ABSTRACT
Cancer is a common complication after kidney transplantation. Kidney transplant recipients (KTR) have a 2- to 4-fold higher risk of developing cancer compared to the general population and post-transplant malignancy is the third most common cause of death in KTR. Moreover, it is well known that certain cancer types are overrepresented after transplantation, especially non-melanoma skin cancer. Immune checkpoint inhibitors (ICI) have revolutionized the treatment of cancer, with remarkable survival benefit in a subgroup of patients. ICI are monoclonal antibodies that block the binding of specific co-inhibitory signaling molecules. Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand programmed cell death ligand 1 (PD-L1) are the main targets of ICI. Solid organ transplant recipients (SOTR) have been excluded from clinical trials owing to concerns about tumor response, allo-immunity, and risk of transplant rejection. Indeed, graft rejection has been estimated as high as 48% and represents an emerging problem. The underlying mechanisms of organ rejection in the context of treatment with ICI are poorly understood. The search for restricted antitumoral responses without graft rejection is of paramount importance. This review summarizes the current knowledge of the use of ICI in KTR, the potential mechanisms involved in kidney graft rejection during ICI treatment, potential biomarkers of rejection, and how to deal with rejection in clinical practice.
Keywords: allograft rejection, cancer, immune checkpoint inhibitors, kidney transplantation
INTRODUCTION
Kidney transplantation is the treatment of choice for patients suffering from end-stage kidney disease, leading to better survival and quality of life compared to dialysis. Long-term outcomes in kidney transplantation have improved significantly due to better pre-transplantation matching techniques, improved surgical techniques, surveillance, and management of infectious and cardiovascular complications [1]. However, this prolonged survival is at the expense of an increased prevalence of cancer in kidney transplant recipients (KTR). The cumulative incidence of cancer rises according to the years after transplantation and reaches >25% after 20 years of transplantation [2]. Moreover, some cancer types are overrepresented in KTR with the greatest standardized incidence ratio observed for Kaposi sarcoma, lip cancer, and non-melanoma skin cancer (NMSC) [2]. Importantly, NMSC has a more aggressive behavior with an increased risk of metastasis and death in KTR. Transplantation as an independent risk factor may negatively affect survival for different cancer types [3]. Factors associated with increased cancer risk are older age, male gender, white ethnicity, past medical history of smoking, a longer time on dialysis, and a previous history of cancer. It is also well recognized that the type, duration, and dose of immunosuppression, higher panel reactive antibody score, higher number of HLA-DR mismatches, and deceased organ donors are associated with an increased cancer risk [4]. Immune checkpoint inhibitors (ICI) have revolutionized the treatment of different cancer types, with remarkable survival benefit in a subgroup of patients [5, 6]. Solid organ transplant recipients (SOTR) have been excluded from clinical trials owing to concerns about tumor response, allo-immunity, organ rejection, and concomitant immunosuppressive therapy. Since the indications of ICI are expected to expand, it is important to determine the risk-benefit ratio of the use of ICI in patients with SOTR.
Immune checkpoints inhibitors
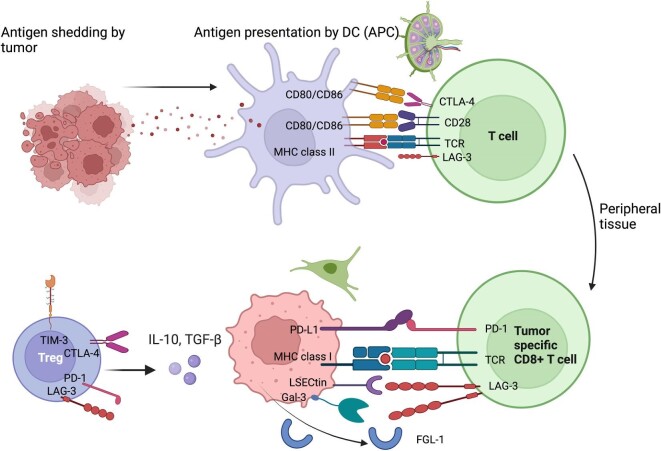
T lymphocytes are the critical players in antitumoral response and allograft rejection. The T cell activation process involves antigen presentation by major histocompatibility complex (MHC) molecules on the antigen-presenting cells (APCs) or tumor cells to the T cell receptor (TCR) on T cells (Fig. 1). Following engagement of the TCR with cognate antigen, CD28 provides the necessary second (co-stimulatory) signal for T cell activation by binding to CD80 (B7-[1]) and CD86 (B7-[2]) on APCs. The interaction with co-stimulatory molecules is tightly regulated by inhibitory checkpoints to avoid collateral damage and autoimmunity. Indeed, activated T cells express multiple co-inhibitory receptors such as lymphocyte-activation gene 3 (LAG-3), programmed cell death protein 1 (PD-1), and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) among others [7]. Programmed cell death ligand 1 (PD-L1), the primary ligand of PD-1, is expressed on different cell types, including T cells, B cells, tumor cells, and tumor-infiltrating myeloid cells. Interaction of PD-1 with PD-L1 on tumor cells induces T cell exhaustion within the tumoral environment (TME), maintains immune tolerance, and favors tumor escape. Relative to PD-1, CTLA-4 acts proximally at the T cell priming sites and limits the extent of T cell activation in secondary lymphoid organs. CTLA-4 also plays a prominent role in the regulation of regulatory T cells (Treg) within the TME [8–11]. LAG-3 is a co-inhibitory molecule expressed by cytotoxic CD8+ T cells and Treg. LAG-3 principally interacts with MHC class II expressed on APC but can also interact with liver sinusoidal endothelial cell lectin, Fibrogen-like protein–1 and galectin-3. Binding of LAG-3 to its ligands leads to inhibition of T cell proliferation, decreased cytokine production, and cytolytic function [12].
Figure 1:
Tumor-associated antigens are presented by APC in secondary lymphoid organs to T cells. Activation of T cells is tightly regulated by immune checkpoint of which CTLA-4 in the most important proximally. In peripheral tissues and at tumoral level PD-1/ PD-L1 pathways exert an inhibitory role on T cells and promotes cancer survival. Tumoral cells also express liver sinusoidal endothelial cell lectin (LSECtin), Galectin-3 (Gal-3), and fibrogen-like protein-1 (FGL-1) that bind LAG-3 to induce T cell anergy. Tumor survival and growth is further enhanced by the presence of tumor-infiltrating regulatory T cells that are known to express higher levels of CTLA-4, PD-1, LAG-3, and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) and to secrete higher levels of IL-10 and TGF-beta, promoting tumoral tolerance within the tumoral microenvironment.
ICI are monoclonal antibodies directed against the immune checkpoints of the immune system. To date, eight ICI have been approved for 17 different malignancies (Table 1) [11]. Moreover, a LAG-3 inhibitor, Relatlimab was recently approved for the treatment of advanced melanoma [13]. ICI are one of the core pillars in the treatment of cancer today, but its usage in SOTR is limited. Indeed, immunosuppressive medications act in direct opposition to ICI, which are used to enhance the adaptive immune response toward cancer antigens. On the other hand, activation of the immune system may lead to an enhanced immune reactivity including autoimmunity and allo-immunity with a greater risk of allograft rejection.
Table 1:
FDA approved ICI therapy and their current indications. Adapted from Wang et al. [73].
| Drug | Target | Date of approval by the FDA | Indications |
|---|---|---|---|
| Ipilimumab | CTLA-4 | 2011 | Melanoma, renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, non-small cell lung cancer, malignant pleural mesothelioma, esophageal cancer |
| Nivolumab | PD-1 | 2014 | Melanoma, non-small cell lung cancer, malignant pleural mesothelioma, renal cell carcinoma, classical Hodgkin lymphoma, squamous cell carcinoma of the head and neck, urothelial carcinoma, colorectal cancer, hepatocellular carcinoma, esophageal cancer, gastric cancer, gastroesophageal junction cancer, esophageal adenocarcinoma |
| Pembrolizumab | PD-1 | 2014 | Melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, classical Hodgkin lymphoma, primary mediastinal large B cell lymphoma, urothelial carcinoma, non-muscle invasive bladder cancer, colorectal cancer, gastric cancer, esophageal cancer, cervical cancer, hepatocellular carcinoma, Merkel cell carcinoma, renal cell carcinoma, endometrial carcinoma, cutaneous squamous cell carcinoma, triple-negative breast cancer |
| Atezolizumab | PD-L1 | 2016 | Non-small cell lung cancer, small cell lung cancer, hepatocellular carcinoma, melanoma, alveolar soft part sarcoma |
| Durvalumab | PD-L1 | 2017 | Non-small cell lung cancer, small cell lung cancer, biliary tract cancer, hepatocellular carcinoma |
| Avelumab | PD-L1 | 2017 | Merkel cell carcinoma, urothelial carcinoma, renal cell carcinoma |
| Cemiplimab | PD-1 | 2019 | Cutaneous squamous cell carcinoma, basal cell carcinoma, non-small cell lung cancer |
| Dostarlimab | PD-1 | 2021 | Endometrial cancer |
| Relatlimab | LAG-3 | 2022 | Melanoma |
Current data on the use of immune checkpoint inhibitors in kidney transplant recipients
The use of ICI in SOTR and KTR is mainly based on case reports, case series, and systematic reviews of the literature [14–21]. Most patients in the published reports were treated with anti-PD-1, were suffering from metastatic melanoma and were started on ICI with a mean of 9 years after transplantation. The rate of rejection is highest among KTR compared to liver, heart, and lung transplant patients and ranges from 41 to 48%. A common feature in the different case reports and case series published is the aggressiveness of the acute allograft rejection under ICI. Most papers report high levels of allograft loss (up to 83%) after rejection, with higher mortality for heart and liver transplants compared to KTR. This is mainly based on the fact that KTR can be hemodialyzed when graft loss occurs [14, 15]. When biopsies have been performed, histological analysis reveals mostly pure T cell-mediated rejection (TCMR) or mixed T cell and antibody-mediated rejection (ABMR). Although different regimens have been proposed ranging from corticosteroids, intravenous immunoglobulins, to thymoglobulin, and ultimately transplantectomy [15, 18], no effective treatment has been reported and response rates are poor.
Factors associated with graft rejection are a previous history of rejection, treatment with low-dose prednisone (<10 mg per day), the use of anti-PD-1 compared to anti-CTLA-4 or anti-PD-L1, and combination therapy (Table 2). Within the different types of anti-PD-1 used, pembrolizumab has the highest rejection rate, compared to nivolumab and cemiplimab [18]. Time after transplantation of at least 8 years, treatment with at least two immunosuppressive drugs, and/or an mTOR inhibitor-based regimen and grafts from deceased kidney donors are associated with a lower rejection rate after ICI treatment in KTR. Interestingly, patients suffering from cutaneous squamous cell carcinoma (cSCC) have the highest cancer response rates compared to other cancer types. Rünger et al., showed that SOTR suffering from cSCC have 59.4% response rates, defined as partial and complete response. Moreover, the ideal response (tumor response without graft rejection) was also highest (50%) in this subgroup of patients [20]. One small retrospective study with seven KTR suffering from advanced cSCC treated with cemiplimab demonstrated a good overall tumoral response (43%) with only one patient experiencing an allograft rejection [22]. Hanna et al. recently published the results of the CONTRAC-1 study. This open-label prospective study included 12 KTR suffering from advanced cSCC treated with cemiplimab. Overall response rate (ORR) was 46% (90% CI, 22 to 73) and no allograft rejection occurred during a median follow-up of 6.8 (range 0.7–29.8) months [23]. This is the first prospective study of KTR to show encouraging results concerning the use of anti-PD-1 for advanced cSCC.
Table 2:
| Risk of rejection | Up to 48% |
|---|---|
| Diagnosis | Rise in serum creatinine and kidney biopsy |
| Median time to graft rejection | 3 weeks |
| Histology of rejected allograft | TCMR or mixed TCMR and ABMR |
| Response to treatment | Poor with up to 70% graft loss |
| Risk factors | Low-dose corticosteroids, history of graft rejection, anti-PD-1 treatment or combination therapy |
| Factors associated with lower risk of rejection | mTOR and at least two immunosuppressants at time of ICI initiation |
KTR: kidney transplant recipients, TCMR: T cell-mediated rejection, ABMR: antibody-mediated rejection. PD-1: programmed cell death-1, mTOR: mammalian target of rapamycin
Most retrospective studies showed similar response rates for all cancer types in KTR compared to the general population. This should be interpreted with caution due to possible publication bias. However, the prospective, phase 1 study of Carroll et al. confirmed the good ORR in this patient population, but lacked a control group [24]. In a recent systematic review, it was also demonstrated that patients with an intact graft had a 1.7-fold higher tumor response rate compared to patients with graft rejection. This is a very interesting finding, but can be related to deleterious treatment with immunosuppression for allograft rejection, the premature stopping of the ICI and possibly death [20].
Mechanisms of allograft rejection in the context of ICI
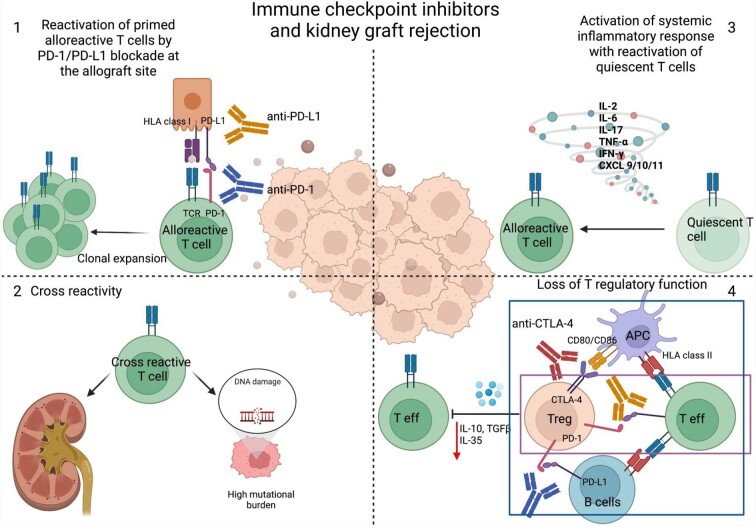
The mechanisms of allograft rejection in the context of ICI are poorly understood and not only explained by the reduction of maintenance immunosuppression as some studies show allograft rejection without prior reduction in immunosuppression (Fig. 2). First, one can imagine that quiescent alloreactive T cells are reactivated by ICI. Indeed, the PD-1/PD-L1 pathway is of utmost importance in maintaining peripheral tolerance in different experimental models of transplantation. In pre-clinical transplant models, blockade of PD-1/PD-L1 pathway in vivo with anti-PD-1 antibodies has been shown to lead to an accelerated rejection characterized by the expansion of alloreactive effector CD8+, Th1 differentiation of CD4+ T cells and decrease in FoxP3 CD4+CD25+ T cells infiltration in affected grafts [10, 25–27]. Interestingly, APC transfected with adenovirus coding for PD-L1 are able to enhance survival in fully mismatched kidney transplant models in rats [28] Furthermore, it is known that human tubular epithelial cells constitutively express PD-L1 and PD-L2, and that the expression can be upregulated by IFN-β and IFN-γ [29]. On a molecular level, KTR with allograft rejection have an upregulation of tissue PD-L1 mRNA compared to KTR with interstitial fibrosis/tubular atrophy or BK nephropathy [30]. This points to a potential protective effect of PD-L1 upregulation during immune activation. In mouse kidney transplant models, the PD-1/PD-L1 pathway was also important to prevent acute rejection immediately after transplantation, and thus was not only related to chronic antigen stimulation of T cells [31]. Ex vivo perfusion of donor kidneys with membrane-anchored-protein PD-L1 (map-PD-L1) in a rat model protected against acute kidney rejection with a reduction in T cell graft infiltration and increase in Treg [32]. However, the PD-1/PD-L1 pathway is only effective when the TCR is engaged. In a pancreatic islet allograft model treated with anti-CD3 antibody, there was a long-standing anergy of CD8+ T cells marked by absence of an inflammatory gene expression. The intragraft CD8+ T cells produced transforming growth factor β (TGFβ) and expressed PD-1 and PD-L1. TGFβ was important for the expression of the inhibitory receptors as blockade of the cytokine led to graft rejection in this model [33]. Not only the expression of PD-L1 in target tissue can protect against allograft rejection, but overexpression of PD-1 on T cells in combination with CTLA-4 blockade can promote allograft tolerance as shown in a fully MHC mismatched cardiac transplant model [34]. Recent evidence for the reactivation of pre-existing alloreactive T cells in ICI-mediated allograft rejection in a patient with melanoma was provided by Dunlap et al. The authors showed an expansion of circulating alloreactive CD8+ T cell clones that accumulated in the kidney allograft during rejection while receiving anti-PD-1 treatment, but were not present in tumor tissues. The expanded CD8+ T cells had a specific transcriptomic profile compatible with an elevated activation and tissue resident-memory signatures by the expression of ZNF683, CXCR3, and HLA-DR [35].
Figure 2:
Potential mechanisms involved in kidney allograft rejection in the context of ICI. (1) Reactivation of alloreactive quiescent T cells by blocking the PD-1/PD-L1 pathway. (2) Cross-reactivity between tumoral neoantigens and kidney allograft antigens. (3) Systemic inflammation can cause overactivation of the immune system, with off-target effects and potential activation of dormant alloreactive T cells. (4) CTLA-4 expressed on Tregs interacts with co-stimulatory molecules CD80/86 preventing APCs from effectively stimulating effector T cells. CTLA-4 can also directly interact with CD80/86 expressed on effector T cells. PD-1 on Treg prevents alloreactive B cells from stimulating other T cells and can inhibit directly T effector cells expressing PD-L1. Blocking both pathways leads to loss of regulatory T cells function and activation of host alloimmune responses.
While rejection is highest under anti-PD-1 therapy, rejection is also seen with the use of anti-CTLA-4. This is not surprising as CTLA-4 is an important regulator of T cell priming in the secondary lymphoid organs and is constitutionally expressed by Treg cells. CTLA-4 knock-out mice develop severe lymphoproliferative disease and die at a young age [36]. On the contrary Belatacept, a CTLA-4Ig, is used for maintenance immunosuppression in kidney transplantation [37]. It is notable from the immune-oncology standpoint that the use of CTLA-4Ig was associated with post-transplant lymphoproliferative disease in Epstein–Barr virus-negative patients, and it is now contraindicated for CTLA-4Ig to be used in Epstein–Barr virus-negative KTR [38].
In ICI-induced allograft rejection, a cross-talk between tumor-related immune response and alloreactivity cannot be ruled out. For instance, one can imagine that KTR who reduce their tumor size but undergo allograft rejection after ICI might also develop T cell repertoires that share common antigenic specificities for tumor and allogeneic peptides. Examining TCR specificity of CD8+ T cells infiltrating the allograft and tumoral tissue may provide evidence of such cross-reactivity.
As parallelism with immune-related adverse events, one can imagine that alteration of the local or systemic cytokine profile can tip the balance toward inflammation leading to tissue and potential allograft damage [39]. Furthermore, the loss of T regulatory function by inhibition of CTLA-4 or PD-1/PD-L1 pathway can lead to loss of tolerance and activation of alloreactive T cells in the context of transplantation.
The tumor itself can have an immunosuppressive function on the host by releasing adenosine, prostaglandin E2 and TGFβ1 [2, 40]. Tumor shrinkage by ICI can therefore indirectly augment host responses toward the allograft.
Biomarkers of allograft rejection in the context of ICI
Several surrogate markers predictive of either rejection or tolerance have been identified in KTR, but their ability to identify ‘high-risk’ or ‘low-risk’ patients before and during administration of ICI remains to be established [41–47]. Increasing evidence points toward the role and involvement of granzyme B expressing regulatory B cells (GZMb-Breg) and TEMRA CD8+ in kidney allograft survival. Based on phenotypical analyses, it has been shown that a higher proportion of Effector Memory expressing CD45RA (TEMRA) CD8+ T cells predict graft failure [35, 36]. On the other hand, a unique expansion of GZMb-Breg has been identified in KTR with operational tolerance and a robust B cell signature of low-risk graft failure has been identified [41, 44].
The ultimate search for noninvasive biomarkers to diagnose allograft rejection has further evolved and those that have been demonstrated to be utile are urinary mRNA levels of CXCL10, CD3 , 18S rRNA, chemokine concentration of CXCL9 and CXCL10 and plasma donor-derived cell-free DNA (dd-cfDNA) measurement [47–50]. dd-cfDNA increases before the rise in plasma creatinine and is monitored for early detection of allograft rejection and/or injury in clinical practice. Levels <1% of total dd-cfDNA are associated with the absence of active rejection, but levels >1% are indicative of active rejection [51].
, 18S rRNA, chemokine concentration of CXCL9 and CXCL10 and plasma donor-derived cell-free DNA (dd-cfDNA) measurement [47–50]. dd-cfDNA increases before the rise in plasma creatinine and is monitored for early detection of allograft rejection and/or injury in clinical practice. Levels <1% of total dd-cfDNA are associated with the absence of active rejection, but levels >1% are indicative of active rejection [51].
The use of these biomarkers in the context of allograft rejection under ICI has been poorly investigated. Moreover, allograft rejection under ICI occurs early so biomarkers of anti-allograft response or allograft injury must be detected early. Hurkmans et al. demonstrated in a case report, the elevation of dd-cfDNA before clinical apparent rejection under nivolumab (anti-PD-1) treatment for metastatic melanoma [52]. The same findings were observed in the preliminary results of an ongoing prospective phase I study (NCT03816332) where dd-cfDNA increases before plasma creatinine rise in two patients with allograft rejection under cemiplimab (anti-PD-1) treatment [53]. Carroll et al. prespecified an exploratory endpoint in their study and measured urinary CXCL-10 concentrations before each nivolumab injection. Higher levels were seen in the two patients who rejected their allograft. They concluded that baseline monitoring of CXCL-10 concentration might be an early predictor of allograft rejection [24]. These reports are of importance and indicate that at least two biomarkers (dd-cfDNA and urinary CXCL-10) can be used for early detection of rejection in KTR under ICI therapy. However, the ultimate goal is to create a risk score to predict allograft rejection before ICI therapy to better inform our patients about the risk of allograft rejection.
So far, the gold standard for diagnosing acute rejection remains kidney biopsy. Adam et al. analyzed gene expression profiles on kidney biopsies of patients with ICI-induced rejection versus ICI-induced interstitial nephritis and showed a significant molecular overlap. However, interferon alpha inducible protein 27 (IFI27), was identified as a potential biomarker of ICI-induced T cell mediated rejection [54]. Some authors suggest anti-PD-L1 staining of the allograft before treatment with ICI, as a positive staining could be associated with a higher risk of rejection after ICI [55–57]. However, this implicates that a biopsy is performed before treatment, with all the risks involved in this already frail population of patients.
Strategies to prevent allograft rejection in the context of ICI
To the best of our knowledge, no guidelines nor sufficient evidence exists to support the use of specific immunosuppressants during ICI therapy [58]. It is tempting to reduce immunosuppression before introduction of ICI to increase tumoral response at the expense of a higher risk of allograft rejection. Several retrospective studies demonstrate the association of mTORi treatment and allograft preservation. A large systematic review on the subject shows that the ideal response (tumoral response without allograft rejection) is highest among patients treated with mTORi [20]. This is not surprising since mTORi have anti-proliferative effects and could uncouple tumoral and allograft responses by maintaining Treg function without impairing the number of IFN-γ secreting T cells [59].
One case report suggests the use of pre-emptive high dose corticosteroids with sirolimus during immunotherapy to prevent graft rejection, the so-called ‘dynamic immunosuppression’ [60]. The effect of this regimen was tested in the CONTRAC-1 study on 12 KTR suffering from advanced cSCC treated with cemiplimab. No patient developed an allograft rejection and the ORR remained good (45%). This regimen, however, should be tested on a larger cohort of patients before becoming the standard of care for KTR suffering from cSCC eligible for cemiplimab, nor do we know if it can be extrapolated for other cancer types [61]. By contrast, in a prospective cohort study by Schenk et al. on eight KTR with advanced skin cancers (melanoma, cSCC, BCC, and Merkel cell carcinoma) treated with nivolumab with or without ipilimumab the patients were maintained on the standardized immunosuppression regimen of tacrolimus (with a target trough level of 2–5 ng/ml) and prednisone 5 mg daily. Out of eight patients enrolled, two experienced allograft rejections after addition of ipilimumab to nivolumab, and the ORR remained 33% [62].
Reduction of immunosuppression before or during ICI therapy may potentially lead to a higher risk of rejection. This question was partially addressed by Carroll et al., who demonstrated that nivolumab was safe and did not impair tumoral response in KTR without pre-emptive reduction in immunosuppression [24]. (Table 3) However, one must note that baseline immunosuppression in their study was already low and variable. Belatacept (CTLA-4Ig) is commonly used as immunosuppression for KTR, but it is unclear whether it could be effectively used to prevent allograft rejection in the setting of ICI use. In non-transplant setting, abatacept, another CTLA-4Ig, has been used to treat life-threatening myocarditis in patients treated with ICI [63]. and its efficacy is being actively tested in a phase 3 clinical trial (NCT05335928) [64]. IL-6 is an important inflammatory cytokine and increased levels have been associated with immune-related adverse events and poor tumoral prognosis in the context of ICI. Anti-IL-6 has been used to treat immune-related adverse events without impairing antitumoral responses [65]. Indeed, IL-6 has an important role in regulating the balance between Th17 and Treg cells [66]. To what extent these results can be extrapolated to KTR requires further investigations [67, 68]. To our knowledge, no specific recommendations for the use of these agents in KTR exist during ICI therapy. Anti-IL-6 therapy is currently being tested in randomized trials for the treatment of TCMR and ABMR [68]. It could be an interesting target to investigate in this specific patient population.
Table 3:
Prospective clinical trials for KTR treated with ICI.
| Study | Nivolumab in transplant patients | Tacrolimus and ICI | CONTRAC-1 |
|---|---|---|---|
| Cancer type | Any cancers (incurable, metastatic solid tumors) | Skin cancers (melanoma, cSCC, BCC, Merkel cell carcinoma) | cSCC |
| Transplant | Kidney | Kidney | Kidney |
| ICI | Nivolumab* | Nivolumab ± Ipilimumab | Cemiplimab |
| Immunosuppression | Keep the same dose | Tac (2–5 ng/ml), pred 5 mg/day | mTORi + dynamic pred |
| Patient # | 17 | 8 | 12 |
| Rejection | 2 (11.7%) | 2 (25%) | 0 (0%) |
| ORR (CR + PR) | 53% | 33% | 45% |
| Registry | ANZCTR CA209-993ISR | NCT03816332 | NCT03565783 |
| Primary institution | Royal Adelaide Hospital, multicenter | Johns Hopkins Hospital, multicenter | Dana Farber Cancer Institute |
| Australia | USA | USA | |
| Reference | Lancet Oncol (2022) | J Clin Oncol (2024) | J Clin Oncol (2024) |
cSCC: cutaneous squamous carcinoma, BCC: basal cell carcinoma, ORR: objective response rate, CR: complete response, PR: partial response, mTORi: mammalian target of rapamycin
Recent evidence from a primary liver cancer mouse model bearing a cardiac allograft showed that dual treatment with a BET protein inhibitor (JQ1) and anti-PD-L1 was associated with better tumoral response without allograft rejection. BET protein inhibition downregulates PD-L1 expression on cardiac myocytes and protected against allograft rejection [69]. These findings are encouraging, but more translational research is necessary to investigate potential use of a BET protein inhibitor in cancer and allograft response to ICI.
Re-transplantation after complete tumoral response
A common feature of all ICI are the long-lasting, and durable responses, even in patients with metastatic solid tumors [11]. The question of re-transplantation in the context of complete tumoral response after immunotherapy is challenging. Only one case report of kidney re-transplantation has been published in the literature so far. The patient was suffering from a metastatic cSCC and was treated with pembrolizumab (anti-PD-1) with a complete tumoral response. However, the patient developed severe TCMR of his first kidney allograft with consequent graft loss. The patient remained in remission 4.5 years after anti-PD-1 treatment and was again transplanted with a kidney from a living unrelated donor. Ten months after his new transplantation there were no signs of tumoral flare nor of allograft rejection [70]. By contrast, less convincing evidence is derived from liver transplant patients treated with ICI for hepatocellular carcinoma (HCC) before liver transplantation. A recent review revealed a rejection rate of 24% (11 out of 45 patients published) with a 36% graft loss (4 out of 11 patients). After graft loss there is a high mortality in this patient population unless urgent re-transplantation is done [71]. The authors postulated that at least 6 weeks between last dose of ICI and re-transplantation should be considered. Whether these results are applicable in KTR needs to be confirmed in larger studies.
It is also unknown whether to continue or interrupt ICI treatment in patients with prolonged responses. Current data in the non-transplant population is limited and long-term treatment with ICI may be associated with the occurrence of new chronic immune-related adverse events [11]. No data are available in SOTR and we do not know how long ICI have to be maintained for metastatic disease. Acute allograft rejection is an early complication, but long-term impact of ICI in SOTR remains elusive. Prospective studies are necessary to answer these important questions as metastatic cancer has changed into a chronic disease.
CONCLUSION
KTR are prone to develop malignancy post-transplantation. The use of ICI in KTR is associated with an increased risk of rejection, but tumor responses seem to be encouraging despite the concomitant use of immunosuppressants. We acknowledge that most data are derived from retrospective studies and case reports. One should carefully weigh the risk and benefit from immunotherapy before starting treatment. Moderate reduction in immunosuppression may be warranted before start of ICI, but at least two immunosuppressants should be used and conversion of tacrolimus to sirolimus in combination with higher dose corticosteroids may be a reasonable treatment option to prevent rejection, without impeding tumoral response. Frequent and close monitoring of kidney function is needed to detect early allograft rejection at the start of ICI treatment. Noninvasive monitoring of kidney rejection could allow us to detect patients at risk of allograft rejection before and during ICI treatment. Ideally, a risk score should be created to help us guide ICI therapies among KTR. Finally, the use of ICI in KTR, should be carefully made by a multidisciplinary team to weigh the potential benefits against the risks.
Contributor Information
Tess Van Meerhaeghe, Departement of Nephrology, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium; Nantes Université, INSERM, Center for Research in Transplantation and Translational Immunology (CR2TI), UMR 1064, Nantes, France.
Naoka Murakami, Division of Renal Medicine, Department of Medicine, Brigham and Women's Hospital, Boston, USA; Harvard Medical School, Boston, USA.
Alain Le Moine, Departement of Nephrology, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium.
Sophie Brouard, Nantes Université, INSERM, Center for Research in Transplantation and Translational Immunology (CR2TI), UMR 1064, Nantes, France.
Ben Sprangers, Biomedical Research Institute, Department of Immunology and Infection, UHasselt, Diepenbeek, Belgium; Department of Nephrology, Ziekenhuis Oost Limburg, Genk, Belgium.
Nicolas Degauque, Nantes Université, INSERM, Center for Research in Transplantation and Translational Immunology (CR2TI), UMR 1064, Nantes, France.
CONFLICT OF INTEREST STATEMENT
B.S. is member of the CKJ Editorial Board. T.V.M., S.B., N.D., and N.M. have no disclosures related to this article
FUNDING
T.V.M. received research grants from FNRS-FRS (grant number 40010386), Fonds Erasme and the European Society of Organ transplantation for her work on immune checkpoint inhibitors in renal transplant patients. S.B. and N.D. are also supported by grants from the IHU-Cesti project (ANR-10-IBHU-005), the ANR project BIKET (ANR-17-CE17-0008), from the LabEX IGO program supported by the National Research Agency via the “Investment into the Future” program (ANR-11-LABX-0016-01), from European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 754995, under the frame of ERA PerMed and from grant from ABM and SFNDT.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this re- search.
REFERENCES
- 1. Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. Ingelfinger JR, ed. N Engl J Med 2021;385:729–43. 10.1056/NEJMra2014530 [DOI] [PubMed] [Google Scholar]
- 2. Au E, Wong G, Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol 2018;14:508–20. 10.1038/s41581-018-0022-6 [DOI] [PubMed] [Google Scholar]
- 3. Miao Y, Everly JJ, Gross TG et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation 2009;87:1347–59. 10.1097/TP.0b013e3181a238f6 [DOI] [PubMed] [Google Scholar]
- 4. Sprangers B, Nair V, Launay-Vacher V et al. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J 2018;11:315–29. 10.1093/ckj/sfx122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang S, Pease DF, Kulkarni AA et al. Real-world outcomes and clinical predictors of immune checkpoint inhibitor monotherapy in advanced lung cancer. Clin Med Insights Oncol 2021;15:117955492110044. 10.1177/11795549211004489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Regan MM, Mantia CM, Werner L et al. Treatment-free survival over extended follow-up of patients with advanced melanoma treated with immune checkpoint inhibitors in CheckMate 067. J Immunother Cancer 2021;9:e003743. 10.1136/jitc-2021-003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma P, Hu-Lieskovan S, Wargo JA et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–23. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu D, Badell IR, Ford ML. Selective CD28 blockade attenuates CTLA-4-dependent CD8+ memory T cell effector function and prolongs graft survival. JCI Insight 2018;3:e96378 96378. 10.1172/jci.insight.96378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka K, Albin MJ, Yuan X et al. PDL1 Is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007;179:5204–10. 10.4049/jimmunol.179.8.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DB, Nebhan CA, Moslehi JJ et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022;19:254–67. 10.1038/s41571-022-00600-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solinas C, Migliori E, De Silva P et al. LAG3: the biological processes that motivate targeting this immune checkpoint molecule in human cancer. Cancers 2019;11:1213. 10.3390/cancers11081213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tawbi HA, Schadendorf D, Lipson EJ et al. Relatlimab and Nivolumab versus Nivolumab in untreated advanced melanoma. N Engl J Med 2022;386:24–34. 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdel-Wahab N, Safa H, Abudayyeh A et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J. Immunother Cancer 2019;7:106. 10.1186/s40425-019-0585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. d'Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transpl 2020;20:2457–65. 10.1111/ajt.15811 [DOI] [PubMed] [Google Scholar]
- 16. Ros J, Matos I, Martin-Liberal J. Immunotherapy in organ-transplanted cancer patients: efficacy and risk of organ rejection. Ann Oncol 2019;30:1173–7. 10.1093/annonc/mdz129 [DOI] [PubMed] [Google Scholar]
- 17. Manohar S, Thongprayoon C, Cheungpasitporn W et al. Systematic review of the safety of immune checkpoint inhibitors among kidney transplant patients. Kidney Int Rep 2020;5:149–58. 10.1016/j.ekir.2019.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murakami N, Mulvaney P, Danesh M et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int 2021;100:196–205. 10.1016/j.kint.2020.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Portuguese AJ, Tykodi SS, Blosser CD et al. Immune checkpoint inhibitor use in solid organ transplant recipients: a systematic review. J Natl Compr Canc Netw 2022;20:406–416.e11. 10.6004/jnccn.2022.7009 [DOI] [PubMed] [Google Scholar]
- 20. Rünger A, Schadendorf D, Hauschild A et al. Immune checkpoint blockade for organ-transplant recipients with cancer: a review. Eur J Cancer 2022;175:326–35. 10.1016/j.ejca.2022.08.010 [DOI] [PubMed] [Google Scholar]
- 21. Cui X, Yan C, Xu Y et al. Allograft rejection following immune checkpoint inhibitors in solid organ transplant recipients: a safety analysis from a literature review and a pharmacovigilance system. Cancer Med 2023;12:5181–94. 10.1002/cam4.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Meerhaeghe T, Baurain JF, Bechter O et al. Cemiplimab for advanced cutaneous squamous cell carcinoma in kidney transplant recipients. Front. Nephrol 2022;2. 10.3389/fneph.2022.1041819. 10.3389/fneph.2022.1041819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanna GJ, Dharanesswaran H, Giobbie-Hurder A et al. Cemiplimab for kidney transplant recipients with advanced cutaneous squamous cell carcinoma. JCO 2024;42:1021–30 Published online January 22, JCO.23.01498. 10.1200/JCO.23.01498. 10.1200/JCO.23.01498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carroll RP, Boyer M, Gebski V et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single-arm, phase 1 study. Lancet Oncol 2022;23:1078–86. 10.1016/S1470-2045(22)00368-0 [DOI] [PubMed] [Google Scholar]
- 25. Koehn BH, Ford ML, Ferrer IR et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol 2008;181:5313–22. 10.4049/jimmunol.181.8.5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tao R, Wang L, Han R et al. Differential effects of B and T lymphocyte attenuator and Programmed Death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol 2005;175:5774–82. 10.4049/jimmunol.175.9.5774 [DOI] [PubMed] [Google Scholar]
- 27. Sandner SE, Clarkson MR, Salama AD et al. Role of the Programmed Death-1 pathway in regulation of alloimmune responses in vivo. J Immunol 2005;174:3408–15. 10.4049/jimmunol.174.6.3408 [DOI] [PubMed] [Google Scholar]
- 28. Peng W, Ran B, Ma Y et al. Dendritic cells transfected with PD-L1 recombinant adenovirus induces T cell suppression and long-term acceptance of allograft transplantation. Cell Immunol 2011;271:73–77. 10.1016/j.cellimm.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 29. Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol 2005;115:184–91. 10.1016/j.clim.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 30. Starke A, Lindenmeyer MT, Segerer S et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int 2010;78:38–47. 10.1038/ki.2010.97 [DOI] [PubMed] [Google Scholar]
- 31. Shim YJ, Khedraki R, Dhar J et al. Early T cell infiltration is modulated by programed cell death-1 protein and its ligand (PD-1/PD-L1) interactions in murine kidney transplants. Kidney Int 2020;98:897–905. 10.1016/j.kint.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo Z, Liao T, Zhang Y et al. Ex vivo anchored PD-L1 functionally prevent in vivo renal allograft rejection. Bioeng Transl Med 2022;7:e10316. 10.1002/btm2.10316. 10.1002/btm2.10316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baas M, Besançon A, Goncalves T et al. TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. eLife 2016;5:e08133. 10.7554/eLife.08133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borges TJ, Murakami N, Lape IT et al. Overexpression of PD-1 on T cells promotes tolerance in cardiac transplantation via ICOS-dependent mechanisms. JCI Insight 2021;6:e142909. 10.1172/jci.insight.142909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dunlap GS, DiToro D, Henderson J et al. Clonal dynamics of alloreactive T cells in kidney allograft rejection after anti-PD-1 therapy. Nat Commun 2023;14:1549. 10.1038/s41467-023-37230-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khattri R, Auger JA, Griffin MD et al. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol 1999;162:5784–91. [PubMed] [Google Scholar]
- 37. Larsen CP, Pearson TC, Adams AB et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-ig with potent immunosuppressive properties. Am J Transpl 2005;5:443–53. 10.1111/j.1600-6143.2005.00749.x [DOI] [PubMed] [Google Scholar]
- 38. Charpentier B, Medina Pestana JO, Del C Rial M et al. Long-term exposure to belatacept in recipients of extended criteria donor kidneys. Am J Transpl 2013;13:2884–91. 10.1111/ajt.12459 [DOI] [PubMed] [Google Scholar]
- 39. Les I, Martínez M, Pérez-Francisco I et al. Predictive biomarkers for checkpoint inhibitor immune-related adverse events. Cancers 2023;15:1629. 10.3390/cancers15051629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dang N, Lin Y, Rutgeerts O et al. Solid tumor–Induced immune regulation alters the GvHD/GvT paradigm after allogenic bone marrow transplantation. Cancer Res 2019;79:2709–21. 10.1158/0008-5472.CAN-18-3143 [DOI] [PubMed] [Google Scholar]
- 41. Danger R, Chesneau M, Paul C et al. A composite score associated with spontaneous operational tolerance in kidney transplant recipients. Kidney Int 2017;91:1473–81. 10.1016/j.kint.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yap M, Boeffard F, Clave E et al. Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8 + T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol 2014;25:1856–68. 10.1681/ASN.2013080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jacquemont L, Tilly G, Yap M et al. Terminally differentiated effector memory CD8 + T cells identify kidney transplant recipients at high risk of graft failure. JASN 2020;31:876–91. 10.1681/ASN.2019080847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chesneau M, Michel L, Dugast E et al. Tolerant kidney transplant patients produce B cells with regulatory properties. J Am Soc Nephrol 2015;26:2588–98. 10.1681/ASN.2014040404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chenouard A, Chesneau M, Bui Nguyen L et al. Renal operational tolerance is associated with a defect of blood Tfh cells that exhibit impaired B cell help. Am J Transpl 2017;17:1490–501. 10.1111/ajt.14142 [DOI] [PubMed] [Google Scholar]
- 46. Baeten D, Louis S, Braud C et al. Phenotypically and functionally distinct CD8+ lymphocyte populations in long-term drug-free tolerance and chronic rejection in human kidney graft recipients. J Am Soc Nephrol JASN 2006;17:294–304. 10.1681/ASN.2005020178 [DOI] [PubMed] [Google Scholar]
- 47. Rabant M, Amrouche L, Lebreton X et al. Urinary C-X-C Motif Chemokine 10 independently improves the noninvasive diagnosis of antibody-mediated kidney allograft rejection. J Am Soc Nephrol JASN 2015;26:2840–51. 10.1681/ASN.2014080797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jackson JA, Kim EJ, Begley B et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transpl 2011;11:2228–34. 10.1111/j.1600-6143.2011.03680.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Filippone EJ, Farber JL. The monitoring of donor-derived cell-free DNA in kidney transplantation. Transplantation 2021;105:509–16. 10.1097/TP.0000000000003393 [DOI] [PubMed] [Google Scholar]
- 50. Suthanthiran M, Schwartz JE, Ding R et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med 2013;369:20–31. 10.1056/NEJMoa1215555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bloom RD, Bromberg JS, Poggio ED et al. Cell-free DNA and active rejection in kidney allografts. JASN 2017;28:2221–32. 10.1681/ASN.2016091034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hurkmans DP, Verhoeven JGHP, de Leur K et al. Donor-derived cell-free DNA detects kidney transplant rejection during nivolumab treatment. J Immunother Cancer 2019;7:182. 10.1186/s40425-019-0653-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schenk KM, Stein JE, Chandra S et al. Nivolumab (NIVO) + tacrolimus (TACRO) + prednisone (PRED) +/- ipilimumab (IPI) for kidney transplant recipients (KTR) with advanced cutaneous cancers. JCO 2022;40:9507–. 10.1200/JCO.2022.40.16_suppl.9507 [DOI] [PubMed] [Google Scholar]
- 54. Adam BA, Murakami N, Reid G et al. Gene expression profiling in kidney transplants with immune checkpoint inhibitor-associated adverse events. CJASN 2021;16:1376–86. 10.2215/CJN.00920121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nguyen LS, Ortuno S, Lebrun-Vignes B et al. Transplant rejections associated with immune checkpoint inhibitors: a pharmacovigilance study and systematic literature review. Eur J Cancer 2021;148:36–47. 10.1016/j.ejca.2021.01.038 [DOI] [PubMed] [Google Scholar]
- 56. Shi XL, Mancham S, Hansen BE et al. Counter-regulation of rejection activity against human liver grafts by donor PD-L1 and recipient PD-1 interaction. J Hepatol 2016;64:1274–82. 10.1016/j.jhep.2016.02.034 [DOI] [PubMed] [Google Scholar]
- 57. Choudhary A, Brinkley DM, Besharati S et al. PD-L1 (Programmed Death Ligand 1) as a marker of acute cellular rejection after heart transplantation. Circ: Heart Failure 2021;14:e008563. 10.1161/CIRCHEARTFAILURE.121.008563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrándiz-Pulido C, Leiter U, Harwood C et al. Immune checkpoint inhibitors in solid organ transplant recipients with advanced skin cancers—emerging strategies for clinical management. Transplantation 2023;107:1452–62. 10.1097/TP.0000000000004459 [DOI] [PubMed] [Google Scholar]
- 59. Esfahani K, Al-Aubodah TA, Thebault P et al. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun 2019;10:4712. 10.1038/s41467-019-12628-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barnett R, Barta VS, Jhaveri KD. Preserved renal-allograft function and the PD-1 pathway inhibitor Nivolumab. N Engl J Med 2017;376:191–2. 10.1056/NEJMc1614298 [DOI] [PubMed] [Google Scholar]
- 61. Hanna GJ, Dharaneeswaran HJ, Giobbie-Hurder A et al. Cemiplimab for kidney organ transplant recipients with advanced cutaneous squamous cell carcinoma: CONTRAC-1. JCO 2023;41:9519–. 10.1200/JCO.2023.41.16_suppl.9519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schenk KM, Deutsch JS, Chandra S et al. Nivolumab + Tacrolimus + Prednisone ± Ipilimumab for kidney transplant recipients with advanced cutaneous cancers. JCO Published online January 22, 2024;JCO.23.01497. 10.1200/JCO.23.01497. 10.1200/JCO.23.01497 [DOI] [PubMed]
- 63. Salem JE, Allenbach Y, Vozy A et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med 2019;380:2377–9. 10.1056/NEJMc1901677 [DOI] [PubMed] [Google Scholar]
- 64. Neilan TG. AbatacepT foR ImmUne checkpoint inhibitor associated myocarditis (ATRIUM): a phase 3, investigator-initiated, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of abatacept in ICI myocarditis. Clinicaltrials.Gov; 2023. Accessed 1 January 2024; https://clinicaltrials.gov/study/NCT05335928
- 65. Verheijden RJ, Van Eijs MJM, May AM et al. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. npj Precis. Onc 2023;7:41. 10.1038/s41698-023-00380-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830–5. 10.1002/eji.201040391 [DOI] [PubMed] [Google Scholar]
- 67. Dimitriou F, Hogan S, Menzies AM et al. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer 2021;157:214–24. 10.1016/j.ejca.2021.08.031 [DOI] [PubMed] [Google Scholar]
- 68. Hailemichael Y, Johnson DH, Abdel-Wahab N et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022;40:509–523.e6. 10.1016/j.ccell.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jordan SC, Ammerman N, Huang E et al. Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts. Am J Transpl 2022;22:28–37. 10.1111/ajt.17207 [DOI] [PubMed] [Google Scholar]
- 70. Miao X, Wu Z, Jiang Y et al. An efficient combination immunotherapy for antitumor immunity without accelerating cardiac allograft rejection. Immunology 2023;169:157–66. 10.1111/imm.13618 [DOI] [PubMed] [Google Scholar]
- 71. Lipson EJ, Naqvi FF, Loss MJ et al. Kidney retransplantation after anti–programmed cell death-1 (PD-1)-related allograft rejection. Am J Transpl 2020;20:2264–8. 10.1111/ajt.15856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Woo SM, Kimchy AV, Sequeira LM et al. Immunotherapy use prior to liver transplant in patients with hepatocellular carcinoma. Current Oncology 2022;29:9813–25. 10.3390/curroncol29120771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang D, Bauersachs J, Berliner D. Immune checkpoint inhibitor associated myocarditis and cardiomyopathy: a translational review. Biology 2023;12:472. 10.3390/biology12030472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this re- search.