Abstract
Several observations indicate that late-G1/S-phase-specific cellular functions may be required for herpes simplex virus (HSV) replication: (i) certain mutant HSV strains are replication impaired during infection of cells in the G0/G1 but not in the G1/S phase of the cell cycle, (ii) several late-G1/S-phase-specific cellular proteins and functions are induced during infection, and (iii) the activity of a cellular protein essential for expression of viral immediate-early (IE) genes, HCF, is normally required during the late G1/S phase of the cell cycle. To test the hypothesis that late-G1/S-phase-specific cellular functions are necessary for HSV replication, HEL or Vero cells were infected in the presence of the cell cycle inhibitors roscovitine (Rosco) and olomoucine (Olo). Both drugs inhibit cyclin-dependent kinase 1 (cdk-1) and cdk-2 (required for cell cycle progression into the late G1/S phase) and cdk-5 (inactive in cycling cells) but not cdk-4 or cdk-6 (active at early G1). We found that HSV replication was inhibited by Rosco and Olo but not by lovastatin (a cell cycle inhibitor that does not inhibit cdk activity), staurosporine (a broad-spectrum protein serine-threonine kinase inhibitor), PD98059 (an inhibitor specific for erk-1 and -2) or iso-Olo (a structural isomer of Olo that does not inhibit cdk activity). The concentrations of Rosco and Olo required to inhibit cell cycle progression and viral replication in both HEL and Vero cells were similar. Inhibition of viral replication was found not to be mediated by drug-induced cytotoxicity. Efforts to isolate Rosco- or Olo-resistant HSV mutants were unsuccessful, indicating that these drugs do not act by inhibiting a single viral target. Viral DNA replication and accumulation of IE and early viral RNAs were inhibited in the presence of cell cycle-inhibitory concentrations of Rosco or Olo. We therefore conclude that one or more cdks active from late G1 onward or inactive in nonneuronal cells are required for accumulation of HSV transcripts, viral DNA replication, and production of infectious virus.
In mammalian cells, the nuclear environment varies considerably during each phase of the cell cycle. Thus, only S-phase nuclei contain all of the transcriptional, enzymatic, structural, and metabolic factors required for semiconservative DNA replication (12). To ensure the replication of their genomes, DNA-containing viruses have developed unique strategies to overcome the problems presented by a changing nuclear environment (12, 33). The simplest strategy is characteristic of the smallest DNA viruses, the parvoviruses, which replicate their genomes only when the infected cell progresses into the S phase (3, 12, 33). The polyomaviruses (including simian virus 40), on the other hand, induce infected cells to progress into the S phase (7, 12, 33). Thus, these small DNA viruses are able to utilize cellular factors present or active in late G1 or early S as a consequence of either spontaneous or induced cell cycle progression. Although these replication strategies are highly successful, support of viral replication is limited to those cells that are able to progress into the S phase. In contrast to these viruses, the alphaherpesviruses, such as herpes simplex virus (HSV), have adopted a strategy that permits genome replication in growth-arrested cells, including terminally differentiated, noncycling neurons, as well as in actively dividing cells. In this sense, HSV replication is cell cycle independent. This does not imply, however, that a cellular function(s) associated with cell cycle progression is not required for HSV replication. Indeed, relationships between HSV infection and cell cycle-related cellular functions are well documented. Thus, HSV replication is blocked at the nonpermissive temperature in five temperature-sensitive cell lines growth arrested in G0/G1 (55, 61). Moreover, HSV has long been known to replicate more efficiently in actively dividing than in growth-arrested cells of most types, and this enhancement of replication efficiency is especially prominent for certain HSV strains with mutations in genes not absolutely required for viral replication (5, 10). For example, the replication impairment of ICP0− mutants can be complemented by cellular functions which are active during progression from G0 to the late G1/S phase of the cell cycle (5). Such complementation is consistent with a model in which during wild-type virus infection, ICP0 substitutes for or induces a cellular activity normally expressed only in the G1 and early S phases of the cell cycle. In a similar vein, HSV mutants that do not express active thymidine kinase (TK) or ribonucleotide reductase are impaired for replication in growth-arrested G0/G1 cells but replicate to wild-type levels in growing cells, which express the cellular counterparts of these viral enzymes in late G1/S (18, 27). At the molecular level, cellular proteins normally expressed only in late G1 and S (proliferating cell nuclear antigen [PCNA], RP-A, DNA polymerase α, and DNA ligase 1) or directly involved in cell cycle regulation (pRb and p53) have been detected in HSV DNA replication compartments of serum-starved cells, which are presumably arrested in G0/G1 (59). E2F DNA binding activity, cyclin-dependent kinase 2 (cdk-2) activity, and cyclin A protein, which are all specific for the late G1, S, or G2 phase of the cell cycle, have been reported to be induced during HSV infection of serum-starved cells (23, 25). Cyclin D3 has been reported to interact with ICP0 in vitro and in vivo when the cyclin was expressed ectopically from the genome of the infecting virus (31). Finally, a cellular protein required for HSV immediate-early (IE) gene expression, HCF, has recently been shown to be an important cell cycle regulator (19).
Based on these and other associations between HSV type 1 (HSV-1) and cell cycle-related functions, we hypothesized that cell cycle-related factors normally active in uninfected cells in the late G1 or early S phase (before the onset of cellular DNA synthesis) may be required for HSV replication. If such factors are indeed required, inhibition of their activities should block viral replication. To test this hypothesis, we measured the effects on viral replication of inhibitors of those cdks whose activities are absolutely required for progression into late G1 and beyond (56). Two such inhibitors have recently been described: olomoucine (Olo) and roscovitine (Rosco) (38, 57). Both are purine derivatives, and they display similar inhibitory profiles. Olo inhibits cdk-1/cyclin B, cdk-2/cyclin A or E, and cdk-5/p25. With the exception of extracellular receptor-activated kinases 1 (erk-1) and erk-2 (which are inhibited at ∼10-fold higher concentrations than the cdk targets), Olo failed to inhibit the 35 other enzymes tested, including protein serine/threonine (S/T) or tyrosine (Y) kinases, phosphatases, topoisomerases, DNA polymerases, and a nucleoside kinase (57). Rosco also inhibits cdk-1/cyclin B, cdk-2/cyclin A or E, cdk-5/p25, and, at a >20-fold higher concentration, erk-1 and -2 but not 25 other kinases (38). Neither Olo nor Rosco significantly inhibits the kinase activity of cdk-4 or -6, whereas the effects of these drugs on cdk-3, -7, and -8 have not been examined (38, 57). Thus, both drugs inhibit cdks that are active, and whose activity is required, from late G1 onward (56). Consequently, Rosco and Olo block cell cycle progression both in late G1/early S (when cdk-2 is required prior to the onset of cellular DNA synthesis) and in M (when cdk-1 is required for cell division) in a wide variety of mammalian cells, with average 50% inhibitory concentrations of 16.0 μM for Rosco and 60.3 μM for Olo (1, 16, 20, 26, 38, 57). An important distinction between the two drugs is that Rosco blocks selected biological effects, whereas Olo only delays them (38, 57).
Here we report that both Rosco and Olo inhibit HSV-1 replication. The inhibitory effects appear to be mediated by inhibition of cellular cdk activity and not of a viral protein in that (i) concentrations of Rosco and Olo that inhibit viral replication are proportional to the dose of each drug that inhibits cdk activity in vitro (ii) the concentrations of Rosco and Olo that inhibit viral replication are similar to those that block cell cycle progression in two cell types (Vero and HEL), (iii) neither Rosco- nor Olo-resistant mutants were detected after extensive passage in the presence of the drugs, (iv) inhibitors of cellular kinases (other than cdks) and inhibitors of cell cycle progression that do not block cdks do not block viral replication, and (v) a structural isomer of Olo that does not inhibit cdk activity does not inhibit HSV replication. In addition, HSV replicates in the presence of otherwise inhibitory concentrations of Olo in two of three Olo-resistant cell lines. Efforts to determine the level of inhibition of viral replication demonstrated that viral DNA replication was significantly reduced in the presence of either drug, and unexpectedly, the accumulation of IE transcripts was also reduced as early as 1 h after adsorption. These results strongly suggest the involvement of a cellular cdk(s) in the replication of a DNA-containing virus that is capable of replicating in noncycling cells. They also suggest the involvement of a cellular cdk in the transcription of viral genes.
MATERIALS AND METHODS
Cells, virus, plasmids, and infections.
Methods used for the propagation and maintenance of HEL and Vero cells have been described previously (30, 50). A plaque-purified low-passage (p9) stock of HSV-1 strain KOS was used throughout these studies and was prepared as previously described (50). The construction of plasmids prpTK, prp8, and prp4 has already been described (30).
For infection of Vero or HEL cells, 1 × 105 to 5 × 105 cells were infected with 2.5 to 3.0 PFU of virus (diluted in serum-free medium) per cell. After adsorption for 1 h at 37°C, the viral inoculum was removed, monolayers were washed twice with cold phosphate-buffered saline (PBS), and standard medium or medium containing the indicated drugs was added. In vivo, Olo does not block cdks completely and its effects are relatively short-lived (20, 38, 57). Thus, for infections in the presence of Olo, the medium was replaced 6 h after infection with an equal volume of fresh Olo-containing medium. Changing the medium at 6 h postinfection (hpi) reproducibly had no effect on the efficiency of HSV-1 replication (data not shown). Infected cells were scraped into the medium at the indicated times after infection (where T = 0 is the time of inoculum addition), and the total volume was transferred to a 5.0-ml tube and frozen at −70°C. After thawing, cells were sonicated for 45 s and the infectious virus was titrated by standard plaque assay. For drug release experiments (see Fig. 3), samples were harvested at the indicated times from 0 to 48 hpi. At 24 hpi, control or Rosco-containing (40 μM for HEL or 100 μM for Vero cells) medium was removed form infected monolayers. After the monolayers had been washed twice with cold PBS to remove the residual drug-containing medium, 2 volumes of control medium or fresh medium containing the same concentration of Rosco was added to each monolayer. Two volumes of medium was used to dilute any residual drug remaining on the monolayers after the washes.
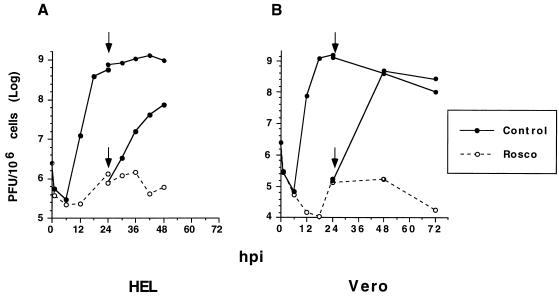
FIG. 3.
Inhibition of HSV replication by Rosco is reversible. HEL (A) or Vero (B) cells (1.5 × 105) in 12-well plates (400 cells/mm2) were infected with 2.5 PFU of HSV-1 strain KOS per cell. After adsorption for 1 h at 37°C, the inoculum was removed, cells were washed twice with cold PBS, and medium containing Rosco (40 μM for HEL, 100 μM for Vero) or control medium containing no drug was added. Infected cells were harvested at 0, 3, 6, 9, 12, 18, and 24 hpi. At 24 hpi, medium was removed from the remaining wells, cells were washed with cold PBS, and medium in Rosco-treated, infected cultures was either changed from Rosco-containing to control medium lacking Rosco (release) or back to Rosco-containing medium (no-release control). The medium in infected cultures lacking Rosco was replaced with fresh medium lacking Rosco (no-treatment control). This medium change at 24 hpi is indicated by the arrows. Cells were harvested at the indicated times after the medium change, and virus was titrated by standard plaque assays. Viral titers are plotted against time postinfection (where time zero is the time of virus addition to cultured cells).
Preparation of drugs.
Olo was purchased from Promega (Madison, Wis.); Rosco and iso-Olo were purchased from Calbiochem (San Diego, Calif.). The stock solutions of these three drugs were 100 mM in dimethyl sulfoxide (DMSO). The stocks of staurosporine (Sigma, St. Louis, Mo.) and PD98059 (Calbiochem) were also diluted in DMSO to final concentrations of 100 μg/ml and 20 mM, respectively. Lovastatin (Lova) was the generous gift of William L. Henckler (Merck & Co., Rahway, N.J.) and was converted to its active lactonic form as previously described (32), except that the final concentration of the stock solution was 10 mM. Phosphonoacetic acid (PAA) was purchased from Sigma, diluted in PBS, neutralized with NaOH, and further diluted with Dulbecco modified Eagle medium (DMEM) to a stock concentration of 100 mg/ml. Stocks of all drugs were aliquoted and kept at −20°C until use. Final dilutions of drugs in DMEM containing 10% fetal bovine serum were prepared immediately before use in the same batch of medium used in no-drug control infections. Except for PD98059, stocks were diluted at least 1:1,000 to obtain the working concentrations of all of the other drugs used. The final concentration of each drug used is indicated in each figure legend.
Fluorescence-activated cell sorter (FACS) analysis.
Vero or HEL cells (2 × 105 to 6 × 105) were seeded in 35-mm-diameter dishes in 3.0 ml of medium containing the indicated concentrations of Rosco, Olo, or Lova. Twenty-four hours later, the medium was removed and the cells were washed with cold PBS. The cells were then treated with 200 μl of a trypsin solution and resuspended in 1.8 ml of DMEM–10% fetal bovine serum. After centrifugation at 800 × g for 10 min, cells were washed with 2.0 ml of cold PBS, centrifuged again, and resuspended in 3.0 ml of 70% ethanol. After fixation on ice for approximately 45 min, cells were centrifuged as described above and resuspended in Telford’s reagent (90 mM EDTA, 2.5 mU of RNase A/ml, 50 μg of propidium iodide/ml, and 0.1% Triton X-100 in PBS) to a final concentration of 1.0 × 106 cells/ml. After incubation in an ice bath for approximately 2 h, the total DNA content was analyzed in a FACSCalibur analyzer using CellQuest software (Becton Dickinson, San Jose, Calif.). Cells were gated by forward scattering (FL-W) to avoid analyzing cell doublets, and limits to G1, S, and G2/M cells were set manually.
Selection of viral mutants.
Selection was performed with Vero cells because they tolerate higher concentrations of Rosco and Olo than do HEL cells (see Fig. 1; data not shown). For PAA selection, 105 Vero cells were infected with 103 PFU of a KOS stock in the presence of PAA at 50 μg/ml. When a viral cytopathic effect (CPE) became evident (∼10 visible plaques in a 35-mm dish), cells were harvested and sonicated, and 100 μl of this stock was used for the second selective passage. From the 2nd passage on, virus was grown in the indicated concentrations of PAA for a total of 11 passages and the virus was harvested in each passage at a 4+ CPE. Except for passage 1, in which the virus required 4 days to replicate, the virus in PAA selection medium was passed every 24 to 72 h. For Rosco selection, 104 PFU of HSV-1 KOS was used to infect 105 Vero cells in the presence of 50 μM Rosco. When a CPE became evident (∼3 visible plaques in a 35-mm dish), cells were harvested as described above and sonicated and 500 μl of this stock was used as the inoculum for the second selective passage. From the second passage on, the virus undergoing selection was harvested at a 4+ CPE or when cells showed signs of drug-induced toxicity, whichever occurred first. If a passage required more than 4 days, the medium was replaced on day 4 with medium containing only 75% of the Rosco concentration used in the first 4 days. Since viral titers had dropped in later passages, the volume of the previous passage stock used as the inoculum was increased to 750 to 1,000 μl (depending on the titer of each passage).
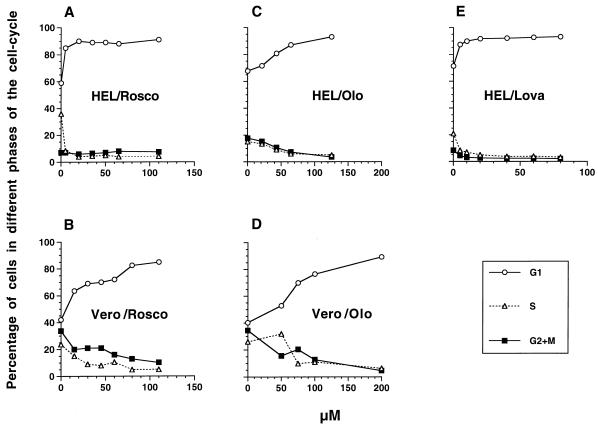
FIG. 1.
Concentrations of Rosco and Olo required to inhibit cell cycle progression in HEL and Vero cells. HEL (A, C, and E) and Vero (B and D) cells (3 × 105 to 6 × 105) were seeded in 35-mm dishes (330 to 600 cells/mm2) in the presence or absence of the indicated concentrations of Rosco (A and B), Olo (C and D), or Lova (E). Because of the short duration of some of Olo biological effects, cultures treated with Olo required a medium change at 6 hpi. After 24 h, cells were harvested by trypsinization and resuspended in Telford’s reagent and cellular DNA content was determined by FACS analysis. The percentages of cells in specific phases of the cell cycle are plotted against the drug concentration.
For Olo selection, the virus was passaged every 24 h for the first eight passages because, since wild-type HSV is able to replicate in the presence of Olo (see Fig. 4), we were concerned that the wild-type virus would outgrow any mutant population during a longer passage. Preliminary results indicated that no Olo-resistant virus had been selected for in these passages; thus, during the final four passages, the virus was grown to a 4+ CPE, which required 3 or more days.
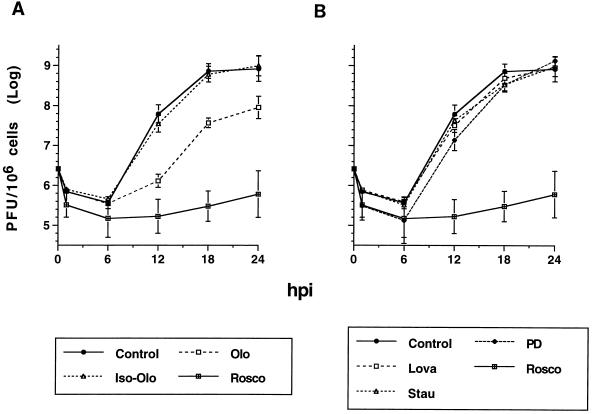
FIG. 4.
Lova, staurosporine, iso-Olo, and PD98059 do not inhibit HSV-1 replication. HEL cells (2 × 105) in 12-well plates (500 cells/mm2) were infected with 2.5 PFU of HSV-1 KOS per cell. After adsorption for 1 h at 37°C, the inoculum was removed, the cells were washed twice with cold PBS, and control medium lacking drugs or containing the indicated concentration of the indicated drug was added. The drug concentrations were: 40 μM Rosco, 75 μM Olo or iso-Olo, 20 μM Lova, 5 ng of staurosporine (Stau)/ml, and 70 μM PD98059 (PD). These concentrations of drugs had no toxic effects on uninfected HEL cells for at least 24 h as determined by microscopic evaluation. Cultures treated with Olo required a medium change at 6 hpi. At the indicated times postinfection, cells were harvested, frozen, thawed, and sonicated and the virus was titrated by standard plaque assay. Viral titers are plotted against time postinfection (where time zero is the time of virus addition to cultured cells). Each time point indicates the average and range of two experiments. Results are presented as two graphs, A and B, for clarity; consequently, the no-treatment (Control) and Rosco-treated (Rosco) controls are shown in both panels.
Probes.
Plasmids prpTK, prp8, prp4 (30), pOHc-Xh (the generous gift of Robert Jordan, University of Pennsylvania School of Medicine), and pTRI-GAPDH (Ambion, Austin, Tex.) were linearized with HindIII, NcoI, XcmI, NruI, and HindIII, respectively. Riboprobes were synthesized by using the Riboprobe in vitro transcription system (Promega) as recommended by the manufacturer, except that 5 μl of [α-32P]GTP (800 Ci/mmol) was used as the label and no unlabeled GTP was included in the transcription mixture. Labeled probes were separated from nonincorporated nucleotides by using NucTrap probe purification columns (Stratagene, La Jolla, Calif.)
Viral DNA replication assays.
HEL cells (9 × 105) were infected with 2.5 PFU of HSV-1 KOS per cell in the presence of 40 μM Rosco or 75 μM Olo or in the absence of a drug. At the indicated times after infection, the medium was removed, monolayers were washed with cold PBS, and cells were scraped into 1.0 ml of DNA extraction buffer (0.5% sodium dodecyl sulfate [SDS] and 50 μg of proteinase K/ml in TEN buffer [10 mM Tris · Cl, 25 mM EDTA, 100 mM NaCl, pH 8.0]). DNA was extracted as previously described (51) and resuspended in TE (10 mM Tris, 1 mM EDTA, pH 7.6) to a final concentration of ∼100 ng/ml. Ten micrograms of DNA from each sample was diluted to 400 μl with TE and alkali denatured with 40 μl of 3N NaOH at 70°C for 50 min. Afterwards, samples were cooled to room temperature and neutralized with 440 μl of 2 M sodium acetate (pH 5.2). Neutralized samples were vacuum slot blotted to a nylon membrane (GeneScreen; New England Nuclear Research Products, Boston, Mass.). DNA was UV cross-linked, prehybridized for 1 h at 75°C in ExpressHyb solution (Clontech, San Francisco, Calif.), and hybridized with a pool of riboprobes specific for TK and infected-cell protein 8 (ICP8) (each at 3 × 106 cpm/ml). After 1 h of hybridization at 75°C in ExpressHyb solution, the membrane was rinsed with 4× SSPE (1× SSPE is 0.15 M NaCl, 0.01 M NaH2PO4, and 0.001 M EDTA, pH 7.4) containing 0.1% SDS and washed twice at room temperature with the same solution for a total of 20 min and once for 15 min at 75°C in 0.2× SSPE–0.2% SDS. The hybridized membrane was exposed to a PhosphorImager cassette (Molecular Dynamics, Sunnyvale, Calif.) overnight and stripped in boiling 0.05× SSPE–1% SDS. After stripping, the membrane was re-exposed, prehybridized in ExpressHyb solution for 1 h at 68°C, and hybridized with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific riboprobe at 2.5 × 106 cpm/ml. After hybridizing for 1 h at 65°C, the membrane was rinsed in 4× SSPE–0.1% SDS, washed three times for a total of 35 min at room temperature in the same solution and once for 5 min at 50°C in 0.1× SSPE–0.1% SDS, and exposed again in the PhosphorImager cassette. Quantitation of the signal was performed by using ImageQuant software (Molecular Dynamics) and the virus-specific signal was standardized to the cell-specific signal.
RNase protection assays.
RNase protection assays were performed by using the DirectProtect kit and following the manufacturer’s instructions (Ambion), with minor modifications. HEL cells (7.5 × 105) were infected with 2.5 PFU of HSV-1 per cell in the presence of 40 μM Rosco or 75 μM Olo or in the absence of either drug. At the indicated times after infection, the medium was removed, the monolayers were washed twice with cold PBS and scraped into 150 μl of RNA extraction buffer (DirectProtect; Ambion), and the resulting cell extracts were transferred to Eppendorf tubes. Aliquots (25 μl) of each sample were annealed with 5 × 105 to 6 × 105 cpm of each of the virus-specific probes at 55°C. Another 25-μl aliquot of each sample was annealed with 5.5 × 105 cpm of the GAPDH-specific probe at 37°C. Preliminary experiments had determined that the amount of probe used was saturating at this cell extract-to-probe ratio. All annealing reactions were performed overnight in a volume of 50 μl. RNase and proteinase digestions were performed in accordance with the manufacturer’s instructions, and the protected fragments were resolved by electrophoresis in 6% denaturing polyacrylamide gels. Dried gels were exposed and analyzed in a PhosphorImager system (Molecular Dynamics).
RESULTS
The concentrations of Rosco and Olo that inhibit cell cycle progression in HEL and Vero cells differ.
Different doses of Rosco or Olo have been shown to be required to inhibit cdk activity in different cell types, depending (presumably) upon the levels of active cdk in each cell type (1, 16, 20, 26, 38, 57). Because inhibition of cdk activity in vivo results in cell cycle arrest, we used FACS analysis to determine the concentrations of each drug needed to arrest cell cycle progression in HEL and Vero cells. Preliminary experiments demonstrated that HEL cells tolerated 75 μM Rosco and 100 μM Olo and that Vero cells tolerated 120 μM Rosco and 200 μM Olo without evidence of toxicity as evaluated by microscopic observation (data not shown). As shown in Fig. 1A, concentrations of Rosco of 20 μM or higher blocked the cell cycle progression of HEL cells. Cells were blocked primarily in G0/G1 (∼85%) and secondarily in G2/M (∼8%). Although 30 μM Rosco had some effect on the cell cycle progression of Vero cells, concentrations as high as 80 μM were required to block ∼85% of these cells in G0/G1, ∼10% being blocked in G2/M (Fig. 1B). Because Olo is known to be less potent than Rosco (26, 38, 57), it was expected that higher does of Olo would be required to block cell cycle progression in both cell lines. Indeed, concentrations of Olo below 20 μM had no major effect on HEL cells. Increasing concentrations of Olo between 20 and 65 μM progressively blocked HEL cell cycle progression more efficiently, with only a minor effect at a higher concentration (Fig. 1C). In contrast, 50 μM Olo had only a modest effect on Vero cell cycle progression. Concentrations of Olo between 50 and 100 μM, however, blocked cell cycle progression incrementally, whereas little additional inhibition was evident at 200 μM (Fig. 1D). HEL cells treated with Lova, which blocks cell cycle progression by indirectly inhibiting transduction of growth-inducing signals across the cytoplasmic membrane, were also examined by FACS analysis. In these tests, Lova proved to be the most efficient drug in blocking cell cycle progression (Fig. 1E). Thus, 5 μM Lova blocked ∼90% of HEL cells in G0/G1, as described for other cell lines (32). Since concentrations of Lova as low as 5 μM had some toxic effect on Vero cells, we did not analyze further the effects of this drug on Vero cells.
These experiments demonstrate that (i) higher concentrations of Rosco and Olo are required to block cell cycle progression in Vero than in HEL cells, (ii) Rosco is more potent than Olo in blocking cell cycle progression in both cell types, and (iii) Lova was the most potent cell cycle inhibitor of HEL cells.
The concentrations of Rosco and Olo that inhibit HSV replication in HEL and Vero cells are similar to those that inhibit cell cycle progression.
If cdk activity were required for HSV replication, viral replication in HEL or Vero cells would be inhibited by concentrations of Rosco and Olo that inhibit cdk activity, as measured by inhibition of cell cycle progression. We therefore analyzed the effects of different concentrations of Rosco and Olo on single-step HSV-1 replication. Viral titers are expressed in PFU/106 cells because this unit allows comparison of titers independently of the number of infected cells and the volume of medium used to overlay the cultures after inoculation. The age and density of monolayers were approximately the same in all cultures prior to infection.
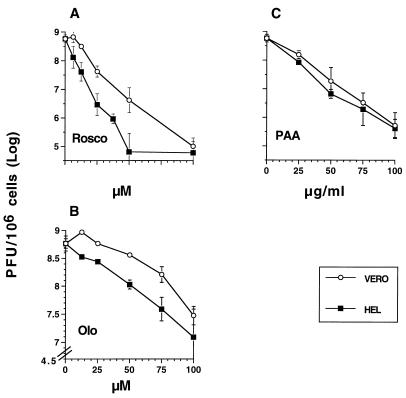
As for inhibition of cell cycle progression in uninfected cells, higher doses of both drugs were required to block HSV replication in Vero than in HEL cells (Fig. 2A and B). Thus, 50 μM Rosco was sufficient to inhibit virus replication completely in HEL cells, whereas 100 μM was required to achieve the same effect in Vero cells (Fig. 2A). In agreement with the different concentrations of the two drugs needed to inhibit cdk activity (38, 57) and cell cycle progression (Fig. 1), Olo was less effective than Rosco in inhibiting HSV replication in both cell types. Thus, 50 μM Olo or less had only modest effects on 24-h viral titers in Vero cells, whereas those same concentrations were sufficient to reduce viral yields significantly in HEL cells (Fig. 2B). Concentrations of Olo between 50 and 100 μM progressively and more efficiently blocked HSV replication in both Vero and HEL cells. Because the concentrations of Rosco and Olo needed to inhibit cell cycle progression are similar to those needed to inhibit viral replication in HEL and Vero cells and because these concentrations are proportional to the concentrations of both drugs required to inhibit cdk activity in vitro (38, 57), we postulate that the inhibition of HSV replication by these drugs occurs through effects on cellular cdk(s) in virus-infected cells. In contrast to Rosco and Olo, PAA, a drug that directly inhibits the activity of an essential virus-encoded function (DNA polymerase), is known to inhibit HSV replication in different cell types at approximately the same concentration (2, 22, 24, 28, 45). Indeed, unlike Rosco and Olo, PAA inhibited viral replication with similar potencies in HEL and Vero cells, although it was slightly more potent in HEL than in Vero cells at all of the concentrations tested (Fig. 2C).
FIG. 2.
Concentrations of Rosco and Olo required to inhibit HSV-1 replication in HEL and Vero cells. HEL or Vero cells (2 × 105) in 12-well plates (500 cells/mm2) were infected with 2.5 PFU of HSV-1 strain KOS per cell. One hour later, the inoculum was removed, the cells were washed twice with cold PBS, and medium containing the indicated concentrations of Rosco (A), Olo (B), or PAA (C) was added. Cultures treated with Olo required a medium change at 6 hpi. After 24 h, cells were harvested and virus was titrated by standard plaque assay. Viral titers at 24 hpi are plotted against the drug concentration. Note that the scales on the y axis differ between panel B and panels A and C. Each time point indicates the average and range of two experiments.
These experiments demonstrate that (i) as for inhibition of cell cycle progression, higher concentrations of Rosco and Olo are required to block HSV replication in Vero cells than in HEL cells, (ii) Rosco is a more potent HSV replication blocker than Olo, and (iii) Rosco inhibits HSV replication as efficiently as does PAA.
Inhibition of HSV replication by Rosco is not a consequence of cytotoxicity.
Although Rosco and Olo did not induce detectable cytopathology, as determined by microscopic evaluation, at the concentrations used to block HSV replication, it remained a possibility that more subtle toxic effects of these drugs may have compromised the ability of cells to support HSV replication. To determine whether this was the case, we performed a Rosco reversal experiment. Rosco was selected for these experiments because it inhibits HSV replication more efficiently than does Olo. Therefore, any increase in viral replication after drug removal would be more obvious. HEL and Vero cells were therefore infected with 2.5 PFU of HSV per cell in the presence of 40 (HEL) or 100 (Vero) μM Rosco, and the medium was replaced 24 h later with fresh medium containing no drug. At selected times before and after the medium change, cells were harvested and the amount of infectious virus was determined by standard plaque assay (Fig. 3). Under these circumstances, the 24-h yield was reduced by 3 (HEL) or 4 (Vero) orders of magnitude, as demonstrated previously for those concentrations of Rosco (Fig. 2), yet resumption of HSV replication was evident in HEL cells 6 h after release from the Rosco-induced block (Fig. 3A). Twenty-four hours after release, viral titers approached (in HEL cells) or reached (in Vero cells) those attained in untreated cultures (Fig. 3B). In contrast, when the medium was replaced with fresh medium containing Rosco, viral titers did not increase during the 24 h after release in either cell type (Fig. 3). Cytotoxicity was evident in cells infected with 2.5 PFU of HSV-1 per cell and treated with Rosco for more than 24 h. Since this toxicity was not observed in Rosco-treated, uninfected cells, we conclude that the combined effect of the drug and the infection is the most likely cause of toxicity. Similar cytotoxicity has been observed during HSV infection in the presence of PAA (24).
We conclude from these experiments that Rosco-induced inhibition of HSV replication is not mediated by irreversible drug-induced toxicity in either HEL or Vero cells.
Inhibition of HSV replication is specific for cdk inhibitors.
Although the specificities of Rosco and Olo have been evaluated so extensively (38, 57) that these drugs are currently used to confirm the involvement of cdks in biological processes (4, 35, 58), it remained a theoretical possibility that the findings presented in Fig. 2 and 3 are a consequence of a block in another cellular protein S/T kinase(s) or of direct inhibition of a virus-encoded function. We therefore performed the following series of experiments to assess these possibilities.
First, we measured HSV replication in HEL cells in the presence and absence of Olo, Rosco, iso-Olo (a structural isomer of Olo that does not inhibit cdk activity) (57, 58), Lova (a cell cycle inhibitor that does not block cdk activity) (32), staurosporine (a broad-spectrum protein S/T kinase inhibitor) (49), or PD98059 (a specific inhibitor of erk-1 and -2) (13). HEL cells were used in these tests because HSV replication was inhibited in these cells by lower concentrations of Rosco or Olo. Thus, any inhibitory effect of the other drugs tested on HSV replication should be more easily detected in HEL cells than in Vero cells. Since the stocks of all of the drugs except Lova were prepared in DMSO, we first determined in preliminary control experiments that a 1:500 dilution of DMSO in medium has no inhibitory effect on HSV replication (data not shown).
In single-cycle growth experiments, Olo inhibited HSV replication by nearly 2 orders of magnitude through 12 hpi but was less inhibitory thereafter (Fig. 4A), consistent with other recognized biological effects of this drug (57). Rosco, on the other hand, blocked HSV replication almost completely throughout the 24-h test period (Fig. 4). A structural isomer of Olo which does not inhibit cdk activity, iso-Olo (57), had no detectable effect on viral replication, suggesting that the observed inhibition of HSV replication by Olo was indeed mediated by inhibition of cellular cdks (Fig. 4A).
Because HSV replicates in growth-arrested cells, we hypothesized that Lova, which, like Rosco and Olo, arrests cells in G1 (but by a different mechanism), would not inhibit HSV replication. As anticipated, 20 μM Lova, which arrests ∼90% of HEL cells in G0/G1 (Fig. 1E), failed to block HSV replication, confirming (53) that simple cell cycle arrest in G0/G1 is not sufficient to block HSV replication (Fig. 4B).
Because Rosco and Olo inhibit erk-1 and -2 (although ∼20- and ∼10-fold less efficiently, respectively, than they inhibit cdks) (38, 57), we investigated whether these kinases are required for HSV replication. As shown in Fig. 4B, PD98059, a drug that specifically inhibits erk-1 and -2 but not cdks (13), did not inhibit HSV replication at a concentration (70 μM) above concentrations that inhibit erk-1 and -2 in vivo (13). Similar results have been obtained with PC12 cells (29). Obvious cytotoxic effects were observed by microscopic evaluation when uninfected HEL cells were treated with concentrations of this drug higher than 70 μM (data not shown).
Although Rosco and Olo are highly cdk-specific drugs (38, 57), we also used a broad-spectrum protein S/T kinase-specific inhibitor to further test our initial hypothesis that the block in HSV replication is mediated by the inhibition of cdks and not by nonspecific inhibition of other protein S/T kinases. Staurosporine, a widely used, broad-spectrum protein S/T kinase inhibitor, did not block HSV replication significantly at a concentration at which it inhibits protein S/T kinases: 5 ng/ml (11 nM) (44, 49) (Fig. 4B). Similar results have been obtained with another broad-spectrum protein S/T kinase inhibitor, K5720, and PC12 cells (29). Higher concentrations of staurosporine, which may inhibit cdk-2 in vivo (i.e., 100 to 200 ng/ml) (9, 44), could not be tested because they are toxic for both HEL and Vero cells (data not shown).
Thus, experiments with inhibitors demonstrated that HSV replication was inhibited only by inhibitors of cdks and not by an isomer of a cdk inhibitor which does not inhibit cdk activity, a cell cycle inhibitor that arrests cells by a mechanism not directly involving cdks, or a broad-spectrum inhibitor of protein S/T kinases.
Rosco and Olo do not target an HSV-1-encoded function: failure to isolate drug-resistant mutants.
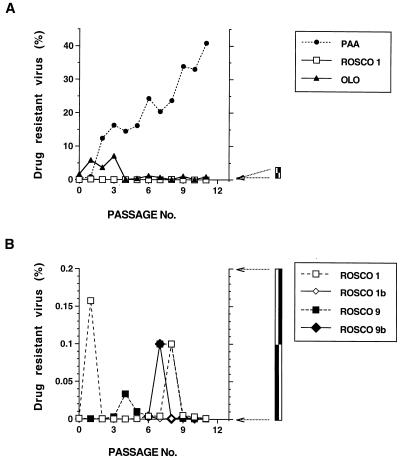
Characteristically, drugs that block HSV replication by direct inhibition of a viral function(s) can be used to select for genetic variants that are resistant to the drug. Therefore, if Rosco or Olo directly inhibits a virus-encoded function, it should be possible to isolate spontaneous drug-resistant mutants by serial passage of the virus in the presence of the drugs. Consequently, we used standard procedures for isolation of drug-resistant mutants to select Rosco- and Olo-resistant HSV-1 mutants. Briefly, HSV-1 was passed several times in the presence of the drugs, starting at subinhibitory concentrations and increasing the concentrations in successive passages. Except for the first passage (when the virus was harvested after a few plaques were visible), the virus was harvested when CPEs were generalized (4+ CPE) or when drug-induced cellular toxicity was evident, whichever occurred first. As a positive control, we conducted a parallel selection with PAA, which specifically targets the virus-encoded DNA polymerase (45). Preliminary tests demonstrated that our plaque-purified HSV-1 KOS stock contained ∼1 PAA-resistant infectious virus per 104 PFU (data not shown), in agreement with previous findings (2, 28). Therefore, 103 PFU of a KOS stock was used to infect 105 Vero cells in the presence of 50-μg/ml PAA in the first passage, and the virus was further passaged in increasing concentrations of PAA for a total of 11 passages (Table 1 and Fig. 5). The concentration of PAA used in the 11th passage was 500 μg/ml, five times the concentration necessary to inhibit the replication of unselected HSV stocks. The 11 passages were completed in 27 selection days (Table 1). For selection in Rosco or Olo, the inocula for the first passages consisted of 104 PFU of HSV-1 KOS (the same stock used for PAA selection), because no Rosco- or Olo-resistant PFU were identified in preliminary experiments. For Rosco selection, a procedure that paralleled that used for PAA selection was followed as closely as possible. However, in the third passage, when the concentration of Rosco was increased from 50 to 75 μM, viral replication was severely impaired, such that the drug concentration was reduced in following passage (Table 1). Consequently, we split the Rosco-selected stock into two parts after passage 6. One half was passaged continuously in low concentrations of Rosco (30 to 50 μM), while we planned to select the other half in increasing concentrations of the drug (Table 1, Rosco 1b and Rosco 1, respectively). As had occurred previously, when the concentration of Rosco was increased (from 50 to 75 μM) in passage 7, viral titers dropped markedly such that we utilized 50 μM for passage 8 and 30 μM for the following two passages (Table 1, Rosco 1). Although the initial basis for splitting the stock undergoing selection proved to be untenable, we continued to pass both stocks under selection to increase the probability of identifying Rosco-resistant variants. Eleven passages in Rosco required 34 selection days (Table 1, Rosco 1); 10 passages in low concentrations of Rosco (all passages after passage 6 were in 30 to 50 μM Rosco) required 30 selection days (Table 1, Rosco 1b). The concentration of Rosco in the final passage for both selection lines was 50 μM, the same concentration that inhibited the replication of unselected HSV- 1 in the first passage (Table 1, Rosco 1 and 1b).
TABLE 1.
Passage of HSV-1 in selective mediaa
| Passage no. | PAA
|
Rosco 1
|
Rosco 1b
|
Rosco 9
|
Rosco 9b
|
Olo
|
Control
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [C]b | Daily titer increasec | Final titerd | [C]e | Daily titer increase | Final titer | [C]e | Daily titer increase | Final titer | [C]e | Daily titer increase | Final titer | [C]e | Daily titer increase | Final titer | [C]e | Daily titer increase | Final titer | [C]e | Daily titer increase | Final titer | |
| 1 | 50 | 60 | 4.5 | 50 | 2 | 3.6 | 50 | <0.5 | 5.7 | 75 | 6 | 5.2 | 0 | 86 | 6.5 | ||||||
| 2 | 75 | 4,417 | 7.5 | 50 | 91 | 5.2 | 50 | 1 | 5.5 | 100 | 262 | 6.3 | 0 | 2,570 | 7.9 | ||||||
| 3 | 75 | 2 | 7.9 | 75 | 0.28 | 4.2 | 75 | 18 | 5.2 | 100 | 48 | 6.8 | 0 | 437 | 8.6 | ||||||
| 4 | 100 | 73 | 7.6 | 50 | <0.5 | 2.3 | 50 | <0.5 | 5.1 | 150 | 130 | 7.3 | 0 | 129 | 8.6 | ||||||
| 5 | 100 | 10 | 7.4 | 50 | 6 | 2.6 | 50 | 29 | 6.1 | 150 | 37 | 7.5 | 0 | 407 | 9.3 | ||||||
| 6 | 100 | 4 | 7.1 | 50 | 2 | 2.6 | 50 | 1 | 5.8 | 150 | 33 | 7.7 | 0h | 59 | 9.0 | ||||||
| 7 | 150 | 219 | 8.1 | 75 | <0.5 | 1.3 | 40g | <0.53 | <1 | 75 | <0.5 | 3.2 | 40g | <0.5 | 4.2 | 150 | 24 | 7.8 | —f | — | — |
| 8 | 200 | 28 | 8.1 | 50 | <0.5 | <1 | 30 | 1,150 | 4.5 | 50 | <0.5 | <1.0 | 30 | 88 | 6.0 | 150 | 7 | 7.7 | — | — | — |
| 9 | 200 | 36 | 8.2 | 30 | 2,076 | 4.0 | 30 | 64 | 6.0 | 30 | 3,458 | 4.2 | 30 | 0.5 | 5.3 | 150 | 417 | 7.3 | — | — | — |
| 10 | 300 | 1 | 7.0 | 30 | 87 | 6.1 | 50 | 2 | 6.1 | 30 | 41 | 6.0 | 50 | 10 | 6.0 | 150 | 0.5 | 6.6 | — | — | — |
| 11 | 500 | 2 | 6.3 | 50 | 2 | 6.1 | — | — | — | 50 | 2 | 6.2 | — | — | — | 150 | 4 | 6.9 | — | — | — |
HSV-1 was passaged 11 times in increasing concentrations of PAA, Rosco, or Olo or in medium without drugs (Control). The concentrations of each drug used, the daily increases in viral titers and the viral titer at harvest are indicated for each passage. Different fractions of each passage were used as inocula during passage in the presence of PAA, Olo, or Rosco. See the text for details.
Concentration of the selection drug in micrograms per milliliter.
Ratio of final titer/inoculum/days required to complete the passage (rounded to the nearest integer, except for ratios of <1).
PFU per milliliter at time of harvest (log).
Micromolar concentration of the selection drug.
—, no drug.
Only four passages in low concentrations of Rosco (30 to 50 μM) were performed after six passages in high concentrations (50 to 75 μM).
Only six passages were performed in the absence of a drug to observe the evolution of viral titers.
FIG. 5.
Absence of Rosco- or Olo-resistant variants of HSV after 11 passages in selective medium. HSV-1 strain KOS was passaged in medium containing either Olo, Rosco, or PAA as described in the text and in Table 1, footnote a. After 11 passages in culture, 1,000 PFU of each passage was used to infect 105 Vero cells in duplicate. One set of infected cells was treated with inhibitory concentrations of the selective drugs (100 μg of PAA/ml, 100 μM Rosco, or 150 μM Olo), while the other set was left untreated (medium without drug). Virus was harvested and titrated at 24 hpi. The medium in cultures treated with Olo was changed at 6 hpi. The percentage of resistant virus after each passage was calculated by using the formula percent resistance = 100 × (PFU in the presence of selection drug/PFU in medium without drug) and is plotted against the passage number. (A) Percentage of virus resistant to Olo, Rosco, or PAA. The percentage of virus resistant to Rosco in the four different Rosco passage series (ROSCO 1, 1b, 9, and 9b) was so low as to be indistinguishable on this scale. Therefore, only the ROSCO 1 selection series was plotted. (B) Percentage of virus resistant to Rosco at different passages in the four Rosco selection series. Note the expanded scale of the y axis. The percentage of virus resistant to Olo or PAA was higher than 0.2% from the first passage on. Thus, the Olo and PAA selection series could not be plotted on this graph. ROSCO 1, selection of HSV-1 KOS in high Rosco concentrations (75, 50, 30, 30, and 50 μM from passages 7 to 11, respectively); ROSCO 1b, selection of HSV-1 KOS in low Rosco concentrations (40, 30, 30, and 50 μM from passages 7 to 10, respectively); ROSCO 9, selection in high Rosco concentrations (75, 50, 30, 30, and 50 μM from passages 7 to 11, respectively) of an HSV-1 stock previously passaged nine times in Olo; ROSCO 9b, selection in low Rosco concentrations (40, 30, 30, and 50 μM from passages 7 to 10, respectively) of an HSV-1 stock previously passaged nine times in Olo. See Table 1 and the text for details.
For Olo selection, the concentration of the drug was increased from 75 to 150 μM from passages 1 to 4 but could not be increased further because Olo is cytotoxic for HSV-1-infected Vero cells when used at 200 μM for more than 24 h (data not shown). Eleven passages in Olo selection required 20 selection days (Table 1, Olo). We entertained the possibility that Rosco blocked viral replication too efficiently to permit selection of resistant mutants. Reasoning that an HSV stock preselected in Olo might be further selected in Rosco, we used 106 PFU from the 9th passage in Olo as the inoculum for 11 successive passages in Rosco (for a total of 20 passages) and followed the protocol described above for Rosco selection of an HSV-1 stock, including the branching in high (Rosco 1) and low (Rosco 1b) drug concentrations after passage 6 (Table 1, Rosco 9 and 9b, respectively).
When all selection lines had undergone 11 passages, 1,000 PFU of each passage selected in PAA, Olo, or Rosco was used to infect 105 Vero cells in the presence or absence of 100-μg/ml PAA, 150 μM Olo, or 100 μM Rosco, respectively. (Viral titers were too low to allow infection with 1,000 PFU in many Rosco passages [selection series 1, passages 1, 3, 4, 5, 6, 7, and 8; selection series 1b, passages 7 and 8; selection series 9, passages 7 and 8]. For these passages, we used the largest inoculum possible, which varied from ∼50 to ∼500 PFU.) Twenty-four hours after infection, the virus was harvested, the percentage of drug-resistant virus in the total yield was calculated by using the formula percent resistant = 100 × (PFU in selection drug/PFU in drug-free medium), and this percentage was plotted as a function of the passage number (Fig. 5A and B). Standard plaque assays could not be used for this purpose because at the concentrations of Rosco required to fully inhibit HSV replication, Vero cells overlaid with methyl cellulose do not form a homogeneous monolayer and are thus unsuitable for plaque counting.
In agreement with previously published results (24), some PAA-resistant virus was detected as early as passage 2 (Fig. 5A). By passage 11, the titers in the presence of PAA were approximately 50% of the titers in the absence of the drug. Unlike selection in PAA, we were unable to detect Rosco- or Olo-resistant mutants of HSV after 11 passages in drug-containing medium (Fig. 5A). Since the differences among the different Rosco selection series (Rosco 1, 1b, 9 and 9b) were too small to discern on the scale of the y axis of Fig. 5A, only Rosco 1 was plotted in this graph. A complete comparison of the four Rosco selection series is shown in Fig. 5B. The expanded scale of the y axis in Fig. 5B shows that no resistant virus was selected in these cultures and, furthermore, that no trend towards development of Rosco resistance was observed either.
We conclude from these experiments that Rosco and Olo blocked HSV replication by inhibition of a cellular function(s), or that the viral functions targeted by these drugs are either numerous or so essential for viral replication that mutations in the genes encoding these functions are lethal.
Viral DNA replication is inhibited in the presence of Rosco and Olo.
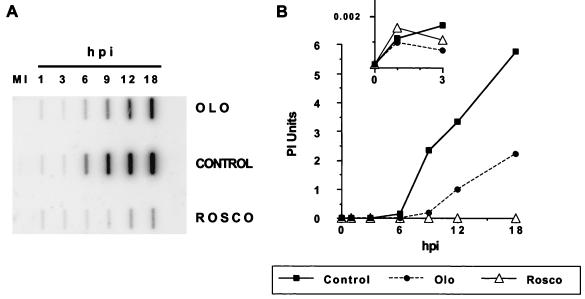
Two recognized cellular targets of Rosco and Olo are cdk-1 and cdk-2, which are required for cell division (mitosis) and DNA replication, respectively. Since several cellular factors are known to be required for HSV DNA replication and cdk-2 is involved in cellular DNA replication (12), we hypothesized that cdk-2 might also participate in viral DNA replication. If this were the case, Rosco and Olo would inhibit viral DNA replication. To test this hypothesis, we infected HEL cells at a multiplicity of 2.5 PFU/cell in the presence or absence of either drug and extracted total DNA at selected times after infection, blotted it onto a nylon membrane, UV cross-linked it, and hybridized it to HSV-specific probes (Fig. 6A). The HSV DNA-specific signal was quantitated and corrected for loading, and the relative amounts of viral DNA at different times postinfection are plotted in Fig. 6B. The total amounts of viral DNA at 18 hpi were 22-, ∼2,000-, and ∼5,300-fold above the amount of DNA detected at 1 hpi (∼0.001 PhosphorImager units) in infections performed in the presence of Rosco or Olo or in the absence of either drug, respectively. Note that the increase in viral DNA in the presence of Rosco is too small to be discernible on the scale of the y axis in Fig. 6B. The small insert in Fig. 6B shows the amounts of viral DNA detected during the interval between 1 and 3 hpi, using expanded scales for both the x and y axes.
FIG. 6.
Viral DNA replication in the presence and absence of Olo or Rosco. HEL cells (9 × 105) in 60-mm dishes (320 cells/mm2) were infected with 2.5 PFU of HSV-1 per cell in the presence of 75 μM Olo (OLO) or 40 μM Rosco (ROSCO) or in the absence of a drug (CONTROL). DNA was extracted from infected cells at 1, 3, 6, 9, 12, and 18 hpi and from mock-infected cells (MI). Five micrograms of total DNA was blotted onto a nitrocellulose membrane, UV cross-linked, and hybridized to labeled HSV-specific riboprobes, and the membrane was exposed in a PhosphorImager (A). The signal hybridized to each slot was quantitated and corrected for loading as measured by GAPDH hybridization, and the amount of viral DNA in each sample at the indicated time after infection was plotted (B). The insert in panel B shows the amounts of viral DNA detected during the interval between 1 and 3 hpi on an expanded scale.
The results shown in Fig. 6 demonstrate that (i) the same amounts of viral DNA entered cells in Olo- or Rosco-treated infections or in the absence of either drug, (ii) lower levels of viral DNA were detected at 6 hpi in the presence of Rosco or Olo than in the absence of either drug, and (iii) the level of viral DNA increased significantly in the presence of Olo at late times after infection but less significantly in the presence of Rosco, in agreement with the extent of viral replication in the presence of these drugs (compare Fig. 6B and 4A).
Accumulation of viral IE and early (E) mRNAs is reduced in the presence of Rosco and Olo.
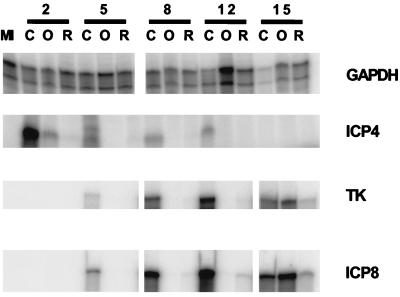
The results presented above could be interpreted to mean that viral DNA replication is a direct target of inhibition by Rosco and Olo. Alternatively (but not exclusively), viral DNA replication could be inhibited indirectly by these drugs through inhibition of viral IE or E gene expression. We therefore analyzed the expression of selected viral IE and E genes at the level of mRNA accumulation in the presence and absence of Rosco or Olo. For this purpose, HEL cells were infected with 2.5 PFU of HSV-1 per cell in the presence and absence of 75 μM Olo or 40 μM Rosco, and total infected cell RNA was extracted at the indicated times postinfection (Fig. 7). Levels of mRNA of a cellular housekeeping gene (that for GAPDH) decreased at late times after infection with wild-type HSV-1 in the absence of either drug, as previously described for other cellular RNAs (30). Levels of GAPDH did not decrease in cells infected in the presence of Rosco or Olo, consistent with the inhibition of viral replication by these drugs (Fig. 3, 4, and 6). In contrast, expression of IE ICP4 mRNA was greatly impaired by Rosco and Olo as early as 2 hpi (Fig. 7). Accumulation of TK and ICP8 mRNAs was also reduced by Rosco or Olo at later times (5, 8, and 12 hpi). At 15 hpi, however, levels of TK and ICP8 mRNAs increased significantly in the Olo-treated cells, consistent with the increased levels of viral replication and viral DNA synthesis that occur in the presence of Olo as noted above (Fig. 3, 4, and 6). A slight increase in the levels of ICP8 and TK RNAs was also observed in the Rosco-treated samples. Notably, an identical reduction of viral transcription was observed after release from a cycloheximide block in the presence of Rosco or Olo (51a). Moreover, no decrease in the half-life of viral mRNAs was apparent under these conditions. Therefore, the low levels of viral transcripts in the presence of Rosco or Olo are a consequence of a block in viral transcription.
FIG. 7.
Viral RNA accumulation in the presence and absence of Olo or Rosco. HEL cells (7.5 × 105) in 60-mm dishes (275 cells/mm2) were infected with 2.5 PFU of HSV-1 per cell in the presence of 75 μM Olo (O) or 40 μM Rosco (R) or in the absence of a drug (C), and total (cellular and viral) RNA was extracted at 2, 5, 8, 12, or 15 hpi, as well as from mock-infected cells (MI). Levels of GAPDH-, ICP4-, TK-, and ICP8-specific RNAs were measured by standard RNase protection assays (ProtectDirect; Ambion) as recommended by the manufacturer, with minor modifications.
We must emphasize that the RNase protection assay was optimized to require minimal handling of the samples, rather than to achieve maximal sensitivity. Thus, the absence of a signal in a given lane implies not necessarily the absence of the measured mRNA but rather the inability to measure low levels of mRNA. Because levels of selected IE and E transcripts are reduced by Rosco and Olo, yet functional IE and E proteins are required for viral DNA replication, we cannot discern whether these drugs block viral DNA replication directly through inhibition of a (cellular) protein kinase essential for this process or indirectly through effects on IE and E gene expression. Moreover, the reduced levels of E transcripts detected can themselves be a secondary effect of the reduced levels of IE transcripts and, consequently, proteins.
DISCUSSION
In these studies, we have demonstrated that two highly specific cdk inhibitors, Rosco and Olo, inhibit the accumulation of selected IE and E transcripts and HSV replication in general. Although each of the findings presented here can be explained by alternative mechanisms, the simplest explanation most consistent with all of the available data is that HSV replication, and accumulation of IE transcripts, requires cellular cdk activity. This hypothesis is based on the following: (i) both Rosco and Olo are highly specific inhibitors of cdks (1, 4, 16, 20, 26, 35, 36, 38, 57, 58), (ii) the concentrations of Rosco and Olo needed to inhibit viral replication were proportional to the concentrations of each inhibitor required to inhibit cdk activity in vitro (38, 57) and similar to the concentrations that inhibit cell cycle progression in vivo (Fig. 1 and 2), (iii) well-characterized inhibitors of other protein kinases did not inhibit viral replication (Fig. 4), and (iv) efforts to isolate Rosco- or Olo-resistant mutant viruses were unsuccessful (Fig. 5) and Rosco- or Olo-resistant mutants were not detected in unselected viral populations (data not shown). Moreover, in experiments not presented here, two of three Olo-resistant cell lines supported HSV-1 replication in the presence of concentrations of Olo that are otherwise inhibitory to viral growth.
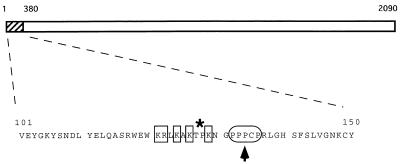
The low levels of IE transcripts detected 1 h after adsorption suggest that cdks are required for transcription of IE genes (Fig. 7). The alternative explanation, that mRNA stability is severely impaired by Rosco or Olo, is not supported by the results of cycloheximide reversal experiments (51a). Two cdks, cdk-7 and -8, are components of the cellular transcription machinery (17, 48). Thus, if cdk-7 and -8 were inhibited by Rosco and Olo, inhibition of these kinases could explain the inhibition of viral transcription by these drugs. Notably, transcription of a cellular housekeeping gene, that for GAPDH, was not affected by either drug (Fig. 7), indicating that if Rosco and Olo inhibit cdk-7 or -8, these kinases are not required for the transcription of this cellular gene. In addition to the cellular transcription complex, only one viral protein (VP16), a cellular transcription factor (oct-1), and a cell cycle regulatory cellular protein (HCF) are required for transcription of IE genes (42). All of these factors are regulated by phosphorylation, and cdks may be involved in this regulation. Thus, phosphorylation of HCF is required for its physical interaction with VP16 (34), and phosphorylation of VP16 is required for activation of IE gene transcription (43). Although VP16 is phosphorylated by cellular casein kinase 2 (CKII) (and perhaps also by protein kinase C [PKC] and PKA) (43), CKII itself is activated by mitogenic stimuli, probably through phosphorylation by cdks (39). The kinases that phosphorylate HCF have not been identified, and several putative phosphorylation site are present in this protein. However, only one good consensus cdk phosphorylation site is present in the entire HCF molecule. Interestingly, this putative phosphorylation site maps to the minimal domain of HCF required for interaction with VP16 and for cell cycle regulation (60), and it is only 7 amino acids distant from the site of a point mutation that disrupts the ability of HCF to activate the transcription of viral IE genes and cell cycle progression (19) (Fig. 8, arrowhead). Finally, oct-1 activity is also regulated by phosphorylation (21), and cdks may be among the protein kinases that phosphorylate oct-1 (47). In addition to the four factors which are essential for IE transcription (VP16, HCF, oct-1, and the cellular transcription complex), ICP0, an HSV IE regulatory protein, is further required to achieve wild-type levels of IE gene transcription. Since the functional significance of ICP0 phosphorylation and the kinases that perform this phosphorylation have yet to be identified, we cannot speculate on the possible involvement of ICP0 in the observed inhibitory effects of Rosco and Olo.
FIG. 8.
Putative cdk phosphorylation site in the amino-terminal domain of HCF. The 50-amino-acid sequence from positions 101 to 150 of HCF contains a unique consensus cdk-2 phosphorylation site. Basic residues are indicated by the squares, the proline-rich segment is indicated by the oval, and the putative cdk site (a threonine followed by a proline) is indicated by the asterisk.
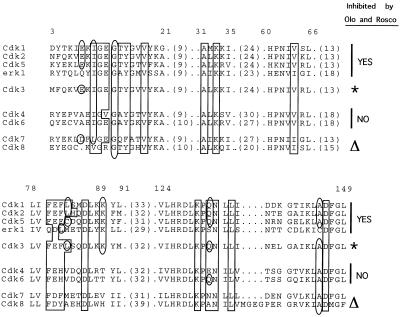
Because our findings indicate that HSV replication requires a cellular cdk(s), the question arises as to which of the many cdks are required. Neither cdk-4 nor cdk-6 is inhibited by Rosco or Olo (38, 57). Therefore, neither can be the cdk which, when inhibited, is responsible for the block in HSV replication in the experiments presented here. cdk-7 and -8 are involved in the phosphorylation of RNA polymerase II (17, 48), which is altered during HSV infection, presumably to favor the transcription of viral rather than cellular genes (46). Moreover, the interaction between cdk-8 and VP16 is required for VP16’s transactivating activity (17). To date, the effects of Rosco or Olo on cdk-7 or -8 in vitro have not been studied; however, neither inhibits activation of cdk-1 in vivo, which requires cdk- 7 activity (38, 57), and cdk-8 kinase activity is not required for transcription in transient transfection assays (17). Finally, analysis of the published structural data on Rosco and Olo bound to cdk-2 (11, 52) indicates that some cdk-2 amino acids that interact with these drugs (e.g., lysine 89) are conserved in all of the other protein kinases known to be inhibited by them (i.e., cdk-1 and -5 and erk-1 and -2) but not in cdks not inhibited by these drugs (i.e., cdk-4 or -6) or in cdk-7 or -8 (Fig. 9). Based on this correlation, cdk-7 and -8 are probably not targets of Rosco and Olo inhibition. Too little is known about the physiology of cdk-3 to speculate on its possible involvement in HSV replication. Based on sequence-structure analysis, however, this kinase is likely to be inhibited by Rosco and Olo (Fig. 9). Finally, cdk-5, a target of Rosco and Olo inhibition, is inactive in nonneuronal cells. In sum, of the cdks known to be active in cycling cells, Rosco and Olo are likely to inhibit only those required and active from late G1/early S (cdk-2 and -3) through S and G2 (cdk-1). Thus, the inhibitory pattern of the two drugs indicates that a cdk(s) normally active from late G1/early S (cdk-1, -2, or -3) or only in neuronal cells (cdk-5) is required for expression of HSV IE transcripts and viral replication. Because HSV-1 replicates in noncycling G0/G1 cells, any late-G1/S cell cycle-related function required for HSV-1 replication (such as cdk-1, -2, or -3) should be induced during infection of G0/G1 cells. Moreover, if cdk-5 is required for HSV replication, this kinase must also be induced during infection of nonneuronal cells. Considering that cdk-2 has been reported to be induced during HSV-2 infection of serum-starved monkey fibroblasts (25) and that cdk-2 is involved in transcriptional regulation (14), it is tempting to speculate that cdk-2 is the cellular enzyme (or one of the cellular enzymes) required for HSV replication and gene expression that is blocked by Rosco and Olo. Moreover, since HSV-1 replicates in neurons, which do not normally express cdk-2 activity, it is necessary to determine whether or not HSV-1 replication in neuronal cells also requires cdk activity. Experiments to address these issues are in progress.
FIG. 9.
Comparison of cdk-2 amino acids that interact with Rosco and Olo among cdks inhibited and not inhibited by these drugs. A comparison of the cdk-2 amino acids that interact with Rosco and Olo in all other recognized cdks, as well as in erk-1 and erk-2, was made by sequence analysis. The sequence of erk-2 (not shown) is identical to that of erk-1 in the relevant region. Alignment of the sequences was performed by using the Pileup program (GCG software package) with a gap weight of 12 and a gap extension weight of 4 (default values for both parameters). Analysis of the conserved residues shown by others to interact with ATP, Olo, or Rosco (11, 55) was performed manually. Only the relevant segments of the sequences are shown; the values in parentheses are the numbers of amino acids not shown. The numbers at the top correspond to the sequence of cdk-1 (cdc2). Boxed amino acids are those identical to the amino acids of cdk-2 that interact with ATP, as determined by Schulze-Gahmen and colleagues (52) and De Azevedo and colleagues (11). Note that nearly all of these amino acids are conserved. Amino acids of cdk-2 that interact with Rosco and Olo, as determined by Schulze-Gahmen and colleagues (52) and De Azevedo and colleagues (11), and their homologs in other cdks are enclosed by ovals; some of these residues also interact with ATP. Note that some amino acids are conserved in cdks known to be inhibited by Rosco and Olo but not in cdks known not to be inhibited by these drugs. The asterisks indicate the cdk predicted by this analysis to be inhibited by Rosco and Olo. The triangles indicate the cdks predicted by this analysis not to be inhibited by Rosco and Olo.
An alternative explanation for the findings reported herein is that Rosco and Olo act through inhibition of a HSV-encoded protein kinase. For this explanation to satisfy all of the available data, however, the putative viral protein kinase must be (i) inhibited by Rosco and Olo as efficiently as cdks are and at similar concentrations (Fig. 1 and 2), (ii) required for expression of HSV IE transcripts (Fig. 7), (iii) essential for HSV replication (Table 1 and Fig. 5), and (iv) resistant to inhibition by staurosporine (Fig. 4). In addition, intracellular concentrations of Rosco and Olo inhibitory for the putative viral protein kinase must be reached with lower extracellular concentrations of the drugs in HEL than in Vero cells to explain the different drug concentrations required to inhibit HSV replication in the two cell types (Fig. 2). Unfortunately, it is not technically possible, at present, to measure intracellular concentrations of either drug. Only two HSV genes (UL13 and US3) encode proteins containing protein S/T kinase motifs (37). A third viral protein, the large subunit of the viral ribonucleotide reductase (the product of the UL39 gene) may possess protein kinase activity (8). None of these three viral proteins is essential for viral replication (18, 37). Moreover, considering the specificity of Rosco and Olo, the structural basis for this specificity, and the lack of homology between cdks and the three putative viral kinases, it is unlikely that Rosco or Olo inhibits any of the three viral protein S/T kinases. Notably, a broad-spectrum protein S/T kinase inhibitor, staurosporine, did not block HSV replication (Fig. 4), consistent with the nearly wild-type phenotypes of HSV mutants with alterations in the genes that encode the three viral protein kinases (18, 37). Finally, the only viral protein known to be required for ICP4 transcription, VP16, has no known enzymatic activity that could be inhibited by Rosco or Olo. Based on these considerations, we postulate that inhibition of IE transcription by Rosco and Olo is not likely mediated by inhibition of a viral function.
We have also entertained the possibility that a cellular kinase other than a cdk is the target of inhibition by Rosco and Olo. However, no other cellular target of these drugs is known, and they do not inhibit most of the cellular kinases that have been implicated in HSV replication, namely, cyclic AMP-activated PKA, CKII, double-stranded RNA-activated PKR, and PKC (6, 15, 40, 43, 54).
Equally as informative as the fact that Rosco and Olo inhibit HSV replication is the fact that other cell cycle and protein kinase inhibitors do not. In addition to the results presented in this report, n-butyrate, which inhibits cell cycle progression in late G1 by inhibiting histone deacetylase, does not inhibit HSV replication (53). In another study, nine unique tyrosine kinase inhibitors inhibited HSV replication by only 10-fold after 24 h, most likely through direct inhibition of a viral function(s) (62). Lova, which inhibits cell cycle progression by blocking the c-Ras-mediated transduction of growth stimulatory signals through the cellular membrane (32), did not block HSV replication in the experiments reported here. Nor did staurosporine, a broad- spectrum protein S/T kinase inhibitor which blocks many protein kinase signaling pathways (49). Although staurosporine is capable of inhibiting cdk-1 in vitro, it only blocks this enzyme in vivo at concentrations ∼20-fold higher than those used in the experiments reported herein (9, 44).
Rosco and Olo also inhibit replication of human cytomegalovirus (HCMV) (4), yet expression of HCMV IE and E genes was not affected by these drugs (4). Moreover, inhibition of HCMV replication by Rosco and Olo could be secondary to the block in cell cycle progression, while blocking of cell cycle progression by itself did not inhibit HSV-1 replication (Fig. 4). Thus, it appears that inhibition of HSV or HCMV replication by the two drugs occurs through different mechanisms. Two other herpesviruses, human herpesvirus 8 and herpesvirus saimiri, encode homologs of the cellular D-type cyclins that activate cellular cdk-6. Although these virus-encoded cyclins may be involved in transformation (41), an alternative function for them may be the activation of late-G1/S-phase-specific cdks during lytic infection.
The failure to isolate Rosco- or Olo-resistant mutants (Table 1 and Fig. 5) suggests that a cdk(s) regulates an essential viral function (such that mutants defective in this function are not viable) or that they regulate multiple viral functions. RNase protection experiments clearly show that transcription of at least two IE genes (ICP4 and ICP0) requires a cdk(s) (Fig. 7 and data not shown). However, the experiments presented here do not address whether a cdk(s) is also required for other events in the HSV replication cycle. Inhibition of E gene transcription (Fig. 7, TK and ICP8) could be secondary to the block in expression of viral IE proteins. Similarly, and by extension, inhibition of E transcription and viral DNA replication (Fig. 6) may simply be the end result of the block in expression of viral DNA replication proteins. Alternatively, inhibition of both E gene expression and DNA replication may be the direct result of cdk inhibition. Experiments designed to address these questions are in progress.
In summary, in the context of the findings presented herein that a cellular function(s) normally expressed in the late G1/S phase of the cell cycle is required for HSV replication, the previously described induction of late-G1/S-phase-specific forms of transcription factors, other cellular proteins, and kinase activities by HSV (23, 25, 59) acquires new functional significance. Whether these cellular functions are active in cells prior to viral infection in vivo or whether they are activated during infection is currently being tested.
ACKNOWLEDGMENTS
This work was supported by Public Health Services grants (R37CA20260 from the National Cancer Institute and PO1NS35138 from the National Institute of Neurological Disorders and Stroke).
We thank Robert Jordan for helpful discussions and ideas, Amy Rosenberg for excellent technical assistance, and Amy Francis, Jennifer Isler, and William Halford for critically reading the manuscript.
REFERENCES
- 1.Abraham R T, Acquarone M, Andersen A, Asensi A, Belle R, Berger F, Bergounioux C, Brunn G, Buquet-Fagot C, Fagot D, et al. Cellular effects of olomoucine, an inhibitor of cyclin-dependent kinases. Biol Cell. 1995;83:105–120. doi: 10.1016/0248-4900(96)81298-6. [DOI] [PubMed] [Google Scholar]
- 2.Becker Y, Asher Y, Cohen Y, Weinberg-Zahlering E, Shlomai J. Phosphonoacetic acid-resistant mutants of herpes simplex virus: effect of phosphonoacetic acid on virus replication and in vitro deoxyribonucleic acid synthesis in isolated nuclei. Antimicrob Agents Chemother. 1977;11:919–922. doi: 10.1128/aac.11.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berns J I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2173–2197. [Google Scholar]
- 4.Bresnahan W A, Boldogh I, Chi P, Thompson E A, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 5.Cai W, Schaffer P A. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J, Chen J-J, Gross M, Roizman B. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole C N. Polyomavirinae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1997–2025. [Google Scholar]
- 8.Cooper J, Conner J, Clements J B. Characterization of the novel protein kinase activity present in the R1 subunit of herpes simplex virus ribonucleotide reductase. J Virol. 1995;69:4979–4985. doi: 10.1128/jvi.69.8.4979-4985.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crissman H A, Gadbois D M, Tobey R A, Bradbury E M. Transformed mammalian cells are deficient in kinase-mediated control of progression through the G1 phase of the cell cycle. Proc Natl Acad Sci USA. 1991;88:7580–7584. doi: 10.1073/pnas.88.17.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daksis J I, Preston C M. Herpes simplex virus immediate early gene expression in the absence of transinduction by Vmw65 varies during the cell cycle. Virology. 1992;189:196–202. doi: 10.1016/0042-6822(92)90695-l. [DOI] [PubMed] [Google Scholar]
- 11.De Azevedo W F, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim S H. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 12.DePamphilis M. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 13.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dynlacht B. Regulation of transcription by proteins that control the cell cycle. Nature. 1997;389:149–152. doi: 10.1038/38225. [DOI] [PubMed] [Google Scholar]
- 15.Elliott G, O’Reilly D, O’Hare P. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology. 1996;226:140–145. doi: 10.1006/viro.1996.0638. [DOI] [PubMed] [Google Scholar]
- 16.Glab N, Labidi B, Qin L X, Trehin C, Bergounioux C, Meijer L. Olomoucine, an inhibitor of the cdc2/cdk2 kinases activity, blocks plant cells at the G1 to S and G2 to M cell cycle transitions. FEBS Lett. 1994;353:207–211. doi: 10.1016/0014-5793(94)01035-8. [DOI] [PubMed] [Google Scholar]
- 17.Gold M O, Tassan J P, Nigg E A, Rice A P, Herrmann C H. Viral transactivators E1A and VP16 interact with a large complex that is associated with CTD kinase activity and contains CDK8. Nucleic Acids Res. 1996;24:3771–3777. doi: 10.1093/nar/24.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein D J, Weller S K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 19.Goto H, Motomura S, Wilson A C, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 20.Graves R, Davies R, Brophy G, O’Beirne G, Cook N. Noninvasive, real-time method for the examination of thymidine uptake events—application of the method to V-79 cell synchrony studies. Anal Biochem. 1997;248:251–257. doi: 10.1006/abio.1997.2088. [DOI] [PubMed] [Google Scholar]
- 21.Grenfell S J, Latchman D S, Thomas N S. Oct-1 and Oct-2 DNA-binding site specificity is regulated in vitro by different kinases. Biochem J. 1996;315:889–893. doi: 10.1042/bj3150889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay J, Subak-Sharpe J H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activity. J Gen Virol. 1976;31:145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- 23.Hilton M J, Mounghane D, McLean T, Contractor N V, O’Neil J, Carpenter K, Bachenheimer S L. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology. 1995;213:624–638. doi: 10.1006/viro.1995.0034. [DOI] [PubMed] [Google Scholar]
- 24.Honess R W, Watson D H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977;21:584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hossain A, Holt T, Ciacci-Zanella J, Jones C. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J Gen Virol. 1997;78:3341–3348. doi: 10.1099/0022-1317-78-12-3341. [DOI] [PubMed] [Google Scholar]
- 26.Iseki H, Ko T C, Xue X Y, Seapan A, Hellmich M R, Townsend C M., Jr Cyclin-dependent kinase inhibitors block proliferation of human gastric cancer cells. Surgery. 1997;122:187–194. doi: 10.1016/s0039-6060(97)90008-8. [DOI] [PubMed] [Google Scholar]
- 27.Jamieson A T, Gentry G A, Subak-Sharpe J H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974;24:465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- 28.Jofre J T, Schaffer P A, Parris D S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977;23:833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan R, Pepe J, Schaffer P A. Characterization of a nerve growth factor-inducible cellular activity that enhances herpes simplex virus type 1 gene expression and replication of an ICP0 null mutant in cells of neural lineage. J Virol. 1998;72:5373–5382. doi: 10.1128/jvi.72.7.5373-5382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keyomarsi K, Sandoval L, Band V, Pardee A B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 33.Knipe D M. Virus-host cell interactions. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 273–299. [Google Scholar]
- 34.Kristie T M, Sharp P A. Purification of the cellular C1 factor required for the stable recognition of the Oct-1 homeodomain by the herpes simplex virus alpha-trans-induction factor (VP16) J Biol Chem. 1993;268:6525–6534. [PubMed] [Google Scholar]
- 35.Kwon Y G, Lee S Y, Choi Y, Greengard P, Nairn A C. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc Natl Acad Sci USA. 1997;94:2168–2173. doi: 10.1073/pnas.94.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malecz N, Foisner R, Stadler C, Wiche G. Identification of plectin as a substrate of p34cdc2 kinase and mapping of a single phosphorylation site. J Biol Chem. 1996;271:8203–8208. doi: 10.1074/jbc.271.14.8203. [DOI] [PubMed] [Google Scholar]
- 37.McGeoch D, Barnett B, McLean C. Emerging functions of alphaherpesvirus genes. Semin Virol. 1993;4:125–134. [Google Scholar]
- 38.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 39.Meisner H, Czech M P. Phosphorylation of transcriptional factors and cell-cycle-dependent proteins by casein kinase II. Curr Opin Cell Biol. 1991;3:474–483. doi: 10.1016/0955-0674(91)90076-b. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell C, Blaho J A, Roizman B. Casein kinase II specifically nucleotidylylates in vitro the amino acid sequence of the protein encoded by the alpha 22 gene of herpes simplex virus 1. Proc Natl Acad Sci USA. 1994;91:11864–11868. doi: 10.1073/pnas.91.25.11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholas J, Cameron K, Honess R. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature. 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 42.O’Hare P. The virion transactivator of herpes simplex virus. Semin Virol. 1993;4:145–156. [Google Scholar]
- 43.O’Reilly D, Hanscombe O, O’Hare P. A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J. 1997;16:2420–2430. doi: 10.1093/emboj/16.9.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omura S, Sasaki Y, Iwai Y, Takeshima H. Staurosporine, a potentially important gift from a microorganism. J Antibiot. 1995;48:535–548. doi: 10.7164/antibiotics.48.535. [DOI] [PubMed] [Google Scholar]
- 45.Overby L, Robishaw E, Schleicher J, Reuter A, Shipkowitz N, Mao J-H. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974;6:360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice S, Long M, Lam V, Spencer C. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts S B, Segil N, Heintz N. Differential phosphorylation of the transcription factor Oct1 during the cell cycle. Science. 1991;253:1022–1026. doi: 10.1126/science.1887216. [DOI] [PubMed] [Google Scholar]
- 48.Roy R, Adamczewski J P, Seroz T, Vermeulen W, Tassan J P, Schaeffer L, Nigg E A, Hoeijmakers J H, Egly J M. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994;79:1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 49.Rüeg U, Burgess G. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 50.Schaffer P A, Aron G M, Biswal N, Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973;52:57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- 51.Schang L M, Kutish G F, Osorio F A. Correlation between precolonization of trigeminal ganglia by attenuated strains of pseudorabies virus and resistance to wild-type virus latency. J Virol. 1994;68:8470–8476. doi: 10.1128/jvi.68.12.8470-8476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.Schang, L. M., and P. A. Schaffer. Unpublished data.
- 52.Schulze-Gahmen U, Brandsen J, Jones H D, Morgan D O, Meijer L, Vesely J, Kim S H. Multiple modes of ligand recognition: crystal structures of cyclin-dependent protein kinase 2 in complex with ATP and two inhibitors, olomoucine and isopentenyladenine. Proteins. 1995;22:378–391. doi: 10.1002/prot.340220408. [DOI] [PubMed] [Google Scholar]
- 53.Shadan F F, Cowsert L M, Villarreal L P. n-Butyrate, a cell cycle blocker, inhibits the replication of polyomaviruses and papillomaviruses but not that of adenoviruses and herpesviruses. J Virol. 1994;68:4785–4796. doi: 10.1128/jvi.68.8.4785-4796.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith R L, Pizer L I, Johnson E M, Jr, Wilcox C L. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 55.Umene K, Nishimoto T. Inhibition of generation of authentic genomic termini of herpes simplex virus type 1 DNA in temperature-sensitive mutant BHK-21 cells with a mutated CCG1/TAF(II)250 gene. J Virol. 1996;70:9008–9012. doi: 10.1128/jvi.70.12.9008-9012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 57.Vesely J, Havlicek L, Strnad M, Blow J J, Donella-Deana A, Pinna L, Letham D S, Kato J, Detivaud L, Leclerc S, et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 58.Wieprecht M, Wieder T, Paul C, Geilen C C, Orfanos C E. Evidence for phosphorylation of CTP:phosphocholine cytidylyltransferase by multiple proline-directed protein kinases. J Biol Chem. 1996;271:9955–9961. doi: 10.1074/jbc.271.17.9955. [DOI] [PubMed] [Google Scholar]
- 59.Wilcock D, Lane D P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991;349:429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- 60.Wilson A C, Freiman R N, Goto H, Nishimoto T, Herr W. VP16 targets an amino-terminal domain of HCF involved in cell cycle progression. Mol Cell Biol. 1997;17:6139–6146. doi: 10.1128/mcb.17.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanagi K, Talavera A, Nishimoto T, Rush M G. Inhibition of herpes simplex virus type 1 replication in temperature-sensitive cell cycle mutants. J Virol. 1978;25:42–50. doi: 10.1128/jvi.25.1.42-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yura Y, Kusaka J, Tsujimoto H, Yoshioka Y, Yoshida H, Sato M. Effects of protein tyrosine kinase inhibitors on the replication of herpes simplex virus and the phosphorylation of viral proteins. Intervirology. 1997;40:7–14. doi: 10.1159/000150515. [DOI] [PubMed] [Google Scholar]