Abstract
Cardiac emergencies in women, such as acute coronary syndromes, acute heart failure, and cardiac arrest, are associated with a high risk of adverse outcomes and mortality. Although women historically have been significantly underrepresented in clinical studies of these diseases, the guideline-recommended treatment for these emergencies is generally the same for both sexes. Still, women are less likely to receive evidence-based treatment compared to men. Furthermore, specific diseases affecting predominantly or exclusively women, such as spontaneous coronary dissection, myocardial infarction with non-obstructive coronary arteries, takotsubo cardiomyopathy, and peripartum cardiomyopathy, require specialized attention in terms of both diagnosis and management. In this clinical consensus statement, we summarize current knowledge on therapeutic management of these emergencies in women. Key statements and specific quality indicators are suggested to achieve equal and specific care for both sexes. Finally, we discuss several gaps in evidence and encourage further studies designed and powered with adequate attention for sex-specific analysis.
Keywords: Acute coronary syndromes, Acute heart failure, Cardiogenic shock, Cardiac arrest, Women, Sex differences
Graphical Abstract
Graphical abstract.
Introduction
Cardiovascular (CV) disease is the leading cause of death in women in Europe and USA; still women are affected 5–7 years later than men.1–3 Multiple studies have demonstrated important sex differences in pathophysiological mechanisms, risk factors, management and outcomes of acute cardiac emergencies such as acute coronary syndromes (ACS), acute heart failure (HF), and cardiac arrest (CA).4–8 Furthermore, women have been underrepresented in randomized clinical trials (RCTs) on optimal treatment of these emergencies, and there is a paucity of knowledge regarding sex-specific dosing and metabolism of various drugs.9
This clinical consensus statement from the Association for Acute CardioVascular Care (ACVC) of the European Society of Cardiology (ESC) in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the Heart Failure Association (HFA), the European Heart Rhythm Association (EHRA), and the ESC Working Group on Cardiovascular Pharmacotherapy (WG CVP) was written with the aim to present current knowledge on the management of these emergencies in women. Areas of uncertainty and controversy are discussed, and specific quality indicators (QI) for measuring the attainment of guideline-indicated care are proposed to ensure optimal care for women with acute cardiac disease. This clinical consensus statement from ESC associations and working group is included in a focused article collection in European Heart Journal Open on Women in Cardiology.
Methods
Authors with expertise in acute cardiac care were selected to contribute to this consensus statement. The participants volunteered to write sections relevant to their expertise and experience. Relevant literature was identified by each writing group, and a first draft of the document was prepared and sent to all co-authors. On the basis of feedback from all co-authors and discussions within the full group, the sections were edited, and a final version of the document was produced. The final document was circulated among all contributors, and consensus was achieved.
The methodology used for development of the sex-specific QIs for measuring the attainment of guideline-indicated care is described in the Supplementary material online, Supplementary Material.
Acute coronary syndromes
Epidemiology and risk factors
The incidence of ACS is lower in women compared to men in all age groups, but the sex difference declines with age.10,11 At all ages, women are more likely to present with non-ST-elevation-ACS, and less likely to present with ST-elevation myocardial infarction (STEMI).4,7
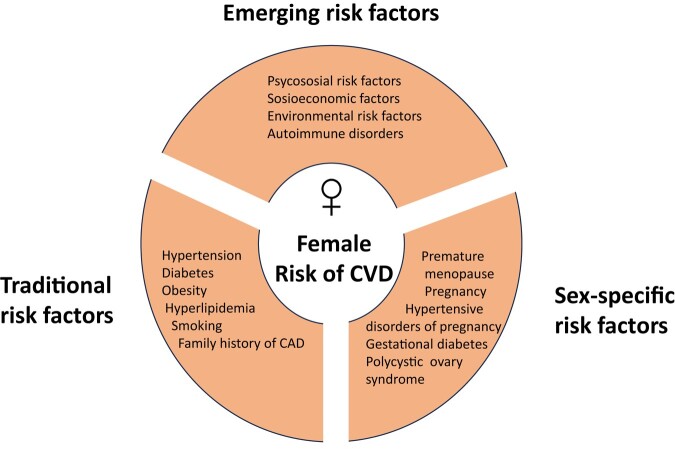
Risk factors for ACS in women are the traditional risk factors, such as hypertension, dyslipidaemia, diabetes, obesity, smoking, but also less recognized factors such as psychosocial and socioeconomic factors.6,7 Sex-specific risk factors also need to be taken into consideration5–7 (Figure 1). Hypertension, diabetes, obesity, and smoking seem to have more impact on the risk for ACS in women compared to men.5–8 Of note, the prevalence and impact of risk factors seem to vary by age and the differences compared with men are less pronounced in the elderly.8 After menopause, the prevalence of CV risk factors in women increases,5 and more than 50% of women with ACS have three or more CV risk factors.6,12 Although the risk of atherosclerotic ACS is significantly lower in women compared to men, ACS should be the initial working diagnosis when women with a clustering of risk factors present with chest pain.
Figure 1.
Risk factors for cardiovascular disease in women.6,7 CAD, coronary artery disease; CVD, cardiovascular disease.
Pathophysiology
Obstructive atherosclerotic coronary artery disease (CAD) is the most frequent cause of ACS in women.13 However, the pathophysiology includes a broader spectrum of aetiologies such as non-obstructive atherosclerotic disease, coronary microvascular dysfunction, coronary spasm, and spontaneous coronary artery dissection (SCAD), requiring different diagnostic and therapeutic strategies.
Clinical presentation and initial diagnostic management
Chest pain is the dominant and most frequent symptom in women diagnosed with ACS, but women often present with additional symptoms such as pain between the shoulder blades, nausea or vomiting, and shortness of breath.14–16 Substantial evidence suggests that women present to the hospital for ACS treatment later than men.7 The reasons for the delay in presentation include lack of awareness, misinterpretation of symptoms, barriers to accessing care, and fear.
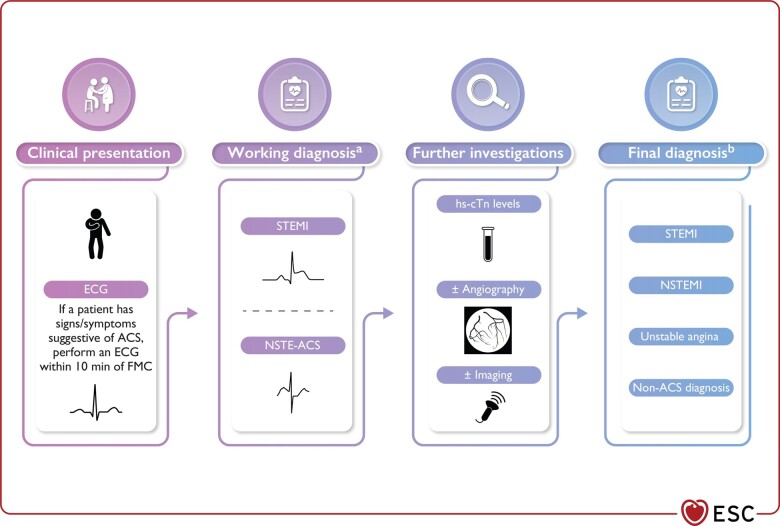
A 12-lead electrocardiogram (ECG) and cardiac troponins are important in the initial triage and diagnosis of women with ACS, guiding the initial management strategy (Figure 2).17 High-sensitivity cardiac troponin levels are on average lower in women than in men, and a lower threshold for troponin concentrations in women have been proposed for clinical decisions in women with ACS.18,19 However, the use of uniform cut-off concentrations remains the standard of care in the 2023 ESC guidelines for the management of ACS.17
Figure 2.
Classification and diagnosis of patients presenting with suspected acute coronary syndrome. Reprinted with permission from Byrne et al.17 ACS, acute coronary syndrome; ECG, electrocardiogram; FMC, first medical contact; hs-cTn, high-sensitivity cardiac troponin; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction.
Treatment
Reperfusion strategies
The 2023 ESC guidelines for management of ACS recommend treating both sexes equally concerning reperfusion.17 Despite clear guideline recommendations, global data report that women are less likely to receive percutaneous coronary intervention (PCI) after admission for ACS, even in the setting of STEMI.7 This may be linked to the fact that obstructive CAD is less frequent in women, although the lack of utilization of angiography and consequently a lower use of primary PCI in female patients, as well as lower transfer of female patients by network systems, have been shown in several studies.20–22 Despite clear guideline recommendations, early invasive management of women with NSTEMI is even more underused.17,23,24
Risk scores, such as the Global Registry of Acute Coronary Events (GRACE) 2.0 score, may be used to select ACS patients for early invasive treatment.17 The GRACE 2.0 risk score for in-hospital mortality does not include sex as a variable, and it was recently shown that the GRACE 2.0 score underestimated in-hospital mortality risk in women.25 Moreover, an updated version of GRACE 3.0 score performed better in women compared to GRACE 2.0 and led to a clinically relevant reclassification of female patients to the high-risk group.25
Pharmacological treatment
The ESC guidelines recommend the same pharmacological treatment for women with atherosclerotic ACS as for men.4,17,26,27 The use of aspirin at the time of ACS is of clear benefit,26,27 and P2Y12 inhibitors have been shown to reduce adverse outcomes.27,28 The benefit of antiplatelet drugs must however be weighed carefully against the risk of bleeding, which is higher in women than in men.29 The Academic Research Consortium on High Bleeding risk (ARC-HBR) definition represents a useful tool for bleeding risk assessment both in women and men undergoing PCI.29 Use of radial access as well as careful consideration of age, weight, and renal function when selecting antithrombotic therapy may reduce the risk of bleeding.17,27,30 De-escalation or shortening of dual antiplatelet therapy (DAPT) are alternative antiplatelet strategies which may be considered in those with HBR.17 The ESC guidelines also recommend adding a proton pump inhibitor to DAPT for patients with HBR.17 Statins, beta-blockers and angiotensin-converting enzyme (ACE) inhibitors are recommended as secondary prophylaxis after ACS in the same way as in men.4,26,27
Prognosis of women with acute coronary syndrome
Sex-related outcomes after ACS vary by age. Younger women appear to have worse short-term and long-term outcomes compared to younger men, but older women have similar outcomes to those of older men.31,32 The reason for these differences are not quite clear, and this and other gaps in evidence are discussed at the end of this article.
Myocardial infarction with non-obstructive coronary arteries
Myocardial infarction with non-obstructive coronary arteries (MINOCA) encompasses a heterogeneous group of underlying causes, both atherosclerotic and non-atherosclerotic.33–35 Myocardial infarction with non-obstructive coronary artery is a working diagnosis, not a final diagnosis, and it is of utmost importance to identify the underlying cause, e.g. non-obstructive atherosclerotic CAD, coronary microvascular dysfunction, coronary spasm, or spontaneous coronary artery dissection (SCAD).34,35 The most recent definition of MINOCA excluded non-ischaemic causes of myocardial injury from the MINOCA definition.33–35 Henceforth, non-ischaemic conditions such as takotsubo cardiomyopathy, myocarditis and non-ischaemic cardiomyopathy are no longer encompassed within the MINOCA definition, but labelled MINOCA mimickers.33–35
The reported prevalence of MINOCA varies widely across studies (1–15%)35 but is significantly higher in women than in men, and higher in younger compared to elderly women.34,35 When the diagnosis is not clear after invasive coronary angiography, echocardiography, cardiac magnetic resonance, intra-coronary imaging, and provocative spasm testing, are useful tools to establish the underlying cause.17,34,36,37 Determining the cause of MINOCA and excluding other possible causes for cardiac troponin elevation have important implications for tailoring secondary prevention measures. Notably, women with non-obstructive CAD are advised aggressive risk factor management.17 The prognosis of MINOCA depends on the underlying cause and comorbidities, may vary. A recent study utilizing data from a nationwide registry found that patients diagnosed with MINOCA and those suffering from myocardial infarction with obstructive CAD had comparable clinical outcomes.38
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection is an important cause of ACS in women, with the peak incidence at 50 years and accounting for 23–36% of ACS events in women under 50–60 years.39–42 Spontaneous coronary artery dissection is also a cause of pregnancy-associated MI occurring primarily in the first few months postpartum and accounting for 23–67% of ACS in this context.43,44
Accurate diagnosis of SCAD is critical, as the management is different to atherosclerotic ACS (Table 1).39,40 Angiographic appearances are often diagnostic39 but in ambiguous cases, intra-coronary imaging with optical coherence tomography (OCT) is useful (Figure 3, Table 1).45
Table 1.
Suggested diagnostic and therapeutic management of SCAD
| Clinical management | Suggested approach |
|---|---|
| Diagnosis | |
| Invasive angiography | Angiographic appearances diagnostic in most cases (Figure 3). Careful technique due to increased risk of iatrogenic dissection |
| Optical coherence tomography (OCT) | Useful and low risk in cases where angiographic appearances are non-diagnostic or to guide PCI |
| Intravascular ultrasound | Lower spatial resolution than OCT. Alternative when OCT unavailable |
| Computed tomography | Lower spatial resolution than angiography. May be helpful where non-invasive follow-up is required of proximal or mid-vessel disease |
| Revascularization | |
| PCI | Reserved for cases with high myocardial jeopardy at presentation (e.g. occlusive SCAD). Long segments of small calibre stents often needed |
| CABG | Reserved as bail-out for high-risk scenarios. Increased risk of early graft failure over time. |
| Conservative | Good outcomes with healing, restoration of coronary architecture and small myocardial injuries in most non-occlusive cases |
| Thrombolysis | Isolated reports of complications. Not the preferred management option in SCAD |
| Medical management | |
| Clopidogrel/P2Y12 inhibitors | In cases managed with PCI, manage according to guidelines. In conservatively managed SCAD, limited observational data suggest increased risk of dual over monotherapy. Clinical trial data awaited |
| Aspirin | Use as long-term prophylaxis is controversial. Clinical trial data awaited |
| Statin | No current evidence to suggest a benefit of statins after SCAD outside primary prevention guidelines |
| Beta-blockers | Use according to guidelines in patients with LVSD after SCAD. Use of beta-blockers and control of hypertension may reduce the risk of recurrent SCAD. Clinical trial data awaited |
| ACE inhibitors/ARB | Use according to guidelines in patients with LVSD post-SCAD or to control hypertension |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CABG, coronary artery bypass grafting; LVSD, left ventricular systolic dysfunction; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
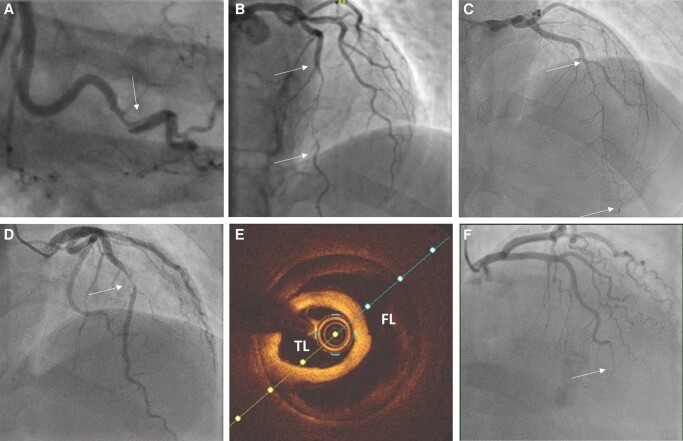
Figure 3.
Angiographic appearances of spontaneous coronary artery dissection: (A) Type 1—characterized by dual lumen appearance and contrast hang-up due to contrast penetration of the false lumen. (B) Type 2A—long narrowing (arrows) often tapering at the distal extent and with recrudescence of a normal calibre vessel distally. (C) Type 2B—long narrowing (arrows) which extends distally with- out restoration of a normal calibre vessel distally. (D) Type 3—shorter stenosis (arrow) angiographically difficult to distinguish from focal atherosclerotic plaque without confirmation by intra-coronary imaging. (E) Optical coherence tomography (OCT) image from case shown in (D). TL, true lumen; FL, false lumen. (F) Type 4—Occlusion, often distally. May be difficult to distinguish from coronary embolus but may have upstream tapering to suggest an underlying occlusive intramural haematoma.
Percutaneous coronary intervention in SCAD is associated with an increased risk of complications, particularly iatrogenic dissection and haematoma extension.46 For this reason, a conservative approach to revascularisation is advised where possible.39,40 However, where PCI is essential (e.g. proximal or mid-vessel occlusive SCAD), improvements in flow are achievable but at the expense of long-stented segments. Following SCAD, optimal medical management is unknown but is now the subject of an ongoing clinical trial.47 Limited observational data suggest that beta-blockers and control of hypertension may be associated with a lower risk of recurrent SCAD.48,49 The role of antiplatelet therapies in conservatively managed SCAD has been questioned, as the pathophysiology of SCAD is thought to relate to the development of a spontaneous intramural haematoma, and it is unclear how medications that prolong bleeding time would reduce future risk in this context.39
Chest pain after SCAD is common and leads to frequent hospital readmissions (27.6% ACS). It is usually non-ischaemic and generally slowly improves but can be challenging to manage. Around 10% of SCAD survivors will have a recurrent event over a 5- to 10-year follow-up.39–42 Despite this, the prognosis from SCAD is good due to the small infarct sizes, as most SCAD is non-occlusive and affects more distal coronary territories.50 Suggested diagnostic strategies and therapeutic management of SCAD are summarized in Table 1.
Acute coronary syndrome during pregnancy
Acute coronary syndromes are overall rare during pregnancy (1.7–6.2/100 000 pregnancies) but is responsible for approximately 20% of maternal CV deaths during this period.51,52 As the birth rate in women >40 years increases, ACS in pregnancy may become more common. The underlying pathophysiological mechanism is most often non-atherosclerotic mechanisms, and SCAD is the most frequent cause.43 The clinical presentation and management of ACS during pregnancy are similar to ACS in non-pregnant women and are detailed in the 2018 ESC Guidelines for the management of cardiovascular diseases (CVD) during pregnancy.52 Foetal monitoring and a multidisciplinary approach are essential. The guidelines recommend primary PCI as the preferred reperfusion therapy for STEMI.52 Intravenous unfractionated heparin and low-dose aspirin appear to be safe.43,52,53 If DAPT is required, clopidogrel is considered safe, but should be maintained for the shortest time possible.52,53 There are very limited safety and efficacy data on bivalirudin, prasugrel, ticagrelor, and glycoprotein IIb/IIIa inhibitors.52,53 Beta-blockers are considered safe (except for atenolol and non-selective beta-blockers),52 although some effects on foetal growth have been observed.54
Targets for equality in the management of acute coronary syndrome
In order to further implement potentially life-saving therapy and procedures, the ESC ACVC established 20 QIs for measuring the attainment of guideline-indicated care.55 These QIs were defined and intended to be used similarly in both men and women. Since no difference across genders is expected, a ‘significant’ imbalance in the rates of QIs between men and women might be interpreted as gender inequality in the quality of care. See Supplementary material online, Table S1 for suggested QIs to measure sex inequality.
Consensus statements on acute coronary syndromes.
Chest pain is the dominant and most frequent symptom in women with ACS, but additional symptoms as shortness of breath and nausea/vomiting are common.
Obstructive atherosclerotic CAD is the most frequent cause of ACS in women. The guideline-recommended treatment is the same as in men, both with respect to revascularization and pharmacological therapy.
Non-obstructive atherosclerotic CAD, microvascular disease, coronary spasm, and SCAD are common causes of ACS in younger women and need specific management.
Use of radial access during invasive angiography, as well as careful tailoring of antithrombotic drugs in relation to age, weight, renal function, and bleeding risk category, are advised in order to reduce the bleeding risk in women.
Beta-blockers are suggested treatment in SCAD. The use of antiplatelets is controversial.
Acute heart failure and cardiogenic shock
Acute HF and cardiogenic shock (CS) represent the most extreme manifestations of CV disease, with significant differences in clinical characteristics and administered treatment between female and male patients.3,56,57 With respect to prognosis, however, most studies report similar or better outcomes in women compared to men when adjusted for comorbidities and age. In the EuroHeart Failure Survey II on acute heart failure, both in-hospital and 1-year mortality were similar (6.6% and 20%, respectively).57
Epidemiology and clinical presentation
Women with acute HF tend to be older and present with more hypertension, atrial fibrillation, diabetes, and valvular heart disease than men. They also have a higher incidence of de novo HF, especially in the setting of acute myocardial infarction (AMI).3,56,57 With respect to hypertension, the Framingham study showed that the risk of developing HF was higher in hypertensive women than men, and that hypertension could be causing 59% of HF cases in women.56,58
Heart failure with preserved ejection fraction (EF) (HFpEF) is the most frequent phenotype. In a recent cohort study, 55% of women presented with HFpEF, 39% with HF with mildly reduced EF, and 29% with HF with reduced EF.3
Management of acute heart failure
As acute HF is a heterogeneous condition, initial management may differ according to the main clinical presentation. Diagnostic workup and treatment according to the clinical presentation are detailed in the 2021 ESC guidelines for diagnosis and treatment of acute and chronic HF and its 2023 Focused Update.59,60 Recommendations are the same for women as for men.
In all HF patients, the key for good outcomes is rapid identification and reversal of any potentially treatable underlying condition with institution of supportive therapies where required. Differences between men and women largely do not affect their management on the cardiac intensive care unit except relating to their physical size. Consideration must however be given to anatomical and physiological differences (body weight and composition, gastrointestinal motility, liver metabolism, and glomerular filtration rate) as these significantly affect pharmacokinetics/dynamics of drugs.27
In acute HF, intravenous diuretics are the cornerstone of treatment. According to guidelines, intravenous vasodilators may be considered to relieve HF symptoms when SBP is >110 mmHg.54 In patients with low cardiac output and hypotension, inotropes may be needed.59,60
Current ESC guidelines recommend early initiation and rapid up-titration of key HF drugs in patients admitted with acute HF, similar in men and women.59,60 Data on the therapeutic effect of several agents in women are however limited, with women remaining underrepresented in clinical trials. This has a potentially significant impact on the generalization of observed results on the female population and underscores the need for more evidence and higher female representation in HF trials. Sex-specific results in some of the HF landmark trials are shown in Supplementary material online, Table S2.
Recent data suggest that women with HF might need lower doses of key disease-modifying agents than men. In the BIOlogy Study to Tailored Treatment in Chronic Heart Failure (BIOSTAT-CHF), the relationship of administered dose of ACE inhibitors and angiotensin receptor blockers (ARBs) on all-cause mortality and hospitalization for HF was investigated.61 As expected, the survival and freedom of hospitalization increased with increasing dose in men. Surprisingly, there was a paradoxical lower risk of death and hospitalization for HF in females that received <50% of the target dose. In addition, women were more likely to have side effects at the same doses. This unexpected finding in available evidence brings into question whether the optimal dose for women may be different to that for men.
Special situations
Two potentially life-threatening conditions in women which require urgent and specific assessment and management warrant particular consideration; takotsubo syndrome (TTS) and peripartum cardiomyopathy (PPCM).
Takotsubo syndrome
Commonly known as stress cardiomyopathy, TTS typically presents in postmenopausal woman. Women comprise approximately 90% of the TTS cases reported with a mean age of 65–75 years in most series, and the risk of developing TTS increases five-fold in women after the age of 55 years.62,63 The presentation may be similar to ACS, and characterized by ECG changes and transient left ventricular (LV) and/or right ventricular wall dysfunction caused by a number of triggers including physical or emotional factors.62,63
A diagnostic algorithm has been proposed where patients presenting with ST-elevation should undergo urgent coronary angiography with left ventriculography to exclude acute STEMI. In those with non-ST-segment elevation, the INterTAK Diagnostic Score can be used (Figure 4).64 Patients with low/intermediate probability (score ≤70) are advised to undergo coronary angiography with left ventriculography, while patients with a high probability (score >70) only require transthoracic echocardiography (TTE).64 Although there are no RCTs to guide therapy, advice includes avoidance of precipitants (including beta-adrenergic agents) and considering the use of levosimendan if haemodynamics indicate.64 Its reversible nature justifies a supportive medical approach to avoid or treat possible complications including acute LV dysfunction aggravated by mitral regurgitation or LV outflow tract obstruction, until full recovery. Mechanical circulatory support (MCS) has been used as ‘bridge to recovery’ in selected cases.65
Figure 4.
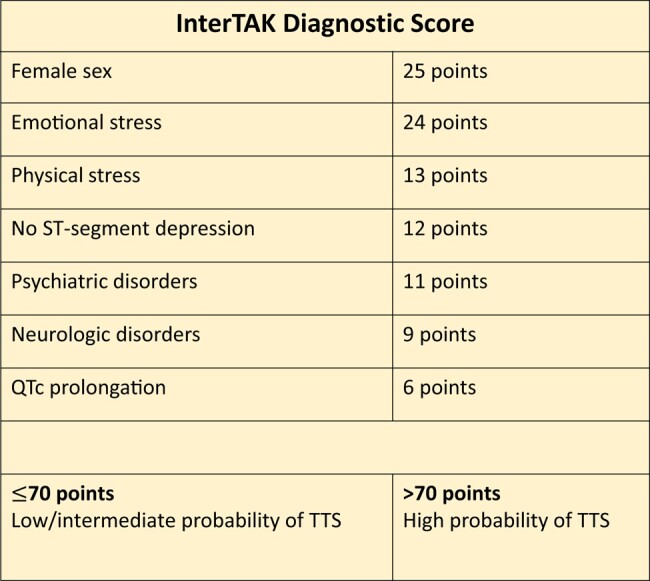
InterTak diagnostic score for Takotsubo syndrome.64
Peripartum cardiomyopathy
Peripartum cardiomyopathy is defined as a new-onset cardiomyopathy during the peripartum episode or up to 6 months postpartum, manifesting as reduced LV ejection fraction without any other cause of HF.66 The presentation may vary from subtle or asymptomatic to CS. The management strategy considers both mother and foetus and includes urgent hospital admission and transfer to an advanced HF centre where MCS and/or cardiac transplantation can be provided. Where CS is present, urgent delivery by caesarean section (irrespective of gestation) should be considered according to current guidelines, while breastfeeding is currently discouraged.52,59 Regarding the initial treatment of HF caused by PPCM, management goals are similar to acute HF in non-pregnant women59,66 whilst avoiding teratogenic agents (see below). Further management of PPCM, including the use of disease-modifying agents and bromocriptine, is detailed in the ESC guidelines for the management of CVD during pregnancy.52 Levosimendan does not seem to increase myocardial oxygen demand and may be considered in patients with severe PPCM.52
General treatment of acute heart failure during pregnancy
Medical treatment of HF in pregnancy is complex because of the teratogenic effect of many commonly used HF medications, which must be avoided (including ACE inhibitors, ARB, angiotensin receptor/neprilysin inhibitors, aldosterone antagonists, sodium-glucose co-transporter-2 inhibitors, and atenolol).52 Loop diuretics, such as furosemide, are considered safe.52 When required, acute vasodilatation may be obtained with nitroglycerine or nitroprusside. According to the 2018 ESC guidelines, hydralazine, in combination with nitrates, may be used after the acute phase.52 Beta-blockers should be initiated with caution and gradually uptitrated. Beta-1-selective drugs are preferred.52 Management of acute HF during pregnancy requires a multidisciplinary approach and referral to an expert centre with a surgical and MCS/transplant program as a backup option.52 Foetal monitoring is advised in all cases.
Cardiogenic shock
The incidence of CS is rising at a higher rate in women compared with men, and several studies suggest that women with AMI are more likely to develop CS than men.67,68 Women also tend to present with an overall higher risk profile (older age, more comorbidities, haemodynamic derangement, vasopressor requirements, and CA).69 There are conflicting data regarding outcomes; recent studies suggest similar mortality rates in women and men after adjusting for baseline differences.67,70
Treatment
Relatively few women (19–31%) have been included in RCTs regarding treatment of CS (Table 2), limiting the generalizability of observed results for women. In the CULPRIT-SHOCK trial, 686 patients (24% women) with CS-AMI and multivessel CAD were randomly assigned to the culprit-lesion-only PCI vs. multivessel PCI.71,72 Sex did not influence mortality or renal failure according to revascularisation strategies (interaction P = 0.11). Hence, revascularization of the culprit lesion only should be the preferred strategy equally among women and men.
Table 2.
Female representation and outcomes in landmark RCTs on management of patients in cardiogenic shock
| Study name | Year | Intervention | Indication | n | Women (%) | Main outcomes in women compared to men |
|---|---|---|---|---|---|---|
| IABP-SHOCK II68 | 2012 | IABP vs. no IABP | AMI-CS | 600 | 31 | Consistent results in men and women with respect to 30-day mortality |
| CULPRIT-SHOCK66,67 | 2017 | Culprit-lesion-only vs. multivessel PCI | AMI-CS | 686 | 24 | No interaction between sex and coronary revascularization strategy regarding mortality or renal failure (interaction P = 0.11) |
| ECLS-SHOCK69 | 2023 | ECLS vs. no ECLS | AMI-CS | 420 | 19 | Consistent results in men and women with respect to 30-day mortality |
AMI-CS, acute myocardial infarction-cardiogenic shock; ECLS, extracorporeal life support; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; RCT, randomized controlled trial.
In pivotal RCTs on MCS in AMI-CS (IABP-SHOCK II and the ECLS-SHOCK), the number of included women were again low, but the results were neutral and similar in men and women (Table 2).73,74 Although no convincing evidence exist, acute MCS may be appropriate in selected patients with AMI-CS according to current guidelines as a bridge to recovery/decision/bridge/transplant.17,59 Published data suggest however that only a minority of MCS recipients are women (33%).67,69 The reasons for this potential under-utilization remain poorly understood. Data regarding complication rates are conflicting, with some studies reporting MCS use in women is associated with increased complication rates (bleeding, vascular, and readmission) and inferring this might impact decision-making to implement MCS, but other studies reporting no difference in complication rates and outcomes.70 Practical differences due to patient size (normothermic flow indices and cannulae/vessel dimensions) do present physical limitations regarding the opportunities for safe peripheral MCS. However, as access routes and technologies change, the recommendations in 2021 HF guidelines, for their use in selected patients, using a predefined algorithm coupled with close monitoring,59 could avoid that sex per se precludes female patients from consideration.69,70
Consensus statements on acute heart failure.
Heart failure with preserved ejection fraction (HFpEF) is the most frequent phenotype in women with HF.
Most studies report similar or better outcomes in women compared to men when adjusted for comorbidities and age.
Takotsubo and peripartum cardiomyopathy are rare causes of HF affecting predominantly or exclusively women and requiring urgent and specific assessment and management.
ESC guidelines recommend early initiation and target-dosage of key disease-modifying drugs, similar in men and women.
It is advised to adjust doses of drugs according to age, body weight, and kidney function.
The therapeutic dose of pharmaceutical agents for treating HF in women is inconclusive and should be re-evaluated in RCTs with equal rates of women vs. men.
Many commonly used HF medications are teratogenic and must be avoided in pregnant women with HF.
It is advised that treatment of CS in women follow the same guidelines as for men.
Cardiac arrest in women
Epidemiology and prognosis
Out-of-hospital cardiac arrest (OHCA) is a leading cause of death in Europe.75 Consistent with other reports, only 35% of patients are women.76,77 Although survival rates have increased in the last decades, mortality after OHCA remains high for both sexes. In Europe, only 8% of all patients with OHCA survive to hospital discharge, but of those admitted with return of spontaneous circulation, up to 50% may be discharged alive.75 Younger age, witnessed arrest, initiation of resuscitation by bystanders, and an initial shockable rhythm, irrespective of sex, are important factors consistently associated with a higher probability of survival.78,79
Female sex is associated with poorer outcomes after OHCA.77,80,81 Not only is overall survival to hospital discharge lower in women but also survival to hospital admission, survival to hospital discharge of patients successfully resuscitated, and survival with a good neurological outcome.76,77,80,81 Outcome differences between sexes can be largely explained by patient characteristics and arrest circumstances. Women resuscitated from OHCA are older, more often live alone when they are older and have less frequently witnessed arrests, and present less often with STEMI and more often with non-cardiac causes of arrest.77,80,81 In a large observational study, 34% of women compared to 52% of men with OHCA presented with an initial shockable rhythm, which is the most important factor associated with survival after OHCA.80 This finding might partly be explained by a longer evolution of arrest related to later recognition of alarming symptoms by women and OHCA by bystanders.
Resuscitation care
The steps to perform cardiopulmonary resuscitation (CPR) are the same in women as in men. However, studies indicate that women may receive suboptimal resuscitation care with less likelihood of undergoing CPR by bystanders, even when OHCA is witnessed.77,80 The potential reasons for inhibiting bystander resuscitation of women are complex and include fear by the public regarding inappropriate touching, or accusations of sexual assault.82 This highlights the importance of the development of focused programs and educational campaigns for the general population.
Post-resuscitation care
Women less frequently undergo invasive coronary angiography after OHCA compared to men,83,84 but this might be explained by other factors and does not necessarily represent real undertreatment.83 Targeted temperature control is also less frequently carried out.84,85 In addition, women receive ‘do-not-resuscitate orders’ and ‘withdrawal of life-sustaining therapy orders’ more often and earlier than men, frequently before the advised 72-h window for neuroprognostication.77,84 The latter may not only contribute to the overall worse outcome of women but also explain the less frequent use of in-hospital procedures after OHCA.86
Women are still significantly underrepresented in recent RCTs on interventions for improving outcomes after OHCA (Table 3). Two recent RCTs failed to show a benefit of performing early angiography in patients presenting without STEMI, and to target a specific blood pressure or oxygen saturation during admission at the intensive care, respectively.87–89 Although there were no differences related to sex, women represented only 19–30% of the included patients (Table 3), limiting the generalizability of these results for women. Similarly, a recent trial on the use of e-CPR for refractory OHCA included only 14 women from a total of 134 randomized patients (10%), highlighting again the insufficient representation of women in RCTs.90
Table 3.
Female representation and outcomes in recent RCTs on management of out-of-hospital cardiac arrest
| Study name | Year | Intervention | Indication | n | Women (%) | Main outcomes in relation to sex |
|---|---|---|---|---|---|---|
| COACT86 | 2019 | Immediate vs. delayed angiography | OHCA without ST-segment elevation | 552 | 20% | Similar results in women and men (no benefit of immediate angiography on survival at 90 days) |
| TOMAHAWK81 | 2021 | Immediate vs. delayed angiography | OHCA without ST-segment elevation | 554 | 30% | Immediate angiography no benefit over delayed with respect to 30-day mortality—no interaction with sex |
| BOX trial82 | 2022 | Oxygen targets | OHCA | 789 | 19% | Similar results in women and men |
| BOX trial83 | 2022 | Blood pressure targets | OHCA | 789 | 19% | Similar results in women and men |
| INCEPTION84 | 2023 | Extracorporeal vs. conventional CPR | Refractory OHCA | 134 | 10% | No subgroup analysis on sex |
| ARREST87 | 2023 | Immediate transfer to CAC vs. standard care | OHCA without ST-segment elevation | 827 | 32% | Similar results in women and men (no reduction in 30-day mortality) |
| TAME88 | 2023 | Mild hypercapnia vs. normocapnia | 1700 | 23% | Similar result in women and men (no effect of hypercapnia on 6 months mortality) |
BOX, Blood Pressure and OXygenation Targets After OHCA; CAC, cardiac arrest centre; COACT, Coronary Angiography After Cardiac Arrest; INCEPTION, Early Initiation of Extracorporeal Life Support in Refractory OHCA; OHCA, out-of-hospital cardiac arrest; RCT, randomized controlled trial; TAME, Targeted Therapeutic Mild Hypercapnia After Resuscitated Cardiac Arrest; TOMAHAWK, Immediate Unselected Coronary Angiography Versus Delayed Triage in Survivors of Out-of-hospital Cardiac Arrest Without ST-segment Elevation.
Cardiac arrest in pregnancy
Although the basic principles of resuscitation for CA apply to pregnant women, some differences should be kept in mind. If arrest occurs beyond 20 weeks of pregnancy, manual displacement of the uterus to the left or left lateral position of the patient can reduce aortocaval compression and is suggested in a scientific statement from the American Heart Association.91
At late stages of pregnancy, emergency caesarean is advised when initial resuscitation fails (within 4 min of CA).53,91 If this is not feasible, rapid maternal transfer to the appropriate clinical setting with uninterrupted resuscitation is advised.
Consensus statements on cardiac arrest.
Women resuscitated from OHCA are older, have less frequently witnessed arrest, and present less often with STEMI and more often with non-cardiac causes of arrest compared to men
In unadjusted analyses, female sex is associated with poorer outcomes after OHCA. In most studies, the sex differences disappear when adjusting for baseline characteristics and treatment
It is advised that management of CA in women follow the general guideline recommendations
It is important to increase awareness of OHCA and the importance of bystander resuscitation in both men and women
Increased representation of women in RCTs on management of OHCA is of urgent importance.
Gaps in evidence and need for further research
Cardiac emergencies in women are associated with a high risk of adverse outcomes and mortality. Still, women are less likely to receive evidence-based treatment compared to men. Despite the recent encouragement to include more women, there is an alarming underrepresentation of women in most RCTs on optimal management of these emergencies.
The first step to improving outcome in women, is to focus attention on sex-specific characteristics. Understanding sex disparities will likely improve the awareness, prevention, recognition, treatment, and outcomes of CVD in women, especially in potentially life-threatening cardiac emergencies. Closing the sex gap in these pathologies requires sex-specific research on:
Pathophysiology of CAD (particularly MINOCA), HF, and CA
Sex-specific clinical risk stratification and decision tools
Development of approaches to shorten interval to diagnosis and treatment of ACS
Optimal target dosage of drugs: antithrombotic and HF agents
Exploration of subpopulations of women who are socially disadvantaged because of race, ethnicity, income level, or education.
We therefore encourage a new era in research, where studies on acute CVD are designed and powered with adequate attention for sex-specific analysis to understand the complex mechanisms of women’s biology. We also underline the need for better consideration of sex hormones as effect-modifiers in healthcare delivery and urge the development of optimal treatments to reduce potentially avoidable deaths among women.
Lead author biography

Professor Sigrun Halvorsen (MD, PhD, FESC) is a clinical cardiologist working as Head of Cardiology at Oslo University Hospital Ullevål, Oslo, Norway, and Professor at the University of Oslo. She is also a member of the Executive Board of the Acute Cardiovascular Care Association of the European Society of Cardiology (ESC), and an associate editor of European Heart Journal Acute Cardiovascular Care. Halvorsen has been involved in writing of several of the recent ESC guidelines, including the 2023 ESC guidelines on management of acute coronary syndromes. Her main research interests are acute coronary syndromes, antithrombotic treatment, and cardiovascular disease in women.
 Professor Antonia Sambola (MD, PhD, FESC) is a cardiologist specialised in Acute Cardiac Care at University Hospital Vall d’Hebron in Barcelona. She is an associate professor at Autonomous University of Barcelona, where she also coordinates the MSc in translational research in cardiovascular disease. She has formed and led the Working Group of cardiovascular disease in Women of the Spanish Society of Cardiology, and the Task Force on Cardiac emergencies in women of Acute Cardiac Care Association-ESC. She is member of the advisory board of the Health Ministry on Government strategies for National Health in cardiovascular disease in women and actively contributes to the National Strategy for Cardiovascular Health in women in Spain.
Professor Antonia Sambola (MD, PhD, FESC) is a cardiologist specialised in Acute Cardiac Care at University Hospital Vall d’Hebron in Barcelona. She is an associate professor at Autonomous University of Barcelona, where she also coordinates the MSc in translational research in cardiovascular disease. She has formed and led the Working Group of cardiovascular disease in Women of the Spanish Society of Cardiology, and the Task Force on Cardiac emergencies in women of Acute Cardiac Care Association-ESC. She is member of the advisory board of the Health Ministry on Government strategies for National Health in cardiovascular disease in women and actively contributes to the National Strategy for Cardiovascular Health in women in Spain.
Supplementary Material
Contributor Information
Antonia Sambola, Department of Cardiology and Research Institute, University Hospital Vall d’Hebron, Universitat Autònoma, CIBER Cardiovascular diseases (CIBER-CV), Barcelona, Spain.
Sigrun Halvorsen, Department of Cardiology, Oslo University Hospital Ulleval, P.O. Box 4956 Nydalen, N-0424 Oslo, Norway; Institue of Clinical Medicine, University of Oslo, P.O. Box 1171 Blindern, N-0318 Oslo, Norway.
David Adlam, Department of Cardiovascular Sciences, University of Leicester, NIHR Leicester Biomedical Research Centre, Leicester, UK.
Christian Hassager, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Susanna Price, Adult Intensive Care Unit, Royal Brompton and Harefield Hospitals, Guy's and St Thomas' NHS Foundation Trust, London, UK.
Giuseppe Rosano, Cardiovascular Clinical Academic Group, St George’s University Hospital, London, UK; Cardiology, San Raffaele Cassino Hospital, Cassino, Italy.
Francois Schiele, Department of Cardiology, University Hospital Besancon, Besancon, France.
Lene Holmvang, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Marta de Riva, Department of Cardiology, Leiden University Medical Center, Leiden, Netherlands.
Amina Rakisheva, Department of Cardiology, City Cardiology Center, Almaty, Kazakhstan; Department of Cardiology, Qonaev City Hospital, Almaty Region, Kazakhstan.
Patrick Sulzgruber, Department of Internal Medicine II, Division of Cardiology, Medical University of Vienna, Vienna, Austria.
Eva Swahn, Department of Cardiology and Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
No funding was provided for this work.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, Gale CP, Maggioni AP, Petersen SE, Huculeci R, Kazakiewicz D, de Benito Rubio V, Ignatiuk B, Raisi-Estabragh Z, Pawlak A, Karagiannidis E, Treskes R, Gaita D, Beltrame JF, McConnachie A, Bardinet I, Graham I, Flather M, Elliott P, Mossialos EA, Weidinger F, Achenbach S; Atlas Writing Group, European Society of Cardiology . European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716–799. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 3. Stolfo D, Uijl A, Vedin O, Stromberg A, Faxen UL, Rosano GMC, Sinagra G, Dahlström U, Savarese G. Sex-based differences in heart failure across the ejection fraction spectrum: phenotyping, and prognostic and therapeutic implications. JACC Heart Fail 2019;7:505–515. [DOI] [PubMed] [Google Scholar]
- 4. Pagidipati NJ, Peterson ED. Acute coronary syndromes in women and men. Nat Rev Cardiol 2016;13:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maas A, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, Kunadian V, Laan E, Lambrinoudaki I, Maclaran K, Panay N, Stevenson JC, van Trotsenburg M, Collins P. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J 2021;42:967–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, Mendelson MA, Wood MJ, Volgman AS, Mieres JH. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes 2018;11:e004437. [DOI] [PubMed] [Google Scholar]
- 7. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Figtree GA, Guerrero M, Kunadian V, Lam CSP, Maas AHEM, Mihailidou AS, Olszanecka A, Poole JE, Saldarriaga C, Saw J, Zühlke L, Mehran R. The Lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet 2021;397:2385–2438. [DOI] [PubMed] [Google Scholar]
- 8. Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med 2019;25:1657–1666. [DOI] [PubMed] [Google Scholar]
- 9. Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ 2020;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, Bønaa KH. Lifelong gender gap in risk of incident myocardial infarction: the Tromso study. JAMA Intern Med 2016;176:1673–1679. [DOI] [PubMed] [Google Scholar]
- 11. Christensen DM, Strange JE, Phelps M, Schjerning AM, Sehested TSG, Gerds T, Gislason G. Age- and sex-specific trends in the incidence of myocardial infarction in Denmark, 2005 to 2021. Atherosclerosis 2022;346:63–67. [DOI] [PubMed] [Google Scholar]
- 12. Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL, Caughey MC. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation 2019;139:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 14. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O'Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;144:e368–e454. [DOI] [PubMed] [Google Scholar]
- 15. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA 2022;327:662–675. [DOI] [PubMed] [Google Scholar]
- 16. van Oosterhout REM, de Boer AR, Maas A, Rutten FH, Bots ML, Peters SAE. Sex differences in symptom presentation in acute coronary syndromes: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan GA, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B; ESC Scientific Document Group . 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720–3826. [DOI] [PubMed] [Google Scholar]
- 18. Lee KK, Ferry AV, Anand A, Strachan FE, Chapman AR, Kimenai DM, Meex SJR, Berry C, Findlay I, Reid A, Cruickshank A, Gray A, Collinson PO, Apple FS, McAllister DA, Maguire D, Fox KAA, Newby DE, Tuck C, Keerie C, Weir CJ, Shah ASV, Mills NL; High-STEACS Investigators . Sex-Specific thresholds of high-sensitivity troponin in patients with suspected acute coronary syndrome. J Am Coll Cardiol 2019;74:2032–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandoval Y, Apple FS, Mahler SA, Body R, Collinson PO, Jaffe AS; International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers . High-sensitivity cardiac troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guidelines for the evaluation and diagnosis of acute chest pain. Circulation 2022;146:569–581. [DOI] [PubMed] [Google Scholar]
- 20. Alabas OA, Gale CP, Hall M, Rutherford MJ, Szummer K, Lawesson SS, Alfredsson J, Lindahl B, Jernberg T. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: national cohort study using the SWEDEHEART registry. J Am Heart Assoc 2017;6:e007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta T, Kolte D, Khera S, Agarwal N, Villablanca PA, Goel K, Patel K, Aronow WS, Wiley J, Bortnick AE, Aronow HD, Abbott JD, Pyo RT, Panza JA, Menegus MA, Rihal CS, Fonarow GC, Garcia MJ, Bhatt DL. Contemporary sex-based differences by age in presenting characteristics, use of an early invasive strategy, and inhospital mortality in patients with non-ST-segment-elevation myocardial infarction in the United States. Circ Cardiovasc Interv 2018;11:e005735. [DOI] [PubMed] [Google Scholar]
- 22. Stehli J, Duffy SJ, Burgess S, Kuhn L, Gulati M, Chow C, Zaman S. Sex disparities in myocardial infarction: biology or bias? Heart Lung Circ 2021;30:18–26. [DOI] [PubMed] [Google Scholar]
- 23. O'Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F, Sabatine MS. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA 2008;300:71–80. [DOI] [PubMed] [Google Scholar]
- 24. Nadarajah R, Ludman P, Laroche C, Appelman Y, Brugaletta S, Budaj A, Bueno H, Huber K, Kunadian V, Leonardi S, Lettino M, Milasinovic D, Gale CP. Sex-specific presentation, care, and clinical events in individuals admitted with NSTEMI: the ACVC-EAPCI EORP NSTEMI Registry of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care 2024;13:36–45. [DOI] [PubMed] [Google Scholar]
- 25. Wenzl FA, Kraler S, Ambler G, Weston C, Herzog SA, Raber L, Muller O, Camici GG, Roffi M, Rickli H, Fox KAA, de Belder M, Radovanovic D, Deanfield J, Lüscher TF. Sex-specific evaluation and redevelopment of the GRACE score in non-ST-segment elevation acute coronary syndromes in populations from the UK and Switzerland: a multinational analysis with external cohort validation. Lancet 2022;400:744–756. [DOI] [PubMed] [Google Scholar]
- 26. Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK; Expert Panel/Writing Group; American Heart Association; American Academy of Family Physicians; American College of Obstetricians and Gynecologists; American College of Cardiology Foundation; Society of Thoracic Surgeons; American Medical Women's Association; Centers for Disease Control and Prevention; Office of Research on Women's Health; Association of Black Cardiologists; American College of Physicians; World Heart Federation; National Heart, Lung, and Blood Institute; American College of Nurse Practitioners . Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007;115:1481–1501. [DOI] [PubMed] [Google Scholar]
- 27. Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, Torp-Pedersen C, Atar D, Lewis BS, Agewall S. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 2017;3:163–182. [DOI] [PubMed] [Google Scholar]
- 28. Lau ES, Braunwald E, Murphy SA, Wiviott SD, Bonaca MP, Husted S, James SK, Wallentin L, Clemmensen P, Roe MT, Ohman EM, Harrington RA, Mega JL, Bhatt DL, Sabatine MS, O'Donoghue ML. Potent P2Y(12) inhibitors in men versus women: a collaborative meta-analysis of randomized trials. J Am Coll Cardiol 2017;69:1549–1559. [DOI] [PubMed] [Google Scholar]
- 29. Chandiramani R, Cao D, Claessen BE, Sorrentino S, Guedeney P, Blum M, Goel R, Roumeliotis A, Krucoff M, Kozuma K, Ge J, Seth A, Makkar R, Bangalore S, Bhatt DL, Angiolillo DJ, Ruster K, Wang J, Saito S, Neumann FJ, Hermiller J, Valgimigli M, Mehran R. Sex-related differences in patients at high bleeding risk undergoing percutaneous coronary intervention: a patient-level pooled analysis from 4 postapproval studies. J Am Heart Assoc 2020;9:e014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andreotti F, Geisler T, Collet JP, Gigante B, Gorog DA, Halvorsen S, Lip GYH, Morais J, Navarese EP, Patrono C, Rocca B, Rubboli A, Sibbing D, Storey RF, Verheugt FWA, Vilahur G. Acute, periprocedural and longterm antithrombotic therapy in older adults: 2022 update by the ESC Working Group on Thrombosis. Eur Heart J 2023;44:262–279. [DOI] [PubMed] [Google Scholar]
- 31. Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, van der Schaar M, Badimon L, Bugiardini R. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med 2018;178:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ricci B, Cenko E, Vasiljevic Z, Stankovic G, Kedev S, Kalpak O, Vavlukis M, Zdravkovic M, Hinic S, Milicic D, Manfrini O, Badimon L, Bugiardini R. Acute coronary syndrome: the risk to young women. J Am Heart Assoc 2017;6:e007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JF; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research . Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 34. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 35. Singh T, Chapman AR, Dweck MR, Mills NL, Newby DE. MINOCA: a heterogenous group of conditions associated with myocardial damage. Heart 2021;107:1458–1464. [DOI] [PubMed] [Google Scholar]
- 36. Reynolds HR, Maehara A, Kwong RY, Sedlak T, Saw J, Smilowitz NR, Mahmud E, Wei J, Marzo K, Matsumura M, Seno A, Hausvater A, Giesler C, Jhalani N, Toma C, Har B, Thomas D, Mehta LS, Trost J, Mehta PK, Ahmed B, Bainey KR, Xia Y, Shah B, Attubato M, Bangalore S, Razzouk L, Ali ZA, Merz NB, Park K, Hada E, Zhong H, Hochman JS. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021;143:624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Occhipinti G, Bucciarelli-Ducci C, Capodanno D. Diagnostic pathways in myocardial infarction with non-obstructive coronary artery disease (MINOCA). Eur Heart J Acute Cardiovasc Care 2021;10:813–822. [DOI] [PubMed] [Google Scholar]
- 38. Choo EH, Chang K, Lee KY, Lee D, Kim JG, Ahn Y, Kim YJ, Chae SC, Cho MC, Kim CJ, Kim HS, Jeong MH; KAMIR-NIH Investigators . Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J Am Heart Assoc 2019;8:e011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adlam D, Alfonso F, Maas A, Vrints C; Writing Committee . European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council . Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, Rose CH. Spontaneous coronary artery dissection: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:961–984. [DOI] [PubMed] [Google Scholar]
- 42. Kim ESH. Spontaneous coronary-artery dissection. N Engl J Med 2020;383:2358–2370. [DOI] [PubMed] [Google Scholar]
- 43. Tweet MS, Lewey J, Smilowitz NR, Rose CH, Best PJM. Pregnancy-associated myocardial infarction: prevalence, causes, and interventional management. Circ Cardiovasc Interv 2020;13:CIRCINTERVENTIONS120008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chan N, Premawardhana D, Al-Hussaini A, Wood A, Bountziouka V, Kotecha D, Swahn E, Palmefors H, Pagonis C, Lawesson SS, Kądziela J, Garcia-Guimarães M, Alfonso F, Escaned J, Macaya F, Santás M, Cerrato E, Maas AHEM, Hlinomaz O, Bogale N, Cortese B, Cheng M, Bolger A, Hussain ST, Samani NJ, Knight M, Cauldwell M, Adlam D. Pregnancy and spontaneous coronary artery dissection: lessons from survivors and nonsurvivors. Circulation 2022;146:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson R, Al-Hussaini A, Joseph S, van Soest G, Wood A, Macaya F, Gonzalo N, Cade J, Caixeta A, Hlinomaz O, Leinveber P, O'Kane P, García-Guimaraes M, Cortese B, Samani NJ, Escaned J, Alfonso F, Johnson T, Adlam D. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovasc Imaging 2019;12:2475–2488. [DOI] [PubMed] [Google Scholar]
- 46. Kotecha D, Garcia-Guimaraes M, Premawardhana D, Pellegrini D, Oliver-Williams C, Bountziouka V, Wood A, Natarajan N, Jackson R, Chan N, Ziaullah J, Rakhit RD, Hoole SP, Johnson TW, Kadziela J, Ludman P, Samani NJ, Maas AHEM, van Geuns RJ, Alfonso F, Adlam D. Risks and benefits of percutaneous coronary intervention in spontaneous coronary artery dissection. Heart 2021;107:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alfonso F, de la Torre Hernandez JM, Ibanez B, Sabate M, Pan M, Gulati R, Saw J, Angiolillo DJ, Adlam D, Sánchez-Madrid F. Rationale and design of the BA-SCAD (beta-blockers and antiplatelet agents in patients with spontaneous coronary artery dissection) randomized clinical trial. Rev Esp Cardiol (Engl Ed) 2022;75:515–522. [DOI] [PubMed] [Google Scholar]
- 48. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 49. Tweet MS, Olin JW. Insights into spontaneous coronary artery dissection: can recurrence be prevented? J Am Coll Cardiol 2017;70:1159–1161. [DOI] [PubMed] [Google Scholar]
- 50. Al-Hussaini A, Abdelaty A, Gulsin GS, Arnold JR, Garcia-Guimaraes M, Premawardhana D, Budgeon C, Wood A, Natarajan N, Mangion K, Rakhit R, Hoole SP, Johnson TW, Berry C, Hudson I, Gershlick AH, Ladwiniec A, Kovac J, Squire I, Samani NJ, Plein S, McCann GP, Adlam D. Chronic infarct size after spontaneous coronary artery dissection: implications for pathophysiology and clinical management. Eur Heart J 2020;41:2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gibson P, Narous M, Firoz T, Chou D, Barreix M, Say L, James M. Incidence of myocardial infarction in pregnancy: a systematic review and meta-analysis of population-based studies. Eur Heart J Qual Care Clin Outcomes 2017;3:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA; ESC Scientific Document Group . 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 53. Egidy Assenza G, Dimopoulos K, Budts W, Donti A, Economy KE, Gargiulo GD, Gatzoulis M, Landzberg MJ, Valente AM, Roos-Hesselink J. Management of acute cardiovascular complications in pregnancy. Eur Heart J 2021;42:4224–4240. [DOI] [PubMed] [Google Scholar]
- 54. Duan L, Ng A, Chen W, Spencer HT, Nguyen J, Shen AY, Lee M-S. beta-Blocker exposure in pregnancy and risk of fetal cardiac anomalies. JAMA Intern Med 2017;177:885–887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schiele F, Aktaa S, Rossello X, Ahrens I, Claeys MJ, Collet JP, Fox KAA, Gale CP, Huber K, Iakobishvili Z, Keys A, Lambrinou E, Leonardi S, Lettino M, Masoudi FA, Price S, Quinn T, Swahn E, Thiele H, Timmis A, Tubaro M, Vrints CJM, Walker D, Bueno H, Halvorsen S, Jernberg T, Jortveit J, Blöndal M, Ibanez B, Hassager C; ESC Scientific Document Group . 2020 update of the quality indicators for acute myocardial infarction: a position paper of the Association for Acute Cardiovascular Care: the study group for quality indicators from the ACVC and the NSTE-ACS guideline group. Eur Heart J Acute Cardiovasc Care 2021;10:224–233. [DOI] [PubMed] [Google Scholar]
- 56. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 57. Nieminen MS, Harjola VP, Hochadel M, Drexler H, Komajda M, Brutsaert D, Dickstein K, Ponikowski P, Tavazzi L, Follath F, Lopez-Sendon JL. Gender related differences in patients presenting with acute heart failure. Results from EuroHeart Failure Survey II. Eur J Heart Fail 2008;10:140–148. [DOI] [PubMed] [Google Scholar]
- 58. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275:1557–1562. [PubMed] [Google Scholar]
- 59. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 60. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Skibelund AK; ESC Scientific Document Group . 2023 focused update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023;44:3627–3639. [DOI] [PubMed] [Google Scholar]
- 61. Santema BT, Ouwerkerk W, Tromp J, Sama IE, Ravera A, Regitz-Zagrosek V, Hillege H, Samani NJ, Zannad F, Dickstein K, Lang CC, Cleland JG, Ter Maaten JM, Metra M, Anker SD, van der Harst P, Ng LL, van der Meer P, van Veldhuisen DJ, Meyer S, Lam CSP; ASIAN-HF Investigators; Voors AA. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 2019;394:1254–1263. [DOI] [PubMed] [Google Scholar]
- 62. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress cardiomyopathy diagnosis and treatment: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:1955–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Napp LC, Westenfeld R, Moller JE, Pappalardo F, Ibrahim K, Bonello L, Wilkins C, Pershad A, Mannino SF, Schreiber TL, Hall PA, Medjamia AM, Haurand JM, Sieweke JT, Schäfer A, Grines CL, Burkhoff D, Moses JW, Ohman EM, O'Neill WW, Kapur NK, Bauersachs J. Impella mechanical circulatory support for Takotsubo syndrome with shock: a retrospective multicenter analysis. Cardiovasc Revasc Med 2022;40:113–119. [DOI] [PubMed] [Google Scholar]
- 66. Bauersachs J, Arrigo M, Hilfiker-Kleiner D, Veltmann C, Coats AJ, Crespo-Leiro MG, De Boer RA, van der Meer P, Maack C, Mouquet F, Petrie MC, Piepoli MF, Regitz-Zagrosek V, Schaufelberger M, Seferovic P, Tavazzi L, Ruschitzka F, Mebazaa A, Sliwa K. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2016;18:1096–1105. [DOI] [PubMed] [Google Scholar]
- 67. Yan I, Schrage B, Weimann J, Dabboura S, Hilal R, Beer BN, Becher PM, Seiffert M, Magnussen C, Schnabel R, Kirchhof P, Blankenberg S Westermann D. Sex differences in patients with cardiogenic shock. ESC Heart Fail 2021;8:1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bukhari S, Fatima S, Elgendy IY. Cardiogenic shock in the setting of acute myocardial infarction: another area of sex disparity? World J Cardiol 2021;13:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alasnag M, Truesdell AG, Williams H, Martinez SC, Qadri SK, Skendelas JP, Jakobleff WA, Alasnag M. Mechanical circulatory support: a comprehensive review with a focus on women. Curr Atheroscler Rep 2020;22:11. [DOI] [PubMed] [Google Scholar]
- 70. Wang AS, Nemeth S, Kurlansky P, Brodie D, Takayama H, Naka Y, Kaku Y, Fried J, Nir U, Takeda K. Sex differences in patients with cardiogenic shock requiring extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2022;164:960–969.e6. [DOI] [PubMed] [Google Scholar]
- 71. Rubini Gimenez M, Zeymer U, Desch S, de Waha-Thiele S, Ouarrak T, Poess J, Meyer-Saraei R, Schneider S, Fuernau G, Stepinska J, Huber K, Windecker S, Montalescot G, Savonitto S, Jeger RV, Thiele H. Sex-specific management in patients with acute myocardial infarction and cardiogenic shock: a substudy of the CULPRIT-SHOCK trial. Circ Cardiovasc Interv 2020;13:e008537. [DOI] [PubMed] [Google Scholar]
- 72. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U; CULPRIT-SHOCK Investigators . PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 73. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K; IABP-SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 74. Thiele H, Freund A, Gimenez MR, de Waha-Thiele S, Akin I, Poss J, Feistritzer HJ, Fuernau G, Graf T, Nef H, Hamm C, Böhm M, Lauten A, Schulze PC, Voigt I, Nordbeck P, Felix SB, Abel P, Baldus S, Laufs U, Lenk K, Landmesser U, Skurk C, Pieske B, Tschöpe C, Hennersdorf M, Wengenmayer T, Preusch M, Maier LS, Jung C, Kelm M, Clemmensen P, Westermann D, Seidler T, Schieffer B, Rassaf T, Mahabadi AA, Vasa-Nicotera M, Meincke F, Seyfarth M, Kersten A, Rottbauer W, Boekstegers P, Muellenbach R, Dengler T, Kadel C, Schempf B, Karagiannidis C, Hopf HB, Lehmann R, Bufe A, Baumanns S, Öner A, Linke A, Sedding D, Ferrari M, Bruch L, Goldmann B, John S, Möllmann H, Franz J, Lapp H, Lauten P, Noc M, Goslar T, Oerlecke I, Ouarrak T, Schneider S, Desch S, Zeymer U; ECLS-SHOCK Investigators . Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock—design and rationale of the ECLS-SHOCK trial. Am Heart J 2021;234:1–11. [DOI] [PubMed] [Google Scholar]
- 75. Grasner JT, Wnent J, Herlitz J, Perkins GD, Lefering R, Tjelmeland I, Koster RW, Masterson S, Rossell-Ortiz F, Maurer H, Böttiger BW, Moertl M, Mols P, Alihodžić H, Hadžibegović I, Ioannides M, Truhlář A, Wissenberg M, Salo A, Escutnaire J, Nikolaou N, Nagy E, Jonsson BS, Wright P, Semeraro F, Clarens C, Beesems S, Cebula G, Correia VH, Cimpoesu D, Raffay V, Trenkler S, Markota A, Strömsöe A, Burkart R, Booth S, Bossaert L. Survival after out-of-hospital cardiac arrest in Europe—results of the EuReCa TWO study. Resuscitation 2020;148:218–226. [DOI] [PubMed] [Google Scholar]
- 76. Kotini-Shah P, Del Rios M, Khosla S, Pugach O, Vellano K, McNally B, Vanden Hoek T, Chan PS. Sex differences in outcomes for out-of-hospital cardiac arrest in the United States. Resuscitation 2021;163:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mody P, Pandey A, Slutsky AS, Segar MW, Kiss A, Dorian P, Parsons J, Scales DC, Rac VE, Cheskes S, Bierman AS, Abramson BL, Gray S, Fowler RA, Dainty KN, Idris AH, Morrison L. Gender-based differences in outcomes among resuscitated patients with out-of-hospital cardiac arrest. Circulation 2021;143:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Minissian MB, Mehta PK, Hayes SN, Park K, Wei J, Bairey Merz CN, Cho L, Volgman AS, Elgendy IY, Mamas M, Davis MB, Reynolds HR, Epps K, Lindley K, Wood M, Quesada O, Piazza G, Pepine CJ. Ischemic heart disease in young women: JACC review topic of the week. J Am Coll Cardiol 2022;80:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, Christenson J, Kudenchuk P, Vaillancourt C, Rea TD, Idris AH, Colella R, Isaacs M, Straight R, Stephens S, Richardson J, Condle J, Schmicker RH, Egan D, May S, Ornato JP; ROC Investigators . Trial of continuous or interrupted chest compressions during CPR. N Engl J Med 2015;373:2203–2214. [DOI] [PubMed] [Google Scholar]
- 80. Blom MT, Oving I, Berdowski J, van Valkengoed IGM, Bardai A, Tan HL. Women have lower chances than men to be resuscitated and survive out-of-hospital cardiac arrest. Eur Heart J 2019;40:3824–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parikh PB, Hassan L, Qadeer A, Patel JK. Association between sex and mortality in adults with in-hospital and out-of-hospital cardiac arrest: a systematic review and meta-analysis. Resuscitation 2020;155:119–124. [DOI] [PubMed] [Google Scholar]
- 82. Perman SM, Shelton SK, Knoepke C, Rappaport K, Matlock DD, Adelgais K, Havranek EP, Daugherty SL. Public perceptions on why women receive less bystander cardiopulmonary resuscitation than men in out-of-hospital cardiac arrest. Circulation 2019;139:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Winther-Jensen M, Hassager C, Kjaergaard J, Bro-Jeppesen J, Thomsen JH, Lippert FK, Køber L, Wanscher M, Søholm H. Women have a worse prognosis and undergo fewer coronary angiographies after out-of-hospital cardiac arrest than men. Eur Heart J Acute Cardiovasc Care 2018;7:414–422. [DOI] [PubMed] [Google Scholar]
- 84. Song J, Ahn S, Kim J, Cho H, Moon S, Choi SH, Park J-H. Sex-related disparities in the in-hospital management of patients with out-of-hospital cardiac arrest. Resuscitation 2022;173:47–55. [DOI] [PubMed] [Google Scholar]
- 85. Kim LK, Looser P, Swaminathan RV, Horowitz J, Friedman O, Shin JH, Minutello RM, Bergman G, Singh H, Wong SC, Feldman DN. Sex-based disparities in incidence, treatment, and outcomes of cardiac arrest in the United States, 2003–2012. J Am Heart Assoc 2016;5:e003704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Perman SM, Siry BJ, Ginde AA, Grossestreuer AV, Abella BS, Daugherty SL, Havranek EP. Sex differences in “do not attempt resuscitation” orders after out-of-hospital cardiac arrest and the relationship to critical hospital interventions. Clin Ther 2019;41:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Desch S, Freund A, Akin I, Behnes M, Preusch MR, Zelniker TA, Skurk C, Landmesser U, Graf T, Eitel I, Fuernau G, Haake H, Nordbeck P, Hammer F, Felix SB, Hassager C, Engstrøm T, Fichtlscherer S, Ledwoch J, Lenk K, Joner M, Steiner S, Liebetrau C, Voigt I, Zeymer U, Brand M, Schmitz R, Horstkotte J, Jacobshagen C, Pöss J, Abdel-Wahab M, Lurz P, Jobs A, de Waha-Thiele S, Olbrich D, Sandig F, König IR, Brett S, Vens M, Klinge K, Thiele H; TOMAHAWK Investigators . Angiography after out-of-hospital cardiac arrest without ST-segment elevation. N Engl J Med 2021;385:2544–2553. [DOI] [PubMed] [Google Scholar]
- 88. Schmidt H, Kjaergaard J, Hassager C, Molstrom S, Grand J, Borregaard B, Roelsgaard Obling LE, Venø S, Sarkisian L, Mamaev D, Jensen LO, Nyholm B, Høfsten DE, Josiassen J, Thomsen JH, Thune JJ, Lindholm MG, Stengaard Meyer MA, Winther-Jensen M, Sørensen M, Frydland M, Beske RP, Frikke-Schmidt R, Wiberg S, Boesgaard S, Lind Jørgensen V, Møller JE. Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med 2022;387:1467–1476. [DOI] [PubMed] [Google Scholar]
- 89. Kjaergaard J, Moller JE, Schmidt H, Grand J, Molstrom S, Borregaard B, Venø S, Sarkisian L, Mamaev D, Jensen LO, Nyholm B, Høfsten DE, Josiassen J, Thomsen JH, Thune JJ, Obling LER, Lindholm MG, Frydland M, Meyer MAS, Winther-Jensen M, Beske RP, Frikke-Schmidt R, Wiberg S, Boesgaard S, Madsen SA, Jørgensen VL, Hassager C. Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med 2022;387:1456–1466. [DOI] [PubMed] [Google Scholar]
- 90. Suverein MM, Delnoij TSR, Lorusso R, Brandon Bravo Bruinsma GJ, Otterspoor L, Elzo Kraemer CV, Vlaar APJ, van der Heijden JJ, Scholten E, den Uil C, Jansen T, van den Bogaard B, Kuijpers M, Lam KY, Montero Cabezas JM, Driessen AHG, Rittersma SZH, Heijnen BG, Dos Reis Miranda D, Bleeker G, de Metz J, Hermanides RS, Lopez Matta J, Eberl S, Donker DW, van Thiel RJ, Akin S, van Meer O, Henriques J, Bokhoven KC, Mandigers L, Bunge JJH, Bol ME, Winkens B, Essers B, Weerwind PW, Maessen JG, van de Poll MCG. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med 2023;388:299–309. [DOI] [PubMed] [Google Scholar]
- 91. Jeejeebhoy FM, Zelop CM, Lipman S, Carvalho B, Joglar J, Mhyre JM, Katz VL, Lapinsky SE, Einav S, Warnes CA, Page RL, Griffin RE, Jain A, Dainty KN, Arafeh J, Windrim R, Koren G, Callaway CW; American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Diseases in the Young, and Council on Clinical Cardiology . Cardiac arrest in pregnancy: a scientific statement from the American Heart Association. Circulation 2015;132:1747–1773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.