Abstract
Colorectal cancer (CRC) is the third most common cancer worldwide. Although the overall incidence of CRC has been decreasing over the past 40 y, early-onset colorectal cancer (EOCRC), which is defined as a CRC diagnosis in patients aged >50 y has increased. In this Perspective, we highlight and summarize the association between diet quality and excess adiposity, and EOCRC. We also explore chronic psychosocial stress (CPS), a less investigated modifiable risk factor, and EOCRC. We were able to show that a poor-quality diet, characterized by a high intake of sugary beverages and a Western diet pattern (high intake of red and processed meats, refined grains, and foods with added sugars) can promote risk factors associated with EOCRC development, such as an imbalance in the composition and function of the gut microbiome, presence of chronic inflammation, and insulin resistance. Excess adiposity, particularly obesity onset in early adulthood, is a likely contributor of EOCRC. Although the research is sparse examining CPS and CRC/EOCRC, we describe likely pathways linking CPS to tumorigenesis. Although additional research is needed to understand what factors are driving the uptick in EOCRC, managing body weight, improving diet quality, and mitigating psychosocial stress, may play an important role in reducing an individual’s risk of EOCRC.
Keywords: colorectal cancer, early-onset colorectal cancer, lifestyle factors, diet quality, excess adiposity, chronic psychosocial stress, Western diet, obesity, cancer prevention
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, and its overall incidence has been decreasing over the past 40 y, except early-onset colorectal cancer (EOCRC), which is defined as a CRC diagnosis among individuals aged >50 y [1]. EOCRC incidence has been on the rise in the United States, with a 63% increase between 1988 and 2015 (7.9 compared with 12.9 cases per 100,000) [2]. The same steady increase of EOCRC experienced in the United States has been observed in other Westernized countries, including Australia and Canada [3,4]. This suggests that similar risk factors and exposures among Westernized nations may be driving the rise in EOCRC incidence.
The etiology of EOCRC remains poorly understood with only 20% of new cases attributable to familial genetic causes [5]. Diet quality, presence of stressors, increase in obesity rates, and sedentary behaviors of Westernized populations has shifted substantially because of urbanization, income growth, technological change (i.e., increased access to TVs, and smartphones), and increased production of ultraprocessed foods [6]. In addition, younger generations are being exposed to these risk behaviors from a very early age, which could be responsible for the increase in EOCRC rates in the past 40 y [7]. The purpose of this Perspective piece is to examine the evidence around diet quality, and excess adiposity as modifiable risk factors for EOCRC development and to discuss opportunities for EOCRC prevention. Second, we explore the role of chronic psychosocial stress (CPS) as a modifiable risk factor for EOCRC and raise awareness of the importance of further investigating it.
Methods
For this brief Perspective review, a PubMed search was conducted using the following keywords: “early-onset colorectal cancer,” “cancer initiation,” “diet quality,” “chronic stress,” “chronic psychosocial stress,” “obesity,” “excess adiposity,” “colorectal adenoma,” “gut dysbiosis,” and “microbiome.” Inclusion criteria for research articles were as follows: 1) adult male and female participants, and 2) intervention or observational study. The following exclusion criteria were applied: 1) reviews, 2) systematic reviews, 3) invited commentaries, 4) conference abstract book, 5) presented findings in individuals aged >50 y only, 6) presented findings in high-risk population only (e.g., Lynch syndrome), and 7) in vitro and/or animal studies.
Diet quality and EOCRC
A Western diet pattern, characterized by a high intake of red and processed meats, refined grains, and foods with added sugars [8], has been linked to various chronic diseases, including obesity, cardiovascular disease, type 2 diabetes, and several cancers [9,10]. Recent studies also suggest that a Western diet pattern may increase the risk of developing CRC, particularly among young adults [11]. An increase in production and consumption of ultraprocessed foods and access to convenience stores that provide energy-dense foods has increased substantially over the past several decades [12]. For example, ultraprocessed foods are rich in sodium, sugar, and saturated fats, and are a key component of a Western diet pattern. On the basis of NHANES data, the consumption of ultraprocessed foods increased from 61.4% to 67.0% [difference, 5.6% (95% CI: 3.5%, 7.7%); P < 0.001 for trend] in United States youths aged 2–19 y between 1999 and 2018 [13]. In adults (aged >19 y), there has been an increase from 53.5% to 57.0% of total calories (P-trend < 0.001) between 2001 and 2018 [14]. Moreover, ultraprocessed food intake is independently associated with chronic disease risk including some cancers [15,16]. On the other hand, the Dietary Approaches to Stop Hypertension (DASH) diet, the Mediterranean Diet, and the Alternative Healthy Eating Index-2010 (AHEI-2010) diet promote a high intake of fruits, vegetables, whole grains, nuts, and legumes, as well as low-fat dairy. These diets also discourage the consumption of red and processed meats, sugar-sweetened beverages, ultraprocessed foods, and sodium. Adherence to the Mediterranean Diet, the DASH diet, and the AHEI-2010 diet are associated with positive health outcomes [17]. Associations between diet quality and EOCRC and diet quality and early-onset colorectal adenoma are discussed below.
Zheng et al. [18], analyzed diet quality and colorectal adenoma in the context of a prospective cohort study of >29,000 women (ages 25–42 y at enrollment). Adherence to a Western diet pattern was compared with adherence to the DASH diet, a Mediterranean Diet (as measured using the alternative Mediterranean Diet score [aMED]), and an AHEI-2010 diet. They found a positive relationship between consuming a Western diet and the presence of distal colon and rectal adenomas before the age of 50 y [multivariable-adjusted odds ratio (OR) Q5 compared with Q1: 1.38; 95% CI: 1.13, 1.68] [18]. They also reported an inverse relationship between the healthier dietary patterns (DASH, aMED, and AHEI-2010) and presence of colon and rectal adenoma before 50 y of age [DASH (OR Q5 compared with Q1: 0.84; 95% CI: 0.69, 1.04, P-trend: 0.04), aMED (OR Q5 compared with Q1: 0.80; 95% CI: 0.65, 0.99, P-trend: 0.07), and AHEI-2010 (OR Q5 compared with Q1: 0.85; 95% CI: 0.69, 1.04, P-trend:0.11)] [18].
The Nurses’ Health Study II, a cohort consisting of over 59,000 women, reported that women who adhered to a Western diet pattern had a significantly higher risk of early-onset (<50 y old) colorectal adenoma (OR Q4 compared with Q1: 1.31; 95% CI: 1.10, 1.56; P-trend: 0.02) than those who followed a healthier dietary pattern, which emphasized intake of fruits, vegetables, whole grains, and lean proteins [19].
Another prospective cohort study of 94,217 women, aged 26–45 y at enrollment, reported that a hyperinsulinemic diet and lifestyle pattern was associated with a higher risk of EOCRC [20]. This study examined both EOCRC and CRC and found a statistically significant positive relationship between poorer diet quality (measured by the empirical dietary and lifestyle index for hyperinsulinemia) [20] and the presence of EOCRC [multivariable hazard ratio (HR): 1.86; 95% CI: 1.12, 3.07], but not CRC (multivariable HR: 1.20; 95% CI: 0.83, 1.73) [20]. The empirical dietary and lifestyle index for hyperinsulinemia is an overall diet quality score ranging from 0 to 42 that is comprised of 21 food groups; a higher score reflects more hyperinsulinemic dietary and lifestyle patterns [21,22]. Lastly, in a population-based case-control study including both males and females (n = 428) greater consumption of sugary drinks (≥7 compared with <1 drink(s)/wk, OR: 2.99; 95% CI: 1.57, 5.68), and a Western diet pattern (OR Q4 compared with Q1: 1.92; 95% CI: 1.01, 3.66) were each associated with increased risk of EOCRC [23].
There are several proposed mechanisms linking colorectal adenoma and EOCRC with overall aspects of diet quality; however, they are complex and multifactorial. One proposed mechanism is the role of dietary factors in modulating the gut microbiome [24]. The human gut microbiome is a complex ecosystem of microorganisms that play a key role in maintaining gut homeostasis and regulating host immune responses [25]. Emerging evidence suggests that a poor-quality diet can lead to a colonic milieu supportive of EOCRC development, characterized by an imbalance in the composition and function of the gut microbiome, which in turn can contribute to the development of EOCRC [26,27]. A second proposed mechanism is the role of dietary factors in promoting chronic inflammation. Chronic inflammation is a key driver of carcinogenesis, and it is considered a hallmark of cancer [[28], [29], [30]]. A poor-quality diet has been shown to induce a proinflammatory state in the gut [31,32]. This can lead to the activation of inflammatory signaling pathways and the production of reactive oxygen species, which can damage DNA and promote the development of EOCRC [27].
In addition, a poor-quality diet intake can lead to the accumulation of adipose tissue and insulin resistance. Both have been linked to an increased risk of EOCRC development [33]. Adipose tissue produces inflammatory cytokines and adipokines, which can promote chronic inflammation and contribute to the development of EOCRC [34,35]. Insulin resistance can lead to hyperinsulinemia and the activation of the insulin/insulin-like growth factor axis, which has been implicated in the development of EOCRC [[36], [37], [38]].
In sum, a poor-quality diet can lead to a colonic milieu, chronic inflammation, and metabolic dysfunction, all of which contribute to the development of EOCRC. The studies reviewed here indicate that avoiding a hyperinsulinemic and Western diet pattern as well as sugary beverages while consuming a diet pattern emphasizing fruits, vegetables, whole grains, and lean proteins, may decrease the risk of early-onset colorectal adenoma and EOCRC. Although more research is needed to fully understand the complex relationship between diet and EOCRC development, these findings highlight the importance of a healthy diet, beginning early in life, as a preventive instrument for EOCRC.
Excess adiposity and EOCRC
Obesity onset in early adulthood (∼18 y) that persists as an individual ages, is positively associated with risk of EOCRC [39]. This raises concern, as obesity among young adults (20–44 y of age) in the United States increased from 32.7% to 40.9% from 2009 to 2020 [40]. In addition, childhood obesity rates have tripled in the United States in the past 30 y, and the country now has some of the highest obesity rates worldwide. One 6 children is obese, and 1 of 3 children is overweight or obese in the United States [41,42]. Obesity has a strong association with the occurrence of chronic medical conditions, and its increasing prevalence at earlier life stages only worsens its negative health impact and implications for disease risk including CRC [43]. Furthermore, obesity is associated with metabolic, hormonal, and immune perturbations, such as insulin resistance, increased systemic inflammation, and oxidative stress, which promote gene mutations that can impact EOCRC tumorigenesis [44]. Therefore, it is important to target excess adiposity to prevent the development of EOCRC. In this section, we briefly discuss the literature examining associations between excess adiposity and EOCRC and excess adiposity and early-onset adenoma. Details regarding the studies reviewed below can also be found in Table 1 [39,[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]].
TABLE 1.
Summary of published studies evaluating excess adiposity and EOCRC
| Author; country | Study design | Study subjects | Summary of findings related to obesity and EOCRC incidence |
|---|---|---|---|
| Rogers et al. (2022); United States [56] | Cross-sectional | 1856 EOCRC cases: % BMI >30 kg/m2: 29.28 % female: 47.3 Mean age, y: 41.11 |
Multivariate Poisson regression models for EOCRC incidence in Utah (2000–2020) among adults aged 18–49. Overall: incidence rate ratio (IRR) 1.00 (95% CI: 0.98, 1.02). Women: IRR 1.01 (95% CI: 0.98, 1.04). Men: IRR 0.99 (95% CI: 0.96, 1.01). |
| Hussan et al. (2020); United States [54] | Cross-sectional | All cancers: 1,272,243 Colorectal only: 1,184,980 %obese: 7.25 |
The number of patients with obesity undergoing CRC resections increased among all age groups, with the highest rise in the 18–49-y group [average annual percent change (AAPC) +13.1%, P < 0.001]. |
| Sung et al. (2019); United States[55] | Cross-sectional | From 1995 to 2014, there were 14,672,409 incident cases for 30 types of cancers for 2,481,416,140 person-years of observation. | The incidences of cancers of the EOCRC increased in younger adults (25–49 y), with the magnitude of the increase’s steeper with younger age (all Pwald <0.05). IRR 25–29-y for EOCRC: 2.41% (95% CI: 0.57, 4.29). |
| Glover et al. (2019); United States [45] | Retrospective, cross-sectional study (Data from Explorys electronic medical record) |
Total n = 34,606,650 of which 8,873,080 were 20–39 y old. ∗Limited to adults 20–39 y of age EOCRC: 1700 % female: 52 No EOCRC: 8,871,400 % female:59 |
EOCRC vs. no EOCRC for obesity: Odds Ratio (OR) 1.819 (95% CI: 1.618, 2.044), P < 0.001. |
| Elangovan et al. (2021); United States [46] | Retrospective, cross-sectional study Commercial database (Explorys, IBM Watson Health) on 57 million individuals ≥18 y who were active patients in the database in the last 5 y. |
Among the 37,483,140 average-risk adults active in the database in the last 5 y, 162,150 individuals had at least one encounter diagnosis of malignant CRC. | Obesity was an independent risk factor for CRC across all age groups. OR between EOCRC and obesity in men only:
|
| Sanford et al. (2020); United States [47] | Cross-sectional (Data from the National Health Institute Survey) |
Overall sample (including CRC and EOCRC): 583,511 individuals, with 3173 cases. EOCRC specific: 321,736 individuals (age 18–49) with 239 cases. |
OR between EOCRC (age 18–49) and BMI > 30 kg/m2: OR 1.39 (95% CI: 1.00, 1.92, P = 0.049). |
| Marx et al. (2022); United States [48] | Cross-sectional | 21 individuals % BMI ≥ 30 kg/m2: 57 % female: 43 |
Identified tumors with high and low differential c-MYC proto-oncogene (MYC) expression. Patients with high-MYC tumors were overweight or obese (n = 4 for low MYC and n = 12 for high-MYC, P = 0.047). |
| Juo et al. (2018); United States [49] | Cross-sectional (Data from the California Cancer Registry Patient Discharge Database) |
EOCRC sample: 112,024 % BMI ≥ 30 kg/m2: 5.7 % BMI ≥ 40 kg/m2: 1.9 |
Obese patients (BMI ≥ 30 kg/m2) had a younger onset age (−4.56 y ± SE 0.18, P < 0.05 when compared with nonobese patients) than nonobese patients. Morbidly obese patients (BMI ≥ 40 kg/m2) had an even younger onset age for EOCRC (−7.75 y ± SE 0.30, P < 0.05 when compared with nonobese). |
| Krigel et al. (2020); United States [58] | Retrospective, cross-sectional study (Single-center endoscopy database to identify symptomatic patients 18–49 y of age who underwent ambulatory colonoscopy) |
Total n = 4333 363 patients (8.4%) with any EOCRC: 315 patients (7.3%) had colorectal adenomas and 48 patients (1.1%) had advanced neoplasia. |
Any neoplasia:
|
| Glenn et al. (2018); United States [52] | Cross-sectional | 156 survivors (106 breast, 50 colorectal) Mean BMI, kg/m2: 26.5 % female: 83 Mean age, y: 49.6 |
Prevalence presented: 24% with obesity (BMI ≥ 30 kg/m2), 31% overweight (BMI ≥ 25 kg/m2), 44% with a normal BMI (BMI < 25 kg/m2), and 1% underweight (BMI <18.5 kg/m2). |
| Wolbert et al. (2018); United States [50] | Cross-sectional |
N = 137 (24 EOCRC, 113 CRC). EOCRC: 24 % female: 50 Mean age, y: 43.1 CRC: 113 % female: 38.1 Mean age, y: 64 |
Number of obese (BMI >30 kg/m2) patients in the younger cohort was statistically higher than the older group (P < 0.001). |
| Kim et al. (2022); South Korea [51] | Cross-sectional | 2,023 with EOCRC and 11,598 without EOCRC at colonoscopy. With EOCRC: 2023 % obese according to BMI: 41.2 % female: 24.7 Mean age, y: 35.7 Without EOCRC: 11,598 % obese according to BMI: 28.7 % female: 40.5 Mean age, y: 35.0031 |
OR between EOCRC and obesity: OR 1.439 (95% CI: 1.133, 1.828), P = 0.003 |
| Lee et al. (2016); South Korea [57] | Cross-sectional | Total n = 2819 Age group: <40 % female: 33 Mean BMI: 23.1 Age group: 40–44 % female: 25 Mean BMI: 23.8 Age group: 45–49 % female: 26.1 Mean BMI: 24 Age group: ≥50 % female: 32.3 Mean BMI: 24 |
Multivariate analysis OR colorectal adenoma in young adults with BMI >30 kg/m2 vs. BMI 18.5–24.9 kg/m2: OR 0.55 (95% CI: 0.24, 1.27, P = 0.161). Univariate analysis OR colorectal advanced adenoma in young adults with BMI >30 kg/m2 vs. BMI < 25 kg/m2: OR 1.16 (95% CI: 0.56, 2.4, P = 0.862). |
| Bohorquez et al. (2016); Colombia [53] | Cross-sectional |
N = 1525 % female: 53.2 Age of onset: % EOCRC (50 y):26.6 % CRC: 73.4 % obese: 33.6 |
No difference in obesity prevalence between CRC and EOCRC (34.7% vs. 30%, respectively; P = 0.478). |
| Clinton et al. (2022); United States [66] | Retrospective predictive study—Machine learning predictive study | 3116 adults aged 35–50 at average-risk for CRC and underwent colonoscopy between 2017 and 2020 at a single center. Prediction outcomes were 1) CRC and 2) CRC or high-risk polyps. |
The most important predictive variables in the regularized discriminant analysis Model for EOCRC or high-risk polyps were income per zip code, the colonoscopy indication, and BMI quartiles. |
| Low et al. (2020); United States [61] | Case-control | EOCRC cases: 651 Mean BMI, kg/m2: 29.5 % female: 8.8 Mean age, y: 44.8 Controls: 67,416 Mean BMI kg/m2: 30 % female: 17.8 Mean age, y: 43.2 |
Obesity was significantly associated with decreased EOCRC odds [odds ratio (OR): 0.7; 95% CI: 0.56, 0.86]. |
| Gausman et al. (2020); United States [60] | Case-control | EOCRC cases: 269 Mean BMI, kg/m2: 27 % female: 46 Mean age, y: 43 Controls: 1122 Mean BMI kg/m2: 28 % female: 55 Mean age, y: 45 |
Did not observe an association between obesity and EOCRC (OR 0.98; 95% CI: 0.95, 1.00). |
| Li et al. (2022); Germany [39] | Case-control | Overall sample combining CRC and EOCRC: 6602 patients with EOCRC/CRC and 7950 matched controls EOCRC specific sample: EOCRC cases: 747 % female: 43 EOCRC Controls: 621 % female: 44 |
OR between EOCRC and obesity classification at 20 y of age: OR 2.56 (95% CI: 1.20, 5.44). OR between EOCRC and obesity classification at 30 y of age: OR 2.06 (95% CI: 1.25, 3.40). OR between EOCRC and obesity classification ∼10 y before diagnosis: OR 1.88 (95% CI: 1.30, 2.73). |
| Schumacher et al. (2021); United States [59] | Case-control | 1032 cases and 5128 controls. EOCRC cases: 1032 % obese according to BMI: 48.5 % female: 50.1 Controls: 5,128 % obese according to BMI: 39.4 % female: 49.9 |
Risk of colorectal adenocarcinoma was significantly associated with obesity (OR 1.41; 95% CI: 1.15, 1.74), but not diabetes, hypertension, or dyslipidemia. Tumor location: obesity significantly associated with risk of colon adenocarcinoma OR 1.56 (95% CI: 1.17, 2.07). No significant interaction was detected between obesity and age (≥40 vs. <40), and obesity and sex. |
| Liu et al. (2019); United States [62] | Prospective cohort study | 85,256 women studied with 114 EOCRC cases observed. | Multivariable relative risk was 1.93 (95% CI: 1.15, 3.25) for women with obesity (BMI ≥ 30.0). |
| Syed et al. (2019); United States [63] | Population-based cohort analysis | Overall sample: 35,493,980. 68,860 patients (0.2%) were identified with CRC, of which 5710 (8.3%) were younger than 50 y old (EOCRC diagnosis). EOCRC specific: % BMI ≥ 30kg/m2: 61 % female: 51 |
OR EOCRC vs. CRC for obesity: OR 1.14 (95% CI: 1.08, 1.20, P < 0.001) OR EOCRC vs. control for obesity: OR 2.88 (95% CI: 2.74, 3.04, P < 0.001) |
| Park et al. (2022); United States [64] | Retrospective observational study | Patients with EOCRC: 3856% female: 47.5 Mean age, y: 43 |
Presented prevalence of new EOCRC only. % with obesity (BMI >30 kg/m2): 26.9 |
| Hussan, et al. (2020); United States [65] | Retrospective cohort study |
N = 30,279 7.4% (2238) with a prior history of bariatric surgery (cases). % female: 77.4 % diagnosed CRC:91.6 92.6% (28,041) were nonbariatric morbidly obese (controls). % female: 57.1 % diagnosed CRC: 92.6 |
The steepest rise in EOCRC resections was after bariatric surgery vs. a lesser increase in morbid obesity controls (AAPC +18.7% and +13.7%, respectively, P < 0.001). |
Abbreviations: AAPC, average annual percent change; AOR, adjusted odds ratio; EOCRC, early-onset colorectal cancer; ERCN, early-onset neoplasia; IRR, incidence rate ratio; MYC, c-MYC proto-oncogene; OR, odds ratio.
Cross-sectional studies
We identified 14 cross-sectional studies; 12 presented results regarding the association between obesity and EOCRC [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]], one evaluated the relationship between obesity and early-onset colorectal adenoma [57], and one presented findings for both EOCRC and early-onset colorectal adenoma [58]. Of the 12 articles investigating the association between obesity and EOCRC, 9 found a significant positive association between obesity and EOCRC [[45], [46], [47], [48], [49], [50], [51],54,55]. However, Glenn et al. [52] reported the prevalence of EOCRC among patients stratified by BMI (kg/m2) category: 24% with obesity (BMI ≥ 30), 31% overweight (BMI between 25 and 29.9), 44% with a normal BMI (BMI between 18.5 and 24.9), and 1% underweight (BMI <18.5). Bohorquez et al. [53] found no difference in CRC or EOCRC prevalence based on BMI category (34.7% of individuals with obesity in the CRC groups compared with 30% of individuals with obesity in the EOCRC group, respectively; P = 0.48). One study examined the relationship between obesity and EOCRC among counties in Utah from 2000 to 2020 [56]. The authors found no significant association between obesity and EOCRC incidence, with an incidence rate ratio of 1.00 (95% CI: 0.98,1.02) [56].
The 2 cross-sectional studies that evaluated the relationship between obesity and early-onset colorectal adenoma also had contradictory findings. Lee et al. [57] did not find a relationship between the presence of early-onset colorectal adenoma in young adults with BMI of ≥30 compared with BMI 18.5–24.9 (OR: 0.55; 95% CI: 0.24, 1.27). Krigel et al. [58] found an increased risk of early-onset colorectal adenoma among individuals with BMI between 30 and 34.9 compared with BMI between 18 and 24.9 (OR: 1.45; 95% CI: 1.02, 2.07), but not for BMI ≥35 compared with BMI between 18 and 24.9 (OR: 1.24; 95% CI: 0.81, 1.89).
Case-control studies
We identified 4 case-control studies examining associations between obesity and EOCRC. Two studies found an increased risk of EOCRC among those with obesity [39,59], 1 found no statistically significant relationship (OR: 0.98; 95% CI: 0.95, 1.00) [60], and 1 found a decrease in risk of developing EOCRC in individuals with obesity (OR: 0.70; 95% CI: 0.56, 0.86) [61]. Li et al. [39], examined 6602 patients with EOCRC/CRC and 7950 matched controls. Of those, 747 were EOCRC cases. They reported that a BMI of ≥30 at 20 y of age was associated with increased odds of EOCRC (OR: 2.56; 95% CI: 1.20, 5.44). Obesity at 30 y of age was also associated with increased odds of EOCRC (OR: 2.06; 95% CI: 1.25, 3.40). Schumacher et al. [59] examined 1032 cases and 5128 controls. They reported that obesity was associated with risk of EOCRC (OR: 1.41; 95% CI: 1.15, 1.74). Conversely, Gausman et al. [60] examined 269 cases and 1122 controls and did not find an association between obesity and EOCRC (OR: 0.98; 95% CI: 0.95, 1.00). Low et al. [61] also reported among 651 cases and 67,416 controls that obesity was associated with decreased odds of EOCRC (OR: 0.7; 95% CI: 0.56, 0.86).
Cohort studies
Four prospective and retrospective cohort studies examined the association between obesity and EOCRC. Two found a significant relationship between obesity and EOCRC [62,63]. Liu et al. [62] conducted an analysis using data from the Nurse’s Health Study II, involving 85,256 women. Their findings showed a multivariable RR of 1.93 (95% CI: 1.15, 3.25) for EOCRC among women with obesity (BMI ≥30.0). Syed et al. [63] conducted an analysis including 68,860 patients with CRC, of which 5710 (8.3%) had EOCRC. They observed higher odds of EOCRC among individuals with obesity but not among those with a CRC diagnosis (OR: 1.14; 95% CI: 1.08, 1.20) [63]. Using medical records, Park et al. [64] conducted a retrospective review of patients with an EOCRC diagnosis (age of enrollment: between 20 and 49 y of age). They identified 3856 patients with EOCRC, but only presented the prevalence of EOCRC cases with BMI ≥30, which was 26.9%. A second retrospective study among 30,279 individuals with gastrointestinal cancers, found a higher prevalence of EOCRC in patients who underwent bariatric weight loss surgery compared with controls with obesity (average annual percent change + 18.7% and + 13.7%, respectively, P < 0.001) [65]. Lastly, in an analysis using patient medical record data and predictive machine learning methods, Hussan et al. [66] reported that the most significant variables in predicting EOCRC or early-onset high-risk colorectal adenoma were income per zip code, the reason for needing a colonoscopy (e.g., functional gastrointestinal symptoms reported by the patient), and higher BMI.
Of the 23 articles reviewed here, 15 showed a significant association between obesity and EOCRC or the presence of early-onset colorectal adenoma. However, 4 studies did not report a significant association between obesity and EOCRC, and one found a protective effect of obesity. Three papers did not investigate the relationship between obesity and EOCRC or early-onset colorectal adenoma, but simply presented prevalence data by BMI category. These discrepancies may be because of the wide range of study designs, and the discrepancy of the sample between studies used to test this hypothesis and a lack of comparable BMI categorization across the studies. There is also the risk of unmeasured confounding, which is especially present in cross-sectional samples.
It is important to note that BMI data are not a reliable measure of an individual’s body composition, as it simply accounts for their height and weight, and it completely ignores their percentage of fat mass compared with muscle mass or body fat distribution. However, BMI is a convenient measure that correlates reasonably well with disease risk for most adults under 65 y old [67]. Its low cost makes it an accessible tool for large-scale studies, as other methods like a dual X-ray absorptiometry scan may not be a feasible approach for large sample size studies because of its high cost and time needed to assess each individual. In addition, further investigation is needed regarding BMI cutoffs based on race and ethnicity. According to Stanford et al. [68], there is a need to redefine BMI values by correlating them with the presence of metabolic risk factors, rather than relying on a single BMI threshold based on historical statistical data from over 60 y ago. More studies are needed to evaluate the relationships of body composition and body fat distribution with EOCRC and early-onset colorectal adenoma.
There are several proposed mechanisms linking obesity with early neoplastic changes (i.e., colorectal adenoma) and EOCRC; however, they are complex and multifactorial. Obesity is associated with metabolic, hormonal, and immune perturbations that can promote gene mutations that drive tumorigenesis. A systematic review and meta-analysis with 6 studies investigating EOCRC diagnosis and potential risk factors classified obesity as a strong risk factor for EOCRC development [69]. In sum, the existing evidence indicates a likely association between excess adiposity and EOCRC and the presence of colorectal adenoma at an earlier age. The high prevalence of child and adult obesity, together with its impact on metabolic, hormonal, and immune disruptions, underscores the importance of addressing obesity as a preventive measure for EOCRC. Promoting a healthy weight across the life course and implementing population-level strategies to reduce obesity, such as policies that incentivize healthy eating, creating supportive environments for healthy eating and physical activity and simplifying efficacious methods for weight management for adolescents and young adults, may help to mitigate the uptick of EOCRC in the United States.
Exploring CPS as a driver of EOCRC
There is anecdotal evidence linking CPS and cancer that goes back almost 2 millennia with early reports describing that cancer was less likely to occur in persons with better mental well-being [70]. CPS dysregulates and augments the release of mediators of the neuroendocrine stress response system, including autonomic nervous system-related catecholamines, epinephrine and norepinephrine, and hypothalamic-pituitary adrenal-axis-related hormones including corticotropin release-factor and adrenocorticotropic hormone and the glucocorticoid, cortisol [70,71]. The prevalence of self-reported psychosocial stress in young adults (ages 19–22) varies widely, with studies reporting rates between 20% [72] and as high as 60% [73]. Moreover, a higher prevalence of the population (ages 18–34) is reporting high levels of stress and at a much earlier age than before [74]. Factors contributing to the rise of stress levels include increased work hours, financial strain, health issues, and social media use.
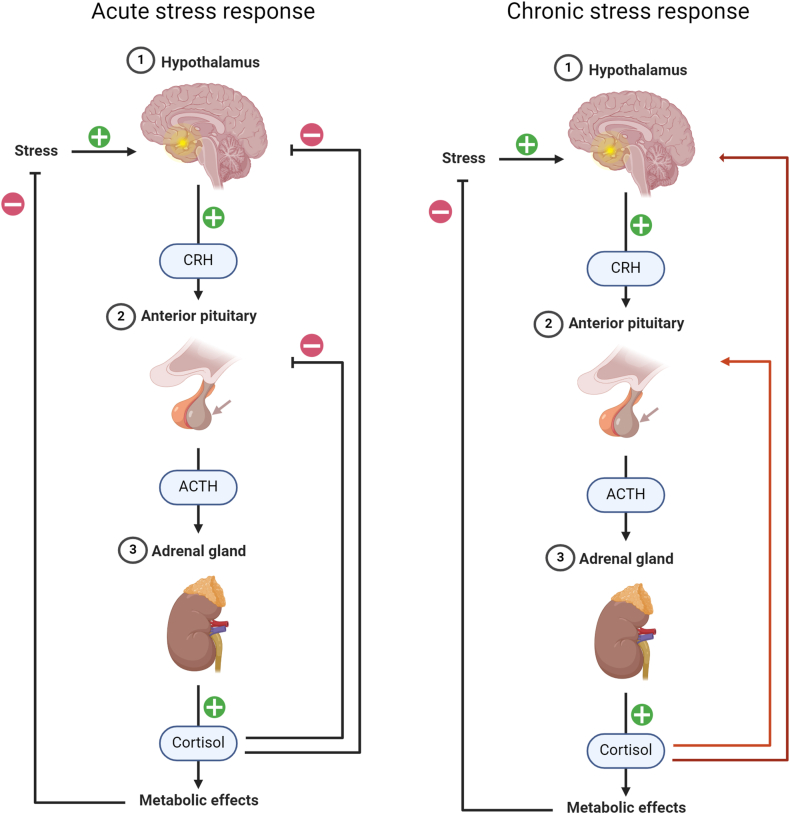
The hypothalamic-pituitary-adrenal axis is a neuroendocrine mechanism that mediates the effects of stressors by regulating numerous physiologic processes, such as metabolism, immune response, and the autonomic nervous system response. Figure 1 illustrates the difference between acute and chronic stress. On the left-hand side of the figure, we present the acute response to a stressor, with the hypothalamic-pituitary-adrenal axis and autonomic nervous system acting together. The autonomic nervous system provides immediate response by releasing the catecholamines, epinephrine, and norepinephrine into the system. The hypothalamic-pituitary-adrenal axis, on the other hand, provides a delayed response, and its final product is the release of the glucocorticoid cortisol. In response to the release of the catecholamines, there is an increase in blood pressure and heart rate. Cortisol’s function is to suppress the production of corticotropin release factor and adrenocorticotropic hormone. In the context of CPS, which is shown on the right side of the figure, this function becomes blunted promoting an overactivation of the hypothalamic-pituitary-adrenal axis. hypothalamic-pituitary-adrenal axis overactivation results in downstream physiologic consequences, such as an increased risk of immune system dysfunction, metabolic diseases, cardiovascular disease, and possibly cancer [75,76].
FIGURE 1.
Hypothalamic-pituitary-adrenal axis response in acute compared with chronic stress. Template adapted from: Camilla Maria Fontana, PhD Student, University of Padova. Template created with Biorender (biorender.com).
Animal studies have connected stress-related increases in epinephrine, norepinephrine, and cortisol to cancer initiation [70,77]. A recent review article by Mravec et al. [70,77] suggested that CPS may affect 7 of the 10 hallmarks of cancer: 1) genome instability and mutation, 2) tumor-promoting inflammation, 3) avoidance to immune destruction, 4) sustained proliferative signaling, 5) resistance to cell death, 6) induction of angiogenesis, and 7) activation of invasion and metastasis. It is thought that the stress (both acute and chronic) acts on these hallmarks either by contributing to the induction of cancer, along with other factors or by potentiating the progression of an already existing cancer and development of metastases. The hallmarks 1 and 2, “genome instability and mutation” and “tumor-promoting inflammation” are implicated in cancer initiation, whereas the remaining are implicated in cancer progression and recurrence.
In addition, CPS, and its downstream effector cortisol, act to control food intake and energy homeostasis with elevated cortisol linked to known EOCRC drivers—poor diet quality, overweight/obesity, increased insulin resistance, and changes in gut composition and metabolic function. Together these CPS-related alternations can promote a milieu that is conducive to the initiation of colon tumorigenesis [78].
Despite the importance of studying the relationship between CPS and EOCRC, there is a lack of research in humans in this area. A search using various terms related to CPS and EOCRC yielded no articles. Further research in humans is needed to determine the extent to which CPS contributes to EOCRC and to develop strategies that could mitigate the negative effects of CPS on health outcomes.
Opportunities
In several of the studies reviewed, poor diet quality was associated with EOCRC and early-onset colorectal adenoma. Interventions designed to increase diet quality from an early age may play a critical role in preventing EOCRC. However, increasing individual-level diet quality is a complex issue that is often influenced by resources and access to food [79]. Redmond et al. [79] provide evidence that multilevel interventions involving community residents, the private sector (e.g., food retailers, food companies), community organizations, and the local government that are implemented community-wide (e.g., schools and childcare settings) can support equitable access to healthy foods that translates to cancer preventive dietary behavior changes.
On the basis of the evidence presented, excess adiposity is a probable risk factor for EOCRC. Although there are many approaches to support healthy weight management, obesity among young adults continues to rise in the United States. In addition, obesity rates are increasing at an earlier age, which could be contributing to the rise in EOCRC development rates. Different weight loss approaches have been extensively tested with moderate overall success indicating a need for innovation. In addition, the recent emergence of glucagon-like peptide 1 agonist medications that produce clinically meaningful weight losses in the range of 4%-20% may also have implications for reducing the incidence of EOCRC among individuals with obesity [80]. Given the fast-moving pace of today’s society weight loss interventions and pharmacologic approaches may be effective for weight management and EOCRC prevention among young adults with obesity.
Although there is a lack of studies directly examining CPS and EOCRC, CPS is often associated with poor diet quality, increased visceral adiposity and insulin resistance, and a shift in gut microbial composition and metabolic function that can support the development of EOCRC [[81], [82], [83]]. Different approaches are being tested to address risk factors associated with CPS. Mindfulness meditation has gained popularity as an approach to managing CPS [84]. Mindfulness meditation is the practice of cultivating a moment-to-moment awareness of internal and external experience in an accepting and open manner. In 1979, Kabat-Zinn developed mindfulness-based stress reduction (MBSR): an intervention to reduce stress, pain, and suffering [84]. Attitudes of open inquiry, patience, suspended judgment, and compassion are encouraged and cultivated during intervention sessions and through daily assignments. Mindfulness approaches, including MBSR is associated with lower perceived stress [85] and decreased lower circulating cortisol concentrations [86,87]. Existing evidence suggests that mindfulness approaches also yield EOCRC preventive effects specific to body weight reduction [88], increased insulin sensitivity [89], and reduction of inflammation [90]. Furthermore, in a prospective study comparing a group that had the habit of meditation compared with nonmeditation, the meditation group showed higher levels of physiologic melatonin compared with the nonmeditation group. This finding is important because melatonin has potential anti-cancer properties [[91], [92], [93]]. Newer implementation methods, including telephone-based applications, such as Calm.com, are allowing for low-cost and easy access to mindfulness programs. This is important because historically groups that are more vulnerable to a Western diet pattern, obesity, and CPS are those that are less likely to have access to mindfulness programs [94]. On its own or combined with dietary lifestyle intervention protocols, stress reduction may be relevant to preventing EOCRC. Different research protocols are currently being executed to test the impact of mindfulness [94,95], but there is still a need for larger and longer trials. It is important to account for multiple factors that can impact CPS among various populations and these need to be accounted for while planning and developing an intervention (i.e., the need for culturally adapted interventions).
Conclusion
The recent increase in EOCRC is a concerning trend that asks for a call-to-action from the nutrition science community. On the basis of the evidence presented here, improving diet quality, and reducing excess body weight are important modifiable risk factors for EOCRC. Interventions aimed at improving diet quality and preventing obesity should be multilevel and strategically implemented in locations targeting younger populations. Although CPS may be contributing to EOCRC, there is a need for more population-based and clinical studies to understand the role of CPS in EOCRC as well as the impact of stress reduction approaches on colon health.
Author contributions
The authors’ responsibilities were as follows – MLO, LT-H: conceptualization; MLO, AB, PW, LT-H: methodology; MLO: writing—original draft preparation; MLO, AB, VMO, BY, EB, LS, KN, PW, LT-H: writing—review and editing; LT-H: supervision; and all authors: read and agreed to the published version of the manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
Supported by NIH R01CA257807 (LT-H), R01CA250390 (LT-H), R01CA204808 (LT-H), T32CA057699 (MLO), and U54MD012523 (LT-H). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Petrick J.L., Barber L.E., Warren Andersen S., Florio A.A., Palmer J.R., Rosenberg L. Racial disparities and sex differences in early- and late-onset colorectal cancer incidence, 2001–2018. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.734998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinicrope F.A. Increasing incidence of early-onset colorectal cancer. N. Eng. J. Med. 2022;386(16):1547–1558. doi: 10.1056/NEJMra2200869. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Torre L.A., Soerjomataram I., Hayes R.B., Bray F., Weber T.K., et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 4.Saad El Din K., Loree J.M., Sayre E.C., Gill S., Brown C.J., Dau H., et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20(1):288. doi: 10.1186/s12885-020-06766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahnen D.J., Wade S.W., Jones W.F., Sifri R., Mendoza Silveiras J., Greenamyer J., et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo. Clin. Proc. 2014;89(2):216–224. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Popkin B.M., Ng S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022;23(1) doi: 10.1111/obr.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlZaabi A., AlHarrasi A., AlMusalami A., AlMahyijari N., Al Hinai K., ALAdawi H., et al. Early onset colorectal cancer: challenges across the cancer care continuum. Ann. Med. Surg. (Lond). 2022;82 doi: 10.1016/j.amsu.2022.104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomou E., Psaltopoulou T., Georgiopoulos G., Siasos G., Kokkou E., Antonopoulos A., et al. Western dietary pattern is associated with severe coronary artery disease. Angiology. 2018;69(4):339–346. doi: 10.1177/0003319717721603. [DOI] [PubMed] [Google Scholar]
- 10.Kopp W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019;12:2221–2236. doi: 10.2147/DMSO.S216791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll K.L., Frugé A.D., Heslin M.J., Lipke E.A., Greene M.W. Diet as a risk factor for early-onset colorectal adenoma and carcinoma: a systematic review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.896330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poti J.M., Braga B., Qin B. Ultra-processed food intake and obesity: what really matters for health-processing or nutrient content? Curr. Obes. Rep. 2017;6(4):420–431. doi: 10.1007/s13679-017-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Martínez Steele E., Du M., Pomeranz J.L., O'Connor L.E., Herrick K.A., et al. Trends in consumption of ultraprocessed foods among US youths aged 2-19 years, 1999-2018. JAMA. 2021;326(6):519–530. doi: 10.1001/jama.2021.10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juul F., Parekh N., Martinez-Steele E., Monteiro C.A., Chang V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022;115(1):211–221. doi: 10.1093/ajcn/nqab305. [DOI] [PubMed] [Google Scholar]
- 15.Baker P., Machado P., Santos T., Sievert K., Backholer K., Hadjikakou M., et al. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020;21(12) doi: 10.1111/obr.13126. [DOI] [PubMed] [Google Scholar]
- 16.Isaksen I.M., Dankel S.N. Ultra-processed food consumption and cancer risk: a systematic review and meta-analysis. Clin. Nutr. 2023;42(6):919–928. doi: 10.1016/j.clnu.2023.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Lacoba R., Pardo-Garcia I., Amo-Saus E., Escribano-Sotos F. Mediterranean diet and health outcomes: a systematic meta-review. Eur. J. Public Health. 2018;28(5):955–961. doi: 10.1093/eurpub/cky113. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X., Hur J., Nguyen L.H., Liu J., Song M., Wu K., et al. Comprehensive assessment of diet quality and risk of precursors of early-onset colorectal cancer. J. Natl. Cancer Inst. 2021;113(5):543–552. doi: 10.1093/jnci/djaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen L.H., Cao Y., Hur J., Mehta R.S., Sikavi D.R., Wang Y., et al. The sulfur microbial diet is associated with increased risk of early-onset colorectal cancer precursors. Gastroenterology. 2021;161(5):1423–1432.e4. doi: 10.1053/j.gastro.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue Y., Hur J., Cao Y., Tabung F.K., Wang M., Wu K., et al. Prospective evaluation of dietary and lifestyle pattern indices with risk of colorectal cancer in a cohort of younger women. Ann. Oncol. 2021;32(6):778–786. doi: 10.1016/j.annonc.2021.03.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rifas-Shiman S.L., Willett W.C., Lobb R., Kotch J., Dart C., Gillman M.W. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001;4(2):249–254. doi: 10.1079/phn200061. [DOI] [PubMed] [Google Scholar]
- 22.Fung T.T., Isanaka S., Hu F.B., Willett W.C. International food group-based diet quality and risk of coronary heart disease in men and women. Am. J. Clin. Nutr. 2018;107(1):120–129. doi: 10.1093/ajcn/nqx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang V.C., Cotterchio M., De P., Tinmouth J. Risk factors for early-onset colorectal cancer: a population-based case-control study in Ontario, Canada. Cancer Causes Control. 2021;32(10):1063–1083. doi: 10.1007/s10552-021-01456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nova E., Gómez-Martinez S., González-Soltero R. The influence of dietary factors on the gut microbiota. Microorganisms. 2022;10(7):1368. doi: 10.3390/microorganisms10071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut. Microbes. 2012;3(1):4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad Kendong S.M., Raja Ali R.A., Nawawi K.N.M., Ahmad H.F., Mokhtar N.M. Gut dysbiosis and intestinal barrier dysfunction: potential explanation for early-onset colorectal cancer. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.744606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21(1):1325. doi: 10.1186/s12885-021-09054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 31.Gill P.A., Inniss S., Kumagai T., Rahman F.Z., Smith A.M. The role of diet and gut microbiota in regulating gastrointestinal and inflammatory disease. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.866059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilal M., Ashraf S., Zhao X. Dietary component-induced inflammation and its amelioration by prebiotics, probiotics, and synbiotics. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.931458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Han Z.P., Zhang S.S., Jing Y.Y., Bu X.X., Wang C.Y., et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J. Biol. Chem. 2011;286(28):25007–25015. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divella R., De Luca R., Abbate I., Naglieri E., Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer. 2016;7(15):2346–2359. doi: 10.7150/jca.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Mansoori L., Al-Jaber H., Prince M.S., Elrayess M.A. Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation. 2022;45(1):31–44. doi: 10.1007/s10753-021-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroith D., Scheinman E.J., Bitton-Worms K. The role of insulin and insulin-like growth factors in the increased risk of cancer in diabetes. Rambam Maimonides Med. J. 2011;2(2) doi: 10.5041/RMMJ.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godsland I.F. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin. Sci (Lond). 2009;118(5):315–332. doi: 10.1042/CS20090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasprzak A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021;22(12):6434. doi: 10.3390/ijms22126434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H., Boakye D., Chen X., Jansen L., Chang-Claude J., Hoffmeister M., et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology. 2022;162(4):1088–1097.e3. doi: 10.1053/j.gastro.2021.12.239. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal R., Yeh R.W., Joynt Maddox K.E., Wadhera R.K. Cardiovascular risk factor prevalence, treatment, and control in US adults aged 20 to 44 years, 2009 to March 2020. JAMA. 2023;329(11):899–909. doi: 10.1001/jama.2023.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djalalinia S., Qorbani M., Peykari N., Kelishadi R. Health impacts of obesity. Pak. J. Med. Sci. 2015;31(1):239–242. doi: 10.12669/pjms.311.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone T.W., McPherson M., Gail Darlington L. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine. 2018;30:14–28. doi: 10.1016/j.ebiom.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glover M., Mansoor E., Panhwar M., Parasa S., Cooper G.S. Epidemiology of colorectal cancer in average risk adults 20-39 years of age: a population-based national study. Dig. Dis. Sci. 2019;64(12):3602–3609. doi: 10.1007/s10620-019-05690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elangovan A., Skeans J., Landsman M., Ali S.M.J., Elangovan A.G., Kaelber D.C., et al. Colorectal cancer, age, and obesity-related comorbidities: a large database study. Dig. Dis. Sci. 2021;66(9):3156–3163. doi: 10.1007/s10620-020-06602-x. [DOI] [PubMed] [Google Scholar]
- 47.Sanford N.N., Giovannucci E.L., Ahn C., Dee E.C., Mahal B.A. Obesity and younger versus older onset colorectal cancer in the United States, 1998-2017. J. Gastrointest Oncol. 2020;11(1):121–126. doi: 10.21037/jgo.2019.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marx O.M., Mankarious M.M., Eshelman M.A., Ding W., Koltun W.A., Yochum G.S. Transcriptome analyses identify deregulated MYC in early onset colorectal cancer. Biomolecules. 2022;12(9):1223. doi: 10.3390/biom12091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juo Y.Y., Gibbons M.A.M., Dutson E., Lin A.Y., Yanagawa J., Hines O.J., et al. Obesity is associated with early onset of gastrointestinal cancers in California. J. Obes. 2018;2018 doi: 10.1155/2018/7014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolbert T., Leigh E.C., Barry R., Thompson E.C., Gress T., Ajmera A., et al. Later stage disease and earlier onset of rectal cancer: epidemiology and outcomes comparison of rectal cancer in a rural Appalachian area to state and national rates. Am. Surg. 2018;84(7):1229–1235. [PubMed] [Google Scholar]
- 51.Kim I., Lee H.H., Ko Y.J., Chang H.E., Cheung D.Y., Lee B.I., et al. Factors associated with the risk of colorectal neoplasia in young adults under age 40. Korean J. Intern. Med. 2022;37(5):969–978. doi: 10.3904/kjim.2021.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glenn B.A., Hamilton A.S., Nonzee N.J., Maxwell A.E., Crespi C.M., Ryerson A.B., et al. Obesity, physical activity, and dietary behaviors in an ethnically-diverse sample of cancer survivors with early onset disease. J. Psychosoc. Oncol. 2018;36(4):418–436. doi: 10.1080/07347332.2018.1448031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohorquez M., Sahasrabudhe R., Criollo A., Sanabria-Salas M.C., Vélez A., Castro J.M., et al. Clinical manifestations of colorectal cancer patients from a large multicenter study in Colombia. Med. (Baltimore). 2016;95(40) doi: 10.1097/MD.0000000000004883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussan H., Patel A., Le Roux M., Cruz-Monserrate Z., Porter K., Clinton S.K., et al. Rising incidence of colorectal cancer in young adults corresponds with increasing surgical resections in obese patients. Clin. Transl. Gastroenterol. 2020;11(4) doi: 10.14309/ctg.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung H., Siegel R.L., Rosenberg P.S., Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3) doi: 10.1016/S2468-2667(18)30267-6. e137–e47. [DOI] [PubMed] [Google Scholar]
- 56.Rogers C.R., Korous K.M., Brooks E., De Vera M.A., Tuuhetaufa F., Lucas T., et al. Early-onset colorectal cancer survival differences and potential geographic determinants among men and women in Utah. Am. Soc. Clin. Oncol. Educ. Book. 2022;42:1–16. doi: 10.1200/EDBK_350241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S.E., Jo H.B., Kwack W.G., Jeong Y.J., Yoon Y.J., Kang H.W. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J. Gastroenterol. 2016;22(10):2981–2992. doi: 10.3748/wjg.v22.i10.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krigel A., Zhou M., Terry M.B., Kastrinos F., Lebwohl B. Symptoms and demographic factors associated with early-onset colorectal neoplasia among individuals undergoing diagnostic colonoscopy. Eur. J. Gastroenterol. Hepatol. 2020;32(7):821–826. doi: 10.1097/MEG.0000000000001720. [DOI] [PubMed] [Google Scholar]
- 59.Schumacher A.J., Chen Q., Attaluri V., McLemore E.C., Chao C.R. Metabolic risk factors associated with early-onset colorectal adenocarcinoma: a case-control study at Kaiser permanente southern California. Cancer Epidemiol. Biomarkers Prev. 2021;30(10):1792–1798. doi: 10.1158/1055-9965.EPI-20-1127. [DOI] [PubMed] [Google Scholar]
- 60.Gausman V., Dornblaser D., Anand S., Hayes R.B., O'Connell K., Du M., et al. Risk factors associated with early-onset colorectal cancer. Clin. Gastroenterol. Hepatol. 2020;18(12):2752–2759.e2. doi: 10.1016/j.cgh.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low E.E., Demb J., Liu L., Earles A., Bustamante R., Williams C.D., et al. Risk factors for early-onset colorectal cancer. Gastroenterology. 2020;159(2):492–501.e7. doi: 10.1053/j.gastro.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu P.H., Wu K., Ng K., Zauber A.G., Nguyen L.H., Song M., et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37–44. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Syed A.R., Thakkar P., Horne Z.D., Abdul-Baki H., Kochhar G., Farah K., et al. Old vs new: risk factors predicting early onset colorectal cancer. World J. Gastrointest. Oncol. 2019;11(11):1011–1020. doi: 10.4251/wjgo.v11.i11.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park L., O'Connell K., Herzog K., Chatila W., Walch H., Palmaira R.L.D., et al. Clinical features of young onset colorectal cancer patients from a large cohort at a single cancer center. Int. J. Colorectal. Dis. 2022;37(12):2511–2516. doi: 10.1007/s00384-022-04286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussan H., Patel A., Akinyeye S., Porter K., Ahnen D., Lieberman D. Bariatric surgery is associated with a recent temporal increase in colorectal cancer resections, most pronounced in adults below 50 years of age. Obes. Surg. 2020;30(12):4867–4876. doi: 10.1007/s11695-020-04902-9. [DOI] [PubMed] [Google Scholar]
- 66.Hussan H., Zhao J., Badu-Tawiah A.K., Stanich P., Tabung F., Gray D., et al. Utility of machine learning in developing a predictive model for early-age-onset colorectal neoplasia using electronic health records. PLOS ONE. 2022;17(3) doi: 10.1371/journal.pone.0265209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nuttall F.Q. Body mass index: obesity, BMI, and health: a critical review. Nutr. Today. 2015;50(3):117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stanford F.C., Lee M., Hur C. Race, ethnicity, sex, and obesity: is it time to personalize the scale? Mayo Clin. Proc. 2019;94(2):362–363. doi: 10.1016/j.mayocp.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H., Boakye D., Chen X., Hoffmeister M., Brenner H. Association of body mass index with risk of early-onset colorectal cancer: systematic review and meta-analysis. Am. J. Gastroenterol. 2021;116(11):2173–2183. doi: 10.14309/ajg.0000000000001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mravec B., Tibensky M., Horvathova L. Stress and cancer. Part I: Mechanisms mediating the effect of stressors on cancer. J. Neuroimmunol. 2020;346 doi: 10.1016/j.jneuroim.2020.577311. [DOI] [PubMed] [Google Scholar]
- 71.Feller L., Khammissa R.A.G., Ballyram R., Chandran R., Lemmer J. Chronic psychosocial stress in relation to cancer. Middle East J. Cancer. 2019;10(1):1–8. [Google Scholar]
- 72.Sahoo S., Khess C.R. Prevalence of depression, anxiety, and stress among young male adults in India: a dimensional and categorical diagnoses-based study. J. Nerv. Ment. Dis. 2010;198(12):901–904. doi: 10.1097/NMD.0b013e3181fe75dc. [DOI] [PubMed] [Google Scholar]
- 73.Fawzy M., Hamed S.A. Prevalence of psychological stress, depression and anxiety among medical students in Egypt. Psychiatry Res. 2017;255:186–194. doi: 10.1016/j.psychres.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 74.American Psychological Association; 2023. Stress in America 2023 [press release] [Google Scholar]
- 75.Cui B., Peng F., Lu J., He B., Su Q., Luo H., et al. Cancer and stress: NextGen strategies, Brain Behav. Immun. 2021;93:368–383. doi: 10.1016/j.bbi.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dial Clin. Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mravec B., Horvathova L., Hunakova L. Neurobiology of cancer: the role of β-adrenergic receptor signaling in various tumor environments. Int. J. Mol. Sci. 2020;21(21):7958. doi: 10.3390/ijms21217958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huttenhower C., Kostic A.D., Xavier R.J. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40(6):843–854. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redmond L.C., Wensel C.R., Estradé M., Fleischhacker S.E., Poirer L., Jock B.W.I., et al. Dietary outcomes of a multilevel, multicomponent, cluster randomized obesity intervention in 6 native American communities in the upper midwest and southwest United States. Curr. Dev. Nutr. 2023;7(6) doi: 10.1016/j.cdnut.2023.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jensterle M., Rizzo M., Haluzík M., Janež A. Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: a narrative review. Adv. Ther. 2022;39(6):2452–2467. doi: 10.1007/s12325-022-02153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khaled K., Tsofliou F., Hundley V., Helmreich R., Almilaji O. Perceived stress and diet quality in women of reproductive age: a systematic review and meta-analysis. Nutr. J. 2020;19(1):92. doi: 10.1186/s12937-020-00609-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aschbacher K., Kornfeld S., Picard M., Puterman E., Havel P.J., Stanhope K., et al. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. doi: 10.1016/j.psyneuen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yau Y.H., Potenza M.N. Stress and eating behaviors. Minerva Endocrinol. 2013;38(3):255–267. [PMC free article] [PubMed] [Google Scholar]
- 84.Kabat-Zinn Jon. Full catastrophe living : using the wisdom of your body and mind to face stress, pain, and illness. :Bantam Books; New York: 2013. [Google Scholar]
- 85.Zhao L., Xu J., Liang F., Li A., Zhang Y., Sun J. Effect of chronic psychological stress on liver metastasis of colon cancer in mice. PLOS ONE. 2015;10(10) doi: 10.1371/journal.pone.0139978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdullah M., Sukartini N., Nursyirwan S.A., Pribadi R.R., Maulahela H., Utari A.P., et al. Gut microbiota profiles in early- and late-onset colorectal cancer: a potential diagnostic biomarker in the future. Digestion. 2021;102(6):823–832. doi: 10.1159/000516689. [DOI] [PubMed] [Google Scholar]
- 88.Fulwiler C., Brewer J.A., Sinnott S., Loucks E.B. Mindfulness-based interventions for weight loss and CVD risk management. Curr. Cardiovasc. Risk Rep. 2015;9(10):46. doi: 10.1007/s12170-015-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia T., Lopes S., Chen L., Roth R., Zinzow H., Jones K., et al. A feasibility study on low-dose mindfulness-based stress reduction intervention among prediabetes and diabetes patients. Complement Ther. Med. 2022;65 doi: 10.1016/j.ctim.2022.102810. [DOI] [PubMed] [Google Scholar]
- 90.Bower J.E., Crosswell A.D., Stanton A.L., Crespi C.M., Winston D., Arevalo J., et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231–1240. doi: 10.1002/cncr.29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Massion A.O., Teas J., Hebert J.R., Wertheimer M.D., Kabat-Zinn J. Meditation, melatonin and breast/prostate cancer: hypothesis and preliminary data. Med. Hypotheses. 1995;44(1):39–46. doi: 10.1016/0306-9877(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 92.Srinivasan V., Spence D.W., Pandi-Perumal S.R., Trakht I., Cardinali D.P. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr. Cancer Ther. 2008;7(3):189–203. doi: 10.1177/1534735408322846. [DOI] [PubMed] [Google Scholar]
- 93.Mehta R., Sharma K., Potters L., Wernicke A.G., Parashar B. Evidence for the role of mindfulness in cancer: benefits and techniques. Cureus. 2019;11(5):e4629. doi: 10.7759/cureus.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Biggers A., Spears C.A., Sanders K., Ong J., Sharp L.K., Gerber B.S. Promoting mindfulness in African American communities. Mindfulness (N Y). 2020;11(10):2274–2282. doi: 10.1007/s12671-020-01480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lima Oliveira M., Biggers A., Oddo V.M., Naylor K.B., Chen Z., Hamm A., et al. Design of a remote time-restricted eating and mindfulness intervention to reduce risk factors associated with early-onset colorectal cancer development among young adults. Nutrients. 2024;16(4):504. doi: 10.3390/nu16040504. [DOI] [PMC free article] [PubMed] [Google Scholar]