Key words: Albendazole, host genotype and sex, in vivo efficacy, mice, microcrystal formulations, Trichinella spiralis infection
Abstract
Albendazole (ABZ) is an anthelmintic pharmaceutical commonly used in the treatment of nematode infections. It is a Class II drug poorly water-soluble, with very low bioavailability, a feature particularly limiting to treat the trichinellosis chronic phase. Microcrystals obtained by controlled precipitation using hydroxyethyl cellulose and chitosan have previously been shown to improve ABZ biopharmaceutical properties. This investigation aimed to test the systems' in vivo efficacy in the CBi-IGE murine model of Trichinella spiralis infection in the infection's different phases and parasite’ stages. Treatment in the enteral phase led to a 90% decrease in the larval muscle load, probably due to its effect on T. spiralis female fecundity. Both microcrystal systems given in the migratory phase halved muscle load in males, a response not observed in females. The chitosan-based microcrystals proved to be the best when administered in the chronic phase of the infection – an increased proportion of L1 dead larvae was found compared to controls, except in CBi+-treated females. Males and females from the highly susceptible CBi+ line presented a significantly different treatment response in this phase. In vivo efficacy depended on the host genotype and sex and was related to the parasite cycle stage in which the formulations were administered.
Introduction
Trichinellosis is a zoonosis produced by the ingestion of raw or undercooked meat from animals infected with larvae of nematodes of the genus Trichinella. Trichinella spiralis belongs to the neglected foodborne parasites recognized as responsible for considerable disease burdens globally (van der Giessen et al., 2021), ranking among the top-five prioritized foodborne parasites of worldwide importance. The impact of foodborne parasites is known to vary considerably between countries and regions. Trichinellosis incidence in human populations is variable and depends largely on the practices related to the ingestion and preparation of the meat of the host species (Murrell, 2016). In Argentina, in the period 2009–2019, outbreaks of trichinellosis were recorded every year (Ministerio de Salud y Desarrollo Social de la Nación, 2019). It is considered an endemic parasitosis and constitutes a serious public health problem, given its high-morbidity rates (Gottstein et al., 2009). Domestic pork still accounts for many outbreaks, mostly in Eastern Europe and Argentina, where traditional small, ‘backyard’ rearing of pigs for household and local consumption often involve high-risk rearing practices, especially feeding with food waste (Ribicich et al., 2009; Murrell, 2016). Humans acquire the infection after ingesting raw or undercooked meat and meat-derived products of different animal origins containing Trichinella-infective larvae in cells of striated muscles (Gottstein et al., 2009). When a new host ingests infected muscle tissues, larvae are released from the nurse cell in the stomach and migrate to the small intestine, where they rapidly develop to the adult stage. Males and females copulate, and on days 6–7 post-infection, females begin releasing newborn larvae (NBL). These NBL migrate into the lymphatic vessels and enter the blood vessels to reach and penetrate striated muscle cells. In this niche, NBL develop to the infective L1 stage in 2 weeks (Pozio, 2018).
It is currently under discussion whether trichinellosis is a disease of low prevalence or it is misdiagnosed. Trichinellosis should ideally be treated at an early stage of infection when the worms are still present in the intestinal mucosa or NBL are migrating from intestinal vessels to the muscle (Gottstein et al., 2009). However, an early clinical diagnosis can be difficult, especially in low-level infections, due to the lack of symptoms and pathognomonic signs. Furthermore, its clinical manifestations overlap with many common diseases, such as influenza and chronic fatigue syndrome (Murrell and Pozio, 2011; Shimoni and Froom, 2015).
Albendazole (ABZ), recommended as a first-line anthelmintic treatment, has a broad-spectrum activity and low cost. However, its effectiveness is reduced by extremely poor aqueous solubility limiting oral absorption and decreasing its bioavailability. A possible way to overcome ABZ low solubility in water is to alter its physical properties to improve solubility and dissolution rate, using methods such as controlled precipitation with different polymers to obtain ABZ microcrystal formulations (Alanazi et al., 2007; Leonardi et al., 2008; Dib et al., 2011; García et al., 2013). Employing this technology, we developed microcrystalline ABZ formulations and characterized them by their physicochemical properties and in vitro biological activity (Priotti et al., 2017). These studies showed that the microcrystalline systems based on hydroxyethyl-cellulose (S4A) and chitosan (S10A) were the best options to optimize oral absorption of the active pharmaceutical ingredient. Furthermore, the pharmacokinetics analysis, using two mouse lines that differ in susceptibility to T. spiralis, demonstrated that microcrystal formulations increased ABZ bioavailability and pharmacokinetic parameters were affected by the host sex and genotype (Codina et al., 2020).
Susceptibility to parasitic diseases is associated with a polygenic genetic basis (Campino et al., 2006; Hernandez-Valladares et al., 2014; Mukherjee et al., 2019). The use of phenotypically defined animal models may help to elucidate the importance of the host-genetic background in resistance to parasitism and identify the genes involved in the trait (Vasconi et al., 2008). The comparative analysis of extreme response phenotypes is useful in studies of the host–parasite relationship (Machado-Silva et al., 2005; McRae et al., 2014, 2016) and has an important role in evaluating the efficacy and safety of new pharmaceutical devices as therapeutic alternatives for medical and veterinary use.
This work aimed to analyse the in vivo anthelmintic efficacy of the ABZ microcrystal formulations S4A and S10A against different stages of T. spiralis in males and females from two mouse lines, which differ in susceptibility to the parasite.
Materials and methods
Animal model
Adult female and male mice (80–90 days old) of the lines CBi/L and CBi+ from the Animal Facilities of the Instituto de Genética Experimental, Facultad de Ciencias Médicas, Universidad Nacional de Rosario (CBi-IGE stock) were used. These lines, obtained by divergent selection for body conformation (Hinrichsen and Di Masso, 2010), are in the 150th generation of selective breeding, and their theoretical inbreeding coefficient is 0.99. The lines differ in body shape (CBi+, obtained by agonistic selection, high body weight-long tail; CBi/L, product of an antagonistic selection, low body weight-long tail) and weight (mean ± s.e.m., g; CBi+ males: 48.3 ± 0.48; CBi + females: 44.9 ± 0.55; CBi/L males: 29.9 ± 0.23; CBi/L females; 27.2 ± 0.21), and characters related to growth. They also show differences in non-correlated characters such as the immune response to different antigens, susceptibility to spontaneous carcinogenesis, resistance/susceptibility to infection with different parasites, indicating that they are a source of significant genetic variation in all the characters analysed (Vasconi et al., 2008; Hinrichsen and Di Masso, 2010). Previous experiments demonstrated that these lines differ in response to challenge with increasing doses of T. spiralis in a primary infection (Vasconi et al., 2015). There was a significant difference in the infection intensity among mouse genotypes. The larval muscle load increased as the dose increased, but the magnitude of this increase was significantly different among genotypes. CBi/L was classified as the most resistant genotype since it showed a very low larval muscle load and little variation in this trait due to dose effect, and CBi+ as the most susceptible.
All the experiments with mice were conducted during the first half of the light cycle. Mice had access to complete balanced food for rodents (Gepsa Feeds, Grupo Pilar S.A., Argentina) and water ad libitum. They were treated following the institutional regulations (Facultad de Ciencias Médicas, Universidad Nacional de Rosario, permit number 1398/2016), which comply with the guidelines issued by the Institute for Laboratory Animal Resources, National Research Council, USA (Guide for the Care and Use of Laboratory Animals, 2011).
Drug formulations
The formulations were obtained by controlled precipitation (a bottom-up method), using hydroxyethyl cellulose or chitosan as stabilizing polymers (Priotti et al., 2017). Hydroxyethyl cellulose, viscosity 145 mPa s (1% in water, 20°C), and chitosan (MW 310–375 kDa, >75% deacetylated), were obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Briefly, ABZ was solubilized (40 mg mL−1) in glacial acetic acid:ethanol and added into the polymeric solutions (0.1% w/v) under stirring. The ratio of ABZ solution and polymeric solution was 1 into 10 for both formulations. After 10 min, each suspension was spray dried in a Mini Spray Dry B-290 (Büchi, Germany).
Formulations or ABZ powder were prepared and administered orally as previously described in detail by Codina et al. (2020).
Parasite
Trichinella spiralis was generously provided by Dr Maria Dalla Fontana (Laboratorio de Zoonosis, Laboratorio Central de la Red Provincial de Laboratorios, Dirección de Bioquímica y Farmacia, Santa Fe, Argentina). It was maintained and passaged in CBi mice since 2006. This strain was genotyped as T. spiralis using multiplex polymerase chain reaction (PCR) (Dr Silvio Krivokapich, Departamento de Parasitología, Administración Nacional de Laboratorios e Institutos de Salud ‘Dr Carlos Malbrán’, Buenos Aires, Argentina, personal communication). L1-infective larvae used in the infection were recovered by artificial digestion from the muscles of mice from line CBi, infected 3–4 months earlier for that purpose, as described previously (Vasconi et al., 2015).
Infection
Mice were infected orally with a single dose of two T. spiralis L1 infective larvae per g of body weight. Since CBi/L and CBi+ mice show significant body weight differences, the equivalent dose was calculated as the number of infective larvae per g of the host's body weight. Each animal was weighed before infection, 24–48 h before treatment, and before sacrifice. During the infection course, general health of the mouse (overall physical condition and behaviour) was monitored three times a week.
Experimental design
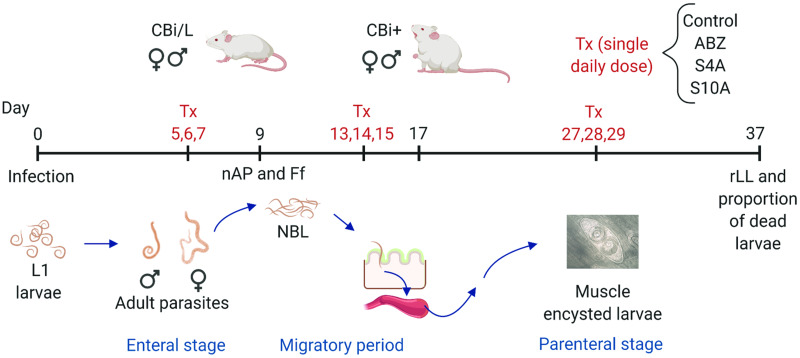
In order to evaluate the efficacy of the treatment against the different stages of T. spiralis, ABZ and the microcrystal formulations S4A and S10A were administered orally in a single dose (30 mg ABZ per kg body weight per day), as already described (Codina et al., 2020), at different stages of the parasite cycle (Fig. 1). Mice (n = 4–5 per line, sex, treatment and date of sacrifice) were non-treated (controls) or treated in the enteral (group 1, days 5, 6 and 7 post-infection, p-i), migratory (group 2, days 13, 14 and 15 p-i) or parenteral (group 3, days 27, 28 and 29 p-i) phases of the infection. In the first two groups, the effects of treatment on both the intestinal and muscular parasitic load were evaluated; in group 3, only the effect on the muscular parasitic load was assessed.
Fig. 1.
Graph outlining the experimental design used. CBi+ and CBi/L mice of both sexes infected with T. spiralis infective L1 larvae were divided into three groups to receive the treatment (Tx) in the enteral (days 5, 6, 7 p-i), migratory (days 13, 14, 15 p-i), or parenteral (days 27, 28, 29 p-i) stages of T. spiralis infection. Within each group, mice were subdivided into four subgroups according to the formulation given daily as a single oral dose (30 mg ABZ/kg body weight/day): control, ABZ, S4A and S10A. Efficacy of the treatments was evaluated by determining the intestinal worm load (nAP) and T. spiralis female fecundity (Ff), and muscle relative Larval Load (rLL) and proportion of dead larvae in the tongue.
Analysis of the formulations’ therapeutic efficacy
Enteral phase
Half the animals from group 1 and their controls were euthanized by CO2 inhalation 2 days after administration of the last anthelmintic dose (day 9 p-i) to estimate intestinal parasitic load (nAP) and T. spiralis female fecundity (Ff), as described by Luebke (2007). Briefly, the small intestine was removed, opened lengthwise and incubated in a Petri dish with sterile saline solution for 4 h, at 37°C, in a 5% CO2 atmosphere. Adult male and female worms were recovered from the suspension by centrifugation and washed with saline. The parasite pellet was resuspended in approximately 2 mL saline and placed in a gridded acrylic plate to count adult worms with a microscope at 40× magnification. nAP was expressed as the total number of adult parasites present in the intestine per mouse. Ff was determined by counting the number of NBL from each isolated female; eight to ten females migrating out of the intestinal wall were identified using a microscopic magnifying glass, collected with a pipette P200 and placed (one per well) in a 96 well plate containing RPMI 1640 plus 10% fetal bovine serum (FBS) and 250 μg/mL gentamicin. They were incubated for 18 h at 37°C in a 5% CO2 atmosphere, and the NBL released in each well were counted. Data were expressed as the average number of NBL per female parasite per mouse.
The remaining treated and control animals were sacrificed on day 37 p-i to assess each treatment's efficacy in reducing the muscular parasitic load, as described below.
Migratory phase
Mice in group 2 and their controls were processed following the same protocol as those in group 1, except that nAP and Ff were studied on day 17 p-i, 2 days after administering the last anthelmintic dose on this phase of the infection.
Parenteral phase
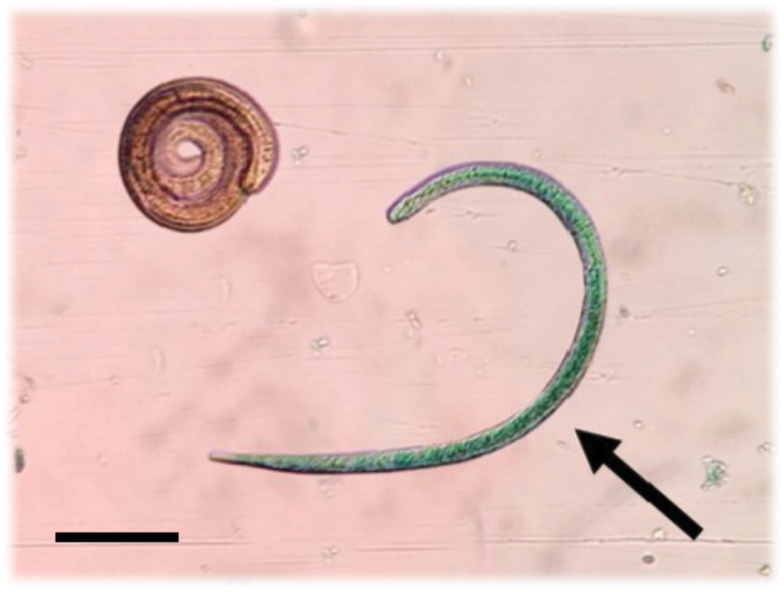
Mice from group 3 and their controls, and the remaining groups 1 and 2 mice, were euthanized at 37 days p-i, 7 days after the last administration of the drug to group 3, to determine the number of muscle encysted larvae in the tongue. This muscle is the preferred site for encystment in mice (Beiting et al., 2007; Luebke, 2007; Vasconi et al., 2015) and allows recovery of encysted larvae even with low levels of infection (Leclair et al., 2003; Picherot et al., 2007). Briefly, the tongue was excised, weighed and subjected to pepsin–HCl artificial digestion. After overnight incubation at 37°C, the digestion was stopped by adding saline solution. Next, the larvae were rinsed with saline several times to remove debris, and the supernatant was gently removed after centrifugation at a very low speed (250–300 g) for 5 min. Finally, the pellet containing all the tongue's encysted larvae was resuspended in 2 mL saline and placed in an acrylic plate to count them under an optical microscope at 40× magnification. Each formulation's anthelmintic efficacy was determined by the number of L1 larvae per g fresh tissue (relative larval load, rLL). The effect of the formulations on the encysted L1 larvae viability was also analysed using the methylene blue supravital staining technique (Randazzo and Costamagna, 2010; Codina et al., 2015). It allows identifying dead and moribund larvae by their blueish coloration due to retraction of the internal structures and chitinous layer's fragmentation that facilitate the dye's penetration. Viability was also corroborated by the lack of movement (the absence of typical movements of constant winding and unrolling) and the characteristic ‘comma’ shape of dead larvae (Fig. 2).
Fig. 2.
Micrograph of T. spiralis L1 larvae recovered after artificial digestion of the tongue. The arrow points to a dead larva showing the characteristic ‘comma shape’, stained with methylene blue. Scale bar, 50 μm.
Statistical analysis
The statistical significance of the differences among treatment groups was evaluated with a one-way analysis of variance (ANOVA), followed by Tukey's post-test for between-groups comparisons, or nonparametric Kruskal–Wallis test, using Dunn's post-test, as appropriate (Sheskin, 2011). Comparison between sexes within genotype and treatment was done with Student's t-test or the nonparametric Mann–Whitney test (Sheskin, 2011). Differences were considered significant if P < 0.05.
Results
Analysis of the therapeutic efficacy
Enteral phase
To evaluate the efficacy of the microcrystal systems compared to pure ABZ, S4A or S10A was administered on days 5, 6 and 7 p-i, in the first stage of the parasite cycle.
Table 1 shows the effect of the formulations on the intestinal parasitic load (nAP) and T. spiralis fecundity (Ff) in mice sacrificed 2 days after administering the last dose. Neither the formulations nor the pure drug modified the intestinal worm load since the determination of nAP on day 9 p-i in treated mice did not differ significantly from the controls in both lines and sexes. The effect of the anthelmintic preparations on T. spiralis Ff could not be evaluated because female worms recovered from the intestines of treated mice were dead or showed an altered morphology. As observed on day 9 p-i ABZ, either pure or as part of a microcrystalline system, penetrated the worms and caused alterations in T. spiralis females' internal structure (Fig. 3), thus reducing the number of NBL that would migrate to encyst in muscles. Although they did not modify the number of recovered intestinal parasites, both raw ABZ and the formulations affected the vitality of the female parasites.
Table 1.
Effect of treatment during the enteral stage of the infection1 on the host intestinal parasite burden and T. spiralis female fecundity
| CBi/L | CBi+ | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Treatment | Variable: nAP2 | |||
| Control | 6.5 (2–17)a | 2.0 (0–5)a | 13.5 (9–19)a | 22.0 (10–27)a |
| ABZ | 7.0 (3–8)a | 1.5 (0–3)a | 13.5 (10–17)a | 8.0 (3–17)a |
| S4A | 6.5 (1–8)a | 2.0 (0–10)a | 19.5 (13–24)a | 17.0 (14–20)a |
| S10A | 7.0 (3–14)a | 0 (0–3)a | 15.0 (5–23)a | 21.0 (20–23)a |
| Variable: Ff3 | ||||
| Control | 29 ± 15.2 | 344 | 50 ± 25.0 | 46 ± 16.6 |
| ABZ | – | – | – | – |
| S4A | – | – | – | – |
| S10A | – | – | – | – |
nAP, total number of intestinal adult parasites; Ff, T. spiralis female fecundity.
nAP differences among treatments within genotype and sex were evaluated with the nonparametric Kruskal–Wallis test followed by Dunn's test for between-groups comparison (nAP).
For each column, differences between groups not sharing the same superscript are significant at the 0.05 level.
Mice were treated on days 5, 6 and 7 p-i and sacrificed two days after the administration of the last dose (9 days p-i).
Median (range).
Mean ± s.e.m.
Only one female recovered.
Fig. 3.
Representative micrographs illustrating T. spiralis female morphology on day 9 post-infection. (A) female recovered from the intestine of a control mouse in which eggs (arrow) and larvae (broken arrow) are observed. (B) Female obtained from the intestine of a treated mouse showing no discernible internal structures. Scale bar, 50 μm.
The effect of the different ABZ formulations on the number of the encysted larvae was analysed on day 37 p-i (Table 2). Muscle rLL was significantly lower in treated animals (CBi/L, ♂ P = 0.01, ♀ P = 0.0001; CBi + , ♂ P = 0.0002, ♀ P = 0.02) compared to the controls. There were no significant differences among the ABZ systems; they all reduced rLL by 90%, regardless of the host's genotype and sex. This finding is remarkable in the susceptible CBi+ host since these mice, when untreated, attain a parasitic muscle burden 5–7 times greater than that of the resistant CBi/L mice.
Table 2.
Effect of treatment during the enteral stage of the infection1 on the number of T. spiralis encysted L1 larvae
| CBi/L | CBi+ | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Treatment | Variable: rLL2,3 | |||
| Control | 247 ± 97.1a | 250 ± 54.2a | 1132 ± 221.7a | 1463 ± 672.0a |
| ABZ | 20 ± 10.1b | 27 ± 6.4b | 83 ± 28.0b | 117 ± 42.9b |
| S4A | 12 ± 9.5b | 9 ± 4.2b | 66 ± 10.2b | 189 ± 117.2b |
| S10A | 19 ± 13.7b | 16 ± 6.8b | 225 ± 119b | 232 ± 76.7b |
Differences among treatments within genotype and sex were evaluated by a one-way ANOVA, using Bonferroni's post-test for comparisons between groups.
For each column, differences between groups not sharing the same superscript are significant at the 0.01 level.
Mice were treated on days 5, 6 and 7 p-i and sacrificed on day 37 p-i.
rLL: relative Larval Load, total number of encysted larvae per g of fresh tissue.
Mean ± s.e.m.
The proportion of dead larvae was similar in the treated and control groups (data not shown), which is expected since the anthelmintic was administered on the enteral phase of infection. By the time NBL reach the muscle and encyst, the active pharmaceutical ingredient has already been metabolized and excreted.
The results of the treatment of this phase of the infection indicate that ABZ, S4A and S10A were equally effective at reducing the number of encysted larvae.
Migratory phase
No adult parasites were found in the intestines of mice treated with ABZ or the formulations S4A and S10A on days 13, 14 and 15 p-i and sacrificed 48 h afterwards. In the CBi-IGE model of T. spiralis infection, the migratory phase begins on days 5–6 p-i and, depending on the host line, by day 13 p-i, few or no worms are recovered from the intestine (Vasconi et al., 2015). Since there is no direct method to measure efficacy in this phase, treatment efficacy was evaluated by the number of larvae reaching the muscles to encyst.
The effect of treatment during the migratory phase on the number of muscle encysted larvae was assessed on day 37 p-i (Table 3). The microcrystals showed a better, although not statistically significant, therapeutic response compared to raw ABZ. Males of both genotypes receiving S4A and S10A halved the larval muscle load compared to controls; however, this response was not observed in the females.
Table 3.
Effect of treatment during the migratory stage of the infection1 on the number of T. spiralis encysted L1 larvae
| CBi/L | CBi+ | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Treatment | Variable: rLL2,3 | |||
| Control | 258 ± 76.7a | 197 ± 33.9a | 1268 ± 462.4a | 1168 ± 287.5a |
| ABZ | 159 ± 36.2a | 151 ± 37.7a | 1107 ± 300.1a | 1081 ± 287.7a |
| S4A | 113 ± 47.8a | 142 ± 30.9a | 669 ± 499.5a | 1596 ± 476.2a |
| S10A | 137 ± 42.9a | 141 ± 31.2a | 669 ± 118.0a | 869 ± 206.2a |
Differences among treatments within genotype and sex were evaluated by a one-way ANOVA, using Bonferroni's post-test for comparisons between groups.
For each column, differences between groups not sharing the same superscript are significant at the 0.05 level.
Mice were treated on days 13, 14 and 15 p-i and sacrificed on day 37 p-i.
rLL: relative Larval Load, total number of encysted larvae per g of fresh tissue.
Mean ± s.e.m.
Parenteral phase
Table 4 shows the effect of administering pure ABZ or ABZ microcrystalline formulations during the chronic phase of infection (days 27, 28 and 29 p-i) on parasite muscle load and proportion of dead larvae. On day 37 p-i, muscle rLL was similar among the groups of the same genotype and sex. The number of dead larvae was, in general, higher in treated mice compared to that in controls. Treated CBi/L hosts, irrespective of sex, showed a higher proportion of dead larvae compared to their controls, this difference being significant for CBi/L males treated with S10A (P = 0.0385). CBi+ males and females did not show the same response to treatment (P < 0.05): while treated males behaved similarly to CBi/L mice and tended to increase the percentage of dead larvae, treated females did not differ from their controls. At this stage of the parasite cycle, the microcrystal system S10A showed better therapeutic efficacy compared to S4A.
Table 4.
Effect of treatment during the chronic stage of infection1 on parasite muscle burden
| CBi/L | CBi+ | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Treatment | Variable: rLL2,3 | |||
| Control | 240 ± 45.5a | 173 ± 75.5a | 998 ± 74.8a | 752 ± 157.9a |
| ABZ | 268 ± 71.6a | 283 ± 94.9a | 1084 ± 201.8a | 794 ± 144.9a |
| S4A | 266 ± 46.7a | 172 ± 45.7a | 1177 ± 154.8a | 662 ± 197.0a |
| S10A | 338 ± 57.6a | 130 ± 26.1a | 1172 ± 287.8a | 883 ± 210.9a |
| Variable: Proportion of dead T. spiralis muscle larvae (%)4,5 | ||||
| Control | 20 (8–29)a | 9 (0–25)a | 24 (14–33)a | 7 (4–14)a |
| ABZ | 28 (0–37)a | 37 (0–68)a | 30 (15–60)a | 7 (5–21)a |
| S4A | 29 (14–37)a | 33 (0–96)a | 38 (7–93)a | 13 (6–20)a |
| S10A | 64 (18–88)b | 50 (6–100)a | 54 (13–74)a | 12 (5–22)a |
Differences among treatments within genotype and sex were evaluated with the nonparametric Kruskal–Wallis test followed by Dunn's test for between-groups comparison.
Differences among groups, within genotype and sex, were evaluated with the non-parametric Kruskal–Wallis test, using Dunn's test for comparisons between groups.
For each variable, within column, groups not sharing the same superscript differ significantly (P < 0.05).
Mice were treated on days 27, 28 and 29 p-i and were sacrificed on day 37 p-i.
rLL: relative larval load, total number of muscle encysted larvae per g of fresh tissue.
Mean ± s.e.m.
Percentage of dead muscle encysted larvae.
Median (range).
Discussion
When an infective agent enters into an organism, three main elements determine the clinical response: the host genotype, the parasite genotype and environmental factors (Incani et al., 2001; Campino et al., 2006; Wilfert and Schmid-Hempel, 2008). Resistance/susceptibility to parasitic infections is therefore determined by a complex equation that combines the three elements. Murine models have proven to be an excellent genetic tool for studying hosts’ response to parasitosis. A defined animal model, such as the mouse lines from the CBi-IGE colony in which most environmental variables can be strictly controlled, may help to elucidate the host genetic background's role in the development and establishment of a parasitic infection and on response to treatment.
ABZ is the drug of choice for treating trichinellosis in recommended doses of 400 mg/day (15 mg/kg/day) twice a day for 8–14 days. However, ABZ formulations commercially available have shown therapeutic failure in the chronic phase, and longer treatments and higher doses are required, enhancing the risk of side- effects. Modifying the properties of solid compounds through micro-crystallization of ABZ allowed us to obtain formulations with better biopharmaceutical properties and potential improved effectiveness (Priotti et al., 2017; Codina et al., 2020), the next step being to test their therapeutic efficacy in vivo in an animal model of T. spiralis infection. The Trichinella/mouse model has been widely used to assay benzimidazole carbamates' anthelmintic effectiveness (McCracken, 1978; López-García et al., 1997; Chung et al., 2001; Casulli et al., 2006; García et al., 2013; Codina et al., 2015). One of the advantages is that it allows, within a short time, testing drugs against different parasite stages and locations within the host. In the present study, our formulations' therapeutic efficacy was analysed in the three major phases of the infection: enteral, migratory and chronic or parenteral.
ABZ, S4A or S10A administered in the enteral phase of infection had no effect on the number of intestinal worms but decreased T. spiralis female worms' vitality, which showed alterations of their internal morphology leading, most likely, to a modification in fecundity with the consequent reduction of muscle encysted larvae. The same morphological alterations were observed in female worms in the in vitro assay that evaluated the formulations' anthelmintic activity (Priotti et al., 2017). Moreover, the significant decrease in muscle parasite load in the chronic phase suggests that an inhibition of reproduction occurred since it is unlikely that the formulations given on days 5–7 p-i would affect migrating larvae. A similar result was reported by Campbell and Cuckler (1964) after feeding infected mice with a diet containing 0.05% thiabendazole on days 4–11 post-infection: the treatment did not eradicate the intestinal worms but did suppress their reproduction. Also, mice under-dosed with ABZ or mebendazole on day 5 post-infection showed an effect on the parasite reproductive process, which disappeared when the drug was cleared from the system (de-la-Rosa et al., 2007).
Notably, ABZ and the formulations given in the enteral phase were equally effective in both lines and sexes: the 90% reduction in muscle relative larval load occurred in resistant and susceptible animals despite the latter having a significantly higher intestinal and muscular parasitic load than the resistant mice.
The migratory phase of infection (Mitreva and Jasmer, 2006) begins when NBL pass to tissues, enter lymphatics, then the general circulation at the thoracic duct, and make their way through the capillaries into the muscle fibres, initiating the muscle phase of infection. Its length depends on how rapidly worms are expulsed, a character partly determined by the genotype of the host (Vallance et al., 1997; Steel et al., 2019). This phase is usually considered the most refractory to treatment, probably because it always involves parasites at different development levels, unlike the intestinal and muscular phases in which the maturation of the stage occurs in a short time. In this period, newly laid larvae can coexist with others in the pre-encysting stage; consequently, their susceptibility to drugs can vary since they are in a different metabolic situation. In this context, despite variations depending on the host, such as longevity of adults in the intestinal intracellular niche, number of NBL laid per female and their survival during migration, the number of L1 larvae recovered on the chronic phase has been used by many researchers as a success index for a given treatment (Denham and Martinez, 1970; Bell et al., 1985; García-Rodriguez et al., 2001; García et al., 2003; Eid et al., 2020).
Although not statistically significant, the formulations given during the migratory phase reduced by half the larval muscle load, suggesting that the microcrystals improve ABZ efficacy in the migratory stage. This tendency to present an improved therapeutic efficacy was observed only in males regardless of the host genotype. Formulations may have affected migrating newborn or pre-encysted larvae, as suggested by the decrease in the number of encysted larvae and the results of a previous in vitro experiment (Priotti et al., 2017). The in vitro experiment exposing T. spiralis females to culture media containing S4A or S10A impacted to varying degrees the mobility of the NBL, which showed complete loss of movement after 2 h and up to 24 h.
Treatment on the chronic phase of trichinellosis is critical, as existing treatments may not eliminate the parasite once the larvae have become established in the muscle cells. These cases usually require repeated or more prolonged treatments that entail a higher probability of suffering side-effects. Hence, the formulations' therapeutic success in this phase would be related to their ability to enter the cyst to kill the larvae. Studies of treatment effectiveness in the chronic phase of infection have yielded mixed results when muscle larvae are fully encapsulated (López-García et al., 1997; Siriyasatien et al., 2003; de la Torre-Iglesias et al., 2014); in fact, neither ABZ nor mebendazole is equally efficacious against all larval stages (Knopp et al., 2012). As stated by Shimoni and Froom (2015), adequate concentrations of the drugs or their active metabolites need to reach larvae that have invaded the muscle to be effective systemically. Adequate concentrations are usually achieved with longer periods and higher anthelminthic doses or developing formulations to enhance the aqueous drug solubility to improve bioavailability (García-Rodríguez et al., 2001; Casulli et al., 2006; Codina et al., 2015). Treatment protocols in this study used a low dose of ABZ in short periods that, in the parenteral phase of infection, was not enough to reduce the number of encysted larvae but did affect their viability. CBi/L males and females treated with S10A showed an increase, significant in males, in the proportion of dead L1 larvae compared to ABZ. A similar effect was observed in CBi+ males, whereas females did not respond to any formulation. It is accepted that parasitic diseases differ in prevalence, course and severity between males and females; biological sex influences physiology, immune responses, drug metabolism, thus affecting the progression of the disease (Nava-Castro et al., 2012; Dkhil, 2015; Hegazy et al., 2019; Lockard et al., 2019). This sex effect was only observed in the resistant line: CBi/L males generally had higher muscle parasite loads compared to females. On the contrary, males and females of the susceptible CBi+ line had similar parasite loads. These results highlight the importance of considering the host characteristics in response to treatment and the need to incorporate sex and genotype as variables in evaluating therapeutic efficacy in preclinical studies (Clayton and Collins, 2014).
Males and females differ in their response to drug treatment, differences that can be critical. In recent years, several studies have described sex-related differences in the progression of diseases and the disposition of drugs in the body (Capece et al., 2000; Afonso-Pereira et al., 2018; Moyer et al., 2019; Valodara and Sr, 2019). In a previous experiment analysing the pharmacokinetics of S4A and S10A (Codina et al., 2020), ABZ bioavailability was significantly increased when administered as microcrystalline formulations. However, host genotype and sex influenced the pharmacokinetic parameters measured: CBi/L females attained a significantly higher Cmax compared to males, whereas no sex effect was observed for this variable in CBi+. Thus, the finding of the formulations' low or lack of efficacy in females treated in the migratory or parenteral phases of the infection was an unexpected outcome suggesting that the metabolism and excretion of ABZ in CBi/L and CBi+ females might be higher than that in males. The active ABZ sulphoxide (ABZSO) metabolite, responsible for the antiparasitic activity against different nematodes, is biotransformed to the inactive metabolite ABZ sulphone (ABZSO2) mainly by CYP3A4, which is expressed more in women than in men, resulting in a higher clearance rate (Wolbold et al., 2003). Fuscoe et al. (2020) reported a similar sex-determined expression of gene Cyp3a2, the rat orthologue of the human gene encoding CYP3A4.
It should also be noted that ABZSO usually presents in two enantiomeric forms with known differences in their pharmacokinetic profile and activity against other helminths. Capece et al. (2000) found differences in the pharmacokinetics of ABZSO enantiomers in sheep of both sexes but not in total-ABZSO or ABZSO2; they also observed a sex difference in the enantiomers Tmax; Cmax values for (−)-ABZSO were similar in both sexes, but peak concentrations differed. Thus, their data suggest another possible sex effect in ABZSO metabolism.
An effect of the ongoing infection on ABZ bioavailability could be ruled out since results reported by García Rodríguez et al. (2009) suggest that although intestinal infection by T. spiralis induces a transient acute inflammation, it is almost completely abrogated after worm expulsion from the gut. This situation prompts the recovery of absorption capacity and oral bioavailability of drugs when administered on the infection's systemic phases. S10A microcrystals, based on chitosan, showed a better therapeutic efficacy compared to ABZ or S4A when administered on the chronic phase of infection. This enhanced efficacy, significant in CBi/L males, is most probably related to the known interactions of chitosan with the immune system. Chitosan is active against a variety of diseases, possessing both antimicrobial and anticancer properties. It has shown in vitro and in vivo anticryptosporidial activity (Mammeri et al., 2018), has effectively reduced symptoms in a mouse model of induced ulcerative colitis, improving intestinal mucosal barrier function and modifying the intestinal microflora (Wang et al., 2019), and has enhanced vaccines’ efficacy as an adjuvant (Sun et al., 2018). Furthermore, chitosan's mucoadhesive properties have been shown to enhance drug absorption (Khan et al., 2019). In fact, a higher bioavailability in a period of 24 h was observed in mice given S10A compared to ABZ and S4A (Codina et al., 2020). The physiological activities of chitosan would depend on both molecular weight and water-solubility of the molecule (Zeng et al., 2008) and the host's genotype.
Our results are consistent with those of a recent publication where nanostructured lipid carriers were coated with chitosan and administered to mice in the three phases of T. spiralis infection (Eid et al., 2020). The authors reported a 2-fold reduction of viable encysted larvae treated with chitosan-coated nanocarriers compared to ABZ suspension. In this study, chitosan-based microcrystals (S10A) increased by 1.4- to 2.3-fold the percentage of dead larvae compared to ABZ. It is important to note that while nanocarriers are prepared with a high proportion of excipient (approximately 92%), the microcrystals require much smaller amounts of the polymer (approximately 20%). This difference makes the microcrystalline formulation promising for translation to a final product.
In summary, the chitosan-based microcrystals showed a better efficacy in the chronic phase of infection when the formulations currently in use are not effective or need to be administered in higher doses or for more extended periods. Our findings suggest that host genotype- and sex-specific effects influenced the response to the formulations when administered after the intestinal phase and should be considered in pre-clinical studies. The observed dependence of the therapeutic efficacy on the different T. spiralis infection phases might respond to several factors such as the host's immune response, T. spiralis stage's susceptibility, drug bioavailability and formulations' components. The results showed that microcrystalline formulations would be suitable systems to treat T. spiralis infection in the chronic phase. More experiments using various administration protocols and analysis of the mechanisms involved should be undertaken in future studies.
Acknowledgements
A.V.C. is grateful to CIUNR (Consejo de Investigaciones, Universidad Nacional de Rosario) for a Research Fellowship. J.P. is grateful to CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas) for a Doctoral Fellowship.
Author contributions
L.I.H. and MCL conceived and designed the study. AVC, JP, MDV and DL conducted data gathering. AVC and JP performed statistical analyses. AVC, JP, MCL and LIH wrote the article.
Financial support
This work was supported by Universidad Nacional de Rosario, Project 1MED447 (LIH), CONICET, Project PIP 112-201001-00194 (MCL), and Ministerio de Ciencia, Tecnología e Innovación Productiva de Santa Fe, Project IO 2017-00220 (LIH).
Ethical standards
The experimental protocol followed the institutional regulations and was approved by the Bioethics Committee of the Facultad de Ciencias Médicas, Universidad Nacional de Rosario (permit number 1398/2016).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001128.
click here to view supplementary material
Conflict of interest
The authors declare there are no conflicts of interest.
References
- Afonso-Pereira F, Dou L, Trenfield SJ, Madla CM, Murdan S, Sousa J, Veiga F and Basit AW (2018) Sex differences in the gastrointestinal tract of rats and the implications for oral drug delivery. European Journal of Pharmaceutical Sciences 115, 339–344. [DOI] [PubMed] [Google Scholar]
- Alanazi FK, El-Badry M, Ahmed MO and Alsarra IA (2007) Improvement of albendazole dissolution by preparing microparticles using spray-drying technique. Scientia Pharmaceutica 75, 63–79. [Google Scholar]
- Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D and Appleton JA (2007) Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. The Journal of Immunology 178, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Bell RG, Wang CH and Ogden RW (1985) Trichinella spiralis: nonspecific resistance and immunity to newborn larvae in inbred mice. Experimental Parasitology 60, 101–110. [DOI] [PubMed] [Google Scholar]
- Campbell WC and Cuckler AC (1964) Effect of thiabendazole upon the enteral and parenteral phases of trichinosis in mice. The Journal of Parasitology 50, 481–488. [PubMed] [Google Scholar]
- Campino S, Kwiatkowski D and Dessein A (2006) Mendelian and complex genetics of susceptibility and resistance to parasitic infections. Seminars in Immunology 18, 411–422. [DOI] [PubMed] [Google Scholar]
- Capece BP, Castells G, Pérez F, Arboix M and Cristòfol C (2000) Pharmacokinetic behaviour of albendazole sulphoxide enantiomers in male and female sheep. Veterinary Research Communications 24, 339–348. [DOI] [PubMed] [Google Scholar]
- Casulli A, Morales MA, Gallinella B, Turchetto L and Pozio E (2006) 2-Hydroxypropyl-beta-cyclodextrin Improves the effectiveness of albendazole against encapsulated larvae of Trichinella spiralis in a murine model. Journal of Antimicrobial Chemotherapy 58, 886–890. [DOI] [PubMed] [Google Scholar]
- Chung MS, Joo KH, Quan FS, Kwon HS and Cho SW (2001) Efficacy of flubendazole and albendazole against Trichinella spiralis in mice. Parasite 8(2 Suppl), S195–S198. [DOI] [PubMed] [Google Scholar]
- Clayton JA and Collins FS (2014) NIH To balance sex in cell and animal studies. Nature 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina AV, García A, Leonardi D, Vasconi MD, Di Masso RJ, Lamas MC and Hinrichsen LI (2015) Efficacy of albendazole: β-cyclodextrin citrate in the parenteral stage of Trichinella spiralis infection. International Journal of Biological Macromolecules 77, 203–206. [DOI] [PubMed] [Google Scholar]
- Codina AV, Priotti J, Leonardi D, Vasconi MD, Hinrichsen LI and Lamas MC (2020) Effect of genotype and sex of the host on the bioavailability of novel albendazole microcrystals based on chitosan and cellulose derivatives. AAPS PharmSciTech 21, 149–157. [DOI] [PubMed] [Google Scholar]
- de-la-Rosa JL, Alvarez N and Gomez-Priego A (2007) Study of the reproductive capacity of Trichinella spiralis recovered from experimentally infected mice under-dosed with albendazole or mebendazole. Tropical Biomedicine 24, 93–97. [PubMed] [Google Scholar]
- de la Torre-Iglesias PM, García-Rodriguez JJ, Torrado G, Torrado S, Torrado-Santiago S and Bolás-Fernández F (2014) Enhanced bioavailability and anthelmintic efficacy of mebendazole in redispersible microparticles with low-substituted hydroxypropylcellulose. Drug Design, Development and Therapy 8, 1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham DA and Martinez AR (1970) Studies with methyridine and Trichinella spiralis 2. The use of the drug to study the rate of larval production in mice. Journal of Helminthology 44, 357–363. [DOI] [PubMed] [Google Scholar]
- Dib A, Palma S, Suárez G, Farías C, Cabrera P, Castro S, Allemandi D, Moreno L, Lanusse C and Sánchez Bruni PSF (2011) Albendazole sulphoxide kinetic disposition after treatment with different formulations in dogs. Journal of Veterinary Pharmacology and Therapeutics 34, 136–141. [DOI] [PubMed] [Google Scholar]
- Dkhil MA (2015) Sex-determined susceptibility and differential MUC2 mRNA expression during the course of murine intestinal eimeriosis. Parasitology Research 114, 283–288. [DOI] [PubMed] [Google Scholar]
- Eid RK, Ashour DS, Essa EA, El Maghraby GM and Arafa MF (2020) Chitosan coated nanostructured lipid carriers for enhanced in vivo efficacy of albendazole against Trichinella spiralis. Carbohydrate Polymers 232, 115826. [DOI] [PubMed] [Google Scholar]
- Fuscoe JC, Vijay V, Hanig JP, Han T, Ren L, Greenhaw JJ, Beger RD, Pence LM and Shi Q (2020) Hepatic transcript profiles of cytochrome P450 genes predict sex differences in drug metabolism. Drug Metabolism and Disposition 48, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodriguez JJ, Torrado J and Bolás F (2001) Improving bioavailability and anthelmintic activity of albendazole by preparing albendazole-cyclodextrin complexes. Parasite 8, S188–S190. [DOI] [PubMed] [Google Scholar]
- García JJ, Bolás F and Torrado JJ (2003) Bioavailability and efficacy characteristics of two different oral liquid formulations of albendazole. International Journal of Pharmaceutics 250, 351–358. [DOI] [PubMed] [Google Scholar]
- García A, Barrera MG, Piccirilli G, Vasconi MD, Di Masso RJ, Leonardi D, Hinrichsen LI and Lamas MC (2013) Novel albendazole formulations given during the intestinal phase of Trichinella spiralis infection reduce effectively parasitic muscle burden in mice. Parasitology International 62, 568–570. [DOI] [PubMed] [Google Scholar]
- García Rodríguez JJ, De Prada I, Torrado Durán JJ and Bolás Fernández F (2009) The effect of intestinal trichinellosis on oral bioavailability of albendazole in mice. Parasitology Research 105, 65–70. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Pozio E and Nöckler K (2009) Epidemiology, diagnosis, treatment, and control of trichinellosis. Clinical Microbiology Reviews 22, 127–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy MM, Elmehankar MS, Azab MS, El-Tantawy NL and Abdel-Aziz A (2019) Sex dichotomy in the course of experimental latent toxoplasmosis. Experimental Parasitology 202, 15–21. [DOI] [PubMed] [Google Scholar]
- Hernandez-Valladares M, Rihet P and Iraqi FA (2014) Host susceptibility to malaria in human and mice: compatible approaches to identify potential resistant genes. Physiological Genomics 46, 1–16. [DOI] [PubMed] [Google Scholar]
- Hinrichsen LI and Di Masso RJ (2010) Use of an original murine model from Argentina in the characterization of complex phenotypes. Journal of Basic and Applied Genetics 21, 1–12. [Google Scholar]
- Incani RN, Morales G and Cesari IM (2001) Parasite and vertebrate host genetic heterogeneity determine the outcome of infection by Schistosoma mansoni. Parasitology Research 87, 131–137. [DOI] [PubMed] [Google Scholar]
- Khan MIH, An X, Dai L, Li H, Khan A and Ni Y (2019) Chitosan-based polymer matrix for pharmaceutical excipients and drug delivery. Current Medicinal Chemistry 26, 2502–2513. [DOI] [PubMed] [Google Scholar]
- Knopp S, Steinmann P, Keiser J and Utzinger J (2012) Nematode infections. Soil-transmitted helminths and Trichinella. Infectious Disease Clinics of North America 26, 341–358. [DOI] [PubMed] [Google Scholar]
- Leclair D, Forbes LB, Suppa S and Gajadhar AA (2003) Evaluation of a digestion assay and determination of sample size and tissue for the reliable detection of Trichinella larvae in walrus meat. Journal of Veterinary Diagnostic Investigation 15, 188–191. [DOI] [PubMed] [Google Scholar]
- Leonardi D, Lamas MC and Olivieri AC (2008) Multiresponse optimization of the properties of albendazole-chitosan microparticles. Journal of Pharmaceutical and Biomedical Analysis 48, 802–807. [DOI] [PubMed] [Google Scholar]
- Lockard RD, Wilson ME and Rodríguez NE (2019) Sex-related differences in immune response and symptomatic manifestations to infection with Leishmania species. Journal of Immunology Research 2019, 4103819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-García ML, Torrado-Durán S, Torrado-Durán J, Martínez-Fernández AR and Bolás-Fernández F (1997) Albendazole versus ricobendazole (albendazole-sulphoxide) against enteral and parenteral stages of Trichinella spiralis in mice. International Journal for Parasitology 27, 781–785. [DOI] [PubMed] [Google Scholar]
- Luebke RW (2007) Nematodes as host resistance models for detection of immunotoxicity. Methods (San Diego, Calif.) 41, 38–47. [DOI] [PubMed] [Google Scholar]
- Machado-Silva JR, Neves RH, Da Silva LO, De Oliveira RMF and Da Silva AC (2005) Do mice genetically selected for resistance to oral tolerance provide selective advantage for Schistosoma mansoni infection? Experimental Parasitology 111, 1–7. [DOI] [PubMed] [Google Scholar]
- Mammeri M, Chevillot A, Thomas M, Polack B, Julien C, Marden JP, Auclair E, Vallée I and Adjou KT (2018) Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum in vitro and in vivo in neonatal mice. Experimental Parasitology 194, 1–8. [DOI] [PubMed] [Google Scholar]
- McCracken RO (1978) Efficacy of mebendazole and albendazole against Trichinella spiralis in mice. Journal of Parasitology 64, 214–219. [PubMed] [Google Scholar]
- McRae KM, Good B, Hanrahan JP, Glynn A, O'Connell MJ and Keane OM (2014) Response to Teladorsagia circumcincta infection in Scottish Blackface lambs with divergent phenotypes for nematode resistance. Veterinary Parasitology 206, 200–207. [DOI] [PubMed] [Google Scholar]
- McRae KM, Good B, Hanrahan JP, McCabe MS, Cormican P, Sweeney T, O'Connell MJ and Keane OM (2016) Transcriptional profiling of the ovine abomasal lymph node reveals a role for timing of the immune response in gastrointestinal nematode resistance. Veterinary Parasitology 224, 96–108. [DOI] [PubMed] [Google Scholar]
- Ministerio de Salud y Desarrollo Social de la Nación, Dirección Nacional de Epidemiología y Análisis de la Situación de Salud (2019) Triquinelosis, Boletín integrado de vigilancia 459, SE 30/2019, 63. Buenos Aires, Argentina. Available at https://bancos.salud.gob.ar/sites/default/files/2020-01/boletin-integrado-vigilancia-n459.pdf.
- Mitreva, M and Jasmer, DP (2006) Biology and genome of Trichinella spiralis. In Hodgkin J (ed.), The C. elegans Research Community, WormBook. Pasadena, CA, pp 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer AM, Matey ET and Miller VM (2019) Individualized medicine: sex, hormones, genetics, and adverse drug reactions. Pharmacology Research & Perspectives 7, e00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Huda S and Sinha Babu SP (2019) Toll-like receptor polymorphism in host immune response to infectious diseases: a review. Scandinavian Journal of Immunology 90, e12771. [DOI] [PubMed] [Google Scholar]
- Murrell KD (2016) The dynamics of Trichinella spiralis epidemiology: out to pasture? Veterinary Parasitology 231, 92–96. [DOI] [PubMed] [Google Scholar]
- Murrell KD and Pozio E (2011) Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerging Infectious Diseases 17, 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals, 8th Edn. Washington, DC, USA: National Academies Press (US). [PubMed] [Google Scholar]
- Nava-Castro K, Hernández-Bello R, Muñiz-Hernández S, Camacho-Arroyo I and Morales-Montor J (2012) Sex steroids, immune system, and parasitic infections: facts and hypotheses. Annals of the New York Academy of Sciences 1262, 16–26. [DOI] [PubMed] [Google Scholar]
- Picherot M, Oswald IP, Cote M, Noeckler K, Le Guerhier F, Boireau P and Vallée I (2007) Swine infection with Trichinella spiralis: comparative analysis of the mucosal intestinal and systemic immune responses. Veterinary Parasitology 143, 122–130. [DOI] [PubMed] [Google Scholar]
- Pozio E (2018) Trichinella and other foodborne Nematodes. In Ortega YR and Sterling CR (eds), Foodborne Parasites. Cham, Switzerland: Springer International Publishing, pp. 175–215. doi: 10.1007/978-3-319-67664-7_9 [DOI] [Google Scholar]
- Priotti J, Codina AV, Leonardi D, Vasconi MD, Hinrichsen LI and Lamas MC (2017) Albendazole microcrystal formulations based on chitosan and cellulose derivatives: physicochemical characterization and In Vitro parasiticidal activity in Trichinella spiralis adult worms. AAPS PharmSciTech 18, 947–956. [DOI] [PubMed] [Google Scholar]
- Randazzo VR and Costamagna SR (2010) Methylene blue test for the determination of viability of free larvae of Trichinella spiralis. Revista Argentina de Microbiología 42, 95–97. [DOI] [PubMed] [Google Scholar]
- Ribicich M, Gamble HR, Bolpe J, Sommerfelt I, Cardillo N, Scialfa E, Gimenez R, Pasqualetti M, Pascual G, Franco A and Rosa A (2009) Evaluation of the risk of transmission of Trichinella in pork production systems in Argentina. Veterinary Parasitology 159, 350–353. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ (2011) Handbook of Parametric and Nonparametric Statistical Procedures, 5th Edn. Boca Raton, USA: Chapman and Hall/CRC. [Google Scholar]
- Shimoni Z and Froom P (2015) Uncertainties in diagnosis, treatment and prevention of trichinellosis. Expert Review of Anti-infective Therapy 13, 1279–1288. [DOI] [PubMed] [Google Scholar]
- Siriyasatien P, Yingyourd P and Nuchprayoon S (2003) Efficacy of albendazole against early and late stage of Trichinella spiralis infection in mice. Journal of the Medical Association of Thailand 86, S257–S262. [PubMed] [Google Scholar]
- Steel N, Faniyi AA, Rahman S, Swietlik S, Czajkowska BI, Chan BT, Hardgrave A, Steel A, Sparwasser TD, Assas MB, Grencis RK, Travis MA and Worthington JJ (2019) TGFβ-activation by dendritic cells drives Th17 induction and intestinal contractility and augments the expulsion of the parasite Trichinella spiralis in mice. PLoS Pathogens 15, e1007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Yu S, Zhao D, Guo S, Wang X and Zhao K (2018) Polysaccharides as vaccine adjuvants. Vaccine 36, 5226–5234. [DOI] [PubMed] [Google Scholar]
- Vallance BA, Blennerhassett PA and Collins SM (1997) Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. American Journal of Physiology – Gastrointestinal and Liver Physiology 272, G321–G327. [DOI] [PubMed] [Google Scholar]
- Valodara AM and Sr KJ (2019) Sexual dimorphism in drug metabolism and pharmacokinetics. Current Drug Metabolism 20, 1154–1166. [DOI] [PubMed] [Google Scholar]
- van der Giessen J, Deksne G, Gómez-Morales MA, Troell K, Gomes J, Sotiraki S, Rozycki M, Kucsera I, Djurković-Djaković O and Robertson LJ (2021) Surveillance of foodborne parasitic diseases in Europe in a one health approach. Parasite Epidemiology and Control 13, e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconi MD, Malfante P, Bassi A, Giudici C, Revelli S, Di Masso R, Font MT and Hinrichsen L (2008) Phenotypic differences on the outcome of the host–parasite relationship: behavior of mice of the CBi stock in natural and experimental infections. Veterinary Parasitology 153, 157–163. [DOI] [PubMed] [Google Scholar]
- Vasconi MD, Bertorini G, Codina AV, Indelman P, Di Masso RJ and Hinrichsen LI (2015) Phenotypic characterization of the response to infection with Trichinella spiralis in genetically defined mouse lines of the CBi-IGE stock. Open Journal of Veterinary Medicine 5, 111–122. [Google Scholar]
- Wang J, Zhang C, Guo C and Li X (2019) Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving Microflora. International Journal of Molecular Sciences 20, 5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert L and Schmid-Hempel P (2008) The genetic architecture of susceptibility to parasites. BMC Evolutionary Biology 8, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M and Zanger UM (2003) Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38, 978–988. [DOI] [PubMed] [Google Scholar]
- Zeng L, Qin C, Wang W, Chi W and Li W (2008) Absorption and distribution of chitosan in mice after oral administration. Carbohydrate Polymers 71, 435–440. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001128.
click here to view supplementary material