This nonrandomized controlled trial analyzes the efficacy and safety of erenumab, an anti–calcitonin gene-related peptide receptor monoclonal antibody, for rosacea-associated erythema and flushing.
Key Points
Question
Can calcitonin gene-related peptide-inhibition improve rosacea-associated erythema and flushing, and is it safe?
Findings
This nonrandomized controlled trial of 30 individuals with rosacea-associated erythema and flushing found that subcutaneous injections of monoclonal antibodies against the calcitonin gene-related peptide receptor administered every 4 weeks for 12 weeks significantly reduced days with flushing and erythema by weeks 9 through 12 compared with baseline.
Meaning
These findings indicate that the calcitonin gene-related peptide pathway may be important in the pathophysiology of erythema and flushing in rosacea and that inhibition of the calcitonin gene-related peptide receptor holds potential in treating rosacea-associated erythema and flushing.
Abstract
Importance
Treatment of erythema and flushing in rosacea is challenging. Calcitonin gene-related peptide (CGRP) has been associated with the pathogenesis of rosacea, raising the possibility that inhibition of the CGRP pathway might improve certain features of the disease.
Objective
To examine the effectiveness, tolerability, and safety of erenumab, an anti–CGRP-receptor monoclonal antibody, for the treatment of rosacea-associated erythema and flushing.
Design, Setting, and Participants
This single-center, open-label, single-group, nonrandomized controlled trial was conducted between June 9, 2020, and May 11, 2021. Eligible participants included adults with rosacea with at least 15 days of either moderate to severe erythema and/or moderate to extreme flushing. No concomitant rosacea treatment was allowed throughout the study period. Visits took place at the Danish Headache Center, Copenhagen University Hospital, Rigshospitalet in Copenhagen, Denmark. Participants received 140 mg of erenumab subcutaneously every 4 weeks for 12 weeks. A safety follow-up visit was performed at week 20. Data analysis occurred from January 2023 to January 2024.
Intervention
140 mg of erenumab every 4 weeks for 12 weeks.
Main Outcomes and Measures
The primary outcome was mean change in the number of days with moderate to extreme flushing during weeks 9 through 12, compared with the 4-week run-in period (baseline). The mean change in number of days with moderate to severe erythema was a secondary outcome. Adverse events were recorded for participants who received at least 1 dose of erenumab. Differences in means were calculated with a paired t test.
Results
A total of 30 participants (mean [SD] age, 38.8 [13.1] years; 23 female [77%]; 7 male [23%]) were included, of whom 27 completed the 12-week study. The mean (SD) number of days with moderate to extreme flushing was reduced by −6.9 days (95% CI, −10.4 to −3.4 days; P < .001) from 23.6 (5.8) days at baseline. The mean (SD) number of days with moderate to severe erythema was reduced by −8.1 days (95% CI, −12.5 to −3.7 days; P < .001) from 15.2 (9.1) days at baseline. Adverse events included transient mild to moderate constipation (10 participants [33%]), transient worsening of flushing (4 participants [13%]), bloating (3 participants [10%]), and upper respiratory tract infections (3 participants [10%]), consistent with previous data. One participant discontinued the study due to a serious adverse event (hospital admission due to gallstones deemed unrelated to the study), and 2 participants withdrew consent due to lack of time.
Conclusions and Relevance
These findings suggest that erenumab might be effective in reducing rosacea-associated flushing and chronic erythema (participants generally tolerated the treatment well, which was consistent with previous data), and that CGRP-receptor inhibition holds potential in the treatment of erythema and flushing associated with rosacea. Larger randomized clinical trials are needed to confirm this finding.
Trial Registration
ClinicalTrials.gov Identifier: NCT04419259
Introduction
Rosacea is a common chronic skin disease affecting 5.5% of the global population.1 The disease primarily affects the facial skin and is characterized by chronic centrofacial redness, episodes or exacerbations of flushing, inflammatory lesions, rhinophyma, and/or ocular symptoms.2 While treatment options for rosacea vary depending on the phenotype, they are most effective for individuals with papules and pustules.2,3,4,5 However, for persistent erythema and flushing, currently available treatments have limited efficacy and patients often experience physical and psychological distress due to visible and debilitating features.5,6,7,8,9 The pathophysiology of rosacea remains incompletely understood, but it is recognized that both the innate and adaptive immune systems are involved, with signaling neuropeptides such as pituitary adenylate cyclase-activating polypeptide-38 and calcitonin gene-related peptide (CGRP), playing a role.10,11,12,13,14 Interestingly, there is an established epidemiological overlap between rosacea and migraine15,16,17,18 and a potential pathophysiological connection has been suggested.15 Both conditions have been associated with increased levels of CGRP in plasma,19 and antibodies against CGRP or its receptors are now approved for treatment of migraine.20 Therefore, we aimed to investigate whether inhibition of the CGRP pathway could also be beneficial in treating rosacea. The purpose of this study was to assess the effectiveness, tolerability, and safety of a subcutaneously administered monoclonal antibody against the CGRP receptor in individuals with rosacea.
Methods
Study Oversight
This nonrandomized controlled trial was approved by the Regional Health Research Ethics Committee of the capital region of Denmark, the Danish Medicines Agency, the Danish Data Protection Agency, and the European Union Drug Regulating Authorities Clinical Trials Database. The study was conducted in accordance with the Helsinki Declaration with later revisions,21 and oral and written informed consent was obtained from all study participants prior to any study procedures. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline and the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline. Please see the trial protocol in Supplement 1 for more information about the trial.
Study Participants
Participants were recruited from the outpatient clinic at the Department of Dermatology and Allergy at Gentofte Hospital in the Capital Region of Denmark through adverts22 and from participation in a previous study.18 Participants were adults aged 18 to 65 years of both sexes with at least a 1-year history of rosacea according to current diagnostic criteria.2 No concomitant rosacea medication was allowed for at least 28 days or 5 half-lives (whichever was longest) prior to study start, and participants had to fill in a daily diary on flushing and erythema from run-in and until 4 weeks after the last dose of erenumab; a compliance of at least 80% in diary completion was required throughout the study. The inclusion criteria were at least 15 days with erythema ratings greater than 3 (ie, moderate to severe) on the Patient Self-Assessment (PSA) and/or greater than 4 (ie, moderate to extreme) on item 2 (rating of overall flushing in the past 24 hours) of the patient-reported flushing assessment in the Flushing Assessment Tool (FAST).23 Exclusion criteria were any cardiovascular disease, including cerebrovascular disease; hypertension or hypotension according to the current classification; any current psychiatric disease unless effectively treated with a stable treatment for at least 2 months; pregnant or breastfeeding women; women of childbearing age who were unable or unwilling to use an acceptable method of effective contraception during the treatment period through 16 weeks posttreatment; and previous treatment with CGRP antibodies.
Study Design and Procedures
This open-label, single-group, nonrandomized controlled trial consisted of a run-in phase (4 weeks) and a treatment phase (12 weeks) and was conducted between June 9, 2020, and May 11, 2021. A total of 5 study visits took place at the Danish Headache Center in Glostrup, Denmark: screening, baseline (first dose), week 4 (second dose), week 8 (third dose), and week 20 (safety follow-up visit 12 weeks after the last dose of erenumab). During the recruitment period, we received feedback from potential participants highlighting their limited availability for on-site study visits due to geographical constraints. In light of this, we made a feasibility-driven decision to exclude the week 12 study visit, given that the primary outcome was reported through daily online diaries from participants’ homes (ie, FAST and PSA), which were the only data collected at weeks 9 through 12. At the screening visit, participants underwent a thorough investigation to ensure that they were eligible for the study, including clinical examination, electrocardiography, pregnancy testing, and blood sampling. Additionally, semistructured interviews were performed to determine subtype and severity of rosacea as well as prevalence and subtype of migraine. The daily flushing and erythema diary was sent via email with a link to an online survey in REDCap version 13.7.4 (Vanderbilt University).
After the 4-week run-in period, participants fulfilling inclusion criteria entered the open-label treatment phase. The open-label phase included 12 weeks of subcutaneous erenumab (140 mg) every 4 weeks delivered at the study site by 1 of the study personnel (N.K.F.W. or T.P.D.). Protocol-specified procedures were performed at each follow-up visit (weeks 4, 8, and 20) (eFigure 1 in Supplement 2).
Outcome Measures
Severity of flushing was assessed by item 2 of the FAST.23 Erythema severity was evaluated with the PSA24 and Clinician Erythema Assessment (CEA; score range 0-4).25 The overall severity of rosacea was assessed using Investigator Global Assessment (IGA; score range, 0-4),26 Rosacea Area and Severity Index (RASI; score range, 0-72),27 and Rosacea Clinical Scorecard (RCS; score range, 0-30).28 Severity of inflammatory lesions was measured using the Inflammatory Lesion Count (ILC; score range, 0 to >125 lesions).
Quality-of-life measurements included the Dermatology Life Quality Index (DLQI; score range, 0-30)29 and Rosacea Quality of Life index (RosaQoL; score range, 0-84).30 Levels of depression and anxiety were assessed by the Hospital Anxiety and Depression Scale (HADS; score range, 0-21)31 and Quick Inventory of Depressive Symptomatology (QIDS; score range 0-27).
FAST and PSA were recorded by daily diaries (at home) whereas additional patient-reported outcomes (HADS, QIDS, DLQI, and RosaQoL) were collected at study visits (weeks 4,8, and 20). The CEA, IGA, RASI, and RCS were evaluated by the physician at study visits.
Outcomes
The primary outcome of this study was the mean change in number of monthly days with moderate to extreme flushing from baseline (4-week pretreatment period) to weeks 9 through 12, based on item 2 of the FAST. Secondary effectiveness outcomes included: (1) mean change in FAST from baseline to weeks 1 to 4 and 5 to 8; (2) proportion of patients with at least 50% reduction in number of days with moderate, severe, or extreme flushing based on the FAST from baseline to weeks 9 through 122; (3) mean (SD) change in CEA, IGA, RCS, RASI, and ILC scores from baseline to weeks 4, 8, and 20; (4) mean change in PSA from baseline to weeks 1 through 4, 5 through 8, and 9 through 12; (5) mean change in quality of life from baseline to weeks 4, 8, and 20 as assessed by the RosaQoL and DLQI; and (6) mean change in depressive symptoms from baseline to week 20 based on the HADS and QIDS. Adverse events were recorded from the first dose of erenumab throughout the study period (recorded at study visits weeks 4, 8, and 20).
Statistical Analysis
The effectiveness outcomes were based on daily erythema and flushing diary entries and analyzed using a complete-case analysis (ie, participants who received all 3 doses of erenumab). Differences in means were calculated with a paired t test. Tolerability and safety analyses included all participants who received at least 1 dose of erenumab. Data are presented as means with SDs, medians with IQRs, and frequencies with percentages. The statistical software used for analyses was R statistical software version 3.6.0 (R Project for Statistical Computing). A 2-sided P < .05 was considered statistically significant. Data analysis occurred from January 2023 to January 2024.
Results
Patients
This study included a total of 30 participants (mean [SD] age 38.8 [13.1] years; 23 female [77%]; 7 male; [23%]) receiving at least 1 dose of erenumab (Table 1). Of these participants, 3 participants discontinued before completing the study and were excluded from final analyses (eFigure 2 in Supplement 2).
Table 1. Baseline Characteristics of Participants Included in the Trial.
| Characteristic | Patients, No. (%) (N = 30) |
|---|---|
| Age, mean (SD), y | 38.8 (13.1) |
| Sex | |
| Female | 23 (77) |
| Male | 7 (23) |
| Weight, mean (SD), kg | 80.0 (17.5) |
| Body mass index, mean (SD)a | 26.5 (5.8) |
| Duration of rosacea, mean (SD), y | 14.5 (10.5) |
| Monthly No. of days with moderate to extreme flushing, mean (SD) | 23.6 (5.7) |
| Previous rosacea treatments | |
| Overall | 26 (87) |
| Topical | |
| Azelaic acid | 4 (13) |
| Brimonidine | 17 (57) |
| Ivermectin | 5 (17) |
| Metronidazole | 9 (30) |
| Systemic | |
| β-Blockers | 2 (7) |
| Doxycycline | 4 (13) |
| Isotretinoin | 3 (10) |
| Tetracycline | 5 (17) |
| Other | |
| Laser or light-based therapy | 3 (10) |
| Sympathectomy | 3 (10) |
| No. of previously failed rosacea treatments | |
| 0-2 | 11 (36) |
| 2 | 6 (20) |
| 3 | 8 (27) |
| ≥4 | 5 (17) |
Body mass index was calculated as weight in kilograms divided by height in meters squared.
The mean (SD) number of days with moderate to extreme flushing per 4 weeks was 23.6 (5.8) days at baseline. Prior to enrollment in the trial, 26 participants (87%) had discontinued at least 1 treatment for rosacea due to lack of effectiveness or tolerability; these included topical treatment with either azelaic acid (4 participants [13%]), brimonidine (17 participants [57%]), ivermectin (5 participants [17%]), and/or metronidazole (9 participants [30%]); as well as oral treatments with β-blockers (2 participants [7%]), doxycycline (4 participants [13%]), isotretinoin (3 participants [10%]), and/or tetracycline (5 participants [17%]). Moreover, 8 participants (27%) had previously tried laser or light-based therapies, and 3 participants (10%) had undergone sympathectomy for their rosacea. A total of 26 participants (87%) had previously failed 1 or more treatments for their rosacea with 13 (43%) previously failing 3 or more rosacea treatments due to lack of efficacy or adverse reactions. For details on each patient, see the eTable in Supplement 3.
Primary Outcome: Flushing
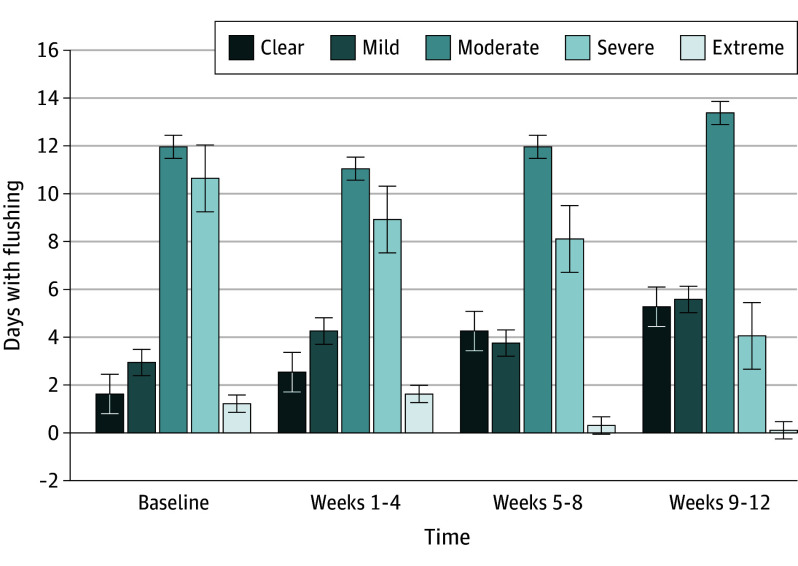
At weeks 9 through 12 (after the third administration of erenumab), the mean reduction in the number of days with moderate to extreme flushing from baseline was −6.9 days (95% CI, −10.4 to −3.4 days; P < .001) (Figure 1 and Table 2). Of the 27 patients who completed the study, 1 patient (4%) achieved at least a 50% decrease in the number of days with moderate to extreme flushing from baseline through weeks 1 to 4, 3 patients (11%) for weeks 5 to 9, and 7 patients (26%) for weeks 9 to 12. Subgroup analysis revealed that in those who were most affected by their flushing (>10 days/month with severe to extreme flushing), there was an decrease from 20.8 days per month with severe to extreme flushing at baseline to 3.8 days per month at weeks 9 through 12 (corresponding to a reduction of 81% [16.9 days]).
Figure 1. Overall Days With Flushing.
The figure provides an overview of change in days with flushing measured by the Flushing Assessment Tool item 2 (self-reported) each day from baseline to weeks 9 through 12.
Table 2. Summary of Outcomes.
| Outcome | Baseline (week −4 to 0) | Weeks 1-4 | P value | Weeks 5-8 | P value | Weeks 9-12 | P value | Week 20 | P value |
|---|---|---|---|---|---|---|---|---|---|
| FAST | |||||||||
| Days, mean (SD) | 23.6 (5.8) | 21.3 (7.2) | .02 | 20.1 (8.8) | .009 | 17.3 (9.7) | <.001 | NA | NA |
| Mean change (95% CI), d | NA | −2.3 (−4.2 to −0.40) | −4.2 (−7.20 to −1.15) | −6.9 (−10.4 to −3.4) | NA | ||||
| PSA | |||||||||
| Days, mean (SD) | 15.2 (9.1) | 13.1 (9.9) | .19 | 10.4 (9.0) | .006 | 7.9 (7.8) | <.001 | NA | NA |
| Mean change (95% CI) | NA | −2.1 (−1.1 to 5.4) | −5.2 d (−8.7 to −1.6) | −8.1 d (−12.5 to −3.7) | NA | ||||
| CEA | |||||||||
| Score, mean (SD) | 2.93 (0.47) | 3.04 (0.52) | .30 | 2.26 (0.81) | <.001 | NA | NA | 1.81 (1.08) | <.001 |
| Mean change (95% CI) | NA | −0.11 (−0.34 to 0.12) | −0.67 (−1.00 to −0.34) | NA | −1.11 (−1.57 to −0.65) | ||||
| IGA | |||||||||
| Score, mean (SD) | 2.96 (0.44) | 3.07 (0.55) | .26 | 2.33 (0.88) | <.001 | NA | NA | 1.85 (1.13) | <.001 |
| Mean change (95% CI) | NA | −0.11 (−0.09 to 0.31) | −0.63 (−0.94 to −0.32) | NA | −1.11 (−1.55 to −0.67) | ||||
| ILC | |||||||||
| Score, mean (SD) | 8.30 (10.64) | 9.00 (15.82) | .81 | 5.19 (9.78) | .15 | NA | NA | 4.63 (8.61) | .10 |
| Mean change (95% CI) | NA | 0.7 (5.37 to 6.78) | −3.11 (−7.40 to 1.18) | NA | −3.67 (−8.08 to 0.75) | ||||
| RASI | |||||||||
| Score, mean (SD) | 10.37 (4.76) | 12.74 (5.45) | .01 | 7.81 (5.51) | .02 | NA | NA | 8.43 (6.83) | .12 |
| Mean change (95% CI) | NA | 2.37 (0.53 to 4.21) | −2.69 (−4.85 to −0.53) | NA | −2.13 (−4.84 to 0.57) | ||||
| RCS | |||||||||
| Score, mean (SD) | 2.05 (0.59) | 2.33 (0.66) | .11 | 1.76 (0.54) | .17 | NA | NA | 1.83 (0.62) | .50 |
| Mean change (95% CI) | NA | 0.29 (−0.01 to 0.64) | 0.24 (−0.59 to 0.11) | NA | −0.11 (−0.45 to 0.23) | ||||
| DLQI | |||||||||
| Score, mean (SD) | 6.22 (4.99) | 5.93 (2.79) | .72 | 4.19 (2.68) | .004 | NA | NA | 3.73 (3.25) | .003 |
| Mean change (95% CI) | NA | −0.30 (−1.96 to 1.36) | −2.08 (−3.74 to −0.42) | NA | −2.73 (−4.43 to −1.02) | ||||
| RosaQoL | |||||||||
| Score, mean (SD) | 48.22 (11.81) | 48.33 (8.61) | .93 | 45.69 (11.74) | .04 | NA | NA | 42.95 (12.46) | .02 |
| Mean change (95% CI) | NA | 0.11 (2.45 to 2.67) | −2.58 (−4.96 to −0.19) | NA | −4.14 (−7.57 to −0.71) | ||||
| HADS anxiety | |||||||||
| Score, mean (SD) | 5.41 (3.82) | NA | NA | NA | NA | NA | NA | 5.52 (3.90) | .60 |
| Mean change (95% CI) | NA | NA | NA | NA | 0.37 (−1.01 to 0.59) | ||||
| HADS depression | |||||||||
| Score, mean (SD) | 2.19 (2.20) | NA | NA | NA | NA | NA | NA | 2.47 (2.42) | .66 |
| Mean change (95% CI) | NA | NA | NA | NA | 0.31 (−0.35 to 0.56) | ||||
| QIDS | |||||||||
| Score, mean (SD) | 5.82 (4.34) | NA | NA | NA | NA | NA | NA | 5.89 (4.18) | .44 |
| Mean change (95% CI) | NA | NA | NA | NA | 0.41 (−1.06 to 0.47) |
Abbreviations: CEA, clinician erythema assessment; DLQI, dermatology life quality index; FAST, flushing assessment tool (item 2); HADS, hospital anxiety and depression scale; IGA, investigator global assessment; ILC, inflammatory lesion count; NA, not applicable; PSA, patient self-assessment; QIDS, quick inventory of depressive symptomatology; RASI, rosacea area and severity assessment; RCS, rosacea clinical scorecard; RosaQoL, rosacea-related quality of life.
Secondary Outcomes
Rosacea Severity
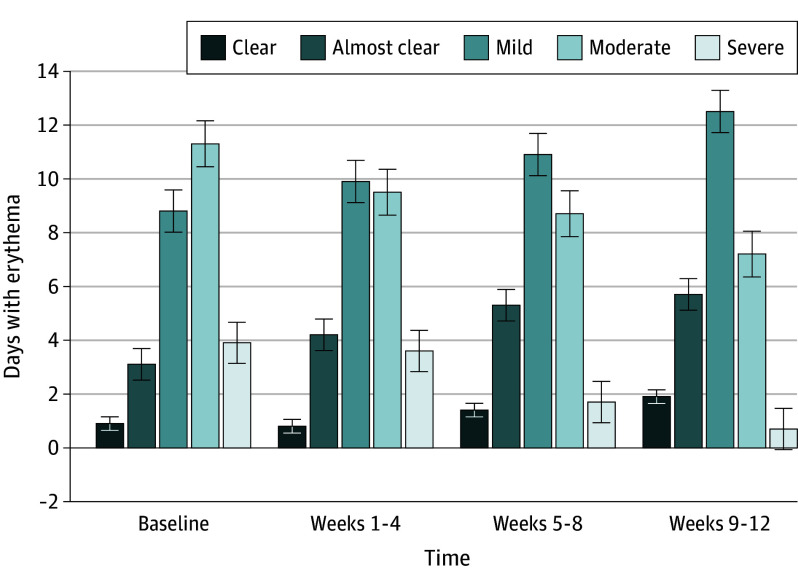
The mean (SD) number of days with moderate to severe erythema (score of 3-4 on the PSA) was 15.2 (9.1) days at baseline with a reduction of −8.1 days (95% CI, −12.5 to −3.7 days; P < .001) at weeks 9 through 12 (Figure 2 and Table 2). The proportion of participants who achieved at least a 50% reduction in PSA from the run-in period was 9 of 27 participants (33%) at weeks 1 through 4, 11 of 27 participants (41%) at weeks 5 through 8, and 15 of 27 participants (56%) at weeks 9 through 12.
Figure 2. Overall Days With Erythema.
The figure provides an overview of change in days with erythema measured by the Patient Self-Assessment each day throughout the study period.
The remaining outcomes were collected at weeks 4, 8, and 20 (12 weeks after last dose of erenumab, which has a half-life of 4 weeks). The mean (SD) CEA score, the physician-reported equivalent to PSA, was 2.93 (0.47) at baseline and was reduced by −0.67 points (95% CI, −1.00 to −0.34 points; P < .001) at week 8 (Table 2). Furthermore, we saw a reduction of −1.11 points (95% CI, −1.57 to −0.65 points; P < .001) at week 20 (12 weeks after last dose). The mean (SD) IGA score (overall rosacea) was 2.96 (0.44) at baseline, with a reduction of −0.63 points (95% CI, −0.94 to −0.32 points; P < .001) at week 8 and a further rection of −1.11 points (95% CI, −1.55 to −0.67 points; P < .001) at week 20. The proportion of patients with an IGA score of 0 or 1 with at least a 2-point reduction was 0 participants (0%) from baseline to week 4, 3 of 27 participants (11%) at week 8, and 8 of 27 participants (30%) at week 20.
At baseline, the mean (SD) RASI score was 10.37 (4.76) with a reduction of −2.69 points (95% CI, −4.85 to −0.53 points; P = .02) at week 8, but no significant change at week 20 (mean change, −2.13 points; 95% CI, −4.84 to 0.57 points; P = .12) (Table 2). The mean (SD) RCS score was 2.05 (0.59) at baseline with no significant change at week 8 (mean change, 0.24 points; 95% CI, −0.59 to 0.11 points; P = .17) or at week 20 (mean change, −0.11 points; 95% CI, −0.45 to 0.23 points; P = .50). The ILC revealed a mean (SD) of 8.30 (10.64) lesions at baseline with no significant change at week 8 (mean change, −3.11 lesions; 95% CI, −7.40 to 1.18 lesions; P = .15) or at week 20 (mean change −3.67 lesions; 95% CI, −8.08 to 0.75 lesions; P = .10).
Quality of Life Measurements
The mean (SD) DLQI score was 6.22 (4.99) at baseline, with a reduction of −2.08 points (95% CI, −3.74 to −0.42 points; P = .004) at week 8 and −2.73 points (95% CI, −4.43 to −1.02 points; P = .003) at week 20 (Table 2). Explorative analyses revealed that improvement in DLQI was not consistent with higher improvement in PSA and FAST scores. The mean (SD) RosaQoL score was 48.22 (11.81) at baseline with a decrease of −2.58 points (95% CI, −4.96 to –0.19 points; P = .04) at week 8 and −4.14 points (95% CI, −7.57 to −0.71 points; P = .02) at week 20.
The mean (SD) HADS score at baseline was 5.41 (3.82) for anxiety and 2.19 (2.20) for depression. The follow-up for HADS was at week 20 (12 weeks after last dose) and we saw no significant change for anxiety (mean change, 0.37 points; 95% CI, −1.01 to 0.59 points; P = .60) or depression (mean change, 0.31 points; 95% CI, −0.35 to 0.56 points; P = .66). The mean (SD) QIDS score was 5.82 (4.34) at baseline and was followed-up at week 20, where we found no significant change (mean change, 0.41 points; 95% CI, −1.06 to 0.47 points; P = .44).
Safety
All 30 participants received at least 1 dose of erenumab and were included in the safety analyses. Of these participants, 25 (83%) experienced at least 1 adverse event (Table 3). The most common adverse events were constipation (10 participants [33%]), transient worsening of flushing (4 participants [13%]), bloating (3 participants [10%]), upper respiratory tract infection (3 participants [30%]), transient worsening of headache or migraine (3 participants [10%]), dry eyes (2 participants [7%]), transient fever (2 participants [7%]), and dry skin (2 participants [7%]). Episodes of constipation were mild to moderate and developed approximately 1 week after the first injection (for 7 of 10 participants), whereas symptoms developed after the second or third injection for the remaining 3 participants. One participant with a history of irritable bowel syndrome and intermittent constipation experienced worsening of constipation throughout the treatment course, although symptoms resolved after the cessation of erenumab. Transient worsening of flushing and/or headache and migraine occurred 1 to 3 days after injection of erenumab and resolved spontaneously. One serious adverse event was reported by a participant who discontinued the study after a hospital admission due to gallstones, which was deemed unlikely to be associated with erenumab.
Table 3. Adverse Events Throughout the Study Period .
| Adverse event | Patients, No. (%) (N = 30)a |
|---|---|
| TEAE | 24 (80) |
| Treatment-related TEAE | 20 (67) |
| Serious TEAE | 0 |
| TEAE leading to study discontinuation | 1 (3) |
| Specific AEs | |
| Constipation | 10 (33) |
| Transient worsening of flushing | 4 (13) |
| Bloating | 3 (10) |
| Upper respiratory tract infection | 3 (10) |
| Transient worsening of headache or migraine | 3 (10) |
| Dry eyes | 2 (7) |
| Transient fever | 2 (7) |
| Dry skin | 2 (7) |
Abbreviations: AE, adverse event; TEAE, treatment-emergent adverse event.
Patients were treated with 140 mg of erenumab every 4 weeks for a period of 12 weeks.
Discussion
In this nonrandomized controlled trial, during the 12-week treatment period, we found that monthly subcutaneous injections of CGRP receptor antibodies was associated with a significant reduction in the number of days with moderate to extreme flushing by −6.9 days (95% CI, −10.4 to −3.4 days; P < .001) from baseline to weeks 9 through 12. Additionally, 23% of the participants experienced at least a 50% reduction in the number of days with moderate to extreme flushing by the end of the treatment period. The number of days with moderate to severe erythema were reduced by −8.1 days (95% CI, −12.5 to −3.7 days; P < .001), and 50% of patients achieved at least 50% reduction in the number of days with moderate to severe erythema by weeks 9 through 12. Subgroup analysis revealed that in those who were most affected by their flushing (>10 days/month with severe to extreme flushing), there was an 81% decrease (corresponding to a reduction of 16.5 days) in days with severe to extreme flushing by weeks 9 through 12. There was a slight decrease in DLQI and RosaQoL scores by week 8 which was sustained at week 20 (12 weeks after the last dose of erenumab). There were no significant differences in the number of inflammatory lesions or depression and anxiety scores throughout the study period.
The release of CGRP can trigger a series of effects such as vasodilation,32,33 edema, increased blood flow with recruitment of inflammatory cells,34,35,36 and inflammation.37 One study12 found elevated CGRP levels in facial skin biopsies from 26 individuals with erythematotelangiectatic rosacea compared with 11 healthy controls and 20 individuals with photoaging. These findings suggest that CGRP might be important for some of the most bothersome rosacea features.
Several treatments have already been approved for rosacea,38,39 although these are mostly effective in those with papules and pustules.40 Despite numerous new therapies being tested, there are currently no effective treatments for the most common rosacea features, erythema and flushing.5 In this study most participants (87%) had previously failed 1 or more treatments for their rosacea with 43% previously failing 3 or more rosacea treatments due to lack of efficacy or adverse reactions.
Erenumab was generally well tolerated, and all adverse effects subsided after stopping treatment. However, mild to moderate constipation (affecting 33% of our population) and bloating (affecting 10% of our population) were common adverse effects, which is consistent with previous trials in participants with headache41,42 as well as empirical data.43 Of note, patients with rosacea are more likely to have gastrointestinal diseases such as irritable bowel syndrome and inflammatory bowel disorders, which could have affected our data.44,45,46 Three participants (10%) withdrew from the study before receiving all doses of erenumab; one patient withdrew due to a serious adverse event (gallstones, which was deemed unrelated to the study drug), and 2 patients withdrew consent due to lack of time for on-site study visits.
While previous studies have suggested an association of rosacea with anxiety and depression,47,48,49,50 our study participants consistently reported a relatively low impact of their disease on skin-related quality of life throughout the study period. Although DLQI and RosaQoL scores showed slight improvements following treatment with erenumab, no significant changes in depression and anxiety measures were observed. This finding may be attributed to the study design, in which we omitted a visit at week 12 (where we may have seen an effect on these data which was no longer present at week 20 [12 weeks after the last dose of erenumab, which has a half-life of 4 weeks]). Consequently, we measured the long-time effect of erenumab rather than short-term improvements. For some outcomes (CEA, IGA, DLQI, and RosaQoL), we saw additional improvements at week 20 (12 weeks after the last dose of the treatment which has a half-life of 4 weeks), and it is unclear whether these improvements are indicative of further improvements that might have occurred at week 12 (where we did not collect these data) or whether they reflect the natural course of the disease. Future study designs should take this information into account, and larger, randomized, double-blind studies could provide more insights into the effects of CGRP pathway inhibition in different populations, including testing different doses of erenumab.
Limitations
This study has limitations. The open-label design and lack of a placebo group are limitations that need to be addressed in future studies to confirm the observed effects of erenumab in rosacea. Another limitation is the relatively short follow-up period of 12 weeks, which limited our ability to assess the sustainability of treatment response over time. Long-term observational studies are needed to address this limitation. Furthermore, quantitative data were not collected immediately after the last dose of erenumab because follow-up was only 3 months after the last dose. On the other hand, the study was conducted in a well-characterized population of patients with rosacea, and erenumab was shown to be well tolerated with adverse effects that were consistent with its known safety profile.
Conclusions
This nonrandomized controlled trial provides evidence to support the potential effectiveness of erenumab in managing chronic redness and flushing in individuals with rosacea, as demonstrated by the reduction in days with moderate to extreme flushing. The results also suggest that erenumab is generally well tolerated, with mild and/or transient adverse effects. Larger randomized, controlled, double-blind studies are necessary to confirm these findings and to investigate the long-term effects of erenumab treatment.
Trial Protocol
eFigure 1. Timeline for the Enrollment, Treatment, and Follow-Up Phase of the Trial
eFigure 2. CONSORT Flow Diagram
eTable. Study Participants
Data Sharing Statement
References
- 1.Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282-289. doi: 10.1111/bjd.16481 [DOI] [PubMed] [Google Scholar]
- 2.Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148-155. doi: 10.1016/j.jaad.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 3.Odom R, Dahl M, Dover J, et al. ; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea . Standard management options for rosacea, part 2: options according to subtype. Cutis. 2009;84(2):97-104. [PubMed] [Google Scholar]
- 4.Steinhoff M, Schmelz M, Schauber J. Facial erythema of rosacea – aetiology, different pathophysiologies and treatment options. Acta Derm Venereol. 2016;96(5):579-586. doi: 10.2340/00015555-2335 [DOI] [PubMed] [Google Scholar]
- 5.van Zuuren EJ, Arents BWM, van der Linden MMD, Vermeulen S, Fedorowicz Z, Tan J. Rosacea: new concepts in classification and treatment. Am J Clin Dermatol. 2021;22(4):457-465. doi: 10.1007/s40257-021-00595-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82(6):1501-1510. doi: 10.1016/j.jaad.2020.01.077 [DOI] [PubMed] [Google Scholar]
- 7.Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011;15(1):33-39. doi: 10.1038/jidsymp.2011.8 [DOI] [PubMed] [Google Scholar]
- 8.Dirschka T, Micali G, Papadopoulos L, Tan J, Layton A, Moore S. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5(2):117-127. doi: 10.1007/s13555-015-0077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574. doi: 10.1080/19381980.2017.1361574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):53-62. doi: 10.1038/jidsymp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinhoff M, Schauber J, Leyden JJ, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6)(suppl 1):S15-S26. doi: 10.1016/j.jaad.2013.04.045 [DOI] [PubMed] [Google Scholar]
- 12.Helfrich YR, Maier LE, Cui Y, et al. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol. 2015;151(8):825-836. doi: 10.1001/jamadermatol.2014.4728 [DOI] [PubMed] [Google Scholar]
- 13.Scala J, Vojvodic A, Vojvodic P, et al. Botulin toxin used in rosacea and facial flushing treatment. Open Access Maced J Med Sci. 2019;7(18):2985-2987. doi: 10.3889/oamjms.2019.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wienholtz NKF, Christensen CE, Zhang DG, et al. Early treatment with sumatriptan prevents PACAP38-induced migraine: a randomised clinical trial. Cephalalgia. 2021;41(6):731-748; Online ahead of print. doi: 10.1177/0333102420975395 [DOI] [PubMed] [Google Scholar]
- 15.Christensen CE, Andersen FS, Wienholtz N, Egeberg A, Thyssen JP, Ashina M. The relationship between migraine and rosacea: systematic review and meta-analysis. Cephalalgia. 2018;38(7):1387-1398. doi: 10.1177/0333102417731777 [DOI] [PubMed] [Google Scholar]
- 16.Egeberg A, Ashina M, Gaist D, Gislason GH, Thyssen JP. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76(3):454-458. doi: 10.1016/j.jaad.2016.08.055 [DOI] [PubMed] [Google Scholar]
- 17.Vera N, Patel NU, Seminario-Vidal L. Rosacea comorbidities. Dermatol Clin. 2018;36(2):115-122. doi: 10.1016/j.det.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Wienholtz NKF, Christensen CE, Zhang DG, et al. Clinical characteristics of combined rosacea and migraine. Front Med (Lausanne). 2022;9(1026447):1026447. doi: 10.3389/fmed.2022.1026447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81(14):1191-1196. doi: 10.1212/WNL.0b013e3182a6cb72 [DOI] [PubMed] [Google Scholar]
- 20.Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. 2021;397(10283):1505-1518. doi: 10.1016/S0140-6736(20)32342-4 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Forsøgsperson. Welcome to Forsøgsperson.dk. 2015. Updated 2014. Accessed February 12, 2024. https://www.forsoegsperson.dk/
- 23.Kawata AK, Revicki DA, Thakkar R, et al. Flushing ASsessment Tool (FAST): psychometric properties of a new measure assessing flushing symptoms and clinical impact of niacin therapy. Clin Drug Investig. 2009;29(4):215-229. doi: 10.2165/00044011-200929040-00001 [DOI] [PubMed] [Google Scholar]
- 24.Tan J, Leoni M. Erythema of rosacea: validation of patient’s self-assessment grading scale. J Drugs Dermatol. 2015;14(8):841-844. [PubMed] [Google Scholar]
- 25.Tan J, Liu H, Leyden JJ, Leoni MJ. Reliability of clinician erythema assessment grading scale. J Am Acad Dermatol. 2014;71(4):760-763. doi: 10.1016/j.jaad.2014.05.044 [DOI] [PubMed] [Google Scholar]
- 26.Webster G, Schaller M, Tan J, Jackson JM, Kerrouche N, Schäfer G. Defining treatment success in rosacea as ‘clear’ may provide multiple patient benefits: results of a pooled analysis. J Dermatolog Treat. 2017;28(5):469-474. doi: 10.1080/09546634.2017.1343435 [DOI] [PubMed] [Google Scholar]
- 27.Wienholtz NKF, Thyssen JP, Christensen CE, et al. Validity and reliability of the Rosacea Area and Severity Index: a novel scoring system for clinical assessment of rosacea severity. J Eur Acad Dermatol Venereol. 2023;37(3):573-580. doi: 10.1111/jdv.18721 [DOI] [PubMed] [Google Scholar]
- 28.Rosacea National Society . Rosacea clinical scorecard 2004. Accessed April 21, 2023. https://www.rosacea.org/physicians/rosacea-clinical-scorecard
- 29.Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9(2):169-180. doi: 10.1111/j.1087-0024.2004.09113.x [DOI] [PubMed] [Google Scholar]
- 30.Nicholson K, Abramova L, Chren MM, Yeung J, Chon SY, Chen SC. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57(2):213-221. doi: 10.1016/j.jaad.2007.01.048 [DOI] [PubMed] [Google Scholar]
- 31.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69-77. doi: 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 32.Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008;71(1):32-38. doi: 10.1016/j.mehy.2008.01.032 [DOI] [PubMed] [Google Scholar]
- 33.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313(5997):54-56. doi: 10.1038/313054a0 [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335(6186):164-167. doi: 10.1038/335164a0 [DOI] [PubMed] [Google Scholar]
- 35.Fujimori A, Saito A, Kimura S, et al. Neurogenic vasodilation and release of calcitonin gene-related peptide (CGRP) from perivascular nerves in the rat mesenteric artery. Biochem Biophys Res Commun. 1989;165(3):1391-1398. doi: 10.1016/0006-291X(89)92758-7 [DOI] [PubMed] [Google Scholar]
- 36.Han SP, Naes L, Westfall TC. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8-37) and CGRP receptor desensitization. Biochem Biophys Res Commun. 1990;168(2):786-791. doi: 10.1016/0006-291X(90)92390-L [DOI] [PubMed] [Google Scholar]
- 37.Cao T, Pintér E, Al-Rashed S, Gerard N, Hoult JR, Brain SD. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J Immunol. 2000;164(10):5424-5429. doi: 10.4049/jimmunol.164.10.5424 [DOI] [PubMed] [Google Scholar]
- 38.van Zuuren EJ. Rosacea. N Engl J Med. 2017;377(18):1754-1764. doi: 10.1056/NEJMcp1506630 [DOI] [PubMed] [Google Scholar]
- 39.Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182(5):1269-1276. doi: 10.1111/bjd.18420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181(1):65-79. doi: 10.1111/bjd.17590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silberstein SD, Reshef S, Cohen JM, et al. Adverse events reported with therapies targeting the CGRP pathway during the first 6 months post-launch: a retrospective analysis using the FDA adverse events reporting system. Adv Ther. 2023;40(2):445-459. doi: 10.1007/s12325-022-02346-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashina M, Goadsby PJ, Reuter U, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39(11):1455-1464. doi: 10.1177/0333102419854082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cullum CK, Do TP, Ashina M, et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: a 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61. doi: 10.1186/s10194-022-01433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spoendlin J, Karatas G, Furlano RI, Jick SS, Meier CR. Rosacea in patients with ulcerative colitis and crohn’s disease: a population-based case-control study. Inflamm Bowel Dis. 2016;22(3):680-687. doi: 10.1097/MIB.0000000000000644 [DOI] [PubMed] [Google Scholar]
- 45.Egeberg A, Weinstock LB, Thyssen EP, Gislason GH, Thyssen JP. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176(1):100-106. doi: 10.1111/bjd.14930 [DOI] [PubMed] [Google Scholar]
- 46.Holmes AD, Spoendlin J, Chien AL, Baldwin H, Chang ALS. Evidence-based update on rosacea comorbidities and their common physiologic pathways. J Am Acad Dermatol. 2018;78(1):156-166. doi: 10.1016/j.jaad.2017.07.055 [DOI] [PubMed] [Google Scholar]
- 47.Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71(5):973-980. doi: 10.1016/j.jaad.2014.05.036 [DOI] [PubMed] [Google Scholar]
- 48.Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Patients with rosacea have increased risk of depression and anxiety disorders: a Danish nationwide cohort study. Dermatology. 2016;232(2):208-213. doi: 10.1159/000444082 [DOI] [PubMed] [Google Scholar]
- 49.Chang HC, Huang YC, Lien YJ, Chang YS. Association of rosacea with depression and anxiety: a systematic review and meta-analysis. J Affect Disord. 2022;299:239-245. doi: 10.1016/j.jad.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 50.Haber R, El Gemayel M. Comorbidities in rosacea: a systematic review and update. J Am Acad Dermatol. 2018;78(4):786-792.e8. doi: 10.1016/j.jaad.2017.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Timeline for the Enrollment, Treatment, and Follow-Up Phase of the Trial
eFigure 2. CONSORT Flow Diagram
eTable. Study Participants
Data Sharing Statement