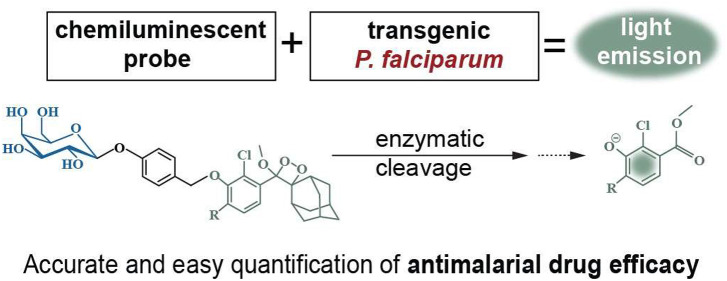
Abstract
Malaria is caused by parasites of the Plasmodium genus and remains one of the most pressing human health problems. The spread of parasites resistant to or partially resistant to single or multiple drugs, including frontline antimalarial artemisinin and its derivatives, poses a serious threat to current and future malaria control efforts. In vitro drug assays are important for identifying new antimalarial compounds and monitoring drug resistance. Due to its robustness and ease of use, the [3H]-hypoxanthine incorporation assay is still considered a gold standard and is widely applied, despite limited sensitivity and the dependence on radioactive material. Here, we present a first-of-its-kind chemiluminescence-based antimalarial drug screening assay. The effect of compounds on P. falciparum is monitored by using a dioxetane-based substrate (AquaSpark β-D-galactoside) that emits high-intensity luminescence upon removal of a protective group (β-D-galactoside) by a transgenic β-galactosidase reporter enzyme. This biosensor enables highly sensitive, robust, and cost-effective detection of asexual, intraerythrocytic P. falciparum parasites without the need for parasite enrichment, washing, or purification steps. We are convinced that the ultralow detection limit of less than 100 parasites of the presented biosensor system will become instrumental in malaria research, including but not limited to drug screening.
Keywords: malaria, Plasmodium falciparum, drug screening, IC50 assay, chemiluminescence, AquaSpark
Malaria is a devastating disease with major impact on public health in endemic regions. 95% of the total malaria case burden is carried by regions in sub-Saharan Africa, with children under the age of five representing the most vulnerable group.1,2 The disease-causing protozoan Plasmodium parasites are transmitted during the bite of infected Anopheles mosquitoes, and following their entry into the bloodstream, parasites migrate to the liver where they infect and proliferate within hepatocytes. Intrahepatic replication is clinically silent, and the disease only manifests after parasites re-enter the blood circulation. The symptomatic blood stage of infection is characterized by repeated rounds of erythrocyte invasion, asexual replication, rupture of the infected red blood cell (RBC), and the consequent release of daughter cells capable of invading new erythrocytes.3
In the late 1950s, the emergence of chloroquine-resistant parasites revealed the need for new antimalarial compounds.4−6 Since then, multiple drugs were developed and introduced, but, worryingly, parasites evolved resistance or partial resistance to nearly all of today’s therapeutic approaches, including the first-line artemisinin-based combination therapies (ACTs).7−9 The current spread of parasites that are partially resistant to artemisinin and its derivatives as well as to the partner drugs used in ACTs is threatening current and future therapeutic strategies. Clearly, the discovery and development of antimalarials with alternative modes of action are needed urgently.
In vitro drug susceptibility assays, which examine the effect of drugs on the growth of intraerythrocytic asexual parasites, are essential tools for monitoring drug resistance and screening for new drug candidates. Various methods using different readout techniques have been described, including the microscopic examination-based WHO microtest,10 isotope incorporation-based assays,11,12 the enzymatic Plasmodium lactate dehydrogenase (pLDH) assay, the ELISA-based assays for detecting either pLDH,13 histidine-rich protein II,14 or P. falciparum aldolase,15 fluorescence-based assays,16−22 hemozoin detection by flow cytometry,23 or rotating-crystal magneto-optical diagnostic techniques24−26 and luciferase-based assays.27−29 Common to all these methods is that they assess the inhibition of asexual parasite development as an end point after drug exposure. The wide range of assay options notwithstanding, all methods are linked to inherent advantages and limitations regarding sensitivity, high-throughput compatibility, the reliance on hazardous chemicals that are difficult to dispose of, applicability to field isolates, and practical handling, such as the possibility to store assay plates between the end point and assay readout.
Typically, assays used to quantify half-maximal inhibitory concentrations (IC50) fail at monitoring the speed at which antimalarial compounds act on parasite viability. To address this important measure of drug efficacy, Sanz et al. developed the so-called parasite reduction ratio (PRR) assay that allows characterizing drug activity using three parameters, (i) the lag time, which describes the time it takes for a compound to achieve its maximum effect, (ii) the log10(PRR), defined as the log10 drop of viable parasites within 48 h in the range of the maximal killing rate, and (iii) the parasite clearance time 99.9% (PCT 99.9%) that quantifies the time required to kill 99.9% of the initial parasites.30 While the PRR assay has proven to be most valuable for evaluating and monitoring drug efficacy, its time- and labor-intensive setup and execution have limited it to low-throughput applications.
The PRR, but also other measures of drug activity, including IC50 assays, are often quantified using incorporation of [3H]-hypoxanthine into DNA of replicating parasites. Due to its robustness and excellent signal-to-noise ratio and despite relying on radioactive material, [3H]-hypoxanthine incorporation remains a gold standard for measuring parasite development and survival.31−33 Alternative quantification methods that perform equally well as the [3H]-hypoxanthine-based assay and do not require the handling of radioactive substances would clearly aid antimalarial drug discovery and development.
Here, we describe the development of a first-of-its-kind chemiluminescence-based malaria drug assay using a luminescent biosensor probe that is activated by P. falciparum parasites expressing a galactosidase transgene. In contrast to fluorescence, chemiluminescence does not require exciting light, thus eliminating the need for an external light source and problems related to overlapping spectra, light scattering, and activation of autofluorescent molecules, which may affect the readout quality of fluorescence-based assays. In addition, luminometers that use photomultiplier tubes to detect luminescence are among the least expensive readout devices on the market, and the sensitivity of chemiluminescence-based assays is generally high due to low background levels.34−36
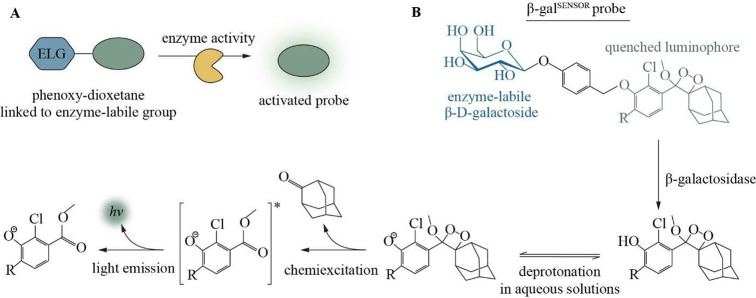
We used a commercially available phenoxy-dioxetane-based chemiluminescent molecule (AquaSpark β-D-galactoside) that, once activated, emits light at 3000-fold higher intensity compared to Schaap’s dioxetanes.35,37 Activation of this probe, henceforth called β-galSENSOR, is initiated by the β-galactosidase-catalyzed removal of a quenching β-D-galactoside substrate. Following the spontaneous elimination of a spacer, the decay of a phenolate intermediate results in the emission of intense green light with an emission maximum at wavelengths between 510 and 550 nm35,38 (Figure 1). In absence of the β-galactosidase enzyme, the β-galSENSOR probe remains highly stable, and the enzymatic activation of the probe works in aqueous solutions without the need for other additives such as complex buffer systems or expensive enzymes.35 Furthermore, and in contrast to most luminescence-based systems, the activity of this β-galSENSOR probe is ATP-independent. Today, phenoxy-dioxetane probes, including the β-galSENSOR probe, are used for the ultrasensitive detection of bacteria such as Listeria monocytogenes or Salmonella spp..(38−41)
Figure 1.
Principle of phenoxy-dioxetane biosensor probes. (A) Schematic of β-galSENSOR probe activation. (B) The β-galSENSOR probe is kept in a stable, nonluminescent state by an enzyme-labile β-galactoside. Removal of this group by β-galactosidase triggers the formation of a phenolate intermediate that undergoes chemiexcitation to emit green light. ELG, enzyme-labile group; hv, photon emission.
To use phenoxy-dioxetane biosensors in antimalarial drug discovery and development, we stably inserted the Escherichia coli β-galactosidase gene, also called lacZ, into the genetic background of different drug-sensitive and multidrug-resistant P. falciparum parasite strains. In combination with the β-galSENSOR probe, these NF54lacZ, F12lacZ, and K1lacZ lines allow screening molecules for antimalarial activity and monitoring drug efficacy using the most easy and cost-effective experimental setup. Upon β-galSENSOR probe addition, parasites expressing β-galactosidase are detected at high sensitivity, offering a new tool for approaches that depend on low parasite numbers including quantification of parasite reduction ratios.
Results
Engineering of P. falciparum Parasites That Process a β-D-Galactoside Biosensor Substrate
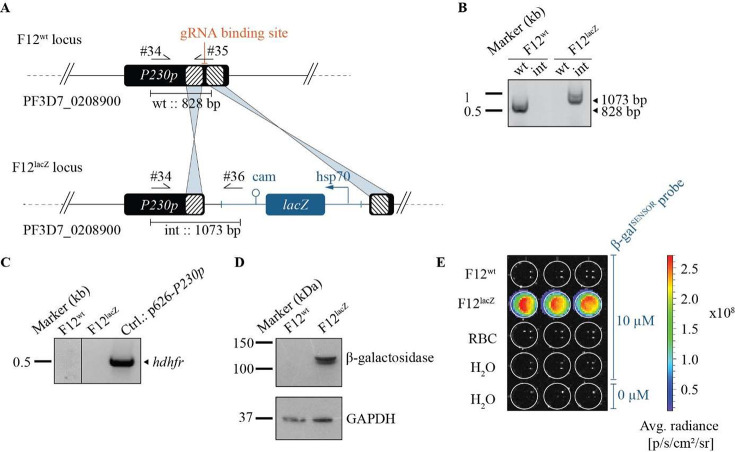
To allow the detection of P. falciparum parasites using the chemiluminescent β-galSENSOR probe (Biosynth AG, cat. #A-8169_P00), we engineered a cell line carrying the bacterial transgene lacZ. In a first step, we modified parasites of the gametocyte-deficient strain F12.42,43 Specifically, we integrated lacZ into the nonessential P230p locus44 of these parasites using a two-plasmid CRISPR/Cas9-based gene editing strategy (Figures 2A,B and S1).45 Parasites were selected to maintain a plasmid carrying expression cassettes for Cas9 and a P230p-targeting guideRNA (gRNA) using the antifolate drug WR99210 in combination with the drug selectable marker human dihydrofolate reductase (hDHFR). The culture was subsequently maintained in the absence of WR99210 to allow for a spontaneous loss of the plasmid and the selection marker. Following limiting dilution cloning, an hDHFR-free, and thus antifolate-sensitive parasite population, was subsequently selected. If not mentioned otherwise, experiments described hereafter were conducted using this clonal F12 line dubbed F12lacZ (Figure 2C). The hsp70 promoter—one of the strongest and constitutively active transcriptional regulators of P. falciparum(46)—controls lacZ expression. As expected, F12lacZ readily express the lacZ-encoded β-galactosidase enzyme (Figure 2D) and is able to process the β-galSENSOR probe (Figure 2E). The concomitant chemiexcitation yields strong luminescence with peak emission between 510 and 550 nm.35,38 We measured luminescence signals either with a multimode plate reader (unit: counts per second (counts/s)) or an IVIS Spectrum In Vivo Imaging System (unit: photons/seconds/cm2/steradian (p/s/cm2/sr); radiance). In absence of β-galactosidase activity, the β-D-galactoside serves as a protective, electron-withdrawing group that keeps the phenoxy-dioxetane molecule in a highly stable and nonluminescent state. Of note, this protection is near-complete, and only negligible luminescence above background was observed upon adding the β-galSENSOR probe to wild type parasites or to parasite-free control conditions (Figure 2E).
Figure 2.
The P. falciparum line F12lacZ expresses β-galactosidase. (A) Schematic of the P230p locus in wild type F12 and F12lacZ parasites. Arrows indicate primers used for diagnostic PCRs. Striped boxes represent the homology regions used for CRISPR/Cas9-based gene editing. The gRNA binding site is indicated. (B) Diagnostic PCR confirms integration of the lacZ expression cassette in F12lacZ. Primers used are shown in (A). wt, wild type; int, integration; kb, kilobases. (C) Diagnostic hdhfr (502 bases) PCR. (D) Western blot showing the expression of β-galactosidase by F12lacZ parasites. Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH, ∼36 kDa) was used as a loading control. kDa, kilodalton. (E) Chemiluminescence emitted from the F12lacZ parasites and controls. Concentration of the β-galSENSOR probe is indicated.
F12lacZ Parasites Are Detected with High Sensitivity
To accurately determine the effect of compounds on cell viability, drug screening assays generally require a linear relationship between reporter activity and the number of viable cells and, ideally, offer a low limit of detection (LOD). Using 200 μM β-galSENSOR probe, parasite number was linearly correlated with emitted radiance. Parasites were detected above the background down to an absolute number of 10 parasites per well of a 384-well plate when probed in phosphate-buffered saline (PBS) and MgCl2 at a final concentration of 1 mM (Figure 3). When probed in culture medium (CM) with a hematocrit (Hc) of 1.25%, 50 parasites per well were detected above background (Figure S2A). Decreasing the concentration of the β-galSENSOR probe to 10 μM increased the LOD to about 200 parasites per well in a 96-well plate when also probed in CM with a Hc of 1.25% (assay conditions) (Figure S2B). As for parasites exposed to 200 μM of the β-galSENSOR probe, F12lacZ parasites emit chemiluminescence that linearly correlates with parasite numbers (Figure S2B).
Figure 3.
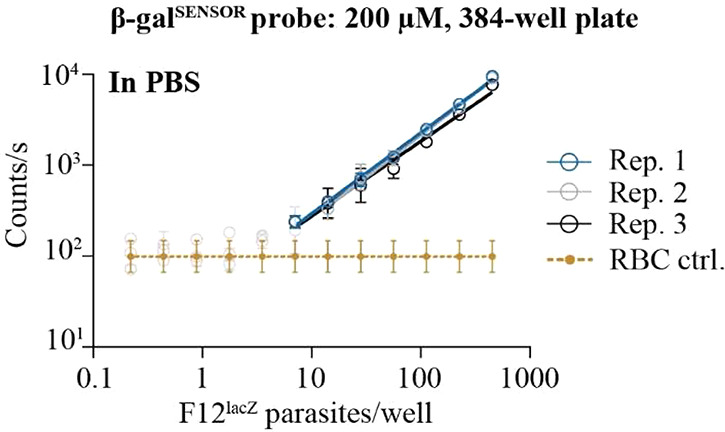
The β-galSENSOR probe allows detecting low numbers of F12lacZ parasites. Mixed-stage F12lacZ parasites are detected at low numbers (10 parasites/well) when incubated with the β-galSENSOR probe at 200 μM, and emitted chemiluminescence that linearly correlates with cell numbers. n = 3; biological replicates (rep.) are shown individually with error bars indicating standard deviations of technical replicates; for the control samples (ctrl.), biological replicates are taken together for simplification, and error bars indicate standard deviations of biological replicates.
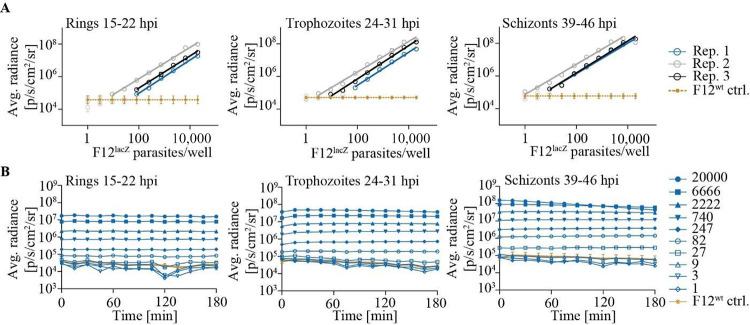
Next, we monitored the LOD and the stability of the luminescence emitted by different F12lacZ asexual parasite stages. Specifically, we serially diluted F12lacZ at three different time points during intraerythrocytic development (15–22 h post invasion (hpi); 24–31 hpi; 39–46 hpi) and added 10 μM β-galSENSOR probe (Figure 4). We monitored luminescence during consecutive time points using stage-matched F12wt parasites as controls. Serial dilution of parasites showed that 100 synchronous F12lacZ parasites can be detected above the background at a probe concentration of 10 μM (Figure 4A). By contrast, a wild type control population (F12wt) remained undetectable under otherwise identical conditions. Generally, the differently staged parasite populations showed similar luminescence intensities and detection limits, and the flux rates emitted from these cultures remained stable for at least 3 h after exposing F12lacZ parasites to the β-galSENSOR probe at 10 μM (Figures 4B and S3). At the highest cell number, schizonts showed decreasing flux over time, indicating that the β-galSENSOR probe reached limiting levels under these conditions. As discussed later, relatively low parasite numbers (≤6000) should thus be used when aiming at an absolute quantification of late stage intraerythrocytic parasites, especially if the assay setup includes a lag time between addition of β-galSENSOR probe and luminescence quantification.
Figure 4.
Luminescence emitted from F12lacZ parasites remains stable. (A) Chemiluminescence emitted by F12lacZ parasites synchronized to different stages of intraerythrocytic development. Linear regression was calculated using values significantly different from the F12wt ctrl. (B) Following addition of the β-galSENSOR probe, luminescence remains stable over a wide range of parasite numbers and stages for at least 180 min. Absolute parasite numbers used per condition are indicated. 20 000 wild type parasites (F12wt) were used as negative controls (ctrl.). For (A), n = 3; biological replicates are shown individually; for the control samples (ctrl.), values were averaged, and error bars indicate standard deviations of biological replicates. For (B), a representative example of three biological replicates is shown. The full data set is shown in supplementary Figure 3.
β-Galactosidase Remains Stable under Nonphysiological Conditions
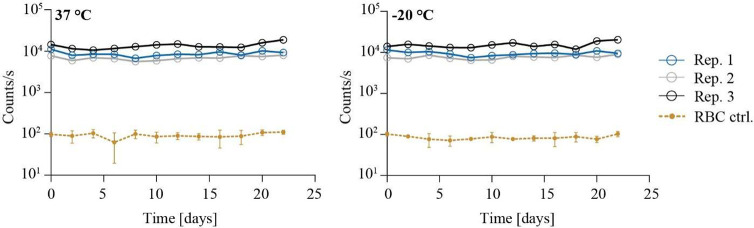
Activity of β-galactosidase is ATP-independent and, in contrast to most luciferase-based assays, is therefore not directly linked to the energy state or the physiological context of cells. Indeed, we observed that β-galactosidase retains its activity after lysis of F12lacZ parasite cultures, i.e., under nonphysiological conditions (Figure 5). In fact, the enzyme readily processes the β-galSENSOR probe even after storing F12lacZ lysates at 37 or −20 °C for more than 3 weeks. It is therefore important to note that the luminescence derived from the lacZ/β-galSENSOR probe pair should not be confused with that of a viability dye. Similar to fluorescence-based assays, such as SYBR Green I-based detection of nucleic acids,19,20 it is therefore important to include appropriate negative controls, as presented hereafter. With regard to handling large amounts of test plates and general flexibility of the assay, the high stability of β-galactosidase is certainly advantageous, as assay plates can be stored at −20 °C without the need to perform readout measurements immediately after the assay end point.
Figure 5.
β-Galactosidase activity can be quantified after storing parasite lysates under different conditions. Viable F12lacZ parasites were lysed, and luminescence was quantified every 2 days following storage of the samples at 37 or −20 °C. n = 3; biological replicates (rep.) are shown individually; for the control samples (ctrl.), values were averaged, and error bars indicate standard deviations of biological replicates.
The lacZ/β-galSENSOR Probe System Allows Probing Antimalarial Drug Efficacy
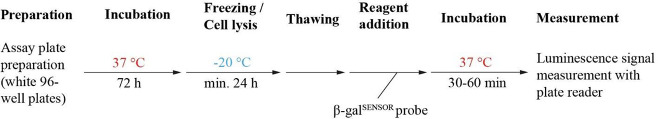
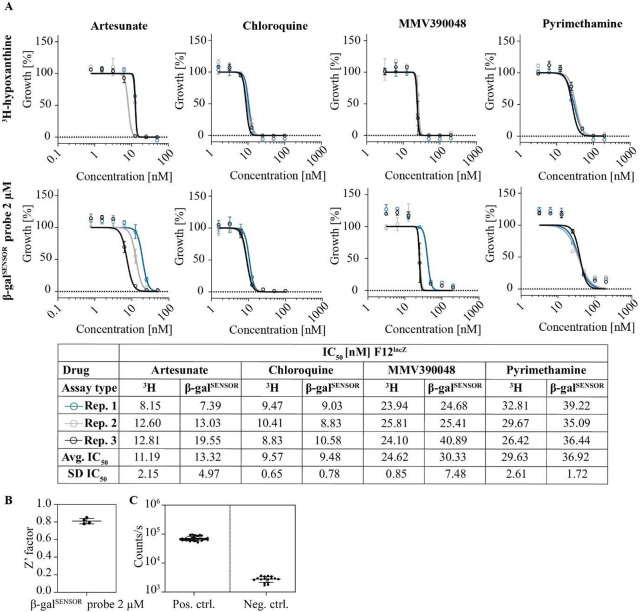
We next examined whether the chemiluminescence emitted from F12lacZ parasites is suitable for determining the drug susceptibility of P. falciparum parasites in a drug screening format. To do so, we probed the effect of the well-characterized antimalarials artesunate (AS), chloroquine (CQ), pyrimethamine (PYRI), and MMV390048 on F12lacZ growth. AS and CQ are routinely used as compound controls in drug screening approaches,47 including the [3H]-hypoxanthine incorporation assay. To evaluate the suitability and performance of the lacZ/β-galSENSOR probe system for drug activity testing, we ran β-galSENSOR probe-based dose–response assays side-by-side with [3H]-hypoxanthine-based quantification of replicating DNA. The assay setup is detailed in Figure 6 and in the Materials and Methods section. Briefly, mixed-stage F12lacZ parasites were exposed to serially diluted test drugs using 96-well microtiter plates and incubated under standard malaria culture conditions for 72 h. The assay plates were stored and RBCs lysed for at least 24 h at −20 °C after the 72 h incubation period (Figure S4). Frozen plates were thawed prior to the addition of the β-galSENSOR probe. Following exposure to the β-galSENSOR probe at 10 or 2 μM, the assay plates were analyzed using a standard plate reader (TECAN, Spark). In parallel, F12lacZ parasites deriving from the same mother culture were treated and analyzed using the [3H]-hypoxanthine incorporation assay and a beta counter (PerkinElmer, MicroBeta). We found the IC50 values determined by the different assay formats to be virtually identical (Figure 7A). As mentioned above, if absolute quantification of intraerythrocytic parasites is not the primary focus, performing the luminescence-based assay with 2 μM of the β-galSENSOR probe allows for robust screening conditions (Z′-factor = 0.81 (standard deviation (SD) = 0.03) with a signal-to-noise ratio of 26.51 (SD = 5.47), n = 4) (Figures 7B,C and S5). Considering the low detection limit of the lacZ/β-galSENSOR probe system, we expected that miniaturization of the assay is feasible. Indeed, we found the system to allow probing of antimalarial effects in the 384-well format simply by reducing the culturing and readout volumes and therefore without affecting the ease of use of the presented assay setup (Figure 8).
Figure 6.
Overview of the IC50 assay protocol using the lacZ/β-galSENSOR probe system.
Figure 7.
lacZ-expressing parasites as a tool for drug screening assays. (A) Comparison of the F12lacZ/β-galSENSOR probe and [3H]-hypoxanthine incorporation assay formats. Biological replicates (rep.) are shown individually. IC50 values (nM) are indicated. The β-galSENSOR probe was used at 2 μM. Avg., average; SD, standard deviation. (B) Assay robustness determined by the Z′-factor, n = 4. (C) Luminescence derived from F12lacZ parasite cultures lysed at the start of the experiment (negative control) and untreated F12lacZ following the 72 h incubation period (positive control).
Figure 8.
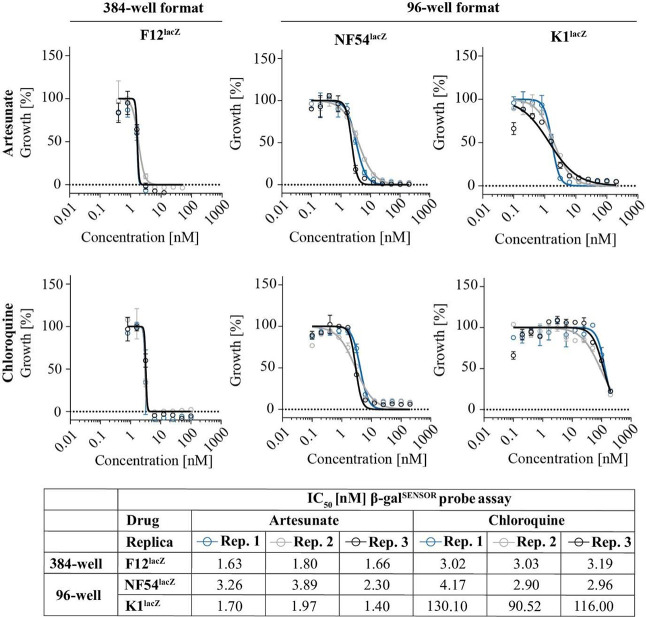
The β-galactosidase-based assay is compatible with the 384-well format and with parasites of different genetic backgrounds. Dose–response experiments performed with the β-galSENSOR probe system using 96- and 384-well plates and in combination with drug sensitive (NF54lacZ) and multidrug-resistant (K1lacZ) parasite lines. 10 μM β-galSENSOR probe; n = 3.
In routine antimalarial drug screening, multiple parasite lines are typically probed in parallel to take strain-specific differences in drug susceptibility into account.48,49 This may include different isolates or strains carrying drug-resistance mutations. To mitigate the drawback of having to use genetically modified parasites, we equipped the widely used drug sensitive and multidrug-resistant P. falciparum lines, NF54 and K1, respectively, with the lacZ reporter cassette used in F12lacZ. These NF54lacZ and K1lacZ parasite lines multiplied normally and showed the expected drug susceptibility/resistance (Figure 8, S6). CQ for instance inhibited K1lacZ with an IC50 of 112.2 nM, i.e., over 30-fold less efficiently compared to the activity of this drug against F12 and NF54 parasites.
Streamlining the Parasite Reduction Ratio (PRR) Assay Using lacZ Reporter Parasites
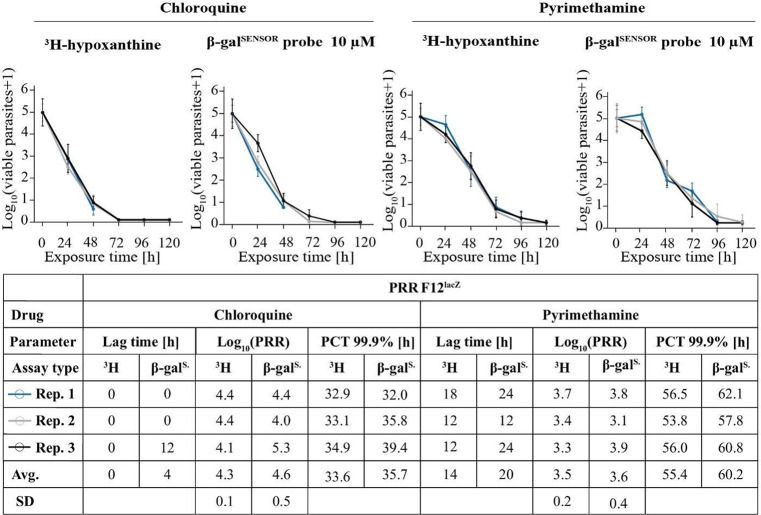
The lacZ/β-galSENSOR probe system presented here may become a valuable tool for many applications, especially those requiring sensitive detection of parasites at low numbers such as the PRR assay. While being labor-intensive and time-consuming, this assay provides important information on the rate at which test compounds kill malaria parasites.30 We therefore set out to test whether the PRR assay would benefit from the chemiluminescence-based detection of F12lacZ parasites (Figure 9). We conducted the PRR assay according to the new PRR assay version 2 published by Walz et al.47 We describe the assay setup in detail in the Materials and Methods section. Briefly, parasite viability is tested after different periods of time under drug pressure (0, 24, 48, 72, 96, or 120 h). After the respective treatment period, the drug is washed off and samples containing 105 parasites are transferred to a 96-well plate and serially diluted to one parasite per well using 4-fold dilution steps. Parasites are then incubated under standard in vitro culturing conditions for 14 days. The subsequent quantification of parasites marks the assay’s end point. Here, we performed PRR assays for two standard drugs, CQ and PYRI, using F12lacZ parasites in combination with either a [3H]-hypoxanthine- or the chemiluminescence-based assay readout.
Figure 9.
The β-galactosidase-based assay allows quantifying the parasite reduction ratio. Side-by-side comparison of the PRR assay performed using [3H]-hypoxanthine incorporation or β-galactosidase-catalyzed luminescence as a readout. Pharmacodynamic parameters derive from a simulation using a previously developed R script (Walz et al.). Avg., average; SD, standard deviation.
The data of these PRR assays are presented in Figure 9 and show that all readout parameters, including the lag time, log10(PRR), and the 99.9% PCT, are highly comparable between the gold standard [3H]-hypoxanthine incorporation readout and the chemiluminescence-based method.
Discussion and Conclusion
The recent discovery of dioxetanes that emit strong luminescence in aqueous solutions upon enzyme-mediated decoupling of a protective group opens up new possibilities for sensitive biomarker detection. Here, we present a lacZ/β-galSENSOR probe system that allows for the highly sensitive, chemiluminescence-based detection of P. falciparum parasites. We describe a protocol for determining and monitoring antimalarial drug activity in vitro. Pairing high sensitivity with ease of use, we believe that the presented chemiluminescence-based system can become a valuable tool including, but not limited to, the discovery of antimalarial drugs.
Requiring lacZ-expressing P. falciparum parasites and a single-component reagent, the β-galSENSOR probe, parasite-catalyzed chemiluminescence can be quantified using a standard plate reader. Time-consuming steps, such as washing required for ELISA- as well as for fluorescence-based assays (SYBR Green I, PICO green), can be omitted. Light scattering and nonselective excitation are drawbacks associated with the use of fluorescent probes, contributing to the current superiority of the [3H]-hypoxanthine incorporation assay.50 While the lacZ/β-galSENSOR probe system does not involve the use of hazardous substances, including radioactive isotopes, the requirement of transgenic lines currently restricts its use to parasite lines created during this study. In attempts to still provide a valuable tool box for antimalarial drug discovery and monitoring, we inserted the lacZ expression cassette into three different parasite stains, F12, NF54, and K1. While the F12lacZ and NF54lacZ lines allow quantifying compound activities in drug-sensitive parasites of different genomic backgrounds, the K1lacZ line is descending from a multidrug-resistant strain.42,43,48,51 Due to the high stability of the ectopically expressed β-galactosidase, it is important to note that the luminescence derived from the lacZ/β-galSENSOR probe pair should not be confused with that of a viability marker. One advantage of this stability is that assay plates can be stored at −20 °C for at least 3 weeks prior to adding the β-galactoside substrate for readout.
Evaluating whether the lacZ/β-galSENSOR probe system allows for high-throughput screening was out of the scope of this study. The assay sensitivity as well as our experiments using the 384-well format, however, indicate that the system is likely amenable to high-throughput formats. While assay miniaturization could reduce costs, the presented assay is already cost-effective in the 96-well format when using 2 μM β-galSENSOR probe (Tab. S1).
We assume that the sensitivity of the lacZ/β-galSENSOR probe system will become an asset to various approaches in malaria research, especially if they require handling small numbers of parasites. One of these applications is the PRR assay. As mentioned above, the PRR assay allows testing recovery of malaria parasites following drug treatment, a measure that is becoming increasingly important for monitoring the action of antimalarials. We thus evaluated the lacZ/β-galSENSOR probe system for PRR assay testing. Compared to the current gold standard—the [3H]-hypoxanthine incorporation method—the chemiluminescence-based readout yielded highly comparable results (Figure 7) for the two tested drugs CQ and PYRI. Of note, the chemiluminescence-based readout inherently differs from quantifying incorporation of a radioactive isotope into replicating parasite DNA. Owed to the high sensitivity at which β-galactosidase can be detected, it is important to distinguish between enzyme activity deriving from viable parasites versus β-galSENSOR probe processed by β-galactosidase present in the initial parasite inoculum. As detailed in the Materials and Methods section, we thus recommend including a control that quantifies residual β-galactosidase activity derived from nonviable parasites. The most recent PRR protocol (version 2) requires a 14-day regrowth phase using the [3H]-hypoxanthine incorporation readout.47 Here, we used the same streamlined protocol for the chemiluminescence-based readout. Given the high sensitivity of the lacZ/β-galSENSOR probe system, it is not surprising that our results indicate the potential for further reduction of the regrowth phase. While this would certainly be beneficial for ramping up the throughput of the PRR assay, more work is needed to confirm our hypothesis.
Compounds inhibiting reporter enzyme activity, rather than test sample viability can cause false-positive hits in drug screening assays. Luciferase is for instance targeted by many compounds,52 and the lacZ/β-galSENSOR probe system presented here will certainly not be an exception to this phenomenon.53,54 This being said, the lacZ/β-galSENSOR probe-based assay may well be used as a counterscreen for luciferase-based assays and vice versa to identify inhibitors of β-galactosidase and luciferase, respectively. This can be conducted without the need to change the luminescence-based readout. In addition, we believe that lacZ-expressing parasites have the potential to become valuable outside drug screening and monitoring, for instance, as sensitive reporters of mosquito infection.
In conclusion, we report that the new generation chemiluminescent β-galSENSOR probe can be used for the sensitive detection of lacZ-expressing P. falciparum parasites. The presented system can be used for antimalarial drug screening and monitoring using straightforward and inexpensive experimental setups without the need for using harmful substances. We thus believe that it will become a valuable tool for different applications in malaria research that require sensitive P. falciparum detection.
Materials and Methods
Transgenic P. falciparum Lines
P. falciparum parasites of strains F12, NF54, and K1 were transfected using standard procedures.55 Two different plasmids with identical functions, p626-P230p (for F12) or pHF-gC-P230p (for NF54 and K1) (containing the Cas9 and the guideRNA expression cassettes),44 were used in combination with the rescue plasmid P230p-lacZ (based on P230p-sfGFP)44 for editing of the P230p locus. The LacZ sequence from E. coli was codon-optimized for P. falciparum (Genewiz). Parasites were selected to maintain a plasmid carrying expression cassettes for Cas9 and a P230p-targeting gRNA using a final concentration of 5 nM of the antifolate drug WR99210 in combination with the drug selectable marker human dihydrofolate reductase (hDHFR). F12lacZ parasites were cloned in 96-well plates using limiting dilution. From the clones obtained, the hDHFR-free clone H12 was used for successive experiments. The same procedure was repeated to obtain NF54lacZ (E1) and K1lacZ (B1) clones. Primer sequences used for diagnostic PCR of the P230p locus were as in ref. (44). Primers used for PCR of the hdhfr gene were CTAAACTGCATCGTTGCTGTG (forward primer hDHFR_F) and CCTGGACATCAGAGAGAACAC (reverse primer hDHFR_R).
Western Blotting
500 μL of packed infected red blood cells at 3–5% parasitaemia were treated with saponin (3 mL 0.15% saponin in PBS) for 10 min on ice in 15 mL Falcon tubes. Parasite pellets were washed twice in ice-cold PBS and resuspended in lysis buffer (2% SDS, 62.5 mM Tris base (pH 6.8), 10% glycerol, 5% β-mercaptoethanol) , prior to adding NuPAGE LDS Sample Buffer (4×, cat. no. #NP0007) to a 1× final concentration. SDS PAGE was performed using 4–12% Bis-Tris gels (Novex, Qiagen) in combination with MOPS running buffer (Novex, Qiagen). Protein blotting was done using a nitrocellulose membrane (GE healthcare #106000169). The membrane was blocked using 5% milk powder in PBS/0.1% Tween 20 (PBS-T) for 1 h and probed using mouse anti-β-galactosidase mAb (Promega, cat.#Z3781), 1:5000, a mouse anti-GAPDH56 diluted 1:10000 in 2% milk powder in PBS-T, and a goat antimouse HRP (Pierce cat. #31430). The membrane was washed five times in PBS-T before SuperSignal West Pico Plus substrate (Thermo Scientific, cat. #34580) was added.
P. falciparum Cell Culture
Parasites were cultured using standard methods.57,58 In brief, the culture medium (CM) consisted of RPMI 1640 (10.44 g/L, ThermoFisher Scientific, cat. #51800–043), supplemented with 25 mM HEPES (5.94 g/L, Sigma-Aldrich, cat. #H4034–500G), NaHCO3 (2.1 g/L, Sigma-Aldrich, cat. no. #31437–500G-R), neomycin (100 mg/L, Sigma-Aldrich, cat. #N6386–100G), Albumax II (5 g/L, Thermo Fisher, cat. # 11021–045), 2 mM choline chloride (279 mg/L, Sigma-Aldrich, cat. #C7527–100G), and 0.36 mM hypoxanthine (50 mg/L, Sigma-Aldrich, cat. # H9377–25G). All components were dissolved in water (Milli-Q purified), stirred for at least 3 h, and then sterile filtered (0.22 μm pore size) with a bottle top filter (Corning, cat# 431118). The parasite cultures were kept at a hematocrit (Hc) of 5% and incubated at 37 °C in a gas mixture consisting of 93% N2, 4% CO2, and 3% O2. Erythrocyte concentrates were obtained from the local blood bank.
Multiplication Rate Analysis
Analysis of the multiplication rates of the transgenic P. falciparum strains F12lacZ, NF54lacZ, and K1lacZ compared to their wild type parental strains was performed using mixed-stage parasite cultures. Starting parasitemia was set at ∼0.3%, and parasitemia was determined by Giemsa-stained blood smears. Parasites were grown under culture conditions with a Hc of 1.25%. 48 h later, parasitemia was determined again and multiplication rates were calculated.
Limit of Detection Experiments
Chemiluminescence detection limits were evaluated using either black 384-well plates (Greiner Bio-One, cat. no. 781086) or white 96-well plates (Greiner Bio-One, cat. no. #655083). The number of parasites was calculated by combining Giemsa-stained blood smears (to quantify parasitemia) and an improved Neubauer chamber (to quantify erythrocytes per volume). Starting with 2250 parasites per well in a 96-well plate (in 100 μL in PBS containing MgCl2 at 1 mM), a 2-fold dilution series was performed to reach a theoretical 1.1 parasites per well. 20 μL of each of these 2-fold dilution steps was transferred to a 384-well plate, resulting in a theoretical 450 to 0.22 parasites per well. Noninfected RBCs at the same Hc were used as a negative control. AquaSpark β-D-galactoside (Biosynth AG, cat. #A-8169_P00), here referred to as β-galSENSOR probe, was dissolved in H2O containing 1 mM MgCl2 to a concentration of 400 μM and incubated at room temperature (RT) for ∼30 min and subsequently added to each well to a final concentration of 200 μM. To determine the LOD under IC50 assay-like conditions, 14 400 parasites per well were serially diluted to a theoretical 0.22 in parasites per well (in 100 μL CM at a Hc of 1.25%) in white 96-well plates. β-galSENSOR probe was diluted to a concentration of 20 μM and added to each well to a final concentration of 10 μM. Chemiluminescence was quantified using the multimode reader Spark from TECAN at 37 °C. Data analysis was done using GraphPad Prism (v 8.2.1). The following GraphPad function was used: [Inhibitor] vs response – Variable slope. The underlying equation is Y = Bottom + (Top – Bottom)/(1 + (X/IC50)^HillSlope). Data were normalized to positive and negative controls prior to nonlinear fit analysis.
Luminescence Stability Experiments
Luminescence stability was quantified using a theoretical parasite per well range of 20 000–1, 3-fold diluted, and lysed in H2O (20 μL per well of a 384-well plate Greiner Bio-One, cat. #781086). 20 000 F12wt parasites were used as negative control. β-galSENSOR probe (Biosynth AG, cat. #A-8169_P00) was dissolved in PBS containing 1 mM MgCl2 to a concentration of 20 μM, incubated at RT for 30–60 min and subsequently added to each well to a final concentration of 10 μM. Chemiluminescence was measured every 15 min (0–180 min) using PerkinElmer’s IVIS Spectrum In Vivo Imaging System Lumina LT Series III at RT. IVIS raw data was extracted using the Living Image Software (v 4.7.2) and analyzed using GraphPad Prism (v 8.2.1).
Assessing β-Galactosidase Activity Stability in Parasite-Lysates
The stability of the ectopically expressed β-galactosidase activity under different storage conditions was evaluated in biological triplicates. To do so, parasite suspensions with a parasitemia of ∼0.3% and an Hc of 1.25% were lysed using one freeze–thaw cycle (room temperature → −20 °C → room temperature). A lysate of uninfected RBCs with the same Hc was used as a negative control. Corresponding lysates were either aliquoted and stored at −20 °C or distributed in technical duplicates to white 96-well plates (Greiner Bio-One, cat. #655083) and incubated with malaria gas at 37 °C. From day 0 to day 22, an aliquot of the lysate stored at −20 °C was taken and distributed in technical duplicates to the 96-well plate every 48 h. β-galSENSOR probe (Biosynth AG, cat. #A-8169_P00) was diluted to 20 μM in H2O containing 1 mM MgCl2 and added 1:1 (100 μL/100 μL) to the corresponding wells. The plate was incubated for ∼30 min at 37 °C prior to reading out luminescence using the multimode reader Spark from TECAN at 37 °C. Plates were stored at 37 °C, and the procedure was repeated using 48 h intervals.
Cost Analysis of [3H]-Hypoxanthine IC50 vs lacZ/β-galSENSOR Probe IC50 Assay
An evaluation and comparison of the costs between the [3H]-hypoxanthine incorporation-based IC50 assay and the lacZ/β-galSENSOR probe IC50 assay is presented in supplementary Table 1.
[3H]-Hypoxanthine IC50 Assays
[3H]-hypoxanthine IC50 assays were performed as reported previously11,59 using parasites cultured in transparent 96-well plates (Falcon, cat. #353072). The highest drug concentrations were 50 nM for AS (Mepha, cat. #11665), 200 nM for PYRI (Sigma-Aldrich, cat. #46706–250MG) and MMV390048, and 100 nM for CQ (Sigma-Aldrich, cat. #C6628–25G). P. falciparum F12lacZ parasites were diluted to 0.3% parasitemia and a Hc of 1.25% in a volume of 200 μL. After 48 h, [3H]-hypoxanthine was added to an end concentration of 0.25 μCi. Parasites were incubated for another 24 h and put at −20 °C for at least 24 h. Plates were then harvested and [3H]-hypoxanthine incorporation counted on a MicroBeta counter from PerkinElmer as described in Desjardins et al.(11) Data was analyzed using Microsoft Excel 2016 (v 16.0.5356.1000) and GraphPad Prism (v 8.2.1). The following GraphPad function was used: [Inhibitor] vs response – Variable slope. The underlying equation is Y = Bottom + (Top – Bottom)/(1 + (X/IC50)^HillSlope). Data were normalized to positive and negative controls prior to nonlinear fit analysis.
lacZ/β-galSENSOR Probe IC50 Assays
IC50 assays using the lacZ/β-galSENSOR probe system were performed in white 96-well plates (Greiner Bio-One, cat. #655083) with highest drug concentrations of 50 nM for AS, 200 nM for PYRI and MMV390048, and 100 nM for CQ. 10 mM drug stocks (in DMSO) were prediluted to 4× working solutions in CM. 2-fold serial dilutions were used, and mixed-stage parasites were incubated at a parasitemia of 0.3% and a Hc of 1.25%. As a negative control, an aliquot of the parasite suspension was immediately stored at −20 °C until the assay was prepared for the readout. The assay plates were placed in a humid malaria gas chamber and incubated at 37 °C for 72 h. After incubation, the plate was stored at −20 °C for at least 24 h. Lysis of samples at −20 °C is strongly recommended, as IC50 values could not be calculated for all drugs if samples were not lysed prior to signal measurement (Figure S4). For the readout, the assay plates and the corresponding controls were thawed. Equal volumes of control samples were added to the test plates. The β-galSENSOR probe (Biosynth AG, cat. #A-8169_P00) was diluted to 4 μM in H2O containing 1 mM MgCl2 and added to all wells of the plate to a final concentration of 2 μM (where indicated, 10 μM end concentration was used). Plates were incubated for 30–60 min at 37 °C, and the luminescence was quantified using the multimode reader Spark from TECAN at 37 °C. Raw data were analyzed using Microsoft Excel 2016 (v 16.0.5356.1000) and GraphPad Prism (v 8.2.1). The following GraphPad was: [Inhibitor] vs response – Variable slope. The underlying equation is Y = Bottom + (Top – Bottom)/(1 + (X/IC50)^HillSlope). Data were normalized to positive and negative controls prior to nonlinear fit analysis using GraphPad Prism.
IC50 assays using the lacZ/β-galSENSOR probe with NF54lacZ or K1lacZ were performed in black 96-well plates (Greiner Bio-One, cat. #655086). The 384-well format assay was evaluated using black plates (Greiner Bio-One, cat. #781086). Highest drug concentrations were 200 nM for AS and CQ in the 96-well format and 50 nM for AS and 100 nM for CQ in the 384-well format. 2-fold serial dilutions were used, and synchronized parasites (8–14 h post invasion) were incubated at a parasitemia of 0.3% and a Hc of 2.5%. As a negative control, parasites treated with 100 nM CQ were used. The assay plates were placed in a humid malaria gas chamber and incubated at 37 °C for 72 h. After incubation, the β-galSENSOR probe was diluted to 20 μM in H2O containing 1 mM MgCl2 and added to all wells of the plate to a final concentration of 10 μM. Plates were incubated for ∼60 min at 37 °C, and chemiluminescence was measured using a PerkinElmer IVIS Spectrum In Vivo Imaging System Lumina LT Series III at RT. IVIS raw data was extracted using the Living Image Software (version 4.7.2) and analyzed using GraphPad Prism (v 8.2.1).
Parasite Reduction Ratio Assay
PRR assays were conducted according to the new PRR assay version 2 protocol published by Walz et al.,47 which was adapted from Sanz et al.30 The [3H]- and β-galSENSOR-based PRR assays were performed using transparent and white 96-well plates, respectively. Compound stock solutions were diluted to 100× the final assay concentration using CM. Mixed-stage parasites at a parasitemia of 0.3% and a Hc of 1.25% were cultured in 5 mL using six-well plates (Falcon, cat. #353046) (equals 1.875 × 106 parasites). Parasites were exposed to test compounds at 10× IC50 and incubated for 24–120 h. The drugs were renewed every 24 h by aspiration of the old supernatant and addition of freshly prepared CM containing the drugs. Every 24 h, 4.5 mL of the treated culture was collected, and the parasites were washed three times using fresh CM (no drug added) and resuspended in 4.5 mL of CM. 267 μL of the washed parasite culture (equivalent to 105 parasites at time point 0 h) was serially diluted in 4-fold steps (67 μL each) into wells of a 96-well plate (in four technical replicates). To avoid edge effects, outer wells were not used and filled with 250 μL of CM instead. Identical plates were prepared for all time points, including the 0 h, initial time point 0. The 96-well plates were incubated for 14 days using standard culture conditions (see Materials and Methods section). The CM was changed twice by removing 100 μL (first medium change) or 150 μL (second medium change) of supernatant and adding 150 μL of fresh CM with a Hc of 1.5% per well. For the [3H]-based PRR assay, tritium-labeled-hypoxanthine solution was added to each well on day 13 to an end concentration of 0.5 μCi. The plates were then incubated for a further 24 h. For the lacZ/β-galSENSOR probe-based PRR assay, the volume per well was adjusted to 100 μL on day 14. For both assay types, the plates were stored at −20 °C for at least 24 h. After the plates were completely thawed, they were analyzed using the respective methods described in Materials and Methods section. Similar to the [3H]-based PRR assay, we defined a threshold value for distinguishing between wells with viable versus nonviable parasites. Specifically, wells were deemed positive for parasite growth if the measured value was above three times the average of the negative control (uninfected RBCs) for the [3H]-hypoxanthine readout and 120-fold for the lacZ/β-galSENSOR probe readout. The data was analyzed using Microsoft Excel 2016 (v 16.0.5356.1000), R (v 4.1.3), and RStudio (v 2022.02.3).
Acknowledgments
The authors would like to acknowledge Biosynth AG for providing gift samples of the AquaSpark β-D-galactoside.
Glossary
Abbreviations
- RBC
red blood cell
- ACT
artemisinin-based combination therapy
- pLDH
Plasmodium lactate dehydrogenase
- IC50
half-maximal inhibitory concentration
- PRR
parasite reduction ratio
- PCT
parasite clearance time
- ELG
enzyme-labile group
- lacZ
Escherichia coli β-galactosidase gene
- hDHFR
human dihydrofolate reductase
- gRNA
guideRNA
- wt
wild type
- int
integration
- LOD
limit of detection
- PBS
phosphate-buffered saline
- CM
culture medium
- Hc
hematocrit
- hpi
hours post invasion
- Rep.
biological replicates
- ctrl.
control sample
- AS
artesunate
- CQ
chloroquine
- PYRI
pyrimethamine
- SD
standard deviation
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.3c00707.
Overview of the two-plasmid CRISPR/Cas9-based gene editing strategy, demonstration of stable luminescence emission from F12lacZ parasites, comparison of the F12lacZ/β-galSENSOR probe assay formats using either 10 or 2 μM β-galSENSOR probe, demonstration of the recommendation of freezing the β-galSENSOR probe assay plates prior to the readout, and table showing the cost effectiveness of the β-galSENSOR probe IC50 assay (PDF)
Author Contributions
Idea and conceptualization : N.M.B.B., K.W., S.W., M.H., J.C., A.H.; conducting experiments: A.H., K.W., K.S., T.B.; manuscript writing: N.M.B.B., A.H., additional support: A.P., B.T., A.W.
This project was supported by the Swiss National Science Foundation (grant number 310030_200683) and the R. Geigy-Stiftung.
The authors declare the following competing financial interest(s): Mario Hupfeld is CEO and co-founder of NEMIS Technologies AG, a company developing diagnostics solutions for the food industry based on the chemiluminescent AquaSpark technology.
Supplementary Material
References
- Ashley E. A.; Pyae Phyo A.; Woodrow C. J. Malaria. Lancet. 2018, 391 (10130), 1608–21. 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World malaria report 2022; World Health Organization: Geneva; 2022.
- Phillips M. A.; Burrows J. N.; Manyando C.; van Huijsduijnen R. H.; Van Voorhis W. C.; Wells T. N. C. Malaria. Nature Reviews Disease Primers. 2017, 3 (1), 17050. 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- Ma N.; Zhang Z.; Liao F.; Jiang T.; Tu Y. The birth of artemisinin. Pharmacol Ther. 2020, 216, 107658 10.1016/j.pharmthera.2020.107658. [DOI] [PubMed] [Google Scholar]
- Klayman D. L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985, 228 (4703), 1049–55. 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17 (10), 1217–20. 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Takala-Harrison S.; Jacob C. G.; Arze C.; Cummings M. P.; Silva J. C.; Dondorp A. M.; Fukuda M. M.; Hien T. T.; Mayxay M.; Noedl H.; Nosten F.; Kyaw M. P.; Nhien N. T.; Imwong M.; Bethell D.; Se Y.; Lon C.; Tyner S. D.; Saunders D. L.; Ariey F.; Mercereau-Puijalon O.; Menard D.; Newton P. N.; Khanthavong M.; Hongvanthong B.; Starzengruber P.; Fuehrer H. P.; Swoboda P.; Khan W. A.; Phyo A. P.; Nyunt M. M.; Nyunt M. H.; Brown T. S.; Adams M.; Pepin C. S.; Bailey J.; Tan J. C.; Ferdig M. T.; Clark T. G.; Miotto O.; MacInnis B.; Kwiatkowski D. P.; White N. J.; Ringwald P.; Plowe C. V. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J. Infect Dis. 2015, 211 (5), 670–9. 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X. Z.; Lane K. D.; Xia L.; Sá J. M.; Wellems T. E. Plasmodium Genomics and Genetics: New Insights into Malaria Pathogenesis, Drug Resistance, Epidemiology, and Evolution. Clin Microbiol Rev. 2019, 32 (4), e00019-19. 10.1128/CMR.00019-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicht K. J.; Mok S.; Fidock D. A. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431–54. 10.1146/annurev-micro-020518-115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann K. H.; Campbell G. H.; Sax L. J.; ema J. E. Drug sensitivity of plasmodium falciparum. An in-vitro microtechnique. Lancet. 1978, 311 (8054), 22–23. 10.1016/S0140-6736(78)90365-3. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E.; Canfield C. J.; Haynes J. D.; Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16 (6), 710–8. 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabbadi N.; Ancelin M. L.; Vial H. J. Use of radioactive ethanolamine incorporation into phospholipids to assess in vitro antimalarial activity by the semiautomated microdilution technique. Antimicrob. Agents Chemother. 1992, 36 (1), 50–5. 10.1128/AAC.36.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druilhe P.; Moreno A.; Blanc C.; Brasseur P. H.; Jacquier P. A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am. J. Trop Med. Hyg. 2001, 64 (5–6), 233–41. 10.4269/ajtmh.2001.64.233. [DOI] [PubMed] [Google Scholar]
- Noedl H.; Wernsdorfer W. H.; Miller R. S.; Wongsrichanalai C. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob. Agents Chemother. 2002, 46 (6), 1658–64. 10.1128/AAC.46.6.1658-1664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritten L.; Matile H.; Brun R.; Wittlin S. A new double-antibody sandwich ELISA targeting Plasmodium falciparum aldolase to evaluate anti-malarial drug sensitivity. Malar J. 2009, 8, 226. 10.1186/1475-2875-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M.; Brun R.; Grieder A. Microscopic and flow cytophotometric analysis of parasitemia in cultures of Plasmodium falciparum vitally stained with Hoechst 33342--application to studies of antimalarial agents. Z. Parasitenkd. 1986, 72 (2), 201–12. 10.1007/BF00931147. [DOI] [PubMed] [Google Scholar]
- Contreras C. E.; Rivas M. A.; Domínguez J.; Charris J.; Palacios M.; Bianco N. E.; Blanca I. Stage-specific activity of potential antimalarial compounds measured in vitro by flow cytometry in comparison to optical microscopy and hypoxanthine uptake. Mem Inst Oswaldo Cruz. 2004, 99 (2), 179–84. 10.1590/S0074-02762004000200011. [DOI] [PubMed] [Google Scholar]
- van Vianen P. H.; Thaithong S.; Reinders P. P.; van Engen A.; van der Keur M.; Tanke H. J.; van der Kaay H. J.; Mons B. Automated flow cytometric analysis of drug susceptibility of malaria parasites. Am. J. Trop Med. Hyg. 1990, 43 (6), 602–7. 10.4269/ajtmh.1990.43.602. [DOI] [PubMed] [Google Scholar]
- Bennett T. N.; Paguio M.; Gligorijevic B.; Seudieu C.; Kosar A. D.; Davidson E.; Roepe P. D. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob. Agents Chemother. 2004, 48 (5), 1807–10. 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein M.; Sriwilaijaroen N.; Kelly J. X.; Wilairat P.; Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48 (5), 1803–6. 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniecki M. L.; Wirth D. F.; Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob. Agents Chemother. 2007, 51 (2), 716–23. 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye D.; Patel V.; Demas A.; LeRoux M.; Ndir O.; Mboup S.; Clardy J.; Lakshmanan V.; Daily J. P.; Wirth D. F. A non-radioactive DAPI-based high-throughput in vitro assay to assess Plasmodium falciparum responsiveness to antimalarials--increased sensitivity of P. falciparum to chloroquine in Senegal. Am. J. Trop Med. Hyg. 2010, 82 (2), 228–30. 10.4269/ajtmh.2010.09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo M.; Sousa C.; Shapiro H. M.; Mota M. M.; Grobusch M. P.; Hänscheid T. A novel flow cytometric hemozoin detection assay for real-time sensitivity testing of Plasmodium falciparum. PLoS One. 2013, 8 (4), e61606 10.1371/journal.pone.0061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butykai A.; Orbán A.; Kocsis V.; Szaller D.; Bordács S.; Tátrai-Szekeres E.; Kiss L. F.; Bóta A.; Vértessy B. G.; Zelles T.; Kézsmárki I. Malaria pigment crystals as magnetic micro-rotors: key for high-sensitivity diagnosis. Sci. Rep. 2013, 3, 1431. 10.1038/srep01431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbán A.; Butykai Á; Molnár A.; Pröhle Z.; Fülöp G.; Zelles T.; Forsyth W.; Hill D.; Müller I.; Schofield L.; Rebelo M.; Hänscheid T.; Karl S.; Kézsmárki I. Evaluation of a novel magneto-optical method for the detection of malaria parasites. PLoS One. 2014, 9 (5), e96981 10.1371/journal.pone.0096981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár P.; Orbán Á; Izrael R.; Babai R.; Marton L.; Butykai Á; Karl S.; Vértessy B. G.; Kézsmárki I. Rapid and quantitative antimalarial drug efficacy testing via the magneto-optical detection of hemozoin. Sci. Rep. 2020, 10 (1), 14025. 10.1038/s41598-020-70860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L.; Miao J.; Wang J.; Li Q.; Cui L. Plasmodium falciparum: development of a transgenic line for screening antimalarials using firefly luciferase as the reporter. Exp Parasitol. 2008, 120 (1), 80–7. 10.1016/j.exppara.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T.; van Brummelen A. C.; Parkinson C. J.; Hoppe H. C. ATP and luciferase assays to determine the rate of drug action in in vitro cultures of Plasmodium falciparum. Malar J. 2012, 11, 369. 10.1186/1475-2875-11-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantoni L.; Duffy S.; Adjalley S. H.; Fidock D. A.; Avery V. M. Identification of MMV malaria box inhibitors of plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob. Agents Chemother. 2013, 57 (12), 6050–62. 10.1128/AAC.00870-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L. M.; Crespo B.; De-Cózar C.; Ding X. C.; Llergo J. L.; Burrows J. N.; García-Bustos J. F.; Gamo F. J. P. falciparum in vitro killing rates allow to discriminate between different antimalarial mode-of-action. PLoS One. 2012, 7 (2), e30949 10.1371/journal.pone.0030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S.; Sarma P.; Sehgal R.; Medhi B. Development in Assay Methods for in Vitro Antimalarial Drug Efficacy Testing: A Systematic Review. Front Pharmacol. 2017, 8, 754. 10.3389/fphar.2017.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M. S.; Engel J. A.; Chua M. J.; Fisher G. M.; Skinner-Adams T. S.; Andrews K. T. Adaptation of the [3H]Hypoxanthine Uptake Assay for In Vitro-Cultured Plasmodium knowlesi Malaria Parasites. Antimicrob. Agents Chemother. 2016, 60 (7), 4361–3. 10.1128/AAC.02948-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho L. P.; Niepoth E.; Mavraj-Husejni A.; Kreidenweiss A.; Herrmann J.; Müller R.; Knaab T.; Burckhardt B. B.; Kurz T.; Held J. Quantification of Plasmodium falciparum HPR-2 as an alternative method to [3H]hypoxanthine incorporation to measure the parasite reduction ratio in vitro. Int. J. Antimicrob Agents. 2023, 62 (3), 106894 10.1016/j.ijantimicag.2023.106894. [DOI] [PubMed] [Google Scholar]
- Dodeigne C.; Thunus L.; Lejeune R. Chemiluminescence as diagnostic tool. A review. Talanta. 2000, 51 (3), 415–39. 10.1016/S0039-9140(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hananya N.; Shabat D. Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water. ACS Cent Sci. 2019, 5 (6), 949–59. 10.1021/acscentsci.9b00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongen H. A.; Hoetelmans R. M.; Bult A.; van Bennekom W. P. Chemiluminescence and immunoassays. J. Pharm. Biomed Anal. 1994, 12 (4), 433–62. 10.1016/0731-7085(94)80027-8. [DOI] [PubMed] [Google Scholar]
- Green O.; Eilon T.; Hananya N.; Gutkin S.; Bauer C. R.; Shabat D. Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes. ACS Cent Sci. 2017, 3 (4), 349–58. 10.1021/acscentsci.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala L.; Sirec T.; Spitz U. Modified Enzyme Substrates for the Detection of Bacteria: A Review. Molecules. 2020, 25 (16), 3690. 10.3390/molecules25163690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz G. M.; Reinau L.; Imhaus A. F.; Hupfeld M.; Fieseler L. AquaSpark(TM) - Rapid Environmental Monitoring of Listeria monocytogenes. Chimia (Aarau). 2020, 74 (10), 791–7. 10.2533/chimia.2020.791. [DOI] [PubMed] [Google Scholar]
- Bromberger B.; Mester P. J. Rapid detection of Listeria monocytogenes in dairy products by a novel chemilumonogenic approach. Food Microbiol. 2023, 109, 104150 10.1016/j.fm.2022.104150. [DOI] [PubMed] [Google Scholar]
- Roth-Konforti M.; Green O.; Hupfeld M.; Fieseler L.; Heinrich N.; Ihssen J.; Vorberg R.; Wick L.; Spitz U.; Shabat D. Ultrasensitive Detection of Salmonella and Listeria monocytogenes by Small-Molecule Chemiluminescence Probes. Angew. Chem., Int. Ed. Engl. 2019, 58 (30), 10361–7. 10.1002/anie.201904719. [DOI] [PubMed] [Google Scholar]
- Kafsack B. F.; Rovira-Graells N.; Clark T. G.; Bancells C.; Crowley V. M.; Campino S. G.; Williams A. E.; Drought L. G.; Kwiatkowski D. P.; Baker D. A.; Cortés A.; Llinás M. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014, 507 (7491), 248–52. 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano P.; Roca L.; Smith D.; Read D.; Carter R.; Day K. Plasmodium falciparum: parasites defective in early stages of gametocytogenesis. Exp Parasitol. 1995, 81 (2), 227–35. 10.1006/expr.1995.1112. [DOI] [PubMed] [Google Scholar]
- Ashdown G. W.; Dimon M.; Fan M.; Sánchez-Román Terán F.; Witmer K.; Gaboriau D. C. A.; Armstrong Z.; Ando D. M.; Baum J. A machine learning approach to define antimalarial drug action from heterogeneous cell-based screens. Sci. Adv. 2020, 6 (39), eaba9338. 10.1126/sciadv.aba9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbal M.; Gorman M.; Macpherson C. R.; Martins R. M.; Scherf A.; Lopez-Rubio J. J. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat. Biotechnol. 2014, 32 (8), 819–21. 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- Toenhake C. G.; Fraschka S. A.; Vijayabaskar M. S.; Westhead D. R.; van Heeringen S. J.; Bártfai R. Chromatin Accessibility-Based Characterization of the Gene Regulatory Network Underlying Plasmodium falciparum Blood-Stage Development. Cell Host Microbe. 2018, 23 (4), 557–69.e9. 10.1016/j.chom.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A.; Duffey M.; Aljayyoussi G.; Sax S.; Leroy D.; Besson D.; Burrows J. N.; Cherkaoui-Rbati M. H.; Gobeau N.; Westwood M. A.; Siethoff C.; Gamo F. J.; Mäser P.; Wittlin S. The Parasite Reduction Ratio (PRR) Assay Version 2: Standardized Assessment of Plasmodium falciparum Viability after Antimalarial Treatment In Vitro. Pharmaceuticals (Basel) 2023, 16 (2), 163. 10.3390/ph16020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzila A.; Mwai L. In vitro selection of Plasmodium falciparum drug-resistant parasite lines. J. Antimicrob. Chemother. 2010, 65 (3), 390–8. 10.1093/jac/dkp449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh M.; Scheurer C.; Sax S.; Bilsland E.; van Schalkwyk D. A.; Wicht K. J.; Hofmann N.; Sharma A.; Bashyam S.; Singh S.; Oliver S. G.; Egan T. J.; Malhotra P.; Sutherland C. J.; Beck H. P.; Wittlin S.; Spangenberg T.; Ding X. C. Identification and deconvolution of cross-resistance signals from antimalarial compounds using multidrug-resistant Plasmodium falciparum strains. Antimicrob. Agents Chemother. 2015, 59 (2), 1110–8. 10.1128/AAC.03265-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiodun O. O.; Gbotosho G. O.; Ajaiyeoba E. O.; Happi C. T.; Hofer S.; Wittlin S.; Sowunmi A.; Brun R.; Oduola A. M. Comparison of SYBR Green I-, PicoGreen-, and [3H]-hypoxanthine-based assays for in vitro antimalarial screening of plants from Nigerian ethnomedicine. Parasitol Res. 2010, 106 (4), 933–9. 10.1007/s00436-010-1743-z. [DOI] [PubMed] [Google Scholar]
- Delves M.; Plouffe D.; Scheurer C.; Meister S.; Wittlin S.; Winzeler E. A.; Sinden R. E.; Leroy D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012, 9 (2), e1001169 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld D. S.; Inglese J.. Interferences with Luciferase Reporter Enzymes. In Assay Guidance Manual; Markossian S., Grossman A., Brimacombe K., Arkin M., Auld D., Austin C., Baell J., Chung T. D. Y., Coussens N. P., Dahlin J. L., Devanarayan V., Foley T. L., Glicksman M., Gorshkov K., Haas J. V., Hall M. D., Hoare S., Inglese J., Iversen P. W., Kales S. C., Lal-Nag M., Li Z., McGee J., McManus O., Riss T., Saradjian P., Sittampalam G. S., Tarselli M., Trask O. J. Jr., Wang Y., Weidner J. R., Wildey M. J., Wilson K., Xia M., Xu X., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda (MD), 2004–Present. [PubMed] [Google Scholar]
- Leitão J. M.; Esteves da Silva J. C. Firefly luciferase inhibition. J. Photochem. Photobiol. B 2010, 101 (1), 1–8. 10.1016/j.jphotobiol.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Thorne N.; Inglese J.; Auld D. S. Illuminating insights into firefly luciferase and other bioluminescent reporters used in chemical biology. Chem. Biol. 2010, 17 (6), 646–57. 10.1016/j.chembiol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss T. S.; Healer J.; Marty A. J.; Duffy M. F.; Thompson J. K.; Beeson J. G.; Reeder J. C.; Crabb B. S.; Cowman A. F. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006, 439 (7079), 1004–8. 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- Daubenberger C. A.; Tisdale E. J.; Curcic M.; Diaz D.; Silvie O.; Mazier D.; Eling W.; Bohrmann B.; Matile H.; Pluschke G. The N’-terminal domain of glyceraldehyde-3-phosphate dehydrogenase of the apicomplexan Plasmodium falciparum mediates GTPase Rab2-dependent recruitment to membranes. Biol. Chem. 2003, 384 (8), 1227–37. 10.1515/BC.2003.135. [DOI] [PubMed] [Google Scholar]
- Haynes J. D.; Diggs C. L.; Hines F. A.; Desjardins R. E. Culture of human malaria parasites Plasmodium falciparum. Nature. 1976, 263 (5580), 767–9. 10.1038/263767a0. [DOI] [PubMed] [Google Scholar]
- Trager W.; Jensen J. B. Human malaria parasites in continuous culture. Science. 1976, 193 (4254), 673–5. 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Snyder C.; Chollet J.; Santo-Tomas J.; Scheurer C.; Wittlin S. In vitro and in vivo interaction of synthetic peroxide RBx11160 (OZ277) with piperaquine in Plasmodium models. Exp Parasitol. 2007, 115 (3), 296–300. 10.1016/j.exppara.2006.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.