Summary
Background
Anlotinib is a new type of tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors 1/2/3, platelet-derived growth factor receptors α/β, and fibroblast growth factor receptors 1–4 and c-Kit, with a broad spectrum of inhibitory effects on tumor angiogenesis and growth. It has been proven effective in HER2-negative metastatic breast cancer, but its efficacy in early-stage triple-negative breast cancer (TNBC) is unknown. This phase 2 study aims to evaluate the efficacy and safety of adding anlotinib to neoadjuvant chemotherapy in patients with TNBC.
Methods
Patients with clinical stage II/III TNBC were treated with 5 cycles of anlotinib (12 mg, d1-14, q3w) plus 6 cycles of taxanes (docetaxel 75 mg/m2 ,d1, q3w or nab-paclitaxel 125 mg/m2, d1 and d8, q3w) and lobaplatin (30 mg/m2, d1, q3w), followed by surgery. The primary endpoint was pathological complete response (pCR; ypT0/is ypN0) and the secondary endpoints include breast pCR (bpCR), axillary pCR (apCR), residual cancer burden (RCB), objective response rate (ORR), survival, and safety. Exploratory endpoints were efficacy biomarkers based on Fudan University Shanghai Cancer Center Immunohistochemical (FUSCC IHC) classification for TNBC and next-generation sequencing (NGS) of DNA from tumor tissue and blood samples of patients with 425-gene panel. This trial is registered with www.chictr.org.cn (ChiCTR2100043027).
Findings
From Jan 2021 to Aug 2022, 48 patients were assessed and 45 were enrolled. All patients received at least one dose of study treatment and underwent surgery. The median age was 48.5 years (SD: 8.7), 71% were nodal involved, and 20% had stage III. In the intention-to-treat population, 26 out of 45 patients achieved pCR (57.8%; 90% CI, 44.5%–70.3%), and 39 achieved residual cancer burden class 0-I (86.7%; 95% CI, 73.2%–94.9%). The bpCR and apCR rate were 64.4% (29/45) and 71.9% (23/32), respectively. No recurrence or metastasis occurred during the short-term follow-up. Based on the FUSCC IHC-based subtypes, the pCR rates were 68.8% (11/16) for immunomodulatory subtype, 58.3% (7/12) for basal-like immune-suppressed subtype and 33.3% (4/12) for luminal androgen receptor subtype, respectively. NGS revealed that the pCR were 77% (10/13) and 50% (14/28) in MYC-amplified and wild-type patients, respectively, and 78% (7/9) and 53% (17/32) in gBRCA1/2-mutated and wild-type patients, respectively. The median follow-up time of the study was 14.9 months (95% CI: 13.5–16.3 months). There was no disease progression or death during neoadjuvant therapy. No deaths occurred during postoperative follow-up. In the safety population (N = 45), Grade 3 or 4 treatment emergent adverse events occurred in 29 patients (64%), and the most common events were neutropenia (38%), leukopenia (27%), thrombocytopenia (25%), anemia (13%), and hypertension (13%), respectively.
Interpretation
The addition of anlotinib to neoadjuvant chemotherapy showed manageable toxicity and encouraging antitumor activity for patients with clinical stage II/III TNBC.
Funding
Chongqing Talents Project, Chongqing Key Project of Technology Innovation and Application Development and Chongqing Outstanding Youth Natural Science Foundation.
Keywords: Anlotinib, Anti-tumor angiogenesis, Triple-negative breast cancer, Neoadjuvant chemotherapy
Research in context.
Evidence before this study
We did systematic literature searches in Pubmed and Embase in the proceedings of the major cancer research conferences reporting results of breast cancer trials, before study activation in January, 2021, using the English search terms “triple-negative”, “breast cancer”, “neoadjuvant”, “platinum” and “anti-angiogenesis”. Anti-angiogenesis agents are mainly used in the treatment of metastatic TNBC, and there are several clinical studies on neoadjuvant therapy. Previous studies have found that anti-angiogenesis drugs, specifically monoclonal antibody-based drugs, can improve the pCR rate of neoadjuvant therapy for TNBC when combined with chemotherapy. There are three studies of platinum salt combined with bevacizumab, GeparSixto, KCSG BR-0905 and CALGB 40603, and pCR rates (ypT0/is ypN0) in TNBC were 53.2% (84/158), 42% (19/45) and 60% (66/110), respectively. But in the CALGB 40603 trial, hypertension, infection, thromboembolic events, bleeding and postoperative complications were more common with bevacizumab. Because of toxicities, only 66% of patients assigned to bevacizumab completed the planned treatment. The neoALTAL trial is the first study to combine a novel, oral, multi-target, anti-angiogenesis TKI, anlotinib, with neoadjuvant chemotherapy of TNBC, which explored the efficacy and safety of adding anlotinib to a combination of taxane and platinum (TP) for TNBC treatment.
Added value of this study
This report presents the primary endpoint of pathological complete response in neoALTAL trial, showing a high rate of a pCR (57.8%), and RCB grade of 0–1 (86.7%) in ITT population with the addition of anlotinib to neoadjuvant chemotherapy. The beneficiaries of this regimen may be patients with the IM subtype of breast cancer with BRCA or RB1 mutations or MYC amplification. More importantly, this combined regimen did not cause serious safety issues, and 91% of patients completed the planned NAC.
Implications of all the available evidence
The neoALTAL trial achieved encouraging anti-tumor activity and controllable toxicity, which supports further evaluation of anlotinib plus taxane and platinum as an alternative neoadjuvant treatment regimen for patients with TNBC, who are not eligible for chemoimmunotherapy or anthracycline-based regimens in randomized studies.
Introduction
Triple-negative breast cancer (TNBC) is a breast cancer subtype with negative expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), characterized by a poor prognosis. Neoadjuvant chemotherapy (NAC) plays an important role in the treatment of early and locally advanced-stage TNBC. A pathologic complete response (pCR) which was defined as pathological stage ypT0/Tis ypN0 at the time of definitive surgery, is a crucial indicator for evaluating NAC efficacy, and patients who achieve a pCR, in general, carry a better prognosis.1
The standard NAC regimen for TNBC is a combination or sequential use of anthracycline and taxane. One meta-analysis showed that the pCR rate using this regimen was ∼40%.2 Several meta-analyses have shown that addition of platinum to the standard regimen increases the rate of pCR and lengthens event-free survival (EFS) or disease-free survival (DFS).1,3 Given the seriousness of cardiac toxicity induced by anthracycline, there is increasing interest in exploring anthracycline-free neoadjuvant or adjuvant chemotherapy regimens for treatment of TNBC. A NAC regimen consisting of taxane and carboplatin is an option for TNBC patients who are not eligible to receive anthracyclines.4
Lobaplatin (LBP) is a platinum-based third-generation anticancer agent approved in China for the treatment of advanced breast cancer, small-cell lung cancer, and chronic myeloid leukemia. A phase IV clinical trial involving 1179 patients showed that the LBP-based chemotherapy had good efficacy and manageable toxicity in the treatment of advanced breast cancer.5 Among the different chemotherapy regimens, such as LBP plus paclitaxel, LBP plus docetaxel, LBP plus gemcitabine, LBP plus vinorelbine, and other LBP-based regimens, LBP plus taxanes showed the highest ORR and DCR (LBP plus docetaxel: 51.7% and 84.6%; LBP plus paclitaxel: 49.6% and 85.6%).5 In a phase III trial involving locoregionally advanced nasopharyngeal carcinoma patients, LBP-based induction chemotherapy combined with concurrent chemoradiotherapy resulted in non-inferior survival and fewer toxic effects than cisplatin-based therapy.6 In addition, a phase II randomized controlled clinical study of our group showed that neoadjuvant therapy with LBP can improve the pCR and ORR rates of TNBC, with tolerable side effects and a trend towards improving long-term survival.7,8
Since the lack of classic molecular targets, TNBC still needs individualized and precise treatment besides chemotherapy. To further improve the pCR rate and achieve better long-term clinical benefits for TNBC patients, clinical researchers are continuously investigating optimized neoadjuvant therapy regimens and promising targeted therapies. In recent years, chemotherapy combined with immune checkpoint inhibitors has become standard regimen for TNBC neoadjuvant therapy due to the improvement of pCR rate and prolongation of survival. However, for patients who are not eligible for immunotherapy, more research is still needed to explore new and effective treatment for them.
Microvascular density in TNBC tissue has been reported to be significantly higher than that in other subtypes. According to NCCN and ESMO guidelines, anti-angiogenic agents, like bevacizumab, plus either paclitaxel or capecitabine are recommended as first-line treatment for metastatic TNBC. Studies have shown that addition of bevacizumab, the most commonly used angiogenic drug, to chemotherapy can improve the chances of a pCR when using NAC of TNBC.9, 10, 11, 12 However, AEs like hypertension, infection, thromboembolic event, bleeding and postoperative complications were commo, and only 66% of patients assigned to bevacizumab completed the planned treatment in the CALGB 40603 trial.12 Novel antiangiogenic agents are urgently needed due to issues such as limited OS prolongation and high incidence of antiangiogenic-associated adverse events. Anlotinib is a new type of tyrosine kinase inhibitor (TKI) that targets vascular endothelial growth factor receptors (VEGFR1/2/3), platelet-derived growth factor receptors (PDGFR-α/β), and fibroblast growth factor receptors (FGFR1-4) and c-Kit, with a broad spectrum of inhibitory effects on tumor angiogenesis and growth.13 Currently, anlotinib has been approved in China as salvage treatment for several cancer types including lung cancer, thyroid cancer and soft tissue sarcoma.14 Evidences are emerging for the efficacy of anlotinib in breast cancer. A randomized trial reported prolonged mPFS of 2.9 months (5.7 vs 2.8 months, hazard ratio = 0.39, 95% CI 0.02–0.87; P = 0.02) with the treatment of chemotherapy plus anlotinib versus chemotherapy alone in patients with locally recurrent or metastatic TNBC. Whereas, the addition of anlotinib did not increase the incidence of all grades of AEs, including grade 3–4 AEs.15 Several clinical studies have also demonstrated that the combination of anlotinib and chemotherapy can be highly effective in the treatment of metastatic breast cancer while exhibiting acceptable toxicity.16,17 The most common treatment-related adverse events (TRAEs) of anlotinib are hypertension (67.7%), fatigue (52.0%), thyroid-stimulating hormone elevation (46.6%), hypertriglyceridemia (44.6%), hand-foot syndrome (43.9%), and hypercholesterolemia (41.8%). The most common grade 3 or higher adverse events among the anlotinib group were hypertension (13.6%), hyponatremia (8.2%), and elevatedγ-glutamyltransferase (5.4%).18
Based on the biological characteristics of TNBC (e.g., high density of blood vessels and rapid proliferation by tumor cells) combined with our previous research results, we designed a single-arm clinical trial to explore the: (i) efficacy and safety of adding anlotinib to a combination of taxane and platinum for TNBC treatment; and (ii) benefits of this regimen through biomarker analysis.
Methods
Ethical approval of the study protocol
Ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital (Southwest Hospital) of Army Medical University (Chongqing, China). This clinical trial was undertaken in accordance with the Declaration of Helsinki 1964 and its later amendments and Good Clinical Practice guidelines. All patients provided written informed consent before enrollment. This trial is registered with www.chictr.org.cn (ChiCTR2100043027).
Study design and participants
This phase-2, single-arm, prospective study of neoadjuvant anlotinib in combination with taxane and lobaplatin for TNBC was carried out at the First Affiliated Hospital of Army Medical University.
Key eligibility criteria included the following: females aged 18–70 years; pathologically confirmed invasive TNBC, which was defined as ER and/or PR positivity rates were less than 1% according to the immunohistochemistry (IHC) results, and the IHC score for HER2 staining was 0 or 1+, or fluorescence in situ hybridization detected no HER2 gene amplification when the score was 2+); stage-II-III disease and untreated previously; measurable disease with Response Evaluation Criteria In Solid Tumors (RECIST) v1.1; and an Eastern Cooperative Oncology Group performance status score of 0–1. Key exclusion criteria included inflammatory breast cancer, uncontrollable hypertension or arrhythmias, and a history of cardiac insufficiency of grade 2 or greater. The full eligibility criteria are detailed in eMethods in Supplement 1.
Procedures
Eligible patients received nab-paclitaxel (125 mg/m2, d1 and d8) or docetaxel (75 mg/m2, d1), which was based on clinician choice, plus lobaplatin (30 mg/m2, d1), both administered via the intravenous route, every 3 weeks for six cycles. Anlotinib (12 mg, p.o., once daily) was administered on days 1–14 of each cycle every 3 weeks for five cycles. The sixth cycle of NAC was administered without anlotinib to allow for a break of ≥3 weeks between the last dose of anlotinib and time of definitive surgery for breast cancer. In cases of tumor progression, the study treatment was discontinued and further local treatment or systemic treatment was permitted at the discretion of the investigator.
Definitive surgery was carried out 2–3 weeks after completing all cycles of NAC. The types of breast surgery (mastectomy, breast-conserving surgery, or mastectomy + reconstruction) and axillary treatment (sentinel lymph-node biopsy [SLNB] or axillary lymph-node dissection [ALND] or SLNB + ALND) were determined by the treating surgeon. Postoperatively, patients who had residual disease were offered adjuvant chemotherapy with capecitabine (initial dose of 1250 mg/m2 [reduced to 1000 mg/m2 if intolerable due to toxicity], b.d.) for 1–14 days, and cycled every 21 days for 6–8 cycles. Follow-up for disease status and survival was scheduled every 3 months for the first 2 years after treatment initiation, then every 6 months for years 3–5, and annually thereafter.
Outcomes
The primary endpoint was the rate of the pathologic complete response (pCR), defined as the absence of residual invasive disease with or without ductal carcinoma in situ in the breast and axilla (ypT0/is ypN0).
Secondary endpoints were: the rate of residual cancer burden (RCB; scored using Symmans’ criteria), breast pathologic complete response (bpCR), and axillary nodal pathologic complete response (apCR); event-free survival (EFS; defined as the time from treatment initiation to any progression, recurrence, or death from any cause); overall survival (OS; defined as the time from treatment initiation to death from any cause); objective response rate (ORR; defined as a partial response or complete response according to RECIST 1.1 based on ultrasound (US)/magnetic resonance imaging (MRI) undertaken at baseline and immediately before surgery); safety; exploratory analyses of biomarkers. When evaluating ORR using the RECIST standard, MRI was preferred, and patients without MRI baseline data were evaluated by US.
Patients who had a pCR (RCB 0) or near pCR (RCB I) were included in a group classified as “RCB 0/I”. The National Cancer Institute Common Terminology Criteria for Adverse Events 5.0 was employed to assess treatment-emergent safety.
Biomarker assessments
Fudan University Shanghai Cancer Center (FUSCC) IHC classification,19 tumor-infiltrating lymphocytes (TILs), neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), next-generation sequencing (NGS) with 425 panel including BRCA germline mutation, were performed, and the sources were tumor tissue and blood samples of patients. The full biomarker assessments are detailed in eMethods in Supplement 1.
Statistical analyses
We used Simon's optimal two-stage design with a one-sided α error of 5% and a power of 80%. Previously reported data indicate the pCR rate of the taxane and platinum (TP) regimen for regional breast cancer or locally advanced breast cancer to be 44% (H0 = 44%).20,21 We expected that the pCR rate for anlotinib combined with the TP regimen would be 65% (H1 = 65%).22,23 Under these assumptions, 13 patients were to be treated in the first stage of the study. At least seven responses were required to continue to the second stage, and 29 more patients would be enrolled in the second stage for a total sample size of 42. Overall, if ≥ 24 responses were observed, then the treatment regimen would be considered a “success”. Assuming a loss to follow-up of 5%, then 45 patients were required.
Efficacy analyses of a pCR were done in the intention-to-treat (ITT) population (i.e., all enrolled patients). The objective response rate (ORR) was reported for patients who underwent US/MRI at baseline and immediately before surgery. Safety analyses were done for all patients who received at least one dose of study treatment (safety population). The primary endpoint pCR were calculated by the Clopper-Pearson method with two-sided 90% confidence intervals (corresponding to the one-sided 0.05 used for sample size calculation). If the lower limit of the 90% confidence intervals (CIs) was greater than the historical control value (H0 hypothesis, 44%), then the trial protocol was considered successful compared to the historical protocol. The other binary endpoints, including bpCR, apCR, RCB, ORR, etc., were calculated by the Clopper-Pearson method with 95% confidence intervals. EFS and OS analyses and associated 95% CIs were estimated using the Kaplan–Meier method. A modified Poisson regression model with robust standard error estimation was used to estimate rate ratios (RRs) and 95% CIs, as well as P values. Descriptive statistics were provided for clinical and demographic characteristics and for AEs. Biomarker analyses were done using univariate analysis. Analyses were undertaken using R 4.0.2 (www.r-project.org). Two-sided P < 0.05 was considered statistically significant. The final date of data acquisition was 23 April 2023.
Role of the funding source
The funding sources of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. XWQ, YZ, JJ and BXW had full access to all of the data in the study. All authors had the final responsibility for the decision to submit the manuscript for publication.
Results
Enrolled population
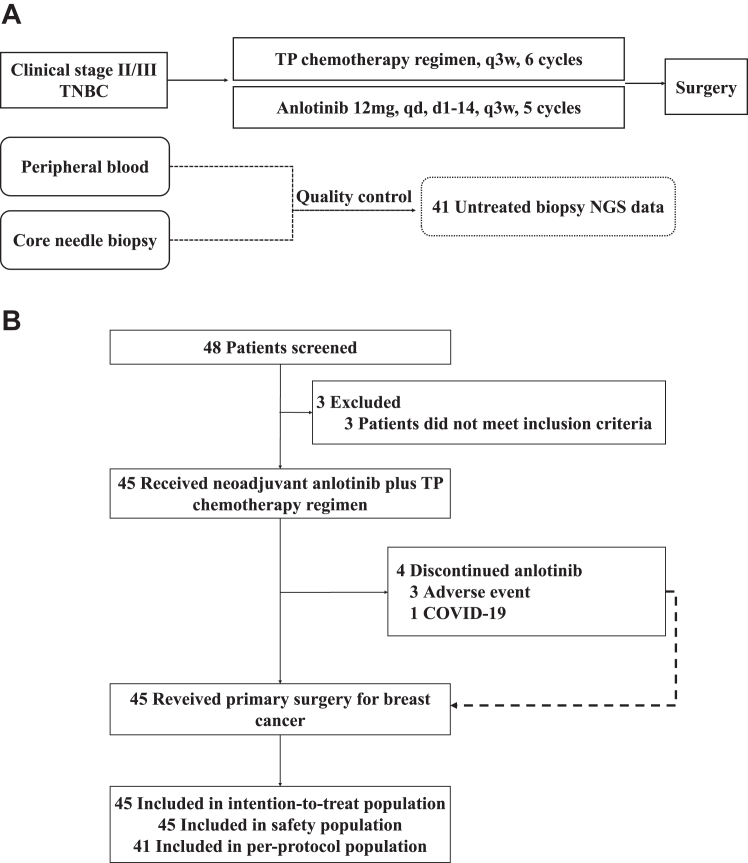
From January 2021 to August 2022, 45 patients with TNBC were enrolled (Fig. 1). The pathologic characteristics of patients at baseline are shown in Supplement 2. The median age was 48.5(SD: 8.7) years. We found that 24 (53%) patients were postmenopausal, 32 (71%) patients had lymph-node metastasis, nine (20%) patients had clinical stage-III breast cancer, and the remainder were stage II. Importantly, 42 (93%) patients were diagnosed with invasive no special type, with the remaining having metaplastic cancer (one patient) and neuroendocrine cancer (two patients). Also, 38 (84%) patients showed high expression (≥30%) of Ki-67. According to the FUSCC IHC (four-subtype) classification, LAR was noted in 12 (27%) patients, IM in 16 (36%) patients, and BLIS in 12 (27%) patients, MES was not detected, and five (11%) patients could not be classified due to an insufficient number of tissue sections.
Fig. 1.
Study design and CONSORT diagram. (A) Study design of the neoALTAL trial; (B) the CONSORT flow diagram. Abbreviations: TNBC, triple-negative breast cancer; TP, taxane and platinum; NGS, next-generation sequencing.
As of April 2023, all 45 patients had completed ≥2 cycles of NAC with a combination of anlotinib and a TP regimen, and underwent surgery. Among them, 41 (91%) patients completed the prescribed five cycles of anlotinib treatment and six cycles of TP chemotherapy; three patients completed only 2–4 cycles of treatment because AEs led to discontinuation, and one patient completed only two cycles due to coronavirus disease-2019 in Supplement 2. The majority of patients underwent both US and MRI examinations, except for 10 who were unable to undergo MRI due to claustrophobia, contrast agent allergies, or other reasons. The follow-up is still ongoing, and the median follow-up time of the study was 14.9 months (95% CI: 13.5–16.3 months). There was no disease progression or death during neoadjuvant therapy. As of April 23, 2023, and there were no deaths during postoperative follow-up.
Assessments of efficacy and biomarkers
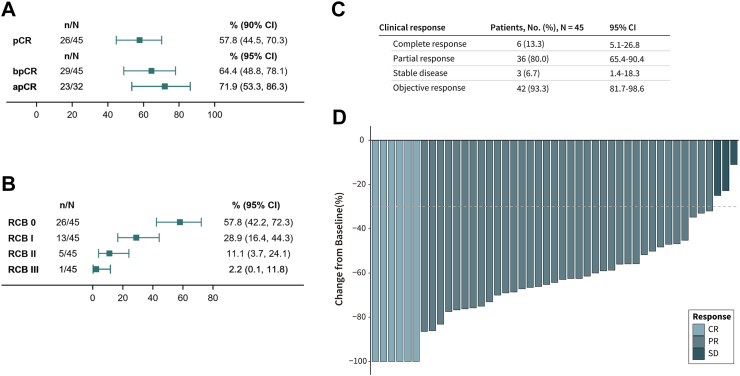
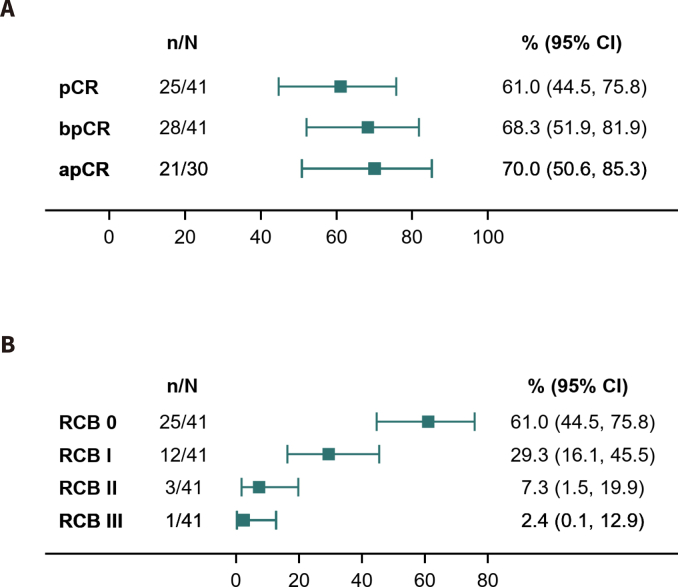
In the first 13 patients enrolled, pCR was noted in seven (53.8%) patients. The pCR threshold for the first stage of Simon's two-stage design was reached, and our trial continued to full accrual. Among the ITT population (N = 45), 26 cases achieved pCR (57.8%, 90% CI: 44.5%–70.3%) and 29 cases achieved bpCR (64.4%, 95 CI: 48.8%–78.1%). Among the 32 patients with lymph-node involvement at baseline, 23 achieved apCR (71.9%, 95% CI: 53.4%–86.3%) (Fig. 2A). Among these 45 patients, 26 had RCB 0 (57.8%, 95% CI: 42.2%–72.3%), 13 had RCB I (28.9%, 95% CI: 16.4%–44.3%), five had RCB II (11.1%, 95% CI: 3.7%–24.1%), and one had RCB III (2.2%, 95% CI: 0.0%–11.8%) (Fig. 2B). The proportion of patients with RCB 0/I was 86.7%. Among the per-protocol population (N = 41), the rates of pCR, bpCR, and apCR were 61.0% (25/41), 68.3% (28/41), and 70.0% (21/30), respectively; 25 had RCB 0 (61.0%), 12 had RCB I (29.3%) in Supplement 3. A 93.3% ORR was observed for the ITT population (95% CI: 81.7%–98.6%); the complete response rate was 13.3% (95% CI: 5.1%–26.8%), and the partial response rate was 80.0% (95% CI: 65.4%–90.4%) (Fig. 2C–D).
Fig. 2.
The pathological complete response (pCR), residual cancer burden (RCB) and clinical response in the intention-to-treat (ITT) population (N = 45). (A) pCR, bpCR and apCR; (B) RCB; (C) clinical response; (D) waterfall plot of clinical response.
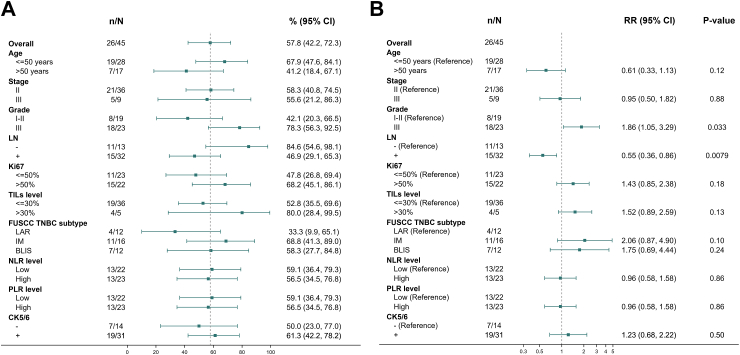
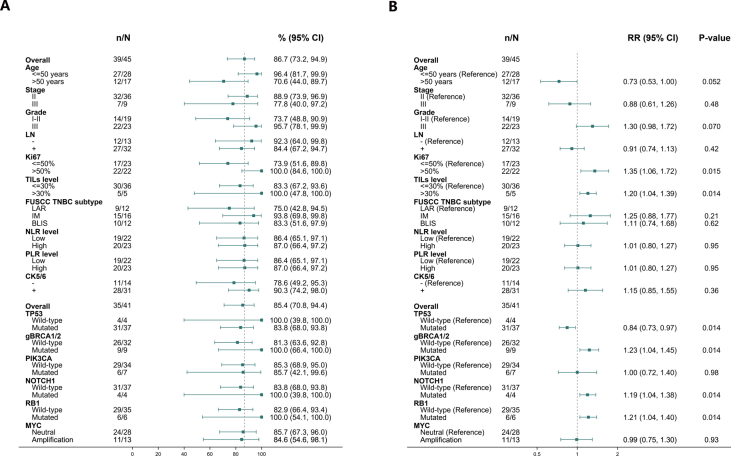
Subgroup analyses were undertaken to explore the association between clinical-pathologic characteristics and pCR rate in the ITT population (Fig. 3). The pCR rate of patients with histology grade III was 78.3% (18/23), higher than that of patients with histology grade I-II (42.1%, 8/19). The pCR rate of patients who were diagnosed initially to be lymph node-negative was 84.6% (11/13), higher than that of patients who were lymph-node positive (47.8% (15/32). Patients with high histological grade and lymph node negative were more likely to achieve pCR. The pCR rate of patients with IM, BLIS, or LAR subtypes were 68.8% (11/16), 58.3% (7/12), and 33.3% (4/12), respectively.
Fig. 3.
Clinicalpathological subgroup analysis. (A) pCR rate and (B) relative risk (RR) of different clinicalpathological characteristics.
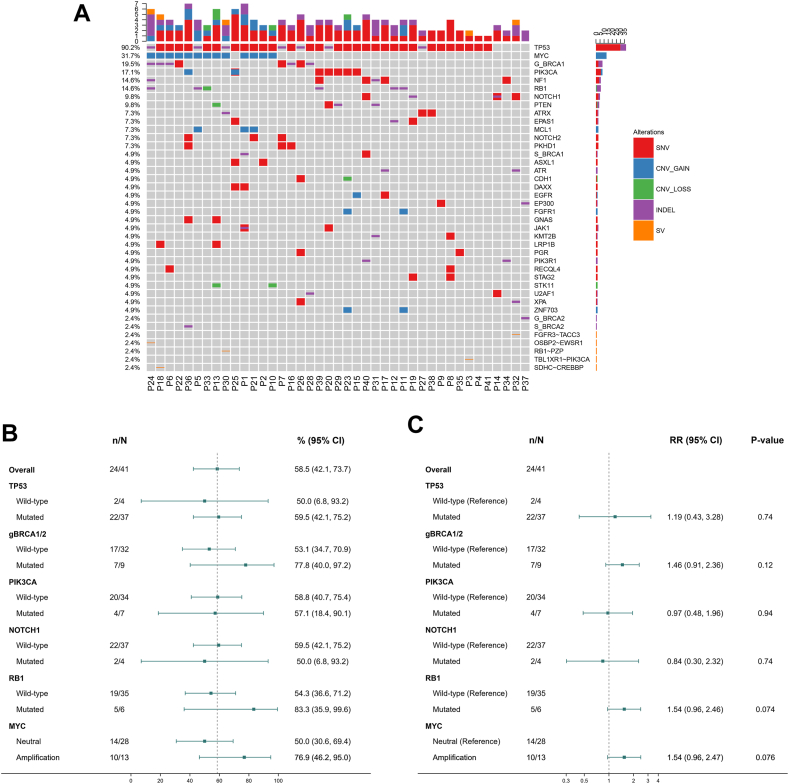
Forty-one patients underwent assessment by NGS. The most common genetic variations were those of TP53 (90.2%), MYC (31.7%), gBRCA1 (19.5%), PIK3CA (17.1%), NF1(14.6%), RB1 (14.6%), and NOTCH (9.8%) (Fig. 4A). Subgroup analyses showed that the pCR rate of patients with MYC amplification was 76.9% (10/13), which was higher than that of patients who without MYC amplification (50.0%, 14/28). The pCR rate of patients with gBRCA mutation was 77.8% (7/9), which was higher than that of patients with gBRCA wild-type (53.1%, 17/32). Five out of 6 RB1 mutation patients achieved pCR, while only 19 out of 35 RB1 wild-type patients achieved pCR. However, the differences stated above were not significant (Fig. 4B–C).
Fig. 4.
The genomic landscape and subgroup analysis. (A) Genomic landscape; (B) pCR rate and (C) relative risk (RR) of different genomic characteristics.
Correlation analyses between clinical-pathologic characteristics, genetic characteristics, and RCB grading showed that the trend was similar to that of a pCR in Supplement 4.
Safety
Among the 45 patients who could be assessed with regard to safety, data on treatment-emergent adverse events (TEAEs) are shown in Table 1. All patients experienced TEAEs, with 89% experiencing hypertension, 76% having anemia, 76% being afflicted with leukopenia, 69% suffering thrombocytopenia, 62% having neutropenia, and 58% suffering hand-foot syndrome. The prevalence of level-3-4 AEs was 64%, with the most common being neutropenia (38% of patients), leukopenia (27%), thrombocytopenia (25%), hypertension (13%), and anemia (13%). No treatment-related deaths occurred.
Table 1.
Treatment-emergent adverse events reported in ≥10% of the safety population (n = 45).
| Treatment-emergent adverse events | Any grade No. (%) |
Grade 1/2 No. (%) |
Grade 3 No. (%) |
Grade 4 No. (%) |
|---|---|---|---|---|
| Any events | 45 (100) | 16 (36) | 21 (47) | 8 (18) |
| Hypertension | 40 (89) | 34 (76) | 6 (13) | 0 |
| Anemia | 34 (76) | 28 (62) | 6 (13) | 0 |
| Leukopenia | 34 (76) | 22 (49) | 9 (20) | 3 (7) |
| Thrombocytopenia | 31 (69) | 20 (44) | 7 (16) | 4 (9) |
| Neutropenia | 28 (62) | 11 (24) | 13 (29) | 4 (9) |
| Hand-foot syndrome | 26 (58) | 26 (58) | 0 | 0 |
| GGT elevation | 22 (49) | 20 (44) | 2 (4) | 0 |
| Increased ALT | 20 (44) | 19 (42) | 1 (2) | 0 |
| Increased AST | 15 (33) | 15 (33) | 0 | 0 |
| Fatigue | 13 (29) | 13 (29) | 0 | 0 |
| Vomiting | 12 (27) | 12 (27) | 0 | 0 |
| Headache | 11 (24) | 11 (24) | 0 | 0 |
| Skin allergy | 10 (22) | 9 (20) | 1 (2) | 0 |
| Inappetence | 9 (20) | 9 (20) | 0 | 0 |
| Oropharyngeal pain | 10 (22) | 9 (20) | 1 (2) | 0 |
| Breast pain | 9 (20) | 9 (20) | 0 | 0 |
| Gingival pain | 9 (20) | 9 (20) | 0 | 0 |
| Diarrhea | 8 (18) | 8 (18) | 0 | 0 |
| Increased ALP | 6 (13) | 6 (13) | 0 | 0 |
| Nausea | 5 (11) | 5 (11) | 0 | 0 |
| Dizzy | 5 (11) | 5 (11) | 0 | 0 |
| Cough | 5 (11) | 5 (11) | 0 | 0 |
| Abdominal pain | 5 (11) | 5 (11) | 0 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
Discussion
The final result of the NeoALTAL trial showed an encouraging pCR rate of 57.8% (90% CI: 44.5%–70.3%). Therefore, it supports the rejection of the H0 hypothesis (44%), implying that the current regimen is better than the historical regimen. Previous trials with regimen of platinum salt combined with bevacizumab or apatinib have reported pCR rates of 42%∼60.9%.10, 11, 12,23These results also compare favorably with pCR rates of neoadjuvant anthracycline-based chemoimmunotherapy trials,24, 25, 26 which have reported pCR rates of 53%∼64.8%. In the NeoPACT study, 83 patients with stage II to III disease and ER and PR less than 1% achieved a pCR rate of 58% using taxane and platinum-based agent combined with pembrolizumab for six cycles.27 Our study used taxane and lobaplatin combined with anlotinib for 6 cycles, and obtained a pCR rate of 57.8%, which is similar to the NeoPACT study. According to the results of pCR rate, it could be interesting to further evaluate of neoadjuvant anlotinib plus taxane and platinum as an alternative regimen for patients with TNBC, who are not eligible for chemoimmunotherapy or anthracycline-based regimens in phase 2–3 randomized studies.
According to the FUSCC IHC classification, the pCR rates of patients with IM, BLIS, or LAR subtypes was 69%, 58%, and 33%, respectively. In the LAR subtype, 33% of patients achieved pCR, whereas historically this subtype had a pCR rate of 10%, which suggests that anlotinib may be beneficial for patients with LAR subtypes with inadequate response to traditional neoadjuvant chemotherapy. However, future research is necessary to clarify the effects and explore possible mechanisms. The difference was not significant, but a clear clinical trend was observed: the breast cancer subtypes IM and BLIS could obtain enhanced tumor-shrinking effects from a combined strategy of chemotherapy and targeted therapy. These two subtypes of breast cancer are also relatively “hot tumors” in TNBC, and are expected to respond well to chemotherapy.28 For breast caner with the BLIS subtype, platinum-containing chemotherapy regimens can achieve greater efficacy because this subtype is prone to deficiency in homologous recombination. Interestingly, the pCR rate in IM subgroup was the highest in our study. Fukumura and colleagues showed that the crosstalk between tumor angiogenesis and immune cells may lead to a “vicious cycle” of tumor growth.29 Liu and colleagues showed that anlotinib use downregulated expression of programmed death-ligand 1 on vascular endothelial cells through inactivation of the protein kinase B pathway, thereby increasing the ratio of CD8/FoxP3 within the tumor and remodeling the immune microenvironment.30 Therefore, we inferred that addition of anlotinib to the treatment of patients with the IM subtype of breast cancer may help normalize vascular-immune crosstalk, thereby achieving more potent anti-tumor effects. Interestingly and excitingly, the combination of antiangiogenic TKI and immunotherapy with/without chemotherapy also showed promising efficacy with manageable toxicity in metastatic TNBC in four studies.17,31, 32, 33 Currently, atezolizumab plus bevacizumab is the standard treatment for advanced unresectable hepatocellular carcinoma. In IMbrave150 trial, serious toxicity was noted in 38% of the patients who received the combination therapy, whereas no new or unexpected toxicity was observed.34 Considering vascular-immune crosstalk, we could try to conduct a new adjuvant treatment trial of TKI, such as anlotinib, combined with immunotherapy and chemotherapy in the sensitive population of TNBC. Our current findings could provide a backbone for applying this strategy to the neoadjuvant treatment of TNBC.
We analyzed the correlation between genetic variations and the efficacy of our combined regimen. pCR was more likely to be achieved in patients with MYC amplification, BRCA mutation, or RB1 mutation than those without these mutations. Studies have shown that MYC amplification shows high expression in basal-like tumors.35 MYC can activate the VEGF signaling pathway, promote tumor angiogenesis, and disease progression. Therefore, we infer that patients with breast cancer with MYC amplification may benefit from anti-vascular therapy. The GeparQuinto trial indicated that compared with patients receiving NAC monotherapy, patients with TNBC and BRCA1/2 mutation were more likely to achieve pCR by receiving NAC combined with anti-vascular therapy (bevacizumab).36 The reason for this observation lies in the connection between DNA repair, hypoxia, and the VEGF signaling pathway in tumor cells. Anti-angiogenesis induces hypoxia and the resulting repair of DNA damage promotes the lethality of BRCA1/2-mutated tumor cells. We employed a small-molecule TKI, anlotinib, for anti-vascular treatment and achieved superior efficacy in patients with the BRCA mutation.
The trial regimen of anlotinib plus taxane and lobaplatin was well tolerated, and 91% of patients completed the planned therapy. These data compare favorably with those from neoadjuvant platinum salt combined with bevacizumab regimen, in which only 66% of patients assigned to bevacizumab completed the planned treatment because of toxicities were reported.9 These data also compare favorably with those from neoadjuvant chemoimmunotherapy regimens, in which toxic effects related treatment discontinuation rates of 23%, 23%, and 12% were reported by the KEYNOTE-522, Impassion031, and NeoPACT trials.25, 26, 27 The hematological toxicities and gastrointestinal reactions are the most common AEs shared by neoadjuvant chemotherapy. The anthracycline drug increases the cardiotoxicity. Chemotherapy combined with anti-angiogenesis drug such as bevacizumab increases hypertension, bleeding, and thrombosis. Chemotherapy combined with immunotherapy increases AEs such as immune related pneumonia, skin adverse reactions, thyroid dysfunction, and vertiginitis. In this study, chemotherapy-related myelosuppression were the most frequently reported grade 3 or 4 adverse events that might be attributed to TP regimen, and have established management strategies in the clinical treatment of cancer. The most common Grade 3 or higher anti-angiogenic therapy related AEs was hypertension with incidences of 13%, which were generally consistent with those reported in previous studies in lung cancer and thyroid carcinoma. In this study, patients with hypertension were effectively controlled by taking antihypertensive drugs, and no patients were interrupted due to this. No perioperative grade 3–4 bleeding events were observed, and no delay in healing at the surgical site was observed in patients. However, in the study of GeparQuinto and ARTemis, four and five grade 3–4 bleeding events were reported, respectively. This may be related to the shorter half-life of anlotinib compared to bevacizumab and the strategy of discontinuing anlotinib in the last cycle of the trial design. Therefore, the addition of anlotinib to taxane and lobaplatin as NAC regimen did not increase the risk of severe toxicity, and the overall safety of this regimen could be managed.
Our study had three main limitations. Firstly, the combination of chemotherapy and antiangiogenic therapy has not been compared to chemotherapy alone for efficacy and safety, so it is not possible to assess the absolute benefit of adding anlotinib to TNBC. Secondly, this trial was conducted at a single center in China with a small sample size, therefore certain subtypes of participants may be overrepresented in this study, such as tumors with grade 1–2, TILs counts<30% and the LAR subtype. Thirdly, the results of the subgroup analysis need to be interpreted with caution due to the small sample size, as well as the lack of long-term follow-up findings. Nevertheless, some obvious trends in clinical-pathologic factors and gene-mutation indicators have implications for making treatment decisions and the direction of future research.
Our study is the first to combine a novel, multi-target, anti-tumor TKI, anlotinib, with NAC for TNBC. Anlotinib plus taxane and lobaplatin achieved encouraging anti-tumor activity and controllable toxicity. The findings of neoALTAL trial support further evaluation of anlotinib plus taxane and platinum as an alternative neoadjuvant treatment regimen for patients with TNBC who are not eligible for chemoimmunotherapy or anthracycline-based regimens in phase 2–3 randomized studies.
Contributors
Dr. XWQ, YZ, XWB and JJ have accessed and verified the data, and all authors were responsible for the decision to submit the manuscript. Dr. YL, JL, JG and QYS are considered co-first authors. Dr. XWQ, YZ, XWB and JJ are considered co-corresponding authors.
Concept and design: XWQ, YL, JL, YZ, JJ, XWB, JG, QYS.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: XWQ, YL, JL, YZ, JG, QYS.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: XWQ, YL, QYS.
Administrative, technical, or material support: All authors.
Supervision: XWQ, YL, JL, YZ, JJ, XWB, JG, QYS.
Data sharing statement
The study's anonymized data will be made available for applications to the Research Administration Department of Southwest Hospital. The trial protocol and statistical analysis will also be available.
Declaration of interests
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by grants from Chongqing Talents Project (grant number 414Z393), Chongqing Key Project of Technology Innovation and Application Development (grant number CSTB2022TIAD-KPX0168) and Chongqing Outstanding Youth Natural Science Foundation (No. CSTB2023NSCQ-JQX0012). We also acknowledge Chunman Wu, the medical manager of Nanjing Geneseeq Technology Inc, for her support in the interpretation of genetic testing data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102585.
Contributor Information
Jun Jiang, Email: jcbd@medmail.com.cn.
Xiuwu Bian, Email: bianxiuwu@263.net.
Yi Zhang, Email: ZY53810@163.com.
Xiaowei Qi, Email: qxw9908@tmmu.edu.cn.
Appendix A. Supplementary data
3
Supplement Figure S1.
The pathological complete response (pCR) and residual cancer burden (RCB) in the per-protocol population (PP). (A) pCR, bpCR and apCR; (B) RCB.
Supplement Figure S2.
Clinicalpathological and genetic subgroups analysis of residual cancer burden (RCB). (A) RCB; (B) relative risk (RR) of different clinicalpathological and genomic characteristics.
References
- 1.Poggio F., Tagliamento M., Ceppi M., et al. Adding a platinum agent to neoadjuvant chemotherapy for triple-negative breast cancer: the end of the debate. Ann Oncol. 2022;33:347–349. doi: 10.1016/j.annonc.2021.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Weng Z.-J., Wu S.-X., Luo H.-S., Du Z.-S., Li X.-Y., Lin J.-Z. Neoadjuvant chemotherapy in early triple-negative breast cancer: a pairwise and network meta-analysis of pathological complete response. Inq J Health Care Organ Provis Financ. 2021;58 doi: 10.1177/00469580211056213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ecancer Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: a phase Ill randomized controlled trial n.d. http://ecancer.org/en/conference/1369-sabcs-2022
- 4.Tian H., Ma D., Tan X., et al. Platinum and taxane based adjuvant and neoadjuvant chemotherapy in early triple-negative breast cancer: a narrative review. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.770663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan M., Yuan P., Ouyang Q., et al. A prospective, open-label, multicenter phase IV clinical trial on the safety and efficacy of lobaplatin-based chemotherapy in advanced breast cancer. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221122715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv X., Cao X., Xia W.-X., et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:716–726. doi: 10.1016/S1470-2045(21)00075-9. [DOI] [PubMed] [Google Scholar]
- 7.Wu X., Tang P., Li S., et al. A randomized and open-label phase II trial reports the efficacy of neoadjuvant lobaplatin in breast cancer. Nat Commun. 2018;9:832. doi: 10.1038/s41467-018-03210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan W., Wu X., Wang S., et al. Lobaplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: a 5-year follow-up of a randomized, open-label, phase II trial. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber B., Loibl S., Eidtmann H., et al. Neoadjuvant bevacizumab and anthracycline–taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44) Ann Oncol. 2013;24:2978–2984. doi: 10.1093/annonc/mdt361. [DOI] [PubMed] [Google Scholar]
- 10.Von Minckwitz G., Schneeweiss A., Loibl S., et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.R., Jung K.H., Im S.-A., et al. Multicentre phase II trial of bevacizumab combined with docetaxel-carboplatin for the neoadjuvant treatment of triple-negative breast cancer (KCSG BR-0905) Ann Oncol. 2013;24:1485–1490. doi: 10.1093/annonc/mds658. [DOI] [PubMed] [Google Scholar]
- 12.Sikov W.M., Berry D.A., Perou C.M., et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syed Y.Y. Anlotinib: first global approval. Drugs. 2018;78:1057–1062. doi: 10.1007/s40265-018-0939-x. [DOI] [PubMed] [Google Scholar]
- 14.Li D., Chi Y., Chen X., et al. Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase IIB trial. Clin Cancer Res. 2021;27:3567–3575. doi: 10.1158/1078-0432.CCR-20-2950. [DOI] [PubMed] [Google Scholar]
- 15.Liu B., Liu L., Ran J., et al. A randomized trial of eribulin monotherapy versus eribulin plus anlotinib in patients with locally recurrent or metastatic breast cancer. ESMO Open. 2023;8 doi: 10.1016/j.esmoop.2023.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu N., Si Y., Yue J., et al. Anlotinib has good efficacy and low toxicity: a phase II study of anlotinib in pre-treated HER-2 negative metastatic breast cancer. Cancer Biol Med. 2021;18:849–859. doi: 10.20892/j.issn.2095-3941.2020.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Sun T., Ouyang Q., Han Y., Xu B. A phase Ib study of TQB2450 plus anlotinib in patients with advanced triple-negative breast cancer. iScience. 2023;26 doi: 10.1016/j.isci.2023.106876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han B., Li K., Wang Q., et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non–small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S., Ma D., Xiao Y., et al. Molecular subtyping of triple-negative breast cancers by immunohistochemistry: molecular basis and clinical relevance. Oncol. 2020;25:e1481–e1491. doi: 10.1634/theoncologist.2019-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni L., Huang C.S., Egle D., et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33:534–543. doi: 10.1016/j.annonc.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Gluz O., Nitz U., Liedtke C., et al. Comparison of neoadjuvant nab-Paclitaxel+Carboplatin vs nab-Paclitaxel+Gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. JNCI J Natl Cancer Inst. 2018;110:628–637. doi: 10.1093/jnci/djx258. [DOI] [PubMed] [Google Scholar]
- 22.Ou K.P., Li Q., Luo Y., et al. [Efficacy and safety of neoadjuvant apatinib in combination with dose-dense paclitaxel and carboplatin in locally advanced triple negative breast cancer patients] Zhonghua Zhongliu Zazhi. 2020;42:966–971. doi: 10.3760/cma.j.cn112152-20200224-00122. [DOI] [PubMed] [Google Scholar]
- 23.Liu J., He M., Ou K., et al. Efficacy and safety of apatinib combined with dose-dense paclitaxel and carboplatin in neoadjuvant therapy for locally advanced triple-negative breast cancer: a prospective cohort study with propensity-matched analysis. Int J Cancer. 2024;154:133–144. doi: 10.1002/ijc.34717. [DOI] [PubMed] [Google Scholar]
- 24.Loibl S., Untch M., Burchardi N., et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorf E.A., Zhang H., Barrios C.H., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion 031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 26.Schmid P., Cortes J., Pusztai L., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P., Stecklein S.R., Yoder R., et al. Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer: NeoPACT phase 2 clinical trial. JAMA Oncol. 2023 doi: 10.1001/jamaoncol.2023.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y.-Z., Ma D., Suo C., et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35:428–440.e5. doi: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S., Qin T., Liu Z., et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11:309. doi: 10.1038/s41419-020-2511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S.-Y., Xu Y., Chen L., et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Mol Cancer. 2022;21:84. doi: 10.1186/s12943-022-01536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L., Wang Z.-H., Ma L.-X., et al. Optimising first-line subtyping-based therapy in triple-negative breast cancer (FUTURE-SUPER): a multi-cohort, randomised, phase 2 trial. Lancet Oncol. 2024;25:184–197. doi: 10.1016/S1470-2045(23)00579-X. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Wang Y., Tian Z., et al. Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat Commun. 2022;13:3011. doi: 10.1038/s41467-022-30569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Olopade O.I. MYC in breast tumor progression. Expert Rev Anticancer Ther. 2008;8:1689–1698. doi: 10.1586/14737140.8.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasching P.A., Loibl S., Hu C., et al. BRCA1/2 mutations and bevacizumab in the neoadjuvant treatment of breast cancer: response and prognosis results in patients with triple-negative breast cancer from the GeparQuinto study. J Clin Oncol. 2018;36:2281–2287. doi: 10.1200/JCO.2017.77.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3