Abstract
Purpose:
To study dry eye medication use and expenditures from 2001 to 2006 using a nationally representative sample of US adults.
Methods:
This study retrospectively analyzed dry eye medication use and expenditures of participants of the 2001 to 2006 Medical Expenditure Panel Survey, a nationally representative subsample of the National Health Interview Survey. After adjusting for survey design and for inflation using the 2009 inflation index, data from 147 unique participants aged 18 years or older using the prescription medications Restasis and Blephamide were analyzed. The main outcome measures were dry eye medication use and expenditures from 2001 to 2006.
Results:
Dry eye medication use and expenditures increased between the years 2001 and 2006, with the mean expenditure per patient per year being $55 in 2001 to 2002 (n = 29), $137 in 2003 to 2004 (n = 32), and $299 in 2005 to 2006 (n = 86). This finding was strongly driven by the introduction of topical cyclosporine emulsion 0.05% (Restasis; Allergan, Irvine, CA). In analysis pooled over all survey years, demographic factors associated with dry eye medication expenditures included gender (female: $244 vs. male: $122, P < 0.0001), ethnicity (non-Hispanic: $228 vs. Hispanic: $106, P < 0.0001), and education (greater than high school: $250 vs. less than high school: $100, P < 0.0001).
Conclusions:
We found a pattern of increasing dry eye medication use and expenditures from 2001 to 2006. Predictors of higher dry eye medication expenditures included female gender, non-Hispanic ethnicity, and greater than a high school education.
Keywords: dry eye syndrome, Medical Expenditure Panel Survey, MEPS, expenditures
Dry eye syndrome (DES) has recently gained recognition as a public health problem.1–3 In the decade between 1970 and 1980, 670 articles were published on DES (search terminology dry eye syndrome, limits humans, and English); this increased to 1485 articles in the 1980s, 2511 articles in the 1990s, and 4887 articles in the last decade. Part of this recognition came from several US population–based and international population–based studies demonstrating that the condition was present in between 5% and 30% of the population aged 50 years or older.1,2,6–17 Another part of the recognition came from understanding that the symptoms of DES, which include constant irritation, foreign body sensation, and blurred vision, interfere with the ability to work and carry out daily functions.18–20 A study using the Impact of Dry Eye Living Questionnaire found that severe dry eye symptoms were correlated with difficulties in physical, social, and mental functioning.21 Such difficulties translate into a relatively lower health-related quality of life compared with the general population—patients with severe dry eye symptoms have health-related quality of life scores in the range of conditions like class III/IV angina.20
An additional event that helped push DES into the limelight was the release of the first Food and Drug Administration–approved prescription medication for DES, cyclosporine emulsion 0.05% (Restasis; Allergan, Irvine, CA). The Food and Drug Administration approved the medication in 2002, and the pharmaceutical company Allergan launched cyclosporine emulsion in the United States in late 2003. As part of its sales strategy, Allergan used direct to consumer marketing and commissioned magazine and television advertisements to reach its target audience; it also heavily promoted cyclosporine emulsion within the eye care community. These activities had the effect of increasing physician and patient awareness of the prevalence of DES, its morbidity, and its potential treatments.
Although there is a sense that the economic implications of DES are substantial, few articles have studied the direct costs associated with DES and other ocular surface disorders. These include costs associated with office visits, prescription medication, over-the-counter medication, alternative or complementary medication, and nonpharmacologic purchases (eg, humidifiers). A retrospective claims analysis evaluating costs in 9065 patients who received topical cyclosporine for DES found a mean health care cost of $336 per patient with a total cost of $3.05 million.22 A retrospective analysis of the annual cost of DES in patients treated by an ophthalmologist in 6 European countries estimated a total annual healthcare cost between 0.27 and 1.10 million US dollars per country. However, this cost did not take into consideration patients who self-treated their condition or were treated by their primary care physician.23
The Medical Expenditure Panel Survey (MEPS) is an annual survey of families and individuals, their medical providers, and employers across the United States. MEPS, which is designed to be representative of the US population, provides the most complete source of data on the cost and use of health care and health insurance coverage.24 Given that prescription cost information is available through the MEPS data set, we examined recent patterns in dry eye medication expenditures. We aimed to confirm our hypothesis that a substantial increase in expenditures has occurred over the past few years, perhaps in response to the increased public and provider awareness of the condition along with the availability of a new prescription medication.
MATERIALS AND METHODS
Sample
The MEPS is a nationally representative subsample of the National Health Interview Survey, a continuous multipurpose and multistage area probability survey of the US civilian noninstitutionalized population living at addressed dwellings. To have an adequate number of persons in important population subgroups, the MEPS oversampled Blacks and Hispanics in all years and began oversampling of Asians in 2002.25 The overall MEPS response rate ranged from 66% in 2001 to 58% in 2006. Sampling weights were applied to ensure that the resulting sample was nationally representative of US households and includes adjustment for oversampling of race/ethnic groups and survey nonresponse.
To obtain dry eye medication expenditures, a comprehensive list of available prescription medications, including name brands, generics, and chemical names, for the study period was first generated and used to identify those MEPS participants who used any medication via the MEPS Prescribed Medicines files. The Prescribed Medicines files contained comprehensive information on medications used by MEPS participants.25 From this list, 2 medications used in the setting of DES were identified: cyclosporine emulsion 0.05%, used to treat aqueous tear deficiency, and sulfacetamide sodium–prednisolone acetate ophthalmic suspension, USP 10%/0.2% (Blephamide), used to treat lipid tear deficiency (blepharitis), among other conditions.
Data from MEPS 2007 were available but were not included in this analysis because the methodology in editing the pharmacy data was changed. Comparison of prescription drug spending before and after 2007 was therefore not recommended by the Agency for Healthcare Research and Quality.26 MEPS initially had an over-the-counter medication section that collected details about nonprescription medication purchases; however, this section was omitted from the questionnaire beginning in 2002.27 Because we were interested in dry eye medication costs in the years since the launch of cyclosporine emulsion, we were unable to include over-the-counter medications in our analysis. For the study period, 147 unique participants aged 18 years or older were found to have used sulfacetamide sodium–prednisolone acetate ophthalmic suspension and/or cyclosporine emulsion and were included in the analysis. Expenditure of these medications for each participant over 2-year intervals was analyzed. The data were adjusted for survey design, and the expenditure was adjusted for inflation using 2009 inflation index.
Demographic Data
Demographic and insurance information of the qualified participants was obtained from the MEPS Full-Year Consolidated Data Files. Demographic data collected included gender, age, race (white, black, other/multiple), ethnicity (Hispanic, non-Hispanic), health insurance status (private, public only, and uninsured), and education level (less than high school, high school, greater than high school). Family income, measured as a percentage, was calculated by dividing total family income by the applicable poverty line (based on family size and composition). The resulting percentages were grouped into 3 categories: low income/poverty (less than 200%), middle income (200% to less than 400%), and high income (400% or more).
Statistical Analyses
All statistical analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC) and SUDAAN 10 (RTI International, Triangle, NC) statistical packages. To account for complex survey design of the MEPS data, analyses were completed with adjustments for sample weights and design effects. We conducted descriptive analyses to evaluate patterns in dry eye medication expenses per person over a 2-year interval. T tests were performed to compare average medication expenditure across different demographic groups. A multivariate linear regression was performed to study demographic variables that predict high dry eye medication expense. The University of Miami Institutional Review Board reviewed and approved this study, which was conducted in accordance with the principles of the Declaration of Helsinki.
RESULTS
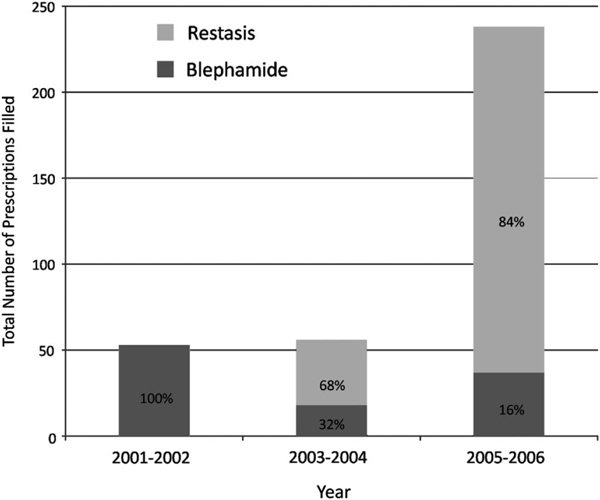
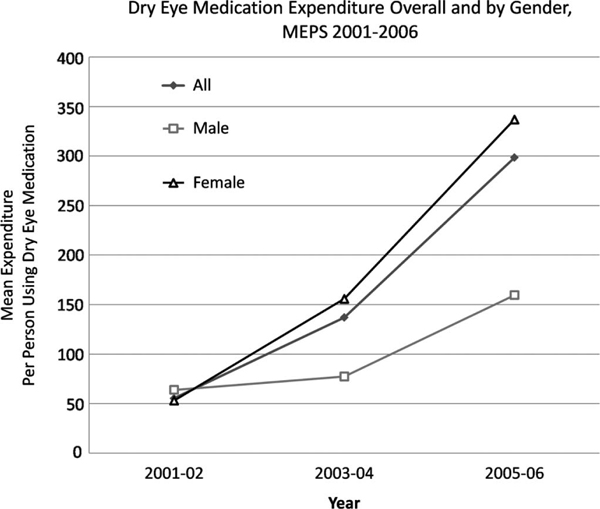
More patients used prescription dry eye medications in 2005 to 2006 (n = 86) compared with the previous 4 years (n = 29 and 32 for 2001–2002 and 2003–2004, respectively), and the total number of prescriptions filled increased with each year (Fig. 1). The cost associated with dry eye prescription medications also increased between 2001 and 2006, with a mean expenditure per patient of $55 in 2001 to 2002, $137 in 2003 to 2004, and $299 in 2005 to 2006 (Fig. 2). The introduction of topical cyclosporine significantly affected both the number of prescriptions filled and the dry eye expenditures because after its introduction, 68% of prescriptions and 80% of expenditures were related to cyclosporine emulsion in 2003 to 2004 and 84% of prescriptions and 92% of expenditures were related to cyclosporine emulsion in 2005 to 2006. The mean cost of sulfacetamide sodium–prednisolone acetate ophthalmic suspension increased from $36.27 in 2001 to 2002 to $54.56 in 2003 to 2004 to $64.43 in 2005 to 2006. Likewise, the mean cost of cyclosporine emulsion increased from $98.98 in 2003 to 2004 to $113.06 in 2005 to 2006. The increase in mean dry eye expenditures over the period, therefore, can be explained by both increased medication usage and cost.
FIGURE 1.
Graphic representation of the total number of dry eye prescriptions filled using the MEPS database, 2001 to 2006.
FIGURE 2.
Graphic representation of mean dry eye medication expenditures per patient (overall and by gender) using the MEPS database, 2001 to 2006.
Several demographic factors were associated with medication expenditures in the treatment of dry eye. Gender had a significant effect, with mean spending for women being double that for men ($244 vs. $122, P < 0.0001) (Table 1, Fig. 2). Similarly, spending for non-Hispanics was double that for the Hispanic population ($228 vs. $106, P < 0.0001). Level of education was also an important factor, with individuals with more than a high school education spending more than those with less than a high school education ($250 vs. $100, P < 0.0001). Race, age, and income status were not found to significantly affect dry eye medication expenditures in our analysis.
TABLE 1.
Mean and Standard Error Cost (in Dollars) Per Prescription of Dry Eye Medications by Demographic Factors, 2001 to 2006 MEPS Data
| Characteristics | N | Mean | SE | P |
|---|---|---|---|---|
|
| ||||
| All | 147 | 217.31 | 23.41 | –– |
| Sex | ||||
| Male | 34 | 122.24 | 6.87 | — |
| Female | 113 | 244.30 | 24.35 | <0.0001 |
| Race | ||||
| White | 134 | 220.51 | 20.63 | White vs. Black = 0.07 |
| Black | 8 | 141.94 | 27.39 | White vs. Other = 0.95 |
| Other | 5 | 214.18 | 95.84 | Black vs. Other = 0.47 |
| Ethnicity | ||||
| Hispanic | 20 | 106.23 | 18.89 | — |
| Non-Hispanic | 127 | 227.99 | 20.78 | <0.0001 |
| Age group, yr | ||||
| 18–44 | 25 | 192.51 | 34.40 | 18–44 vs. 45–64 = 0.78 |
| 45–64 | 53 | 206.44 | 27.06 | 18–44 vs. 65+ = 0.38 |
| 65+ | 69 | 235.88 | 34.50 | 45–64 vs. 65+ = 0.51 |
| Insurance type | ||||
| Private insurance | 111 | 225.06 | 23.01 | Private vs. public = 0.57 |
| Public insurance only | 29 | 194.26 | 45.82 | Private vs. uninsured = 0.02* |
| Uninsured | 7 | 166.56 | 7.84 | Public vs. uninsured = 0.56* |
| Education | ||||
| Less than HS | 27 | 100.18 | 15.82 | <HS vs. HS = 0.05 |
| HS | 43 | 204.54 | 46.43 | <HS vs. >HS = <0.0001 |
| Greater than HS | 77 | 250.52 | 21.78 | HS vs. >HS = 0.36 |
| Poverty | ||||
| Low income/poverty | 33 | 219.62 | 37.10 | Low vs. middle = 0.14 |
| Middle income | 40 | 168.49 | 25.46 | Low vs. high = 0.64 |
| High income | 74 | 240.57 | 38.41 | Middle vs. high = 0.06 |
Bold values represent factors significantly associated with increased dry eye expenditures.
Statistical analyses for the uninsured group are reported but are considered unstable due to small sample size. HS, high school; SE, standard error.
In a multivariable linear regression analysis considering all demographic factors, gender and education remained significant predictors of dry eye medication expenditures. Female gender was associated with a $159 higher mean expenditure compared with male gender (P = 0.0004). Greater than high school education was associated with a $145 higher mean expenditure compared with less than a high school education (P = 0.0016). Although not significant in our univariable analysis, with adjustment for all other covariates, those in the 65 and older age group spent $107 more on dry eye medications than those in the 45- to 64-year-old group (P = 0.04).
DISCUSSION
In this nationally representative study of patterns in prescription dry eye medication expenditures from 2001 to 2006, we found that the number of patients treated with prescription dry eye medications and their associated expenditures increased between these years. This finding was strongly driven by the introduction of cyclosporine emulsion in 2003. Considering demographic factors, female gender, non-Hispanic ethnicity, and a greater than high school education were factors significantly associated with a higher mean yearly expenditure for DES in our univariate models.
Although studies have suggested that the economic implications of DES are substantial,28 limited data are available to support this statement. Fiscella et al22 analyzed claims data from a proprietary research database containing pharmacy claims data on over 13 million individuals. They identified 9065 subjects that had one or more prescriptions filled for topical cyclosporine emulsion between January 1, 2004, and December 31, 2005. The mean yearly prescription cost by the health insurance plans was $336, and the mean out-of-pocket prescription cost for the patient was $98. This compares favorably with our findings because the cost analysis above includes both patient and insurance expenditures combined.
Putting these numbers in the context of other chronic ocular and nonocular diseases, a recent MEPS study found that patients with glaucoma spent a mean of $556 per year on prescription glaucoma medications in 2006 (adjusted for inflation using 2009 inflation index).29 Similarly, another article using the MEPS database found that people with spine problems spent a mean of $397 per year on prescription medications in 2006.30 The findings in this study suggest that although DES is not a blinding condition, individuals are willing to spend a nontrivial amount of money per year to alleviate the discomfort associated with this disorder. It is also important to note that the expenditures presented in this study do not incorporate the costs of nonprescription medications and doctor’s visits and therefore the total amount of money spent on the disease is likely to be significantly higher.
We found that several demographic factors affected the expenditures of dry eye medications, including gender, ethnicity, and education. The presence of gender and ethnic disparities in medical expenditures has been described in other conditions, including mental health31 and hypertension management.32 An association between higher expenditures and higher education levels has been reported in systemic lupus erythematosus.33 Although the etiologies behind these discrepancies are not clear, it is important to recognize the role of demographic factors when considering the myriad determinants of health.
As with all retrospective studies, the study findings must be considered bearing in mind its limitations. One limitation is that information on nonprescription medications was not available in the MEPS database, and we could therefore only estimate costs associated with prescription dry eye medications. As many more patients use over-the-counter medications to treat DES, we failed to include patients with less severe forms of the disease in our analysis. Furthermore, because of changes within MEPS that started in 2007,26 medication information for this year was not included in the analysis. Another limitation is that the sample size in the present analysis was relatively small, limiting our ability to examine trends in dry eye medication expenditures and in our comparisons in subgroups of interest (eg, the uninsured). Because of the relatively small sample size, it should not be assumed that our analytic sample of dry eye medication users are nationally representative despite the fact that they were obtained from a population-based survey. However, if present patterns continue, there will be a growing number of persons in the MEPS who will use these medications, facilitating future subgroup analyses. Furthermore, both cyclosporine emulsion and sulfacetamide sodium–prednisolone acetate ophthalmic suspension can be used to treat ocular surface disorders other than DES. Because we did not have diagnosis information linked to medication use, it is possible that we included patients treated for ocular surface conditions other than DES in our analysis. Finally, we acknowledge that other medications are used to treat subtypes of DES, including corticosteroids and tetracycline derivates; we chose not to include these in our analysis, given their multiple indications for use. Despite these limitations, there is no other ongoing population-based studies that look specifically at drug medication cost patterns; therefore, the analysis of the MEPS provides us with the best expenditure estimates for newly introduced ocular medications.
In summary, we found a pattern of increased dry eye medication use and expenditure from 2001 to 2006. Women, non-Hispanics, and those with greater than a high school education had higher expenditures compared with their counterparts. Additional research is necessary to understand the underlying reasons for the difference in dry eye medication expenditures by patient characteristics.
Acknowledgments
Supported by a grant from the National Eye Institute (1R21EY019096) and an unrestricted grant from the Research to Prevent Blindness.
Footnotes
The authors state that they have no proprietary interest in the products named in this article.
REFERENCES
- 1.The epidemiology of dry eye disease: report of the epidemiology subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:93–107. [DOI] [PubMed] [Google Scholar]
- 2.Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001;45(suppl 2):S199–S202. [DOI] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol. 2002;506(pt B):989–998. [DOI] [PubMed] [Google Scholar]
- 4.Begley CG, Chalmers RL, Mitchell GL, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20:610–618. [DOI] [PubMed] [Google Scholar]
- 5.Schein OD, Muñoz B, Tielsch JM, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. [DOI] [PubMed] [Google Scholar]
- 6.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268. [DOI] [PubMed] [Google Scholar]
- 7.Bandeen-Roche K, Munoz B, Tielsch JM, et al. Self-reported assessment of dry eye in a population-based setting. Invest Ophthalmol Vis Sci. 1997; 38:2469–2475. [PubMed] [Google Scholar]
- 8.Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–825. [DOI] [PubMed] [Google Scholar]
- 9.McCarty CA, Bansal AK, Livingston PM, et al. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 10.Chia EM, Mitchell P, Rochtchina E, et al. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2003;31:229–232. [DOI] [PubMed] [Google Scholar]
- 11.Hikichi T, Yoshida A, Fukui Y, et al. Prevalence of dry eye in Japanese eye centers. Graefes Arch Clin Exp Ophthalmol. 1995;233:555–558. [DOI] [PubMed] [Google Scholar]
- 12.Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115:1982–1988. [DOI] [PubMed] [Google Scholar]
- 13.Uchino M, Dogru M, Uchino Y, et al. Japan Ministry of Health study onprevalence of dry eye disease among Japanese high school students. Am J Ophthalmol. 2008;146:925–929 e2. [DOI] [PubMed] [Google Scholar]
- 14.Shimmura S, Shimazaki J, Tsubota K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea. 1999;18:408–411. [DOI] [PubMed] [Google Scholar]
- 15.Sahai A, Malik P. Dry eye: prevalence and attributable risk factors in a hospital-based population. Indian J Ophthalmol. 2005;53:87–91. [DOI] [PubMed] [Google Scholar]
- 16.Lekhanont K, Rojanaporn D, Chuck RS, et al. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25:1162–1167. [DOI] [PubMed] [Google Scholar]
- 17.Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86:1347–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46:46–50. [DOI] [PubMed] [Google Scholar]
- 19.Miljanovic B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005; 8:168–174. [DOI] [PubMed] [Google Scholar]
- 22.Fiscella RG, Lee JT, Walt JG, et al. Utilization characteristics of topical cycolsporine and punctal plugs in a managed care database. Am J Manag Care. 2008;14:S107–S112. [PubMed] [Google Scholar]
- 23.Clegg JP, Guest JF, Lehman A, et al. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13:263–274. [DOI] [PubMed] [Google Scholar]
- 24.MEPS Home. Available at: http://www.meps.ahrq.gov/mepsweb/. Accessed June 16, 2011.
- 25.Data overview, Agency for Healthcare Research and Quality. Available at: http://www.meps.ahrq.gov/mepsweb/data_stats/data_overview.jsp. Accessed June 16, 2011.
- 26.MEPS HC-113: 2007 Full Year Consolidated Data File. Available at: MEPS HC-113: 2007 Full Year Consolidated Data File. Available at: http://www.meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h113/h113doc.shtml. Accessed November 30, 2011.
- 27.Summary of questionnaire sections. Panel 6 Round 3 and Panel 7 Round 1. Available at: http://www.meps.ahrq.gov/mepsweb/survey_comp/hc_ques_sections.jsp. Accessed June 16, 2011.
- 28.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14:S102–S106. [PubMed] [Google Scholar]
- 29.Lam BL, Zheng DD, Davila EP, et al. Trends in glaucoma medication expenditure: medical expenditure panel survey 2001–2006. Arch Ophthalmol. 2011;129:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin BI, Turner JA, Mirza SK, et al. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997–2006. Spine (Phila Pa 1976). 2009;34:2077–2084. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Rizzo J. Racial and ethnic disparities in use of psychotherapy: evidence from U.S. national survey data. Psychiatr Serv. 2010;61:364–372. [DOI] [PubMed] [Google Scholar]
- 32.Basu R, Franzini L, Krueger PM, et al. Gender disparities in medical expenditures attributable to hypertension in the United States. Womens Health Issues. 2010;20:114–125. [DOI] [PubMed] [Google Scholar]
- 33.Sutcliffe N, Clarke AE, Taylor R, et al. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2001;40:37–47. [DOI] [PubMed] [Google Scholar]