilities are not always readily available and due to the risk of working with these agents, there are concerns over how to protect laboratorians performing the critical research.
Although several obstacles do exist to for development of prototype alphavirus countermeasures, much work has already provided a wealth of valuable information that will be critical. First, relatively consistent correlates of protection (NAbs) have been identified for several alphaviruses, which could accelerate vaccine development across the genera. Second, and most importantly, a range of vaccine platforms exist or are currently under development that could be rapidly applied to different alphaviruses. This baseline knowledge provides the foundation to develop the alphavirus prototype pathogen profile for increased preparedness to respond to this group of viruses.
Contributor Information
Ann M Powers, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, Colorado, USA.
Lauren E Williamson, The Vanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Robert H Carnahan, The Vanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
James E Crowe, Jr., The Vanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, Tennessee, USA Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Pathology, Microbiology and Immunology, Vanderbilt University, Nashville, Tennessee, USA.
Jennifer L Hyde, Department of Microbiology, University of Washington, Seattle, Washington, USA.
Colleen B Jonsson, Department of Microbiology, Immunology and Biochemistry, College of Medicine, University of Tennessee Health Science Center, Memphis, Tennessee, USA.
Farooq Nasar, Emerging Infectious Diseases Branch and Viral Disease Branch, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA; Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, Texas, USA.
Scott C Weaver, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, Texas, USA; World Reference Center for Emerging Viruses and Arboviruses, Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston, Texas, USA.
Notes
Acknowledgments. We thank Daved Fremont, Ilya Frolov, Mark Heise, William Klimstra, Margaret Macdonald, Thomas Morrison, and Jonathan Smith for numerous helpful discussions in formulating the ideas in this paper. This article is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Disclaimers. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention, the official policy or position of the US Department of Defense, the Department of the Army, BigHat Biosciences, or Henry M. Jackson Foundation.
Financial support. Funding in support of this work was provided by the NIAID/NIH to J. E. C., L. E. W., and S. C. W. (R24 AI120942) and by NIH grant 5U19AI142762 to CBJ.
Supplement sponsorship. This article appears as part of the supplement “Pandemic Preparedness at NIAID: Prototype Pathogen Approach to Accelerate Medical Countermeasures—Vaccines and Monoclonal Antibodies,” sponsored by the National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD.
References
- 1. Nasar F, Palacios G, Gorchakov RV, et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A 2012; 109:14622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffin DE, Weaver SC. Alphaviruses. In: Howley PM, Knipe DM, Whelan S, eds. Fields Virology, Volume 1: Emerging Viruses. Philadelphia: Wolters Lluwer, pp 2021:194–245. [Google Scholar]
- 3. Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med 2018; 69:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long KC, Ziegler SA, Thangamani S, et al. Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg 2011; 85:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weaver SC, Salas R, Rico-Hesse R, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet 1996; 348:436–40. [DOI] [PubMed] [Google Scholar]
- 6. Ortiz DI, Kang W, Weaver SC. Susceptibility of Ae. aegypti (Diptera: Culicidae) to infection with epidemic (subtype IC) and enzootic (subtypes ID, IIIC, IIID) Venezuelan equine encephalitis complex alphaviruses. J Med Entomol 2008; 45:1117–25. [DOI] [PubMed] [Google Scholar]
- 7. Yuen KY, Bielefeldt-Ohmann H. Ross river virus infection: a cross-disciplinary review with a veterinary perspective. Pathogens 2021; 10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang R, Kim AS, Fox JM, et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018; 557:570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma H, Kim AS, Kafai NM, et al. LDLRAD3 is a receptor for Venezuelan equine encephalitis virus. Nature 2020; 588:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark LE, Clark SA, Lin C, et al. VLDLR and ApoER2 are receptors for multiple alphaviruses. Nature 2021; 602:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voss JE, Vaney MC, Duquerroy S, et al. Glycoprotein organization of chikungunya virus particles revealed by X-ray crystallography. Nature 2010; 468:709–12. [DOI] [PubMed] [Google Scholar]
- 12. Kim DY, Atasheva S, Frolova EI, Frolov I. Venezuelan equine encephalitis virus nsP2 protein regulates packaging of the viral genome into infectious virions. J Virol 2013; 87:4202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nalca A, Fellows PF, Whitehouse CA. Vaccines and animal models for arboviral encephalitides. Antiviral Res 2003; 60:153–74. [DOI] [PubMed] [Google Scholar]
- 14. Broeckel R, Haese N, Messaoudi I, Streblow DN. Nonhuman primate models of chikungunya virus infection and disease (CHIKV NHP model). Pathogens 2015; 4:662–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rusnak JM, Dupuy LC, Niemuth NA, Glenn AM, Ward LA. Comparison of aerosol- and percutaneous-acquired Venezuelan equine encephalitis in humans and nonhuman primates for suitability in predicting clinical efficacy under the animal rule. Comp Med 2018; 68:380–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reed DS, Lind CM, Sullivan LJ, Pratt WD, Parker MD. Aerosol infection of cynomolgus macaques with enzootic strains of Venezuelan equine encephalitis viruses. J Infect Dis 2004; 189:1013–7. [DOI] [PubMed] [Google Scholar]
- 17. Jackson AC, SenGupta SK, Smith JF. Pathogenesis of Venezuelan equine encephalitis virus infection in mice and hamsters. Vet Pathol 1991; 28:410–8. [DOI] [PubMed] [Google Scholar]
- 18. Smith DR, Schmaljohn CS, Badger C, et al. Comparative pathology study of Venezuelan, eastern, and western equine encephalitis viruses in non-human primates. Antiviral Res 2020; 182:104875. [DOI] [PubMed] [Google Scholar]
- 19. Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses—an overview. Nat Rev Rheumatol 2012; 8:420–9. [DOI] [PubMed] [Google Scholar]
- 20. Levi LI, Vignuzzi M. Arthritogenic alphaviruses: a worldwide emerging threat? Microorganisms 2019; 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halsey ES, Siles C, Guevara C, et al. Mayaro virus infection, Amazon basin region, Peru, 2010–2013. Emerg Infect Dis 2013; 19;1839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suchowiecki K, Reid SP, Simon GL, Firestein GS, Chang A. Persistent joint pain following arthropod virus infections. Curr Rheumatol Rep 2021; 23:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W, Foo SS, Rulli NE, et al. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc Natl Acad Sci U S A 2014; 111:6040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haese NN, Broeckel RM, Hawman DW, Heise MT, Morrison TE, Streblow DN. Animal models of chikungunya virus infection and disease. J Infect Dis 2016; 214:S482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hawman DW, Stoermer KA, Montgomery SA, et al. Chronic joint disease caused by persistent chikungunya virus infection is controlled by the adaptive immune response. J Virol 2013; 87:13878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poo YS, Rudd PA, Gardner J, et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl Trop Dis 2014; 8:e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ziegler SA, Lu L, da Rosa AP, Xiao SY, Tesh RB. An animal model for studying the pathogenesis of chikungunya virus infection. Am J Trop Med Hyg 2008; 79:133–9. [PubMed] [Google Scholar]
- 28. Morrison TE, Oko L, Montgomery SA, et al. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol 2011; 178:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roy CJ, Adams AP, Wang E, et al. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis 2014; 209:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pal P, Fox JM, Hawman DW, et al. Chikungunya viruses that escape monoclonal antibody therapy are clinically attenuated, stable, and not purified in mosquitoes. J Virol 2014; 88:8213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Labadie K, Larcher T, Joubert C, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 2010; 120:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fields BN, Knipe DM, Howley PM eds. Virology. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 33. Lindsey NP, Staples JE, Fischer M. Eastern equine encephalitis virus in the United States, 2003–2016. Am J Trop Med Hyg 2018; 98:1472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gardner CL, Burke CW, Tesfay MZ, Glass PJ, Klimstra WB, Ryman KD. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: impact of altered cell tropism on pathogenesis. J Virol 2008; 82:10634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trobaugh DW, Gardner CL, Sun C, et al. RNA viruses can hijack vertebrate microRNAs to suppress innate immunity. Nature 2014; 506:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips AT, Rico AB, Stauft CB, et al. Entry sites of Venezuelan and western equine encephalitis viruses in the mouse central nervous system following peripheral infection. J Virol 2016; 90:5785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ronca SE, Dineley KT, Paessler S. Neurological sequelae resulting from encephalitic alphavirus infection. Front Microbiol 2016; 7:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryman KD, Klimstra WB. Host responses to alphavirus infection. Immunol Rev 2008; 225:27–45. [DOI] [PubMed] [Google Scholar]
- 39. Long KM, Ferris MT, Whitmore AC, et al. γδ T cells play a protective role in chikungunya virus-induced disease. J Virol 2016; 90:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paessler S, Yun NE, Judy BM, et al. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology 2007; 367:307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Albe JR, Ma H, Gilliland TH, et al. Physiological and immunological changes in the brain associated with lethal eastern equine encephalitis virus in macaques. PLoS Pathog 2021; 17:e1009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trefry JC, Rossi FD, Accardi MV, et al. The utilization of advance telemetry to investigate critical physiological parameters including electroencephalography in cynomolgus macaques following aerosol challenge with eastern equine encephalitis virus. PLoS Negl Trop Dis 2021; 15:e0009424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams JA, Long SY, Zeng X, et al. Eastern equine encephalitis virus rapidly infects and disseminates in the brain and spinal cord of infected cynomolgus macaques following aerosol challenge. PLoS Negl Trop Dis 2022; 16:e0010081. doi: 10.1371/journal.pntd.0010081. eCollection 2022 May. PMID: 35533188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trobaugh DW, Sun C, Dunn MD, Reed DS, Klimstra WB. Rational design of a live-attenuated eastern equine encephalitis virus vaccine through informed mutation of virulence determinants. PLoS Pathog 2019; 15:e1007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berge TO, Banks IS, Tigertt WD. Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in Guinea pig heart cells. Am J Epidemiol 1961; 73:209–18. [Google Scholar]
- 46. Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine 1986; 4:157–62. [DOI] [PubMed] [Google Scholar]
- 47. Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine 1996; 14:337–43. [DOI] [PubMed] [Google Scholar]
- 48. Atasheva S, Wang E, Adams AP, et al. Chimeric alphavirus vaccine candidates protect mice from intranasal challenge with western equine encephalitis virus. Vaccine 2009; 27:4309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Erasmus JH, Seymour RL, Kaelber JT, et al. Novel insect-specific Eilat virus-based chimeric vaccine candidates provide durable, mono- and multivalent, single-dose protection against lethal alphavirus challenge. J Virol 2018; 92:e01274–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torres-Ruesta A, Chee RS, Ng LFP. Insights into antibody-mediated alphavirus immunity and vaccine development landscape. Microorganisms 2021; 9:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg 2000; 62:681–5. [DOI] [PubMed] [Google Scholar]
- 52. Wang E, Volkova E, Adams AP, et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine 2008; 26:5030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang E, Kim DY, Weaver SC, Frolov I. Chimeric chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J Virol 2011; 85:9249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roy CJ, Adams AP, Wang E, et al. A chimeric Sindbis-based vaccine protects cynomolgus macaques against a lethal aerosol challenge of eastern equine encephalitis virus. Vaccine 2013; 31:1464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McKinney RW, Berge TO, Sawyer WD, Tigertt WD, Crozier D. Use of an attenuated strain of Venezuelan equine encephalomyelitis virus for immunization in man. Am J Trop Med Hyg 1963; 12:597–603. [DOI] [PubMed] [Google Scholar]
- 56. Jahrling PB, Stephenson EH. Protective efficacies of live attenuated and formaldehyde-inactivated Venezuelan equine encephalitis virus vaccines against aerosol challenge in hamsters. J Clin Microbiol 1984; 19:429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. Assessment of immunogenic potential of vero adapted formalin inactivated vaccine derived from novel ECSA genotype of chikungunya virus. Vaccine 2009; 27:2513–22. [DOI] [PubMed] [Google Scholar]
- 58. Noranate N, Takeda N, Chetanachan P, Sittisaman P AAN, Anantapreecha S. Characterization of chikungunya virus-like particles. PLoS One 2014; 9:e108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ko SY, Akahata W, Yang ES, et al. A virus-like particle vaccine prevents equine encephalitis virus infection in nonhuman primates. Sci Transl Med 2019; 11:eaav3113. [DOI] [PubMed] [Google Scholar]
- 60. Akahata W, Yang ZY, Andersen H, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med 2010; 16:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chang LJ, Dowd KA, Mendoza FH, et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet 2014; 384:2046–52. [DOI] [PubMed] [Google Scholar]
- 62. Mallilankaraman K, Shedlock DJ, Bao H, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis 2011; 5:e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dupuy LC, Richards MJ, Livingston BD, Hannaman D, Schmaljohn CS. A multiagent alphavirus DNA vaccine delivered by intramuscular electroporation elicits robust and durable virus-specific immune responses in mice and rabbits and completely protects mice against lethal Venezuelan, western, and eastern equine encephalitis virus aerosol challenges. J Immunol Res 2018; 2018:8521060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shaw C, Panther L, August A, et al. Safety and immunogenicity of a mRNA-based chikungunya vaccine in a phase 1 dose-ranging trial. Int J Infect Dis 2019; 79:17. [Google Scholar]
- 65. Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science 1991; 254:856–60. [DOI] [PubMed] [Google Scholar]
- 66. Griffin D. Roles and reactivities of antibodies to alphaviruses. Semin Virol 1995; 6:249–55. [Google Scholar]
- 67. Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev 1997; 159:155–61. [DOI] [PubMed] [Google Scholar]
- 68. Metcalf TU, Baxter VK, Nilaratanakul V, Griffin DE. Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. J Virol 2013; 87:2420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rabinowitz SG, Adler WH. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. I. Passive transfer of protection with immune serum and immune cells. J Immunol 1973; 110:1345–53. [PubMed] [Google Scholar]
- 70. Couderc T, Khandoudi N, Grandadam M, et al. Prophylaxis and therapy for chikungunya virus infection. J Infect Dis 2009; 200:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kose N, Fox JM, Sapparapu G, et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci Immunol 2019; 4:eaaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. August A, Attarwala HZ, Himansu S, et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against chikungunya virus. Nat Med 2021; 27:2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mendoza QP, Stanley J, Griffin DE. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J Gen Virol 1988; 69(Pt 12):3015–22. [DOI] [PubMed] [Google Scholar]
- 74. Long F, Fong RH, Austin SK, et al. Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity. Proc Natl Acad Sci U S A 2015; 112:13898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williamson LE, Gilliland T Jr, Yadav PK, et al. Human antibodies protect against aerosolized eastern equine encephalitis virus infection. Cell 2020; 183:1884–1900.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fox JM, Long F, Edeling MA, et al. Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell 2015; 163:1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Earnest JT, Basore K, Roy V, et al. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J Exp Med 2019; 216:2282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Powell LA, Miller A, Fox JM, et al. Human mAbs broadly protect against arthritogenic alphaviruses by recognizing conserved elements of the Mxra8 receptor-binding site. Cell Host Microbe 2020; 28:699–711.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pereboev AV, Razumov IA, Svyatchenko VA, Loktev VB. Glycoproteins E2 of the Venezuelan and eastern equine encephalomyelitis viruses contain multiple cross-reactive epitopes. Arch Virol 1996; 141:2191–205. [DOI] [PubMed] [Google Scholar]
- 80. Smith JL, Pugh CL, Cisney ED, et al. Human antibody responses to emerging Mayaro virus and cocirculating alphavirus infections examined by using structural proteins from nine new and old world lineages. mSphere 2018; 3:e00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhou QF, Fox JM, Earnest JT, et al. Structural basis of chikungunya virus inhibition by monoclonal antibodies. Proc Natl Acad Sci U S A 2020; 117:27637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jin J, Simmons G. Antiviral functions of monoclonal antibodies against chikungunya virus. Viruses 2019; 11:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jin J, Liss NM, Chen DH, et al. Neutralizing monoclonal antibodies block chikungunya virus entry and release by targeting an epitope critical to viral pathogenesis. Cell Rep 2015; 13:2553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Selvarajah S, Sexton NR, Kahle KM, et al. A neutralizing monoclonal antibody targeting the acid-sensitive region in chikungunya virus E2 protects from disease. PLoS Negl Trop Dis 2013; 7:e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smith SA, Silva LA, Fox JM, et al. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe 2015; 18:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim AS, Austin SK, Gardner CL, et al. Protective antibodies against eastern equine encephalitis virus bind to epitopes in domains A and B of the E2 glycoprotein. Nat Microbiol 2019; 4:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boere WA, Harmsen T, Vinje J, Benaissa-Trouw BJ, Kraaijeveld CA, Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol 1984; 52:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hunt AR, Roehrig JT. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of western equine encephalitis virus. Virology 1985; 142:334–46. [DOI] [PubMed] [Google Scholar]
- 89. Pal P, Dowd KA, Brien JD, et al. Development of a highly protective combination monoclonal antibody therapy against chikungunya virus. PLoS Pathog 2013; 9:e1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Williamson LE, Reeder KM, Bailey K, et al. Therapeutic alphavirus cross-reactive E1 human antibodies inhibit viral egress. Cell 2021; 184:4430–46.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim AS, Kafai NM, Winkler ES, et al. Pan-protective anti-alphavirus human antibodies target a conserved E1 protein epitope. Cell 2021; 184:4414–29.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ahn A, Klimjack MR, Chatterjee PK, Kielian M. An epitope of the Semliki Forest virus fusion protein exposed during virus-membrane fusion. J Virol 1999; 73:10029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li L, Jose J, Xiang Y, Kuhn RJ, Rossmann MG. Structural changes of envelope proteins during alphavirus fusion. Nature 2010; 468:705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sahoo B, Gudigamolla NK, Chowdary TK. Acidic pH-induced conformational changes in chikungunya virus fusion protein E1: a spring-twisted region in the domain I-III linker acts as a hinge point for swiveling motion of domains. J Virol 2020; 94:e01561–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meyer WJ, Gidwitz S, Ayers VK, Schoepp RJ, Johnston RE. Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH. J Virol 1992; 66:3504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schmaljohn AL, Kokubun KM, Cole GA. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology 1983; 130:144–54. [DOI] [PubMed] [Google Scholar]
- 97. Fuller SD, Berriman JA, Butcher SJ, Gowen BE. Low pH induces swiveling of the glycoprotein heterodimers in the Semliki Forest virus spike complex. Cell 1995; 81:715–25. [DOI] [PubMed] [Google Scholar]
- 98. Gibbons DL, Ahn A, Liao M, Hammar L, Cheng RH, Kielian M. Multistep regulation of membrane insertion of the fusion peptide of Semliki Forest virus. J Virol 2004; 78:3312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fong RH, Banik SS, Mattia K, et al. Exposure of epitope residues on the outer face of the chikungunya virus envelope trimer determines antibody neutralizing efficacy. J Virol 2014; 88:14364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Quiroz JA, Malonis RJ, Thackray LB, et al. Human monoclonal antibodies against chikungunya virus target multiple distinct epitopes in the E1 and E2 glycoproteins. PLoS Pathog 2019; 15:e1008061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fox JM, Roy V, Gunn BM, et al. Optimal therapeutic activity of monoclonal antibodies against chikungunya virus requires Fc-FcgammaR interaction on monocytes. Sci Immunol 2019; 4:eaav5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rezza G, Weaver SC. Chikungunya as a paradigm for emerging viral diseases: evaluating disease impact and hurdles to vaccine development. PLoS Negl Trop Dis 2019; 13:e0006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Powers AM. Vaccine and therapeutic options to control chikungunya virus. Clin Microbiol Rev 2017; 31:e00104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, Ferro C, Haddow AD, Weaver SC. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol 2011; 6:721–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kuhn RJ, Griffin DE, Owen KE, Niesters HG, Strauss JH. Chimeric Sindbis-Ross River viruses to study interactions between alphavirus nonstructural and structural regions. J Virol 1996; 70:7900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I. Recombinant Sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol 2003; 77:9278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Erasmus JH, Auguste AJ, Kaelber JT, et al. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat Med 2017; 23:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hallengard D, Kakoulidou M, Lulla A, et al. Novel attenuated chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J Virol 2014; 88:2858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Volkova E, Frolova E, Darwin JR, Forrester NL, Weaver SC, Frolov I. IRES-dependent replication of Venezuelan equine encephalitis virus makes it highly attenuated and incapable of replicating in mosquito cells. Virology 2008; 377:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]