Abstract
INTRODUCTION
Recent Alzheimer's disease (AD) clinical trials have used cerebrospinal fluid (CSF) biomarker levels for screening and enrollment. Preliminary evidence suggests that AD risk is related to impaired renal function. The impact of kidney function on commonly used AD biomarkers remains unknown.
METHODS
Participants in studies conducted at the Goizueta Alzheimer's Disease Research Center (N = 973) had measurements of serum creatinine and CSF AD biomarkers. General linear models and individual data were used to assess the relationships between biomarkers and eGFR.
RESULTS
Lower estimated glomerular filtration rate (eGFR) was associated with lower amyloid beta (Aβ)42/tau ratio (p < 0.0001) and Aβ42 (p = 0.002) and higher tau (p < 0.0001) and p‐tau (p = 0.0002). The impact of eGFR on AD biomarker levels was more robust in individuals with cognitive impairment (all p‐values were < 0.005).
DISCUSSION
The association between eGFR and CSF AD biomarkers has a significant impact that varies by cognitive status. Future studies exploring this impact on the pathogenesis of AD and related biomarkers are needed.
Highlights
There is a significant association between Alzheimer's disease (AD) cerebrospinal fluid (CSF) biomarkers and both estimated glomerular filtration rate (eGFR) and mild cognitive impairment (MCI).
Kidney function influences CSF biomarker levels in individuals with normal cognitive function and those with MCI.
The impact of kidney function on AD biomarker levels is more pronounced in individuals with cognitive impairment.
The variation in CSF tau levels is independent of cardiovascular factors and is likely directly related to kidney function.
Tau may have a possible role in both kidney and cognitive function.
Keywords: Alzheimer's disease, biomarkers, cerebrospinal fluid analysis, kidney function, neurodegenerative diseases
1. BACKGROUND
Alzheimer's disease (AD) biomarkers such as amyloid beta 1‐42 (Aβ42), tau, phosphorylated tau (p‐tau), and more specifically the Aβ42/tau ratio play a crucial role in the diagnosis of AD. 1 In addition, these biomarkers are commonly used in clinical trials for screening and enrollment, and the use of a combination of these biomarkers has been found to yield improved case identifications. 2 , 3 , 4 , 5 The variation in levels of these AD biomarkers by demographics and comorbidities is still being explored, with recent evidence suggesting that consideration of these variations is important. 6 , 7 , 8 Kidney function may impact levels of disease markers, especially on plasma protein–based markers. 9 Whether there is a similar impact on AD biomarkers commonly measured in cerebrospinal fluid (CSF) remains unclear. Preliminary evidence has suggested that impaired kidney function is associated with cognitive impairment based on neuropsychological assessments. 10 , 11 , 12 A recent analysis of the Whitehall II prospective study suggested a connection between a lower estimated glomerular filtration rate (eGFR) and the risk of clinical dementia diagnosis. 13 It has also been suggested that the risk of AD is related to impaired renal function and its impact on levels of AD biomarkers in the plasma. 14 , 15 Because the CSF is a highly controlled microenvironment, studying the association of kidney function with CSF biomarkers offers great insight into the kidney–brain axis. 12 , 16
In this study, we aimed to investigate the association between estimated glomerular filtration rate (eGFR), as a measure of kidney function (especially considering it has previously been suggested as a biomarker for risk of cognitive impairment) 17 and the levels of AD biomarkers in the CSF—specifically, the Aβ42/tau ratio and other individual AD biomarkers such as Aβ42, tau, and p‐tau)—in individuals with and without cognitive impairment. By analyzing the data from participants enrolled in two research protocols, we sought to determine whether eGFR is associated with alterations in the levels of AD biomarkers in the CSF. We also explored potential interactions between kidney function, AD biomarker levels, and mild cognitive impairment (MCI), a common precursor to AD. This might provide critical insights into the impact of renal health on cognitive decline especially in individuals in the early symptomatic stages of AD.
RESEARCH IN CONTEXT
Systematic review: The literature review was conducted using sources such as PubMed and Google Scholar. We found that both research studies and clinical evaluations of Alzheimer's disease (AD) rely heavily on AD biomarker settings. Although kidney function is known to impact levels of plasma proteins, the impact on levels of commonly used AD biomarkers in the cerebrospinal fluid (CSF) is not clear. Given that lower kidney function is associated with risk of cognitive decline, it is important to study the association between AD biomarkers and kidney function.
Interpretation: Our results demonstrate that kidney function may have a significant associaiton with AD‐biomarker measurements in the CSF which is stronger in in the early symptomatic stages of AD.
Future directions: The stronger impact of kidney function in individuals with mild cognitive impairment needs to be further explored, as it may offer insight into mechanisms for the increased risk of AD in high‐risk populations for kidney disease such as older adults, African Americans, or individuals with diabetes or hypertension.
2. METHODS
2.1. Design, setting, and participants
We conducted a cross‐sectional study by analyzing data from participants in studies conducted within the Goizueta Alzheimer's Disease Research Center (ADRC) who had simultaneous measurement of their serum creatinine during their visit for CSF collection. The sample included 973 participants who were 50 years old or older and were enrolled in two observational protocols that were approved by the Emory IRB (Institutional Review Board). All participants signed a written informed consent and underwent identical lumbar puncture procedures, CSF analysis, and kidney function measurements: The Brain Stress Hypertension and Aging program (B‐SHARP; N = 375) and ADRC Clinical Research in Neurology (CRIN; N = 598). CRIN is an ADRC‐affiliated research protocol offered to individuals undergoing lumbar puncture as part of their evaluation at the Emory Cognitive Neurology clinic. B‐SHARP sample participants were identified either through a referral from the Goizueta ADRC or through strategic community partnerships with grass‐roots health education organizations, health fairs, advertisements, and mail‐out announcements. An appropriate study informant, defined as an individual who has regular contact with the participant for at least once a week (in person or by telephone), was also identified. The potential study participants attended a screening visit, during which they underwent cognitive testing. Demographics (age, sex, race, education), anthropometrics (weight and height), medical diagnosis, and income levels were collected at baseline by interview. A study physician performed the clinical evaluation and lumbar punctures (LP). MCI was determined by cognitive assessments and an interview with a clinician. Each evaluation was reviewed by the study physician (I.H.). MCI was defined as having the following: Montreal Cognitive Assessment (MoCA) score of less than 26, subjective memory concerns, Clinical Dementia Rating (CDR) sum of boxes score of 0.5 and memory box score of 0.5, education‐adjusted cutoff score on the Logical Memory task (delayed paragraph A only) of the Wechsler Memory Scale – Revised (Alzheimer's Disease Neuroimaging Initiative) of less than 11 for 16 or more years of education, less than 9 for 8 to 15 years of education, and less than 6 for 7 or fewer years of education (the maximum score is 25), and preserved instrumental activities of daily living (IADL; Functional Activities Questionnaire) score of 7 or less. LP procedures and pre‐analytical protocols are identical in all our ADRC‐affiliated research and clinical protocols. Following a fast of no less than 6 hours, CSF samples were collected via lumbar puncture using 24G Sprotte atraumatic spinal needles. Samples were collected in sterile polypropylene tubes, separated into 0.5 mL aliquots, and stored at −80°C. CSF Biomarkers, Aβ1‐42, tau, and p‐tau181, were measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics (INNO‐BIA AlzBio3; Ghent, Belgium; for research use– only reagents) immunoassay kit– based reagents. Kidney function was measured by calculating the eGFR. We calculated the eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation, 18 which was the method most widely used to calculate eGFR; it was calculated using this equation: 41 × min (Scr/κ, 1)α × max (Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if African American] (where Scr is serum creatinine in µmol/L, κ is 61.9 for females and 79.6 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1).
2.2. AD biomarkers measurement
CSF Aβ42, total tau, and p‐tau181 were measured using the xMAP Luminex platform with INNO‐BIA AlzBio3 immunoassay (Fujirebio) in a single laboratory (AKESOgen; Norcross, GA) following the manufacturer's protocols. Standard samples were run in duplicate for each plate (range pg/mL: 55‐2060 Aβ42, 28‐1610 total tau, 13‐234 p‐tau181), and each test sample was run in triplicate. Average coefficient of variation was 9.6% for Aβ42, 9.2% for total tau, and 8.9% for p‐tau181. Lots of reagents used during these experiments were #404212 and #405221. Plasma AD biomarkers were measured using the Simoa Platform Version 2 Advantage Kit (Quanterix Corp) and were used in a fully automated two‐step sandwich immunoassay as described previously. 19
2.3. Statistical analysis
Statistical analysis was performed with SAS statistical software version 9.4 M6. The statistical significance was defined as a two‐sided p < 0.05. We used t‐test or chi‐square test to compare between the characteristics of study participants, which are presented as means (SDs) or numbers (percentages). All data distributions were checked for normality. Although the two protocols had identical procedures for the biomarkers and kidney function, we conducted an individual participant data metanalysis with a general linear model and “protocol” as the random effect to assess the associations between AD biomarkers and eGFR using the combined data. 20 In the full‐sample analysis we did not adjust for age, sex, and race, since eGFR was calculated from age, sex, and race; to prevent collinearity, these factors are not included in the model. We also performed additional analyses on a subsample that had data available to use for an analysis that is adjusted for hypertension, diabetes, and a separate analysis looking at the association with the plasma levels of these biomarkers. In addition to the eGFR/biomarker measurement analysis, we conducted an interaction analysis (eGFR*MCI) to assess if the associations differed by cognitive status.
3. RESULTS
3.1. Participant characteristics
The study's sample included 973 participants, consisting of 613 women (63%) and 360 men (37%). The mean age was 66.52 (SD: 8.83). The participant distribution included 227 African American patients (23.33%) and 746 White patients (76.67%). Among the participants, 413 patients (42.45%) were diagnosed with MCI. The mean eGFR was 78 (SD: 16.70) mL/min/1.73 m2, with no statistically significant difference between participants with normal cognition (NC) ad participants with MCI. As expected, we found that individuals in the MCI group exhibited a substantially lower average Aβ42 level, and higher average tau and p‐tau levels (Table S1). The key characteristics of the study participants are provided in Table 1. Characteristics of participants in each protocol can be found in Table S2.
TABLE 1.
Descriptive characteristics of the pooled sample.
| Characteristic | Combined sample | NC | MCI | p‐value a |
|---|---|---|---|---|
| N | 973 | 560 (57.6%) | 413 (42.4%) | |
| Age, years, mean (SD) | 66.52 (8.83) | 64.7 ± 8.3 | 69.0 ± 8.9 | <0.001 |
| Sex (%) | ||||
| Female | 613 (63%) | 383 (68.4%) | 230 (55.7%) | <0.001 |
| Male | 360 (37%) | 177 (31.6%) | 183 (44.3%) | |
| Race (%) | ||||
| AA | 227 (23.33) | 114 (20.4%) | 113 (27.4%) | 0.011 |
| White | 746 (76.67%) | 446 (79.6%) | 300 (72.6%) | |
| eGFR, mean (SD) | 78.00 (16.70) | 79.2 (15.9) | 76.3 (17.6) | 0.007 |
| Aβ42, pg/mL, mean (SD) | 385.15 (178.22) | 457.2 (184.1) | 287.5 (111) | <0.001 |
| tau, pg/mL, mean (SD) | 66.75 (43.29) | 51.3 (23.4) | 87.6 (53.9) | <0.001 |
| p‐tau, pg/mL, mean (SD) | 31.52 (22.08) | 24.9 (11.9) | 40.6 (28.6) | <0.001 |
| Aβ42/tau ratio, mean (SD) | 8.06 (6.09) | 10.7 (6.4) | 4.5 (3.1) | <0.001 |
Abbreviations: AA, African American; Aβ42, amyloid beta42; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; N, sample size; NC, normal cognition; p‐tau, phosphorylated tau; SD, standard deviation; tau, tau protein.
p‐values obtained through a pooled analysis using t‐test for continuous data and chi‐square for discrete data.
3.2. Association of eGFR with CSF AD biomarkers in the full sample
We observed a significant association between eGFR and the Aβ42/tau ratio (0.033, p < 0.0001). We also found significant associations between eGFR and all three CSF AD biomarkers: Aβ42 (slope per unit increase: 0.75, p = 0.002), tau (−0.39, p < 0.0001), and p‐tau (−0.13, p = 0.0002). The results show that for every unit increase in eGFR, there is a corresponding change in CSF AD biomarkers as follows: an increase in both the Aβ42/tau ratio and Aβ42, and a decrease in both tau and p‐tau (Table 2).
TABLE 2.
Association of CSF biomarkers with estimated glomerular filtration rate from the pooled sample. a
| Biomarker | Slope b per unit of eGFR (95% CI) | p‐value | MCI adjusted slope c per unit of eGFR (95% CI) | p‐value |
|---|---|---|---|---|
| Aβ42, pg/mL | 0.75, (0.27, 1.23) | 0.002 | 0.52 (0.03, 1.01) | 0.04 |
| tau, pg/mL | −0.39, (−0.55, −0.23) | <0.001 | −0.23 (−0.38, −0.09) | 0.001 |
| p‐tau, pg/mL | −0.13, (−0.19, −0.06) | 0.0002 | −0.07 (−0.14, −0.01) | 0.02 |
| Aβ42/tau ratio | 0.033, (0.02, 0.05) | <0.001 | 0.03 (0.01, 0.04) | 0.002 |
Abbreviations: Aβ42, amyloid beta42; 95% CI%, 95% confidence Interval; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; ‐p‐tau, phosphorylated tau; tau, tau protein.
p‐values were obtained from the meta‐analysis using linear mixed‐effects models and “study” as a random effect.
Change is the beta coefficient for the change in the CSF biomarker levels per change in eGFR.
Change is the beta coefficient for the change in the CSF biomarker levels per change in eGFR adjusted for MCI.
Furthermore, our analysis showed that each unit increase in eGFR corresponded to an approximate increase of 0.52 in Aβ42 levels, and for tau and p‐tau each unit increase in eGFR corresponded with a decrease in levels of tau by 0.23 and by 0.07 for p‐tau levels in the CSF, even after adjusting for MCI status (Table 2).
3.3. The impact of MCI on the association between CSF AD biomarkers and eGFR
Our subsequent analysis aimed to explore the potential interaction among CSF AD biomarkers, eGFR, and MCI. We found a notable difference in the variation of CSF AD biomarkers with eGFR based on cognitive status, where the variation in AD biomarker levels was significant only in the MCI group. However, in the NC group, the variation with eGFR did not reach statistical significance (Table 3). This observation indicates that the impact of eGFR on AD biomarker levels is more pronounced in individuals with cognitive impairment (Figure 1).
TABLE 3.
Interaction between MCI, CSF biomarkers, and eGFR from the pooled sample. a
| Biomarkers | Effects | β | 95% CI | p‐value b |
|---|---|---|---|---|
| Aβ42, pg/mL | eGFR*MCI | 1.37 | (0.4, 2.33) | 0.005 |
| tau, pg/mL | eGFR*MCI | −0.30 | (−0.59, −0.01) | 0.04 |
| p‐tau, pg/mL | eGFR*MCI | −0.13 | (−0.25, 0) | 0.05 |
| Aβ42/tau ratio | eGFR*MCI | 0.03 | (0, 0.06) | 0.05 |
Abbreviations: β, beta coefficient for interaction between CSF biomarkers; 95% CI, 95% confidence Interval; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; p‐tau, phosphorylated tau; tau, tau protein; Aβ42, amyloid beta 42.
Model includes the interaction term eGFR*MCI and uses “study” as a random effect.
p‐values obtained through meta‐analysis of the data from both studies (B‐SHARP and CRIN).
FIGURE 1.
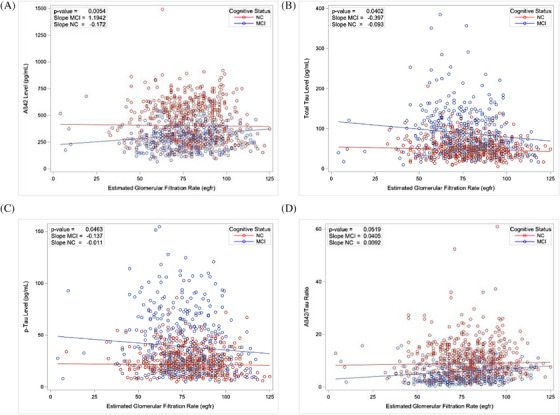
Impact of mild cognitive impairment (MCI) on the association between cerebrospinal fluid (CSF) Alzheimer's disease (AD) biomarker levels and estimated glomerular filtration rate (eGFR). Interaction analysis performed using linear mixed models to assess differences in the relationship between CSFAD biomarker levels: (A) amyloid beta (Aβ)42, (B) tau, (C) phosphorylated tau (p‐tau), and (D) Aβ42/tau ratio and eGFR based on cognitive status. The slope and intercept are plotted separately for individuals with MCI and those with normal cognition (NC). The p‐value presented is for the interaction term (eGFR*MCI); it suggests a significant difference in the association between these variables based on cognitive status.
In addition, we found that after adjusting for hypertension and diabetes, which was available only for a subsample (N = 375, 50.74% with hypertension and 12.76% with diabetes), the association between the Aβ42/tau ratio, tau, and p‐tau with eGFR remained significant except for Aβ42 (Table S3).
Finally, we conducted an analysis to explore the association of eGFR with variations in levels of AD plasma biomarker levels, which was also available for only a subset of the total sample. We found similar associations for plasma p‐tau (p < 0.0001) as in the CSF. We present these results in the online supplement (Table S4).
4. DISCUSSION
In our present study of a large cohort of older adults with CSF biomarker quantifications, we found that kidney function was associated with the levels of AD biomarkers in the CSF. Although these associations remained significant after adjusting for cognitive status, we observed significant interactions between MCI and eGFR, where the impact of this association is more pronounced in individuals with MCI. The significant association between CSF biomarkers and both eGFR and MCI, even after adjusting for confounders, reinforces the notion from previous studies of a more direct influence of kidney health on AD‐related pathology. 21 Earlier studies have consistently indicated a link between kidney function, cognitive impairment, and dementia. 10 , 11 , 12 , 13 Lower eGFR has been found to be associated with specific cognitive domains, specifically the domain for executive function. 22 In addition, recent studies have found that the kidneys play a physiological role in the clearance of Aβ from the blood and brain in both humans and animals, 23 , 24 as well as tau having a physiological role in the kidneys. 25 Furthermore, studies have suggested that Aβ from the periphery might be contributing to AD pathogenesis by increasing the levels of Aβ crossing the blood–brain barrier (BBB) and inducing Aβ‐related pathologies in the brain. 26 , 27 Therefore, given that it has been established that lower Aβ42 levels in the CSF or plasma are associated with increased amyloid deposition in the brain by many clinical studies, 26 , 28 , 29 , 30 , 31 , 32 and the amyloid hypothesis that suggests AD is due to the imbalance of Aβ, 33 a possible explanation for our findings is that the decline in kidney function resulted in decreased clearance and therefore accumulation of AD pathology. 34 , 35 Another possible explanation is that the decline in kidney function contributed to an increase in the production of AD biomarkers in the brain. In both scenarios, the association of kidney function with AD biomarkers needs further exploration. It is also worth noting that the associations between kidney function and CSF tau and Aβ42/tau ratio were significant after adjusting for hypertension and diabetes, suggesting that our findings are independent of the common risk factors for kidney function loss. Our finding that adjusting for hypertension and diabetes renders the Aβ42‐kidney function association not significant may imply that it is related to kidney disease risk factors. This supports previous studies that demonstrated an association between increased plasma levels of Aβ and vascular disease in both the brain and the periphery including hypertension and diabetes, 27 especially considering we found similar significant associations with plasma AD biomarkers such as p‐tau. However, it is important to note that these analyses were conducted on only a subsample and hence the results should be interpreted with caution.
This is to our knowledge one of the few first large studies that reported the association of kidney function with CSF AD biomarkers and its interaction with cognitive status. This study highlights the need to incorporate kidney function in studies of AD risk factors and the need to consider renal health when interpreting the results of AD biomarkers 36 , 37 within clinical evaluations and trials, particularly during the early symptomatic stages of AD. This also adds an additional factor to our understanding of the complex multifactorial processes contributing to AD and its biomarkers.
4.1. Limitations
This study is limited by its cross‐sectional design. Considering that the AD biomarkers were measured at a single point, whether kidney function would influence these measures in a longitudinal study remains an important and unexplored phenomenon We also had few participants with an eGFR in the lower range and none of participants with dementia. Our intent was to assess the associations between CSF AD biomarkers and kidney function independent of having kidney disease or dementia.
4.2. Future directions
It is important that future studies investigate the associations with other known comorbidities such as microvascular dysfunction, vitamin D metabolism, erythropoietin variation, and alterations in the renin–angiotensin system and their effects on AD biomarker variations.
4.3. Conclusion
In conclusion, our study provided insights into the complex association between kidney function, AD biomarkers, and cognitive impairment. The findings suggest a significant association between kidney function and variation in levels of AD biomarkers in the CSF (Aβ42, tau, and p‐tau), which were stronger in individuals with MCI. This suggests that kidney function should be considered in the context of interpreting AD biomarkers in clinical evaluations and clinical trials, especially in patients in the early symptomatic stage of AD. Future studies need to further explore the impact of kidney function and other comorbidities on the interpretation of AD biomarkers.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONSENT STATEMENT
The Emory Institutional Review Board approved the study protocol, and each participant provided written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank all study participants for their contributions to our research. We acknowledge the B‐SHARP (Brain, Stress, Hypertension and Aging Research Program) team and the Emory Goizueta Alzheimer's Disease Research Center (ADRC) staff. This work was supported by National Institutes of Health/National Institute on Aging (NIH/NIA) grants (AG051633, AG057470‐01, AG042127), and the Alzheimer's Drug Discovery Foundation grant (20150603; PI: Ihab Hajjar). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Hajjar I, Neal R, Yang Z, Lah JJ. Alzheimer's disease cerebrospinal fluid biomarkers and kidney function in normal and cognitively impaired older adults. Alzheimer's Dement. 2024;16:e12581. 10.1002/dad2.12581
REFERENCES
- 1. Papaliagkas V, Kalinderi K, Vareltzis P, Moraitou D, Papamitsou T, Chatzidimitriou M. CSF biomarkers in the early diagnosis of mild cognitive impairment and Alzheimer's disease. Int J Mol Sci. 2023;24(10):8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouwman FH, Schoonenboom NS, Verwey NA, et al. CSF biomarker levels in early and late onset Alzheimer's disease. Neurobiol Aging. 2009;30(12):1895‐1901. [DOI] [PubMed] [Google Scholar]
- 3. Hampel H, Teipel SJ, Fuchsberger T, et al. Value of CSF β‐amyloid1‐42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9(7):705‐710. [DOI] [PubMed] [Google Scholar]
- 4. Jack C Jr, Bennett D, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattsson N, Insel PS, Donohue M, et al. Predicting reduction of cerebrospinal fluid β‐amyloid 42 in cognitively healthy controls. JAMA Neurol. 2015;72(5):554‐560. [DOI] [PubMed] [Google Scholar]
- 6. Garrett SL, McDaniel D, Obideen M, et al. Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Netw Open. 2019;2(12):e1917363‐e1917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sundermann EE, Panizzon MS, Chen X, et al. Sex differences in Alzheimer's‐related Tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang J, Brody EN, Murthy AC, et al. Impact of kidney function on the blood proteome and on protein cardiovascular risk biomarkers in patients with stable coronary heart disease. J Am Heart Assoc. 2020;9(15):e016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basit S, Damholt MB, Wohlfahrt J, Boyd HA. Is kidney disease associated with both Alzheimer's disease and vascular dementia? Epidemiology/risk and protective factors in MCI and dementia. Alzheimers Dement. 2020;16:e042527. [Google Scholar]
- 11. Xu H, Garcia‐Ptacek S, Trevisan M, et al. Kidney function, kidney function decline, and the risk of dementia in older adults: a registry‐based study. Neurology. 2021;96(24):e2956‐e2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C‐Y, He F‐F, Su H, Zhang C, Meng X‐F. Association between chronic kidney disease and Alzheimer's disease: an update. Metab Brain Dis. 2020;35:883‐894. [DOI] [PubMed] [Google Scholar]
- 13. Singh‐Manoux A, Oumarou‐Ibrahim A, Machado‐Fragua MD, et al. Association between kidney function and incidence of dementia: 10‐year follow‐up of the Whitehall II cohort study. Age Ageing. 2022;51(1):afab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun H‐L, Yao X‐Q, Lei L, et al. Associations of blood and cerebrospinal fluid Aβ and tau levels with renal function. Mol Neurobiol. 2023:1‐9. [DOI] [PubMed] [Google Scholar]
- 16. Xie Z, Tong S, Chu X, Feng T, Geng M. Chronic kidney disease and cognitive impairment: the kidney‐brain axis. Kidney Diseases. 2022;8(4):275‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns CM, Knopman DS, Tupper DE, et al. Prevalence and risk of severe cognitive impairment in advanced chronic kidney disease. J Gerontol 2018;73(3):393‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS. CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration): a new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hajjar I, Yang Z, Okafor M, et al. Association of plasma and cerebrospinal fluid Alzheimer disease biomarkers with race and the role of genetic ancestry, vascular comorbidities, and neighborhood factors. JAMA Netw Open. 2022;5(10):e2235068‐e2235068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheu C‐F, Suzuki S. Meta‐analysis using linear mixed models. Behav Res Methods Instrum Comput. 2001;33(2):102‐107. [DOI] [PubMed] [Google Scholar]
- 21. Shi Y, Liu Z, Shen Y, Zhu H. A novel perspective linkage between kidney function and Alzheimer's disease. Front Cell Neurosci. 2018;12:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zammit AR, Katz MJ, Lai JY, Zimmerman ME, Bitzer M, Lipton RB. Association between renal function and cognitive ability domains in the Einstein aging study: a cross‐sectional analysis. J Gerontol. 2015;70(6):764‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghiso J, Calero M, Matsubara E, et al. Alzheimer's soluble amyloid β is a normal component of human urine. FEBS Lett. 1997;408(1):105‐108. [DOI] [PubMed] [Google Scholar]
- 24. Tian D‐Y, Cheng Y, Zhuang Z‐Q, et al. Physiological clearance of amyloid‐beta by the kidney and its therapeutic potential for Alzheimer's disease. Mol Psychiatry. 2021;26(10):6074‐6082. [DOI] [PubMed] [Google Scholar]
- 25. Vallés‐Saiz L, Peinado‐Cahuchola R, Ávila J, Hernández F. Microtubule‐associated protein tau in murine kidney: role in podocyte architecture. Cell Mol Life Sci. 2022;79(2):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bu X, Xiang Y, Jin W, et al. Blood‐derived amyloid‐β protein induces Alzheimer's disease pathologies. Mol Psychiatry. 2018;23(9):1948‐1956. [DOI] [PubMed] [Google Scholar]
- 27. Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β‐amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016;6(1):26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grimmer T, Riemenschneider M, Förstl H, et al. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65(11):927‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS β‐amyloid in Alzheimer's disease. Science. 2010;330(6012):1774‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmqvist S, Mattsson N, Hansson O, Initiative AsDN . Cerebrospinal fluid analysis detects cerebral amyloid‐β accumulation earlier than positron emission tomography. Brain. 2016;139(4):1226‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strozyk D, Blennow K, White L, Launer L. CSF Aβ 42 levels correlate with amyloid‐neuropathology in a population‐based autopsy study. Neurology. 2003;60(4):652‐656. [DOI] [PubMed] [Google Scholar]
- 32. Tapiola T, Alafuzoff I, Herukka S‐K, et al. Cerebrospinal fluid β‐amyloid 42 and tau proteins as biomarkers of Alzheimer‐type pathologic changes in the brain. Arch Neurol. 2009;66(3):382‐389. [DOI] [PubMed] [Google Scholar]
- 33. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353‐356. [DOI] [PubMed] [Google Scholar]
- 34. Kelly DM, Rothwell PM. Disentangling the relationship between chronic kidney disease and cognitive disorders. Front Neurol. 2022;13:830064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y‐J, Zhou H‐D, Zhou X‐F. Clearance of amyloid‐beta in Alzheimer's disease: progress, problems and perspectives. Drug Discovery Today. 2006;11(19‐20):931‐938. [DOI] [PubMed] [Google Scholar]
- 36. Stocker H, Beyer L, Trares K, et al. Association of kidney function with development of Alzheimer disease and other dementias and dementia‐related blood biomarkers. JAMA Netw Open. 2023;6(1):e2252387‐e2252387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Syrjanen JA, Campbell MR, Algeciras‐Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


