Abstract
As highly social animals, Indo-Pacific humpback dolphins (Sousa chinensis) exhibit community differentiation. Nevertheless, our understanding of the external and internal factors influencing these dynamics, as well as their spatiotemporal variations, is still limited. In the present study, variations in the social structure of an endangered Indo-Pacific humpback dolphin population in Xiamen Bay, China, were monitored over two distinct periods (2007–2010 and 2017–2019) to analyze the effects of habitat utilization and the composition of individuals within the population. In both periods, the population demonstrated a strikingly similar pattern of social differentiation, characterized by the division of individuals into two main clusters and one small cluster. Spatially, the two primary clusters occupied the eastern and western waters, respectively, although the core distribution area of the eastern cluster shifted further eastward between the two periods. Despite this distribution shift, the temporal stability of the social structure and inter-associations within the eastern cluster remained unaffected. A subset of 16 individuals observed in both periods, comprising 51.6% and 43.2% of the population in each respective period, emerged as a foundational element of the social structure and may be responsible for sustaining social structure stability, especially during the 2007–2010 period. These observations suggest that the composition of dominant individuals, an internal factor, had a more substantial influence on the formation of the social network than changes in habitat use, an external factor. Consequently, the study proposes distinct conservation measures tailored to each of the two main clusters.
Keywords: Social differentiation, Social structure, Sousa chinensis, Dynamics, Conservation
INTRODUCTION
Social structure is an important ecological concept that integrates behavioral interactions and ecological relationships among conspecifics (Lehmann & Boesch, 2004; Whitehead, 1997). This structure can affect many aspects of a population, including its growth, genetics, and movements (Whitehead, 2009). Consequently, exploring the social structure of group-living animals is vital for understanding their ecological characteristics and for informing their conservation and management strategies (Chan et al., 2022; Davies et al., 2012; Parra et al., 2011).
Social networks represent static depictions of dynamic societies. However, the patterns of association within these networks are subject to continual fluctuations over time and space (Aureli et al., 2008; Cantor et al., 2012). Thus, network analysis is a powerful tool in sociality studies (Sosa et al., 2021; Titcomb et al., 2015), particularly for determining the spatiotemporal dynamics of fission-fusion populations (James et al., 2009). Differences in ranging behavior can influence social interactions spatially (Clutton-Brock, 1989; Titcomb et al., 2015), as proximity facilitates communication among individuals (Kossinets & Watts, 2006). Moreover, stochastic processes such as birth, death, and movement can shape interaction patterns within social systems over time (Lehmann & Boesch, 2004). Therefore, an effective description of social patterns needs to encompass an appropriate spatiotemporal scale (Cantor et al., 2012).
Indo-Pacific humpback dolphins (Sousa chinensis), which inhabit coastal areas, exhibit a fission-fusion social pattern. Populations of S. chinensis in the Pearl River Estuary (Chan et al., 2022; Dungan et al., 2012) and eastern Taiwan Strait (Sousa chinensis taiwanensis) (Dungan et al., 2016; Wang et al., 2015a) are reported to form distinct clusters and display strong intra-cluster associations. In contrast, populations in the waters of Hong Kong and Zhanjiang appear to show more casual and short-term associations (Jefferson, 2000; Jefferson & Karczmarski, 2001; Xu et al., 2012). The social structure of Sousa populations is primarily influenced by factors such as resource availability, male mating opportunities, and parental requirements of nursing females (Gowans et al., 2007; Hunt et al., 2019). Nevertheless, the degree to which external factors, such as habitat use, drive the differentiation of clusters and their subsequent impact on the social structure, is yet to be extensively investigated.
In this study, we investigated the social structure of an endangered S. chinensis population in Xiamen Bay, China, where they are considered residents (Chen et al., 2008; Huang & Liu, 2000; Zeng et al., 2020). Prior research identified consistent patterns of social differentiation within this population, dividing individuals into two discrete clusters (Chen et al., 2011; Wang et al., 2015b). However, the spatiotemporal dynamics of this social structure over an extended time frame (10 years) and the internal-external influencing factors have not yet been fully explored. To address this gap, we explored and compared the spatiotemporal dynamics of the social structure of this endangered S. chinensis population between 2007–2010 and 2017–2019, focusing on the effects of external factors on ranging patterns and core habitat use, as well as the impact of internal factors on the composition of individuals. Based on existing research results (Chen et al., 2011; Wang et al., 2015b), we hypothesized that similar social interactions would persist across the two periods. Furthermore, given the previous identification of two distinct clusters (Chen et al., 2011; Wang et al., 2015b), we hypothesized that habitat use would be a contributing factor to this cluster segregation.
MATERIALS AND METHODS
Study area
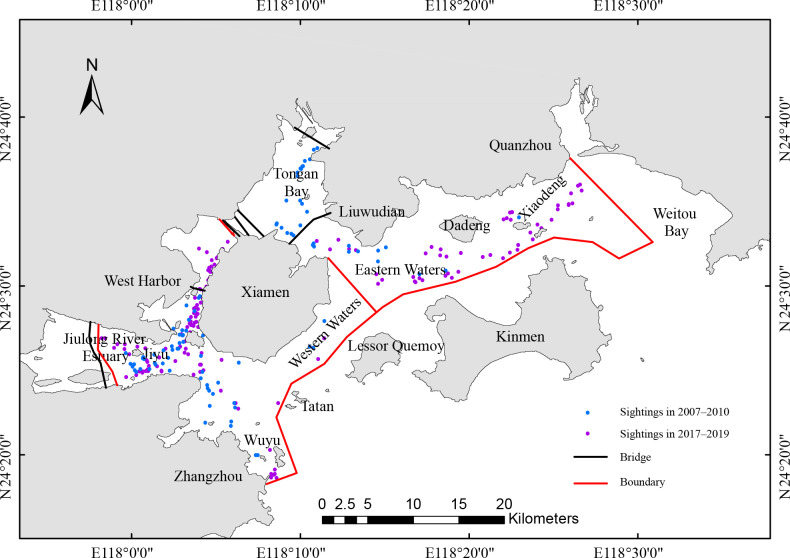
The study area comprised Xiamen Bay, Zhangzhou, Kinmen, and Quanzhou, covering about 700 km2 along the southeastern coast of China. Characterized by semidiurnal tidal variations, the region is influenced by the Jiulong River, especially to the west of Xiamen, which provides a crucial freshwater source (Chen et al., 2011). The area also serves as one of the main habitats for S. chinensis in China (Chen et al., 2018). The study area was subdivided into eastern (area 1) and western waters (area 2) (Figure 1).
Figure 1.
Study area in Xiamen Bay and adjacent waters
Bold red line defines the boundary of the study area, with the area divided into eastern (area 1) and western (area 2) parts.
Data collection
Vessel-based surveys were conducted during two periods: 2007–2010 and 2017–2019. Weather permitting, the research vessel followed pre-designated transects at a speed of 10–15 km/h. The survey vessel, measuring 25 m in length, was equipped with a 3 m high viewing platform. A minimum of two experienced observers searched for dolphins either with the naked eye or through 10×50 mm binoculars. Upon locating dolphins, the vessel approached slowly, occasionally halting the engine. Digital photographs were captured using a Canon EOS 1Dx Mark II camera with a 100–400 (or 28–300) mm zoom lens (Canon, Japan) (Chen et al., 2018). Overall, 202 and 179 field surveys were conducted in 2007–2010 and 2017–2019, respectively, resulting in 76 and 154 dolphin group sightings along the 6 297.9 km and 7 620.5 km survey tracks, respectively (Table 1, Supplementary Figure S1). Due to the long sampling interval between the two periods, the datasets from each period were analyzed independently to explore the spatiotemporal dynamics of the social structure.
Table 1. Survey efforts in Xiamen Bay from 2007 to 2019.
| Year | No. (d) | Survey track (km) | Sampling effort (h) |
| 2007 | 74 | 1 700.7 | 367.4 |
| 2008 | 78 | 2 859.6 | 330.1 |
| 2009 | 32 | 1 152.1 | 128.3 |
| 2010 | 18 | 585.5 | 68.2 |
| 2017 | 14 | 585.9 | 65.1 |
| 2018 | 111 | 4 782.7 | 522.6 |
| 2019 | 54 | 2 251.9 | 268.5 |
| Total | 381 | 13 918.4 | 1 750.2 |
Photo-identification
Individual identification of the dolphins was based on distinct characteristics such as body color, spots, dorsal fin shape and defects, and trauma-related injuries. At the start of the first survey, each newly identified individual was assigned a unique catalog number. Of note, the numbering systems for individuals in 2007–2010 and 2017–2019 were independent of each other. Subsequently, photographs from each survey were compared with the most recent catalog. Dolphins were recorded as new individuals only if they exhibited obvious differences. To prevent double counting, photographs were taken of the dorsal fin of each dolphin on both sides where possible. The photographs were graded as ‘excellent’, ‘good’, or ‘poor’ according to the clarity, contrast, angle, and size of the dorsal fin within the frame (Chen et al., 2018; Hunt et al., 2017). To maximize identification accuracy, only photographs rated as excellent or good were used. Additionally, individuals identified in both the 2007–2010 and 2017–2019 periods were compared to determine the presence of identical individuals (IIs).
Association patterns
Individuals were considered to be associated when they were sighted in the same group within a 150 m radius of each other (Whitehead, 2008a). The coefficient of association between the individuals was estimated using the half-weight association index (HWI), with the value varying from 0 (two dolphins never sighted in association) to 1 (two dolphins always sighted in association) (Bejder et al., 1998). The HWI was calculated using the following formula:
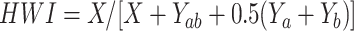 |
1 |
where X is the number of sightings of dolphin a and b in one group; Yab is the number of sightings of dolphin a and b in different groups; Ya is the number of sightings of dolphin a; Yb is the number of sightings of dolphin b (Cairns & Schwager, 1987). Analysis was conducted using SOCPROG v2.9 (Whitehead, 2009). The sampling interval was set to 1 d (Whitehead, 2008a). If a group was sighted more than one time on the same day, only the first sighting was used for analysis (Cantor et al., 2012). For all analyses, except for movement analysis, only individuals sighted at least five times during the study period were selected to avoid spurious associations.
Social differentiation (S) and correlation between the true and estimated association indices (r) were calculated based on the maximum-likelihood method (Table 2) (Whitehead, 2008b). Preferred or avoided associations were determined using a Monte Carlo permutation test (Bejder et al., 1998). The permutation test option “permute groups within samples” was selected in SOCPROG v2.9. The null hypothesis was that individuals associated with others at random. A higher standard deviation (SD) and coefficient of variation (CV) of HWI in the real datasets than those at random indicated that social preferences were occurring among the populations, and the null hypothesis was rejected (Whitehead, 2008a). A two-tailed test was used to determine significance, with the critical value set to 0.05 (Whitehead, 2008b). The tests were run with 10 000 random permutations, which increased by 1 000 trials per permutation until the P-values stabilized.
Table 2. Definitions of social association parameters.
| Parameter | Definition |
| Social differentiation (S) | S is the proportion of time spent together between individuals and a measure of social structure variability. S value of less than 0.3 indicates a homogenous society, greater than 0.5 indicates a well-differentiated society, and greater than 2 indicates extremely differentiated societies in the population (Whitehead, 2009). |
| Correlation between true and estimated association indices (r) | r represents the power of the result to detect the true social system. A value less than 0.4 indicates a general result, greater than 0.8 indicates a reliable result (Whitehead, 2008b). |
| Modularity (Q) | Q, for a defined set of clustered individuals, is the difference between the observed and expected proportion of associations within clusters. A modularity value above 0.3 indicates a credible result for individuals divided into their respective communities (Newman, 2004). |
Cluster division, hierarchical cluster analysis, and social network parameters
The potential communities in the population were assessed using Newman’s modularity (Q) method (Table 2) (Newman, 2006). Generally, the association indices were higher between individuals in the same community than those in different communities.
A sociogram was visualized using NETDRAW v2.158 (Borgatti, 2002) to illustrate the social connections among individuals. Four network parameters were applied, namely, strength, eigenvector centrality, reach, and clustering coefficient. Strength was the sum of the association indices of a dolphin with all other individuals, with higher values representing a preference for larger groups (Bouveroux et al., 2019). Eigenvector centrality was the measure of the connectedness of an individual in the network, with higher values representing an individual with high gregariousness (an individual’s tendency to form associations (Pepper et al., 1999)) and/or an individual associated with individuals with high gregariousness (Sosa et al., 2021). Reach was the indirect connectedness of an individual in the population, with higher values indicating that the individual was indirectly associated with many other individuals (Bouveroux et al., 2019; Sosa et al., 2021). The clustering coefficient was the measure of how well an individual’s associates were themselves associated (Titcomb et al., 2015). The values of the four parameters are significantly greater than expected at random in highly interconnected and cohesive social networks. Significance was assessed using two-tailed permutation tests with 10 000 Bejder matrix permutations, as described in Lusseau et al. (2008).
Average-linkage hierarchical cluster analysis was applied to explore the relationships among individuals, displayed as a dendrogram (Whitehead, 2008a). The cophenetic correlation coefficient (CCC) was used to determine the credibility of the dendrogram. The CCC value varies from 0 to 1, with a value above 0.8 indicating an acceptable dendrogram (Whitehead, 2009).
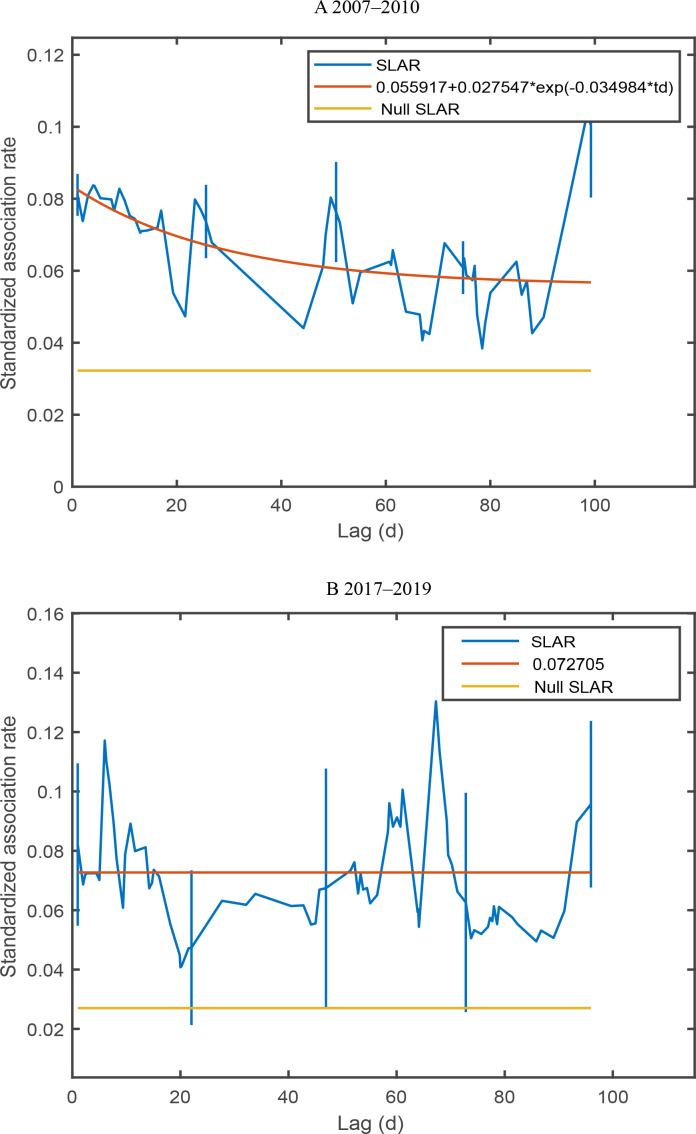
Temporal patterns of association
Changes in association over time were assessed using standardized lagged association rate (SLAR) analysis (Whitehead, 1995). The standardized null association rate (SNAR) was also calculated, depicting the theoretical SLAR under random association conditions (Whitehead, 2008a). Standard errors (SE) of SLAR and SNAR were determined using a jackknife procedure (Whitehead, 1995). Fission-fusion dynamics are characterized by three elements: constant/preferred companionships, casual acquaintances, and rapid dissociations (Whitehead, 1995). Consequently, four theoretical models delineating temporal association patterns were incorporated into the analysis. The best model was selected based on the lowest quasi-Akaike’s Information Criterion (QAIC) score (Whitehead, 2007). A difference in the QAIC of the other models and the optimal model (ΔQAIC) of less than two suggested substantial support for the model, while a ΔQAIC value less than seven indicated a less well-supported model and a ΔQAIC value above 10 indicated an unsupported model (Burnham & Anderson, 2002).
Spatial patterns
We investigated the overlap in habitat ranges among distinct clusters of dolphins, segregated using Newman’s modularity method, to assess the impact of geographic area on their social structure. First, the range sizes of the different clusters were estimated using the fixed kernel (Parra, 2006) and minimum complex polygon (MCP) approaches (Hayne, 1949). For the fixed kernel method, smoothing parameters were estimated using least-squares cross-validation, providing the least-biased estimates for the 95% and 50% habitat range areas (Seaman et al., 1999). Second, the overlap between the two clusters was calculated using the intersect tool in ArcMap 10.2. The proportion of habitat range overlap was determined following the method outlined in Cederlund & Sand (1994) and Atwood & Weeks (2003).
Movement analyses
Movement analysis was used to evaluate how dolphins moved across the survey areas. Movements between the western and eastern waters were estimated using the lagged identification rate (LIR). This parameter represented the probability of a dolphin being identified in either the western or eastern waters at any given time and was determined based on a single identification conducted after a given time lag (Whitehead, 2001). The LIRs were calculated for the same and different areas. Based on the LIRs, movements within and between areas were assessed (Whitehead, 2009). The SEs of the LIRs were estimated using a bootstrap procedure. The best model was confirmed based on the lowest QAIC value, as described above (Whitehead, 2007).
RESULTS
Survey effort
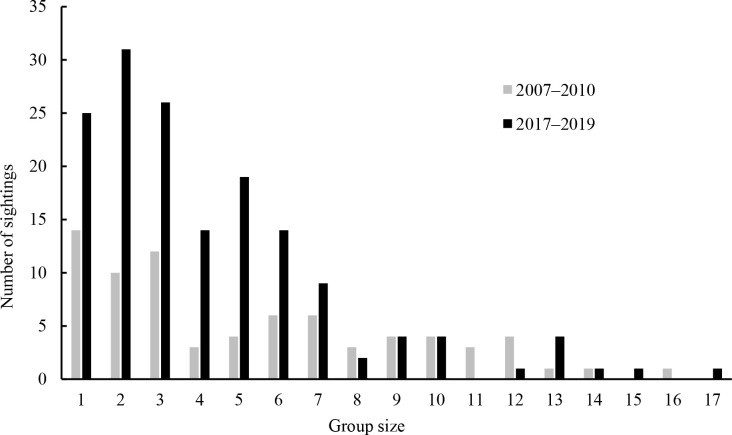
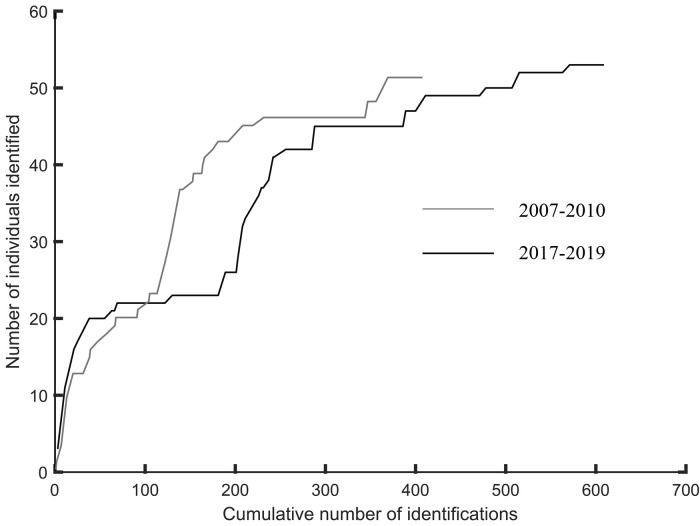
Group size ranged from 1 to 16 in 2007–2010, with an average of 5.35 (SE=3.89) and from 1 to 17 in 2017–2019, with an average of 4.24 (SE=3.19) (Figure 2). During the study, 52 and 55 individuals were successfully identified in 2007–2010 and 2017–2019, respectively, including 16 individuals identified in both periods (Supplementary Table S1). The discovery curve almost reached stability, indicating the high rate at which the individuals entered the dataset (Figure 3). Notably, 60% (n=31) and 65% (n=37) of the identified individuals were sighted at least five times in 2007–2010 and 2017–2019, respectively. These individuals were chosen for further association and network analyses.
Figure 2.
Frequency distribution of group sizes of Indo-Pacific humpback dolphins in Xiamen Bay over sampling years 2007–2010 and 2017–2019
Figure 3.
Discovery curve of number of individual Indo-Pacific humpback dolphins identified vs cumulative number of identifications in Xiamen Bay over sampling years 2007–2010 and 2017–2019
Association patterns
Overall mean HWI values among dolphins were 0.21 (SD=0.08, range: 0.05–0.30) and 0.12 (SD=0.04, range: 0.04–0.17), with a maximum HWI of 0.67 (SD=0.19, range: 0.24–0.92) and 0.61 (SD=0.18, range: 0.17–0.9) in 2007–2010 and 2017–2019, respectively. The estimated S scores were 1.052 (SE=0.100) and 1.216 (SE=0.119), respectively, for the two periods, indicating a well-differentiated society for the dolphin population in Xiamen Bay. The r values in 2007–2010 and 2017–2019 were 0.856 (SE=0.019) and 0.815 (SE=0.018), respectively, suggesting a precise representation of the true pattern. In 2007–2010, the SD and CV values of the association indices were higher for the real values than those at random (HWIreal SD=0.24414, HWIrandom SD=0.24334, P=0.0094; HWIreal CV=1.16326, HWIrandom CV=1.15960, P=0.0124; Table 3), indicating that some dolphins preferentially associated with others during the study period. However, the proportion of non-zero association indices was not lower for the real value than that at random (real=0.60860, random=0.60716, P=0.8370; Table 3), suggesting that avoided associations did not occur during this period. Similar preferential associations (HWIreal SD=0.16880, HWIrandom SD=0.16517, P=0.0010; HWIreal CV=1.43462, HWIrandom CV=1.40602, P=0.0000) and avoided associations (real=0.47297, random=0.47747, P=0.0240; Table 3) were detected in 2017–2019.
Table 3. Tests for preferred and avoided associations.
| Period | Parameter | Real | Random | P |
| SD: Standard deviation. CV: Coefficient of variation. P: P-value. | ||||
| 2007–2010 | Mean association index | 0.20988 | 0.20985 | |
| SD | 0.24414 | 0.24334 | 0.0094 | |
| CV | 1.16326 | 1.15960 | 0.0124 | |
| Proportion non-zero elements | 0.60860 | 0.60716 | 0.8370 | |
| Mean non-zero elements | 0.34485 | 0.34562 | 0.8667 | |
| SD non-zero elements | 0.22667 | 0.22491 | 0.0002 | |
| CV non-zero elements | 0.65729 | 0.65075 | 0.0004 | |
| 2017–2019 | Mean association index | 0.11766 | 0.11747 | |
| SD | 0.16880 | 0.16517 | 0.0010 | |
| CV | 1.43462 | 1.40602 | 0.0000 | |
| Proportion non-zero elements | 0.47297 | 0.47747 | 0.0240 | |
| Mean non-zero elements | 0.24877 | 0.24604 | 0.0110 | |
| SD non-zero elements | 0.16621 | 0.15969 | 0.0010 | |
| CV non-zero elements | 0.66812 | 0.64905 | 0.0030 | |
Social cluster and network
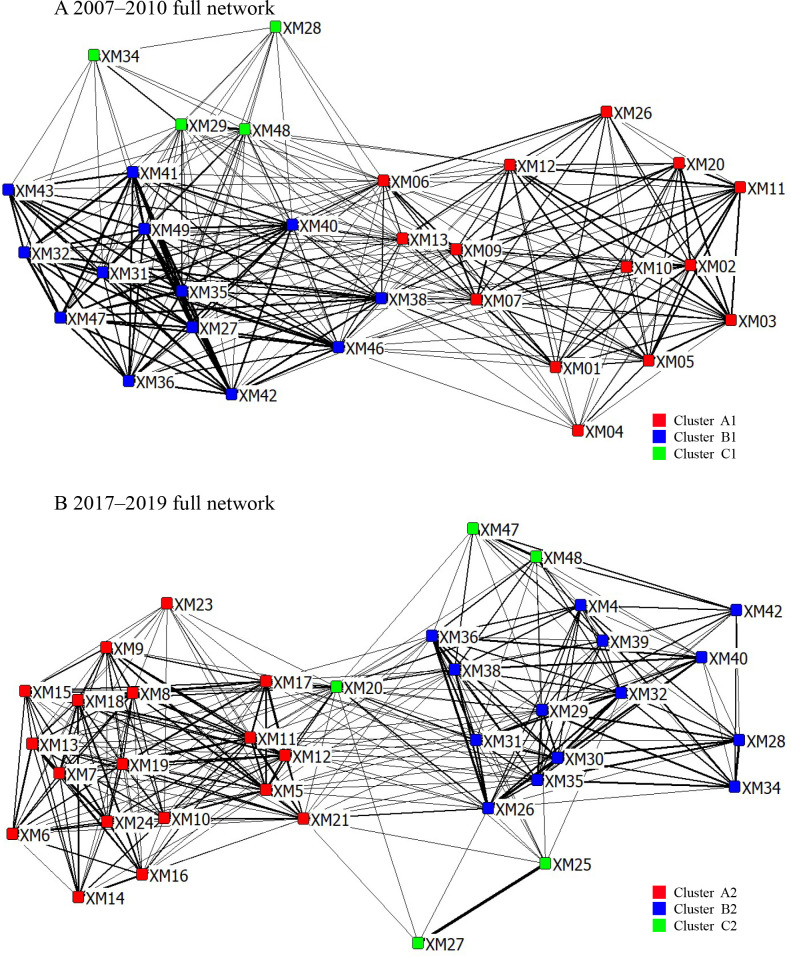
Using Newman’s eigenvector method, the dolphin population was divided into three clusters, exhibiting maximum Q-values of 0.400 (modularity=1; from gregariousness) and 0.447 in 2007–2010 and 2017–2019, respectively. The maximum modularity was higher than 0.3, suggesting that the three clusters (two main clusters and one small cluster) were a credible division. Specifically, in 2007–2010, Cluster A1 consisted of 14 individuals, Cluster B1 consisted of 13 individuals, and Cluster C1 consisted of four individuals. Visualization of the social network showed the division of the clusters (Figure 4A). In 2017–2019, the three clusters contained 18 (Cluster A2), 14 (Cluster B2), and five (Cluster C2) individuals (Figure 4B). Moreover, the CCC values exceeded 0.8 in both periods (0.93987 in 2007–2010; 0.9002 in 2017–2019), thereby validating the dendrogram as a credible estimate (Supplementary Figure S2).
Figure 4.
Sociogram of Indo-Pacific humpback dolphins consisting of two main clusters and one small cluster
Each node represents an individual dolphin. Numbers within each node represent individual dolphin ID codes. Edge-thickness is proportional to dyadic association index. A: 2007–2010 full network, cluster A1 in red, cluster B1 in blue, and cluster C1 in green. B: 2017–2019 full network, cluster A2 in red, cluster B2 in blue, and cluster C2 in green.
In 2007–2010 and 2017–2019, characterization of the social network of the population showed that the reach and clustering coefficient were not significantly different from random, while eigenvector centrality was significantly lower than expected (Table 4). The dataset was restricted to contain only II sightings, and no individuals were excluded in 2007–2010. The tests for significant differences in the four network parameters were consistent with the findings in the full network. However, eigenvector centrality was not significantly different from what was expected in 2017–2019 for II sightings (Table 4).
Table 4. Social network measures calculated for Indo-Pacific humpback dolphins in Xiamen Bay (mean±SE).
| Strength (SE) | P | Eigenvector centrality (SE) | P | Reach (SE) | P | Clustering coefficient (SE) | P | |
| SE: Standard error. P: P-value. *: P<0.025 or P>0.975. | ||||||||
| 2007–2010 full network | 6.30 (2.28) | 0.81 | 0.15 (0.11) | 0.00* | 44.68 (21.04) | 0.56 | 0.44 (0.10) | 0.33 |
| 2007–2010 IIs-presence | 6.69 (2.33) | 0.91 | 0.15 (0.10) | 0.00* | 50.04 (22.48) | 0.31 | 0.45 (0.09) | 0.84 |
| 2017–2019 full network | 4.24 (1.31) | 1.00* | 0.15 (0.06) | 0.02* | 19.62 (6.98) | 0.80 | 0.28 (0.06) | 0.16 |
| 2017–2019 IIs-presence | 4.47 (1.32) | 0.95 | 0.16 (0.06) | 0.02* | 21.66 (7.03) | 0.77 | 0.33 (0.06) | 0.97 |
Temporal patterns of association
In 2007–2010 and 2017–2019, the SLARs exceeded the null association rates with a time lag (Figure 5), implying that the association patterns among individuals were nonrandom over time. The four exponential models were fitted to the data. The best model (∆QAIC 0–2) with the lowest QAIC in 2007–2010 was the “preferred companions and casual acquaintances” model. The best model for 2017–2019 indicated temporal association patterns, characterized by models of “preferred companions” and “casual acquaintances”. In 2007–2010, the “preferred companions” model was not supported (ΔQAIC>10), and the other two models showed low levels of support. In 2017–2019, the remaining two models were also less well supported (Table 5).
Figure 5.
SLAR and null association rate plotted against time lag with best-fitting model for Indo-Pacific humpback dolphins in Xiamen Bay
A: 2007–2010; B: 2017–2019. SLAR: Standardized lagged association rate.
Table 5. Model fitting to SLARs among Indo-Pacific humpback dolphins in Xiamen Bay.
| Period | Model formula | Parameter value (SE) | QAIC | ΔQAIC | ||
| QAIC: Quasi-Akaike information criterion. ΔQAIC: Variation of QAIC between current and best fit model. g: SLAR. td: Time in days. | ||||||
| 2007–2010 | Preferred companions | g(td)=a1 | a1=0.0701 (0.0051) | 12 081.6177 | 28.4464 | No support |
| Casual acquaintances | g(td)=a2e(-a1td) |
a1=0.0041 (0.0016) a2=0.0791 (0.0042) |
12 055.2454 | 2.0741 | Less support | |
| Preferred companions and casual acquaintances | g(td)=a2+a3e(-a1td) |
a1=0.0350 (4.4459) a2=0.0559 (0.0479) a3=0.0275 (0.0664) |
12 053.1731 | 0 | Best | |
| Two levels of casual acquaintances | g(td)=a3e(-a1td)+a4e(-a2td) |
a1=1.0160 (1.0574) a2=0.0040 (0.9204) a3=0.0063 (0.0739) a4=0.0785 (0.0717) |
12 059.1157 | 5.9444 | Less support | |
| 2017–2019 | Preferred companions | g(td)=a1 | a1=0.0727 (0.0097) | 3 110.5746 | 0 | Best |
| Casual acquaintances | g(td)=a2e(-a1td) |
a1=0.0008 (0.0024) a2=0.0753 (0.0145) |
3 112.2883 | 1.7137 | Substantial support | |
| Preferred companions and casual acquaintances | g(td)=a2+a3e(-a1td) |
a1=9.3991 (31.0645) a2=0.0724 (0.0098) a3=117.176 (946.1045) |
3 114.3512 | 3.7766 | Less support | |
| Two levels of casual acquaintances | g(td)=a3e(-a1td)+a4e(-a2td) |
a1=0.0008 (68.6991) a2=0.0008 (0.0205) a3=–0.6352 (179.5280) a4=0.7105 (2.6708) |
3 116.2883 | 5.7137 | Less support | |
Spatial patterns
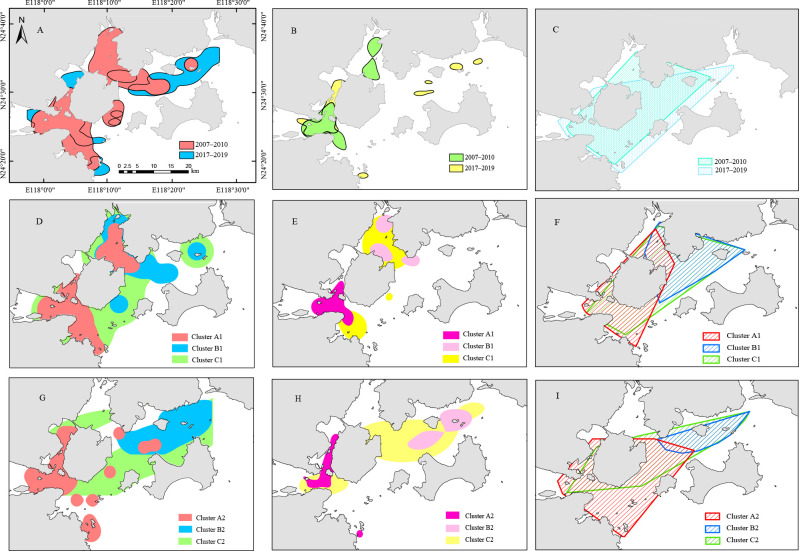
The western waters consistently served as the primary habitat throughout the study. In the 2007–2010 period, Tongan Bay was identified as the core habitat, whereas in 2017–2019, the dolphins showed a preference for the waters around Dadeng and Xiaodeng (Figure 6A–C). This shift in habitat preference is particularly evident within the 50% kernel range, as illustrated in Figure 6B.
Figure 6.
95% kernel, 50% kernel, and MCP range use by Indo-Pacific humpback dolphins in Xiamen Bay
A: 95% kernel range; B: 50% kernel range; C: MCP range; D: 95% kernel range of each cluster from 2007 to 2010; E: 50% kernel range of each cluster from 2007 to 2010; F: MCP range of each cluster from 2007 to 2010; G: 95% kernel range of each cluster from 2017 to 2019; H: 50% kernel range of each cluster from 2017 to 2019; I: MCP range of each cluster from 2017 to 2019.
The fixed kernel (95% and 50% kernel range) and MCP range analyses revealed distinct habitat preferences for each cluster during 2007–2010. Notably, cluster A1 predominantly occupied the western waters, cluster B1 primarily resided in the eastern waters, and cluster C1 utilized most of the study area (Figure 6D–F). The three clusters showed similar habitat use characteristics in the 2017–2019 period, with cluster A2 preferring the western waters, cluster B2 preferring the eastern waters, and cluster C2 exhibiting no specific habitat preference (Figure 6G–I).
The 95% kernel range sizes of cluster A1, cluster B1, and cluster C1 were 269.25 km2, 244.13 km2, and 535.08 km2, the 50% kernel range sizes were 58.91 km2, 51.95 km2, and 181.22 km2, and the MCP range sizes were 233.63 km2, 222.78 km2, and 335.89 km2, respectively. The spatial overlap between the clusters changed greatly. The MCP habitat range overlap between cluster A1 and cluster B1 was 6.22%, while cluster C1 used 60.06% of the habitat of cluster B1. The overlap ratio of the 95% kernel range among the three clusters was 12.33%–32.57%. Of note, there was no overlap in the 50% kernel range between clusters A1 and B1. In 2017–2019, the MCP and 95% and 50% kernel ranges between cluster A2 and cluster B2 were 0.58%, 1.53%, and 0%, between cluster A2 and cluster C2 were 27.36%, 5.58%, and 2.79%, and between cluster B2 and cluster C3 were 100%, 24.41%, and 17.34%, respectively.
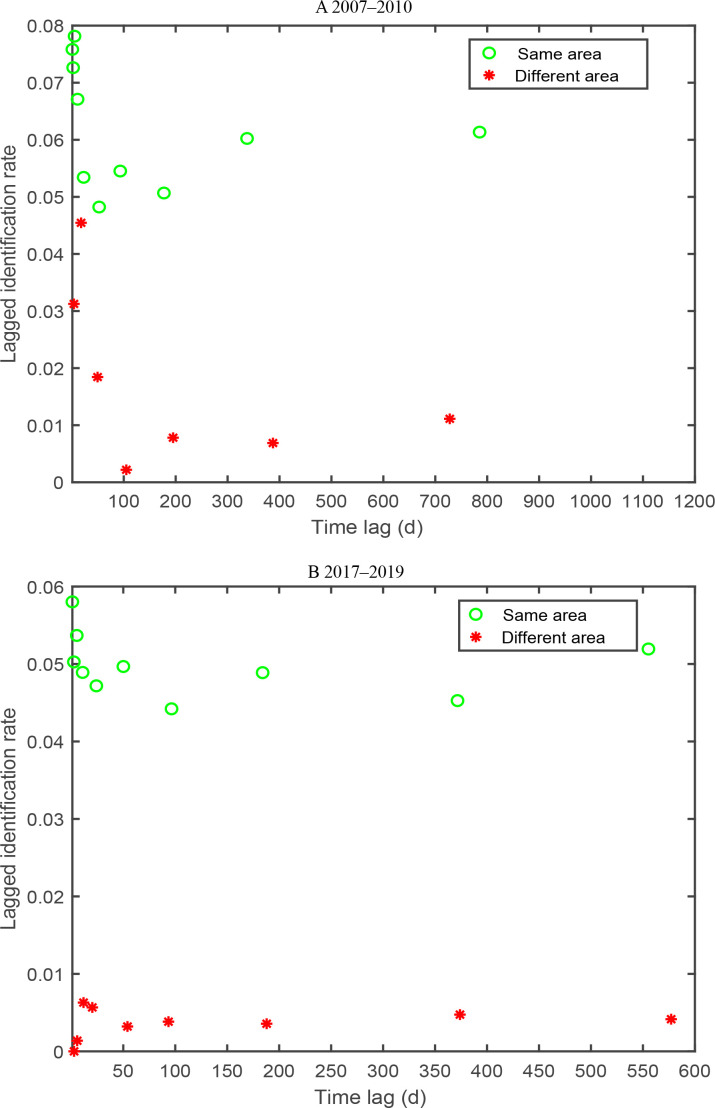
Movements throughout the study area
Generally, the LIRs plotted against the time lag suggested that the probability of one dolphin being identified in the eastern or western waters and then being identified in the other waters was higher than that for the other waters in 2007–2010 and 2017–2019 (Figure 7). The LIRs for western waters to western waters were the highest, indicating that individuals in the western waters spent most of their time in that area during the study period (Figure 7). The LIRs for the western waters to eastern waters were the lowest, suggesting that individuals in the western waters rarely moved to other waters. The best-fitting models for the two areas (either area 1 to 2, or area 2 to 1) exhibited similar results, and were fully mixed and fitted to the datasets in the two periods. The chi-square test for goodness-of-fit was significant (P=0.00) for the models for the overall LIRs for individuals staying within and between areas, and the LIRs for the same study area were far higher than the LIRs for the different study areas (Figure 7).
Figure 7.
LIR against time lag within/between areas for Indo-Pacific humpback dolphins in Xiamen Bay
A: 2007–2010; B: 2017–2019. LIR: Lagged identification rate.
DISCUSSION
Comparisons with other populations and species
The social structure in cetaceans, particularly in the family Delphinidae, varies from stable to fluid patterns. For instance, short-finned pilot whales (Globicephala macrorhynchus Gray, 1846; Mahaffy et al., 2015) and killer whales (Orcinus orca (Linnaeus, 1758); Reisinger et al., 2017) display relatively stable social structures, with individuals forming disconnected components. In contrast, bottlenose dolphins (Tursiops truncatus (Montagu, 1821)) have a fluid (fission-fusion) society, with frequently changing or stable associations among individuals (Connor et al., 2000). Furthermore, certain species display an intermediate form of association. For instance, individuals may belong to stable units and show strong associations or exhibit no long-term associations at all, such as Risso’s dolphins (Grampus griseus (G. Cuvier, 1812); Hartman et al., 2008).
Humpback dolphins live in a fission-fusion society, characterized by nonrandom relationships (Hunt et al., 2019; Parra et al., 2011). The nonrandom associations among S. chinensis in Xiamen are consistent with findings in Zhanjiang (Xu et al., 2012), Hong Kong (Dungan et al., 2012), and Taiwan (Dungan et al., 2016), China. Similar association patterns have also been reported in Indian Ocean humpback dolphins (S. plumbea (G. Cuvier, 1829); Karczmarski, 1999) and Australian humpback dolphins (Sousa sahulensis Jefferson and Rosenbaum, 2014; De Biasi Cagnazzi et al., 2011). In addition, the social divisions in Xiamen are similar to previous research on the same population, revealing two discrete clusters (Chen et al., 2011; Wang et al., 2015b), as well as in S. chinensis populations in Hong Kong, showing two clusters in the western and northern parts of Lantau, respectively (Dungan et al., 2012), and in S. sahulensis populations near Queensland, showing two geographically distinct clusters (De Biasi Cagnazzi et al., 2011). However, the social structure in Xiamen is also distinct from those observed in Hong Kong and Queensland, marked by the emergence of two main clusters and one small cluster in 2007–2010 (four individuals) and 2017–2019 (five individuals). This smaller cluster moved throughout the study area, apparently serving as a link between the two primary clusters in Xiamen. We speculate that the individuals within this cluster exhibit wider movement to increase their mating opportunities. The formation of fission-fusion dolphin societies is strongly influenced by various factors such as resource availability and predation risk (Connor et al., 1998; Gowans et al., 2007; Van Schaik, 1989; Wittemyer et al., 2005). In Xiamen, S. chinensis are considered apex predators without natural threats. Therefore, the observed higher clustering and more stable associations within this population may facilitate the dissemination and exchange of information during foraging and feeding (Perrin & Lehmann, 2001; Lusseau et al., 2003; Möeller, 2012). This is especially relevant given the significant decline in fishery resources in Xiamen Bay (Huang et al., 2010; You et al., 2016; Zhang et al., 2022). Consequently, resource availability is likely a primary factor driving the fission-fusion sociality of the Xiamen humpback dolphins.
Effect of area use and identical individuals on social patterns
In the study population, the two main clusters were predominantly located in the eastern and western waters, each with its own distinct core habitat (50% kernel density estimate). This geographical segregation may be due to the narrowing of the strait, akin to the pattern observed in humpback dolphins in Queensland, Australia (De Biasi Cagnazzi et al., 2011), or different habitat preferences and use, as observed in bottlenose dolphins (Tursiops truncatus gephyreus Lahille, 1908) in southern Brazil (Genoves et al., 2018). We speculate that the social segregation of the Xiamen population may be the result of long-term adaptation to different environmental pressures, i.e., high anthropogenic development in western waters and relatively pristine environment in eastern waters (Wang et al., 2015b). The western cluster seems to have adapted to the presence of human activities, consistently using this area from 2007 to 2010 and again from 2017 to 2019. Conversely, the eastern cluster exhibited a notable shift over the same period, moving eastward from Tongan Bay (2007–2010) to the Dadeng-Xiaodeng waters (2017–2019). This shift may be in response to the construction and operation of three bridges in Tongan Bay between 2008 and 2010, prompting the cluster to seek a quieter environment in the Dadeng-Xiaodeng waters. Of concern, the construction of a new airport in the Dadeng waters currently presents a potential threat, and its impact on the eastern cluster will require continuous observation and monitoring.
The structure of dolphin social networks is often maintained by pivotal adult individuals, as observed in bottlenose dolphins in Doubtful Sound, New Zealand, and Shannon Estuary, Ireland (Baker et al., 2018; Lusseau et al., 2003). In this population, 16 IIs were identified in both study periods, with 15 of them being adults in 2007–2010. Most of these adults occupied central positions in the network, signifying their crucial role in connecting associated individuals. However, their prominence in the network appeared to diminish in 2017–2019. Considering that the interval between the two sampling periods was 7 to 12 years, closely aligning with the 9–12-year sexual maturity cycle of S. chinensis (Jefferson et al., 2012), it is reasonable to infer that the roles of adult S. chinensis are in a state of continual flux, suggesting a gradual shift wherein newly matured dolphins assume the central roles once held by their older counterparts.
Conservation implications
Since the 1950s, numerous marine engineering projects in Xiamen Bay have significantly impacted the survival and habitat conditions of the local dolphin population. Land reclamation, in particular, has led to the permanent loss of habitat (Zeng et al., 2020), with an estimated 154 km2 of land reclaimed as of 2017 (Qin et al., 2019). Furthermore, at least 16 dolphin deaths were recorded between 2002 and 2004 (Chen et al., 2008), some of which were confirmed to be attributable to underwater blasting (Wang et al., 2003). In response to these impacts, various conservation measures have been adopted, including relevant legislation, nature reserve establishment, fishing activity restrictions, and fish stocking programs. These measures have proven effective in mitigating harm from human activities and enhancing habitat quality.
Currently, both the western and eastern clusters of S. chinensis face different threats, i.e., the western waters are burdened with heavy vessel traffic, while the eastern waters are impacted by mariculture and airport construction. The western waters host over 10 terminals, frequented by ferries and cargo ships. For instance, the tourist route between Xiagu Wharf and Gulangyu Sanqiutian Wharf operates a round-trip every 20 min. In 2022 alone, more than 290 000 ships passed through Xiamen Port. While traffic density may signify the degree of bulk risk, each vessel is a potential source of stress for the dolphins (Ng & Leung, 2003). Such disturbances may cause the dolphins to extend their dive durations, increase their swimming speeds, and make evasive maneuvers (Ng & Leung, 2003). Notably, high-speed vessels present a dual threat: they can cause direct physical harm to dolphins and the noise generated can interfere with dolphin communication, disrupting their social interactions (Chen et al., 2008; Ng & Leung, 2003).
Throughout the latter half of the previous century, extensive mariculture activities were undertaken in Xiamen Bay and its adjacent waters. These activities not only reduced the habitat range available to the dolphins (Wang et al., 2015b) but also contributed to increased water pollution, impacting the local marine ecosystem. Mariculture activities in Xiamen Bay have markedly declined since 2006, particularly in the western waters. By 2021, the area dedicated to mariculture had diminished to 0.35 km², representing less than 5% of its peak extent, with most remaining mariculture activities concentrated in the eastern waters. In addition, the construction of a new airport between Dadeng Island and Xiaodeng Island, a common sighting area for S. chinensis, poses a new challenge. The airport, slated for completion and operation in 2026, involves a reclamation area of 14.38 km2 (Zhao, 2015), which would result in the permanent loss of habitat for S. chinensis. Given the distinct threats in the western and eastern waters, the two main clusters of dolphins should be considered as separate management units for conservation purposes. In the western waters, the existing restriction on shipping speeds to a maximum of 10 knots must be stringently enforced. In the eastern waters, a ban on mariculture is recommended to extend and improve the habitat range and quality for the dolphins. Moreover, the potential impacts of the construction and operation of the new airport need to be fully assessed prior to its commencement.
In conclusion, many factors have affected the survival of the dolphin population in Xiamen over the past few decades. The findings of this study contribute to a deeper understanding of this population, offering valuable insights to support its protection. Overall, different conservation measures should be applied to the eastern and western waters, and the management of the reserve needs to be further strengthened to facilitate population recovery.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for field surveys was granted by Xiamen Rare Marine Species National Nature Reserve.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTIONS
Y.L., B.Y.C., and G.Y. conceived and designed the research; Y.L., B.Y.C., and X.R.X. conducted field surveys and collected samples; Y.L. and B.Y.C. analyzed the data; Y.L., B.Y.C., T.A.J., and H.F. wrote the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors are particularly grateful to Na Liang (Nanjing Normal University) and Min Xu (Pharmaron Company Limited) for helpful discussions. Many thanks are also given to Sheng Chen and Shuang-Lin Chen (Xiamen Xiangbosheng Marine Environmental Protection Service Company Limited) for help with fieldwork.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32030011, 31630071), National Key Research and Development Program of China (2022YFF1301600), and Priority Academic Program Development of Jiangsu Higher Education Institutions
Contributor Information
Bing-Yao Chen, Email: chby2632@163.com.
Guang Yang, Email: gyang@njnu.edu.cn.
References
- Atwood TC, Weeks HP Spatial home-range overlap and temporal interaction in eastern coyotes: the influence of pair types and fragmentation. Canadian Journal of Zoology. 2003;81(9):1589–1597. doi: 10.1139/z03-144. [DOI] [Google Scholar]
- Aureli F, Schaffner CM, Boesch C, et al Fission-fusion dynamics: new research frameworks. Current Anthropology. 2008;49(4):627–654. doi: 10.1086/586708. [DOI] [Google Scholar]
- Baker I, O'Brien J, McHugh K, et al Bottlenose dolphin (Tursiops truncatus) social structure in the Shannon Estuary, Ireland, is distinguished by age- and area-related associations. Marine Mammal Science. 2018;34(2):458–487. doi: 10.1111/mms.12462. [DOI] [Google Scholar]
- Bejder L, Fletcher D, Bräger S A method for testing association patterns of social animals. Animal Behaviour. 1998;56(3):719–725. doi: 10.1006/anbe.1998.0802. [DOI] [PubMed] [Google Scholar]
- Borgatti SP. 2002. NetDraw: Graph Visualization Software. Cambridge: Harvard Analytic Technologies.
- Bouveroux T, Kirkman SP, Conry D, et al The first assessment of social organisation of the Indian Ocean humpback dolphin (Sousa plumbea) along the south coast of South Africa. Canadian Journal of Zoology. 2019;97(10):855–865. doi: 10.1139/cjz-2018-0244. [DOI] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. New York: Springer.
- Cairns SJ, Schwager SJ A comparison of association indices. Animal Behaviour. 1987;35(5):1454–1469. doi: 10.1016/S0003-3472(87)80018-0. [DOI] [Google Scholar]
- Cantor M, Wedekin LL, Guimarães PR, et al Disentangling social networks from spatiotemporal dynamics: the temporal structure of a dolphin society. Animal Behaviour. 2012;84(3):641–651. doi: 10.1016/j.anbehav.2012.06.019. [DOI] [Google Scholar]
- Cederlund G, Sand H Home-range size in relation to age and sex in moose. Journal of Mammalogy. 1994;75(4):1005–1012. doi: 10.2307/1382483. [DOI] [Google Scholar]
- Chan SCY, Karczmarski L, Lin WZ, et al An unknown component of a well-known population: socio-demographic parameters of Indo-Pacific humpback dolphins (Sousa chinensis) at the western reaches of the Pearl River Delta region. Mammalian Biology. 2022;102(4):1149–1171. doi: 10.1007/s42991-022-00335-2. [DOI] [Google Scholar]
- Chen BY, Gao HL, Jefferson TA, et al Survival rate and population size of Indo-Pacific humpback dolphins (Sousa chinensis) in Xiamen Bay, China. Marine Mammal Science. 2018;34(4):1018–1033. doi: 10.1111/mms.12510. [DOI] [Google Scholar]
- Chen BY, Zheng DM, Ju JF, et al Range patterns of resident Indo-Pacific humpback dolphins (Sousa chinensis, Osbeck 1765) in Xiamen, China: implications for conservation and management. Zoological Studies. 2011;50(6):751–762. [Google Scholar]
- Chen BY, Zheng DM, Zhai FF, et al Abundance, distribution and conservation of Chinese white dolphins (Sousa chinensis) in Xiamen, China. Mammalian Biology. 2008;73(2):156–164. doi: 10.1016/j.mambio.2006.12.002. [DOI] [Google Scholar]
- Clutton-Brock TH Review lecture: mammalian mating systems. Proceedings of the Royal Society B. 1989;236(1285):339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Connor RC, Mann J, Tyack PL, et al Social evolution in toothed whales. Trends in Ecology & Evolution. 1998;13(6):228–232. doi: 10.1016/s0169-5347(98)01326-3. [DOI] [PubMed] [Google Scholar]
- Connor RC, Wells RS, Mann J, et al. 2000. The bottlenose dolphin: social relationships in a fission-fusion society. In: Mann J, Connor RC, Tyack PL, et al. Cetacean Societies: Field Studies of Dolphins and Whales. Chicago: University of Chicago Press, 91–127.
- Davies NB, Krebs JR, West SA. 2012. An Introduction to Behavioural Ecology. 4th ed. Hoboken: Wiley-Blackwell.
- De Biasi Cagnazzi D, Harrison PL, Ross GJB, et al Abundance and site fidelity of Indo-Pacific humpback dolphins in the Great Sandy Strait, Queensland, Australia. Marine Mammal Science. 2011;27(2):255–281. doi: 10.1111/j.1748-7692.2009.00296.x. [DOI] [Google Scholar]
- Dungan SZ, Hung SK, Wang JY, et al Two social communities in the Pearl River Estuary population of Indo-Pacific humpback dolphins (Sousa chinensis) Canadian Journal of Zoology. 2012;90(8):1031–1043. doi: 10.1139/z2012-071. [DOI] [Google Scholar]
- Dungan SZ, Wang JY, Araújo CC, et al Social structure in a critically endangered Indo-Pacific humpback dolphin (Sousa chinensis) population. Aquatic Conservation:Marine and Freshwater Ecosystems. 2016;26(3):517–529. doi: 10.1002/aqc.2562. [DOI] [Google Scholar]
- Genoves RC, Fruet PF, Di Tullio JC, et al Spatiotemporal use predicts social partitioning of bottlenose dolphins with strong home range overlap. Ecology and Evolution. 2018;8(24):12597–12614. doi: 10.1002/ece3.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans S, Würsig B, Karczmarski L The social structure and strategies of delphinids: predictions based on an ecological framework. Advances in Marine Biology. 2007;53:195–294. doi: 10.1016/S0065-2881(07)53003-8. [DOI] [PubMed] [Google Scholar]
- Hartman KL, Visser F, Hendriks AJE Social structure of Risso’s dolphins (Grampus griseus) at the Azores: a stratified community based on highly associated social units. Canadian Journal of Zoology. 2008;86(4):294–306. doi: 10.1139/Z07-138. [DOI] [Google Scholar]
- Hayne DW Calculation of size of home range. Journal of Mammalogy. 1949;30(1):1–18. doi: 10.2307/1375189. [DOI] [Google Scholar]
- Huang LM, Xie YJ, Zhang YZ, et al Current fisheries resources assessment in Xiamen coastal waters. Journal of Jimei University:Natural Science. 2010;15(2):81–87. [Google Scholar]
- Huang ZG, Liu WH. 2000. Chinese White Dolphin and Other Cetaceans. Xiamen: Xiamen University Press. (in Chinese)
- Hunt TN, Allen SJ, Bejder L, et al Assortative interactions revealed in a fission-fusion society of Australian humpback dolphins. Behavioral Ecology. 2019;30(4):914–927. doi: 10.1093/beheco/arz029. [DOI] [Google Scholar]
- Hunt TN, Bejder L, Allen SJ, et al Demographic characteristics of Australian humpback dolphins reveal important habitat toward the southwestern limit of their range. Endangered Species Research. 2017;32:71–88. doi: 10.3354/esr00784. [DOI] [Google Scholar]
- James R, Croft DP, Krause J Potential banana skins in animal social network analysis. Behavioral Ecology and Sociobiology. 2009;63(7):989–997. doi: 10.1007/s00265-009-0742-5. [DOI] [Google Scholar]
- Jefferson TA Population biology of the Indo-Pacific hump-backed dolphin in Hong Kong waters. Wildlife Monographs. 2000;144:1–65. [Google Scholar]
- Jefferson TA, Hung SK, Robertson KM, et al Life history of the Indo-Pacific humpback dolphin in the Pearl River Estuary, southern China. Marine Mammal Science. 2012;28(1):84–104. doi: 10.1111/j.1748-7692.2010.00462.x. [DOI] [Google Scholar]
- Jefferson TA, Karczmarski L. 2001. Sousa chinensis. Mammalian Species, 655: 1–9.
- Jefferson TA, Rosenbaum HC Taxonomic revision of the humpback dolphins (Sousa spp. ), and description of a new species from Australia. Marine Mammal Science. 2014;30(4):1494–1541. doi: 10.1111/mms.12152. [DOI] [Google Scholar]
- Karczmarski L Group dynamics of humpback dolphins (Sousa chinensis) in the Algoa Bay region, South Africa. Journal of Zoology. 1999;249(3):283–293. doi: 10.1111/j.1469-7998.1999.tb00765.x. [DOI] [Google Scholar]
- Kossinets G, Watts DJ Empirical analysis of an evolving social network. Science. 2006;311(5757):88–90. doi: 10.1126/science.1116869. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Boesch C To fission or to fusion: effects of community size on wild chimpanzee (Pan troglodytes verus) social organisation. Behavioral Ecology and Sociobiology. 2004;56(3):207–216. [Google Scholar]
- Lusseau D, Schneider K, Boisseau OJ, et al The bottlenose dolphin community of Doubtful Sound features a large proportion of long-lasting associations: can geographic isolation explain this unique trait? Behavioral Ecology and Sociobiology. 2003;54(4):396–405. doi: 10.1007/s00265-003-0651-y. [DOI] [Google Scholar]
- Lusseau D, Whitehead H, Gero S Incorporating uncertainty into the study of animal social networks. Animal Behaviour. 2008;75(5):1809–1815. doi: 10.1016/j.anbehav.2007.10.029. [DOI] [Google Scholar]
- Mahaffy SD, Baird RW, McSweeney DJ, et al High site fidelity, strong associations, and long-term bonds: Short-finned pilot whales off the island of Hawai ‘i. Marine Mammal Science. 2015;31(4):1427–1451. doi: 10.1111/mms.12234. [DOI] [Google Scholar]
- Möeller LM Sociogenetic structure, kin associations and bonding in delphinids. Molecular Ecology. 2012;21(3):745–764. doi: 10.1111/j.1365-294X.2011.05405.x. [DOI] [PubMed] [Google Scholar]
- Newman ME Analysis of weighted networks. Physical Review E. 2004;70(5):056131. doi: 10.1103/PhysRevE.70.056131. [DOI] [PubMed] [Google Scholar]
- Newman MEJ Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(23):8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SL, Leung S Behavioral response of Indo-Pacific humpback dolphin (Sousa chinensis) to vessel traffic. Marine Environmental Research. 2003;56(5):555–567. doi: 10.1016/S0141-1136(03)00041-2. [DOI] [PubMed] [Google Scholar]
- Parra GJ Resource partitioning in sympatric delphinids: space use and habitat preferences of Australian snubfin and Indo-Pacific humpback dolphins. Journal of Animal Ecology. 2006;75(4):862–874. doi: 10.1111/j.1365-2656.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- Parra GJ, Corkeron PJ, Arnold P Grouping and fission-fusion dynamics in Australian snubfin and Indo-Pacific humpback dolphins. Animal Behaviour. 2011;82(6):1423–1433. doi: 10.1016/j.anbehav.2011.09.027. [DOI] [Google Scholar]
- Pepper JW, Mitani JC, Watts DP General gregariousness and specific social preferences among wild chimpanzees. International Journal of Primatology. 1999;20(5):613–632. doi: 10.1023/A:1020760616641. [DOI] [Google Scholar]
- Perrin N, Lehmann L Is sociality driven by the costs of dispersal or the benefits of philopatry? A role for kin-discrimination mechanisms. The American Naturalist. 2001;158(5):471–483. doi: 10.1086/323114. [DOI] [PubMed] [Google Scholar]
- Qin YF, Ye L, Chen SM Study on coastline changes of Xiamen city based on remote sensing images. E3S Web of Conferences. 2019;136:05003. doi: 10.1051/e3sconf/201913605003. [DOI] [Google Scholar]
- Reisinger RR, Beukes C, Hoelzel AR, et al Kinship and association in a highly social apex predator population, killer whales at Marion Island. Behavioral Ecology. 2017;28(3):750–759. doi: 10.1093/beheco/arx034. [DOI] [Google Scholar]
- Seaman DE, Millspaugh JJ, Kernohan BJ, et al Effects of sample size on kernel home range estimates. The Journal of Wildlife Management. 1999;63(2):739–747. doi: 10.2307/3802664. [DOI] [Google Scholar]
- Sosa S, Sueur C, Puga-Gonzalez I Network measures in animal social network analysis: their strengths, limits, interpretations and uses. Methods in Ecology and Evolution. 2021;12(1):10–21. doi: 10.1111/2041-210X.13366. [DOI] [Google Scholar]
- Titcomb EM, O'Corry-Crowe G, Hartel EF, et al Social communities and spatiotemporal dynamics of association patterns in estuarine bottlenose dolphins. Marine Mammal Science. 2015;31(4):1314–1337. doi: 10.1111/mms.12222. [DOI] [Google Scholar]
- Van Schaik CP. 1989. The ecology of social relationships amongst female primates. In: Standen V, Foley RA. Comparative Socioecology the Behavioural Ecology of Humans and Other Mammals. Oxford: Blackwell Scientific, 195–218.
- Wang D, Liu RJ, Zhao QZ, et al Pathological anatomy and analysis of death causes for a Chinese white dolphin. Acta Theriologica Sinica. 2003;23(2):183–184. [Google Scholar]
- Wang JY, Yang SC, Hung SK Diagnosability and description of a new subspecies of Indo-Pacific humpback dolphin, Sousa chinensis (Osbeck, 1765), from the Taiwan Strait. Zoological Studies. 2015a;54:36. doi: 10.1186/s40555-015-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Wu FX, Turvey ST, et al Social organization and distribution patterns inform conservation management of a threatened Indo-Pacific humpback dolphin population. Journal of Mammalogy. 2015b;96(5):964–971. doi: 10.1093/jmammal/gyv097. [DOI] [Google Scholar]
- Whitehead H Investigating structure and temporal scale in social organizations using identified individuals. Behavioral Ecology. 1995;6(2):199–208. [Google Scholar]
- Whitehead H Analysing animal social structure. Animal Behaviour. 1997;53(5):1053–1067. doi: 10.1006/anbe.1996.0358. [DOI] [Google Scholar]
- Whitehead H Analysis of animal movement using opportunistic individual identifications: application to sperm whales. Ecology. 2001;82(5):1417–1432. doi: 10.1890/0012-9658(2001)082[1417:AOAMUO]2.0.CO;2. [DOI] [Google Scholar]
- Whitehead H Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Communications in Statistics-Simulation and Computation. 2007;36(6):1233–1246. doi: 10.1080/03610910701569531. [DOI] [Google Scholar]
- Whitehead H. 2008a. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. Chicago: University of Chicago Press.
- Whitehead H Precision and power in the analysis of social structure using associations. Animal Behaviour. 2008b;75(3):1093–1099. doi: 10.1016/j.anbehav.2007.08.022. [DOI] [Google Scholar]
- Whitehead H SOCPROG programs: analysing animal social structures. Behavioral Ecology and Sociobiology. 2009;63(5):765–778. doi: 10.1007/s00265-008-0697-y. [DOI] [Google Scholar]
- Wittemyer G, Douglas-Hamilton I, Getz WM The socioecology of elephants: analysis of the processes creating multitiered social structures. Animal Behaviour. 2005;69(6):1357–1371. doi: 10.1016/j.anbehav.2004.08.018. [DOI] [Google Scholar]
- Xu XR, Zhang ZH, Ma LG, et al Site fidelity and association patterns of Indo-Pacific humpback dolphins off the east coast of Zhanjiang, China. Acta Theriologica. 2012;57(2):99–109. doi: 10.1007/s13364-011-0058-5. [DOI] [Google Scholar]
- You TF, Chen XY, Lin JX, et al Investigation on nekton resources of spring in the west sea areas of Xiamen. Journal of Fisheries Research. 2016;38(5):386–393. [Google Scholar]
- Zeng QH, Lin WZ, Dai YF, et al Modeling demographic parameters of an edge-of-range population of Indo-Pacific humpback dolphin in Xiamen Bay, China. Regional Studies in Marine Science. 2020;40:101462. doi: 10.1016/j.rsma.2020.101462. [DOI] [Google Scholar]
- Zhang XY, Chen B, Ding SX, et al Analysis of Ecosystem structure and function changes in Xiamen Bay in the past 10 years based on Ecopath model. Journal of Ecology and Rural Environment. 2022;38(2):217–224. [Google Scholar]
- Zhao DY Study on flood and tide protection criteria for artificial island berms of Xiamen Xiang'an New Airport Reclamation Project. Technology Innovation and Application. 2015;24:2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.