Abstract
Background:
Combined hormonal contraceptives (CHCs), containing estrogen and progestin, are associated with an increased risk of venous thromboembolism (VTE) and arterial thromboembolism (ATE) compared with nonuse. Few studies have examined whether nonoral formulations (including the combined hormonal patch, combined vaginal ring and combined injectable contraceptives) increase the risk of thrombosis compared with combined oral contraceptives (COCs).
Objectives:
The objectives were to examine the risk of VTE and ATE among women using nonoral CHCs compared to women using COCs.
Methods:
We searched the PubMed database for all English language articles published from database inception through May 2016. We included primary research studies that examined women using the patch, ring or combined injectables compared with women using levonorgestrel-containing or norgestimate-containing COCs. Outcomes of interest included VTE (deep venous thrombosis or pulmonary embolism) or ATE (acute myocardial infarction or ischemic stroke). We assessed the quality of each individual piece of evidence using the system developed by the United States Preventive Services Task Force.
Results:
Eight studies were identified that met inclusion criteria. Of seven analyses from six studies examining VTE among patch users compared with levonorgestrel- or norgestimate-containing COC users, two found a statistically significantly elevated risk among patch users (risk estimates 2.2–2.3), one found an elevated risk that did not meet statistical significance (risk estimate 2.0), and four found no increased risk. Of three studies examining VTE among ring users compared with levonorgestrel COC users, one found a statistically significantly elevated risk among patch users (risk estimate 1.9) and two did not. Two studies did not find an increased risk for ATE among women using the patch compared with norgestimate COCs. We did not identify any studies examining combined injectable contraceptives.
Conclusion:
Limited Level II-2 good to fair evidence demonstrated conflicting results on whether women using the patch or the ring have a higher risk of VTE than women using COCs. Evidence did not demonstrate an increased risk of ATE among women using the patch. Overall, any potential elevated risk likely represents a small number of events on a population level. Additional studies with standard methodology are needed to further clarify any associations and better understand mechanisms of hormone-induced thrombosis among users of nonoral combined hormonal contraception.
Keywords: Nonoral combined hormonal contraception, Patch, Ring, Venous thromboembolism, Arterial thromboembolism
1. Introduction
Combined hormonal contraceptives (CHCs), containing estrogen and progestin, are important methods in the array of contraceptives available to women. Globally, combined oral contraceptives (COCs) are the third most widely used contraceptive method and are used by over 100 million women [1,2]. Nonoral formulations of CHCs, including the combined hormonal patch, combined vaginal ring and combined injectable contraceptives, offer similar benefits and side effect profiles and may increase ease of use by eliminating need for daily intervention [3–5].
The elevated relative risk of thrombosis among women using CHCs compared with nonusers is well established [6]. Risks include venous thromboembolism (VTE), such as deep venous thrombosis (DVT) or pulmonary embolism (PE), and arterial thromboembolism (ATE), such as acute myocardial infarction (AMI) or ischemic stroke. Estrogen can promote coagulation through multiple effects on the procoagulant, anticoagulant and fibrinolytic pathways [7]. In addition, there is increasing evidence that different progestins may also independently and variably affect hemostatic factors and thrombosis risk [7,8]. The relevant safety question for women choosing CHCs is whether certain formulations have differential risks of thrombosis. Among COCs, formulations with <50 mcg ethinyl estradiol containing levonorgestrel (LNG) appear to have the lowest risk of VTE [9]. This systematic review was conducted to examine the risk of VTE and ATE with use of nonoral CHCs. Specifically, the review sought to identify evidence comparing risks among women using nonoral CHCs with women using LNG-containing or norgestimate (NGM)-containing COCs.
2. Materials and methods
We conducted this systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10].
2.1. Literature search
We searched the PubMed database for all relevant articles published from database inception through May 2016 (see Appendix A for search strategy). We searched for all primary research articles published in any language. We also searched reference lists of identified articles and relevant review articles for additional citations of interest. We did not consider unpublished studies, abstracts of conference presentations or dissertations.
2.2. Selection criteria
Articles were included in this review if they were primary reports on studies examining venous or arterial thromboembolic events among users of nonoral CHCs compared with users of COCs. The nonoral CHCs of interest included the combined hormonal patch, the combined vaginal ring and the combined injectable contraceptives (containing medroxyprogesterone acetate + estradiol cypionate or norethisterone enanthate + estradiol valerate). The specific reference group of interest was users of LNG-containing COCs because these have been generally found to have the lowest risk of thromboembolic complications [8]. Some studies compared users of the patch to users of NGM-containing COCs because the progestin in the patch is a metabolite of NGM. We included these studies because the risk of VTE among users of NGM COCs has been found to be similar to LNG COCs, and therefore, we considered this to be a useful reference group [8]. Outcomes of interest included venous thromboembolic events (e.g., DVT, PE or cerebral venous sinus thrombosis) or arterial thromboembolic events (e.g., AMI or ischemic stroke).
2.3. Study quality assessment and data synthesis
Two authors (N.T. and M.D.) summarized and systematically assessed the evidence. We assessed the quality of each individual piece of evidence using the system developed by the United States Preventive Services Task Force [11]. We focused on methodologic features specific to our research question by study design. For cohort studies, we assessed adequacy of sample size, exposure assessment and timing of exposure, validation of outcome assessment and adequate control for potential confounders (including exclusion of women with other risk factors for thrombosis). For case–control studies, we assessed the selection of cases and controls, diagnostic criteria used for both groups, exposure assessment and adequate control for potential confounders. Summary measures were not calculated due to the small number of studies of each contraceptive method with the same reference groups.
3. Results
The search identified 504 articles. After reviewing the titles and abstracts of these articles, as well as the full articles when necessary, we determined that eight articles met criteria for inclusion in this review (Table 1) [12–19]. Of the included articles, seven reported outcomes among patch users [12,14–19] and three reported outcomes among ring users [12,13,18]. If there were multiple articles reporting on the same study, only the most recent report was included [14,16] and the older reports were excluded [20–22]. Three studies were excluded because the reference group was users of multiple types of COCs [23] or nonhormonal users [24,25]. We did not identify any studies examining combined injectable contraceptives.
Table 1.
Studies examining risk of venous or arterial thromboembolism among users of nonoral combined hormonal contraceptives.
| Author, Year, Location, Support | Study design, Study period | Population | Exclusions | CHC information | Outcome information | Results | Strengths | Weaknesses | Quality Grading | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jick [19], 2006 United States Johnson & Johnson |
Retrospective cohort 2000–2005 |
Women ages 15–44 PharMetrics database Patch: 77,231 woman-years LNG COCs: 299,536 woman-years |
History of significant head injury, major surgery, severe trauma, pregnancy, cancer, renal failure, chronic cardiovascular disease, inflammatory/autoimmune conditions | Prescription claims | Diagnosis codes from hospitalizations plus prolonged anticoagulant therapy Review of computer records for appropriate tests and procedures |
Venous events:
|
Use of large, nationwide database Excluded women with certain CVST risk factors |
May have included previous users of other pills, no CHC history reported CHC information from claims data only Outcomes not verified by medical record review Small number of events |
Level II-2, fair | ||||||||||||||||||||||||||||||||||||
| Jick [17], 2007 United States Johnson & Johnson, Pharmaceutical Research & Development |
Retrospective cohort 2002–2005 |
Women ages 15–44 PharMetrics database Patch: 58,752 woman-years NGM COCs: 88,571 woman-years |
History of stroke, history of VTE, pregnancy, major trauma, major surgery | Prescription claims | Diagnosis codes from hospitalizations Review of computer records for appropriate tests and procedures |
Arterial events:
|
Use of large, nationwide database Excluded women with certain ATE risk factors |
May have included previous users of other pills, no CHC history reported CHC information from claims data only Outcomes not verified by medical record review No information on BMI, smoking Small numbers of events Crude estimates |
Level II-2, fair | ||||||||||||||||||||||||||||||||||||
| Dore [14], 2010 (includes data from 1 earlier study [20]) United States i3 Drug Safety, Johnson & Johnson, Pharmaceutical Research & Development |
Case–control 2002–2006 | Women ages 15–44 Normative Health Information database Cases: first ever inpatient or outpatient VTE, or from National Death Index Controls: randomly selected from database matched by year of birth, pattern of drug use |
Trauma, pregnancy, major surgery, postoperative complications, anticoagulant therapy | Prescription claims | Diagnosis codes Adjudicated using medical records |
Venous events:
OR (new users): 1.8 (0.8–3.8) OR (not new initiators): 2.2 (1.1–4.5)
* Matched OR accounted for birth year and new initiator status, confounders assessed but not included because not significant
|
VTE outcomes confirmed by medical record review Excluded women with certain VTE risk factors |
CHC information from claims data only Small numbers for some comparisons of interest |
Level II-2, good | ||||||||||||||||||||||||||||||||||||
| Jick [15], 2010 United States Johnson & Johnson Pharmaceutical Research & Development |
Nested case–control 2002–2006 (PharMetrics/IMS) 2002–2007 (MarketScan) |
Women ages 15–44, new users PharMetrics/IMS and MarketScan databases Cases: first ever VTE, nonfatal Controls: matched by year of birth and index date |
Major surgery, trauma, epilepsy, pregnancy, previous anticoagulation, cancer, coronary artery disease, ulcerative colitis | Prescription claims | Diagnosis codes plus anticoagulant treatment | Venous events:
*
Confounders assessed but not included because not significant
Results similar when stratified by new users and non-new users |
Analyses conducted in 2 separate large nationwide databases Excluded women with certain ATE and VTE risk factors |
No information on smoking and limited information on BMI CHC information from claims data only Outcomes not verified by medical record review |
Level II-2, good | ||||||||||||||||||||||||||||||||||||
| Jick [16], 2010 (includes data from 2 earlier studies) [21,22] United States Johnson & Johnson Pharmaceutical Research & Development |
Case–control 2002–2007 |
Women ages 15–44, new users PharMetrics/IMS database Cases: first ever VTE Controls: matched by year of birth and index date |
Major surgery, trauma, epilepsy, pregnancy, cancer, renal failure, chronic inflammation | Prescription claims | Diagnosis codes plus anticoagulant treatment | Venous events:
* Unadjusted; authors state adjustment for various medical conditions did notmaterially change results
|
Use of large, nationwide database Excluded women with certain VTE risk factors |
CHC information from claims data only Outcomes not verified by medical record review Unadjusted ORs |
Level II-2, fair | ||||||||||||||||||||||||||||||||||||
| Lidegaard [18], 2012 Denmark Gynecological Clinic, Juliane Marie Centre, Rigshospitalet |
Retrospective cohort 2001–2010 |
Women ages 15–49 Patch: 6178 WY Ring: 50,334 WY LNG COC: 231,675 WY |
History of VTE or ATE, cancer, hysterectomy, bilateral oophorectomy, sterilized, coagulation disorder, pregnancy | Prescription claims | Diagnosis codes from hospitalizations or from national death registry Anticoagulant treatment prescriptions |
Venous events (confirmed):
* Adjusted for age, calendar year, and education.
|
Use of large, nationwide database Examined history of contraceptive use from 1995–2010 Excluded women with certain ATE and VTE risk factors |
Did not control for smoking, BMI CHC and outcomeinformation from claims data only, not verified by medical record review |
Level II-2, good | ||||||||||||||||||||||||||||||||||||
| Dinger [13], 2013 Austria France Germany Italy Russia United States Organon NV (Merck &Co) |
Prospective cohort 2007–2012 |
Women using CHCs referred by physician network Ring: N=16,864 COC: N=16,431 (overall COCs, number for LNG COC not stated)Followed at 6 and 12 months and then yearly up to 4 years |
None | Physician identification of women prescribed CHCs Participant questionnaire for CHC history |
Participant questionnaire Followed up with physicians for confirmation of events Medical record review for diagnostic studies Verified by independent blinded adjudication |
Venous events:
* Adjusted for age, BMI, duration of CHC use, family history VTE
|
Cohort identified by physicians prescribing contraceptives VTE outcomes confirmed by physician and diagnostic studies, verified by independent blinded adjudication Low loss to follow-up (3%) |
Did not exclude or adjust for certain thrombosis risk factors Included new users, switchers and restarters Information on specific contraceptive use obtained by questionnaireReported as-treated results (did not differ from intention-to-treat results) |
Level II-2, good | ||||||||||||||||||||||||||||||||||||
| Bergendal [12], 2014 Sweden Janssen-Cilag, Novartis, Organon, Schering, Wyeth, AFA Insurance, Center for Gender Medicine Karolinska Institutet, the Medical Products Agency |
Case–control 2003–2009 |
Women ages 18–54 Cases: women with first ever DVT or PE from inpatient or outpatient hospitals Controls: randomly selected from population register, matched by birth year |
Previous VTE, pregnancy, malignancy | Participant telephone interview | Identified by study coordinator or registry from hospitals [30] Confirmed by review of radiologic tests and anticoagulant treatment [30] |
Venous events:
* Adjusted for BMI, immobilization, and smoking; excluding BMI>30 and severely immobilized
|
Excluded women with certain VTE risk factors VTE outcomes confirmed by review of testing and anticoagulant treatment Memory support aids for contraceptive types |
Information on contraceptive use obtained by phone interview Differential participation rate between cases (90%) and controls (69%) Small numbers and wide CIs |
Level II-2, good |
Abbreviations: aOR, adjusted odds ratio; ATE, arterial thromboembolism; BMI, body mass index; CHC, combined hormonal contraceptive; CI, confidence interval; COC, combined oral contraceptive; CVST, cerebral venous sinus thrombosis; DVT, deep venous thrombosis; HR, hazard ratio; IRR, incidence rate ratio; LNG, levonorgestrel; PE, pulmonary embolism; NGM, norgestimate; VTE, venous thromboembolism; WY, woman-years.
3.1. Studies examining users of the patch
3.1.1. Venous events
There were six articles that described venous events among patch users compared with COC users (Fig. 1) [12,14–16,18,19]. Two were cohort studies [18,19], and the remaining were case-control studies. Four studies used LNG COCs as the reference group [12,15,18,19], and two used NGM COCs as the reference group [14,16].
Fig. 1.

Risk of venous thromboembolism among patch users compared with combined oral contraceptive users. aReference group is norgestimate-containing combined oral contraceptives bReference group is levonorgestrel-containing combined oral contraceptives.
One cohort study found an elevated rate of VTE among patch users compared with LNG COC users, with an adjusted rate ratio of 2.31 [95% confidence interval (CI) 1.02–5.23] [18]. The other cohort study found no instances of cerebral venous sinus thrombosis among patch users compared with an incidence of 0.7/100,000 woman-years among LNG COC users [19]. One of the case–control studies also found an elevated odds of VTE among patch users compared with NGM COC users [odds ratio (OR) 2.2; 95% CI 1.2–4.0) [14]. However, among new users (no prior use of CHCs), the point estimate remained elevated but no longer met statistical significance (OR 1.8; 95% CI 0.8–3.8). A case–control analysis from a large administrative database found a point estimate that was elevated to a similar degree but that did not meet statistical significance (OR 2.0; 95% CI 0.9–4.1) [15]. In contrast, a similar analysis of a different database did not find a statistically significant effect (OR 1.3; 95% CI 0.8–2.1) [15]. Two additional case–control studies did not find increased odds of VTE among patch users compared with COC users, with ORs of 1.0 (95% CI 0.1–11.0) and 1.2 (95% CI 0.9–1.8), respectively [12,16].
3.1.2. Arterial events
There were two articles reporting risk of arterial events among patch users compared with COC users (Fig. 2) [14,17]. One was a cohort study [17], and one was a case–control study [14]. Both studies compared patch users to NGM COC users, and neither found a statistically significant difference in AMI or ischemic stroke. The point estimates for AMI ranged from 0.2 to 1.6, and all CIs included 1; the point estimates for ischemic stroke ranged from 0.8 to 1.2, and all CIs included 1.
Fig. 2.
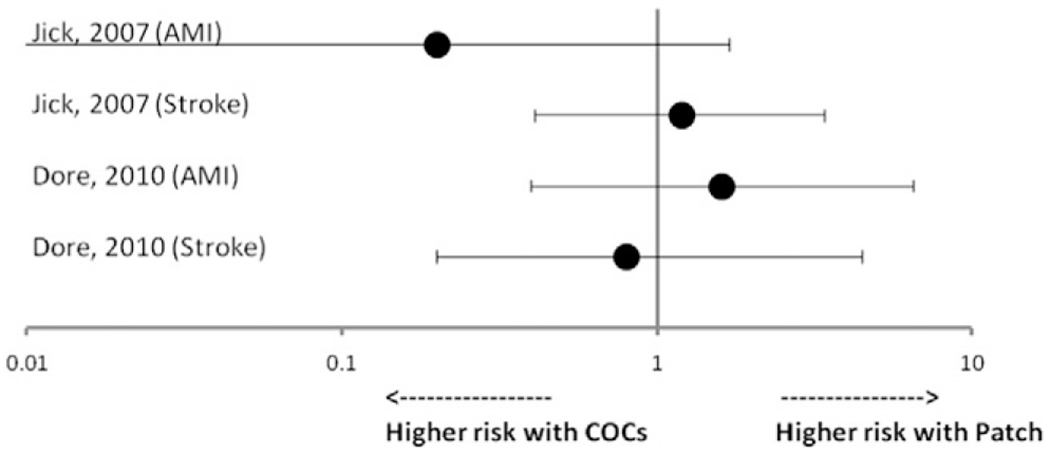
Risk of arterial thromboembolism among patch users compared with norgestimate-containing combined oral contraceptive users.
3.2. Studies examining users of the ring
3.2.1. Venous events
There were three articles which reported venous outcomes among vaginal ring users compared to COC users (Fig. 3) [12,13,18]. Two were cohort studies [13,18], and one was a case–control study [12]. All studies used LNG COCs as the reference group for comparisons. One of the cohort studies reported an elevated rate ratio of confirmed VTEs among ring users compared with LNG COC users (adjusted rate ratio 1.90; 95% CI 1.33–2.71) [18]. The other cohort study and the case–control study did not find statistically significant differences in risk of VTE between ring users and LNG COC users, with point estimates ranging from 1.0 to 1.5 [12, 13].
Fig. 3.
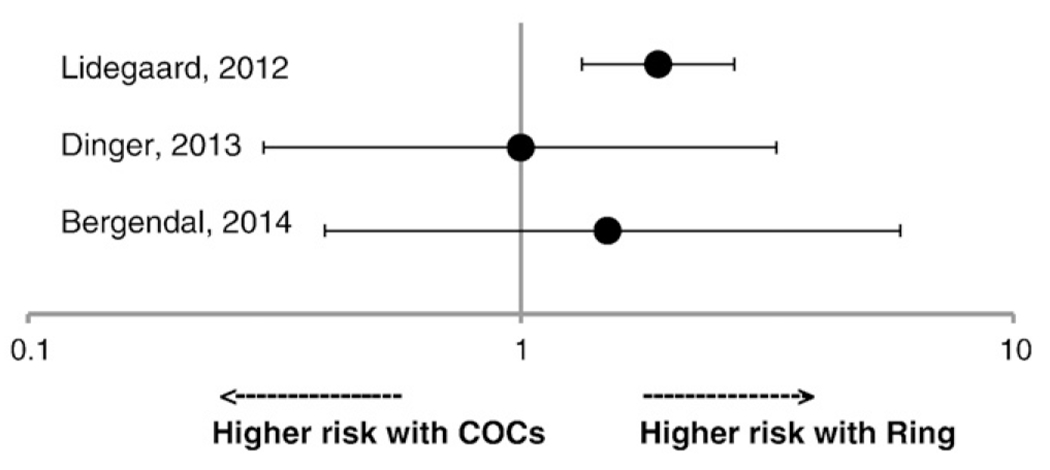
Risk of venous thromboembolism among ring users compared with levonorgestrel-containing combined oral contraceptive users.
3.2.2. Arterial events
There were no studies describing arterial events among ring users that met inclusion criteria. Results from Dinger et al. are not included here because the incidence of arterial events among ring users was compared to a reference group of women using multiple types of COCs [13].
4. Discussion
This systematic review identified six articles that found conflicting results on whether women using the patch have a higher risk of VTE than women using LNG or NGM COCs. The review also identified three articles which found conflicting results on whether women using the ring have a higher risk of VTE than women using LNG COCs. The review identified two articles which did not find an increased risk for ATE among women using the patch compared with NGM COCs. The review did not identify any studies that met inclusion criteria describing combined injectable contraceptives.
There are several limitations to this body of evidence which should be considered when interpreting results. Several of the studies used data obtained from insurance claims not verified by medical records, which may be subject to misclassification [15,16,18,19]. Several studies had small numbers for the comparisons of interest, resulting in lower precision around point estimates [12,14,17]. Two studies presented results that were not adjusted for potential confounders such as obesity [16,17], although the authors of one of the studies stated that adjustment did not materially change results [16]. Three studies did not limit analyses to new users of CHCs, which may introduce bias if long-term users are at lower risk for VTE [13,17,19].
Potential reasons for discordant results for relative risk of VTE between studies include differences in population, study design, funding source, and ascertainment and confirmation of contraceptive use and outcomes. With regard to the studies examining the patch, the study that found the highest risk estimate was the only cohort study and the only study not funded by pharmaceutical companies [18]. However, the study relied only on diagnostic codes and prescription data for exposure and outcome information, which may have artificially increased results if women using the patch were more likely to be evaluated and diagnosed with VTE. The case–control study that found no increased risk of VTE was based on two events among cases and one event among controls [12]. The remaining case–control studies found either a statistically significantly increased risk or a similarly elevated point estimate that did not meet statistical significance.
With regard to the studies examining the ring, the same study that found the highest relative risk for patch use and VTE also found a statistically significantly increased risk for ring use and VTE [18]; this could again be explained by higher likelihood of evaluation and diagnosis among ring users. The other two studies did not find statistically significantly increased risk of VTE with ring use [12,13]. One study did not exclude or adjust for certain key thrombosis risk factors such as recent pregnancy or history of VTE [13]. Theoretically, this might have led to attenuated estimates because the group of nonusers may have had a higher baseline risk of VTE.
The discordance of the results highlights a need for additional studies with standard methodology to better compare results across studies and better understanding of potential associations between these methods and thrombosis. The mechanisms whereby nonoral CHCs may impact thrombosis risk are not well understood. Historically, estrogen levels were thought to be the critical factor in thrombosis risk, and progressive decreases in ethinyl estradiol levels resulted in reduced risks [7]. Levels of estrogen differ based on route of administration. The oral route of hormone administration results in characteristic peaks and troughs of serum concentrations [26]. The nonoral routes result in more steady serum hormone levels. Among women using the patch, the maximum levels of ethinyl estradiol are lower than among women using COCs; however, the overall exposure to ethinyl estradiol is higher [26]. Among women using the ring, the maximum levels and the overall exposure to ethinyl estradiol are lower than among women using COCs [26]. However, it is not clear whether the different serum hormone levels achieved by nonoral routes may correlate with thrombosis risk.
Estrogen impacts the clotting cascade in several ways including effects on the procoagulant, anticoagulant and fibrinolytic pathways [7]. Most proteins involved in hemostasis are synthesized in the liver. Because estrogen is metabolized in the liver, nonoral administration of hormones might diminish impacts by avoiding the first-pass effect on liver metabolism in theory [7]. However, changes in the hemostatic system are observed with all routes of hormone administration. Several studies have found that the patch induced unfavorable changes in thrombotic markers [27–29]. Studies of thrombotic markers in ring users have found conflicting results, with some finding favorable and others finding prothrombotic effects [27,28]. Further, thrombosis formation is multifactorial, and it is not known how these hormonally induced changes in hemostatic factors may impact thrombosis risk.
In addition to the well-documented effects of estrogen on thrombosis, there has been increasing attention on the role that the progestin component in COCs may play in the development of thrombosis. The combined hormonal patch contains norelgestromin, which is a metabolite of NGM. COCs containing NGM have not been associated with higher risk of VTE compared with COCs containing LNG [8]. However, similar to the estrogen exposure, women using the patch have an overall higher exposure to NGM than women using COCs. The combined vaginal ring contains etonogestrel, which is not found in COCs and therefore not directly comparable.
Any communication of risk with use of these methods should include discussion of relative versus absolute risks. If there is a modest increase in relative risk of thrombosis among patch or ring users compared to COC users, this represents an overall small excess number of events at the population level. The incidence of thrombosis among users of the patch and ring remained low in these studies and may have accounted for only a small number of excess events. The incidence of VTE among patch and ring users was approximately 8–10 per 10,000 woman-years [13,18]. The incidence of ATE among patch users was approximately 2–14 per 100,000 women-years [17]. Further, these risks must be balanced with the risks of non-use including unintended pregnancy and pregnancy-related morbidity.
In summary, this systematic review identified limited Level II-2 good to fair evidence on risk of thrombosis with use of the patch and the ring compared with COC use. Studies demonstrated conflicting results on whether users of the patch have an increased risk of VTE compared with users of LNG or NGM COCs. Evidence did not demonstrate an increased risk of ATE among users of the patch. Limited evidence also demonstrated conflicting results on whether users of the ring have an increased risk of VTE compared with LNG COCs. Any elevated risk is likely small and represents only a slight increase in absolute numbers of events at the population level. Nonoral CHCs remain a safe and viable option in the contraceptive method mix. Nonetheless, additional studies are needed to better understand the relationship between nonoral CHCs and thrombosis.
Acknowledgements
This review was supported by resources from the Department of Reproductive Health and Research at the World Health Organization, the Centers for Disease Control and Prevention, the US Agency for International Development, and the National Institute of Child Health and Human Development.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the World Health Organization or US Centers for Disease Control and Prevention.
Appendix A. Search Strategy for nonoral combined hormonal contraceptives and thromboembolism
(((Contraceptive Agents, Female[Mesh] AND patch) OR ortho evra OR evra OR norelgestromin OR (Contraceptive Devices, Female[Mesh] AND ring) OR nuvaring OR CVR OR (ring AND vagina*) OR (((combin* AND inject*) AND contracept*) OR ((((once a month OR monthly) AND inject*) AND contracept*) OR cyclofem OR lunelle OR mesigyna OR cycloprovera)))) AND ((((“venous thrombosis”[MeSH Terms] OR (“venous”[All Fields] AND “thrombosis” [All Fields]) OR “venous thrombosis”[All Fields] OR (“deep”[All Fields] AND “vein”[All Fields] AND “thrombosis” [All Fields]) OR “deep vein thrombosis”[All Fields]) OR DVT[All Fields] OR (“venous thromboembolism” [MeSH Terms] OR (“venous”[All Fields] AND “thromboembolism” [All Fields]) OR “venous thromboembolism”[All Fields]) OR ((“veins” [MeSH Terms] OR “veins”[All Fields] OR “venous”[All Fields]) AND (“thromboembolism”[MeSH Terms] OR “thromboembolism”[All Fields] OR (“thromboembolic”[All Fields] AND “event”[All Fields]) OR “thromboembolic event”[All Fields])) OR VTE[All Fields] OR PE[All Fields] OR (“pulmonary”[All Fields] AND “embolus”[All Fields]) OR “pulmonary embolus”[All Fields])) OR ((“cerebrovascular disorders”[MeSH Terms] OR (“cerebrovascular”[All Fields] AND “disorders”[All Fields]) OR “cerebrovascular disorders”[All Fields]) OR (“stroke”[MeSH Terms] OR “stroke”[All Fields]) OR (((“brain”[MeSH Terms] OR “brain”[All Fields]) OR (“cerebrum”[MeSH Terms] OR “cerebrum”[All Fields] OR “cerebral”[All Fields] OR “brain”[MeSH Terms] OR “brain”[All Fields])) AND ((“infarction”[MeSH Terms] OR “infarction”[All Fields]) OR (“ischaemia”[All Fields] OR “ischemia”[MeSH Terms] OR “ischemia “[All Fields]) OR (“embolism”[MeSH Terms] OR “embolism”[All Fields]) OR (“thrombosis”[MeSH Terms] OR “thrombosis”[All Fields]))) OR (“myocardial infarction”[MeSH Terms] OR (“myocardial”[All Fields] AND “infarction”[All Fields]) OR “myocardial infarction” [All Fields] OR (“heart”[All Fields] AND “attack”[All Fields]) OR “heart attack”[All Fields]) OR (“myocardial infarction” [MeSH Terms] OR (“myocardial”[All Fields] AND “infarction” [All Fields]) OR “myocardial infarction”[AllFields]))).
References
- [1].Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab 2013;27:3–12. [DOI] [PubMed] [Google Scholar]
- [2].United Nations Department of Economic and Social Affairs Population Division. World contraceptive patterns 2013. Available at http://www.un.org/en/development/desa/population/publications/pdf/family/worldContraceptivePatternsWallChart2013.pdf 2013. [Accessed August 8, 2016].
- [3].Audet MC, Moreau M, Koltun WD, Waldbaum AS, Shangold G, Fisher AC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. J Am Med Assoc 2001;285:2347–54. [DOI] [PubMed] [Google Scholar]
- [4].De Aguilar MA, Altamirano L, Leon DA, De Fung RC, Grillo AE, Gonzalez JD, et al. Current status of injectable hormonal contraception, with special reference to the monthly method. Adv Contracept 1997;13:405–17. [DOI] [PubMed] [Google Scholar]
- [5].Kerns J, Darney P. Vaginal ring contraception. Contraception 2011;83:107–15. [DOI] [PubMed] [Google Scholar]
- [6].Lidegaard O, Milsom I, Geirsson RT, Skjeldestad FE. Hormonal contraception and venous thromboembolism. Acta Obstet Gynecol Scand 2012;91:769–78. [DOI] [PubMed] [Google Scholar]
- [7].Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thromb Res 2010;126:5–11. [DOI] [PubMed] [Google Scholar]
- [8].Martinez F, Ramirez I, Perez-Campos E, Latorre K, Lete I. Venous and pulmonary thromboembolism and combined hormonal contraceptives. Systematic review and meta-analysis. Eur J Contracept Reprod Health Care 2012;17:7–29. [DOI] [PubMed] [Google Scholar]
- [9].de Bastos M, Stegeman BH, Rosendaal FR, Van Hylckama VA, Helmerhorst FM, Stijnen T, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev 2014;3:CD010813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US preventive services task force: a review of the process. Am J Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- [12].Bergendal A, Persson I, Odeberg J, Sundstrom A, Holmstrom M, Schulman S, et al. Association of venous thromboembolism with hormonal contraception and thrombophilic genotypes. Obstet Gynecol 2014;124:600–9. [DOI] [PubMed] [Google Scholar]
- [13].Dinger J, Mohner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol 2013;122:800–8. [DOI] [PubMed] [Google Scholar]
- [14].Dore DD, Norman H, Loughlin J, Seeger JD. Extended case–control study results on thromboembolic outcomes among transdermal contraceptive users. Contraception 2010;81:408–13. [DOI] [PubMed] [Google Scholar]
- [15].Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010;81:16–21. [DOI] [PubMed] [Google Scholar]
- [16].Jick SS, Hagberg KW, Kaye JA. ORTHO EVRA and venous thromboembolism: an update. Contraception 2010;81:452–3. [DOI] [PubMed] [Google Scholar]
- [17].Jick SS, Jick H. The contraceptive patch in relation to ischemic stroke and acute myocardial infarction. Pharmacotherapy 2007;27:218–20. [DOI] [PubMed] [Google Scholar]
- [18].Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. Br Med J 2012;344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jick SS, Jick H. Cerebral venous sinus thrombosis in users of four hormonal contraceptives: levonorgestrel-containing oral contraceptives, norgestimate-containing oral contraceptives, desogestrel-containing oral contraceptives and the contraceptive patch. Contraception 2006;74:290–2. [DOI] [PubMed] [Google Scholar]
- [20].Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007;109:339–46. [DOI] [PubMed] [Google Scholar]
- [21].Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users oforal contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007;76:4–7. [DOI] [PubMed] [Google Scholar]
- [22].Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006;73:223–8. [DOI] [PubMed] [Google Scholar]
- [23].Sidney S, Cheetham TC, Connell FA, Ouellet-Hellstrom R, Graham DJ, Davis D, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception 2013;87:93–100. [DOI] [PubMed] [Google Scholar]
- [24].Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, case–control study. World Health Organization collaborative study of cardiovascular disease and steroid hormone contraception. Contraception 1998;57:315–24. [PubMed] [Google Scholar]
- [25].Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. New Engl J Med 2012;366:2257–66. [DOI] [PubMed] [Google Scholar]
- [26].van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005;72:168–74. [DOI] [PubMed] [Google Scholar]
- [27].Jensen JT, Burke AE, Barnhart KT, Tillotson C, Messerle-Forbes M, Peters D. Effects of switching from oral to transdermal or transvaginal contraception on markers of thrombosis. Contraception 2008;78:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fleischer K, van Vliet HA, Rosendaal FR, Rosing J, Tchaikovski S, Helmerhorst FM. Effects of the contraceptive patch, the vaginal ring and an oral contraceptive on APC resistance and SHBG: a cross-over study. Thromb Res 2009;123:429–35. [DOI] [PubMed] [Google Scholar]
- [29].Kluft C, Meijer P, LaGuardia KD, Fisher AC. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception 2008;77:77–83. [DOI] [PubMed] [Google Scholar]
- [30].Bergendal A, Bremme K, Hedenmalm K, Larfars G, Odeberg J, Persson I, et al. Risk factors for venous thromboembolism in pre-and postmenopausal women. Thromb Res 2012;130:596–601. [DOI] [PubMed] [Google Scholar]


