Abstract
Objective:
To determine whether serum trace metals are related to abnormal cognition in Alzheimer’s disease (AD).
Methods:
We studied serum lead (Pb), cadmium (Cd), mercury (Hg), and arsenic(As) in 89 patients with AD and in 118 cognitively normal individuals. We analyzed the results of the blood tests and the food intake.
Results:
Serum Pb levels correlated with word list recall (P = .039) and word list recognition (P = .037). Without age adjustment, serum Cd levels (P = .044) were significantly higher in the AD group. After stratified age adjustment, the levels of selected trace metals did not differ significantly between AD and normal individuals. Food intakes regarding selected trace metals were not significantly different between the 2 groups.
Conclusions:
In this study, serum Pb, Cd, Hg, and As levels were not directly related to abnormal cognition in AD. Serum Pb levels were significantly negatively correlated with verbal memory scores.
Keywords: heavy metal toxicity, Alzheimer’s disease, lead, cadmium
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia among older people. Although the greatest known risk factor for AD is advancing age, the precise etiology of AD remains elusive. Ongoing efforts seek to understand the cause or causes of AD, yet the picture remains complex. Some studies have suggested a role of oxidative stress in the pathogenesis of AD, 1,2 and there are increasing evidences for an imbalance in transition metal homeostasis in AD. 3 –5 Additionally, several studies suggest that trace elements may be important in the pathogenesis of AD. 6 –12 Those results that the homeostasis of these metals is perturbed in AD and that metal ions are concentrated in senile plaques, neurofibrillary tangles, and cerebrospinal fluid (CSF) supports this notion. 13
Several metals have been proposed as pathogenic cofactors in AD. Among the various toxic heavy metals, cadmium (Cd), lead (Pb), and mercury (Hg) are especially prevalent in nature because of their high industrial use. These metals serve no biological function and their presence in tissues reflects contact between the organism and its environment. They are cumulative poisons and are toxic even at a low dose. 14 Arsenic (As), typically considered a heavy metal, shares many toxic characteristics with the other heavy metals. Previous research has shown that As exposure induces changes that coincide with most of the developmental, biochemical, pathological, and clinical features of AD and associated disorders. 15
Lead is the most historically pervasive and well-established neurotoxic pollutant. 16 Experimental evidence indicates that Pb can cause white matter damage, cell death, and changes in cellular architecture. 17 The Pb is thought to be interfere with functions essential for neuronal homeostasis, such as inhibiting glycolytic enzymes in neurotransmitter metabolism. 18
Oxidative stress appears to play a major role in chronic Cd-induced hepatic and renal toxicity. 19 A decrease in the turnover of brain acetylcholine in Pb- or Cd-treated animals was found in some studies. 20,21 The lateral choroid plexus sequesters Hg, Cd, As, and Pb. This is probably an important mechanism to protect the CSF and the brain from fluxes of heavy metals in the blood. However, Hg and Cd can directly damage the choroid plexus. 22
In vitro models showed that inorganic Hg reproduces all the pathological changes seen in AD, and in animal models, inorganic Hg produced changes similar to those seen in AD. 23
Although toxicity and the resulting threat to human health are a function of the concentration of a contaminant, chronic exposure to As, Cd, Hg, and Pb at relatively low levels can cause adverse effects. 24 For decades, these selected toxic metals have been included in surveys. 24 –27 The Korea Food and Drug Administration (KFDA) has been responsible for monitoring metal contents, especially for these selected toxic metals (As, Cd, Hg, and Pb). 28,29
The primary aim of this study was to investigate whether levels of serum trace metals in patients with AD differed from those in cognitively normal elderly individuals. Therefore, we measured the levels of trace metals in sera from a group of cognitively normal elderly individuals and a group of individuals with AD. A secondary aim was to determine whether serum trace metals are related to abnormal cognition in the elderly individuals. To this end, we administered the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K) to assess cognition, and we assessed the relationship between cognitive ability and selected serum trace metal levels.
Methods
In all, 89 patients with probable AD (diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association guidelines) 30 and 118 healthy elderly individuals were recruited from 1 specialized dementia clinic and 1 care center for elderly individuals, and we evaluated their trace substance exposure status.
Criteria for exclusion included any conditions known to affect trace metal levels and biological variables of oxidative stress, assessed on the basis of previous medical history and laboratory screening tests. Individuals with mild cognitive impairment (MCI) were excluded by established clinical consensus criteria, 31 and CERAD-K total score 1 (cutoff score 59.5, sensitivity 79.2%, and specificity 71.0%) was also used accessorily. 32 All patients with AD underwent neurologic and extensive neuropsychological evaluation as well as routine laboratory tests. Magnetic resonance imaging (MRI) was performed to rule out frontotemporal dementia or vascular dementia.
Blood was sampled using disposable stainless steel needles after disinfecting the part in which blood was collected with 70% isopropyl alcohol. Because the general public, in which concentration would be low, are included in the samples, in particular, the effect of pollution which can occur during sampling and inspection on the results of analysis is relatively more important. Then, the blood was sampled in Serum Separation Tube (SST, 5 mL each) for the analysis of serum. They were not in fasted state. The serum was centrifuged at 2500 rpm for 5 minutes, and the collected samples were kept at −70°C until they were used in test. The standard solution of each target material (Pb, As, and Cd) used in this study is Perkin-Elmer’s multi-element calibration standard 3 (10 mg/kg; Perkin-Elmer, NY). Kinto Chemical ’s solution (1000 mg/L; Kinto Chemical, Tokyo) was used as the standard solution of Hg.
For sample preparation and analysis, the triple distilled water (Milli-Q system, Millipore, Massachusetts) was used. In this study, nitric acid (67% v/v) and hydrogen peroxide (31% v/v) that would be used in the Microwave Digestion System, DONGWOO FINE-CHEM’s (Seoul, Korea) semiconductor level was used.
For the accurate validation of the analysis method, a certificated reference material (Lyphochel Whole Blood Metals Control Level 1; Biorad, Berkeley, California) was used in the test.
Inductively coupled plasma-mass spectrometry system (Perkin-Elmer, NY) was used for measurement. The gold-Amalgamated Direct Mercury Analyzer 80, manufactured by Milestone (Sorisole, Italy), was used for analysis of Hg. Analysis of all samples was performed at Advanced Analysis Center, Korea Institute of Science and Technology, Seoul, Korea.
The control sample consisted of elderly volunteers with no clinical evidence of neurologic or psychiatric disease. Neither participants nor patients had taken any trace metal-containing supplements for at least 30 days prior to the study.
To verify whether there are significant effects of selected trace metal-related food intake on serum level or cognitive function of both the groups, we evaluated selected trace metal-related food intake. To compare the trace metal-related food intake, we selected 10 high trace metal-containing foods per trace metal using the total diet study 33 data, which are established in the KFDA.
Food intake data were calculated by computerized analysis program CAN Pro 3.0 (2002). In analyzing differences in food intake, a generalized linear model was used for adjust age and sex.
The study was approved by the Sanggye Paik hospital internal review board which conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000), and all participants or legal guardians provided their written informed consent.
Cognitive Evaluation
Dementia was defined according to the diagnostic features given in the Diagnostic and Statistical Manual of Mental Disorders, (Fourth Edition, Text Revision). The CERAD-K was used for the evaluation of cognitive functions. A detailed description of the CERAD-K can be found elsewhere. 34
The AD was diagnosed according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s disease and Related Disorders Association (NINCDS-ADRDA). Vascular dementia was excluded according to the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignementen Neurosciences (NINDS-AIREN) criteria.
Statistics
Selected trace metal levels were compared between the AD group and the control group by using a t test. A bivariate correlation test was conducted between CERAD subscales and each trace metal. In order to control for the potentially confounding effects of age and years of education, partial correlations were also conducted.
High (≥75th percentile), medium, and low (≤25th percentile) selected trace metals were classified on the basis of serum levels. The odds ratio (OR) of selected serum trace metal levels associated with AD was calculated. A stratified analysis was used to account for age differences between the AD and the healthy elderly individual groups.
All statistical tests were performed using SAS 4.0 and Medcalc software. 35,36 In all cases, a 2-tailed P < .05 was considered as the threshold for statistical significance.
Results
We studied the serum levels of selected trace metals in 89 patients with AD (mean age, 77.83 years; 41 men, 48 women) and in 118 cognitively normal control individuals (mean age, 69.93 years; 50 men, 68 women; see Table 1). Cognitive functions were also assessed in both the groups.
Table 1.
Comparison of Sociodemographic Data and Cognitive Function Test Results Between Alzheimer’s Disease and Control (Normal Elderly Individuals) Groups.a
| Normal Elderly Group (n = 118) | AD Group (n = 89) | P Value | |
|---|---|---|---|
| Age | 69.93 ± 5.89 | 77.83 ± 6.65 | <.001b |
| Sex, M/F | 50/68 | 41/48 | .596 |
| Years of education | 7.66 ± 4.35 | 6.94 ± 4.40 | .252 |
| J1 Verbal fluency test: animal category | 13.30 ± 4.36 | 7.99 ± 4.05 | <.001b |
| J2 Modified Boston Naming test | 10.52 ± 2.59 | 7.21 ± 3.47 | <.001b |
| J3 MMSE | 24.86 ± 3.38 | 16.84 ± 5.51 | <.001b |
| J4 Word list memory | 14.87 ± 3.94 | 8.21 ± 3.94 | <.001b |
| J5 Constructional praxis | 9.56 ± 1.81 | 7.48 ± 2.51 | <.001b |
| J6 Word list recall | 4.69 ± 2.09 | 1.55 ± 1.70 | <.001b |
| J7 Word list recognition | 8.27 ± 1.88 | 4.23 ± 3.10 | <.001b |
| J8 Constructional recall | 5.68 ± 2.78 | 1.33 ± 1.71 | <.001b |
Abbreviations: AD, Alzheimer’s disease; F, female; M, male; MMSE, Mini-Mental State Examination.
a Values are given as means ± standard error of the mean.
b Significant difference between groups.
Patient and control groups differed significantly in their mean Mini-Mental State Examination (MMSE) scores and other CERAD subscores (Table 1). Serum Cd levels were significantly higher in the AD group (0.046 ± 0.029 µg/dL, P = .044) than in the control group.
There was a significant difference in the age distribution between the AD and the control groups (P < .001). Therefore, the correlation between age and serum trace metal levels was also examined. Serum Hg levels had a significant negative correlation with age (r = −.249; P = .004), but other trace metals (Pb, Cd, and As) were not significantly correlated with age.
Therefore, in order to compensate for any effects of demographic differences between the 2 groups, a stratified analysis was performed. Ages were stratified in 5-year intervals, and individuals from each group were matched according to age. As a result, 131 people (control 67; AD 64) were selected. Between these 2 groups, there were no significant age differences (P = .087). After the stratified analysis, the concentrations of the selected serum trace metals were again compared between the 2 groups (Table 2). Serum levels of the selected trace metals did not differ significantly between the AD group and the normal control group.
Table 2.
Comparison of Serum Heavy Metal Levels Between AD and Control Group (µg/L; Stratified to Adjust for Age Differences).a
| Normal Elderly Group (n = 67) | AD Group (n = 64) | P Value | |
|---|---|---|---|
| Age | 73.36 ± 5.29 | 74.97 ± 5.37 | .087 |
| Sex, M/F | 30/37 | 30/34 | .810 |
| Years of education | 7.45 ± 4.58 | 7.50 ± 4.18 | .952 |
| MMSE | 24.24 ± 3.84 | 17.92 ± 5.08 | <.001 |
| Pb | 1.96 ± 1.31 | 1.52 ± 1.41 | .065 |
| Cd | 0.040 ± 0.022 | 0.048 ± 0.030 | .084 |
| As | 28.66 ± 11.25 | 28.08 ± 10.23 | .309 |
| Hg | 1.54 ± 1.04 | 1.46 ± 0.81 | .488 |
Abbreviations: AD, Alzheimer’s disease; F, female; M, male; MMSE, Mini-Mental State Examination; Pb, lead; Cd, cadmium; As, arsenic; Hg, mercury.
a Values are mean ± standard error of the Mean.
Bivariate correlations were conducted to assess the relationship between trace metal levels and CERAD subscores (Table 3). These correlations were calculated across both AD and control groups. Serum Cd levels were significantly negatively correlated with scores on the verbal fluency test (J1, P = .024) and MMSE (J3, P = .028). Serum Pb levels were significantly negatively correlated with scores on word list recognition (J7, P = .025). Serum As and Hg had no significant positive or negative correlation with any CERAD subscore.
Table 3.
Correlations Between Serum Copper Levels and Cognitive Functions.
| J1 | J2 | J3 | J4 | J5 | J6 | J7 | J8 | ||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Pearson correlation | −.042 | −.114 | −.09 | −.086 | .061 | −.149a | −.17a | −.106 |
| Sig (2-tailed) | .565 | .115 | .213 | .233 | .404 | .039 | .019 | .143 | |
| Cd | Pearson correlation | −.115 | −.057 | −.087 | −.013 | −.052 | −.073 | −.055 | −.059 |
| Sig (2-tailed) | .113 | .431 | .228 | .854 | .472 | .312 | .446 | .417 | |
| As | Pearson correlation | −.017 | −.035 | .046 | −.031 | .056 | −.031 | −.001 | .047 |
| Sig (2-tailed) | .815 | .627 | .523 | .665 | .438 | .667 | .99 | .517 | |
| Hg | Pearson correlation | −.052 | .005 | −.013 | −.077 | .061 | −.121 | −.117 | −.039 |
| Sig (2-tailed) | .473 | .946 | .862 | .291 | .401 | .095 | .105 | .596 |
Abbreviations: Pb, lead; Cd, cadmium; As, arsenic; Hg, mercury; MMSE, Mini-Mental State Examination; J1, verbal fluency test: animal category; J2, Modified Boston Naming test; J3, MMSE; J4, word list memory; J5, constructional praxis; J6, word list recall; J7, word list recognition; J8, constructional recall; Sig, significant.
a Correlation is significant at the .05 level (2-tailed).
After correcting for differences in age and education level with partial correlation analysis, we again investigated the correlation between trace metals and cognition. Serum Pb levels were negatively correlated with scores on Word List Recall (r = −.149, P = .039; Figure 1) and Word List Recognition (r = −.17, P = .019; Figure 2) tests.
Figure 1.
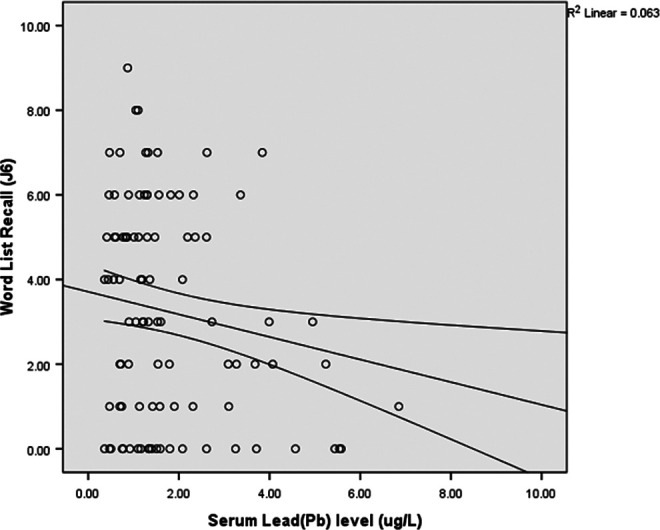
Correlations between serum lead levels and scores on the Word List Recall test (r = −.149; P = .039).
Figure 2.
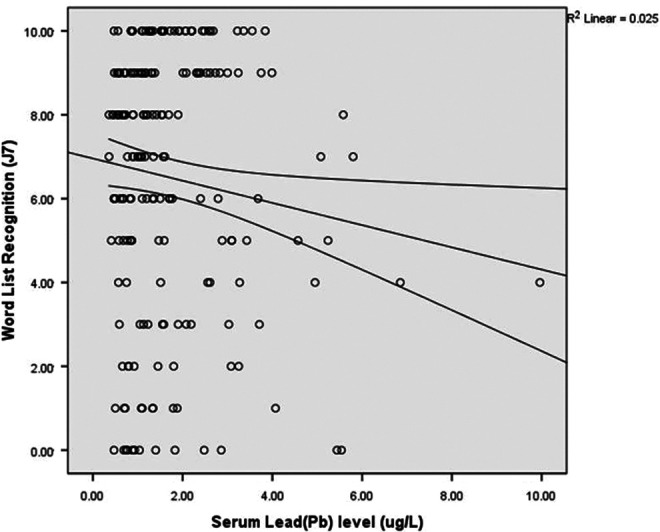
Correlations between serum lead levels and scores on the Word List Recognition test (r = −.17; P = .019).
The odds of AD in the upper 25th percentile serum Cd level group and the lower 25th percentile group were not statistically significant (OR, 1.68; 95% confidence interval [CI], 0.76-3.69, P = .19). The odds of AD in the upper 50th percentile serum Cd level group and the lower 50th percentile group were also not statistically significant (OR, 1.10; 95% CI, 0.63-1.91, P = .71).
The upper 25th percentile serum Pb level group did not have significantly higher odds of AD than the lower 25th percentile group (OR, 0.65; 95% CI, 0.29-1.42; P = .28). Additionally, the upper 50th percentile serum Pb level group did not have significantly higher odds of AD than the lower 50th percentile group (OR, 0.94; 95% CI, 0.54-1.63; P = .84).
According to the results of surveillance program for the establishment of safety guidelines for food products in Korea, 33 we measured intakes of 10 leading foods ranked by each selected trace metals. But there were no significant differences between AD and normal groups in intake grams per day for all chosen foods (Table 4).
Table 4.
Comparison About Leading Foods Intake Grams Per Day Ranked by Each Selected Trace Metals Between Normal Elderly Group and Alzheimer’s Disease Group.
| Normal Elderly Group (n = 118) | AD (n = 89) | P Value | |
|---|---|---|---|
| Mean ± SD, g/d | Mean ± SD, g/d | ||
| Seaweeda,b,d | 0.1 ± 0.9 | 0.2 ± 1.8 | .9665 |
| Anchovya,b,d | 3.6 ± 4.6 | 3.3 ± 5.1 | .8691 |
| Short-necked clama,b,d | 1.4 ± 6.8 | 1.0 ± 3.0 | .7775 |
| Lavera,b,d | 0.9 ± 4.0 | 0.8 ± 1.4 | .7712 |
| Squidb,c,d | 0.6 ± 2.1 | 1.0 ± 3.5 | .1167 |
| Peacha,b | 33.1 ± 67.1 | 23.3 ± 47.6 | .7329 |
| Spinacha,b | 3.2 ± 7.4 | 3.1 ± 7.3 | .2219 |
| Crabb,d | 2.4 ± 14.5 | 2.1 ± 15.4 | .7273 |
| Mackerelc,d | 5.2 ± 9.7 | 4.0 ± 8.3 | .3706 |
| Common eelb,c | 0.4 ± 4.3 | 1.1 ± 6.8 | .6596 |
| Soybean oila | 2.2 ± 1.8 | 2.5 ± 2.3 | .1296 |
| Turnip juicea | 1.9 ± 5.4 | 2.1 ± 5.1 | .1722 |
| Sweet potatoa | 8.9 ± 25.7 | 10.4 ± 28.7 | .7113 |
| Rice winea | 19.1 ± 70.2 | 2.7 ± 15.6 | .2391 |
| Powdered red pepperb | 1.3 ± 1.2 | 1.2 ± 1.1 | .9643 |
| Glutinous riceb | 3.7 ± 6.0 | 3.1 ± 7.9 | .5148 |
| Polished ricec | 53.9 ± 31.2 | 51.5 ± 29.9 | .2886 |
| Hair tail fishc | 2.2 ± 7.8 | 1.7 ± 6.9 | .7326 |
| Fish pastec | 1.2 ± 5.3 | 2.1 ± 5.8 | .3262 |
| Walleye Pollackc | 3.2 ± 9.5 | 3.2 ± 8.3 | .4888 |
| Flatfishc | 0.2 ± 2.1 | 0.1 ± 1.2 | .6952 |
| Canned tunac | 1.0 ± 4.6 | 0.6 ± 3.6 | .6582 |
| Croakerc | 3.3 ± 11.8 | 3.0 ± 11.1 | .7492 |
| Common octopusc | 0.3 ± 2.4 | 0.5 ± 2.8 | .5576 |
| Tangleweedd | 0.1 ± 0.3 | 0.1 ± 0.4 | .1142 |
Abbreviations: AD, Alzheimer’s disease; SD, standard deviation.
a 10 leading foods selected by high lead contents.
b 10 leading foods selected by high cadmium contents.
c 10 leading foods selected by high mercury contents.
d 10 leading foods selected by high arsenic contents.
Discussion
There were no differences in selected trace metal levels between AD and cognitively normal groups. Although we couldn’t find diagnostic implication for AD diagnosis, there are some clinically meaningful analysis results with Pb and Cd.
Lead
In adults, Pb exposure is associated with impaired cognitive function in cross-sectional and longitudinal studies 37 –41 In our study, there was no significant difference in serum Pb levels between the 2 groups, but there was a significant negative correlation between serum Pb levels and verbal memory scores. Previous studies showed similar results. Bleecker et al 42 found that chronic Pb exposure affects encoding/storage and retrieval of verbal information. Weisskopf et al 43 also found that blood Pb levels were cross-sectionally associated with performance only on a vocabulary test.
In workers with high blood Pb concentrations, some evidence suggested mild impairment of attention, verbal memory, and linguistic processing. 44 One study of 535 former organo-Pb manufacturing workers with past mixed exposures to organic and inorganic Pb, peak tibia Pb levels, which measured tibial lead concentration by x-ray fluorescence, predicted longitudinal declines for 6 tests of verbal memory and learning, visual memory, executive ability, and manual dexterity. 40
Some explanations can be proposed for the relationship between verbal memory and serum Pb levels. The Pb has been reported to affect specific areas in the brain such as the hippocampus and frontal cortex. 45 Exposure to Pb may impair the processes of encoding and response execution, in addition to the rate of scanning items in short-term verbal memory. 46 A more likely explanation may be that neural systems subserving language functions are more sensitive than other cognitive functions to perturbations from the effects of Pb. These factors may explain the inverse association we found between blood Pb and vocabulary score.
Other studies have investigated the relationship between Pb and other cognitive functions in addition to verbal memory. Nervous system symptoms such as irritability, poor attention and concentration, forgetfulness, depressed effect, and sleep disturbance are common after lower doses. 47,48 Even at very low levels, Pb is associated with impaired cognitive function in children. 49
Chronic, low-dose exposure to Pb may adversely affect cognitive function in older age in several ways. Several possible mechanisms that could result in structural changes to the brain support the hypothesis that there is a relationship between Pb and cognitive decline. The Pb could increase apoptosis, 50 cause changes in cellular architecture, increase oxidative stress, or enhance vascular or inflammatory mechanisms. 51 The Pb alters the permeability of the blood–brain barrier 52 and accumulates in astroglia that are essential for the maintenance of the neuronal environment. 17 The Pb exposure is now thought to interfere with several calcium-dependent processes and activate protein kinase C, which has been implicated in neurotoxicity. 53 Circulating blood Pb could affect synaptic transmission and plasticity and thereby impede, for example, information storage mechanisms or processing speed, which has been suggested to weaken performance on cognitive tests. 54
In future studies, more incisive measurements offered by imaging technology (eg, structural MRI volumetric analysis) would provide the means to evaluate longitudinal changes in volume as a function of cumulative Pb dose and to evaluate whether changes in structural volumes are associated with changes in understanding the locus of change in the brain.
Although there were no differences in serum Pb levels between the AD and the control groups, circulating serum Pb levels may predict performance on some cognitive tests.
The MCI is more prevalent than AD and is receiving increasing attention not only as a possible intermediate stage on the path to AD but also as an important deficit in its own right. 55 Significant benefits could result from improved understanding of modifiable risk factors for subclinical cognitive impairment, because this condition may be the most amenable to prevention and treatment. 43 Exposure to Pb may be one such modifiable risk factor.
Even if Pb has a subtle effect in accelerating cognitive aging, given the pervasiveness of Pb exposure in Korea and the world, widespread reductions in exposure could have a substantial impact on the burden of cognitive impairment in the population. Because Pb is difficult to excrete, older adults, by virtue of their longer life spans, generally have accrued higher Pb exposures than younger adults, regardless of whether these exposures originated from occupational or, more commonly, nonoccupational sources. 56
Our results suggest that exposure to Pb in a nonoccupational setting is associated with accelerated decline in cognition.
Cadmium
In this study, serum Cd levels in the AD and control groups were significantly different when the data were not adjusted for age. After age adjustment, however, there was no significant difference. In studies by Bomboi et al 57 and Gerhardsson et al, 58 there was no significant difference in Cd levels between AD and control groups. In the studies by Bocca et al 59 and Basun et al, 60 although the serum Cd level was significantly higher in the AD group than in the control group, 61 it was also found that the Cd concentration was high in the liver tissue and blood of patients with AD. Cognitive functions as assessed by CERAD were not related to the Cd levels in the blood in either of the groups. This may indicate that blood Cd concentrations lower than the level known to produce acute toxicity do not affect cognitive functions.
The following mechanisms can be proposed about the relation between Cd and AD. Neurotoxicity has been reported in lactating pups of dams exposed to Cd, 62 but the relationship remains controversial because Cd does not pass the blood–brain barrier. Thus, the mechanism is not known. Whether there is a direct effect (for example, toxicity may be due to the interference of Cd with Zn metabolism and third member of the metallothionein in the brain 63 ) is at present not clear. Another explanation posits that Cd could be taken up via the olfactory bulb, as described for Ni. 64
In this study, there was no relation between Cd and AD. Nonetheless, investigating the effects of Cd remains important. We are frequently exposed to Cd in everyday life. Although some individuals are primarily exposed in the workplace, for most people the main route of exposure is through the diet. 24 In muscles and other organs, Cd accumulates from lifetime exposure to food and tobacco smoke. 60
We presume that this study will provide promising preliminary data for future research. Studies into the effects of selected trace metals in dementia should continue. Metals could be connected with the risk factors for dementia or the pathophysiology of dementia.
Limitations
This study was cross-sectional in design and therefore subject to the limitations of that design. Further longitudinal analysis is needed to investigate whether the effect of Pb is persistent.
Serum Pb represents the acute Pb dose. Chronic Pb exposure can be measured in the tibia using (109)Cd-induced K-shell x-ray fluorescence. Higher tibia Pb is consistently associated with poorer cognitive function and with lower brain volume. 51
Considering MMSE, at least some of the have had clinical MCI in normal elderly group.
We could not evaluate the effects of smoking. Inhalation of tobacco smoke contributes significantly to the Cd concentration in kidney. The kidney Cd concentration is approximately 2 times higher in smokers than in nonsmokers. Among nonsmokers, a slight increase in the blood Cd concentration can usually be seen with age.
The concentration of Cd in the blood is a useful indicator of ongoing exposure in recent months but it may also be partly related to lifetime accumulation.
In spite of these limitations, efforts to find an association between metals and cognitive functions are valuable. Sustainable development of research into metals and AD could improve our understanding of the pathophysiology and progression of AD.
Conclusion
In this study, serum Pb, Cd, Hg, and As levels were not directly related to abnormal cognition in AD. The most interesting finding that emerged from this investigation was the significant negative correlation between serum Pb levels and some CERAD subscores related to verbal memory.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant (11162KFDA701) from Korea Food & Drug Administration in 2011.
References
- 1. Casado A, Encarnación López-Fernández M, Concepción Casado M, de La Torre R. Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer dementias. Neurochem Res. 2008;33(3):450–458. [DOI] [PubMed] [Google Scholar]
- 2. Delibas N, Ozcankaya R, Altuntas I. Clinical importance of erythrocyte malondialdehyde levels as a marker for cognitive deterioration in patients with dementia of Alzheimer type: a repeated study in 5-year interval. Clin Biochem. 2002;35(2):137–141. [DOI] [PubMed] [Google Scholar]
- 3. Adlard PA, Bush AI. Metals and Alzheimer's disease. J Alzheimers Dis. 2006;10(2-3):145–163. [DOI] [PubMed] [Google Scholar]
- 4. Bonda DJ, Lee HG, Blair JA, Zhu X, Perry G, Smith MA. Role of metal dyshomeostasis in Alzheimer's disease. Metallomics. 2011;3(3):267–270. doi:10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hegde ML, Anitha S, Latha KS, et al. First evidence for helical transitions in supercoiled DNA by amyloid beta peptide (1-42) and aluminum: a new insight in understanding Alzheimer's disease. J Mol Neurosci. 2004;22(1-2):19–31. [DOI] [PubMed] [Google Scholar]
- 6. Dani SU. Arsenic for the fool: an exponential connection. Sci Total Environ. 2010;408(8):1842–1846. doi:10.1016/j.scitotenv.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 7. Duce JA, Bush AI. Biological metals and Alzheimer's disease: implications for therapeutics and diagnostics. Prog Neurobiol. 2010;92(1):1–18. doi:10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 8. Gharibzadeh S, Hoseini SS. Arsenic exposure may be a risk factor for Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2008;20(4):501. doi:10.1176/appi.neuropsych.20.4.501. [DOI] [PubMed] [Google Scholar]
- 9. Haraguchi T, Ishizu H, Takehisa Y, et al. Lead content of brain tissue in diffuse neurofibrillary tangles with calcification (DNTC): the possibility of lead neurotoxicity. NeuroReport. 2001;12(18):3887–3890. [DOI] [PubMed] [Google Scholar]
- 10. Lam PK, Kritz-Silverstein D, Barrett Connor E, et al. Plasma trace elements and cognitive function in older men and women: the Rancho Bernardo study. J Nutr Health Aging. 2008;12(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McClain CJ, McClain M, Barve S, Boosalis MG. Trace metals and the elderly. Clin Geriatr Med. 2002;18(4):801–818. [DOI] [PubMed] [Google Scholar]
- 12. Mutter J, Naumann J, Schneider R, Walach H. Mercury and Alzheimer's disease [in German]. Fortschr Neurol Psychiatr. 2007;75(9):528–538. doi:10.1055/s-2007-959237. [DOI] [PubMed] [Google Scholar]
- 13. Atwood CS, Huang X, Moir RD, Tanzi RE, Bush AI. Role of free radicals and metal ions in the pathogenesis of Alzheimer's disease. Met Ions Biol Syst. 1999;36:309–364. [PubMed] [Google Scholar]
- 14. Chowdhury BA, Chandra RK. Biological and health implications of toxic heavy metal and essential trace element interactions. Prog Food Nutr Sci. 1987;11(1):55. [PubMed] [Google Scholar]
- 15. Gong G, O'Bryant SE. The arsenic exposure hypothesis for Alzheimer disease [published online May 13, 2010]. Alzheimer Dis Assoc Disord. 2010. doi:10.1097/WAD.0b013e3181d71bc7. [DOI] [PubMed] [Google Scholar]
- 16. Weuve J, Korrick SA, Weisskopf MA, et al. Cumulative exposure to lead in relation to cognitive function in older women. Environ Health Perspect. 2009;117(4):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khalil N, Morrow LA, Needleman H, Talbott EO, Wilson JW, Cauley JA. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology. 2009;23(1):10–19. doi:10.1037/a0013757. [DOI] [PubMed] [Google Scholar]
- 18. Yun S, Hoyer S. Effects of low-level lead on glycolytic enzymes and pyruvate dehydrogenase of rat brain in vitro: relevance to sporadic Alzheimer's disease? J Neural Transm. 2000;107(3):355–368. [DOI] [PubMed] [Google Scholar]
- 19. Patra RC, Swarup D, Senapati SK. Effects of cadmium on lipid peroxides and superoxide dismutase in hepatic, renal and testicular tissue of rats. Vet Hum Toxicol. 1999;41(2):65. [PubMed] [Google Scholar]
- 20. Costa L, Fox D. A selective decrease of cholinergic muscarinic receptors in the visual cortex of adult rats following developmental lead exposure. Brain Res. 1983;276(2):259–266. [DOI] [PubMed] [Google Scholar]
- 21. Webster WS, Valois AA. The toxic effects of cadmium on the neonatal mouse CNS. J Neuropathol Exp Neurol. 1981;40(3):247. [DOI] [PubMed] [Google Scholar]
- 22. Gerhardsson L, Lundh T, Londos E, Minthon L. Cerebrospinal fluid/plasma quotients of essential and non-essential metals in patients with Alzheimer's disease. J Neural Transm. 2011;118(6):957–962. doi:10.1007/s00702-011-0605-x. [DOI] [PubMed] [Google Scholar]
- 23. Mutter J, Curth A, Naumann J, Deth R, Walach H. Does inorganic mercury play a role in Alzheimer's disease? A systematic review and an integrated molecular mechanism. J Alzheimers Dis. 2010;22(2):357–374. doi:10.3233/jad-2010-100705. [DOI] [PubMed] [Google Scholar]
- 24. Llobet J, Falco G, Casas C, Teixido A, Domingo J. Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. J Agric Food Chem. 2003;51(3):838–842. [DOI] [PubMed] [Google Scholar]
- 25. Alkorta I, Hernández-Allica J, Becerril J, Amezaga I, Albizu I, Garbisu C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev Environ Sci Biotechnol 2004;3(1):71–90. [Google Scholar]
- 26. Chigbo FE, Smith RW, Shore FL. Uptake of arsenic, cadmium, lead and mercury from polluted waters by the water hyacinth Eichornia crassipes Environ Pollut Series A Ecol Biol. 1982;27(1):31–36. [Google Scholar]
- 27. Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT. Effects of micronutrients on metal toxicity. Environ Health Perspect. 1998;106(suppl 1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hwang Y, Park S, Park G, Choi S, Kim M. Total arsenic, mercury, lead, and cadmium contents in edible dried seaweed in Korea. Food Addit Contamin Part B. 2010;3(1):7–13. [DOI] [PubMed] [Google Scholar]
- 29. Lee HS, Cho YH, Park SO, et al. Dietary exposure of the Korean population to arsenic, cadmium, lead and mercury. J Food Compos Anal. 2006;19(suppl):S31–S37. [Google Scholar]
- 30. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–939. [DOI] [PubMed] [Google Scholar]
- 31. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. [DOI] [PubMed] [Google Scholar]
- 32. Seo EH, Lee DY, Lee JH, et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry. 2010;18(9):801–809. [DOI] [PubMed] [Google Scholar]
- 33. KFDA. Dietary Intake and Risk Assessment of Contaminants in Korean Foods. Seoul, Korea: Korea Food and Drug Administration; 2009. [Google Scholar]
- 34. Lee JH, Lee KU, Lee DY, et al. Development of the Korean Version of the consortium to establish a registry for Alzheimer's disease assessment packet (CERAD-K). J Gerontol B Psychol Sci Soc Sci. 2002;57(1):P47–P53. [DOI] [PubMed] [Google Scholar]
- 35. SAS Institute. SAS Procedures Guide. Version 8. Vol 1. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 36. Schoonjans F. Medcalc Statistics for Biomedical Research: Software Manual [computer program]. Mariakerke, Belgium: Medcalc Statistical Software; 2003. [Google Scholar]
- 37. Mantere P, Hanninen H, Hernberg S, Luukkonen R. A prospective follow-up study on psychological effects in workers exposed to low levels of lead. Scand J Work Environ Health. 1984;10(1):43–50. [DOI] [PubMed] [Google Scholar]
- 38. Schroter C, Schroter H, Huffmann G. Neurologic and psychiatric manifestations of lead poisoning in adults (case report and review of the literature) [in German]. Fortschr Neurol Psychiatr. 1991;59(10):413–424. doi:10.1055/s-2007-1000716. [DOI] [PubMed] [Google Scholar]
- 39. Schwartz BS, Caffo B, Stewart WF, et al. Evaluation of cumulative lead dose and longitudinal changes in structural magnetic resonance imaging in former organolead workers. J Occup Environ Med. 2010;52(4):407–414. doi:10.1097/JOM.0b013e3181d5e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwartz BS, Stewart W, Bolla K, et al. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology. 2000;55(8):1144–1150. [DOI] [PubMed] [Google Scholar]
- 41. Weisskopf MG, Hu H, Mulkern RV, et al. Cognitive deficits and magnetic resonance spectroscopy in adult monozygotic twins with lead poisoning. Environ Health Perspect. 2004;112(5):620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bleecker ML, Ford DP, Celio MA, Vaughan CG, Lindgren KN. Impact of cognitive reserve on the relationship of lead exposure and neurobehavioral performance. Neurology. 2007;69(5):470–476. doi:10.1212/01.wnl.0000266628.43760.8c. [DOI] [PubMed] [Google Scholar]
- 43. Weisskopf MG, Proctor SP, Wright RO, et al. Cumulative lead exposure and cognitive performance among elderly men. Epidemiology. 2007;18(1):59–66. [DOI] [PubMed] [Google Scholar]
- 44. Stollery BT, Banks HA, Broadbent DE, Lee WR. Cognitive functioning in lead workers. Br J Ind Med. 1989;46(10):698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang W, Chen J, Wei QY, Chen XM. Effects of Brn-3a protein and RNA expression in rat brain following low-level lead exposure during development on spatial learning and memory. Toxicol Lett. 2006;164(1):63–70. [DOI] [PubMed] [Google Scholar]
- 46. Williamson A, Teo R. Neurobehavioural effects of occupational exposure to lead. Br J Ind Med. 1986;43(6):374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gordon J, Taylor A, Bennett P. Lead poisoning: case studies. Br J Clin Pharmacol. 2002;53(5):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tüzün M, Tüzün D, Salan A, Hekimoglu B. Lead encephalopathy: CT and MR findings. J Comput Assist Tomogr. 2002;26(3):479. [DOI] [PubMed] [Google Scholar]
- 49. Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect. 2004;112(9):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M. Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo. Neurosci Lett. 2002;329(1):45–48. [DOI] [PubMed] [Google Scholar]
- 51. Stewart WF, Schwartz BS. Effects of lead on the adult brain: a 15-year exploration. Am J Ind Med. 2007;50(10):729–739. doi:10.1002/ajim.20434. [DOI] [PubMed] [Google Scholar]
- 52. Strużyńska L, Walski M, Gadamski R, Dabrowska-Bouta B, Rafałowska U. Lead-induced abnormalities in blood-brain barrier permeability in experimental chronic toxicity. Mol Chem Neuropathol. 1997;31(3):207–224. [DOI] [PubMed] [Google Scholar]
- 53. Hwang KY, Lee BK, Bressler JP, Bolla KI, Stewart WF, Schwartz BS. Protein kinase C activity and the relations between blood lead and neurobehavioral function in lead workers. Environ Health Perspect. 2002;110(2):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403. [DOI] [PubMed] [Google Scholar]
- 55. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985. [DOI] [PubMed] [Google Scholar]
- 56. Vig EK, Hu H. Lead toxicity in older adults. JAm Geriatr Soc. 2000; 48 (11):1501. [PubMed] [Google Scholar]
- 57. Bomboi G, Marchione F, Sepe-Monti M, et al. Correlation between metal ions and clinical findings in subjects affected by Alzheimer's disease. Annali Ist Super Sanita. 2005;41(2):205–212. [PubMed] [Google Scholar]
- 58. Gerhardsson L, Lundh T, Minthon L, Londos E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25(6):508–515. [DOI] [PubMed] [Google Scholar]
- 59. Bocca B, Forte G, Petrucci F, et al. Monitoring of chemical elements and oxidative damage in patients affected by Alzheimer's disease. Ann Ist Super Sanita. 2005;41(2):197–203. [PubMed] [Google Scholar]
- 60. Basun H, Lind B, Nordberg M, Nordstrom M, Bjorksten KS, Winblad B. Cadmium in blood in Alzheimer's disease and non-demented subjects: results from a population-based study. Biometals. 1994;7(2):130–134. [DOI] [PubMed] [Google Scholar]
- 61. Basun H, Forssell LG, Wetterberg L, Winblad B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer's disease. J Neural Transm Park Dis Dement Sect. 1991;3(4):231–258. [PubMed] [Google Scholar]
- 62. Andersson H, Petersson-Grawe K, Lindqvist E, Luthman J, Oskarsson A, Olson L. Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol Teratol. 1997;19(2):105–115. [DOI] [PubMed] [Google Scholar]
- 63. Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19(4-5):529–535. [PubMed] [Google Scholar]
- 64. Tjälve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 2009;79(6):347–356. [DOI] [PubMed] [Google Scholar]


