Abstract
This cross-sectional study investigated the association between glaucoma and B vitamin dietary intake. A total of 5025 enrolled individuals participated in self-reported glaucoma questionnaire and 3264 participated in International Society Geographical and Epidemiological Ophthalmology (ISGEO) criteria. In self-reported glaucoma, the risk of having self-reported glaucoma was lower in the third quartile of vitamin B1 intake (odds ratio [odds ratio [OR] 0.63, 95% confidence interval [CI] 0.40–0.97), and P trend (P trend = 0.004) for vitamin B12 was significant; in males, the third quartile of vitamin B1 intake (OR 0.44, 95% CI 0.24–0.83) and the fourth quartile of vitamin B2 intake (OR 0.39, 95% CI 0.17–0.89) were associated with a lower risk. In glaucoma based on ISGEO criteria, the increase of niacin intake (OR 0.94, 95% CI 0.89–0.99) was negatively associated with the odds of self-reported glaucoma. After sex-stratified analysis, the third quartile of vitamin B6 intake (OR 0.21, 95% CI 0.08–0.60) in males were associated with reduced odds of glaucoma. The restricted cubic spline analysis revealed a nonlinear association of vitamin B2 (p for nonlinearity = 0.04) and B9 (p for nonlinearity = 0.024) intake with glaucoma diagnosed by ISGEO criteria in females.
Keywords: Glaucoma, Vitamin B, Dietary intake, Cross-sectional study
Subject terms: Diseases, Risk factors
Introduction
The global prevalence of glaucoma, a serious irreversible eye-blinding disease, is about 3.5% in the population over 40 years of age. Primary open-angle glaucoma (POAG), a common type of glaucoma in European populations, has a global prevalence of about 3.1%1,2. With the aging of the population, the prevalence of glaucoma will increase significantly in the future, resulting in social, medical, and economic burdens3. However, the mechanisms underlying retinal ganglion cell (RGC) apoptosis in glaucoma remain unclear. Increased intraocular pressure (IOP) is a leading factor of glaucomatous visual impairment and the effective intervention point4,5.
Currently, most treatments are based on reducing IOP, which reduces damage to RGCs6. Oxidative stress is one of the mechanisms of RGC damage. Free radical species can interfere with the tricarboxylic acid cycle and mitochondrial metabolic pathways, leading to RGC death7,8. However, specific nutrients may act as antioxidants to improve and protect nerve cell function9–11. Niacin (vitamin B3), as a precursor of total nicotinamide adenine dinucleotide (NAD), can slow mitochondrial dysfunction and lessen optic nerve damage in glaucoma12,13. Serum homocysteine levels are elevated in patients with POAG and pseudoexfoliation glaucoma (PEXG)14,15. Serum vitamin B6, B9, and B12 levels are closely related to serum homocysteine concentrations, which can induce oxidative stress to accelerate the degeneration and apoptosis of RGCs16. Kang et al.17 explored whether increased intake of vitamins B6 and B12 and folic acid (vitamin B9) could reduce the risk of exfoliative glaucoma (EG) by reducing the oxidative stress effect of homocysteine, and reported that a higher total intake of folic acid was associated with a reduced EG risk. Oxidative stress markers are significantly increased in the aqueous humor in different glaucoma types18. B vitamins, as antioxidants, can protect the function of nerve cells by reducing oxidative stress in RGCs.
In this study, we investigated the potential impact of the daily intake of vitamins B1, B2, B6, B12, niacin, and folic acid on glaucoma using the National Health and Nutrition Examination Survey (NHANES), a large population-based study in the United States of America (US) with the aim to understand the association between vitamin B intake and glaucoma prevalence.
Materials and methods
Sample population
We used publicly available data from the 2005–2006 and 2007–2008 NHANES, which entailed conducting cross-sectional interviews and examinations of approximately 10,000 US non-institutionalized civilians in every cycle. The NHANES uses a stratified multi-stage sampling design and weighting scheme to accurately determine disease prevalence in the US population. All NHANES protocols were approved by the National Center for Health Statistics Ethics Review Board of the Centers for Disease Control (Protocol #2005-06, Continuation of Protocol #2005-06), and all survey participants provided written informed consent. The study followed the tenets of the Declaration of Helsinki.
We analyzed the public data from 2005 to 2008 in NHANES from 7081 participants aged over 40 years. The self-reported glaucoma exclusion criteria were results of at least one dietary interview for lacking B vitamins, no response to self-glaucoma interview and taking nutritional supplements; this led to the exclusion of 63931 and 1386 participants, respectively. Thus, 5025 eligible participants were included in the self-rated glaucoma primary outcome analysis. The secondary outcome was based on the International Society Geographical and Epidemiological Ophthalmology (ISGEO) criteria for glaucoma diagnosis based on retinal imaging and frequency doubling technology (FDT). Additional exclusion criteria based on self-rated glaucoma were incomplete, insufficient, or unreliable FDT analysis, and insufficient or unreliable retinal imaging. Accordingly, we excluded 1,560 and 201 participants, respectively. We included 3264 eligible participants in the secondary outcome analysis.
Assessment of glaucoma
The primary outcome was the severity of self-reported glaucoma. Responses were obtained using a visual questionnaire. When asked, “Have you ever been told by an ophthalmologist that you had glaucoma, sometimes called high pressure in eyes?”. participants who refused to answer or stated they did not know the answer, were excluded; 331 answered “yes.”
The secondary outcome variable was glaucoma diagnosis using the ISGEO criteria based on retinal imaging and FDT. Retinal imaging refers to technicians using a Canon Non-Mydriatic Retinal Camera CR6-45 NM to capture 45°-non-mydriatic digital retina images in a dark room. The participants were required to focus on the target for proper positioning. Two digital images per eye are captured at the same time, with the first image centered on the macula and the second image centered on the optic nerve. The images are first shipped to the University of Wisconsin for scoring. In 2012, ophthalmologists at Johns Hopkins University re-read retinal images with a cup-disc ratio (CDR) of 0.6 or higher to look for other indicators indicating the presence of glaucoma. The N-30-5 FDT screening protocol was designed to test visual field loss owing to eye diseases, especially glaucoma, using the Humphrey Matrix Visual Field Instrument. Each participant was tested twice, including 19 visual field locations in each eye. Each visual field location was tested until participants responded. A positive FDT result was obtained when at least two positions fell below a 1% threshold level in the first and second tests, and at least one failure position was the same in both tests (2-2-1 algorithm). Incomplete or unreliable test results were excluded from the study.
Combined with the results of the NHANES examination, we adopted classifications 1 and 2 of the ISGEO diagnostic criteria19,20: (1) positive FDT results in at least one eye with a cup-disc ratio (CDR) ≥ 97.5th percentile in the same eye or with a CDR asymmetry ≥ 97.5th percentile for the NHANES participants with normal visual function (normal visual field); and (2) CDR ≥ 99.5th percentile in either eye or CDR asymmetry between eyes ≥ 99.5th percentile for the NHANES participants with normal visual function (normal visual field), regardless of the FDT results. We diagnosed 108 participants with glaucoma based on the ISGEO criteria.
Assessment of dietary B vitamins
The main predictors were the daily intake of vitamins B1, B2, B6, B12, niacin, and folic acid derived from the NHANES Dietary Interview-Total Nutrient Intakes. This information was used to estimate the type and amount of food and drink consumed 24-h before the interview (midnight to midnight) and to calculate the intake of energy, nutrients, and other food components. All NHANES participants underwent two 24-h dietary recall interviews. The first dietary recall interview was conducted at the mobile examination center, and the second was conducted via telephone 3–10 days later. We used the average of the two 24-h dietary intakes as the final dietary intake data, and participant lacking the second 24-h dietary intake data used the first 24-h dietary intake as the final data.
Assessment of covariates
We included age, sex, race, educational level, smoke, total energy intake, caffeine intake, diabetes, and cataract surgery as covariates12,21,22. Vitamins B2 and B6 are closely related because the interconversion of some vitamin B6 species requires the vitamin B2 forms flavin mononucleotide (FMN) and flavin dinucleotide (FAD) as cofactors23. At the same time, vitamins B2 and B6 are necessary cofactors for converting tryptophan into niacin24. Folic acid and vitamin B12 coordinate and play an important role in the treatment of megaloblastic anemia (MA) and in improving the metabolism of the central nervous system25. Considering the interaction between B vitamins, additional covariates of different B vitamins are added: vitamin B2: add vitamin B6; niacin: add vitamin B2 and vitamin B6; vitamin B6: add vitamin B2; folic acid: add vitamin B12; vitamin B12: add folic acid.
Data analyses
Descriptive statistics were used to assess the baseline characteristics of the study population. Age, total energy intake, caffeine intake, and B vitamins intake were analyzed as continuous variables, whereas sex, race, educational level, household income, smoking status, diabetes status, and cataract surgery as categorical variables. The distributions of these variables between participants with and without self-reported glaucoma were compared using design-adjusted Rao–Scott (Pearson-type) χ2 and Wald tests for categorical and continuous variables, respectively. All data were weighted by NHANES to produce weighted estimates representing the US population.
Logistic regression modeling was used to examine the association between daily dietary vitamin B consumption and glaucoma with B vitamins as continuous and categorical variable in quartiles. The reference of quartiles was determined based on recommended daily allowances (RDAs), proposed by the National Institutes of Health, which was sufficient to meet the nutritional needs of 97–98% healthy individuals and was commonly used to plan for a nutritionally adequate diet for individuals in Supplement Table S126. Supplemental Table S1 provides the overall RDAs based on the male-to-female ratio of self-reported participants to facilitate the determination of the overall reference value. Trend analysis was performed by modeling the median within the quartiles as a continuous covariate. Crude model is an unadjusted model. Model I was adjusted for socio-demographic characteristics and some factors where p values were less than 0.05, including age, sex, race, and education level. Model II was adjusted comprehensively, including age, sex, race, educational level, smoke, total energy intake, caffeine intake, diabetes, cataract surgery, and interacted B vitamins. Additionally, we performed multivariable-adjusted restricted cubic splines (RCS) with 3 knots at the 10th, 50th, and 90th percentiles to examine potential non-linear associations between the dietary intake of B vitamins and glaucoma prevalence after controlling for all confounders. The test level was α = 0.05, and P < 0.05 was considered statistically significant. The analyses were performed using Stata 16.1 (Stata Corp LP, College Station, TX, USA) and R software version 4.2.2.
Results
The NHANES database included 5025 participants aged over 40 years, with reliable glaucoma questionnaire responses and B vitamin dietary interview results from 2005 to 2008. The female individuals comprised 49.19% (2472/5025) of the population. A total of 331 (6.59%) participants had self-reported glaucoma. The database included 3264 patients with reliable retinal imaging, FDT visual field results, and B vitamin dietary interviews to assess glaucoma characteristics based on the ISGEO criteria, including 108 participants with glaucoma (3.31%). The flowchart of participants selection is presented in Fig. 1. Table 1 presents the demographic characteristics of participants with and without self-reported glaucoma. Compared with participants without glaucoma, participants with self-reported glaucoma were older (P < 0.001), had a more distinct race-ethnic distribution (P = 0.004), lower levels of education (P = 0.006), higher rates of smoking (P < 0.001), diabetes (P < 0.001), cataract surgery (P < 0.001), higher intake levels of daily total energy (P < 0.001), caffeine intake (P = 0.008), and lower intake levels of B vitamins (all P < 0.01).
Figure 1.
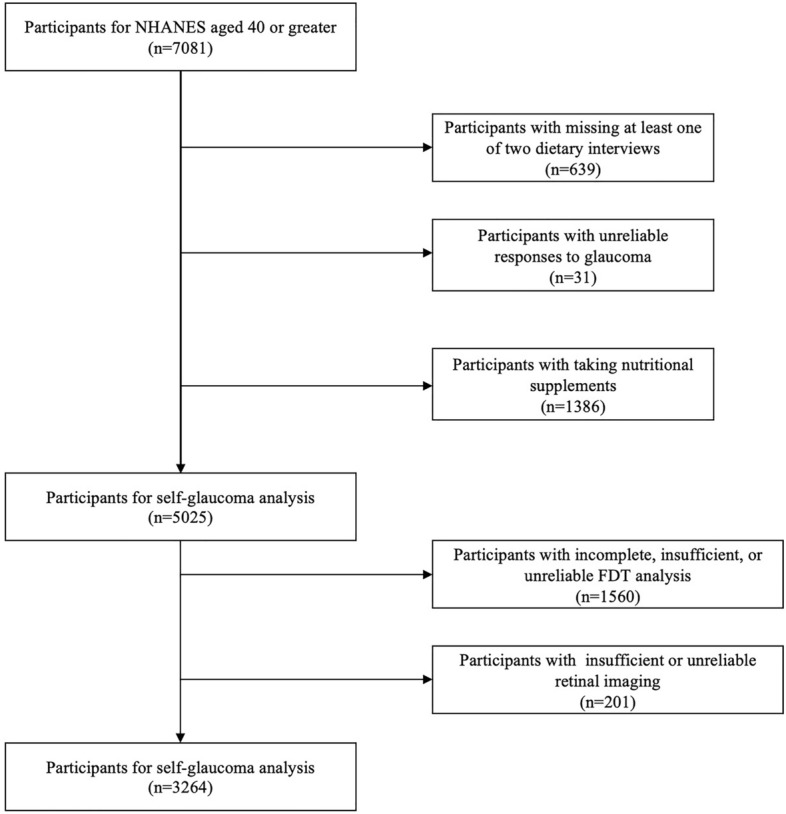
Flowchart of the study population.
Table 1.
Demographic and characteristics of participants with or without self-reported glaucoma.
| Characteristic | Self-reported glaucoma | P value* | |
|---|---|---|---|
| Yes (n = 331) | No (n = 4694) | ||
| Age (mean ± SD, years) | 66.21 ± 12.35 | 55.84 ± 11.91 | < 0.001** |
| Sex%(SE) | 0.761 | ||
| Male | 49.49 (3.53) | 48.51 (0.93) | |
| Female | 50.51 (3.53) | 51.49 (0.93) | |
| Race/ethnicity%(SE) | 0.004 | ||
| Mexican American | 4.63 (0.84) | 6.21 (0.25) | |
| Other Hispanic | 2.43 (0.54) | 3.36 (0.27) | |
| Non-hispanic white | 69.80 (2.79) | 74.59 (0.67) | |
| Non-hispanic black | 18.59 (2.01) | 10.77 (0.38) | |
| Other race | 4.56 (1.81) | 5.06 (0.44) | |
| Education level%(SE) | 0.006 | ||
| Less than 9th grade | 12.89 (1.92) | 8.08 (0.38) | |
| 9–11th grade | 12.86 (2.08) | 12.00 (0.53) | |
| High school grade | 26.36 (3.29) | 26.70 (0.83) | |
| Some college | 28.21 (3.32) | 28.01 (0.85) | |
| College graduate or above | 16.69 (2.78) | 25.22 (0.86) | |
| Annual household income%(SE) | 0.198 | ||
| < $35,000 | 52.90 (3.57) | 48.73 (0.93) | |
| ≥ $35,000 | 47.10 (3.57) | 51.27 (0.93) | |
| Smoking status%(SE) | < 0.001 | ||
| Never | 46.45 (3.52) | 47.78 (0.93) | |
| Former | 40.70 (3.50) | 29.21 (0.84) | |
| Current | 12.86 (2.38) | 23.02 (0.79) | |
| Diabetes%(SE) | < 0.001 | ||
| Yes | 23.74 (2.74) | 11.11 (0.52) | |
| No | 76.26 (2.74) | 88.89 (0.52) | |
| Cataract surgery%(SE) | < 0.001 | ||
| Yes | 31.49 (3.17) | 8.61 (0.44) | |
| No | 68.51 (3.17) | 91.39 (0.44) | |
| Alcohol intake%(SE) | 0.201 | ||
| Yes | 63.64 (3.28) | 68.79 (0.84) | |
| No | 31.14 (3.16) | 26.12 (0.79) | |
| Not recorded | 5.23 (1.26) | 5.09 (0.40) | |
| Vitamin B1 (mean ± SD, mg) | 1.41 ± 0.64 | 1.61 ± 0.80 | < 0.001** |
| Vitamin B2 (mean ± SD, mg) | 1.94 ± 0.82 | 2.23 ± 1.08 | < 0.001** |
| Niacin (mean ± SD, mg) | 20.66 ± 7.94 | 24.47 ± 11.67 | < 0.001** |
| Vitamin B6 (mean ± SD, mg) | 1.72 ± 0.83 | 1.97 ± 1.05 | < 0.001** |
| Folic acid (mean ± SD, mcg) | 344.14 ± 149.47 | 397.72 ± 202.47 | < 0.001** |
| Vitamin B12 (mean ± SD, mcg) | 5.35 ± 4.82 | 6.54 ± 7.16 | 0.009** |
| Daily total energy (mean ± SD, kcal) | 1739.39 ± 617.78 | 2041.77 ± 825.05 | < 0.001** |
| Caffeine intake (mean ± SD, mg) | 169.54 ± 228.13 | 207.61 ± 222.24 | 0.008** |
SE standard error
*Design-adjusted Rao-Scott Chi-squared test, **Adjusted Wald test.
The quartiles of the daily intake of B vitamins are presented in Table 2.
Table 2.
Quartile categories of vitamin B intake.
| Nutrient | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Overall | ||||
| Vitamin B1 (mg/day) | < 1.011 | ≥ 1.011 | ≥ 1.386 | ≥ 1.855 |
| Vitamin B2 (mg/day) | < 1.339 | ≥ 1.339 | ≥ 1.877 | ≥ 2.52 |
| Niacin (mg/day) | < 15.014 | ≥ 15.014 | ≥ 20.729 | ≥ 28.433 |
| Vitamin B6 (mg/day) | < 1.179 | ≥ 1.179 | ≥ 1.658 | ≥ 2.285 |
| Folic acid (mcg/day) | < 241.5 | ≥ 241.5 | ≥ 336 | ≥ 462.75 |
| Vitamin B12 (mcg/day) | < 2.515 | ≥ 2.515 | ≥ 4.31 | ≥ 7.308 |
| Male | ||||
| Vitamin B1 (mg/day) | < 1.159 | ≥ 1.159 | ≥ 1.601 | ≥ 2.097 |
| Vitamin B2 (mg/day) | < 1.509 | ≥ 1.509 | ≥ 2.129 | ≥ 2.834 |
| Niacin (mg/day) | < 17.615 | ≥ 17.615 | ≥ 24.343 | ≥ 32.605 |
| Vitamin B6 (mg/day) | < 1.369 | ≥ 1.369 | ≥ 1.914 | ≥ 2.669 |
| Folic acid (mcg/day) | < 270 | ≥ 270 | ≥ 375 | ≥ 514 |
| Vitamin B12 (mcg/day) | < 2.945 | ≥ 2.945 | ≥ 4.983 | ≥ 8.463 |
| Female | ||||
| Vitamin B1 (mg/day) | < 0.902 | ≥ 0.902 | ≥ 1.208 | ≥ 1.58 |
| Vitamin B2 (mg/day) | < 1.223 | ≥ 1.223 | ≥ 1.664 | ≥ 2.173 |
| Niacin (mg/day) | < 13.286 | ≥ 13.286 | ≥ 17.709 | ≥ 23.462 |
| Vitamin B6 (mg/day) | < 1.048 | ≥ 1.048 | ≥ 1.45 | ≥ 1.961 |
| Folic acid (mcg/day) | < 219.5 | ≥ 219.5 | ≥ 300 | ≥ 401 |
| Vitamin B12 (mcg/day) | < 2.25 | ≥ 2.25 | ≥ 3.665 | ≥ 6.195 |
We examined the association between daily B vitamins dietary intake and self-reported glaucoma prevalence (Table 3). Meanwhile, we conducted a further sex-stratification analysis (male: Table 4; female: Supplement Table S2). Overall analysis showed that vitamin B1 consumption at the third quartile (crude model: OR 0.55, 95% CI 0.36–0.82 P = 0.004; model I: OR 0.58, 95% CI 0.38–0.89, P = 0 0.013; model II: OR 0.63, 95% CI 0.40–0.97, P = 0.036) had significantly decreased odds of self-reported glaucoma compared with that at the second quartile in all models. Additionally, the odds of self-reported glaucoma showed a decreasing trend with higher quartiles of vitamin B12 intake (crude model: P trend < 0.001; model I: P trend < 0.001; model II: P trend = 0.004).
Table 3.
Association between daily dietary intake of B vitamins and self-reported glaucoma prevalence.
| Crude model OR (95% CI) |
P value | Model I OR (95% CI) |
P value | Model II OR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Vitamin B1 | ||||||
| Continuous | 0.67 (0.53, 0.84) | < 0.001 | 0.73 (0.56, 0.97) | 0.029 | 0.84 (0.61, 1.14) | 0.26 |
| Q1 | 1.03 (0.70, 1.51) | 0.872 | 1.10 (0.74, 1.65) | 0.64 | 1.09 (0.72, 1.66) | 0.69 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.55 (0.36, 0.82) | 0.004 | 0.58 (0.38, 0.89) | 0.013 | 0.63 (0.40, 0.97) | 0.036 |
| Q4 | 0.53 (0.35, 0.80) | 0.002 | 0.63 (0.40, 1.01) | 0.056 | 0.74 (0.45, 1.23) | 0.244 |
| p trend | < 0.001 | 0.011 | 0.122 | |||
| Vitamin B2 | ||||||
| Continuous | 0.73 (0.63, 0.85) | < 0.001 | 0.80 (0.67, 0.95) | 0.012 | 0.85 (0.69, 1.06) | 0.156 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.94 (0.64, 1.37) | 0.746 | 1.02 (0.68, 1.53) | 0.907 | 1.07 (0.71, 1.63) | 0.737 |
| Q3 | 0.68 (0.45, 1.02) | 0.065 | 0.73 (0.47, 1.14) | 0.165 | 0.81 (0.51, 1.28) | 0.369 |
| Q4 | 0.48 (0.31, 0.72) | < 0.001 | 0.59 (0.37, 0.96) | 0.033 | 0.72 (0.39, 1.32) | 0.289 |
| p trend | < 0.001 | 0.009 | 0.118 | |||
| Niacin | ||||||
| Continuous | 0.96 (0.95, 0.98) | < 0.001 | 0.98 (0.96, 0.99) | 0.007 | 0.99 (0.97, 1.02) | 0.521 |
| Q1 | 0.99 (0.68, 1.44) | 0.962 | 0.87 (0.59, 1.29) | 0.495 | 0.71 (0.47, 1.08) | 0.114 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.66 (0.44, 0.98) | 0.038 | 0.73 (0.48, 1.11) | 0.142 | 0.85 (0.54, 1.32) | 0.461 |
| Q4 | 0.48 (0.31, 0.74) | 0.001 | 0.65 (0.39, 1.07) | 0.088 | 1.04 (0.56, 1.92) | 0.899 |
| p trend | < 0.001 | 0.051 | 0.505 | |||
| Vitamin B6 | ||||||
| Continuous | 0.76 (0.65, 0.89) | 0.001 | 0.81 (0.67, 0.96) | 0.018 | 0.96 (0.79, 1.16) | 0.665 |
| Q1 | 1.17 (0.80, 1.72) | 0.42 | 1.10 (0.74, 1.65) | 0.635 | 1.03 (0.68, 1.58) | 0.884 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.76 (0.50, 1.16) | 0.204 | 0.77 (0.50, 1.20) | 0.25 | 0.87 (0.56, 1.34) | 0.518 |
| Q4 | 0.63 (0.42, 0.94) | 0.024 | 0.68 (0.44, 1.05) | 0.081 | 0.93 (0.59, 1.47) | 0.756 |
| p trend | 0.005 | 0.038 | 0.63 | |||
| Folic acid | ||||||
| Continuous | 1.00 (1.00, 1.00) | < 0.001 | 1.00 (1.00, 1.00) | 0.002 | 1.00 (1.00, 1.00) | 0.228 |
| Q1 | 1.45 (0.97, 2.15) | 0.067 | 1.37 (0.90, 2.08) | 0.145 | 1.21 (0.75, 1.97) | 0.431 |
| Q2 | 1.27 (0.85, 1.90) | 0.251 | 1.16 (0.76, 1.77) | 0.481 | 1.08 (0.70, 1.67) | 0.714 |
| Q3 | Ref | Ref | Ref | |||
| Q4 | 0.62 (0.40, 0.96) | 0.032 | 0.65 (0.42, 1.03) | 0.065 | 0.78 (0.50, 1.21) | 0.264 |
| p trend | 0.001 | 0.01 | 0.155 | |||
| Vitamin B12 | ||||||
| Continuous | 0.96 (0.93, 0.99) | 0.025 | 0.96 (0.93, 1.00) | 0.036 | 0.98 (0.95, 1.01) | 0.238 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 1.00 (0.67, 1.51) | 0.987 | 1.07 (0.70, 1.63) | 0.753 | 1.14 (0.74, 1.75) | 0.559 |
| Q3 | 0.89 (0.60, 1.32) | 0.569 | 0.88 (0.58, 1.33) | 0.532 | 1.00 (0.65, 1.54) | 0.994 |
| Q4 | 0.55 (0.36, 0.84) | 0.006 | 0.54 (0.34, 0.85) | 0.008 | 0.70 (0.42, 1.16) | 0.162 |
| p trend | < 0.001 | < 0.001 | 0.004 | |||
Model I adjusted for age, sex, race and educational level. Model II adjusted for age, sex, race, educational level, smoking, diabetes, cataract surgery, daily total energy, caffeine intake and interacted vitamin b.
Table 4.
Association between daily dietary intake of B vitamins and self-reported glaucoma prevalence in males.
| Crude model OR (95% CI) |
P value | Model I OR (95% CI) |
P value | Model II OR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Vitamin B1 | ||||||
| Continuous | 0.47 (0.32, 0.69) | < 0.001 | 0.55 (0.36, 0.83) | 0.005 | 0.68 (0.43, 1.08) | 0.101 |
| Q1 | 0.99 (0.58, 1.67) | 0.961 | 1.09 (0.62, 1.91) | 0.773 | 1.00 (0.56, 1.78) | 0.992 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.33 (0.18, 0.61) | < 0.001 | 0.39 (0.21, 0.71) | 0.002 | 0.44 (0.24, 0.83) | 0.012 |
| Q4 | 0.30 (0.16, 0.57) | < 0.001 | 0.42 (0.22, 0.82) | 0.011 | 0.56 (0.27, 1.13) | 0.105 |
| p trend | < 0.001 | < 0.001 | 0.023 | |||
| Vitamin B2 | ||||||
| Continuous | 0.61 (0.48, 0.78) | < 0.001 | 0.66 (0.51, 0.86) | 0.002 | 0.72 (0.53, 0.97) | 0.029 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.72 (0.42, 1.25) | 0.245 | 0.67 (0.37, 1.20) | 0.177 | 0.75 (0.41, 1.41) | 0.376 |
| Q3 | 0.65 (0.37, 1.17) | 0.151 | 0.68 (0.36, 1.27) | 0.223 | 0.85 (0.41, 1.74) | 0.655 |
| Q4 | 0.25 (0.13, 0.47) | < 0.001 | 0.29 (0.14, 0.59) | 0.001 | 0.39 (0.17, 0.89) | 0.025 |
| p trend | < 0.001 | 0.002 | 0.051 | |||
| Niacin | ||||||
| Continuous | 0.94 (0.92, 0.96) | < 0.001 | 0.96 (0.94, 0.98) | < 0.001 | 0.98 (0.94, 1.02) | 0.275 |
| Q1 | Ref | Ref | Ref | |||
| Q 2 | 0.65 (0.38, 1.12) | 0.119 | 0.75 (0.42, 1.36) | 0.35 | 1.03 (0.54, 1.96) | 0.94 |
| Q3 | 0.35 (0.19, 0.62) | < 0.001 | 0.48 (0.25, 0.92) | 0.026 | 0.89 (0.42, 1.87) | 0.753 |
| Q4 | 0.28 (0.15, 0.53) | < 0.001 | 0.47 (0.23, 0.96) | 0.037 | 1.36 (0.47, 3.90) | 0.569 |
| p trend | < 0.001 | 0.024 | 0.617 | |||
| Vitamin B6 | ||||||
| Continuous | 0.62 (0.48, 0.79) | < 0.001 | 0.69 (0.53, 0.89) | 0.004 | 0.96 (0.75, 1.23) | 0.733 |
| Q1 | 1.29 (0.75, 2.21) | 0.354 | 1.11 (0.64, 1.94) | 0.706 | 0.84 (0.48, 1.47) | 0.537 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.48 (0.26, 0.89) | 0.019 | 0.48 (0.26, 0.91) | 0.024 | 0.55 (0.29, 1.06) | 0.075 |
| Q4 | 0.44 (0.24, 0.81) | 0.008 | 0.49 (0.26, 0.93) | 0.028 | 0.89 (0.46, 1.74) | 0.737 |
| p trend | < 0.001 | 0.005 | 0.656 | |||
| Folic acid | ||||||
| Continuous | 1.00 (1.00, 1.00) | < 0.001 | 1.00 (1.00, 1.00) | 0.001 | 1.00 (1.00, 1.00) | 0.296 |
| Q1 | 1.78 (1.01, 3.12) | 0.045 | 1.57 (0.85, 2.88) | 0.147 | 1.05 (0.51, 2.16) | 0.904 |
| Q2 | 0.97 (0.53, 1.75) | 0.91 | 0.85 (0.47, 1.54) | 0.589 | 0.64 (0.34, 1.21) | 0.166 |
| Q3 | Ref | Ref | Ref | |||
| Q4 | 0.57 (0.30, 1.09) | 0.09 | 0.63 (0.33, 1.19) | 0.155 | 0.80 (0.43, 1.47) | 0.467 |
| p trend | 0.007 | 0.027 | 0.678 | |||
| Vitamin B12 | ||||||
| Continuous | 0.93 (0.87, 1.00) | 0.036 | 0.94 (0.88, 1.00) | 0.061 | 0.97 (0.92, 1.02) | 0.296 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 1.01 (0.58, 1.76) | 0.965 | 1.09 (0.62, 1.91) | 0.771 | 1.22 (0.68, 2.18) | 0.508 |
| Q3 | 0.58 (0.32, 1.05) | 0.072 | 0.59 (0.32, 1.10) | 0.094 | 0.76 (0.41, 1.43) | 0.399 |
| Q4 | 0.31 (0.15, 0.62) | 0.001 | 0.33 (0.16, 0.69) | 0.002 | 0.48 (0.22, 1.06) | 0.069 |
| p trend | 0.002 | 0.002 | 0.093 | |||
Model I adjusted for age, race and educational level. Model II adjusted for age, race, educational level, smoking, diabetes, cataract surgery, daily total energy, caffeine intake and interacted vitamin b.
In males, there was a significant negative association with the odds of self-reported glaucoma observed in the third quartile (crude model: OR 0.33, 95% CI 0.18–0.61 P < 0.001; model I: OR 0.39, 95% CI 0.21–0.71, P = 0 0.002; model II: OR 0.44, 95% CI 0.24–0.83, P = 0.012) quartile of vitamin B1 compared with that in the second quartile in the fully adjusted model. Trend analysis showed the adjusted odds of glaucoma were reduced with higher vitamin B1 consumption (crude model: P trend < 0.001; model I: P trend < 0.001; model II: P trend = 0.023).We also found vitamin B2 intake as a continuous variable, with a 28% reduction in glaucoma risk for every 1 mg increase (crude model: OR 0.61, 95% CI 0.48–0.78 P < 0.001; model I: OR 0.66, 95% CI 0.51–0.86, P = 0 0.002; model II: OR 0.72, 95% CI 0.53–0.97, P = 0.029). Furthermore, high quartile vitamin B2 intake was significantly associated with the risk of glaucoma (crude model: OR 0.25, 95% CI 0.13–0.47 P < 0.001; model I: OR 0.29, 95% CI 0.14–0.59, P = 0.001; model II: OR 0.39, 95% CI 0.17–0.89, P = 0.025). No association was observed between the daily consumption of other B vitamins and the odds of glaucoma prevalence. Moreover, we did not find an association between intake B vitamins and self- reported glaucoma in females.
We examined the association between the daily dietary intake of B vitamins and glaucoma diagnosis using the ISGEO criteria (Table 5). Additionally, we conducted a sex-stratification analysis (male: Table 6; female: Supplement Table S3). Overall analysis suggested that niacin analyzed as a continuous variable was associated with lower odds of glaucoma in all models (crude model: OR 0.97, 95% CI 0.94–0.99, P = 0.011; model I: OR 0.97, 95% CI 0.95–1.00, P = 0.048; model II: OR 0.94, 95% CI 0.89–0.99, P = 0.031).
Table 5.
Association between daily B vitamins and glaucoma diagnosed by ISGEO criteria.
| Crude model OR (95% CI) |
P value | Model I OR (95% CI) |
P value | Model II OR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Vitamin B1 | ||||||
| Continuous | 0.86 (0.59, 1.25) | 0.425 | 0.91 (0.59, 1.39) | 0.665 | 0.94 (0.55, 1.60) | 0.822 |
| Q1 | 0.48 (0.24, 0.98) | 0.044 | 0.50 (0.24, 1.05) | 0.066 | 0.50 (0.24, 1.05) | 0.069 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.48 (0.24, 0.98) | 0.043 | 0.51 (0.25, 1.04) | 0.064 | 0.51 (0.24, 1.08) | 0.074 |
| Q4 | 0.46 (0.23, 0.93) | 0.03 | 0.50 (0.24, 1.05) | 0.068 | 0.50 (0.23, 1.08) | 0.077 |
| p trend | 0.189 | 0.198 | 0.419 | |||
| Vitamin B2 | ||||||
| Continuous | 0.83 (0.63, 1.09) | 0.183 | 0.89 (0.67, 1.18) | 0.417 | 1.07 (0.74, 1.53) | 0.717 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.86 (0.43, 1.75) | 0.685 | 0.87 (0.41, 1.84) | 0.711 | 0.85 (0.39, 1.84) | 0.676 |
| Q3 | 0.81 (0.38, 1.70) | 0.571 | 0.84 (0.39, 1.81) | 0.652 | 0.92 (0.39, 2.20) | 0.857 |
| Q4 | 0.55 (0.25, 1.20) | 0.133 | 0.61 (0.27, 1.40) | 0.243 | 0.73 (0.25, 2.17) | 0.571 |
| p trend | 0.161 | 0.168 | 0.665 | |||
| Niacin | ||||||
| Continuous | 0.97 (0.94, 0.99) | 0.011 | 0.97 (0.95, 1.00) | 0.048 | 0.94 (0.89, 0.99) | 0.031 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.82 (0.43, 1.59) | 0.561 | 0.79 (0.40, 1.53) | 0.483 | 0.86 (0.42, 1.76) | 0.688 |
| Q3 | 0.53 (0.26, 1.10) | 0.089 | 0.52 (0.25, 1.06) | 0.074 | 0.48 (0.22, 1.02) | 0.057 |
| Q4 | 0.45 (0.21, 0.95) | 0.036 | 0.49 (0.22, 1.06) | 0.07 | 0.36 (0.11, 1.21) | 0.099 |
| p trend | 0.034 | 0.07 | 0.165 | |||
| Vitamin B6 | ||||||
| Continuous | 0.92 (0.69, 1.22) | 0.57 | 0.94 (0.67, 1.32) | 0.73 | 0.91 (0.61, 1.37) | 0.664 |
| Q1 | 0.79 (0.40, 1.58) | 0.508 | 0.81 (0.40, 1.64) | 0.56 | 0.84 (0.41, 1.72) | 0.63 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.50 (0.23, 1.11) | 0.088 | 0.51 (0.22, 1.16) | 0.106 | 0.49 (0.21, 1.14) | 0.097 |
| Q4 | 0.71 (0.35, 1.43) | 0.331 | 0.73 (0.34, 1.58) | 0.429 | 0.69 (0.28, 1.69) | 0.417 |
| p trend | 0.364 | 0.344 | 0.374 | |||
| Folic acid | ||||||
| Continuous | 1.00 (1.00, 1.00) | 0.344 | 1.00 (1.00, 1.00) | 0.431 | 1.00 (1.00, 1.00) | 0.408 |
| Q1 | 0.87 (0.41, 1.83) | 0.709 | 0.87 (0.40, 1.85) | 0.704 | 0.81 (0.35, 1.92) | 0.637 |
| Q2 | 1.14 (0.56, 2.33) | 0.709 | 1.08 (0.53, 2.21) | 0.825 | 1.09 (0.53, 2.25) | 0.814 |
| Q3 | Ref | Ref | Ref | |||
| Q4 | 0.90 (0.43, 1.87) | 0.78 | 0.92 (0.42, 1.98) | 0.823 | 0.98 (0.44, 2.15) | 0.955 |
| p trend | 0.841 | 0.751 | 0.834 | |||
| Vitamin B12 | ||||||
| Continuous | 1.00 (0.97, 1.03) | 0.93 | 1.00 (0.97, 1.03) | 0.99 | 1.00 (0.98, 1.03) | 0.643 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.97 (0.46, 2.04) | 0.931 | 1.05 (0.48, 2.29) | 0.906 | 1.04 (0.45, 2.39) | 0.922 |
| Q3 | 1.03 (0.49, 2.16) | 0.929 | 1.07 (0.49, 2.33) | 0.87 | 1.17 (0.47, 2.87) | 0.74 |
| Q4 | 0.99 (0.50, 1.99) | 0.987 | 1.02 (0.47, 2.19) | 0.961 | 1.24 (0.45, 3.43) | 0.679 |
| p trend | 0.928 | 0.782 | 0.581 | |||
Model I adjusted for age, sex, race and educational level. Model II adjusted for age, sex, race, educational level, smoking, diabetes, cataract surgery, daily total energy, caffeine intake and interacted vitamin b.
Table 6.
Association between daily B vitamins and glaucoma diagnosed by ISGEO criteria in males.
| Crude model OR (95% CI) |
P value | Model I OR (95% CI) |
P value | Model II OR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Vitamin B1 | ||||||
| Continuous | 0.71 (0.45, 1.13) | 0.145 | 0.81 (0.49, 1.32) | 0.399 | 0.68 (0.39, 1.20) | 0.182 |
| Q1 | 1.35 (0.54, 3.39) | 0.525 | 1.42 (0.54, 3.74) | 0.475 | 1.51 (0.55, 4.14) | 0.419 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.60 (0.19, 1.89) | 0.386 | 0.67 (0.22, 2.11) | 0.497 | 0.58 (0.19, 1.81) | 0.35 |
| Q4 | 0.75 (0.29, 1.92) | 0.547 | 1.00 (0.35, 2.80) | 0.996 | 0.84 (0.29, 2.45) | 0.75 |
| p trend | 0.11 | 0.247 | 0.193 | |||
| Vitamin B2 | ||||||
| Continuous | 0.88 (0.65, 1.20) | 0.433 | 0.99 (0.72, 1.35) | 0.948 | 1.23 (0.82, 1.83) | 0.318 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 1.29 (0.53, 3.10) | 0.574 | 1.23 (0.53, 2.90) | 0.628 | 1.27 (0.55, 2.95) | 0.58 |
| Q3 | 0.83 (0.30, 2.33) | 0.727 | 0.85 (0.31, 2.33) | 0.757 | 0.94 (0.26, 3.41) | 0.924 |
| Q4 | 0.72 (0.30, 1.72) | 0.463 | 0.91 (0.37, 2.21) | 0.828 | 1.13 (0.39, 3.30) | 0.822 |
| p trend | 0.267 | 0.408 | 0.873 | |||
| Niacin | ||||||
| Continuous | 0.96 (0.93, 0.99) | 0.016 | 0.98 (0.94, 1.01) | 0.183 | 0.93 (0.86, 1.01) | 0.085 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.57 (0.21, 1.53) | 0.268 | 0.62 (0.24, 1.58) | 0.317 | 0.53 (0.19, 1.51) | 0.235 |
| Q3 | 0.66 (0.28, 1.58) | 0.353 | 0.81 (0.32, 2.00) | 0.64 | 0.62 (0.21, 1.89) | 0.405 |
| Q4 | 0.35 (0.13, 0.98) | 0.047 | 0.55 (0.20, 1.49) | 0.237 | 0.36 (0.04, 2.94) | 0.337 |
| p trend | 0.05 | 0.295 | 0.457 | |||
| Vitamin B6 | ||||||
| Continuous | 0.89 (0.58, 1.36) | 0.593 | 0.97 (0.62, 1.52) | 0.893 | 0.84 (0.48, 1.47) | 0.534 |
| Q1 | 1.17 (0.45, 3.04) | 0.748 | 1.11 (0.43, 2.86) | 0.83 | 1.27 (0.45, 3.55) | 0.651 |
| Q2 | Ref | Ref | Ref | |||
| Q3 | 0.27 (0.10, 0.72) | 0.009 | 0.26 (0.09, 0.76) | 0.014 | 0.21 (0.08, 0.60) | 0.004 |
| Q4 | 0.69 (0.26, 1.79) | 0.44 | 0.76 (0.27, 2.18) | 0.611 | 0.47 (0.14, 1.59) | 0.225 |
| p trend | 0.111 | 0.216 | 0.036 | |||
| Folic acid | ||||||
| Continuous | 1.00 (1.00, 1.00) | 0.254 | 1.00 (1.00, 1.00) | 0.445 | 1.00 (1.00, 1.00) | 0.324 |
| Q1 | 0.88 (0.31, 2.47) | 0.81 | 0.80 (0.28, 2.27) | 0.68 | 0.84 (0.25, 2.81) | 0.78 |
| Q2 | 1.13 (0.44, 2.93) | 0.796 | 1.04 (0.41, 2.63) | 0.929 | 1.02 (0.40, 2.56) | 0.969 |
| Q3 | Ref | Ref | Ref | |||
| Q4 | 0.68 (0.24, 1.94) | 0.472 | 0.70 (0.23, 2.15) | 0.537 | 0.66 (0.19, 2.26) | 0.512 |
| p trend | 0.264 | 0.288 | 0.249 | |||
| Vitamin B12 | ||||||
| Continuous | 0.98 (0.93, 1.04) | 0.517 | 0.99 (0.94, 1.04) | 0.588 | 0.99 (0.95, 1.04) | 0.826 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.91 (0.34, 2.40) | 0.848 | 0.94 (0.35, 2.49) | 0.897 | 0.86 (0.30, 2.51) | 0.787 |
| Q3 | 0.49 (0.17, 1.41) | 0.183 | 0.50 (0.17, 1.52) | 0.224 | 0.46 (0.12, 1.72) | 0.247 |
| Q4 | 0.62 (0.23, 1.65) | 0.337 | 0.65 (0.24, 1.80) | 0.409 | 0.65 (0.17, 2.54) | 0.534 |
| p trend | 0.227 | 0.213 | 0.282 | |||
Model I adjusted for age, race and educational level. Model II adjusted for age, race, educational level, smoking, diabetes, cataract surgery, daily total energy, caffeine intake and interacted vitamin b.
In males, vitamin B6 intake at the third quartile (crude model: OR 0.27, 95% CI 0.10–0.72, P = 0.009; model I: OR 0.26 95% CI 0.09–0.76, P = 0.014; model II: OR 0.21, 95% CI 0.08–0.60, P = 0.031) quartile had significantly decreased odds of glaucoma compared with that at the second quartile in all models. We observed no association between other B vitamins and the odds of glaucoma. Sex-stratified analysis indicated no significant association between vitamin B and the odds of glaucoma. After trend analysis, we discovered a significant P trend value for vitamin B6 in model II (model II: P trend = 0.036). Sex-stratified analysis indicated no significant association between vitamin B and the odds of glaucoma in females.
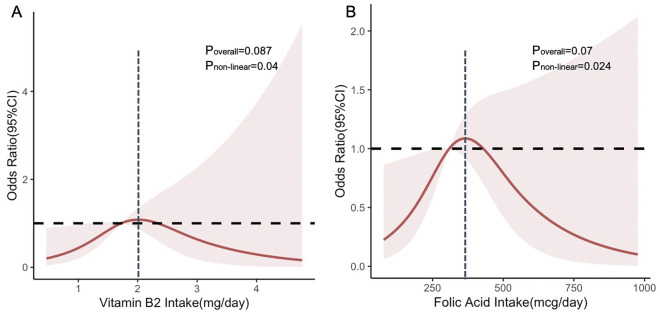
Restricted cubic spline regression revealed a nonlinear association (Fig. 2) between vitamin B2, B9 intake and glaucoma diagnosed by ISGEO criteria in females after controlling for all confounders. Within the higher range of vitamin B2 and folic acid intake, there was a slight decrease in glaucoma prevalence. The highest risk was reached at approximately 2.021 mg/day (vitamin B2) and 366.133 mcg/day (folic acid), and then gradually decreased (vitamin B2: P for nonlinearity = 0.040; folic acid: P for nonlinearity = 0.024). However, no nonlinear relationship was observed between the intake of other B vitamins and glaucoma (Supplement Fig. S1–S6).
Figure 2.
Restricted cubic spline regression of the association between B vitamin intake and the odds ratio of glaucoma based on ISGEO criteria in females after controlling for age, race, educational level, smoking, diabetes, cataract surgery, daily total energy, caffeine intake and interacted vitamin b. A Vitamin B1; B Folic acid. ISGEO International Society Geographical and Epidemiological Ophthalmology.
Discussion
This was a study on glaucoma based on self-report and ISGEO criteria associated with the dietary intake of B vitamins in a population of Americans aged over 40 years. In logistic regression analysis, reference values of quartiles were determined based on RDAs. This analysis allowed us to compare the risk of glaucoma in people who consume different levels of B vitamins and that in people who consume the RDAs. The overall analysis revealed that daily consumption of vitamin B1 was related to glaucoma prevalence as diagnosed by self-report. Moreover, there was a significant P trend for vitamin B12 intake. After sex-stratified analysis, the association between vitamin B1 and self-reported glaucoma persisted in males, and we also found that high vitamin B2 intake was associated with a reduced risk of self-reported glaucoma. Further research found that the daily intake of niacin was associated with glaucoma prevalence according to the overall analysis based on ISGEO criteria. Furthermore, higher intake of vitamin B6 was associated with a reduced risk of glaucoma based on ISGEO criteria in males. Restricted cubic spline regression revealed a nonlinear association between vitamin B2, folic acid intake, and glaucoma based on ISGEO criteria in females after controlling for all variables.
Vitamins B1 and B2 can function as coenzymes in amino acid metabolism, cell division and growth, DNA synthesis, and repair in human cells10. In addition, vitamin B1 is involved in glucose metabolism and neurotransmitter synthesis, and has an antioxidant effect on nerve cells27. Sulbutiamine, a synthetic derivative of vitamin B1, exerts protective effects on RGCs28. Vitamin B2 is effective in migraine treatment, which is often a predisposing factor for glaucoma attack10,28. Our study found that when vitamin B1 intake was in the third (1.386–1.855 mg/day) quartile, compared to that in the second quartile, which is the range of RDAs, the odds of self-reported glaucoma decreased. When all the adjustments were made, the connection did not disappear. The Rotterdam study reported that medium and high doses of vitamin B1 had the strongest protective effect on POAG, which supported our conclusion29. According to a Korean study on the association between nutrient intake and POAG, a low intake of vitamins B1 and B2 may be associated with an increased likelihood of glaucoma30. In our study, the association between the intake of vitamins B1, B2 and self-reported glaucoma appeared to be stronger in males. In females, vitamin B2 intake was nonlinearly associated with ISGEO-diagnosed glaucoma, and glaucoma risk decreased with higher intake.
Several studies have reported an association between serum homocysteine levels and normal-tension glaucoma, POAG, and PEXG31,32. Folic acid and vitamins B6 and B12 can help improve homocysteine metabolism, thereby reducing vascular endothelial function injury, ganglion cell apoptosis, extracellular matrix alterations, lysyl oxidase activity, and oxidative stress33. Our findings showed a significant P trend for vitamin B12 intake in self-reported glaucoma analysis. This result implied that the risk of self-reported glaucoma gradually decreases with vitamin B12 intake from Q1 to Q4. Our findings suggested that in males, higher vitamin B6 intake has a negative relationship with the odds of glaucoma based on ISGEO criteria at the third quartile (1.914–2.669 mg/day) compared to that in the second quartile (the range where the RDAs are located). In addition, Giaconi et al.34 and Coleman et al.35 did not observe a correlation between folic acid intake and POAG in older women, which was similar to our conclusion from the logistic regression analysis. However, in further analysis of females, we found a non-linear relationship between folate intake and glaucoma based on ISGEO criteria.
Niacin, vitamin B3, has been confirmed to have neuroprotective effects in cell and animal experiments, can increase mitochondrial size and dynamics and provide effectiveness for neuroprotective therapy for glaucoma36. A study based on NHANES in South Korea, which analyzed 24-h recalled dietary data and identified glaucoma by ophthalmic examination, found that high levels of dietary niacin were associated with a reduced risk of glaucoma in people over 40 years of age. The results of Taechameekietichai et al.12 and Lee et al.13 support the association between niacin intake and glaucoma. Our findings found that every 1 mg increase in dietary niacin intake was associated with a 6 percent reduction in the risk of glaucoma diagnosed by ISGEO criteria. However, after sex- stratification analysis, the relationship was not significant.
This study conducted a sex-stratified analysis of the association between vitamin B and glaucoma and found differences between the two sexes. We observed an association between higher intakes of vitamin B1, B2 and the risk of glaucoma on self-reported criteria in males. In glaucoma diagnosed based on ISGEO criteria, dietary vitamin B6 intake was associated with the risk of glaucoma in males, while vitamin B2 and folic acid were non-linearly associated with the risk of glaucoma in females. This suggested that sex hormones play a role in metabolism of B vitamins and might influence glaucoma risk37. Our study could not prove a sex-specific effect of B vitamins on glaucoma; hence, more research would be needed to explore the mechanisms behind phenomenon.
The strength of our study design is the reliable data sample obtained from the US population between 2005 and 2008, which could be adjusted for potential confounding factors, rendering it representative and persuasive. Additionally, our reference values were used as RDAs proposed by the National Institutes of Health, which had certain clinical significance. However, our study also had some limitations. NHANES is a cross-sectional study, although, we did not observe valid evidence of a causal relationship between B vitamins and glaucoma prevalence. Moreover, the possibility of dietary changes owing to glaucoma onset cannot be ruled out. The main outcome variable in this study was self-reported glaucoma, and glaucoma diagnosis was based solely on participant questionnaire responses. This method lacked rigorous ophthalmic examinations and could have resulted in information bias during data analysis. However, some studies have reported high consistency between self-reported glaucoma and participant medical records in glaucoma diagnosis38,39. Furthermore, our secondary outcome variable was glaucoma based on categories 1 and 2 of the ISGEO criteria. The ISGEO standard included fundus vertical CDR assessment and FDT examinations, which partially reduced information bias and misclassification bias incidence, despite the absence of IOP values. Optic neuropathy predates visual field changes in fundus imaging in glaucoma40. Therefore, the ISGEO criteria may not apply to patients with mild glaucoma. In addition, the prevalence of self-reported glaucoma was 6.59%, and that of ISGEO standard diagnosis was 3.31%. The former was likely to include a significant proportion of false positive cases, the latter might be closer to the current prevalence of glaucoma1,2,41. Daily intake levels of B vitamins calculated based on nutrient sources from two 24-h dietary interviews, may have been influenced by recall and information bias. In addition, bioavailability varies among individuals. Therefore, serum vitamin levels should be investigated, and prospective cohort studies should be designed to provide more objective evidence.
Based on this large cross-sectional study, we concluded that on the self-reported criteria, vitamin B1 and B12 intake was associated with the odds of glaucoma; while in males, higher intake of vitamin B1 and B2 had negative relationship with glaucoma risk. On the ISGEO criteria, the risk of glaucoma decreased with the increase in niacin intake; while in males, there was a significant association between vitamin B6 intake and glaucoma; in females, dietary intake of vitamin B2 and folic acid had obviously nonlinear relationship with the odds of glaucoma.
Supplementary Information
Acknowledgements
We appreciate the extraordinary work of the National Health and Nutrition Examination Survey (NHANES).
Author contributions
J.H. designed the study and contributed to writing this article. L.T. and Z.J. revised this article. Y.W. and S.G. collected and analyzed data. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Data availability
Data can be available from the database website https://www.cdc.gov/nchs/nhanes.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-58526-5.
References
- 1.Jayaram H, Kolko M, Friedman DS, Gazzard G. Glaucoma: Now and beyond. Lancet. 2023;402(10414):1788–1801. doi: 10.1016/S0140-6736(23)01289-8. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, et al. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 3.Kang JM, Tanna AP. Glaucoma. Med. Clin. N. Am. 2021;105(3):493–510. doi: 10.1016/j.mcna.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S, Low-Pressure Glaucoma Study Group A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 2011;151(4):671–681. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N. Engl. J. Med. 2009;360(11):1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jutley G, Luk SM, Dehabadi MH, Cordeiro MF. Management of glaucoma as a neurodegenerative disease. Neurodegener. Dis. Manag. 2017;7(2):157–172. doi: 10.2217/nmt-2017-0004. [DOI] [PubMed] [Google Scholar]
- 7.Pinazo-Durán MD, et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging. 2014;9:637–652. doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013;13(1):12–15. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Jung KI, Kim YC, Park CK. Dietary niacin and open-angle glaucoma: The Korean national health and nutrition examination survey. Nutrients. 2018;10(4):387. doi: 10.3390/nu10040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramdas WD, Schouten JSAG, Webers CAB. The effect of vitamins on glaucoma: A systematic review and meta-analysis. Nutrients. 2018;10(3):359. doi: 10.3390/nu10030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutolo MG, Albanese G, Rusciano D, Pescosolido N. Oral administration of forskolin, homotaurine, carnosine, and folic acid in patients with primary open angle glaucoma: Changes in intraocular pressure, pattern electroretinogram amplitude, and foveal sensitivity. J. Ocul. Pharmacol. Therap. 2016;32(3):178–183. doi: 10.1089/jop.2015.0121. [DOI] [PubMed] [Google Scholar]
- 12.Taechameekietichai T, Chansangpetch S, Peerawaranun P, Lin SC. Association between daily niacin intake and glaucoma: National health and nutrition examination survey. Nutrients. 2021;13(12):4263. doi: 10.3390/nu13124263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SY, et al. Associations between niacin intake and glaucoma in the national health and nutrition examination survey. J. Glaucoma. 2023;32(6):443–450. doi: 10.1097/IJG.0000000000002216. [DOI] [PubMed] [Google Scholar]
- 14.Xu F, Zhang L, Li M. Plasma homocysteine, serum folic acid, serum vitamin B12, serum vitamin B6, MTHFR and risk of pseudoexfoliation glaucoma: A meta-analysis. Graefe's Arch. Clin. Exp. Ophthalmol. 2012;250(7):1067–1074. doi: 10.1007/s00417-011-1877-4. [DOI] [PubMed] [Google Scholar]
- 15.Ghanem AA, Mady SM, Elawady HE, Arafa LF. Homocysteine and hydroxyproline levels in patients with primary open-angle glaucoma. Curr. Eye Res. 2012;37(8):712–718. doi: 10.3109/02713683.2012.669512. [DOI] [PubMed] [Google Scholar]
- 16.Roedl JB, et al. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J. Neural Transm. 2007;114(5):571–575. doi: 10.1007/s00702-006-0598-z. [DOI] [PubMed] [Google Scholar]
- 17.Kang JH, Loomis SJ, Wiggs JL, Willett WC, Pasquale LR. A prospective study of folate, vitamin B6, and vitamin B12 intake in relation to exfoliation glaucoma or suspected exfoliation glaucoma. JAMA Ophthalmol. 2014;132(5):549–559. doi: 10.1001/jamaophthalmol.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benoist d'Azy C, Pereira B, Chiambaretta F, Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: A systematic review and meta-analysis. PLoS ONE. 2016;11(12):e0166915. doi: 10.1371/journal.pone.0166915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am. J. Ophthalmol. 2014;158(6):1121–1129. doi: 10.1016/j.ajo.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, et al. Relationship between high dose intake of vitamin B12 and glaucoma: Evidence from NHANES 2005–2008 among United States adults. Front. Nutr. 2023;10:1130032. doi: 10.3389/fnut.2023.1130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, et al. Habitual coffee consumption increases risk of primary open-angle glaucoma: A Mendelian randomization study. Ophthalmology. 2022;129(9):1014–1021. doi: 10.1016/j.ophtha.2022.04.027. [DOI] [PubMed] [Google Scholar]
- 23.McCormick DB. Two interconnected B vitamins: Riboflavin and pyridoxine. Physiol. Rev. 1989;69(4):1170–1198. doi: 10.1152/physrev.1989.69.4.1170. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health. Niacin. Webpage in NIH. Accessed November 26, 2022. https://ods.od.nih.gov/factsheets/list-all (2020).
- 25.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5(11):949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health. Webpage in NIH. 2020. Accessed November 26. https://ods.od.nih.gov/factsheets/list-all (2022).
- 27.Calderón-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Therap. 2020;26(1):5–13. doi: 10.1111/cns.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang KD, et al. Sulbutiamine counteracts trophic factor deprivation induced apoptotic cell death in transformed retinal ganglion cells. Neurochem. Res. 2010;35(11):1828–1839. doi: 10.1007/s11064-010-0249-5. [DOI] [PubMed] [Google Scholar]
- 29.Ramdas WD, et al. Nutrient intake and risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Epidemiol. 2012;27(5):385–393. doi: 10.1007/s10654-012-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JY, et al. Relationships between obesity, nutrient supply and primary open angle glaucoma in Koreans. Nutrients. 2020;12(3):878. doi: 10.3390/nu12030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roedl JB, et al. Increased homocysteine levels in tear fluid of patients with primary open-angle glaucoma. Ophthalmic Res. 2008;40(5):249–256. doi: 10.1159/000127832. [DOI] [PubMed] [Google Scholar]
- 32.Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am. J. Ophthalmol. 2003;136(1):41–46. doi: 10.1016/S0002-9394(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 33.Ajith TA, Ranimenon Homocysteine in ocular diseases. Clin. Chim. Acta Int. J. Clin. Chem. 2015;450:316–321. doi: 10.1016/j.cca.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Giaconi JA, et al. The association of consumption of fruits/vegetables with decreased risk of glaucoma among older African-American women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2012;154(4):635–644. doi: 10.1016/j.ajo.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman AL, et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2008;145(6):1081–1089. doi: 10.1016/j.ajo.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Tribble JR, et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 2021;43:101988. doi: 10.1016/j.redox.2021.101988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: What we know and what we need to know. Curr. Opin. Ophthalmol. 2010;21(2):91–99. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popovic M, et al. Discrepancies in physician-patient agreement in reporting ocular history. Can. J. Ophthalmol. 2016;51(5):378–381. doi: 10.1016/j.jcjo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 39.MacLennan PA, McGwin G, Jr, Searcey K, Owsley C. Medical record validation of self-reported eye diseases and eye care utilization among older adults. Curr. Eye Res. 2013;38(1):1–8. doi: 10.3109/02713683.2012.733054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucy KA, Wollstein G. Structural and functional evaluations for the early detection of glaucoma. Expert Rev. Ophthalmol. 2016;11(5):367–376. doi: 10.1080/17469899.2016.1229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tham YC, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be available from the database website https://www.cdc.gov/nchs/nhanes.