Abstract
Chronic primary systemic vasculitis (PSV) comprises a group of heterogeneous diseases that are broadly classified by affected blood vessel size, clinical traits and the presence (or absence) of anti-neutrophil cytoplasmic antibodies (ANCA) against proteinase 3 (PR3) and myeloperoxidase (MPO). In small vessel vasculitis (SVV), ANCA are not present in all patients, and they are rarely detected in patients with vasculitis involving medium (MVV) and large (LVV) blood vessels. Some studies have demonstrated that lysosome-associated membrane protein-2 (LAMP-2/CD107b) is a target of ANCA in SVV, but its presence and prognostic value in childhood MVV and LVV is not known. This study utilized retrospective sera and clinical data obtained from 90 children and adolescents with chronic PSV affecting small (SVV, n = 53), medium (MVV, n = 16), and large (LVV, n = 21) blood vessels. LAMP-2-ANCA were measured in time-of-diagnosis sera using a custom electrochemiluminescence assay. The threshold for seropositivity was established in a comparator cohort of patients with systemic autoinflammatory disease. The proportion of LAMP-2-ANCA-seropositive individuals and sera concentrations of LAMP-2-ANCA were assessed for associations with overall and organ-specific disease activity at diagnosis and one-year follow up. This study demonstrated a greater time-of-diagnosis prevalence and sera concentration of LAMP-2-ANCA in MVV (52.9% seropositive) and LVV (76.2%) compared to SVV (45.3%). Further, LAMP-2-ANCA-seropositive individuals had significantly lower overall, but not organ-specific, disease activity at diagnosis. This did not, however, result in a greater reduction in disease activity or the likelihood of achieving inactive disease one-year after diagnosis. The results of this study demonstrate particularly high prevalence and concentration of LAMP-2-ANCA in chronic PSV that affects large blood vessels and is seronegative for traditional ANCA. Our findings invite reconsideration of roles for autoantigens other than MPO and PR3 in pediatric vasculitis, particularly in medium- and large-sized blood vessels.
Keywords: lysosome-associated membrane protein-2, anti-neutrophil cytoplasmic antibodies, childhood-onset primary vasculitis, autoantibodies, Takayasu’s arteritis, polyarteritis nodosa
1. Introduction
Chronic primary systemic vasculitis (PSV) is an umbrella term for a family of heterogeneous diseases that are commonly characterized by inflammation and damage in blood vessel walls. PSV in children and adolescents has an average age of onset of 10–14 years and is particularly rare (<23/100,000 cases annually in North America) compared to the disease in adults (onset > 50 years) [1,2,3]. In both pediatric- and adult-onset vasculitis, different disease subtypes are broadly classified under the predominant size—small, medium, and large—of the affected blood vessels and consideration of differing clinical features, histologic analysis of affected tissues, and the presence (or absence) of anti-neutrophil cytoplasmic antibodies (ANCA) [4,5].
ANCA are a family of autoantibodies that can target distinct autoantigens in the cytoplasmic (c-ANCA) and perinuclear (p-ANCA) region of neutrophils and, to a lesser extent, monocytes [6]. ANCA seropositivity is predominantly observed in chronic PSV that affects small vessels, and in these cases, it targets one of two antigens: proteinase 3 (PR3) and myeloperoxidase (MPO). Seropositivity and specificity of ANCA for PR3 or MPO has some proven utility in the diagnosis and differentiation of “ANCA-associated vasculitis (AAV)” subtypes and more recently were demonstrated to have power in predicting disease-associated risks in adult-onset disease [4]. Similar diagnostic and prognostic tools do not exist for “ANCA-negative” vasculitis [7], which in children includes ~10–30% of cases of small-vessel vasculitis and the majority, if not all, forms of vasculitis affecting medium to large blood vessels [8].
Beyond PR3 and MPO, some studies have demonstrated that lysosome-associated membrane protein-2 (LAMP-2/CD107b) is an antigenic target of ANCA. LAMP-2 is a heavily glycosylated lysosome and plasma membrane protein that, in contrast to MPO and PR3, is expressed in almost every cell and tissue type in the body [9]. One epitope of LAMP-2 has 100% amino acid homology with type I fimbriated bacterial adhesion protein, FimH. Kain et al. [10] demonstrated that FimH-immunized rats produce ANCA against human LAMP-2 and spontaneously develop microvascular injury, glomerulonephritis, and lung damage. Previous reports have demonstrated an increase in LAMP-2 protein in sera from adults with a medium-sized vessel vasculitis subtype called polyarteritis nodosa (PAN) [11]. Elevated circulating concentrations of anti-LAMP-2 autoantibodies (LAMP-2-ANCA) have been observed in adults with small-vessel ANCA-associated vasculitis (AAV) and pauci-immune crescentic glomerulonephritis [12], as well as children with AAV [13]. Subsequent to this, elevated circulating concentrations of LAMP-2 protein [11,14] and LAMP-2-ANCA [13,15] were detected in adults with chronic PSV affecting medium- and large-sized blood vessels. Stemming from these collective reports, we hypothesized that LAMP-2-ANCA would be present in childhood-onset chronic PSV subtypes that affect medium to large blood vessels and have no detectable concentrations of circulating PR3-ANCA or MPO-ANCA.
2. Results
2.1. Greater Prevalence and Elevated Sera Concentration of LAMP-2-ANCA in PR3- and MPO- ANCA-Negative Vasculitis Subtypes Affecting Medium to Large Blood Vessels
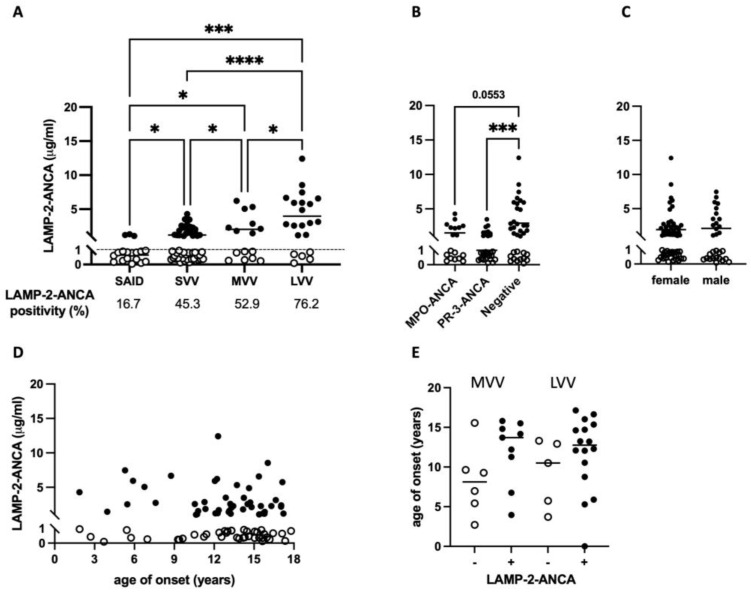
A total of 90 children and adolescents newly diagnosed with chronic primary systemic vasculitis (PSV) affecting small- (small-vessel vasculitis, SVV, n = 53), medium- (MVV, n = 16), and large- (LVV, n = 21) sized blood vessels were included in this study (Table 1). The median age at symptom onset (13.3 years, range 1.9–17.8 years) and biological sex at birth (66.7% female) were comparable between individuals with vasculitis affecting vessels of different size: SVV median onset 14.2 years, 66.0% female; MVV median onset 11.2 years, 62.5% female; and LVV median onset 12.6 years, 71.4% female. Compared to individuals with a systemic inflammatory disease lacking autoimmune features (SAID cohort; see Methods), a greater percentage of participants with SVV, MVV, and LVV were LAMP-2-ANCA-seropositive, with the greatest prevalence observed in chronic PSV subtypes, where larger vessels were involved: 45.3% (24/53) of SVV patients are LAMP-2-ANCA-seropositive compared to 52.9% (9/16) in MVV and 76.2% (16/21) in LVV (Table 2). The concentration of sera LAMP-2-ANCA followed a similar trend in that a significantly higher concentration of LAMP-2-ANCA were detected in individuals with SVV (p = 0.0225), MVV (p = 0.0056) and LVV (p = 0.0001) compared to the SAID cohort (Figure 1A). Among participants with PSV, significantly higher concentrations of LAMP-2-ANCA were detected in individuals with MVV (2027.8 ng/mL, range 237.7–6210.9 ng/mL) compared to those with SVV (1197.1 ng/mL, range 134.0–4281.9 ng/mL) (p = 0.0207). Likewise, significantly higher concentrations of sera LAMP-2-ANCA were present in individuals with LVV (3965.7 ng/mL, range 103.8–12,413.7 ng/mL) compared to both SVV (p < 0.0001) and MVV (p = 0.0418). Focusing on seropositive participants only, the mean concentrations of LAMP-2-ANCA detected in our cohort were 1976.3 ng/mL in SVV, 3172.9 ng/mL in MVV and 5040.5 ng/mL in LVV.
Table 1.
Pediatric chronic primary systemic vasculitis (PSV) cohort.
| Small Vessel (n = 53) |
Medium Vessel (n = 16) |
Large Vessel (n = 21) |
|
|---|---|---|---|
| PSV subtype, n (%) | |||
| GPA/limGPA | 43 (81.1) | - | - |
| MPA | 5 (9.4) | - | - |
| EGPA | 2 (3.8) | - | - |
| ANCA + GN | 1 (1.9) | - | - |
| PAN/cPAN | - | 14 (87.5) | - |
| TA | - | - | 19 (90.5) |
| UPV | 2 (3.8) | 2 (12.5) | 2 (9.5) |
| Sex, n (%) | |||
| Female | 35 (66.0) | 10 (62.5) | 15 (71.4) |
| Male | 18 (34.0) | 6 (37.5) | 6 (28.6) |
| No significant difference in representation of males/females between groups (p = 0.8400) based on chi-squared test | |||
| Age (years) of symptom onset—median (range) | * 14.2 (1.9–17.3) | 11.2 (2.7–15.8) | 12.6 (3.7–17.1) |
| 1 ANCA Antigen Positivity, n (%) | |||
| Proteinase 3 (PR3) | 29 (55.8) | 1 (6.7) | - |
| Myeloperoxidase (MPO) | 18 (34.6) | - | - |
| PR3 and MPO | 2 (3.8) | - | - |
| Neither | 3 (5.8) | 14 (93.3) | 18 (100) |
| Disease activity (pVAS) at diagnosis | |||
| Total pVAS, median (range) | 19 (6–50) | 8 (1–18) | 16 (6–26) |
| Subcomponent pVAS, median (range) | |||
| Renal | 10 (0–12) | 0 (0–6) | 4 (0–12) |
| Cardiovascular | 0 (0–4) | 0 | 4 (0–6) |
| Pulmonary | 0 (0–6) | 0 (0–4) | 0 (0–6) |
| Upper respiratory tract | 4 (0–6) | 0 (0–6) | 0 (0–4) |
| 2 Induction Treatment, n (%) | |||
| 3 Immune-suppressing agents | 38 (73.0) | 4 (25.0) | 2 (9.5) |
| 4 Disease-modifying agents | 6 (11.5) | 6 (37.5) | 11 (52.4) |
| 5 Biologic agents | - | 1 (6.3) | 6 (28.6) |
| Corticosteroids | 48 (92.3) | 12 (75.0) | 20 (95.2) |
| 2 Maintenance Treatment, n (%) | |||
| 3 Immune-suppressing agents | 16 (37.2) | 4 (28.6) | 4 (20.0) |
| 4 Disease-modifying agents | 14 (32.5) | 9 (64.3) | 10 (50.0) |
| 5 Biologic agents | - | 2 (14.3) | 8 (40.0) |
| Corticosteroids | 41 (95.3) | 10 (71.4) | 19 (95.0) |
1 Percentage of patients with ANCA calculated based on available data for 52/53 SVV cases, 15/16 MVV cases and 18/21 LVV cases, 2 Percentage of patients on each induction treatment (for 3–6 months following diagnosis) is calculated based on available data within the SVV (52/53), MVV (16/16), and LVV (21/21) groups and likewise for maintenance treatment (initiated after induction treatment is complete) within the SVV (43/53), MVV (14/16), and LVV (20/21) groups. 3 Immune-suppressing agents are most commonly cyclophosphamide and rituximab. 4 Disease-modifying agents are most commonly azathioprine, methotrexate, and mycophenolate mofetil. 5 Biologic agents are most commonly infliximab and tocilizumab. GPA, granulomatosis with polyangiitis; limGPA, limited GPA; MPA, microscopic polyangiitis; EGPA, eosinophilic GPA; GN, glomerulonephritis; PAN, polyarteritis nodosa; cPAN, cutaneous PAN; TA, Takayasu’s arteritis; UPV, unclassifiable primary vasculitis; pVAS, pediatric vasculitis activity score. * Significant difference in median age between SVV and MVV (p = 0.0209), but not SVV and LVV (p = 0.0666) or MVV and LVV (p = 0.5357) based on Mann–Whitney tests.
Table 2.
LAMP-2-ANCA seropositivity in pediatric chronic primary systemic vasculitis (PSV).
| PSV Total (n = 90) |
Small Vessel (n = 53) |
Medium Vessel (n = 16) |
Large Vessel (n = 21) |
|||||
|---|---|---|---|---|---|---|---|---|
| a LAMP-2-ANCA | − | + | − | + | − | + | − | + |
| n | n = 41 | n = 49 | n = 29 | n = 24 | n = 7 | n = 9 | n = 5 | n = 16 |
| % | 45.6% | 54.4% | 54.7% | 45.3% | 41.2% | 52.9% | 23.8% | * 76.2% |
| b Sex, n (%) | ||||||||
| Female | 26 (63.4) | 34 (69.4) | 18 (62.1) | 17 (70.8) | 4 (57.1) | 6 (66.7) | 4 (80.0) | 11 (68.8) |
| Male | 15 (36.6) | 15 (30.6) | 11 (37.9) | 7 (29.2) | 3 (42.9) | 3 (33.3) | 1 (20.0) | 5 (31.2) |
| c Age of onset, years | ||||||||
| Median | 13.8 | 13.2 | 14.5 | 13.1 | 8.1 | 13.7 | 10.5 | 13.2 |
| Range | 1.9–17.3 | 1.9–17.2 | 1.9–17.8 | 1.9–17.2 | 2.7–15.5 | 4.0–15.8 | 3.7–13.3 | 5.3–17.1 |
| d ANCA antigen positivity, n (%) | ||||||||
| PR3 | 19 (22.4) | 11 (12.9) | 18 (34.6) | 11 (21.2) | 1 (5.9) | − | − | − |
| MPO | 9 (10.6) | 9 (10.6) | 9 (17.3) | 9 (17.3) | − | − | − | − |
| PR3 and MPO | 1 (1.2) | 1 (1.2) | 1 (1.9) | 1 (1.9) | − | − | − | − |
| Neither | 12 (14.1) | 23 (27.1) | 1 (1.9) | 2 (3.8) | 6 (35.3) | 8 (47.1) | 5 (27.8) | 13 (72.2) |
a LAMP-2-ANCA-seronegative indicated by (−) and -seropositive indicated by (+). b Percentage calculated from the total seronegative or seropositive participants of the same vessel size; no statistically significant differences (chi-squared test) between seropositive and seronegative individuals in the PSV (0.5494), SVV (0.5024), MVV (0.6963), and LVV (0.6269) groups. c No statistically significant differences between seropositive and seronegative individuals in the PSV (0.7235, Mann–Whitney test), SVV (0.1479, Mann–Whitney test), MVV (0.1146, unpaired t-test), and LVV (0.1085, unpaired t-test) groups. d Percentage calculated based on available data for participants within the PSV (85/90), SVV (52/53), MVV (15/16), and LVV (18/21) groups. * Significant difference in the prevalence of seropositive individuals between SVV and LVV (p = 0.0162) based on chi-squared test. Across all groups, p = 0.0545 for the prevalence of seropositivity; SVV vs. MVV, p = 0.4415; MVV vs. LVV, p = 0.1993.
Figure 1.
Sera LAMP-2-ANCA in chronic primary systemic vasculitis of childhood. (A) LAMP-2-ANCA concentration (y-axis; μg/mL) in sera from children and adolescents (x-axis) with systemic autoinflammatory disease (n = 18, SAID) and chronic primary systemic vasculitis (PSV) affecting small- (SVV, n = 53), medium- (MVV, n = 16) and large- (LVV, n = 21) sized blood vessels. Horizontal dotted line marks the threshold for LAMP-2-ANCA seropositivity in samples with greater than 1 μg/mL LAMP-2-ANCA. (B,C) LAMP-2-ANCA concentration (y-axis; μg/mL) in the pediatric chronic PSV cohort grouped (x-axis) based on (B) positivity for MPO-ANCA (n = 18), PR3-ANCA (n = 30), or neither MPO- or PR3-ANCA (negative, n = 35), and (C) biological sex. (D) Concentration of LAMP-2-ANCA (y-axis; μg/mL) in pediatric chronic PSV participants plotted against age of symptom onset (x-axis; years). (E) Age of symptom onset (y-axis; years) in LAMP-2-ANCA-seropositive and -seronegative patients with MVV and LVV (x-axis). Bars show means in (A–C), and medians in (E). LAMP-2-ANCA-seropositive (+) and -seronegative (−) samples indicated with closed and open circles, respectively. * Denotes statistical significance with p < 0.05, *** p < 0.001, **** p < 0.0001.
Consistent with a greater number of LAMP-2-ANCA-seropositive individuals with MVV and LVV compared to SVV, LAMP-2-ANCA seropositivity was observed in the majority of individuals (23/35; 65.7%) seronegative for MPO-ANCA and PR3-ANCA, and by comparison, in fewer individuals seropositive for PR3-ANCA (11/30; 36.7%) (Table 2) or MPO-ANCA (9/18; 50%). Similarly, the concentration of LAMP-2-ANCA in individuals seronegative for MPO- and PR3-ANCA was significantly higher compared to LAMP-2-ANCA titers in PR3-ANCA- (p = 0.0008, Figure 1B) and MPO-ANCA-positive patients (p = 0.0553, Figure 1B). These data contrast with several previous reports [16,17,18] in which LAMP-2-ANCA titers, although not correlated with titers of MPO- or PR3-ANCA, were commonly detected in individuals also seropositive for either MPO- or PR3-ANCA.
Together, these results demonstrate that a greater majority of cases of pediatric-onset medium- and large-vessel vasculitis compared to small-vessel vasculitis in our cohort were not only seropositive for autoantibodies against LAMP-2 (exceeding 1 μg/mL) but had significantly elevated concentrations of circulating LAMP-2-ANCA; this was especially evident in cases of LVV. The observed differences in both LAMP-2-ANCA positivity (Table 2) and concentration in small-, medium- and large-vessel vasculitis was not associated with biological sex (Figure 1C, p = 0.8400) or the age of symptom onset (Figure 1D,E). Paradoxically, MVV and LVV are considered “ANCA-negative” subtypes of chronic PSV as they are rarely associated with autoantibodies against PR3 or MPO that are common to SVV.
2.2. LAMP-2-ANCA Seropositivity Is Associated with Lower Overall Disease Activity at Diagnosis
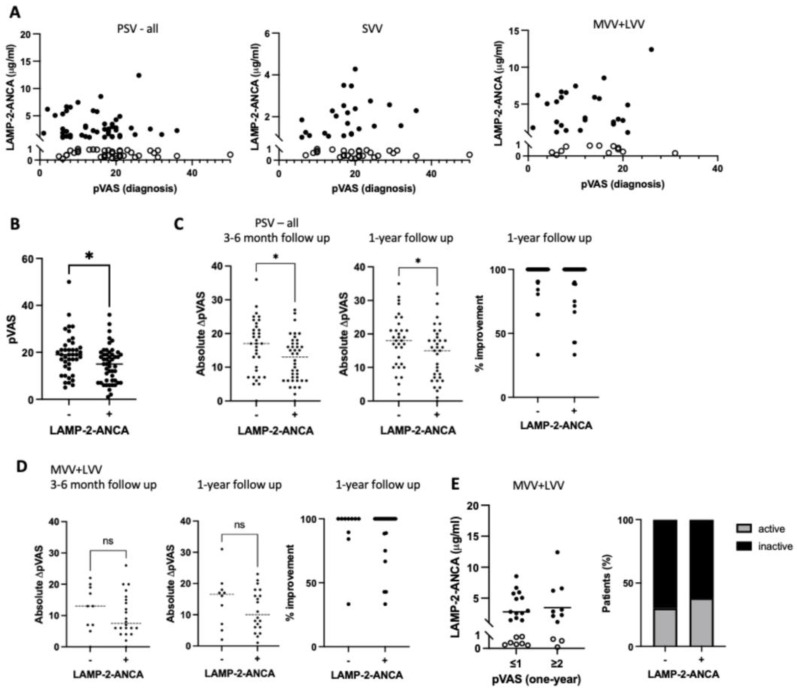
To gain insight into the potential for clinical utility of LAMP-2-ANCA measures in MVV and LVV, we asked if seropositivity or concentration of LAMP-2-ANCA at diagnosis could inform the present state of vasculitis-specific (inflammatory) activity or the ability to attain inactive disease within the first year following diagnosis. Results indicate that LAMP-2-ANCA concentration was not associated with generalized inflammation, as indicated by the concentration of C-reactive protein (CRP) or with disease-specific (inflammatory) activity at diagnosis measured by pVAS (Figure 2A). When considering seropositivity independently of LAMP-2-ANCA concentration, however, we observed significantly lower overall disease activity at diagnosis in LAMP-2-ANCA-seropositive (median pVAS = 15) versus -seronegative (median pVAS = 19) individuals (p = 0.0176), irrespective of the size of affected blood vessels (Figure 2B and Table 3).
Figure 2.
Measures of sera LAMP-2-ANCA positivity and concentration relative to disease activity at diagnosis and follow-up. (A) Concentration of LAMP-2-ANCA (y-axis; μg/mL) plotted against the pediatric vasculitis activity score (pVAS) (x-axis) at the time of diagnosis for all participants with chronic primary systemic vasculitis (PSV—all) and stratified according to the involvement of small (SVV) or medium- and large-sized (MVV + LVV) vessels. (B) Pediatric vasculitis activity score (pVAS) (y-axis, bar shows median) at diagnosis in LAMP-2-ANCA-seronegative (−) and -seropositive (+) participants (x-axis). (C) Reduction in disease activity between all LAMP-2-ANCA-seronegative (−) and -seropositive (+) PSV participants (x-axis) shown by absolute change in pediatric vasculitis activity score (pVAS) (y-axis) from diagnosis to (left panel) the completion of 3–6 months of induction therapy (seropositive, n = 40; seronegative, n = 34) and (middle panel) one-year follow-up (seropositive, n = 32; seronegative, n = 36). Improvement in disease activity expressed as percentage of maximal improvement from diagnosis to one-year follow-up. (D) Reduction in disease activity between LAMP-2-ANCA-seronegative (−) and seropositive (+) MVV/LVV participants (x-axis) shown by absolute change in pediatric vasculitis activity score (pVAS) (y-axis) from diagnosis to (left panel) the completion of 3–6 months of induction therapy (seropositive, n = 22; seronegative, n = 10) and (middle panel) one-year follow-up (seropositive, n = 20; seronegative, n = 10). Improvement in disease activity expressed as percentage of maximal improvement from diagnosis to one-year follow-up. (E) Concentration of LAMP-2-ANCA (y-axis; μg/mL, bar shows mean) at diagnosis in participants with medium-/large-sized vessel vasculitis (MVV + LVV) with inactive (pVAS at one-year ≤ 1) or active (pVAS at one-year ≥ 2) disease one-year later (left panel). Similarly (right panel), percentage (x-axis) of seronegative (−) and seropositive (+) MVV/LVV participants with inactive (black) or active (gray) disease one-year post-diagnosis. Bars show medians in (B,C) and means in (D). LAMP-2-ANCA-seropositive (+) and -seronegative (−) samples indicated with closed and open circles, respectively. ns indicates non-significant. * Denotes statistical significance with p < 0.05.
Table 3.
Disease activity at diagnosis and 1-year follow up stratified by LAMP-2-ANCA seropositivity.
| PSV Total | Small Vessel | Medium Vessel | Large Vessel | |||||
|---|---|---|---|---|---|---|---|---|
| a LAMP-2-ANCA | − | + | − | + | − | + | − | + |
| Disease activity (total pVAS), median (range) | ||||||||
| b at diagnosis | 19 (5–50) | * 15 (1–36) | 20 (7–50) | 18 (6–36) | 8 (5–31) | 7 (1–18) | 19 (6–20) | 15 (6–26) |
| c at 1-year | 0 (0–4) | 0 (0–12) | 0 (0–6) | 0 (0–12) | 0 (0) | 1 (0–4) | 2 (3–4) | 0 (0–12) |
| d Inactive disease (total pVAS ≤ 1), n (%) | ||||||||
| at 1-year | 26 (37.1) | 25 (35.7) | 19 (48.7) | 12 (30.8) | 5 (38.5) | 4 (30.8) | 2 (11.1) | 9 (50.0) |
a LAMP-2-ANCA-seronegative indicated by (−) and seropositive indicated by (+). b At diagnosis, pVAS available for n = 90 PSV patients. * Significant difference in disease activity (p = 0.0176) across all groups based on unpaired t-test. c At 1 year post-diagnosis, pVAS available for n = 70 PSV patients: n = 39 SVV (15 LAMP2 ANCA+), n = 13 MVV (8 LAMP2 ANCA+), n = 18 LVV (13 LAMP2 ANCA+). d Inactive disease defined as total pVAS ≤ 1 at one-year post-diagnosis. Percentage is relative to the total number of seronegative or seropositive participants of the same vessel size.
Using a subset of participants with follow-up data after induction therapy (3–6 months post-diagnosis, n = 74) and one-year post-diagnosis (n = 70), we comparatively analyzed LAMP-2-ANCA seropositivity and concentration as being informative for disease trajectory based on two measures: overall improvement in disease activity (i.e., reduction in pVAS) and achievement of inactive disease. Chronic PSV patients showed marked improvement over the first 12 months of disease, with even the least improved patients achieving a minimum 33.3% decline in disease activity (pVAS). Individuals seronegative for LAMP-2-ANCA showed a greater improvement compared to LAMP-2-ANCA-seropositive individuals after induction therapy (Figure 2C left panel, p = 0.023) and one-year post-diagnosis (Figure 2C middle panel, p = 0.038). However, when the reduction in disease activity was calculated relative to pVAS at diagnosis (lower in LAMP-2-ANCA-seropositive patients, Figure 2B), no significant differences in improvement related to LAMP-2-ANCA status were observed (right panels in Figure 2C,D). Focusing on the achievement of inactive disease (pVAS ≤ 1), our results showed a comparable number of seropositive (13/21, 61.9%) and seronegative participants (7/10, 70.0%) with inactive disease or sustained disease activity one-year following diagnosis (Figure 2E right panel and Table 3) and no significant difference in LAMP-2-ANCA concentration at diagnosis between individuals (p = 0.5472, MVV + LVV, Figure 2E, left panel) that went on to achieve inactive disease one -year post-diagnosis.
Although our data show a relationship between LAMP-2-ANCA positivity and lower disease activity at diagnosis, neither diagnostic LAMP-2-ANCA seropositivity nor concentration was informative for overall disease activity following therapy induction or at one-year after diagnosis.
2.3. LAMP-2-ANCA Seropositivity and Concentration Are Not Significantly Associated with the Extent or Type of Organ Involvement
Given the observed relationship between LAMP-2-ANCA seropositivity and overall lower disease activity (pVAS) at diagnosis, we asked if the involvement of multiple, single, or particular organ systems were driving this association. In our cohort at the time of diagnosis, we observed 71.1% of patients with kidney involvement (renal pVAS ≥ 2), 37.7% of patients with upper respiratory tract involvement (ear, nose, throat (ENT) pVAS ≥ 2), 27.8% of patients with pulmonary system involvement (chest pVAS ≥ 2), and 23.4% of patients with cardiovascular involvement (cardio pVAS ≥ 2). A multivariate logistic regression model, adjusted for age at diagnosis and biological sex at birth, was used to evaluate the association between organ involvement and vessel size. As expected, we observed a significant association between vessel size and organ involvement. Compared to individuals with LVV, MVV less frequently affected the cardiovascular (OR 0.024, 95% CI 0.001–0.163, p = 0.001) and renal (OR 0.169, 95% CI 0.035–0.707, p = 0.019) systems; SVV had less cardiovascular (OR 0.023, 95% CI 0.004–0.097, p < 0.0001) system involvement, but higher involvement of the upper respiratory tract (URT) (OR 12.212, 95% CI 2.936–84.855, p = 0.003) and pulmonary system (OR 6.011, 95% CI 1.456–41.134, p = 0.027) involvement. The results of a regression model, adjusted for vessel size, assessing the relationship between the presence or absence of LAMP-2-ANCA and the involvement of more than one of these organ systems (p = 0.173) or a single organ (p = 0.281) were inconclusive. Furthermore, the results did not provide clear evidence of an association between LAMP-2-ANCA seropositivity/seronegativity and type of organ system involvement (Table 4).
Table 4.
Association between organ system involvement and LAMP-2-ANCA seropositivity.
| Organ System | LAMP-2-ANCA |
b Adjusted OR (95% CI) |
Standard Error | p-Value | |
|---|---|---|---|---|---|
| Seronegative (n = 36) a | Seropositive (n = 41) a | ||||
| Cardiovascular | 7 (19.4%) | 14 (34.1%) | 0.82 (0.15, 3.73) | 0.79 | 0.800 |
| Renal | 29 (81.0%) | 35 (85.4%) | 1.70 (0.46, 6.91) | 0.68 | 0.429 |
| Pulmonary | 16 (44.4%) | 9 (22.0%) | 0.49 (0.16, 1.46) | 0.56 | 0.198 |
| URT | 19 (52.8%) | 13 (31.7%) | 0.58 (0.19, 1.79) | 0.57 | 0.339 |
a Number of PSV patients with involvement of URT, cardiovascular, renal, and pulmonary systems. b The reference category is LAMP-2-ANCA seronegativity. OR; odds ratio, CI; confidence interval, URT; upper respiratory tract.
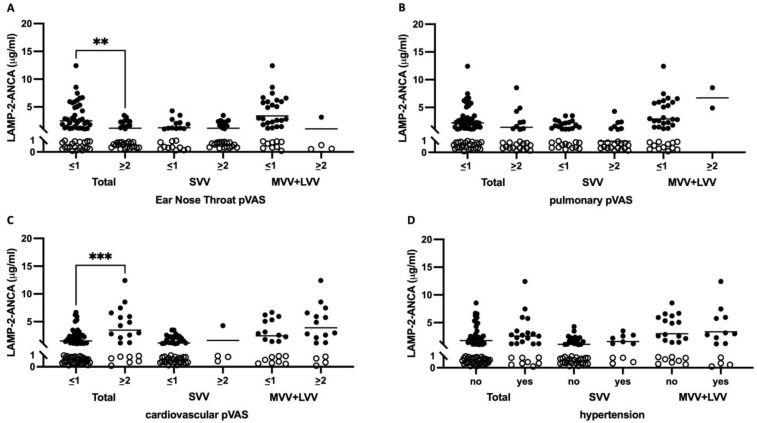
We next observed significantly lower LAMP-2-ANCA concentrations in individuals with (mean LAMP-2-ANCA 1154.4 ng/mL) versus without (mean LAMP-2-ANCA 2498.6 ng/mL; p = 0.0043) URT involvement (Figure 3A), but not the pulmonary system as a whole (Figure 3B). In contrast, and despite no association between ANCA titers and hypertension (Figure 3D) [19], we observed significantly elevated LAMP-2-ANCA concentrations in individuals with cardiovascular involvement (mean LAMP-2-ANCA 3475.6 ng/mL) versus those individuals without this manifestation (mean LAMP-2-ANCA 1538.9 ng/mL; p = 0.0003) (Figure 3C). When using a multivariate linear regression model adjusted for vessel size, biological sex, and age at diagnosis, however, the results neither supported an association between the level of organ involvement (multi or single) and LAMP-2-ANCA concentration nor significant differences in LAMP-2-ANCA concentration between individuals with and without cardiovascular (p = 0.392), renal (p = 0.541), pulmonary (p = 0.511) and URT (p = 0.901) involvement.
Figure 3.
Organ system involvement and LAMP-2-ANCA titers in PSV. (A–C) Sera LAMP-2-ANCA concentration (y-axis; μg/mL) versus disease activity (component pVAS ≤ 1 is inactive and ≥2 is active) in (A) upper respiratory tract (ear–nose–throat component of pVAS), (B) pulmonary system, (C) cardiovascular system, or relative to (D) the presence of hypertension (yes/no) in children and adolescents with chronic primary systemic vasculitis (x-axis, total, n = 25) affecting small (SVV, n = 11) and medium/large (MVV + LVV, n = 14) vessels. LAMP-2-ANCA-seropositive and -seronegative samples indicated with closed and open circles, respectively. Bars show means. ** Denotes statistical significance with p < 0.01, *** p < 0.001.
3. Discussion
Chronic primary systemic vasculitis (PSV) is a heterogeneous group of diseases characterized by inflammation and damage to blood vessels that vary in size and location. Pediatric PSV affecting small blood vessels is commonly associated with autoantibodies (ANCA) against proteinase 3 (PR3) or myeloperoxidase (MPO). Herein, we demonstrate an increased prevalence and concentration of ANCA that are specific for LAMP-2 in children with PR3/MPO-ANCA-negative vasculitis affecting medium (MVV) and large (LVV) blood vessels. Our findings may prompt reconsideration of the presence and potential of monitoring autoantibody seropositivity against LAMP-2, or other autoantigens, in subtypes of MVV and LVV previously regarded as seronegative for ANCA.
PR3 and MPO are the predominant antigens of interest in small-vessel vasculitis and autoantibodies (ANCA) to these aid with classification of AAV subtypes granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). There is conflicting data on the utility of ANCA titers to inform disease activity, and an as yet unknown role for MPO- or PR3-ANCA in organ-specific disease processes. Only recently, more than 40 years after their presence in vasculitis was discovered [20], a role for ANCA specificity towards PR3 and MPO in predicting disease outcomes in ANCA-associated SVV is being recognized. The presence of PR3-ANCA is associated with a higher risk of severe inflammatory lung disease, multi-organ involvement, and disease relapse; whereas MPO-ANCA are associated with more severe, renal-limited disease at presentation [21,22,23]. Not all patients, however, follow such patterns of disease, and there is evidence that other factors, such as type of organ involvement (e.g., renal versus non-renal) and genetic associations also impact risk [24,25]. ANCA specificity for LAMP-2 or other autoantigens, particularly those expressed in affected tissues, may have an additional prognostic role. Although the prevalence of LAMP-2-ANCA has been debated in PR3/MPO-ANCA-associated vasculitis (AAV) and AAV-related kidney disease, overlapping seropositivity for LAMP-2-ANCA with MPO- or PR3-ANCA in SVV is consistently observed [10,13,16,18,26].
The value of LAMP-2-ANCA seropositivity as a biomarker may be greatest among AAV patients who have MPO/PR3-ANCA-negative SVV, but more so in individuals with middle vessel vasculitis (MVV), namely, polyarteritis nodosa (PAN) and large vessel vasculitis (LVV), namely Takayasu’s arteritis (TAK). Due to the rarity of pediatric PAN and TAK combined with the absence of PR3- and MPO-ANCA, predictive biomarkers and specific disease activity markers are lacking. Determining levels of disease activity in PAN and TAK patients has proved challenging for several reasons: vascular imaging of medium- or large-sized vessels is frequently invasive and may not reliably differentiate between active inflammation versus damage; biopsy of affected vessels is often too risky and not feasible on a repeated basis; and traditional markers of inflammation (C-reactive protein and erythrocyte sedimentation rate) are non-specific and may not be elevated in organ-limited disease.
Our findings of elevated LAMP-2-ANCA seropositivity in pediatric PAN and TAK are consistent with reports in adults with PAN and TAK of elevated sera LAMP-2 protein [11,14], as well as elevated sera LAMP-2-ANCA [15,19]. In AAV, seropositivity for MPO or PR3-ANCA, rather than serial measures of titer, appear to have greater clinical value in predicting disease course [23,24,25,27]. In adult-onset PAN, Li et al. demonstrated a positive correlation between sera LAMP-2 protein concentration, C-reactive protein concentration, and overall disease activity in adult PAN [11]. The observed absence of a correlation between LAMP-2-ANCA titers and disease activity in this study was consistent with several previous reports on adult SVV [16,17,18] and our own observations in pediatric SVV [13]. Further, our work revealed that neither seropositivity nor titer at diagnosis was informative to disease course, with similar improvements in disease activity observed in LAMP-2-ANCA-seronegative and -seropositive participants.
In 2013, Kawakami et al. observed positive perinuclear (p) ANCA staining in adult cases of cutaneous polyarteritis nodosa and proposed that there are as yet undiscovered autoantigens [15]. More recently, Mukherjee et al. provided strong evidence for the presence of ANCA towards an unidentified antigenic target in sputa from adults with MPO/PR3-ANCA-negative eosinophilic granulomatosis with polyangiitis (EGPA). Importantly, their study demonstrated that ANCA reactivity was not observed in sera, emphasizing the importance of investigations in the affected tissue [28]. Additional studies support the existence of ANCA/autoantigens in MPO/PR3-ANCA-negative vasculitides including identification of elastase-ANCA in adult ANCA-negative glomerulonephritis [29] and the presence of alpha-enolase, a potential cytosolic autoantigen, in 82% of adults with predominantly ANCA-negative EGPA [30].
Our study has certain limitations. Despite having the largest reported pediatric chronic PSV study cohort, it may not be reflective of all populations. Further, the sample size may be underpowered for some analyses. Notably, it may not have been possible to observe any associations between organ system involvement and LAMP-2-ANCA, given that our multivariate logistic regression model did not reveal associations between MPO/PR3-ANCA status and particular organ systems as would be expected, given that a majority of cases have renal and pulmonary/URT involvement at diagnosis [31,32,33].
Distinguishing between the presence (or absence) and the antigenic target of autoimmune processes has important implications for therapeutic decision-making. Our data suggest that LAMP-2-ANCA may be important in childhood-onset PSV, where ANCA against classical targets, PR3 or MPO, are commonly absent. Although traditionally studied in the context of renal involvement, our data highlight a high prevalence and concentration of LAMP-2-ANCA in subtypes of PSV with extrarenal manifestations. Although substantive gaps in the understanding of LAMP-2-ANCA utility and pathogenicity remain, this report and others argue for continued examination of LAMP-2-ANCA, particularly in the context of medium- and large-vessel vasculitides that are commonly considered seronegative for autoantibodies.
4. Materials and Methods
4.1. Study Cohorts and Biosamples
The children and youth with chronic PSV that are described in this study (n = 90; Table 1) were enrolled in the Pediatric Vasculitis Initiative (PedVas), for which eligibility criteria have been described previously [32,34]. The Children’s and Women’s Research Ethics Board of the University of British Columbia [H12-00894] and the respective ethics review boards at participating PedVas centers gave their approval to the research protocol. Participants contributed blood in serum separation tubes (BD Biosciences, Franklin Lakes, NJ, USA) at the time of diagnosis. These sample were processed to sera according to a standard protocol and stored at −80 °C.
Individuals also included in the study were children and youth (n = 18; 38.9% female, median age of symptom onset 8.3 years, range 1.2–16.1 years) receiving care for a systemic autoinflammatory disease (SAID) characterized by recurrent episodes of uncontrolled inflammation in the absence of infection or autoimmunity (i.e., no detectable autoantibodies or autoreactive immune cells) [35]. Cohort characteristics are summarized in Supplementary Table S1. Approval from the Children’s and Women’s Research Ethics Board of the University of British Columbia was obtained to record diagnostic and demographic data in a research database (H14-00272) [36] and to collect and store (as described for PSV patients) sera (H15-00351). Sera were used to establish a range of LAMP-2-ANCA concentrations associated with systemic inflammation, as indicated by concentrations of human C-reactive protein (CRP ELISA kit) according to the manufacturer’s instructions (ThermoFisher, Waltham, MA, USA).
4.2. Clinical Data for Chronic PSV Participants
Clinical data were entered by participating centers on a web-based clinical data registry [31,32] and used to formally classify chronic PSV patients (n = 90) into disease subtypes under the broader designation of small- (SVV, n = 53), medium- (MVV, n = 16), and large- (LVV, n = 21) vessel vasculitis. A subset of relevant registry data is summarized in Table 1. The majority of cases of SVV met EULAR/PRINTO/PRES criteria for granulomatosis with polyangiitis (GPA, 43/53, 81.1%) [37,38]. MPA patients fulfilled the pediatric modified algorithm of the EMA [39] and EGPA patients met the ACR or Lanham criteria [37,40]. MVV subtypes included: polyarteritis nodosa (PAN, n = 11; EULAR/PRINTO/PRES criteria [37]), cutaneous PAN (cPAN, n = 3) and two cases of unclassified medium vessel vasculitis (uMVV, n = 2). Patients with LVV were predominantly Takayasu’s arteritis (TA, n = 19/21, 90.5%; EULAR/PRINTO/PRES criteria [37]) with two cases of unclassified large-vessel vasculitis (uLVV, n = 2). ANCA positivity and specificity for proteinase 3 (PR3) and myeloperoxidase (MPO) were entered in the registry by participating centers. As expected, the majority of cases of SVV were positive for MPO- or PR3-ANCA (49/52 cases with available data; 94.2%)
4.3. Quantification of Active Disease in Chronic PSV Participants
Disease activity at the time of sample/data collection (diagnosis) was calculated from the registry data using the pediatric vasculitis activity score (pVAS; range 0–63, where zero indicates inactive disease), which is a cumulative weighted score of disease activity across nine organ systems [41]. For individuals with follow-up data, improvement at one-year post-diagnosis was calculated as: % improvement = 100% × ((pVAS at diagnosis) − (pVAS at 1-year))/(pVAS at diagnosis). All participants had active disease at the time of diagnosis (and sample/data collection), with a median overall disease activity (median total pVAS) score equal to 17 (range 1–50). Using subcomponent scores of pVAS, analyses were focused on four critical organs/organ systems that drive treatment decisions early in the disease course and are frequently involved in SVV, MVV, and LVV, namely, kidneys (max. pVAS = 12), upper respiratory tract (max. pVAS = 6), pulmonary system (max. pVAS = 6), and cardiovascular system (max. pVAS = 6), where a subcomponent pVAS ≥ 2 indicated organ involvement [41].
4.4. Meso Scale Discovery (MSD) Electrochemiluminescence Assay to Detect Serum Anti-LAMP-2-Antibodies
Recombinant human (rh) LAMP-2 protein (R&D Systems, Minneapolis, MN, USA) was diluted in phosphate-buffered saline (PBS) and added (30 μL at 2.5 μg/mL) to 96-well electrochemiluminescence assay plates (Meso Scale Discovery, Rockville, MD, USA). Plates were sealed and incubated at 4 °C overnight, blocked (150 μL blocker A solution; RT, 1 h, 700 rpm) and washed (3 × 150 μL/well PBS + 0.05% Tween 20 (PBST)). A standard curve was generated with anti-human-LAMP-2 (H4B4) monoclonal antibody (BioLegend, San Diego, CA, USA, cat #354302) serially diluted from 1–4000 ng/mL in blocker A solution. Standards and sera (50 μL/well of 1:10 dilution) were added (1 h, RT, shaking), then wells were washed (3 × 150 μL/well PBST). A biotin-conjugated anti-IgG Fc (multi-species) antibody (ThermoFisher Scientific, Waltham, MA, USA, cat #7102852100) was added (50 μL/well at 1 μg/mL in blocker A; RT, 1 h, shaking) followed by strep-SULFO-TAG (50 μL/well at 0.5 μg/mL in blocker A; RT, 1 h, shaking) and 150 μL/well of MSD Gold Read buffer B with washing between steps (3 × 150 μL/well TBST). Plates were read using an MSD QuickPlex SQ 120 MM electrochemiluminescence instrument and a standard curve generated using a 4-parameter logistical curve fit algorithm within MSD Discovery Workbench Software (version 4.0.12). The assay was validated with sera, courtesy of Dr. Renate Kain at the University of Vienna, from young-onset ANCA-associated vasculitis patients known to be seropositive for anti-LAMP-2 antibodies [10,13,42] and a subset of participants with an SAID (n = 5) or a chronic PSV (n = 44; predominantly SVV) that were previously assessed for sera LAMP-2-ANCA concentrations using an indirect, in-house colorimetric ELISA [13]. Compared to the traditional colorimetric ELISA, the MSD platform displayed 10–60 times the linear range and improvement in the signal-to-noise ratio with a broad dynamic range. Using this assay, sera LAMP-2-ANCA concentrations in the majority (15/18; 83.3%) of individuals with an SAID were detected at less than or equal to 1000 ng/mL, with an average concentration of 661.0 ng/mL (range 44.4–1311.4 ng/mL) (Figure 1A) and informed the threshold of seropositivity for LAMP-2-ANCA at >1000 ng/mL in this study.
4.5. Statistical Analysis
GraphPad Prism v9.0 statistical software (GraphPad Software, La Jolla, CA, USA) was used to conduct the statistical analyses. Group differences were examined by one-way ANOVA and/or two-tailed t-tests. Pearson’s correlation coefficient was used to evaluate correlations. Where indicated, group differences were analyzed by Kruskal–Wallis, Mann–Whitney, or chi-squared statistical tests. R (R Foundation for Statistical Computing, version 4.2.2, Vienna, Austria) was used for multivariate analyses. A 95% confidence interval was used for all analyses, and a p-value equal to or less than 0.05 was regarded as significant.
Acknowledgments
The authors would like to thank Stephanie Hughes (UBC) for excellent technical assistance and advice on assay design, implementation, and analysis. The authors thank Renate Kain (University of Vienna) for providing anti-LAMP-2 antibody-positive sera for assay validation. The authors would like to acknowledge all participating patients and their families, without whom this study would not be possible. The authors thank the PedVas site investigators and clinical coordinators for their dedicated work: Else Bosman, BC Children’s Hospital, University of British Columbia, Vancouver, BC, Canada; Rae Yeung and Emily Hickey, Hospital for Sick Children, Toronto ON, Canada; Linda Wagner-Weiner, University of Chicago Comer Children’s Hospital, Chicago, IL, USA; Suzanne Li and Justine Griswold, Joseph M. Sanzari Children’s Hospital, Hackensack University Medical Center, Hackensack, NJ, USA; Alan Rosenberg and Joan Dietz, Royal University Hospital and University of Saskatchewan, SK, Canada; Melissa Elder and Heather Bell-Brunson, University of Florida, Gainesville, FL, USA; Susanne Benseler and Michaela Grice, Alberta Children’s Hospital, Calgary, AB, Canada; Susan Shenoi and Nidhi Naik, Seattle Children’s Hospital, Seattle, WA, USA; Stacey Tarvin and Jessica Lee, Riley Children’s Hospital, Indianapolis, IN, USA; Neil Martin and Yvonne McCafferty, Royal Hospital for Children, Glasgow, UK; Kathryn Cook and Jessica Kracker, Akron Children’s Hospital, Akron, OH, USA; Eslam Al-Abadi and Ruth Howman, IWK Health Centre and Dalhousie University, Halifax, NS, Canada;, Birmingham Children’s Hospital NHS Foundation Trust, Birmingham, UK; Karen James and Suzy Richins, University of Utah’s Primary Children’s Medical Center, Salt Lake City, UT, USA; Adam Huber and Victoria Dempsey, IWK Health Centre, Halifax, NS, Canada; Flora McErlane and Caroline Miller, Great North Children’s Hospital, Newcastle, UK; Marek Bohm and Heather Rostron, Leed’s Children’s Hospital, Leeds, UK; Dirk Foell and Antje Hellige, University Children’s Hospital Munster, Munster, Germany; Eileen Baildam and Michelle Andrews, Alder Hey Children’s NHS Foundation Trust Hospital, Liverpool, UK; Paul Dancey and Rana Aslanova, Janeway Children’s Health, St. John’s, NL, Canada; Phil Riley and Ann McGovern, CMFT Manchester, Manchester, UK; Roberta Berard and Michelle Diebold, London Health Sciences Centre, London, ON, Canada; Sirirat Charuvanij, Siriraj Hospital, Bangkok, Thailand; Vidya Sivaraman and Joanne Drew, Nationwide Children’s Hospital, Columbus, OH, USA; Kathryn Bailey and Danielle Miller, Oxford University Hospitals, Oxford, UK.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25073771/s1.
Author Contributions
All authors (T.H.A., K.K.T., S.K.M., K.M.G., G.A.A., B.B.-P., D.A.C., K.A.M. and K.L.B.) contributed to the conceptualization of the project, writing, and review of the manuscript. T.H.A., K.K.T., S.K.M. and K.M.G. were responsible for the acquisition and analysis of data. T.H.A., K.K.T. and S.K.M. prepared figures and tables. G.A.A. was responsible for methodology implementation. D.A.C., K.A.M., B.B.-P. and K.L.B. were responsible for funding acquisition and project oversight. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study complies with the Declaration of Helsinki and received approval from the Children’s and Women’s Research Ethics Board of the University of British Columbia (H12-00894; renewed annually since 2012 and most recently on 23 February 2024) and the respective ethics review boards at participating PedVas centers. Diagnostic and demographic data were recorded in a research database with approval from the Children’s and Women’s Research Ethics Board of the University of British Columbia (H14-00272) as described previously (18) with biosamples collected under a linked (ethics board-approved) research study (H15-00351).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. Gabriel Alejandro Alfaro was an employee of Meso Scale Diagnostics, LLC, Rockville, MD, USA.
Funding Statement
This project has been supported by Canadian Institutes of Health Research grants (TR2-119188 to DC and PJT-180302 to KLB). THA is supported by funds from a Global Affairs Canada Go-Global Study in Canada Scholarship (THA). KT is supported by a Centre for Blood Research graduate award and a Women+ and Children’s Health Sciences Diversity Award. SM is supported by a Canadian Institutes of Health Research Graduate Student Scholarship (CGS-M) and a BC Children’s Hospital Graduate Student Award. KMG is supported by a University of British Columbia Four-Year Fellowship for PhD students. DC is supported by the Arthritis Society Canada through the Ross Petty Arthritis Society Chair. KLB is supported by a BC Children’s Hospital Salary Award and a Michael Smith Foundation for Health Research Scholar Award.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Okazaki T., Shinagawa S., Mikage H. Vasculitis syndrome-diagnosis and therapy. J. Gen. Fam. Med. 2017;18:72–78. doi: 10.1002/jgf2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss P.F. Pediatric vasculitis. Pediatr. Clin. N. Am. 2012;59:407–423. doi: 10.1016/j.pcl.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner-Medwin J.M., Dolezalova P., Cummins C., Southwood T.R. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197–1202. doi: 10.1016/S0140-6736(02)11279-7. [DOI] [PubMed] [Google Scholar]
- 4.Lionaki S., Blyth E.R., Hogan S.L., Hu Y., Senior B.A., Jennette C.E., Nachman P.H., Jennette J.C., Falk R.J. Classification of antineutrophil cytoplasmic autoantibody vasculitides: The role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–3462. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva de Souza A.W. Autoantibodies in systemic vasculitis. Front. Immunol. 2015;6:184. doi: 10.3389/fimmu.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitching A.R., Anders H.J., Basu N., Brouwer E., Gordon J., Jayne D.R., Kullman J., Lyons P.A., Merkel P.A., Savage C.O.S., et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers. 2020;6:71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 7.Quartuccio L., Treppo E., Urso L., Del Frate G., Mescia F., Alberici F., Vaglio A., Emmi G. Unmet needs in ANCA-associated vasculitis: Physicians’ and patients’ perspectives. Front. Immunol. 2023;14:1112899. doi: 10.3389/fimmu.2023.1112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter R.W., Welsh N., Farrah T.E., Gallacher P.J., Dhaun N. ANCA associated vasculitis. BMJ. 2020;369:m1070. doi: 10.1136/bmj.m1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskelinen E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006;27:495–502. doi: 10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Kain R., Exner M., Brandes R., Ziebermayr R., Cunningham D., Alderson C.A., Davidovits A., Raab I., Jahn R., Ashour O., et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat. Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N., Zhu B., Zhu Q., Heizati M., Wu T., Wang G., Yao X., Luo Q., Liu S., Liu S. Serum lysosomal-associated membrane protein-2 levels are increased in small and medium-vessel vasculitis, especially in polyarteritis nodosa. Clin. Exp. Rheumatol. 2019;37((Suppl. S117)):79–85. [PubMed] [Google Scholar]
- 12.Peschel A., Basu N., Benharkou A., Brandes R., Brown M., Rees A.J., Kain R. Autoantibodies to hLAMP-2 in ANCA-negative pauci-immune focal necrotizing GN. J. Am. Soc. Nephrol. 2014;25:455–463. doi: 10.1681/ASN.2013030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson K.M., Kain R., Luqmani R.A., Ross C.J., Cabral D.A., Brown K.L. Autoantibodies Against Lysosome Associated Membrane Protein-2 (LAMP-2) in Pediatric Chronic Primary Systemic Vasculitis. Front. Immunol. 2021;11:624758. doi: 10.3389/fimmu.2020.624758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z., Hu C., Sun F., Li J., Yang Y., Tian X., Zeng X. Study on the association of serum pentraxin-3 and lysosomal-associated membrane protein-2 levels with disease activity in Chinese Takayasu’s arteritis patients. Clin. Exp. Rheumatol. 2019;37((Suppl. S117)):109–115. [PubMed] [Google Scholar]
- 15.Kawakami T., Ishizu A., Arimura Y., Soma Y. Serum anti-lysosomal-associated membrane protein-2 antibody levels in cutaneous polyarteritis nodosa. Acta Derm. Venereol. 2013;93:70–73. doi: 10.2340/00015555-1418. [DOI] [PubMed] [Google Scholar]
- 16.Moiseev S., Zykova A., Bulanov N., Gitel E., Novikov P., Bulanova M., Kronbichler A., Jayne D. Is There a Role for LAMP-2 Autoantibodies in Patients with Antineutrophil Cytoplasmic Antibody-associated Vasculitis? J. Rheumatol. 2020;47:636–638. doi: 10.3899/jrheum.191082. [DOI] [PubMed] [Google Scholar]
- 17.Kain R., Tadema H., McKinney E.F., Benharkou A., Brandes R., Peschel A., Hubert V., Feenstra T., Sengölge G., Stegeman C., et al. High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis. J. Am. Soc. Nephrol. 2012;23:556–566. doi: 10.1681/ASN.2011090920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth A.J., Brown M.C., Smith R.N., Badhwar A.K., Parente O., Chung H., Bunch D.O., McGregor J.G., Hogan S.L., Hu Y., et al. Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J. Am. Soc. Nephrol. 2012;23:545–555. doi: 10.1681/ASN.2011030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu B., Cai X., Zhu Q., Wu T., Liu S., Liu S., Hong J., Li N. The Association of Serum Anti-Lysosomal-Associated Membrane Protein-2 Antibody with Vasculitis Combined with Hypertension. Int. J. Hypertens. 2022;2022:9656560. doi: 10.1155/2022/9656560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball G.V. The history of ANCA-associated vasculitis. Rheum. Dis. Clin. N. Am. 2010;36:439–446. doi: 10.1016/j.rdc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Hilhorst M., van Paassen P., Tervaert J.W. Proteinase 3-ANCA Vasculitis versus Myeloperoxidase-ANCA Vasculitis. J. Am. Soc. Nephrol. 2015;26:2314–2327. doi: 10.1681/ASN.2014090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen Tervaert J.W. Should proteinase-3 and myeloperoxidase anti-neutrophil cytoplasmic antibody vasculitis be treated differently: Part 2. Nephrol. Dial. Transpl. 2019;34:384–387. doi: 10.1093/ndt/gfy406. [DOI] [PubMed] [Google Scholar]
- 23.Fussner L.A., Hummel A.M., Schroeder D.R., Silva F., Cartin-Ceba R., Snyder M.R., Hoffman G.S., Kallenberg C.G., Langford C.A., Merkel P.A., et al. Factors Determining the Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3. Arthritis Rheumatol. 2016;68:1700–1710. doi: 10.1002/art.39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemna M.J., Damoiseaux J., Austen J., Winkens B., Peters J., van Paassen P., Cohen Tervaert J.W. ANCA as a predictor of relapse: Useful in patients with renal involvement but not in patients with nonrenal disease. J. Am. Soc. Nephrol. 2015;26:537–542. doi: 10.1681/ASN.2013111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilhorst M., Arndt F., Joseph Kemna M., Wieczorek S., Donner Y., Wilde B., Thomas Epplen J., van Paassen P., Cohen Tervaert J.W. HLA-DPB1 as a Risk Factor for Relapse in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Cohort Study. Arthritis Rheumatol. 2016;68:1721–1730. doi: 10.1002/art.39620. [DOI] [PubMed] [Google Scholar]
- 26.Kain R., Rees A.J. What is the evidence for antibodies to LAMP-2 in the pathogenesis of ANCA associated small vessel vasculitis? Curr. Opin. Rheumatol. 2013;25:26–34. doi: 10.1097/BOR.0b013e32835b4f8f. [DOI] [PubMed] [Google Scholar]
- 27.Tomasson G., Grayson P.C., Mahr A.D., Lavalley M., Merkel P.A. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—A meta-analysis. Rheumatology. 2012;51:100–109. doi: 10.1093/rheumatology/ker280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee M., Thomas S.R., Radford K., Dvorkin-Gheva A., Davydchenko S., Kjarsgaard M., Svenningsen S., Almas S., Felix L.C., Stearns J., et al. Sputum Antineutrophil Cytoplasmic Antibodies in Serum Antineutrophil Cytoplasmic Antibody-Negative Eosinophilic Granulomatosis with Polyangiitis. Am. J. Respir. Crit. Care Med. 2019;199:158–170. doi: 10.1164/rccm.201804-0809OC. [DOI] [PubMed] [Google Scholar]
- 29.Seidowsky A., Hoffmann M., Ruben-Duval S., Mesbah R., Masy E., Kyndt X., Maouad B., Billion S., Noël L.H., Vanhille P., et al. Elastase-ANCA-associated idiopathic necrotizing crescentic glomerulonephritis—A report of three cases. Nephrol. Dial. Transplant. 2007;22:2068–2071. doi: 10.1093/ndt/gfm169. [DOI] [PubMed] [Google Scholar]
- 30.Moodie F.D., Leaker B., Cambridge G., Totty N.F., Segal A.W. Alpha-enolase: A novel cytosolic autoantigen in ANCA positive vasculitis. Kidney Int. 1993;43:675–681. doi: 10.1038/ki.1993.97. [DOI] [PubMed] [Google Scholar]
- 31.Cabral D.A., Uribe A.G., Benseler S., O’Neil K.M., Hashkes P.J., Higgins G., Zeft A.S., Lovell D.J., Kingsbury D.J., Stevens A., et al. Classification, presentation, and initial treatment of Wegener’s granulomatosis in childhood. Arthritis Rheum. 2009;60:3413–3424. doi: 10.1002/art.24876. [DOI] [PubMed] [Google Scholar]
- 32.Cabral D.A., Canter D.L., Muscal E., Nanda K., Wahezi D.M., Spalding S.J., Twilt M., Benseler S.M., Campillo S., Charuvanij S., et al. Comparing Presenting Clinical Features in 48 Children with Microscopic Polyangiitis to 183 Children Who Have Granulomatosis with Polyangiitis (Wegener’s): An ARChiVe Cohort Study. Arthritis Rheumatol. 2016;68:2514–2526. doi: 10.1002/art.39729. [DOI] [PubMed] [Google Scholar]
- 33.Morishita K.A., Moorthy L.N., Lubieniecka J.M., Twilt M., Yeung R.S.M., Toth M.B., Shenoi S., Ristic G., Nielsen S.M., Luqmani R.A., et al. Early Outcomes in Children with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2017;69:1470–1479. doi: 10.1002/art.40112. [DOI] [PubMed] [Google Scholar]
- 34.Gill E.E., Smith M.L., Gibson K.M., Morishita K.A., Lee A.H.Y., Falsafi R., Graham J., Foell D., Benseler S.M., Ross C.J., et al. Different Disease Endotypes in Phenotypically Similar Vasculitides Affecting Small-to-Medium Sized Blood Vessels. Front. Immunol. 2021;12:638571. doi: 10.3389/fimmu.2021.638571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marino A., Tirelli F., Giani T., Cimaz R. Periodic fever syndromes and the autoinflammatory diseases (AIDs) J. Transl. Autoimmun. 2020;3:100031. doi: 10.1016/j.jtauto.2019.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker L.B., Niemietz I., Mangat P., Belen M., Tekano J., Cabral D.A., Guzman J., Houghton K.M., Morishita K.A., Chan M.O., et al. Children with systemic autoinflammatory diseases have multiple, mixed ethnicities that reflect regional ethnic diversity. Clin. Exp. Rheumatol. 2021;39((Suppl. S132)):124–128. doi: 10.55563/clinexprheumatol/8cjqiy. [DOI] [PubMed] [Google Scholar]
- 37.Ozen S., Pistorio A., Iusan S.M., Bakkaloglu A., Herlin T., Brik R., Buoncompagni A., Lazar C., Bilge I., Uziel Y., et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann. Rheum. Dis. 2010;69:798–806. doi: 10.1136/ard.2009.116657. [DOI] [PubMed] [Google Scholar]
- 38.Fries J.F., Hunder G.G., Bloch D.A., Michel B.A., Arend W.P., Calabrese L.H., Fauci A.S., Leavitt R.Y., Lie J.T., Lightfoot R.W., Jr., et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum. 1990;33:1135–1136. doi: 10.1002/art.1780330812. [DOI] [PubMed] [Google Scholar]
- 39.Watts R., Lane S., Hanslik T., Hauser T., Hellmich B., Koldingsnes W., Mahr A., Segelmark M., Cohen-Tervaert J.W., Scott D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007;66:222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanham J.G., Elkon K.B., Pusey C.D., Hughes G.R. Systemic vasculitis with asthma and eosinophilia: A clinical approach to the Churg-Strauss syndrome. Medicine. 1984;63:65–81. doi: 10.1097/00005792-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Dolezalova P., Price-Kuehne F.E., Özen S., Benseler S.M., Cabral D.A., Anton J., Brunner J., Cimaz R., O’Neil K.M., Wallace C.A., et al. Disease activity assessment in childhood vasculitis: Development and preliminary validation of the Paediatric Vasculitis Activity Score (PVAS) Ann. Rheum. Dis. 2013;72:1628–1633. doi: 10.1136/annrheumdis-2012-202111. [DOI] [PubMed] [Google Scholar]
- 42.Kain R., Matsui K., Exner M., Binder S., Schaffner G., Sommer E.M., Kerjaschki D. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: The lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J. Exp. Med. 1995;181:585–597. doi: 10.1084/jem.181.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.