Abstract
In this study, we investigated the induction of cellular gene expression by the Epstein-Barr Virus (EBV) latent membrane protein 1 (LMP1). Previously, LMP1 was shown to induce the expression of ICAM-1, LFA-3, CD40, and EBI3 in EBV-negative Burkitt lymphoma (BL) cells and of the epidermal growth factor receptor (EGF-R) in epithelial cells. We now show that LMP1 expression also increased Fas and tumor necrosis factor receptor-associated factor 1 (TRAF1) in BL cells. LMP1 mediates NF-κB activation via two independent domains located in its C-terminal cytoplasmic tail, a TRAF-interacting site that associates with TRAF1, -2, -3, and -5 through a PXQXT/S core motif and a TRADD-interacting site. In EBV-transformed B cells or transiently transfected BL cells, significant amounts of TRAF1, -2, -3, and -5 are associated with LMP1. In epithelial cells, very little TRAF1 is expressed, and only TRAF2, -3, and -5, are significantly complexed with LMP1. The importance of TRAF binding to the PXQXT/S motif in LMP1-mediated gene induction was studied by using an LMP1 mutant that contains alanine point mutations in this motif and fails to associate with TRAFs. This mutant, LMP1(P204A/Q206A), induced 60% of wild-type LMP1 NF-κB activation and had approximately 60% of wild-type LMP1 effect on Fas, ICAM-1, CD40, and LFA-3 induction. In contrast, LMP1(P204A/Q206A) was substantially more impaired in TRAF1, EBI3, and EGF-R induction. Thus, TRAF binding to the PXQXT/S motif has a nonessential role in up-regulating Fas, ICAM-1, CD40, and LFA-3 expression and a critical role in up-regulating TRAF1, EBI3, and EGF-R expression. Further, D1 LMP1, an LMP1 mutant that does not aggregate failed to induce TRAF1, EBI3, Fas, ICAM-1, CD40, and LFA-3 expression confirming the essential role for aggregation in LMP1 signaling. Overexpression of a dominant form of IκBα blocked LMP1-mediated TRAF1, EBI3, Fas, ICAM-1, CD40, and LFA-3 up-regulation, indicating that NF-κB is an important component of LMP1-mediated gene induction from both the TRAF- and TRADD-interacting sites.
Epstein-Barr virus (EBV) infection of resting human B lymphocytes in vitro results in continuous cell proliferation and transformation into lymphoblastoid cell lines (LCLs). These latently infected cells express several EBV-encoded nuclear and membrane proteins (reviewed in reference 36). The EBV latent membrane protein 1 (LMP1) has a critical role in EBV-induced B-cell transformation. LMP1 has transforming properties in rodent fibroblast cell lines (2, 57) and is essential for the ability of EBV to transform B cells, as demonstrated by genetic analyses with recombinant EBV (32). LMP1 is also expressed in most EBV-associated malignancies, including lymphoproliferative disease, Hodgkin’s disease, and nasopharyngeal carcinoma (reviewed in reference 50).
Accumulating evidence supports the model that LMP1 mimics a constitutively activated tumor necrosis factor receptor (TNFR). LMP1 directly interacts and constitutively associates in cells with cytoplasmic signaling molecules of the TNFR family, TNFR-associated factor 1 (TRAF1), TRAF2, and TRAF3, and TNFR-associated death domain protein, TRADD (Fig. 1) (5, 10, 31, 34, 46, 52). In B lymphocytes, expression of LMP1 induces effects similar to those observed after cross-linking of CD40, a member of the TNFR family. These effects include increased expression of cell surface markers and adhesion molecules and activation of NF-κB (3, 26, 36, 40, 45, 58, 59). LMP1 is also similar to CD40 in induction of stress-activated protein kinases and in effects on B-cell growth (13, 16, 19, 37, 38).
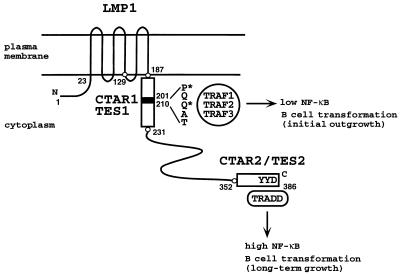
FIG. 1.
Schematic representation of LMP1. LMP1 consists of a 23-aa N-terminal cytoplasmic domain, six hydrophobic transmembrane domains separated by short reverse turns, and a 200-aa CTD. The two signaling domains, LMP1 aa 187 to 231 (CTAR1/TES1) and aa 352 to 386 (CTAR2/TES2), are represented by empty boxes; the core of the TRAF binding site, aa 201 to 210, is represented by a black box. Residues directly implicated in TRAF or TRADD binding are indicated. Residues marked with an asterisk were mutated to alanine in the LMP1(P204A/Q206A) mutant. D1 LMP1 comprises the last two transmembrane domains and the CTD, i.e., LMP1 aa 129 to 386.
LMP1 aggregation at the plasma membrane is essential for signaling and for B-lymphocyte growth transformation (15, 16, 19, 26, 29, 32, 42, 58). LMP1 constitutively signals because the six transmembrane domains (Fig. 1) enable ligand-independent, continuous aggregation in the plasma membrane. As a consequence of LMP1 aggregation, TRAFs and TRADD constitutively associate with the LMP1 carboxyl-terminal cytoplasmic domain (CTD) (10, 31). In contrast to LMP1, TNFRs associate with TRAFs or TRADD in response to ligand binding (25, 39, 53, 55).
While the important function of the 23 amino-terminal cytoplasmic amino acids (aa) appears to be in orienting the first transmembrane domains (29), two regions of the 200-aa CTD are critical for NF-κB activation and for B-lymphocyte growth transformation (Fig. 1). The region consisting of the membrane-proximal 45 residues of the cytoplasmic tail (aa 187 to 231) mediates less than 30% of the LMP1-induced NF-κB activation and has the functional designation CTAR1 (carboxyl-terminal activation region 1) (26, 45). This domain is both necessary and sufficient for initial B-lymphocyte transformation and also is designated TES1 (transformation effector site 1) (30, 33, 35). CTAR1/TES1 mediates the association of LMP1 with TRAFs (10). In LCLs, at least 50% of TRAF3 or TRAF1 associates with this site, whereas TRAF2 associates to a lesser extent. Point mutations within the LMP1 PXQXT/S core motif that is important in TRAF interaction reduce NF-κB induction by CTAR1/TES1 (10). Our previous studies suggest that TRAF2 or TRAF1-TRAF2 heterodimers mediate NF-κB activation by CTAR1/TES1, while TRAF3 may act as a negative modulator by displacing TRAF1 and TRAF2 from the LMP1 CTD (10, 34).
The distal region of LMP1 CTD, encompassing aa 352 to 386 and designated CTAR2/TES2, is the major NF-κB-inducing domain (15, 26, 45) and mediates the association of LMP1 with TRADD (31). An LMP1 CTAR2/TES2 double-point mutant that fails to interact with TRADD is defective in NF-κB activation and in B-lymphocyte transformation, while a second CTAR2/TES2 double-point mutant that is wild type in NF-κB activation is also wild type in B-lymphocyte transformation (31).
Although CTAR1/TES1 and CTAR2/TES2 both activate NF-κB, they are not functionally equivalent. CTAR1/TES1 is a weak activator of NF-κB in epithelial cells but is sufficient for initial transformation, whereas CTAR2/TES2 is a strong activator of NF-κB and thus far found to be insufficient for transformation in the absence of CTAR1/TES1 (30). Both domains can induce A20 expression in C33A epithelial cells, but only CTAR1/TES1 can induce epidermal growth factor receptor (EGF-R) expression in these cells (44). Hence, CTAR1/TES1 has different effects on gene induction than CTAR2/TES2. The studies reported here were undertaken to investigate the specific role of TRAF binding to CTAR1/TES1 in LMP1-mediated cell gene induction.
MATERIALS AND METHODS
Plasmids.
Plasmids pcLMP1 and pcLMP1(P204A/Q206A) were constructed by inserting an EcoRI fragment of pSG5 LMP1 and pSG5 LMP1(P204A/Q206A) (10), respectively, in the EcoRI site of pcDNA3 (Invitrogen). pcDNA3-based expression vectors use the cytomegalovirus (CMV) immediate-early promoter/enhancer, and the pSG5-based expression vectors use the simian virus 40 (SV40) early promoter. The Flag-tagged IκBα expression vector with serines 32 and 36 mutated to alanines, pCMV4 IκBα S32AS36A, was provided by D. W. Ballard (Vanderbilt University) (6). The green fluorescent protein (GFP) expression vector, pEGFP-C1, was obtained from Clontech.
Cell lines and transfections.
IB4 is an EBV-transformed LCL. BJAB and BL41 are EBV-negative Burkitt lymphoma (BL) cell lines. C33A is an epithelial cell line derived from a human cervical carcinoma. 293 is a human embryonal kidney cell line. Cell lines were grown in RPMI 1640 (B-cell lines) or Dulbecco’s modified Eagle medium (DMEM) (293 and C33A) supplemented with 10% fetal calf serum. For transient expression, 4 × 106 to 107 cells were transfected by electroporation with a Bio-Rad electroporator at 200 V (C33A), 210 V (BJAB), or 220 V (BL41) and 960 μF at room temperature in 400 μl of RPMI 1640 medium containing 10% fetal calf serum (R10) and cultivated for 16 to 24 h. Transfection efficiency ranged from 20 to 40% for C33A cells, 40 to 70% for BJAB cells, and approximately 5% for BL41 cells, as assessed by cotransfection with GFP expression vector and fluorescence-activated cell sorting (FACS) analysis. In some experiments, BL41 cells were electroporated with a BTX electroporator by one pulse at 200 V for 65 ms in 400 μl of ice-cold R10 and then placed on ice for 5 min before incubation in R10 at 37°C. Under these conditions, up to 15% of BL41 cells could be transfected. To establish BJAB or C33A cells stably expressing LMP1 or LMP1(P204A/Q206A), cells were electroporated with pcLMP1, pcLMP1(P204A/Q206A), or pcDNA3 vector control and selected 2 days after electroporation in complete medium supplemented with 3 (BJAB) or 1 (C33A) mg of Geneticin (Gibco BRL) per ml. Transfected BJAB were plated in 96-well plates, and G418-resistant cells were screened by immunoblotting. LMP1-expressing BJAB cells were cloned by limiting dilution. Macroscopically visible resistant C33A colonies were individually picked and expanded for immunoblot analysis.
Immunoprecipitations and immunoblots.
LCLs or transfected cells obtained 16 to 18 h posttransfection were washed in phosphate-buffered saline and lysed for 30 min on ice in 0.5% Nonidet P-40 (NP-40) lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 3% glycerol, 1.5 mM EDTA) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, pepstatin [1 μg/ml], leupeptin [1 μg/ml]). LCL lysates were prepared by Dounce homogenization. Cell lysates were centrifuged for 15 min at 14,000 × g and precleared with protein G-Sepharose beads (Pharmacia) for 1 to 2 h. Cleared lysates were incubated with anti-Flag M2 affinity gel (International Biotechnologies Inc.) for 2 h at 4°C. Beads were then washed five times with 1 ml of 0.5% NP-40 lysis buffer, and bound proteins were recovered by boiling in sodium dodecyl sulfate (SDS) sample buffer. Eluted proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membrane for immunoblotting. TRAFs and EGF-R were detected with rabbit polyclonal antisera recognizing TRAF1 (H-132 or S-19), TRAF2 (C-20), TRAF3 (H-122), TRAF5 (H-257), TRAF6 (H-274), or EGF-R (1005) or with goat polyclonal antisera recognizing TRAF4 (N-16), TRAF5 (C-19), or TRAF6 (C-20) at 1 μg/ml (Santa Cruz Biotechnology). TRAF5 antibody H-257 was found to also detect TRAF3 in immunoprecipitation experiments but not upon immunoblotting. EBI3 was detected with affinity-purified EBI3 rabbit antisera (9). Binding of rabbit or goat antibodies was detected with horseradish peroxidase-conjugated protein A (1:7,500 dilution; Amersham) or horseradish peroxidase-conjugated donkey anti-goat antibodies (1:5,000 dilution in Tris-buffered saline–0.5% milk; Santa Cruz Biotechnology), respectively. Wild-type and mutant LMP1 were detected with anti-LMP1 monoclonal antibody S12 followed by sheep anti-mouse antibodies conjugated to horseradish peroxidase (1:5,000 dilution; Amersham). Flag-tagged proteins were detected by with anti-Flag monoclonal antibody M2 or M5 (International Biotechnology Inc.).
Cell surface immunofluorescence and FACS analysis.
Cell surface immunofluorescence of transfected cells was performed by two-color FACS analysis. Cells (5 × 106 cells per transfection) were electroporated with wild-type or mutant LMP1 expression vector, together with 3 μg of GFP reporter plasmid; 16 to 24 h posttransfection, cells (106 per staining) were washed in phosphate-buffered saline supplemented with 0.2% fetal bovine serum and 0.01% sodium azide and then incubated for 30 min on ice with phycoerythrin (PE)-conjugated monoclonal antibodies CD40-PE (Serotec), CD54 (ICAM-1)-PE (Pharmingen), CD58 (LFA-3)-PE (Serotec), and CD95 (Fas)-PE (Pharmingen). Cells were then washed and analyzed on a FACScan or FACScalibur flow cytometer (Becton Dickinson) with Cellquest software. Viable cells were gated by forward and side scatter. Transfected (GFP-positive) cells were further gated (green fluorescence; FL1) and surface expression in these cells was detected as red fluorescence (FL2). A minimum of 5,000 GFP-positive cells were analyzed.
NF-κB assays.
293 cells were transfected with 350 ng of the luciferase reporter driven by three NF-κB binding sites from the major histocompatibility complex class I promoter (45), 350 ng of pGK-β-galactosidase, and 375 ng of pcDNA3-based expression plasmids by using Superfect (Qiagen). The day before transfection, 293 cells (5 × 105 per well) were plated in a six-well plate in 2 ml of medium. Immediately prior to transfection, 1 ml of medium was removed. Fifteen microliters of Superfect were added to the DNA diluted in 150 μl of DMEM, and complexes were allowed to form for 10 min. Superfect-DNA complexes were added dropwise to the cells. Following a 3- to 4-h incubation, the transfection medium was replaced with 1 ml of culture medium. The cells were harvested 20 to 24 h after addition of the Superfect-DNA to the cultures. The transfection was performed in duplicate, and the cells were pooled for subsequent analysis. One half of the cells was used to determine luciferase and β-galactosidase activities as instructed by the manufacturers (Promega and Tropix, respectively); the other half was lysed in lysis buffer (1% NP-40, 1% deoxycholate, 50 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 400 μM Na3VO4, 1 mM NaF, 20 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride. Insoluble material was removed by centrifugation, and protein determinations were made by using the bicinchoninic acid protein assay reagent (Pierce). Equivalent amounts of protein were analyzed by Western blot with anti-LMP1 monoclonal antibody S12.
RESULTS
LMP1(P204A/Q206A) is markedly impaired in TRAF association but induces 60% of wild-type LMP1 NF-κB activation.
Previous studies found that mutation of either P204 or Q206 to alanine within the PXQXT/S motif in the context of CTAR1/TES1 (Fig. 1) abrogated TRAF2 binding, reduced TRAF1 binding, but had almost no effect on TRAF3 binding. LMP1(1–231) mutants with P204A or Q206A were significantly impaired in NF-κB activation but still retained 10 to 15% of LMP1(1–231) NF-κB activation (10). To construct a putative null mutation for TRAF binding and CTAR1/TES1 signaling, both P204 and Q206 were mutated to alanine within full-length LMP1 to yield LMP1(P204A/Q206A). LMP1(P204A/Q206A) was similar to wild-type LMP1 in intracellular localization in EBV-negative BL cell line (BJAB) (data not shown). LMP1(P204A/Q206A) was nearly as stable as wild-type LMP1. Usually, no more than twofold LMP1(P204A/Q206A) expression vector was required to achieve protein expression equivalent to that of wild-type LMP1, as assessed by Western blotting (data not shown). Nevertheless, the double-point mutation had a profound effect on the ability of TRAFs to associate with LMP1. While high amounts of the endogenous TRAF1, TRAF2, and TRAF3 coimmunoprecipitated with wild-type Flag-tagged LMP1 (Flag-LMP1) from transiently transfected BJAB cells, TRAF1 and TRAF2 did not coimmunoprecipitate with Flag-LMP1(P204A/Q206A) and only a trace of TRAF3 associated with Flag-LMP1(P204A/Q206A) (Fig. 2A, left, lanes 5 and 6). Although CTAR2/TES2 mediates NF-κB activation through a TRADD-TRAF2 interaction (31), no TRAF2 binding to CTAR2/TES2 could be detected in the Flag-LMP1(P204A/Q206A) immunoprecipitate under the experimental conditions used.
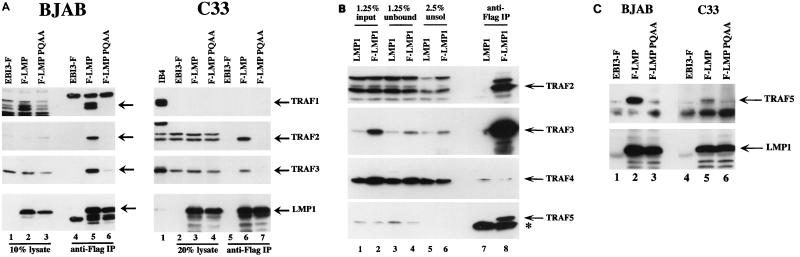
FIG. 2.
Effect of the P204A/Q206A mutation on TRAFs binding to LMP1. (A) BJAB cells (107 per transfection) or C33A cells (4 × 106 per transfection) were electroporated with 20 μg of plasmid pSG5 expressing Flag-LMP1 (F-LMP1) (10), 20 (left) or 30 (right) μg of pSG5 Flag-LMP1(P204A/Q206A) (F-LMP PQAA) (10), or 20 μg of pSG5 EBI3-Flag (EBI3-F) (9) as a control. Eighteen hours posttransfection, cells were lysed in 0.5% NP-40 lysis buffer, and cell extracts were submitted to immunoprecipitation (IP) with anti-Flag affinity gel. A fraction of the cell lysate obtained before immunoprecipitation (left, lanes 1 to 3; right, lanes 2 to 4) and total immunoprecipitates (left, lanes 4 to 6; right, lanes 5 to 7) were analyzed by SDS-PAGE on an 8% gel and subjected to Western blot analysis with rabbit polyclonal antibodies recognizing TRAF1 (S-19 or H-132), TRAF2 (C-20), or TRAF3 (H-122), with anti-Flag antibody M5 (left), or with anti-LMP1 monoclonal antibody S12 (right). LMP1 and LMP1(P204A/Q206A) display similar solubilities under the conditions of extraction used here, so that the amount of LMP1 protein observed in the cell lysate fraction is representative of the whole LMP1 protein content (data not shown). Cell extract from 5 × 105 IB4 cells was used as a control for TRAF1 detection (right, lane 1). The positions of the TRAF1 doublet and of TRAF2, TRAF3, and LMP1 are indicated. (B) NP-40 cell extracts from 80 × 106 cells from a recombinant LCL expressing an N-terminal Flag-tagged LMP1 protein (F-LMP1) or of a control IB4 LCL expressing non-Flag-tagged LMP1 (LMP1) were subjected to immunoprecipitation with M2 anti-Flag affinity gel. A fraction of the cell lysates obtained before (input; lanes 1 and 2) or after (unbound; lanes 3 and 4) immunoprecipitation, or of the unsoluble fraction (unsol; lanes 5 and 6), and the total immunoprecipitates (lanes 7 and 8) were analyzed by SDS-PAGE on a 10% gel and subjected to Western blot analysis with rabbit polyclonal antibodies recognizing TRAF2 (C-20), TRAF3 (H-122), and TRAF4 (N-16) and with goat polyclonal antibodies recognizing TRAF5 (C-19). The positions of the TRAFs are marked; immunoglobulin heavy chain is indicated by an asterisk. Western blotting with anti-Flag antibody Ms showed that more than 50% of LMP1 was solubilized and precipitated (data not shown). (C) BJAB cells (107 per transfection) or C33A cells (5 × 106 per transfection) were electroporated with 25 μg of pSG5 Flag-LMP1 (F-LMP1), 35 μg of pSG5 Flag-LMP1(P204A/Q206A) (F-LMP PQAA), or 25 μg of pSG5 EBI3-Flag (EBI3-F) as a control. Eighteen hours posttransfection, cells were lysed in 0.5% NP-40 lysis buffer, and cell extracts were submitted to immunoprecipitation with anti-Flag affinity gel. Immunoprecipitates were analyzed by SDS-PAGE on a 10% gel and subjected to Western blot analysis with rabbit polyclonal antibodies recognizing TRAF5 (H-257) or with anti-Flag antibody M5. The positions of TRAF5 and LMP1 are indicated.
LMP1(P204A/Q206A) association with TRAFs was also evaluated in the C33A epithelial cell line and in the 293 kidney cell line. TRAF1 was not detectable in lysates from transfected C33A cells and was barely detectable in 293 cell lysates (Fig. 2A [right] and data not shown). TRAF2 and TRAF3 efficiently coimmunoprecipitated with Flag-LMP1 from transiently transfected C33A or 293 cells but did not coimmunoprecipitate with Flag-LMP1(P204A/Q206A). Little or no TRAF1 was detected in the Flag-LMP1 immunoprecipitates from epithelial cells, consistent with the barely detectable level of TRAF1 in these cells (Fig. 2A [right, lane 6] and data not shown). Thus, TRAF2 efficiently coimmunoprecipitated with wild-type LMP1 from transfected C33A and 293 cells in the near absence of TRAF1. This was somewhat surprising given the weak binding of TRAF2 to the LMP1 CTD measured in a glutathione S-transferase binding assay and the low level of coimmunoprecipitation of TRAF2 with LMP1 observed in LCLs (10).
Recently, in an in vitro glutathione S-transferase binding assay, TRAF5 was shown to bind to CTAR1/TES1 but not CTAR2/TES2, while TRAF6 failed to show any detectable binding to the LMP1 CTD (5, 27). To investigate whether LMP1 associates with TRAF5 in vivo, LMP1 was immunoprecipitated from extracts of an LCL transformed by a recombinant EBV encoding Flag-LMP1 (10), and the amount of associated TRAF5 was evaluated by Western blotting. A significant amount of TRAF5 coprecipitated with Flag-LMP1, but no TRAF5 signal was detected in the Flag immunoprecipitate from a control non-Flag-tagged LMP1 EBV-transformed LCL (Fig. 2B, lanes 7 and 8). The extent of TRAF5 association with LMP1 was less than that observed for TRAF3 or TRAF1 (Fig. 2B; compare the amount of TRAF5 or TRAF3 present in the cell lysate before immunoprecipitation [lane 2] to that present in the unbound fraction [lane 4] and in the Flag immunoprecipitate [lane 8]; also data not shown for TRAF1). TRAF6 could not be detected in the lysate or in the Flag-LMP1 immunoprecipitate from LCLs (data not shown). Although TRAF4 was detected in the LCL extracts, no specific TRAF4 signal was detected in the Flag immunoprecipitate (Fig. 2B). TRAF5 association with LMP1 was also investigated in transiently transfected BJAB and C33A cells. In transfected cells of both cell lines, endogenous TRAF5 coimmunoprecipitated with Flag-LMP1 but not with Flag-LMP1(P204A/Q206A) (Fig. 2C). Thus, the P204A/Q206A mutation in the context of full-length LMP1 abrogates the association in vivo of TRAF1, TRAF2, TRAF3, and TRAF5 with LMP1 under the experimental conditions used here.
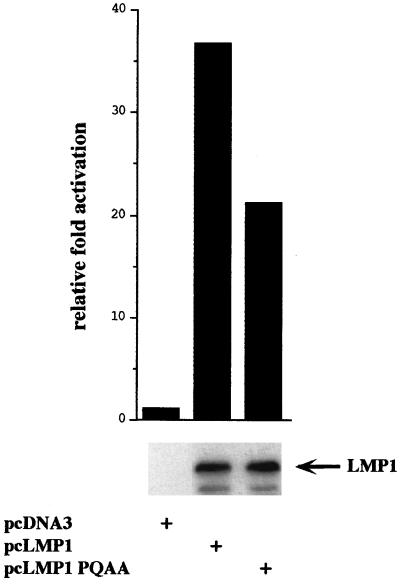
LMP1(P204A/Q206A) induced about 60% as much NF-κB activation as LMP1 when expressed at levels comparable to those of LMP1 in 293 cells (Fig. 3). This result is consistent with previous data for LMP1 mutants deleted for the CTAR1/TES1 or the TRAF binding site, which showed that CTAR1/TES1 is less active in NF-κB activation than CTAR2/TES2 and that deletion of CTAR1/TES1 only modestly decreased NF-κB activation (30, 45).
FIG. 3.
NF-κB induction by LMP1 and LMP1(P204A/Q206A) in 293 cells. 293 cells (5 × 105) were transfected with 350 ng of the luciferase reporter driven by three NF-κB binding sites from the major histocompatibility complex class I promoter, 350 ng of pGK-β-galactosidase, and 375 ng of pcDNA-based expression plasmids. Data are presented as luciferase activity normalized to β-galactosidase activity and adjusted to 1 for pcDNA3 control-transfected cells. 293 lysates were normalized for protein content and analyzed for LMP1 expression by immunoblot analysis using anti-LMP1 monoclonal antibody S12.
LMP1(P204A/Q206A) cannot induce EGF-R expression in stably transfected C33A cells.
LMP1 or LMP1(1–231) can induce EGF-R expression in C33A epithelial cells, while LMP1 deleted of aa 187 to 351 cannot, compatible with the notion that the TRAF binding site (LMP1 aa 199 to 210) mediates EGF-R induction (44). To more precisely assess the involvement of the TRAF signaling pathway in EGF-R induction, C33A cells were stably transfected with wild-type LMP1 or LMP1(P204A/Q206A) and tested by immunoblotting for EGF-R induction (Fig. 4). While six pcDNA3 vector control-transfected clones lacked EGF-R expression, EGF-R expression was detected in all five C33A clones transfected with the pcDNA3-LMP1 expression vector. The level of EGF-R expression varied among these clones (Fig. 4 [lanes 3, 4, 9, and 10] and data not shown). In contrast, 8 of 10 clones of C33A cells transfected with the pcDNA3-LMP1(P204A/Q206A) expression vector failed to express EGF-R, and the two other clones had barely detectable EGF-R expression (Fig. 4 [lanes 5, 6, 11, and 12] and data not shown). The failure of LMP1(P204A/Q206A) to induce EGF-R expression in C33A cells was not due to lower LMP1(P204A/Q206A) expression. By immunoblotting, LMP1(P204A/Q206A) was expressed at levels similar to or higher than those of wild-type LMP1 (Fig. 4 and data not shown). Thus, these data more precisely link LMP1 induction of EGF-R expression in C33A cells to TRAF-mediated signaling.
FIG. 4.
Effect of the P204A/Q206A mutation on EGF-R induction in C33A cells. Whole-cell extracts from four independent C33A clones stably transfected with either pcDNA3 (vector; lanes 1, 2, 7, and 8), pcDNA3 expressing wild-type LMP1 (WT; lanes 3, 4, 9, and 10), or LMP1(P204A/Q206A) (PQAA; lanes 5, 6, 11, and 12) were immunoblotted with rabbit polyclonal antibodies recognizing EGF-R or with anti-LMP1 monoclonal antibody S12. Numbers at the left show positions of standard molecular weight proteins (in thousands). The intensity of the 120-kDa cross-reactive protein observed with EGF-R antibodies paralleled that of the Ponceau staining and is indicative of the total amount of protein loaded. Due to the very low amount of LMP1 protein present in lane 4, the S12 blot shown on the left was overexposed compared to the blot shown on the right. Immunoblot analysis was performed after over 40 days of selection.
Efficient LMP1-mediated induction of TRAF1 and EBI3 expression in BL cells is dependent on TRAF binding to the PXQXT/S motif within CTAR1/TES1.
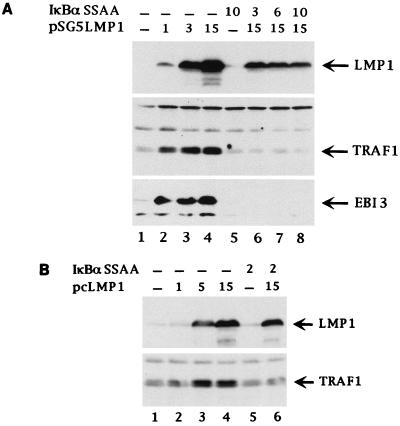
TRAF1 is not expressed in most tissues and is induced in B lymphocytes by EBV infection (46). We noted that transient or stable transfection of BJAB or BL41 BL cells with LMP1 expression vectors specifically induced TRAF1 expression. Transient transfection of BJAB cells with the SV40 promoter-driven pSG5 LMP1 expression vector resulted in increased TRAF1 expression compared to the low level observed in pSG5 vector control-transfected cells (Fig. 5A, lane 1 to 4). Similarly, stable expression of LMP1 in BJAB cells under control of the metallothionein or CMV promoter resulted in higher levels of TRAF1 expression (Fig. 5B, lanes 2, 6, and 7). LMP1-induced TRAF1 expression levels were similar to those observed in EBV-transformed B lymphocytes (Fig. 5B; compare TRAF1 signal in lanes 2, 6, and 7 with TRAF1 signal in IB4 LCL in lane 3). In contrast, stable expression of LMP1(P204A/Q206A) in BJAB cells did not alter TRAF1 expression, despite similar levels of LMP1 and LMP1(P204A/Q206A) expression (Fig. 5B, lanes 8 and 9). Transient expression of LMP1(P204A/Q206A) in BJAB cells also did not usually result in significant increases in TRAF1 expression (Fig. 5A, lanes 5 to 7). High-level LMP1(P204A/Q206A) transient expression or transient expression of an LMP1 mutant deleted of CTAR1/TES1 (Flag-tagged LMP1Δ187–351 [31]) weakly induced TRAF1 expression in some experiments (data not shown). TRAF1 induction was cell type specific, since neither transient nor stable LMP1 expression in C33A cells, nor transient LMP1 expression in 293 cells, caused detectable increased TRAF1 expression (Fig. 2A, right, and data not shown).
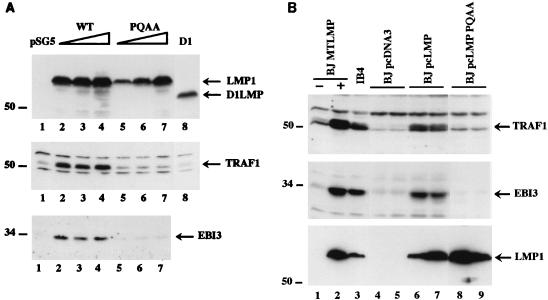
FIG. 5.
Immunoblot analysis of TRAF1 and EBI3 induction by LMP1 mutants in transfected BJAB cells. (A) BJAB cells (107 per transfection) were transiently transfected with increasing amounts (10, 20, and 40 μg) of pSG5 vector expressing wild-type LMP1 (WT) or LMP1(P204A/Q206A) (PQAA) or with 20 μg of pSG5 vector expressing D1 LMP1 (D1) (42). Cells extracts (106 cells per lane) obtained 20 h posttransfection were immunoblotted with a rabbit polyclonal antibody recognizing TRAF1 (H-132), with affinity-purified rabbit polyclonal anti-EBI3 antibodies, or with anti-LMP1 monoclonal antibody S12. The positions of LMP1 and its derivatives, of the TRAF1 doublet, and of EBI3 are indicated on the right. Numbers at the left show positions of standard molecular weight proteins (in thousands). Ponceau S staining of the blot showed equal total protein loading per lane (data not shown). Expression of a higher amount of D1 LMP1 did not result in significant induction of TRAF1 (data not shown). (B) Cell extracts (106 cells per lane) from independent BJAB clones stably expressing wild-type LMP1 or LMP1(P204A/Q206A) were analyzed by immunoblotting for TRAF1 and EBI3 expression as described above. BJ MTLMP (lanes 1 and 2) are stably transfected BJAB cell lines expressing (+) or not expressing (−) LMP1 under the control of a metallothionein promoter (59); lanes 4 and 5 contain two pcDNA3 vector control-derived BJAB clones; BJ pcLMP (lanes 6 and 7) and BJpcLMP PQAA (lanes 8 and 9) stably express wild-type LMP1 and LMP1(P204A/Q206A), respectively, under the control of a CMV promoter. IB4 (lane 3) is an EBV-transformed LCL. The positions of LMP1, TRAF1 doublet, and EBI3 are indicated on the right. Numbers at the left show positions of standard molecular weight proteins (in thousands). Ponceau S staining of the blot showed equal total protein loading per lane (data not shown).
Aggregation of the cytoplasmic tail of LMP1 was required for TRAF1 induction by LMP1. Transient expression of D1 LMP1 (the last two transmembrane domains and the CTD, aa 129 to 386; [Fig. 1]) in BJAB cells failed to induce TRAF1 expression (Fig. 5A, lane 8). D1 LMP1 does not aggregate in the plasma membrane but has a diffuse distribution in cytoplasmic membranes (42). Similarly to these results in BJAB cells, LMP1 was able to induce TRAF1 expression in transiently transfected BL41 cells but LMP1(P204A/Q206A) and D1 LMP1 were unable to do so (data not shown).
Stable LMP1 expression in BJAB, BL41, or Louckes BL cell lines induces the expression of EBI3, an EBV-induced interleukin-12 p40-homologous cytokine that associates with interleukin-12 p35 to form a heterodimeric cytokine (9, 11). While transient or stable LMP1 expression in BJAB cells up-regulated EBI3 expression as detected on immunoblots, transient or stable LMP1(P204A/Q206A) and transient D1 expression did not (Fig. 5 and data not shown). High-level expression of LMP1(P204A/Q206A) induced little or no EBI3 (data not shown). However, as observed with TRAF1, weak EBI3 induction could be observed in BJAB cells after transient transfection with Flag-tagged LMP1Δ187–351 (data not shown). Altogether, these data indicate that efficient LMP1 up-regulation of TRAF1 and EBI3 expression in B lymphocytes is dependent on the TRAF binding to CTAR1/TES1. CTAR2/TES2 can induce only low-level TRAF1 and EBI3 expression.
TRAF binding to CTAR1/TES1 is not required for LMP1 up-regulation of ICAM-1, Fas, CD40, or LFA-3.
BL41 cells stably expressing LMP1 have higher levels of CD40, ICAM-1, and LFA-3 than vector-derived control clones (59). Similarly, transient cotransfection of LMP1 and GFP expression vectors in BL41 cells resulted in substantial up-regulation of ICAM-1, CD40, and LFA-3 expression on the GFP-positive cells (Fig. 6). Levels of induction of ICAM-1, CD40, and LFA-3 observed in BL41 cells by transient transfection are similar to those found in DG75 BL cells following induction of LMP1 expression under control of a tetracycline-regulated promoter (14). We now find that transient or stable LMP1 expression in BL41 cells also induces Fas expression (Fig. 6 and data not shown). BJAB cells constitutively express high levels of CD40, LFA-3, and Fas, and these proteins were not further up-regulated by LMP1 expression (data not shown). However, LMP1 expression did up-regulate ICAM-1 expression on BJAB cells (data not shown).
FIG. 6.
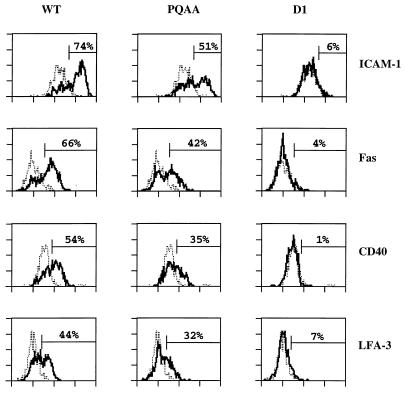
FACS analysis of cell surface molecule induction by wild-type and mutant LMP1 in transiently transfected BL41 cells. BL41 cells (5 × 106) were electroporated with pSG5 vector alone or pSG5 vector expressing wild-type LMP1 (WT; 20 μg), LMP1(P204A/Q206A) (PQAA; 30 μg), or D1 LMP1 (D1; 20 μg), together with 3 μg of GFP reporter plasmid. Twenty-two hours posttransfection, one fraction of the cells was stained for ICAM-1, Fas, CD40, and LFA-3 cell surface expression and examined by two-color FACS analysis as indicated in Materials and Methods; a second fraction was analyzed by immunoblotting for LMP1 expression. FL2 fluorescence of GFP-positive cells only is represented (X axis, fluorescence intensity; y axis, cell number). The dotted line corresponds to vector control-transfected cells; the thick line represents LMP1- or LMP1 mutant-transfected cells. To quantify the relative efficiency of wild-type and mutant LMP1 proteins to up-regulate surface expression of these markers, a gate was set so that 5% of the vector control-transfected cells scored positive for the surface marker analyzed. The percentages on each graph corresponds to the fraction of LMP1-expressing cells scoring positive. Data from one representative experiment of three are represented. Wild-type and mutants LMP1 proteins were expressed at similar levels, as assessed by immunoblotting with anti-LMP1 monoclonal antibody S12.
In contrast to wild-type LMP1, D1 LMP1 failed to induce significant ICAM-1, Fas, CD40, or LFA-3 surface expression in BL41 cells (Fig. 6). LMP1(P204A/Q206A) induced all four markers, although less efficiently than wild-type LMP1 (Fig. 6). LMP1, LMP1(P204A/Q206A), and D1 LMP1 were expressed at approximately similar levels, as assessed by LMP1 immunoblotting (data not shown). On average, LMP1(P204A/Q206A) had 70% of the wild-type LMP1 effect on ICAM-1 up-regulation (n = 7), 62% of the effect on Fas up-regulation (n = 6), 65% of the effect on CD40 up-regulation (n = 4), and 56% of the effect on LFA-3 up-regulation (n = 5). LMP1 and LMP1(P204A/Q206A) also weakly induced Fas expression, as observed by immunoblot analysis of stable C33A transfectants (data not shown). Thus, ICAM-1, Fas, CD40, and LFA-3 up-regulation does not specifically require TRAF binding to the PXQXT/S motif within CTAR1/TES1, although it contributes to maximal induction.
LMP1-mediated protein induction is NF-κB dependent.
Since the efficiency by which LMP1 and LMP1 mutants induce ICAM-1, Fas, CD40, and LFA-3 surface expression correlates with their relative ability to induce NF-κB, NF-κB activation is likely to be an important mediator of the up-regulation of these four cell surface antigens. To more directly assess the role of NF-κB in LMP1-mediated gene induction, we evaluated the extent to which a dominant mutant of IκBα which is resistant to activation-induced degradation, IκBα S32AS36A (6), would abrogate these LMP1-induced effects.
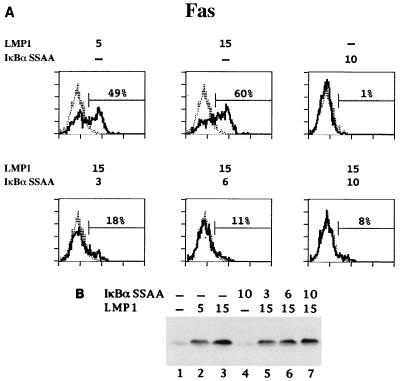
BL41 cells were transiently cotransfected with GFP expression vector and increasing amounts of pSG5 LMP1 (0, 5, and 15 μg) and pCMV4 IκBα S32AS36A (0, 3, 6, and 10 μg). ICAM-1, Fas, CD40, and LFA-3 levels were analyzed by FACS. Expression of IκBα S32AS36A alone consistently caused a very small decrease in ICAM-1, Fas, CD40, and LFA-3 expression on BL41 cells (Fig. 7A and data not shown). Cell viability and transfection efficiency were unaffected (data not shown). Transfection of BL41 cells with 5 μg of pSG5 LMP1 induced increased ICAM-1, Fas, CD40, and LFA-3 levels, and 15 μg of pSG5 LMP1 induced even higher levels (Fig. 7A and data not shown). Coexpression of increasing amounts of IκBα S32AS36A resulted in a dose-dependent inhibition of LMP1 induction of ICAM-1, Fas, CD40, and LFA-3 surface expression, with over 90% inhibition when 10 μg of pCMV4 IκBα S32AS36A was cotransfected (Fig. 7A and data not shown). Under these conditions, IκBα S32AS36A coexpression did not significantly affect LMP1 levels (Fig. 7B). Thus, NF-κB activation is an essential mediator of LMP1 induction of ICAM-1, Fas, CD40, and LFA-3 expression.
FIG. 7.
Effect of IκBα S32AS36A overexpression on LMP1-mediated Fas up-regulation. BL41 cells were transfected with 3 μg of GFP expression vector and the indicated amounts (in micrograms) of pSG5 LMP1 and pCMV4 IκBα S32AS36A. Total DNA transfected was kept constant by addition of vector DNA. (A) Approximately 20 h posttransfection, one fraction of the cells was tested for Fas, ICAM-1, CD40, and LFA-3 surface expression by two-color FACS analysis as described for Fig. 6. Only data for Fas are shown. (B) Whole-cell lysates (5 × 105 cells per lane) were analyzed by immunoblotting for LMP1 expression with anti-LMP1 monoclonal antibody S12.
We next evaluated the importance of NF-κB activation in TRAF1 and EBI3 induction. Transfection of BJAB cells with only 1 μg of pSG5 LMP1 resulted in significant TRAF1 and EBI3 induction (Fig. 8A, lane 2). In contrast, cotransfection of BJAB cells with as much as 15 μg of pSG5 LMP1 together with 3 to 10 μg of IκBα S32AS36A expression vector completely inhibited TRAF1 or EBI3 induction (Fig. 8A, lanes 6 to 8). Although coexpression of IκBα S32AS36A decreased LMP1 expression, LMP1 levels were higher than the levels observed following transfection with 1 μg of pSG5 LMP1 (Fig. 8A, lanes 2 and 6 to 8). Cell surface ICAM-1 expression was analyzed in parallel by FACS. Increased LMP1 expression was accompanied by dose-dependent increased ICAM-1 surface expression. Transfection of 3 or 6 μg of IκBα S32AS36A expression vector resulted in over 90% inhibition of ICAM-1 induction, and cotransfection of 10 μg resulted in 100% inhibition of ICAM-1 induction (data not shown).
FIG. 8.
Effect of IκBα S32AS36A overexpression on TRAF1 and EBI3 induction by LMP1. BJAB cells (5 × 106 per transfection) were transiently transfected with 3 μg of GFP expression vector and the indicated amounts (in micrograms) of pSG5 LMP1 (A) or pcDNA LMP1 (B) and of pCMV4 IκBα S32AS36A. Total DNA transfected was maintained constant by addition of vector DNA. Cells extracts (5 × 105 cells per lane) obtained 20 to 22 posttransfection were subjected to immunoblot analysis with a rabbit polyclonal antibody recognizing TRAF1 (H-132), with affinity-purified rabbit polyclonal anti-EBI3 antibodies, or with anti-LMP1 monoclonal antibody S12. The positions of LMP1, the TRAF1 doublet, and EBI3 are indicated on the right.
Cotransfection of BJAB cells with a CMV promoter-driven LMP1 expression vector and pCMV4 IκBα S32AS36A had less effect on LMP1 expression levels. Nevertheless, IκBα S32AS36A coexpression clearly inhibited LMP1-induced TRAF1, EBI3, or ICAM-1 expression (Fig. 8B and data not shown). Thus, NF-κB activation is also essential for LMP1-induced EBI3 and TRAF1 expression.
DISCUSSION
These experiments demonstrate new roles for LMP1 in up-regulating cell gene expression and evaluate the importance of TRAF association with the CTAR1/TES1 PXQXT/S site and NF-κB activation in LMP1 signaling. LMP1 induces Fas expression. LMP1 may thereby cause EBV-infected B lymphocytes to display high levels of Fas, be more sensitive to Fas ligand, and promote apoptosis. Although TRADD interaction with CTAR2/TES2 could also, in itself, have a proapoptotic effect, we have so far been unable to demonstrate such an effect. While LMP1 cytotoxicity has been demonstrated in several cell lines including BALB/c 3T3 cells (17), LMP1-induced apoptosis has been observed only in RHEK-1 cells (43). The absence of a net apoptotic effect in most cells is probably due to LMP1 activation of NF-κB and up-regulation of antiapoptotic genes including bcl-2 and A20 (20, 40). The latter effects enable LMP1 to protect BL cells from apoptosis (20).
We now show that LMP1 stimulates TRAF1 expression. TRAF1 interacts with CTAR1/TES1 in B lymphocytes and has a coactivating effect on NF-κB induction (10). Thus, TRAF1 up-regulation contributes to high-level NF-κB activation, and this is likely to be important in the antiapoptotic and transforming effects of LMP1. Since LMP1 has both pro- and antiapoptotic effects, the net LMP1 effect may depend on the cell type and stage of differentiation and activation, as well as the presence of Fas ligand and other environmental factors.
A significant amount of TRAF5 has now been demonstrated to be constitutively associated with LMP1 in EBV-transformed B cells, and that association is dependent on the PXQXT/S motif in CTAR1/TES1. Since both TRAF2 and TRAF5 can activate NF-κB and bind to the same site on LMP1, the negative effect of overexpression of a dominant negative mutant form of TRAF2 on NF-κB induction by CTAR1/TES1 (34) is probably not only an effect on TRAF2 binding; TRAF5 probably also mediates NF-κB activation from this site.
The LMP1(P204A/Q206A) double-point mutation almost completely disrupted the ability of LMP1 to associate with TRAFs and enabled a more precise genetic linkage of LMP1 effects with TRAF association. This LMP1 mutant was impaired in induction of EGF-R, TRAF1, and EBI3 expression, compatible with the notion that TRAF aggregation through CTAR1/TES1 has a unique activity that is not readily provided by the rest of LMP1, despite its ability to engage TRADD. In contrast, the expression of ICAM-1, Fas, CD40, and LFA-3 was induced by LMP1(P204A/Q206A) at a level that was about 60% of that obtained by wild-type LMP1, which correlates with the 60% of wild-type NF-κB activation mediated by the TRADD binding site (31). These latter data are in agreement with previous deletion analyses showing that both CTAR1/TES1 and CTAR2/TES2 can independently mediate ICAM-1 and CD40 induction in various cell lines and that both are required for optimal induction of these two cell surface molecules (26).
The results described here provide additional evidence for a role of the TRAFs in gene induction mediated by TNFRs. TRAFs have been implicated in CD40-mediated gene induction. Overexpression of dominant negative mutant forms of TRAF3 or TRAF5 was shown to block CD40-mediated CD23 induction (7, 28) In addition, mutation of T-234 to alanine within the PXQXT motif in CD40 cytoplasmic tail disrupts TRAF2, TRAF3, and TRAF5 binding and abolishes or decreases CD40-mediated ICAM-1, LFA-1, Fas, CD23, B7.1, and LT-α induction in B lymphocytes (18, 21, 23). . However, mutation of T-234 to alanine disrupted not only TRAF binding but also Jak3 binding, and therefore Jak3 may be responsible for some of CD40’s effects on gene induction (18). TRAF signaling from LMP1 or CD40 has also been implicated in NF-κB-mediated interleukin-6 induction in epithelial cells. Both CTAR1/TES1 and CTAR2/TES2 contribute to interleukin-6 induction. Mutations within the PXQXT/S motif or overexpression of dominant negative mutants of TRAF2 or TRAF3 inhibited interleukin-6 induction by CTAR1/TES1 (12).
Although CTAR1/TES1 and CTAR2/TES2 appear to contribute differentially to the LMP1-induced gene effects studied here, it is not clear how these distinct effects are biochemically mediated. CTAR1/TES1 and CTAR2/TES2 activate NF-κB through direct and indirect interactions with TRAF2, respectively, and might, at this stage, be predicted to be comparable in their effects (31, 34). However, the data presented here and previously (44), support a unique role for the LMP1 TRAF binding site in EGF-R, TRAF1, and EBI3 up-regulation and probably in resting B-lymphocyte growth transformation. The unique role for CTAR1/TES1 could be in activation of distinct NF-κB/Rel family members. We showed that CTAR1/TES1 engages TRAF5 in vivo, but TRAF5 appears to be similar to TRAF2 in downstream effects (1, 28, 47, 54). TRAF2 and TRAF5 also mediate stress-activated protein kinase activation from TNFRs (41, 48, 49, 54), and LMP1 also induces stress-activated protein kinases (13, 19, 37). Recent studies suggest that CTAR1/TES1 and CTAR2/TES2 may differ in these as yet poorly characterized pathways (13, 37). Alternatively, the unique ability of CTAR1/TES1 to associate with TRAF3 at a high level may explain the difference. TRAF3 has been implicated in down-modulating NF-κB activity and in affecting cell death in HT29 cells (10, 51, 55) and is likely to mediate other activities. Analysis of the TRAF1, EBI3, and EGF-R promoters may be useful in determining the functional differences between CTAR1/TES1 and CTAR2/TES2.
The data presented here cast the role of TRAF1 in LMP1 signaling in a somewhat different light than previous experiments using LCLs or B lymphoblasts. In such cells, LMP1 appears to largely engage TRAF2 through TRAF1, as a TRAF1-TRAF2 heterodimer (10). Furthermore, in B lymphocytes, TRAF1 is expressed at a high level in response to LMP1 and can synergize with LMP1 in NF-κB activation (10). We now show that in the near absence of TRAF1 in epithelial cells, TRAF2 can associate at a high level with LMP1. Signaling from CTAR1/TES1 through TRAF2, and TRAF5 is therefore likely to be centrally important for the effects of LMP1 on epithelial cell growth, including effects on early lesions of nasopharyngeal carcinoma.
Overexpression of a nondegradable IκBα S32AS36A construct abolishes all of the LMP1-mediated protein induction that we have studied, confirming an important role for NF-κB activation in LMP1-mediated signaling. NF-κB sites have been identified in the Fas promoter and in the ICAM-1 promoter (4, 8, 56). The NF-κB site in the ICAM-1 promoter is required for TNF-α-induced ICAM-1 expression (22). NF-κB was not previously known to have a role in CD40 and LFA-3 gene induction, and the role may be indirect since the results of a computer search indicate that the human CD40 and LFA-3 promoters do not have obvious NF-κB sites.
ACKNOWLEDGMENTS
Dean Ballard provided the IκBα S32AS36A expression vector. David Davidson provided advice on BL41 electroporation. Fred Wang provided the BJAB cell lines that are stably transfected with LMP1 under the control of the metallothionein promoter.
This work was supported by PHS grant CA47006 from the National Cancer Institute (E.K.) and by the Institut National de la Santé et la Recherche Médicale and Université Paris-Sud (O.D.). E.C.M. was supported by NIH training grant AI 07061-20.
ADDENDUM
After submission of this paper, Miller et al. (44a) reported that mutations of the LMP1 PXQXT/S motif impaired the ability of LMP1 to induce EGF-R induction in C33A epithelial cells.
REFERENCES
- 1.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, Yagita H, Okumura K, Inoue J, Watanabe T. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J Biol Chem. 1997;272:2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 2.Baichwal V R, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of the Epstein-Barr virus. Oncogene. 1988;2:461–467. [PubMed] [Google Scholar]
- 3.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 4.Behrmann I, Walczak H, Krammer P H. Structure of the human APO-1 gene. Eur J Immunol. 1994;24:3057–3062. doi: 10.1002/eji.1830241221. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur S R, Cheng G, Baltimore D, Thorley-Lawson D A. Localisation of the major NF-κB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J Biol Chem. 1997;272:19777–19784. doi: 10.1074/jbc.272.32.19777. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I-κBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Cleary A M, Ye Z S, Hong D I, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Liu C, Koopman W J, Mountz J D. Characterization of the human Fas gene. Exon/intron organization and promoter region. J Immunol. 1995;154:1239–1245. [PubMed] [Google Scholar]
- 9.Devergne O, Hummel M, Koeppen H, Le Beau M, Nathanson E C, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos A G, Stack M, Dawson C W, Kaye K M, Hodgkin L, Sihota S, Rowe M, Young L S. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 13.Eliopoulos A G, Young L S. Activation of the c-jun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 14.Floettmann J E, Ward K, Rickinson A B, Rowe M. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 (LMP1) analyzed using tetracycline-regulated expression in B cell lines. Virology. 1996;223:29–40. doi: 10.1006/viro.1996.0452. [DOI] [PubMed] [Google Scholar]
- 15.Floettmann J E, Rowe M. Epstein-Barr virus latent membrane protein 1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C-terminus and requires oligomerisation for NF-κB activation. Oncogene. 1997;15:1851–1858. doi: 10.1038/sj.onc.1201359. [DOI] [PubMed] [Google Scholar]
- 16.Gires O, Zimber-Strobl U, Gonella R, Ueffing M, Marshall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerschmidt W, Sugden B, Baichwal V R. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J Virol. 1989;63:2469–2475. doi: 10.1128/jvi.63.6.2469-2475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanissian S H, Geha R S. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6:379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- 19.Hatzivassiliou E, Miller W E, Raab-Traub N, Kieff E, Mosialos G. A fusion of the EBV latent membrane protein 1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-κB and stress-activated protein kinase. J Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 20.Henderson S, Rowe M, Gregory C, Croom C D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of Bcl-2 expression by Epstein-Barr virus latent membrane protein protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 21.Hostager B S, Hsing Y, Harms D E, Bishop G A. Different CD40-mediated signaling events require distinct structural features. J Immunol. 1996;157:1047–1053. [PubMed] [Google Scholar]
- 22.Hou J, Baichwal V, Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1994;91:11641–11645. doi: 10.1073/pnas.91.24.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsing Y, Hostager B S, Bishop G A. Characterization of CD40 signaling determinants regulating nuclear factor-κB activation in B lymphocytes. J Immunol. 1997;159:4898–4906. [PubMed] [Google Scholar]
- 24.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 25.Hsu H, Shu H B, Pan M P, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor-1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 26.Huen D S, Henderson S A, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 27.Ishida T, Mizushima S-I, Azuma S, Kabayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J-I. Identification of TRAF6, a novel tumor necrosis factor receptor-associated protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 28.Ishida T, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumi K M, Kaye K M, Kieff E. Epstein-Barr virus recombinant molecular genetics analysis of the LMP1 amino-terminal cytoplasmic domain reveals a probable structural role, with no component essential for primary B-lymphocyte growth transformation. J Virol. 1994;68:4369–4376. doi: 10.1128/jvi.68.7.4369-4376.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izumi K M, Kaye K M, Kieff E D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izumi K M, Kieff E D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye K M, Izumi K M, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B lymphocyte transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaye K M, Izumi K M, Mosialos G, Kieff E. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B lymphocyte transformation: fibroblast cocultivation complements a critical function within the terminal 155 residues. J Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaye K M, Devergne O, Harada J N, Izumi K M, Yalamanchili R, Kieff E, Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye, K. M., K. M. Izumi, E. Johannsen, and E. Kieff. Unpublished results.
- 36.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Virology. 3rd ed. Philadelphia, Pa: Raven Press, Ltd.; 1996. pp. 2343–2396. [Google Scholar]
- 37.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilger E, Kilger E, Gries O, Ueffling M, Kolch W, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhne M R, Robbins M, Hambor J E, Mackey M F, Kosaka Y, Nishimura T, Gigley J P, Noelle R J, Calderhead D M. Assembly and regulation of the CD40 receptor complex in human B cells. J Exp Med. 1997;186:337–342. doi: 10.1084/jem.186.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor-κB. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 41.Lee S Y, Reichlin A, Santana A, Kokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 42.Liebowitz D, Mannick J, Takada K, Kieff E. Phenotypes of Epstein-Barr virus LMP1 deletion mutants indicate transmembrane and amino-terminal cytoplasmic domains necessary for effects in B-lymphoma cells. J Virol. 1992;66:4612–4616. doi: 10.1128/jvi.66.7.4612-4616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J J, Chen J Y, Hsu T Y, Yu W C, Su I J, Yang C S. Induction of apoptosis in epithelial cells by Epstein-Barr virus latent membrane protein 1. J Gen Virol. 1996;77:1883–1892. doi: 10.1099/0022-1317-77-8-1883. [DOI] [PubMed] [Google Scholar]
- 44.Miller W E, Mosialos G, Kieff E, Raab-Traub N. Epstein-Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-κB activation. J Virol. 1997;71:586–594. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Miller W E, Cheshire J L, Raab-Traub N. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol Cell Biol. 1998;18:2835–2844. doi: 10.1128/mcb.18.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell T, Sugden B. Stimulation of NF-κB mediated transcription by mutant derivatives of the latent membrane protein of the Epstein-Barr virus. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 47.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware C F, Yagita H, Okumura K. TRAF5, an activator of NF-kappaB and putative signal transducer for the lymphotoxin-beta receptor. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 48.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a non cytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 49.Reinhard C, Shamoon B, Shyamala V, Williams L T. Tumor necrosis factor α-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997;16:1080–1092. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S, editors. Virology. 3rd ed. Philadelphia, Pa: Raven Press, Ltd.; 1996. pp. 2397–2446. [Google Scholar]
- 51.Rothe M, Sarma V, Dixit V M, Goeddel D V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 52.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP1’s association with TRAF1, TRAF2, and TRAF3. J Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shu H B, Takeuchi M, Goeddel D V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinases cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at the TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanArsdale T L, VanArsdale S L, Force W R, Walter B N, Mosialos G, Kieff E, Reed J C, Ware C F. Lymphotoxin-beta receptor signaling complex: role of tumor necrosis factor receptor-associated factor 3 recruitment in cell death and activation of nuclear factor kappaB. Proc Natl Acad Sci USA. 1997;94:2460–2465. doi: 10.1073/pnas.94.6.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voraberger G, Schafer R, Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991;147:2777–2786. [PubMed] [Google Scholar]
- 57.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 58.Wang D, Liebowitz D, Wang F, Gregory C, Rickinson A B, Larson R, Springer T, Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]