Abstract
Demographic histories are frequently a product of the environment, as populations expand or contract in response to major environmental changes, often driven by changes in climate. Meso‐ and bathy‐pelagic fishes inhabit some of the most temporally and spatially stable habitats on the planet. The stability of the deep‐pelagic could make deep‐pelagic fishes resistant to the demographic instability commonly reported in fish species inhabiting other marine habitats, however the demographic histories of deep‐pelagic fishes are unknown. We reconstructed the historical demography of 11 species of deep‐pelagic fishes using mitochondrial and nuclear DNA sequence data. We uncovered widespread evidence of population expansions in our study species, a counterintuitive result based on the nature of deep‐pelagic ecosystems. Frequency‐based methods detected potential demographic changes in nine species of fishes, while extended Bayesian skyline plots identified population expansions in four species. These results suggest that despite the relatively stable nature of the deep‐pelagic environment, the fishes that reside here have likely been impacted by past changes in climate. Further investigation is necessary to better understand how deep‐pelagic fishes, by far Earth's most abundant vertebrates, will respond to future climatic changes.
Keywords: bathypelagic, climate change, mesopelagic, population ecology, population genetics
In this study, we tested the hypothesis that a stable environment should lead to stable demographic histories for the species occupying that environment. We used molecular data to investigate the demographic histories of 11 species of fishes residing in the deep‐pelagic, one of the most stable environments on the planet. Contrary to expectations, we inferred multiple changes in effective population size in the study species.

1. INTRODUCTION
The demographic history of a species is strongly influenced by the environment it inhabits (Alheit & Hagen, 1997; Avise, 2000; Grant, 2015). Major changes in the environment can alter the distribution and size of suitable habitat for a species, reducing or increasing the species' range. Population sizes expand or contract in response to these fluctuations in habitat suitability (Avise, 2000; Nye et al., 2009). Evidence for the environment's control over population dynamics can be seen across taxonomic groups in terrestrial and marine habitats around the world (Almada et al., 2012; Eytan & Hellberg, 2010; Grant, 2015; Robalo et al., 2012).
Given that changes in environmental conditions strongly influence population size, species inhabiting unstable environments should be characterized by unstable population sizes. On the other hand, species inhabiting temporally stable environments should be less susceptible to frequent population expansions or contractions due to global or regional climatic events. Studies have supported this notion, finding genetic diversity to be greater in species inhabiting more stable environments than closely related species in environments more subject to change (Carnaval et al., 2009; Gugger et al., 2013).
The open ocean mesopelagic (200–1000 m depth) and bathypelagic (1000 m to approximately 100 m above the sea floor) domains (deep‐pelagic, cumulatively) are arguably the most temporally and spatially stable environments on the planet. In terms of physical characteristics like temperature, there is a strong latitudinal homogeneity in the environment that increases with depth (Robison, 2009). In comparison to shallower habitats, temperature change occurs slowly and the magnitude of change is less (Abraham et al., 2013; Clark et al., 2006, 2009; Levitus et al., 2012; Mora et al., 2013; Robison, 2009). Regarding age, the deep‐pelagic milieu has existed longer than continents; while the latter have shifted position, uplifted, submerged, and fractionated, the former have remained relatively unchanged, inter‐ocean connectivity notwithstanding. Based on the age and stability of the environment, as well as our current understanding of the manner in which habitat influences demography, the population sizes of the fishes inhabiting the deep‐pelagic would be expected to be stable over time.
If the demographic histories of deep‐pelagic fishes include population size fluctuations, it is difficult to predict which physical factors could drive this instability. Molecular analyses are frequently used to reconstruct and infer historical demography, however molecular investigations into the historical demography of deep‐sea organisms are few and have focused on deep‐benthic species (Etter et al., 2005; Sakuma et al., 2014; Varela et al., 2012). The deep‐benthic environment, benthic habitat found below 200 m depth, is much more heterogeneous than the deep‐pelagic and likely under differing environmental pressures (Sutton et al., 2017; Thurber et al., 2014; Watling et al., 2013). Recent publications based on the fossil record have reported local changes in deep‐pelagic fish abundance and community composition (Lin et al., 2023; Salvatteci et al., 2022). These observations could be indicative of range changes and fluctuations in population size. Correlations between climate have been made to these findings, but the precise mechanism driving the phenomenon is not yet known.
The physical factors influencing the historical demography of marine fishes inhabiting the less homogenous coastal and epipelagic (upper 200 m of the ocean water column) zones are better understood. Studies have consistently shown population size changes that correspond with major changes in these environments that would not be expected in the more homogenous, ‘sheltered’ deep‐pelagic domain. A plurality of these studies indicates widespread population expansions in shallow‐dwelling fishes following the last glacial maximum (Avise, 2000; Eytan & Hellberg, 2010; Grant, 2015; Robalo et al., 2012). Two factors are frequently cited to explain increases in geographic range and a corresponding increase in population size: an increase in global sea‐surface temperatures and sea‐level rise that dramatically increased shelf habitats (Avise, 2000; Eytan & Hellberg, 2010; Grant, 2015) During this period of great change for shallow marine habitats, the deep‐pelagic experienced far smaller changes in temperature, and the amount of deep‐pelagic habitat would have increased negligibly (Abraham et al., 2013; Clark et al., 2006, 2009; Levitus et al., 2012; Robison, 2009).
If the demographic histories of deep‐pelagic fishes reveal large‐scale population fluctuations, it is possible that physical conditions external to the deep‐pelagic domain drive these dynamics. One habit shared by many deep‐pelagic fishes could be responsible, diel vertical migration.
Most mesopelagic fish species perform diel vertical migrations, a nocturnal migration to the shallower and more variable epipelagic waters and a diurnal return to mesopelagic depths (Barham, 1966; Sutton, 2013). Vertical migrations by bathypelagic fishes, while not unknown are much less common (Cook et al., 2013). An analysis of the distribution of mesopelagic fishes found that the ranges of vertically migrating species were more likely to change in response to large‐scale changes in climate than the ranges of species that do not vertically migrate (Hsieh et al., 2009). This could be a result of the greater influence of atmospheric heating on the upper ocean than deep waters. If the changes in surface waters are no longer physiologically tolerable to vertically migrating fishes, then these species would no longer persist in their former range. If vertical migratory behavior alone drives demography in deep‐pelagic fishes, vertical migrators should be characterized by population expansions and/or contractions, while the population sizes of non‐vertical migrators should be relatively stable over time.
Given the current lack of knowledge regarding deep‐pelagic fish historical demography, we sought to investigate the demographic history of 11 species inhabiting this environment, using two sets of molecular based analyses: frequency‐based tests and gene tree‐based analyses. These tests can infer demographic events such as population expansion and complement one another. Knowledge of the demographic history of deep‐pelagic fishes serves two key purposes. First, it will provide insight into the ecological processes driving population dynamics in the world's largest and most environmentally stable ecosystem. These insights provide the basis for an ideal case study to test the hypothesis that a stable environment should in turn lead to stable demographic histories. Second, understanding how deep‐pelagic fishes responded to past climatic events will allow us to make predictions about how they will respond to future changes in climate.
2. METHODS
2.1. Sampling and sequence generation
We selected 11 deep‐pelagic species that span phylogenetic lineages, life histories, and vertical migration behavior (see Table 1). Samples were obtained by trawling with a MOCNESS (Multiple Opening and Closing Net and Environmental Sensing System) in discrete depth zones from the surface to 1500 m depth in the northern Gulf of Mexico (GOM) (see Figure 1 for sampling locations). Upon collection and identification of vouchers at sea, a ~1 cm strip of lateral muscle tissue was preserved in 95% non‐denatured ethanol, stored at −20°C while at sea, and moved to long‐term storage at −80°C when back on land. Voucher specimens are housed in the Ocean Ecology Lab at Nova Southeastern University pending accession into a permanently curated fish collection.
TABLE 1.
Life history traits and taxonomic data for the study species.
| Species | Family | Order | Diel vertical migrators | Upper depth of occurrence | Lower depth of occurrence | Total depth range | References |
|---|---|---|---|---|---|---|---|
| Bathophilus pawneei | Stomiidae | Stomiiformes | Yes | 0 | 1500 | 1500 | McEachran and Fechhelm (1998) and Sutton and Hopkins (1996) |
| Chauliodus sloani | Stomiidae | Stomiiformes | Yes | 0 | 1800 | 1800 | McEachran and Fechhelm (1998), Clarke (1983) and Sutton and Hopkins (1996) |
| Cyclothone alba | Gonostomatidae | Stomiiformes | No | 300 | 600 | 300 | McEachran and Fechhelm (1998) and Miya and Nemoto (1986) |
| Cyclothone pseudopallida | Gonostomatidae | Stomiiformes | No | 300 | 900 | 600 | McEachran and Fechhelm (1998) and Miya and Nemoto (1986) |
| Diplospinus multistriatus | Gempylidae | Perciformes | Yes | 100 | 1000 | 900 | McEachran and Fechhelm (1998) and Clarke and Wagner (1976) |
| Ditropichthys storeri | Cetomimidae | Stephanoberyciformes | No | 650 | 2150 | 1500 | McEachran and Fechhelm (1998) and Paxton (1989) |
| Photostomias guernei | Stomiidae | Stomiiformes | Yes | 15 | 800 | 785 | Clarke (1983) and Sutton and Hopkins (1996) |
| Scopelogadus mizolepis | Melamphaidae | Stephanoberyciformes | Yes | 100 | 1000 | 900 | McEachran and Fechhelm (1998), Clarke (1983) and Clarke and Wagner (1976) |
| Sigmops elongatus | Gonostomatidae | Stomiiformes | Yes | 50 | 1200 | 1150 | McEachran and Fechhelm (1998) and Lancraft et al. (1988) |
| Sternoptyx pseudobscura | Sternoptychidae | Stomiiformes | No | 800 | 1500 | 700 | McEachran and Fechhelm (1998) |
| Stomias affinis | Stomiidae | Stomiiformes | Yes | 50 | 850 | 800 | McEachran and Fechhelm (1998) and Sutton and Hopkins (1996) |
FIGURE 1.
Map of the sampling locations. Station locations are indicated by the black dots, and depth is indicated according to color.
DNA was extracted from tissues using a Qiagen DNEasy Blood & Tissue extraction kit (Germantown, MD, USA). We generated DNA sequence data from the mitochondrial gene cytochrome oxidase I (COI) as well as three nuclear DNA exons (PLAG, ENC, and MYH). PCR was performed using Promega GoTAQ (Madison, Wisconsin, USA) (see Table A2 for primers used). Following amplification, all PCR products were cleaned using a standard PEG protocol (Glenn, 2019). Amplicons were Sanger‐sequenced on an ABI 3730 capillary sequencer at Yale Keck Biotechnology Resource Laboratory. Sequences were cleaned and edited in Sequencher v5.1. Nuclear markers were phased using Phase v2.1 to resolve heterozygous sites (Stephens & Scheet, 2001, 2005). The sequences were then aligned using MAFFT in Geneiousv9.1.8 (Kearse et al., 2012).
2.2. Frequency‐based analyses
We calculated Tajima's D, Fu's F s, and R 2 for each marker (Fu, 1997; Ramos‐Onsins & Rozas, 2002; Tajima, 1989). Comparisons of the statistical power of frequency‐based tests have shown that F s and R 2 are the most capable of detecting population growth (Ramos‐Onsins & Rozas, 2002). They complement one another as well, with F s excelling at population growth detection in large sample sizes, while R 2 performs better with small sample sizes. A significant and large negative F s value suggests population growth, while a significant and small positive R 2 value indicates population growth. Tajimas's D points to population growth and/or a selective sweep when significant and negative.
All of the frequency‐based tests were performed in DNAsp v6 (Rozas et al., 2017). Ambiguity codes were replaced with Ns to allow for calculation in DNAsp. Significance of Tajima's D results are determined by the test itself. The significance of all three tests was also determined using coalescent simulations with 1000 replicates implemented in DNAsp.
2.3. Gene tree‐based analysis
The second set of tests makes use of the topologies and branch lengths of gene trees to infer changes in population size over time using the coalescent. We performed these analyses in BEAST v2.4.7 (Bouckaert et al., 2014) to generate extended Bayesian skyline plots (EBSPs). EBSPs utilize coalescent theory and a Markov Chain Monte Carlo Algorithm to infer and visualize demographic changes in a dataset. The Bayesian skyline plot is preferable to earlier skyline plot methods as it models both genealogy and demographic history simultaneously, which reduces error rates from uncertainty in estimates of node time (Heled & Drummond, 2008; Ho & Shapiro, 2011).
Nuclear and mitochondrial genes were included in the analysis for each species. The chain length was set to 50,000,000 sampling every 1000. COI rates were fixed, while the nuclear rates were allowed to vary. The partitioning scheme and substitution models were set based on PartitionFinder v2.0 results (Lanfear et al., 2012). A second set of trees was created using the same methodology, with the exception of the selection of substitution models. All partitions were set to the RBS substitution model (Bouckaert et al., 2014). RBS is a reversible‐jump based substitution model for nucleotide data. This substitution model does not require a fixed substitution model to be assigned to each partition at the beginning of the analysis. Instead, it allows five different substitution models to be explored through the run, to find the substitution model with the best fit to the dataset.
After running in BEAST, log files for both sets of trees were inspected using Tracer v 1.7.1 (Rambaut et al., 2014). The most strongly supported EBSP analysis, based on ESS values, was selected and used for the inference of each species' demographic history. The posterior estimate of the number of population size changes provided a test for a rejection of constant population size. A stable demographic history can be rejected in species that do not include a possibility of zero demographic events in this posterior estimate. Finally, the trees files were uploaded to Rstudio v 0.99.484 (Studio 2012). The Rscript “plotEBSP”, provided with the EBSP tutorial (http://www.beast2.org/files/2016/01/ebsp2‐tut.zip), was used to generate and visualize the extended Bayesian skyline plots to understand the nature of these demographic events (Heled, 2010).
We treated each species as a single population for the purpose of these analyses. The accuracy of EBSP results require that the sequences were derived from a single population. The small size of the sampling area (Figure 1) and lack of geographic barriers for dispersal, make this a reasonable assumption. This assumption is supported by genome wide, temporal (multi‐year) analyses of three species within the deep‐pelagic family Myctophidae that reside in the northern Gulf of Mexico (Bernard et al., 2022). Very little instraspecific structure was found, and the authors characterize the GOM lanternfishes as largely panmictic. The samples used in our study were collected alongside those used in the Myctophid research. Given the overlap in habitat, range, and life history traits between lanternfishes and our study species, we believe it is reasonable to assume panmictic populations for our study species within the northern Gulf of Mexico.
2.4. Population dynamics and vertical migration
We placed species into two categories; those that had undergone an inferred population size change and those that had not. Species placed into the “inferred population size change” group were categorized as such if the inference was uncovered in both the frequency‐based and gene tree‐based analyses. We further divided species into vertical migrators and non‐vertical migrators. The migration type for each species can be found in Table 1. A chi‐squared test was used to test for a correlation between inferred population size changes and vertical migration.
3. RESULTS
3.1. Summary
The number of sequences generated for each species and marker varied according to sample availability and our ability to achieve amplification. The number of unique sequences obtained for each gene ranged from 10 to 97 (Table 2). The COI dataset included a low of 10 of sequences (Bathophilus pawneei) and a high of 97 sequences (Chauliodus sloani). The PLAG dataset included a low of 10 sequences (B. pawneei, Cyclothone pseudopallida, and Photostomias guernei) and a high of 17 sequences (Sternoptyx pseudobscura) (see Figure 2 for Photo of P. guernei). The ENC dataset included a low of 12 sequences (Diplospinus multistriatus) and a high of 16 sequences (S. pseudobscura). Finally, the MYH dataset included 11 sequences (S. pseudobscura) and 15 sequences (Stomias affinis). We used two genes for analysis in nine species, three genes in one species, and four genes in one species (Table 2). All sequences have been deposited in Genbank (Accession numbers listed in Table A1).
TABLE 2.
Results of frequency‐based statistics analysis.
| Species | COI | PLAG | ENC | MYH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Of sequences | Tajima's D | R 2 | F s | # Of sequences | Tajima's D | R 2 | F s | # Of sequences | Tajima's D | R 2 | F s | # Of sequences | Tajima's D | R 2 | F s | |
| Bathophilus pawneei | 10 | 1.21 | .206 | 1.761 | 10 | −0.395 | .146 | −0.07 | NA | NA | NA | NA | NA | NA | NA | NA |
| Chauliodus Sloani | 97 | −2.124 | .027 | −33.567 | NA | NA | NA | NA | 19 | −2.162 | .046 | −10.151 | NA | NA | NA | NA |
| Cyclothone alba | 12 | −1.831 | .2 | −1.008 | 12 | −1.591 | .068 | −4.89 | NA | NA | NA | NA | NA | NA | NA | NA |
| Cyclothone pseudopallida | 14 | −0.026 | .133 | −0.68 | 10 | −0.023 | .137 | 0.216 | NA | NA | NA | NA | NA | NA | NA | NA |
| Diplospinus multistriatus | 12 | −1.141 | .267 | −0.476 | 12 | −0.163 | .126 | 0.2 | 12 | −1.863 | .073 | −5.836 | NA | NA | NA | NA |
| Ditropichthys storeri | 11 | −0.796 | .137 | −0.865 | 10 | −2.186 | .074 | −5.778 | NA | NA | NA | NA | NA | NA | NA | NA |
| Photostomias guernei | 12 | −1.83 | .096 | −3.216 | 12 | −1.346 | .086 | −2.582 | NA | NA | NA | NA | NA | NA | NA | NA |
| Scopelogaus mizolepis | 11 | −0.786 | .182 | −2.995 | 11 | −1.165 | .121 | −0.097 | NA | NA | NA | NA | NA | NA | NA | NA |
| Sigmops elongatus | 12 | −1.141 | .227 | −0.476 | 12 | −1.494 | .096 | −2.383 | NA | NA | NA | NA | NA | NA | NA | NA |
| Sternoptyx pseudobscura | 13 | −1.149 | .227 | −0.537 | 17 | −1.993 | .046 | −9.189 | 16 | −2.41 | .059 | −6.027 | 15 | −1.346 | .074 | −2.209 |
| Stomias affinis | 11 | −1.673 | .07 | −8.668 | NA | NA | NA | NA | NA | NA | NA | NA | 11 | −0.477 | .115 | 0.43 |
Note: Tajima's D values that were significant based on the two‐tailed test are dark gray. Significant values determined through coalescent simulations are highlighted in light gray.
FIGURE 2.

Photo of Photostomias guernei. Photo by Dante Fenolio.
3.2. Frequency‐based analyses
Frequency‐based analyses recovered population expansions in 9 of our 11 sampled species (Table 2). In four species (C. sloani, S. pseudobscura, Cyclothone alba, and P. guernei) more than half of the frequency‐based tests for all markers inferred population size changes. Weaker support was present in another five species (D. multistriatus, Ditropichthys storeri, Scopelogadus mizolepis, Sigmops elongatus, and S. affinis), where less than half of the markers tested produced significant results. No evidence for demographic change was present in B. pawneei or C. pseudopallida.
3.3. Gene tree based analysis
We were able to reject a stable demographic history in four of the 11 species; C. alba, C. sloani, P. guernei, and S. pseudobscura, based on the posterior estimate of the number of population size changes generated in the analyses (Table 3). The estimates suggest a minimum one demographic event and maximum of three demographic events. Population expansions were inferred in every case based on the EBSPs (Figure 3). These four species also shared the strongest evidence for population size changes based on the frequency‐based analyses.
TABLE 3.
Posterior estimate of population sizes changes.
| Study species | Reject constant population | Posterior estimate of population size changes |
|---|---|---|
| Bathophilus pawneii | No | [0, 3] |
| Chauliodus Sloani | Yes | [1, 3] |
| Cyclothone alba | Yes | [1,3] |
| Cyclothone pseudopallida | No | [0,3] |
| Diplospinus multistriatus | No | [0,3] |
| Ditropichthys storeri | No | [0,3] |
| Photostomias guernei | No | [1,3] |
| Scopelogaus mizolepis | No | [0,3] |
| Sigmops elongatus | No | [0,3] |
| Sternoptyx pseudobscura | Yes | [1,3] |
| Stomias affinis | No | [0,3] |
Note: These estimates were generated using the gene tree based analyses and provide a test to reject a stable demographic history.
FIGURE 3.
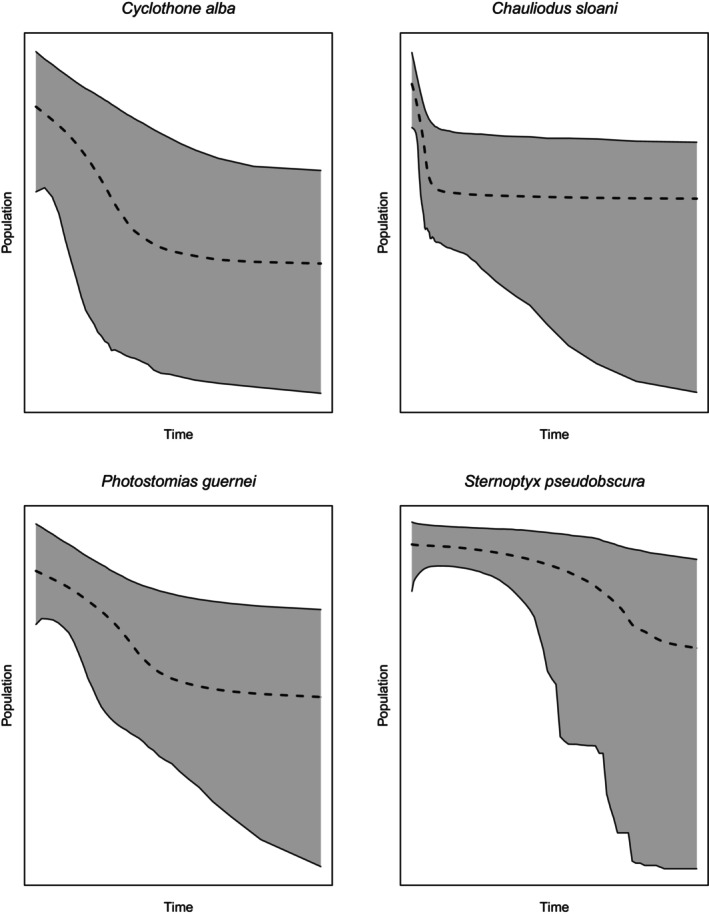
Extended Bayesian skyline plots. The x‐axis represents time, with the left side of the axis being the most recent time point. The y‐axis represents relative population size on a log scale. The gray area displays the 95% central posterior density.
3.4. Vertical migration and population dynamics
The chi‐squared test did not provide support for a relationship between vertical migration and population size changes (p‐value .8190, Table 4). Of the four species with strong support for population expansions, two (C. sloani and P. guernei) are vertical migrators while two (C. alba and S. pseudobscura) do not vertically migrate.
TABLE 4.
Chi‐squared test for significance of vertical migration on the inference of recent population size changes.
| Pop size change inferred | No pop size change inferred | |||
|---|---|---|---|---|
| Vertical migrator | 4 | 3 | Chi‐squared | 0.0524 |
| Non vertical migrator | 2 | 2 | p‐Value | 0.819 |
4. DISCUSSION
Historic changes in population size are frequently inferred for marine fishes and attributed to major ecological events and past climatic change (Avise, 2000; Grant, 2015). Previous molecular studies however, have largely focused on marine species inhabiting shallower environments that are more variable over time in terms of physical conditions such as temperature in comparison to the deep‐pelagic. Given the temporal and abiotic stability of the deep‐pelagic environment, population sizes of fishes inhabiting this environment might also be predicted to be stable, so that no effective population size changes would be inferred from genetic examination. Nonetheless, we uncovered multiple lines of evidence suggesting population expansions in four of the 11 study species, while demographic events were inferred in an additional five species using frequency‐based tests.
4.1. Interpretation of frequency‐based statistics
Departures from neutrality frequently occur due to past demographic events, however other factors such as selective sweeps and reproductive skew may lead to departures from neutrality as well (Birkner et al., 2013; Eldon et al., 2015; Montano, 2016). This has been shown to be true in several marine fishes (Eldon et al., 2015; Niwa et al., 2016). We sought to avoid such issues by employing a multilocus approach, including both mitochondrial and nuclear genes. Selection could be leading to a positive result on a single gene, however it would be unlikely to be acting on multiple unrelated genes in concert.
4.2. Both frequency‐based statistics and EBSPs suggest population expansions
Population expansions were indicated using two different types of analyses that were largely in agreement, however the EBSPs suggested fewer instances of demographic change. It is not surprising that these tests would not agree in every case. Simulated datasets demonstrate that the EBSP analyses are prone to false negatives when using fewer than eight loci (Heled & Drummond, 2008). Given the number of loci sequenced, it seems likely that our EBSPs were more conservative in their inference of population expansions than the frequency‐based tests.
The generation of sequence data was complicated due to the evolutionary distance separating these species, over 200 million years in some cases (Near et al., 2012). Finding primer sets that successfully amplified genes in multiple study species was the limiting to factor to the number of sequences included in this investigation. In the future, the use of high‐throughput sequencing would greatly expand the number of genes available for analyses and provide greater resolution to the demographic histories of the fishes inhabiting the deep‐pelagic, likely increasing the number of demographic expansions that are inferred.
4.3. Genetic evidence furthers recent findings based on the fossil record
While molecular techniques can infer demographic history directly, analyses of the fossil record can uncover trends in local species abundance and community composition over time, which can be indicative of larger demographic trends. Several recent publications have used the fossil record to recreate prehistoric deep‐pelagic fish communities. Salvatteci et al. (2022) identified fossilized vertebrae to compare the community structure of fishes inhabiting Humboldt Current at two points in time (the last interglacial and the Holocene) (Salvatteci et al., 2022). The study includes two deep‐pelagic species, with one species increasing and the other decreasing in frequency in the Holocene sample. Lin et al. 2023) utilized fossilized otoliths to investigate trends in deep‐pelagic fishes in the Warm Pacific Pool over the last 460 thousand years (Lin et al., 2023). They included species from five major deep‐pelagic families and found temporal changes in the number of otoliths present in the fossil record as well as the community composition. While some species remained well represented throughout the record others fluctuated greatly. These inferred changes in community composition and abundance could be a record of large‐scale fluctuations in range and population sizes in deep‐pelagic fishes. Given our widespread inference of population instability, it seems likely that the fossil‐based analyses are identifying the same phenomenon.
4.4. Potential drivers of deep‐pelagic fish population dynamics
Our widespread inference of population expansions in deep‐pelagic fishes was unexpected. Demographic events are typically attributed to major climate changes that alter the environment, in turn increasing or decreasing the amount of optimal habitat for a given species (Avise, 2000; Grant, 2015). Populations expand or contract in response to these alterations in optimal habitat. Because the deep‐pelagic domain has been a relatively stable habitat in terms of its size and temperature for millions of years, it would seem likely that the demographic histories of deep‐pelagic fishes would be characterized by a lack of expansions/contractions (Clark et al., 2009; Levitus et al., 2012; Robison, 2009). Instead, we uncovered a minimum of four cases of population expansion (identified by both frequency‐based statistics and gene tree‐based analysis) and possibly nine cases of population expansion (based on frequency‐based statistics alone).
We proposed and tested one potential driver of population size change in deep‐pelagic fishes, diel vertical migration. We hypothesized that the obligate use of the more volatile epipelagic domain by vertically migrating species may increase their likelihood of undergoing population fluctuations relative to species that do not vertically migrate. Hsieh et al. (2009) provide support for the hypothesis as they reported the larval distribution of fish species with vertically migrating adults changed more rapidly/frequently than non‐vertically migrating species (Hsieh et al., 2009). This could be attributed to short‐term changes in surface water conditions that impact vertically migrating species but are unfelt by those adults that remain at depth. If vertical migratory habits were the primary driver of population dynamics in deep‐pelagic fishes, the demographic histories of vertically migrating species would be characterized by population expansions/contractions, while non‐vertically migrating species should be less variable over time. Based on our chi‐squared test, we were unable to detect any difference in population dynamics between these two groups. Of the four species with the strongest evidence for population expansions, two are vertical migrators and two are non‐vertical migrators. We find vertical migration, to be an unlikely driver of population dynamics in deep‐pelagic fishes.
Another feature of deep‐pelagic fish biology might explain population dynamics in the fishes inhabiting this environment, a pelagic larval phase, where the larvae of most deep‐pelagic fishes reside in the upper 200 m (Bowlin, 2016; Johnson et al., 2009; Moser, 1996). Two lines of evidence support the hypothesis that the physiological tolerances of the larvae residing in the epipelagic domain drive population dynamics in deep‐pelagic fishes: long‐term monitoring of larval distribution and deep‐pelagic patterns of distribution.
Long‐term monitoring efforts in transition zones between tropical and subpolar regions have shown that physical conditions, such as sea surface temperature, are key predictors of larval community composition. Furthermore, physical changes in these environments alter the larval composition of the community (Ahlstrom, 1969; Netburn & Koslow, 2018; Sassa et al., 2004; Urias‐Leyva et al., 2018). Aceves‐Medina et al. (2004) found that the distribution of larvae was congruent with that of the adults. This suggests that as sea surface conditions alter larval distributions, the ranges of adults would change accordingly.
The second piece of evidence for larval control on demography comes from the distribution patterns of deep‐pelagic fishes. Most deep‐pelagic fishes can broadly be classified as warm‐water or cold‐water species, and many species have latitudinal biogeographic boundaries (see Table A3 for range description of study species) (Olson, 2001; Pearcy, 1991; Randall, 1981). Within oceanic basins, latitudinal differences in temperature decrease by depth (Vecchione et al., 2015). By 1000 m depth the temperature is a near uniform 5°C throughout most of the world's oceans (Helfman et al., 2009; Tyus, 2011). It is therefore noteworthy that even some non‐vertically migrating bathypelagic groups such as the whale fishes exhibit strong latitudinal biogeographic boundaries (Paxton, 1989). It seems unlikely that the distribution trends exhibited by deep‐pelagic fishes can be explained by physiological constraints on the adults of these species given the relative homogeneity of the environment. Rather, a given species range is constrained to regions with surface waters tolerable to their larvae. If correct periods of warm SST in high latitudes would increase available habitat to deep‐pelagic larval fishes and lead to population expansions.
Finally, trophic dynamics could potentially drive changes in population size in deep‐pelagic fishes. Vertically migrating fishes in the upper mesopelagic typically forage in surface waters where the food web is supported by recent in situ primary production (Gloeckler et al., 2018; Sutton & Hopkins, 1996). In contrast, some non‐migratory fishes that reside in the lower mesopelagic and the bathypelagic largely rely on a suspended, particle‐based food web composed of degraded particulate organic matter originating in the epipelagic (Crichton et al., 2023; Eduardo et al., 2023; Gloeckler et al., 2018; Hannides et al., 2020). Thus, the amount of carbon available to non‐migratory consumers in the lower mesopelagic and bathypelagic zones is directly linked to the amount of primary production in surface waters and the rate at which it can reach deeper depths.
Changes in ocean temperatures can greatly alter the amount of the particulate organic carbon (POC) that reaches this environment, in turn affecting the amount of food available to support this community (Crichton et al., 2023; John et al., 2013; Olivarez Lyle & Lyle, 2006). Crichton et al. (2023) suggest that bacteria may drive this phenomenon. As oceans warm the metabolic rates of bacteria increase, leading to greater consumption of sinking organic material, and less POC reaching deep waters (Crichton et al., 2023).
The availability of organic carbon at depth may be evident in the fossil record (Boscolo‐Galazzo et al., 2021). Deep‐pelagic formaninifera diversity and abundance increases as ocean temperatures decrease, a feature attributed to a greater volume of food reaching this habitat (Boscolo‐Galazzo et al., 2021; Crichton et al., 2023). This increase in food availability would be expected to positively benefit population sizes for the other members of the deep‐pelagic food web, including fish species. If food availability shapes deep‐pelagic fish demographics, periods of global warming would lead to population contractions while periods of global cooling would lead to population expansions. This feature would be more pronounced in deeper dwelling species.
5. CONCLUSIONS
Insights into the nature of deep‐pelagic fish population dynamics are currently lacking. Our results demonstrate that despite the long‐term stability of the global mesopelagic and bathypelagic domains, the population sizes of the fishes that reside within them are not static in nature. It seems likely that previous changes to the environment, potentially as a result of large‐scale changes in climate, have impacted the fish community residing in the deep‐pelagic. As we continue to investigate the particular environmental factors that influence demographic changes in these fishes, we will better be able to predict how populations of these fishes will behave in the face of future climate change.
AUTHOR CONTRIBUTIONS
Max D. Weber: Conceptualization (lead); data curation (lead); investigation (equal); writing – original draft (lead). Travis M. Richards: Investigation (equal); writing – review and editing (equal). Tracey T. Sutton: Funding acquisition (lead); investigation (equal); writing – review and editing (equal). Joshua E. Carter: Investigation (equal); writing – review and editing (equal). Ron I. Eytan: Funding acquisition (supporting); investigation (equal); resources (lead); supervision (lead); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by a grant from The Gulf of Mexico Research Initiative, and in part by the National Oceanic and Atmospheric Administration's RESTORE Science Program (NA19NOS4510193). Data are publicly available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org. We thank M. Hellberg and two anonymous reviewers for their helpful comments on previous versions of the manuscript. We thank D. Fenolio for the high quality photo of one of our study species (Dante@anotheca.com).
TABLE A1.
List of GenBank accession numbers.
| Species | Gene | Accession number | Specimen ID |
|---|---|---|---|
| Bathophilus pawneei | COI | OP131918 | DPND_1336 |
| Bathophilus pawneei | COI | OP131919 | DPND_1900 |
| Bathophilus pawneei | COI | OP131920 | DPND_1914 |
| Bathophilus pawneei | COI | OP131921 | DPND_1985 |
| Bathophilus pawneei | COI | OP131922 | DPND_2001 |
| Bathophilus pawneei | COI | OP131923 | DPND_2002 |
| Bathophilus pawneei | COI | OP131924 | DPND_2267 |
| Bathophilus pawneei | COI | OP131925 | RIE_0008 |
| Bathophilus pawneei | COI | OP131926 | RIE_0313 |
| Bathophilus pawneei | COI | OP131927 | RIE_0516 |
| Chauliodus sloani | COI | OP132420 | DPND_1241 |
| Chauliodus sloani | COI | OP132421 | DPND_1556 |
| Chauliodus sloani | COI | OP132422 | DPND_1557 |
| Chauliodus sloani | COI | OP132423 | DPND_1605 |
| Chauliodus sloani | COI | OP132424 | DPND_1669 |
| Chauliodus sloani | COI | OP132425 | DPND_1695 |
| Chauliodus sloani | COI | OP132426 | DPND_1877 |
| Chauliodus sloani | COI | OP132427 | DPND_1895 |
| Chauliodus sloani | COI | OP132428 | DPND_1896 |
| Chauliodus sloani | COI | OP132429 | DPND_1937 |
| Chauliodus sloani | COI | OP132430 | DPND_1997 |
| Chauliodus sloani | COI | OP132431 | DPND_2027 |
| Chauliodus sloani | COI | OP132432 | DPND_2037 |
| Chauliodus sloani | COI | OP132433 | DPND_2097 |
| Chauliodus sloani | COI | OP132434 | DPND_2181 |
| Chauliodus sloani | COI | OP132435 | DPND_2208 |
| Chauliodus sloani | COI | OP132436 | DPND_2260 |
| Chauliodus sloani | COI | OP132437 | DPND_2387 |
| Chauliodus sloani | COI | OP132438 | DPND_2418 |
| Chauliodus sloani | COI | OP132439 | DPND_2490 |
| Chauliodus sloani | COI | OP132440 | DPND_2528 |
| Chauliodus sloani | COI | OP132441 | DPND_2699 |
| Chauliodus sloani | COI | OP132442 | DPND_2731 |
| Chauliodus sloani | COI | OP132443 | DPND_2756 |
| Chauliodus sloani | COI | OP132444 | DPND_2757 |
| Chauliodus sloani | COI | OP132445 | DPND_2813 |
| Chauliodus sloani | COI | OP132446 | DPND_2958 |
| Chauliodus sloani | COI | OP132447 | DPND_3621 |
| Chauliodus sloani | COI | OP132448 | DPND_3682 |
| Chauliodus sloani | COI | OP132449 | DPND_3774 |
| Chauliodus sloani | COI | OP132450 | DPND_3789 |
| Chauliodus sloani | COI | OP132451 | DPND_3790 |
| Chauliodus sloani | COI | OP132452 | DPND_3937 |
| Chauliodus sloani | COI | OP132453 | DPND_3938 |
| Chauliodus sloani | COI | OP132454 | DPND_4109 |
| Chauliodus sloani | COI | OP132455 | DPND_4119 |
| Chauliodus sloani | COI | OP132456 | DPND_4120 |
| Chauliodus sloani | COI | OP132457 | DPND_4121 |
| Chauliodus sloani | COI | OP132458 | DPND_4138 |
| Chauliodus sloani | COI | OP132459 | DPND_4139 |
| Chauliodus sloani | COI | OP132460 | DPND_4212 |
| Chauliodus sloani | COI | OP132461 | DPND_4278 |
| Chauliodus sloani | COI | OP132462 | DPND_4465 |
| Chauliodus sloani | COI | OP132463 | DPND_4528 |
| Chauliodus sloani | COI | OP132464 | DPND_4529 |
| Chauliodus sloani | COI | OP132465 | DPND_4546 |
| Chauliodus sloani | COI | OP132466 | DPND_4551 |
| Chauliodus sloani | COI | OP132467 | DPND_4649 |
| Chauliodus sloani | COI | OP132468 | DPND_4693 |
| Chauliodus sloani | COI | OP132469 | DPND_4694 |
| Chauliodus sloani | COI | OP132470 | DPND_4695 |
| Chauliodus sloani | COI | OP132471 | DPND_4736 |
| Chauliodus sloani | COI | OP132472 | DPND_4920 |
| Chauliodus sloani | COI | OP132473 | DPND_4946 |
| Chauliodus sloani | COI | OP132474 | DPND_5014 |
| Chauliodus sloani | COI | OP132475 | DPND_5015 |
| Chauliodus sloani | COI | OP132476 | DPND_5050 |
| Chauliodus sloani | COI | OP132477 | DPND_5126 |
| Chauliodus sloani | COI | OP132478 | DPND_5302 |
| Chauliodus sloani | COI | OP132479 | DPND_5334 |
| Chauliodus sloani | COI | OP132480 | DPND_5335 |
| Chauliodus sloani | COI | OP132481 | DPND_5398 |
| Chauliodus sloani | COI | OP132482 | DPND_5426 |
| Chauliodus sloani | COI | OP132483 | DPND_5427 |
| Chauliodus sloani | COI | OP132484 | DPND_5467 |
| Chauliodus sloani | COI | OP132485 | DPND_5468 |
| Chauliodus sloani | COI | OP132486 | DPND_5500 |
| Chauliodus sloani | COI | OP132487 | DPND_5573 |
| Chauliodus sloani | COI | OP132488 | DPND_5574 |
| Chauliodus sloani | COI | OP132489 | DPND_5593 |
| Chauliodus sloani | COI | OP132490 | DPND_5610 |
| Chauliodus sloani | COI | OP132491 | DPND_5634 |
| Chauliodus sloani | COI | OP132492 | DPND_5665 |
| Chauliodus sloani | COI | OP132493 | DPND_5675 |
| Chauliodus sloani | COI | OP132494 | DPND_5680 |
| Chauliodus sloani | COI | OP132495 | DPND_5682 |
| Chauliodus sloani | COI | OP132496 | DPND_5723 |
| Chauliodus sloani | COI | OP132497 | DPND_5741 |
| Chauliodus sloani | COI | OP132498 | RIE_18 |
| Chauliodus sloani | COI | OP132499 | RIE_236 |
| Chauliodus sloani | COI | OP132500 | RIE_510 |
| Chauliodus sloani | COI | OP132501 | RIE_576 |
| Chauliodus sloani | COI | OP132502 | RIE_642 |
| Chauliodus sloani | COI | OP132503 | RIE_690 |
| Chauliodus sloani | COI | OP132504 | RIE_698 |
| Chauliodus sloani | COI | OP132505 | RIE_912 |
| Chauliodus sloani | COI | OP132506 | RIE_913 |
| Chauliodus sloani | COI | OP132507 | RIE_914 |
| Chauliodus sloani | COI | OP132508 | RIE_948 |
| Chauliodus sloani | COI | OP132509 | RIE_0994 |
| Chauliodus sloani | COI | OP132510 | DPND_5114 |
| Chauliodus sloani | COI | OP132511 | DPND_4579 |
| Chauliodus sloani | COI | OP132512 | DPND_2788 |
| Chauliodus sloani | COI | OP132513 | DPND_3505 |
| Chauliodus sloani | COI | OP132514 | DPND_4717 |
| Chauliodus sloani | COI | OP132515 | DPND_4008 |
| Chauliodus sloani | COI | OP132516 | DPND_3987 |
| Cyclothone alba | COI | OP131971 | RIE_0349 |
| Cyclothone alba | COI | OP131972 | RIE_0350 |
| Cyclothone alba | COI | OP131973 | RIE_0351 |
| Cyclothone alba | COI | OP131974 | RIE_0031 |
| Cyclothone alba | COI | OP131975 | RIE_0252 |
| Cyclothone alba | COI | OP131976 | RIE_0253 |
| Cyclothone alba | COI | OP131977 | RIE_0254 |
| Cyclothone alba | COI | OP131978 | RIE_0255 |
| Cyclothone alba | COI | OP131979 | RIE_0344 |
| Cyclothone alba | COI | OP131980 | RIE_0345 |
| Cyclothone alba | COI | OP131981 | RIE_0347 |
| Cyclothone alba | COI | OP131982 | RIE_0348 |
| Cyclothone pseudopallida | COI | OP131984 | RIE_0359 |
| Cyclothone pseudopallida | COI | OP131985 | RIE_0357 |
| Cyclothone pseudopallida | COI | OP131986 | RIE_0077 |
| Cyclothone pseudopallida | COI | OP131987 | RIE_0354 |
| Cyclothone pseudopallida | COI | OP131988 | RIE_0239 |
| Cyclothone pseudopallida | COI | OP131989 | RIE_0360 |
| Cyclothone pseudopallida | COI | OP131990 | RIE_0477 |
| Cyclothone pseudopallida | COI | OP131991 | RIE_0493 |
| Cyclothone pseudopallida | COI | OP131992 | RIE_0544 |
| Cyclothone pseudopallida | COI | OP131993 | DPND_4547 |
| Cyclothone pseudopallida | COI | OP131994 | DPND_4483 |
| Cyclothone pseudopallida | COI | OP131995 | RIE_0238 |
| Cyclothone pseudopallida | COI | OP131996 | RIE_0358 |
| Cyclothone pseudopallida | COI | OP131997 | RIE_0492 |
| Diplospinus multistriatus | COI | OP132142 | DPND_2718 |
| Diplospinus multistriatus | COI | OP132143 | RIE_0886 |
| Diplospinus multistriatus | COI | OP132144 | RIE_0887 |
| Diplospinus multistriatus | COI | OP132145 | RIE_0171 |
| Diplospinus multistriatus | COI | OP132146 | RIE_0240 |
| Diplospinus multistriatus | COI | OP132147 | RIE_0413 |
| Diplospinus multistriatus | COI | OP132148 | RIE_0495 |
| Diplospinus multistriatus | COI | OP132149 | RIE_0713 |
| Diplospinus multistriatus | COI | OP132150 | RIE_0882 |
| Diplospinus multistriatus | COI | OP132151 | RIE_0883 |
| Diplospinus multistriatus | COI | OP132152 | RIE_0884 |
| Diplospinus multistriatus | COI | OP132153 | RIE_0885 |
| Ditropichthys storeri | COI | OP131959 | DPND_2989 |
| Ditropichthys storeri | COI | OP131960 | DDPND_3302 |
| Ditropichthys storeri | COI | OP131961 | DPND_3911 |
| Ditropichthys storeri | COI | OP131962 | DPND_4130 |
| Ditropichthys storeri | COI | OP131963 | DPND_4210 |
| Ditropichthys storeri | COI | OP131964 | DPND_1466 |
| Ditropichthys storeri | COI | OP131965 | DPND_2251 |
| Ditropichthys storeri | COI | OP131966 | RIE_0438 |
| Ditropichthys storeri | COI | OP131967 | RIE_0522 |
| Ditropichthys storeri | COI | OP131968 | RIE_0243 |
| Ditropichthys storeri | COI | OP131969 | DPND_4431 |
| Photostomias guernei | COI | OP132211 | RIE_0506 |
| Photostomias guernei | COI | OP132212 | RIE_0514 |
| Photostomias guernei | COI | OP132213 | RIE_0551 |
| Photostomias guernei | COI | OP132214 | RIE_0081 |
| Photostomias guernei | COI | OP132215 | RIE_0107 |
| Photostomias guernei | COI | OP132216 | RIE_0176 |
| Photostomias guernei | COI | OP132217 | RIE_0337 |
| Photostomias guernei | COI | OP132218 | RIE_0400 |
| Photostomias guernei | COI | OP132219 | RIE_0401 |
| Photostomias guernei | COI | OP132220 | RIE_0459 |
| Photostomias guernei | COI | OP132221 | RIE_0460 |
| Photostomias guernei | COI | OP132222 | RIE_0461 |
| Scopelogadus mizolepis | COI | OP132154 | DPND_2511 |
| Scopelogadus mizolepis | COI | OP132155 | DPND_2512 |
| Scopelogadus mizolepis | COI | OP132156 | DPND_4271 |
| Scopelogadus mizolepis | COI | OP132157 | DPND_1298 |
| Scopelogadus mizolepis | COI | OP132158 | DPND_1299 |
| Scopelogadus mizolepis | COI | OP132159 | DPND_1379 |
| Scopelogadus mizolepis | COI | OP132160 | DPND_1380 |
| Scopelogadus mizolepis | COI | OP132161 | DPND_2220 |
| Scopelogadus mizolepis | COI | OP132162 | RIE_0041 |
| Scopelogadus mizolepis | COI | OP132163 | RIE_0518 |
| Scopelogadus mizolepis | COI | OP132164 | RIE_0954 |
| Sigmops elongatus | COI | OP132179 | RIE_0278 |
| Sigmops elongatus | COI | OP132180 | RIE_0279 |
| Sigmops elongatus | COI | OP132181 | RIE_0280 |
| Sigmops elongatus | COI | OP132182 | RIE_0002 |
| Sigmops elongatus | COI | OP132183 | RIE_0017 |
| Sigmops elongatus | COI | OP132184 | RIE_0097 |
| Sigmops elongatus | COI | OP132185 | RIE_0182 |
| Sigmops elongatus | COI | OP132186 | RIE_0204 |
| Sigmops elongatus | COI | OP132187 | RIE_0205 |
| Sigmops elongatus | COI | OP132188 | RIE_0206 |
| Sigmops elongatus | COI | OP132189 | RIE_0244 |
| Sigmops elongatus | COI | OP132190 | RIE_0277 |
| Sternoptyx pseudobscura | COI | OP132242 | RIE_0415 |
| Sternoptyx pseudobscura | COI | OP132243 | RIE_0417 |
| Sternoptyx pseudobscura | COI | OP132244 | RIE_0078 |
| Sternoptyx pseudobscura | COI | OP132245 | RIE_0195 |
| Sternoptyx pseudobscura | COI | OP132246 | RIE_0196 |
| Sternoptyx pseudobscura | COI | OP132247 | RIE_0197 |
| Sternoptyx pseudobscura | COI | OP132248 | RIE_0200 |
| Sternoptyx pseudobscura | COI | OP132249 | RIE_0201 |
| Sternoptyx pseudobscura | COI | OP132250 | RIE_0223 |
| Sternoptyx pseudobscura | COI | OP132251 | RIE_0224 |
| Sternoptyx pseudobscura | COI | OP132252 | RIE_0226 |
| Sternoptyx pseudobscura | COI | OP132253 | DPND_3772 |
| Sternoptyx pseudobscura | COI | OP132254 | DPND_4225 |
| Stomias affinis | COI | OP132165 | RIE_0237 |
| Stomias affinis | COI | OP132166 | RIE_0517 |
| Stomias affinis | COI | OP132167 | DPND_3645 |
| Stomias affinis | COI | OP132168 | DPND_1314 |
| Stomias affinis | COI | OP132169 | DPND_1543 |
| Stomias affinis | COI | OP132170 | DPND_1639 |
| Stomias affinis | COI | OP132171 | RIE_0917 |
| Stomias affinis | COI | OP132172 | RIE_0466 |
| Stomias affinis | COI | OP132173 | RIE_0577 |
| Stomias affinis | COI | OP132174 | DPND_1201 |
| Stomias affinis | COI | OP132175 | DPND_1315 |
| Bathophilus pawneei | PLAG1 | OP149759 | DPND_1336 |
| Bathophilus pawneei | PLAG1 | OP149760 | DPND_1336 |
| Bathophilus pawneei | PLAG1 | OP149761 | DPND_1900 |
| Bathophilus pawneei | PLAG1 | OP149762 | DPND_1900 |
| Bathophilus pawneei | PLAG1 | OP149763 | DPND_1914 |
| Bathophilus pawneei | PLAG1 | OP149764 | DPND_1914 |
| Bathophilus pawneei | PLAG1 | OP149765 | DPND_1985 |
| Bathophilus pawneei | PLAG1 | OP149766 | DPND_1985 |
| Bathophilus pawneei | PLAG1 | OP149767 | DPND_2001 |
| Bathophilus pawneei | PLAG1 | OP149768 | DPND_2001 |
| Bathophilus pawneei | PLAG1 | OP149769 | DPND_2002 |
| Bathophilus pawneei | PLAG1 | OP149770 | DPND_2002 |
| Bathophilus pawneei | PLAG1 | OP149771 | DPND_2267 |
| Bathophilus pawneei | PLAG1 | OP149772 | DPND_2267 |
| Bathophilus pawneei | PLAG1 | OP149773 | RIE_313 |
| Bathophilus pawneei | PLAG1 | OP149774 | RIE_313 |
| Bathophilus pawneei | PLAG1 | OP149775 | RIE_516 |
| Bathophilus pawneei | PLAG1 | OP149776 | RIE_516 |
| Bathophilus pawneei | PLAG1 | OP149777 | RIE_8 |
| Bathophilus pawneei | PLAG1 | OP149778 | RIE_8 |
| Cyclothone alba | PLAG1 | OP149779 | RIE_252 |
| Cyclothone alba | PLAG1 | OP149780 | RIE_252 |
| Cyclothone alba | PLAG1 | OP149781 | RIE_253 |
| Cyclothone alba | PLAG1 | OP149782 | RIE_253 |
| Cyclothone alba | PLAG1 | OP149783 | RIE_254 |
| Cyclothone alba | PLAG1 | OP149784 | RIE_254 |
| Cyclothone alba | PLAG1 | OP149785 | RIE_255 |
| Cyclothone alba | PLAG1 | OP149786 | RIE_255 |
| Cyclothone alba | PLAG1 | OP149787 | RIE_31 |
| Cyclothone alba | PLAG1 | OP149788 | RIE_31 |
| Cyclothone alba | PLAG1 | OP149789 | RIE_344 |
| Cyclothone alba | PLAG1 | OP149790 | RIE_344 |
| Cyclothone alba | PLAG1 | OP149791 | RIE_345 |
| Cyclothone alba | PLAG1 | OP149792 | RIE_345 |
| Cyclothone alba | PLAG1 | OP149793 | RIE_347 |
| Cyclothone alba | PLAG1 | OP149794 | RIE_347 |
| Cyclothone alba | PLAG1 | OP149795 | RIE_348 |
| Cyclothone alba | PLAG1 | OP149796 | RIE_348 |
| Cyclothone alba | PLAG1 | OP149797 | RIE_349 |
| Cyclothone alba | PLAG1 | OP149798 | RIE_349 |
| Cyclothone alba | PLAG1 | OP149799 | RIE_350 |
| Cyclothone alba | PLAG1 | OP149800 | RIE_350 |
| Cyclothone alba | PLAG1 | OP149801 | RIE_351 |
| Cyclothone alba | PLAG1 | OP149802 | RIE_351 |
| Cyclothone pseudopallida | PLAG1 | OP149803 | RIE_238 |
| Cyclothone pseudopallida | PLAG1 | OP149804 | RIE_238 |
| Cyclothone pseudopallida | PLAG1 | OP149805 | RIE_239 |
| Cyclothone pseudopallida | PLAG1 | OP149806 | RIE_239 |
| Cyclothone pseudopallida | PLAG1 | OP149807 | RIE_357 |
| Cyclothone pseudopallida | PLAG1 | OP149808 | RIE_357 |
| Cyclothone pseudopallida | PLAG1 | OP149809 | RIE_358 |
| Cyclothone pseudopallida | PLAG1 | OP149810 | RIE_358 |
| Cyclothone pseudopallida | PLAG1 | OP149811 | RIE_359 |
| Cyclothone pseudopallida | PLAG1 | OP149812 | RIE_359 |
| Cyclothone pseudopallida | PLAG1 | OP149813 | RIE_360 |
| Cyclothone pseudopallida | PLAG1 | OP149814 | RIE_360 |
| Cyclothone pseudopallida | PLAG1 | OP149815 | RIE_477 |
| Cyclothone pseudopallida | PLAG1 | OP149816 | RIE_477 |
| Cyclothone pseudopallida | PLAG1 | OP149817 | RIE_492 |
| Cyclothone pseudopallida | PLAG1 | OP149818 | RIE_492 |
| Cyclothone pseudopallida | PLAG1 | OP149819 | RIE_493 |
| Cyclothone pseudopallida | PLAG1 | OP149820 | RIE_493 |
| Cyclothone pseudopallida | PLAG1 | OP149821 | RIE_544 |
| Cyclothone pseudopallida | PLAG1 | OP149822 | RIE_544 |
| Diplospinus multistriatus | PLAG1 | OP149823 | DPND_2718 |
| Diplospinus multistriatus | PLAG1 | OP149824 | DPND_2718 |
| Diplospinus multistriatus | PLAG1 | OP149825 | RIE_171 |
| Diplospinus multistriatus | PLAG1 | OP149826 | RIE_171 |
| Diplospinus multistriatus | PLAG1 | OP149827 | RIE_240 |
| Diplospinus multistriatus | PLAG1 | OP149828 | RIE_240 |
| Diplospinus multistriatus | PLAG1 | OP149829 | RIE_413 |
| Diplospinus multistriatus | PLAG1 | OP149830 | RIE_413 |
| Diplospinus multistriatus | PLAG1 | OP149831 | RIE_495 |
| Diplospinus multistriatus | PLAG1 | OP149832 | RIE_495 |
| Diplospinus multistriatus | PLAG1 | OP149833 | RIE_713 |
| Diplospinus multistriatus | PLAG1 | OP149834 | RIE_713 |
| Diplospinus multistriatus | PLAG1 | OP149835 | RIE_882 |
| Diplospinus multistriatus | PLAG1 | OP149836 | RIE_882 |
| Diplospinus multistriatus | PLAG1 | OP149837 | RIE_883 |
| Diplospinus multistriatus | PLAG1 | OP149838 | RIE_883 |
| Diplospinus multistriatus | PLAG1 | OP149839 | RIE_884 |
| Diplospinus multistriatus | PLAG1 | OP149840 | RIE_884 |
| Diplospinus multistriatus | PLAG1 | OP149841 | RIE_885 |
| Diplospinus multistriatus | PLAG1 | OP149842 | RIE_885 |
| Diplospinus multistriatus | PLAG1 | OP149843 | RIE_886 |
| Diplospinus multistriatus | PLAG1 | OP149844 | RIE_886 |
| Diplospinus multistriatus | PLAG1 | OP149845 | RIE_887 |
| Diplospinus multistriatus | PLAG1 | OP149846 | RIE_887 |
| Ditropichthys storeri | PLAG1 | OP149847 | DPND_1466 |
| Ditropichthys storeri | PLAG1 | OP149848 | DPND_1466 |
| Ditropichthys storeri | PLAG1 | OP149849 | DPND_2251 |
| Ditropichthys storeri | PLAG1 | OP149850 | DPND_2251 |
| Ditropichthys storeri | PLAG1 | OP149851 | DPND_2989 |
| Ditropichthys storeri | PLAG1 | OP149852 | DPND_2989 |
| Ditropichthys storeri | PLAG1 | OP149853 | DPND_3302 |
| Ditropichthys storeri | PLAG1 | OP149854 | DPND_3302 |
| Ditropichthys storeri | PLAG1 | OP149855 | DPND_3911 |
| Ditropichthys storeri | PLAG1 | OP149856 | DPND_3911 |
| Ditropichthys storeri | PLAG1 | OP149857 | DPND_4130 |
| Ditropichthys storeri | PLAG1 | OP149858 | DPND_4130 |
| Ditropichthys storeri | PLAG1 | OP149859 | DPND_4210 |
| Ditropichthys storeri | PLAG1 | OP149860 | DPND_4210 |
| Ditropichthys storeri | PLAG1 | OP149861 | RIE_243 |
| Ditropichthys storeri | PLAG1 | OP149862 | RIE_243 |
| Ditropichthys storeri | PLAG1 | OP149863 | RIE_438 |
| Ditropichthys storeri | PLAG1 | OP149864 | RIE_438 |
| Ditropichthys storeri | PLAG1 | OP149865 | RIE_522 |
| Ditropichthys storeri | PLAG1 | OP149866 | RIE_522 |
| Photostomias guernei | PLAG1 | OP149867 | RIE_107 |
| Photostomias guernei | PLAG1 | OP149868 | RIE_107 |
| Photostomias guernei | PLAG1 | OP149869 | RIE_176 |
| Photostomias guernei | PLAG1 | OP149870 | RIE_176 |
| Photostomias guernei | PLAG1 | OP149871 | RIE_337 |
| Photostomias guernei | PLAG1 | OP149872 | RIE_337 |
| Photostomias guernei | PLAG1 | OP149873 | RIE_400 |
| Photostomias guernei | PLAG1 | OP149874 | RIE_400 |
| Photostomias guernei | PLAG1 | OP149875 | RIE_401 |
| Photostomias guernei | PLAG1 | OP149876 | RIE_401 |
| Photostomias guernei | PLAG1 | OP149877 | RIE_459 |
| Photostomias guernei | PLAG1 | OP149878 | RIE_459 |
| Photostomias guernei | PLAG1 | OP149879 | RIE_461 |
| Photostomias guernei | PLAG1 | OP149880 | RIE_461 |
| Photostomias guernei | PLAG1 | OP149881 | RIE_506 |
| Photostomias guernei | PLAG1 | OP149882 | RIE_506 |
| Photostomias guernei | PLAG1 | OP149883 | RIE_514 |
| Photostomias guernei | PLAG1 | OP149884 | RIE_514 |
| Photostomias guernei | PLAG1 | OP149885 | RIE_551 |
| Photostomias guernei | PLAG1 | OP149886 | RIE_551 |
| Photostomias guernei | PLAG1 | OP149887 | RIE_552 |
| Photostomias guernei | PLAG1 | OP149888 | RIE_552 |
| Photostomias guernei | PLAG1 | OP149889 | RIE_81 |
| Photostomias guernei | PLAG1 | OP149890 | RIE_81 |
| Scopelogadus mizolepis | PLAG1 | OP149891 | DPND_1298 |
| Scopelogadus mizolepis | PLAG1 | OP149892 | DPND_1298 |
| Scopelogadus mizolepis | PLAG1 | OP149893 | DPND_1299 |
| Scopelogadus mizolepis | PLAG1 | OP149894 | DPND_1299 |
| Scopelogadus mizolepis | PLAG1 | OP149895 | DPND_1379 |
| Scopelogadus mizolepis | PLAG1 | OP149896 | DPND_1379 |
| Scopelogadus mizolepis | PLAG1 | OP149897 | DPND_1380 |
| Scopelogadus mizolepis | PLAG1 | OP149898 | DPND_1380 |
| Scopelogadus mizolepis | PLAG1 | OP149899 | DPND_2220 |
| Scopelogadus mizolepis | PLAG1 | OP149900 | DPND_2220 |
| Scopelogadus mizolepis | PLAG1 | OP149901 | DPND_2511 |
| Scopelogadus mizolepis | PLAG1 | OP149902 | DPND_2511 |
| Scopelogadus mizolepis | PLAG1 | OP149903 | DPND_2512 |
| Scopelogadus mizolepis | PLAG1 | OP149904 | DPND_2512 |
| Scopelogadus mizolepis | PLAG1 | OP149905 | DPND_4271 |
| Scopelogadus mizolepis | PLAG1 | OP149906 | DPND_4271 |
| Scopelogadus mizolepis | PLAG1 | OP149907 | RIE_41 |
| Scopelogadus mizolepis | PLAG1 | OP149908 | RIE_41 |
| Scopelogadus mizolepis | PLAG1 | OP149909 | RIE_518 |
| Scopelogadus mizolepis | PLAG1 | OP149910 | RIE_518 |
| Scopelogadus mizolepis | PLAG1 | OP149911 | RIE_954 |
| Scopelogadus mizolepis | PLAG1 | OP149912 | RIE_954 |
| Sigmops elongatus | PLAG1 | OP149913 | RIE_17 |
| Sigmops elongatus | PLAG1 | OP149914 | RIE_17 |
| Sigmops elongatus | PLAG1 | OP149915 | RIE_182 |
| Sigmops elongatus | PLAG1 | OP149916 | RIE_182 |
| Sigmops elongatus | PLAG1 | OP149917 | RIE_204 |
| Sigmops elongatus | PLAG1 | OP149918 | RIE_204 |
| Sigmops elongatus | PLAG1 | OP149919 | RIE_205 |
| Sigmops elongatus | PLAG1 | OP149920 | RIE_205 |
| Sigmops elongatus | PLAG1 | OP149921 | RIE_206 |
| Sigmops elongatus | PLAG1 | OP149922 | RIE_206 |
| Sigmops elongatus | PLAG1 | OP149923 | RIE_244 |
| Sigmops elongatus | PLAG1 | OP149924 | RIE_244 |
| Sigmops elongatus | PLAG1 | OP149925 | RIE_277 |
| Sigmops elongatus | PLAG1 | OP149926 | RIE_277 |
| Sigmops elongatus | PLAG1 | OP149927 | RIE_278 |
| Sigmops elongatus | PLAG1 | OP149928 | RIE_278 |
| Sigmops elongatus | PLAG1 | OP149929 | RIE_279 |
| Sigmops elongatus | PLAG1 | OP149930 | RIE_279 |
| Sigmops elongatus | PLAG1 | OP149931 | RIE_280 |
| Sigmops elongatus | PLAG1 | OP149932 | RIE_280 |
| Sigmops elongatus | PLAG1 | OP149933 | RIE_2 |
| Sigmops elongatus | PLAG1 | OP149934 | RIE_2 |
| Sigmops elongatus | PLAG1 | OP149935 | RIE_97 |
| Sigmops elongatus | PLAG1 | OP149936 | RIE_97 |
| Sternoptyx pseudobscura | PLAG1 | OP149937 | DPND_3772 |
| Sternoptyx pseudobscura | PLAG1 | OP149938 | DPND_3772 |
| Sternoptyx pseudobscura | PLAG1 | OP149939 | RIE_195 |
| Sternoptyx pseudobscura | PLAG1 | OP149940 | RIE_195 |
| Sternoptyx pseudobscura | PLAG1 | OP149941 | RIE_196 |
| Sternoptyx pseudobscura | PLAG1 | OP149942 | RIE_196 |
| Sternoptyx pseudobscura | PLAG1 | OP149943 | RIE_197 |
| Sternoptyx pseudobscura | PLAG1 | OP149944 | RIE_197 |
| Sternoptyx pseudobscura | PLAG1 | OP149945 | RIE_200 |
| Sternoptyx pseudobscura | PLAG1 | OP149946 | RIE_200 |
| Sternoptyx pseudobscura | PLAG1 | OP149947 | RIE_201 |
| Sternoptyx pseudobscura | PLAG1 | OP149948 | RIE_201 |
| Sternoptyx pseudobscura | PLAG1 | OP149949 | RIE_223 |
| Sternoptyx pseudobscura | PLAG1 | OP149950 | RIE_223 |
| Sternoptyx pseudobscura | PLAG1 | OP149951 | RIE_224 |
| Sternoptyx pseudobscura | PLAG1 | OP149952 | RIE_224 |
| Sternoptyx pseudobscura | PLAG1 | OP149953 | RIE_225 |
| Sternoptyx pseudobscura | PLAG1 | OP149954 | RIE_225 |
| Sternoptyx pseudobscura | PLAG1 | OP149955 | RIE_226 |
| Sternoptyx pseudobscura | PLAG1 | OP149956 | RIE_226 |
| Sternoptyx pseudobscura | PLAG1 | OP149957 | RIE_415 |
| Sternoptyx pseudobscura | PLAG1 | OP149958 | RIE_415 |
| Sternoptyx pseudobscura | PLAG1 | OP149959 | RIE_417 |
| Sternoptyx pseudobscura | PLAG1 | OP149960 | RIE_417 |
| Sternoptyx pseudobscura | PLAG1 | OP149961 | RIE_537 |
| Sternoptyx pseudobscura | PLAG1 | OP149962 | RIE_537 |
| Sternoptyx pseudobscura | PLAG1 | OP149963 | RIE_538 |
| Sternoptyx pseudobscura | PLAG1 | OP149964 | RIE_538 |
| Sternoptyx pseudobscura | PLAG1 | OP149965 | RIE_539 |
| Sternoptyx pseudobscura | PLAG1 | OP149966 | RIE_539 |
| Sternoptyx pseudobscura | PLAG1 | OP149967 | RIE_78 |
| Sternoptyx pseudobscura | PLAG1 | OP149968 | RIE_78 |
| Sternoptyx pseudobscura | PLAG1 | OP149969 | RIE_915 |
| Sternoptyx pseudobscura | PLAG1 | OP149970 | RIE_915 |
| Chauliodus sloani | ENC1 | OP149971 | DPND_1556 |
| Chauliodus sloani | ENC1 | OP149972 | DPND_1556 |
| Chauliodus sloani | ENC1 | OP149973 | DPND_1669 |
| Chauliodus sloani | ENC1 | OP149974 | DPND_1669 |
| Chauliodus sloani | ENC1 | OP149975 | DPND_1695 |
| Chauliodus sloani | ENC1 | OP149976 | DPND_1695 |
| Chauliodus sloani | ENC1 | OP149977 | DPND_1877 |
| Chauliodus sloani | ENC1 | OP149978 | DPND_1877 |
| Chauliodus sloani | ENC1 | OP149979 | DPND_1895 |
| Chauliodus sloani | ENC1 | OP149980 | DPND_1895 |
| Chauliodus sloani | ENC1 | OP149981 | DPND_1896 |
| Chauliodus sloani | ENC1 | OP149982 | DPND_1896 |
| Chauliodus sloani | ENC1 | OP149983 | DPND_1937 |
| Chauliodus sloani | ENC1 | OP149984 | DPND_1937 |
| Chauliodus sloani | ENC1 | OP149985 | DPND_2027 |
| Chauliodus sloani | ENC1 | OP149986 | DPND_2027 |
| Chauliodus sloani | ENC1 | OP149987 | DPND_2037 |
| Chauliodus sloani | ENC1 | OP149988 | DPND_2037 |
| Chauliodus sloani | ENC1 | OP149989 | DPND_2097 |
| Chauliodus sloani | ENC1 | OP149990 | DPND_2097 |
| Chauliodus sloani | ENC1 | OP149991 | DPND_2208 |
| Chauliodus sloani | ENC1 | OP149992 | DPND_2208 |
| Chauliodus sloani | ENC1 | OP149993 | DPND_2260 |
| Chauliodus sloani | ENC1 | OP149994 | DPND_2260 |
| Chauliodus sloani | ENC1 | OP149995 | DPND_2387 |
| Chauliodus sloani | ENC1 | OP149996 | DPND_2387 |
| Chauliodus sloani | ENC1 | OP149997 | DPND_2418 |
| Chauliodus sloani | ENC1 | OP149998 | DPND_2418 |
| Chauliodus sloani | ENC1 | OP149999 | DPND_2490 |
| Chauliodus sloani | ENC1 | OP150000 | DPND_2490 |
| Chauliodus sloani | ENC1 | OP150001 | DPND_2528 |
| Chauliodus sloani | ENC1 | OP150002 | DPND_2528 |
| Chauliodus sloani | ENC1 | OP150003 | DPND_2669 |
| Chauliodus sloani | ENC1 | OP150004 | DPND_2669 |
| Chauliodus sloani | ENC1 | OP150005 | DPND_2756 |
| Chauliodus sloani | ENC1 | OP150006 | DPND_2756 |
| Chauliodus sloani | ENC1 | OP150007 | DPND_2757 |
| Chauliodus sloani | ENC1 | OP150008 | DPND_2757 |
| Diplospinus multistriatus | ENC1 | OP150009 | DPND_2718 |
| Diplospinus multistriatus | ENC1 | OP150010 | DPND_2718 |
| Diplospinus multistriatus | ENC1 | OP150011 | RIE_171 |
| Diplospinus multistriatus | ENC1 | OP150012 | RIE_171 |
| Diplospinus multistriatus | ENC1 | OP150013 | RIE_240 |
| Diplospinus multistriatus | ENC1 | OP150014 | RIE_240 |
| Diplospinus multistriatus | ENC1 | OP150015 | RIE_413 |
| Diplospinus multistriatus | ENC1 | OP150016 | RIE_413 |
| Diplospinus multistriatus | ENC1 | OP150017 | RIE_495 |
| Diplospinus multistriatus | ENC1 | OP150018 | RIE_495 |
| Diplospinus multistriatus | ENC1 | OP150019 | RIE_713 |
| Diplospinus multistriatus | ENC1 | OP150020 | RIE_713 |
| Diplospinus multistriatus | ENC1 | OP150021 | RIE_882 |
| Diplospinus multistriatus | ENC1 | OP150022 | RIE_882 |
| Diplospinus multistriatus | ENC1 | OP150023 | RIE_883 |
| Diplospinus multistriatus | ENC1 | OP150024 | RIE_883 |
| Diplospinus multistriatus | ENC1 | OP150025 | RIE_884 |
| Diplospinus multistriatus | ENC1 | OP150026 | RIE_884 |
| Diplospinus multistriatus | ENC1 | OP150027 | RIE_885 |
| Diplospinus multistriatus | ENC1 | OP150028 | RIE_885 |
| Diplospinus multistriatus | ENC1 | OP150029 | RIE_886 |
| Diplospinus multistriatus | ENC1 | OP150030 | RIE_886 |
| Diplospinus multistriatus | ENC1 | OP150031 | RIE_887 |
| Diplospinus multistriatus | ENC1 | OP150032 | RIE_887 |
| Sternoptyx pseudobscura | ENC1 | OP150033 | DPND_3772 |
| Sternoptyx pseudobscura | ENC1 | OP150034 | DPND_3772 |
| Sternoptyx pseudobscura | ENC1 | OP150035 | RIE_195 |
| Sternoptyx pseudobscura | ENC1 | OP150036 | RIE_195 |
| Sternoptyx pseudobscura | ENC1 | OP150037 | RIE_196 |
| Sternoptyx pseudobscura | ENC1 | OP150038 | RIE_196 |
| Sternoptyx pseudobscura | ENC1 | OP150039 | RIE_197 |
| Sternoptyx pseudobscura | ENC1 | OP150040 | RIE_197 |
| Sternoptyx pseudobscura | ENC1 | OP150041 | RIE_200 |
| Sternoptyx pseudobscura | ENC1 | OP150042 | RIE_200 |
| Sternoptyx pseudobscura | ENC1 | OP150043 | RIE_201 |
| Sternoptyx pseudobscura | ENC1 | OP150044 | RIE_201 |
| Sternoptyx pseudobscura | ENC1 | OP150045 | RIE_224 |
| Sternoptyx pseudobscura | ENC1 | OP150046 | RIE_224 |
| Sternoptyx pseudobscura | ENC1 | OP150047 | RIE_225 |
| Sternoptyx pseudobscura | ENC1 | OP150048 | RIE_225 |
| Sternoptyx pseudobscura | ENC1 | OP150049 | RIE_226 |
| Sternoptyx pseudobscura | ENC1 | OP150050 | RIE_226 |
| Sternoptyx pseudobscura | ENC1 | OP150051 | RIE_415 |
| Sternoptyx pseudobscura | ENC1 | OP150052 | RIE_415 |
| Sternoptyx pseudobscura | ENC1 | OP150053 | RIE_417 |
| Sternoptyx pseudobscura | ENC1 | OP150054 | RIE_417 |
| Sternoptyx pseudobscura | ENC1 | OP150055 | RIE_537 |
| Sternoptyx pseudobscura | ENC1 | OP150056 | RIE_537 |
| Sternoptyx pseudobscura | ENC1 | OP150057 | RIE_538 |
| Sternoptyx pseudobscura | ENC1 | OP150058 | RIE_538 |
| Sternoptyx pseudobscura | ENC1 | OP150059 | RIE_539 |
| Sternoptyx pseudobscura | ENC1 | OP150060 | RIE_539 |
| Sternoptyx pseudobscura | ENC1 | OP150061 | RIE_78 |
| Sternoptyx pseudobscura | ENC1 | OP150062 | RIE_78 |
| Sternoptyx pseudobscura | ENC1 | OP150063 | RIE_915 |
| Sternoptyx pseudobscura | ENC1 | OP150064 | RIE_915 |
| Sternoptyx pseudobscura | MYH6 | OP150065 | DPND_4225 |
| Sternoptyx pseudobscura | MYH6 | OP150066 | DPND_4225 |
| Sternoptyx pseudobscura | MYH6 | OP150067 | RIE_195 |
| Sternoptyx pseudobscura | MYH6 | OP150068 | RIE_195 |
| Sternoptyx pseudobscura | MYH6 | OP150069 | RIE_196 |
| Sternoptyx pseudobscura | MYH6 | OP150070 | RIE_196 |
| Sternoptyx pseudobscura | MYH6 | OP150071 | RIE_197 |
| Sternoptyx pseudobscura | MYH6 | OP150072 | RIE_197 |
| Sternoptyx pseudobscura | MYH6 | OP150073 | RIE_200 |
| Sternoptyx pseudobscura | MYH6 | OP150074 | RIE_200 |
| Sternoptyx pseudobscura | MYH6 | OP150075 | RIE_201 |
| Sternoptyx pseudobscura | MYH6 | OP150076 | RIE_201 |
| Sternoptyx pseudobscura | MYH6 | OP150077 | RIE_223 |
| Sternoptyx pseudobscura | MYH6 | OP150078 | RIE_223 |
| Sternoptyx pseudobscura | MYH6 | OP150079 | RIE_224 |
| Sternoptyx pseudobscura | MYH6 | OP150080 | RIE_224 |
| Sternoptyx pseudobscura | MYH6 | OP150081 | RIE_226 |
| Sternoptyx pseudobscura | MYH6 | OP150082 | RIE_226 |
| Sternoptyx pseudobscura | MYH6 | OP150083 | RIE_415 |
| Sternoptyx pseudobscura | MYH6 | OP150084 | RIE_415 |
| Sternoptyx pseudobscura | MYH6 | OP150085 | RIE_417 |
| Sternoptyx pseudobscura | MYH6 | OP150086 | RIE_417 |
| Sternoptyx pseudobscura | MYH6 | OP150087 | RIE_537 |
| Sternoptyx pseudobscura | MYH6 | OP150088 | RIE_537 |
| Sternoptyx pseudobscura | MYH6 | OP150089 | RIE_538 |
| Sternoptyx pseudobscura | MYH6 | OP150090 | RIE_538 |
| Sternoptyx pseudobscura | MYH6 | OP150091 | RIE_539 |
| Sternoptyx pseudobscura | MYH6 | OP150092 | RIE_539 |
| Sternoptyx pseudobscura | MYH6 | OP150093 | RIE_78 |
| Sternoptyx pseudobscura | MYH6 | OP150094 | RIE_78 |
| Stomias affinis | MYH6 | OP150095 | DPND_1201 |
| Stomias affinis | MYH6 | OP150096 | DPND_1201 |
| Stomias affinis | MYH6 | OP150097 | DPND_1302 |
| Stomias affinis | MYH6 | OP150098 | DPND_1302 |
| Stomias affinis | MYH6 | OP150099 | DPND_1314 |
| Stomias affinis | MYH6 | OP150100 | DPND_1314 |
| Stomias affinis | MYH6 | OP150101 | DPND_1315 |
| Stomias affinis | MYH6 | OP150102 | DPND_1315 |
| Stomias affinis | MYH6 | OP150103 | DPND_1408 |
| Stomias affinis | MYH6 | OP150104 | DPND_1408 |
| Stomias affinis | MYH6 | OP150105 | DPND_1543 |
| Stomias affinis | MYH6 | OP150106 | DPND_1543 |
| Stomias affinis | MYH6 | OP150107 | DPND_1639 |
| Stomias affinis | MYH6 | OP150108 | DPND_1639 |
| Stomias affinis | MYH6 | OP150109 | DPND_3645 |
| Stomias affinis | MYH6 | OP150110 | DPND_3645 |
| Stomias affinis | MYH6 | OP150111 | RIE_466 |
| Stomias affinis | MYH6 | OP150112 | RIE_466 |
| Stomias affinis | MYH6 | OP150113 | RIE_577 |
| Stomias affinis | MYH6 | OP150114 | RIE_577 |
| Stomias affinis | MYH6 | OP150115 | RIE_917 |
| Stomias affinis | MYH6 | OP150116 | RIE_917 |
TABLE A2.
List of primers used.
| Gene | Primer name | Primer sequence |
|---|---|---|
| COI | FISH1F | TCAACCAACCACAAAGACATTGGCAC |
| COI | FISH1R | TAGACTTCTGGGTGGCCAAAGAATCA |
| COI | FISH2F | TCGACTAATCATAAAGATATCGGCAC |
| COI | FISH2R | ACTTCAGGGTGACCGAAGAATCAGAA |
| COI | BOLD_COI_Forward | TTCTCCACCAACCACAARGAYATYGG |
| COI | BOLD_COI_Reverse | CACCTCAGGGTGTCCGAARAAYCARAA |
| COI | FISHCOI_F | TCAACYAATCAYAAAGATATYGGCAC |
| COI | FISHCOI_R | ACTTCYGGGTGRCCRAARAATCA |
| ENC | Perc_ENC_F | TTCCTRGAGAGAAACCTTCACC |
| ENC | Perc_ENC_R | GAYGGAGARGCNGGGAGGCAGCC |
| PLAG | Perc_PLAG_F | CATGAYCCYAACAARGARGCCTT |
| PLAG | Perc_PLAG_R | TGRCARCCCATGCCCATAGCTG |
| MYH | Perc_MYH_F | ACYAARAGRGTYATYCAGTACT |
| MYH | Perc_MYH_R | CCRAKGGMRTAGTAGACYTGRTC |
TABLE A3.
Range description of study species.
| Species | Range description | Oceans inhabited | Latitudes inhabited | Citations | Notes |
|---|---|---|---|---|---|
| Bathophilus pawneei | Circumglobal; Tropical | Atlantic, Indian, Pacific | 36° N–34° S | Agustin (2018) | |
| Chauliodus sloani | Circumglobal; Tropical and Polar | Atlantic, Indian, Pacific | 50° N–50° S | Mundy (2005) |
Less common but records exist from individuals as far as 70° N–56° S (Priede, 2017) |
| Cyclothone alba | Circumglobal; Tropical | Atlantic, Indian, Pacific | 40° N–40° S | Miya and Nemoto (1986) | |
| Cyclothone pseudopallida | Circumglobal; Tropical and Polar | Atlantic, Indian, Pacific | 65° N–30° S | Mundy (2005) | |
| Diplospinus multistriatus | Circumglobal; Tropical | Atlantic, Indian, Pacific | 40° N–40° S | Mundy (2005) | |
| Ditropichthys storeri | Circumglobal; Tropical | Atlantic, Indian, Pacific | 48° N–43° S | Paxton (1989) | |
| Photostomias guernei | Non circumglobal; Tropical | Atlantic | 40° N–3° N | Kenaley (2009) | |
| Scopelogadus mizolepis | Circumglobal; Tropical | Atlantic, Indian, Pacific | 40° N–22° S | Mundy (2005) | |
| Sigmops elongatus | Circumglobal; Tropical and Polar | Atlantic, Indian, Pacific | 65° N–35° S | Torres (2018) | |
| Sternoptyx pseudobscura | Circumglobal; Tropical | Atlantic, Indian, Pacific | 40° N–40° S | Mundy (2005) and Zamarro and Lloris (1999) | One record from 42° N and 47° N |
| Stomias affinis | Circumglobal; Tropical | Atlantic, Indian, Pacific | 35° N–39° S | Priede (2017) |
Weber, M. D. , Richards, T. M. , Sutton, T. T. , Carter, J. E. , & Eytan, R. I. (2024). Deep‐pelagic fishes: Demographic instability in a stable environment. Ecology and Evolution, 14, e11267. 10.1002/ece3.11267
DATA AVAILABILITY STATEMENT
DNA sequences: available on Genbank. The accession numbers and corresponding sample data are located in Table A1.
REFERENCES
- Abraham, J. P. , Baringer, M. , Bindoff, N. L. , Boyer, T. , Cheng, L. J. , Church, J. A. , Conroy, J. L. , Domingues, C. M. , Fasullo, J. T. , Gilson, J. , Goni, G. , Good, S. A. , Gorman, J. M. , Gouretski, V. , Ishii, M. , Johnson, G. C. , Kizu, S. , Lyman, J. M. , Macdonald, A. M. , … Willis, J. K. (2013). A review of global ocean temperature observations: Implications for ocean heat content estimates and climate change. Reviews of Geophysics, 51, 450–483. [Google Scholar]
- Aceves‐Medina, G. , Jiménez‐Rosenberg, S. P. A. , Hinojosa‐Medina, A. , Funes‐Rodríguez, R. , Saldierna‐Martínez, R. J. , & Smith, P. E. (2004). Fish larvae assemblages in the Gulf of California. Journal of Fish Biology, 65, 832–847. [Google Scholar]
- Agustin, L. Q. (2018). Bathophilus pawneei . http://fishbase.org/summary/Bathophilus‐pawneei
- Ahlstrom, E. H. (1969). Mesopelagic and bathypelagic fishes in the California current region. California Cooperative Oceanic Fisheries Investigations Reports, 13, 39–44. [Google Scholar]
- Alheit, J. , & Hagen, E. (1997). Long‐term climate forcing of European herring and sardine populations. Fisheries Oceanography, 6, 130–139. [Google Scholar]
- Almada, V. C. , Almada, F. , Francisco, S. M. , Castilho, R. , & Robalo, J. I. (2012). Unexpected high genetic diversity at the extreme northern geographic limit of Taurulus bubalis (Euphrasen, 1786). PLoS One, 7, e44404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise, J. C. (2000). Phylogeography: The history and formation of species. Harvard University Press. [Google Scholar]
- Barham, E. G. (1966). Deep scattering layer migration and composition: Observations from a diving saucer. Science, 151, 1399–1403. [DOI] [PubMed] [Google Scholar]
- Bernard, A. M. , Finnegan, K. A. , Sutton, T. T. , Eytan, R. I. , Weber, M. D. , & Shivji, M. S. (2022). Population genomic dynamics of mesopelagic lanternfishes Diaphus dumerilii, Lepidophanes guentheri, and Ceratoscopelus warmingii (Family: Myctophidae) in the Gulf of Mexico. Deep Sea Research Part I: Oceanographic Research Papers, 185, 103786. [Google Scholar]
- Birkner, M. , Blath, J. , & Eldon, B. (2013). Statistical properties of the site‐frequency spectrum associated with Λ‐coalescents. Genetics, 195, 1037–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo‐Galazzo, F. , Crichton, K. A. , Ridgwell, A. , Mawbey, E. M. , Wade, B. S. , & Pearson, P. N. (2021). Temperature controls carbon cycling and biological evolution in the ocean twilight zone. Science, 371, 1148–1152. [DOI] [PubMed] [Google Scholar]
- Bouckaert, R. , Heled, J. , Kühnert, D. , Vaughan, T. , Wu, C. H. , Xie, D. , Suchard, M. A. , Rambaut, A. , & Drummond, A. J. (2014). BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10, e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlin, N. M. (2016). Ontogenetic changes in the distribution and abundance of early life history stages of mesopelagic fishes off California. University of California. [Google Scholar]
- Carnaval, A. C. , Hickerson, M. J. , Haddad, C. F. , Rodrigues, M. T. , & Moritz, C. (2009). Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science, 323, 785–789. [DOI] [PubMed] [Google Scholar]
- Clarke, T. A. (1983). Sex ratios and sexual differences in size among mesopelagic fishes from the central Pacific Ocean. Marine Biology, 73, 203–209. [Google Scholar]
- Clark, P. U. , Archer, D. , Pollard, D. , Blum, J. D. , Rial, J. A. , Brovkin, V. , Mix, A. C. , Pisias, N. G. , & Roy, M. (2006). The middle Pleistocene transition: Characteristics, mechanisms, and implications for long‐term changes in atmospheric pCO2 . Quaternary Science Reviews, 25, 3150–3184. [Google Scholar]
- Clark, P. U. , Dyke, A. S. , Shakun, J. D. , Carlson, A. E. , Clark, J. , Wohlfarth, B. , Mitrovica, J. X. , Hostetler, S. W. , & McCabe, A. (2009). The last glacial maximum. Science, 325, 710–714. [DOI] [PubMed] [Google Scholar]
- Clarke, T. A. , & Wagner, P. J. (1976). Vertical distribution and other aspects of the ecology of certain mesopelagic fishes taken near Hawaii. Fishery Bulletin, 74(3), 635–645. [Google Scholar]
- Cook, A. B. , Sutton, T. T. , Galbraith, J. K. , & Vecchione, M. (2013). Deep‐pelagic (0–3000 m) fish assemblage structure over the mid‐Atlantic ridge in the area of the Charlie‐Gibbs fracture zone. Deep Sea Research Part II: Topical Studies in Oceanography, 98, 279–291. [Google Scholar]
- Crichton, K. A. , Wilson, J. D. , Ridgwell, A. , Boscolo‐Galazzo, F. , John, E. H. , Wade, B. S. , & Pearson, P. N. (2023). What the geological past can tell us about the future of the ocean's twilight zone. Nature Communications, 14, 2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eduardo, L. N. , Lucena‐Frédou, F. , Lanco Bertrand, S. , Lira, A. S. , Mincarone, M. M. , Nunes, G. T. , Frédou, T. , Soares, A. , le Loc'h, F. , Pelage, L. , Schwamborn, R. , Travassos, P. , Martins, K. , Lira, S. M. A. , Figueiredo, G. A. A. , Júnior, T. V. , Ménard, F. , & Bertrand, A. (2023). From the light blue sky to the dark deep sea: Trophic and resource partitioning between epipelagic and mesopelagic layers in a tropical oceanic ecosystem. Science of the Total Environment, 878, 163098. [DOI] [PubMed] [Google Scholar]
- Eldon, B. , Birkner, M. , Blath, J. , & Freund, F. (2015). Can the site‐frequency spectrum distinguish exponential population growth from multiple‐merger coalescents? Genetics, 199, 841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter, R. , Rex, M. A. , Chase, M. R. , & Quattro, J. M. (2005). Population differentiation decreases with depth in deep‐sea bivalves. Evolution, 59, 1479–1491. [PubMed] [Google Scholar]
- Eytan, R. I. , & Hellberg, M. E. (2010). Nuclear and mitochondrial sequence data reveal and conceal different demographic histories and population genetic processes in Caribbean reef fishes. Evolution: International Journal of Organic Evolution, 64, 3380–3397. [DOI] [PubMed] [Google Scholar]
- Fu, Y.‐X. (1997). Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics, 147, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn, T. (2019). PEG precipitation of PCR products . http://labs.mcdb.lsa.umich.edu/labs/olsen/files/PCR.pdf
- Gloeckler, K. , Choy, C. A. , Hannides, C. C. S. , Close, H. G. , Goetze, E. , Popp, B. N. , & Drazen, J. C. (2018). Stable isotope analysis of micronekton around Hawaii reveals suspended particles are an important nutritional source in the lower mesopelagic and upper bathypelagic zones. Limnology and Oceanography, 63, 1168–1180. [Google Scholar]
- Grant, W. S. (2015). Problems and cautions with sequence mismatch analysis and Bayesian skyline plots to infer historical demography. Journal of Heredity, 106, 333–346. [DOI] [PubMed] [Google Scholar]
- Gugger, P. F. , Ikegami, M. , & Sork, V. L. (2013). Influence of late Quaternary climate change on present patterns of genetic variation in valley oak, Quercus lobata Née. Molecular Ecology, 22, 3598–3612. [DOI] [PubMed] [Google Scholar]
- Hannides, C. C. , Popp, B. N. , Close, H. G. , Benitez‐Nelson, C. R. , Cassie, A. , Gloeckler, K. , Wallsgrove, N. , Umhau, B. , Palmer, E. , & Drazen, J. C. (2020). Seasonal dynamics of midwater zooplankton and relation to particle cycling in the North Pacific Subtropical Gyre. Progress in Oceanography, 182, 102266. [Google Scholar]
- Heled, J. (2010). Extended Bayesian skyline plot tutorial . tutorial.east.bio.ed.ac.uk/Tutorials
- Heled, J. , & Drummond, A. J. (2008). Bayesian inference of population size history from multiple loci. BMC Evolutionary Biology, 8, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman, G. , Collette, B. B. , Facey, D. E. , & Bowen, B. W. (2009). The diversity of fishes: Biology, evolution, and ecology. John Wiley & Sons. [Google Scholar]
- Ho, S. Y. , & Shapiro, B. (2011). Skyline‐plot methods for estimating demographic history from nucleotide sequences. Molecular Ecology Resources, 11, 423–434. [DOI] [PubMed] [Google Scholar]
- Hsieh, C. H. , Kim, H. J. , Watson, W. , Di Lorenzo, E. , & Sugihara, G. (2009). Climate‐driven changes in abundance and distribution of larvae of oceanic fishes in the southern California region. Global Change Biology, 15, 2137–2152. [Google Scholar]
- John, E. H. , Pearson, P. N. , Coxall, H. K. , Birch, H. , Wade, B. S. , & Foster, G. L. (2013). Warm ocean processes and carbon cycling in the Eocene. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 371, 20130099. [DOI] [PubMed] [Google Scholar]
- Johnson, G. D. , Paxton, J. R. , Sutton, T. T. , Satoh, T. P. , Sado, T. , Nishida, M. , & Miya, M. (2009). Deep‐sea mystery solved: Astonishing larval transformations and extreme sexual dimorphism unite three fish families. Biology Letters, 5, 235–239. 10.1098/rsbl.2008.0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , Buxton, S. , Cooper, A. , Markowitz, S. , Duran, C. , Thierer, T. , Ashton, B. , Meintjes, P. , & Drummond, A. (2012). Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenaley, C. P. (2009). Revision of Indo‐Pacific species of the loosejaw dragonfish genus Photostomias (Teleostei: Stomiidae: Malacosteinae). Copeia, 2009, 175–189. [Google Scholar]
- Lancraft, T. M. , Hopkins, T. L. , & Torres, J. J. (1988). Aspects of the ecology of the mesopelagic fish Gonostoma elongatum (Gonostomatidae, Stomiiformes) in the eastern Gulf of Mexico. Marine Ecology Progress Series. Oldendorf, 49(1), 27–40. [Google Scholar]
- Lanfear, R. , Calcott, B. , Ho, S. Y. , & Guindon, S. (2012). PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29, 1695–1701. [DOI] [PubMed] [Google Scholar]
- Levitus, S. , Antonov, J. I. , Boyer, T. P. , Baranova, O. K. , Garcia, H. E. , Locarnini, R. A. , Mishonov, A. V. , Reagan, J. R. , Seidov, D. , Yarosh, E. S. , & Zweng, M. M. (2012). World ocean heat content and thermosteric sea level change (0–2000 m), 1955–2010. Geophysical Research Letters, 39. [Google Scholar]
- Lin, C. H. , Wei, C. L. , Ho, S. L. , & Lo, L. (2023). Ocean temperature drove changes in the mesopelagic fish community at the edge of the Pacific Warm Pool over the past 460,000 years. Science Advances, 9(27), eadf0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran, J. , & Fechhelm, J. D. (1998). Fishes of the Gulf of Mexico. University of Texas Press. [Google Scholar]
- Miya, M. , & Nemoto, T. (1986). Life history and vertical distribution of the mesopelagic fish Cyclothone alba (family Gonostomatidae) in Sagami Bay, Central Japan. Deep Sea Research Part A. Oceanographic Research Papers, 33(8), 1053–1068. [Google Scholar]
- Montano, V. (2016). Coalescent inferences in conservation genetics: Should the exception become the rule? Biology Letters, 12, 20160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora, C. , Wei, C. L. , Rollo, A. , Amaro, T. , Baco, A. R. , Billett, D. , Bopp, L. , Chen, Q. , Collier, M. , Danovaro, R. , Gooday, A. J. , Grupe, B. M. , Halloran, P. R. , Ingels, J. , Jones, D. O. B. , Levin, L. A. , Nakano, H. , Norling, K. , Ramirez‐Llodra, E. , … Yasuhara, M. (2013). Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biology, 11, e1001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, H. G. (1996). The early life stages of fishes in the California current region. California Cooperative Oceanic Fisheries Investigations Atlas, 33, 1505. [Google Scholar]
- Mundy, B. C. (2005). Checklist of the fishes of the Hawaiian Archipelago. Bishop Museum Bulletins in Zoology, 6, 1–704. [Google Scholar]
- Near, T. J. , Eytan, R. I. , Dornburg, A. , Kuhn, K. L. , Moore, J. A. , Davis, M. P. , Wainwright, P. C. , Friedman, M. , & Smith, W. L. (2012). Resolution of ray‐finned fish phylogeny and timing of diversification. Proceedings of the National Academy of Sciences of the United States of America, 109, 13698–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netburn, A. N. , & Koslow, J. A. (2018). Mesopelagic fish assemblages across oceanic fronts: A comparison of three frontal systems in the southern California current ecosystem. Deep Sea Research Part I: Oceanographic Research Papers, 134, 80–91. [Google Scholar]
- Niwa, H.‐S. , Nashida, K. , Yanagimoto, T. , & Grant, H. e. W. S. (2016). Reproductive skew in Japanese sardine inferred from DNA sequences. ICES Journal of Marine Science, 73, 2181–2189. [Google Scholar]
- Nye, J. A. , Link, J. S. , Hare, J. A. , & Overholtz, W. J. (2009). Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Marine Ecology Progress Series, 393, 111–129. [Google Scholar]
- Olivarez Lyle, A. , & Lyle, M. W. (2006). Missing organic carbon in Eocene marine sediments: Is metabolism the biological feedback that maintains end‐member climates? Paleoceanography, 21. [Google Scholar]
- Olson, D. B. (2001). Biophysical dynamics of western transition zones: A preliminary synthesis. Fisheries Oceanography, 10, 133–150. [Google Scholar]
- Paxton, J. R. (1989). Synopsis of the whalefishes (family Cetomimidae) with descriptions of four new genera. Records of the Australian Museum, 41, 135–206. [Google Scholar]
- Pearcy, W. G. (1991). Biology of the transition region. NOAA Technical Report NMFS, 105, 39–55. [Google Scholar]
- Priede, I. G. (2017). Deep‐sea fishes: Biology, diversity, ecology and fisheries. Cambridge University Press. [Google Scholar]
- Rambaut, A. , Drummond, A. , & Suchard, M. (2014). Tracer v1. 6 . http://beast.bio.ed.ac.uk.Tracer
- Ramos‐Onsins, S. E. , & Rozas, J. (2002). Statistical properties of new neutrality tests against population growth. Molecular Biology and Evolution, 19, 2092–2100. [DOI] [PubMed] [Google Scholar]
- Randall, J. E. (1981). Examples of antitropical and antiequatorial distribution of Indo‐West‐Pacific fishes. Pacific Science, 35, 197–209. [Google Scholar]
- Robalo, J. I. , Castilho, R. , Francisco, S. M. , Almada, F. , Knutsen, H. , Jorde, P. E. , Pereira, A. M. , & Almada, V. C. (2012). Northern refugia and recent expansion in the North Sea: The case of the wrasse Symphodus melops (Linnaeus, 1758). Ecology and Evolution, 2, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison, B. H. (2009). Conservation of deep pelagic biodiversity conservación de la biodiversidad pelágica profunda. Conservation Biology, 23, 847–858. 10.1111/j.1523-1739.2009.01219.x [DOI] [PubMed] [Google Scholar]
- Rozas, J. , Ferrer‐Mata, A. , Sánchez‐DelBarrio, J. C. , Guirao‐Rico, S. , Librado, P. , Ramos‐Onsins, S. E. , & Sánchez‐Gracia, A. (2017). DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution, 34, 3299–3302. [DOI] [PubMed] [Google Scholar]
- Sakuma, K. , Ueda, Y. , Hamatsu, T. , & Kojima, S. (2014). Contrasting population histories of the deep‐sea demersal fish, Lycodes matsubarai, in the Sea of Japan and the Sea of Okhotsk. Zoological Science, 31, 375–382. [DOI] [PubMed] [Google Scholar]
- Salvatteci, R. , Schneider, R. R. , Galbraith, E. , Field, D. , Blanz, T. , Bauersachs, T. , Crosta, X. , Martinez, P. , Echevin, V. , Scholz, F. , & Bertrand, A. (2022). Smaller fish species in a warm and oxygen‐poor Humboldt current system. Science, 375, 101–104. [DOI] [PubMed] [Google Scholar]
- Sassa, C. , Kawaguchi, K. , Oozeki, Y. , Kubota, H. , & Sugisaki, H. (2004). Distribution patterns of larval myctophid fishes in the transition region of the western North Pacific. Marine Biology, 144, 417–428. [Google Scholar]
- Stephens, M. , & Scheet, P. (2001). Estimating population haplotype frequencies from pooled DNA samples using PHASE algorithm. American Journal of Human Genetics, 68, 978–989.11254454 [Google Scholar]
- Stephens, M. , & Scheet, P. (2005). Accounting for decay of linkage disequilibrium in haplotype inference and missing‐data imputation. The American Journal of Human Genetics, 76, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, T. (2013). Vertical ecology of the pelagic ocean: Classical patterns and new perspectives. Journal of Fish Biology, 83, 1508–1527. [DOI] [PubMed] [Google Scholar]
- Sutton, T. T. , Clark, M. R. , Dunn, D. C. , Halpin, P. N. , Rogers, A. D. , Guinotte, J. , Bograd, S. J. , Angel, M. V. , Perez, J. A. A. , Wishner, K. , Haedrich, R. L. , Lindsay, D. J. , Drazen, J. C. , Vereshchaka, A. , Piatkowski, U. , Morato, T. , Błachowiak‐Samołyk, K. , Robison, B. H. , Gjerde, K. M. , … Heino, M. (2017). A global biogeographic classification of the mesopelagic zone. Deep Sea Research Part I: Oceanographic Research Papers, 126, 85–102. [Google Scholar]
- Sutton, T. T. , & Hopkins, T. (1996). Trophic ecology of the stomiid (Pisces: Stomiidae) fish assemblage of the eastern Gulf of Mexico: Strategies, selectivity and impact of a top mesopelagic predator group. Marine Biology, 127, 179–192. [Google Scholar]
- Tajima, F. (1989). Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics, 123, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber, A. R. , Sweetman, A. K. , Narayanaswamy, B. E. , Jones, D. O. B. , Ingels, J. , & Hansman, R. L. (2014). Ecosystem function and services provided by the deep sea. Biogeosciences, 11, 3941–3963. [Google Scholar]
- Torres, A. G. (2018). Sigmops elongatus .
- Tyus, H. M. (2011). Ecology and conservation of fishes. CRC Press. [Google Scholar]
- Urias‐Leyva, H. , Aceves‐Medina, G. , Avendaño‐Ibarra, R. , Saldierna‐Martínez, R. J. , Gómez‐Gutiérrez, J. , & Robinson, C. J. (2018). Regionalization in the distribution of larval fish assemblages during winter and autumn in the Gulf of California. Latin American Journal of Aquatic Research, 46, 20–36. [Google Scholar]
- Varela, A. I. , Ritchie, P. A. , & Smith, P. J. (2012). Low levels of global genetic differentiation and population expansion in the deep‐sea teleost Hoplostethus atlanticus revealed by mitochondrial DNA sequences. Marine Biology, 159, 1049–1060. [Google Scholar]
- Vecchione, M. , Falkenhaug, T. , Sutton, T. , Cook, A. , Gislason, A. , Hansen, H. Ø. , Heino, M. , Miller, P. I. , Piatkowski, U. , Porteiro, F. , Søiland, H. , & Bergstad, O. A. (2015). The effect of the North Atlantic Subpolar Front as a boundary in pelagic biogeography decreases with increasing depth and organism size. Progress in Oceanography, 138, 105–115. [Google Scholar]
- Watling, L. , Guinotte, J. , Clark, M. R. , & Smith, C. R. (2013). A proposed biogeography of the deep ocean floor. Progress in Oceanography, 111, 91–112. [Google Scholar]
- Zamarro, J. , & Lloris, D. (1999). New northern limit for the distribution of Sternoptyx pseudobscura Baird, 1971 (Pisces: Sternoptychidae) in the Atlantic .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
DNA sequences: available on Genbank. The accession numbers and corresponding sample data are located in Table A1.


