Abstract
The mechanism of interfacial folding and membrane insertion of designed peptides is explored by using an implicit membrane generalized Born model and replica-exchange molecular dynamics. Folding/insertion simulations initiated from fully extended peptide conformations in the aqueous phase, at least 28 Å away from the membrane interface, demonstrate a general mechanism for structure formation and insertion (when it occurs). The predominately hydrophobic peptides from the synthetic WALP and TMX series first become localized at the membrane-solvent interface where they form significant helical secondary structure via a helix–turn–helix motif that inserts the central hydrophobic residues into the membrane interior, and then fluctuations occur that provide a persistent helical structure throughout the peptide and it inserts with its N-terminal end moving across the membrane. More specifically, we observed that: (i) the WALP peptides (WALP16, WALP19, and WALP23) spontaneously insert in the membrane as just noted; (ii) TMX-1 also inserts spontaneously after a similar mechanism and forms a transmembrane helix with a population of ≈50% at 300 K; and (iii) TMX-3 does not insert, but exists in a fluctuating membrane interface-bound form. These findings are in excellent agreement with available experimental data and demonstrate the potential for new implicit solvent/membrane models together with advanced simulation protocols to guide experimental programs in exploring the nature and mechanism of membrane-associated folding and insertion of biologically important peptides.
Keywords: implicit solvation, replica-exchange molecular dynamics, TMX-1, TMX-3, WALP
Biological membranes provide a unique hydrophilic and hydrophobic environment in which a protein may undergo a thermodynamically driven conformational transition from a water-soluble form to a membrane-bound state (1–3). Membrane insertion is a key process in the biological functioning of peptides and proteins such as bacterial toxins (4), antimicrobial peptides (5), and fusion peptides (6). Because of the importance of the processes of membrane association and insertion to our understanding of function in these systems, it is essential to understand the thermodynamic balance between the water-soluble state and the membrane-bound state.
The conformational and energetic changes of these peptides/proteins at the molecular level during the spontaneous insertion process, generally, remain poorly understood. However, recent experiments using designed (synthetic) model peptides have yielded new insights into these events (6–11). However, a principal experimental difficulty in designing peptides to study spontaneous insertion arises from the insoluble and aggregation-prone nature of these highly nonpolar molecules in aqueous solution (8, 11). Considering the biological importance of these systems and the processes that control their functioning, modern methods from theory and computational biology should aim to assist experiments in understanding membrane insertion at the molecular level.
Computational approaches using a detailed atomistic representation of the aqueous/lipid environment are attractive and of considerable current interest (12, 13) in considering questions involving membrane-associated peptides and proteins, and particular successes are evident (14, 15). However, the straightforward application of these techniques to the spontaneous insertion process is severely inhibited, mainly because of the prohibitive computational cost associated with the fully detailed representation, even though recent calculations suggest that such brute-force simulations may be possible on a limited scale (16).
An alternative to atomically detailed models are coarse-grain approaches that use simplified aqueous/lipid environments. A number of such models have been developed and proven useful in lending insight into aspects of membrane insertion (17, 18). Examples include a simplified peptide/membrane model in which a linked chain of hard spheres interacts with a mean-field lipid bilayer within a mean-field approximation for the bilayer (19, 20). Using similar models, Longo and coworkers performed Monte Carlo simulations to study the insertion process for two membrane peptides, Magainin2 and M2δ (17), an HIV-1 fusion peptide and related mutants (21), as well as the Alzheimer's amyloid β peptide and several of its mutants (22). However, the nature of their model restricted their exploration to short monomeric helix-forming peptides. Lopez et al. (18) performed molecular dynamics (MD) simulations of a modified nanotube using a coarse-grain model for water and lipid molecules as well as the nanotube, and they found that the insertion of the nanotube was assisted by transleaflet lipid flips. The application of these approaches has provided insights into aspects of peptide association with and insertion into biological membranes; however, they have been limited either in the scope of systems one can study or the detail in which processes can be explored.
Recently, we extended the generalized Born (GB) continuum electrostatics theory to include the mean influence of both water and the biological membrane implicitly (23, 24). This approach enables the study of many of the problems noted above with a fully atomically detailed model of the peptide or protein. By combining our membrane GB model with advanced computational sampling methods, like replica-exchange (REX) MD (25, 26), we have shown that it is possible to fold and assemble simple helical membrane peptides (24) and predict the structure of small membrane-bound proteins, while reproducing the solid-state NMR properties reasonably well (27). In the present study, we apply these theoretical models and computational methods to membrane interfacial folding and insertion of designed peptides within the WALP (10) and TMX (8, 11) series (see Table 1).
Table 1. Amino acid sequences of WALP and TMX peptides.
| Peptides | Sequence | |||
|---|---|---|---|---|
| WALP16 | GWWLALALAL | ALAWWA | ||
| WALP19 | GWWLALALAL | ALALALWWA | ||
| WALP23 | GWWLALALAL | ALALALALAL | WWA | |
| TMX-1 | WNALAAVAAA | LAAVAAALAA | VAASKSKSKS | K |
| TMX-3* | GGWAALAAHA | APALAAALAH | AAASRSRSRS | R |
| TMX-3s* | GGWAALAAHA | APALAAALAH | AAASR | |
| TMX-3sH2L | GGWAALAALA | ALALAAALAL | AAASR | |
| TMX-3sA2L* | GGWLLLLLHL | LPLLLLLLLH | LLLSR | |
The N terminus of each peptide is blocked by an acetyl group and its C terminus by an N-methyl amide group.
The His residues in TMX-3 and its mutants are modeled as a neutral form.
The WALP peptides developed by Killian and coworkers (10, 28) comprise a series of synthetic tryptophan-flanked transmembrane (TM) peptides with different hydrophobic lengths that were designed to study the influence of hydrophobic mismatch on peptide backbone structure, orientation, and extent of membrane incorporation. These peptides are water-insoluble and tend to aggregate in aqueous solution. When codissolved with lipid molecules in organic solvent, however, the WALP peptides are incorporated in membranes and form continuous α-helices (28). White and coworkers have designed synthetic TM peptides called TMX-1 (8) and TMX-3 (11) to study interfacial folding and membrane insertion and related energetics. TMX-1 forms an aggregate in solution under all experimentally attainable conditions, but inserts at low concentrations. In contrast, TMX-3 exists in an unordered (monomeric) conformation and prefers to stay at the membrane interface at low concentrations, whereas it inserts across the bilayer and adopts a helical TM conformation at high concentrations. The aims of the present study are to explore the folding and insertion mechanism of these systems and to examine the efficacy of our membrane GB model and validate its accuracy for studying interfacial folding and membrane insertion. Finally, we will suggest a possible route to TMX-3 variants that spontaneously insert even at low concentrations.
Methods
In Table 1 the sequences of the peptides studied in this work are given. The WALP series of peptides consist of sequences of highly hydrophobic residues (26), whereas the TMX-1 and TMX-3 family contain a number of polar and basic residues that enhance the solubility. Additionally, the TMX-3 peptides developed by Ladokhin and White (11) possess a central proline residue. In addition to the peptides already studied experimentally, we examine the properties of TMX-3 variants, the TMX3s series.
Our studies were performed by using the gbsw module (a GB model with a simple switching function) (23, 24) in the charmm biomolecular simulation program (29). The formalism and implementation of the membrane GB model, based on a numerical volume integration method (30), have been described (23, 24). All MD simulations were performed with a time step of 2 fs by using the all-atom parameter set param22 for proteins (31), including dihedral cross-term corrections (32). A 20-Å cutoff distance was applied to both the nonbonded interactions and the GB terms. For the GB calculations we used a smoothing length of 0.6 Å at the dielectric boundary, defined by the optimized Poisson–Boltzmann atomic radii for proteins (33, 34), with 24 radial integration points up to 20 Å and 38 angular integration points (23, 24).
The physical parameters representing the membrane in our GB model used 0.04 kcal/(mol·Å2) for the surface tension coefficient, representing the nonpolar solvation energy, 23 Å (WALP peptides) and 25 Å (TMX peptides) for the thickness of the membrane hydrophobic core, and 5 Å for a membrane smoothing length over which the hydrophobic region is gradually changed to the solvent region. The planar membrane is perpendicular to the Z axis and centered at Z = 0. It should be noted that no parameters were optimized for the insertion simulations.
The starting configuration of each peptide was generated by positioning a fully extended conformation (in the XY plane) at Z = 45 Å, i.e., ≈28 Å away from the membrane interface. To increase conformational space sampling efficiency we used 32 replicas that were distributed over an exponentially spaced temperature range from 300 to 800 K (WALP and TMX-3s peptides) or 1,000 K (TMX-1 and TMX-3). Langevin dynamics with a friction coefficient of 5.0 ps–1 for heavy atoms was used to maintain the target temperatures and ensure random drifts of the peptides. To prevent the peptides from drifting away from the bilayer, periodic boundary conditions were applied with an edge dimension of 110 Å.
The REX simulations lasted 14 ns (WALP16), 18 ns (WALP19), 20 ns (WALP23), and 30 ns (TMX peptides). A REX step was attempted every 2 ps; the pairwise exchange ratio was >40% for each run. The MMTSB Tool Set (35), which is available from http://mmtsb.scripps.edu, was used to control the REX simulations. The coordinates were saved every 2 ps for further analysis.
We note that our simulations do not attempt to account for the temperature-dependent properties of the bilayer and aqueous phase, and thus membrane dissolution does not occur. Furthermore, the viscosity of the membrane/aqueous-phase environment is not modeled, and thus the time scale of insertion and interfacial folding events are distorted. However, for the purposes of exploring the insertion mechanism and conformational changes ≈300 K, where the membrane and aqueous-phase model parameters are most appropriate, the present model should be adequate.
Results and Discussion
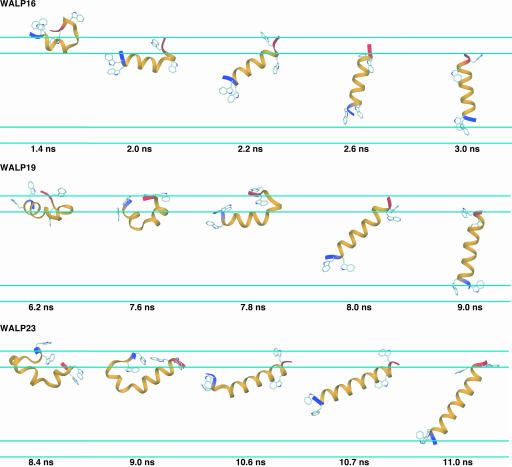
Membrane Insertion of the WALP Peptides. Three main features dominate the mechanism by which the WALP peptides insert into the membrane environment from an aqueous phase. First, as shown in Fig. 1, α-helix formation is enhanced at the membrane interface before complete insertion, such that the backbone atoms are not directly exposed to the low dielectric hydrophobic region (for the insertion process see Movies 1–3, which are published as supporting information on the PNAS web site). Complete insertion requires that >80% of the sequence forms a continuous α-helix. Second, formation of a continuous α-helix occurs by thermal fluctuations of α-helical hairpin conformations at the interface or partially buried in the membrane. WALP16 and WALP23 slightly vary in their insertion properties with regard to the burial of the peptide into the low-dielectric environment before full incorporation. The α-helical hairpin conformation resides mostly at the membrane interface for WALP16, whereas in WALP23 the α-helical hairpin dips deeply into the membrane with both termini positioned at the membrane interface (most likely because of its extensive hydrophobic central core, see Fig. 1). Third, insertion always occurs from the N terminus. In fact, we observed many cases in which the insertion is delayed until the N terminus forms an α-helical conformation (data not shown). Clearly, preferential insertion of the N terminus does not arise from a particular amino acid distribution because the sequences of the WALP peptides are nearly symmetric (see Table 1). One can rationalize this observation in terms of the solvation preference of both termini; carbonyl groups at the C terminus have larger dipole moments and thus desolvation is disfavored compared with the amide groups of the N terminus. This argument is consistent with previous experimental and theoretical studies indicating that helical stability is greater at the N terminus than the C terminus (36).
Fig. 1.
Characteristic conformations for one replica of each of the WALP peptides illustrate the conformational and configurational changes that occur during membrane insertion. The N terminus is blue, and the C terminus is red. Trp residues are shown in ball-and-stick representations, and the cyan lines represent the 5-Å membrane smoothing regions.
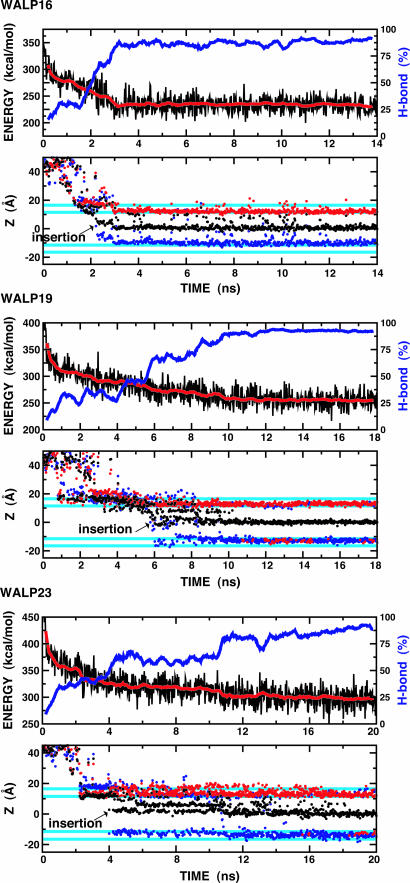
The convergence and time course of the insertion simulations can be examined by monitoring the total potential energy at the lowest temperature (300 K) of the replica ensemble. As shown in Fig. 2, the efficient REX sampling technique drives a rapid configurational change over the initial 2 ns of the simulations, with a quasi-static state being reached after ≈3 ns (WALP16), 10 ns (WALP19), or 14 ns (WALP23). The potential energy profiles appear to correlate well with the fraction of α-helical hydrogen bonds (H bonds) formed by peptide replicas occupying the lowest-temperature ensemble; most structures in this state are >90% H-bonded (a “continuous α-helix”). Fig. 2 clearly shows that all three WALP peptides spontaneously insert in the membrane and form continuous TM α-helices. This finding is consistent with the experimental data that showed 90% insertion for all three peptides in a lipid of 23-Å hydrophobic thickness (28).
Fig. 2.
Time series for several properties for each WALP peptide from the lowest temperature (300 K) ensemble during the REX simulations. (Upper) Profiles of total potential energy (black) as a function of time and its running average over 400-ps windows. The blue lines represent a running average of backbone H-bond fractions, defined as the number of H bonds in each configuration divided by the total number of H bonds in a continuous α-helix. Hydrogen bonds are defined by dOiHNi+4 ≤ 2.8 Å and 120° ≥ θO-H-N ≤ 180°, where dOiHNi+4 is the distance between the carbonyl oxygen of residue i, Oi, and the amide hydrogen of residue i + 4, HNi+4, and θO-H-N is the angle between Oi, HNi+4, and Ni+4. (Lower) Time series of the Z component of the center of mass of each peptide (black) and the Z coordinates of Cα atoms of the first (blue) and last (red) residue of each peptide. The cyan lines represent the 5-Å membrane smoothing region over which the hydrophobic region is gradually changed to the solvent region. The arrow in each plot indicates the insertion point during the simulations.
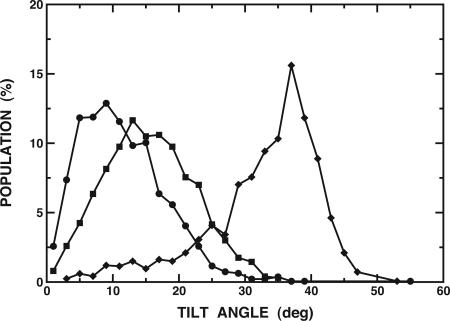
The average distances between first and last Cα atoms of WALP peptides in TM α-helical conformations are ≈22.5 Å (WALP16), 27.0 Å (WALP19), and 33.0 Å (WALP23). Because the membrane hydrophobic thickness is 23 Å in the present simulations, there is a certain “hydrophobic mismatch” for WALP19 and WALP23. Each peptide responds to this mismatch by tilting its helical access relative to the membrane normal. The extent of helical tilt and its distribution were calculated and are shown in Fig. 3. The average tilt angles and fluctuations are 11.5 ± 6.4° (WALP16), 15.5 ± 6.7° (WALP19), and 32.7 ± 8.5° (WALP23). The tilt angles show a strong dependence on the membrane hydrophobic thickness in our membrane model (24), i.e., WALP19 and WALP23 tilted more than WALP16 to overcome the energetically unfavorable mismatch. In contrast, the available experimental data suggest that the tilt angles are quite similar to each other and relatively small (≈5°) in a lipid bilayer of ≈23-Å hydrophobic thickness (37). In the case of WALP23, the experimental tilt angle changes from 5.5° in a 23-Å hydrophobic thickness lipid to 8.2° in a 19.5-Å lipid, suggesting that tilting of these peptides in a lipid bilayer may be energetically unfavorable (37). Although it might be difficult to directly compare these experimental data with the present simulation results because of unknown oligomer states (37), we speculate that the WALP peptides under the conditions of the experiments may exist as an oligomer, because the dependence on the membrane thickness of the tilt angle of an oligomer appears to be much less sensitive than in monomeric peptides (24, 38). Clearly, more experiments are required to resolve this issue.
Fig. 3.
Distribution of the helical tilt for WALP16 (•), WALP19 (▪), and WALP23 (♦) at 300 K, calculated from the last 4 ns of each REX simulation. The average tilt and fluctuations are 11.5 ± 6.4° (WALP16), 15.5 ± 6.7° (WALP19), and 32.7 ± 8.5° (WALP23). The tilt angle is defined by the angle between the membrane interface and the principal axis of the backbone atoms.
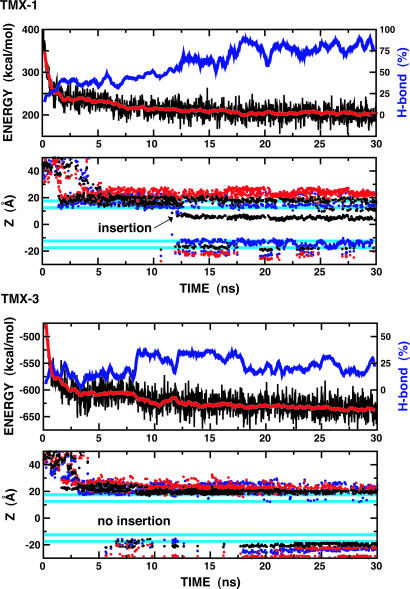
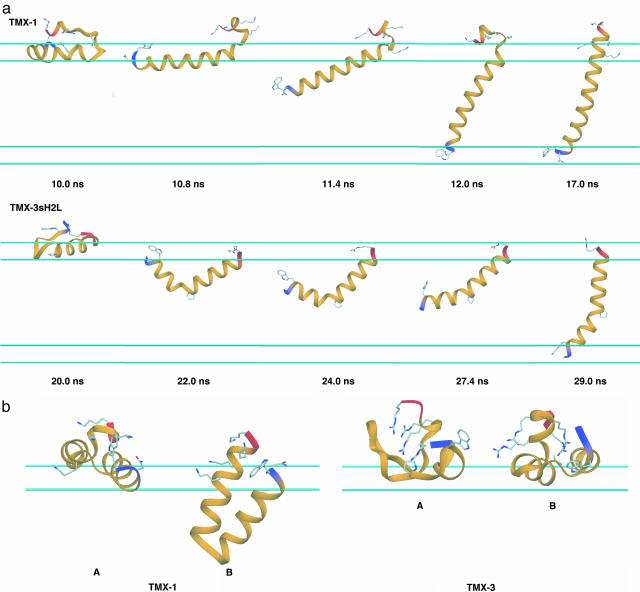
Interfacial Folding and Insertion of the TMX Peptides. TMX-1 spontaneously inserts across the membrane and forms a TM α-helix during our simulations, as shown in Fig. 4. Similar to the WALP peptides, its potential energy profile appears to correlate well with the fraction of H bonds formed and a continuous α-helix comprising >80% of the sequence is required for complete insertion. Complete insertion occurred for only a fraction of the replicas in this system, which shared a common mechanism as shown in Fig. 5a. Despite its size, the insertion mechanism for TMX-1 is similar to that observed for WALP16. Although TMX-1 has a polar Asn residue near the N terminus, N-terminal insertion occurs preferentially, in part because of the Lys-Ser repeats at the C terminus. Because the C-terminal-led insertion appears to be very difficult, as shown in the WALP peptides, it will be interesting to explore the influence of reversing the amino acid sequence of TMX-1. In the quasi-static state (after ≈18 ns, see Fig. 4), the lowest-temperature ensemble contains 52% TM α-helical conformations and 48% noninserted α-helical hairpin conformations at the interface (A) or in the membrane (B), as shown in Fig. 5b. The average potential energy values of these conformations (averaged over 1 ns of dynamics and relative to the TM state) are 0.0 ± 14.9 (TM), 5.8 ± 14.9 (A in Fig. 5b), and 5.0 ± 14.9 (B in Fig. 5b) kcal/mol, respectively. Although more extensive calculations are required to determine the free energy difference, including entropic contributions, these simple calculations demonstrate that both inserted and noninserted forms are thermodynamically accessible at 300 K. Interestingly, experiment suggests that at least 50% of TMX-1 inserts spontaneously across vesicle membranes at low concentrations and its conformation is predominantly α-helical for both the inserted and noninserted interface-bound forms (8), which is consistent with our observations. This agreement, together with the results displayed in Fig. 5, leads us to suggest that the stable interface-bound conformations may not be straight α-helices. In fact, a straight α-helix is only observed as a transient conformation to a TM structure and its formation may be a rate-determining step in the TMX-1 insertion.
Fig. 4.
Time series of total potential energy, backbone H-bond fractions, and Z coordinates for the TMX-1 and TMX-3 peptides at the lowest temperature (300 K). The same color scheme and calculation methods as in Fig. 2 are used.
Fig. 5.
Insertion and interfacial folding of the TMX peptides. (a) Characteristic conformations for one replica of TMX-1 and TMX-3sH2L to illustrate the conformational and configurational changes during membrane insertion. The N terminus is blue, and the C terminus is red. Asn, Trp, and Lys residues of TMX-1 and Trp, Pro, and Arg residues of TMX-3sH2L are shown as ball-and-stick models. The cyan lines represent the 5-Å membrane smoothing regions. (b) Interfacial conformations of TMX-1 and TMX-3. At the membrane interface, TMX-1 shows both highly helical surface-bound (A) or membrane-bound (B) α-helical hairpin conformations. TMX-3 shows both very low helical (A, ≈7% H bond) and relatively high helical (A, ≈44% H bond) conformations. See Movies 4 and 5, which are published as supporting information on the PNAS web site.
In contrast to TMX-1, TMX-3 does not insert, but exists mostly in a membrane interface-bound form, as shown in Fig. 4. Its potential energy profile does not correlate well with the fraction of H bonds formed. While the H-bond frequency oscillates, the potential energy continues to decrease slowly even after 30 ns of simulation for each replica. Fig. 5b shows two representative conformations from the lowest-temperature ensemble, e.g., conformation A has very low helicity (≈7%) and conformation B has relatively high helicity (≈44%). Experimentally, TMX-3 is known to exist in an unordered (monomeric) conformation with helix content of ≈25% in aqueous solution and prefers to reside at the membrane interface without inserting, at low peptide concentrations (11). The helical content of the interface-bound conformation was reported to be ≈70% for negatively charged membranes (11). Thus, our TMX-3 simulation is consistent with experiment in terms of the insertion event, but the absolute helicity in the surface-bound form appears to be lower than that from experiment. Simulation of the shorter C-terminal peptide (TMX-3s, see Table 1 and Fig. 6, which is published as supporting information on the PNAS web site) showed enhanced helicity, up to 58% on average, at the membrane interface but still did not insert. Based on the TMX-1 and TMX-3s simulations, we suggest that the four C-terminal Arg residues in TMX-3 discourage α-helix formation at the membrane interface, at least in our model.
Designing a peptide sequence showing a specific partitioning ratio between TM or interface-bound conformations requires a knowledge of the thermodynamic balance between its water-soluble, interface-bound, and TM states. In this context, TMX-3 represents a good starting peptide for the development of these thermodynamic data. In the present study, instead of TMX-3, TMX-3s was used to generate two mutants (TMX-3sH2L and TMX-3sA2L, see Table 1) because of the aforementioned C-terminal Arg residues in TMX-3. This modification is fully justified by the fact that Lys or Arg residues are used only to provide aqueous-phase solubility. Our simulations show that two His-to-Leu substitutions in TMX-3s (TMX-3sH2L) were sufficient to induce insertion of this peptide (see Fig. 6). As shown in Fig. 5A, the insertion mechanism is quite similar to WALP23. Structures from the lowest-temperature ensemble after 28 ns contain 60% noninserted α-helical hairpin conformations and 40% TM α-helical conformations (nearly the same ratio persisted in simulations up to 42 ns). Based on the Wimley–White interfacial hydrophobicity scale (7), Ladokhin and White (11) estimated that seven Ala-to-Leu substitutions in TMX-3 would result in TMX-3's interfacial/TM ratio to shift to 1:60, favoring membrane insertion. To avoid ambiguity in choosing the Ala to be mutated, simulations were performed with all 14 Ala-to-Leu mutations (TMX-3sA2L). The result is shown in Fig. 6. TMX-3sA2L spontaneously inserted into the membrane with a similar mechanism as seen for TMX-3sH2L and yielded an interfacial/TM ratio of 17:83 in the lowest-temperature ensemble (after 25 ns). This ratio is in qualitative agreement with their prediction.
Conclusions
We have explored membrane insertion and interfacial folding for the WALP and TMX series of peptides by using REX-MD and an implicit membrane GB model. All three WALP peptides (WALP16, WALP19, and WALP23) showed spontaneous N-terminal-led insertion through the formation of a continuous α-helix arising from thermal fluctuations of α-helical hairpin conformations formed at the interface or in the membrane. A similar insertion mechanism was observed for the TMX peptides. In most simulations, a straight α-helix was not observed as a stable interface-bound conformation but existed as a transient conformation before membrane insertion as a TM helix. We suggest that the formation of such a straight α-helix may be a rate-determining step in insertion.
The behavior of TMX-1 and TMX-3 was markedly different. Consistent with experiment, TMX-1 spontaneously inserted and formed a TM helix whose population was ≈50% at 300 K, and TMX-3 did not insert, but existed predominately as membrane interface-bound conformations. The conformation associated with TMX-1 or TMX-3 (TMX-3s) during the REX simulations suggest a revision in traditional membrane insertion/association free energy calculations is necessary to include the influence the conformational changes that occur between peptide-membrane association and insertion, i.e., the presence of helical hairpins at the interface. A more detailed free energy description of this process could be obtained by combining extensive umbrella sampling methods with the current implicit solvent and membrane GB model and REX MD. Furthermore, pH-dependent free energy profiles could be obtained by combining these techniques with constant pH MD simulations (39).
Our calculations provide predictions of the interface/TM ratio for two TMX-3 mutants, i.e., two His-to-Leu substitutions and all 14 Ala-to-Leu mutations. Future experiments should help clarify whether these predictions are accurate. Such comparisons will aid us in characterizing the molecular-level conformational changes and related energetics of these peptides during spontaneous membrane insertion or surface binding.
Supplementary Material
Acknowledgments
We thank Angel E. Garcia for stimulating discussions that led us to initiate this work and Jianhan Chen and Alexey S. Ladokhin for their helpful comments. This work was supported by National Institutes of Health Grant RR12255 and the Center for Theoretical Biological Physics through National Science Foundation Grant PHY0216576. Support for computational infrastructure from Department of Defense Grant DAMD17-03-2-0012 is greatly appreciated.
Author contributions: C.L.B. designed research; W.I. performed research; and W.I. and C.L.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MD, molecular dynamics; GB, generalized Born; REX, replica exchange; TM, transmembrane.
References
- 1.White, S. H. & Wimley, W. C. (1999) Annu. Rev. Biophys. Biomol. 28, 319–365. [DOI] [PubMed] [Google Scholar]
- 2.Popot, J. L. & Engelman, D. M. (2000) Annu. Rev. Biochem. 69, 881–922. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain, A. K., Faham, S, Yohannan, S. & Bowie, J. U. (2003) Adv. Protein Chem. 63, 19–46. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt, C. K., Meysick, K. C. & O'Brien, A. D. (1999) Emerg. Infect. Dis. 5, 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zasloff, M. (2002) Nature 415, 389–395. [DOI] [PubMed] [Google Scholar]
- 6.Han, X., Bushweller, J. H., Cafiso, D. S. & Tamm, L. K. (2001) Nat. Struct. Biol. 8, 715–720. [DOI] [PubMed] [Google Scholar]
- 7.Wimley, W. C. & White, S. H. (1996) Nat. Struct. Biol. 3, 842–848. [DOI] [PubMed] [Google Scholar]
- 8.Wimley, W. C. & White, S. H. (2000) Biochemistry 39, 4432–4442. [DOI] [PubMed] [Google Scholar]
- 9.Ladokhin, A. S. & White, S. H. (2001) J. Mol. Biol. 309, 543–552. [DOI] [PubMed] [Google Scholar]
- 10.de Planque, M. R. R. & Killian, J. A. (2003) Mol. Membr. Biol. 20, 271–284. [DOI] [PubMed] [Google Scholar]
- 11.Ladokhin, A. S. & White, S. H. (2004) Biochemistry 43, 5782–5791. [DOI] [PubMed] [Google Scholar]
- 12.Roux, B. (2002) Curr. Opin. Struct. Biol. 12, 182–189. [DOI] [PubMed] [Google Scholar]
- 13.Im, W. & Roux, B. (2002) J. Mol. Biol. 319, 1177–1197. [DOI] [PubMed] [Google Scholar]
- 14.Bernèche, S. & Roux, B. (2001) Nature 414, 73–77. [DOI] [PubMed] [Google Scholar]
- 15.Tieleman, D. P., Leontiadou, H., Mark, A. E. & Marrink, S. J. (2003) J. Am. Chem. Soc. 125, 6382–6383. [DOI] [PubMed] [Google Scholar]
- 16.Nymeyer, H., Woolf, T. & Garcia, A. (2005) Proteins, in press. [DOI] [PubMed]
- 17.Maddox, M. W. & Longo, M. L. (2002) Biophys. J. 82, 244–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez, C. F., Nielsen, S. O., Moore, P. B. & Klein, M. L. (2004) Proc. Natl. Acad. Sci. USA 101, 4431–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milik, M. & Skolnick, J. (1993) Proteins 15, 10–25. [DOI] [PubMed] [Google Scholar]
- 20.Baumgaertner, A. (1996) Biophys. J. 71, 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maddox, M. W. & Longo, M. L. (2002) Biophys. J. 83, 3088–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mobley, D. L., Cox, D. L., Singh, R. R., Longo, M. W. & Maddox M. L. (2004) Biophys. J. 86, 3585–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Im, W., Lee, M. S. & Brooks, C. L., III (2003) J. Comput. Chem. 24, 1691–1702. [DOI] [PubMed] [Google Scholar]
- 24.Im, W., Feig, M. & Brooks, C. L., III (2003) Biophys. J. 85, 2900–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansmann, U. H. E. (1997) Chem. Phys. Lett. 281, 140–150. [Google Scholar]
- 26.Sugita, Y. & Okamoto, Y. (1999) Chem. Phys. Lett. 314, 141–151. [Google Scholar]
- 27.Im, W. & Brooks, C. L., III (2004) J. Mol. Biol. 337, 513–519. [DOI] [PubMed] [Google Scholar]
- 28.de Planque, M. R. R., Goormaghtigh, E., Greathouse, D. V., Koppe, R. E., II, Kruijtzer, J. A. W., Liskamp, R. M. J., de Kruijff, B. & Killian, J. A. (2001) Biochemistry 40, 5000–5100. [DOI] [PubMed] [Google Scholar]
- 29.Brooks, B. R., Bruccoleri, R. E., Olafson, B. D., States, D. J., Swaminathan, S. & Karplus, M. (1983) J. Comput. Chem. 4, 187–217. [Google Scholar]
- 30.Lee, M. S., Salsbury, F. R., Jr., & Brooks, C. L., III (2002) J. Chem. Phys. 116, 10606–10614. [Google Scholar]
- 31.MacKerell, A. D., Jr., Bashford, D., Bellot, M., Dunbrack, R. L., Evanseck, J. D., Field, M. J., Fischer, S., Gao, J., Guo, H., Ha, S., et al. (1998) J. Phys. Chem. B 102, 3586–3616. [DOI] [PubMed] [Google Scholar]
- 32.MacKerell, A. D., Jr., Feig, M. & Brooks, C. L., III (2004) J. Comput. Chem. 25, 1400–1415. [DOI] [PubMed] [Google Scholar]
- 33.Nina, M., Beglov, D. & Roux, B. (1997) J. Phys. Chem. B 101, 5239–5248. [Google Scholar]
- 34.Nina, M., Im, W. & Roux, B. (1999) Biophys. Chem. 78, 89–96. [DOI] [PubMed] [Google Scholar]
- 35.Feig, M., Karanicola, J. & Brooks, C. L., III (2004) J. Comput. Graph. Model. 22, 3777–3795. [Google Scholar]
- 36.Young, W. S. & Brooks, C. L., III (1996) J. Mol. Biol. 259, 560–572. [DOI] [PubMed] [Google Scholar]
- 37.Strandberg, E., Ozdirekcan, S., Rijkers, D. T. S., van der Wel, P. C. A., Koeppe, R. E., II, Liskamp, R. M. J. & Killian, J. A. (2004) Biophys. J. 86, 3709–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovacs, F. A., Denny, J. K., Song, Z., Quine, J. R. & Cross, T. A. (2000) J. Mol. Biol. 295, 117–125. [DOI] [PubMed] [Google Scholar]
- 39.Lee, M. S., Salsbury, F. R., Jr., & Brooks, C. L., III (2004) Proteins 56, 738–752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.