Abstract
Metabolic syndrome (MS) is a multifaceted pathological condition characterized by the atypical accumulation of various metabolic components such as central obesity or excess weight, hyperlipidemia, low-density lipoprotein (LDL), hypertension, and insulin resistance. Recently, MS has been recognized as a notable contributor to heart and circulatory diseases. In addition, with increasing research, the impact of MS on tendon repair and disease has gradually emerged. Recent studies have investigated the relationship between tendon healing and diseases such as diabetes, dyslipidemia, obesity, and other metabolic disorders. However, diabetes mellitus (DM), hypercholesterolemia, obesity, and various metabolic disorders often coexist and together constitute MS. At present, insulin resistance is considered the major pathological mechanism underlying MS, central obesity is regarded as the predominant factor responsible for it, and dyslipidemia and other metabolic diseases are known as secondary contributors to MS. This review aims to evaluate the current literature regarding the impact of various pathological conditions in MS on tendon recovery and illness, and to present a comprehensive overview of the effects of MS on tendon recovery and diseases, along with the accompanying molecular mechanisms.
Keywords: metabolic syndrome, tendon, diabetes mellitus, hyperlipidemia, obesity, hypertension
Introduction
Metabolic syndrome (MS) is a complex multifactorial condition associated with hypertension, hyperglycemia, and gout that was first identified by Kylin in the 1920s.1 Following almost 30 years of research, Vague discovered a correlation between upper body obesity and metabolic disorders associated with cardiovascular disease and diabetes.2 Subsequently, Reaven introduced the term “X syndrome” in his 1988 Banting, underscoring the syndrome’s clinical significance.3 Over the following 10 years, Kaplan decided to rename the term “fatal quartet”,4 and other academics coined the phrase “insulin resistance syndrome”.5,6 MS is caused by several factors—including lifestyle choices, environmental factors, and genetics.7 The immune and metabolic systems maintain a dynamic balance and depend on one another to function. Throughout human evolution, our bodies have formed common pathways linking immune and metabolic responses. MS is comprised of endothelial dysfunction, hypertension, obesity, atherosclerosis, dyslipidemia, diabetes mellitus (DM), and other conditions. Its development is intricate and not yet fully understood, although obesity and insulin resistance represent significant risk factors.8–12 For instance, it is widely recognized that individuals with MS have a considerably higher rate of cardiovascular disease.13–16 Furthermore, metabolic disorders and skeletal muscle diseases have strong associations with MS. This disease may stimulate the progression of osteoarthritis (OA), exacerbate the condition, and raise the possibility of fractures.17–19 MS impacts not only bones and cartilage but also the proper maintenance of balance within the internal bodily environment and the restoration of damaged tendons.20 Further exploration of MS is warranted, owing to the currently limited understanding of the various tendon-related diseases caused by it and its associated complications. This review seeks to enhance public understanding of internal homeostasis and the mechanisms governing the repair of tendon damage in patients with MS. Theoretical insights from this study will hopefully inform future targeted interventions for tendon-related diseases in patients with MS.
Current Definition of MS
Owing to the rising prevalence of MS, researchers worldwide have increasingly acknowledged that it constitutes a set of metabolism-related disorders rather than a distinct ailment, which can make it highly detrimental to afflicted individuals. Therefore, formulating a clinically pragmatic definition of MS is critical. As a result, scholars worldwide have been striving to devise ways to further enhance and standardize the definition of MS. New insights and suggestions are constantly being provided through extensive epidemiological surveys and clinical studies. Consequently, a number of scientific groups have proposed different perspectives.
Differences in the definitions for MS have arisen across various scientific groups and research organizations. In 1998, the World Health Organization (WHO) proposed diagnostic criteria for MS that centered around insulin resistance (IR).21 These criteria included impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), DM, and IR (which was defined as a glucose utilization rate, as determined by the high insulin glucose clamp technique, lower than the lower quartile). Two of the following conditions also needed to be present: high blood pressure; hypertriglyceridemia; and low levels of high-density lipoprotein (HDL), central adiposity, and microalbuminuria. The WHO definition links key components, such as IR, obesity, and hypertension. The key criterion for this definition is IR. If all of the other criteria are met but IR is not present, MS cannot be diagnosed. This definition relies on the high-insulin-positive glucose clamp technique to confirm insulin resistance. However, in a clinical setting, quick and simple assessments are preferable, and the high-insulin-positive glucose clamp technique is not commonly used. Consequently, this definition has not been widely adopted in clinical practice.
In 1999, the European Group for the Study of Insulin Resistance (EGIR) recommended modifying the WHO’s definition of MS and proposed its own definition. It required the fulfillment of at least two of the following prerequisites: IR, augmented central obesity, heightened triglyceride levels, diminished HDL cholesterol, hypertensive conditions, or elevated fasting plasma glucose levels.22 The EGIR definition is essentially the same as the WHO’s, using IR as the core requirement; however, it replaces the waist-hip ratio with waist measurement alone and removes microproteinuria as a diagnostic criterion.
In 2001, the National Cholesterol Education Program Adult Treatment Panel III (NCEP: ATP III) established its own definition of MS. Its criteria include the presence of central obesity, elevated triglyceride levels, reduced HDL cholesterol, hypertension, and increased fasting blood glucose levels.23 For a patient to be classified as having MS, three of these conditions must be met. NCEP: ATP III linked central obesity, IR-based hypertension, and core elements of atherogenic dyslipidemia. This approach enabled researchers to efficiently use clinically feasible laboratory tests and study MS from both a clinical and epidemiological perspective. Importantly, NCEP: ATP III does not require any single mandatory criterion to be met, but only requires three of the five conditions for diagnosis. Consequently, it is frequently used as a definitional criterion in many clinical settings.
In 2003, the American Association of Clinical Endocrinology (AACE) developed its definition for MS that included three of: obesity, elevated plasma triglycerides, low high-density lipoproteinemia, heightened glucose levels following glucose stimulation, and additional risk factors.24 Notably, the AACE’s definition incorporates high-risk contributors such as a familial predisposition to cardiovascular disease, the presence of polycystic ovary syndrome (PCOS), a sedentary behavior pattern, and advanced age—thus providing a more holistic conceptualization of MS.
In 2005, the International Diabetes Federation (IDF) promoted the first globally harmonized definition of MS within the international academic community, by combining existing diagnostic criteria.25 Its core criterion was central obesity, with waist circumference and triglyceride levels serving as diagnostic indicators of central obesity. It was based on the presence of any two of the following indicators: high triglyceride and low HDL levels, increased blood pressure, and elevated fasting blood glucose levels. Central obesity is a compulsory criterion in the IDF definition that sets diverse standards for distinct nationalities, regions, and ethnic groups. It is important to note that this definition does not emphasize IR, but rather prioritizes fasting plasma glucose concentrations.
Overall, MS is a collection of metabolism-linked disorders that remains in need of a consistent definition and an all-encompassing treatment plan to ensure patient welfare. Researchers worldwide are dedicated to enhancing and perfecting the definition of MS, as well as exploring more efficient treatment approaches.
Effect of the Components of MS on Tendon Disease
Effect of Obesity on Tendon Disease
Obesity presents an increasing challenge in modern society. Economic progress and improved living standards have significantly altered global dietary patterns, increasing preferences for high-calorie and high-fat foods. Meanwhile, sedentary lifestyles and insufficient physical exercise exacerbate obesity. The IDF has stated that obesity is a significant contributor to MS worldwide, and its prevalence is rapidly increasing.25 Obesity is a complex illness linked to several metabolic irregularities. Excess energy is stored as fat when the body consumes more calories than it burns, resulting in weight gain. For a considerable time, there has been a popular belief that obesity is solely a cosmetic issue. However, it significantly endangers human health. This condition frequently coexists with other metabolic abnormalities, including IR and fat accumulation. As a result, it is one of the primary triggers of MS—which is characterized by a complex pathogenesis.
Research has found that patients with obesity who have body mass indexes (BMIs) of > 30 have a higher incidence of postoperative re-tearing following rotator cuff surgery than normal-weight patients, for both small incision and arthroscopic procedures.26 Furthermore, obesity reduces patient mobility and decreases Constant-Murley, as well as disabilities of the arm, shoulder, and hand (DASH) scores. One possible explanation for this phenomenon is that excess weight causes the upper arm to bear additional mechanical stress on the rotator cuff tendons. Most rotator cuff tears occur in the tendons responsible for smaller loads, such as the supraspinatus tendon, which have only limited exposure to external forces. The impact of elevated mechanical stress generated by obesity on postoperative outcomes following rotator cuff surgery is controversial.27 One study found that the hospitalization period and duration of arthroscopic surgery for rotator cuff repair were found to be longer and led to poorer functional outcomes in patients with obesity.28
Interestingly, another study found no noteworthy correlation between BMI and postoperative rotator cuff surgery outcomes in patients with obesity.29 In cases where patients have weight-bearing tendon injuries, obesity may impede postsurgical recovery. These tendons are critical components of the shoulder joint that provide support and stability during movement. Unfortunately, they can sustain damage caused by a range of causes—including overuse, trauma, and age. The biomechanical properties of the flexor hallucis longus tendon have been shown to be impaired following injury in obese type II diabetic mice when compared to lean control mice. This impairment may be attributable to increased mechanical stress on weight-bearing tendons.30 Weight-bearing tendons in patients with obesity may experience higher mechanical stresses. Obesity results in a higher body weight and causes increased stress on the muscular and skeletal systems. Injured tendons may require greater tension and stress to complete the repair process. However, the tendons of patients who are overweight may not be able to withstand the additional mechanical stress, which can negatively impact the repair process.
In a recent investigation, scholars examined the medical records of 500 individuals who underwent surgical interventions for Achilles’ tendon injuries at five Italian medical facilities between 2003 and 2021.31 Among them, 95 had documented medical conditions such as diabetes, obesity, and hypercholesterolemia. The study demonstrated that obesity increases the risk of Achilles tendinopathy and that adipokines play a vital role (Figure 1). Furthermore, patients with MS frequently experience inflammatory reactions in their adipose tissues, which obstruct the tendon healing process through long-range effects.32 Obesity is also associated with chronic inflammation. Inflammatory factors released from adipose tissue throughout the body trigger a systemic inflammatory response.33 This ongoing state of inflammation can complicate and impair the inflammatory response during tendon repair, resulting in a more complex and challenging repair process.32,34 Having a BMI > 25 significantly increases a patient’s susceptibility to tendon disorders involving the Achilles tendon, posterior tibial tendon, or gastrocnemius muscle.35 A high-fat diet (HFD) can also affect gene expression, thus specifically influencing tendon cell proliferation, collagen production, and gene expression profiles.36 Moreover, a number of studies have indicated that tendons go through significant transformations as BMI rises, resulting in increased stiffness, thickness, and decreased elasticity (Table 1).37–39 HFD can also impact the mechanical properties of tendons.40–42 Nevertheless, there is inconclusive evidence regarding specifically how obesity can impact tendon properties.30,43
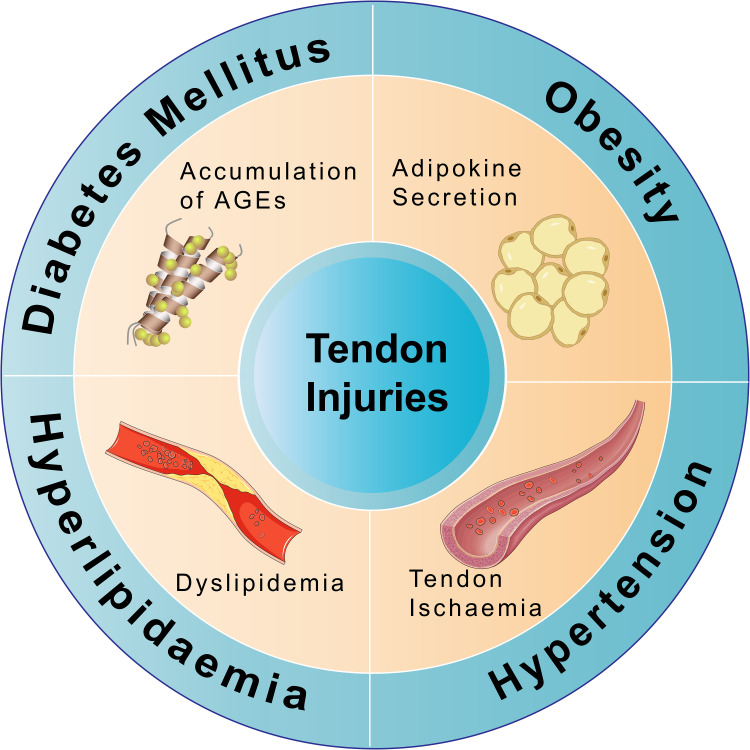
Figure 1.
The effects of different components of MS on tendon injuries. In diabetes, the accumulation of AGEs significantly impairs tendon adhesion healing, exhibits poor histological characteristics with hyperglycemia, reduces the failed load, and diminishes the stiffness of the reparative structure, consequently resulting in tendon injuries. Obesity increases the risk of tendon injuries, which is closely associated with adipokine secretion. Hyperlipidemia results in dyslipidemia, primarily due to the accumulation of cholesterol by-products, which can impair normal blood circulation in the tendons, leading to tendon injuries. Hypertension-induced peripheral blood supply insufficiency leads to ischemia of the tendons, increasing the risk of tendon injury.
Abbreviation: AGEs, advanced glycation end products.
Table 1.
The Impact of Different Components of MS on Different Tendons
| Components of MS | Tendon | Impact on Tendons | Ref |
|---|---|---|---|
| Obesity | Rotator cuff, Achilles | Increased in hardness Increased in thickness Decreased in elasticity |
[38–40] |
| DM | Rotator cuff, Achilles | Decreased in intensity Decreased in elasticity Decreased in failure payload |
[44,45] |
| Hyperlipidemia | Rotator cuff, Achilles | Increase in thickness Increased in elasticity Increased in hardness |
[46,47] |
| Hypertension | Rotator cuff | Vascular Damage | [48] |
Effect of Diabetes on Tendon Disease
Diabetes is a global metabolic disease with high incidence and mortality. The increasing prevalence of chronic diabetes has a significant impact on patient health. DM represents an important component of MS. Over the past several years, there has been growing evidence suggesting that DM is closely associated with tendon healing, which increases the risk of tendon injury.
In a study of injured tendons in rats with type 2 DM (T2DM), it was found that T2DM had adverse effects on tendon repair, leading to changes in collagen and matrix metalloproteinases.49 Nam Su Cho et al studied 355 patients who underwent arthroscopic rotator cuff repair, including 64 with DM. The results showed that patients with DM had a significantly higher rate of retear compared to those without, and patients with DM who had well-controlled blood glucose levels experienced significantly better recovery following rotator cuff repair compared to those with poor blood glucose control.50 On ultrasound, the tendon fibers of patients with DM were found to be disorganized and calcified within the Achilles tendon (Table 1).44 This may be associated with significant impairment of sustained hyperglycemia and tendon adhesion healing, poor histological characteristics of hyperglycemia, and reduced failure loads and stiffness of repair structures when compared to those with normal blood glucose (Table 1 and Figure 1).45 In addition to poor tendon healing, research has also found that patients with DM are more likely to experience shoulder joint stiffness.51 One study reported that 36% of insulin-dependent patients with DM developed stiffness of the shoulder, which was much higher than the 3% for the general population.52 Other studies have shown a link between DM and shoulder stiffness, which may be caused by capsular contracture, and claimed that the longer DM develops the more likely the patient is to develop periarthritis as well.51,53,54
Effect of Hyperlipidemia on Tendon Disease
Hyperlipidemia is a serious health problem that is clinically linked to heart disease; however, few people realize that hyperlipidemia can also have a significant impact on tendon disorders.55 Tendons are crucial connective tissues that establish connections between muscles and bones, but also play crucial roles in transmitting strength and maintaining bodily stability. When hyperlipidemia occurs, elevated levels of lipids in the bloodstream can result in endothelial injury and inflammation, causing cardiovascular diseases.56,57 As tissues with high metabolic rates, tendons also require sufficient blood supplies providing them with oxygen and nutrients. However, when the body is hyperlipidemic, the accumulation of cholesterol byproducts can impair proper blood circulation to tendons (Figure 1).58 Insufficient blood supply may restrict the physiological functions of tendons, leading to fatigue and injuries.
The lipid deposits caused by hyperlipidemia not only occur in blood vessels but also the skin and tendons. These can result in xanthomas, with Achilles’ tendon xanthomas being the most common.59 During the formation of tendon xanthomas, low-density lipoprotein (LDL) accumulates within tendons, where it is subsequently ingested by macrophages—a process similar to the development of arteriosclerosis.60 In individuals with Achilles tendon xanthomas, macrophages are in an inflammatory state. The persistent presence of inflammation can disrupt the integrity and functionality of tendon tissues, thereby increasing the patient’s susceptibility to tendon injury.61 One clinical study identified a correlation among patients with hyperlipidemia—linking the thickness of the Achilles tendon, blood lipid levels, and vascular intima thickness. This implied that an increased Achilles tendon thickness could serve as a significant indicator of atherosclerosis (Table 1).46 However, that study did not explore the influence of other factors on Achilles’ tendon thickness; thus, its results findings may have been due to multiple factors. Ozgurtas et al found an elevated presence of triglycerides, total cholesterol, and LDL in patients suffering from Achilles’ tendon rupture. Comparing them to a healthy control population, they found significant abnormal reductions in HDL levels.62 In another study, lipid-lowering therapy improved symptoms in > 50% of patients with Achilles tendon rupture.63 Aside from detrimental effects on the Achilles’ tendon, elevated total triglyceride, LDL, cholesterol, and diminished HDL levels were also observed in patients with rotator cuff injuries, implying a potential correlation with hyperlipidemia.64 Some experiments in animal models have also shown that hypercholesterolemia can affect tendon healing. Michael et al examined various animal models of hyperlipidemia and discovered that hyperlipidemic environments markedly augmented tendon stiffness in rats and mice, with a substantial increase in tendon elastic modulus observed in hyperlipidemic monkeys and mice (Table 1). This was attributed to the inherent influence of hyperlipidemia on supraspinatus tendons; however, the precise mechanism remains unclear and merits further exploration.47
Effect of Hypertension on Tendon Disease
Hypertension, a prevalent cardiovascular disorder affecting a significant number of individuals worldwide, is often associated with vascular dysfunction and hemodynamic changes that exert detrimental effects on the cardiovascular system.65 Vasoconstriction and increased blood viscosity can result in poor blood flow to tendons. Although there is currently insufficient research regarding the effects of hypertension on tendon healing, evidence suggests that hypertension may have a negative impact on tendons.66 In a study on hypertension and rotator cuff tears, patients with hypertension were twice as likely to experience significant rotator cuff tears compared to normotensive individuals.67 There are multiple etiologies for rotator cuff tears, with all involving blood supply as a critical factor. Hypertension is a significant cause of reduced peripheral blood supply that increases the likelihood of rotator cuff tears in patients with hypertension (Figure 1). Another study indicated that elevated blood pressure could lead to vascular damage and subsequent tendon ruptures (Table 1).48 Research conducted by Zhao et al identified a correlation between hypertension and rotator cuff tears, although the mechanisms influencing their onset and progression remain unclear.68
Molecular Mechanism of the Effects of MS on Tendon Disease
HbA1c May Be Used as a Marker of Tendon-Related Pathology
Hemoglobin A1c (HbA1c) level reflects glucose control in the human body. This complex is composed of hemoglobin and blood sugar. In hyperglycemia, HbA1c levels are elevated. In addition to measuring the current glucose concentration, the average glucose concentration over the preceding 2–3 months can be obtained by measuring the HbA1c concentration. Elevated HbA1c has been recognized as a significant indicator of tendinous pathology risk.69,70 Some scholars have established that tendon elongation is inversely related to glycated hemoglobin in the normal physiological range.71 Skovgaard et al used HbA1c levels as a predictive marker to evaluate the likelihood of tendinopathic conditions in patients with DM.20 Their results showed that patients with elevated HbA1c levels had a 3-fold higher risk of experiencing lower limb tendon injuries than healthy controls. An increase in HbA1c may affect tendon structure and make tendon tissues more prone to injury and damage. Therefore, HbA1c may serve as a biomarker of tendon-related pathologies.
CFTR Impact on TDSCs
Tendon-derived stem cells (TDSCs) are a specialized cell group present in human tendon tissues that can help form the extracellular matrix (ECM) and promote tendon differentiation.72–74 They have important biological functions and promising medical applications. One of the key functions of TDSCs is to form the ECM, which is a complex molecular framework with structural support and signal conduction abilities. The ECM plays a vital role in maintaining tendon tissue integrity and functional stability. TDSCs are also crucial homeostasis-sustaining cells in tendons and facilitate tendon repair following injuries.75 In patients with DM, high glucose levels regulate TDSC proliferation and differentiation, thereby inhibiting tendon healing following injuries.72,76 Hence, elucidating the functions of TDSCs in terms of regulating glucose levels is essential to promoting tendon healing in patients with DM.
Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel-regulating protein on the cell membrane. Its malfunction can impact intercellular signaling and contribute to various diseases, most notably cystic fibrosis.77,78 Research shows CFTR possesses mechanosensitivity.79 TDSCs are also mechanosensitive, and mechanical stimuli can induce TDSC gene expression and protein synthesis to facilitate tendon differentiation.80 Further research has demonstrated that CFTR can promote TDSC proliferation and moderate differentiation.81 Curcumin, an agonist of CFTR, has been shown to benefit tendon repair, further confirming the significant role of CFTR in tendon repair.82–84 Studies have also linked CFTR to DM.85,86 One confirmed that high glucose levels inhibited TDSC proliferation and differentiation through the let-7b-5p/CFTR pathway, contributing to poor tendon healing in patients with DM (Figure 2A).87 Under the high glucose levels present in cases of DM, CFTR may adversely impact TDSCs and consequently affect tendon healing. Hence, further analysis of the interplay between CFTR and TDSCs can provide novel insights into tendon biology and disease mechanisms, as well as new strategies for the treatment and regeneration of tendons following injury events.
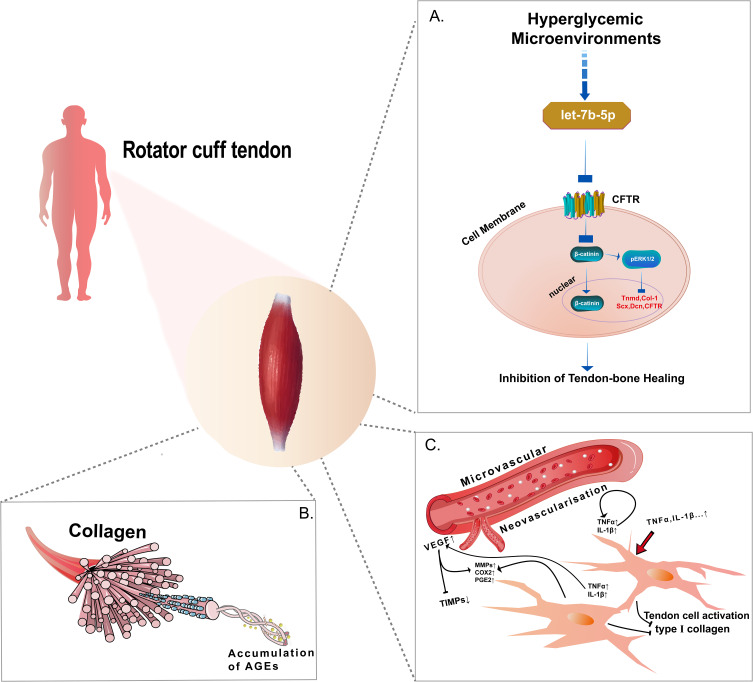
Figure 2.
The molecular mechanisms by which MS affects tendon disease. For example, in the case of the rotator cuff tendon. (A) The high sugar microenvironment inhibits the proliferation and differentiation of TDSCs through the let-7b-5p/CFTR pathway, thereby contributing to the occurrence and development of poor tendon-bone healing in DM. (B) Tendon tissue exhibits a natural triple helical structure, and accumulated AGEs can crosslink with surrounding collagen, thereby affecting the biomechanical properties. (C) Pro-inflammatory cytokines such as TNF-α and IL-1 b induce the expression of pro-inflammatory cytokines, inflammatory mediators (COX-2, PGE2), degrading enzymes (MMPs), expression of angiogenesis (VEGF), inhibition of type I collagen expression, which may affect the biomechanics and mechanical properties of tendons and thus influence tendon repair.
Abbreviations: CFTR, the cystic fibrosis transmembrane conductance regulator; AGEs, advanced glycation end products; TNF-α, tumor necrosis factor Alpha; VEGF, vascular endothelial growth factor.
AGEs Impact on Tendon
Advanced glycation end products (AGEs) are harmful substances that form inside living organisms, mainly as a result of non-enzymatic glycation reactions between sugars and proteins.88 The consequential formation of these substances within the body may lead to tissue damage, including damage to structures such as tendons.89 AGEs are crucial metabolites of DM that accumulate in various bodily tissues.90,91 While the ECM in patients with DM accumulates AGEs in significant amounts, the main component of the tendon ECM is Col-I. Studies have reported that tendon tissue possesses an inherent triple-helical arrangement and that accumulated AGEs can link with the surrounding collagen.91,92 Fessel et al demonstrated that lateral molecular attachments via AGEs could curtail sliding of collagen fibers in the caudal tendons of rats.93 Experimental evidence has suggested that glycosylation significantly diminishes the sliding and viscoelastic properties of tendon fibers.94 Lee and Veres demonstrated that cross-linking of AGEs also significantly diminished the mechanical plasticity of bovine caudal tendons.91 It has also been suggested that cross-linking can fill the voids of the ECM, which may affect its biomechanical properties.95 Thus, in cases of DM, the existence of such cross-links results in stiffened cartilage and tendons, thereby reducing their biomechanical strength (Figure 2B). Furthermore, AGEs in the ECM can also engage with a variety of cytokines, proteinaceous substances, and other substances, leading to biochemical impacts that can affect the mechanical properties of tendons.90,93,96 One study discovered that collagen glycosylation levels were significantly higher in rabbit tendons and that collagen cross-linking was meaningfully augmented in a high-glucose environment. This suggests a possible correlation between high glucose levels and the morphological changes that take place in tendon tissues.97 However, the same study determined that AGEs do not impact the mechanical properties of tendons, implying that tendon function may be affected through a different mechanism.98
Expression of TNF-α in Tendon
Tumor necrosis factor alpha (TNF-α), an inflammatory mediator produced by a number of cell types, is primarily secreted by macrophages and T lymphocytes. This cytokine is released by cells when the body encounters an infection or injury to induce an inflammatory response aimed at eradicating pathogens and repairing the damage. However, excessive inflammation can lead to further tissue damage and exacerbation of the condition.
The functions of TNF-α are not limited to inflammation; it also plays a role in the regulation of cell growth, differentiation, and death. During the processes of cellular growth and differentiation, TNF-α can have either a promoting or inhibitory effect, significantly impacting tissue development and repair. Studies have revealed TNF-α’s role in promoting the secretion of inflammatory factors, including interleukin (IL)-6, IL-10, and the matrix-degrading enzyme MMP-1, by tendon cells.99 Wu et al discovered that the rate of cell death increases during the early stages of tendon healing, suggesting that TNF-α plays a role in tendon inflammation and degeneration, and can induce apoptosis under certain conditions.100 An increase in apoptosis can cause severe damage to tissues with fewer cells such as tendons, potentially leading to a variety of tendon-related diseases. An increase in TNF-α expression and caspase-3 activity has also been observed in equine tendon samples in inflammatory states, which further corroborates its role in promoting apoptosis.101 The apoptosis induced by TNF-α not only disrupts the homeostasis of the tendon but may also lead to the emergence of various tendon diseases.
Expression of VEGF in Tendon
Vascular endothelial growth factor (VEGF) belongs to a family of proteins known for their roles in promoting angiogenesis and facilitating tissue repair following injuries. It enhances the proliferation and migration of endothelial cells, thereby stimulating the formation of new blood vessels. In addition to its involvement in vascular growth and repair, VEGF is also closely associated with metabolic processes. Studies have indicated that VEGF plays a significant role in DM and its related complications, particularly angiogenic dysfunction and hypoxia-related complications.102 Within tendon tissues, VEGF expression correlates closely with vascular density and the tendon repair process (Figure 2C). After tendon injury, VEGF expression is upregulated to foster new blood vessel formation and supply ample blood flow for tendon healing. VEGF can also promote the proliferation and migration of tendon cells, thereby accelerating their repair. The presence of residual microvasculature within the tendon tissue may also influence the level to which the VEGF gene is expressed.103 Moderate expression of VEGF is beneficial for tendon health; however, sustained overexpression of VEGF can be detrimental to tendon healing. A study by Korntner et al suggested that excessive angiogenesis or vascular damage caused by an overactive inflammatory response represented a significant impediment to wound healing that led to vascular scarring and functional anomalies.104 This could potentially affect the biomechanical and mechanical properties of the tendon, thereby impacting the tendon repair process.
Establishment of Animal Models of MS
It is crucial to establish animal models of MS to investigate its influence on tendinous structures and the corresponding molecular mechanisms for the prevention, control, or treatment of MS. Although rodents have traditionally been the primary animal models in MS research, additional models that may exhibit more resemblances to humans should be considered.7 Among rodent models, the two most frequently used model types are monogenic and diet-induced obesity (DIO) animal models.105,106 Rodent models lacking leptin or leptin receptors are currently the most commonly used single-gene animal models. The following are also widely used: db/db mice, Zucker obese rats, ob/ob mice, and Zucker DM fat (ZDF) rats.107 The ob/ob murine model, similar to the db/db one, has a high propensity for obesity with reduced glucose tolerance, hyperinsulinemia, and increased IR.108 Disordered tendon fibers and uneven deposition of glycoproteins have been reported in the tendons of ob/ob mice.109 In Zucker obese murine models, researchers have also noted a disruption in the normal alignment of collagen fibrils within the tendinous structures.110 These alterations may impact the mechanical performance of tendons and lead to the development of tendon pathologies.
The Goto-Kakizaki (GK) rat model can present the initial characteristics of hyperglycemia and DM complications at two weeks of age and is recognized as one of the optimal models for research on T2DM.111 In GK rats with DM, T2DM has adverse effects on neurological and vascular trophic pathways, possibly leading to compromised Achilles tendon repair.111 Single-gene animal models may demonstrate certain MS components during growth, but they do not adequately reflect the complete pathogenesis of the syndrome. Moreover, modeling them is an expensive and challenging task. DIO animal models are created solely through food induction and are selected from Sprague-Dawley (SD) rats, Wistar rats, and C57BL/6J mice—with the SD rat model being the most prevalent. These animals are fed a diet high in fat, sugar, and salt to simulate the development of obesity and MS in humans. This results in central obesity, IR, hyperglycemia, and dyslipidemia which exhibit similar characteristics to those observed in humans. The supraspinatus tendon of SD mice fed a high-fat diet showed sparse arrangement and microtears of collagen fibers at 2 weeks, and significant tearing and infiltration of inflammatory cells in tendon collagen fibers at 12 weeks.112 By integrating the advantages and disadvantages of both monogenic animal models and DIO animal models, the selection of an appropriate animal model during experimental processes can further reflect the impact of MS on tendons.
Conclusion
The influence of each component of MS on tendinopathy indicates that MS is an important risk factor for the early onset, progression, and poor prognostic outcomes of tendon diseases. However, the specific mechanisms whereby MS exerts its detrimental effects on tendinopathy remain unclear. In future studies, we will investigate MS as an independent subject and further examine its impact on tendinopathy.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This review was supported by China Postdoctoral Science Foundation (2023M732927), the Science and Technology Innovation Cooperation Special Programme of Sichuan Province (2022YFS0609-C1), Luzhou Science and Technology Plan Project (2021-SYF-25), Industry-University-Research Cooperation Foundation (2021CXYZ01) and Scientific Research Project of Southwest Medical University (2021ZKMS051, 2022QN018), Central Guiding of Local Science and Technology Development Special Project in Sichuan Province (2023ZYD0072).
Abbreviations
MS, metabolic syndrome; DM, diabetes mellitus; OA, osteoarthritis; WHO, the World Health Organisation; IR, insulin resistance; IGT, impaired glucose tolerance; IFG, impaired fasting glucose; GEIR, the European Group for the Study of Insulin Resistance; NCEP: ATP III, the National Cholesterol Education Program Adult Treatment Panel III; AACE, the American Association of Clinical Endocrinology; PCOS, polycystic ovary syndrome; IDF, the International diabetes federation; BMI, body mass index; DASH, Disabilities of the Arm, Shoulder and Hand; HFD, high-fat diet; T2DM, type 2 diabetes mellitus; HbA1c, hemoglobin A1c; TDSCs, tendon-derived stem cells; ECM, extracellular matrix; CFTR, the cystic fibrosis transmembrane conductance regulator; AGEs, advanced glycation end products; TNF-α, tumor necrosis factor Alpha; VEGF, vascular endothelial growth factor; DIO, diet-induced obesity; ZDF, Zucker diabetes mellitus fat; GK, GotoKakizaki; SD, Sprague Dawley.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kylin E. Studien ueber das hypertonie-hyperglykamie-hyperurikamiesyndrom. Zentralblatt Fuer Innere Med. 1923;44:105–127. [Google Scholar]
- 2.Vague J. Sexual differentiation, a factor affecting the forms of obesity. Presse Medl. 1947;53:339–340. [PubMed] [Google Scholar]
- 3.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595 [DOI] [PubMed] [Google Scholar]
- 4.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149(7):1514–1520. doi: 10.1001/archinte.1989.00390070054005 [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173 [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes. 1992;41(6):715–722. doi: 10.2337/diab.41.6.715 [DOI] [PubMed] [Google Scholar]
- 7.Cornier MA, Dabelea D, Hernandez TL, et al. The MS. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Després JP, Lemieux I. Abdominal obesity and MS. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 9.Anderson PJ, Critchley JA, Chan JC, et al. Factor analysis of the MS: obesity vs insulin resistance as the central abnormality. Int J Obes Relat Metab Disord. 2001;25(12):1782–1788. doi: 10.1038/sj.ijo.0801837 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Tokunaga K, Shimomura I, et al. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1994;107(2):239–246. doi: 10.1016/0021-9150(94)90025-6 [DOI] [PubMed] [Google Scholar]
- 11.Stern M. Natural history of macrovascular disease in type 2 diabetes. Role of Insulin Resistance. Diabetes Care. 1999;22(3):C2–C5. [PubMed] [Google Scholar]
- 12.Huang PL. A comprehensive definition for MS. Dis Model Mech. 2009;2(5–6):231–237. doi: 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakka HM, Laaksonen DE, Lakka TA, et al. The MS and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709 [DOI] [PubMed] [Google Scholar]
- 14.Malik S, Wong ND, Franklin SS, et al. Impact of the MS on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E [DOI] [PubMed] [Google Scholar]
- 15.Gami AS, Witt BJ, Howard DE, et al. MS and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032 [DOI] [PubMed] [Google Scholar]
- 16.Dekker JM, Girman C, Rhodes T, et al. MS and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112(5):666–673. doi: 10.1161/CIRCULATIONAHA.104.516948 [DOI] [PubMed] [Google Scholar]
- 17.Zhuo Q, Yang W, Chen J, Wang Y. MS meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729–737. doi: 10.1038/nrrheum.2012.135 [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Lv X, Wei D, Yue F, Guo J, Zhang T. MS and the risk of bone fractures: a Meta-analysis of prospective cohort studies. Bone. 2016;84:52–56. doi: 10.1016/j.bone.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 19.Babagoli M, Soleimani M, Baghdadi S, Vatan MS, Shafiei SH. Does MS increase the risk of fracture? A systematic review and meta-analysis. Arch Osteoporos. 2022;17(1):118. doi: 10.1007/s11657-022-01149-y [DOI] [PubMed] [Google Scholar]
- 20.Skovgaard D, Siersma VD, Klausen SB, et al. Chronic hyperglycemia, hypercholesterolemia, and MS are associated with risk of tendon injury. Scand J Med Sci Sports. 2021;31(9):1822–1831. doi: 10.1111/sms.13984 [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of DM and its complications. Part 1: diagnosis and classification of DM provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: [DOI] [PubMed] [Google Scholar]
- 22.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999;16(5):442–443. [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 24.Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25–26 August 2002, Washington, DC. Diabetes Care. 2003;26(4):1297–1303. doi: 10.2337/diacare.26.4.1297 [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J. MS--A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 26.Ateschrang A, Eggensperger F, Ahrend MD, Schröter S, Stöckle U, Kraus TM. Obesity causes poorer clinical results and higher re-tear rates in rotator cuff repair. Arch Orthop Trauma Surg. 2018;138(6):835–842. doi: 10.1007/s00402-018-2921-1 [DOI] [PubMed] [Google Scholar]
- 27.Abate M, Salini V, Andia I. How Obesity Affects Tendons? Adv Exp Med Biol. 2016;920:167–177. [DOI] [PubMed] [Google Scholar]
- 28.Warrender WJ, Brown OL, Abboud JA. Outcomes of arthroscopic rotator cuff repairs in obese patients. J Shoulder Elbow Surg. 2011;20(6):961–967. doi: 10.1016/j.jse.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 29.Namdari S, Baldwin K, Glaser D, Green A. Does obesity affect early outcome of rotator cuff repair? J Shoulder Elbow Surg. 2010;19(8):1250–1255. doi: 10.1016/j.jse.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 30.David MA, Jones KH, Inzana JA, Zuscik MJ, Awad HA, Mooney RA. Tendon repair is compromised in a high fat diet-induced mouse model of obesity and type 2 diabetes. PLoS One. 2014;9(3):e91234. doi: 10.1371/journal.pone.0091234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliva F, Marsilio E, Asparago G, et al. Achilles tendon rupture and dysmetabolic diseases: a multicentric, epidemiologic study. J Clin Med. 2022;11(13):3698. doi: 10.3390/jcm11133698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins KH, Herzog W, MacDonald GZ, et al. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol. 2018;9:112. doi: 10.3389/fphys.2018.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abate M. How obesity modifies tendons (implications for athletic activities). Muscles Ligaments Tendons J. 2014;4(3):298–302. doi: 10.32098/mltj.03.2014.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battery L, Maffulli N. Inflammation in overuse tendon injuries. Sports Med Arthrosc Rev. 2011;19(3):213–217. doi: 10.1097/JSA.0b013e31820e6a92 [DOI] [PubMed] [Google Scholar]
- 35.Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007;28(9):996–999. doi: 10.3113/FAI.2007.0996 [DOI] [PubMed] [Google Scholar]
- 36.Bolam SM, Konar S, Park YE, et al. A high-fat diet has negative effects on tendon resident cells in an in vivo rat model. Int Orthop. 2022;46(5):1181–1190. doi: 10.1007/s00264-022-05340-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abate M, Oliva F, Schiavone C, Salini V. Achilles tendinopathy in amateur runners: role of adiposity (Tendinopathies and obesity). Muscles Ligaments Tendons J. 2012;2(1):44–48. [PMC free article] [PubMed] [Google Scholar]
- 38.Abate M, Schiavone C, Di Carlo L, Salini V. Achilles tendon and plantar fascia in recently diagnosed type II diabetes: role of body mass index. Clin Rheumatol. 2012;31(7):1109–1113. doi: 10.1007/s10067-012-1955-y [DOI] [PubMed] [Google Scholar]
- 39.Wearing SC, Hooper SL, Grigg NL, Nolan G, Smeathers JE. Overweight and obesity alters the cumulative transverse strain in the Achilles tendon immediately following exercise. J Bodyw Mov Ther. 2013;17(3):316–321. doi: 10.1016/j.jbmt.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 40.Grewal N, Thornton GM, Behzad H, et al. Accumulation of oxidized LDL in the tendon tissues of C57BL/6 or apolipoprotein E knock-out mice that consume a high fat diet: potential impact on tendon health. PLoS One. 2014;9(12):e114214. doi: 10.1371/journal.pone.0114214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eriksen C, Svensson RB, Scheijen J, et al. Systemic stiffening of mouse tail tendon is related to dietary advanced glycation end products but not high-fat diet or cholesterol. J Appl Physiol. 2014;117(8):840–847. doi: 10.1152/japplphysiol.00584.2014 [DOI] [PubMed] [Google Scholar]
- 42.Boivin GP, Platt KM, Corbett J, et al. The effects of high-fat diet, branched-chain amino acids and exercise on female C57BL/6 mouse Achilles tendon biomechanical properties. Bone Joint Res. 2013;2(9):186–192. doi: 10.1302/2046-3758.29.2000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rios JL, Ko L, Joumaa V, et al. The mechanical and biochemical properties of tail tendon in a rat model of obesity: effect of moderate exercise and prebiotic fibre supplementation. J Biomech. 2019;88:148–154. doi: 10.1016/j.jbiomech.2019.03.031 [DOI] [PubMed] [Google Scholar]
- 44.Batista F, Nery C, Pinzur M, et al. Achilles tendinopathy in DM. Foot Ankle Int. 2008;29(5):498–501. doi: 10.3113/FAI.2008.0498 [DOI] [PubMed] [Google Scholar]
- 45.Bedi A, Fox AJ, Harris PE, et al. DM impairs tendon-bone healing after rotator cuff repair. J Shoulder Elbow Surg. 2010;19(7):978–988. doi: 10.1016/j.jse.2009.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiortsis DN, Argyropoulou MI, Xydis V, Tsouli SG, Elisaf MS. Correlation of Achilles tendon thickness evaluated by ultrasonography with carotid intima-media thickness in patients with familial hypercholesterolemia. Atherosclerosis. 2006;186(1):228–229. doi: 10.1016/j.atherosclerosis.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Hast MW, Abboud JA, Soslowsky LJ. Exploring the role of hypercholesterolemia in tendon health and repair. Muscles Ligaments Tendons J. 2014;4(3):275–279. doi: 10.32098/mltj.03.2014.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmes GB, Mann RA. Possible epidemiological factors associated with rupture of the posterior tibial tendon. Foot Ankle. 1992;13(2):70–79. doi: 10.1177/107110079201300204 [DOI] [PubMed] [Google Scholar]
- 49.Ahmed AS, Schizas N, Li J, et al. Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol. 2012;113(11):1784–1791. doi: 10.1152/japplphysiol.00767.2012 [DOI] [PubMed] [Google Scholar]
- 50.Cho NS, Moon SC, Jeon JW, Rhee YG. The influence of DM on clinical and structural outcomes after arthroscopic rotator cuff repair. Am J Sports Med. 2015;43(4):991–997. doi: 10.1177/0363546514565097 [DOI] [PubMed] [Google Scholar]
- 51.Thomas SJ, McDougall C, Brown ID, et al. Prevalence of symptoms and signs of shoulder problems in people with DM. J Shoulder Elbow Surg. 2007;16(6):748–751. doi: 10.1016/j.jse.2007.02.133 [DOI] [PubMed] [Google Scholar]
- 52.Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995;77(5):677–683. doi: 10.1302/0301-620X.77B5.7559688 [DOI] [PubMed] [Google Scholar]
- 53.Balci N, Balci MK, Tüzüner S. Shoulder adhesive capsulitis and shoulder range of motion in type II DM: association with diabetic complications. J Diabetes Complications. 1999;13(3):135–140. doi: 10.1016/S1056-8727(99)00037-9 [DOI] [PubMed] [Google Scholar]
- 54.Bridgman JF. Periarthritis of the shoulder and DM. Ann Rheum Dis. 1972;31(1):69–71. doi: 10.1136/ard.31.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soslowsky LJ, Fryhofer GW. Tendon Homeostasis in Hypercholesterolemia. Adv Exp Med Biol. 2016;920:151–165. [DOI] [PubMed] [Google Scholar]
- 56.An L, Gao L, Ning M, et al. [Correlation between decreased plasma miR-29a and vascular endothelial injury induced by hyperlipidemia]. Korrelation zwischen verringertem Plasma-miR-29a-Wert und durch Hyperlipidämie induzierten vaskulären endothelialen Läsionen. Herz. 2023;48(4):301–308. doi: 10.1007/s00059-022-05121-x [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM, Bhatt DL, Pradhan AD, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–1301. doi: 10.1016/S0140-6736(23)00215-5 [DOI] [PubMed] [Google Scholar]
- 58.Rothman RH, Parke WW. The vascular anatomy of the rotator cuff. Clin Orthop Relat Res. 1965;41:176–186. [PubMed] [Google Scholar]
- 59.Sijbrands EJ. Xanthomas and atheromas. Atherosclerosis. 2017;263:315. doi: 10.1016/j.atherosclerosis.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 60.Tsouli SG, Kiortsis DN, Argyropoulou MI, Mikhailidis DP, Elisaf MS. Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest. 2005;35(4):236–244. doi: 10.1111/j.1365-2362.2005.01484.x [DOI] [PubMed] [Google Scholar]
- 61.Taylor B, Cheema A, Soslowsky L. Tendon pathology in hypercholesterolemia and familial hypercholesterolemia. Curr Rheumatol Rep. 2017;19(12):76. doi: 10.1007/s11926-017-0704-2 [DOI] [PubMed] [Google Scholar]
- 62.Ozgurtas T, Yildiz C, Serdar M, Atesalp S, Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta. 2003;331(1–2):25–28. doi: 10.1016/S0009-8981(03)00075-5 [DOI] [PubMed] [Google Scholar]
- 63.Klemp P, Halland AM, Majoos FL, Steyn K. Musculoskeletal manifestations in hyperlipidaemia: a controlled study. Ann Rheum Dis. 1993;52(1):44–48. doi: 10.1136/ard.52.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1493–1497. doi: 10.1007/s11999-009-1151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 66.Giri A, O’Hanlon D, Jain NB. Risk factors for rotator cuff disease: a systematic review and meta-analysis of diabetes, hypertension, and hyperlipidemia. Ann Phys Rehabil Med. 2023;66(1):101631. doi: 10.1016/j.rehab.2022.101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gumina S, Arceri V, Carbone S, et al. The association between arterial hypertension and rotator cuff tear: the influence on rotator cuff tear sizes. J Shoulder Elbow Surg. 2013;22(2):229–232. doi: 10.1016/j.jse.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 68.Zhao J, Luo M, Liang G, et al. What factors are associated with symptomatic rotator cuff tears: a meta-analysis. Clin Orthop Relat Res. 2022;480(1):96–105. doi: 10.1097/CORR.0000000000001949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranger TA, Wong AM, Cook JL, Gaida JE. Is there an association between tendinopathy and DM? A systematic review with meta-analysis. Br J Sports Med. 2016;50(16):982–989. doi: 10.1136/bjsports-2015-094735 [DOI] [PubMed] [Google Scholar]
- 70.Otoshi K, Takegami M, Sekiguchi M, et al. Chronic hyperglycemia increases the risk of lateral epicondylitis: the locomotive syndrome and health outcome in aizu cohort study (LOHAS). Springerplus. 2015;4:407. doi: 10.1186/s40064-015-1204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hansen M, Couppe C, Hansen CS, et al. Impact of oral contraceptive use and menstrual phases on patellar tendon morphology, biochemical composition, and biomechanical properties in female athletes. J Appl Physiol. 2013;114(8):998–1008. doi: 10.1152/japplphysiol.01255.2012 [DOI] [PubMed] [Google Scholar]
- 72.Shi L, Rui YF, Li G, Wang C. Alterations of tendons in diabetes mellitus: what are the current findings? Int Orthop. 2015;39(8):1465–1473. doi: 10.1007/s00264-015-2775-x [DOI] [PubMed] [Google Scholar]
- 73.Kalyanaraman H, Schwaerzer G, Ramdani G, et al. Protein Kinase G activation reverses oxidative stress and restores osteoblast function and bone formation in male mice with type 1 diabetes. Diabetes. 2018;67(4):607–623. doi: 10.2337/db17-0965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marin C, Luyten FP, Van der Schueren B, Kerckhofs G, Vandamme K. The impact of type 2 diabetes on bone fracture healing. Front Endocrinol. 2018;9:6. doi: 10.3389/fendo.2018.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y, Sun Y, Xu Y, et al. Single-Cell Integration Analysis of Heterotopic Ossification and Fibrocartilage Developmental Lineage: endoplasmic Reticulum Stress Effector Xbp1 Transcriptionally Regulates the Notch Signaling Pathway to Mediate Fibrocartilage Differentiation. Oxid Med Cell Longev. 2021;2021:7663366. doi: 10.1155/2021/7663366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin YC, Li YJ, Rui YF, et al. The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget. 2017;8(11):17518–17528. doi: 10.18632/oncotarget.15418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16(1):45–56. doi: 10.1038/nrg3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang WK, Wang D, Duan Y, Loy MM, Chan HC, Huang P. Mechanosensitive gating of CFTR. Nat Cell Biol. 2010;12(5):507–512. doi: 10.1038/ncb2053 [DOI] [PubMed] [Google Scholar]
- 79.Wang HN, Huang YC, Ni GX. Mechanotransduction of stem cells for tendon repair. World J Stem Cells. 2020;12(9):952–965. doi: 10.4252/wjsc.v12.i9.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11(4):223–233. doi: 10.1038/nrrheum.2015.26 [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Xu J, Xu L, et al. Cystic fibrosis transmembrane conductance regulator mediates tenogenic differentiation of tendon-derived stem cells and tendon repair: accelerating tendon injury healing by intervening in its downstream signaling. FASEB J. 2017;31(9):3800–3815. doi: 10.1096/fj.201601181R [DOI] [PubMed] [Google Scholar]
- 82.Buhrmann C, Mobasheri A, Busch F, et al. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011;286(32):28556–28566. doi: 10.1074/jbc.M111.256180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang D, Gao P, Lin H, Geng H. Curcumin improves tendon healing in rats: a histological, biochemical, and functional evaluation Connect. Tissue Res. 2016;57:20–27. doi: 10.3109/03008207.2015.1087517 [DOI] [PubMed] [Google Scholar]
- 84.Córdova A, Drobnic F, Noriega-González D, Caballero-García A, Roche E, Alvarez-Mon M. Is curcumine useful in the treatment and prevention of the tendinopathy and myotendinous junction injury? A Scoping Review. Nutrients. 2023;15(2):384. doi: 10.3390/nu15020384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hasan S, Soltman S, Wood C, Blackman SM. The role of genetic modifiers, inflammation and CFTR in the pathogenesis of Cystic fibrosis related diabetes. J Clin Transl Endocrinol. 2021;27:100287. doi: 10.1016/j.jcte.2021.100287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hart NJ, Aramandla R, Poffenberger G, et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight. 2018;3(8):e98240. doi: 10.1172/jci.insight.98240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao T, Hong J, Qi F, et al. A hyperglycemic microenvironment inhibits tendon-to-bone healing through the let-7b-5p/CFTR pathway. Comput Math Methods Med. 2022;2022:8268067. doi: 10.1155/2022/8268067 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced glycation end-products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells. 2022;11(8):1312. doi: 10.3390/cells11081312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki A, Yabu A, Nakamura H. Advanced glycation end products in musculoskeletal system and disorders. Methods. 2022;203:179–186. doi: 10.1016/j.ymeth.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 90.Avery NC, Bailey AJ. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol. 2006;54(7):387–395. doi: 10.1016/j.patbio.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 91.Lee JM, Veres SP. Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon. J Appl Physiol. 2019;126(4):832–841. doi: 10.1152/japplphysiol.00430.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. 2017;13(2):110–122. doi: 10.1038/nrrheum.2016.213 [DOI] [PubMed] [Google Scholar]
- 93.Fessel G, Li Y, Diederich V, et al. Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS One. 2014;9(11):e110948. doi: 10.1371/journal.pone.0110948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gautieri A, Passini FS, Silván U, et al. Advanced glycation end-products: mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017;59:95–108. doi: 10.1016/j.matbio.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 95.Naresh MD, Brodsky B. X-ray diffraction studies on human tendon show age-related changes in collagen packing. Biochim Biophys Acta. 1992;1122(2):161–166. doi: 10.1016/0167-4838(92)90319-9 [DOI] [PubMed] [Google Scholar]
- 96.Sell DR, Monnier VM. Age-related association of tail tendon break time with tissue pentosidine in DBA/2 vs C57BL/6 mice: the effect of dietary restriction. J Gerontol a Biol Sci Med Sci. 1997;52(5):B277–84. doi: 10.1093/gerona/52a.5.b277 [DOI] [PubMed] [Google Scholar]
- 97.Kent MJ, Light ND, Bailey AJ. Evidence for glucose-mediated covalent cross-linking of collagen after glycosylation in vitro. Biochem J. 1985;225(3):745–752. doi: 10.1042/bj2250745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schulze-Tanzil G, Al-Sadi O, Wiegand E, et al. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports. 2011;21(3):337–351. doi: 10.1111/j.1600-0838.2010.01265.x [DOI] [PubMed] [Google Scholar]
- 99.John T, Lodka D, Kohl B, et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J Orthop Res. 2010;28(8):1071–1077. doi: 10.1002/jor.21079 [DOI] [PubMed] [Google Scholar]
- 100.Wu YF, Chen CH, Cao Y, Avanessian B, Wang XT, Tang JB. Molecular events of cellular apoptosis and proliferation in the early tendon healing period. J Hand Surg Am. 2010;35(1):2–10. doi: 10.1016/j.jhsa.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 101.Hosaka Y, Sakamoto Y, Kirisawa R, et al. Distribution of TNF receptors and TNF receptor-associated intracellular signaling factors on equine tendinocytes in vitro. Jpn J Vet Res. 2004;52(3):135–144. [PubMed] [Google Scholar]
- 102.Sun L, Yuan Q, Cao N, et al. VEGF genetic polymorphisms may contribute to the risk of diabetic nephropathy in patients with diabetes mellitus: a meta-analysis. Scientif World J. 2014;2014:624573. doi: 10.1155/2014/624573 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Stoll C, John T, Endres M, et al. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: implications for tendon tissue engineering. J Orthop Res. 2010;28(9):1170–1177. doi: 10.1002/jor.21109 [DOI] [PubMed] [Google Scholar]
- 104.Korntner S, Lehner C, Gehwolf R, et al. Limiting angiogenesis to modulate scar formation. Adv Drug Deliv Rev. 2019;146:170–189. doi: 10.1016/j.addr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 105.Doulberis M, Papaefthymiou A, Polyzos SA, et al. Rodent models of obesity. Minerva Endocrinol. 2020;45(3):243–263. doi: 10.23736/S0391-1977.19.03058-X [DOI] [PubMed] [Google Scholar]
- 106.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;58. doi: 10.1002/0471141755.ph0561s58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fuchs T, Loureiro MP, Macedo LE, Nocca D, Nedelcu M, Costa-Casagrande TA. Animal models in metabolic syndrome. Modelos animais na síndrome metabólica. Rev Col Bras Cir. 2018;45(5):e1975. doi: 10.1590/0100-6991e-20181975 [DOI] [PubMed] [Google Scholar]
- 108.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diab Rev. 2014;10(2):131–145. doi: 10.2174/1573399810666140508121012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu YF, Wang HK, Chang HW, Sun J, Sun JS, Chao YH. High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway. Sci Rep. 2017;7:44199. doi: 10.1038/srep44199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biancalana A, Veloso LA, Gomes L. Obesity affects collagen fibril diameter and mechanical properties of tendons in Zucker rats. Connect Tissue Res. 2010;51(3):171–178. doi: 10.3109/03008200903191312 [DOI] [PubMed] [Google Scholar]
- 111.Ahmed AS, Li J, Abdul AM, et al. Compromised neurotrophic and angiogenic regenerative capability during tendon healing in a rat model of type-II diabetes. PLoS One. 2017;12(1):e0170748. doi: 10.1371/journal.pone.0170748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu K, Zhang L, Ren Z, et al. Evaluating the role of type 2 diabetes mellitus in rotator cuff tendinopathy: development and analysis of a novel rat model. Front Endocrinol. 2022;13:1042878. doi: 10.3389/fendo.2022.1042878 [DOI] [PMC free article] [PubMed] [Google Scholar]