Abstract
Interleukin-12 is an important regulator of other cytokines. Although interleukin-12 is considered to act primarily on lymphocytes, provoking a shift from T helper 2 to T helper 1 cells and an increase in lymphocyte-derived tumor necrosis factor α, we hypothesized that interleukin-12 might also affect tumor necrosis factor α secretion from skin cells. In this study, keratinocytes were treated with ultraviolet-B, ultraviolet-A, or sham irradiation, without or with exogenous interleukin-12. Remarkably, the exogenous interleukin-12 totally blocked ultraviolet-B-induced tumor necrosis factor α production. Both ultraviolet-A and ultraviolet-B were capable of inducing interleukin-12 production. To determine the molecular mechanism of this effect, we used a chloramphenicol acetyl transferase reporter under the control of a 1.2 kb fragment of the wild-type (−308G) human tumor necrosis factor α promoter and found significant suppression of promoter activity with interleukin-12. Studies using the −308A variant of the human tumor necrosis factor α promoter showed much higher promoter activity overall, but also a greater sensitivity to suppression by interleukin-12. The mechanism did not involve blockage of the interleukin-1 receptor, because interleukin-12 did not suppress interleukin-1-mediated induction of collagenase mRNA. To determine the role of endogenous interleukin-12, we found that anti-interleukin-12 antibodies enhanced ultraviolet-B-induced tumor necrosis factor α secretion. Thus, interleukin-12 strongly inhibits tumor necrosis factor α production by noninflammatory skin cells, mostly or entirely through inhibition of gene transcription via an element within the first 1.2 kb of the tumor necrosis factor α promoter. The result is a shift in tumor necrosis factor α production from noninflammatory cells to T helper 1 cells. Because tumor necrosis factor α is central to the pathogenesis of several photosensitive skin diseases and certain forms of immune suppression, interleukin-12 may have important physiologic, pathophysiologic, and therapeutic roles.
Keywords: cytokines, fibroblasts, lupus, Th1, Th2
Interleukin-12 (IL-12) is an important regulator of other cytokines. It is a heterodimeric molecule produced primarily by antigen-presenting cells and plays a key role in promoting T helper 1 (Th1) responses (Adorini, 1999), including activation of Th1 clones (Kremer et al, 1996). As part of this shift, IL-12 typically increases the secretion of tumor necrosis factor α (TNFα), aTh1 cytokine, from inflammatory cells such as T cells (Kostense et al, 1998; Nagayama et al, 2000; Ma, 2001). Ultraviolet-B (UVB) irradiation depresses expression and secretion of IL-12 from antigen-presenting cells, and UV stimulates lymphoid organs to secrete the IL-12 p40 homodimer, a natural antagonist of biologically active IL-12 (Schmitt and Ullrich, 2000). Interestingly, UV-induced immune suppression and tolerance induction are reversed by recombinant interleukin-12 (Schmitt et al, 1995; Schwarz et al, 1996). Nearly all studies have examined the role of IL-12 in altering the immune responses of inflammatory cells (DeKruyff et al, 1995; Marshall et al, 1995; Matsuo et al, 1996), although one recent study showed that IL-12 directly suppresses IL-10 secretion from irradiated keratinocytes and blunts the rise in plasma TNFα levels that typically occur after UV irradiation of mice (Schmitt et al, 2000). The latter result cannot be explained from the fact that IL-12 has stimulatory or neutral effects on TNFα secretion by immune cells.
UV exerts many medically important effects on the immune system in susceptible individuals, in part by altering the production of specific cytokines, particularly TNFα. For example, contact hypersensitivity that develops on regions of skin painted with dinitrofluorobenzene is suppressed by prior local cutaneous irradiation with UVB. To establish a link with TNFα, investigators have shown that UVB in the presence of autocrine or paracrine sources of IL-1α induces TNFα production; local intradermal injection of TNFα impairs the induction of contact hypersensitivity to dinitrofluorobenzene; and most importantly, systemic administration of anti-TNFα antibodies abolishes this immunosuppressive effect of UV exposure (Bromberg et al, 1992; Yoshikawa et al, 1992; Werth and Zhang, 1999). Mice genetically deficient in TNF-receptor 2 (p75) lack the ability to impair contact hypersensitivity induction after UVB (Kurimoti and Streilein, 1999). Of interest, studies in humans have shown that most skin cancer patients exposed to UVB and dinitrofluorobenzene fail to develop contact hypersensitivity, suggesting a role for UVB sensitivity in the origin or proliferation of these neoplasms (Yoshikawa et al, 1990).
UV radiation also plays a substantial role in certain photosensitive autoimmune diseases. Lupus erythematosus (Sullivan et al, 1997), subacute cutaneous lupus erythematosus (Werth et al, 2000), pediatric dermatomyositis (Pachman et al, 2000), and adult dermatomyositis (Werth et al, 2002) are each associated with the −308A polymorphism in the TNFα promoter. This polymorphism enhances TNFα production, particularly after stimulation with UVB, consistent with a role in the pathogenesis of photosensitivity (Werth et al, 2000). The increase in TNFα production after UVB irradiation is part of a larger set of changes that involve both nonimmune and immune cells. (Kock et al, 1990; Fujisawa et al, 1997) UVB also stimulates keratinocytes and fibroblasts to secrete IL-1, IL-6, IL-8, IL-10, and IL-15 (Rivas and Ullrich, 1992; Kondo et al, 1993; de Vos et al, 1994; Enk et al, 1995; Grewe et al, 1995; Mohamadzadeh et al, 1995; Chung et al, 1996; Eberlein-Konig et al, 1998). Furthermore, studies suggest that UVB radiation generally shifts the immune response in the skin from aTh1 cell response to a Th2 cell predominance, both in terms of the Th2-associated cytokines produced (IL-4, IL-10) and because of interference with antigen presentation to Th1 cells (Brown et al, 1995; Ullrich, 1996).We now hypothesize that IL-12 might be an important inhibitor of TNFα secretion from non-T-cell sources, and may thereby affect responses of nonimmune cells to UV irradiation. As a model system, we used wavelength-specific stimulation of TNFα secretion from keratinocytes and fibroblasts exposed to UVB in the presence of paracrine or exogenous IL-1α, as previously described (Werth and Zhang, 1999).
MATERIALS AND METHODS
Cultured cells
Normal human fibroblasts were obtained from the American Type Culture Collection (ATCC, Rockville, MD, Catalog #1828-CRL). Genotype analysis according to prior methods (Sullivan et al, 1997) indicated the −308G (wild-type) TNFα promoter polymorphism. These cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Normal neonatal human keratinocytes were cultured from foreskins and grown in MCDB 153 medium (Sigma, M-7403, St. Louis, MO) supplemented with 30 µM CaCl2, bovine pituitary extract, epidermal growth factor, insulin, hydrocorticone, ethanolamine, phosphoethanolamine, high amino acids, penicillin, and streptomycin. Adult keratinocytes were obtained from Clonetics (San Diego, CA). Fibroblasts and keratinocytes were plated in triplicate Petri dishes (60 mm diameter, Corning), and grown to 90% confluence before irradiation or cytokine addition.
Light sources and radiometry
The UVB source was a bank of two FS-40 sunlamps (Lights of America, Walnut, CA), with a peak irradiance of 313 nm, equipped with a cellulose triacetate filter to remove wavelengths below 290 nm, as previously described (Werth and Zhang, 1999). UVB doses were measured with an International Light UV IL-443 UVB meter. The filtered UVB light source measured by spectroradiometric measurement at the time of the experiments showed 0.64% UVC, 44.51% UVB, 19.43% UVA, and 35.42% visible and near infrared (Vis + NIR). The UVA source was a 1000 W xenon lamp solar simulator (Solar Light, Philadelphia, PA), which was used with a UG5 internal filter and an external UG11 filter to remove long wavelengths and a 3 mm WG335 Schott (UVA) filter to allow only longer UV wavelengths (Werth and Zhang, 1999). UVA and UVA1 doses were verified with an IL 1400 A Research Radiometer (International Light, Newburyport, MA). The solar simulator withWG335 Schott filter showed 0.0036% UVC, 0.016% UVB, 96.63% UVA (11.28% UVA2 and 88.72% UVA1), 3.35% Vis + NIR.
Chemicals
Cytokines (IL-1α, IL-12) and cytokine enzyme-linked immunosorbent assay (ELISA) kits (TNFα, IL-12 p70) were purchased from Pierce-Endogen (Rockford, Ill.) Anti-IL-12 and anti-interferon-γ (anti-INFγ) antibodies were purchased from R&D Systems (Minneapolis, MN). All other chemicals were obtained from Fisher (Pittsburgh, PA) or Sigma.
Radiation protocols
Radiation doses were 10, 20, and 30 mJ UVB per cm2, and 5, 10, and 20 J UVA per cm2. Cells receivings ham irradiation treatment (0 mJ per cm2) went through the same procedure, but covered with aluminum foil. After irradiation, cells were immediately returned to DMEM/10% FBS, with or without addition of the following reagents: IL-1α (10 ng IL-1α per ml) for the fibroblast experiments, IL-12 (0–10 ng per ml), anti-γ-IFN antibody (1 µg per ml), and anti-IL-12 antibody (10 µg per ml) for both fibroblasts and keratinocytes. Conditioned media were harvested 24 h after irradiation of cells and assayed for either TNFα or IL-12 levels, using standard ELISA kits (R&D Systems). Three Petri dishes were used for each irradiation dose. Cytotoxicity of UV was assessed using try pan-blue staining of cells 24 h after irradiation and was always < 5%.
Transfection of cultured cells
To assess transcriptional activity, we used two promoter-reporter plasmids, each containing one of the −308 polymorphic forms of the TNFα promoter region (1173 bp) fused to a chloramphenicol acetyl transferase (CAT) reporter gene, as previously described (Werth et al, 2000). The wild-type construct contains a G at position −308 (−308G), and the variant construct was made by introducing an A at position −308 (−308A) by site-directed mutagenesis, to ensure an otherwise identical promoter sequence. In addition, a promoterless CAT construct was used to assess assay background. Each CAT construct was spliced into a pSV vector (Promega, Madison, WI). A β-gal construct (Promega), driven by SV40 promoter, was spliced into the same pSV vector to be used as a marker for transfection efficiency.
Murine fibroblast-like 3T3 cells (N.T.H., Bethesda, MD) and keratinocyte cells were cultured at 37°C for approximately 24 h to 60%–70% confluence, followed by transfection. Transfections were done using FuGENE 6 Transfection Reagent (Boehringer Mannheim, Indianapolis, IN). After transfection, cells were incubated in complete medium for 24 h. Cultured cells were then placed in phosphate-buffered saline (PBS), maintained at 35°C–37°C in a thermostatically controlled water bath, and irradiated at a distance of 40 cm with the Petri dish cover removed, using the same irradiation protocols as described above.
CAT assay
Cells were harvested 24 h after irradiation and extracted with lysis buffer (CAT assay kit, Boehringer Mannheim). CAT and β-gal were quantitated by ELISA (Boehringer Mannheim), and CAT results were normalized to β-gal.
Assessment of collagenase mRNA
As a control for IL-1α action, we assessed collagenase mRNA levels by northern blot, as previously described (Werth et al, 1997).
Statistical analysis
Comparisons of several groups simultaneously were performed by initially using analysis of variance (anova).When the anova indicated differences amongst the groups, pairwise comparisons of each experimental group versus the control group were performed using the Dunnett q′ statistic. Unless otherwise indicated, summary statistics are reported as means ± SEM, n = 3. Absent error bars in graphical displays of summary statistics indicate SEM values smaller than the drawn symbols.
RESULTS
IL-12 blocks TNFα production from UV-irradiated keratinocytes and fibroblasts
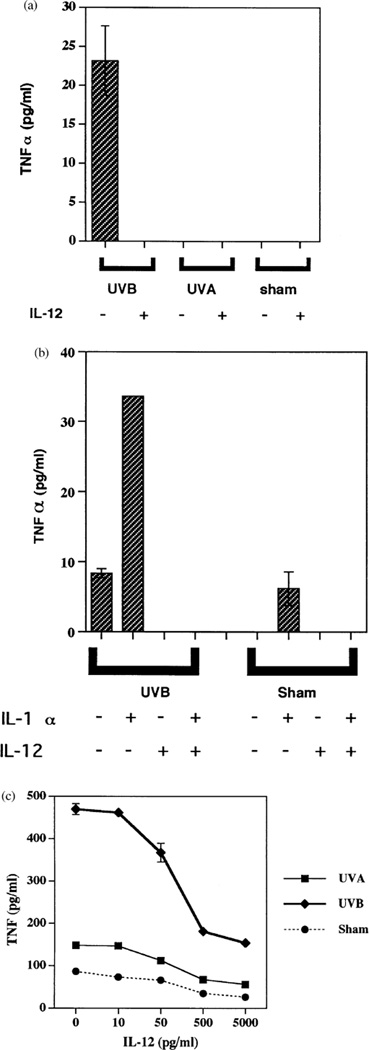
As we previously reported (Werth and Zhang, 1999), UVB but not UVA stimulated the secretion of TNFα from cultured neonatal keratinocytes (Fig 1a). Addition of exogenous IL-12 (10 ng per ml) immediately after UVB irradiation, however, completely blocked TNFα release from these cells (Fig 1a). Similarly, addition of IL-12 to cultured fibroblasts stimulated with UVB, exogenous IL-1α, or the combination reduced TNFα secretion to undetectable levels (Fig 1b). Addition of different concentrations of IL-12 to adult keratinocytes after UVB irradiation showed inhibition of TNFα secretion beginning at a dose of 50 pg per ml, with progressively greater inhibition thereafter (Fig 1c). This result confirms our underlying hypothesis, proving that IL-12 strongly inhibits TNFα secretion from these nonimmune cells. This is in contrast to immune cells, where IL-12 has stimulatory or neutral effects on TNFα production (Kostense et al, 1998; Nagayama et al, 2000; Ma, 2001).
Figure 1. IL-12 inhibits UVB-induced TNFα production by cell.
(a) IL-12 inhibits UVB-induced neonatal keratinocyte TNFα production. Neonatal keratinocytes were irradiated with 30 mJ per cm2 of UVB, 5 J per cm2 of UVA, or sham irradiated, and then given media without (minus symbols) or with (plus symbols) 10 ng IL-12 per ml, followed by 24 h of incubation at 37°C. Displayed are TNFα concentrations in the conditioned media. The TNF ELISA is sensitive to < 2 pg per ml. (b) IL-12 inhibits UVB-induced fibroblast TNFα production. Fibroblasts were irradiated with 30 mJ per cm2 of UVB, or sham irradiated, and then given media without (minus symbols) or with (plus symbols) 10 ng IL-1α per ml, 10 ng IL-12 per ml, followed by 24 h of incubation at 37°C. Displayed are TNFα concentrations in the conditioned media. (c) IL-12 inhibits UVB-induced adult keratinocyte TNFα production in a dose-dependent manner. Adult keratinocytes were irradiated with 30 mJ per cm2 of UVB, 5 J per cm2 of UVA, or sham irradiated, and then given media with 0–5000 pg IL-12 per ml, followed by 24 h of incubation at 37°C. Displayed are TNFα concentrations in the conditioned media.
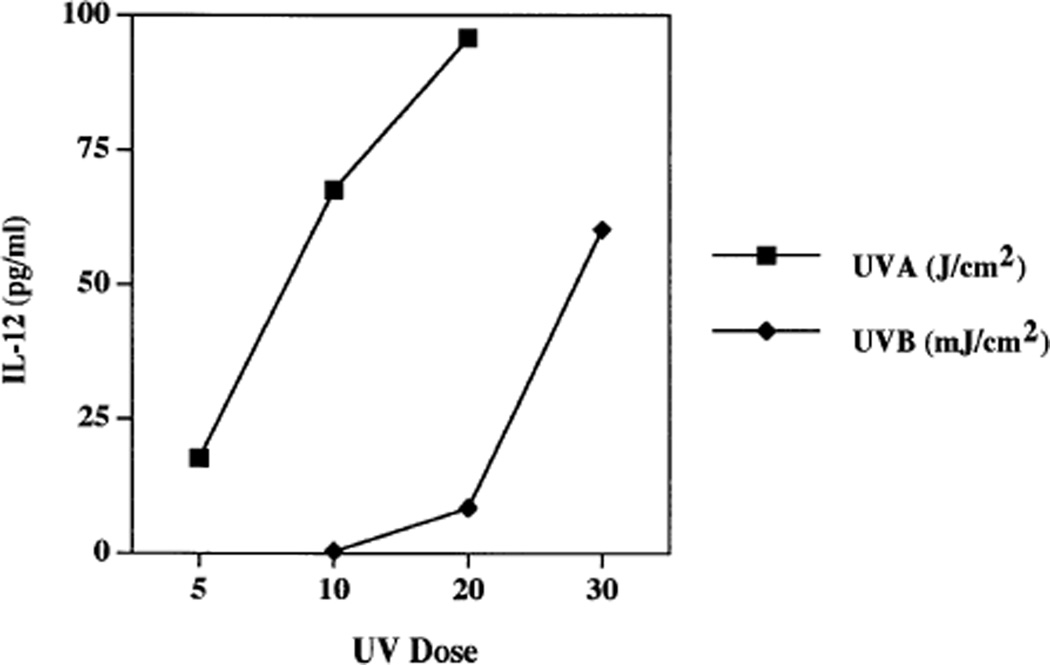
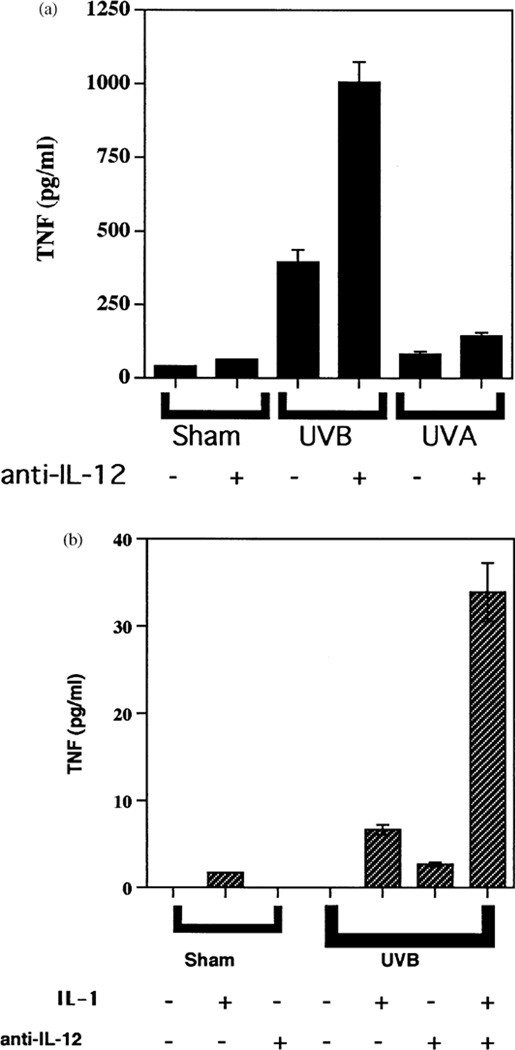
Because UVB, but not UVA, stimulates the secretion of TNFα by keratinocytes and fibroblasts (Werth and Zhang, 1999), and because IL-12 is a strong inhibitor of TNFα secretion (Fig 1), we next determined if there might be wavelength-specific induction of IL-12 secretion. Instead, we found that UVB and UVA each significantly induced secretion of the active IL-12 p70 heterodimer, in a dose-responsive fashion (Fig 2). This induction of IL-12 release raised the possibility that endogenously secreted IL-12 might regulate cellular output of TNFα. Thus, we examined TNFα secretion in the absence or presence of anti-IL-12 antibodies. TNFα levels from adult keratinocytes increased to 2.5 of the control value in the presence of UVB + anti-IL-12 antibodies relative to UVB alone (Fig 3a). Levels of TNFα seen in these experiments with adult keratinocytes (Figs 1c, 3a) were higher than those seen with neonatal keratinocytes (Fig 1a), but IL-12 consistently suppressed TNFα output from both cell types. In similar experiments with fibroblasts, TNFα increased 4-fold in the presence of UVB + IL-1α + anti-IL-12 antibodies relative to UVB + IL-1α alone (Fig 3b). Nevertheless, addition of anti-IL-12 antibodies to keratinocytes irradiated with UVA (5 J per cm2) had no effect on TNFα secretion (Fig 3a). Anti-IL-12 antibodies added to UVA-irradiated, IL-1α-treated fibroblasts failed to induce any detectable TNFα secretion (data not shown). These results indicate that the amount of autocrine or paracrine IL-12 produced by these cells after UVB irradiation is sufficient to partially suppress TNFα production, but that the inability of UVA to induce TNFα release by keratinocytes and fibroblasts is unrelated to endogenous IL-12.
Figure 2. UVA and UVB induce IL-12 release from human keratinocytes.
Keratinocytes were irradiated with UVA (5, 10, and 20 J per cm2) or UVB (10, 20, and 30 mJ per cm2), followed by 24 h of incubation at 37°C. Displayed are IL-12 concentrations in the conditioned media.
Figure 3. Anti-IL-12 antibodies increase TNFα production from UVB-irradiated cells.
(a) Anti-IL-12 antibodies increase TNFα release from UVB-irradiated adult keratinocytes. Adult keratinocytes were irradiated with 30 mJ per cm2 of UVB, 5 J per cm2 of UVA, or sham irradiated, and then given media without (minus symbols) or with (plus symbols) 10 µg anti-IL-12 antibody per ml followed by 24 h of incubation at 37°C. Displayed are TNFα concentrations in the conditioned media. (b) Anti-IL-12 antibodies increase TNFα release from UVB-irradiated fibroblasts. Fibroblasts were irradiated with 30 mJ per cm2 of UVB, or sham irradiated, and then given media without (minus symbols) or with (plus symbols) 10 ng IL-1α per ml, 10 µg anti-IL-12 antibody per ml followed by 24 h of incubation at 37°C. Displayed are TNFα concentrations in the conditioned media.
Lack of role for IFNγ or IL-1α signaling in the suppression of UVB-induced TNFα production by IL-12
IFNγ is stimulated by IL-12 and mediates some IL-12 effects (Seder et al, 1993). Nevertheless, we found that anti-γ-IFN antibodies had no effect on IL-12 suppression of TNFα (data not shown).
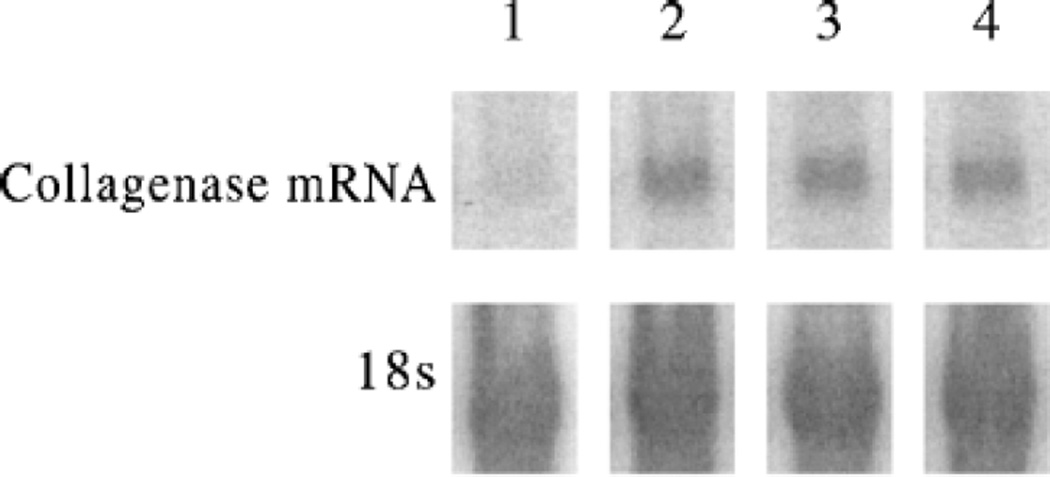
Induction of TNFα secretion by UVB is substantially enhanced in the presence of IL-1α, which keratinocytes secrete but which has to be provided exogenously to fibroblasts (Werth and Zhang, 1999). Thus, it is possible that IL-12 could suppress TNFα release by inhibiting IL-1α signaling. To explore this possibility, we examined collagenase mRNA, which is induced by IL-1α and by TNFα. Fibroblasts exposed to IL-1α (Fig 4, lane 2) showed increased collagenase mRNA relative to unirradiated, untreated sham cells (Fig 4, lane 1). Addition of 500 pg IL-12 per ml and IL-1α (Fig 4, lane 3) gave collagenase mRNA levels similar to IL-1α in the presence of anti-TNFα antibodies and IL-1α (Fig 4, lane 4). Anti-TNFα antibodies blocked some of the collagenase upregulation in UV-irradiated cells, but left IL-1α-mediated induction intact (data not shown). This result demonstrates that IL-12 does not block IL-1α mediated upregulation of collagenase mRNA, suggesting that IL-12 is affecting TNFα secretion independent of effects on IL-1α or its receptor.
Figure 4. IL-12 has no effect on IL-1α-mediated increase of collagenase.
Quantitative comparison of collagenase message levels. RNA was extracted from cultured control fibroblast monolayers (lane 1) 24 h after IL-1α (lanes 2–4), ±IL-12 (500 pg per ml) (lane 3), ±anti-TNFα antibody (lane 4), and analyzed by northern blot. The filters were hybridized sequentially with a collagenase cDNA probe and then rehybridized with the 18S probe.
IL-12 inhibits TNFα promoter activity
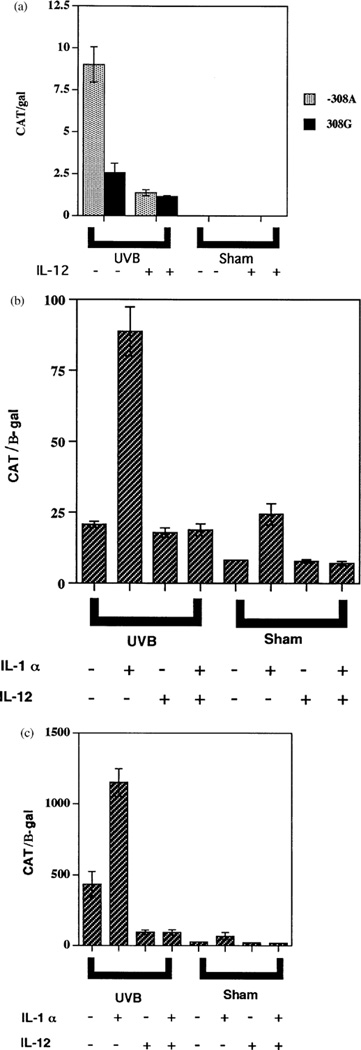
To evaluate the molecular mechanism for IL-12-induced suppression of TNFα release, we transiently transfected two TNFα promoter constructs (−308G wild-type and −308A variant) into human adult keratinocytes and mouse 3T3 fibroblasts. The transfected cells were irradiated with UVB (30 mJ per cm2) or sham, ±IL-12, and in the case of 3T3 cells, ±IL-1α. In transfected keratinocytes, UVB irradiation increased the activity of both TNFα promoter constructs, but −308A was 3.5 times more active than −308G (Fig 5a), consistent with our prior results (Werth et al, 2000). Addition of IL-12 (500 pg per ml) inhibited UVB-induced promoter activity by 55% (−308G) and 85% (−308 A; Fig 5a). In the transfected 3T3 fibroblasts, UVB in combination with IL-1α produced large increases in CAT activity relative to IL-1α alone, UVB alone, or untreated cells, and the −308 A construct was again substantially more active than −308G (compare Fig 5b, c). Addition of IL-12 produced large suppressions of both promoter constructs under nearly all conditions examined (Fig 5b, c). These results indicate that IL-12 acts on the TNFα promoter, on an element within the first 1173 bp.
Figure 5. IL-12 inhibits the −308A and −308G TNFα promoter.
Relative activities of TNFα−308G/β-gal construct following transfection into cells. Cells were irradiated with 30 mJ per cm2 of UVB or sham irradiated, and then given media without (minus symbols) or with (plus symbols) 10 ng IL-12 per ml, 10 ng IL-1α per ml (for 3T3 cells only), followed by 24 h of incubation at 37°C. Displayed are normalized levels of CAT expressed by the TNFα promoter construct. The bars represent means (±SEM) of triplicate transfections. (a) Human adult keratinocytes transfected with −308A and −308G TNFα promoter. (b) 3T3 cells transfected with −308G TNFα promoter. (c) 3T3 cells transfected with −308A TNFα promoter.
DISCUSSION
We have shown here that exogenous IL-12 blocks UVB-induced TNFα secretion by keratinocytes and fibroblasts. Furthermore, through addition of anti-IL-12 antibodies (Fig 3), we have found large regulatory effects of endogenous IL-12 on TNFα secretion in the absence of exogenously added IL-12. The increase in TNFα secretion seen upon addition of anti-IL-12 antibodies to irradiated keratinocytes and fibroblasts (Fig 3) also suggests that induction of endogenous IL-12 occurs prior to TNFα release. Many lines of evidence indicate that keratinocyte- or fibroblast-derived TNFα participates in UV-induced skin diseases (Werth et al, 2000; 2002), and our new results suggest that these processes can be controlled by exogenous or endogenous IL-12.
Prior literature and these studies provide some clues about the molecular mechanisms by which exogenous and endogenous IL-12 inhibits TNFα production. IL-12 was previously found to inhibit gene transcription and release of IL-10 from UVB-irradiated keratinocytes (Schmitt et al, 2000b). Importantly, UV-induced DNA damage has been reported to be the major mediator by which UV induces the release of TNFα and IL-10 (Nishigori et al, 1996; Kibitel et al, 1998), and IL-12 might inhibit UVB-induced apoptosis and DNA damage through induction of nucleotide-excision repair enzymes (Schwarz et al, 2002). Putting these results together, it is possible that IL-12 blocks UVB-induced release of TNFα and IL-10 through stimulation of DNA repair. Consistent with this idea, direct induction of DNA repair in vivo protects skin from UV-induced upregulation of both IL-10 and TNFα (Wolf et al, 2000).
At the level of the TNFα gene itself, our promoter transfection studies show a substantial effect of IL-12 at the level of transcription, via elements within the first 1173 bp of the TNFα promoter. The greater IL-12 sensitivity of the −308A promoter variant relative to −308G suggests that IL-12 may alter a transcription factor that binds either uniquely or more avidly to one of the polymorphic alleles. Nevertheless, the precise molecular steps linking UV irradiation and DNA damage to altered TNFα promoter activity are not known.
This inhibitory effect of IL-12 on keratinocytes and fibroblasts, which are nonimmune cells, contrasts with its effect on immune cells, where IL-12 is stimulatory or neutral on TNFα output (Adorini, 1999; Xing et al, 2000). Prior work has shown that IL-12 shifts inflammatory cells fromTh2 to Th1 (Adorini, 1999), and that IL-12 is required to maintain Th1 responses (Stobie et al, 2000). Thus, incorporating our results, IL-12 shifts the overall pattern of TNFα output away from nonimmune cells such as keratinocytes and fibroblasts and specifically towards Th1 immune cells. This shift could serve important global regulatory roles, such as decreasing TNFα-mediated apoptosis of keratinocytes, thereby diminishing one source of self-antigen and enhancing immune responses against exogenous infections.
As summarized before, several photosensitive diseases have been associated with the −308A TNFα promoter variant, and studies in vitro show a substantially enhanced response of this promoter variant to UVB (Werth et al, 2000). Nevertheless, many patients with each of these diseases do not carry the −308A polymorphism, suggesting that other regulatory factors could be involved. Based on our findings, such factors could include IL-10 polymorphisms and genetic variants of factors that are known to regulate IL-12 or responses to IL-12.
From the standpoint of pathogenesis and therapeutics of photosensitive diseases, an important difference between UVB and UVA at these doses is that UVB increased the secretion of both TNFα (Fig 1a, b) and IL-12 (Fig 2), whereas UVA stimulated secretion of only IL-12 (Fig 2) without increasing TNFα (Fig 1a). Because we have now found that IL-12, both in physiologic and supraphysiologic doses, inhibits TNFα secretion from nonimmune cells, it is likely that UVA or direct administration of IL-12 could lessen or eliminate some of the TNFα-mediated effects of UVB. This model provides an attractive explanation for the prior finding that prior irradiation with UVA can blunt the ability of UVB to inhibit contact hypersensitivities in mice (Reeve et al, 1998); this effect of UVB is mediated by TNFα (Bromberg et al, 1992; Yoshikawa et al, 1992). In addition, a few studies have suggested that UVA is therapeutic for some patients with lupus erythematosus, particularly photosensitive forms such as subacute cutaneous lupus erythematosus (McGrath, 1994); our results imply that IL-12 might be helpful as well. Conversely, in situations where IL-12 has been found to be therapeutic, such as cutaneous T cell lymphoma or enhancement of vaccine responses, selective UVA therapy might also provide a benefit.
We have found that IL-12 suppresses TNFα production by keratinocytes and fibroblasts. Because TNFα is involved in the pathogenesis of certain photosensitive skin diseases (Werth et al, 2000), IL-12 and stimuli, such as UVA, that specifically increase IL-12 could play important physiologic, pathophysiologic, and therapeutic roles.
Acknowledgments
We thank Kevin Jon Williams, M.D. (Department of Medicine, Thomas Jefferson School of Medicine, Philadelphia, PA), and Paul Stein, Ph.D. (University of Pennsylvania), for critical review of the manuscript and Pamela Jensen, Ph.D. (University of Pennsylvania), for assistance with growing keratinocytes. The work is supported in part by grants from the Lupus Foundation of the Delaware Valley, Lupus Research Institute, a V.A. Merit Review Grant, and the National Institutes of Health (1K24AR002207-01).
Footnotes
This work was presented at the Society of Investigative Dermatology on May 11, 2001 and is published in abstract form in the Journal of Investigative Dermatology 117: 498, 2001.
REFERENCES
- Adorini L. Interleukin-12, a key cytokine inTh1-mediated autoimmune diseases. Cell Mol Life Sci. 1999;55:10–25. doi: 10.1007/s000180050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JS, Chavin KD, Kunkel SL. Anti-tumor necrosis factor antibodies suppress cell-mediated immunity in vivo. J Immunol. 1992;148:3412–3417. [PubMed] [Google Scholar]
- Brown EL, Rivas JM, Ullrich SE, Young CR, Norris SJ, Kripke ML. Modulation of immunity to Borrelia burgdorferi by ultraviolet irradiation: differential effect on Th1 and Th2 immune responses. Eur J Immunol. 1995;25:3017–3022. doi: 10.1002/eji.1830251105. [DOI] [PubMed] [Google Scholar]
- Chung JH, Youn SH, Koh WS, Eun HC, Cho KH, Park KC, Youn JI. Ultraviolet B irradiation-enhanced interleukin (IL)-6 production and mRNA expression are mediated by IL-1α in cultured human keratinocytes. J Invest Dermatol. 1996;106:715–720. doi: 10.1111/1523-1747.ep12345608. [DOI] [PubMed] [Google Scholar]
- DeKruyff RH, Fang Y, Wolf SF, Utmetsu DT. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen presenting cells. J Immunol. 1995;154:2578–2587. [PubMed] [Google Scholar]
- Eberlein-Konig B, Jager C, Przybilla B. Ultraviolet B radiation-induced production of interleukin 1 alpha and interleukin 6 in a human squamous carcinoma cell line is wavelength-dependent and can be inhibited by pharmacological agents. Br J Dermatol. 1998;139:415–421. doi: 10.1046/j.1365-2133.1998.02404.x. [DOI] [PubMed] [Google Scholar]
- Enk CD, Sredni D, Blauvelt A, Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154:4851–4856. [PubMed] [Google Scholar]
- Fujisawa H, Wang B, Kondo S, Shivji GM, Sauder DN. Costimulation with ultraviolet B and interleukin-1 alpha dramatically increases tumor necrosis factor-α production in human dermal fibroblasts. J Interferon Cytokine Res. 1997;17:307–313. doi: 10.1089/jir.1997.17.307. [DOI] [PubMed] [Google Scholar]
- Grewe M, Gyufko K, Krutmann J. Interleukin-10 production by cultured human keratinocytes: regulation by ultraviolet B and ultraviolet A1 radiation. J Invest Dermatol. 1995;104:3–6. doi: 10.1111/1523-1747.ep12613446. [DOI] [PubMed] [Google Scholar]
- Kibitel J, Hejmadi V, Alas L, O’Connor A, Sutherland BM, Yarosh D. UV-DNA damage in mouse and human cells induces the expression of tumor necrosis factor α. Photochem Photobiol. 1998;67:541–546. [PubMed] [Google Scholar]
- Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, Luger TA. Human keratinocytes are a source for tumor necrosis factor α: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Kono T, Sauder DN, Mckenzie RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol. 1993;101:690–694. doi: 10.1111/1523-1747.ep12371677. [DOI] [PubMed] [Google Scholar]
- Kostense S, Sun WH, Cottey R, et al. Interleukin 12 administration enhances Th1 activity but delays recovery from influenza A virus infection in mice. Antiviral Res. 1998;38:117–130. doi: 10.1016/s0166-3542(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Kremer IB, Hilkens CM, Sylva-Steenland RM, Koomen CW, Kapsenberg ML, Bos JD, Teunissen MB. Reduced IL-12 production by monocytes upon ultraviolet-B irradiation selectively limits activation of T helper-1 cells. J Immunol. 1996;157:1913–1918. [PubMed] [Google Scholar]
- Kurimoti I, Streilein JW. Tumor necrosis factor-α impairs contact hypersensitivity induction after ultraviolet B radiation via TNF-receptor 2. Exp Dermatol. 1999;8:495–500. doi: 10.1111/j.1600-0625.1999.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Ma X. TNF-α and IL-12: a balancing act in macrophage functioning. Microbes Infection. 2001;3:121–129. doi: 10.1016/s1286-4579(00)01359-9. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Secrist H, DeKruyff RH, Wolf SF, Umetso DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- Matsuo R, Kobayashi M, Herndon DN, Pollard RB, Suzuki F. Interleukin-12 protects thermally injured mice from herpes simplex virus type 1 infection. J Leukoc Biol. 1996;59:623–630. doi: 10.1002/jlb.59.5.623. [DOI] [PubMed] [Google Scholar]
- McGrath H., Jr Ultraviolet-A1 irradiation decreases clinical disease activity and autoantibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1994;12:129–135. [PubMed] [Google Scholar]
- Mohamadzadeh M, Takashima A, Dougherty I, Knop J, Bergstresser PR, Cruz PD., Jr Ultraviolet B radiation up-regulates the expression of IL-15 in human skin. J Immunol. 1995;155:4492–4496. [PubMed] [Google Scholar]
- Nagayama H, Sato K, Kawasaki H, et al. IL-12 responsiveness and expression of IL-12 receptor in human peripheral blood monocyte-derived dendritic cells. J Immunol. 2000;165:59–66. doi: 10.4049/jimmunol.165.1.59. [DOI] [PubMed] [Google Scholar]
- Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc Natl Acad Sci USA. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, Chen EH. TNFα-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor α, disease duration, and pathologic calcifications. Arthr Rheumatol. 2000;43:2368–2377. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Reeve VE, Bosnic M, Boehm-Wilcox C, Nishimura N, Ley RD. Ultraviolet A radiation (320–400 nm) protects hairless mice from immunosuppression induced by ultraviolet B radiation (280–320 nm) or cis-urocanic acid. Int Arch Allergy Immunol. 1998;115:316–322. doi: 10.1159/000069463. [DOI] [PubMed] [Google Scholar]
- Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- Schmitt DA, Ullrich SE. Exposure to ultraviolet radiation causes dendritic cells/macrophages to secrete immune-suppressive IL-12p40 homodimers. J Immunol. 2000;165:3162–3167. doi: 10.4049/jimmunol.165.6.3162. [DOI] [PubMed] [Google Scholar]
- Schmitt DA, Walterscheid JP, Ullrich SE. Reversal of ultraviolet radiation-induced immune suppression by recombinant interleukin-12: suppression of cytokine production. Immunology. 2000;101:90–96. doi: 10.1046/j.1365-2567.2000.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Grabbe S, Aragane Y, et al. Interleukin-12 prevents ultraviolet B-induced local immunosuppression and overcomes UVB-induced tolerance. J Invest Dermatol. 1996;106:1187–1191. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Stander S, Berneburg M, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nature Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobie L, Guranathan S, Prussin C, Sacks DL, Glaichenhaus N, Wu CY, Seder RA. The role of antigen and IL-12 in sustaining Th1memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc Natl Acad Sci USA. 2000;97:8427–8432. doi: 10.1073/pnas.160197797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, Wooten C, Schmeckpeper BJ, Goldman D, Petri MA. A promoter polymorphism of tumor necrosis factor α associated with systemic lupus erythematosus in African-Americans. Arthr Rheum. 1997;40:2207–2211. doi: 10.1002/art.1780401215. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Does exposure to UV radiation induce a shift to a Th-2-like immune reaction? Photochem Photobiol. 1996;64:254–258. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- de Vos S, Brach M, Budnik A, Grewe M, Herrmann F, Krutmann J. Post-transcriptional regulation of interleukin-6 gene expression in human keratinocytes by ultraviolet B radiation. J Invest Dermatol. 1994;103:92–96. doi: 10.1111/1523-1747.ep12391818. [DOI] [PubMed] [Google Scholar]
- Werth VP, Zhang W. Wavelength-specific synergy between ultraviolet radiation and interleukin-1α in the regulation of matrix-related genes: mechanistic role for tumor necrosis factor-α. J Invest Dermatol. 1999;113:196–201. doi: 10.1046/j.1523-1747.1999.00681.x. [DOI] [PubMed] [Google Scholar]
- Werth VP, Williams KJ, Fisher EA, Bashir M, Rosenbloom JR, Shi X. UVB irradiation alters cellular responses to cytokines: role in extracellular matrix gene expression. J Invest Dermatol. 1997;108:290–294. doi: 10.1111/1523-1747.ep12286462. [DOI] [PubMed] [Google Scholar]
- Werth VP, Zhang W, Dortzbach K, Sullivan K. Association of a promoter polymorphism of TNFα with subacute cutaneous lupus erythematosus and distinct photoregulation of transcription. J Invest Dermatol. 2000;115:726–730. doi: 10.1046/j.1523-1747.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- Werth VP, Callen JP, Ang G, Sullivan KE. Associations of tumor necrosis factor-α (TNFα) and HLA polymorphisms with adult dermatomyositis: implications for a unique pathogenesis. J Invest Dermatol. 2002;119:617–620. doi: 10.1046/j.1523-1747.2002.01869.x. [DOI] [PubMed] [Google Scholar]
- Wolf P, Maier H, Mullegger RR, et al. Topical treatment with liposomes containing T4 endonuclease V protects human skin in vivo from ultraviolet-induced upregulation of interleukin-10 and tumor necrosis factor-α. J Invest Dermatol. 2000;114:149–156. doi: 10.1046/j.1523-1747.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- Xing Z, Zganiacz A, Santosuosso M. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-α and nitric oxide from macrophages via IFN-α induction. J Leukoc Biol. 2000;68:897–902. [PubMed] [Google Scholar]
- Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Kurimoto I, Streilein JW. Tumour necrosis factor-α mediates ultraviolet light B-enhanced expression of contact hypersensitivity. Immunology. 1992;76:264–271. [PMC free article] [PubMed] [Google Scholar]