Abstract
Tumor-specific tumor-infiltrating lymphocytes (TILs) can be in vitro expanded and have the ability to induce complete and durable tumor regression in some patients with melanoma following adoptive cell therapy (ACT). In this preclinical study, we investigated the feasibility of expanding TIL from sarcomas, as well as performing functional in vitro analyses on these. TILs were expanded in vitro by the use of IL2 stimulation with or without the addition of 4-1BB and CD3 antibodies. Phenotypical and functional analyses were mainly performed by flow cytometry. TILs were expanded from 25 of 28 (89%) tumor samples from patients with 9 different sarcoma subtypes. TILs were predominantly αβ T-cells of effector memory subtype with CD4+ dominance. In particular, CD8+ TIL highly expressed LAG3 and to a lesser degree PD-1 and BTLA. In total, 10 of 20 TIL cultures demonstrated in vitro recognition of autologous tumor. In some cases, the fraction of tumor-reactive T cells was more than 20%. 4-1BB stimulation augmented expansion kinetics and favored CD8+ occurrence. In conclusion, TIL expansion from sarcoma is feasible and expanded TILs highly express LAG3 and comprise multifunctional tumor-reactive T-cells.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02568-x) contains supplementary material, which is available to authorized users.
Keywords: Tumor-infiltrating lymphocytes, TIL, Sarcoma, Adoptive cell transfer, T cell expansion, 4-1BB
Introduction
Sarcomas are rare malignant tumors arising from cells of mesenchymal origin. More than 80 histological subtypes exist, and sarcomas can occur at virtually any anatomical site [1]. This makes sarcomas extremely diverse with regard to prognosis but also leaves a large unexplored potential for improving treatment options. Surgery is the first choice of treatment for localized sarcoma, but despite early intervention around half of all patients will develop metastases and eventually succumb to the disease [2]. Chemotherapy is the standard treatment option for metastasized sarcoma, but responses are short term, and the estimated overall median survival is approximately 12 months [2].
Accumulating evidence supports that the immune system plays a role in sarcomas as has been shown in other tumors. Tumor-infiltrating lymphocytes (TILs) are common in sarcomas, but the prognostic implications of this are not clearly understood [3]. High T-cell infiltration has been shown to correlate with improved prognosis in gastrointestinal stromal tumors (GIST) [4] and Ewing sarcoma [5], but in a combined analysis of various soft tissue sarcomas (STS) only CD20+ TILs were found to be an independent positive prognostic indicator [6]. Another study has shown that PD1 expression by TILs and PDL1 expression by tumor cells are associated with a poor prognosis in STS patients, which indicates that this immunomodulatory mechanism could also play an important part in tumor immune escape in sarcoma patients [7].
Results from clinical studies of PD1 blocking are also not unanimous. In a recent study, Tawbi et al. showed that treatment with pembrolizumab could induce objective responses in 4 of 10 patients with undifferentiated pleomorphic sarcoma (UPS) and 2 of 10 patients with liposarcoma [8], but in another study by Toulmonde et al. no patients (n = 16) with UPS showed response to pembrolizumab in combination with cyclophosphamide [9].
Adoptive cell transfer (ACT) with TILs is a complex immunotherapeutic treatment strategy first described to mediate cancer regression in patients with metastatic melanoma 30 years ago [10]. For melanoma patients receiving ACT with TILs along with preparative lymphodepleting chemotherapy and followed by high-dose IL2, around 20% experience complete and durable responses [11]. To our knowledge, no clinical data have been published on TIL-based ACT for sarcoma patients; however, some ongoing clinical trials are currently exploring this field (NCT03449108 and NCT03725605). Also, a pilot trial using lymphocytes genetically engineered to express a T-cell receptor specific for the tumor antigen NY-ESO-1 showed objective clinical response in 11 of 18 patients with synovial cell sarcoma [12].
In this study, we assessed the feasibility of expanding TILs from multiple sarcoma subtypes to numbers needed for clinical application and analyzed TIL phenotype and ability to recognize tumor cells in vitro. We hypothesize that TIL-based ACT can be a beneficial treatment option for patients with otherwise incurable sarcoma.
Materials and methods
Collection of tumor specimens
We collected tissue samples from patients undergoing elective surgery for primary or recurrent sarcoma. To allow later pathological analyses, the tumor had to be at least 5 cm in diameter. Immediately after removal of the tumor, a small sample of 1–2 cm3 was transferred to our laboratory facilities for further processing. Experiments were conducted in a laboratory dedicated for exploratory research using established standard operating protocols.
The study protocol was approved by the local ethics committee (H-15007073), and the Danish Data Protection Agency was notified (HEH-2015-057). All included patients were provided with both oral and written information about the study protocol and have signed informed consent.
Expansion of tumor-infiltrating lymphocytes from sarcoma specimens
Within one hour of resection, the tumor specimen was cut into small fragments of 1–2 mm3 and placed in individuals wells in two 24-well culture plates (Nunc) with 2 mL culture medium (CM) containing IL2 (a full description of the media contents is provided in the supplementary). Three times a week, half of the medium was removed, and fresh medium was added. Cell cultures were split, when confluent. TIL cultures were established by pooling expanded TILs from 48 fragments according to the “Young TIL method” [11, 13]. In the following the term, “Young TILs” refers to TILs following this initial expansion. When an estimated number of 100 × 106 was reached, or after a maximum of eight weeks, TIL cultures were manually counted by use of trypan blue staining and a hemocytometer and afterward cryopreserved at − 140 °C in a medium containing heat-inactivated human AB serum (Sigma-Aldrich) and 10% DMSO using thermo-conductive freezing containers.
To mimic conditions from clinical protocols, we further expanded TILs with a small-scale Rapid Expansion Protocol (REP) [14]. Cryopreserved Young TILs were thawed and rested for 2 days in CM before setting up the REP in culture flasks containing a 1:1 mixture of CM and AIM-V (Gibco 12055-083) with 10% human AB serum, 30 ng/mL anti-CD3 (clone OKT3), and 6000 IU/mL IL-2, along with 105 TILs and irradiated (40 Gy) allogeneic PBMC feeder cells in a ratio of 1:200. The REP lasted 14 days, and fresh AIM-V and IL-2 were added during expansion. On day 14, REP TILs were counted and cryopreserved.
For the last eight patient samples, we further investigated the effect of 4-1BB and CD3 stimulation during expansion. In this sub-study, we initiated four TIL cultures from eight tumor fragments each and expanded TILs in CM along with either anti-4-1BB, anti-CD3, or the combination of the two along with standard expansion in CM serving as control. TILs from all fragments were pooled and cryopreserved, when an estimated number of 50 × 106 was reached, or after a maximum of four weeks.
For 4-1BB stimulation, we used 10 µg/mL of Urelumab® (BMS-663513, clone 10C7; Bristol-Myers Squibb), and for CD3 stimulation we used 30 ng/mL of an agonistic anti-CD3 antibody (clone OKT3, Orthoclone TAB-019). Urelumab® is a fully human IgG4 monoclonal agonistic anti-4-1BB antibody.
Tumor cell lines and fresh tumor digest
Autologous tumor cells were generated from the same tumor specimens as TIL cultures in R10 medium containing 10% fetal bovine serum (FBS). Tumor cell lines (TCL) were established both from tumor fragments and from the cells suspended in the medium following tumor processing and expanded in culture flasks in tumor medium. When TCL cultures had been established, they were cryopreserved at − 140 °C in a medium containing FBS and 10% DMSO. Fresh tumor digest (FTD) was generated from a portion of tumor fragments incubated overnight in enzyme digest media. Following incubation, the cell suspension was passed through a 70-µm filter and washed in RPMI 1640 before cryopreservation.
TIL phenotype analysis
The phenotypes of TILs were determined by flow cytometry using a FACS Canto II flow cytometer with Diva Software (BD Biosciences). Cryopreserved TILs were thawed and tested, either on the same day or on the following day. Cells were washed in PBS (Lonza BE17-512F) and stained for approximately 30 min with fluorescence-conjugated antibodies against CD3, CD4, CD8, CD25, CD45RO, CD56, γδ-TCR, BTLA, PD-1, 7AAD, CCR7, LAG3, TIM-3 (a full list of antibodies is provided in the supplementary). To determine if TIL cultures contained regulatory T cells, we additionally stained for CD25 and FoxP3. Intracellular staining of FoxP3 was performed after permeabilization and fixation of cells as described in Sect. 2.6. To limit the in vitro effect of IL2, TILs for Treg analyses were rested 6 days without IL2.
Elispot INFγ measurement
Tumor recognition by TILs was assessed using an Elispot assay to quantify IFNγ-releasing effector T cells among TILs, when co-cultured with autologous (if available) or allogenic HLA-matched TCLs. The assay was set up in 96-well plates (Merck Millipore) coated with anti-IFNγ antibody 1-D1K (Mabtech 3420-3-1000) and blocked with X-vivo (Lonza BE02-053Q). 1 × 105 TILs were added in triplicates and incubated for four hours or overnight at 37 °C in 5% CO2 in the presence or absence (negative control) of 1 × 104 tumor cells. After incubation, cells and medium were discarded, and the wells were washed, followed by application of biotinylated secondary antibody 7-B6-1-Biotin (Mabtech 3420-6-1000) and incubated for two hours at room temperature. Afterwards, wells were washed again, and Streptavidin-ALP (Mabtech 3310-10) was added to each well, before another one hour of incubation. Finally, wells were washed, and the enzyme substrate BCIP/NBT (Mabtech 3650-10) was added and incubated for 2 to 5 min, until the reaction was terminated, when dark violet spots appeared. The spots were counted using an ImmunoSpot 2.0 Analyzer (CTL).
Intracellular cytokine staining
To further describe the anti-tumor reactivity of the expanded TILs, we performed flow cytometry measuring CD107a expression and intracellular cytokine release following co-culturing with tumor cells. TILs were incubated for 5 to 7 h at 37 °C and 5% CO2, either alone (negative control) or in co-culture with a TCL, an IFNγ-prestimulated TCL or FTD. Before incubation, GolgiPlug (BD Biosciences 51-2301KZ) and anti-CD107a (BD Biosciences 562623) were added. After incubation, cells were washed before adding NIR live/dead (Life Technologies L10119), anti-CD3 (BD Biosciences 345764), anti-CD4 (Biolegend 317432), and anti-CD8 (Life Technologies Q10009). Following 20 min of incubation with the fluorescence-conjugated antibodies, cells were washed and incubated overnight in a fixation/permabilization solution (eBioscience). The following day cells were washed in permabilization buffer (eBioscience), before adding fluorescence-conjugated antibodies against TNFα (BD Biosciences 554514) and IFNγ (BD Biosciences 557643). Cells were acquired using a FACS Canto II flow cytometer with Diva Software (BD Biosciences).
Cytolysis using xCelligence
The impedance-based technology xCelligence (Acea Biosciences) was used to visualize the real-time cytolytic effect of highly reactive TILs on autologous TCLs. This technology measures an electric current by electrodes in the bottom of the incubation plates. When the tumor cells die and thus detach from the plastic, it creates changes in the impedance, and these data are translated by the software into a cell index used as a measurement for cell growth and cell death [15, 16]. TCLs were thawed and kept in culture, until their growth pattern was deemed appropriate by visual assessment. TCLs were added at day 0, and a titration of TILs (4:1, 2:1, and 1:1) was added, when the cell index indicated growth of TCLs (usually day 2 or 3). To be able to detect specific cytotoxicity mediated by CD8+ TILs, the MHC class 1 blocker W6/32 (Biolegend 311412) or the corresponding IgG isotype was added to the control wells in a final concentration of 20 µg/mL.
Proximity extension assay
To assess the cytokines profile of TILs, we analyzed supernatants from selected samples with the Olink multiplex immuno-oncology panel. This panel analyzes 92 protein biomarkers selected for their involvement in immune responses to cancer. Briefly, a pair of oligonucleotide-labeled antibodies will bind pairwise to the target protein, and the close proximity of these probes will form a new PCR target sequence that can subsequently be detected and quantified using standard real-time PCR. Data are reported as normalized protein expression levels (NPX) that allow to identify changes and differences between samples. NPX values cannot be translated directly into concentrations, so the data presented can only be used to describe a difference in the pattern in cytokine production. Detailed assay information is available on the manufacturer’s Web site (www.olink.com).
Statistics
Figures and statistical comparison between groups were made in GraphPad Prism 8.0.0. Comparisons between two groups were performed with the Mann–Whitney test for unpaired samples, not assuming a Gaussian distribution. Comparisons between multiple groups were performed with the Friedman test for paired samples, not assuming a Gaussian distribution.
Results
Patient characteristics and distribution of sarcoma subtypes
Tumor samples from 30 patients were collected in the project. Two samples were later determined to have benign histology, and the two patients were excluded. Characteristics of the remaining 28 patients with nine different sarcoma subtypes are shown in Table 1.
Table 1.
Patient characteristics
| Patient ID | Pathology | Localization | Type | Malignancy grade | Previous treatment | Sex | Age |
|---|---|---|---|---|---|---|---|
| SAR-03 | GIST | Stomach | Primary | High | None | Male | 66 |
| SAR-15 | GIST | Stomach | Primary | Intermediate | None | Male | 66 |
| SAR-06 | Leiomyosarcoma | Leg | Primary | High | None | Female | 70 |
| SAR-23 | Leiomyosarcoma | Leg | Primary | Intermediate | None | Male | 56 |
| SAR-26 | Myofibroblasticsarcoma | Thigh | Primary | High | None | Female | 89 |
| SAR-04 | Myxofibrosarcoma | Arm | Primary | Intermediate | None | Male | 76 |
| SAR-05 | Myxofibrosarcoma | Thorax | Primary | Intermediate | None | Male | 80 |
| SAR-12 | Myxofibrosarcoma | Thorax | Primary | High | None | Female | 56 |
| SAR-18 | Myxofibrosarcoma | Thorax | Primary | High | None | Female | 28 |
| SAR-28 | Myxofibrosarcoma | Gluteus | Primary | Intermediate | None | Male | 41 |
| SAR-25 | Myxoid liposarcoma | Thigh | Primary | Intermediate | None | Male | 24 |
| SAR-02 | Myxoid liposarcoma | Thigh | Primary | Intermediate | None | Male | 36 |
| SAR-07 | Myxoid liposarcoma | Thigh | Metastasis | Intermediate | None | Male | 45 |
| SAR-13 | Myxoid liposarcoma | Thigh | Primary | Intermediate | None | Female | 31 |
| SAR-16 | Myxoid liposarcoma | Calf | Primary | Low | None | Female | 55 |
| SAR-19 | Myxoid liposarcoma | Thigh | Primary | Intermediate | None | Male | 32 |
| SAR-08 | Osteosarcoma | Arm | Metastasis | High | Chemotherapy | Male | 73 |
| SAR-09 | Osteosarcoma | Thigh | Primary | High | Chemotherapy | Male | 74 |
| SAR-10 | Osteosarcoma | Calf | Primary | High | Chemotherapy | Male | 64 |
| SAR-17 | Pleomorphic liposarcoma | Thorax | Primary | High | None | Female | 77 |
| SAR-29 | Pleomorphic liposarcoma | Foot | Primary | Intermediate | None | Male | 67 |
| SAR-30 | Pleomorphic liposarcoma | Arm | Primary | High | None | Female | 46 |
| SAR-11 | Sarcomatoid carcinoma | Inguen | Primary | - | None | Female | 68 |
| SAR-01 | UPS | Thigh | Primary | High | None | Male | 70 |
| SAR-14 | UPS | Leg | Primary | High | None | Female | 74 |
| SAR-21 | UPS | Gluteus | Primary | High | None | Female | 74 |
| SAR-24 | UPS | Thigh | Primary | High | None | Male | 63 |
| SAR-22 | Undifferentiated sarcoma | Shoulder | Primary | High | None | Female | 52 |
TIL expansion and kinetics
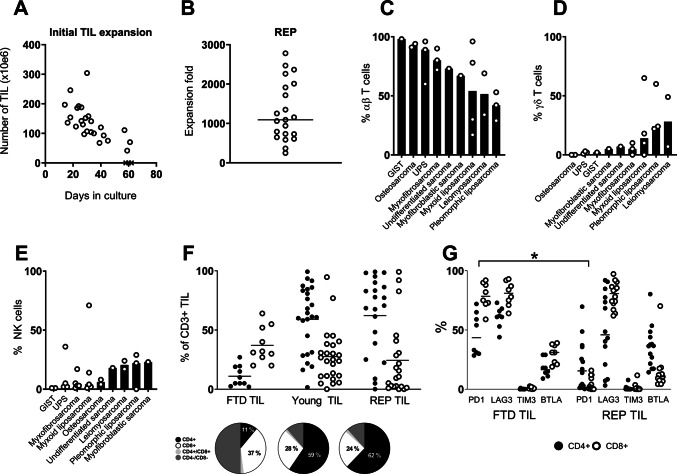
Young TIL cultures were established from all included sarcoma subtypes with a success rate of 25 of 28 (89%) tumor samples. During a median expansion time of 30 days (range 16–61 days), we obtained a median of 130 × 106 TILs (range 42 × 106–304 × 106) (Fig. 1a).
Fig. 1.
TILs were expanded from sarcoma specimens, and phenotypic characteristics were analyzed using flow cytometry. Young TIL cultures were harvested, when a number of approximately 100 × 106 cells was reached a Scatter plot showing expansion kinetics (total TIL yield and expansion time) of the initial TIL expansion (Young TIL). The four subgroups are represented with a different symbol. b Scatter plot showing expansion rates following the Rapid Expansion Protocol (REP). Each open circle represents one patient sample. The horizontal line is showing median expansion fold. c Bar plot showing median percentage of αβ T cells in the Young TIL population for each sarcoma subtype. Each open circle represents one patient sample (data not available for patients 1–5). d Bar plot showing median percentage of γδ T cells in the Young TIL population for each sarcoma subtype (data not available for patients 1–5). e Bar plot showing median percentage of NK cells in the Young TIL population for each sarcoma subtype. f Scatter plot showing CD4 and CD8 expression by T cells among FTD TILs, Young TILs, and REP TILs. g Scatter plot showing expression of PD-1, LAG3, BTLA, and TIM-3 on CD4+ and CD8+ T cells in FTD and among REP TILs (* = P < 0.05).
TILs were further expanded in a REP to scale up expansion rates to clinically relevant numbers. Across all nine sarcoma subtypes, REP generated a median expansion fold of 1092 (range 248–2780) (Fig. 1b). Of note, the lowest expansion folds were obtained predominantly for TILs from cultures with the longest initial expansion time and was not associated with sarcoma subtype.
We saw a trend towards a faster TIL expansion from patients with UPS, myxofibrosarcoma, and myofibroblastic sarcoma. TIL growth from GIST was significantly slower than for any other sarcoma subtype (Mann–Whitney p = 0.01, Supplementary Figure S1). TIL cultures from osteosarcoma, UPS, and myxofibrosarcoma consisted mainly of αβ T cells, while cultures from leiomyosarcoma, myxoid liposarcoma, and pleomorphic liposarcoma were more diverse, encompassing higher fractions of γδ T cells and NK cells (Fig. 1c–e). Following REP, this diversity disappeared, thus creating a uniform cell culture consisting of almost exclusively αβ T cells (Supplementary Figure S2).
T cell subsets and phenotype
CD4 and CD8 expression in young TIL and REP TIL populations varied extensively between different patients, but in the majority of cultures the largest fraction was CD4+. High numbers of CD4−CD8− T cells were observed in the uncultured TILs from FTD, but this could be caused by cleavage of CD4+ during the enzymatic digest [17, 18]. The fraction of CD8+ TILs should not be affected by this (Fig. 1f).
Young TIL and REP TIL populations mainly comprised T effector memory cells (TEM), defined as being CD3+ CD45RO+ and CCR7− (or CCR7-low), which corresponds to previous findings [13, 19]. A smaller fraction of TILs were more differentiated T-effector cells (CD3+CD45RO−CCR7−), while very few were T central memory cells (CD3+ CD45RO+ CCR7+) (Supplementary files, figure S2).
Expression of immune regulatory molecules on TILs may contribute to tumor immune escape, which can be reversed by blocking selected receptor/ligand interactions with immune checkpoint inhibitors. To test if this could potentially influence the expanded TILs, we assessed the surface expression of selected regulatory molecules on FTD TILs and REP TILs (Fig. 1g). PD-1 and LAG3 were highly expressed on FTD CD8+ TILs (median 79% and 81%, respectively), and while LAG3 expression persisted following REP (median 81%), PD-1 was significantly downregulated on both CD8+and CD4+ TILs (Mann–Whitney, p < 0.05). BTLA was mainly expressed on FTDCD8+ TILs but shifted to being expressed on CD4+ TILs after REP. TIM-3 expression was almost non-existing.
Autologous tumor cell lines
We were successful in expanding cell lines from 21 of 25 tumor samples, from which we were also able to expand TILs. Some cell lines, however, could not be re-established after freezing. Cells lines were adherent to the plastic surface of the culture flasks and varied markedly in morphology and growth kinetics. For tumor cells lines recognized by autologous TILs, the mesenchymal nature of cells was studied by morphological evaluation by a dedicated sarcoma pathologist after cells were paraffin embedded and HE stained. Tumor origin was assessed by cell morphology and mitotic activity (Supplementary files, figure S3). In this way, we could verify seven established sarcoma cell lines with the remaining being less certain. All established cell lines will in the following be referred to as tumor cell lines (TCL).
Tumor-reactive T-cells in expanded TIL cultures
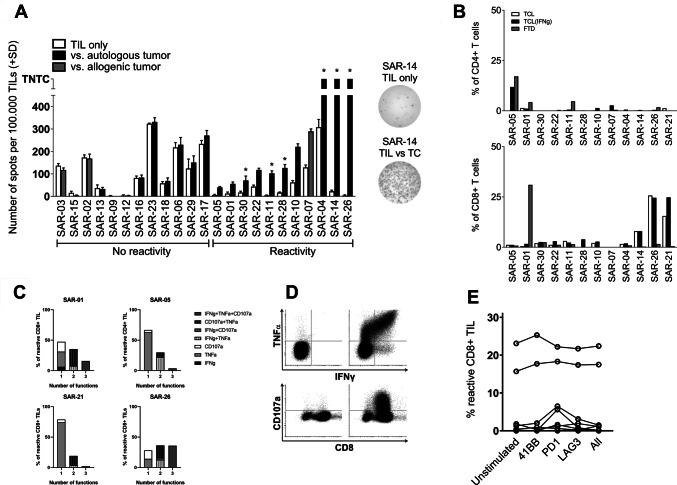
In 10 of 20 tested samples, we found increased numbers of IFNγ-releasing Young TILs using Elispot upon stimulation with autologous TCLs compared with negative controls (Fig. 2a). Among three patients without available autologous tumors, we identified one patient with TIL reactivity against an HLA-matched allogenic TCL.
Fig. 2.
Recognition of tumor cells was analyzed in triplicates using Elispot. If any reactivity was detected, TIL recognition of tumor cells was subsequently analyzed using flow cytometry. a Bar plot showing number of spots (mean + SD) in IFNγ Elispot upon co-culture of TILs with either autologous TCL (black) or allogenic HLA-matched TCL (light grey). Samples are grouped by increasing reactivity determined by the number of spots in wells co-cultured with TILs and TCL compared to the number of spots in the negative controls containing only TILs (white). TCLs that were confirmed by pathologist to be sarcoma cells are marked with an *. Of note, SAR-01 revealed little reactivity against TCL in Elispot, but high reactivity against FTD using ICS, indicating that the TCL sample is not representative for the heterogeneity of the in vivo tumor. b Bar plot showing percentage of tumor-reactive T cells within the CD4+ and CD8+ subsets, when co-cultured with TCL, IFNγ-treated TCL, or FTD analyzed with flow cytometry. Reactivity is determined by production of either TNFα or IFNγ, or expression of CD107a, or a combination of these. All data are from Young TILs.c Scatter plot showing fraction of reactive CD8+ TILs (as determined above) when co-cultured with FTD with and without addition of stimulating or blocking antibodies. Data from each patient are connected. d Representative example of flow cytometry data from a patient with highly reactive TILs (SAR-26): on the left the negative control (i.e., only TILs) and on the right TILs co-cultured with TCL. E) Venn diagrams showing specification of functionality of reactive CD8+ TILs (i.e., production of IFNγ, TNFα, and/or expression of CD107a) in the three samples with high numbers of reactive CD8+ TILs
If reactivity was detected by Elispot or if FTD was available, TIL cultures were subsequently tested in an intracellular cytokine staining (ICS) assay. Six samples had more than 3% reactive TILs, and in four of these, reactivity exceeded 10%. Most of the reactive TILs were CD8+ T cells, but in one sample we observed a high number of reactive CD4+ T cells (Fig. 2b).
Multifunctionality of T-cells is defined as T-cells with more than one function, including production of cytokines/chemokines and degranulation, and is associated with enhanced immune response to HIV and other viral infections [20]. Following TIL-based ACT, the tumor-reactive CD8+ T-cells persisting in the peripheral blood have been demonstrated to be mostly multifunctional [21]. We investigated the multifunctionality of the four TIL samples with high numbers of tumor-reactive CD8+ TIL (SAR-01, SAR-21, and SAR-26) and the one sample with high numbers of tumor-reactive CD4+ TIL (SAR-05). In two samples (SAR-01 and SAR-26), the majority of the tumor-reactive TILs were multifunctional (51% and 72%, respectively). The tumor-reactive CD4+ TILs in SAR-05 were mainly monofunctional with 61% only producing TNFα. In general, we saw higher expression of CD107a than production of TNFα and IFNγ (Fig. 2c, d).
To investigate the functional impact of immune regulatory and co-stimulatory surface molecules on TILs, we performed parallel experiments with the addition of inhibitory monoclonal antibodies targeting LAG3 (BMS) and PD-1 (BMS), as well as an agonistic antibody targeting 4-1BB (BMS). Neither LAG3 blocking nor 4-1BB stimulation during co-culture with autologous tumor changed tumor recognition of the REP TILs. PD-1 blocking tended to increase the fraction of tumor-reactive TILs in three cases, but no statistically significant changes were found (Fig. 2e).
4-1BB stimulation increases total yield and number of CD8+ T cells
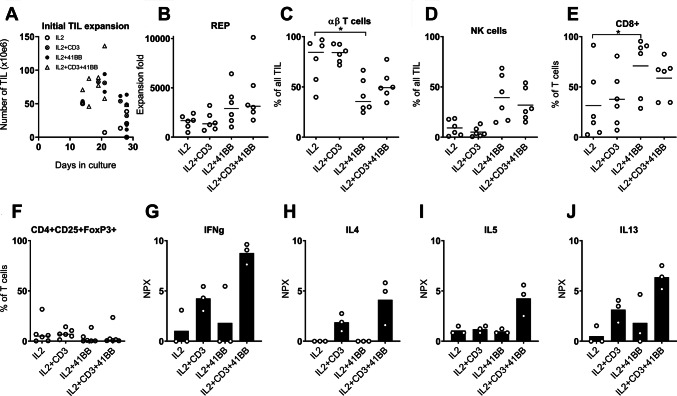
We further tested the effect of 4-1BB and CD3 stimulation during expansion in eight samples. Expansion rate varied significantly (Friedman, p < 0.05) with the highest total yield seen in cultures stimulated with the combination of CD3 and 4-1BB antibodies (Fig. 3a).
Fig. 3.
Eight samples were stimulated with 4-1BB and/or CD3 antibodies during expansion to test the effect of these on expansion time and phenotypic characteristics. Significant variation (Friedman; p < 0.05) from the control group with only IL2 stimulation are shown with a *. a Scatter plot showing the total yield and expansion time of Young TILs for the four analyte groups. b Dot plot showing expansion fold from the REP for the four analyte groups. Only the six samples with TIL from all analyte groups are shown (for SAR-30, we used TIL grown from 48 fragments for the IL2 group). c Dot plot showing percentage of αβ T cells in the Young TIL populations for the four analyte groups. As above, only six samples are shown. d Dot plot showing percentage of NK cells in the Young TIL populations for the four analyte groups. As above, only six samples are shown. e Dot plot showing expression of CD8+ on T cells in the Young TIL population. f Dot plot showing percentage of regulatory T cells in the REP TIL population. g–j Bar plots showing median NPX values as measures of IFNγ, IL4, IL5, and IL-13 concentrations in the supernatants on day 5 of expansion. Each open circle represents one patient sample
This variation in expansion rate was also observed, when TILs were further expanded in the REP (Friedman, p < 0.05), and was even more pronounced, when correcting for number of CD3+ TILs in the starting TIL cultures. The highest expansion fold was seen in cultures stimulated with 4-1BB antibody alone (median 3500; 1680–6420) and the combination of CD3 and 4-1BB antibodies (median 3020; 1730–10110) (Fig. 3b).
Young TILs stimulated with the 4-1BB antibody alone had significantly smaller fractions of αβ T cells (Fig. 3c) compared with the control group with only IL2 stimulation (Friedman, p < 0.05). This difference was caused by an increased fraction of NK cells (Fig. 3d) while the fraction of γδ T cells did not change significantly (Supplementary files, figure S4A). Following REP, the vast majority of TILs were αβ T cells, regardless of previous stimulation (Supplementary files, figure S4B).
We found a significant increase in the fraction of CD8+ T cells in TIL cultures stimulated with 4-1BB antibody alone when compared with IL2 stimulation alone (Friedman, p < 0.05) (Fig. 3e). This corresponds well with previous findings in melanoma TILs [22, 23]. In general, only a small fraction of TILs had phenotypical characteristics usually associated with regulatory T cells (Treg), i.e., being CD4+ CD25+ FoxP3+ (Fig. 3f).
Looking further into other phenotypical features of the expanded TILs, we saw a trend towards fewer PD-1+ T cells in the subgroup stimulated with CD3 and 4-1BB antibodies in combination, most pronounced in the CD4+ subpopulation (Friedman, p = 0.16). No differences in expression of CD27, CD28, 4-1BB, LAG3, BTLA, and TIM-3 were observed (Supplementary files, figure S4C).
To characterize the functional influence of 4-1BB and CD3 stimulation, we looked into the cytokines secreted into the growth media of three patients (SAR-22, SAR-26, and SAR-28) using the Proximity Extension Assay (PEA), including 92 protein biomarkers. After 5 days of culture, we found significantly increased concentrations of both Th1-associated cytokines like IFNγ and the Th2-associated cytokines IL4, IL5, and IL-13 in cultures stimulated with the combination of 4-1BB and CD3 antibodies (Fig. 3g–j). Cytokine signatures in 4-1BB stimulated cultures were very similar to the control with only IL-2 stimulation. Other significant cytokines with varying concentration between analyte groups are shown in supplementary files, figures S4D−G.
4-1BB stimulation could increase functional capacity of expanded T cells
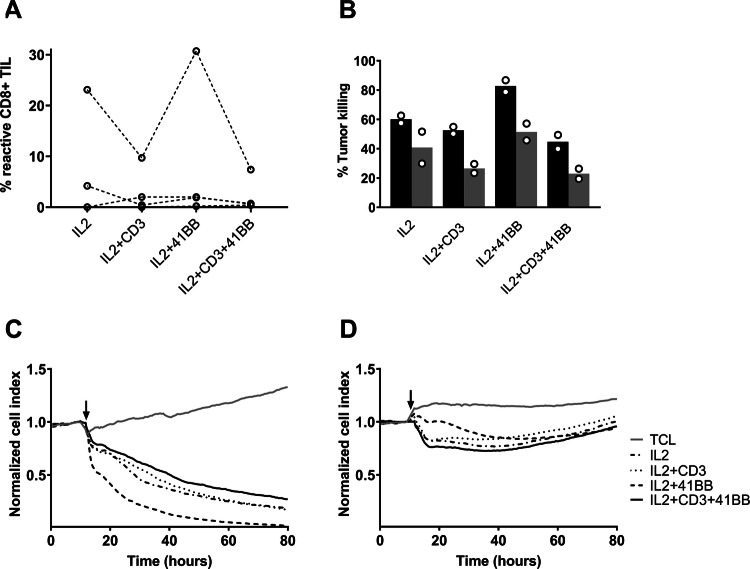
In one sample with already high reactivity (SAR-26), we found an increased fraction of tumor-reactive CD3+ CD8+ cells when TILs had been 4-1BB stimulated compared with the only IL2 stimulated (Fig. 4a). In this sample, CD3 stimulation alone and in combination with 4-1BB stimulation resulted in decreased tumor recognition. No differences in reactivity were observed in three other tested samples.
Fig. 4.
TILs expanded with 4-1BB and/or anti-CD3stimulation were tested for reactivity using flow cytometry and compared with TILs expanded with only IL-2 stimulation. Subsequently, TIL cytolysis of tumor cells was assessed using xCelligence. a Scatter plot showing percentage of tumor-reactive CD8+ T cells in the REP TIL population. Each open circle represents one patient sample. Samples from the same patient are connected with a line. b Bar plot of cytolysis of SAR-26 tumor cells following co-culture with TIL measured by Xcelligence. Bars show the percentage killing of tumor cells after 24 h of co-culture with TIL (black bars) and percentage killing after 24 h of co-culture with TIL and the HLA class I blocking agent W6/32 (gray bars). The assay was performed in duplicates each indicated by an open circle. c Graph showing an example of cytolysis from a TIL sample with high reactivity (SAR-26) as a function of time using xCelligence. The arrow indicates the time point for addition of the REP TILs. d Graph showing an example of minimal/lacking cytolysis from a TIL sample with low reactivity (SAR-28) as a function of time using xCelligence. The arrow indicates the time point for addition of the REP TILs
Furthermore, we saw indications that 4-1BB-stimulated TILs from this patient (SAR-26) also had increased tumor-killing capacity determined by xCelligence (Fig. 4b–d). Addition of the HLA class I blocking agent W6/32 resulted in decreased tumor killing, indicating that this tumor killing was at least partly caused by TCR-HLA class I interaction.
We also tested whether addition of different stimulating or inhibitory antibodies would affect cytotoxic capacity, when added to the co-culture, but found no effect of either PD-1 blocking, LAG3 blocking, 4-1BB stimulation, or any combination of these (Supplementary files, figure S5).
Discussion
In this study, we show that it is indeed feasible to expand TILs from multiple different sarcoma subtypes to numbers needed for clinical application. We also show that approximately half of TIL cultures, representing eight of nine sarcoma subtypes, comprise T-cells capable of recognizing autologous (or allogenic) sarcoma tumor cells in vitro, in some cases to an extent comparable to what has been seen in melanoma samples [24].
Growth kinetics varied significantly between samples, also within the same sarcoma subtype, but we saw a trend towards a shorter expansion time in myxofibrosarcoma and UPS, compared with other subtypes. This adds important information to the ongoing discussion about UPS responses to immunotherapy. A recent report shows objective response in 4 of 10 patients with UPS following treatment with pembrolizumab [8], while another report showed no responders in 16 patients with UPS following treatment with pembrolizumab [9]. Our in vitro results indicate that some UPS tumors contain highly tumor-reactive CD8+ T cells, which may also influence efficacy of other immunotherapeutic drugs.
To determine the occurrence of potentially targetable immune regulatory molecules, we identified selected exhaustion markers in both FTD and cultured TILs. We observed high expression of LAG3 on CD8+ TILs, while PD-1 and BTLA were expressed to a lower degree and mainly on CD4+ TILs. In the recognition assays, however, specific blocking of these immune checkpoints did not significantly change the tumor reactivity in short-term co-culture assays. The functional consequences of expression of these markers on cultured TILs are not clearly understood but might be explained by their transient expression upon in vitro activation [25, 26]. Thus, these markers may not per se be markers of dysfunctional/exhausted cells [27]. Still, expression of LAG3 on TILs would be expected to have a negative impact on effector function of especially CD4+ T-cells, because of the high affinity binding to MHC class II [28], and preliminary results from an ongoing clinical study of simultaneously blocking PD-1 and LAG3 in heavily pretreated melanoma patients (NCT01968109) indicate that LAG3 expression on immune infiltrates correlates with enhanced response [29].
ACT based on ex vivo-expanded TILs is a powerful treatment option for patients with malignant melanoma, but has yet to show convincing clinical results in other cancers. Recent preclinical studies have reported that TILs can be expanded from various malignancies like ovarian cancer [30], head and neck cancer (HNSCC) [31], renal cell carcinoma [32], pancreatic cancer [33], triple-negative breast cancer [34], non-small cell lung cancer [35], bladder cancer [36], and bone sarcomas [37]. In all these studies, except HNSCC, the TILs expanded with IL2 have been predominantly CD4+, thus phenotypically different from melanoma derived TILs, which comprise a larger fraction of CD8+ TILs [38–40].
The role of CD4+ TILs in ACT remains controversial, and responses seen following ACT using CD8+ enriched TILs suggest that CD4+ TILs are not necessary to induce objective responses in melanoma patients [41]. On the other hand, significant tumor regression has been reported in melanoma patients following infusion of MHC class II-restricted TILs [42] and in one patient with cholangiocarcinoma treated with mutation antigen-specific CD4+ TILs [43]. T-cell responses in this study were predominately CD8+ mediated, but CD4+ responses also occurred. In one patient (SAR-05), we observed highly reactive CD4+ TIL when co-cultured with FTD and IFNγ-pre-stimulated TCL, but not against non-treated TCL. This could be due to the presence of antigen-presenting cells in the FTD and upregulation of MHC class II molecules on tumor cells by IFNγ stimulation. The CD4+ response in this patient was largely dominated by TNFα production, which has been suggested to dampen CD8+ -mediated antitumor reactivity [44].
A key to harness the potential of ACT for non-melanoma malignancies could be to improve in vitro expansion techniques. Recent studies have shown great potential by selectively expanding and infusing T cells, which are specifically directed towards known targets on the cancer cells. Robbins et al. reported objective clinical response in patients with synovial sarcoma and melanoma following ACT with genetically modified autologous T cells expressing T cell receptors targeting NY-ESO-1 [12], and Zacharakis et al. demonstrated that selective expansion of neoepitope-reactive TILs can lead to complete durable regression in metastatic breast cancer [45]. These methods bring enormous potential to the future of ACT but are both limited to targets that can be identified on cancer cells, such as well-described shared antigens or neoepitopes determined by whole exome sequencing of the tumor. In contrast, unselected in vitro expanded TILs are highly heterogeneous with multiple tumor-reactive CD8+ T cell clonotypes that have been shown to be present in both the infusion products and persist in the periphery following ACT [21].
A promising approach to improve the quality of expanded TILs, while preserving the polyclonality, is ex vivo manipulation of the tumor microenvironment by 4-1BB stimulation during TIL expansion, which has been shown to increase the number of CD3+ CD8+ TILs in melanoma. The mechanisms behind this are partly due to direct stimulation of CD8+ TILs, which express 4-1BB when activated [46], but are likely to also be influenced by activation of dendritic cells resident in the tumor fragments, thus strengthening the ongoing presentation of antigens [23]. In this study we report that this approach is also feasible for expanding TILs from sarcoma specimens, resulting in a more favourable phenotype and a higher total yield, resulting in shorter expansion times compared with the standard methods. However, 4-1BB stimulation does not convert non-tumor-reactive or low tumor-reactive TILs to highly tumor-reactive TILs.
While 4-1BB stimulation is a potent way to increase number and functionality of CD8+ TILs, it may also affect the regulatory T cells in the TME. IL-2 stimulation induces expression of 4-1BB on Tregs, and thus, the combination of IL-2 and 4-1BB stimulation could potentially act in synergy to expand Tregs [47]. Additionally, 4-1BB co-stimulation of T cells has been shown to increase production of both IFNγ and IL-13 in mouse models [48]. In our material, we found no significant differences in fractions of CD4+ TILs with a Treg-like phenotype between analyte groups. Interestingly, we found that stimulation with the combination of 4-1BB and CD3 antibodies increased both the Th1-associated cytokine IFNγ and the Th2-associated cytokines IL4, IL5, and IL-13. The counteracting actions of these cytokines suggest that double stimulation might not be beneficial for TILs. Previously, double 4-1BB and CD3 stimulation has been demonstrated to increase expansion and tumor reactivity of melanoma TIL [49], but in our study, the only sample with high reactivity (SAR26) showed decreased reactivity and functionality when stimulated with both 4-1BB and CD3. An explanation for this difference could be that anti-CD3 was continuously added in the present study in contrast to only being added on day 1.
Despite the beneficial impact on phenotype and functionality of TILs in this and other studies, 4-1BB stimulation did not convert non-reactive or low-reactive TILs to highly reactive TILs. This fact emphasizes that the tumor immune infiltrate at the time of surgery is a crucial parameter for successful TIL expansion. Treatment with a PD-1 blocking antibody has been shown to increase T cell infiltration of the tumor in mouse models [50], and clinical studies are currently investigating TIL-based ACT combined with checkpoint blockade, BRAF inhibitor treatment, or local treatment of the tumor prior to surgery.
We have in this study shown that it is feasible to expand TILs from the majority of the included sarcoma subtypes, with the exception of GIST. However, expansion kinetics was less uniform, showing highly varying expansion time to obtain a clinically sufficient number of TILs. Based on our trials with melanoma and ovarian cancer, we know that this time frame is critical, because the physical condition of patients with late-stage cancer can change rapidly. In order to address this critical time parameter, we found that the addition of a 4-1BB agonist augmented expansion kinetics markedly by reducing expansion time, increasing the likelihood of sufficient growth, and resulting in a favored CD8+ occurrence. Further optimization of TIL expansion kinetics and reactivity by agonistic targeting of tumor-reactive TILs is ongoing.
In conclusion, we show that it is feasible to expand TILs from sarcoma patients to clinically relevant numbers and that these TILs can recognize autologous tumor cells in an in vitro setting. Based on this, we conclude that TIL-based ACT could potentially benefit sarcoma patients with otherwise limited treatment options.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the technicians at CCIT-DK and the staff at the Department of Oncology at Herlev Hospital, and the Department of Orthopedic Surgery at Rigshospitalet, for practical assistance in carrying out this study. The authors would also like to thank Bristol-Myers Squibb for kindly providing the agonistic anti-4-1BB antibody (BMS-663513), the blocking PD-1 antibody (PD1.5C4), and the blocking LAG3 antibody (BMS-986016) used in this study.
Abbreviations
- ACT
Adoptive cell therapy
- BTLA
B- and T-lymphocyte attenuator
- CM
Culture medium
- DMSO
Dimethyl sulfoxide
- FACS
Fluorescence-activated cell sorter
- FTD
Fresh tumor digest
- GIST
Gastrointestinal stromal tumor
- HIV
Human immunodeficiency virus
- ICS
Intracellular cytokine staining
- IL2
Interleukin-2
- INFγ
Interferon-γ
- LAG3
Lymphocyte activation gene 3
- MHC
Major histocompatibility complex
- NY-ESO-1
New York esophageal squamous cell carcinoma 1
- PBMC
Peripheral blood mononuclear cell
- PBS
Phosphate-buffered saline
- PD1
Programmed cell death protein 1
- REP
Rapid expansion protocol
- TCL
Tumor cell line
- TCR
T cell receptor
- TIL
Tumor-infiltrating lymphocyte
- TIM3
T-cell immunoglobulin and mucin-domain containing-3
- TME
Tumor microenvironment
- TNFα
Tumor necrosis factor α
- UPS
Undifferentiated pleomorphic sarcoma
Author contributions
All authors contributed to the study design and were involved in data acquisition and interpretation of data. MN, IMS, and NJ drafted the manuscript. All authors contributed to the revision of the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by Aase & Ejnar Danielsen’s Foundation; Fabrikant Einar Willumsens Mindelegat; Højmosegårdlegatet; Tømrermester Jørgen Holm og Hustru Elisa f. Hansens Mindelegat; Dagmar Marshalls Fond; and a Herlev Hospital research grant.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
The authors of this paper report on their T cell assays transparently and comprehensively as per field-wide consensus, allowing the community a full understanding and interpretation of presented data as well as a comparison of data between groups. The electronic supplementary materials of this publication include a MIATA checklist. For more details, see miataproject.org.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Inge Marie Svane and Niels Junker have contributed equally to this work.
References
- 1.ESMO Guidelines Committee and EURACAN Soft tissue and visceral sarcomas : ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 2.Italiano A, Mathoulin-pelissier S, Le CA, Terrier P. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–1054. doi: 10.1002/cncr.25538. [DOI] [PubMed] [Google Scholar]
- 3.D’Angelo SP, Shoushtari AN, Agaram NP, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357–365. doi: 10.1016/j.humpath.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusakiewicz S, Semeraro M, Sarabi M, et al. Immune in filtrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 5.Berghuis D, Santos SJ, Baelde HJ, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8+ T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223:347–357. doi: 10.1002/path.2819. [DOI] [PubMed] [Google Scholar]
- 6.Sorbye SW, Kilvaer T, Valkov A, et al. Prognostic impact of lymphocytes in soft tissue Sarcomas. PLoS ONE. 2011;6:1–10. doi: 10.1371/journal.pone.0014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JR, Moon YJ, Kwon KS, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS ONE. 2013;8:1–9. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone. Lancet Oncol. 2017;2045:1–9. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toulmonde M, Penel N, Adam J, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas. JAMA Oncol. 2017;4:93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins PF, Kassim SH, Tran TLN, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2014;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donia M, Junker N, Ellebaek E, et al. Characterization and comparison of ‘standard’ and ‘young’ tumour-infiltrating lymphocytes for adoptive cell therapy at a Danish Translational Research Institution. Scand J Immunol. 2011;75:157–167. doi: 10.1111/j.1365-3083.2011.02640.x. [DOI] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA (2009) Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. 27:417–428. 10.1055/s-0029-1237430.Imprinting [DOI] [PMC free article] [PubMed]
- 15.Erskine CL, Henle AM, Knutson KL. Determining optimal cytotoxic activity of human Her2neu specific CD8 T cells by comparing the Cr51 release assay to the xCELLigence system protocol. J Vis Exp. 2012 doi: 10.3791/3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peper J, Schuster H, Löffler MW, et al. An impedance-based cytotoxicity assay for real-time and label-free assessment of T-cell-mediated killing of adherent cells. J Immunol Methods. 2014;405:192–198. doi: 10.1016/j.jim.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Abuzakouk M, FeigheryO´Farrelly CC. Collagenase and Dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods. 1996;194:211–216. doi: 10.1016/0022-1759(96)00038-5. [DOI] [PubMed] [Google Scholar]
- 18.Grange C, Létourneau J, Forget MA, et al. Phenotypic characterization and functional analysis of human tumor immune infiltration after mechanical and enzymatic disaggregation. J Immunol Methods. 2011;372:119–126. doi: 10.1016/j.jim.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 20.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 21.Donia M, Kjeldsen JW, Andersen R, et al. PD-1+ polyfunctional T cells dominate the periphery after tumor-infiltrating lymphocyte therapy for cancer. Clin Cancer Res. 2017;23:5779–5789. doi: 10.1158/1078-0432.CCR-16-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chacon JA, Wu RC, Sukhumalchandra P, et al. Co-Stimulation through 4–1BB/CD137 improves the expansion and function of CD8+ melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS ONE. 2013 doi: 10.1371/journal.pone.0060031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chacon JA, Sarnaik AA, Chen JQ, et al. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor- in filtrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21:611–621. doi: 10.1158/1078-0432.CCR-14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donia M, Hansen M, Sendrup SL, et al. Methods to improve adoptive T-cell therapy for melanoma: IFN-γ enhances anticancer responses of cell products for infusion. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.336. [DOI] [PubMed] [Google Scholar]
- 25.Bae J, Lee SJ, Park C, et al. Trafficking of LAG-3 to the surface on activated T cells via its cytoplasmic domain and protein kinase C signaling. J Immunol. 2014;193:3101–3112. doi: 10.4049/jimmunol.1401025. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe N, Gavrieli M, Sedy J, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 27.Legat A, Speiser DE, Pircher H, et al. Inhibitory receptor expression depends more dominantly on differentiation and activation than “ exhaustion ” of human CD8 T cells. Front Immunol. 2013;4:1–15. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huard B, Prigent P, Tournier M, et al. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 29.Ascierto P, Bono P, Bhatia S, et al (2017) Efficacy of BMS-986016, a Monoclonal Antibody That Targets Lymphocyte Activation Gene-3 (LAG-3), in Combination With Nivolumab in Pts With Melanoma
- 30.Westergaard MCW, Andersen R, Chong C, et al. Tumour-reactive T cell subsets in the microenvironment of ovarian cancer. Br J Cancer. 2019 doi: 10.1038/s41416-019-0384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Junker N, Andersen MH, Wenandy L, et al. Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer. Cytotherapy. 2011;13:822–834. doi: 10.3109/14653249.2011.563291. [DOI] [PubMed] [Google Scholar]
- 32.Andersen R, Christine M, Westergaard W, et al. T-cell responses in the microenvironment of primary renal cell carcinoma—implications for adoptive cell therapy. Cancer Immunol Res. 2018;6:222–235. doi: 10.1158/2326-6066.CIR-17-0467. [DOI] [PubMed] [Google Scholar]
- 33.Sakellariou-Thompson D, Forget M-A, Creasy C, et al. 4–1BB agonist focuses CD8+ tumor-infiltrating T-cell growth into a distinct repertoire capable of tumor recognition in pancreatic cancer. Clin Cancer Res. 2017;23:7263–7276. doi: 10.1158/1078-0432.CCR-17-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harao M, Forget M-A, Roszik J, et al. 4–1BB—enhanced expansion of CD8+ TIL from triple-negative breast cancer unveils mutation-specific CD8+ T cells. Cancer Immunol Res. 2017;5:439–446. doi: 10.1158/2326-6066.CIR-16-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Avi R, Farhi R, Ben-Nun A, et al. Establishment of adoptive cell therapy with tumor infiltrating lymphocytes for non-small cell lung cancer patients. Cancer Immunol Immunother. 2018;67:1221–1230. doi: 10.1007/s00262-018-2174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poch M, Hall M, Joerger A, et al. Expansion of tumor infiltrating lymphocytes (TIL) from bladder cancer. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1476816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Théoleyre S, Mori K, Cherrier B, et al. Phenotypic and functional analysis of lymphocytes infiltrating osteolytic tumors: use as a possible therapeutic approach of osteosarcoma. BMC Cancer. 2005;5:1–10. doi: 10.1186/1471-2407-5-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itzhaki O, Hovav E, Ziporen Y, et al. Establishment and large-scale expansion of minimally adoptive transfer therapy. J Immunother. 2011;34:212–220. doi: 10.1097/CJI.0b013e318209c94c. [DOI] [PubMed] [Google Scholar]
- 39.Besser MJ, Shapira-frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2656. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 40.Andersen R, Donia M, Ellebaek E, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-in filtrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 41.Dudley ME, GrossLanghan CMM, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman KM, Prieto PA, Devillier LE, et al. Tumor-specific CD4 + Melanoma Tumor-infiltrating Lymphocytes. J Immunother. 2012;35:400–408. doi: 10.1097/CJI.0b013e31825898c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran E, Turcotte S, Gros A, et al (2014) Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science (80) 9:641–646 [DOI] [PMC free article] [PubMed]
- 44.Donia M, Andersen R, Kjeldsen JW, et al. Aberrant expression of MHC class II in melanoma attracts inflammatory tumor-specific CD4+ T-cells, which dampen CD8+ T-cell antitumor reactivity. Cancer Res. 2015;75:3747–3760. doi: 10.1158/0008-5472.CAN-14-2956. [DOI] [PubMed] [Google Scholar]
- 45.Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24:724–730. doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye Q, Song DG, Poussin M, et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014;20:44–55. doi: 10.1158/1078-0432.CCR-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elpek KG, Yolcu ES, Franke DDH, et al. Ex vivo expansion of CD4+CD25+FoxP3+ T Regulatory Cells Based on Synergy between IL-2 and 4–1BB Signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 48.Shin SM, Kim YH, Choi BK, et al. 4–1BB triggers IL-13 production from T cells to limit the polarized, Th1-mediated inflammation. J Leukoc Biol. 2007;81:1455–1465. doi: 10.1189/jlb.1006619. [DOI] [PubMed] [Google Scholar]
- 49.Tavera R, Forget M, Kim Y, et al. Utilizing T-cell activation signals 1, 2 and 3 for tumor-infiltrating lymphocytes (TIL) expansion: the advantage over the sole use of interleukin-2 in cutaneous and uveal melanoma. J Immunother. 2018;41:399–405. doi: 10.1097/CJI.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodumudi KN, Siegel J, Weber AM, et al. Immune checkpoint blockade to improve tumor infiltrating lymphocytes for adoptive cell therapy. PLoS ONE. 2016;11:1–13. doi: 10.1371/journal.pone.0153053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.