Abstract
Background
Disordered autonomic nervous system regulation and supraspinal pain inhibition have been repeatedly described in chronic pain. We aimed to explore the effects of δ-9-tetrahydrocannabinol (THC), an emerging treatment option, on autonomic nervous system and central pain modulation measures in patients with chronic pain.
Methods
Twelve male patients with chronic radicular neuropathic pain participated in a randomized, double-blind, crossover, placebo-controlled, single-administration trial. Low/high frequency (LF/HF) heart rate variability (HRV) ratio and conditioned pain modulation (CPM) response were measured and resting-state functional magnetic resonance imaging (MRI) was performed at baseline and after sublingual administration of either 0.2 mg/kg oral THC or placebo.
Results
THC significantly reduced the LF/HF ratio compared with placebo (interaction effect F(1,11) = 20.5; p < 0.005) and significantly improved CPM responses (interaction effect F(1,9) = 5.2; p = 0.048). The THC-induced reduction in LF/HF ratio correlated with increased functional connectivity between the rostral ventrolateral medulla and the dorsolateral prefrontal cortex [T(10) = 6.4, cluster p-FDR < 0.005].
Conclusions
THC shifts the autonomic balance towards increased parasympathetic tone and improves inhibitory pain mechanisms in chronic pain. The increase in vagal tone correlates with connectivity changes in higher-order regulatory brain regions, suggesting THC exerts top-down effects. These changes may reflect a normalizing effect of THC on multiple domains of supraspinal pain dysregulation.
Clinical Trial Registry Number
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-024-01085-0.
Key Points
| Chronic pain can be extremely debilitating and difficult to treat, and is also a complex neurobiological phenomenon, involving the entire neural system. |
| In recent years, the use of medical cannabis to treat chronic pain has gained much attention; however, the neurobiological mechanisms underlying cannabis-induced pain relief are complex, multimodal, and remain poorly understood. |
| This article presents a double-blind, randomized trial that demonstrates that the main psychoactive ingredient in medical cannabis, δ-9-tetrahydrocannabinol (THC), exerts a balancing effect on autonomic nervous system activity and central nervous system pain modulation, with corresponding changes in functional brain connectivity in male patients with chronic neuropathic pain. These findings could potentially contribute to understanding chronic pain dysregulation mechanisms and support the medicinal use of cannabis as well as other re-emerging treatments. |
Introduction
Chronic pain is one of the most common problems encountered in healthcare [1]. Normally, pain perception is modulated by an array of biological, environmental, cognitive and affective factors that heavily influence the subjective experience of pain [2]. These in turn are supported by a network of cortical and subcortical brain areas that can facilitate or inhibit nociceptive afferent brain input via brainstem nuclei [3]. In chronic pain conditions, there is altered modulation of such inhibitory pathways [4], as well as dysfunction of the autonomic nervous system (ANS) [5]. The ANS comprises the sympathetic and parasympathetic nervous systems, in which the latter is dominated by the vagus nerve, involved in many physiological processes, including cardiovascular function and gastrointestinal responses, and has recently been shown to also be involved in pain regulation [6, 7]. Although the ANS and supraspinal pain modulatory systems do not necessarily interact directly [8], all display abnormal activity patterns in chronic pain states. A recent meta-analysis concluded, based on heart rate variability (HRV) findings from chronic pain patients, that the ANS imbalance specifically implicates dysregulation of the parasympathetic arm of the ANS [9]. Notably, relief of chronic pain by injection of a local anesthetic results in an improvement in vagal cardiovascular control measures [10].
Higher central nervous system modulation and processing of the autonomic activity involves a set of neural structures in the brain stem, and limbic and cortical areas [11]. HRV dynamics are mediated through efferent outflow from medulla nuclei [12]. Specifically, neurons within the rostral ventrolateral medulla (RVLM) play a pivotal role in the maintenance and control of the tonic sympathetic activity [13, 14]. This activity includes high-frequency (HF) and low-frequency (LF) components, representing vagal and sympathetic influences, accordingly. The LF/HF ratio assesses sympathetic-parasympathetic balance, providing a better insight into stimulus-response relations. The RVLM is a crucial region in the brainstem that plays a key role in regulating cardiovascular functions, impacting HRV (both LF and HF). Thus, understanding RVLM-HRV interaction helps decipher autonomic regulation, crucial when assessing drug effects (e.g. cannabis), where changes in the LF/HF ratio better reflect overall autonomic dynamics [11]. Descending excitatory and inhibitory inputs from higher brain regions are demonstrated from the hypothalamus, amygdala, and regions in the prefrontal cortex (PFC) [15, 16]. These neuronal centers play critical roles in the control of both the emotional-cognitive aspects of pain processing and autonomic responses, as described in animal models as well as in human neuroimaging studies. Numerous resting state functional magnetic resonance imaging (fMRI) studies have shown that these brain networks show altered activation in chronic pain patients [17, 18], and this remains one of the most widely used functional neuroimaging methods used in imaging chronic pain [19, 20].
The function of other pain regulatory systems, in particular the inhibitory pathway, can be evaluated by the experimental paradigm of conditioned pain modulation (CPM), which estimates the reduction in pain perception that occurs when a participant is exposed to pain and an additional conditioning pain stimulus is applied, a phenomenon also known as ‘pain inhibits pain’ [21, 22]. Patients with chronic pain exhibit an impairment in the inhibitory pathway, resulting in lower CPM efficiency [23].
Cannabis and its main psychoactive component δ-9-tetrahydrocannabinol (THC) have gained increased interest over recent years as accumulating evidence has shown their safety and efficacy in treating chronic pain conditions [24–26]. Cannabis treatment for patients with chronic pain provides short- and long-term benefits in pain reduction along with improvements in quality of life and wellbeing [27]. A recent comprehensive review concluded that there was conclusive or substantial evidence that cannabinoids are effective for the treatment of pain in adults [28], although others have shown more ambiguous results [29]. Cannabis can potentially affect both arms of the ANS, sympathetic and parasympathetic, although its effects on the hemodynamics and interplay amidst the two systems parts are inconclusive [30]. THC given to healthy participants resulted in an increase in sympathetic cardiac activity, alongside a decrease in vagal cardiac activity [31, 32]. Contrarily, a study conducted on regular recreational users of cannabis showed an opposite effect [33].
To our knowledge, there is no evidence of the effect of cannabis or THC on the autonomic nervous control and on CPM paradigm measures in chronic pain patients. Thus, in this study, we aimed to investigate the effect of THC on supraspinal pain modulation as reflected by the inhibitory pathway activity, as well as on parasympathetic autonomic tone as reflected by the sympathovagal balance. In order to achieve this, we tested the effect of THC on the CPM response as well as HRV indices in male patients with chronic pain, in a double-blind, placebo-controlled trial assessing the effect of a single dose of THC versus placebo in patients with chronic neuropathic radicular pain. Physiologically, radicular pain is pain evoked by ectopic discharges emanating from a dorsal root or its ganglion [34]. In that sense, it is a classic example of neuropathic pain, i.e. pain emanating from lesion or dysfunction of the somatosensory nervous system. In addition, patients underwent resting state fMRI scans before and after receiving sublingual THC/placebo.
Methods
Study Participants
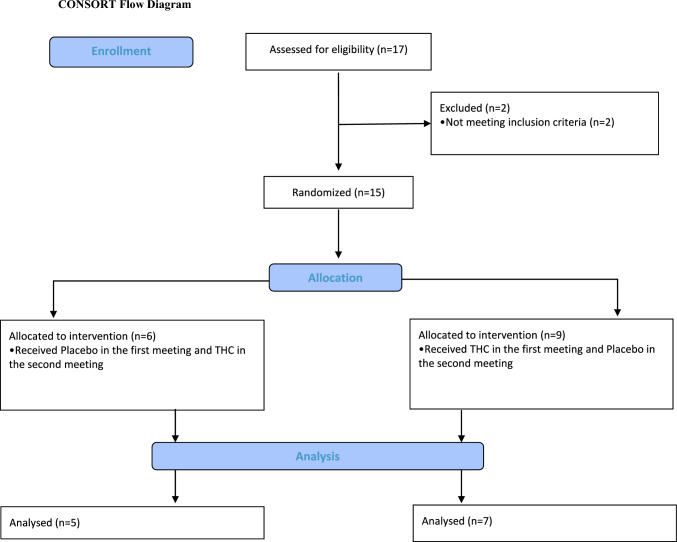
Patients were recruited by their treating physicians from the Institute of Pain Medicine, Tel-Aviv Sourasky Medical Center. Inclusion criteria were established neuropathic lower limb radicular pain for over 6 months and medium to high chronic pain (over 40 on a 100-point visual analog scale), with no other significant comorbidities or chronic pain syndromes. Women were excluded due to evidence that menstruation-related hormonal fluctuations may alter pain sensitivity and ANS regulation [35, 36]. Seventeen male participants provided written informed consent to be included in this study. Two participants were excluded because, on further examination, they did not fulfill the inclusion criteria. Three participants were not included in the final analyses due to data acquisition problems. Twelve patients with chronic lumbar radicular pain completed the study (27–40 years of age, mean age 33.9 ± 3.6 years, all males). Importantly, all patients were cannabis-naïve based on direct questioning, although we did not conduct a toxicological screening prior to participation. Full data for two participants of the CPM part were not completed and therefore they were excluded from the CPM analysis. Participants’ demographic and clinical data, as well as detailed inclusion and exclusion criteria, are available in Appendix Tables 1a and 1b. The study was approved by the Tel-Aviv Sourasky Medical Center Institutional Review Board (Clinical Trial Registration: clinicaltrials.gov/study/NCT02560545). The CONSORT diagram is shown in Fig. 1.
Fig. 1.
Study CONSORT diagram. CONSORT Consolidated Standards of Reporting Trials
Study Procedure
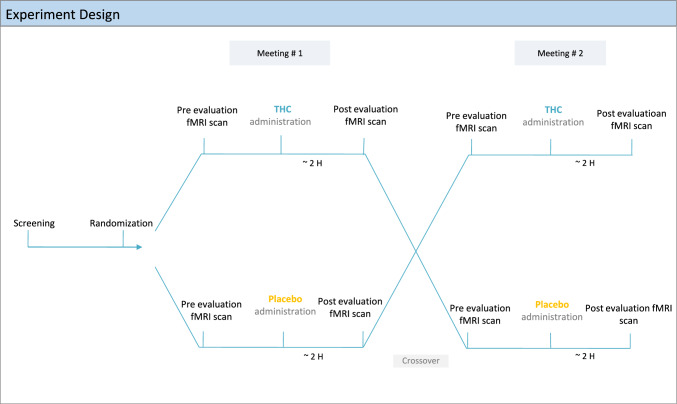
Patients participated in two counterbalanced meetings of a crossover, randomized, double-blind, placebo-controlled trial. In each meeting, patients received THC oil or placebo hemp oil (similar in color and smell to cannabis oil) sublingually (0.2 mg/kg, average THC dose = 15.3 ± 2.1 mg; Panaxia Pharmaceutical Industries Ltd, Israel), and were instructed to keep the formulation sublingually in an upright position for 2 min. Of note, the oral formulation used consisted of isolated THC with no other trace substances, and therefore was independent of cultivar effects. Randomization was performed by a dedicated physician using a sealed envelope website (sealedenvelope.com/). The experimental design is depicted in Fig. 2.
Fig. 2.
Experiment design: Twelve patients with chronic lumbar radicular pain participated in two meetings of a randomized, double-blind, counterbalanced, placebo-controlled trial. In each meeting, they received a sublingual dose of either 0.2 mg/kg THC oil or placebo. Patients underwent clinical evaluation and an fMRI scan pre and post drug/placebo administration. fMRI functional magnetic resonance imaging, THC δ-9-tetrahydrocannabinol
In each session, patients underwent baseline clinical evaluation, including heart rate (HR) and blood pressure (BP) measurements, and HRV assessment (Nexfin, BMEYE, Amsterdam, The Netherlands). HRV was sampled, calculated, and monitored by a three-lead electrocardiogram (ECG) for a period of 5 min after patients were asked to rest in a supine position, task-free, for 15 min. The CPM paradigm was applied, with a noxious heat stimulus as the test stimulus (Ts), delivered first alone and then concomitantly with a conditioning stimulus. Patients rated the pain intensity of the Ts continuously using a computerized visual analog scale (COVAS, Medoc, Israel), ranging from 0 (no pain at all) to 100 (the most intense pain imaginable). Subsequently, patients underwent a non-task resting state fMRI scan, lasting 6 min, and patients were instructed to keep their eyes closed, rest, and relax, but not to fall asleep. After the scan, patients received the treatment (THC/placebo). One hour post drug administration, the same procedure was repeated. The second fMRI scan was thus started about 2 h post drug administration, in accordance with THC sublingual absorption, usually showing maximal plasma concentrations after 2–3 h [37]. The meetings were separated by at least 1 week in order to enable a THC washout period (average weeks interval = 2.8 ± 3.4). Participants were not questioned as to the perceived allocation to THC or placebo per session.
Conditioned Pain Modulation (CPM) Paradigm
The Ts was a tonic noxious heat stimulus (TSA II, Neurosensory Analyzer; Medoc, Israel) delivered to the dominant volar forearm for 120 s at PAIN60 intensity. PAIN60 was determined individually as the temperature that induces pain at the intensity of 60 in a 0–100 scale. The temperature increase and decrease rate was 0.3 °C/s from a baseline temperature of 31 °C. The conditioning stimulus was immersion of the left foot into a cold water bath (8 °C) for 120 s or until the patient could not tolerate the stimulus and removed his leg.
Functional Magnetic Resonance Imaging (fMRI) Data Acquisition
fMRI data were acquired with a 3T MRI scanner (Magnetom Prisma, Siemens, Munich, Germany), with a 20-channel head coil, located at the Wohl Institute for Advanced Imaging at the Tel Aviv Sourasky Medical Center. Functional scans were performed with T2*-weighted echoplanar images (44 axial interleaved slices, repetition time [TR] 3000 ms, echo time [TE] 35 ms, field of view [FOV] 220 mm, in-plane matrix resolution 96 × 96, voxel size 2.3 × 2.3 × 3.0 mm, slice thickness 3 mm, flip angle 90°). Anatomical scan consisted of T1-weighted magnetization-prepared rapid gradient echo structural images (TR 1860 ms, TE 2.74 ms, FOV 256 mm, in-plane matrix resolution 256 × 256, voxel size 1 × 1 × 1 mm, slice thickness 1 mm, flip angle 8°).
Data Analysis
Physiologic Measurements
Statistical analyses for physiologic measurements were performed using STATISTICA 10 (TIBCO Software Inc., Palo Alto, CA, USA). Within-subject repeated measures analysis of variance was employed to ascertain significant interaction and simple main effects between the treatment (THC, placebo) and the state (pre, post) for cardiovascular measures (HR, BP and HRV) and CPM.
Heart Rate Variability (HRV) Analysis
The HRV measures that were calculated were low frequency (LF), high frequency (HF), and LF/HF ratio, which reflects the balance between the two components of the autonomic system. A three-lead surface ECG was sampled for 5 min and digitized at 500 Hz by an analog-to-digital converter using the Windaq pro software (WinDaq, version 2.27; DataQ Instruments, Akron, OH, USA). The assessment of the power spectral analyses of R-R intervals was performed using the Welch periodogram method for power spectral density calculation. Band pass filter (BPF) was used for respiration and noise reduction. A Hanning window in the time domain was adopted to attenuate spectral leakage (512 samples). Two subsets of the frequency domain were used for the RR interval, low-frequency band (LFRR: 0.04–0.15 Hz) and high-frequency band (HFRR: 0.15–0.4 Hz). LF and HF were also normalized as the relative value of each power component in proportion to the total power minus the very LF (VLF) component (local software, using MathLab 2018 [38]).
For each participant, in each treatment condition, HRV changes were determined as the delta between the HRV score before intervention and the HRV score after intervention.
CPM Analysis
CPM response was calculated by subtracting the mean pain intensity ratings of the Ts alone from the mean pain intensity ratings of the Ts during the conditioning stimulus. Thus, greater CPM response is presented as a negative value. For each patient, the difference between baseline and the intervention stages was calculated [8]. The whole paradigm was completed by 12 participants but due to recording problems, two participants did not include the whole dataset and were excluded.
fMRI Data Analysis
Preprocessing and Functional Connectivity Analysis
Functional analyses were performed using Statistical Parametric Mapping (SPM12) software (fil.ion.ucl.ac.uk/spm/software/spm12/) and the Functional Connectivity toolbox [39] (nitrc.org/projects/conn). Preprocessing included the following; the first 18 s of the functional data were discarded to allow steady-state magnetization. Functional images were slice-time corrected, realigned to the middle scan, motion-corrected, and normalized according to standard Montreal Neurological Institute (MNI) space. Spatial smoothing was performed using a 6-mm full width at half maximum Gaussian kernel. In order to reduce noise, functional volumes were bandpass filtered at 0.008–0.15 and the component-based method (CompCor) was used for noise signals such as white matter, CSF, and movement artifacts that were taken as confounders. In addition, images that were regarded as movement outliers were regressed out. Outliers were detected using the ART toolbox (nitrc.org/projects/artifact_detect/) and defined as volumes with a movement > 2 mm or signal intensity changes > 9 SD.
Functional connectivity was performed using a seed-based analysis looking for temporal correlations of the resting-state blood oxygenation level-dependent (BOLD) signal time series between the RVLM as the seed region and the rest of the brain. The region of interest (ROI) was defined using the left RVLM peak coordinates from an fMRI study of the sympathetic nerve activity [40]. A 2 mm radius sphere was generated around the coordinates (MNI coordinates − 5, − 42, − 52).
For each participant, first-level correlation maps were produced by extracting the residual BOLD time course from the seed and computing the Pearson correlation coefficients between that time course and the time course of all other voxels. Correlation coefficients were converted to normally distributed z scores using the Fisher transformation to allow second-level general linear model analyses.
To examine HRV-related changes in connectivity, first-level connectivity maps for each participant, at each state (pre, post), were entered into a whole-brain regression analysis with HRV changes as a covariate. The states were contrasted (post > pre) in order to examine the change in the treatment state (post) compared with the baseline state (pre). In this analysis, reported clusters survived a height threshold of uncorrected p < 0.001 and an extent threshold of false discovery rate (FDR)-corrected p < 0.05 at the cluster level.
Results
HRV
THC administration significantly reduced the LF/HF ratio compared with placebo, from 1.53 ± 0.27 to 0.96 ± 0.21 (interaction effect F(1,11) = 20.5, p = 0.001; simple effect p = 0.001) (Fig. 3 and Table 1). The decrease in LF/HF ratio was mainly driven by the significant increase observed in the HF component following THC administration, from 34.0 ± 3.5 to 46.3 ± 4.1 (normalized unit) [interaction effect F(1,11) = 27.2, p < 0.001; simple effect p = 0.009) (Fig. 3). However, the cardiovascular measures of HR and BP did not change significantly post THC administration compared with placebo (Table 1).
Fig. 3.
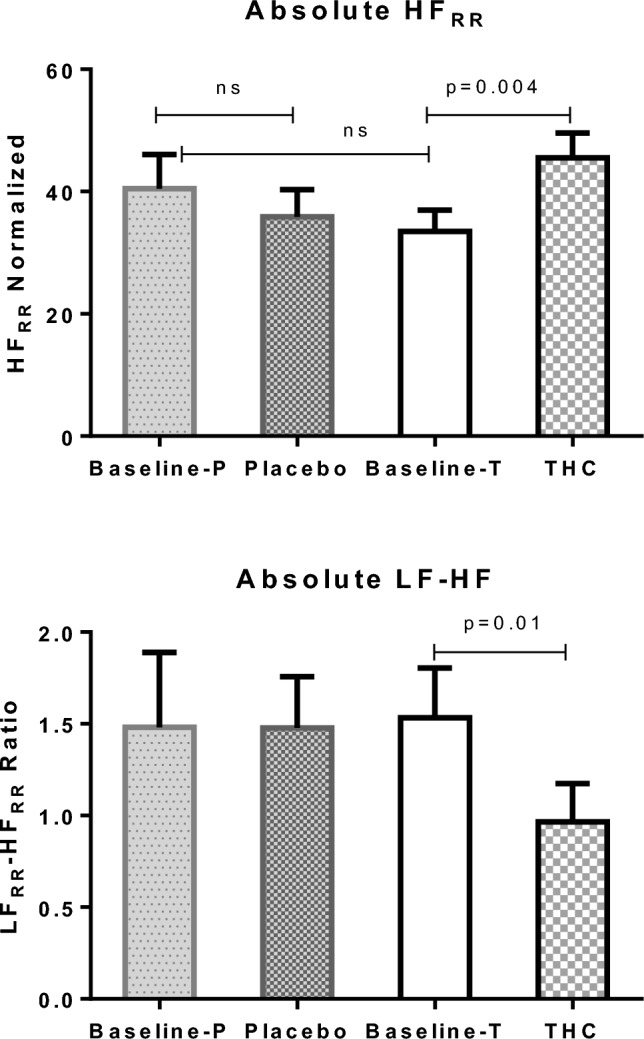
Mean absolute data of the normalized HFRR (upper graph) and ratio between the LFRR to HFRR values (lower graph). The p value represents the result of the two-tailed paired t-test. Error bars represent the SEM. HF high frequency, HFRR high-frequency domain of RR intervals, LF low frequency, LFRR low-frequency domain of the RR intervals, NS non-significant, P placebo, SEM standard error of the mean, T THC, THC δ-9-tetrahydrocannabinol
Table 1.
Hemodynamic and heart variability data, time domain, and frequency domain
| Parameter | Placebo | THC | ||
|---|---|---|---|---|
| Baseline | Drug | Baseline | Drug | |
| HR bpm | 70 ± 3 | 68 ± 3 | 72 ± 4 | 70 ± 3 |
| Systolic BP mmHg | 125 ± 6 | 123 ± 5 | 124 ± 4 | 123 ± 6 |
| Diastolic BP | 74 ± 4 | 75 ± 2 | 73 ± 2 | 74 ± 3 |
| VAS score | 52 ± 3.5 | 44 ± 3 | 53 ± 4.5 | 35 ± 4a,b |
| HRV frequency domain | ||||
| LFn (nu) | 35 ± 3.5 | 38.7 ± 2.1 | 40 ± 2.6 | 34 ± 2.4 |
| HFn (nu) | 40 ± 5.7 | 36.5 ± 4.8 | 34 ± 3.5 | 46 ± 4.1a,b |
| LF to HF ratio | 1.48 ± 0.4 | 1.47 ± 0.28 | 1.53 ± 0.27 | 0.96 ± 0.21a,b |
| Delta: drug–baseline | ||||
| Δ LFn (nu) | 3.8 ± 2.7 | −5.86 ± 2.4b | ||
| Δ HFn (nu) | −4.5 ± 3.5 | 12 ± 2.36b | ||
| Δ LF to HF ratio | −0.004 ± 0.2 | −0.57 ± 0.2b | ||
| HRV time domain | ||||
| RMSSD ms | 34 ± 8 | 39 ±11 | 31 ± 5 | 51 ± 12a,b |
| PNN50% | 10 ± 4 | 11 ± 5 | 11 ± 3 | 18 ± 6 |
Data are expressed as mean ± SEM
bpm beats per minute, Delta changes from baseline for each intervention, LF low frequency, HF high frequency, HR heart rate, HRV heart rate variability, nu normalized unit, RMSSD root mean square of successive differences between normal heartbeats, PNN50% proportion of adjacent NN intervals that differ from each other by more than 50 ms (both these time-domain data are indices of vagal cardiac control), SEM standard error of the mean, VAS visual analog scale
ap < 0.05 compared with baseline
bp < 0.05 compared with placebo
CPM
THC significantly improved the CPM response. The mean pain reports subtraction between the Ts and the Ts during the conditioned stimulus changed from − 9.8 ± 6.2 at baseline to − 25.7 ± 7.4 post THC administration (interaction effect F(1,9) = 5.2, p = 0.048; simple effect p = 0.002) [Fig. 4]. Placebo administration had no significant effect on the CPM response.
Fig. 4.
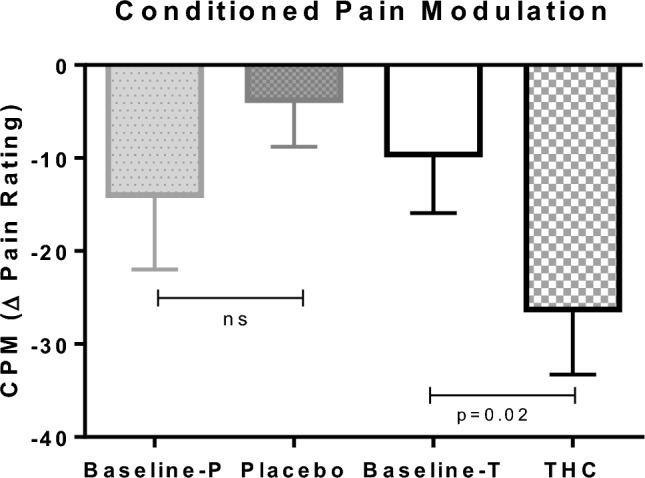
CPM after THC/placebo administration. Compared with placebo, THC significantly increased CPM. Error bars represent the SEM. Within-subject repeated measures ANOVA was employed to ascertain significant interaction and simple main effects between the treatment (THC, placebo) and the state (pre, post). Interaction effect F(1,9) = 5.2, p = 0.048; simple effect p = 0.02. ANOVA analysis of variance, CPM conditioned pain modulation, SEM standard error of the mean, P placebo, T THC, THC δ-9-tetrahydrocannabinol
Functional Connectivity (FC)
In order to examine the brain regions that may associate with the HRV changes, the LF/HF ratio was used as a covariate in a seed-to-whole-brain functional connectivity regression analysis. The RVLM was used as the seed ROI and the changes in functional connectivity were tested via the contrast between the pre and post THC administration during the resting state fMRI scans (pre < post).
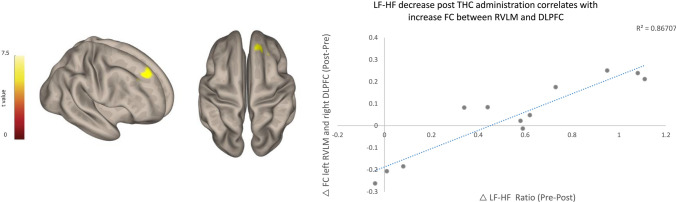
An increase in functional connectivity between the RVLM and the dorsolateral PFC (DLPFC) was found in correlation to the reduction in the LF/HF ratio after THC administration (right DLPFC, MNI coordinates 18 38 38; 145 voxels, T(10) = 6.4, cluster p-FDR = 0.000071) (Fig. 5), whereas in the placebo condition, no association was found when testing the RVLM functional connectivity and the LF/HF ratio changes.
Fig. 5.
LF-HF ratio decrease post THC administration correlates with an increase in functional connectivity between RVLM and DLPFC. Seed-to-whole-brain functional connectivity regression analysis was performed with left RVLM ROI (− 5, − 42, − 52, 2 mm) 30. Increased functional connectivity was found between RVLM and right DLPFC correlated with LF-HF ratio reduction after THC administration. Right DLPFC, MNI coordinates 18 38 38; 145 voxels, T(10) = 6.4, cluster p-FDR = 0.000071. DLPFC dorsolateral prefrontal cortex, FDR false discovery rate, HF high frequency, LF low frequency, MNI Montreal Neurological Institute, ROI region of interest RVLM rostral ventrolateral medulla, THC δ-9-tetrahydrocannabinol
Discussion
The aim of this study was to examine the changes that THC induces on behavioral and brain measures of the autonomic system, and on the efficiency of inhibitory pain modulation mechanisms in patients with chronic pain. To this end, we applied a double-blind, placebo-controlled trial using HRV, CPM paradigm and resting state fMRI assessed before and after a single dose of THC versus placebo in male patients with chronic neuropathic pain.
The LF/HF ratio was significantly reduced only after THC administration, but not after placebo. Based on the concept that a lower LF/HF ratio reflects parasympathetic dominance, whereas a high LF/HF ratio indicates the contrary, toward sympathetic dominance [41], we conclude that THC shifted the autonomic system towards a higher parasympathetic activity as expressed by cardiovascular control (increased HF band). Since chronic pain conditions are characterized by lower parasympathetic activity (i.e., sympathetic dominance) [7, 11], and are specifically associated with a significant increase in the LF/HF ratio compared with healthy controls [9], our results of a reduced LF/HF ratio signifying increased parasympathetic activity may reflect THC modulation of the autonomic system in the direction of normalizing existing aberrations in patients with chronic pain.
The CPM response was significantly improved following THC administration, compared with placebo, meaning patients reported a higher reduction in experienced pain when the Ts was delivered together with the conditioning stimulus. This observation signifies a more efficient inhibitory modulation of peripheral pain stimuli. It has been shown, including in meta-analyses, that CPM is impaired in patients with chronic pain [42], and that different factors could alter the CPM response in various chronic pain conditions [43, 44]. Such factors include the specific type of pain syndrome [44] (e.g. fibromyalgia vs. chronic low back pain [45]), opioid, and other medication use [44]; use of non-invasive neuromodulation; and, relevant to this case, autonomic cardiovascular measures [46]. Interestingly, healthy participants do not exhibit changes in CPM response after THC administration [47, 48]. Our results therefore indicate that THC promotes activation in the inhibitory pain pathways and improves their function in patients with chronic neuropathic radicular pain.
Interactions between the autonomic and nociceptive systems can be found at the levels of the periphery, spinal cord, brainstem, midbrain and forebrain [49, 50]. It has been suggested that the impairments in descending inhibitory control in chronic pain conditions relates to central sensitization resulting from sustained ongoing pain [4], and that the latter also influences the imbalances observed in activity of the ANS [51, 52].
Subsequently, the RVLM, a part of the medulla in the brainstem, was chosen as the ROI due to its key role, through neuronal connections, in controlling the tonic sympathetic activity [13, 14]. We found that the decrease in the LF/HF ratio after THC administration correlated with an increase in functional connectivity between the RVLM and the DLPFC. The DLPFC is a region within the PFC and is generally identified with maintenance and regulation of top-down modulation of various processes, including the CPM response [53]. Moreover, this region has been shown to display anatomical and functional alterations in chronic pain conditions [53, 54]. It has been suggested that the output of the human RVLM at rest may provide a tonic modulatory role during experimental tonic muscle pain, and is held in check by active inhibition from several higher-order brain regions, including the DLPFC, withdrawal of which can lead to increases in pain related muscle sympathetic nerve activity and BP [55]. Interestingly, a brain imaging study investigating the associations between fMRI time series and HRV found that the right DLPFC was involved in modulation of the parasympathetic system activity [15]. Additionally, accumulating data from recent studies of non-invasive brain stimulation of the DLPFC have demonstrated that this stimulation results in attenuation of sympathetic activity [56–58]. The organization of higher brain regions in the PFC and brainstem nuclei was conceptualized in a neurovisceral model, based largely on animal studies, where it posits the PFC regulates and inhibits the activity of limbic structures and brainstem regions, which control vagal tone and the activity of the autonomic system [16]. Specifically, according to the neurovisceral model, regulation through the PFC and the RVLM is related to direct control of the sympathetic arm, while other brainstem nuclei, such as the nucleus ambiguous (NA) and dorsal vagal motor nucleus (DVN), are related to control of the parasympathetic arm. However, our results could not determine whether the changes documented in the RVLM connectivity caused inhibition in the sympathetic activity resulting in higher parasympathetic activity, and/or impacted the other brainstem nuclei, which in turn affected the parasympathetic system.
THC is a potent partial agonist of the CB1 and CB2 receptors of the endocannabinoid system [59]. The distribution of these receptors in the nervous system consists of particularly dense expression of CB1 receptors throughout the cortex and especially the PFC, with relatively low expression in the brainstem [60]. Considering this pattern, the increased functional connectivity between the RVLM and DLPFC is more likely to reflect a THC modulation of the DLPFC, which exerts top-down regulation on the RVLM and the autonomic response. This suggestion should be further investigated with methods and paradigms that can allow for drug-specific locality and directionality effects. In addition, bottom-up effects cannot be ruled out and might still also contribute to the autonomic changes through THC effects on CB1 receptors in the brain stem, spinal cord or the peripheral nervous systems [61].
Taken together, our results of increased connectivity of the DLPFC and the RVLM resting state functional connectivity associated with increased parasympathetic activity may indicate that THC modulates and influences the autonomic response through key substantial brain regions for controlling and regulation of the ANS, known to be plausibly malfunctioning in chronic pain conditions. Further fMRI studies with high resolution should investigate other brainstem nuclei and their potential contributions for the effect of THC on the ANS in healthy individuals and patients with chronic pain.
Limitations
While we included patients who were reportedly cannabis-naïve we did not test participants for cannabinoids or other drugs of abuse prior to inclusion. In addition, we did not directly question participants as to whether they thought they received a cannabis or placebo intervention, which would have made it possible to at least partly control for incomplete blinding and the effects of expectation. This study has two major limitations. Sample size was relatively small, as the complex logistics of conducting extensive psychophysiological tests and four fMRI scans (two of which were pharmaco-fMRI scans) in patients with clinical ongoing pain was extremely demanding. In addition, crucially, women were excluded from this study due to a concern regarding menstruation-induced fluctuations in pain sensitivity, therefore larger-scale studies with bigger sample size cohorts should examine whether these results are reproducible and in a heterogeneous population including women before assuming generalizability. Future investigations should include other chronic conditions to better understand whether our results represent a pervasive neuronal mechanism of THC effects on chronic pain. Moreover, high fMRI resolution studies should further investigate other brainstem nuclei and their involvement in the effect of THC on the autonomic system. The three considered measures in this study were not performed simultaneously, which may affect our conclusions.
Conclusions
To conclude, in this study, THC administration in the context of chronic clinical neuropathic pain, in this case radicular neuropathic pain, resulted in changes in the sympathovagal balance, shifting the balance toward a parasympathetic dominance. In addition, THC improved the CPM response in patients, indicating enhanced inhibitory modulation of pain. Lastly, the increase in parasympathetic control was associated with increased connectivity between the RVLM and DLPFC, indicating enhanced higher-order prefrontal modulation. It therefore seems that THC has a normalizing effect on multiple supraspinal pain modulatory pathways, known to be abnormal in chronic pain.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Tel Aviv University.
Conflict of interest
Libat Weizman, Haggai Sharon, Lior Dayan, Joumana Espaniol, Silviu Brill, Hadas Nahman-Averbuch, Talma Hendler, and Giris Jacob report no competing interests.
Availability of data and material
Anonymized data are available upon request by contacting the corresponding author.
Ethics approval
This study was approved by the Tel Aviv Sourasky Medical Center’s Institutional Review Board.
Consent to participate
Written informed consent was obtained from all participants in this trial.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
LW, TH, HS and GJ conceived and designed the trial. HS, LD and SB recruited and assessed the patients, wrote the clinical study protocol, and participated as acting supervising clinicians during the trials. LW and JE collected the data. HS, LW, HNA and GJ analyzed the data. LW, HS and GJ wrote the manuscript.
Footnotes
Libat Weizman and Haggai Sharon contributed equally to this work.
References
- 1.Dahlhamer J, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Investig. 2010;120(11):3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Kwon M, et al. The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Pract. 2014;14(7):656–667. doi: 10.1111/papr.12145. [DOI] [PubMed] [Google Scholar]
- 5.Koenig J, et al. Pneumogastric (vagus) nerve activity indexed by heart rate variability in chronic pain patients compared to healthy controls: a systematic review and meta-analysis. Pain Physician. 2016;19(1):E55–78. doi: 10.36076/ppj/2016.19.E55. [DOI] [PubMed] [Google Scholar]
- 6.Brodal P. The central nervous system: structure and function. Oxford: Oxford University Press; 2004. [Google Scholar]
- 7.Chakravarthy K, et al. Review of the uses of vagal nerve stimulation in chronic pain management. Curr Pain Headache Rep. 2015;19(12):54. doi: 10.1007/s11916-015-0528-6. [DOI] [PubMed] [Google Scholar]
- 8.Dayan L, et al. Increased sympathetic outflow induces adaptation to acute experimental pain. Pain Pract. 2018;18(3):322–330. doi: 10.1111/papr.12606. [DOI] [PubMed] [Google Scholar]
- 9.Tracy LM, et al. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain. 2016;157(1):7–29. doi: 10.1097/j.pain.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 10.Storella RJ, et al. Relief of chronic pain may be accompanied by an increase in a measure of heart rate variability. Anesth Analg. 1999;89(2):448–450. doi: 10.1097/00000539-199908000-00037. [DOI] [PubMed] [Google Scholar]
- 11.Beissner F, et al. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dampney RA, et al. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29(4):261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 13.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74(2):323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 14.Dampney RA, et al. What drives the tonic activity of presympathetic neurons in the rostral ventrolateral medulla? Clin Exp Pharmacol Physiol. 2000;27(12):1049–1053. doi: 10.1046/j.1440-1681.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 15.Napadow V, et al. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage. 2008;42(1):169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Pfannmöller J, Lotze M. Review on biomarkers in the resting-state networks of chronic pain patients. Brain Cogn. 2019;131:4–9. doi: 10.1016/j.bandc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Malfliet A, et al. Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur J Pain. 2017;21(5):769–786. doi: 10.1002/ejp.1003. [DOI] [PubMed] [Google Scholar]
- 19.Kumbhare DA, Elzibak AH, Noseworthy MD. Evaluation of chronic pain using magnetic resonance (MR) neuroimaging approaches: what the clinician needs to know. Clin J Pain. 2017;33(4):281–290. doi: 10.1097/AJP.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 20.Thorp SL, et al. Functional connectivity alterations: novel therapy and future implications in chronic pain management. Pain Physician. 2018;21(3):E207–E214. doi: 10.36076/ppj.2018.3.E207. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy DL, et al. Reliability of conditioned pain modulation: a systematic review. Pain. 2016;157(11):2410–2419. doi: 10.1097/j.pain.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131–137. doi: 10.1097/SPC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 23.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 24.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 25.Sharon H, Brill S. Cannabis-based medicines for chronic pain management: current and future prospects. Curr Opin Anaesthesiol. 2019;32(5):623–628. doi: 10.1097/ACO.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 26.Sharon H, et al. Medical cannabis for refractory cancer-related pain in a specialised clinical service: a cross-sectional study. BMJ Support Palliat Care. 2023 doi: 10.1136/spcare-2023-004421. [DOI] [PubMed] [Google Scholar]
- 27.Haroutounian S, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32(12):1036–1043. doi: 10.1097/AJP.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 28.The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington DC: National Academy of Sciences; 2017. [PubMed]
- 29.Pantoja-Ruiz C, et al. Cannabis and pain: a scoping review. Braz J Anesthesiol. 2022;72(1):142–151. doi: 10.1016/j.bjane.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh M, Naderi S. Cannabis and cardiovascular disease. Curr Atheroscler Rep. 2019;21(6):21. doi: 10.1007/s11883-019-0783-9. [DOI] [PubMed] [Google Scholar]
- 31.Pabon E, et al. Acute effects of oral delta-9-tetrahydrocannabinol (THC) on autonomic cardiac activity and their relation to subjective and anxiogenic effects. Psychophysiology. 2022;59(2):e13955. doi: 10.1111/psyp.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nayak SK, et al. Analysis of heart rate variability to understand the effect of cannabis consumption on Indian male paddy-field workers. Biomed Signal Process Control. 2020;62:102072. doi: 10.1016/j.bspc.2020.102072. [DOI] [Google Scholar]
- 33.Schmid K, et al. The effects of cannabis on heart rate variability and well-being in young men. Pharmacopsychiatry. 2010;43(4):147–150. doi: 10.1055/s-0030-1248314. [DOI] [PubMed] [Google Scholar]
- 34.Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain. 2009;147(1–3):17–19. doi: 10.1016/j.pain.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Hirshoren N, et al. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab. 2002;87(4):1569–1575. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- 36.Vincent K, Tracey I. Sex hormones and pain: the evidence from functional imaging. Curr Pain Headache Rep. 2010;14(5):396–403. doi: 10.1007/s11916-010-0139-1. [DOI] [PubMed] [Google Scholar]
- 37.Robson GW, Robson PJ. A phase I, double blind, three-way crossover study to assess the pharmacokinetic profile of cannabis based medicine extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers (GWPK0215) J Cannabis Ther. 2004;3(4):121–152. doi: 10.1300/J175v03n04_02. [DOI] [Google Scholar]
- 38.Jacob G, et al. Vagal and sympathetic function in neuropathic postural tachycardia syndrome. Hypertension. 2019;73(5):1087–1096. doi: 10.1161/HYPERTENSIONAHA.118.11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 40.Macefield VG, Gandevia SC, Henderson LA. Neural sites involved in the sustained increase in muscle sympathetic nerve activity induced by inspiratory capacity apnea: a fMRI study. J Appl Physiol (1985) 2006;100(1):266–273. doi: 10.1152/japplphysiol.00588.2005. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13(10):936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Goubert D, et al. Effect of pain induction or pain reduction on conditioned pain modulation in adults: a systematic review. Pain Pract. 2015;15(8):765–777. doi: 10.1111/papr.12241. [DOI] [PubMed] [Google Scholar]
- 44.Petersen KK, et al. Assessment of conditioned pain modulation in healthy participants and patients with chronic pain: manifestations and implications for pain progression. Curr Opin Support Palliat Care. 2019;13(2):99–106. doi: 10.1097/SPC.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 45.Gerhardt A, et al. Conditioned pain modulation in patients with nonspecific chronic back pain with chronic local pain, chronic widespread pain, and fibromyalgia. Pain. 2017;158(3):430–439. doi: 10.1097/j.pain.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 46.Chalaye P, et al. The role of cardiovascular activity in fibromyalgia and conditioned pain modulation. Pain. 2014;155(6):1064–1069. doi: 10.1016/j.pain.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 47.van Amerongen G, et al. Effect profile of paracetamol, Δ9-THC and promethazine using an evoked pain test battery in healthy subjects. Eur J Pain. 2018;22(7):1331–1342. doi: 10.1002/ejp.1222. [DOI] [PubMed] [Google Scholar]
- 48.Dunn KE, et al. Within-subject, double-blinded, randomized, and placebo-controlled evaluation of the combined effects of the cannabinoid dronabinol and the opioid hydromorphone in a human laboratory pain model. Neuropsychopharmacology. 2021;46(8):1451–1459. doi: 10.1038/s41386-021-01007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res. 2001;11(6):343–349. doi: 10.1007/BF02292765. [DOI] [PubMed] [Google Scholar]
- 50.Benarroch EE. Pain-autonomic interactions. Neurol Sci. 2006;27(Suppl 2):S130–S133. doi: 10.1007/s10072-006-0587-x. [DOI] [PubMed] [Google Scholar]
- 51.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev. 2004;28(4):395–414. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Randich A, Maixner W. Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev. 1984;8(3):343–367. doi: 10.1016/0149-7634(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 53.Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. 2017;18(9):1027–1035. doi: 10.1016/j.jpain.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macefield VG, Henderson LA. Identifying increases in activity of the human RVLM through MSNA-coupled fMRI. Front Neurosci. 2020;13:496481. doi: 10.3389/fnins.2019.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunoni AR, et al. Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology. 2013;38(1):58–66. doi: 10.1016/j.psyneuen.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Carnevali L, et al. Effects of prefrontal transcranial direct current stimulation on autonomic and neuroendocrine responses to psychosocial stress in healthy humans. Stress. 2020;23(1):26–36. doi: 10.1080/10253890.2019.1625884. [DOI] [PubMed] [Google Scholar]
- 58.Remue J, et al. The effect of a single HF-rTMS session over the left DLPFC on the physiological stress response as measured by heart rate variability. Neuropsychology. 2016;30(6):756–766. doi: 10.1037/neu0000255. [DOI] [PubMed] [Google Scholar]
- 59.Vemuri VK, Makriyannis A. Medicinal chemistry of cannabinoids. Clin Pharmacol Ther. 2015;97(6):553–558. doi: 10.1002/cpt.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- 61.Pacher P, Bátkai S, Kunos G. Cardiovascular pharmacology of cannabinoids. Handb Exp Pharmacol. 2005;168:599–625. doi: 10.1007/3-540-26573-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.