ABSTRACT
Objective
Antiseizure medications (ASMs) are frequently used for other indications, such as migraine, pain syndromes, and psychiatric disorders. Possible teratogenic effects are therefore of wide concern and the risks imposed by the medications must be weighed against the risk with the disorder treated. It is our objective to update family practitioners on the implications of starting ASM for women with epilepsy during childbearing age. We hypothesized that clinicians would prescribe ASM based on avoiding teratogenesis and treating associated comorbidities simultaneously.
Methods
The study cohort was derived from women veterans with epilepsy (WVWE) prescribed ASM who received Veterans Health Administration care for at least 3 years in Veterans Health Administration between fiscal years (FY)01 and FY19. Regimens were classified as monotherapy or polytherapy. Multivariant logistic regression examined the association between demographics, military characteristics, physical/psychiatric comorbidities, neurological care, and use of each ASM.
Results
Among 2,283 WVWE, in ages between 17 and 45, the majority (61%) received monotherapy in FY19. Commonly prescribed ASM included 29% gabapentin, 27% topiramate, 20% lamotrigine, 16% levetiracetam, and 8% valproate (VPA). Comorbid diagnosis of headache predicted use of topiramate and VPA, bipolar disease predicted use of LMT and VPA, pain predicted gabapentin, and schizophrenia was associated with VPAs use. Women receiving levetiracetam and lamotrigine were significantly more likely to receive neurology care previously.
Conclusion
The presence of medical comorbidities influences the selection of ASM. VPAs use in WVWE during childbearing age continues, despite the high teratogenic risk, especially in women with bipolar disorder and headaches. Multidisciplinary care integrating family practice doctors, mental health, and neurology can prevent the enduring problem of teratogenesis in women taking ASM.
INTRODUCTION
Epilepsy is one of the most common neurological conditions, estimated to affect approximately three million people in the United States and 70 million worldwide.1 Women with epilepsy (WWE) face a variety of clinical challenges, particularly with respect to reproductive health, including birth control, conception, and maternal and fetal complications during pregnancy and postpartum treatment of seizures.
Over the past two decades, there has been the fastest rate of growth among women veterans seeking care at the Veterans Administration Health Care system,2 and currently, the number is over half a million.
International seizure medication pregnancy registries have provided valuable information regarding the risk of major congenital malformation (MCM) of development, which seems to be a consequence of seizure medication therapy and not epilepsy itself.3 The highest risk is related to cognitive impairment and autism spectrum disorder in children of women exposed to valproate (VPA) in utero.4,5
This article gives an overview of antiseizure medication (ASM) prescription trends in women veterans with epilepsy (WVWE) during fiscal years 2019 (FY19).
MATERIALS AND METHODS
Data for this study included inpatient, outpatient, and pharmacy data from the national Defense Health Agency and Veterans Health Administration (VHA) systems, accessed via the DoD and DVA Infrastructure for Clinical Intelligence (DaVINCI). These data provided diagnoses based on International Classification of Diseases Ninth and Tenth Revisions clinical modification (ICD-9/10 CM) and pharmacy data required for this study. Additional data sources included the Veterans Services Network (VETSNET) to identify epilepsy-related service-connected disability (SCD) and the Joint DVAs Affairs and DoD Mortality Data Repository through 2018.
Cohort Development
This analysis is derived from a larger study examining phenotypes of comorbidity before and after the identification of epilepsy in health care data. That study required longitudinal data and included veterans who received at least 1 year of DoD care in FY00–19, received at least 2 years of care in the VHA during FY02–18, and entered VA care between FY02 and FY14. Veterans who had only one convulsion diagnosis (e.g., ICD-9 diagnosis 780.3 or ICD-10 diagnosis G56), or those with possible, probable, or definite psychogenic non-epileptic seizures were excluded. Because our focus on concerns was related to teratogenicity of ASMs, we restricted analyses for this study to WWE who were of childbearing potential (17–45 years of age), who were alive on October 1, 2019, and who were treated for epilepsy in FY19 (N = 2,283; Fig. 1).
FIGURE 1.
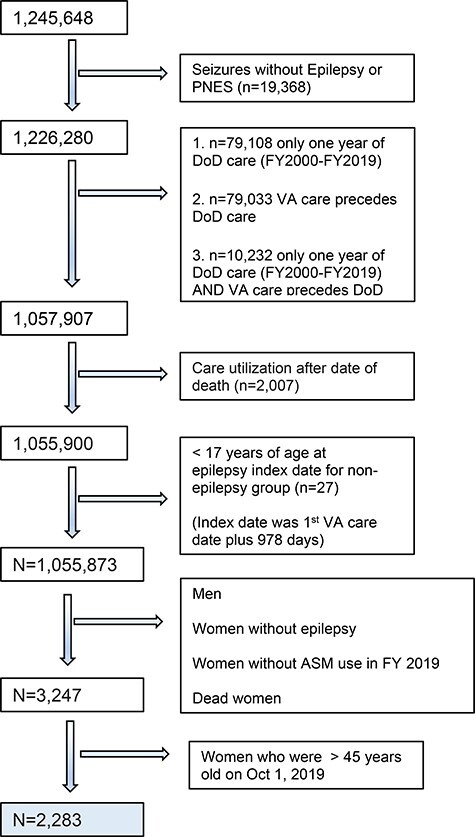
Cohort flow chart.
Epilepsy Identification
We identified veterans with epilepsy as those who met one of the following criteria: (1) At least one epilepsy specific diagnosis ICD-9/10 CM code (345.x, G40) and more than 1 year of ASM prescription; (2) more than one epilepsy specific or convulsion diagnosis ICD-9/10 CM code (780.3X, R56) and any duration of ASM prescription; or (3) receipt of SCD for epilepsy.
Covariates
We identified demographic characteristics in VHA inpatient and outpatient data, and data from DaVINCI. Race/ethnicity was classified as White, African American/Black, Hispanic, Asian, Native American/Pacific Islander, and unknown. Age in 2019 was classified as 17–29, 30–39, and 40–45, which are approximately quartiles of age in the broader post-9/11 veteran population. Military characteristics considered included service branch (army, air force, marines, and navy/coast guard), rank (enlisted vs. officer/warrant officer), and deployment status (yes/no).
Clinical characteristics included polytherapy (vs. monotherapy), VA SCD, receipt of neurology care, and physical and psychiatric comorbidities diagnosed before 2019. Veteran with epilepsy (VWE) who received two or more ASMs were classified as having polytherapy. Service-connected disability was identified using VETSNET data. We identified care in neurology clinics based on MEPRS 4 Codes (DoD) and Clinic Stop Codes (VHA). Physical and psychiatric health conditions were identified in VHA and DoD inpatient and outpatient data using ICD-9/10 codes. To qualify, each condition was required to have either one inpatient diagnosis or two outpatient diagnoses at least 7 days apart. Conditions include those related to cardiovascular/cerebrovascular disease (smoking history, hypertension, diabetes mellitus, and cardiac disease), mental health (depression, bipolar disorder, schizophrenia, anxiety, post-traumatic stress disorder, and suicide related behavior), neurological conditions/symptoms (headache, back and neck pain, and brain tumor), and other neurological conditions not including epilepsy. ICD-9/10 codes for these conditions are provided in Appendix 1.
Statistical Analysis
All analyses were conducted in SAS 9.4. Bivariate analyses were used to examine the independent predictors of use for each ASM used in 2019. A multivariable logistic regression model for each of the five ASM was used to identify predictors of use in FY19, such as demographics, military characteristics, and physical/psychiatric comorbidities. The adjusted odds of ASM medication use and 95% CI [adjusted odds ratios (aOR), 95% CI] were calculated. A P-value of <.05 indicated statistical significance.
RESULTS
The descriptive analysis (Table I) demonstrated that the final cohort included 2,283 women, with majority (67.4%) of age between 30 and 39 years. White patients comprised 57.9% of the cohort, while 24.9%, 9.9%, and 7.3% were Black, Hispanic, and other racial/ethnicity, respectively. Approximately, 45% had been previously deployed.
TABLE I.
Veteran Women with Epilepsy Cohort Characteristics (N = 2,283) by Anti-Seizure Medication
| Gabapentin | Lamotrigine | Levetiracetam | Topiramate | Valproate | |
|---|---|---|---|---|---|
| (n = 829) | (n = 569) | (n = 447) | (n = 768) | (n = 230) | |
| % (n) | % (n) | % (n) | % (n) | % (n) | |
| Monotherapy | 47.53 (394) | 44.46 (253) | 36.02 (161) | 42.97 (330) | 35.22 (81) |
| Service-connected disability for epilepsy | 14.35 (119) | 23.02 (131) | 25.28 (113) | 19.92 (153) | 17.83 (41) |
| TBI recorded in VA or DoD system | 48.37 (401) | 47.80 (272) | 45.86 (205) | 49.35 (379) | 57.83 (133) |
| Age: | |||||
| 17–29 years | 7.24 (60) | 10.54 (60) | 13.65 (61) | 9.64 (74) | 11.30 (26) |
| 30–39 years | 67.67 (561) | 69.95 (398) | 66.22 (296) | 64.97 (499) | 64.35 (148) |
| 40–45 years | 25.09 (208) | 19.51 (111) | 20.13 (90) | 25.39 (195) | 24.35 (56) |
| Race/ethnicity: | |||||
| White | 61.28 (508) | 64.15 (365) | 53.02 (237) | 55.21 (424) | 60.43 (139) |
| Asian | 1.69 (14) | 1.76 (10) | 1.12 (5) | 1.82 (14) | 1.74 (4) |
| African American/Black | 23.52 (195) | 19.86 (113) | 29.08 (130) | 25.65 (197) | 26.09 (60) |
| Hispanic | 8.69 (72) | 8.08 (46) | 11.86 (53) | 10.94 (84) | 8.26 (19) |
| Native American/Pacific Islander | 2.17 (18) | 3.16 (18) | 1.79 (8) | 2.73 (21) | 1.30 (3) |
| Unknown | 2.65 (22) | 2.99 (17) | 3.13 (14) | 3.65 (28) | 2.17 (5) |
| Married | 41.01 (340) | 37.79 (215) | 38.26 (171) | 43.23 (332) | 36.52 (84) |
| Branch of service: | |||||
| Army | 54.89 (455) | 50.79 (289) | 51.68 (231) | 49.87 (383) | 60.00 (138) |
| Air force | 18.58 (154) | 20.39 (116) | 19.24 (86) | 22.92 (176) | 16.52 (38) |
| Marines | 7.00 (58) | 8.08 (46) | 8.05 (36) | 6.12 (47) | 8.26 (19) |
| Navy/coast guard | 19.30 (160) | 20.74 (118) | 20.58 (92) | 21.09 (162) | 14.78 (34) |
| Unknown | 0.24 (2) | 0.00 (0) | 0.45 (2) | 0.00 (0) | 0.43 (1) |
| Rank: | |||||
| Enlisted | 56.57 (469) | 55.36 (315) | 55.48 (248) | 53.91 (414) | 49.57 (114) |
| Officer/warrant | 2.17 (18) | 2.28 (13) | 2.24 (10) | 3.52 (27) | 3.48 (8) |
| Unknown | 41.25 (342) | 42.36 (241) | 42.28 (189) | 42.58 (327) | 46.96 (108) |
| Component: | |||||
| Guard | 6.27 (52) | 4.92 (28) | 4.70 (21) | 5.08 (39) | 6.52 (15) |
| Reserve | 4.83 (40) | 4.75 (27) | 2.68 (12) | 2.60 (20) | 3.48 (8) |
| Active | 83.84 (695) | 86.99 (495) | 87.02 (389) | 88.41 (679) | 84.78 (195) |
| Unknown | 5.07 (42) | 3.34 (19) | 5.59 (25) | 3.91 (30) | 5.22 (12) |
| Deployment history | 47.65 (395) | 44.82 (255) | 46.76 (209) | 42.97 (330) | 45.65 (105) |
Table II shows the aOR associated with age, race ethnicity, and military characteristics associated with receipt of each ASM. Younger age (17–29 vs. 30–39 years) was associated with significantly higher odds of receiving levetiracetam (LEV) (aOR = 1.5, 95%CI: 1.0–2.3), while Black race was associated with significantly lower odds of receiving lamotrigine (LTG) (aOR = 0.6, 95%CI: 0.5–0.9) compared to Whites. Military characteristics were not associated with ASM use.
TABLE II.
Logistic Regression Age and ethnicity impact on ASM prescribing paterns Demographic and Military Characteristics
| Study groups (aOR-95% CI) | Gabapentin, n = 829 | Lamotrigine, n = 569 | Levetiracetam, n = 447 | Topiramate, n = 768 | Valproate, n = 230 |
|---|---|---|---|---|---|
| Age group (reference group: 30–39) | |||||
| 17–29 vs. 30–39 | 0.7(0.5–1.1) | 0.9(0.6–1.3) | 1.5(1.0–2.3) | 1.3(0.9–1.8) | 1.2(0.7–2.0) |
| 40–45 vs. 30–39 | 1.1(0.9–1.4) | 0.8(0.6–1.0) | 0.8(0.6–1.2) | 1.1(0.8–1.4) | 1.3(0.9–1.8) |
| Race/ethnicity (reference group: White) | |||||
| Asian | 1.1(0.5–2.2) | 0.8(0.4–1.8) | 0.6(0.2–1.8) | 1.6(0.7–3.3) | 1.2(0.4–3.7) |
| Black | 1.0(0.8–1.2) | 0.6(0.5–0.9) | 1.1(0.8–1.5) | 1.1(0.8–1.4) | 1.2(0.8–1.7) |
| Hispanic | 0.8(0.5–1.1) | 0.7(0.5–1.0) | 1.1(0.7–1.7) | 1.2(0.9–1.7) | 0.8(0.5–1.4) |
| Native/Alaskan/PI | 0.9(0.5–1.7) | 1.4(0.7–2.6) | 0.7(0.3–1.7) | 1.6(0.8–2.9) | 0.5(0.1–1.6) |
| Unknown | 0.9(0.5–1.6) | 0.6(0.3–1.2) | 0.9(0.5–1.9) | 1.2(0.7–2.2) | 0.8(0.3–2.2) |
| Branch of service (reference group: army) | |||||
| Air force | 0.9(0.7–1.2) | 0.9(0.7–1.3) | 0.8(0.6–1.1) | 1.3(1.0–1.7) | 0.8(0.6–1.3) |
| Marines | 1.2(0.8–1.7) | 1.0(0.7–1.5) | 0.9(0.6–1.5) | 1.0(0.7–1.5) | 1.1(0.6–1.9) |
| Navy/coast guard | 1.1(0.9–1.5) | 1.1(0.8–1.4) | 0.7(0.5–1.0) | 1.2(0.9–1.6) | 0.7(0.4–1.1) |
| Rank (reference group: officer/warrant officer) | |||||
| Enlisted | 0.8(0.4–1.4) | 0.6(0.3–1.2) | 0.8(0.4–1.7) | 1.7(0.9–3.0) | 1.8(0.8–4.3) |
| Deployed vs. not deployed | 1.2(0.8–1.6) | 0.9(0.6–1.3) | 1.2(0.8–1.8) | 0.9(0.7–1.3) | 1.6(0.9–2.9) |
N =2843.
Abbreviation: aOR, adjusted odds ratios; CI, 95% confidence intervals. The bold numbers represent statistically significant results. N = 2,283.
Clinical characteristics associated with ASM use are provided in Table III. All ASMs were associated with significantly elevated odds of polytherapy. Levetiracetam use was significantly associated with having epilepsy SCD (aOR = 1.5, 95%CI: 1.1–2.0), having neurology care in 2019 (aOR = 2.1, 95%CI: 1.7–2.8), and having neurology care before 2019 (aOR = 1.8, 95%CI: 1.3–2.5).
TABLE III.
Logistic Regression Predicting ASM Prescriptions: Clinical Characteristics
| Study groups (aOR-95% CI) | Gabapentin, n = 829 | Lamotrigine, n = 569 | Levetiracetam, n = 447 | Topiramate, n = 768 | Valproate, n = 230 |
|---|---|---|---|---|---|
| Polytherapy vs. monotherapy | 3.0(2.4–3.7) | 2.9(2.4–3.7) | 4.5(3.5–5.7) | 3.1(2.5–3.8) | 3.1(2.2–4.1) |
| Service connected for epilepsy | 0.7(0.6–0.9) | 1.2(0.9–1.6) | 1.5(1.1–2.0) | 1.1(0.8–1.4) | 0.9(0.6–1.4) |
| Neurology care in 2019 | 0.6(0.5–0.7) | 1.2(0.9–1.4) | 2.1(1.7–2.8) | 1.1(0.8–1.3) | 1.2(0.9–1.7) |
| Neurology care before 2019 | 0.8(0.7–1.0) | 0.9(0.7–1.2) | 1.8(1.3–2.5) | 1.0(0.8–1.3) | 0.9(0.6–1.3) |
| Smoking ever | 1.1(0.9–1.4) | 0.9(0.7–1.1) | 0.8(0.6–1.1) | 0.9(0.8–1.2) | 0.9(0.7–1.3) |
| Comorbidities | |||||
| Anxiety | 1.5(1.1–1.9) | 0.9(0.7–1.2) | 0.8(0.6–1.0) | 1.2(0.9–1.6) | 1.0(0.6–1.5) |
| Depression | 1.5(1.1–2.1) | 0.9(0.6–1.3) | 0.8(0.6–1.2) | 0.9(0.7–1.3) | 0.8(0.5–1.5) |
| Bipolar | 0.7(0.5–0.8) | 2.3(1.8–2.9) | 0.7(0.5–0.9) | 0.9(0.7–1.1) | 1.5(1.1–2.1) |
| Schizophrenia | 0.9(0.5–1.4) | 0.7(0.4–1.2) | 0.5(0.2–1.1) | 1.2(0.7–1.9) | 1.9(1.1–3.3) |
| PTSD | 1.0(0.8–1.2) | 0.9(0.7–1.2) | 0.9(0.7–1.2) | 0.9(0.8–1.3) | 1.3(0.9–1.9) |
| Suicide | 1.2(0.9–1.5) | 0.2(0.9–1.5) | 1.0(0.7–1.4) | 0.8(0.7–1.1) | 1.3(0.9–1.9) |
| Headache | 0.8(0.6–1.1) | 0.7(0.5–0.9) | 0.5(0.3–0.7) | 3.7(2.5–5.4) | 1.4(0.8–2.4) |
| Pain (neck, back) | 1.7(1.3–2.4) | 0.9(0.7–1.3) | 0.7(0.5–1.0) | 0.9(0.7–1.3) | 0.9(0.5–1.4) |
| Brain tumor | 0.5(0.3–1.2) | 0.7(0.3–1.4) | 1.2(0.6–2.6) | 0.9(0.5–1.9) | 0.6(0.2–1.9) |
| Other neurological (not epilepsy) | 1.4(0.9–2.0) | 0.6(0.4–1.0) | 1.2(0.7–1.9) | 0.9(0.6–1.3) | 1.1(0.6–1.9) |
| Stroke | 0.7(0.5–0.9) | 0.9(0.7–1.2) | 1.3(0.9–1.8) | 1.3(0.9–1.7) | 0.9(0.6–1.4) |
| Diabetes | 0.9(0.6–1.2) | 0.8(0.5–1.2) | 1.9(1.2–2.8) | 0.7(0.5–1.0) | 1.0(0.6–1.7) |
| Hypertension | 1.0(0.8–1.3) | 0.9(0.7–1.3) | 1.1(0.8–1.5) | 0.9(0.7–1.1) | 0.8(0.5–1.2) |
| Cardiac | 1.1(0.9–1.4) | 0.8(0.7–1.1) | 1.0(0.7–1.4) | 0.9(0.7–1.1) | 1.2(0.8–1.6) |
N = 2283.
Abbreviation: aOR, adjusted odds ratios; CI, 95% confidence intervals. The bold numbers represent statistically significant results. N = 2,283.
In addition, medical comorbidities played a significant role predicting ASM prescription. Anxiety and depression were more common among VWE receiving gabapentin (GAP), bipolar disorder was more common among VWE receiving VPA and LTG, schizophrenia was more common among VWE receiving VPA, headache was more common among VWE receiving topiramate (TOP), and back pain was more common among those receiving GAP.
DISCUSSION
This study found that, among WVWE those who are of child-bearing potential, approximately 38% received polytherapy, 10% received VPA, and 34% received TOP—medications with evidence of teratogenicity. This important finding demonstrates use of teratogenic ASM and polypharmacy are still commonly used in WWE during childbearing age. The selection of the medication was associated with age, comorbidity, and type of care received, suggesting that these patterns may be modifiable.
The ASM use in pregnancy is associated with MCM to five times that of the general population and is dose dependent with VPA being at ≥1,500 mg or greater.6 Teratogenesis is recognized across multiple, prospective, observational pregnancy registries including the Australian Pregnancy Register, the EURAP: Prospective observational study of pregnancies with antiepileptic drugs, the North American Antiepileptic Drug Pregnancy Registry, and the UK and Ireland Epilepsy Pregnancy Register.7 The most common malformations, include neural tube, cardiac, urogenital, and craniofacial defects, are more frequently associated with VPA.8 Currently, the FDA warns that the use of VPA should be avoided in women of childbearing age unless other medications have failed or are deemed unacceptable.
Notably, the most common malformation associated with barbiturates [e.g., phenobarbital (PB)] and carbamazepine is cardiac, whereas TOP is linked with cleft palate/lip. Valproate is associated with neural tube, cardiac, and urogenital malformations at near equal distribution.9
Data from Medical Expenditure Panel Survey were analyzed between the years 2004 and 2015. This nationally representative analysis of 395,292 women of childbearing age revealed several notable trends in ASM prescription.9–11 For instance, there has been a significant increase in odds of prescription of LEV as well as decrease in odds of prescription of PHT and PB. The prescription of LTG did not significantly change during the study period. This finding is in contrast to an increase in use previously reported during the periods 2009–2013.9 Regardless, LTG was the third most prescribed ASM overall after TPM and CBZ. However, VPA did not significantly change, while TPM change was more sporadic.10
The ASM selection should consider seizure type, epilepsy syndrome, drug interactions, adverse events, and comorbidities. A growing number of ASM are currently available; however, LEV and LTM appear to possess lower risk for both anatomical and behavioral teratogenesis.12 The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study enrolled 351 pregnant women with epilepsy (PWWE), and 105 healthy pregnant women (HPW), most PWWE were on monotherapy (74%), which were primarily LTG (43%) or LEV (37%).13 The study demonstrated no significant differences for fetal loss (2.8% vs. 0%) or MCMs (5.2% vs. 1.9%). Most pregnancies in WWE do not have severe adverse outcomes.14 However, specific monotherapies appear to affect fetal growth with the greatest reduction in birth weight among infants born to PWWE exposed to TOP monotherapy.15 Valproate poses a special risks for malformations16 and cognitive impairment in the fetus.17 This is emphasized by a 2016 Cochrane meta-analysis that showed the malformation prevalence of monotherapy is 1.47% for GAP, 1.77% for LEV, 2.39% for oxcarbazepine, 4.28% for TOP, 4.93% for carbamazepine, 6.26% for phenytoin (PTH), 7.10% for PB, and 10.93% for VPA.18
Our study shows ASM prescribing patterns receiving care at the VA during childbearing age is primarily driven by non-neurologists. An overall of 62.0% (95% CI = 55.2%–67.5%) of adults with active epilepsy visited a neurologist or epilepsy specialist in the past year and 79.8% (95% CI = 73.4%–85.0%) visited a general doctor in the civilian sector over the past year.19
In our study, 37.5% had a neurology or epilepsy specialist visit in 2019, although ∼72.4% had a neurology or epilepsy specialist visit before 2019; 39% of our cohort received polytherapy. Polytherapy has been associated with impaired fertility20 and higher risk of teratogenesis,20 especially when exposed to polytherapy during the first trimester.21,22
The most common ASM prescribed included GAP 29%, TOP 27%, LTG 20%, and LEV 16%. Comorbid diagnosis of headache was a predictor of TOP use, bipolar disorder was associated with prescription of LMT and VPA, and schizophrenia was associated with VPA. These findings are the opposite of what was described in Iraq and Afghanistan war veteran with epilepsy, where multivariable analysis revealed no significant associations between ASM choice except for bipolar disorder.23 This difference may be because of a larger cohort, inclusion of all post-9/11 WVWE, or changes in prescribing that occurred over time.
Contraception can fail with the use of certain ASM, such as TOP at doses of 200 mg or higher, carbamazepine, PTH, and PB that accelerate the hepatic metabolism oral hormonal contraceptives resulting in the contraception failure. It is recommended that these women should use a high-dose contraceptive and use an additional form of contraception to prevent an unwanted pregnancy.24 In addition, the estrogen component of oral contraceptive induces the glucuronidation pathway, which results in a significant reduction of the LTG level causing breakthrough seizures and patients may also experience toxic symptoms during the hormone week-free of the cycle as they may need higher LTG dose. Since, this effect is not present in every WWE, the dose adjustment should be monitored according to the LMT level.
Patients with epilepsy (PWE) have a 2–5 times increased risk of developing any psychiatric disorder, and one in three patients with epilepsy have a lifetime psychiatric diagnosis.25 Psychiatric comorbidities represent a poor prognostic marker as they have been associated with a poor response to treatment (drugs and surgery), increased morbidity, and mortality. Antiseizure medications such as LTG and oxcarbazepine can have a potential beneficial effect on depression treatment, while TOP and PB may play an iatrogenic effect.26 Pregabalin and GAP reduce the severity and psychological symptoms of anxiety.27,28 It is important to select the appropriate ASM with the awareness of dual benefit if possible.
Findings indicated that WWE who received neurology/epilepsy specialty care were more likely to receive LTG, and LEV-ASM was considered safer for women of child-bearing potential.29 These findings support the need of increased awareness and education to family practice doctors who manage a large percentage of these patients. There are several studies containing relatively small samples demonstrating the declining use of ASM with greater teratogenesis,11,30 a recently published nationally representative analysis revealed several new trends of ASM over the past 12 years, with significant increase in odds of prescribing LEV and avoiding PTH and PB.10
We have come a long way improving the care of WWE, but effort and wiliness to advocate for research, education, and access to care need to continue. Our mission is to be a better practitioner and keep up to date with different resources to patients and physicians to improve care.
CONCLUSIONS
WWE should be counseled early and regularly about reproductive health. Practitioners should be familiar with the relevant information and able to discuss the implications of prescribing ASMs. Including potential of interactions of oral contraception, the risk of teratogenesis and neurodevelopment dysfunction with ASM use, and the need to maintain folic acid supplementation.
Our study has several limitations. First, our study focusses on WVWE and such may not be generalizable to the general U.S. population. Second, while we previously validated the use of algorithm for identifying patients with epilepsy with high positive predictive value in VHA data, administrative data do not allow in-depth examination of epilepsy. In addition, our analysis may include individuals with erroneous diagnosis such as psychogenic non-epileptic seizures. Finally, in the study, the newest ASMs such as lacosamide and clobazam were not included in the study.
ACKNOWLEDGMENTS
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA Health Services Research and Development Service (DHI 09-237). The funding agency had no role in data collection, analysis, or manuscript development. The authors acknowledge and appreciate the support from the Veterans Health Care System and the VA Environmental Epidemiology Service in providing the OEF/OIF roster file. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Appendix 1. ICD 9/10 Codes
| Condition | ICD 9 code | ICD 10 code |
|---|---|---|
| Smoking history | V15.82 | Z87.891 |
| Anxiety | 300 | F41.9 |
| Depression | 296.2 | F32.9 |
| Bipolar disorder | 296.7 | F31.9 |
| Schizophrenia | 295.9 | F20.0 |
| Posttraumatic stress disorder | 309.8 | F43.10 |
| Suicide-related behavior | E950–E959 | Z91.5 |
| Headache | 339 | G44.89 |
| Neck or back pain | 723.1 | M47.9 |
| Brain tumor | 191.9 | C71.9 |
| Other neurological conditions (not epilepsy) | 349.9 | G96.9 |
| Stroke/transient ischemic attack | 430.0 | I63.9 |
| Diabetes | 250.0 | E11.9 |
| Hypertension | 796.2 | I10 |
| Cardiovascular conditions | 429.2 | I25.10 |
Contributor Information
Maria Raquel Lopez, VA Epilepsy Centers of Excellence, Miami Veterans Health Care System, Miami, FL 33125, USA; Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL 33125, USA.
Anne C VanCott, VA Pittsburgh Healthcare System, University of Pittsburgh, University Drive C, Pittsburgh, PA 15240, USA; Department of Neurology, University of Pittsburgh, Pittsburgh, PA 15240, USA.
Megan E Amuan, Department of Medicine, Texas A&M Health Science Center, Bryan, TX 78229, USA; Center for Health Quality, Outcomes and Economic Research, Bedford VA Medical Center, Bedford, MA 01730, USA.
Samin Panahi, Division of Epidemiology, Department of Medicine, School of Medicine, University of Utah, Salt Lake City, UT 84132, USA.
Amy Henion, Division of Epidemiology, Department of Medicine, School of Medicine, University of Utah, Salt Lake City, UT 84132, USA.
Mary Jo Pugh, Division of Epidemiology, Department of Medicine, School of Medicine, University of Utah, Salt Lake City, UT 84132, USA; Internal Medicine, Division of Epidemiology, Research Career Scientist VA, Salt Lake City, UT 84132, USA.
FUNDING
This work was supported by the Congressionally Directed Medical Research Program W81XWH-18-1-0247. This work was supported in part by the VA HSR&D Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation (CIN 13-414); Dr Pugh also received support by Health Services Research and Development Service Research Career Scientist Award (IK6HX002608; RCS 17-297).
CONFLICT OF INTEREST STATEMENT
Mary Jo Pugh received support from DHI 09-237 and IIR 11-067, Anne Van Cott receives support from R01 NS081772, while the remaining authors have no conflicts of interest.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author. All data are freely accessible.
CLINICAL CARE REGISTRATION
None declared.
INSTITUTIONAL REVIEW BOARD (HUMAN SUBJECTS)
IRB_00111927 from University of Utah approved the protocol on the federal grant – Phenotypes of Comorbidity in Epilepsy: Variation by TBI Severity and Deployment Status 9.22.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC)
Not applicable.
INSTITUTIONAL CLEARANCE
Not applicable.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
M.A. and S.P. collected and analyzed the data and drafted the original manuscript. M.J. designed this research, reviewed, and edited the manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1. Tian N, Boring M, Kobau R, Zack MM, Croft JBJM: Active epilepsy and seizure control in adults—United States, 2013 and 2015. Morb Mortal Wkly Rep 2018; 67(15): 437–42.doi: 10.15585/mmwr.mm6715a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sullivan-Baca E, Modiano YA, Miller BI, Fadipe M, Van Cott AC, Haneef Z: Characterizing women veterans receiving seizure care in the veterans affairs healthcare system. Epilepsy Res 2022; 180: 106849.doi: 10.1016/j.eplepsyres.2021.106849 [DOI] [PubMed] [Google Scholar]
- 3. Meador KJ, Pennell PB, May RC, et al. : Changes in antiepileptic drug-prescribing patterns in pregnant women with epilepsy. Epilepsy Behav 2018; 84: 10–4.doi: 10.1016/j.yebeh.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christensen J, Grønborg TK, Sørensen MJ, et al. : Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013; 309(16): 1696–703.doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meador KJ, Baker GA, Browning N, et al. : Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013; 12(3): 244–52.doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomson T, Battino D, Perucca E: Teratogenicity of antiepileptic drugs. Curr Opin Neurol 2019; 32(2): 246–52.doi: 10.1097/WCO.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 7. Tomson T, Battino D, Bromley R, et al. : Management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epileptic Disord 2019; 21(6): 497–517.doi: 10.1684/epd.2019.1105. [DOI] [PubMed] [Google Scholar]
- 8. Tomson T, Battino D, Perucca E: Teratogenicity of antiepileptic drugs. Curr Opin Neurol 2019; 32(2): 246–52.doi: 10.1097/wco.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 9. Kim H, Faught E, Thurman DJ, Fishman J, Kalilani L: Antiepileptic drug treatment patterns in women of childbearing age with epilepsy. JAMA Neurol 2019; 76(7): 783–90.doi: 10.1001/jamaneurol.2019.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ajinkya S, Fox J, Lekoubou A: Contemporary trends in antiepileptic drug treatment among women of childbearing age with epilepsy in the United States: 2004–2015. J Neurol Sci 2021; 427: 117500.doi: 10.1016/j.jns.2021.117500. [DOI] [PubMed] [Google Scholar]
- 11. Johannessen Landmark C, Larsson PG, Rytter E, Johannessen SI: Antiepileptic drugs in epilepsy and other disorders—a population-based study of prescriptions. Epilepsy Res 2009; 87(1): 31–9.doi: 10.1016/j.eplepsyres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12. Meador KJ, Loring DW: Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology 2016; 86(3): 297–306.doi: 10.1212/WNL.0000000000002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meador K, Cohen M, Loring D, Brown C, Robalino C, Pennell P: Fetal antiseizure medication effects on neuropsychological outcomes at age 3 years in the MONEAD Study (2165) Neurology. 2021; 96(15 Suppl): 2165. doi: 10.101/jamaneurol.2021,5487. [DOI] [Google Scholar]
- 14. Meador KJ, Pennell PB, May RC, et al. : Fetal loss and malformations in the MONEAD study of pregnant women with epilepsy. Neurology 2020; 94(14): e1502–11.doi: 10.1212/WNL.0000000000008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Marter LJ, Pennell PB, Brown C, et al. : Neonatal outcomes in the MONEAD study of pregnant women with epilepsy. J Pediatr 2021; 7: 100073.doi: 10.1016/j.ympdx.2021.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vajda FJ, O’Brien TJ, Graham JE, Hitchcock AA, Lander CM, Eadie MJJE: Pregnancy after valproate withdrawal—fetal malformations and seizure control. Epilepsia 2020; 61(5): 944–50.doi: 10.1111/epi.16505. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Meador KJ: Epilepsy and pregnancy. Continuum 2022; 28(1): 34–54. doi: 10,1212/CON.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weston J, Bromley R, Jackson CF, et al. : Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev 2016; 11(11): CD010224. doi: 10.1002/14651858CD1024.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kobau R, Sapkota S, Pennell PB, Croft JBJE: Epilepsy by the numbers—from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav 2020; 112: 107348. doi: 10.1016/j.yebeh.2020.107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keni RR, Jose M, Sarma PS, Thomas SVJN: Teratogenicity of antiepileptic dual therapy: dose-dependent, drug-specific, or both? Neurology 2018; 90(9): e790–6.doi: 10.1212/WNL.0000000000005031. [DOI] [PubMed] [Google Scholar]
- 21. Margulis AV, Mitchell AA, Gilboa SM, et al. : Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynecol 2012; 207(5): 405.e1–405.e7.doi: 10.1016/j.ajog.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wyszynski D, Nambisan M, Surve T, Alsdorf R, Smith C, Holmes LJN: Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology 2005; 64(6): 961–5.doi: 10.1212/01.WNL.0000154516.43630.C5. [DOI] [PubMed] [Google Scholar]
- 23. Rohde NN, Baca CB, Van Cott AC, et al. : Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav 2015; 46: 133–9.doi: 10.1016/j.yebeh.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 24. Harden CL, Leppik IJN: Optimizing therapy of seizures in women who use oral contraceptives. Neurology 2006; 67(12 Suppl. 4): S56–8.doi: 10.1212/WNL.67.12_suppl_4.S56. [DOI] [PubMed] [Google Scholar]
- 25. Mula M, Kanner AM, Jette N, Sander JW: Psychiatric comorbidities in people with epilepsy. Neurol Clin Pract 2021; 11(2): e112–20.doi: 10.1212/CPJ.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanner AM: Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry 2003; 54(3): 388–98.doi: 10.1016/S0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- 27. Baldwin DS, Ajel K, Masdrakis VG, Nowak M, Rafiq R: Pregabalin for the treatment of generalized anxiety disorder: an update. Neuropsychiatr Dis Treat 2013; 9: 883–92.doi: 10.2147/NDT.S36453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pollack MH, Matthews J, Scott EL: Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry 1998; 155(7): 992–3.doi: 10.1176/ajp.155.7.992. [DOI] [PubMed] [Google Scholar]
- 29. Harden CL, Meador KJ, Pennell PB, et al. : Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy ofNeurology and American Epilepsy Society. Neurology 2009; 73(2): 133–41.doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ackers R, Besag FM, Wade A, Murray ML, Wong IC: Changing trends in antiepileptic drug prescribing in girls of child-bearing potential. Arch Dis Child 2009; 94(6): 443–7.doi: 10.1136/adc.2008.144386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. All data are freely accessible.


