Abstract
Chemotherapy is still the backbone of systemic treatment in the majority of cancers. However, immunotherapies, especially those based on checkpoint inhibition, are additional therapy options for many. For this, functional T cells are a mandatory requirement. The aim of this prospective study was to investigate the influence of chemotherapy on the cellular immune status of individual patients. Peripheral blood samples of 26 patients with solid malignancies undergoing chemotherapy were analyzed for lymphocyte populations and their subsets in a longitudinal approach. Chemotherapy decreased total B lymphocyte counts [median value (25–75 percentile): before chemotherapy 76/µl (39–160) vs. after chemotherapy 49/µl (24–106); p = 0.001]. Among B cells, specific subsets decreased particularly [naïve B cells (49/µl (21–111) vs. 25/µl (13–56); p = 0.001], memory B cells [3/µl (2–8) vs. 2/µl (1–4); p = 0.001], and class-switched B cells [11/µl (6–20) vs. 6/µl (3–12); p = 0.011]. In contrast, chemotherapy had no influence on the total numbers of CD4 + and CD8 + T lymphocytes or on their subsets (T helper cells 1, 2, and 17 as well as cytotoxic T cells in early, intermediate, late, terminal effector and exhausted status as well as both T-cell types with naïve, center memory, effector memory, activated, or regulatory phenotype). Furthermore, the count of natural killer (NK) lymphocytes showed no significant change before and after chemotherapy. In summary, this study shows a decrease of B lymphocytes during systemic chemotherapy, but no relevant effect on T lymphocytes, NK lymphocytes and their subsets. This could support the idea of an effective additive T-cell-dependent immunotherapy to chemotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02449-y) contains supplementary material, which is available to authorized users.
Keywords: Chemotherapy, B cells, T cells, NK cells, Cancer, Lymphocyte subsets
Introduction
Until a few years ago, the main therapeutic options for solid tumors were chemotherapy and radiation. More recently, targeted therapies, such as monoclonal antibodies, specific inhibitors of intracellular metabolic pathways, and modulation of cellular immunity, have become additional and often preferable options for systemic cancer therapy. One of the most significant side effects of systemic chemotherapy is the risk of infection during leukocytopenia. Furthermore, immunosuppression may thwart physiological antineoplastic reaction against survival mechanisms of tumor cells [1, 2, 3]. However, it is not well understood how far this side effect impairs the efficacy of potential concordant or subsequent treatment strategies that target the activation of the patient´s cellular immune system. In particular, the impact on checkpoint inhibitors such as pembrolizumab, nivolumab, or atezolizumab is not known. These indirect activators of cytotoxic T lymphocytes have established a very important role in various lines of treatment after or in combination with cytotoxic therapy of a number of different tumors. Therefore, it is important to know what effect classical chemotherapy may have on lymphocytes and their subsets, in particular on the composition of active cytotoxic as well as immunosuppressive regulatory cells.
In the present study, we evaluated the recovery of T, B, and natural killer (NK) cell compartments after cytotoxic treatment for solid malignancies. For this purpose, we measured the numbers of various lymphocyte subpopulations before and after a first-line chemotherapy using flow cytometry.
Patients and methods
Study population and trial design
We included 26 patients with solid malignancy, who were treated at University Medical Center Augsburg and did not have any prior antineoplastic treatment. Patients with additional chronic disease (especially hepatitis or HIV), inherent or acquired immunodeficiency, autoimmune disorder or any prior cytotoxic therapy were excluded. Medical information about age, sex, diagnosis, and protocol of chemotherapy (with or without additional radiotherapy) were obtained as well as data regarding episodes of infections, clinical response rate, and administration of granulocyte-colony stimulating factor (G-CSF) during the study period. Blood samples (EDTA-blood) were taken before the start of and after a 12-week period of chemotherapy. Blood of blood donors from the blood bank at University Medical Center Augsburg was used as control. In addition, a few older, healthy volunteers were recruited for the control group to guarantee a better comparability with the patients.
Analysis of lymphocytes and subsets
All blood samples were processed within 24 h. Samples were analyzed by flow cytometry using FC500 from Beckman Coulter after cell staining with commercial fluoreszeinisothiocyanat (FITC-), phycoerythrin (PE-), phycoerythrin Texas red-X (ECD-), and phycoerythrin–cyanin (PC5- and PC7-) labeled antibodies purchased from Beckman Coulter (Brea, California, USA) and Biolegend (San Diego, California, USA). For all lymphocyte subsets, percentages were determined and absolute numbers were calculated. Lymphocytes were identified using forward scatter and side scatter. To divide lymphocytes into different subsets, established standard and well-described gating strategies were used [4].
B lymphocytes were identified by the presence of CD19 (CD19-PC7 IM3628) and were further divided into naïve (IgD + CD27-; IgD-FITC B30652, CD27-ECD B26603), memory (IgD + CD27 +), class switched (IgD- CD27 +), and transitional (CD24hi CD38hi; CD24-PE IM1428U, CD38-PC5 A07780) subsets. The gating strategy of B lymphocytes is shown in Fig. 1.
Fig. 1.
Flowcytometric gating strategy of B-cell subsets. Lymphocytes were identified using forward and side scatter; B lymphocytes were defined by the presence of CD19 and further divided into naïve (IgD + CD27-), memory (IgD + CD27 +), class switched (IgD- CD27 +), and transitional (CD24hi CD38hi) subsets
T lymphocytes defined by positivity for CD8 or CD4 were subdivided into naïve (CD62L + CD45RA +), memory T cells (CD4 + CD45RA- CD45RO + /CD8 + CD45RA- CD45RO +), which were further divided into central memory (CD62L + CD45RA-), effector memory (CD62L- CD45RA-), effector memory RA + (EMRA) (CD62L- CD45RA +) and activated memory (HLA-DR + or CD69 +) cells, and regulatory (FoxP3 +) cells.
Furthermore, types 1, 2, and 17 CD4 + T-helper (Th1/Th2/Th17) cells were identified using antibodies against CXCR3, CCR4, CCR5 and CCR6. Th1 cells were defined as CD4 + CXCR3 + CCR4- CCR5 + CCR6-, Th2 cells as CD4 + CXCR3- CCR4 + CCR5- CCR6- and Th17 cells as CD4 + CXCR3- CCR4 + CCR5- CCR6 + .
Within cytotoxic CD8 + T lymphocytes, activated subsets in early (CD28 + CD27 +), intermediate (CD28- CD27 +) and late (CD28- CD27-) status were identified as well as exhausted (CD279 +) and terminal effector (CD279- CD57 +) cells. NK-like T cells were defined as CD56 + CD3 + .
NK lymphocytes were detected as CD56 + cells and subdivided into 3 NK subsets (CD56 + CD16 + , CD56dim CD16bright, and CD56bright CD16dim) [5, 6, 7].
Detailed information on fluorochrome-antibody conjugates of T cells and NK cells as well as the specific gating strategies for theses lymphocytes and their subgroups can be found in de supplement part (Fig. S1 and Table S1).
Statistical analysis
Results are given as median values with interquartile ranges for descriptive analysis if not otherwise indicated. Wilcoxon test for associated samples was used for all statistical tests (two-tailed) to detect different numbers of lymphocytes and their subsets before and after chemotherapy. Benjamini–Hochberg procedure was applied for multiple testing correction and p values < 0.022 were regarded as statistically significant. Data were analyzed with SPSS for Windows (IBM SPSS Statistics 24, Armonk, New York, USA).
Results
Population characteristics
The median age was 63 (48–80) years, 14 patients were male, and 12 were female. A broad variety of different tumor histologies were included. Most patients were diagnosed with lung cancer, 9 with non-small cell lung cancer (NSCLC), and 2 with small cell lung cancer (SCLC), followed by colorectal cancer, pancreatic cancer, sarcomas, and others. 18 patients were in metastatic situation. Patients received either platinum-containing or platinum-free chemotherapy. The specific chemotherapeutic agents administered as well as demographic and disease characteristics of patients and healthy controls are shown in Table 1.
Table 1.
Demographic and disease characteristics of patients at baseline and healthy control group
| Variables | Results | Control group |
|---|---|---|
| Age; median (range) | 63 (48–80) | 58 (43–80) |
| Gender | ||
| Male; n (%) | 14 (54) | 6 (50) |
| Female; n (%) | 12 (46) | 6 (50) |
| Histology | ||
| NSCLC; n (%) | 9 (35) | |
| SCLC; n (%) | 2 (8) | |
| Colorectal; n (%) | 4 (15) | |
| Pancreatic; n (%) | 2 (8) | |
| Sarcoma; n (%) | 2 (8) | |
| Other; n (%) | 7 (27) | |
| Stage | ||
| Metastatic; n (%) | 18 (69) | |
| Non-metastatic; n (%) | 8 (31) | |
| Chemotherapy | ||
| Containing platinum; n (%) | 20 (77) | |
| No platinum; n (%) | 6 (23) | |
| Carboplatin, Etoposid; n (%) | 5 (19) | |
| FOLFIRI; n (%) | 3 (12) | |
| Cisplatin, pemetrexed; n (%) | 3 (12) | |
| Carboplatin, pemetrexed; n (%) | 3 (12) | |
| FOLFIRINOX; n (%) | 2 (8) | |
| FLOT; n (%) | 1 (4) | |
| Cisplatin, 5-FU; n (%) | 1 (4) | |
| Paclitaxel; n (%) | 1 (4) | |
| Cisplatin, gemcitabine; n (%) | 1 (4) | |
| Carboplatin, Gemcitabine; n (%) | 1 (4) | |
| Gemicatibene, paclitaxel; n (%) | 1 (4) | |
| Cisplatin, vinorelbine; n (%) | 1 (4) | |
| Cisplatin, paclitaxel; n (%) | 1 (4) | |
| Doxorubicin, cyclophosphamide; n (%) | 1 (4) | |
| Doxorubicin; n (%) | 1 (4) | |
| Response | ||
| CR; n (%) | 4 (15) | |
| PR; n (%) | 7 (27) | |
| SD; n (%) | 9 (35) | |
| PD; n (%) | 6 (23) | |
| G-CSF | ||
| Yes; n (%) | 16 (62) | |
| No; n (%) | 10 (38) | |
| Radiation | ||
| Yes; n (%) | 8 (31) | |
| No; n (%) | 18 (69) | |
| Infection | ||
| Yes; n (%) | 9 (35) | |
| No; n (%) | 17 (65) | |
NSCLC non-small cell lung cancer, SCLC small cell lung cancer, FOLFIRI folinic acid, fluorouracil, irinotecan, FOLFIRINOX folinic acid, fluorouracil, irinotecan, oxaliplatin, CR complete remission, PR partial remission, SD stable disease, PD progressive disease, G-CSF granulocyte-colony stimulating factor
Tumor response to therapy was evaluated after 4–6 cycles of chemotherapy. 11 patients achieved a complete or partial response. 9 patients had stable disease, and 6 had progressive disease.
G-CSF was administered to 16 of the 26 patients, 8 of them received additional radiation, and 9 were diagnosed with infection during the chemotherapy period.
Lymphocyte measurements
The numbers of all analyzed lymphocyte subsets before and after chemotherapy are shown in Table 2. In addition, differences between sex, response rate, patients with or without G-CSF administration, radiation or infectious complications during therapy, and between platinum-containing and platinum-free protocols were analyzed (Supplement, Table S2).
Table 2.
Changes of lymphocyte subsets during chemotherapy
| Total population | ||||||
|---|---|---|---|---|---|---|
| Before chemotherapy | After chemotherapy | P value | Control group | P-value (patients vs. control) | ||
| (a) B lymphocytes | ||||||
| B Lymphocytes | 0.076 [0.039; 0.160] | 0.049 [0.024; 0.106] | 0.001 | 0.230 [0.173; 0.270] | 0.000 | |
| Naive | 0.049 [0.021; 0.111] | 0.025 [0.013; 0.056] | 0.001 | 0.115 [0.087; 0.189] | 0.002 | |
| Memory | 0.003 [0.002; 0.008] | 0.002 [0.000; 0.004] | 0.001 | 0.005 [0.004; 0.065] | n.s. | |
| Class switch | 0.011 [0.006; 0.020] | 0.006 [0.003; 0.012] | 0.011 | 0.030 [0.023; 0.042] | 0.001 | |
| Transitory | 0.002 [0.000; 0.005] | 0.000 [0.000; 0.002] | n.s. | 0.006 [0.003; 0.006] | 0.005 | |
| (b) CD8 + T lymphocytes | ||||||
| CD8 + Lymphocytes | 0.214 [0.122; 0.374] | 0.233 [0.120; 0.319] | n.s. | 0.275 [0.212; 0.415] | n.s. | |
| CD4 + CD8+ | 0.005 [0.002; 0.013] | 0.003 [0.002; 0.012] | n.s. | 0.006 [0.001; 0.009] | n.s. | |
| Memory | 0.059 [0.040; 0.126] | 0.086 [0.034; 0.128] | n.s. | 0.101 [0.051; 0.137] | n.s. | |
| Central memory | 0.028 [0.015; 0.062] | 0.025 [0.016; 0.048] | n.s. | 0.004 [0.002; 0.014] | 0.000 | |
| Naive | 0.043 [0.017; 0.091] | 0.034 [0.016; 0.066] | n.s. | 0.005 [0.002; 0.015] | 0.001 | |
| Effector memory | 0.054 [0.037; 0.110] | 0.067 [0.030; 0.119] | n.s. | 0.121 [0.074; 0.172] | 0.007 | |
| EMRA | 0.050 [0.023; 0.110] | 0.068 [0.031; 0.120] | n.s. | 0.140 [0.106; 0.187] | 0.003 | |
| Early | 0.082 [0.048; 0.168] | 0.078 [0.052; 0.174] | n.s. | 0.176 [0.087; 0.262] | n.s. | |
| Intermediate | 0.010 [0.006; 0.185] | 0.014 [0.008; 0.030] | n.s. | 0.026 [0.013; 0.045] | 0.001 | |
| Late | 0.044 [0.024; 0.117] | 0.054 [0.024; 0.126] | n.s. | 0.081 [0.033; 0.151] | n.s. | |
| Exhausted | 0.055 [0.034; 0.107] | 0.068 [0.036; 0.118] | n.s. | 0.140 [0.078; 0.266] | 0.006 | |
| Terminal effector | 0.033 [0.021; 0.114] | 0.037 [0.015; 0.101] | n.s. | 0.026 [0.013; 0.092] | n.s. | |
| HLA-DR+ | 0.066 [0.031; 0.131] | 0.076 [0.032; 0.153] | n.s. | 0.007 [0.003; 0.025] | 0.000 | |
| CD69+ | 0.037 [0.018; 0.065] | 0.043 [0.022; 0.773] | n.s. | 0.054 [0.036; 0.070] | n.s. | |
| Regulatory | 0.000 [0.000; 0.002] | 0.001 [0.000; 0.002] | n.s. | 0.000 [0.000; 0.001] | n.s. | |
| CD25+ | 0.001 [0.000; 0.001] | 0.001 [0.000; 0.001] | n.s. | 0.000 [0.000; 0.001] | n.s. | |
| (c) CD4 + T lymphocytes | ||||||
| CD4 + Lymphocytes | 0.587 [0.367; 0.802] | 0.443 [0.236; 0.679] | n.s. | 0.595 [0.442; 1.128] | n.s. | |
| Memory | 0.317 [0.226; 0.547] | 0.301 [0.157; 0.376] | n.s. | 0.333 [0.258; 0.512] | n.s. | |
| Central memory | 0.220 [0.141; 0.331] | 0.178 [0.103; 0.270] | n.s. | 0.079 [0.061; 0.193] | 0.007 | |
| Naive | 0.193 [0.065; 0.330] | 0.104 [0.035; 0.272] | n.s. | 0.216 [0.106; 0.365] | n.s. | |
| Effector memory | 0.119 [0.068; 0.203] | 0.099 [0.068; 0.198] | n.s. | 0.273 [0.200; 0.385] | 0.010 | |
| EMRA | 0.008 [0.002; 0.020] | 0.005 [0.002; 0.016] | n.s. | 0.050 [0.036; 0.088] | 0.001 | |
| HLA-DR+ | 0.058 [0.043; 0.082] | 0.046 [0.037; 0.085] | n.s. | 0.018 [0.008; 0.030] | 0.001 | |
| CD69+ | 0.049 [0.027; 0.117] | 0.035 [0.021; 0.071] | n.s. | 0.034 [0.020; 0.039] | n.s. | |
| Th1 | 0.042 [0.018; 0.069] | 0.030 [0.012; 0.060] | n.s. | 0.025 [0.011; 0.063] | n.s. | |
| Th2 | 0.049 [0.025; 0.091] | 0.042 [0.023; 0.076] | n.s. | 0.040 [0.023; 0.071] | n.s. | |
| Th17 | 0.033 [0.020; 0.054] | 0.031 [0.021; 0.050] | n.s. | 0.049 [0.034; 0.071] | n.s. | |
| Regulatory | 0.027 [0.015; 0.047] | 0.012 [0.010; 0.033] | n.s. | 0.017 [0.011; 0.026] | n.s. | |
| CD25+ | 0.008 [0.005; 0.014] | 0.009 [0.004; 0.014] | n.s. | 0.003 [0.001; 0.003] | 0.000 | |
| CD4/CD8 | 2.403 [1.685; 3.842] | 1.824 [1.328; 3.482] | n.s. | 2.276 [1.641; 3.390] | n.s. | |
| (d) NK cells | ||||||
| NK Cells | 0.131 [0.093; 0.240] | 0.131 [0.073; 0.232] | n.s. | 0.240 [0.153; 0.463] | n.s. | |
| CD56 + CD16+ | 0.036 [0.017; 0.083] | 0.043 [0.012; 0.072] | n.s. | 0.160 [0.108; 0.255] | 0.000 | |
| CD56bright CD16dim | 0.003 [0.001; 0.007] | 0.003 [0.001; 0.006] | n.s. | 0.014 [0.008; 0.027] | 0.000 | |
| CD56dim CD16bright | 0.004 [0.002; 0.018] | 0.006 [0.002; 0.010] | n.s. | 0.019 [0.015; 0.026] | 0.020 | |
| NK/T | 0.046 [0.025; 0.098] | 0.053 [0.021; 0.083] | n.s. | 0.005 [0.020; 0.135] | n.s. | |
(a) B lymphocytes (b) CD8 + T lymphocytes (c) CD4 + T lymphocytes (d) NK cells. Numbers are median values/ml and interquartile ranges in square brackets. P < 0.022 = significant
n.s not significant, EMRA effector memory RA+
B lymphocytes
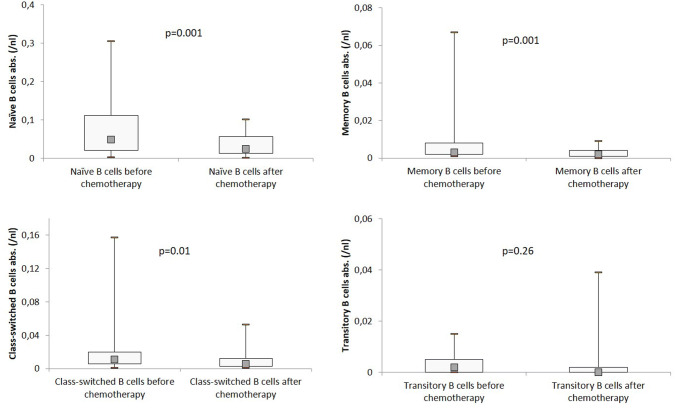
The total number of B lymphocytes decreased significantly during the chemotherapy period, from 76 to 49/µl (p < 0.001) (Fig. 2). There was also a significant decrease observed for naïve (49/µl vs. 25/µl; p < 0.001), memory (3/µl vs. 2/µl; p < 0.001), and class-switched B lymphocytes (11/µl vs. 6/µl; p = 0.011). Only the amount of transitory B cells did not decrease significantly (Fig. 3).
Fig. 2.
B cells, T cells, and NK cells before and after chemotherapy. Distribution of total numbers of B cells, T cells and NK cells in the study population, analyzed by flow cytometry before and after chemotherapy. Boxplots show median values as well as interquartile range. P < 0.022 = significant
Fig. 3.
B-cell subsets before and after chemotherapy. Distribution of total numbers of B-cell subsets in the study population, analyzed by flow cytometry before and after chemotherapy. Boxplots show median values as well as interquartile range. P < 0.022 = significant
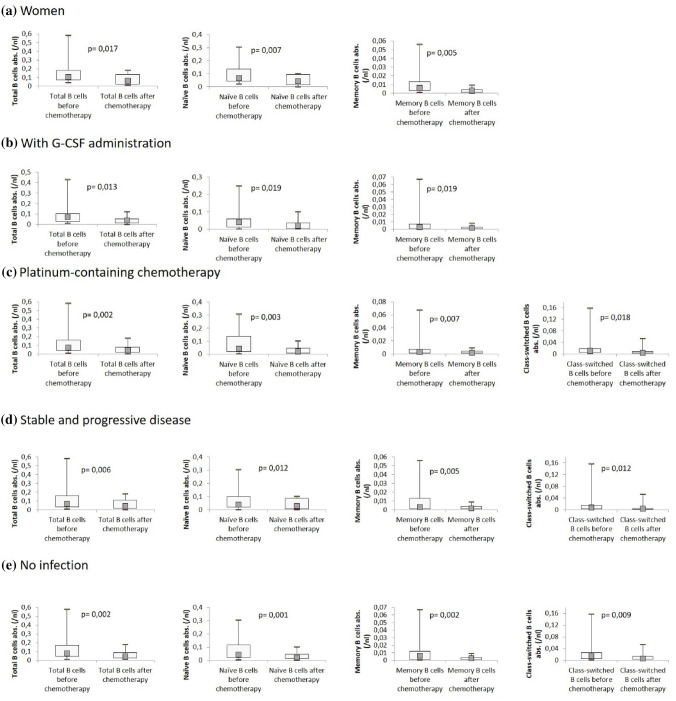
We saw differences between male and female patients. While there was no significant decline of any B-cell subsets in men, women showed a decrease of total (105/µl vs. 60/µl; p = 0.017), naïve (67/µl vs. 44/µl; p = 0.005), and memory B cells (6/µl vs. 2/µl; p = 0.007). Patients who achieved at least a partial remission did not show significant changes in B-lymphocyte subsets, whereas patients in stable or progressive disease showed significant changes in total (66/µl vs. 44/µl; p = 0.006), naïve (41/µl vs. 27/µl; p = 0.012), memory (3/µl vs. 1/µl; p = 0.005), and class-switched (8/µl vs. 4/µl; p = 0.012) B cells. The differences between total (79/µl vs. 46/µl; p = 0.002), naïve (44/µl vs. 23/µl; p = 0.001), memory (6/µl vs. 3/µl; p = 0.002), and class-switched (15/µl vs. 6/µl; p = 0.009) B cells before and after chemotherapy were significant for patients without any infection during the therapy period compared to patients who underwent an infection. Receiving platinum-containing therapy was associated with a significant decrease of total (73/µl vs. 45/µl; p = 0.002), naïve (42/µl vs. 23/µl; p = 0.003), memory (3/µl vs. 2/µl; p = 0.007), and class switched (11/µl vs. 4/µl; p = 0.018) B cells. No significant changes were observed in patients receiving platinum-free chemotherapy.
In patients who did not undergo radiotherapy significant changes of naïve (56/µl vs. 33/µl; p = 0.013) and memory (3/µl vs. 2/µl; p = 0.010), B cells were observed, whereas in the radiation group, only class switched B cells declined significantly (10/µl vs. 6/µl; p = 0.018). The administration of G-CSF made no difference. Decline of naïve (G-CSF group: 41/µl vs. 21/µl; p = 0.019//no-G-CSF group: 84/µl vs. 53/µl; p = 0.017) and memory B cells (G-CSF group: 3/µl vs. 2/µl; p = 0.019//no-G-SCF group: 6/µl vs. 4/µl; p = 0.013) was comparable in patients who received or did not receive G-CSF, and both groups did not show any difference in class switched or transitory B lymphocytes. Significant changes of B-cell subsets in the subgroups are shown in Fig. 4.
Fig. 4.
B-cell subsets before and after chemotherapy for subgroups with significant changes. Distribution of total numbers of B-cell subsets in the subgroups, analyzed by flow cytometry before and after chemotherapy. a Women, b with G-CSF administration, c platinum-containing chemotherapy, d stable and progressive disease, e no infection. Boxplots show median values as well as interquartile range. P < 0.022 = significant
T lymphocytes
Total number of CD4 + and CD8 + as well as CD4 CD8 double-positive lymphocytes and CD4/CD8 ratio did not show significant changes before and after chemotherapy (CD8 + cells: 21/µl vs. 23/µl; CD4-cells 59/µl vs. 44/µl). In addition, the different T-cell subsets did not reveal significant changes during the therapy period in the whole study population (Fig. 2).
Regarding the different T-cell subsets, there were no significant changes after the administration of chemotherapy according to sex, G-CSF administration and infections. In the platinum-containing chemotherapy group, a significant decline of terminal effector CD8 + cells (37/µl vs. 27/µl; p = 0.013) was found. Response rate was not linked to significant differences in T-cell subsets before and after therapy except for regulatory CD4 + cells (28/µl vs. 12/µl; p = 0.001) in patients with stable or progressive disease. In addition, radiation was not associated with significant changes in T-cell subsets before and after chemotherapy, except for a decrease in naïve CD4 + cells (131/µl vs. 61/µl; p = 0.017) in patients who did receive radiotherapy.
NK lymphocytes
NK cells in the whole study population showed no significant changes before and after chemotherapy (Fig. 2). Regarding subgroups, a significant decrease of CD56 + CD16 + cells was found in platinum-receiving patients (29/µl vs. 19/µl; p = 0.006).
Control group
Comparing the patient samples to a control group of 12 healthy volunteers, we did not observe significant differences in the number of total CD4 + cells (patients: 19/µl vs. control: 21/µl), total CD8 + cells (patients: 13/µl vs. control: 23/µl), and total NK cells (patients: 16/µl vs. control: 25/µl). B cells (patients: 16/µl vs. control 28/µl; p = 0.002) with most of their subsets, as well as different CD4 + lymphocyte, CD8 + lymphocyte and NK cell subsets showed significantly lower values in cancer patients (Table 2).
Discussion
In this prospective study, we observed significant changes of B-cell but not of T and NK cell count in patients with solid tumors before and after the administration of 4–6 cycles of a first-line chemotherapy. In view of the increasing importance of immune therapies following or in combination with cytostatic treatment, this is a very important finding.
T- and B-lymphocytic infiltrations of solid tumors are a common observation and may be prognostically relevant in some entities [8, 9]. The role of B cells in cancer therapy has been the focus of recent studies. By antigen presentation, B cells activate T cells [10] and the cytotoxic potential of activated B cells leads to a direct impairment of tumor development [11, 12]. For example, in breast cancer, a tumor infiltration of B lymphocytes of up to 40% was reported [13–15].
In our study, chemotherapy was associated with a significant decrease of absolute numbers of B lymphocytes in the peripheral blood. Transitional B cells, that are defined as immature cells and have left the bone marrow only a short time before [16], were the only population of B cells that did not show a significant decrease after chemotherapy. For all other B-cell subsets, a significant decline was demonstrated. This decline, however, was concordant for all subsets and did not lead to shifted percentages of the subsets in relation to the whole B cell count. Thus, an influence of chemotherapy on the total B-cell population could be seen, but there were no specific subsets that were more sensitive to chemotherapy than others. Whether the cause for the decline of B cells is a higher susceptibility for chemotherapy or a slower recovery remains unclear.
Especially, in the subgroup of patients showing no or only a low response (stable or progressive disease) to chemotherapy, the reported decline of B-cell subsets was observed, while patients with partial or complete response showed constant values of B lymphocytes. These findings are in accordance with the predescribed correlation of tumor-infiltrating B cells with relapse and survival rates in melanoma [17], cervical [18] and lung cancer [19], respectively.
Patients who did not have infections during chemotherapy period showed significantly lower counts of the different B-cell subsets after chemotherapy. It may be hypothesized that infectious conditions lead to an increased regeneration of B cells.
The significant decline of total, naïve, and memory B cells that were seen for women, but not for men is not consistent with other studies. Sex-dependent differences of lymphocytes in healthy subjects have been described for T and NK cells, but not for B cells [20]. Wichman et al. showed longer lasting changes of B, T, and NK cells in men after abdominal surgery [21].
Interestingly, patients who received platinum-containing chemotherapy regimens showed significant changes of total, naïve, memory, and class-switched B cells in contrast to patients without platinum administration, although cisplatin, carboplatin, and oxaliplatin as alkylating agents are less myelosuppressive as, for example, topoisomerase inhibitors and microtubule inhibitors [22]. However, it has to be considered that almost all patients received a combination therapy and platinum agents were often combined with strongly myelosuppressive chemotherapeutics. Another point that should be taken into account when focussing on different chemotherapeutics is the induction of immunogenic cell death (ICD) which is observed, for example, after application of drugs such as doxorubicine or paclitaxel [23]. In our study population, the chemotherapeutic drugs were very heterogeneous and the number of patients that received ICD-inducing agents was to small to perform reasonable statistical analysis.
The effect of cytotoxic chemotherapy on lymphocyte subsets has up to now mainly be analyzed in patients with hematological malignancies. Eyrich et al. showed that B cells of children who underwent chemotherapy for hematological neoplasias were more severely affected than T cells and that T cells show a more rapid regeneration [24]. For T-cell regeneration thymic function seems to play an important role, which is why T-cell regeneration is faster in children than in adults [25, 26]. Van Tilburg et al. showed that B-cell subgroups of patients with hematological neoplasias were significantly reduced after chemotherapy and recovered after a time period of 3–6 months with exception of memory B cells [7]. These data on patients with hematological malignancies undergoing chemotherapy are in most parts consistent with our findings in patients with solid malignancies. Nevertheless, it has to be taken into account that the chemotherapeutic agents used for hematological neoplasias differ from the ones used for solid malignancies, and therefore, for example, the above-mentioned impact of immunogenic cell death could make a difference. For making a statement on regeneration of B cells, T cells, and NK cells after cessation of chemotherapy, our data do not reflect a sufficient time period.
Previous studies regarding B cells in the peripheral blood of cancer patients showed inconsistent results with increased or decreased counts compared to healthy control groups [27, 28]. In our study, B cells and most subsets showed significantly lower values in patients compared to a healthy control group.
Apart from a few exceptions, significant changes in T-cell and T-cell subsets were neither seen in the whole study population nor in the specific subgroups after the administration of chemotherapy. The few significant changes found did not show a specific pattern.
T cells are affected by the presence of cancer which leads to significant drops of CD4 + cells and NK cells, but not of CD8 + cells in patients with NSCLC [29], and to significant drops of CD4 + , CD8 + and NK cells in hepatocellular carcinoma (HCC) patients [28]. In patients with colorectal cancer, Choi et al. did not find significant differences in CD4 + and CD8 + cells between patients and a healthy control group [30]. In our study population, significant differences between untreated cancer patients and the control group could be seen only for subsets of CD4 + lymphocytes, CD8 + lymphocytes, and NK cells, but not in the total amount of these lymphocytes. These results are at least in parts consistent with the described findings of other studies.
As T cells play an essential role in the function of checkpoint inhibitors, our findings match the response that is observed when checkpoint inhibitors are administered after chemotherapy. Currently, checkpoint inhibitors are for the most part approved for second- or third-line therapies for most tumor entities and are, therefore, very frequently applied after a series of chemotherapy cycles. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are inhibitory surface markers playing a pivotal role in cancer immunotherapy. Both immune checkpoints are intended to stop potential autoreactive T lymphocytes at different steps of T-cell evolution: CTLA-4 at the very beginning, shortly after naïve T cells have been stimulated in lymph nodes, and PD-1 at a later evolutional state of effector T cells in peripheral tissues [31]. In cancer patients, these inhibitory pathways are stimulated, and therefore, no antitumor immune response is elicited. The potential that there is an attenuated antitumor effect by immune checkpoint inhibitors administered after chemotherapy due to potentially decreased lymphocyte subsets could be excluded by our data. Regarding the stable counts of T cells after chemotherapy, a combination of checkpoint inhibitors and classical chemotherapy, as currently approved, for example, for the treatment of non-small cell lung cancer, is a therapeutic option, whose biological rationale may be supported by our data.
B cells also express inhibitory surface markers such as PD-1 and CTLA-4 [12, 32]. How much this expression influences an antitumor immune response and subsequently plays a role in checkpoint inhibition therapy is not well understood. In case of a PD-1/CTLA-4-dependent tumor control of B cells, the administration of chemotherapy with the subsequent decrease of different B-cell subsets might play a role for the efficacy of parallel or consecutively administered checkpoint inhibitors. Certainly, the impact of T cells on tumor control seems to be of more relevance with regard to checkpoint inhibition therapy than the impact of B cells.
In summary, we showed that chemotherapy has an effect on B cells in cancer patients almost irrespective of subtype, while T cells, NK cells, and their subsets seem to be not quantitatively affected. As immune therapies mainly based on T-cell activation [33], these findings could support the idea of effective combinations of chemotherapy and immune therapies such as checkpoint inhibitors in simultaneous or sequential protocols, although to prove this hypothesis and to rule out an impact of hidden variables, studies on bigger numbers of patients would be necessary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Gernot Müller, Professor for computational statistics and data analysis, University of Augsburg, for his statistical support, Prof. Bruno Märkl, director of the Institute of Pathology and Molecular Diagnostics, University Medical Center Augsburg, for his help with data interpretation, and Mrs. Tatajana Lensjaka on behalf of all laboratory staff for the technical support in our laboratory.
Abbreviations
- CD
Cluster of differentiation
- CR
Complete remission
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- ECD
Phycoerythrin texas red-X
- EDTA
Ethylenediaminetetraacetic acid
- FITC
Fluoreszeinisothiocyanat
- FOLFIRI
Folinic acid, fluorouracil, irinotecan
- FOLFIRINOX
Folinic acid, fluorouracil, irinotecan, oxaliplatin
- G-CSF
Granulocyte-colony stimulating factor
- ICD
Immunogenic cell death
- NSCLC
Non-small cell lung cancer
- PC
Phycoerythrin–cyanin
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PE
Phycoerythrin
- PR
Partial remission
- SCLC
Small cell lung cancer
- SD
Stable disease
Author contributions
JW: study design, data acquisition and interpretation, and manuscript drafting. AS: data acquisition and interpretation. MT: manuscript drafting. A-KS: manuscript drafting. AR: study design, data acquisition, and interpretation, manuscript drafting
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The study was approved by the local medical ethical committee (institutional review board, reference number BKF 2016/09) and written informed consent was obtained. All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Patients and healthy blood donors consented to the use of their blood samples and data for research and publications.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68(13):5405–5413. doi: 10.1158/0008-5472.Can-07-5206. [DOI] [PubMed] [Google Scholar]
- 4.Streitz M, Miloud T, Kapinsky M, Reed MR, Magari R, Geissler EK, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transpl Res. 2013;2(1):17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol Orlando Fla). 2009;133(1):95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Finak G, Langweiler M, Jaimes M, Malek M, Taghiyar J, Korin Y, et al. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci Rep. 2016;6:20686. doi: 10.1038/srep20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Tilburg CM, van Gent R, Bierings MB, Otto SA, Sanders EA, Nibbelke EE, et al. Immune reconstitution in children following chemotherapy for haematological malignancies: a long-term follow-up. Br J Haematol. 2011;152(2):201–210. doi: 10.1111/j.1365-2141.2010.08478.x. [DOI] [PubMed] [Google Scholar]
- 8.Flammiger A, Bayer F, Cirugeda-Kuhnert A, Huland H, Tennstedt P, Simon R, et al. Intratumoral T but not B lymphocytes are related to clinical outcome in prostate cancer. APMIS. 2012;120(11):901–908. doi: 10.1111/j.1600-0463.2012.02924.x. [DOI] [PubMed] [Google Scholar]
- 9.Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4 + and CD8 + T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol (Baltimore, Md: 1950) 2010;184(7):4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol (Baltimore, Md: 1950). 2009;183(5):3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–1466. [PubMed] [Google Scholar]
- 14.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52(12):715–738. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139(1):33–41. doi: 10.1016/S0304-3835(98)00379-6. [DOI] [PubMed] [Google Scholar]
- 16.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105(11):4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–1080. doi: 10.1158/0008-5472.Can-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedergaard BS, Ladekarl M, Nyengaard JR, Nielsen K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol. 2008;108(1):106–111. doi: 10.1016/j.ygyno.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–5227. doi: 10.1158/1078-0432.Ccr-08-0133. [DOI] [PubMed] [Google Scholar]
- 20.Rovati B, Mariucci S, Poma R, Tinelli C, Delfanti S, Pedrazzoli P. An eight-colour flow cytometric method for the detection of reference values of lymphocyte subsets in selected healthy donors. Clin Exp Med. 2014;14(3):249–259. doi: 10.1007/s10238-013-0239-4. [DOI] [PubMed] [Google Scholar]
- 21.Wichmann MW, Muller C, Meyer G, Adam M, Angele MK, Eisenmenger SJ, et al. Different immune responses to abdominal surgery in men and women. Langenbeck’s Arch Surg. 2003;387(11–12):397–401. doi: 10.1007/s00423-002-0346-2. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Lam MS, Birkeland A, Riffel A, Montana L, Sullivan ME, et al. Cell-based assays for profiling activity and safety properties of cancer drugs. J Pharmacol Toxicol Methods. 2006;54(3):313–319. doi: 10.1016/j.vascn.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Diederich M. Natural compound inducers of immunogenic cell death. Arch Pharmacal Res. 2019 doi: 10.1007/s12272-019-01150-z. [DOI] [PubMed] [Google Scholar]
- 24.Eyrich M, Wiegering V, Lim A, Schrauder A, Winkler B, Schlegel PG. Immune function in children under chemotherapy for standard risk acute lymphoblastic leukaemia—a prospective study of 20 paediatric patients. Br J Haematol. 2009;147(3):360–370. doi: 10.1111/j.1365-2141.2009.07862.x. [DOI] [PubMed] [Google Scholar]
- 25.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Age, thymopoiesis, and CD4 + T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332(3):143–149. doi: 10.1056/nejm199501193320303. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg K, Annett G, Kashyap A, Lenarsky C, Forman SJ, Parkman R. The effect of thymic function on immunocompetence following bone marrow transplantation. Biol Blood Marrow Transpl. 1995;1(1):18–23. [PubMed] [Google Scholar]
- 27.Lin JC, Shih YL, Chien PJ, Liu CL, Lee JJ, Liu TP, et al. Increased percentage of B cells in patients with more advanced hepatocellular carcinoma. Hum Immunol. 2010;71(1):58–62. doi: 10.1016/j.humimm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Spacek J, Vocka M, Netikova I, Skalova H, Dundr P, Konopasek B, et al. Immunological examination of peripheral blood in patients with colorectal cancer compared to healthy controls. Immunol Invest. 2018;47(7):643–653. doi: 10.1080/08820139.2018.1480030. [DOI] [PubMed] [Google Scholar]
- 29.Wang WJ, Tao Z, Gu W, Sun LH. Variation of blood T lymphocyte subgroups in patients with non- small cell lung cancer. Asian Pacific J Cancer Prevention. 2013;14(8):4671–4673. doi: 10.7314/APJCP.2013.14.8.4671. [DOI] [PubMed] [Google Scholar]
- 30.Choi J, Maeng HG, Lee SJ, Kim YJ, Kim DW, Lee HN, et al. Diagnostic value of peripheral blood immune profiling in colorectal cancer. Ann Surg Treat Res. 2018;94(6):312–321. doi: 10.4174/astr.2018.94.6.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi: 10.1097/coc.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grace JY (2016) B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 747–757 [DOI] [PMC free article] [PubMed]
- 33.Fessas P, Lee H, Ikemizu S, Janowitz T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44(2):136–140. doi: 10.1053/j.seminoncol.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaften für Hämatologie und Medizinische Onkologie, October 11th–14th 2019, Berlin, Germany. Oncol Res Treat 42(suppl 4):XIV + 346 (2019) (Abstract) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.