Human genetic phenotypes associated with loss or gain of function implicate the Nav1.7 channel as a promising target for novel analgesics.1,2 However, the expression of NaV1.7 on autonomic afferent c-type fibers, on sympathetic efferent fibers, and cardiovascular–related autonomic adverse effects reported in gain-of-function mutation phenotypes2,3 create concern for potential Nav1.7 antagonists. Recent clinical assessment of a Nav1.7 antagonist suggested that cardiovascular adverse events could be related to on-target activity.4 The clinical pharmacodynamic relationship between efficacy and cardiovascular adverse events or the predictive value of nonclinical models has not been fully explored. As such, the effects of MK-2075,5 a small-molecule selective Nav1.7 inhibitor (human and rhesus half-maximal inhibitory concentration [IC50]=85 and 161 nmol/L, respectively), were assessed in rhesus monkey (non-human primate [NHP]) and in phase I clinical studies to understand the pharmacodynamics and cardiovascular safety of Nav1.7 blockade.
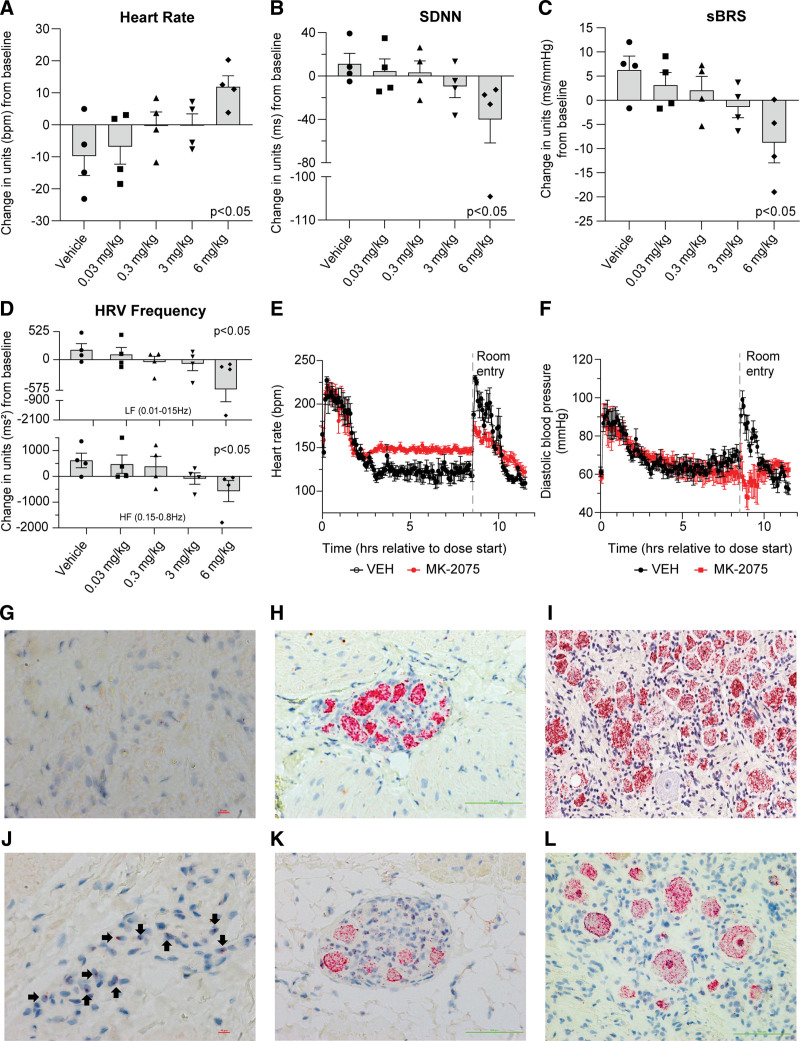
MK-2075 was highly selective against peripheral Nav channels (Nav1.4 and Nav1.6 IC50 >300 µmol/L), cardiac ion channels (hERG, IKs, and Nav1.5 IC50 >30 µmol/L), a broad panel of 114 potential off-targets (IC50 >10 µmol/L). Moreover, NHP whole-body autoradiography demonstrated no brain penetrance. The Figure shows the concentration-dependent decrease in heart rate variability (HRV, Figure B) and spontaneous baroreceptor sensitivity (Figure C) with physiologically meaningful trends occurring at unbound plasma concentrations as low as 0.18 µmol/L (0.3 mg/kg) and statistically significant decreases at 2.3 µmol/L (6 mg/kg). Frequency domain HRV analysis (Figure D) showed concomitant decreases in both high- and low-frequency domains, suggestive of an MK-2075–dependent effect on sympathetic and parasympathetic tone. Supratherapeutic exposures of MK-2075 (50 mg/kg; maximum plasma concentration after rest article administration=169 µmol/L or 16 µmol/L unbound; 100-fold over the rhesus in vitro Nav1.7 IC50) demonstrated complete loss of all HRV indices, paradoxical decreases in heart rate and blood pressure on animal handling (Figure E and F), and one animal exhibited a brief loss of consciousness on postural change; all are indicative of compromised cardiovascular reflexes. Consistent with these being on-target effects, a Nav1.7 inhibitory peptide analogue of JzTx-V with greater NaV1.7 specificity elicited similar effects on HRV in NHPs. In situ hybridization (RNAscope, Figure G–L) on NHP or commercially sourced human tissue demonstrated Nav1.7 colocalized with markers for cardiac and stellate ganglia but not sinoatrial node (NHP) and cardiac/sympathetic ganglia and sinoatrial node/right atrium (human). Clinical testing of MK-2075 was conducted on healthy adult male participants in 2 protocol number (PN) studies: PN001 where participants were given up to 30 mg IV over 8 hours and PN005 where participants were given up to 8 mg IV over 2 hours. Overall, adverse events were generally mild, with the most frequently reported being paresthesia, and there was a low incidence of cardiovascular-related observations. One PN001 participant experienced orthostatic hypotension 5 hours into the 30 mg/8 h IV infusion. Maximum plasma concentration after rest article administration exposures (0.36 µmol/L unbound) was ≈4-fold the in vitro NaV1.7 IC50 at that time. The orthostatic hypotension resolved after the completion of drug administration. Five other reports of self-limited, presyncope symptoms at lower doses were reported but were often associated with study procedures and without concomitant changes in vital signs. One PN005 participant lost consciousness during protocol-driven orthostatic hypotension testing 1 hour after the start of the 8 mg/2 h IV infusion (≈126 nmol/L unbound). The participant spontaneously regained consciousness after ≈20 seconds. Review of the temporal telemetry ECG showed a period of severe bradycardia with two episodes of sinus arrest of ≈4 to 5 seconds each, followed by a brief junctional escape rhythm and then return to normal sinus rhythm. The MK-2075 infusion was stopped immediately, and the participant remained asymptomatic thereafter. One additional participant receiving 8 mg MK-2075 exhibited a ≈70% reduction in spontaneous baroreceptor sensitivity slope at the 2-hour measurement point that was unaccompanied by clinical signs and resolved 2 hours later. In PN005, there were no mean changes in HRV or spontaneous baroreceptor sensitivity nor were there any effects on afferent sensory function to cold (cold water bath)- or heat (Peltior thermode)-induced painful stimuli, or olfactory sensory function assessed by Sniffin’ Sticks.
Figure.
Effects of MK-2075 on cardiac autonomic nervous system balance and reflex control through the assessment of HRV and sBRS in telemetered rhesus monkey at 2 to 6 hours postdose (A–D). Adult male rhesus monkeys (n=4) were given increasing subcutaneous doses of MK-2075 and continuous ECG was evaluated for changes in heart rate (HR, A), the time domain HRV parameter, standard deviation of N-N intervals (SDNN, B), baroreceptor effectiveness index (sBRS, C), and both high-frequency (HF) and low-frequency (LF) domain heart rate variability (HRV, D). To further clarify persistent effects during the diurnal phase, continuous data extracted as 15-minute means were aggregated into a 4-hour time period (2–6 hours postdose). A dose-dependent trend in SDNN, sBRS, and HRV decreases can be observed with a statistically significant (P<0.05) difference in all parameters at the 6 mg/kg SC dose. Statistical significance from vehicle was determined for change from baseline data using a linear mixed-effects model (Y=Group×Time+ID+error) where Group×Time capture the fixed group and time effects and their interactions; ID characterizes the between-subject random effects. E and F, Effect of supratherapeutic doses of MK-2075 on heart rate and diastolic blood pressure. Adult male rhesus monkeys (n=5) were given a continuous 8-hour intravenous infusion through jacketed infusion pumps to determine the effect of MK-2075 on HR (E) and diastolic blood pressure (F) parameters at supratherapeutic exposures (50 mg/kg over 8 hours). Increased HR and no change in diastolic pressure were observed during the infusion. Note that postinfusion handling procedures (noted as “room entry” on the graphs) caused an expected increase in HR and diastolic blood pressure in vehicle-treated animals (VEH), but the HR increase was attenuated and the diastolic blood pressure paradoxically decreased after administration of MK-2075 suggestive of compromised cardiovascular autonomic regulation. G through L, In situ hybridization Nav1.7 mRNA expression in rhesus and human tissues. G, H, and I represent Nav1.7 staining in formalin-fixed paraffin-embedded rhesus sinoatrial node, cardiac ganglia, and dorsal root ganglia, and J, K, and L represent Nav1.7 staining in human sinoatrial node, cardiac ganglia, and dorsal root ganglia, respectively. Nav1.7 is not detected in rhesus sinoatrial node, but mildly expressed in human sinoatrial node (40×). It highly expressed in cardiac ganglia and dorsal root ganglia of both rhesus and human subjects (20×).
This study reports for the first time that acute Nav1.7 inhibition could lead to autonomic effects in NHP and human subjects, and that these may be observed with no therapeutic margin to analgesic pharmacology on the basis of quantitative sensory testing. Dose-dependent decreases in HRV and spontaneous baroreceptor sensitivity occurred in NHPs, and clinical events of syncope were observed that were suggestive of Nav1.7-dependent cardiac autonomic dysfunction.
It is tempting to speculate that these results and those of others,4 which contrast the lack of effects in NaV1.7 null individuals, serve as a reminder of the potential phenotypic differences between acute pharmacological block versus genetic deficiency and the important role developmental compensation may play in the latter.
Animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (2011) and received facility approval. Clinical studies were approved by an institutional ethics review committee and subjects gave informed consent. Nonclinical data may be available on reasonable request and completion of required agreements.
ARTICLE INFORMATION
Acknowledgments
The authors thank A. Houghton, D. Henze, R. Briscoe, and C. Burgey for their helpful scientific dialog and perspective, H. Regan, T. Detwiler, S. Gruver, B. Rockafellow, and M. Syrylo for the conduct of the telemetered non-human primate experiments, P. Fanelli for help with data analysis, S. Wang for the conduct of the statistical analysis, and D. Gilberto and A. Bone for veterinarian surgical support and care.
Author contributions:
Conceptualization, Drs Regan, Morissette, and A. Struyk; Investigation, Drs Regan and Kraus, M. Vavrek, Drs Wang and A. Struyk; Supervision, Dr A. Stoch; Writing – original draft, Drs Regan, Morissette, and A. Struyk; Writing – review and editing, Drs Regan, Morissette, and Kraus, M. Vavrek, Drs Wang, de Hoon, Depre, T. Lodeweyck, Demeyer, Laethem, A. Stoch, and A. Struyk.
Sources of Funding
None.
Disclosures
Drs Regan, Morissette, and Kraus, M. Vavrek, and Drs Wang, T. Laethem, A. Stoch, and A. Struyk are employed by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ. L. Arrington is employed by Amgen Inc. but was employed by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, at the time of the study. Drs de Hoon, Dupre, and T. Lodeweyck are employed by the University of Leuven, Belgium. Dr Demeyer is employed by the Military Hospital Queen Astrid, Belgium.
Nonstandard Abbreviations and Acronyms
- hERG
- human ether-a-go-go ion channel
- HRV
- heart rate variability
- IC50
- half-maximal inhibitory concentration
- NHP
- non-human primate
C.P. Regan and P. Morisette contributed equally.
For Sources of Funding and Disclosures, see page 1396.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Pierre Morissette, Email: pierre_morissette@merck.com.
Richard L. Kraus, Email: richard_kraus@merck.com.
Erjia Wang, Email: erjia.wang@merck.com.
Leticia Arrington, Email: leticia.arrington@gmail.com.
Marissa Vavrek, Email: marissa_vavrek@merck.com.
Jan de Hoon, Email: jan.dehoon@uzleuven.be.
Marleen Depre, Email: marleen.depre@uzleuven.be.
Tine Laethem, Email: tine_laethem@merck.com.
REFERENCES
- 1.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26–39. doi: 10.1002/ana.22485 [DOI] [PubMed] [Google Scholar]
- 3.Minett MS, Nassar MA, Clark AK, Passmore G, Dickenson AH, Wang F, Malcangio M, Wood JN. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun. 2012;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothenberg ME, Tagen M, Chang JH, Boyce-Rustay J, Friesenhahn M, Hackos DH, Hains A, Sutherlin D, Ward M, Cho W. Safety, tolerability, and pharmacokinetics of GDC-0276, a novel NaV1.7 inhibitor, in a first-in-human, single- and multiple-dose study in healthy volunteers. Clin Drug Investig. 2019;39:873–887. doi: 10.1007/s40261-019-00807-3 [DOI] [PubMed] [Google Scholar]
- 5.Ballard JE, Pall PS, Vardigan J, Zhao F, Holahan MA, Zhou X, Jochnowitz N, Kraus RL, Klein RM, Henze DA, et al. Translational pharmacokinetic-pharmacodynamic modeling of NaV1.7 inhibitor MK-2075 to inform human efficacious dose. Front Pharmacol. 2021;12:786078. doi: 10.3389/fphar.2021.786078 [DOI] [PMC free article] [PubMed] [Google Scholar]