Extract
Bronchiectasis is a chronic inflammatory disease [1, 2]. Although it is recognised that bronchiectasis is composed of multiple phenotypes and endotypes, inflammation has classically been regarded as neutrophilic and patients with higher levels of neutrophilic inflammation have been shown to have worse clinical outcomes [3–5].
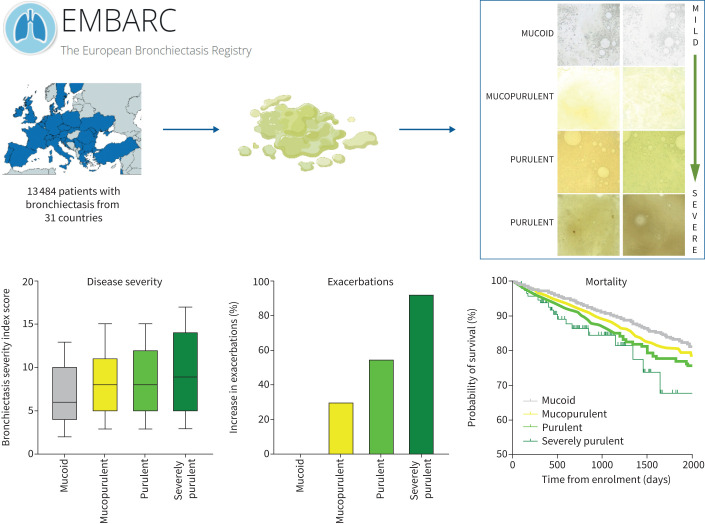
Graphical abstract
We enrolled 13 484 patients with bronchiectasis from 31 countries. Assessment of sputum colour at baseline was used to investigate the relationship with disease severity and outcomes. We show a strong relationship between sputum colour and exacerbations and hospitalisation for severe exacerbations. Increasing sputum purulence is a marker of disease outcome in bronchiectasis.
Abstract
Background
A validated 4-point sputum colour chart can be used to objectively evaluate the levels of airway inflammation in bronchiectasis patients. In the European Bronchiectasis Registry (EMBARC), we tested whether sputum colour would be associated with disease severity and clinical outcomes.
Methods
We used a prospective, observational registry of adults with bronchiectasis conducted in 31 countries. Patients who did not produce spontaneous sputum were excluded from the analysis. The Murray sputum colour chart was used at baseline and at follow-up visits. Key outcomes were frequency of exacerbations, hospitalisations for severe exacerbations and mortality during up to 5-year follow-up.
Results
13 484 patients were included in the analysis. More purulent sputum was associated with lower forced expiratory volume in 1 s (FEV1), worse quality of life, greater bacterial infection and a higher bronchiectasis severity index. Sputum colour was strongly associated with the risk of future exacerbations during follow-up. Compared to patients with mucoid sputum (reference group), patients with mucopurulent sputum experienced significantly more exacerbations (incident rate ratio (IRR) 1.29, 95% CI 1.22–1.38; p<0.0001), while the rates were even higher for patients with purulent (IRR 1.55, 95% CI 1.44–1.67; p<0.0001) and severely purulent sputum (IRR 1.91, 95% CI 1.52–2.39; p<0.0001). Hospitalisations for severe exacerbations were also associated with increasing sputum colour with rate ratios, compared to patients with mucoid sputum, of 1.41 (95% CI 1.29–1.56; p<0.0001), 1.98 (95% CI 1.77–2.21; p<0.0001) and 3.05 (95% CI 2.25–4.14; p<0.0001) for mucopurulent, purulent and severely purulent sputum, respectively. Mortality was significantly increased with increasing sputum purulence, hazard ratio 1.12 (95% CI 1.01–1.24; p=0.027), for each increment in sputum purulence.
Conclusion
Sputum colour is a simple marker of disease severity and future risk of exacerbations, severe exacerbations and mortality in patients with bronchiectasis.
Shareable abstract
Sputum colour is a simple non-invasive marker of airway inflammation that identifies patients with bronchiectasis at higher risk of exacerbation, hospitalisation and mortality https://bit.ly/3HczGxO
Introduction
Bronchiectasis is a chronic inflammatory disease [1, 2]. Although it is recognised that bronchiectasis is composed of multiple phenotypes and endotypes, inflammation has classically been regarded as neutrophilic and patients with higher levels of neutrophilic inflammation have been shown to have worse clinical outcomes [3–5].
Although there are composite severity and prognostic assessment tools for bronchiectasis, there are currently no direct measures of inflammation [5–7]. Direct measurement of neutrophilic inflammation is currently only possible in the research sphere and is difficult to implement in clinical practice [4, 8].
Therefore the most accessible measure of neutrophilic inflammation in bronchiectasis is thought to be the assessment of sputum colour [9, 10]. The green colour associated with sputum purulence in chronic inflammatory lung diseases is thought to result from accumulation of myeloperoxidase (MPO) released from granulocytes. The increase of sputum purulence therefore provides a non-invasive assessment of the extent of neutrophilic inflammation [11, 12].
Since colour perception is subjective, the assessment of sputum colour in clinical practice requires to be standardised. In 2009, Murray et al. [9] developed a sputum colour chart for bronchiectasis patients based on sputum photographs which classifies samples into four groups. This study demonstrated an association between sputum colour and the presence of bacterial infection [9]. Goeminne et al. [10] extended these findings, demonstrating that neutrophilic inflammation and protease activity in sputum was directly related to sputum colour using this method. Existing data have, however, been derived from single-centre studies with small sample sizes. Prior studies did not investigate a possible correlation between the sputum colour chart and patients’ clinical outcomes. Prior studies are therefore insufficient to establish that sputum colour assessment could be used for patient phenotyping in routine clinical practice.
To investigate the value of sputum colour assessment in identifying positive airway bacterial cultures, disease severity and risk of future exacerbations, we incorporated baseline and annual assessment of sputum colour using a standardised sputum colour chart into the data collected as part of the European Bronchiectasis Registry (EMBARC) [13]. We tested whether a single assessment of sputum colour could identify the risk of airway infection, severity of disease and risk of future exacerbations.
Methods
EMBARC is a prospective observational study of patients with computed tomography (CT)-confirmed bronchiectasis conducted across more than 30 countries worldwide [13, 14]. The registry includes European Union (EU) countries, non-EU European countries and several non-European countries (Israel, India, Kyrgyzstan and Pakistan) [14, 15]. The study is approved by the ethical committee in the host country (UK) and by institutional review boards or ethics committees in all countries and regions in which the study is conducted. A detailed protocol of the study and baseline patient characteristics have been previously published [13, 14].
Data collection
Patient enrolment commenced in January 2015 and recruitment is open-ended and ongoing. Patients enrolled up to April 2022 were included for the purposes of this analysis. Patient data were collected annually using a standardised case report form. Comprehensive clinical data incorporating demographics, comorbidities, medications, aetiological testing, microbiology, radiology, lung function and disease history were recorded. Spirometry was performed according to American Thoracic Society/European Respiratory Society standards [16]. Aetiology was recorded by the investigators and verified using data on results of aetiological testing. Microbiology data from clinically indicated sputum samples sent during clinical stability and exacerbation were collected and patients classified according to whether they had specific bacteria isolated in any sample in the 12 months prior to the baseline visit. Radiological severity was evaluated in the patient's most recent CT scan using the modified Reiff score as previously described [17]. Disease severity was evaluated using the bronchiectasis severity index (BSI) with a sensitivity analysis performed using the FACED tool [7]. Exacerbations were defined as use of antibiotics for acute respiratory symptoms and were recorded from a combination of patient history, hospital and prescription records [18]. Severe exacerbations were defined as exacerbations requiring hospitalisation. Symptoms were evaluated using the Quality of Life Questionnaire-Bronchiectasis (QOL-B) version 3.1 using validated translations [19, 20].
Sputum colour assessment
Baseline and annual follow-up visits were performed during clinical stability. Sputum samples from these visits were used for sputum colour assessment by either the physician or patient using the chart developed by Murray et al. [9] (figure 1). The study user guide asked the physicians to make an assessment of sputum colour on a fresh sputum sample wherever possible. If this was not possible, patients were asked to report the most common/typical colour of their sputum during stable state (away from both exacerbations and antibiotic courses). The reliability of this scoring system for assessment by both patients and physicians was established in the original validation study with an intraclass correlation coefficient of 0.83. The online data collection tool provided the sputum colour chart (figure 1) to allow scoring by investigators at sites. A numerical scoring system from 1 (mucoid) to 4 (severely purulent) was used as originally described.
FIGURE 1.
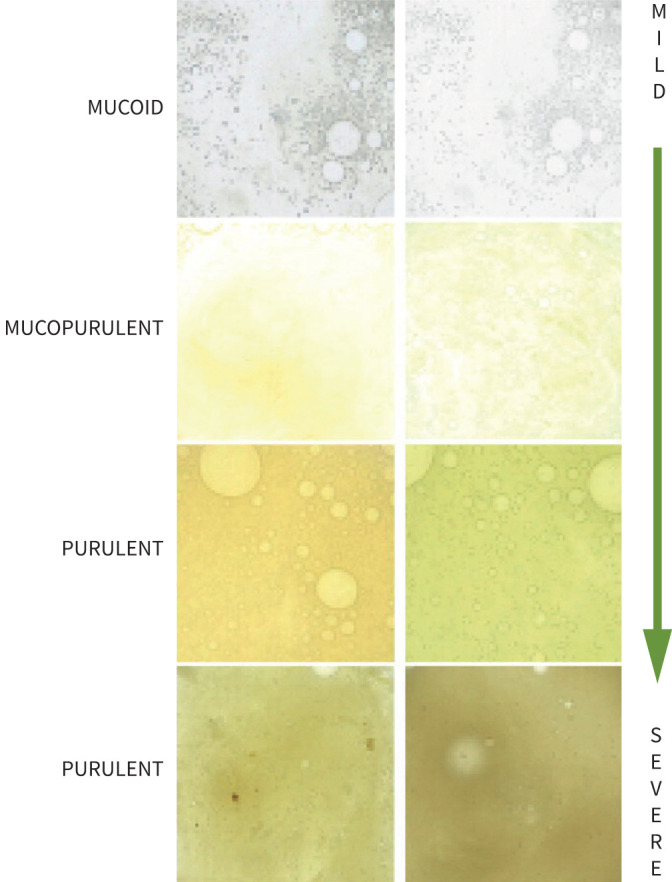
The Murray sputum colour chart. Reproduced from [9] with permission.
For the purposes of this analysis any patient unable to produce sputum at baseline was excluded, but data from this subgroup are shown for comparison.
Long-term clinical outcomes
Data are collected for up to 5 years on an annual basis for calculation of clinical outcomes. Since patient enrolment began in 2015, patients have up to 5 years of follow-up at the time of writing, although the dataset includes patients enrolled through to 2021/2022 who have not yet had a follow-up visit. Statistical analysis of relevant end-points takes account of the duration of follow-up. Relevant clinical outcomes were survival, exacerbation frequency and risk of hospitalisation due to severe exacerbations.
Statistical analysis
Summary data are presented as median (interquartile range (IQR)). Comparisons of more than two groups were performed by the Kruskal–Wallis test. Exacerbation frequency and frequency of severe exacerbations requiring hospital admission over time were studied using a negative binomial model with time in study as an offset. Survival analysis was performed using Cox proportional hazards regression [14]. The proportional hazards assumption was assessed with log–log plots.
We observed a strong relationship between sputum colour and Pseudomonas aeruginosa infection and so present adjusted analyses of these models with P. aeruginosa infection included as a covariate. In addition, an analysis was performed adjusting the negative binomial model for prior exacerbations to determine if sputum colour was associated with exacerbation risk after adjusting for prior exacerbation history. All analyses utilised SPSS version 27 (IBM, Armonk, NY, USA) or Prism version 9 (GraphPad, Boston, MA, USA).
Results
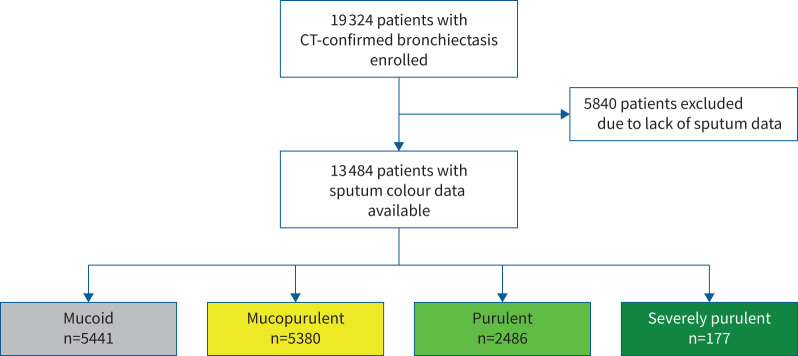
19 324 patients were enrolled from 31 countries. 13 484 patients reported daily sputum expectoration, had a sputum colour grade using the Murray chart performed and were, therefore, included in the analysis. This is shown in figure 2; a detailed description of the cohort is reported elsewhere [14]. Table 1 shows the characteristics of the patients at enrolment according to sputum colour at baseline (visit 1) including the characteristics of patients excluded as they did not regularly produce sputum.
FIGURE 2.
Flow of patients through the study. CT: computed tomography.
TABLE 1.
Patient characteristics according to baseline sputum colour#
|
Grade 1
(mucoid) |
Grade 2
(mucopurulent) |
Grade 3
(purulent) |
Grade 4
(severely purulent) |
Non-sputum-producing patients | |
| Patients | 5441 (28.2) | 5380 (27.8) | 2486 (12.9) | 177 (0.9) | 5840 (30.2) |
| Demographics | |||||
| Age (years) | 66 (55–74) | 66 (55–74) | 66 (56–73) | 67 (57–75) | 65 (53–73) |
| Female | 3039 (55.9) | 3123 (58.0) | 1425 (57.3) | 97 (54.8) | 3712 (63.6) |
| BMI (kg·m−2) | 24.6 (21.4–28.4) | 24.4 (21.4–28.2) | 24.2 (21.0–28.0) | 23.7 (20.7–28.5) | 24.4 (21.4–28.3) |
| Never-smoker | 3038 (55.8) | 3000 (55.8) | 1367 (55.0) | 89 (50.3) | 3325 (56.9) |
| Ex-smoker | 2031 (37.3) | 2052 (38.1) | 989 (39.8) | 77 (43.5) | 2156 (36.9) |
| Current smoker | 372 (6.8) | 328 (6.1) | 130 (5.2) | 11 (6.2) | 359 (6.1) |
| Comorbidity | |||||
| Cardiovascular disorders | 1685 (31.0) | 1729 (32.1) | 773 (31.1) | 64 (36.2) | 1672 (28.6) |
| Stroke | 169 (3.1) | 185 (3.4) | 73 (2.9) | 11 (6.2) | 171 (2.9) |
| Diabetes | 593 (10.9) | 573 (10.7) | 261 (10.5) | 22 (12.4) | 595 (10.2) |
| Liver disease | 36 (0.7) | 24 (0.4) | 16 (0.6) | 3 (1.7) | 43 (0.7) |
| Chronic renal failure | 181 (3.3) | 184 (3.4) | 102 (4.1) | 9 (5.1) | 218 (3.7) |
| COPD | 1428 (26.2) | 1450 (27.0) | 712 (28.6) | 49 (27.7) | 1243 (21.3) |
| Asthma | 1665 (30.6) | 1666 (31.0) | 732 (29.4) | 46 (26.0) | 1655 (28.3) |
| Osteoporosis | 649 (11.9) | 713 (13.3) | 337 (13.6) | 30 (16.9) | 634 (10.9) |
| Depression | 654 (12.0) | 763 (14.2) | 392 (15.8) | 48 (27.1) | 617 (10.6) |
| Solid tumour | 515 (9.5) | 530 (9.9) | 270 (10.9) | 19 (10.7) | 547 (9.4) |
| Aetiology | |||||
| Idiopathic | 1934 (35.5) | 1964 (36.5) | 876 (35.2) | 66 (37.3) | 2117 (36.3) |
| Post-infective | 1173 (21.6) | 1193 (22.2) | 571 (23.0) | 29 (16.4) | 1200 (20.5) |
| Tuberculosis | 538 (9.9) | 396 (7.4) | 149 (6.0) | 10 (5.6) | 533 (9.1) |
| Immunodeficiency | 177 (3.3) | 201 (3.7) | 110 (4.4) | 4 (2.3) | 214 (3.7) |
| ABPA | 179 (3.3) | 177 (3.3) | 79 (3.2) | 7 (4.0) | 226 (3.9) |
| Rheumatoid arthritis | 112 (2.1) | 159 (3.0) | 63 (2.5) | 8 (4.5) | 148 (2.5) |
| Others | 1328 (24.4) | 1290 (24.0) | 638 (25.7) | 53 (29.9) | 1402 (24.0) |
| Clinical status | |||||
| Sputum volume (mL·day−1) | 10 (5–20) | 15 (10–30) | 20 (10–50) | 30 (10–66) | 0 (0–0) |
| mMRC dyspnoea score¶ | |||||
| 0 | 1425 (26.2) | 1069 (19.9) | 416 (16.7) | 34 (19.2) | 1864 (31.9) |
| 1 | 1776 (32.6) | 1727 (32.1) | 759 (30.5) | 42 (23.7) | 1945 (33.3) |
| 2 | 1188 (21.8) | 1343 (25.0) | 595 (23.9) | 34 (19.2) | 1172 (20.1) |
| 3 | 710 (13.0) | 859 (16.0) | 457 (18.4) | 37 (20.9) | 560 (9.6) |
| 4 | 279 (5.1) | 338 (6.3) | 219 (8.8) | 30 (16.9) | 207 (3.5) |
| Treatment | |||||
| Long-term macrolide treatment | 768 (14.1) | 995 (18.5) | 547 (22.0) | 36 (20.3) | 733 (12.6) |
| Other long-term oral antibiotic treatment | 228 (4.2) | 292 (5.4) | 202 (8.1) | 13 (7.3) | 196 (3.4) |
| Inhaled antibiotic treatment | 313 (5.8) | 523 (9.7) | 357 (14.4) | 31 (17.5) | 167 (2.9) |
| Inhaled corticosteroids | 2900 (53.3) | 3008 (55.9) | 1376 (55.3) | 86 (48.6) | 2739 (46.9) |
Data are presented as n (%) or median (interquartile range). BMI: body mass index; ABPA: allergic bronchopulmonary aspergillosis; mMRC modified Medical Research Council. #: all variables measured at baseline visit. ¶: totals for mMRC dyspnoea score do not add up to 100% due to missing data.
Patients with more purulent sputum have a higher daily sputum volume, a higher modified Medical Research Council dyspnoea score, and a greater likelihood of prescription of macrolides, long-term antibiotics and inhaled antibiotics. No strong relationship was found between sputum colour and age, smoking status, or the presence of asthma or COPD as underlying conditions (table 1). Supplementary table S1 shows the relationships with aetiology, where the most common aetiologies did not have a greater odds of having purulent sputum compared to idiopathic bronchiectasis. Patients with primary ciliary dyskinesia or immunodeficiency had significantly higher odds of purulent sputum (supplementary table S1).
Relationship between sputum colour and severity of disease
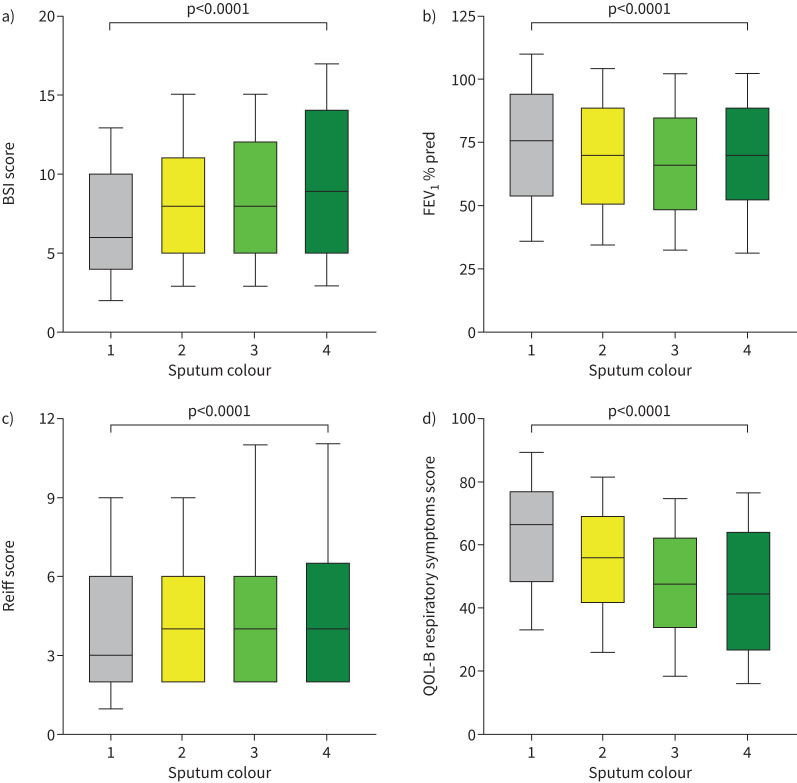
Figure 3 shows the association between sputum colour and cross-sectional markers of bronchiectasis disease severity using the BSI. The BSI increased with increasing sputum colour from median (IQR) 6 (4–10) in patients with mucoid sputum to 8 (5–11) in patients with mucopurulent sputum, 8 (5–12) in patients with purulent sputum and 9 (5–14) in patients with severely purulent sputum. Similar results were seen with the FACED score (supplementary figure S1). Examining severity groups, we included 12 229 patients with complete data for analysis. Severe bronchiectasis classified by the BSI was observed in 1531 (31.5%) patients with mucoid sputum, 1992 (41.0%) patients with mucopurulent sputum, 1091 (46.3%) patients with purulent sputum and 81 (51.3%) patients with severely purulent sputum (supplementary table S2). Differences in disease severity were all statistically significant (p<0.0001).
FIGURE 3.
Disease severity and its relationship with sputum colour: a) bronchiectasis severity index (BSI) score, b) forced expiratory volume in 1 s (FEV1) percentage predicted, c) Reiff score and d) Quality of Life Questionnaire-Bronchiectasis (QOL-B) respiratory symptoms score. Sputum colour is graded as 1=mucoid, 2=mucopurulent, 3=purulent and 4=severely purulent. Box plots show median and interquartile range with whiskers at the 10–90th percentiles.
Forced expiratory volume in 1 s (FEV1) percentage predicted was significantly lower in patients with more purulent sputum, with median values of 75.7%, 70.2%, 65.9% and 70.4% (p<0.0001) for mucoid, mucopurulent, purulent and severely purulent sputum, respectively (figure 3b). There were less obvious differences in radiological severity, with a median Reiff radiological severity score of 3 in patients with mucoid sputum and 4 in patients with mucopurulent to severely purulent sputum (p<0.0001) (figure 3c). The median (IQR) values of the QOL-B respiratory symptoms score were also strongly related to sputum colour, with patients with mucoid sputum having the best quality of life (66.7 (48.1–77.8)), followed by patients with mucopurulent sputum (55.6 (40.7–70.4)), purulent sputum (48.1 (33.3–63.0)) and severely purulent sputum (44.4 (25.9–64.9)) (p<0.0001) (figure 3d). Patients with purulent sputum also reported a larger daily sputum volume. Median (IQR) sputum volume was 10 (5–20) mL in patients with mucoid sputum and 15 (10–30), 20 (10–50) and 30 (10–66) mL in patients with mucopurulent, purulent and severely purulent sputum, respectively.
Relationship between sputum colour and sputum culture
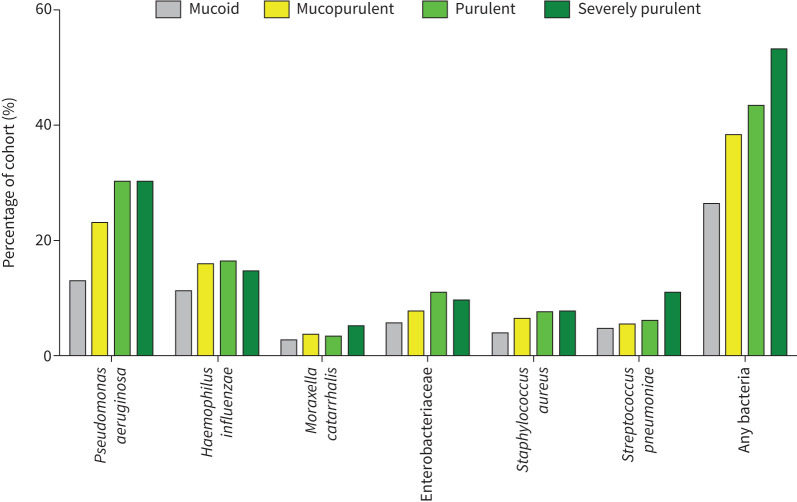
12 229 patients with complete data were included in this analysis. There was an increase in the percentage of patients with sputum samples positive for bacteria as sputum purulence increased. Figure 4 shows the frequency of the most common bacteria isolated in sputum at baseline. The frequency of additional pathogens is shown in supplementary table S3. 1279 (26.3%) patients with mucoid sputum had a positive sputum for bacteria compared to 1863 (38.4%) with mucopurulent sputum, 1026 (43.5%) with purulent sputum and 84 (53.2%) with severely purulent sputum (p<0.0001). Approximately a third of patients with purulent sputum and severely purulent sputum had isolation of P. aeruginosa. The EMBARC registry includes a data field for chronic P. aeruginosa infection and this also showed a clear increase with increasing sputum purulence, from 797 (16.4%) in patients with mucoid sputum to 57 (36.1%) in patients with severely purulent sputum (p<0.0001).
FIGURE 4.
Percentage of all patients with positive sputum cultures for selected respiratory pathogens or any bacteria at baseline.
Significant differences were observed across sputum purulence categories for P. aeruginosa (p<0.0001), Haemophilus influenzae (p<0.0001), Moraxella catarrhalis (p=0.01), Enterobacteriaceae (p<0.0001), Staphylococcus aureus (p<0.0001) and Streptococcus pneumoniae (p<0.0001). Aspergillus species were observed in 110 (2.3%) patients with mucoid sputum, 129 (2.7%) with mucopurulent sputum, 98 (4.2%) with purulent sputum and five (3.2%) with severely purulent sputum (p<0.0001).
Relationship between sputum colour and exacerbations in the previous year
In the 12 months prior to the baseline visit there were 33 724 exacerbations in 12 229 patients with a valid sputum colour measurement and 6298 severe exacerbations requiring hospitalisation. The median (IQR) exacerbation frequency was 2 (1–4) per year.
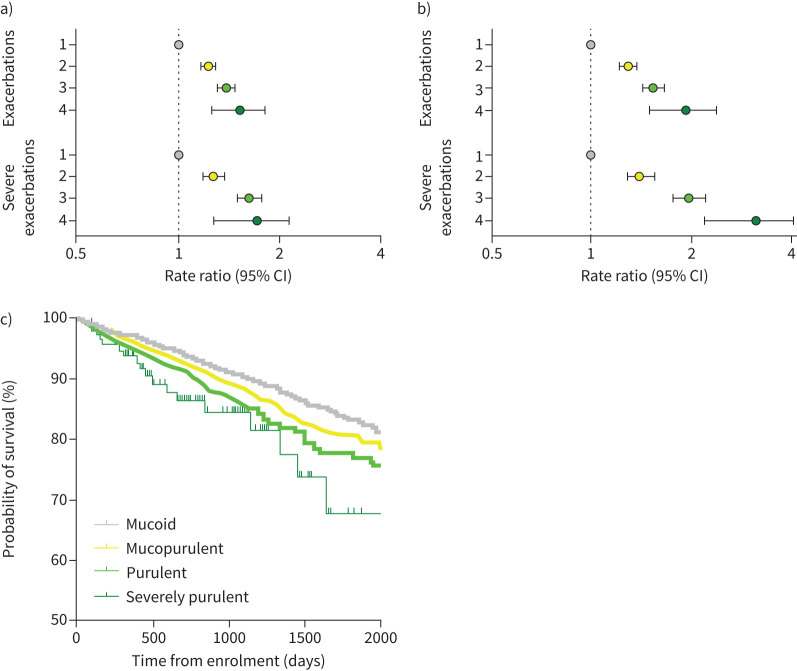
Compared to patients with mucoid sputum (reference group), exacerbation rates were increased with increasing sputum purulence, with rate ratios of 1.23 (95% CI 1.17–1.29), 1.38 (95% CI 1.31–1.47) and 1.51 (95% CI 1.26–1.81) for mucopurulent, purulent and severely purulent sputum, respectively. For severe exacerbations the relationship was even stronger, with rate ratios of 1.28 (95% CI 1.19–1.37), 1.63 (95% CI 1.50–1.77) and 1.68 (95% CI 1.31–2.15) for patients with mucopurulent, purulent and severely purulent sputum, respectively. This is shown in figure 5a.
FIGURE 5.
a, b) Forest plots of the frequency of exacerbations and severe exacerbations across the four sputum colour groups (1=mucoid sputum (reference group), 2=mucopurulent sputum, 3=purulent sputum and 4=severely purulent sputum): a) in the previous year and b) during follow-up. c) Kaplan–Meier survival curve.
At baseline, across the whole cohort, 2579 (21.1%) patients had no exacerbations, 2242 (18.3%) had 1 exacerbation per year, 2138 (17.5%) patients had 2 exacerbations per year and 5270 (43.1%) had ≥3 exacerbations per year. The proportion of patients with ≥3 exacerbations per year markedly increased with increasing sputum purulence from 36.0% (n=1751) in patients with mucoid sputum to 45.8% (n=2225) in patients with mucopurulent sputum, 51.0% (n=1202) in patients with purulent sputum and 58.2% (n=92) in patients with severely purulent sputum (p<0.0001).
Sputum colour and long-term clinical outcomes
Long-term clinical outcomes were evaluated over a cumulative total of 29 830 years of patient follow-up (range 0–5 years per patient).
Sputum colour was strongly associated with the risk of future exacerbations. Compared to patients with mucoid sputum (reference group), patients with mucopurulent sputum experienced significantly more exacerbations (incident rate ratio (IRR) 1.29, 95% CI 1.22–1.38; p<0.0001), while the rates were even higher for patients with purulent sputum (IRR 1.55, 95% CI 1.44–1.67; p<0.0001) and severely purulent sputum (IRR 1.91, 95% CI 1.52–2.39; p<0.0001) (figure 5b). The relationship between sputum colour and exacerbations was evaluated in subgroups of patients based on disease severity using the BSI, P. aeruginosa infection status, baseline FEV1 and prior exacerbation history, showing clear associations between increasing sputum purulence and increasing exacerbations independent on these other criteria (supplementary table S4).
For hospitalisation, increasing sputum colour was also associated with worse clinical outcomes with rate ratios (compared to patients with mucoid sputum) of 1.41 (95% CI 1.29–1.56; p<0.0001), 1.98 (95% CI 1.77–2.21; p<0.0001) and 3.05 (95% CI 2.25–4.14; p<0.0001) for patients with mucopurulent, purulent and severely purulent sputum, respectively (figure 5b).
We performed a number of sensitivity analyses. Sputum colour remained strongly associated with future exacerbations even after adjustment for prior exacerbations. Compared to the reference group (mucoid sputum) the adjusted rate ratios for exacerbation frequency were 1.21 (95% CI 1.14–1.29), 1.31 (95% CI 1.21–1.42) and 1.68 (95% CI 1.33–2.11) for mucopurulent, purulent and severely purulent sputum, respectively. Sputum colour was also clearly associated with future exacerbation risk in patients using long-term macrolide or inhaled antibiotics at baseline (supplementary table S4). Since P. aeruginosa is a strong predictor of future hospitalisation and sputum purulence was strongly associated with P. aeruginosa infection, an analysis was conducted adjusting for P. aeruginosa in the negative binomial model. This shows rate ratios of 1.20 (95% CI 1.10–1.30), 1.53 (95% CI 1.39–1.68) and 1.74 (95% CI 1.33–2.29) for mucopurulent, purulent and severely purulent sputum, respectively. In an additional analysis, sputum colour was also associated with a shorter time to the first severe exacerbation following baseline (supplementary table S5).
There were 582 deaths during follow-up. Mortality was significantly increased with increasing sputum purulence (hazard ratio 1.12, 95% CI 1.01–1.24; p=0.027), indicating a 12% increased risk of death for each 1-point increase in sputum purulence. For patients with purulent sputum at baseline compared to patients with mucoid sputum the corresponding hazard ratios were 1.02 (95% CI 0.85–1.23) for mucopurulent sputum, 1.26 (95% CI 1.01–1.57; p=0.039) for purulent sputum and 1.60 (95% CI 0.90–2.86; p=0.11) for severely purulent sputum. The Kaplan–Meier survival curve is shown in figure 5c.
Visit-to-visit repeatability of sputum colour
7632 patients were included in the analysis of repeated sputum colour assessment over time, on the basis of having at least two assessments 12 months apart. Sputum colour showed a degree of stability from visit to visit in the stable state within individuals. We analysed the levels of agreement between baseline and year 1 sputum colour assessments within individuals. Of those with mucoid sputum at baseline, 89.3% of patients had mucoid sputum at their next follow-up visit. The corresponding figures were 88.2% for mucopurulent sputum at both visits, 78.6% for purulent sputum and 79.7% for severely purulent sputum. The Sankey diagram is shown in supplementary figure S2.
Discussion
Bronchiectasis is a chronic inflammatory disorder [10]. Airway inflammation in bronchiectasis is a key component of the vicious vortex of disease and is believed to play a key role in disease progression [21]. Sputum colour represents a potentially clinically useful, simple and non-invasive marker of airway inflammation [9, 10]. Our study shows that sputum colour as determined by a validated sputum colour chart is strongly associated with disease severity measured by the BSI, lower FEV1, worse quality of life and greater radiological severity. Patients with purulent sputum are more likely to have positive sputum cultures for multiple bacteria and particularly P. aeruginosa, an organism that is known to be associated with greater risk of hospitalisation, mortality and FEV1 decline [22]. Patients with greater sputum purulence experience more frequent exacerbations and a higher risk of severe exacerbations during follow-up. Importantly, this increased risk is seen even when patients are matched for severity of disease or prior exacerbation history. When we examined subgroups of patients defined by their prior exacerbation history (0, 1, 2 or ≥3 exacerbations in the previous year), patients with more purulent sputum still experienced more exacerbations. Similar results were observed for severe exacerbation risk. Finally, survival was also lower for patients with increased sputum purulence. Taken together our results suggest that sputum purulence can be used as a relatively simple risk stratification tool in clinical practice to identify patients at greater risk of severe disease, positive sputum cultures and future exacerbations.
Our study is observational and it cannot prove that any of these relationships are causal. It is unlikely that the presence of purulent sputum, in itself, is causing any of these poor outcomes. It is most likely that purulent sputum is a reflection of underlying lung inflammation and that this inflammation directly contributes to lung injury, while the resulting exacerbations contribute to disease progression [12, 23]. Sputum colour therefore represents a potentially clinically useful and non-invasive marker of airway inflammation.
Previous work has shown that sputum purulence reflects the presence of neutrophilic inflammation [11, 12]. MPO, an enzyme contained within the azurophilic granules of neutrophils, is responsible for the green colour in purulent sputum. MPO itself generates the toxic metabolite hypochlorite which contributes to oxidative stress and inflammation in the airways [24]. MPO is a key component of neutrophil extracellular traps (NETs) which are believed to be the dominant mechanism of neutrophilic inflammation in the bronchiectasis airway and which have also been linked with disease severity and future risk of exacerbations [12, 25]. NETs release multiple granule and cytoplasmic proteins associated with DNA and histones which together amplify lung inflammation and protease-mediated degradation of lung tissue [12]. Neutrophil elastase, which has been strongly linked to disease progression in bronchiectasis, is released from the same granules as MPO and increases with increasing sputum purulence [3, 8]. The relationships between neutrophilic inflammation and exacerbations and survival seen in this study mirror those seen with laboratory assays of neutrophilic inflammation such as elastase and NETs, and confirm, in a much larger cohort, that neutrophilic inflammation is a key prognostic marker in bronchiectasis.
Recognition that bronchiectasis is an inflammatory disease has led to the development of novel anti-inflammatory treatment strategies including dipeptidyl peptidase-1 inhibition, a treatment that reduces neutrophilic inflammation by blocking neutrophil serine protease activation in the bone marrow [26]. This was shown to prolong the time to first exacerbation in a phase 2 trial [26]. A phase 3 trial is ongoing. In that trial, sputum purulence was used as an inclusion criteria in an effort to enrol patients with significant neutrophilic inflammation and a higher risk of exacerbations. Our data would support that sputum colour can be used as such a marker for trials and may in future be useful to identify patients for anti-inflammatory treatment.
There is a strong relationship between sputum purulence and airway infection, which is the major driver of neutrophil recruitment to the airway, and this was clearly shown in our data where positive sputum cultures greatly increased as sputum purulence increased. Given this relationship, sputum purulence may be a promising biomarker to identify patients that would respond to antibiotic treatment. In an analysis of the AIR-BX trials of inhaled aztreonam, sputum purulence was significantly reduced during the on-cycles of treatment [27]. More importantly, patients with purulent sputum (identified on self-reported questionnaire) had a significant improvement in quality of life on treatment, while patients without sputum purulence did not [27]. Conversely, patients without sputum purulence had a significantly increased frequency of exacerbations and adverse events with inhaled antibiotic treatment [27]. This suggests that antibiotic treatment is most likely to be effective in patients with higher levels of neutrophilic inflammation and bacterial load, a concept now supported by several studies in bronchiectasis [28–31].
Sputum colour identifies patients at increased risk but it is clear from our data that patients with mucoid sputum can still experience exacerbations, including severe exacerbations, and may have severe bronchiectasis. It is increasingly recognised that bronchiectasis is a heterogeneous disease with multiple endotypes [21]. Endotyping of this subgroup of patients without clear neutrophilic inflammation is important. Recent data suggest around 20% of bronchiectasis patients without asthma have eosinophilic inflammation, which would not be expected to produce sputum purulence and may therefore explain some of the burden of disease in the subgroup [28, 32–34]. Other treatable traits are likely to be identified in this subgroup through ongoing research into inflammatory endotypes [35].
Our study has unique strengths, including the very large sample size, systematic collection of data and inclusion of a large number of countries. Limitations of this analysis include the real-life nature of the data collection. Although the sputum colour chart was provided to all sites through the online data collection system, monitoring how this was used across so many sites is not feasible and variation in practice is inevitable. Sputum characteristics may vary by time of day, or depending on airway clearance techniques or other interventions [36]. Sputum cultures were not mandatory and so the analysis of sputum bacteria is limited by whether or not patients had sputum cultures performed for clinical reasons. The frequency of bacteria in our study therefore likely underestimates the true prevalence of pathogens. Other sputum parameters such as plugging on CT and rheology parameters such as viscosity may provide additional information but were not evaluated in this study.
In summary we show that sputum colour is a clinically useful non-invasive inflammatory biomarker that is associated with disease severity and identifies patients at risk of future exacerbations.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01554-2023.Supplement (373.4KB, pdf)
Shareable PDF
Footnotes
Conflict of interest: S. Aliberti reports grants or contracts from Insmed Incorporated, Chiesi, Fisher & Paykel and GlaxoSmithKline, royalties or licences from McGraw Hill, consulting fees from Insmed Incorporated, Insmed Italy, Insmed Ireland Ltd, Zambon SpA, AstraZeneca UK Ltd, AstraZeneca Pharmaceutical LP, CSL Behring GmbH, Grifols, Fondazione Internazionale Menarini, Moderna, Chiesi, MCD Italia Srl, Brahms, PhysioAssist SAS and GlaxoSmithKline SpA, payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline SpA, Thermofisher Scientific, Insmed Italy, Insmed Ireland, Zambon and Fondazione Internazionale Menarini, and participation on a data safety monitoring board or advisory board for Insmed Incorporated, Insmed Italy, AstraZeneca UK Ltd and MSD Italia Srl. F.C. Ringshausen reports grants or contracts from the German Center for Lung Research (DZL), German Center for Infection Research (DZIF), IMI (EU/EFPIA), iABC Consortium (including Alaxia, Basilea, Novartis and Polyphor), Mukoviszidose Institute, Novartis, Insmed Germany, Grifols, Bayer and InfectoPharm, consulting fees from Parion, Grifols, Zambon, Insmed and Helmholtz-Zentrum für Infektionsforschung, payment or honoraria for lectures, presentations, manuscript writing or educational events from I!DE Werbeagentur GmbH, Interkongress GmbH, AstraZeneca, Insmed, Grifols and Universitätsklinikum Frankfurt am Main, payment for expert testimony from Social Court Cologne, support for attending meetings and/or travel from the German Kartagener Syndrome and Primary Ciliary Dyskinesia (PCD) Patient Advocacy Group Mukoviszidose eV, participation on a data safety monitoring board or advisory board for Insmed, Grifols and Shionogi, and leadership or fiduciary roles as Coordinator of the ERN-LUNG Bronchiectasis Core Network, Chair of the German Bronchiectasis Registry PROGNOSIS, Member of the SteerCo of the European Bronchiectasis Registry EMBARC, Member of the SteerCo of the European Nontuberculous Mycobacterial Pulmonary Disease Registry EMBARC-NTM, Co-Speaker of the Medical Advisory Board of the German Kartagener Syndrome and PCD Patient Advocacy Group, Speaker of the Respiratory Infections and TB group of the German Respiratory Society (DGP), Speaker of the Cystic Fibrosis group of German Respiratory Society (DGP), PI of the German Center for Lung Research (DZL), Member of the Protocol Review Committee of the PCD-CTN, and Member of the Physician Association of the German Cystic Fibrosis Patient Advocacy Group; F.C. Ringshausen has other financial or non-financial interests with AstraZeneca, Boehringer Ingelheim, Celtaxsys, Corbus, Insmed, Novartis, Parion, University of Dundee, Vertex and Zambon. R. Dhar reports payment or honoraria for lectures, presentations, manuscript writing or educational events from LUPIN, CIPLA and Glenmark. C.S. Haworth reports payment or honoraria for lectures, presentations, manuscript writing or educational events from 30 Technology, CSL Behring, Chisi, Insmed, Janssen, LifeArc, Meiji, Mylan, Pneumagen, Shionogi, Vertex and Zambon. M.R. Loebinger reports consulting fees from Armata, 30T, AstraZeneca, Parion, Insmed, Chiesi, Zambon, Electromed, Recode, AN2 and Boehringer Ingelheim, payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, and a leadership or fiduciary role as ERS Infection Group Chair. K. Dimakou reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Novartis, Boehringer Ingelheim, GlaxoSmithKline, Norma Hellas, Chiesi, AstraZeneca and Zambon, support for attending meetings and/or travel from Novartis, Boehringer Ingelheim, GlaxoSmithKline, Norma Hellas, Chiesi, AstraZeneca and Menarini, and participation on a data safety monitoring board or advisory board for Novartis, GlaxoSmithKline and Chiesi. M.L. Crichton reports consulting fees from Boxer Capital LLC. A. De Soyza reports grants or contracts from AstraZeneca, Pfizer, GlaxoSmithKline and Novartis, consulting fees from AstraZeneca, Insmed, GlaxoSmithKline, Boehringer, 30T and Bayer, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Pfizer, GlaxoSmithKline and Novartis. M. Vendrell reports grants or contracts from Chiesi, payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed and Publi Creation, support for attending meetings and/or travel from Zambon, Chiesi, Novartis, Behring and Gebro, participation on a data safety monitoring board or advisory board for Insmed, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Insmed and Novartis. P-R. Burgel reports grants or contracts from GlaxoSmithKline and Vertex, consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, Insmed, MSD, Vertex, Viatris and Zambon, and support for attending meetings and/or travel from AstraZeneca and Chiesi. S. Skrgat reports honoraria for educational events, invited lectures and presentations supported by Sanofi, AstraZeneca, Medis, Berlin-Chemie and Chiesi, and participation on a data safety monitoring board or advisory board for AstraZeneca. A. de Roux reports consulting fees from Adboard Insmed, payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, AstraZeneca, GlaxoSmithKline, Berlin-Chemie and Boehringer Ingelheim, support for attending meetings and/or travel from AstraZeneca, and advisory board participation with Insmed. A. Bossios reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chiesi, and leadership or fiduciary roles as Secretary of ERS Assembly 5 (Airway diseases, asthma, COPD and chronic cough), Vice-chair of the Nordic Severe Asthma Network (NSAN)-NORDSTAR and on the Steering Committee of ERS CRC Severe Asthma (SHARP). P. Kauppi reports support for attending meetings and/or travel from the Nordic Respiratory Academy, participation on a data safety monitoring board or advisory board for the Swedish Orphan Biovitrium, leadership or fiduciary roles for the Finnish Respiratory Society and Finnish Tuberculosis Foundation Grant Committee, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Theravance. M. Murris reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Pfizer, payment for expert testimony from Vertex, support for attending meetings and/or travel from Zambon, and participation on a data safety monitoring board or advisory board for Zambon and Viatris. D. Obradovic reports a leadership or fiduciary role as president of the Serbian Society of Intensive Care Medicine. A. Amorim reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Zambon Group, and support for attending meetings and or/travel from Zambon Group, Boehringer Ingelheim and Novartis Farma. E. Van Braeckel reports grants or contracts from Insmed, Boehringer Ingelheim and Zambon. A. Shoemark reports consulting fees from Spirovant and Translate Bio, payment or honoraria for lectures, presentations, manuscript writing or educational events from Translate Bio, Ethris and Insmed, leadership or fiduciary roles in ERS CRCs (EMBARC, BEATPCD, AMR). M. Shteinberg reports consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Kamada and Zambon, payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Teva, Novartis, Kamada and Sanofi, support for attending meetings and/or travel from Novartis, Actelion, Boehringer Ingelheim, GlaxoSmithKline and Rafa, participation on a data safety monitoring board or advisory board for Bonus Therapeutics, Israel, leadership or fiduciary roles in EMBARC, Israel Pulmonology Society, and Israel Society for TB and Mycobacterial Diseases, receipt of equipment, materials, drugs, medical writing, gifts or other services from Trudell Medical International, and is Associate Editor of the American Journal of Respiratory and Critical Care Medicine. P.C. Goeminne reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, GlaxoSmithKline and Chiesi, support for attending meetings and/or travel from Chiesi, and participation on a data safety monitoring board or advisory board for Boehringer, GlaxoSmithKline and Pfizer. T. Welte reports grants from the German Ministry of Research and Education (BMBF), consulting fees and lecture honoraria from AstraZeneca, Chiesi, Grifols, GlaxoSmithKline, Insmed, MSD, Novartis, Pfizer and Sanofi, leadership roles for the German Center for Lung Research (DZL) – Board of Directors, Novartis Foundation – Board of Directors, Chairman of the Board of Trustees for CAPNETZ Foundation, German Lung Foundation, German Sepsis Foundation, and is Past President of the ERS, German Society of Pneumology and German Sepsis Society. F. Blasi reports grants or contracts from AstraZeneca, Chiesi, GlaxoSmithKline and Insmed, consulting fees from Menarini, GlaxoSmithKline and Om Pharma, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti, Grifols, Insmed, Menarini, Novartis, OM Pharma, Pfizer, Sanofi, Viatris, Vertex and Zambon. E. Polverino reports grants or contracts from Grifols, consulting fees from Insmed, Bayer, Chiesi and Zambon, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Chiesi, Grifols, GlaxoSmithKline, Insmed, Menarini and Zambon, and support for attending meetings and/or travel from Insmed, Pfizer and Moderna. J.D. Chalmers reports grants or contracts from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed, LifeArc and Novartis, and consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, Insmed, Grifols, Novartis, Boehringer Ingelheim, Pfizer, Janssen, Antabio and Zambon, and is Chief Editor of the European Respiratory Journal. The remaining authors have no potential conflicts of interest to disclose.
This article has an editorial commentary: https://doi.org/10.1183/13993003.00152-2024
Support statement: This work was supported by the Innovative Medicines Initiative and the European Federation of Pharmaceutical Industries and Associations companies under the European Commission-funded Horizon 2020 Framework Program and by Inhaled Antibiotic for Bronchiectasis and Cystic Fibrosis (grant 115721). EMBARC3 is funded by the European Respiratory Society through the EMBARC3 Clinical Research Collaboration. EMBARC3 is supported by project partners Armata, AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Grifols, Insmed, Janssen, Lifearc and Zambon. J.D. Chalmers is supported by the GlaxoSmithKline/Asthma and Lung UK Chair of Respiratory Research. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Oriano M, Gramegna A, Terranova L, et al. Sputum neutrophil elastase associates with microbiota and Pseudomonas aeruginosa in bronchiectasis. Eur Respir J 2020; 56: 2000769. doi: 10.1183/13993003.00769-2020 [DOI] [PubMed] [Google Scholar]
- 2.Aliberti S, Goeminne PC, O'Donnell AE, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med 2021; 10: 298–306. doi: 10.1016/S2213-2600(21)00277-0 [DOI] [PubMed] [Google Scholar]
- 3.Shoemark A, Cant E, Carreto L, et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur Respir J 2019; 53: 1900303. doi: 10.1183/13993003.00303-2019 [DOI] [PubMed] [Google Scholar]
- 4.Gramegna A, Aliberti S, Sibila O, et al. Sputum neutrophil elastase in bronchiectasis: a Southern European cohort study. Eur Respir J 2020; 56: 2001702. doi: 10.1183/13993003.01702-2020 [DOI] [PubMed] [Google Scholar]
- 5.Sibila O, Laserna E, Shoemark A, et al. Heterogeneity of treatment response in bronchiectasis clinical trials. Eur Respir J 2022; 59: 2100777. doi: 10.1183/13993003.00777-2021 [DOI] [PubMed] [Google Scholar]
- 6.McLeese RH, Spinou A, Alfahl Z, et al. Psychometrics of health-related quality of life questionnaires in bronchiectasis: a systematic review and meta-analysis. Eur Respir J 2021; 58: 2100025. doi: 10.1183/13993003.00025-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonnell MJ, Aliberti S, Goeminne PC, et al. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016; 71: 1110–1118. doi: 10.1136/thoraxjnl-2016-208481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med 2017; 195: 1384–1393. doi: 10.1164/rccm.201605-1027OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray MP, Pentland JL, Turnbull K, et al. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 361–364. doi: 10.1183/09031936.00163208 [DOI] [PubMed] [Google Scholar]
- 10.Goeminne PC, Vandooren J, Moelants EA, et al. The Sputum Colour Chart as a predictor of lung inflammation, proteolysis and damage in non-cystic fibrosis bronchiectasis: a case-control analysis. Respirology 2014; 19: 203–210. doi: 10.1111/resp.12219 [DOI] [PubMed] [Google Scholar]
- 11.Stockley RA, Bayley D, Hill SL, et al. Assessment of airway neutrophils by sputum colour: correlation with airways inflammation. Thorax 2001; 56: 366–372. doi: 10.1136/thorax.56.5.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021; 9: 873–884. doi: 10.1016/S2213-2600(20)30504-X [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, Aliberti S, Polverino E, et al. The EMBARC European Bronchiectasis Registry: protocol for an international observational study. ERJ Open Res 2016; 2: 00081-2015. doi: 10.1183/23120541.00081-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data from the European Bronchiectasis Registry (EMBARC). Lancet Respir Med 2023; 11: 637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 15.Dhar R, Singh S, Talwar D, et al. Clinical outcomes of bronchiectasis in India: data from the EMBARC/Respiratory Research Network of India registry. Eur Respir J 2022; 61: 2200611. doi: 10.1183/13993003.00611-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall GL, Filipow N, Ruppel G, et al. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J 2021; 57: 2000289. doi: 10.1183/13993003.00289-2020 [DOI] [PubMed] [Google Scholar]
- 17.Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol 1995; 165: 261–267. doi: 10.2214/ajr.165.2.7618537 [DOI] [PubMed] [Google Scholar]
- 18.Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. doi: 10.1183/13993003.00051-2017 [DOI] [PubMed] [Google Scholar]
- 19.Quittner AL, O'Donnell AE, Salathe MA, et al. Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax 2015; 70: 12–20. doi: 10.1136/thoraxjnl-2014-205918 [DOI] [PubMed] [Google Scholar]
- 20.Quellhorst L, Barten-Neiner G, de Roux A, et al. Psychometric validation of the German translation of the Quality of Life Questionnaire-Bronchiectasis (QOL-B) – data from the German Bronchiectasis Registry PROGNOSIS. J Clin Med 2022; 11: 441. doi: 10.3390/jcm11020441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. doi: 10.1016/S0140-6736(18)31767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med 2021; 9: 885–896. doi: 10.1016/S2213-2600(20)30557-9 [DOI] [PubMed] [Google Scholar]
- 23.Rigauts C, Aizawa J, Taylor S, et al. Rothia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur Respir J 2021; 59: 2101293. doi: 10.1183/13993003.01293-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law SM, Gray RD. Neutrophil extracellular traps and the dysfunctional innate immune response of cystic fibrosis lung disease: a review. J Inflamm 2017; 14: 29. doi: 10.1186/s12950-017-0176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finch S, Shoemark A, Dicker AJ, et al. Pregnancy zone protein is associated with airway infection, neutrophil extracellular trap formation, and disease severity in bronchiectasis. Am J Respir Crit Care Med 2019; 200: 992–1001. doi: 10.1164/rccm.201812-2351OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 2020; 383: 2127–2137. doi: 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]
- 27.Crichton ML, Lonergan M, Barker AF, et al. Inhaled aztreonam improves symptoms of cough and sputum production in patients with bronchiectasis: a post hoc analysis of the AIR-BX studies. Eur Respir J 2020; 56: 2000608. doi: 10.1183/13993003.00608-2020 [DOI] [PubMed] [Google Scholar]
- 28.Keir HR, Shoemark A, Huang JTJ, et al. SPLUNC1 is a novel marker of disease severity and airway infection in bronchiectasis. Eur Respir J 2021; 58: 2101840. doi: 10.1183/13993003.01840-2021 [DOI] [PubMed] [Google Scholar]
- 29.Bedi P, Cartlidge MK, Zhang Y, et al. Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: a proof-of-concept randomised controlled trial. Eur Respir J 2021; 58: 2004388. doi: 10.1183/13993003.04388-2020 [DOI] [PubMed] [Google Scholar]
- 30.Sibila O, Laserna E, Shoemark A, et al. Airway bacterial load and inhaled antibiotic response in bronchiectasis. Am J Respir Crit Care Med 2019; 200: 33–41. doi: 10.1164/rccm.201809-1651OC [DOI] [PubMed] [Google Scholar]
- 31.Chalmers JD, Cipolla D, Thompson B, et al. Changes in respiratory symptoms during 48 weeks treatment with ARD-3150 (inhaled liposomal ciprofloxacin) in bronchiectasis: results from the ORBIT-3 and -4 studies. Eur Respir J 2020; 55: 2000110. doi: 10.1183/13993003.00110-2020 [DOI] [PubMed] [Google Scholar]
- 32.Shoemark A, Shteinberg M, De Soyza A, et al. Characterisation of eosinophilic bronchiectasis: a European multicohort study. Am J Respir Crit Care Med 2022; 205: 894–902. doi: 10.1164/rccm.202108-1889OC [DOI] [PubMed] [Google Scholar]
- 33.Aliberti S, Sotgiu G, Blasi F, et al. Blood eosinophils predict inhaled fluticasone response in bronchiectasis. Eur Respir J 2020; 56: 2000453. doi: 10.1183/13993003.00453-2020 [DOI] [PubMed] [Google Scholar]
- 34.Oriano M, Gramegna A, Amati F, et al. T2-high endotype and response to biological treatments in patients with bronchiectasis. Biomedicines 2021; 9: 772. doi: 10.3390/biomedicines9070772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalmers JD, Aliberti S, Altenburg J, et al. Transforming clinical research and science in bronchiectasis: EMBARC3, a European Respiratory Society Clinical Research Collaboration. Eur Respir J 2023; 61: 2300769. doi: 10.1183/13993003.00769-2023 [DOI] [PubMed] [Google Scholar]
- 36.Herrero-Cortina B, Lee AL, Oliveira A, et al. European Respiratory Society statement on airway clearance techniques in adults with bronchiectasis. Eur Respir J 2023; 62: 2202053. doi: 10.1183/13993003.02053-2022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01554-2023.Supplement (373.4KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01554-2023.Shareable (1.6MB, pdf)