Abstract
Background
Inhibition of the mitogen-activated protein kinase (MAPK) pathway as well as programmed death 1 receptor (PD-1) blockade was shown to prolong overall survival (OS) in patients with advanced B-Raf proto-oncogene (BRAF)-mutant melanoma. However, due to the lack of head-to-head trials, it remains unclear if one of these therapeutic approaches should be preferred in first-line therapy. Here, we present a retrospective analysis comparing anti-PD-1 monotherapy with BRAF/MAPK/ERK kinase (MEK) combined inhibition used as first-line agents in a real-world clinical setting.
Patients and methods
Clinical data, routine blood counts and lactate dehydrogenase (LDH) levels of 301 patients with unresectable or metastatic melanoma harboring an activating mutation in BRAF (V600E/K) were included. Of these, 106 received anti-PD-1 antibodies, while 195 patients were treated with a selective BRAF inhibitor combined with an MEK inhibitor as palliative first-line therapy. Patients were sub-grouped according to previously described predictive and prognostic markers.
Results
OS was significantly longer in patients receiving anti-PD-1 monotherapy compared to patients receiving combined MAPK inhibitors. Subsequent therapies were comparable among these groups. The difference in OS was less pronounced in patients with high LDH levels and visceral metastatic spread.
Conclusion
First-line treatment with a PD-1 blocking antibody might be associated with longer OS than first-line inhibition of the MAPK pathway in patients with advanced melanoma harboring mutant BRAF. These hypothesis-generating data need to be confirmed or rejected in prospective, randomized trials.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02311-1) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, BRAF, First-line treatment, PD-1, MAPK
Introduction
Approximately, 50% of all melanomas harbor an activating mutation in B-Raf proto-oncogene (BRAF) affecting the codon 600, with consequent activation of the mitogen-activated protein kinase (MAPK) pathway [1]. In patients with advanced BRAFV600E/K-mutated melanomas, inhibition of the MAPK pathway has shown a high response rate and prolongation of overall survival (OS) [2]. Therapeutically, combinations of selective BRAF inhibitors (BRAFi) (vemurafenib or dabrafenib) and MAPK/ERK kinase (MEK) inhibitors (MEKi) (cobimetinib or trametinib) have become a standard of care in these patients [3, 4]. Programmed death 1 receptor (PD-1) blockade with monoclonal antibodies (nivolumab, pembrolizumab) has demonstrated an objective response rate (ORR) of ~ 40% and to prolong survival in patients with advanced melanoma [5, 6]. Here, the efficacy of this form of therapy was shown both in patients with mutated BRAF and in patients without such an activating mutation [7]. Thus, patients with advanced melanoma harboring mutated BRAFV600E/K can benefit from MAPK inhibitors (MAPKi) as well as PD-1 blockade. However, it remains unclear which, if any, modality should be used as preferred first-line therapy to yield maximum patient benefit. No prospective head-to-head data are currently available to address this everyday challenge in the treatment of patients with BRAF-mutated advanced melanoma.
Exploratory analyses of prospective clinical trials as well as retrospective biomarker studies have identified several markers associated with benefit from MAPKi or PD-1 blockade. For instance, elevated lactate dehydrogenase (LDH) is associated with worse outcome both in the context of MAPKi and PD-1 blockade [8, 9]. For programmed death ligand 1 (PD-L1) expression, a positive correlation with the ORR of PD-1 blockade and OS has been observed [5, 10, 11]. Patients considered to be PD-L1 negative, however, still show clinical responses. For patients receiving MAPKi, controversial data on the predictive value of PD-L1 expression exist [12–14]. To date, it remains unclear if any biomarker exists that is predictive for either therapeutic approach that could help to guide the treatment of BRAFV600-mutant melanoma patients regarding first-line therapy and prospective, clinical trials are still underway.
To optimize patient benefit, data that may support the use of either PD-1 blockade or MAPKi as first-line therapy in patients with advanced BRAF-mutated melanoma, are urgently needed. With data from prospective clinical trials pending, we conducted a multicenter retrospective analysis to investigate the outcome of patients undergoing either measure as first-line therapy.
Materials and methods
Patient cohort
Patients with advanced (unresectable or metastatic) melanoma harboring mutant BRAF (V600E/K) were included in this retrospective analysis. Participating clinical sites provided data of patients who received either dual MAPKi (vemurafenib plus cobimetinib or dabrafenib plus trametinib) or single-agent PD-1 blocking antibody (nivolumab or pembrolizumab) as first-line therapy for advanced disease. No further restrictions applied regarding patient selection. The American Joint Committee on Cancer (AJCC) 2009 classification was used to categorize patients [15]. Demographic, as well as clinical data were collected for all identified patients from patient records. Data were obtained as documented at the start of first-line therapy. Laboratory values were obtained within 4 weeks before start of systemic therapy. Elevated LDH was defined as any value above the local upper limit of normal (ULN).
Definition of end points and data acquisition
OS was calculated from the start of first-line therapy. Patients who were still living were censored at the last documented follow-up. Patients who died from reasons other than progressive melanoma were censored at the date of death. Median follow-up was calculated by inverted censoring [16]. Patients were categorized according to the pattern of metastasis into three different groups as reported previously [9]: (i) unresectable or distant soft tissue and/or lymph node metastases only, (ii) pulmonary metastases and (iii) visceral metastases other than or in addition to lung.
Statistical analysis
Statistical analyses were performed by applying the Chi square test or Wilcoxon rank sum test using SPSS (IBM, version 23). Survival analysis was conducted using the Kaplan–Meier method; the log-rank test was used for curve comparison. The Cox proportional-hazards model was used for multivariate analyses. Differences were considered statistically significant for p values < 0.05.
Results
Study population
Clinicopathological characteristics of patients included in this study are shown in Table 1. All patients showed an activating somatic mutation in BRAF (BRAFV600E/K). In the PD-1 group, 65 of 106 (61.9%) received pembrolizumab, while the remaining 41 patients (38.1%) were treated with nivolumab. In patients receiving dual MAPKi as first-line therapy, dabrafenib plus trametinib was employed in 80.5% of patients, while 19.5% received vemurafenib combined with cobimetinib. Distribution of sex was similar between the PD-1 and MAPKi groups. In contrast, patients with visceral metastases and elevated LDH were significantly more common in the MAPKi group. Importantly, a similar proportion of patients in either cohort received the alternate treatment modality as subsequent therapy (Table 1). In the MAPKi group, 33.3% of patients received PD-1 blockade subsequently, while 34.9% patients in the PD-1 group received dual MAPKi therapy at a later stage.
Table 1.
Clinicopathological characteristics of patients with metastatic malignant melanoma included in this study
| All | PD-1 | MAPKi | p | |
|---|---|---|---|---|
| Individual patients | n = 301 (%) | n = 106 (%) | n = 195 (%) | |
| First-line therapy received | ||||
| Pembrolizumab | 65 (21.6) | 65 (61.9) | n.a | |
| Nivolumab | 41 (13.6) | 41 (38.1) | ||
| Dabrafenib/trametinib | 157 (52.2) | 157 (80.5) | ||
| Vemurafenib/cobimetinib | 38 (12.6) | 38 (19.5) | ||
| Subsequent therapy with | ||||
| Anti-PD-1 monotherapy | 60 (19.9) | 1 (0.9) | 59 (30.3) | |
| Dual MAPKi | 49 (16.3) | 37 (34.9) | 12 (6.2) | |
| Anti-CTLA-4 monotherapy | 28 (9.3) | 5 (4.7) | 23 (11.8) | < 0.0001# |
| Dual anti CTLA-4/PD-1 | 29 (9.6) | 5 (4.7) | 24 (12.3) | |
| Unknowna | 20 (6.6) | 2 (1.9) | 18 (9.2) | |
| Other/none | 115 (38.2) | 56 (52.8) | 59 (30.3) | |
| BRAF-status | ||||
| Mutant (V600E/K) | 301 (100) | 106 (100) | 195 (100) | n.a |
| Sex | ||||
| Male | 184 (61.1) | 67 (63.2) | 117 (60.0) | 0.59# |
| Female | 117 (38.9) | 39 (36.8) | 78 (40.0) | |
| Age (years) | ||||
| (Median, [range]) | 58 [18–88] | 60 [21–86] | 58 [18–88] | 0.008 ## |
| Elevated LDH | ||||
| Yes | 104 (34.6) | 20 (18.9) | 84 (43.1) | < 0.0001# |
| No | 197 (65.4) | 86 (81.1) | 111 (56.9) | |
| Metastatic pattern | ||||
| Lymph node or soft tissue only | 51 (16.9) | 22 (20.8) | 29 (14.9) | 0.0031 # |
| Pulmonary | 35 (11.6) | 20 (18.9) | 15 (7.7) | |
| Other visceral | 215 (71.4) | 64 (60.4) | 151 (77.4) | |
Bold values indicate p-values lower than 0.05
n.a. not applicable, #by Χ2 test, ##by Wilcoxon rank sum test.
aUnknown if subsequent PD-1 blockade or MAPKi was received
Overall survival according to first-line treatment
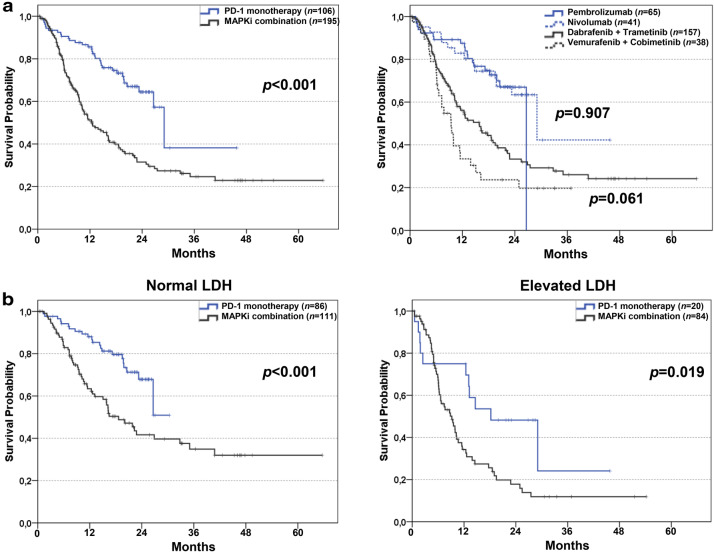
As shown in Fig. 1a, left panel, Kaplan–Meier estimates of OS were compared between patients in the PD-1 group (n = 106) and in the MAPKi group (n = 195). Median OS was 29.1 months [95% confidence interval (CI) 24.9–33.2] in the PD-1 group and 12.4 months (95% CI 8.6–14.4) in the MAPKi group (p < 0.001). Median follow-up for survival was 22.3 (95% CI 20.7–23.8) for the PD-1 group and 25.8 months (95% CI 16.4–35.1) for the MAPKi group. Figure 1a, right panel, shows OS after subgrouping according to the specific treatment patients received. No differences were found within the PD-1 (nivolumab vs. pembrolizumab) and MAPKi (dabrafenib plus trametinib vs. vemurafenib plus cobimetinib) treatment groups.
Fig. 1.
Overall survival of BRAF-mutated melanoma patients stratified by first-line therapy. a Kaplan–Meier curves for OS of BRAF mutated patients receiving any PD-1 blocking antibody as monotherapy (n = 106; 35.2%) or any dual MAPKi (n = 195; 64.8; a left). Overall survival of BRAF mutated patients receiving pembrolizumab (n = 65; 21.6%) or nivolumab (n = 41; 13.6%) or vemurafenib plus cobimetinib (n = 38; 12.6%) or dabrafenib plus trametinib (n = 157; 52.2%) as first-line therapy (a right). b OS is shown for patients with normal LDH levels at the start of any PD-1 blocking antibody monotherapy (n = 86; 43.7%) or any dual MAPKi (n = 111; 56.3%; b left). OS is shown for patients with elevated LDH levels at the start of any PD-1 blocking antibody monotherapy (n = 20; 19.2%) or any dual MAPKi (n = 84; 80.8%; b right). Vertical lines indicate censored values; p values were calculated by log rank testing
Demographic and clinical subgroup analyses of overall survival
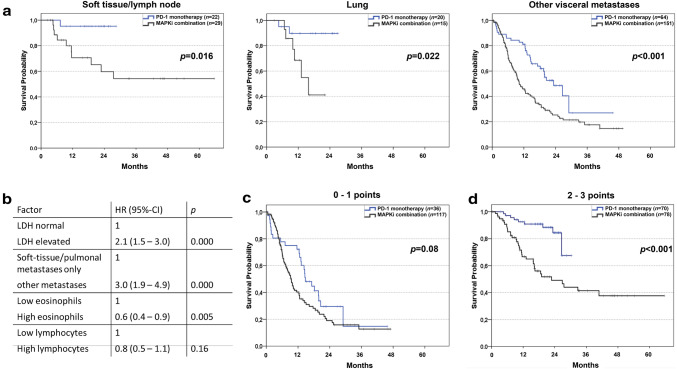
Differences in patients’ characteristics between treatment groups are shown in Table 1. Sex and age distribution were similar for both groups. For patients with normal LDH, regardless of first-line therapy, median OS was 26.9 months, while patients with elevated LDH showed a median OS of 9.9 months (data not shown). Figure 1b, left panel, shows OS in patients with normal LDH after stratification according to therapy. When LDH levels were normal, median OS was significantly shorter for MAPKi-treated patients than for patients receiving PD-1 blockade (18.6 vs. 26.6 months, p < 0.001). In patients with elevated LDH, a similar observation was made (Fig. 1b, right panel). Median OS was 9.2 months in patients with elevated LDH receiving MAPKi as compared to 18.2 months in patients receiving PD-1 blockade (p = 0.019). As depicted in Fig. 2a, OS was significantly longer in anti-PD-1-treated than in MAPKi-treated patients, regardless of the metastatic pattern.
Fig. 2.
Subgroup analyses of overall survival based on metastatic pattern or age groups. a Kaplan–Meier curves for OS are shown for BRAF mutated melanoma patients treated with PD-1 blocking antibodies (n = 22; 43.1%) or dual MAPKi (n = 29; 56.9%) with metastases located in lymph nodes/soft tissue (a left), patients treated with PD-1 blocking antibodies (n = 20; 57.1%) or dual MAPKi (n = 15; 42.9%) with metastases limited to lung (a middle) or other visceral organs (a right) treated with PD-1 blocking antibodies (n = 64; 29.8%) or dual MAPKi (n = 151; 70.2%). b Table showing hazard ratios (HR) for death after stratification of all 301 patients according to four biomarkers. Hazard ratios, 95% CI and p values were calculated by multivariate Cox proportional hazards model. c Factors identified as independent by multivariate Cox proportional hazards model (LDH levels, metastatic pattern, eosinophil levels) were used to develop a point model where patients are stratified according to the total number of favorable factors (normal LDH, no visceral metastases other than lung, eosinophils count > 1.5%). Kaplan–Meier curves for OS are shown for BRAF mutated melanoma patients treated with PD-1 blocking antibodies (n = 36; 23.5%) or dual MAPKi (n = 117; 76.5%) with 0 or 1 points. d Kaplan–Meier curves for OS are shown for BRAF mutated melanoma patients treated with PD-1 blocking antibodies (n = 70; 47.3%) or dual MAPKi (n = 78; 52.7%) with two or three points. Vertical lines indicate censored values; p values were calculated by log rank testing (a, c, d)
Biomarker-based subgroup analyses of overall survival
We and others have previously reported biomarkers predicting outcome in patients undergoing PD-1 blockade or MAPKi [8, 9]. By analyzing several biomarkers, including peripheral eosinophils (Supplementary Fig. 1a) and lymphocytes (Supplementary Fig. 1b), we could not identify a biomarker-defined patient subgroup in which MAPKi was associated with improved survival compared to PD-1 blockade. In multivariate analysis of the overall cohort without stratification by first-line therapy, LDH, metastatic pattern and frequency of peripheral eosinophils were found to be independently associated with patient prognosis (Fig. 2b). Next, we tested the association of OS and models incorporating three independent prognostic factors (normal LDH, no visceral metastasis other than lung, eosinophils count > 1.5%). As depicted in Fig. 2d, patients with two to three favorable markers showed superior OS when receiving PD-1 blockade as first-line therapy as compared to MAPKi, while OS was similar in patients with zero to one favorable markers after stratification by first-line therapy (Fig. 2c).
Discussion
In this study, we observed an association between first-line therapy with PD-1 blocking agents with OS in advanced BRAFV600E/K-mutant melanoma. This association was found in comparison to MAPKi-treated patients and was evident in several analyzed subgroups. In patients with poor prognostic features, first-line PD-1 blockade and combined MAPKi were associated with similar OS. Importantly, the proportion of patients subsequently receiving the alternate therapeutic regime was comparable between both groups. Although this is a retrospective study, it provides hypothesis-generating data on treatment sequence in advanced BRAFV600-mutant melanoma.
So far, only a single study addressing a similar question has been published [17]. In contrast to our study, Johnson et al. found no association with OS when comparing patients who received PD-1 blockade prior to or after MAPKi. However, their cohort contained patients receiving BRAFi monotherapy as well as combined MAPKi and included ~ 50% of patients who were not treatment naïve. Furthermore, patients were included who had received combined ipilimumab and nivolumab as well as atezolizumab, an anti-PD-L1 antibody with limited data in patients with metastatic melanoma. In contrast, we have gathered data from a more homogenous group treated with approved drugs as first-line therapy. Since ipilimumab combined with nivolumab has been approved most recently, we did not include such patients due to limited follow-up time. Comparing landmark OS rates in our MAPKi group to data from prospective clinical trials [18], survival rates were lower in our study. This difference might be explained by the enrollment criteria needed to be fulfilled in these trials and differences regarding subgrouping patients according to LDH and metastatic spread for OS analyses. Due to these differences, a direct comparison between OS data is not possible and warrants further investigations.
Since there is no evidence to support a specific treatment sequence for BRAF-positive patients [19], the European Society of Medical Oncology (ESMO) [20] and National Comprehensive Cancer Network (NCCN) melanoma guidelines (Version 2.2018) give no recommendation about the sequencing of systemic treatments. Recommendations for first-line therapy in BRAFV600-mutant metastatic melanoma are currently extrapolated from prospective clinical trials and based on patient-centered factors. In addition to patient preference, expected treatment-related adverse events (AE), disease-related symptoms and the extent of metastatic spread were suggested to guide clinicians when recommending PD-1 blockade or MAPKi to patients [21, 22]. In our cohort, these considerations are reflected by a high representation of patients with elevated LDH and visceral metastases among those who received MAPKi as first-line therapy. Even though we could not observe superior OS in this subgroup, the higher response rate as compared to PD-1 blockade needs to be considered in treatment recommendations. In patients with symptomatic metastases, MAPKi can quickly induce remission and, for instance, provide pain relief [23]. Besides these clinical features, tumor biology could impact treatment outcome of MAPKi and PD-1 blockade. For instance, a patient with a PD-L1-positive, BRAFV600-mutated melanoma harboring an additional Rac family small GTPase 1 (RAC1)P29S mutation is prone to receive immunotherapy and not targeted therapy first line [4, 24]. Thus, first-line therapy in BRAFV600-mutant melanoma needs to be discussed in a patient-by-patient manner integrating clinical and molecular data until prospective clinical trials like National Clinical Trial (NCT) 02224781 or NCT 02631447 can provide data confirming or dismissing any possible advantage for patients undergoing a specific treatment schedule.
As of today, single agent PD-1 blockade remains the standard of care for patients with advanced melanoma. While adding the cytolytic T lymphocyte-associated antigen-4 (CTLA-4) blocking agent ipilimumab to nivolumab yields a numerically higher response rate and better OS in the prospective CheckMate 067, the trial was not designed to show statistical superiority of the combination [4]. However, ongoing trials employing new combinations aim at replacing PD-1 monotherapy. For instance, NCT 03470922 is designed to show improved progression-free survival (PFS) of the lymphocyte activation gene 3 (LAG3) blocker relatlimab, combined with nivolumab compared to nivolumab. Although early data on relatlimab are promising [25], the recent failure of combined indoleamine 2,3-dioxygenase (IDO) inhibition and pembrolizumab [26] indicates that PD-1 monotherapy has set the bar high for new combinations. Until new data and drugs become available, PD-1 monotherapy will continue to play a major role in melanoma therapy.
The retrospective nature of our study implies several limitations. Mainly, patients with poor prognostic features (elevated LDH, visceral metastases) are significantly enriched in the MAPKi cohort. Confounding can be avoided by stratification in prospective clinical trials like NCT 02224781, comparing dual MAPKi with combined PD-1/CTLA-4 blockade in previously untreated patients with advanced melanoma. To address this issue, OS analyses in our study were conducted in subgroups from both cohorts sharing the same prognostic feature. Due to the disbalance in the frequency of some prognostic features, some of these subgroups are rather small. However, our study supports the practice that patients without poor prognostic features could benefit more from first-line PD-1 blockade than from dual MAPKi.
Conclusion
Our data show significantly better OS for advanced BRAF-mutant melanoma patients receiving PD-1 blockade as first-line therapy compared to patients receiving MAPKi in all subgroups. Limitations apply due to the retrospective nature of our study. Additionally, it should be acknowledged that other parameters apart from patient survival, such as symptom control, must be considered when making treatment decisions for treatment-naïve patients with advanced BRAFV600E/K-mutant melanoma. Our data from a large multicenter cohort provide additional information to guide the decision-making process until prospective clinical trials addressing the treatment sequence in BRAF-mutant melanoma are reported.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Giorgos Kyriakakis (First Department of Internal Medicine, Laikon General Hospital, National and Kapodistrian University of Athens, Greece) for provision of clinical data. We thank Christopher Shipp (The Natural and Medical Sciences Institute at the University of Tübingen, Reutlingen, Germany) for proofreading the manuscript.
Abbreviations
- BRAF
B-Raf proto-oncogene
- BRAFi
BRAF inhibitors
- CI
Confidence interval
- CTLA-4
Cytolytic T lymphocyte-associated antigen-4
- HR
Hazard ratio
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated protein kinase
- MAPKi
MAPK inhibitors
- MEK
MAPK/ERK kinase
- MEKi
MEK inhibitors
- NCT
National Clinical Trial
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed death 1 receptor
- PD-L1
Programmed death ligand 1
- ULN
Upper limit of normal
Author contributions
BS, AM, MHGF, CG, DS, CB and BW designed the study. BS, AM, MHGF and BW analyzed all data and prepared the manuscript. BS, MHGF, CG, JCH, EAR, AG, RG, KCK, EL, PTD, HG, GM, PAA, SMG, JM and BW contributed to patient data acquisition. All authors supported the preparation of the manuscript. All authors have read and approved the final manuscript.
Funding
No specific funding was received for this study.
Compliance with ethical standards
Conflict of interest
Bastian Schilling reports advisory roles for or has received honoraria from Pierre Fabre Pharmaceuticals, Incyte, Novartis, Roche, Bristol-Myers Squibb (BMS) and Merck Sharp & Dohme (MSD), research funding from BMS, Pierre Fabre Pharmaceuticals and MSD, and travel support from Novartis, Roche, BMS, Pierre Fabre Pharmaceuticals and Amgen. Christoffer Gebhardt reports advisory roles for or has received honoraria from Pierre Fabre Pharmaceuticals, Beiersdorf, BMS, MSD, Novartis, Roche and Sysmex, and travel support from BMS, MSD, Novartis and Roche. Jessica C. Hassel reports advisory roles for Pierre Fabre and Sanofi, and honoraria from BMS, MSD, Novartis, Roche and Pfizer. Anja Gesierich reports advisory roles for BMS, MSD, Novartis, Roche and Pfizer, has received honoraria from MSD and BMS, and travel support from BMS, MSD, Novartis and Roche. Ralf Gutzmer reports advisory roles for Roche, BMS, MSD, Amgen, Almirall, Leo, Pfizer, Novartis, GlaxoSmithKline (GSK), Incyte, Merck and Pierre-Fabre, has received honoraria from Roche, BMS, MSD, GSK, Novartis, Merck, Almirall, Amgen, Galderma, Astra-Zeneca and Pierre-Fabre, research funding from Novartis, Johnson & Johnson and Pfizer, honoraria from Roche, BMS, MSD, GSK, Novartis, Merck, Almirall, Amgen, Galderma, Astra-Zeneca and Pierre-Fabre, and travel support from Roche, BMS, MSD, GSK, Novartis, Merck, Almirall, Amgen, Galderma, Astra-Zeneca and Pierre-Fabre. Elisabeth Livingstone reports advisory roles for Roche, BMS, Novartis and Actelion, has received honoraria from Roche, BMS, MSD, Amgen, Novartis, Boehringer-Ingelheim (BI) and medac, and travel support from Roche, BMS, MSD, Amgen, Novartis, BI and medac. Panagiotis T. Diamantopoulos reports honoraria from Novartis and Roche, and travel support from Roche, Novartis, Janssen and Amgen. Helen Gogas reports honoraria from BMS, Roche, MSD, Novartis, Amgen and Pierre Fabre, and research funding from BMS, Roche, MSD and Novartis. Simone M. Goldinger reports advisory roles for Roche, Novartis, MSD and BMS, and has received travel support from Roche, Novartis, MSD and BMS. Joanna Mangana reports advisory roles for Merck and Pfizer, has received research funding from BMS, and travel support from MSD. Claus Garbe reports advisory roles for Amgen, BMS, MSD, Philogen, Roche and LEO Pharma, has received honoraria from Novartis, Amgen, BMS, MSD, Philogen, Roche and LEO Pharma, and research funding from Novartis, BMS and Roche. Dirk Schadendorf has received honoraria from Roche Pharma, BMS, Novartis, Merck Serono, MSD, Amgen, Incyte, LEO, Pfizer, Pierre Fabre, Array, Astra Zeneca, Regeneron, Philogen, Sanofi and Mologen, and research funding from Novartis and BMS. Christian Blank reports advisory roles for BMS, MSD, Roche, Novartis, GSK, Lilly, Pfizer and Genmab, and has received research funding from Novartis and BMS. Benjamin Weide has received honoraria from Merck/MSD, BMS, Philogen, Curevac, Roche, Novartis and Amgen, research funding from MSD and BMS, and travel support from MSD and BMS. All other authors declare no conflicts of interest.
Ethical approval
Retrospective collection of anonymous patient data was performed during routine clinical care. This study was approved by the Ethics Committee, University of Tübingen (approvals 524/2012BO2 and 234/2015BO2).
Informed consent
For anonymous publication of retrospective data acquired during routine clinical care, individual informed consents were not obtained.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bastian Schilling, Alexander Martens and Marnix H. Geukes Foppen contributed equally.
References
- 1.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long GV, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 7.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long GV, et al. Long-term outcomes in patients with BRAF V600–mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2018;36(7):667–673. doi: 10.1200/JCO.2017.74.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weide B, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbognin L, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10(6):e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daud AI, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaper-Gerhardt K, et al. PD-L1 status does not predict the outcome of BRAF inhibitor therapy in metastatic melanoma. Eur J Cancer. 2018;88:67–76. doi: 10.1016/j.ejca.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Wongchenko MJ, et al. Association of programmed death ligand-1 (PD-L1) expression with treatment outcomes in patients with BRAF mutation-positive melanoma treated with vemurafenib or cobimetinib combined with vemurafenib. Pigment Cell Melanoma Res. 2018;31(4):516–522. doi: 10.1111/pcmr.12670. [DOI] [PubMed] [Google Scholar]
- 14.Massi D, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann Oncol. 2015;26(9):1980–1987. doi: 10.1093/annonc/mdv255. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DB, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-PD-1 before and after BRAF inhibition. J Immunother. 2017;40(1):31–35. doi: 10.1097/CJI.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schadendorf D, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. 2017;82:45–55. doi: 10.1016/j.ejca.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Atkins MB. What is optimal first-line treatment of unresectable or advanced BRAF-mutant melanoma? Clin Adv Hematol Oncol. 2016;14(6):417–419. [PubMed] [Google Scholar]
- 20.Dummer R, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–v132. doi: 10.1093/annonc/mdv297. [DOI] [PubMed] [Google Scholar]
- 21.Silva IP, Long GV. Systemic therapy in advanced melanoma: integrating targeted therapy and immunotherapy into clinical practice. Curr Opin Oncol. 2017;29(6):484–492. doi: 10.1097/CCO.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 22.Ascierto PA, et al. The impact of patient characteristics and disease-specific factors on first-line treatment decisions for BRAF-mutated melanoma: results from a European expert panel study. Melanoma Res. 2018;28(4):333–340. doi: 10.1097/CMR.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dréno B, et al. Quality-of-life (QOL) assessment in patients (pts) with metastatic melanoma receiving vemurafenib (V) and cobimetinib (C) J Clin Oncol. 2015;33(15_suppl):9021–9021. doi: 10.1200/jco.2015.33.15_suppl.9021. [DOI] [Google Scholar]
- 24.Van Allen EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ascierto PA, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti-LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti-PD-1/PD-L1 therapy. J Clin Oncol. 2017;35(15_suppl):9520–9520. doi: 10.1200/JCO.2017.35.15_suppl.9520. [DOI] [Google Scholar]
- 26.Long GV, et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J Clin Oncol. 2018;36(15_suppl):108–108. doi: 10.1200/JCO.2018.36.15_suppl.108. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.